Abstract
Objective:
In this study, an experiment was conducted to examine whether noise exposure produced acute changes in cardiovascular responses, and whether these responses differed based on psycho-acoustic parameters to noises of low to high intensity.
Methods:
Thirty healthy subjects were enrolled. Three industrial noises were binaurally presented with a supra-aural earphone. The sound levels of noise were <55, 75, and 90 dB. Each noise was continued for 20 min and the electrocardiogram was simultaneously recorded.
Results:
The results showed a statistically significant increase in systolic blood pressure (SBP) at the 90 dB sound level. The study estimated a blood pressure increase of 0.85 mmHg/10 dB and 0.71 mmHg/10 dB in SBP and diastolic blood pressure (DBP), respectively. These results suggest that exposure to noise, particularly high-frequency noise, negatively impacts blood pressure. The tonality and fluctuation strength of noise especially impacts systolic blood pressure.
Conclusions:
The psycho-acoustic parameters of noise should be considered when evaluating the impact of noise exposure.
Keywords: Blood pressure, Heart rate, Hypertension, Noise, Psycho-acoustic parameters
1. Introduction
Noise is a persistent environmental problem, and because it has so many sources, including modes of transport, industry and neighbors, it is a prominent feature of the environment. Noise exposure is associated with a number of health effects which manifest in psychosocial responses such as annoyance, disturbance of daily activities, and reductions in sleep and job performance; as well as physiological responses such as hearing loss, hypertension, and ischemic heart disease1,2).
Presently, the only noise-induced occupational disease with sufficient supporting evidence is noise-induced hearing loss. The role of long-term noise exposure in hearing loss has long been recognized, and research has progressed to identifying dose-response relationships. However, there is less agreement on the non-auditory effects of noise, and concern about such effects is a relatively recent development. Potential effects include impaired performance, detrimental physiological responses, annoyance, etc. Environmental noise causes subjective discomfort, which is reported as noise annoyance3,4). Noise annoyance, one of the negative effects of noise, receives a great deal of attention from scientists. Annoyance is one of the most common negative effects of noise, and it has been proposed as an indicator of other adverse noise effects, although no empirical evidence or theoretical underpinning for the latter suggestion has been provided5). Noise level and noise annoyance have also both been shown to be associated with cardiovascular disorders6,7).
Cardiovascular disease is unequivocally recognized as the leading cause of mortality in most developed countries. Environmental noise exposure (sound level) also has physiological health effects, of which high blood pressure and ischemic heart diseases are the most investigated8-11). In addition to the direct pathways to and from the cerebral cortex, a variety of indirect connections travel from the inner ear to the brain centers that control the body's physiological, emotional, and behavioral responses. Importantly, recent research has indicated that chronic exposure to noise is associated with increased risk of cardiovascular diseases such as coronary heart disease and hypertension, especially in occupational settings12-15). In addition, studies in which industrial noise was the source of experimental noise exposure revealed increases primarily in the exposed subjects' norepinephrine levels and adrenaline levels16-18). The general stress model is the proposed biological mechanism explaining the physiological dysfunction and potential long-term health effects caused by chronic noise exposure.
In experimental studies, the stress mechanism hypothesis has been tested using short-term exposure to noise and biological stress-response measures such as blood pressure, heart rate variability, salivary amylase, and cortisol. The results of these studies have been mixed. While Lee et al.19) found no changes in blood pressure across noise-exposed groups, Lusk et al.20) and Zamanian et al.21) observed increases in blood pressure as a result of noise exposure.
Many occupational studies have suggested that individuals chronically exposed to continuous noise of at least 85 dB have higher blood pressure than those not exposed to such noise22). Noise assessments in work environments have primarily been directed toward the risk of hearing damage. This is also the purpose behind the use of the equivalent A-weighted dB sound level (dBA) as an exposure measure, since this has been found to be a reasonably good predictor of the risk of hearing damage. However, noise may also be a serious problem in work environments where it does not constitute a risk of hearing damage, but rather is a source of annoyance. In these cases, the use of dBA has rather weak support from empirical studies. One problem is that annoyance and the risk of hearing damage have different relationships with physical noise parameters such as sound level, frequency, intermittency, exposure time, etc. Some of these parameters may have harmful effects, such as increasing annoyance, without affecting the risk of hearing damage23). The presence of strong, low-frequency noise components is one such example24,25). In addition, noise annoyance is influenced by many factors other than the physical characteristics of the noise. The same type of noise obviously causes different degrees of annoyance in different situations. Because the causative characteristics of noise annoyance are not well-defined, there is a lack of established methods for the evaluation of annoying noise in work environments. For instance, several studies indicate that tonal components should be taken into account when assessing the likelihood of noise annoyance26).
The quantitative relationship between a physical noise stimulus and the psychological response is important. A fairly high correlation can be seen between an evaluation index based on energy, such as LAeq, and the psychological response to the noise27). A broadband noise that includes a prominent tone component, however, is perceived to be noisier than the broadband noise only, even though the two have the same LAeq value. That is, when a broadband noise includes a prominent tone component, predictions made using an energy-based evaluation index will likely underestimate psychological responses. Therefore, some studies have not been able to substantially correlate an evaluation index such as LAeq and the psychological response28). A comprehensive model is needed to ensure that research on sound includes the critical variables that make noise a significant source of human stress. A weak relationship between annoyance and the equivalent noise level (Leq) has also been found in other studies29,30). However, it is possible that different acoustic factors not fully detected by the Leq are also important for noise perception and annoyance. When emitted from technical products, noises with tonal components, howling sounds, and modulated signals are often the cause of complaints. The perception and evaluation of sound events containing such components have become increasingly important, and how they influence physiological responses has become an interesting question.
The aim of this study was to examine: (1) whether noise exposure produced acute changes in cardiovascular responses; and (2) whether these responses differed based on psycho-acoustic parameters. Using a panel study design, in which subjects were monitored on multiple visits during no-noise and noise exposure scenarios, the changes in autonomic cardiac function were evaluated by measuring blood pressure and heart rate.
2. Subjects and Methods
This study investigated the effect of industrial noise on cardiovascular function. Blood pressure and heart rate were measured in 30 healthy young adults who listened to three types of noise, with each type of noise synchronized with respect to intensity variations.
2.1 Subjects
To test hearing acuity, a pure tone audiogram was performed by a trained health care professional for each subject at the beginning of the study, using an audiometer which produces sounds at various volumes and frequencies. Subjects listened to the sounds through headphones and responded by pressing a button when sounds are heard. All subjects were found to have normal hearing acuity, with the threshold of less than 15 dB for frequencies of 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz.
Thirty healthy college students (15 male, 15 female) were enrolled in this study. Mean age of the subjects was 20.8±0.6 years. All subjects reported no medical history of neurological disease, hypertension or heart disease. The subjects received financial compensation for their participation in the experiment. The study procedures were approved by the Central Regional Research Ethics Center (Taiwan), and informed consent was obtained from all subjects.
2.2 Presentation of noise
Three different kinds of sound were recorded at industrial sites. The designations A, B, and C refer to the sound recorded at a distance of about 1 meter away from a diesel generator, piston pump, and automatic stamping press, respectively. Noise A was characterized by high frequency sounds, noise B by lower frequencies, and noise C had an average value of low, medium, and high frequencies. Recording and playback were first carried out using a 01dB Symphonie system (ACOEM Group, France). Sections of 120 s of the recorded noise were then played back using a continuous loop. The test sounds were generated using a built-in sound adapter on a personal computer, with a 22-kHz sound output and a 16-bit digital-to-analogue conversion.
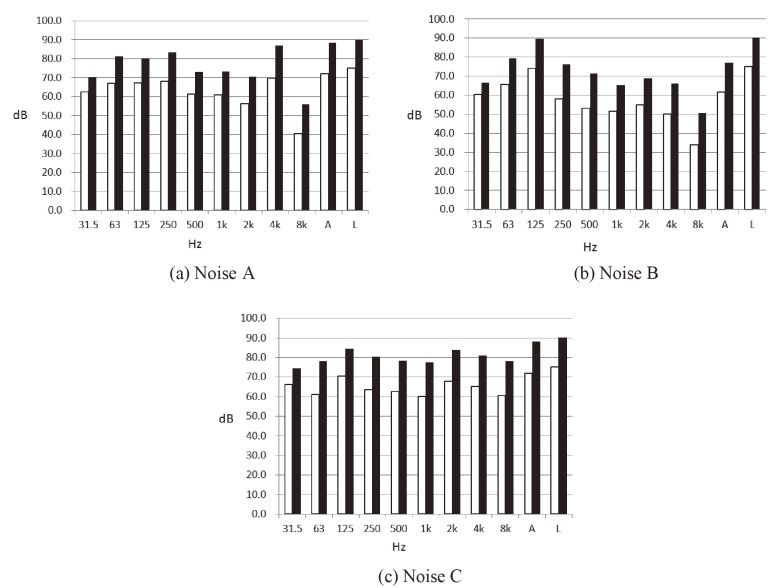
In the experimental design, subjects were exposed to a 20 min sound test sample while sitting in front of a desk and staring at the computer screen. The sounds were presented to both ears using a pair of TDH-39 headphones (Telephonics®, USA). Each kind of sound test intensity was reproduced at an energy-equivalent continuous sound level (Leq) of about 75 dB and 90 dB via headphones. Random factorial combinations of noise level (75 dB, 90 dB) and noise type (A, B, C) variables constituted the six experimental sessions, i.e. 71.9 dBA, 88.5 dBA, 61.8 dBA, 77.3 dBA, 72.1 dBA, 88.0 dBA. Fig. 1 shows the sound frequencies of A, B, and C noises at the intensity levels of 75 dB and 90 dB. In this study, sound measurements made using A-weighting are shown with dBA to show that the information is 'A' weighted decibels, while measurements made without frequency weighting are shown with dB to show that the information is un-weighted (or L-weighted) decibels.
Fig. 1.
Sound frequencies of noises A, B, and C at 75 dB and 90 dB. □ A-weighting, ■ L-weighting. "A" and "L" columns represent the total A-weighted and L-weighted sound level, respectively.
2.3 Physiological response measurements
The subjects were seated in a quiet (<55 dB or 43 dBA) room, which was air-conditioned to minimize any influence of hot temperatures or high humidity. Subjects were requested to sit quietly for 10 min in order to adapt to the test room. Resting values of systolic and diastolic blood pressure and heart rate were assessed while subjects were in a seated position with the left arm kept at heart level during measurement, using a validated and automated manometer, an HEM-1000 (OMRON Corporation, Japan). During each session, measurements were taken immediately before noise-exposure (pre-test), and immediately following noise exposure (i.e., beginning of the "post-test" period). A 24-min break was allowed between sessions (Table 1). Each subject was assigned a random presentation order for the six sessions, and each subject completed all sessions in two separate days.
Table 1.
Study protocol and sampling scheme
| Time Period | Adaption 10 min | Pre-Test 2 min | During 20 min | Post-Test 2 min | Resting 10 min | |
|---|---|---|---|---|---|---|
| Statistical analysis was performed by comparing the differences in cardiovascular parameters before (pre-test) and after noise exposure (post-test). V indicates that a cardiovascular measurement was taken. | ||||||
| Noise exposure scenario | ||||||
| Noise A (75 dB) | No noise | No noise | Stimulus | No noise | No noise | |
| Noise A (90 dB) | No noise | No noise | Stimulus | No noise | No noise | |
| Noise B (75 dB) | No noise | No noise | Stimulus | No noise | No noise | |
| Noise B (90 dB) | No noise | No noise | Stimulus | No noise | No noise | |
| Noise C (75 dB) | No noise | No noise | Stimulus | No noise | No noise | |
| Noise C (90 dB) | No noise | No noise | Stimulus | No noise | No noise | |
| Cardiovascular sampling | ||||||
| SBP | V | V | ||||
| DBP | V | V | ||||
| HR | V | V | ||||
2.4 Psycho-acoustic analysis
Many factors come into play in the sound quality evaluation process. Traditional objective measuring and analysis methods such as A-weighted sound pressure and FFT analysis are not sufficient for analyzing sound. Loudness is the most important tool for objectively determining sound quality parameters, or metrics. It attempts to understand how the human ear experiences sounds by properly weighting the different parts of the sound signal. In general, sharpness is increased by adding higher frequency content, and decreased by adding lower frequency content. Sharpness is a measure of the high frequency content of a sound, and the greater the proportion of high frequencies, the 'sharper' the sound. Roughness is a complex effect which quantifies the subjective perception of the rapid amplitude modulation of a sound. Fluctuation strength is caused by signal variations with very low modulation frequencies or amplitudes, and is generally perceived as an irritating property. Tone-to-noise ratio is a measure describing the amount of pure tones in the signal. Prominence ratio is a description of the amount of noise in a critical band in relation to the noise in the adjacent bands. The twin metrics of prominence ratio and tone-to-noise ratio can be used to quantify the presence of distinctly audible tones in a sound signal.
The analysis of the psycho-acoustic parameters of loudness, sharpness, roughness, fluctuation strength, tone-to-noise ratio, and prominence ratio were carried out at the Institute of Labor, Occupational Safety and Health in Taiwan. The parameters were analyzed from 30-s long sound sequences recorded in an industrial factory, using a B&K 4165 microphone (Brüel & Kjær Company, Denmark) positioned on a dummy head. The calculations were carried out using a Sound Quality Head and Torso Simulator 4100 (Brüel & Kjær Company) and Sound Quality Program Type 7698 (Brüel & Kjær Company). Loudness was calculated according to the standardized method described in ISO 532B31)/DIN 45 63132), while other sound quality parameters could be determined based on the Zwicker loudness calculation. However, both the prominence ratio and the tone-to-noise ratio were calculated according to ANSI S1.13-199533). Table 2 shows the psycho-acoustic parameters of the three noises examined in the study.
Table 2.
Psycho-acoustic parameters of noises A, B, and C.
| Psycho-acoustic parameter | A noise | B noise | C noise |
|---|---|---|---|
| Loudness (sone) | 53.2 | 38.4 | 60.5 |
| Sharpness (acum) | 1.71 | 1.42 | 2.25 |
| Roughness (asper) | 0.475 | 0.469 | 0.752 |
| Fluctuation Strength (vacil) | 1.83 | 1.59 | 2.33 |
| Tone-to-Noise Ratio (dB) | 4.43 | <0.1 | <0.1 |
| Prominence Ratio (dB) | 4.2 | <0.1 | <0.1 |
2.5 Statistical analysis
We analyzed two periods: (1) the 2 min period immediately preceding noise exposure (pre-test); and (2) the 2 min period immediately following noise exposure (post-test, refer to Table 1). The descriptive statistic is presented as mean values±standard deviation (SD) for numeric variables. Parametric differences between the groups were tested using Student's t-test and one-way ANOVA. Pearson correlation analysis was performed to test the association between noise-level variables and blood pressure and heart rate. Based on the results of the univariate analyses, variables significantly related to blood pressure and heart rate were included in a multiple linear regression model. A p-value <.05 was accepted as significant. The statistical software IBM® SPSS 16 (IBM Corporation, USA) was used for all data analyses.
3. Results
Table 3 shows the demographic characteristics of the 30 subjects. Significant differences in the mean values for systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were identified between male and female young adults. Men are generally at greater risk for cardiovascular and renal disease than age-matched, premenopausal women. As shown in Table 3, blood pressure was higher in males than in females at similar ages, while heart rate was statistically and significantly higher in females than in males. Three subjects felt slight discomfort with the loudest noises, but all participants completed the entire experimental procedure without any intolerability.
Table 3.
Pre-test cardiovascular measurements.
| Characteristic | Male | Female | Total | p-value |
|---|---|---|---|---|
| All results are presented as mean value ± standard deviation * p-value < 0.05 when males and females are compared using Student's t-test. | ||||
| No. of subjects | 15 | 15 | 30 | |
| Age (yr) | 20.9±0.6 | 20.8±0.5 | 20.8±0.6 | 0.459 |
| Systolic blood pressure (mmHg) | 119.4±11.6 | 106.3±10.4 | 112.9±12.8 | <0.001* |
| Diastolic blood pressure (mmHg) | 69.0±9.9 | 65.4±8.7 | 67.2±9.5 | 0.011* |
| Heart rate (b/min) | 72.9±10.2 | 82.8±11.6 | 77.9±11.9 | <0.001* |
All 30 subjects completed all six sessions, with a total of 60 person-days of monitoring (30 subjects over 2 days each). A total of 360 blood pressure and heart rate measurements were obtained, including measurements taken before and after noise exposure. For each individual, a difference value was calculated by subtracting the pre-test sample result from the post-test sample result for all of the measured cardiovascular parameters. The average SBP, DBP, and HR measurements showed a difference between the noises of different intensities (Table 4). Comparisons between background sound (<55 dB, pre-test) and stimulus sounds (75 dB or 90 dB, post-test) revealed that the difference values for both SBP and DBP were increased when subjects were exposed to greater noise (SBP: 3.9±0.9, DBP: 2.7±0.7 at 90dB). Student's t-test analysis revealed a statistically significant difference (p=0.040) in DBP but not SBP at 75 dB. However, at 90 dB both SBP (p<0.001) and DBP (p<0.001) were significantly increased. HR was not significantly altered at either noise level.
Table 4.
Pre- and post-test differences in cardiovascular parameters for all noises.
| Difference | 75dB | 90dB | |
|---|---|---|---|
| All values are presented as mean (standard error mean). SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate. * p-value < 0.05 compared to exposure to background noise (< 55 dB) using Student's t-test. | |||
| SBP | |||
| Male | 0.9 (1.5) | 4.6 (1.3) * | |
| Female | 1.6 (1.2) | 3.1 (1.1) * | |
| Total | 1.3 (1.0) | 3.9 (0.9) * | |
| DBP | |||
| Male | 1.6 (1.3) | 4.2 (1.0) * | |
| Female | 1.5 (0.8) | 1.2 (0.9) | |
| Total | 1.6 (0.8) * | 2.7 (0.7) * | |
| HR | |||
| Male | 0.4 (0.8) | -0.6 (0.8) | |
| Female | -1.1 (0.8) | -0.4 (0.9) | |
| Total | -0.4 (0.6) | -0.5 (0.6) | |
A simple linear regression analysis of the 360 blood pressure and heart rate measurements taken in 3 noise levels estimated a 0.85 mmHg/10 dB increase in SBP (y=0.08549x-0.1396, r2=0.0541, p<0.001), and a 0.71 mmHg/10 dB increase in DBP (y=0.07141x-0.0017, r2=0.0541, p<0.001), where the y value represents the change of blood pressure and x represents the dB level increments. A Pearson correlation analysis was then performed to study the correlation between sound level (dB) and blood pressure or heart rate. A positive correlation was observed between noise level and SBP and DBP levels, with a statistically significant increase in the blood pressure level.
Three types of noise (A, B, and C) were tested at 75 dB and 90 dB, and correlations between the noise types and energy levels and cardiac parameters were tested using one-way ANOVA using data from all experimental subjects (Table 5). All three types of noise provoked a significant increase in SBP at 90 dB; however, at 75 dB only noise A had a significant effect. DBP was considerably less sensitive to noise exposure, and a significant increase was only seen following exposure to noise C at 90 dB. None of the noises had a significant effect on HR.
Table 5.
Difference in cardiovascular parameters between pre- and post-test for different types of noise.
| A | B | C | |||||
|---|---|---|---|---|---|---|---|
| 75dB | 90dB | 75dB | 90dB | 75dB | 90dB | ||
| All values are presented as mean (standard error mean). SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate * p-value < 0.05 compared to exposure to background noise (< 55 dB) using one-way ANOVA. | |||||||
| Total (N=30) | |||||||
| SBP | 3.6 (1.7) * | 4.3 (1.6) * | -0.6 (1.6) | 3.5 (1.3) * | 0.8 (1.7) | 3.9 (1.6) * | |
| DBP | 2.7 (1.4) | 1.8 (1.2) | 1.3 (1.3) | 2.5 (1.4) | 0.8 (1.2) | 3.9 (1.0) * | |
| HR | 0.6 (1.1) | -0.2 (1.4) | -0.4 (0.7) | -0.4 (0.9) | -1.5 (1.0) | -0.8 (0.8) | |
| Male (N=15) | |||||||
| SBP | 2.4 (2.3) | 4.6 (2.8) | -1.1 (2.5) | 4.6 (2.0) * | 1.3 (3.1) | 4.6 (2.4) | |
| DBP | 4.5 (2.4) | 3.3 (1.8) | 0.7 (1.9) | 4.1 (1.9) * | -0.3 (2.2) | 5.2 (1.6) * | |
| HR | 2.1 (1.6) | 1.0 (1.7) | -0.7 (1.1) | -2.2 (1.2) | -0.2 (1.5) | -0.5 (1.4) | |
| Female (N=15) | |||||||
| SBP | 4.8 (2.4) | 3.9 (1.7) * | -0.2 (2.0) | 2.3 (1.7) | 0.4 (1.4) | 3.2 (2.1) | |
| DBP | 0.9 (1.1) | 0.2 (1.4) | 1.8 (2.0) | 0.9 (1.9) | 1.8 (1.1) | 2.7 (1.2) * | |
| HR | -0.9 (1.5) | -1.5 (2.1) | -0.1 (1.0) | 1.5 (1.1) | -2.7 (1.4) | -1.0 (0.8) | |
Additionally, we identified differences in the sensitivity of men and women to different types of noise. Exposure to noise A at 90 dB significantly increased SBP in women, but not in men. Conversely, we found that 90 dB of noise B had a significant effect on men, and provoked significant increases in both SBP and DBP. However, both sexes exhibited a significant increase in DBP following exposure to noise C at 90 dB, and a marginally significant increase in SBP (men p=0.067; women p=0.167).
Discussion
The current study investigated the effects of noise exposure on cardiovascular parameters in 30 young adults. We found that various types of noise exposure were associated with small but significant increases in both in SBP and DBP. These results agree with the results of a meta-analysis conducted by Passchier-Vermeer34) evaluating 21 occupational studies, which reported increases in the mean SBP and DBP following noise exposure (3.9 mmHg and 1.7 mmHg, respectively). Here, we found that the both SBP and DBP increased significantly following exposure to 90 dB sound. Previously, studies by Chang et al.35) and Lusk et al.20) simultaneously measured SBP and DBP and noise levels as subjects went about their daily activities during work and non-work days. Chang et al. observed that a 5 dBA increase in the 24 h average environmental noise exposure significantly increased SBP and DPB in both young men and women. Lusk et al. found statistically significant associations between noise exposure and SBP and DBP. Here, we found that the general effect of noise on cardiovascular parameters seemed to be slightly greater in men than women. However, the results of this study showed that the intensity of the noise did not significantly affect HR.
When comparing the physiological influences of different experimental sound types, noise A appears to have greater effects on SBP than noises B and C, and significantly increases SBP even at 75 dB (Table 5). Analysis of psycho-acoustic parameters found that noise A is characterized by high tone-to-noise and prominence ratios (Table 2), suggesting that these attributes may have strong effects on SBP. In contrast, Noise C, which is characterized by large values in loudness, sharpness, roughness, and fluctuation strength had a greater impact on DBP. Noises with tonal components and howling sounds are often the cause of listener complaints. The perception and evaluation of sounds containing such components have become increasingly important, and the psycho-acoustic parameter tonality was introduced in order to quantify the perception of tonal content. These noise characteristics could be related to the level of stress.
Two possible mechanisms in the genesis of hypertension resulting from noise exposure are 1) sympatheticotonia-induced endothelial lesion, and 2) stress-induced hormone release. In the former model, acute noise exposure can activate an immediate sympathetic reflex, and then accelerate the development of structural changes in the cardiovascular system, ultimately resulting in sustained elevation of blood pressure36-39). Conversely, several studies have shown higher levels of stress hormones such as cortisol in relation to chronic noise exposure9). Stress-related activation of neuroendocrine pathways through high cortisol levels can give rise to metabolic syndrome, resulting in hypertension and other cardiovascular effects. Both pathways may quite reasonably lead to hypertension as a result of noise exposure.
In addition, previous studies suggest that noise-induced cardiovascular effects must be seen as the consequence of stress. Noise-related stress can arise in several ways. The physiological and psychological pathways might be distinguished. In the present study, acute cardiovascular changes were found. This marked a common physiologic stress reaction of short duration that occurred as a consequence of the activation of the autonomous nervous and hormone system. It appeared that the acute effects referred to were the same as the effects caused by an ordinary stress reaction.
The current study therefore established that the increase in the workers' systolic blood pressure was the result of exposure to noise. This is in agreement with the study by Lee et al.40) on workers at a metal manufacturing company in Busan, Korea, which established a significant relationship between chronic noise exposure and an increase in SBP. This study also agrees with the studies of Tomei et al.41) and Chang et al.42), and also confirmed an earlier study43) which found noise exposure did not significantly increase DBP. Nonetheless, several other studies12,44,45), which found that exposure to noise significantly increased diastolic blood pressure, can still be partially supported. The current study determined that depending on the sound characteristics, noise might increase DBP when some of the psycho-acoustic parameters, such as loudness, sharpness, roughness, and fluctuation strength, were high.
Using the blood pressure values presented here and the noise levels (dB), we calculated the blood pressure change (mm Hg) per 10 dB increase in noise level for SBP and DBP. Kempen et al.8) studied the influence of noise exposure on blood pressure for occupational and road and air traffic noise and estimated an increase of 0.51 mmHg/5 dBA. Dzhambov's systematic review with meta-analysis46) showed a 0.48 mmHg increase in SBP and a 0.22 mmHg increase in DBP per 5 dB increase in road traffic noise (RTN) at schools/kindergartens; and a 0.20 mmHg increase in SBP and a 0.03 mmHg in DBP per 5 dB increase in RTN at home.
With respect to the effect of sound frequency on physiological responses, our results showed that low-frequency noises had little effect on the subjects. The sound level with linear weighting of noises A, B, and C was 75 dB and 90 dB; however the A-weighting of noise A was 71.9 dBA and 88.5 dBA; noise B was 61.8 dBA and 77.3 dBA; and noise C was 72.1 dBA and 88.0 dBA. The larger the difference between the sound levels using L-weighting (i.e. unweighting or dB) and A-weighting (dBA), the lower the frequency. The difference in L-weighted vs. A-weighted sound levels for noise B was the greatest of the three noises (13.2 dB and 12.7 dB), demonstrating that noise B had the lowest frequency of the noises used in this experiment. Accordingly, noise B caused minimal physical responses in experimental subjects. In line with our results, Walker et al.47) tested the cardiovascular and stress responses to short-term exposures to low (31.5-125 Hz) and high (500-2000 Hz) frequency noises at 75 dBA, and found no significant (p<0.05) changes in blood pressure. These results suggest that noise exposure negatively impacts blood pressure, especially when high-frequency noises are involved. Thus, noise frequency should be considered when evaluating the potential cardiovascular health impacts of exposure.
The physiological and psychological effects of industrial noise are one of its most important areas of impact. Increases in workers' blood pressure and heart rate have been detected after exposure to high levels of noise. Our study suggests that workers should not be exposed to sound levels greater than 75 dB, as this noise level had the smallest effects on SBP, although diastolic blood pressures had the lowest values at about 90 dB. However, a previous experimental laboratory study found that blood pressure and heart rate increased after exposure to white noise greater than 110 dB48). The main limitation of our present study was the small number of subjects, and a study with a larger number of subjects is recommended to validate these results.
Conclusions
This study adds to our understanding of the acoustic characteristics that drive cardiovascular autonomic responses to noise exposure. Noise exposure may be associated with elevated blood pressure in young adults, and our results suggest that noise tonality and fluctuation strength may particularly impact SBP. In future studies, the dominant noise parameters should be considered when evaluating the effects of noise on cardiovascular health.
Acknowledgments: This study was funded by the Institute of Labor, Occupational Safety and Health and the Ministry of Science and Technology, Taiwan. The authors would like to thank Yen-Ju Lin, a student in our graduate program who made contributions to this study.
Conflicts of interest: None declared.
References
- 1). Passchier W, Knottnerus A, Albering H, Walda I. Public Health Impact of Large Airport. Rev Environ Health 2000; 15 (1-2): 83-96. [DOI] [PubMed] [Google Scholar]
- 2). Stansfeld S, Haines M, Brown B. Noise and health in the urban environment. Rev Environ Health 2000; 15 (1-2): 43-82. [DOI] [PubMed] [Google Scholar]
- 3). European Commission Working Group on Dose-Effect Relations. . Position paper on dose response relationships between transportation noise and annoyance. In: EU's future noise policy, WG2 - dose/effect. Luxembourg: Office for Official Publications of the European Communities; 2002. [Google Scholar]
- 4). Miedema HME, Oudshoorn CGM. Annoyance from transportation noise: relationships with exposure metrics DNL and DENL and their confidence intervals. Environ Health Perspect 2001; 109: 409-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Miedema HME. Annoyance caused by environmental noise: Element for evidence-based noise policies. Journal for Social Issues 2007; 63 (1): 41-57. [Google Scholar]
- 6). Ndrepepa A, Twardella D. Relationship between noise annoyance from road traffic noise and cardiovascular diseases: a meta-analysis. Noise Health 2011; 13 (52): 251-259. [DOI] [PubMed] [Google Scholar]
- 7). Babisch W. Transportation noise and cardiovascular risk: Updated review and synthesis of epidemiological studies indicate that the evidence has increased. Noise Health 2006; 8 (30): 1-29. [DOI] [PubMed] [Google Scholar]
- 8). van Kempen E, Babisch W. The quantitative relationship between road traffic noise and hypertension: a meta-analysis. J Hypertens 2012; 30: 1075-1086. [DOI] [PubMed] [Google Scholar]
- 9). Babisch W. 2011. Cardiovascular effects of noise. Noise Health 2011; 13 (52): 201-204. [DOI] [PubMed] [Google Scholar]
- 10). Basner M, Babisch W, Davis A, et al. Auditory and non-auditory effects of noise on health. Lancet 2014; 383 (9925): 1325-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Münzel T, Gori T, Babisch W, Basner M. Cardiovascular effects of environmental noise exposure. Eur Heart J 2014; 35 (13): 829-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). van Kempen EE, Kruize H, Boshuizen HC, Ameling CB, Staatsen BA, de Hollander AE. The association between noise exposure and blood pressure and ischemic heart disease: a meta-analysis. Environ Health Perspect 2002; 110: 307-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Jarup L, Babisch W, Houthuijs D, et al. ; HYENA study team.. Hypertension and exposure to noise near airports: the HYENA study. Environ Health Perspect 2008; 116 (3): 329-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Davies HW, Teschke K, Kennedy SM, Hodgson MR, Hertzman C, Demers PA. Occupational exposure to noise and mortality from acute myocardial infarction. Epidemiology 2005; 16: 25-32. [DOI] [PubMed] [Google Scholar]
- 15). Chang TY, Liu CS, Hwang BF, et al. Acute effects of noise exposure on 24-h ambulatory blood pressure in hypertensive adults. J Hypertens 2015; 33 (3): 507-514; discussion 514. [DOI] [PubMed] [Google Scholar]
- 16). Buczyński A, Kedziora J. The effect of acoustic stimulus of different continuity pattern on plasma concentration of catecholamines in humans during submaximal exercise. Acta Physiol Pol 1983; 34 (5-6): 595-600. [PubMed] [Google Scholar]
- 17). Tafalla RJ, Evans GW. Noise, physiology, and human performance: the potential role of effort. Journal of Occupational Health Psychology 1997; 2: 148-155. [DOI] [PubMed] [Google Scholar]
- 18). Fruhstorfer B, Pritsch MG, Fruhstorfer H, Sturm G, Wesemann W. Effects and after-effects of daytime noise load. In New advances in noise research, part II. Proceeding of the 5th international congress on Noise as a Public Health Problem in Stockholm 1988; 5: 81-91. [Google Scholar]
- 19). Lee GS, Chen ML, Wang GY. Evoked response of heart rate variability using short - duration white noise. Auton Neurosci 2010; 155 (1-2): 94-97. [DOI] [PubMed] [Google Scholar]
- 20). Lusk SL, Gillespie B, Hagerty BM, Ziemba RA. Acute effects of noise on blood pressure and heart rate. Arch Environ Health 2004; 59 (8): 392-399. [DOI] [PubMed] [Google Scholar]
- 21). Zamanian Z, Rostami R, Hasanzadeh J, Hashemi H. Investigation of the effect of occupational noise exposure on blood pressure and heart rate of steel industry workers. J Environ Public Health 2013; 2013: 256060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Lang T, Fouriaud C, Jacquinet-Salord MC. Length of occupational noise exposure and blood pressure. Int Arch Occup Environ Health 1992; 63: 369-372. [DOI] [PubMed] [Google Scholar]
- 23). Landstrom U. Exposure parameters involved in low frequency noise annoyance. Report from the Workshop "Assessing and controlling community low frequency with components", Copenhagen, Sweden 1995. [Google Scholar]
- 24). Kjellberg A, Goldstein M. Loudness assessment of band noise of varying bandwidth and spectral shape. An evaluation of various frequency weighting networks. Journal of Low Frequency Noise, Vibration and Active Control 1985; 4 (1): 12-26. [Google Scholar]
- 25). Persson K, Bjorkman M. Annoyance due to low frequency noise and the use of the dBA scale. J Sound Vibration 1988; 127: 491-497. [Google Scholar]
- 26). Saeki T, Yamaguchi S, Tamesue T, Oimatsu K. A method to predict psychological response of noisiness to an acoustical stimulus with a prominent frequency component. J Sound Vibration 2004; 277: 299-307. [Google Scholar]
- 27). Namba S, Kuwano S, Fastl H. On the judgment of loudness, noisiness and annoyance with actual and artificial noises. J Sound Vibration 1988; 127 (3): 457-465. [Google Scholar]
- 28). Namba S, Kuwano S, Kinoshita K, Kurakata K. Loudness and timbre of broad-band noise mixed with frequency-modulated sounds. Journal of Acoustical Society of Japan 1992; 13 (1): 49-58. [Google Scholar]
- 29). Miedema HME, Vos H. Noise sensitivity and reactions to noise and other environmental conditions. J. Acoust. Soc. Am. 2003; 113: 1492-1504. [DOI] [PubMed] [Google Scholar]
- 30). Job RFS. Community response to noise - A review of factors influencing the relationship between noise exposure and reaction. J. Acoust. Soc. Am. 1988; 83: 991-1001. [Google Scholar]
- 31).ISO 532, Acoustics - Method for calculating loudness level, 1975.
- 32).DIN 45631, Berechnung des Lautstärkepegels und der Lautheit aus dem Geräuschspektrum - Verfahren nach Zwicker (Calculation of loudness level and loudness from the sound spectrum - Zwicker method), 1991.
- 33).ANSI S1.13, American National Standard Methods for the Measurement of Sound Pressure Level in Air, 1995.
- 34).Passchier-Vermeer W. Noise and health. The Hague: Health Council of the Netherlands, 1993; publication no. A93/02E [Google Scholar]
- 35). Chang TY, Lai YA, Hsieh HH, Lai JS, Liu CS. Effects of environmental noise exposure on ambulatory blood pressure in young adults. Environ Res 2009; 109 (7): 900-905. [DOI] [PubMed] [Google Scholar]
- 36). Andrén L, Hansson L, Björkman M, Jonsson A. Noise as a contributory factor in the development of elevated arterial pressure. A study of the mechanisms by which noise may raise blood pressure in man. Acta Med Scand 1980; 207 (6): 493-498. [DOI] [PubMed] [Google Scholar]
- 37). Casto R, Nguyen T, Printz MP. Characterization of cardiovascular and behavioral responses to alerting stimuli in rats. Am J Physiol 1989; 256 (5 Pt 2): R1121-R1126. [DOI] [PubMed] [Google Scholar]
- 38). Baudrie V, Tulen JHM, Blanc J, Elghozi JL. Autonomic components of the cardiovascular responses to an acoustic startle stimulus in rats. J Auton Pharmacol 1997; 17: 303-309. [DOI] [PubMed] [Google Scholar]
- 39). Johsson A, Hansson L. Prolonged exposure to a stressful stimulus (noise) as a cause of raised blood-pressure in man. Lancet 1977; 1 (8002): 86-87. [DOI] [PubMed] [Google Scholar]
- 40). Lee JH, Kang W, Yaang SR, Choy N, Lee C. Cohort study for the effect of chronic noise exposure on blood pressure among male workers in Busan, Korea. Am J Ind Med 2009; 52: 509-517. [DOI] [PubMed] [Google Scholar]
- 41). Tomei F, Fantini S, Tomao E, Baccolo TP, Rosati MV. Hypertension and chronic exposure to noise. Arch Environ Health 2000; 55: 319-325. [DOI] [PubMed] [Google Scholar]
- 42). Chang TY, Jain RM, Wang CS, Chan CC. Effects of occupational noise exposure on blood pressure. J Occup Environ Med 2003; 45 (12): 1289-1296. [DOI] [PubMed] [Google Scholar]
- 43). Attarchi M, Dehghan F, Safakhah F, Nojomi M, Moham S. Effect of exposure to occupational noise and shift working on blood pressure in rubber manufacturing company workers. Ind Health 2012; 50: 205-213. [DOI] [PubMed] [Google Scholar]
- 44). Ising H, Braun C. Acute and chronic endocrine effects of noise: review of the research conducted at the Institute for water, soil and air hygiene. Noise Health 2000; 2: 7-24. [PubMed] [Google Scholar]
- 45). Motamedzade M, Ghazaiee S. Combined effects of noise and shift work on physiological parameters in a chemical industry workers. J Hamedan Univ Med Sci 2003; 10 (1): 39-46. [Google Scholar]
- 46). Dzhambov AM, Dimitrova DD. Children's blood pressure and its association with road traffic noise exposure - A systematic review with meta-analysis. Environmental Research 2017; 152: 244-255. [DOI] [PubMed] [Google Scholar]
- 47). Walker ED, Brammer A, Cherniack MG, Laden F, Cavallari JM. Cardiovascular and stress responses to short-term noise exposures-A panel study in healthy males. Environ Res 2016; 150: 391-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Holand S, Girard A, Laude D, Meyer-Bisch C, Elghozi JL. Effects of an auditory startle stimulus on blood pressure and heart rate in humans. J Hypertens 1999; 17: 1893-1897. [DOI] [PubMed] [Google Scholar]