Abstract
The RNA-binding protein tristetraprolin (TTP) binds to adenosine-uridine AU-rich elements in the 3′-untranslated region of messenger RNAs and facilitates rapid degradation of the target mRNAs. Therefore, it regulates the expression of multiple cancer and immunity-associated transcripts. Furthermore, a lack of TTP in cancer cells influences cancer progression and predicts poor survival. Although the functions of TTP on cancer cells have previously been researched, the mechanism of TTP on the interaction between cancer cells with their microenvironment remains undiscovered. In this study, we admed to determine the role of cancer cell TTP during the interaction between tumor and immune cells, specifically regulatory T cells (Tregs). We evaluate the capability of TTP to modulate the antitumor immunity of GC and explored the underlying mechanism. The overexpression of TTP in GC cells dramatically increased peripheral blood mononuclear lymphocyte (PBML) -mediated cytotoxicity against GC cells. Increased cytotoxicity against TTP-overexpressed GC cells by PBMLs was determined by Treg development and infiltration. Surprisingly, we found the stabilization of programmed death-ligand 1 (PD-L1) mRNA was declining while TTP was elevated. The PD-L1 protein level was reduced in TTP-abundant GC cells. PD-L1 gas been found to play a pivotal role in Treg development and functional maintenance in immune system. Taken together, our results suggest the overexpression of TTP in GC cells not only affects cell survival and apoptosis but also increases PBMLs -mediated cytotoxicity against GC cells to decelerate tumor progression. Moreover, we identified PD-L1 as a critical TTP-regulated factor that contributes to inhibiting antitumor immunity.
Keywords: immunity, PD-L1, Tregs, tristetraprolin, tumor progression
INTRODUCTION
Gastric cancer (GC) has a high frequency and contributes to the overall cancer mortality worldwide. In 2012, GC reportedly occupied third place among cancer-related deaths globally (Siegel et al., 2012). Although taking surgical operation on time prior to the spread of tumor cells was available to reduce mortality, GC progression and metastasis are still caused of high mortality. Hence, it is urgent to elucidate the potential molecular pathways concerning tumor progression and metastasis, which may be beneficial to finding effective therapeutic approaches.
Posttranscriptional regulation is the key point of gene expression regulation, and 3′-untranslated regions (3′-UTRs) are closely related to posttranscriptional regulation (Perez-Ortin et al., 2013). Adenosine-uridine (AU)-rich elements (AREs), located in the 3′-UTRs of certain gene transcripts, have a vital influence on the stability of messenger RNA (mRNA) and mediate gene expression (Sanduja et al., 2011). Tristetraprolin (TTP), regarded as one of the best-characterized RNA-binding proteins, is capable of recognizing and binding directly to AREs of target mRNAs and subsequently influence their stability (Brooks and Blackshear, 2013; Guo et al., 2017a). Therefore, it mediates the expression of multiple cancer and immunity-associated transcripts (Guo et al., 2017a; Jeltsch and Heissmeyer, 2016; Sanduja et al., 2012). Mounting evidence suggests that TTP is involved in regulating the progression and metastasis of several types of malignant tumors at different levels, including proliferation, migration, invasion, and apoptosis (Guo et al., 2017a; 2017b). The quantity and activity of TTP are reduced in several cancers and its expression and basic function in GC have previously been studied (Deng et al., 2016). However, there are still few studies focused on the underlying mechanism of tumor progression and metastasis of GC cells are still lacking, and the impact of TTP on the metastatic potential of GC cells remains unknown. More importantly, the role of TTP in the interaction of tumors with their microenvironment needs to be investigated further.
The progression and metastasis of a malignant tumor are dominated by multiple factors and associated with the microenvironment (Schreiber et al., 2011; Seo et al., 2015). Immune system dysfunction has gained increasing attention in tumorigenesis. Tumor immune escape has a direct correlation with its metastatic potential (Friedl and Wolf, 2003; Robert, 2013). The escape of malignant cells from local and systemic immune control contributes to the subsequent migration and metastasis to lymph nodes or the secondary sites (Becht et al., 2016). The status change of the immune system and microenvironment in situ results in the tumor immune escape (Giraldo et al., 2014; Junttila and de Sauvage, 2013).
Immune cell infiltration into tumors is a critical determinant of tumor progression. Tregs, belonging to the subset of CD4+ helper T lymphocytes characterized by the CD4+ CD25+ phenotype, has been recognized to possess the capacity of suppressing proliferation and cytokine secretion of effector T lymphocytes through immunoregulation (Hatam et al., 2012). It is widely accepted that tumorigenesis is strongly influenced by the interaction between tumor cells and host cytotoxic effector cells (Tripathi and Guleria, 2015). Tregs are responsible for inhibiting the cytotoxicity of effector T cells. Several studies have demonstrated a closed link between Treg infiltration and tumor immune escape (Li et al., 2016; Pollack et al., 2017). High Treg number in GC tissue is associated with poor prognosis (Das et al., 2006; Geng et al., 2015).
Recruitment of peripheral Tregs is a key step for tumor immune escape, which is determined by a sophisticated regulatory network in vivo. Programmed cell death ligand-1 (PD-L1 or CD274) is a novel costimulatory molecule that is reported to suppress the immune response and lead to immune escape (Hirai et al., 2017). Moreover, recent research has shown that PD-L1 plays a pivotal role in Treg development and function maintenance (Liu et al., 2017). Thus, PD-L1 is one of the leading causes of Treg infiltration in the tumor microenvironment (Heeren et al., 2015; Tian et al., 2016).
Damnification of immune surveillance leads to metastasis and indicates adverse clinical outcomes. The intention of this study is to evaluate the influences of TTP in GC cells and concentrate on the antitumor immunity of GC mediated by TTP. In this study, we attempted to prove that TTP negatively contributes to Treg infiltration and enhances the cytotoxicity of effector T cells. The declining Tregs number is caused by reduced PD-L1 levels. TTP inhibits PD-L1 expression through regulating its mRNA stability. TTP may therefore be a target for GC treatment.
MATERIALS AND METHODS
Patients and tissue samples
All subjects provided informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of The Affiliated Wuxi No.2 People’s Hospital of Nanjing Medical University. All the experimental methods in the current study were carried out in accordance with the approved guidelines by the Ethics Committee of The Affiliated Wuxi No.2 People’s Hospital of Nanjing Medical University.
Paraffin-embedded GC specimens from 144 patients undergoing surgical excision of gastric carcinoma at the Affiliated Wuxi No.2 People’s Hospital of Nanjing Medical University between July 2009 and October 2012 were collected for immunohistochemistry. None of the patients involved in the study had received radiotherapy, chemotherapy or other medical intervention before the gastrectomy. All patients were followed up for more than 5 years.
Cell culture
Human GC cells MGC-803 and BGC-823 were purchased from the Chinese Academy of Medical Sciences and stored in the Department of General Surgery and Center of Translational Medicine. GC cells were cultured Dulbecco’s modified Eagle’s medium (DMEM) (Gibco-BRL, USA) supplemented with 10% fetal bovine serum (FBS) (HyClone, USA). Cells were kept at 37°C in a humidified incubator with 5% CO2.
RNA preparation and qRT-PCR
Total RNA was extracted and isolated by TRIzol Reagent (Invitrogen, USA). RNA samples were reverse transcribed into cDNA using the PrimeScript RT Reagent Kit (Takara, China). mRNA level were analyzed by qRT-PCR using the QuantiFast SYBR Green PCR Kit (Qiagen, Germany). The following primers were used: TTP forward, 5′-TTCGCCCACT GCAACCTC-3′ and reverse, 5′-CGCCCACTCTCTGAGAAGGTC-3′; PD-L1 forward, 5′-TCAATGCCCCATACA ACAAA-3′ and reverse, 5′-TGCTTGTCCAGATGACTTCG-3′; Foxp3 forward, 5′-TCCCAGAGTTCCTCCACAAC-3′ and reverse, 5′-GCAAGA CAGTGGAAACCTCAC-3′; GAPDH forward, 5′-AAGGTGAA GGTCGGAGTCAA-3′ and reverse, 5′-AATGAAGGGGTCATT GATGG-3′. The results of the target genes were quantified by the 2−ΔΔCT method, with GAPDH expression as internal control, and all samples were measured in triplicate.
Plasmid construction and DNA transfection
The pcDNA-TTP plasmid and pcDNA 3.1(+) empty vector were constructed according to the paper. The pcDNA-TTP plasmid was transfected into MGC-803 and BGC-823 cells separately using Lipofectamine 2000 (Invitrogen, China) to produce MGC-803/TTP and BGC-823/TTP cells. G418 (Invitrogen, China) was added to select stably-transfected cells. After screening for two weeks, stable transfectants were tested by qRT-PCR or western blotting to ensure overexpression of TTP and for further amplification afterwards. The control cell lines MGC-803/pcDNA and BGC-823/ pcDNA were produced and selected by transfection with the empty pcDNA3.1 (+) vector in the same way.
Protein extraction, antibodies, and western blotting
Total protein of cells was extracted by radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotechnology, USA) with phenylmethylsulfonyl fluoride (PMSF) (1 mM, final concentration). Extracted proteins were quantitated and then separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After blotting onto polyvinylidene difluoride (PVDF) membranes, target proteins were detected using specific antibodies and corresponding secondary antibodies. Immobilonδ Western Chemiluminescent HRP Substrate (Millipore, USA) was used for visualization. Target protein expression was normalized to β–actin. The following primary antibodies were applied: TTP (ab33058, Abcam), PD-L1 (66248-I-Ig Proteintech Group, Inc), Bcl2 (2872S, CST), cleavage of caspase 3 (9664S, CST), and GAPDH (60004-1-Ig, Proteintech, China).
Human peripheral blood mononuclear lymphocytes (PBMLs) isolation
PBMLs were separated from peripheral blood of healthy donors, The donors were selected by excluding anyone with autoimmune diseases or taking immunosuppressive drugs was excluded from this study. Ficoll-Hypaque density gradient centrifugation (Cedarlane, Canada) was used to separate PBMCs. After isolation, PBMCs were resuspended in RPMI-1640 (Gibco-BRL, USA) with 10% FBS for 2 h to make monocytes (MO) adhere to plastic wells, and PBMLs in the supernatants were harvested for application (Seo et al., 2015).
PBML-mediated cytotoxicity assay of GC cells
Approximately 1 × 105 GC cells were seeded into 96-well microplates and then 1×106 PBMLs were added and cocultured at 37°C for 16 h. The number of both GC cells and PBMLs in each plate is guaranteed to be consistent in the beginning. The lactate dehydrogenase (LDH) assay was applied to assess cell cytotoxicity (Seo et al., 2015). LDH leakage into the medium was quantified by a Cytotoxicity LDH Assay Kit (Dojindo Laboratories, Japan). Three replicate wells for spontaneous (GC cell monoculture in DMEM with 10% FBS, PBML monoculture in RPMI-1640 with 10% FBS) and maximum release (GC cell monoculture in DMEM with 1% Triton X-100) were measured in parallel. The ratio of total lysed cancer cells was calculated as follows: cytotoxicity ratio = (experimental LDH - PBMLs spontaneous LDH – GC cells spontaneous LDH)/(GC cells maximum LDH - GC cells spontaneous LDH)(Seo et al., 2015).
Flow Cytometry
A total of 5 × 103 GC cells were seeded into 96-well plates per well. For co-culture experiments, 1 × 105 PBMLs were added and cocultured at 37°C for 72 h. After cultivation, the cocultured PBMLs were employed for flow cytometric analysis using following fluorochrome-labelled antibodies: CD4-FITC (555748, BD Biosciences, Germany), CD25-PE (555749, BD Biosciences, Germany), Foxp3-PerCPCy5.5 (561493, BD Biosciences, Germany). For intracellular detection of Foxp3, cells were fixed and permeabilized using the Foxp3 staining kit (BD Biosciences, Germany). Tregs are identified by CD4+ CD25+ Foxp3+.
Luciferase assays
Cells (5 × 106) were transfected with plasmid constructs using Lipofectamine 2000 (Invitrogen). Twenty-four hours after transfection, the transfected cells were harvested and quantified by a Dual-Luciferase Reporter Assay Kit (Biovision).
Exposure to human PD-L1 neutralizing antibody and human recombinant PD-L1 (rPD-L1) protein
Human PD-L1 neutralizing antibody was provided by Hengrui Pharmaceutical Co, Shanghai, China. rPD-L1 was purchased from Sino Biological Inc, Beijing, China. Cells were incubated with 10 ug/ml of human PD-L1 neutralizing antibody or 100 ng/ml of rPD-L1 for further experiments.
Immunohistochemical staining assay
For immunohistochemistry, each paraffin-embedded specimen was sectioned for at least three pieces (4 μm). Staining of TTP, PD-L1, and forkhead/winged-helix transcription factor 3 (Foxp3) was done using the MaxVision HRP-Polymer anti-Mouse/Rabbit IHC Kit and diaminobenzidine (DAB) kit (Maixin Biotech, China). Hematoxylin was used to counterstain the sections subsequently. To calculate the expression of TTP, PD-L1 in tumor cells, and Foxp3 in Tregs, a semi-quantitative approach on the basis of both staining intensity and percentage of staining cells was employed. The intensity (I) of staining was graded on a scale of 0–3: 0, negative staining; 1, weak staining; 2, moderate staining; 3, strong staining. The percentage of staining cells (R) was scored as follows: 1, (0–10% staining cells); 2, (11–50% staining cells); 3, (51–75% staining cells); 4, (76–100% staining cells). The final immunohistochemistry score (IHS) was calculated with the formula ∑R*I. Samples with scores ≥ 3 were assessed as positive.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Science (SPSS) 20.0 (SPSS Inc, USA). Fisher’s exact test was used to analyze the correlation between TTP, PD-L1, or Foxp3 and clinicopathological characteristics. The correlations of the expression levels of TTP, PD-L1, and Foxp3 were examined by Spearman correlation coefficient. Other data were evaluated by Student’s t-test or one-way analysis of variance (ANOVA). Kaplan–Meier curves and the log-rank test were performed for the survival analysis. P value < 0.05 was considered statistically significant.
RESULTS
TTP expression is conversely related to cell survival of GC cells
The absence of TTP was previously observed in a mass of malignant tumors and was connected to cell proliferation and invasiveness (Milke et al., 2013). Cell viability is a crucial factor in tumorigenesis (Mohamed et al., 2017). To determine whether TTP exerted critical role in modulating viability of GC cells, MGC-803 and BGC-823 cells were transfected with TTP expression plasmids pcDNA-TTP to construct stably-transfected cell lines, negative controls, MGC-803/TTP and BGC-823/TTP, respectively. As negative controls, MGC-803 and BGC-823 cells were transfected with empty vector pcDNA3.1 (+) to construct MGC-803/pcDNA and BGC-823/pcDNA respectively. Overexpression of TTP in MGC-803/TTP and BGC-823/TTP cells was confirmed by qRT-PCR and western blotting analysis (Figs. 1A and 1C). We used trypan blue dye exclusion assay to measure the viability rate of GC cells. As shown in Fig 1B, overexpression of TTP in GC cells could significantly reduce the viability rate (P = 0.04, P = 0.013). Then, we evaluated B cell lymphoma-2 (Bcl-2) and cleavage of caspase 3 as a predictor for apoptosis by western blotting analysis. As shown in Fig. 1C, TTP overexpression significantly decreased the protein level of Bcl-2 and increased the protein level of cleavage of caspase 3 in both MGC-803 and BGC-823 cells. To sum up, our data indicated that TTP overexpression could promote apoptosis and reduce cell survival in both MGC-803 and BGC-823 cells apart from its known role in cell proliferation.
Fig. 1. TTP overexpression reduced cell survival and promoted apoptosis in both MGC-803 and BGC-823 cells. MGC-803 and BGC-823 cells were transfected with pcDNA-TTP or empty vector pcDNA3.1 (+).
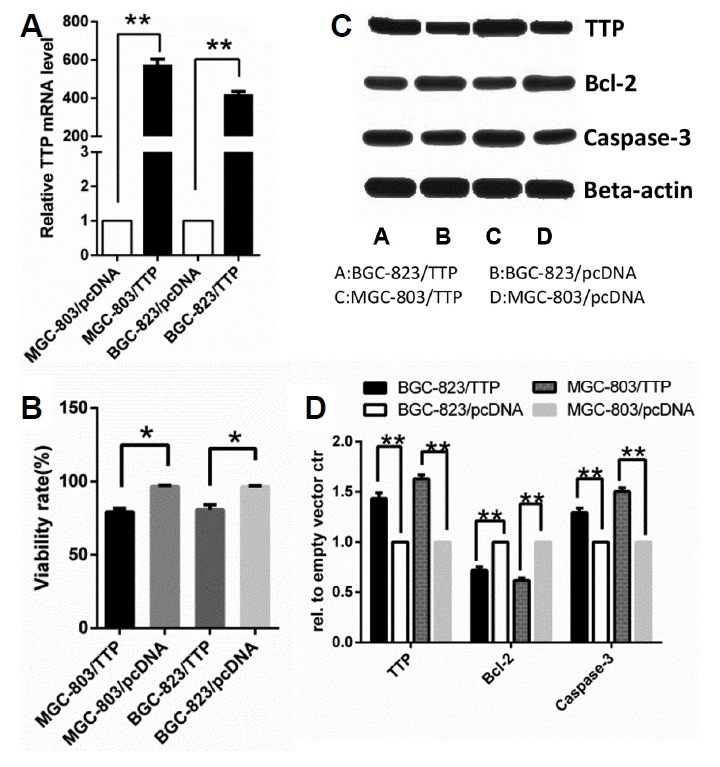
(A) Relative expression of TTP mRNA in MGC-803/TTP and BGC-823/TTP cell lines and corresponding control group was examined by qRT-PCR. An empty vector ctr clone was used as the control. (B) The viability rate of GC cells was measured by trypan blue dye exclusion assay. (C) Expression of TTP protein level was examined by western blotting. Bcl-2 and cleavage of caspase 3 expression in MGC-803/TTP and BGC-823/TTP and the corresponding control group were analyzed by western blotting. GAPDH and β-actin were used as internal controls for qRT-PCR and western blotting analysis, respectively. (D) Quantifications of western blotting results was processed by Image J software. All data were represented as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01.
Overexpression of TTP in GC cells enhances PBML-mediated cytotoxicity of GC cells
It is widely accepted that tumorigenesis is strongly determined by the cytotoxicity of effector T lymphocytes and related to immune surveillance (Eckert et al., 2016; Finn, 2017; Tan et al., 2017). We cocultured the GC cell lines MGC-803 and BGC-823 with PBML at different E: T ratios at 37°C for 16 h. Human PBMLs were separated from peripheral blood of healthy donors. LDH release assay was applied to detect cytotoxicity after cocultivation, as shown in Fig. 2A, the cytotoxicity of PBML against GC cells depended on the E: T, and increased E: T ratio could enhance the cytotoxicity activity. According to the results, we chose E: T at 10:1 as the best ratio for follow-up experiments. To investigate whether TTP had an effect on antitumor immunity, we evaluated the effects of TTP on PBML-mediated cytotoxicity against MGC-803 and BGC-823 cells. Human PBMLs were separated from peripheral blood of healthy donors and were added to the MGC-803/TTP and BGC-823/TTP cells or the control group by E: T at 10:1. After addition, the mixture was cocultured at 37°C for 16 h for PBML-mediated cytotoxicity assay. As shown in Fig. 2B, the cytotoxicity of PBMLs against MGC-803/TTP was 61.5 ± 4.24% while the control was 28.5 ± 3.14%. The cytotoxicity of PBMLs against BGC-823/TTP was 52.8 ± 5.65% while the control was 28.1 ± 3.85%. TTP overexpression significantly increased PBML-mediated cytotoxicity against both MGC-803 and BGC-823 cells (P < 0.05). These results suggested that TTP contributed to regulation of antitumor immunity by increasing PBML-mediated cytotoxicity.
Fig. 2. Effects of TTP on PBML-mediated cytotoxicity against GC cells.
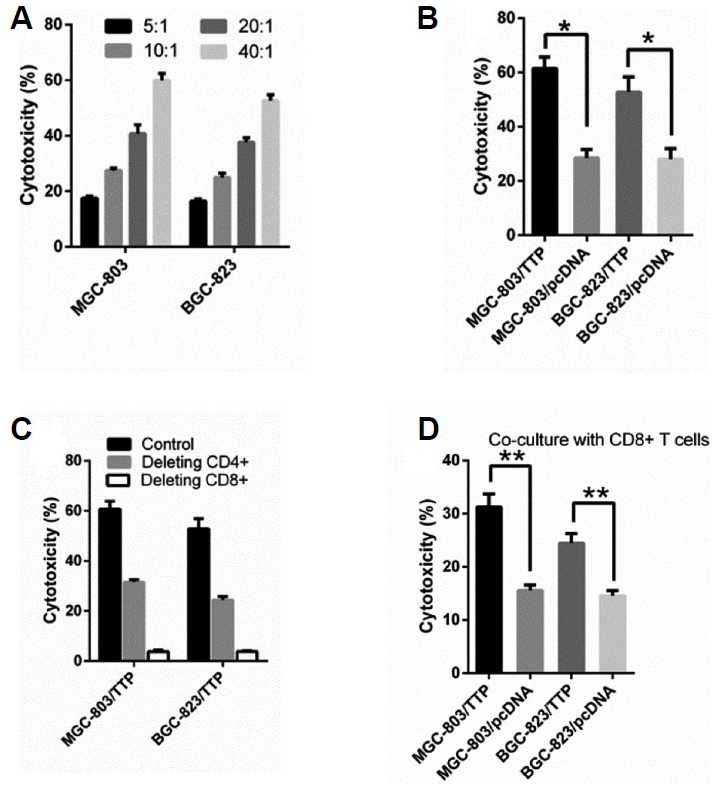
The transfected MGC-803 and BGC-823 cells were precultured in 96-well plates and PBMLs were added to the precultured cells and cocultured at 37°C for 16 h for the cytotoxicity assay. (A) The cytotoxicity of PBML against GC cells depended on the E: T and exists dose-dependent relationships exists. (B) TTP overexpression enhanced the cytotoxicity against MGC-803 and BGC-823 cells when cocultured with PBMLs. (C) The cytotoxicity was reduced when adding depleting antibodies against CD4 and CD8 into the co-culture system. (D) TTP overexpression enhanced the cytotoxicity against MGC-803 and BGC-823 cells when cocultured with purified CD8+ T cells. All data were represented as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01.
As shown by the results above, we cannot determine which subtypes of PBMLs are responsible for lysing the GC cells. Therefore, we conducted a series of experiments to clarify which subtypes affect and regulate the cytotoxicity. T lymphocytes can be divided into CD4+ and CD8+ groups. PBMLs were treated with depleting antibodies against CD4 or CD8 (20 ug/ml) and then cocultured with MGC-803/TTP and BGC-823/TTP cells at 37°C for 16 h. As shown in Fig. 2C, the cytotoxicity decreased extensively when CD8+ T cells were depleted and decreased modestly when CD4+ T cells were depleted. The results meant both CD4+ and CD8+ T cells had an influence on the TTP-mediated cytotoxicity. Hence, we performed further research to determine how TTP enhances the cytotoxicity against GC cells.
Overexpression of TTP in GC cells enhances CD8+ T cell cytotoxicity
CD8+ T cells are shown to be responsible for lysing the GC cells in Fig. 2C. To determine whether TTP could interact with CD8+ T cells directly to adjust the cytotoxicity, CD8+ T cells were isolated from PBMLs using a magnetic cell separation system (MACS). Purified CD8+ T cells were added to the MGC-803/TTP and BGC-823/TTP cells or the control group by E: T at 10:1. After addition, the mixture was cocultured at 37°C for 16 h for PBML-mediated cytotoxicity assay. As shown in Fig. 2D, the cytotoxicity of CD8+ T cells against GC cell lines overexpressing TTP was higher than the control. TTP overexpression significantly increased CD8+ T cell-mediated cytotoxicity against both MGC-803 (P = 0.03) and BGC-823 cells (P = 0.04). However, the cytotoxicity was lower than that mediated by PBMLs. The phenomenon inferred that CD4+ T cells may be involved in the cytotoxicity regulated by TTP. These results suggested that TTP contributed to regulation of antitumor immunity by promoting CD8+ T cell activation per se directly subsequently enhanced its cytotoxicity.
TTP abundance in GC cells decreases infiltration with Tregs
Tumor microenvironmental status, including tumor-infiltrating immune cells is a determinant for cell survival and tumorigenesis (Ai et al., 2017). Tregs are involved in the development of GC according to the existing experimental conclusion (Curiel et al., 2004; Geng et al., 2015; Hatam et al., 2012; Hou et al., 2014). Following our studies of PBML-mediated cytotoxicity of GC cells by TTP, we next explored and evaluated the functional significance of this finding. As discussed above, Tregs play a critical role in mediating the cytotoxicity of effector T cells. We had a reasonable hypothesis that TTP may enhance antitumor immunity by connecting with Tregs. To verify whether TTP enhances the cytotoxicity by reducing the induction of FoxP3+ Tregs, we next examined the percentage change of Tregs in PBMLs after cocultivation with transfected GC cells. PBMLs were added to the MGC-803/TTP and BGC-823/TTP cells or the control group and cocultured at 37°C for 72 h. The cocultured PBMLs were harvested for detection of Tregs. Frequencies of Tregs in PBMLs were measured by flow cytometry with antibodies against CD4, CD25 and Foxp3 after cocultivation. The data in Fig. 3A shows that the percent of Tregs in MGC-803/TTP was 6.56 ± 0.84%, while the control was 11.53 ± 0.9%. The percent of Tregs in BGC-823/TTP was 8.67 ± 0.86%, while the control was 13.43 ± 0.75%. The frequencies of Tregs in PBMLs coclutured with MGC-803/TTP and BGC-823/TTP cells were distinctly lower than the control group (P < 0.05). Foxp3 is the specific molecular marker of Tregs located on chromosome Xp11.23 and is established to detect the number of Tregs (Geng et al., 2015; Hou et al., 2014; Yuan et al., 2011). To detect the infiltration of Tregs into tumor cells, the cocultured GC cells were harvested for qRT-PCR for mRNA expression of Foxp3. The mRNA level of Foxp3 can indirectly indicate the infiltrating Tregs number. As shown in Fig. 3B, the Foxp3 mRNA was lower in MGC-803/TTP and BGC-823/TTP than the control groups (P < 0.05). Thus, TTP overexpression observably reduced induction and infiltration of Tregs into MGC-803/TTP and BGC-823/TTP cells compared with the control group.
Fig. 3. TTP overexpression in GC cells decreased infiltration of Tregs. PBMLs were added to MGC-803/TTP and BGC-823/TTP cells or the control group separately and then cocultured at 37°C for 72 h.
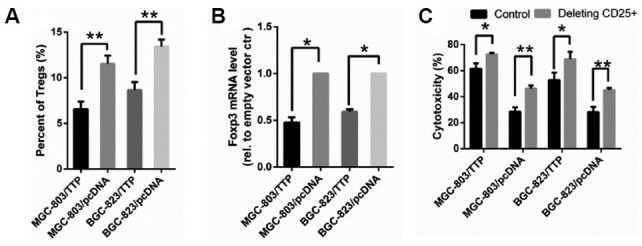
(A) After cocultivation, PBMLs were harvested for detection of Tregs by flow cytometry. (B) After cocultivation, to measure the Tregs infiltration into tumor cells, the cocultured GC cells were harvested for qRT-PCR for mRNA expression of Foxp3. Foxp3 level cocultivation with GC cells transfected with empty vector was used as the control. (C) Depleting antibodies against CD25 were added to the co-culture system and the cytotoxicity change trend was observed. All data were represented as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01.
To further define whether Tregs were involved in the increasing cytotoxicity mediated by TTP, Tregs were depleted by treating with depleting antibodies against CD25 (20 ug/ml). Then, we measured the cytotoxicity after cocultivation. As shown in Fig. 3C, the cytotoxicity was increased after addition of depleting antibodies, which proved that increased cytotoxicity of PBMLs on TTP overexpressing GC cells was due to less abundant Tregs. In conclusion, TTP increases PBML-mediated cytotoxicity by regulating Tregs generation and infiltration in GC cells.
TTP negatively regulates PD-L1 expression in GC cells
The change of tumor microenvironment was a typical indicator of patient survival. PD-L1 was also responsible for Treg infiltration and tumor immune escape (Peligero et al., 2015). Surprisingly, existing studies showed that PD-L1 expression was critically increased in GC tissue (Cho et al., 2017; Hou et al., 2014; Yuan et al., 2016; Zhang et al., 2016), thereby, we reasonably assumed that TTP could down-regulate PD-L1 expression to affect immune function. To investigate whether TTP could regulate the level of PD-L1, we detected PD-L1 level by qRT-PCR and western blotting analysis. As shown in Figs. 4A and 4B, overexpression of TTP in MGC-803/TTP and BGC-823/TTP cells were confirmed by qRT-PCR and western blotting analysis above, TTP was shown to have an inverse correlation with PD-L1 expression compared to levels in control cells.
Fig. 4. TTP regulated the expression of PD-L1 in GC cells.
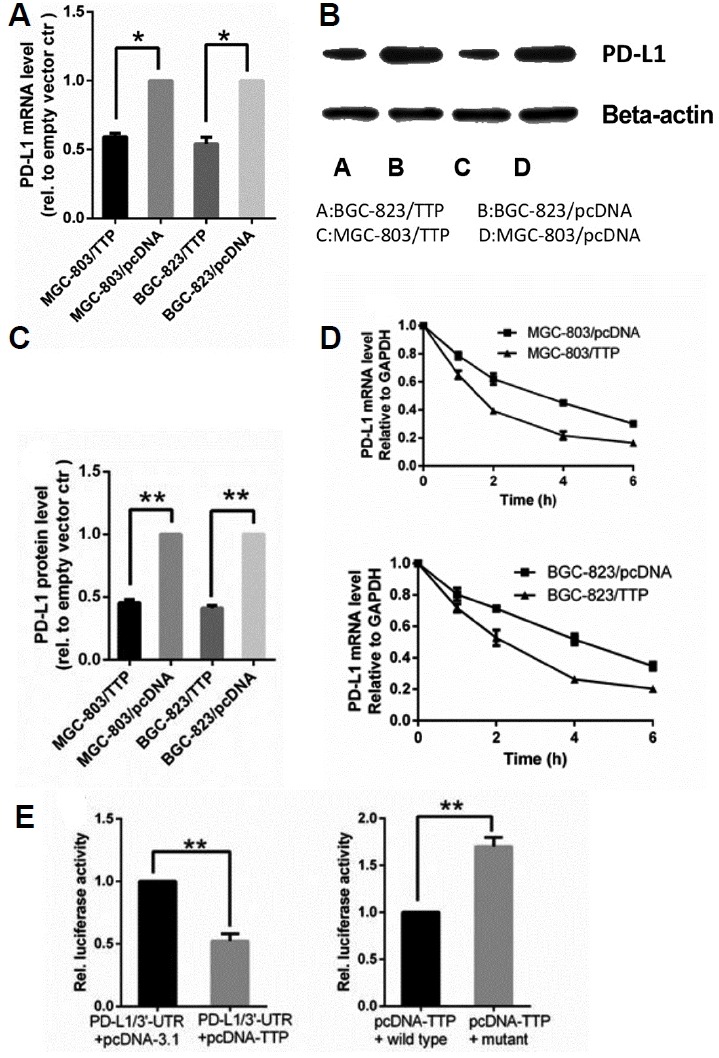
(A) PD-L1 mRNA level in MGC-803/TTP and BGC-823/TTP and corresponding control group was assessed by qRT-PCR. (B) Expression of PD-L1 protein in MGC-803/TTP and BGC-823/TTP and corresponding control group was analyzed by western blotting. (C) Quantifications of western blotting results were processed by Image J software. (D) PD-L1 mRNA stability in MGC-803/TTP and BGC-823/TTP and corresponding control group was analyzed by qRT-PCR after blocking de novo transcription by adding 5 μg/ml actinomycin D (Act D). The remaining PD-L1 mRNA in each cell population and at each time point was determined by cross-normalization using the internal controls. The normalized level of PD-L1 mRNA at time 0 was set at 100%. (E) TTP overexpression inhibited luciferase reporter containing the PD-L1 3′-UTR. AREs in 3′-UTR was required for TTP to down-regulate PD-L1 expression. Renilla luciferase activity was normalized firefly activity. GAPDH and β-actin were used as internal controls for qRT-PCR and western blotting analysis, respectively. All data were represented as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01.
TTP induces destabilization of PD-L1 mRNA
The result above proved that TTP can downregulate PD-L1 expression. Referring to documents, we found that PD-L1 has several ATTTA pentamers in its 3′-UTR (Kataoka et al., 2016). Act D was applied to inhibit the transcription following transfection to examine whether TTP could regulate the stability of PD-L1. Total cellular RNA was harvested after 0, 1, 2, 4, and 6 h, and PD-L1 mRNA levels were measured at these different times by qRT-PCR. As shown in Fig. 4D, upon MGC-803 transfected with TTP, the PD-L1 mRNA decay with a half-life of 1.58 h compared to the half-life of 3.4 h in the control. While BGC-823 transfected with TTP, the half-life of PD-L1 mRNA was 2.1 h compared to 4.2 h in the control. This result indicated that TTP regulated PD-L1 expression in an ARE- dependent manner.
To confirm whether TTP binds to the AREs in the 3′-UTR of PD-L1 directly, we constructed a luciferase reporter linked to a fragment of the 3′-UTR of PD-L1 containing six ATTTA pentamers in the plasmid pGL3 (pGL3/PD-L1 3′-UTR). We transfected MGC-803 cells with luciferase reporters containing the full-length PD-L1 3′-UTR, along with the transfection of plasmids pcDNA-TTP or pcDNA-3.1, as shown in Fig. 4E, TTP overexpression inhibited the luciferase activity. Thus it was concluded that TTP mediated the 3′-UTR of PD-L1. Each ATTTA motif was replaced with ATGCA in mutant to further explore whether TTP could bind to the AREs in the 3′-UTR. When MGC-803 cells were transfected with pcDNA-TTP and pGL3/PD-L1 3′-UTR or the mutant, the luciferase activity was markedly reduced in the wild-type as compared with the mutant (Fig. 4E). TTP has been confirmed to binds to the 3′-UTR of PD-L1 mRNA directly to trigger its degradation and downregulates PD-L1 expression.
PD-L1 affects induction of Tregs and PBML-mediated cytotoxicity
In this part, we speculated that TTP could enhance antitumor immunity by down-regulating PD-L1 expression. To verify that PD-L1 contributed to inducing Tregs and inhibiting PBML-mediated cytotoxicity, we increased its level with human recombinant PD-L1 (rPD-L1) protein (100 ng/ml) in the co-culture system. Indeed, as shown in Fig. 5A, rPD-L1 significantly decreased PBML-mediated cytotoxicity. rPD-L1 abrogated the strengthening effect of TTP on antitumor immunity. We also depleted the function of PD-L1 using a neutralizing antibody against PD-L1 (10 ug/ml) in the coculture system. After cocultivation, we measured the percentage of Tregs in the PBMLs. Adding the PD-L1 neutralizing antibody could help reduce the induction of Tregs mediated by TTP (Fig. 5B). To sum up, TTP suppresses the induction of Tregs and increased PBML-mediated cytotoxicity in vitro, and that PD-L1 is involved in the TTP-mediated strengthening of antitumor immunity. Our team also reported that TTP negatively regulates interleukin (IL)-33 expression, and we conducted research to determine whether IL-33 participated in this process. Treatment with rIL-33 had no significance in the induction of Tregs or PBML-mediated cytotoxicity (data not shown).
Fig. 5. PD-L1 is involved in the TTP-mediated strengthening of antitumor immunity.
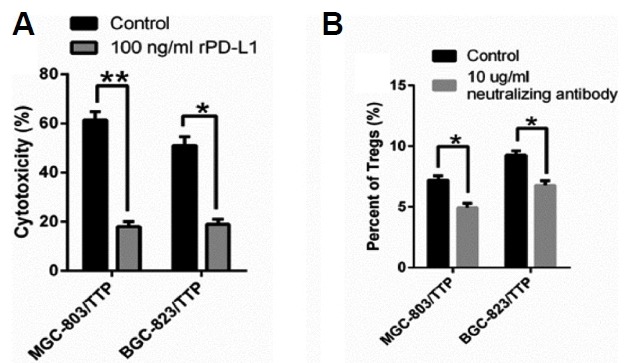
(A)The cytotoxicity was measured in the presence of rPD-L1 (100 ng/ml). (B) The percentage of Tregs in the PBMLs was reduced with the addition of PD-L1 neutralizing antibody (10 ug/ml). All data were represented as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01.
TTP expression is inversely correlated with PD-L1 expression and infiltration of Foxp3+ Tregs in GC tissue
To further understand the role of TTP, PD-L1, and Foxp3+ Tregs in the relationship between the three in GC tissue, we measured the expression of TTP, PD-L1, and Foxp3 in 144 cases of GC specimens by immunohistochemistry score (IHS). As shown in Table 1, the percentage of TTP positive staining in GC tissues was 34.7% (n = 50, IHS > 3) while PD-L1 positive staining was 68.1% (n = 98, IHS > 3). The Foxp3+ positive staining was observed in 72.2% (n =104, IHS > 3) of total specimens. The positive proportion of TTP, PD-L1, and Foxp3 was in agreement with previous studies (Figs. 6A and 6B). We further analyzed the correlations between TTP, PD-L1, or Foxp3 expression and clinicopathological parameters to explore potential role of TTP in cancer and immunity. TTP expression showed significant correlation with invasion depth (P < 0.01), lymph node metastasis (P < 0.01), distant metastasis (P = 0.032), and TMN stage (P < 0.01). The overexpression of PD-L1 was confirmed to have positive association with invasion depth (P < 0.01), differentiation (P < 0.01), lymph node metastasis (P < 0.01), and TMN stage (P < 0.01). Moreover, the high rate of Foxp3+ Tregs in GC tissues was also found to be positively associated with invasion depth (P < 0.01), differentiation (P = 0.011), lymph node metastasis (P < 0.01), and TMN stage (P < 0.01) likewise (Table 1).
Table 1.
Correlation between TTP, PD-L1, or Foxp3 expression and clinicopathological features
| Clinicopathological features | n | TTP | P value | PD-L1 | P value | Foxp3 | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| − | + | + | − | + | − | |||||
| Sex | ||||||||||
| Male | 76 | 46 | 30 | 0.224 | 48 | 28 | 0.212 | 50 | 26 | 0.093 |
| Female | 68 | 48 | 20 | 50 | 18 | 54 | 14 | |||
| Age | ||||||||||
| ≥60 | 84 | 54 | 30 | 0.860 | 54 | 30 | 0.281 | 58 | 26 | 0.350 |
| <60 | 60 | 40 | 20 | 44 | 16 | 46 | 14 | |||
| Invasion depth | ||||||||||
| Muscle/serosa | 116 | 86 | 30 | <0.01** | 88 | 28 | <0.01** | 94 | 22 | <0.01** |
| Mucosa | 28 | 8 | 20 | 10 | 18 | 10 | 18 | |||
| Differentiation | ||||||||||
| Well/Moderate | 48 | 32 | 16 | 0.854 | 22 | 26 | <0.01** | 28 | 20 | 0.011* |
| Poor/Undifferentiated | 96 | 62 | 34 | 76 | 20 | 76 | 20 | |||
| Lymph node metastasis | ||||||||||
| Yes | 92 | 72 | 20 | <0.01** | 76 | 16 | <0.01** | 82 | 10 | <0.01** |
| No | 52 | 22 | 30 | 22 | 30 | 22 | 30 | |||
| Distant metastasis | ||||||||||
| Yes (M1) | 18 | 16 | 2 | 0.032* | 16 | 2 | 0.057 | 16 | 2 | 0.157 |
| No (M0) | 126 | 78 | 48 | 82 | 44 | 88 | 38 | |||
| Size | ||||||||||
| ≤5 cm | 74 | 46 | 28 | 0.485 | 56 | 18 | 0.051 | 56 | 18 | 0.359 |
| >5 cm | 70 | 48 | 22 | 42 | 28 | 48 | 22 | |||
| TNM stage | ||||||||||
| I + II | 62 | 30 | 32 | <0.01** | 30 | 32 | <0.01** | 32 | 30 | <0.01** |
| III + IV | 82 | 64 | 18 | 68 | 14 | 72 | 10 | |||
Fig. 6. Expression of TTP, PD-L1, and Foxp3 is correlated with overall survival of GC.
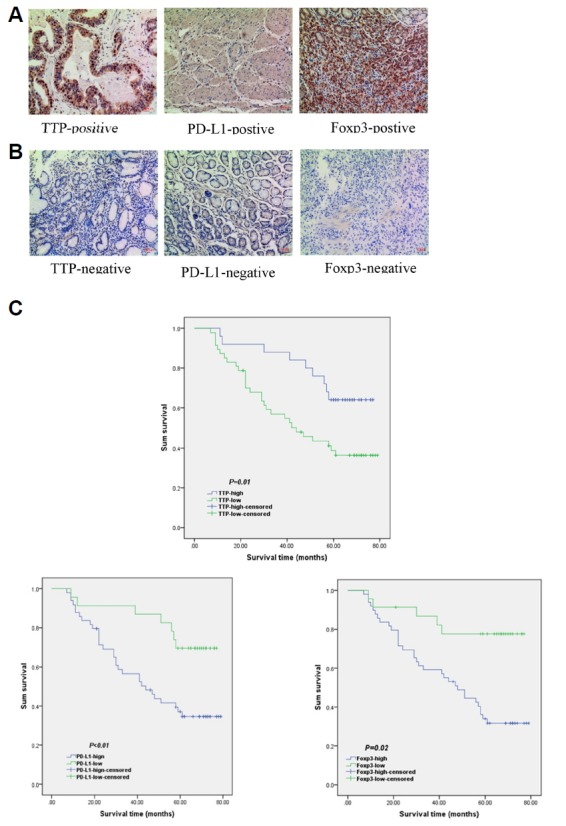
(A) Representative images of TTP, PD-L1, and Foxp3 positive immunohistochemical staining. (B) Representative images of TTP, PD-L1, and Foxp3 negative immunohistochemical staining. (C) Kaplan–Meier curves and log-rank test results for the overall survival for GC patients.
In addition, as discussed above, TTP was found to reduce PD-L1 expression and Tregs infiltration. We evaluated the correlation between TTP and PD-L1/ FOXP3+ Tregs by Spearman correlation analysis. As a result, a negative correlation was confirmed between TTP and PD-L1, or FOXP3+ Tregs, the correlation coefficients (r) were −0.799 (P < 0.01) and −0.335 (P = 0.04), respectively. Besides, PD-L1 expression was also correlated with FOXP3+ Tregs (r = 0.445, P < 0.01) positively (Table 2).
Table 2.
Correlation between TTP, PD-L1 and Foxp3 expression in GC tissues
| Spearman correlation analysis (n = 72) | TTP | PD-L1 | Foxp3+ Tregs |
|---|---|---|---|
| TTP | |||
| r | 1.000 | −0.799 | −0.335 |
| P | – | <0.01** | 0.04* |
| PD-L1 | |||
| r | −0.799 | 1.000 | 0.445 |
| P | <0.01** | – | <0.01** |
| Foxp3+ Tregs | |||
| r | −0.335 | 0.445 | 1.000 |
| P | 0.04* | <0.01** | – |
Survival analysis
All patients were followed up for more than 5 years. We first evaluated the relationship of TTP, PD-L1, and Foxp3 with 5-year overall survival (OS) by Kaplan-Meier analysis in 144 cases of GC tissues. As shown in Fig. 6C, the mean survival time was 47.16 months in TTP-negative cases and 63.84 months in TTP-positive cases (P = 0.001). The mean survival time was 46.84 months in PD-L1-postive cases and 65.83 months in PD-L1-negative cases (P < 0.01). In the meantime, Foxp3+ Tregs −positive infiltration was verified to reduce survival time from 63.5 months to 48.59 months (P < 0.01). In our study, we found further evidence to support that TTP, PD-L1, and Foxp3+ Tregs are all correlated with OS.
Moreover, Cox regression univariate analysis was applied to indicate invasion depth (P < 0.01), lymph node metastasis (P < 0.01), distant metastasis (P < 0.01), TNM stage (P < 0.01), TTP-low expression (P = 0.001), PD-L1 (P < 0.01), and Foxp3 (P < 0.01) overexpression are negative factors for OS. Furthermore, Cox regression multivariate analysis confirmed that invasion depth (HR = 7.734, P < 0.01), distant metastasis (HR = 0.381, P < 0.01), and TNM stage (HR = 0.242, P = 0.012) were three independent prognostic factors our study (Table 3). All of the above data indicate that low TTP expression was associated with PD-L1 and Treg expression, inducing poor prognosis of patients with GC.
Table 3.
Univariate and multivariate analysis for overall survival in GC patients
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
| ||||
| HR (95.0 % CI) | P value | HR (95.0 % CI) | P value | |
| Sex | 0.897 (0.572–1.408) | 0.637 | - | |
| Male vs Female | ||||
| Age | 0.695 (0.410–1.058) | 0.084 | - | - |
| ≥60 vs <60 | ||||
| Invasion depth | 0.072 (0.018–0.296) | <0.01** | 7.735(1.833–32.693) | <0.01** |
| Muscle/serosa vs Mucosa | ||||
| Differentiation | 1.475 (0.869–2.504) | 0.150 | - | - |
| Well/moderate vs Poor | ||||
| Lymph node metastasis | 5.584 (2.861–10.896) | <0.01** | 0.840 (0.246–2.874) | 0.781 |
| Yes vs No | ||||
| Distant metastasis | 5.163 (2.893–9.214) | <0.01** | 0.381(0.207–0.703) | <0.01** |
| Yes vs No | ||||
| Size | 1.561 (0.992–2.456) | 0.054 | ||
| ≤5 cm vs >5 cm | ||||
| TNM stage | 0.146 (0.064–0.336) | <0.01** | 0.242(0.081–0.729) | 0.012* |
| I + II vs III + IV | ||||
| TTP | 2.415 (1.420–4.107) | 0.001* | 1.574 (0.825–3.005) | 0.169 |
| Positive vs Negative | ||||
| PD-L1 | 0.331 (0.185–0.593) | <0.01** | 1.424 (0.667–3.042) | 0.361 |
| Positive vs Negative | ||||
| Foxp3 | 0.299 (0.153–0.582) | <0.01** | 0.967 (0.411–2.278) | 0.939 |
| Positive vs Negative | ||||
DISCUSSION
The expression of TTP, which is obviously down-regulated in GC cells, is confirmed to have a link with different hallmarks of GC, including cell proliferation, migration, and invasion (Deng et al., 2016). Therefore, investigation into the role of TTP in GC is vital for obtaining a better understanding of the regulation mechanism of tumor progression and metastasis, which is beneficial to clinical treatment. However, the results of existing research on this topic remain to be explored. In this study, our aim was to determine the role of TTP in the progression and metastatic potential of GC cells. We focused more attention on the underlying mechanism by which TTP mediated antitumor immunity. There was no evidence that TTP directly regulated antitumor immunity in GC cells ahead of our study.
We have reached a consensus that the microenvironment has a profound influence on tumorigenesis, and immune escape is determined by microenvironment conditions (Becht Giraldo et al., 2016; Iovanna and Closa, 2017). A classical track of tumor progression and metastasis is that malignant cells obtain the capacity to escape from local and systemic immune surveillance and, sequentially, to invade and metastasize to the secondary sites (Eckert et al., 2016; Seo et al., 2015). The cytotoxicity of effector T cells against GC cells is associated with immune escape (Liu et al., 2016). Tregs are critical in host defense against various cancers. According to previous review, Tregs contribute to immune escape and predict poor outcome in many cancers including GC (Hou et al., 2014; Geng et al., 2015; Yuan et al., 2011).
In the present study, we demonstrate that overexpression of TTP decreased survival and promoted apoptosis of both MGC-803 and BGC-823 GC cells. Specifically, we observed reduced Bcl-2 expression and increased caspase 3 activity in TTP-transferred cells. We further provided evidence that TTP can enhance the cytotoxicity of effector T cells against GC cells. During the past decades, it has been reported that Tregs in the tumor microenvironment can inhibit anti-tumor immune responses. Therefore, we attempted to connect the enhanced cytotoxicity in TTP-transferred cells with Tregs infiltration. This proved that TTP is correlative to the number of Tregs in the tumor microenvironment.
The maintenance of immune homeostasis is mediated by various signaling molecules under the control of intricate mechanisms (Newman et al., 2016). The PD-L1 and Treg pathways play a pivotal role in the maintenance of peripheral immune tolerance (Hatam et al., 2012). We also uncovered that TTP appeared to regulate PD-L1 expression in an ARE-dependent manner. TTP was found to have an affinity to bind to AREs in the 3′-UTR of the transcript. Act D experiment and luciferase assays were performed to confirm that TTP negatively regulated PD-L1 expression through destabilizing and degrading its mRNA. Thereby, restoration of PD-L1 strengthened the immunosuppression and inhibited the antitumor immunity. This phenomenon was further evidence that PD-L1 played a role in TTP mediating antitumor immunity.
The most significant findings of this study are the congregation of Foxp3+ Tregs and PD-L1 in the tumor microenvironment of GC, and the correlation between TTP expression and PD-L1 expression or the number of Foxp3+ Tregs in GC tissues was also analyzed. We concluded that a negative correlation existed between TTP and PD-L1or Foxp3 expression.
In summary, our study revealed that TTP in GC cells is functionally related to the regulation of tumor progression and metastasis in GC. TTP can not only reduce cell viability and promote apoptosis but also enhance antitumor immunity. As discussed above, TTP reduces the expression of PD-L1, which in turn inhibits Treg induction and infiltration into GC cells and subsequently increases Treg-mediated cytotoxicity against GC cells. TTP has been proven to affect the functions and hallmarks of GC cells as well as acting on the immune system to suppress tumor progression. Additionally, the significantly negative correlation between TTP and PD-L1 or Foxp3+ Tregs suggests that TTP, PD-L1 and Tregs may be the new targets for the therapy of GC. We anticipate that our findings will help to offer better understanding of the values of TTP on antitumor immunity.
ACKNOWLEDGMENTS
This work was supported by Grants from Wuxi Science and Education Project (QNRC026), the Natural Science Foundation of Jiangsu Province, China (No. BK20170206) and Wuxi Municipal Bureau on Science and Technology (No. YGZXM14035).
REFERENCES
- Ai R., Tao Y., Hao Y., Jiang L., Dan H., Ji N., Zeng X., Zhou Y., Chen Q. Microenvironmental regulation of the progression of oral potentially malignant disorders towards malignancy. Oncotarget. 2017;8:81617–81635. doi: 10.18632/oncotarget.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becht E., Giraldo N.A., Dieu-Nosjean M.C., Sautes-Fridman C., Fridman W.H. Cancer immune contexture and immunotherapy. Curr Opin Immunol. 2016;39:7–13. doi: 10.1016/j.coi.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Brooks S.A., Blackshear P.J. Tristetraprolin (TTP): interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim Biophys Acta. 2013;1829:666–679. doi: 10.1016/j.bbagrm.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J., Lee J., Bang H., Kim S.T., Park S.H., An J.Y., Choi M.G., Lee J.H., Sohn T.S., Bae J.M., et al. Programmed cell death-ligand 1 expression predicts survival in patients with gastric carcinoma with microsatellite instability. Oncotarget. 2017;8:13320–13328. doi: 10.18632/oncotarget.14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel T.J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P., Evdemon-Hogan M., Conejo-Garcia J.R., Zhang L., Burow M., et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Das S., Suarez G., Beswick E.J., Sierra J.C., Graham D.Y., Reyes V.E. Expression of B7-H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infection. Journal of immunology (Baltimore, Md : 1950) 2006;176:3000–3009. doi: 10.4049/jimmunol.176.5.3000. [DOI] [PubMed] [Google Scholar]
- Deng K., Wang H., Shan T., Chen Y., Zhou H., Zhao Q., Xia J. Tristetraprolin inhibits gastric cancer progression through suppression of IL-33. Sci Rep. 2016;6:24505. doi: 10.1038/srep24505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert A.W., Wickenhauser C., Salins P.C., Kappler M., Bukur J., Seliger B. Clinical relevance of the tumor microenvironment and immune escape of oral squamous cell carcinoma. J Trans Med. 2016;14:85. doi: 10.1186/s12967-016-0828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn O.J. Human tumor antigens yesterday, today, and tomorrow. Cancer Immunol Res. 2017;5:347–354. doi: 10.1158/2326-6066.CIR-17-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nature reviews Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- Geng Y., Wang H., Lu C., Li Q., Xu B., Jiang J., Wu C. Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significance. Int J Clin Oncol. 2015;20:273–281. doi: 10.1007/s10147-014-0701-7. [DOI] [PubMed] [Google Scholar]
- Giraldo N.A., Becht E., Remark R., Damotte D., Sautes-Fridman C., Fridman W.H. The immune contexture of primary and metastatic human tumours. Curr Opin Immunol. 2014;27:8–15. doi: 10.1016/j.coi.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Guo J., Qu H., Chen Y., Xia J. The role of RNA-binding protein tristetraprolin in cancer and immunity. Med Oncol. 2017a;34:196. doi: 10.1007/s12032-017-1055-6. [DOI] [PubMed] [Google Scholar]
- Guo J., Wang H., Jiang S., Xia J., Jin S. The cross-talk between tristetraprolin and cytokines in cancer. Anticancer Agents Med Chem. 2017b;17:1477–1486. doi: 10.2174/1871520617666170327155124. [DOI] [PubMed] [Google Scholar]
- Hatam L.J., Devoti J.A., Rosenthal D.W., Lam F., Abramson A.L., Steinberg B.M., Bonagura V.R. Immune suppression in premalignant respiratory papillomas: enriched functional CD4+Foxp3+ regulatory T cells and PD-1/PD-L1/L2 expression. Clin Cancer Res. 2012;18:1925–1935. doi: 10.1158/1078-0432.CCR-11-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren A.M., Koster B.D., Samuels S., Ferns D.M., Chondronasiou D., Kenter G.G., Jordanova E.S., de Gruijl T.D. High and interrelated rates of PD-L1+CD14+ antigen-presenting cells and regulatory T cells mark the microenvironment of metastatic lymph nodes from patients with cervical cancer. Cancer Immunol Res. 2015;3:48–58. doi: 10.1158/2326-6066.CIR-14-0149. [DOI] [PubMed] [Google Scholar]
- Hirai M., Kitahara H., Kobayashi Y., Kato K., Bou-Gharios G., Nakamura H., Kawashiri S. Regulation of PD-L1 expression in a high-grade invasive human oral squamous cell carcinoma microenvironment. Int J Oncol. 2017;50:41–48. doi: 10.3892/ijo.2016.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J., Yu Z., Xiang R., Li C., Wang L., Chen S., Li Q., Chen M., Wang L. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol. 2014;96:284–291. doi: 10.1016/j.yexmp.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Iovanna J.L., Closa D. Factors released by the tumor far microenvironment are decisive for pancreatic adenocarcinoma development and progression. Oncoimmunology. 2017;6:e1358840. doi: 10.1080/2162402X.2017.1358840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch K.M., Heissmeyer V. Regulation of T cell signaling and autoimmunity by RNA-binding proteins. Curr Opin Immunol. 2016;39:127–135. doi: 10.1016/j.coi.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Junttila M.R., de Sauvage F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- Kataoka K., Shiraishi Y., Takeda Y., Sakata S., Matsumoto M., Nagano S., Maeda T., Nagata Y., Kitanaka A., Mizuno S., et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature. 2016;534:402–406. doi: 10.1038/nature18294. [DOI] [PubMed] [Google Scholar]
- Li Z., Dong P., Ren M., Song Y., Qian X., Yang Y., Li S., Zhang X., Liu F. PD-L1 Expression Is Associated with Tumor FOXP3(+) Regulatory T-Cell infiltration of breast cancer and poor prognosis of patient. J Cancer. 2016;7:784–793. doi: 10.7150/jca.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Lu J., Tian H., Du W., Zhao L., Feng J., Yuan D., Li Z. Increased expression of PDL1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol Med Rep. 2017;15:1063–1070. doi: 10.3892/mmr.2017.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Bakthavatsalam R., Meng Z., Li Z., Li W., Perkins J.D., Reyes J. PD-L1 signal on liver dendritic cells is critical for Foxp3(+)CD4(+)CD25(+) Treg and liver tolerance induction in mice. Transplant Proc. 2013;45:1853–1855. doi: 10.1016/j.transproceed.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Liu X., Yang Z., Latchoumanin O., Qiao L. Antagonizing programmed death-1 and programmed death ligand-1 as a therapeutic approach for gastric cancer. Therapeutic advances in gastroenterology. 2016;9:853–860. doi: 10.1177/1756283X16658251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milke L., Schulz K., Weigert A., Sha W., Schmid T., Brune B. Depletion of tristetraprolin in breast cancer cells increases interleukin-16 expression and promotes tumor infiltration with monocytes/macrophages. Carcinogenesis. 2013;34:850–857. doi: 10.1093/carcin/bgs387. [DOI] [PubMed] [Google Scholar]
- Mohamed M.S., Bishr M.K., Almutairi F.M., Ali A.G. Inhibitors of apoptosis: clinical implications in cancer. Apoptosis. 2017;22:1487–1509. doi: 10.1007/s10495-017-1429-4. [DOI] [PubMed] [Google Scholar]
- Newman R., McHugh J., Turner M. RNA binding proteins as regulators of immune cell biology. Clin Exp Immunol. 2016;183:37–49. doi: 10.1111/cei.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peligero C., Argilaguet J., Guerri-Fernandez R., Torres B., Ligero C., Colomer P., Plana M., Knobel H., Garcia F., Meyerhans A. PD-L1 blockade differentially impacts regulatory T cells from HIV-infected individuals depending on plasma viremia. PLoS Pathogens. 2015;11:e1005270. doi: 10.1371/journal.ppat.1005270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Ortin J.E., Alepuz P., Chavez S., Choder M. Eukaryotic mRNA decay: methodologies, pathways, and links to other stages of gene expression. J Mol Biol. 2013;425:3750–3775. doi: 10.1016/j.jmb.2013.02.029. [DOI] [PubMed] [Google Scholar]
- Pollack S.M., He Q., Yearley J.H., Emerson R., Vignali M., Zhang Y., Redman M.W., Baker K.K., Cooper S., Donahue B., et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. 2017 doi: 10.1002/cncr.30726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J. [Biology of cancer metastasis]. Bulletin du Cancer. 2013;100:333–342. doi: 10.1684/bdc.2013.1724. [DOI] [PubMed] [Google Scholar]
- Sanduja S., Blanco F.F., Dixon D.A. The roles of TTP and BRF proteins in regulated mRNA decay. Wiley interdisciplinary reviews. RNA. 2011;2:42–57. doi: 10.1002/wrna.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanduja S., Blanco F.F., Young L.E., Kaza V., Dixon D.A. The role of tristetraprolin in cancer and inflammation. Frontiers in bioscience (Landmark edition) 2012;17:174–188. doi: 10.2741/3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (New York, NY) 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Seo G.S., Jiang W.Y., Chi J.H., Jin H., Park W.C., Sohn D.H., Park P.H., Lee S.H. Heme oxygenase-1 promotes tumor progression and metastasis of colorectal carcinoma cells by inhibiting antitumor immunity. Oncotarget. 2015;6:19792–19806. doi: 10.18632/oncotarget.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Tan M.P., Dolton G.M., Gerry A.B., Brewer J.E., Bennett A.D., Pumphrey N.J., Jakobsen B.K., Sewell A.K. Human leucocyte antigen class I-redirected anti-tumour CD4(+) T cells require a higher T cell receptor binding affinity for optimal activity than CD8(+) T cells. Clin Exp Immunol. 2017;187:124–137. doi: 10.1111/cei.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M., Zhang Y., Liu Z., Sun G., Mor G., Liao A. The PD-1/PD-L1 inhibitory pathway is altered in pre-eclampsia and regulates T cell responses in pre-eclamptic rats. Sci Rep. 2016;6:27683. doi: 10.1038/srep27683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S., Guleria I. Role of PD1/PDL1 pathway, and TH17 and treg cells in maternal tolerance to the fetus. Biomed J. 2015;38:25–31. doi: 10.4103/2319-4170.143511. [DOI] [PubMed] [Google Scholar]
- Yuan J., Zhang J., Zhu Y., Li N., Tian T., Li Y., Li Y., Li Z., Lai Y., Gao J., et al. Programmed death-ligand-1 expression in advanced gastric cancer detected with RNA in situ hybridization and its clinical significance. Oncotarget. 2016;7:39671–39679. doi: 10.18632/oncotarget.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X.L., Chen L., Zhang T.T., Ma Y.H., Zhou Y.L., Zhao Y., Wang W.W., Dong P., Yu L., Zhang Y.Y., et al. Gastric cancer cells induce human CD4+Foxp3+ regulatory T cells through the production of TGF-beta1. World J Gastroenterol. 2011;17:2019–2027. doi: 10.3748/wjg.v17.i15.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng B., Zhu D., Su Z., Li Z., Yu Z. Tristetraprolin exerts tumor suppressive functions on the tumorigenesis of glioma by targeting IL-13. Int Immunopharmacol. 2016;39:63–70. doi: 10.1016/j.intimp.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Zhang M., Dong Y., Liu H., Wang Y., Zhao S., Xuan Q., Wang Y., Zhang Q. The clinicopathological and prognostic significance of PD-L1 expression in gastric cancer: a meta-analysis of 10 studies with 1,901 patients. Sci Rep. 2016;6:37933. doi: 10.1038/srep37933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X.T., Xiao X.Q., Dai J.J. Corrigendum to “Sodium butyrate down-regulates tristetraprolin-mediated cyclin B1expression independent of the formation of processing bodies” [Int. J. Biochem. Cell Biol. 69 (2015) 241–248] Int J Biochem Cell Biol. 2016;74:161. doi: 10.1016/j.biocel.2016.02.024. [DOI] [PubMed] [Google Scholar]


