Abstract
BACKGROUND/OBJECTIVE
There is intense interest in soy isoflavone as a hormone replacement therapy for the prevention of postmenopausal osteoporosis. A new kind of isoflavone-enriched whole soy milk powder (I-WSM) containing more isoflavones than conventional whole soy milk powder was recently developed. The aim of this study was to investigate the effects of I-WSM on bone metabolism in ovariectomized mice.
MATERIALS/METHODS
Sixty female ICR mice individually underwent ovariectomy (OVX) or a sham operation, and were randomized into six groups of 10 animals each as follows: Sham, OVX, OVX with 2% I-WSM diet, OVX with 10% I-WSM diet, OVX with 20% I-WSM diet, and OVX with 20% WSM diet. After an 8-week treatment period, bone mineral density (BMD), calcium, alkaline phosphatase (ALP), tartrate-resistant acid phosphatase (TRAP) 5b, osteocalcin (OC), procollagen 1 N-terminal propeptide (P1NP), and osteoprotegenin (OPG) were analyzed.
RESULTS
BMD was significantly lower in the OVX group compared to the Sham group but was significantly higher in OVX + 10% I-WSM and OVX + 20% I-WSM groups compared to the OVX group (P < 0.05). Serum calcium concentration significantly increased in the OVX + 10% and 20% I-WSM groups. Serum ALP levels were significantly lower in the OVX + 10% and 20% I-WSM groups compared to the other experimental groups (P < 0.05). OC was significantly reduced in the OVX group compared to the Sham group (P < 0.05), but a dose-dependent increase was observed in the OVX groups supplemented with I-WSM. P1NP and OPG levels were significantly reduced, while TRAP 5b level was significantly elevated in the OVX group compared with the Sham group, which was not affected by I-WSM (P < 0.05).
CONCLUSIONS
This study suggests that I-WSM supplementation in OVX mice has the effect of preventing BMD reduction and promoting bone formation. Therefore, I-WSM can be used as an effective alternative to postmenopausal osteoporosis prevention.
Keywords: Functional food, soybeans, isoflavones, ovariectomy, bone remodeling
INTRODUCTION
Postmenopausal osteoporosis is usually associated with aging and decreased levels of the ovarian sex hormone estrogen. The main clinical manifestation of this metabolic disorder is weakening of bones and increased risk of fractures [1]. This has become a serious public health problem in the current aging society. The first-line therapy for postmenopausal osteoporosis is estrogen replacement therapy (ERT), which can prevent bone loss and promote bone formation [2]. However, data also suggest that ERT increases the risk of breast cancer, endometrial cancer, vaginal bleeding, coronary heart disease, stroke, and venous thromboembolism [3,4,5]. These serious side effects limit the application of ERT. Thus, there is increasing interest in the use of plant-derived estrogens, known as phytoestrogens.
Phytoestrogens are a large group of polyphenolics, i.e., non-steroidal plant compounds with a chemical structure such as 17 β-estradiol, which can bind to estrogen receptors and mimic the actions of estrogens on target organs, thereby exerting many health benefits when used under hormone-dependent conditions [6,7]. The main classes of phytoestrogens are isoflavones, lignans, coumestanes, flavonoids, and stilbenes [8,9]. Among them, isoflavones have the most potent estrogenic activity; genistein, daidzein, and glycitein, in particular, are the most active isoflavones found in soybeans. Epidemiological and experimental studies have confirmed that isoflavones have a bone-sparing effect in postmenopausal women [10,11,12] and in ovariectomized rat models [13,14]. However, the effect of isoflavones on bone metabolism in postmenopausal women is not always positive. Previous randomized controlled trials have shown that the effects of isoflavones supplementation can differ depending on the race/ethnicity of the subjects, research design, variety of isoflavone supplements, dosage, duration of exposure, and responsiveness of postmenopausal women to isoflavones supplementation [15]. Recently, the chemical forms of isoflavones have been reported to be important since they can influence the bioavailabilities and, thus biological activities of isoflavones [7].
A recently developed type of isoflavone-enriched whole soy milk powder (I-WSM) was shown to contain about 8.8 times more isoflavones than conventional whole soy milk powder (WSM). Thus, I-WSM is expected to be more effective than WSM in preventing and treating postmenopausal osteoporosis, and this study was conducted to verify this idea.
MATERIALS AND METHODS
Preparation of I-WSM
I-WSM was produced by Uwell Bio Co. Ltd. (Gangneung, Korea). Soybeans used in this study were cultivated in the Yeongwol region of Gangwon province in 2015. Washed whole soybeans were soaked for 7 h in water at a soybeans:water ratio of 1:9. After 7 h of soaking, soybeans were grounded by using a blender (K&S Co., LTD., Whasung, Korea). The slurry was heated at 90℃ for 1 h, followed by cooling at 55℃. To hydrolyze carbohydrates, the slurry was added to 2% (w/w) termamyl (Novozymes, Denmark) and then incubated at 55℃ for 5 h. The slurry was then centrifuged at 3,500 x g for 20 min to separate soy milk cake residues, and the resulting soy milk was obtained. To hydrolyze protein, soy milk was added to 1% (w/w) alcalase (Novozymes, Copenhagen, Denmark) and incubated at 55℃ for 5 h, followed by addition of 1% (w/w) flavourzyme (Novozymes) and incubation at 55℃ for 5 h. Next, soy milk was boiled at 100℃ for 30 min to inactivate enzymes. The final produced soy milk was freeze-dried by using a vacuum freeze-dryer (Operon, Kimpo, Korea) and grounded to a particle size of less than 75 µm for final use. The resulting powder was used as an isoflavone-enriched whole soy milk powder (I-WSM) and stored at −20℃ until further use. The isoflavone content of this powder was analyzed by using HPLC (Agilent, CA, USA). The concentration of total isoflavones was 1,660 µg/g of I-WSM, which was about 8.8 times higher than the isoflavone content of 187.8 µg/g of WSM.
Animals, diet, and study design
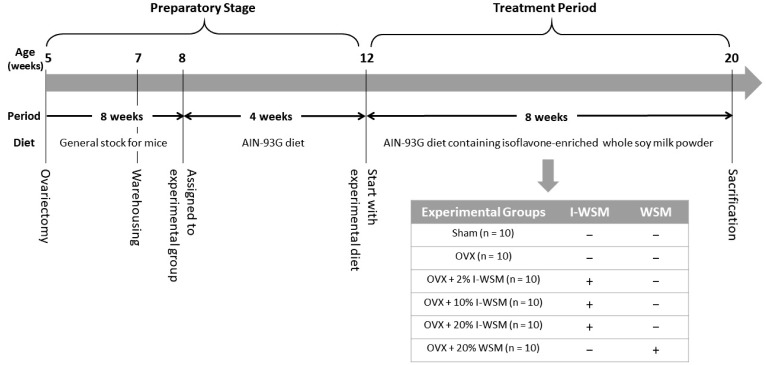
The study design is shown in Fig. 1 (n = 10). A total of 60 7-week-old female ICR mice were purchased from Dooyeol Biotech Co. Ltd. (Seoul, Korea). Each mouse underwent either an ovariectomy (OVX, n = 50) or were sham-treated (n = 10) at 5 weeks of age. The mice were housed in polycarbonate cages located in temperature-controlled rooms (23 ± 3℃) with a relative humidity of 50 ± 10%, ventilation number of 10–15 times, illumination of 150–300 Lux, and a 12 h light/dark cycle. During the adaptation period, all animals were given free access to food from Cargill Agri Purina, Inc. (Seongnam, Korea) and water.
Fig. 1. Experimental design.
Sham, sham-operated; OVX, ovariectomized; I-WSM, isoflavone-enriched whole soy milk powder diet; WSM, whole soy milk powder. The mice were allocated to six different treatment groups.
After 7 days of acclimation, the mice were randomly assigned to six experimental groups: Sham, OVX, OVX with 2% I-WSM diet (OVX + 2% I-WSM), OVX with 10% I-WSM diet (OVX + 10% I-WSM), OVX with 20% I-WSM diet (OVX + 20% I-WSM), and OVX with 20% WSM diet (OVX + 20% WSM). The manufacturer of WSM, which is a meal replacement supplement, claims that it can replace up to 30% of daily food intake. The OVX + 20% I-WSM and OVX + 20% WSM groups were formed in order to compare the effects when I-WSM and WSM replaced 20% of daily food intake, and the OVX + 2% I-WSM and OVX + 10% I-WSM groups were for comparing the effects in relation to I-WSM dosage. The number of animals in each experimental group was 10. After the experimental groups were classified, the mice were fed a basic diet (AIN-93G diet, Research Diets Inc., New Brunswick, NJ, USA) for 4 weeks to induce osteoporosis. Then, the experimental animals were fed I-WSM or a WSM-containing experimental diet for 8 weeks. The experimental diets were prepared by mixing 2% I-WSM, 10% I-WSM, 20% I-WSM, or 20% WSM into AIN-93G diet. The daily food intake in each group was measured, and the mice were weighed weekly.
These animal studies were conducted according to the protocols with approval from the Animal Care and Use Committee of Hallym University (approval number: Hallym 2017-23).
Specimen collection and biochemical assessment
After 8 weeks on the experimental diet, the mice were anesthetized with tribromoethanol diluted with tertiary amyl alcohol and blood was drawn from the orbital vein. Blood samples were centrifuged at 3,000 rpm for 20 min at 4℃ to prepare the serum. The separated serum stored at −70℃ was subjected to biochemical analysis for the contents of calcium (Ca), phosphorous (P), alkaline phosphatase (ALP), tartrate-resistant acid phosphatase (TRAP) 5b, osteocalcin (OC), procollagen 1 N-terminal propeptide (P1NP), and osteoprotegenin (OPG). Serum levels of Ca, P, and ALP were measured by a blood chemistry autoanalyzer (KoneLAb 20 XT, Thermo Fisher Scientific, Vantaa, Finland). Serum levels of P1NP, OPG, and OC were measured using relevant ELISA kits (Elabscience Biotechnology Inc., Houston, TX, USA), and serum levels of TRAP 5b were measured using an ELISA kit (EIAab, Hubei, China), in accordance with the manufacturer's instructions.
Measurement of bone mineral density
The femurs of mice were dissected to remove soft tissues and were then weighed. Femoral bone mineral densities (BMD) were measured using a bone densitometer (PIXImus™, GE Healthcare, Little Chalfont, England).
Histomorphological Study
The femurs were fixed in 4% paraformaldehyde solution for 24 h. After fixation, they were demineralized for 24 h in Calci-Clear Rapid (National Diagnostics, Atlanta, GA, USA), treated according to a general tissue-processing procedure, embedded in paraffin, and then cut to a thickness of 10 µm. The bone tissues were stained with hematoxylin and eosin (H&E) and examined under a light microscope (Axiomager, Carl Zeiss, Jena, Germany).
Statistical Analysis
Statistical analysis was performed with SAS for Windows version 9.1 software (SAS Institute, Cary, NC, USA). All data are presented as a mean ± standard error of the mean (SEM). Differences in measured parameters among the experimental groups were analyzed by one-way analysis of variance and Duncan's multiple range tests. Differences were significant for P < 0.05.
RESULTS
Body weight and food intake
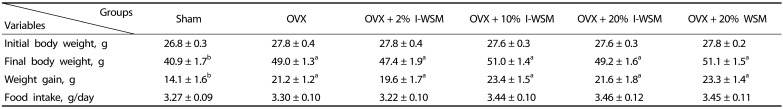
As shown in Table 1, final body weight and body weight gain were significantly higher in the OVX group compared to the sham group (P < 0.01). There was no significant difference in final body weight among the groups treated with OVX. Food intake of the experimental animals did not differ among the six groups.
Table 1. Body weight and food intake of mice treated with isoflavone-enriched whole soy milk powder (I-WSM).
Values are expressed as mean ± SE. Sham, sham-operated; OVX, ovariectomized; I-WSM, isoflavone-enriched whole soy milk powder; WSM, whole soy milk powder. Statistical difference between the experimental groups based on one-way analysis of variance and Duncan's multiple range tests at P < 0.05. Means with different alphabetical superscripts are significantly different (P < 0.05).
Effects of I-WSM on BMD and morphology of femurs in ovariectomized mice
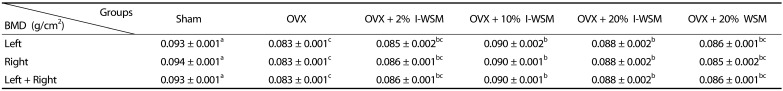
After the treatments, BMDs of the femurs extracted from the sacrificed animals were measured, and the results are shown in Table 2. The BMDs of the left and right femurs were 0.093 ± 0.001 g/cm2 in the Sham group and 0.083 ± 0.001 g/cm2 in the OVX group, indicating that the OVX group had significantly lower BMD than the Sham group (P < 0.001). This result indicates that ovariectomies led to reduction of bone densities. Compared to the OVX group, the OVX + 2% I-WSM group did not show any difference in BMD, whereas the OVX + 10% and 20% I-WSM groups showed significant increases in BMD. The OVX + 20% WSM and OVX groups showed no difference in BMD. These results show that greater than 10% I-WSM supplementation prevented reduction of BMD in ovariectomized postmenopausal animals and that I-WSM was more helpful in maintaining BMD after menopause than WSM. However, BMD of the OVX + 20% I-WSM group was lower than that of the OVX + 10% I-WSM group. These results suggest that the increase in BMD due to I-WSM supplementation was not in a dose-dependent manner. Further studies are needed to determine the levels of I-WSM supplementation that may be helpful in increasing BMD.
Table 2. Effects of isoflavone-enriched whole soy milk powder on bone mineral density of femurs in ovariectomized mice.
Values are expressed as mean ± SE. Sham, sham-operated; OVX, ovariectomized; I-WSM, isoflavone-enriched whole soy milk powder; WSM, whole soy milk powder. Statistical difference between the experimental groups based on one-way analysis of variance and Duncan's multiple range tests at P < 0.05. Means with different alphabetical superscripts are significantly different (P < 0.05).
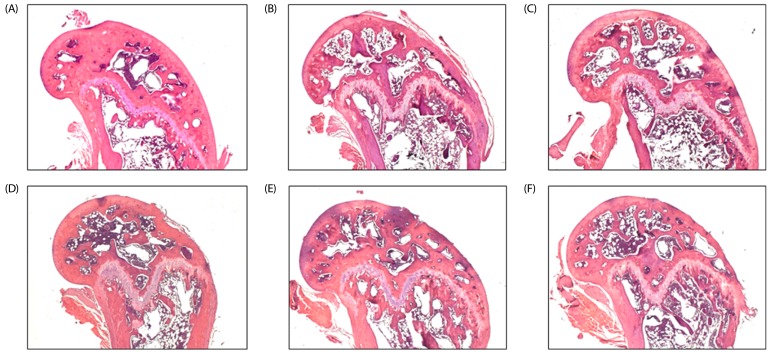
As shown in Fig. 2, the density of the femoral cortical and trabecular bone was significantly lower in the OVX group compared to the Sham group. However, the magnitude of the reduction tended to be lower in the OVX + I-WSM groups.
Fig. 2. Effects of isoflavone-enriched whole soy milk powder on femur bone morphology in ovariectomized mice.
(A) G1: The femur in mice of the Sham group. (B) G2: The femur in mice of the OVX group. (C) G3: The femur in mice of the OVX + 2% I-WSM group. (D) G4: The femur in mice of the OVX + 10% I-WSM group. (E) G5: The femur in mice of the OVX + 20% I-WSM group. (E) G6: The femur in mice of the OVX + 20% WSM group. All pictures were stained with H & E and examined under x40 magnification.
Effects of I-WSM on serum levels of calcium and phosphorous in ovariectomized mice
Serum levels of Ca and P in the experimental groups were measured in order to investigate the effect of I-WSM on mineral homeostasis, and the results are shown in Table 3. Serum Ca concentration of the OVX group was lower than that of the Sham group; however, this value was not statistically significant. In contrast, the concentrations of Ca in the OVX + 10% I-WSM, OVX + 20% I-WSM, and OVX + 20% WSM groups were significantly higher compared with the OVX group. Serum P concentration was significantly reduced by ovariectomy but then recovered to be the same level as the Sham group upon I-WSM or WSM supplementation.
Table 3. Serum levels of calcium and phosphorous in ovariectomized mice treated with isoflavone-enriched whole soy milk powder for 8 weeks.
Values are expressed as mean ± SE. Sham, sham-operated; OVX, ovariectomized; I-WSM, isoflavone-enriched whole soy milk powder; WSM, whole soy milk powder. Statistical difference between the experimental groups based on one-way analysis of variance and Duncan's multiple range tests at P < 0.05. Means with different alphabetical superscripts are significantly different (P < 0.05).
Effects of I-WSM on serum levels of bone turnover markers in ovariectomized mice
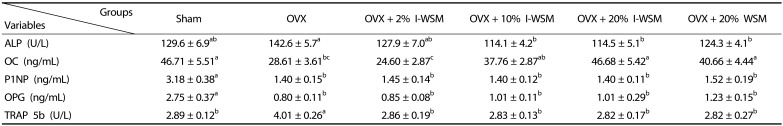
Serum levels of bone turnover markers in the experimental groups are shown in Table 4. In this study, ALP, OC, P1NP, and OPG were measured as bone formation markers, and TRAP 5b was measured as a bone resorption marker. ALP, an enzyme secreted from osteoblasts, reflects the activity of osteoblasts and is an important index of osteogenesis. It has been reported that an increase in serum ALP level is associated with an increase in osteoblast activity after ovariectomy-induced osteoporosis [16]. In this study, serum levels of ALP were significantly increased in the OVX group compared to the Sham group. Serum levels of ALP were significantly lower in the OVX + 10% I-WSM, OVX + 20% I-WSM, and OVX + 20% WSM groups than in the OVX group. OC, a protein produced in osteoblasts, reflects the activity of osteoblasts and is an important indicator of bone formation. The OC level was significantly reduced in the OVX group compared to the Sham group, but dose-dependently elevated in the OVX groups upon I-WSM supplementation. In OVX mice supplemented with 20% I-WSM or 20% WSM, the OC level increased to the same level as the Sham group. OPG is an osteoclastogenesis inhibitory factor that inhibits activation of osteoclasts and promotes osteoclast apoptosis [17], and P1NP has recently emerged as a better marker of bone formation than ALP [18]. In this study, P1NP and OPG levels were significantly reduced by ovariectomy and did not recover to the same levels as the Sham group, even in OVX groups supplemented with I-WSM or WSM.
Table 4. Serum levels of bone turnover markers in ovariectomized mice treated with isoflavone-enriched whole soy milk powder for 8 weeks.
Values are expressed as mean ± SE. Sham, sham-operated; OVX, ovariectomized; I-WSM, isoflavone - enriched whole soy milk powder; WSM, whole soy milk powder; ALP, alkaline phosphatase; OC, osteocalcin; P1NP, procollagen type 1 amino-terminal propeptide; TRAP 5b, tartrate-resistant acid phosphatase 5b; OPG, osteoprotegerin. Statistical difference between the experimental groups based on one-way analysis of variance and Duncan's multiple range tests at P < 0.05. Means with different alphabetical superscripts are significantly different (P < 0.05)
Under normal circumstances, TRAP is highly expressed by osteoclasts. TRAP expression is increased under certain pathological conditions such as osteoporosis [19]. TRAP 5b level was significantly elevated in the OVX group compared to the Sham group, whereas it decreased to the same level as the Sham group in OVX groups supplemented with I-WSM or WSM.
The results above indicate that changes in ALP, OS, and TRAP 5b in OVX mice can be prevented by supplementation with I-WSM or WSM. However, changes in P1NP and OPG due to OVX did not recover, even upon supplementation with I-WSM or WSM. Therefore, in the context postmenopausal bone metabolism, isoflavone supplementation seemed to have a greater effect on bone formation markers than on bone resorption markers, which may vary depending on the type of bone formation marker under investigation.
DISCUSSION
Estrogens are important regulators of body composition and lipid metabolism in females [20]. After menopause, estrogen is no longer secreted, which often results in obesity [21]. This study was conducted to determine the estrogenic effects of soy isoflavone, a phytoestrogen that affects bone metabolism, in ovariectomized female mice in vivo. In our study, OVX ICR mice were chosen as an animal model. A bilateral OVX ICR mouse model was utilized to mimic the conditions in postmenopausal women [22]. It has already been demonstrated that ovariectomized mice and rats show significant increases in visceral adipose tissue mass as well as an increased risk of obesity compared to Sham controls [23,24]. In this study, the body weight of the OVX group was significantly higher than that of the Sham group, although there was no difference in food intake among the experimental groups. OVX groups supplemented with I-WSM and WSM also had a significantly higher body weight than the Sham group. This differs from other studies, in which an isoflavone-enriched diet was shown to inhibit weight gain in OVX rats [24]. This may be due to differences in the duration and dose of isoflavone supplementation and age at ovariectomy.
It has been reported that OVX and OVX-induced obesity are associated with reduced bone formation [24,25,26]. In agreement with data from the literature [24,25,26], we observed ovariectomy-induced bone loss in the animal model in this study (Table 2). Previous studies have shown that an isoflavone-rich diet exhibits bone protective effects in OVX animals. In this study, isoflavone supplementation inhibited reduction of BMD in OVX mice (Fig 2, Table 2). In addition, although the gap was relatively small, I-WSM supplementation seemed to be more effective in improving BMD of OVX mice compared to WSM supplementation. However, improvement of femoral BMD by I-WSM supplementation in OVX animals was not completely dose-dependent. Diets with 10% I-WSM or 20% I-WSM had the effect of inhibiting OVX-induced BMD reduction compared to a diet with 2% I-WSM. However, the respective effects of 10% and 20% I-WSM on the femoral BMD of OVX mice did not significantly differ, while being slightly higher in the 10% I-WSM supplementation group. Therefore, further studies are needed to determine the optimal level of I-WSM supplementation to prevent postmenopausal osteoporosis.
Previous studies have suggested that isoflavones could exert estrogen-like effects in estrogen-deficient animal models and postmenopausal women [5,12,27]. Studies by Wang [28] have showed that genistein supplementation promotes bone mineralization by increasing bone Ca, P, and Mg, thereby preventing osteoporosis by adjusting serum calcitonin. Qi and Zheng [5] reported that genistein supplementation (5 mg/g body weight) for 10 weeks increased serum levels of Ca and P in ovariectomized rats. In this study, following treatment with I-WSM for 8 weeks, serum Ca and P levels in OVX mice increased (Table 3). Particularly, serum Ca levels in the OVX + 10% I-WSM, OVX + 20% I-WSM, and OVX + 20% WSM groups were higher compared to the Sham group (P < 0.05).
In this study, several bone turnover markers related to bone formation and bone resorption were analyzed. Maintenance of bone mass is achieved through a balance between the osteogenic action of osteoblasts and the bone resorption action of osteoclasts [16]. After menopause, bone resorption occurs faster than osteogenesis, which causes loss of bone mass [29]. Bone turnover markers have been studied for decades. Biochemical monitoring of bone metabolism involves the measurement of enzymes and proteins secreted during bone formation and bone resorption. Although BMD is the best diagnostic tool to diagnose osteoporosis and bone turnover markers are used more as adjunctive factors, the effectiveness of bone turnover markers has been verified in numerous recent reports [30,31,32]. Bone turnover markers change more rapidly than BMD or risk of fracture and are thus useful for early analysis of osteoporosis treatment effects [32]. The serum bone formation markers analyzed in this study were ALP, OC, PINP, and OPG. ALP has been clinically available for several years as a marker for bone metabolism [33]. In bones, ALP is present in the cell membrane of osteoblasts and is an indicative markers of osteogenic activity [16]. OC, a non-collagen bone matrix protein, is produced by osteoblasts and is implicated in bone mineralization [33]. As the bone matrix is mineralized by the deposition of newly formed OC, a portion of it is released into the blood [33,34]. Therefore, serum OC level is considered as a specific marker of osteoblast function related to bone formation, as it correlates with the bone formation rate [35]. Procollagen type 1 contains N- and C-terminal extensions, which are removed by specific proteases during conversion of procollagen to collagen. These extensions are the C- and N-terminal propeptides of procollagen type 1 (P1CP and P1NP). It was reported that P1NP is a very sensitive marker of the bone formation rate in osteoporosis [30]. OPG is also known as osteoclastogenesis inhibitory factor or tumor necrosis factor receptor superfamily member 11B. OPG production is stimulated in vivo by the female sex hormone estrogen. OPG has been reported to inhibit osteoclast activation and promote osteoclast apoptosis [17]. OPG specifically acts on bone, increasing BMD and bone volume [36]. Moreover, OPG has been used experimentally to reduce bone resorption in women with postmenopausal osteoporosis and in patients with lytic bone metastases [37]. In this study, the levels of ALP, OC, P1NP, and OPG were all altered to indicate osteoporosis after ovariectomy (Table 4). ALP level was not significantly different between the OVX and Sham groups, whereas it significantly decreased in the OVX + 10% I-WSM and OVX + 20% I-WSM groups compared to the Sham group. Serum levels of OC decreased significantly in the OVX group compared to the Sham group, but increased dose-dependently by I-WSM supplementation and were recovered to the same level as the Sham group. Serum levels of P1NP and OPG showed significant reductions in OVX to 44% and 29%, respectively, compared with the Sham group, and this decrease was not reversed even after I-WSM supplementation. It was reported in three previous randomized controlled trials that supplementation with soy isoflavone had no significant effect on serum P1NP [38,39,40]. In contrast to our OC results, Fu et al. [12] reported in systematic reviews and meta-analyses that phytoestrogen treatment increased BMD and significantly reduced bone turnover markers such as ALP and OC. In this study, I-WSM seemed to influence bone formation in OVX-induced postmenopausal animal models. However, these effects were significantly different depending on the type of bone formation marker. In other words, regarding the four bone formation markers analyzed in this study, I-WSM supplementation in OVX-ICR mice was most effective in altering OC, slightly effective in altering ALP, and ineffective in altering PINP and OPG levels.
TRAP 5b, which has been reported as a sensitive marker of bone resorption, was also analyzed in this study [41,42]. Human serum contains two forms of TRAP, namely 5a and 5b. Unlike 5b, the 5a variant contains sialic acid. In this study, the level of TRAP 5b was 138.8% higher than that of the Sham group following OVX (Table 4). However, this dramatically decreased to Sham level after I-WSM supplementation. Thus, I-WSM supplementation in OVX menopausal mice seemed to be effective in preventing bone resorption.
In conclusion, this study examined the effect of I-WSM on bone metabolism in ovariectomized menopausal mice. Our results suggest that I-WSM supplementation made a moderate contribution to prevention of BMD reduction in OVX mice, maintenance of serum Ca and P levels, reduction of a bone resorption marker (TRAP5b), and elevation of a bone formation marker (OC). Therefore, I-WSM can be used as an effective alternative to postmenopausal osteoporosis prevention. This study is important to reaffirming the supplemental effect of isoflavone on bone metabolism in postmenopausal animal models and confirming the functional properties of soybean milk products fortified with isoflavones through a special process. However, I-WSM did not demonstrate significant improvement compared to WSM in terms of BMD, serum Ca and P concentrations, and bone turnover markers. These results do not seem to meet expectations, considering that I-WSM has an 8.8 times higher isoflavone content than WSM. This discrepancy is probably because the effect of isoflavone on postmenopausal BMD becomes saturated over a certain concentration or due to the difference between I-WSM and WSM in terms of components than isoflavone. Further studies will have to be conducted on the optimal concentration, availability, and mechanism of action of isoflavone-containing substances in preventing postmenopausal osteoporosis.
Footnotes
This work was supported by the Ministry of Trade, Industry and Energy (MOTIE) and Korea Institute for Advancement of Technology (KIAT) through the Research and Promoting Regional Specialized Industry (R0005131) and the Center for Efficacy Assessment and Development of Functional Foods and Drugs at Hallym University (B0008864), Korea.
CONFLICT OF INTEREST: The authors declare no potential of interests.
References
- 1.Lecart MP, Reginster JY. Current options for the management of postmenopausal osteoporosis. Expert Opin Pharmacother. 2011;12:2533–2552. doi: 10.1517/14656566.2011.618123. [DOI] [PubMed] [Google Scholar]
- 2.Seckin B, Pekcan MK, Inal HA, Gulerman C. The relationship between breast density and bone mineral density in never users of postmenopausal hormone therapy. Aging Clin Exp Res. 2017;29:537–541. doi: 10.1007/s40520-016-0599-1. [DOI] [PubMed] [Google Scholar]
- 3.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Gambacciani M, Levancini M. Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Prz Menopauzalny. 2014;13:213–220. doi: 10.5114/pm.2014.44996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi S, Zheng H. Combined effects of phytoestrogen genistein and silicon on ovariectomy-induced bone loss in rat. Biol Trace Elem Res. 2017;177:281–287. doi: 10.1007/s12011-016-0882-1. [DOI] [PubMed] [Google Scholar]
- 6.Pilšáková L, Riečanský I, Jagla F. The physiological actions of isoflavone phytoestrogens. Physiol Res. 2010;59:651–664. doi: 10.33549/physiolres.931902. [DOI] [PubMed] [Google Scholar]
- 7.Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet. 2013;38:15–25. doi: 10.1007/s13318-012-0112-y. [DOI] [PubMed] [Google Scholar]
- 8.Moutsatsou P. The spectrum of phytoestrogens in nature: our knowledge is expanding. Hormones (Athens) 2007;6:173–193. [PubMed] [Google Scholar]
- 9.Cos P, De Bruyne T, Apers S, Vanden Berghe D, Pieters L, Vlietinck AJ. Phytoestrogens: recent developments. Planta Med. 2003;69:589–599. doi: 10.1055/s-2003-41122. [DOI] [PubMed] [Google Scholar]
- 10.Huang HY, Yang HP, Yang HT, Yang TC, Shieh MJ, Huang SY. One-year soy isoflavone supplementation prevents early postmenopausal bone loss but without a dose-dependent effect. J Nutr Biochem. 2006;17:509–517. doi: 10.1016/j.jnutbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Mei J, Yeung SS, Kung AW. High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J Clin Endocrinol Metab. 2001;86:5217–5221. doi: 10.1210/jcem.86.11.8040. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Shu XO, Li H, Yang G, Li Q, Gao YT, Zheng W. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med. 2005;165:1890–1895. doi: 10.1001/archinte.165.16.1890. [DOI] [PubMed] [Google Scholar]
- 13.Kim MS, Lee YS. Effects of soy isoflavone and/or estrogen treatments on bone metabolism in ovariectomized rats. J Med Food. 2005;8:439–445. doi: 10.1089/jmf.2005.8.439. [DOI] [PubMed] [Google Scholar]
- 14.Bitto A, Burnett BP, Polito F, Marini H, Levy RM, Armbruster MA, Minutoli L, Di Stefano V, Irrera N, Antoci S, Granese R, Squadrito F, Altavilla D. Effects of genistein aglycone in osteoporotic, ovariectomized rats: a comparison with alendronate, raloxifene and estradiol. Br J Pharmacol. 2008;155:896–905. doi: 10.1038/bjp.2008.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, Nicolaidis C, Walker M, Humphrey L. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 16.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergh JJ, Xu Y, Farach-Carson MC. Osteoprotegerin expression and secretion are regulated by calcium influx through the L-type voltage-sensitive calcium channel. Endocrinology. 2004;145:426–436. doi: 10.1210/en.2003-0319. [DOI] [PubMed] [Google Scholar]
- 18.Klepzig M, Jonas D, Oremek GM. Procollagen type 1 amino-terminal propeptide: a marker for bone metastases in prostate carcinoma. Anticancer Res. 2009;29:671–673. [PubMed] [Google Scholar]
- 19.Ljusberg J, Wang Y, Lång P, Norgård M, Dodds R, Hultenby K, Ek-Rylander B, Andersson G. Proteolytic excision of a repressive loop domain in tartrate-resistant acid phosphatase by cathepsin K in osteoclasts. J Biol Chem. 2005;280:28370–28381. doi: 10.1074/jbc.M502469200. [DOI] [PubMed] [Google Scholar]
- 20.Campbell SE, Febbraio MA. Effects of ovarian hormones on exercise metabolism. Curr Opin Clin Nutr Metab Care. 2001;4:515–520. doi: 10.1097/00075197-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Misso ML, Jang C, Adams J, Tran J, Murata Y, Bell R, Boon WC, Simpson ER, Davis SR. Differential expression of factors involved in fat metabolism with age and the menopause transition. Maturitas. 2005;51:299–306. doi: 10.1016/j.maturitas.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Chen JC, Hsu CW, Chang WH. Effects of nano calcium carbonate and nano calcium citrate on toxicity in ICR mice and on bone mineral density in an ovariectomized mice model. Nanotechnology. 2009;20:375102. doi: 10.1088/0957-4484/20/37/375102. [DOI] [PubMed] [Google Scholar]
- 23.Zoth N, Weigt C, Laudenbach-Leschowski U, Diel P. Physical activity and estrogen treatment reduce visceral body fat and serum levels of leptin in an additive manner in a diet induced animal model of obesity. J Steroid Biochem Mol Biol. 2010;122:100–105. doi: 10.1016/j.jsbmb.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 24.Zheng W, Rogoschin J, Niehoff A, Oden K, Kulling SE, Xie M, Diel P. Combinatory effects of phytoestrogens and exercise on body fat mass and lipid metabolism in ovariectomized female rats. J Steroid Biochem Mol Biol. 2018;178:73–81. doi: 10.1016/j.jsbmb.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Wang X, Chiba H, Higuchi M, Nakatani T, Ezaki O, Cui H, Yamada K, Ishimi Y. Combined intervention of soy isoflavone and moderate exercise prevents body fat elevation and bone loss in ovariectomized mice. Metabolism. 2004;53:942–948. doi: 10.1016/j.metabol.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Hertrampf T, Gruca MJ, Seibel J, Laudenbach U, Fritzemeier KH, Diel P. The bone-protective effect of the phytoestrogen genistein is mediated via ER α-dependent mechanisms and strongly enhanced by physical activity. Bone. 2007;40:1529–1535. doi: 10.1016/j.bone.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial. Arch Intern Med. 2011;171:1363–1369. doi: 10.1001/archinternmed.2011.330. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Murphy PA. Isoflavone content in commercial soybean foods. J Agric Food Chem. 1994;42:1666–1673. [Google Scholar]
- 29.Davey RA, Hahn CN, May BK, Morris HA. Osteoblast gene expression in rat long bones: effects of ovariectomy and dihydrotestosterone on mRNA levels. Calcif Tissue Int. 2000;67:75–79. doi: 10.1007/s00223001100. [DOI] [PubMed] [Google Scholar]
- 30.Nishizawa Y, Nakamura T, Ohta H, Kushida K, Gorai I, Shiraki M, Fukunaga M, Hosoi T, Miki T, Chaki O, Ichimura S, Nakatsuka K, Miura M Committee on the Guidelines for the Use of Biochemical Markers of Bone Turnover in Osteoporosis Japan Osteoporosis Society. Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004) J Bone Miner Metab. 2005;23:97–104. doi: 10.1007/s00774-004-0547-6. [DOI] [PubMed] [Google Scholar]
- 31.Taku K, Melby MK, Kurzer MS, Mizuno S, Watanabe S, Ishimi Y. Effects of soy isoflavone supplements on bone turnover markers in menopausal women: systematic review and meta-analysis of randomized controlled trials. Bone. 2010;47:413–423. doi: 10.1016/j.bone.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Fu SW, Zeng GF, Zong SH, Zhang ZY, Zou B, Fang Y, Lu L, Xiao DQ. Systematic review and meta-analysis of the bone protective effect of phytoestrogens on osteoporosis in ovariectomized rats. Nutr Res. 2014;34:467–477. doi: 10.1016/j.nutres.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev. 2005;26:97–122. [PMC free article] [PubMed] [Google Scholar]
- 34.Vasikaran SD. Utility of biochemical markers of bone turnover and bone mineral density in management of osteoporosis. Crit Rev Clin Lab Sci. 2008;45:221–258. doi: 10.1080/10408360801949442. [DOI] [PubMed] [Google Scholar]
- 35.Miller PD, Hochberg MC, Wehren LE, Ross PD, Wasnich RD. How useful are measures of BMD and bone turnover? Curr Med Res Opin. 2005;21:545–553. doi: 10.1185/030079905x41390. [DOI] [PubMed] [Google Scholar]
- 36.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 37.Bateman TA, Countryman S. Osteoprotegerin and bone loss associated with spaceflight. Drug Discov Today. 2002;7:456–457. doi: 10.1016/s1359-6446(02)02260-2. [DOI] [PubMed] [Google Scholar]
- 38.Brink E, Coxam V, Robins S, Wahala K, Cassidy A, Branca F PHYTOS Investigator. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo controlled study. Am J Clin Nutr. 2008;87:761–770. doi: 10.1093/ajcn/87.3.761. [DOI] [PubMed] [Google Scholar]
- 39.Lydeking-Olsen E, Beck-Jensen JE, Setchell KD, Holm-Jensen T. Soymilk or progesterone for prevention of bone loss-a 2 year randomized, placebo-controlled trial. Eur J Nutr. 2004;43:246–257. doi: 10.1007/s00394-004-0497-8. [DOI] [PubMed] [Google Scholar]
- 40.Nikander E, Metsä-Heikkilä M, Ylikorkala O, Tiitinen A. Effects of phytoestrogens on bone turnover in postmenopausal women with a history of breast cancer. J Clin Endocrinol Metab. 2004;89:1207–1212. doi: 10.1210/jc.2003-031166. [DOI] [PubMed] [Google Scholar]
- 41.Halleen JM, Alatalo SL, Suominen H, Cheng S, Janckila AJ, Väänänen HK. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J Bone Miner Res. 2000;15:1337–1345. doi: 10.1359/jbmr.2000.15.7.1337. [DOI] [PubMed] [Google Scholar]
- 42.Halleen JM. Tartrate-resistant acid phosphatase 5B is a specific and sensitive marker of bone resorption. Anticancer Res. 2003;23:1027–1029. [PubMed] [Google Scholar]