Abstract
Neurodegeneration can result in memory loss in the central nervous system (CNS) and impairment of taste and smell in the peripheral nervous system (PNS). The neurodegeneration seen in Parkinson’s disease (PD) is characterized by functional loss of dopaminergic neurons. Recent studies have also found a role for dopaminergic neurons in regulating taste memory rewards in insects. To investigate how taste memories and sugar sensitivity can be affected in PD, we utilized the DJ-1β mutant fruit fly, DJ-1βex54, as a PD model. We performed binary choice feeding assays, electrophysiology and taste-mediated memory tests to explore the function of the DJ-1β gene in terms of sugar sensitivity as well as associative taste memory. We found that PD flies exhibited an impaired ability to discriminate sucrose across a range of sugar concentrations, with normal responses at only very high concentrations of sugar. They also showed an impairment in associative taste memory. We highlight that the taste impairment and memory defect in DJ-1βex54 can be recovered by the expression of wild-type DJ-1β gene in the dopaminergic neurons. We also emphasized the role of dopaminergic neurons in restoring taste memory function. This impaired memory property of DJ-1βex54 flies also allows them to be used as a model system for finding supplementary dietary foods that can improve memory function. Here we provide evidence that the associative taste memory of both control and DJ-1βex54 flies can be enhanced with dietary supplementation of the medicinal plant, omija.
Keywords: DJ-1β, dopaminergic neuron, Schisandra chinensis (omija), taste memory, taste sensitivity
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative condition that is characterized by the loss of motor function. The reduction or loss of dopaminergic (DA) neurons from substantia nigra of the brain is a hallmark of PD (Damier et al., 1999).
PD also causes defects in odor perception (Ansari and Johnson, 1975), and this odor impairment can be used as a diagnostic feature (Tissinhg et al., 2011). Researchers also considered whether PD patients have impairments in taste perception. While a 2005 study did not find a significant difference in the taste sensitivity between control and PD patients groups (Sienkiewicz et al., 2005), a later study in 2009 of 25 non-demented PD patients and 74 control patients found a direct correlation between taste impairment and PD (Shah et al., 2009).
Mutations in various genes underlie PD, and one of the autosomal recessive candidate genes is DJ-1 (Dawson and Dawson, 2003). Drosophila melanogaster has two homologues of DJ-1 gene, DJ-1α and DJ-1β. DJ-1β is expressed in almost all parts of the fly body including the brain, while DJ-1α is expressed strongly in the testis and weakly in the brain (Menzies et al., 2005; Meulener et al., 2005). In humans, DJ-1 is expressed ubiquitously. Both DJ-1 gene homologues in Drosophila shares great similarity to human DJ-1. DJ-1α and DJ-1β share 56% and 52% identity and 70% and 69% similarity, respectively, with human DJ-1 (Meulener et al., 2005). The function of DJ-1 in both mammals and fruit flies is to protect tissues from oxidative stress, and defects in this gene cause animals to be more susceptible to various environmental toxins (Meulener et al., 2005; Park et al., 2005; Yokota et al., 2003). Mutations in the DJ-1 gene result in early onset autosomal recessive Parkinson’s disease in humans as well as flies (Bonifati et al., 2003; Meulener et al., 2005; Park et al., 2005).
Here, we use the fly as a model system to investigate sugar sensitivity and associative taste memory. Taste perception in flies is mediated by taste organs including the labellum, pharynx, leg, margin of wings and ovipositor (Lee and Poudel, 2014). These organs contain gustatory receptors that sense sweet and bitter tastants in the sensilla. Each sensillum has 2–4 gustatory receptor neurons (GRNs). We performed a binary choice feeding assay as well as tip recordings to evaluate any differences between control and PD model flies. We found that the DJ-1β mutant is defective in associative taste memory and this defect is fully recovered by expression of wild-type DJ-1β in dopaminergic neurons.
The memory impairment of DJ-1β mutant flies and the ease of performing of the associative taste memory assay make this a good model system to screen for dietary supplements that can improve memory. We sought to identify a natural product with medicinal value that can recover the taste sensitivity and memory deficit of PD model flies. The medicinal value of plants and other natural products is mainly described and practiced by traditional Chinese and Ayurveda medicine (Chopra et al., 2002; Devinsky et al., 2005). Schisandra chinensis, commonly known as omija in Korea, is a plant that has been identified as having medicinal value. The berries of omija are rich in phenolic contents with a high proportion of phytochemicals like anthocyanins and flavonoids (Kim et al., 2009). The presence of different phytochemicals in omija like polyphenol, lignin, organic acid (citric, malic, fumaric and tartaric acid) and essential oils (Panossian and Wikman, 2007) has contributed to its medicinal value. The polyphenol present in omija is proposed to be the mediator of the antioxidant properties of omija (Wu et al., 2011), as polyphenol has a redox property that acts as a reducing agent (Rice-Evans et al., 1997).
The anti-microbial and anti-oxidative properties of omija have been described in many literatures (Jung et al., 2000). This anti-oxidative property of omija and the anti-oxidative role of DJ-1 (Taira et al., 2004) raised the possibility that omija extract could be useful in recovering the physiological defect seen in DJ-1βex54 flies. Here we present the use of omija as food supplement to increase taste memory in both control and DJ-1βex54 flies.
MATERIALS AND METHODS
Fly stocks
DJ-1βex54, and UAS-DJ-1β flies were provided by Dr. J. Chung and Dr. K.S. Cho respectively (Park et al., 2005; Hwang et al., 2013). H. Amrein provided the Gr5a-GAL4 flies (Dunipace et al., 2001).
Chemical sources
Caffeine, sucrose and sulforhodamine B were purchased from Sigma-Aldrich Company (USA). Brilliant blue FCF was obtained from Wako Pure Chemical Industry, Ltd (Japan). Omija extract (PB2892.3) was purchased from the Korea Research Institute of Bioscience and Biotechnology (Korea). The leaves and flowers of omija were extracted using 99.99% methyl alcohol (HPLC grade).
Two-way choice behavioral assay
We performed a two-way choice assay as previously described (Poudel et al., 2017). Briefly, 50–70 adult flies (3–6 days old) were starved for 18 h in a humidified chamber. We then prepared two types of test mixtures, with one mixture always containing 2 mM sucrose, and the other mixture containing 2, 4, 6, 8, or 10 mM sucrose. We mixed the two test mixtures with two dyes: one with blue (brilliant blue FCF, 0.125 mg/ml) and the other with red (sulforhodamine B, 0.2 mg/ml). The two test mixtures were distributed in a 72-well microtiter dish in an alternated fashion, then the starved flies were introduced into the dish. The fly-containing microtiter dishes were kept in a dark humidified chamber and the flies were allowed to feed for 90 min. Flies were sacrificed by freezing and stored at −20°C until we analyzed the color of their abdomens using a microscope, which allowed us to determine which food they ate. We counted the number of flies that had abdomens that were blue (NB), red (NR), or purple (NP). The preference index (P.I.) was calculated according to the following equation: (NB-NR)/(NR+NB+NP) or (NR-NB)/(NR+NB+NP), depending on the dye/tastant combinations. P.I.s of −1.0 and 1.0 indicated a complete preference for 2 mM sucrose alone and the other concentration of sucrose, respectively. A P.I. of 0.0 indicated no bias between the two food alternatives.
Tip recording assay
We performed tip recordings as previously described (Poudel et al., 2017), using different concentrations of sucrose. We first immobilized 3–7-day-old flies by placing them on ice. We then inserted a reference glass electrode filled with Ringer’s solution into the thorax of the fly, and extended the electrode toward the proboscis. We stimulated the sensilla with tastants dissolved in the buffer solution of the recording pipette (10–20 μm tip diameter). We used 30 mM tricholine citrate (TCC) as the electrolyte for recording. We also tested 10 mM caffeine with 1 mM KCl from S-type sensilla. The recording electrode was connected to a preamplifier (TastePROBE, Syntech, Germany), and we collected and amplified the signals 10× using a signal connection interface box (Syntech) in conjunction with a 100–3000 Hz band-pass filter. Recordings of action potentials were made using a 12-kHz sampling rate and analyzed using Autospike 3.1 software (Syntech). Spike sorting was used as an indicator of the spike amplitudes that correspond to the action potentials of sucrose-sensitive neurons not to include relatively small amplitudes of water spikes.
Taste memory assay
Taste memory assay was performed by modifying previous methods (Masek et al., 2015). Three to four day old adult flies were starved for 12–18 h. The flies were fixed onto a glass slide using nail polish and ice was used to anesthetize flies while fixing. Between 10–15 flies were used per each assay. The flies were kept at 25°C in a 60% humidified incubator to recover for at least 2 h. The experiment was divided into three different phases. The first phase is the pretest in which the flies were presented with 500 mM sucrose stimuli to the leg. Flies that showed positive proboscis extension to this present were used in the next phases. The next phase was the training phase where the flies were presented with 500 mM sucrose stimuli at the leg while simultaneously being punished with presentation of an aversive taste, i.e. by giving 50 mM caffeine stimuli at the labellum. Training was repeated 15 times for each fly. Data for the training binned into three five-trial. After training was completed, the flies were given 500 mM sucrose stimuli at the leg at different time intervals (0, 5, 15, 30, 45 and 60 min), and the proboscis extension response was noted. Memory tests were also performed for both control and DJ-1βex54 flies after feeding on normal or 0.1% omija-supplemented food for 8–10 days. All the compared genotypes were assessed on the same day.
Feeding quantification assay
Feeding quantification was performed as previously described (Wong et al., 2009) for both control and DJ-1βex54 flies. One to two days’ old flies were fed for eight days on normal food or food that was supplemented with 0.1% omija. Then we measured the quantity of food consumed during different time periods (15 min, 30 min, 1 h, 4 h, and 24 h). To quantify food consumption, we made special food containing 1% sucrose, 1% blue dye (brilliant blue), and 1% agarose. At 8 a.m. (1 h before the lights turn on in the fly incubator), the flies were allowed to feed on the special food for the specified time periods. Then, the flies were homogenized in 1 ml PBST solution (1× phosphate buffer saline with 0.2% triton X-100). Five flies were homogenized in each tube. The homogenate was centrifuged at 10,000 rpm for 5 min and optical density (OD) was measured at 630 nm. PBST solution was used as blank. To generate a standard curve, 1% sucrose plus 1% blue dye was serially diluted to 10−2, 10−3, 10−4 and 10−5.
Statistical Analysis
All error bars represent standard error of the means (SEMs). Single factor analysis of variance (ANOVA) with Scheffe’s analysis as a post hoc test was used to compare multiple sets of data. Asterisks indicate statistical significance compared with the control (*P < 0.05, **P < 0.01).
RESULT AND DISCUSSION
Deficits in peripheral sensations, such as vision (Almer et al., 2012), pain (Seiss et al., 2003), odor (Ansari and Johnson, 1975), and taste (Shah et al., 2009), have been described in PD patients. We chose DJ-1βex54 mutant flies as a PD fly model, because this mutant is fully viable and fertile until the late adult stage, compared with others such as the pink and parkin mutants. To explore the role of the DJ-1β gene in taste sensory perception, we performed behavioral feeding assays as well as electrophysiological recordings with DJ-1βex54 mutant and wild-type control flies to determine their responses across a range of sucrose concentrations (Fig. 1). In the behavioral feeding assays, flies were given a choice between feeding on 2 mM sucrose on one side of the behavioral apparatus or the same or higher concentration of sucrose on the other side. Wild-type flies showed a strong preference for a two-fold higher concentration of sucrose and an almost complete preference for a three-fold higher concentration (Fig. 1A). However, DJ-1βex54 mutants showed defects in preference for two- to six-fold higher concentrations of sucrose over 2 mM sucrose, compared with control (Fig. 1A). DJ-1βex54 mutants showed normal preference to a seven-fold higher concentration of sucrose (Fig. 1A). To further clarify this defect, we carried out electrophysiological recording from the sugar sensing sensillum, L6, which shows a strong response to sucrose (Hiroi et al., 2002) (Figs. 1B and 1C). The recordings were performed with 3, 10, and 50 mM sucrose, which represent low, intermediate and high concentrations of sucrose. We found a reduced response to 3 mM sucrose in the DJ-1βex54 mutant flies when compared with control flies (7.5 ± 2.3 spikes/sec for control and 1.2 ± 0.5 spikes/sec for DJ-1βex54 mutants) (Figs. 1B and 1C). DJ-1βex54 mutant flies even showed reduced nerve responses to the high concentration of 50 mM sucrose (57.8 ± 3.7 spikes/sec for control and 38.1 ± 3.5 spikes/sec for DJ-1βex54). These results indicate that DJ-1βex54 flies have significant defects in their behavioral preference up to 50 mM sucrose and the physiological responses from sugar-sensing sensilla across a range of sucrose concentrations, including high concentrations. Furthermore, we fully rescued this reduced taste sensitivity of DJ-1βex54 flies by expressing the wild-type cDNA of DJ-1β into dopaminergic neurons using a TH-GAL4 driver line (Fig. 1A–C). In previous studies, the defects of DJ-1β mutants, such as loss of dopamine neurons, motor deficit, and decreased survival rates, were only observed under oxidative stress (Park et al., 2005). However, we found that the reduced taste sensitivity of DJ-1β mutants can be observed under normal food conditions.
Fig. 1. Sugar sensitivity in DJ-1βex54.
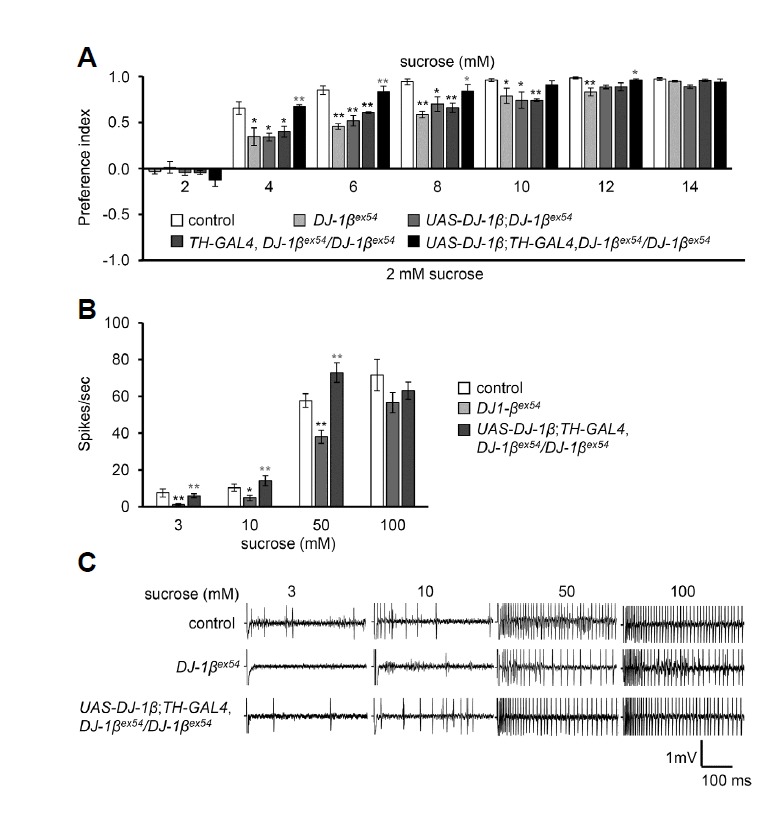
(A) Binary choice feeding assay was performed for different concentrations of sucrose versus 2 mM sucrose with control, DJ-1βex54, and rescue flies. n = 4–6. (B) Tip recordings from L6 sensillum with 3 mM, 10 mM, 50 mM and 100 mM sucrose from control, DJ-1βex54, and rescue flies. n = 10. (C) Representative sample traces of (B). The error bars represent SEMs. The asterisks indicate significant differences when compared to control (black asterisk) and mutant parent lines (gray asterisk) (*P < 0.05, **P < 0.01) using single factor ANOVA with Scheffe’s analysis as a post hoc test to compare the two sets of data.
Defects in learning and memory are an important feature of neurodegenerative disease. Neurotransmitters like dopamine and serotonin play an important role in learning and memory. Dopamine functions in taste memory (Masek et al., 2015), whereas serotonin is required for place memory (Sitaraman et al., 2008). PD is characterized by the loss of dopaminergic neurons (Damier et al., 1999), which lends the suggestion that PD model flies may have impairments in taste memory. In Drosophila melanogaster, dopaminergic neurons are associated with various activities like locomotion, sleep, appetite and aversive memory (Andretic and Hirish, 2000; Ganguly-Fitzgerald et al., 2006; Masek et al., 2015). To discover whether DJ-1βex54 flies show defects in taste memory, we performed taste memory assays with control and DJ-1βex54 flies. In this assay, flies are trained to associate a normally attractive stimulus, sucrose, with an aversive stimulus, caffeine, by pairing the two stimuli together (Lee et al., 2009). We used 500 mM sucrose and 50 mM caffeine as the stimuli because there is no difference in taste sensitivity from L6 and S6 sensilla between control and the DJ-1βex54 flies at these concentrations (Fig. 2A). First, we selected the flies that showed proboscis extension responses (PER) to sucrose 100% of the time (Fig. 2B). The flies were then given 15 training trials consisting of presentation of 500 mM sucrose to the leg followed shortly with presentation of 50 mM caffeine to the proboscis. One training session consisted of five trials, and the averaged data from the three sessions is shown in Fig. 2B. The majority of the flies showed almost complete PER retraction at the end of training (Fig. 2B). Next, we tested associative memory by presenting 500 mM sucrose at different time intervals. Flies that have formed an association of sucrose with the aversive taste in caffeine show reduced PER to sucrose immediately following training, and this association is lost over time. The DJ-1βex54 mutants show increased PER responses compared to control during the test phase, indicating that the mutant flies were defective in their ability to form associative memories (Fig. 2B).
Fig. 2. Associative taste memory assay displayed by DJ-1βex54.
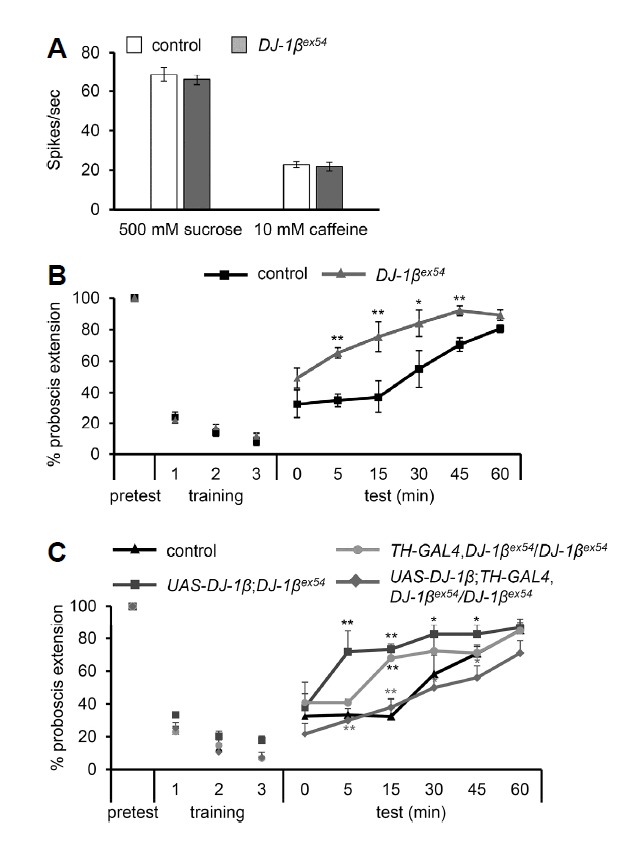
Average frequency of spikes/sec for 500 mM sucrose and 10 mM caffeine from L6 and S6 sensilla, respectively, in control and DJ-1βex54 flies. n = 10–14. (B) Both control and DJ-1βex54 mutant flies were trained with an aversive compound (i.e. 50 mM caffeine) for 15 trials and were then tested with 500 mM sucrose for acquisition of the taste memory at different time intervals. n = 7. (C) Recovery of taste memories of the DJ-1βex54 mutant flies by expressing the wild-type transgene of DJ-1β using a TH-GAL4 driver. The parental fly lines, UAS-DJ-1β;DJ-1βex54, and TH-GAL4,DJ-1βex54 were tested as well. n = 4. The error bars represent SEMs. The asterisks indicate significant differences when compared to control (black asterisk) and mutant parent lines (gray asterisk) (*P < 0.05, **P < 0.01) using single factor ANOVA with Scheffe’s analysis as a post hoc test to compare the two sets of data.
Since dopamine functions in taste memories, we examined whether DJ-1β function in dopaminergic neurons is important for formation of associative taste memories. To achieve this, we expressed wild-type DJ-1β in dopaminergic neurons of DJ-1βex54 mutant flies using a TH-GAL4 driver line (Friggi-Grelin et al., 2003) (Fig. 2C). These flies, along with their parent lines (UAS-DJ-1β;DJ-1βex54, and TH-GAL4,DJ-1βex54), were trained to associate sucrose with an aversive tastant, caffeine, and then tested for memory retention at different time intervals with presentation of sucrose alone (Fig. 2C). We found that expression of wild-type DJ-1β in dopaminergic neurons recovered the taste memory deficit of DJ-1βex54 mutant flies. PER responses of the UAS-DJ-1β;DJ-1βex54 and TH-GAL4,DJ-1βex54 parental fly lines matched those of the DJ-1βex54 mutant flies. These results indicate that DJ-1β function in dopaminergic neurons is important for the formation of associative taste memories.
In our aging society, memory loss is increasingly becoming a major health problem, with few effective solutions. The associative taste memory assay described above could be a useful tool for screening dietary health supplements for improving memory. We performed a small scale screen of about ten natural compounds to identify supplements that may be useful for improving memory. From this screen, we discovered that omija, a very popular berry in Korea normally taken as a refreshing beverage or in the form of omija tea, and has been published to have many medicinal uses (Jung et al., 2000; Kim et al., 2009) may be useful in improving associative memory. To test this, we fed control and DJ-1βex54 flies omija-supplemented food for 8–10 days, and then tested their ability in the associative taste memory assay. We found that both control and DJ-1βex54 flies fed on an omija diet showed improved associative taste memory compared to those fed a normal diet (Fig. 3A and 3B). The control flies fed omija showed a significant improvement in memory at 45 min, while DJ-1βex54 flies fed omija showed significant memory improvement from very beginning at 0 min and 15 min. The enhancement of associate taste memory seen with omija supplementation may be due to the antioxidant properties of omija (Meena et al., 2012). Future studies to confirm this and to identify the single component in omija that is responsible are warranted.
Fig. 3. Effect of Schisandra chinensis (omija) in improving taste associative memory.
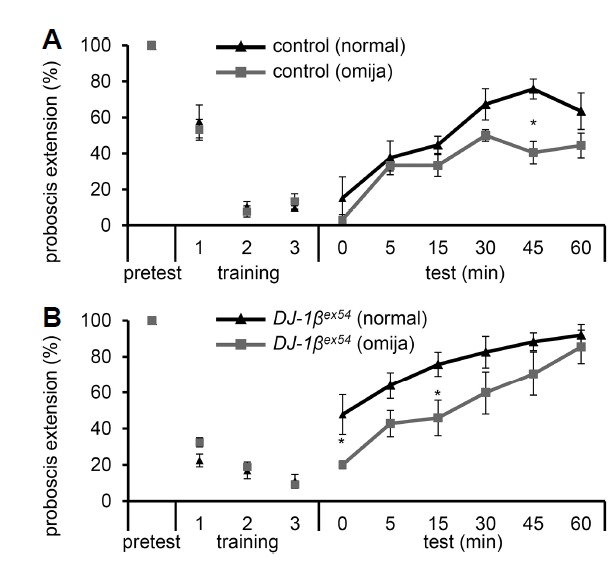
(A, B) Taste memory assay for control (A) and DJ-1βex54 mutant (B) flies after feeding on normal or 0.1% omija-supplemented food. n = 5–6. The error bars represent SEMs. The asterisks indicate significant differences between flies feeding on normal food when compared to feeding on omija-supplemented food in the control and DJ-1βex54 mutant flies (*P < 0.05) using single factor ANOVA with Scheffe’s analysis as a post hoc test to compare the two sets of data.
The addition of omija to the fly food could impart a novel taste quality and cause flies to restrict their food intake. Caloric restriction could artificially affect the results of the taste memory task; therefore, to ensure that the alterations in associative memory seen with omija supplementation are not due to differences in food intake, we quantified the amount of food ingested at different time intervals and examined any difference in intake between normal cornmeal and omija-supplemented food (Fig. 4A). We found no significant difference in ingestion of the two foods, with the exception that DJ-1βex54 mutant flies ingest significantly more omija-supplemented food than normal food at only the 15 min time point (Fig. 4A). Importantly, this suggests that omija-supplemented food is not aversive and does not cause caloric restriction. Furthermore, we tested taste sensitivity after feeding omija to DJ-1βex54 by performed tip recordings and found no clear difference in responses to sucrose in flies fed omija-supplemented versus normal food (Fig. 4B).
Fig. 4. Effect of omija in ingestion and taste sensitivity.
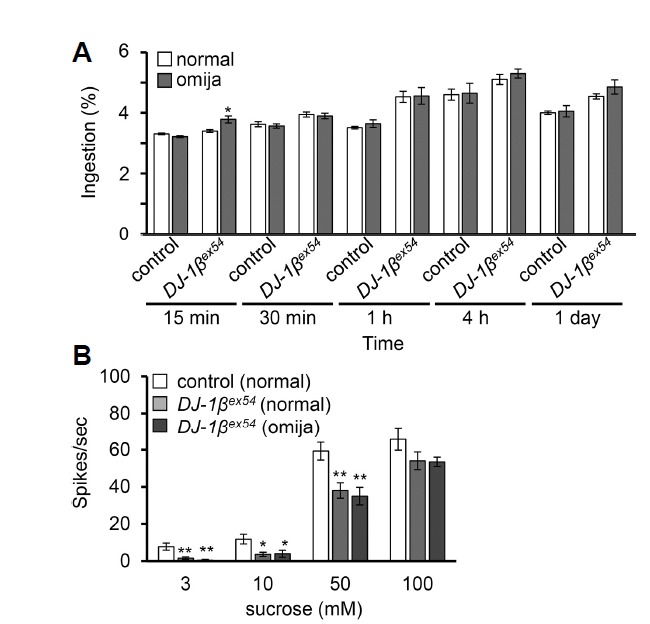
(A) Quantification of food intake of control and DJ-1βex54 mutant flies. Flies were fed 1% sucrose after feeding on normal food or normal food supplemented with 0.1% omija for 8 days. (B) Tip recordings from DJ-1βex54 flies after feeding on omija-supplemented food.
Our present results suggest that loss of function mutations in the DJ-1β gene result in loss of taste sensitivity to sucrose and associative taste memory impairment. We also highlight the role of dopaminergic neurons in taste memory using the PD fly model. Furthermore, we uncovered a role of the medicinal plant, omija, in improving the associative taste memory of both wild-type and DJ-1βex54 flies.
ACKNOWLEDGMENTS
We thank Dr. Melissa Fowler for comments. We thank J. Chung, K. S. Cho, H. Amrein and the Bloomington stock center for fly stocks. S. Poudel was supported by Global Scholarship Program for Foreign Graduate Students at Kookmin University in Korea. This work is supported by grants to Y. Lee from the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A1A2058094 and 2016R1D1A1B03931273).
REFERENCES
- Almer Z., Klein K.S., Marsh L., Gerstenhaber M., Repka M.X. Ocular motor and sensory function in Parkinson’s disease. Ophthalmology. 2012;119:178–182. doi: 10.1016/j.ophtha.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R., Hirsh J. Circadian modulation of dopamine receptor responsiveness in Drosophila melanogaster. Proc Natl Acad Sci, USA. 2000;97:1873–1878. doi: 10.1073/pnas.97.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari K.A., Johnson A. Olfactory function in patients with Parkinson’s disease. J Chronic Dis. 1975;28:493–497. doi: 10.1016/0021-9681(75)90058-2. [DOI] [PubMed] [Google Scholar]
- Bonifati V., Rizzu P., Van Baren M.J., Schaap O., Breedveld G.J., Krieger E., Dekker M.C., Squitieri F., Ibanez P., Joosse M., et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Chopra A., Doiphode V.V. Ayurvedic medicine: core concept, therapeutic principles, and current relevance. Med Clin. 2002;86:75–89. doi: 10.1016/s0025-7125(03)00073-7. [DOI] [PubMed] [Google Scholar]
- Damier P., Hirsch E.C., Agid Y., Graybiel A.M. The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Dawson T.M., Dawson V.L. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Denvinsky O., Steven C., Shachter S.C., Pacia S.V. Traditional chinese medicine. Complementary and Alternative Therapies for Epilepsy. 2005:177–182. [Google Scholar]
- Dunipace L., Meister S., McNealy C., Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr Biol. 2001;11:822–835. doi: 10.1016/s0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F., Coulom H., Meller M., Gomez D., Hirsh J., Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. Dev Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Ganguly-Fitzgerald I., Donlea J., Shaw P.J. Waking experience affects sleep need in Drosophila. Science. 2006;313:1775–1781. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- Hiroi M., Marion-Poll F., Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zool Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- Hwang S., Song S., Hong Y.K., Choi G., Suh Y.S., Han S.Y., Lee M., Park S.H., Lee J.H., Lee S., et al. Drosophila DJ-1 decreases neural sensitivity to stress by negatively regulating Daxx-like protein through dFOXO. PLoS Genet. 2013;9:e1003412. doi: 10.1371/journal.pgen.1003412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Joo M.H., Yoo S.H. Structural identification and antioxidant properties of major anthocyanin extracted from Omija (Schizandra chinensis) fruit. J Food Sci. 2009;74:C134–140. doi: 10.1111/j.1750-3841.2009.01049.x. [DOI] [PubMed] [Google Scholar]
- Jung G.T., Ju I.O., Choi J.S., Hong J.S. The antioxidative, antimicrobial and nitrite scavenging effects of Schizandra chinensis RUPRECHT (Omija) seed. Korean J Food Sci and Technol. 2000;32:928–935. [Google Scholar]
- Lee Y., Poudel S. Taste sensation in Drosophila melanogaster. Hanyang Med Rev. 2014;34:130–136. [Google Scholar]
- Lee Y., Moon S.J., Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci USA. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek P., Worden K., Aso Y., Rubin G.M., Keene A.C. A dopamine-modulated neural circuit regulating aversive taste memory in Drosophila. Curr Biol. 2015;25:1535–1541. doi: 10.1016/j.cub.2015.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena H., Pandey H.K., Pandey P., Arya M.C., Ahmed Z. Evaluation of antioxidant activity of two important memory enhancing medicinal plants Baccopa monnieri and Centella asiatica. Indian J Pharmacol. 2012;44:114. doi: 10.4103/0253-7613.91880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies F.M., Yenisetti S.C., Min K.T. Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol. 2005;15:1578–1582. doi: 10.1016/j.cub.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Meulener M., Whitworth A.J., Armstrong-Gold C.E., Rizzu P., Heutink P., Wes P.D., Pallanck L.J., Bonini N.M. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- Panossian A., Wikman G. Pharmacology of Schisandra chinensis Bail.: an overview of Russian research and uses in medicine. J Ethnopharmacol. 2008;118:183–212. doi: 10.1016/j.jep.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Park J., Kim S.Y., Cha G.H., Lee S.B., Kim S., Chung J. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Poudel S., Kim Y., Gwak J.S., Jeong S., Lee Y. Gustatory receptor 22e is essential for sensing chloroquine and strychnine in Drosophila melanogaster. Insect Biochem Mol. 2017;88:30–36. doi: 10.1016/j.ibmb.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C., Miller N., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- Seiss E., Praamstra P., Hesse C., Rickards H. Proprioceptive sensory function in Parkinson’s disease and Huntington’s disease: evidence from proprioception-related EEG potentials. Exp Brain Res. 2003;148:308–319. doi: 10.1007/s00221-002-1291-6. [DOI] [PubMed] [Google Scholar]
- Shah M., Deeb J., Fernando M., Noyce A., Visentin E., Findley L.J., Hawkes C.H. Abnormality of taste and smell in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:232–237. doi: 10.1016/j.parkreldis.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Sienkiewicz-Jarosz H., Scinska A., Kuran W., Ryglewicz D., Rogowski A., Wrobel E., Korkosz A., Kostowski W., Bienkowski P. Taste responses in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;76:40–46. doi: 10.1136/jnnp.2003.033373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D., Zars M., LaFerriere H., Chen Y.C., Sable-Smith A., Kitamoto T., Rottinghaus G.E., Zars T. Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci USA. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira T., Saito Y., Niki T., Iguchi-Ariga S.M., Takahashi K., Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissingh G., Berendse H.W., Bergmans P., DeWaard R., Drukarch B., Stoof J.C., Wolters E.C. Loss of olfaction in de novo and treated Parkinson’s disease: possible implications for early diagnosis. Mov Disord. 2001;16:41–46. doi: 10.1002/1531-8257(200101)16:1<41::aid-mds1017>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Wong R., Piper M.D., Wertheim B., Partridge L. Quantification of food intake in Drosophila. PloS One. 2009;4:e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Yu X., Jing H. Optimization of phenolic antioxidant extraction from Wuweizi (Schisandra chinensis) pulp using random-centroid optimazation methodology. Int J Mol Sci. 2011;12:6255–6266. doi: 10.3390/ijms12096255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Sugawara K., Ito K., Takahashi R., Ariga H., Mizusawa H. Down regulation of DJ-1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochem Biophys Res Commun. 2003;312:1342–1348. doi: 10.1016/j.bbrc.2003.11.056. [DOI] [PubMed] [Google Scholar]


