Abstract
Objectives:
Rotating shift work has been reported to increase the risk of cardiovascular diseases. Vascular endothelial dysfunction and platelet activation are among the leading causes of thrombus formation in patients with myocardial infarction or stroke. Endothelial function has been shown to be impaired immediately after night-shift work; however, it is not known whether platelets are also activated. The aim of this study was to investigate the acute impact of night-shift work on platelet function.
Methods:
This observational study included 11 healthy medical staff members (seven women, median age 32 years). We examined each subject's platelet aggregation rates and the serum concentrations of eicosanoid mediators after night-shift work and on day-shift work without preceding night-shift work (baseline).
Results:
Platelet aggregation did not differ from baseline levels after night-shift work. However, serum cyclooxygenase (COX)-metabolized eicosanoid mediators, particularly thromboxane (Tx) B2 (a stable metabolite of TxA2 and the most important marker of platelet activation), were significantly higher after the night-shift than at baseline (median 65.3 vs 180.4 ng/ml).
Conclusions:
Although platelet aggregation did not increase, there was an increase in serum COX-metabolized eicosanoid mediators such as TxB2 in healthy medical staff after night-shift work. This platelet hypersensitivity may be one of the mechanisms underlying the significant association between night-shift work and adverse cardiovascular outcomes.
Keywords: Cyclooxygenase, Eicosanoids, Platelet aggregation, Thromboxane B2
Introduction
Various physiological functions of the human body exhibit circadian rhythms, including the autonomic nervous and endocrine systems1,2). Rotating shift work, which disrupts normal circadian rhythms, has recently been reported to be associated with an increased risk of cardiovascular and cerebrovascular diseases3-5). Platelet activation arises from various causes, including vascular endothelial dysfunction, and is one of the most important causes of thrombus formation in the vessel walls of patients with atherosclerotic diseases such as myocardial infarction or stroke. Previous investigations have demonstrated circadian variation in vascular endothelial function6-8), and have shown that night-shift work disturbs circadian rhythms and impairs brachial endothelial function and coronary microcirculation9-11), suggesting that these factors may play a role in the mechanisms underlying cardiovascular and cerebrovascular events associated with rotating shift work. Similarly, it has been suggested that there may be circadian variation in platelet function12,13). However, the acute impact of night-shift work on platelet function remains unclear. To determine whether platelet function was greater after night-shift work than at the beginning of day-shift work, we examined the platelet aggregation rate using both the conventional light transmission method and a highly sensitive light scattering method. We also measured the serum concentration of eicosanoid mediators (bioactive lipids derived from metabolites of 20-carbon arachidonic acid [AA]), which play important roles in platelet activation.
Subjects and Methods
This observational study compared the platelet aggregation rate, the serum concentration of eicosanoid mediators, hemodynamic data, and laboratory variables after night-shift work with those on day-shift work in healthy medical staff at the University of Tokyo Hospital, Tokyo, Japan. Written informed consent was obtained from all subjects, and the study protocol was approved by the institutional review board at the Graduate School of Medicine and Faculty of Medicine, University of Tokyo, complying with the principles of the World Medical Association's Declaration of Helsinki (IRB number 10767).
Recruitment and subjects
The subjects were recruited between July and December 2015, mainly through advertisements on notice boards of the Department of Clinical Laboratory, University of Tokyo Hospital. The inclusion criteria were as follows: (i) men and women aged >20 years, (ii) medical staff working at the University of Tokyo Hospital who were engaged in rotating shift work, (iii) never smoked, (iv) felt subjectively well, and (v) provided written informed consent. The following exclusion criteria were applied: (i) pregnancy or lactation; (ii) known history of (or currently receiving drugs for) cardiovascular disease, diabetes mellitus, hypertension, or a hematologic disease; (iii) history of any other severe disease, such as cancer or inflammation; or (iv) use of nonsteroidal anti-inflammatory drugs or antiallergic drugs within the 10 days prior to blood collection.
The required sample size was calculated using the free sample size-calculating software G*Power version 3.1.9.2 (University of Kiel, Germany). To achieve a power of 80%, statistical significance at the 0.05 level, and an effect size of 0.8, the required sample size was determined to be 15.
Study protocol
To assess the platelet aggregation rate and serum concentration of eicosanoid mediators, fasting venous blood was collected from each subject at the following times: (1) on day-shift work without previous night-shift work (baseline), and (2) after night-shift work (post-shift). Using paired data for each subject decreased the influence of variability between subjects and increased the likelihood of detecting differences between the baseline and post-shift conditions. The subjects were asked to refrain from drinking any beverages containing caffeine or glucose and eating any food after midnight on the test day. Blood was collected between 8:00 and 9:00 A.M. to minimize changes in platelet activation associated with circadian variations. The blood samples were carefully drawn from an antecubital vein using a 21-G butterfly needle. A 9-ml sample was collected in a vacuum tube containing 1 ml of 3.2% sodium citrate for platelet aggregation studies, and a 6-ml sample was collected in a vacuum tube containing thrombin and silica for serum eicosanoid analysis. Additional blood samples were also obtained to assess the complete blood count, prothrombin time, activated partial thromboplastin time, and the concentrations of fibrinogen, glucose, hemoglobin A1c, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides. Blood pressure and heart rate were measured immediately before each blood sample collection. The subjects were also asked about how long they slept the previous night and their shift work history.
Measurement of platelet aggregation rate
The citrated samples were centrifuged at 100 ×g for 15 min at 23°C to obtain platelet-rich plasma. The remaining samples were centrifuged at 1700 ×g for an additional 10 min at 23°C to isolate platelet-poor plasma. The interval between blood collection and aggregation assay was standardized to 1 h. Platelet aggregation rate was measured in the platelet-rich plasma at 37°C using a PA-200 aggregometer (Kowa, Tokyo, Japan). This simultaneously assessed platelet aggregation by two different methods: the light transmission method14), in which the magnitude of platelet aggregation is expressed as the percentage increase in the light transmission of platelet-rich plasma relative to that of platelet-poor plasma (%LT); and the light scattering method, which measures scattering from small, medium, and large platelet aggregates15,16). The platelet aggregation rate was assessed in the absence of any agonists as spontaneous platelet aggregation (SPA); or in the presence of adenosine 5'-diphosphate (ADP; MC Medical Co., Tokyo, Japan), collagen (Takeda Austria GmbH, Linz, Austria), or epinephrine (Arkray Factory Co., Shiga, Japan) for 11 min. Final concentrations of each agonist were 0.5 μM for ADP, 0.1 μg/ml for collagen, and 0.1 and 0.25 μM for epinephrine. We measured the maximum light intensity scattered by the small-sized aggregates (SMAX), the maximum of the sum of light intensities scattered by all three aggregate sizes (TMAX) over an 11-min period, the area under the light intensity curve of the small-sized aggregates (SAUC), and the sum of the areas under the light intensity curves for all three aggregate sizes (TAUC).
Measurement of eicosanoid mediators in serum
An internal standard method was used to measure the serum concentrations of eicosanoid mediators. A mixture containing each of the following 12 internal standards at 0.2 μg/ml was prepared: tetranor-prostaglandin (PG) E metabolite-d6 (tetranor-PGEM-d6), PGE2-d4, PGD2-d4, PGF2α-d4, 6-keto-PGF1α-d4, thromboxane (Tx) B2-d4, leukotriene (LT) C4-d5, LTB4-d4, 15 (S) -hydroxyeicosatetraenoic acid (HETE) -d8, 5 (S) -HETE-d8, and 12 (S) -HETE-d8, as well as 4.0 μg/ml of AA-d8. Then 200 μl of each serum sample was diluted with 1 ml of methanol, and 10 μl of the internal standard mixture was added. The samples were mixed well, centrifuged (20,000 ×g, 10 min, 4°C), and the supernatants were diluted with 3 ml of 0.03% formic acid in water. Solid-phase extraction of eicosanoids was performed using Oasis HLB 1 ml (10 mg) extraction cartridges (Waters) set on a vacuum manifold. The cartridges were preconditioned using 150 μl of methanol followed by 1 ml of 0.03% formic acid in water. The samples were loaded into the columns, and then serially washed with 1 ml of 0.03% formic acid in water, 15% methanol in 0.03% formic acid/water, and 3 ml of petroleum ether. The samples (analytes) were then eluted with 200 μl of methanol. The eluent was evaporated by vacuum evaporator (EC-95C3T, Sakuma, Tokyo, Japan) and reconstituted in 20 μl of methanol. The samples were analyzed by liquid chromatography coupled to a triple quadrupole mass spectrometer, LCMS-8050, equipped with an electrospray ion source (Shimadzu, Kyoto, Japan), into which 5 μl of the samples was injected for analysis. Chromatographic separation was achieved on a reversed-phase column (Kinetex C8, 2.1 × 150 mm, 2.6 μm; Phenomenex, Torrance, CA) at a flow rate of 0.4 ml/min. For mobile phases A and B, 0.1% formic acid in water and acetonitrile were used, respectively. The sample cooler and column oven temperatures were set at 5°C and 40°C, respectively. Methanol was used as the sample solvent. The mass spectrometer was operated in selected reaction monitoring mode. Nitrogen gas was used for nebulizer and drying gas, and argon gas was used for collision-induced dissociation. All compounds were quantified using internal standard methods. LabSolutions Ver. 5.80 and Insight (Shimadzu) software packages were used for data processing, which included peak detection, peak area integration, generation of a calibration curve, and evaluation of linearity and accuracy. The results of the automatic peak integration were manually inspected and corrected if necessary.
Statistical analysis
The distribution of each variable was tested for normality using the Shapiro-Wilk test. As previously reported, the platelet aggregation rate assessed by the light scattering method exhibited a skewed distribution16). The log-transformed values, which followed a normal distribution, were therefore used for statistical analysis. Values are expressed as median and interquartile range (IQR). Post-shift and baseline hemodynamic and laboratory data, platelet aggregation rates, and serum concentrations of eicosanoids in each subject were compared using paired t-tests or the Wilcoxon signed-rank test when the differences were not normally distributed. Correlations between the increase in serum TxB2 concentration after night-shift work and clinical and laboratory factors were determined by univariate linear regression analysis, or by Spearman's rank correlation when the data were not normally distributed. Independent t-tests were used to analyze the effect of gender on the increase in serum TxB2 concentrations after night-shift work. All hypothesis tests were two-tailed. Differences were considered statistically significant at p-value < 0.05. Data were analyzed with IBM SPSS statistics version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Fifteen subjects were selected among the 21 volunteers who expressed interest in participating. All completed the study; however, following the analysis four of the subjects reported that they had taken nonsteroidal anti-inflammatory drugs or antiallergic drugs within the 10 days preceding the study. We therefore excluded them from the analysis. Thus, the final study subjects comprised 11 medical staff (four men and seven women), consisting of 10 medical technologists and 1 physician. None was taking any medications. The median (IQR) age and body mass index was 32 years (29-42 years) and 21.4 kg/m2 (19.7-21.8 kg/m2). The median period they had worked night shifts was 8.3 years (IQR, 6-16 years). The median hours slept during night-shift work was significantly less than that at baseline (Table 1). There was no significant difference in any hemodynamic or laboratory variable between baseline and after night-shift work (Table 1 and 2).
Table 1.
Sleeping hours, hemodynamics, and laboratory data at baseline and after night-shift work (n = 11)
| Parameter | Baseline median (IQR) | After the shift median (IQR) | Post-shift—baseline mean (95% CI) | p-value |
|---|---|---|---|---|
| SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; LDL, low-density lipoprotein; HDL, high-density lipoprotein; IQR, interquartile range; post-shift—baseline, difference in parameter values between baseline and after night-shift work; CI, confidence interval. †Wilcoxon signed-rank test; all other variables were compared using paired t-tests. | ||||
| Sleep duration, hour | 6.0 (5.0-7.0) | 3.0 (4.0-7.0) | 3.0 (2.0 to 4.0) | <.001 |
| SBP, mmHg | 113.0 (100.0-121.0) | 112.0 (101.0-128.0) | 0.2 (-7.0 to 7.0) | .954 |
| DBP, mmHg | 75.0 (65.0-81.0) | 74.0 (69.0-77.0) | 0.6 (-5.2 to 6.5) | .814 |
| HR, beats/min | 73.0 (64.0-82.0) | 65.0 (62.0-72.0) | 6.3 (-0.2 to 12.7) | .055 |
| Fasting glucose, mg/dl | 90.0 (84.0-96.0) | 92.0 (91.0-94.0) | 3.0 (-5.0 to 7.0) | .246† |
| Total cholesterol, mg/dl | 188.0 (163.0-235.0) | 203.0 (167.0-214.0) | 1.0 (-21.0 to 18.0) | .722† |
| LDL cholesterol, mg/dl | 109.0 (99.0-130.0) | 118.0 (85.0-136.0) | 3.1 (-12.1 to 18.3) | .660 |
| HDL cholesterol, mg/dl | 70.3 (60.8-94.6) | 79.3 (60.9-93.8) | -1.8 (-5.6 to 2.1) | .336 |
| Triglycerides, mg/dl | 57.0 (52.0-86.0) | 54.0 (50.0-63.0) | 5.3 (-7.5 to 18.1) | .380 |
Table 2.
Complete blood count and coagulation tests at baseline and after night-shift work (n = 11)
| Parameter | Baseline median (IQR) | After the shift median (IQR) | Post-shift—baseline mean (95% CI) | p-value |
|---|---|---|---|---|
| PDW, platelet distribution width; MPV, mean platelet volume; IPF, immature platelet fraction; PT, prothrombin time; aPTT, activated partial thromboplastin time; IQR, interquartile range; post-shift—baseline, difference in parameter values between baseline and after night-shift work; CI, confidence interval. Baseline and after shift median values were compared using a paired t-test. | ||||
| Leukocyte count, ×109/L | 5.0 (4.6-5.7) | 4.9 (4.6-5.9) | -0.1 (-0.6 to 0.5) | .762 |
| Hematocrit, % | 42.6 (38.7-44.3) | 40.2 (37.7-42.4) | 1.0 (-0.2 to 2.1) | .101 |
| Reticulocyte, % | 1.21 (1.04-1.40) | 1.14 (1.12-1.40) | 0.0 (-0.1 to 0.1) | .927 |
| Platelet count, ×109/L | 220.0 (203.0-238.0) | 225.0 (208.0-232.0) | 0.0 (-1.1 to 1.0) | .970 |
| PDW, fL | 11.4 (10.7-12.6) | 11.8 (10.2-12.6) | 0.0 (-0.4 to 0.5) | .854 |
| MPV, fL | 10.3 (10.0-10.5) | 10.2 (9.5-10.7) | 0.0 (-0.2 to 0.3) | .688 |
| IPF, % | 4.2 (2.5-4.6) | 4.3 (2.6-4.9) | -0.2 (-0.5 to 0.2) | .359 |
| PT, % | 106.0 (101.0-115.0) | 102.0 (99.0-108.0) | 4.1 (-1.1 to 9.3) | .111 |
| aPTT, s | 32.9 (31.2-36.3) | 33.4 (31.9-36.9) | -0.5 (-1.4 to 0.4) | .225 |
| Fibrinogen, mg/dl | 253.0 (204.0-311.0) | 242.0 (202.0-308.0) | 8.6 (-18.4 to 35.7) | .493 |
Platelet aggregation rates
We initially considered platelet aggregation after stimulation by stirring without agonists (i.e., SPA). SPA is considered to have occurred if SMAX is ≥2.5×104 mV × count17). We examined the primary aggregation process, as shown by the SMAX and SAUC values, and the overall platelet aggregation rate shown by TMAX, TAUC, and %LT, and compared these parameters between baseline and after night-shift work. At baseline, SPA was negative in all subjects, apart from one with hysteromyoma (data not shown). SMAX and SAUC did not differ between baseline and after night-shift work (Supplementary Table 1). Similarly, there was no significant difference in TMAX, TAUC, or %LT between the two time points (Table 3 and Supplementary Table 1). There was also no difference in any of these values between baseline and after night-shift work when the samples were stimulated with ADP, collagen, or epinephrine (Table 3 and Supplementary Table 1). This suggests that night-shift work did not affect platelet aggregation rates.
Table 3.
Comparison of platelet aggregation rates estimated by the light transmission method between baseline and after night-shift work (n = 11)
| Agonist | Baseline %LT median (IQR) | Post-shift %LT median (IQR) | %LT (post-shift—baseline) mean (95% CI) | p-value |
|---|---|---|---|---|
| SPA, spontaneous platelet aggregation; ADP, adenosine 5'-diphosphate; %LT, percentage increase in the light transmission of platelet-rich plasma relative to that of platelet-poor plasma; IQR, interquartile range; post-shift—baseline, difference in parameter values between baseline and after night-shift work; CI, confidence interval. †Wilcoxon signed-rank test. SPA was assessed using a paired t-test. | ||||
| None (SPA) | 1 (1-2) | 1 (1-1) | 0.09 (-0.5 to 0.7) | .756 |
| ADP (0.5 μM) | 17 (7-20) | 13 (10-16) | -1 (-13 to 4) | .397† |
| Collagen (0.1 μg/ml) | 7 (6-16) | 7 (5-25) | -1 (-8 to 35) | .553† |
| Epinephrine (0.1 μM) | 6 (5-22) | 7 (6-10) | 0 (-56 to 3) | .766† |
| Epinephrine (0.25 μM) | 10 (6-73) | 13 (8-42) | -1 (-60 to 24) | 1.000† |
Serum eicosanoid mediators
Serum TxB2, which is dependent on cyclooxygenase (COX) activity, has been shown to be a useful platelet activation marker18). In this study, we performed multiplex quantitative analysis of serum eicosanoids, including COX metabolites, using liquid chromatography-tandem mass spectrometry (LC-MS/MS)19). The results for the major eicosanoid mediators with concentrations greater than 5 ng/ml are shown in Table 4; and the results for all of the tested mediators are shown in Supplementary Table 2. As expected, we detected relatively high baseline concentrations of the COX-metabolized eicosanoid mediators TxB2 and 12-HHT in the serum samples. Additionally, we found a high baseline concentration of the 12-lipoxygenase (LOX) -metabolized eicosanoid mediator 12-HETE, which is consistent with 12-LOX expression in platelets.
Table 4.
Comparison of serum concentrations of major eicosanoid mediators between baseline and after night-shift work (n = 11)
| Parameter | Baseline median (IQR) | After the shift median (IQR) | Post-shift—baseline mean (95% CI) | p-value |
|---|---|---|---|---|
| TxB2, thromboxane B2; 12-HHT, 12-hydroxyheptadecatrienoic acid; HETE, hydroxyeicosatetraenoic acid; IQR, interquartile range; post-shift—baseline, difference in parameter values between baseline and after night-shift work; CI, confidence interval. Baseline and after shift median values were compared using a paired t-test. | ||||
| TxB2, ng/ml | 65.3 (23.1-169.4) | 180.4 (93.5-258.9) | 99.7 (14.4 to 184.9) | .026 |
| 12-HHT, ng/ml | 44.4 (17.2-107.7) | 116.7 (67.1-156.9) | 57.3 (1.2 to 113.5) | .046 |
| 12-HETE, ng/ml | 24.0 (11.9-46.5) | 32.3 (20.9-80.5) | 28.2 (-3.5 to 60.1) | .075 |
| 15-HETE, ng/ml | 1.8 (1.0-5.3) | 4.9 (3.5-8.4) | 2.8 (0.1 to 5.4) | .043 |
| 11-HETE, ng/ml | 2.6 (0.9-5.9) | 6.9 (3.8-9.6) | 3.6 (0.6 to 6.6) | .025 |
Following night shift work, serum concentrations of TxB2 and 12-HHT were significantly elevated over baseline values (Fig. 1 and Table 4). This result contrasted with the lack of change in platelet aggregation reported above. Serum concentrations of other COX-metabolized eicosanoid mediators, such as PGE2, PGF2α, and PGD2 were also significantly higher after night-shift work than at baseline (Supplementary Table 2).
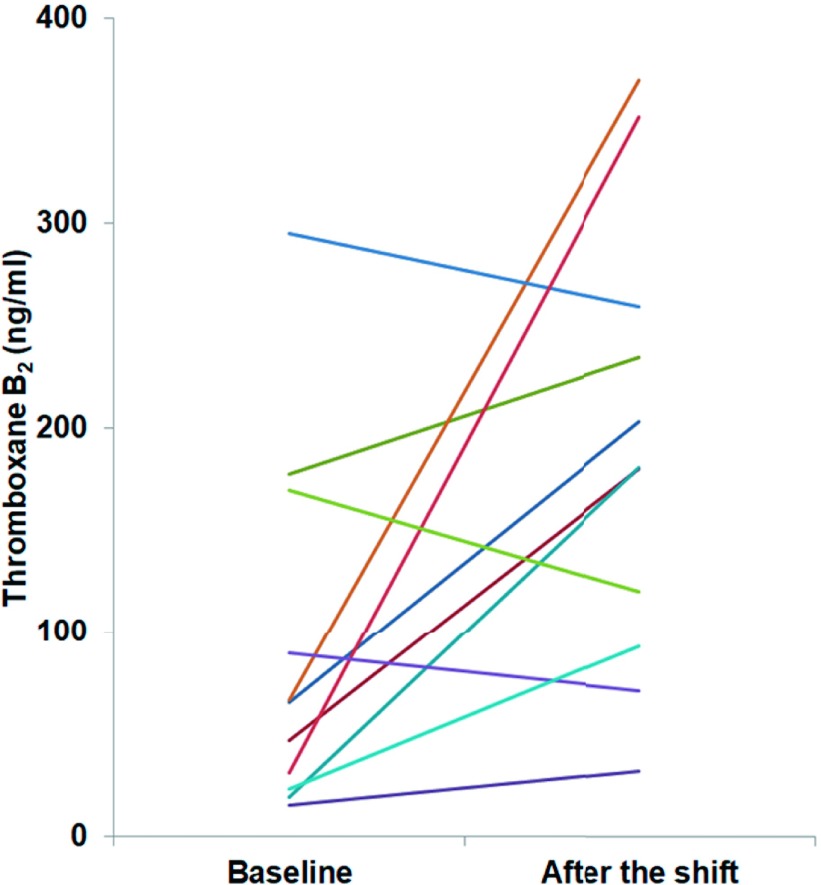
Fig. 1.
Serum thromboxane B2 concentrations for each subject at baseline and after night-shift work (n = 11)
There were no significant relationships between clinical and laboratory parameters and the increase in serum TxB2 after night-shift work compared with baseline levels (Table 5). Moreover, no significant difference in the increase in serum TxB2 concentrations after a night shift was observed between males and females (men vs. women, median (IQR) 115.8 (-19.7-281.1) vs. 56.5 (-18.2-137.5) ng/ml, p =.632). Other COX-metabolized eicosanoid mediators, 15-HETE and 11-HETE, were also found in serum (Table 4). Importantly, the concentrations of these mediators increased after night-shift work, as was the case with TxB2 and 12-HHT.
Table 5.
Linear regression analysis of the association between clinical and laboratory factors and the increase in serum thromboxane B2 level after night-shift work (n = 11)
| Parameter | r | p-value |
|---|---|---|
| BMI, body mass index; HbA1c, hemoglobin A1c; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; LDL, low-density lipoprotein; HDL, high-density lipoprotein; PT, prothrombin time; aPTT, activated partial thromboplastin time. †Spearman's rank correlation; all other samples were assessed using univariate linear regression analysis. | ||
| Age | -.234 | .488† |
| Duration of night-shift work | -.380 | .248 |
| BMI | -.350 | .291 |
| HbA1c | .318 | .341 |
| Sleep duration during shift | .483 | .133 |
| Decrease in sleep duration during shift compared to baseline | .053 | .877 |
| SBP at baseline | -.485 | .131 |
| DBP at baseline | -.558 | .074 |
| HR at baseline | .183 | .591 |
| Fasting glucose at baseline | .138 | .685† |
| LDL cholesterol at baseline | .105 | .760 |
| HDL cholesterol at baseline | .403 | .218 |
| Triglycerides at baseline | .082 | .811† |
| Leukocyte count at baseline | -.142 | .677 |
| Hematocrit at baseline | .237 | .482 |
| Platelet count at baseline | -.286 | .394 |
| PT at baseline | .369 | .264 |
| aPTT at baseline | .040 | .907 |
| Fibrinogen at baseline | -.211 | .534 |
Discussion
To our knowledge, this is the first report to show an acute impact of night-shift work on serum concentrations of eicosanoid mediators. Our study showed no increase in platelet aggregation rate after night-shift work, but found that the concentrations of COX-metabolized eicosanoid mediators in serum increased, primarily due to platelet activation elicited during blood coagulation. This change may partially explain the pathophysiology underlying the increased cardiovascular risk observed in shift workers.
Many epidemiological studies have sought to elucidate this pathophysiology20-22). Previous investigations9-11),23) have shown that impairment of vascular endothelial function is a major contributor to this increased risk. A properly functioning endothelium exhibits antiplatelet activity that prevents platelet aggregation24), whereas an impaired endothelium tends to cause pathologic thrombosis that can lead to myocardial infarction and stroke25). Flow-mediated dilation of the brachial artery, a marker of endothelial function, has been reported to be decreased after night-shift work10,23). However, it has not been established whether platelet activity increases after night-shift work. In this study, we demonstrated that night-shift work did indeed have an impact on platelet function.
First, we examined the effect of night-shift work on the rates of SPA and platelet aggregation induced by a low-dose agonist. SPA and small aggregate formation in low concentrations of ADP or epinephrine have been reported to be elevated in patients with arterial or venous thrombotic diseases such as anterior ischemic optic neuropathy, retinal vein occlusion26), or acute coronary syndrome27,28) due to platelet hypersensitivity resulting from platelet stimulation. Thus, rates of SPA and platelet aggregation induced by a low-dose agonist can be used to document platelet sensitivity to platelet stimuli ex vivo. In this study, these rates did not differ between baseline and after night-shift work. Contrary to expectations, these results indicated that platelet sensitivity was not increased after night-shift work when the platelet aggregation rate was used as an index. However, the subjects of this study were healthy, and it remains unclear whether the platelet aggregation rate would be further increased after night-shift work in patients with pathological conditions that impair endothelial function and increase baseline platelet aggregation rate, such as diabetes mellitus29) or hypertension30).
Second, we examined serum concentrations of eicosanoid mediators to evaluate platelet hypersensitivity resulting from the COX pathway. In activated platelets, AA is released from membrane phospholipids and oxygenated by COX-1 to produce pro-aggregatory eicosanoids (mainly TxA2). TxA2 is rapidly hydrolyzed to the biologically inactive TxB2. Serum TxB2 is mainly produced in the blood collection tube by platelets activated by thrombin, a product of the coagulation cascade. Therefore the concentration of serum TxB2 is thought to reflect the ability of platelets to synthesize TxA2 and ex-vivo responsiveness to thrombin31). In this study, serum concentrations of TxB2 and other COX-metabolized eicosanoid mediators were higher after night-shift work than at baseline, indicating that platelet sensitivity assessed using these indicators was enhanced after night-shift work. In contrast, no increase in the rate of platelet aggregation induced by a low-dose agonist was observed after night-shift work. In other words, there was a discrepancy between the synthesis of TxB2 by platelets activated by thrombin during blood coagulation, and the rate of platelet aggregation induced by a low-dose agonist. In this context, it should be noted that TxB2 biosynthesis and platelet aggregation were observed under different experimental conditions. We examined TxB2 biosynthesis in whole blood, containing white and red blood cells in addition to platelets, as well as thrombin produced during the coagulation process. Conversely, we examined platelet aggregation in isolated platelets and also in the presence of low-dose ADP, collagen, or epinephrine. The activation threshold for the former pathway may be lower than for the latter pathway. Furthermore, there are TxA2-dependent and TxA2-independent pathways associated with platelet aggregatory reactivity. Indeed, a discrepancy has been reported between ex vivo platelet aggregation and TxB2 production in aggregated platelets18), supported by reports that serum TxB2 and agonist-induced platelet aggregation did not correlate with each other in patients treated with aspirin18,32,33).
Nevertheless, this study revealed that platelet sensitivity assessed by serum TxB2 concentration increased after night-shift work. The measurement of TxB2 concentrations can be performed using commercial kits, and sampling serum specimens is not difficult in daily medical practice. Therefore, the serum level of TxB2 may be a useful marker of platelet activation in clinical settings.
What are the determinants of platelet hypersensitivity? Amir et al.10) investigated the influence of night-shift work on brachial artery endothelial function and demonstrated that the decrease in function was independently related to the number of sleeping hours during night-shift work and the length of shift work history. In the present study, we found that neither these factors nor other clinical and laboratory factors such as age, blood pressure, fasting glucose, and LDL cholesterol were associated with an increase in serum TxB2 concentrations after night-shift work (Table 5). Serum TxB2 levels decreased after night-shift work in three of the four subjects with the highest baseline levels of serum TxB2 (Fig. 1). Therefore, the unadulterated effects of night-shift work on serum TxB2 levels may not have been examined in subjects whose baseline serum TxB2 levels were already enhanced due to factors that were not investigated in this study. Night-shift work also induces fatigue through psychological or physical stressors. To examine the effect of night-shift work-induced fatigue on brachial artery endothelial function, previous studies had utilized either the self-reported shift difficulty score10) or plasma levels of epinephrine and norepinephrine11). Nevertheless, further investigations are required to assess the influence of these parameters on platelet function after night-shift work. Night-shift work has also been known to promote not only sleep restriction and fatigue but also disturbances in normal physiological circadian rhythms. Heart rate has been reported to exhibit circadian variation and to decrease during sleep, and then peak during the early morning34). Amir et al.10) reported that heart rates tended to be lower after night-shift work, suggesting that circadian misalignment had been induced. However, it is not clear whether circadian misalignment caused platelet hypersensitivity in that study. Further study is needed to simultaneously investigate platelet function alongside indicators such as body temperature that can be used monitor disturbances in the circadian rhythm.
In this study, we applied multiplex quantitative analysis using LC-MS/MS to serum eicosanoid mediators, allowing us to assay not only COX metabolites but also a broad range of eicosanoid mediators. Unexpectedly, in addition to TxB2 serum 15-HETE and 11-HETE were found to increase significantly after night-shift work. Because the serum samples were prepared by coagulation of whole blood, platelets were not necessarily the source of these mediators35,36), and their presence remains to be explained. However, given a recent report that plasma levels of 15-HETE and 11-HETE were significantly increased in acute coronary syndrome37), these mediators may make novel prothrombotic markers.
This study had several limitations. In accordance with previous studies, agonists were applied at 0.5 μM, 0.1 μg/ml, and 0.1 and 0.25 μM for ADP, collagen, and epinephrine, respectively27,28,38). However, agonist-induced platelet aggregation rates varied among the subjects. To examine whether platelet aggregation was truly activated by night-shift work, further studies that examine platelet aggregation using at least two concentrations of each agonist are required. Additionally, we determined that at least 15 subjects were required to examine the effect of shift work; unfortunately, four of our subjects were later excluded from the study. Moreover, among the major eicosanoid mediators, serum 12-HETE concentrations did not significantly differ after night-shift work in this study. Therefore, further studies with a larger population are needed to confirm the results of the present study. An investigation is also needed to determine whether platelet sensitivity is enhanced not only after night-shift work but also after day-shift work. In this study, we compared platelet function at the beginning of day-shift work and after night-shift work, and measured platelet function in all subjects between 8:00 and 9:00 A.M. to avoid the influence of circadian variation on platelet function. However, platelet aggregation is reported to be decreased in the evening after day-shift work is ended, compared with morning levels12,13). A subject's platelet function may differ between the beginning of a day-shift work day, after day-shift work, and after night-shift work because of circadian variation, but it is important to establish whether stress from work enhances platelet sensitivity. This study focused on the short-term effect of night-shift work on platelet function; further investigation is needed to clarify when platelet function returns to baseline level. Several studies have reported conflicting results regarding the effects of sex differences or menstrual cycles on platelet function39). Consequently, further studies are needed to examine the effect of these factors on platelet function after night-shift work. Furthermore, whether platelet activation also increases after night-shift work in patients with increased baseline platelet function, such as those with diabetes mellitus or hypertension, should also be clarified. The results of such studies would help in establishing appropriate intervals between night shifts according to the comorbidities of staff members.
In conclusion, this study revealed that serum concentrations of COX-metabolized eicosanoid mediators, but not platelet aggregation parameters, increased after night-shift work compared with day-shift work in the same subject. Increased platelet hypersensitivity monitored by serum COX-metabolized eicosanoid mediators rather than platelet aggregation may be related to the mechanisms that underlie the association between night-shift work and adverse cardiovascular outcomes. Because serum samples can be easily obtained in clinical settings, serum eicosanoid mediators may represent promising prothrombotic markers.
Acknowledgement: We are indebted to all participants. We appreciate the professional support of Nobuko Kanno. This work was partly supported by a restart research grant from the University of Tokyo (Tomoko Nakao), a grant-in-aid for Scientific Research C (17K09227) (Tomoko Nakao), a grant-in-aid for Scientific Research C (15K08296) (Yoshihiro Kita), and a grant-in-aid for Scientific Research A (16H02637) (Yutaka Yatomi) from the Japanese Society for the Promotion of Science.
Conflicts of interest: The Department of Lipidomics is funded in part by Shimadzu Corporation, Kyoto, Japan; and one item of equipment (LCMS-8050) was kindly provided by Shimadzu Corporation, Kyoto, Japan.
Supplementary material: This article contains supplementary material (Apendix), which is available in the online version (doi: 10.1539/joh.2018-0027-FS).
Supplementary Materials
References
- 1). Maemura K, Takeda N, Nagai R. Circadian rhythms in the CNS and peripheral clock disorders: role of the biological clock in cardiovascular diseases. J Pharmacol Sci 2007; 103 (2): 134-138. [DOI] [PubMed] [Google Scholar]
- 2). Nicolaides NC, Charmandari E, Chrousos GP, et al. . Circadian endocrine rhythms: the hypothalamic-pituitary-adrenal axis and its actions. Ann N Y Acad Sci 2014; 1318: 71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Fujino Y, Iso H, Tamakoshi A, et al. ; Japanese Collaborative Cohort Study Group.. A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers. Am J Epidemiol 2006; 164 (2): 128-135. [DOI] [PubMed] [Google Scholar]
- 4). Brown DL, Feskanich D, Sánchez BN, et al. . Rotating night shift work and the risk of ischemic stroke. Am J Epidemiol 2009; 169 (11): 1370-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Vyas MV, Garg AX, Iansavichus AV, et al. . Shift work and vascular events: systematic review and meta-analysis. BMJ 2012; 345: e4800. (doi: 10.1136/bmj.e4800). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Ringqvist A, Caidahl K, Petersson AS, et al. . Diurnal variation of flow-mediated vasodilation in healthy premenopausal women. Am J Physiol Heart Circ Physiol 2000; 279 (6): H2720-H2725. [DOI] [PubMed] [Google Scholar]
- 7). Al Mheid I, Corrigan F, Shirazi F, et al. . Circadian variation in vascular function and regenerative capacity in healthy humans. J Am Heart Assoc 2014; 3 (3): e000845. (doi: 10.1161/JAHA.114.000845). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Otto ME, Svatikova A, Barretto RB, et al. . Early morning attenuation of endothelial function in healthy humans. Circulation 2004; 109 (21): 2507-2510. [DOI] [PubMed] [Google Scholar]
- 9). Sekine T, Daimon M, Hasegawa R, et al. . The impact of sleep deprivation on the coronary circulation. Int J Cardiol 2010; 144 (2): 266-267. [DOI] [PubMed] [Google Scholar]
- 10). Amir O, Alroy S, Schliamser JE, et al. . Brachial artery endothelial function in residents and fellows working night shifts. Am J Cardiol 2004; 93 (7): 947-949. [DOI] [PubMed] [Google Scholar]
- 11). Kubo T, Fukuda S, Hirata K, et al. . Comparison of coronary microcirculation in female nurses after day-time versus night-time shifts. Am J Cardiol 2011; 108 (11): 1665-1668. [DOI] [PubMed] [Google Scholar]
- 12). Feuring M, Wehling M, Ruf A, et al. . Circadian variation of platelet function measured with the PFA-100. Platelets 2009; 20 (7): 466-470. [DOI] [PubMed] [Google Scholar]
- 13). Scheer FA, Michelson AD, Frelinger AL 3rd, et al. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS One 2011; 6 (9): e24549. (doi: 10.1371/journal.pone.0024549). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Cattaneo M, Cerletti C, Harrison P, et al. . Recommendations for the Standardization of Light Transmission Aggregometry: A Consensus of the Working Party from the Platelet Physiology Subcommittee of SSC/ISTH. J Thromb Haemost 2013; 11 (6): 1183-1189. [DOI] [PubMed] [Google Scholar]
- 15). Ozaki Y, Satoh K, Yatomi Y, et al. . Detection of platelet aggregates with a particle counting method using light scattering. Anal Biochem 1994; 218 (2): 284-294. [DOI] [PubMed] [Google Scholar]
- 16). Toshima H, Sugihara H, Hamano H, et al. . Spontaneous platelet aggregation in normal subject assessed by a laser light scattering method: an attempt at standardization. Platelets 2008; 19 (4): 293-299. [DOI] [PubMed] [Google Scholar]
- 17). Suzuki S, Kudo H, Koyama T. Assessment of spontaneous platelet aggregation using laser light scattering in healthy subjects: an attempt to standardize. Int J Lab Hematol 2014; 36 (6): 676-685. [DOI] [PubMed] [Google Scholar]
- 18). Ohmori T, Yatomi Y, Nonaka T, et al. . Aspirin resistance detected with aggregometry cannot be explained by cyclooxygenase activity: involvement of other signaling pathway (s) in cardiovascular events of aspirin-treated patients. J Thromb Haemost 2006; 4 (6): 1271-1278. [DOI] [PubMed] [Google Scholar]
- 19). Yamada M, Kita Y, Kohira T, et al. . A comprehensive quantification method for eicosanoids and related compounds by using liquid chromatography/mass spectrometry with high speed continuous ionization polarity switching. J Chromatogr B Analyt Technol Biomed Life Sci 2015; 995-996: 74-84. [DOI] [PubMed] [Google Scholar]
- 20). Szosland D. Shift work and metabolic syndrome, diabetes mellitus and ischaemic heart disease. Int J Occup Med Environ Health 2010; 23 (3): 287-291. [DOI] [PubMed] [Google Scholar]
- 21). Morris CJ, Purvis TE, Hu K, et al. . Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A 2016; 113 (10): E1402-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Ellingsen T, Bener A, Gehani AA. Study of shift work and risk of coronary events. J R Soc Promot Health 2007; 127 (6): 265-267. [DOI] [PubMed] [Google Scholar]
- 23). Shimada K, Fukuda S, Maeda K, et al. . Aromatherapy alleviates endothelial dysfunction of medical staff after night shift work: preliminary observations. Hypertens Res 2011; 34 (2): 264-267. [DOI] [PubMed] [Google Scholar]
- 24). Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord 2015; 15: 130. (doi: 10.1186/s12872-015-0124-z). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Tatsumi K, Mackman N. Tissue Factor and Atherothrombosis. J Atheroscler Thromb 2015; 22 (6): 543-549. [DOI] [PubMed] [Google Scholar]
- 26). Kuhli-Hattenbach C, Hellstern P, Kohnen T, et al. . Platelet activation by ADP is increased in selected patients with anterior ischemic optic neuropathy or retinal vein occlusion. Platelets 2017; 28 (7): 720-723. [DOI] [PubMed] [Google Scholar]
- 27). Eto K, Takeshita S, Ochiai M, et al. . Platelet aggregation in acute coronary syndromes: use of a new aggregometer with laser light scattering to assess platelet aggregability. Cardiovasc Res 1998; 40 (1): 223-229. [DOI] [PubMed] [Google Scholar]
- 28). Miyamoto S, Ogawa H, Soejima H, et al. . Increased rate of formation of small-sized platelet aggregates in patients with acute coronary syndromes. Jpn Circ J 2000; 64 (9): 647-652. [DOI] [PubMed] [Google Scholar]
- 29). Hara K, Omori K, Sumioka Y, et al. . Spontaneous platelet aggregation evaluated by laser light scatter in patients with type 2 diabetes: effects of short-term improved glycemic control and adiponectin. Transl Res 2012; 159 (1): 15-24. [DOI] [PubMed] [Google Scholar]
- 30). de Meirelles LR, Mendes-Ribeiro AC, Santoro MM, et al. . Inhibitory effects of endogenous L-arginine analogues on nitric oxide synthesis in platelets: role in platelet hyperaggregability in hypertension. Clin Exp Pharmacol Physiol 2007; 34 (12): 1267-1271. [DOI] [PubMed] [Google Scholar]
- 31). Maree AO, Fitzgerald DJ. Variable platelet response to aspirin and clopidogrel in antherothrombotic disease. Circulation 2007; 115 (16): 2196-2207. [DOI] [PubMed] [Google Scholar]
- 32). Maree AO, Curtin RJ, Dooley M, et al. . Platelet response to low-dose enteric-coated aspirin in patients with stable cardiovascular disease. J Am Coll Cardiol 2005; 46 (7): 1258-1263. [DOI] [PubMed] [Google Scholar]
- 33). Gurbel PA, Bliden KP, DiChiara J, et al. . Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation 2007; 115 (25): 3156-3164. [DOI] [PubMed] [Google Scholar]
- 34). Tochikubo O, Mizushima S, Watanabe J, et al. . Base heart rate during sleep in hypertensive and normotensive subjects. J Hypertens 2001; 19 (6): 1131-1137. [DOI] [PubMed] [Google Scholar]
- 35). Bailey JM, Bryant RW, Whiting J, et al. . Characterization of 11-HETE and 15-HETE, together with prostacyclin, as major products of the cyclooxygenase pathway in cultured rat aorta smooth muscle cells. J Lipid Res 1983; 24 (11): 1419-1428. [PubMed] [Google Scholar]
- 36). Rosolowsky M, Campbell WB. Synthesis of hydroxyeicosatetraenoic (HETEs) and epoxyeicosatrienoic acids (EETs) by cultured bovine coronary artery endothelial cells. Biochim Biophys Acta 1996; 1299 (2): 267-277. [DOI] [PubMed] [Google Scholar]
- 37). Zu L, Guo G, Zhou B, et al. . Relationship between metabolites of arachidonic acid and prognosis in patients with acute coronary syndrome. Thromb Res 2016; 144: 192-201. [DOI] [PubMed] [Google Scholar]
- 38). Yang L, Yatomi Y, Satoh K, et al. . Inhibitory effects of beraprost on platelet aggregation: comparative study utilizing two methods of aggregometry. Thromb Res 1999; 94 (1): 25-32. [DOI] [PubMed] [Google Scholar]
- 39). Patti G, De Caterina R, Abbate R, et al. ; Working Group on Thrombosis of the Italian Society of Cardiology.. Platelet function and long-term antiplatelet therapy in women: is there a gender-specificity? A 'state-of-the-art' paper. Eur Heart J 2014; 35 (33): 2213-2223b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.