Abstract
Objective
To examine the trends and survival for women with early-stage epithelial ovarian cancer who underwent adequate lymphadenectomy during surgical treatment.
Methods
This is a retrospective observational study examining the Surveillance, Epidemiology, End Results program between 1988 and 2013. We evaluated 21,537 cases of stage I–II epithelial ovarian cancer including serous (n=7,466), clear cell (n=6,903), mucinous (n=4,066), and endometrioid (n=3,102) histology. A time-trend analysis of the proportion of patients who underwent adequate pelvic lymphadenectomy (≥8 per Gynecologic Oncology Group [GOG] criteria, ≥12 per Collaborative Group Report [CGR] criteria for bladder cancer, and >22 per Mayo criteria for endometrial cancer) and a survival analysis associated with adequate pelvic lymphadenectomy were performed.
Results
There were significant increases in the proportion of women who underwent adequate lymphadenectomy: GOG criteria 3.6% to 28.6% (1988–2010); CGR criteria 2.4% to 22.4% (1988–2013); and Mayo criteria 0.7% to 9.5% (1988–2013) (all, p<0.05). On multivariable analysis, adequate lymphadenectomy was independently associated with improved cause-specific survival compared to inadequate lymphadenectomy: GOG criteria, adjusted-hazard ratio (HR)=0.75, CGR criteria, adjusted-HR=0.77, and Mayo criteria, adjusted-HR=0.85 (all, p<0.05). Compared to inadequate lymphadenectomy, adequate lymphadenectomy was significantly associated with improved cause-specific survival for serous (HR range=0.67–0.73), endometrioid (HR range=0.59–0.61), and clear cell types (HR range=0.66–0.73) (all, p<0.05) but not in mucinous type (HR range=0.80–0.91; p>0.05).
Conclusion
Quality of lymphadenectomy during the surgical treatment for early-stage epithelial ovarian cancer has significantly improved. Adequate lymphadenectomy is associated with a 15%–25% reduction in ovarian cancer mortality compared to inadequate lymphadenectomy.
Keywords: Ovarian Neoplasms, Early-stage, Lymph Node Excision, Adequate, Trend, Survival
INTRODUCTION
While the incidence has been steadily decreasing, ovarian cancer remains the most deadly gynecologic malignancy in the United States [1,2]. The standard treatment approach for ovarian cancer remains surgery [3]. Surgical quality is an important factor associated with survival for women with ovarian cancer [4,5]. While conducting a maximal cytoreductive surgery to remove all gross disease is the general principal for advanced-stage ovarian cancer, surgical staging is more important in the management of apparent early-stage disease to identify occult metastasis [3]. A key element of staging includes assessment of pelvic and para-aortic lymph node metastasis by performance of comprehensive lymphadenectomy [3].
As 5%–20% of women with apparent early-stage ovarian cancer have occult nodal disease, performance of lymphadenectomy is paramount in identifying women with more advanced-stage tumors [6]. Women with apparent early-stage ovarian cancer in whom lymphatic metastasis are diagnosed via staging lymphadenectomy are upstaged to advanced-stage disease and require adjuvant therapy [3,7]. Previous studies have shown that use of lymphadenectomy is associated with decreased mortality in early-stage ovarian cancer [8,9]. Therefore, performing an adequate lymphadenectomy is paramount in the management of early-stage ovarian cancer.
The adequacy of lymphadenectomy is generally based upon the number of nodes removed. While quality metrics for adequate lymphadenectomy have been defined for various malignancies [10,11,12], studies to examine the importance of adequate lymphadenectomy on survival of women with apparent early-stage ovarian cancer are largely lacking. The objective of our study was to examine the trends in performance and association with survival of adequate lymphadenectomy for women with early-stage ovarian cancer.
MATERIALS AND METHODS
This is a retrospective observational study examined the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program [13]. The SEER program is the largest population-based tumor registry in the United States, and launched in 1973 and covers approximately 28% of the United State population from 11 states and 7 areas. The SEER database is deidentified and publicly available. The study was deemed exempt by the Institutional Review Board at the University of Southern California. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were utilized to outline the results of the observational study [14].
Women with stage I–II epithelial ovarian cancer who had information for the extent of pelvic lymphadenectomy between 1988 and 2013 were analyzed. We examined the 4 most common histologic subtypes of epithelial ovarian cancer: serous, endometrioid, clear cell, and mucinous. Exclusion criteria of the study were women with stage III–IV disease, non-epithelial histology types, epithelial ovarian cancer but not the aforementioned 4 histologic types, lack of information for pelvic lymphadenectomy, borderline ovarian tumors, and metastatic tumors to the ovary. Cases between 1973 and 1987 were also excluded due to lack of detailed information for the extent of lymphadenectomy in the SEER program.
SEER*Stat8.3.2 (IMS Inc., Calverton, MD, USA) was used to extract the SEER18 cases. This was generated by the query for “Ovary” limited to “female sex” and “malignancy.” The International Classification of Diseases for Oncology third edition site/histology validation and the World Health Organization (WHO) histological classification codes were used for identifying the eligible histology types of ovarian cancer as previously described [15,16,17]. Lymphadenectomy performance, the surrogate index of pelvic lymphadenectomy, was based on the coding for the “Regional Nodes” that was introduced in 1988 in the database [13].
Among the eligible cases, patient demographics (age at diagnosis, race, year of diagnosis, registry area, and marital status), tumor characteristics (cancer stage, histology types, tumor differentiation grade, and tumor size), performance of lymphadenectomy, and survival (cause-specific survival) were abstracted from the database. For lymphadenectomy, use of pelvic lymphadenectomy (yes vs. no) and the number of sampled lymph node among the lymphadenectomy cases were abstracted. The database does not have information for para-aortic lymphadenectomy during the study period.
Recorded cancer stage was based on the American Joint Committee on Cancer 7th surgical-pathological staging classification schema [18]. Cause-specific survival was defined as the time interval between the date of ovarian cancer diagnosis and the date of death from the ovarian cancer. Women who were alive at the last follow-up or who died of other causes were censored. In the SEER program, cause of death is linked with the National Death Index and the state mortality records [19].
To date, there is no established definition for adequate lymphadenectomy specific for ovarian cancer. Thus, in this study adequacy of lymphadenectomy was assessed using various consensus guidelines: Gynecologic Oncology Group (GOG) criteria [12], Collaborative Group Report (CGR) criteria [10], and the Mayo criteria [11]. The GOG Surgical Procedure Manual defines adequate pelvic lymphadenectomy as the removal of at least 8 lymph nodes [12]. The CGR defines removal of at least 10–14 lymph nodes during pelvic lymphadenectomy for bladder cancer [10], and we used the cutoff of 12 lymph nodes for adequate lymphadenectomy in this study. The Mayo criteria define that >22 lymph nodes to be removed for adequate pelvic lymphadenectomy for endometrial cancer (based on the mean number minus one standard division) [11]. We utilized the criteria from endometrial and bladder cancers because pelvic lymphatic chains are the regional lymph nodes of these tumors, and pelvic lymphadenectomy is a part of standard surgical approach in indicated cases. Inadequate lymphadenectomy in our study was defined as the sampled lymph nodes that do not meet the adequate lymphadenectomy among staged cases.
The primary objective of our analysis was to examine the trends of the proportion of women with early-stage epithelial ovarian cancer who underwent adequate pelvic lymphadenectomy. The secondary objective of analysis was to assess the association between adequate pelvic lymphadenectomy and cause-specific survival of women with stage I–II epithelial ovarian cancer.
In order to examine annual trends in performance of adequate lymphadenectomy, Joinpoint Trend Software (version 4.4.0.0; National Cancer Institute, Bethesda, MD, USA) was used to determine potential changes in temporal trends as described previously [20,21,22]. Time duration was grouped every 1 year to provide percent frequency of collected variables. The results were analyzed with linear segmented regression test, and log-transformation was performed to determine annual percent change of the slope with 95% confidence interval (CI) [23].
Binary logistic regression models were used to identify the independent clinico-pathological factors associated with adequate lymphadenectomy. Patient demographics and tumor characteristics were entered in the final model, and the magnitude of statistical significance was expressed with adjusted-odds ratio (OR) with 95% CI. The Hosmer-Lemeshow test was used to assess the goodness-of-fit in the final model, and a p-value of 0.05 or greater was considered a good-fit model [24].
Survival curves were constructed with the Kaplan-Meier method, and statistical differences between the curves were assessed by means of a log-rank test. Cox proportional hazards regression models were used to examine the independent association between lymphadenectomy and cause-specific survival; women who underwent adequate lymphadenectomy were compared to those who underwent inadequate lymphadenectomy. In this multivariable analysis, patient demographics, tumor characteristics, and surgical performance were entered in the final model, and the magnitude of statistical significance was expressed with adjusted-hazard ratio (HR) with 95% CI.
In a sensitivity analysis, performance and survival estimates of adequate lymphadenectomy were examined for each histologic subtypes (serous, endometrioid, clear cell, and mucinous). This was based on the rationale that biology and outcome of ovarian cancer differ across the histology types [25,26,27,28,29]. In addition, the impact of age and lymphadenectomy on survival was examined based on the assumption that older women, defined as 60 years or older per the WHO, are less likely to undergo radical staging surgery for ovarian cancer.
In the multivariable model, the variance inflation factor was determined among covariates in multivariable analysis, and a value of 2 or larger was interpreted as multicollinearity in this study [30]. All statistical analyses were based upon 2-tailed hypotheses, and a p-value of less than 0.05 was considered statistical significant. Statistical Package for Social Sciences (version 24.0; IBM SPSS, Armonk, NY, USA) was used for the analysis.
RESULTS
Among 109,456 cases of ovarian cancer diagnosed during the study period, 100,370 cases had information for pelvic lymph lymphadenectomy. Of those, 69,721 cases were stage III–IV disease or unknown stage, and the remaining 30,649 cases were stage I–II disease with known pelvic lymphadenectomy. After excluding 9,112 cases of other histology types, 21,537 women with stage I–II epithelial ovarian cancer of the 4 aforementioned histology types with available pelvic lymphadenectomy information met the inclusion criteria.
Patient demographics are shown in Table 1. The most common histology type was serous (n=7,466), followed by clear cell (n=6,903), mucinous (n=4,066), and endometrioid (n=3,102). The median age at diagnosis was 56 years, and the majority of the study population was White (n=16,044, 74.5%) and resided in the Western U.S. (n=11,679, 54.2%). The majority of ovarian cancer patients in the study had stage I disease (n=15,714, 73.0%) and had high-grade tumors (n=6,589, 30.6%).
Table 1. Patient demographics.
| Characteristic | Value (n=21,537) | |
|---|---|---|
| Age (yr) | 56 (IQR 20) | |
| <40 | 2,337 (10.9) | |
| 40–49 | 4,490 (20.8) | |
| 50–59 | 6,085 (28.3) | |
| 60–69 | 4,299 (20.0) | |
| ≥70 | 4,326 (20.1) | |
| Race | ||
| White | 16,044 (74.5) | |
| Black | 1,087 (5.0) | |
| Hispanic | 2,115 (9.8) | |
| Asian | 1,939 (9.0) | |
| Others | 352 (1.6) | |
| Year | ||
| Before 1990 | 735 (3.4) | |
| 1990–1999 | 5,050 (23.4) | |
| 2000–2009 | 10,834 (50.3) | |
| 2010 or later | 4,918 (22.8) | |
| Registry | ||
| West | 11,679 (54.2) | |
| Central | 4,391 (20.4) | |
| East | 5,467 (25.4) | |
| Marital status | ||
| Single | 4,296 (19.9) | |
| Others | 16,485 (76.5) | |
| Unknown | 756 (3.5) | |
| Cancer stage | ||
| I | 15,714 (73.0) | |
| II | 5,823 (27.0) | |
| Histology type | ||
| Serous | 7,466 (34.7) | |
| Endometrioid | 3,102 (14.4) | |
| Clear cell | 6,903 (32.1) | |
| Mucinous | 4,066 (18.9) | |
| Grade | ||
| 1 | 4,532 (21.0) | |
| 2 | 5,667 (26.3) | |
| 3 | 6,589 (30.6) | |
| Unknown | 4,749 (22.1) | |
| Tumor size (cm) | ||
| ≤10 | 7,684 (35.7) | |
| >10 | 7,206 (33.5) | |
| Unknown | 6,647 (30.9) | |
| No. of lymph nodes | 11 (IQR 14)* | |
| 0 | 8,422 (39.1) | |
| 1–5 | 3,441 (16.0) | |
| 6–10 | 2,852 (13.2) | |
| 11–15 | 2,189 (10.2) | |
| 16–20 | 1,678 (7.8) | |
| 21–25 | 1,074 (5.0) | |
| 26–30 | 735 (3.4) | |
| 31–35 | 465 (2.2) | |
| 35–40 | 271 (1.3) | |
| >40 | 412 (1.9) | |
Number (%) or median (IQR) is shown.
IQR, interquartile range.
*Median of removed lymph node among resected cases.
Performance of pelvic lymphadenectomy was assessed (Table 1). There were 13,115 (60.9%, 95% CI=60.2–61.5) women who underwent pelvic lymphadenectomy. Among those who underwent pelvic lymphadenectomy, the median number of sampled lymph nodes was 11 (interquartile range [IQR]=14). Among the entire cohort including staged and unstaged cases, adequate lymphadenectomy was seen in 8,489 (39.4%) women per the GOG criteria, 6,349 (29.5%) women per the CGR criteria, and 2,488 (11.6%) women per the Mayo criteria during the study period. Inadequate lymphadenectomy was seen in 4,628 (21.5%) women per the GOG criteria, 6,768 (31.4%) women per the CGR criteria, and 10,629 (49.4%) women per the Mayo criteria during the study period.
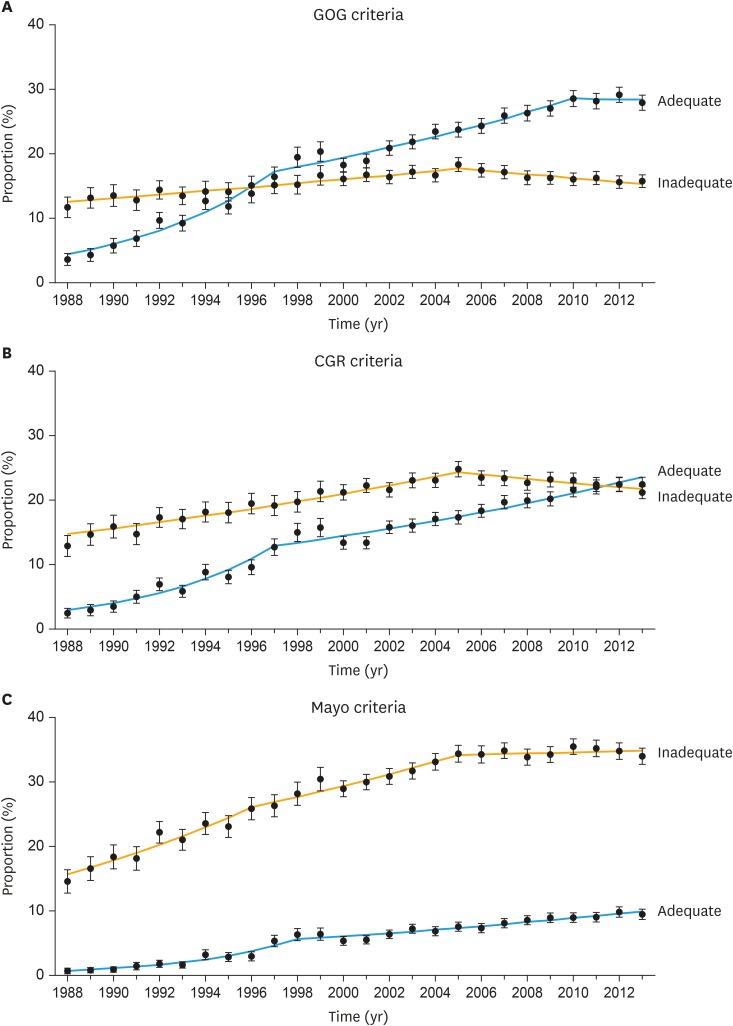
The trend of adequate lymphadenectomy was examined per calendar year (Fig. 1). There were significant increases in the proportion of women who underwent adequate lymphadenectomy during the study period across all 3 criteria: the GOG criteria 3.6% to 28.6% between 1988–2010 (Fig. 1A); the CGR criteria 2.4% to 22.4% between 1988–2013 (Fig. 1B); and the Mayo criteria 0.7% to 9.5% between 1988–2013 (Fig. 1C) (all, p<0.05). Proportion of adequate lymphadenectomy exceeded inadequate counterpart in 1996 per the GOG criteria and in 2012 per the CGR criteria. On multivariable analysis (Table 2), recent year diagnosis was the most significant factor for adequate lymphadenectomy in the 3 models (all, p<0.001). Older women were less likely to have adequate lymphadenectomy.
Fig. 1. Trends of performance of pelvic lymphadenectomy for stage I–II epithelial ovarian cancers. Dots represent actual values and bars represent 95% CI. Performance of lymphadenectomy is shown per (A) the GOG criteria, (B) the CGR criteria for bladder cancer, and (C) the Mayo Clinic criteria for endometrial cancer. In the GOG criteria, APC values for adequate lymphadenectomy were 16.2 (95% CI=12.2–20.4; p<0.05) between 1988–1997, 3.9 (95% CI=3.1–4.8; p<0.05) between 1997–2010, and −0.3 (95% CI=−5.3 to 5.1; p=0.92) between 2010–2013; and APC values for inadequate lymphadenectomy were 2.0 (95% CI=1.7–2.4; p<0.05) between 1988–2005, and −1.7 (95% CI=−2.4 to −1.0; p<0.05) between 2005–2013. In the CGR criteria, APC values for adequate lymphadenectomy were 18.5 (95% CI=13.1–24.2; p<0.05) between 1988–1997, and 3.2 (95% CI=4.5–12.5; p<0.05) between 1997–2013; and APC values for inadequate lymphadenectomy were 3.0 (95% CI=2.6–3.4; p<0.05) between 1988–2005 and −1.4 (95% CI=−2.2 to −0.7; p<0.05) between 2005–2013. In the Mayo criteria, APC values for adequate lymphadenectomy were 22.5 (95% CI 16.8–28.5, p<0.05) between 1988–1998, and 3.9 (95% CI=3.1–4.7; p<0.05) between 1998–2013; and APC values for inadequate lymphadenectomy were 6.5 (95% CI=4.7–8.4; p<0.05) between 1988–1996 and 3.1 (95% CI=2.1–4.1; p<0.05) between 1996–2005, and 0.2 (95% CI=−0.5 to 0.9; p=0.49) between 2005–2013.
APC, annual percent change; CGR, Collaborative Group Report; CI, confidence interval; GOG, Gynecologic Oncology Group.
Table 2. Clinico-pathological factors for adequate lymphadenectomy (multivariable analysis).
| Characteristic | GOG criteria | CGR criteria | Mayo criteria | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age (yr) | |||||||
| <40 | 1.00 | 1.00 | 1.00 | ||||
| 40–49 | 1.11 (0.97–1.28) | 0.13 | 1.18 (1.03–1.35) | 0.015 | 1.23 (1.04–1.46) | 0.016 | |
| 50–59 | 1.10 (0.96–1.25) | 0.18 | 1.16 (1.02–1.32) | 0.022 | 1.15 (0.98–1.36) | 0.09 | |
| 60–69 | 0.98 (0.84–1.13) | 0.73 | 1.02 (0.88–1.17) | 0.83 | 0.96 (0.80–1.14) | 0.61 | |
| ≥70 | 0.70 (0.60–0.81) | <0.001 | 0.74 (0.63–0.85) | <0.001 | 0.72 (0.59–0.88) | 0.001 | |
| Race | |||||||
| White | 1.00 | 1.00 | 1.00 | ||||
| Black | 0.63 (0.53–0.76) | <0.001 | 0.75 (0.62–0.89) | 0.001 | 0.75 (0.58–0.96) | 0.024 | |
| Hispanic | 0.90 (0.79–1.02) | 0.10 | 0.92 (0.81–1.04) | 0.17 | 1.01 (0.87–1.12) | 0.90 | |
| Asian | 0.86 (0.75–0.97) | 0.017 | 0.93 (0.82–1.05) | 0.21 | 0.88 (0.76–1.02) | 0.09 | |
| Others | 0.76 (0.57–1.01) | 0.06 | 0.80 (0.61–1.05) | 0.11 | 0.83 (0.59–1.18) | 0.30 | |
| Year | |||||||
| Before 1990 | 1.00 | 1.00 | 1.00 | ||||
| 1990–1999 | 2.67 (1.91–3.72) | <0.001 | 2.35 (1.61–3.44) | <0.001 | 2.23 (1.16–4.28) | 0.016 | |
| 2000–2009 | 4.28 (3.09–5.94) | <0.001 | 3.59 (2.46–5.22) | <0.001 | 3.70 (1.94–7.04) | <0.001 | |
| 2010 or later | 6.33 (4.53–8.84) | <0.001 | 5.40 (3.70–7.90) | <0.001 | 5.05 (2.64–9.64) | <0.001 | |
| Registry | |||||||
| West | 1.00 | 1.00 | 1.00 | ||||
| Central | 0.76 (0.69–0.84) | <0.001 | 0.73 (0.66–0.80) | <0.001 | 0.67 (0.59–0.77) | <0.001 | |
| East | 0.80 (0.73–0.88) | <0.001 | 0.77 (0.71–0.84) | <0.001 | 0.65 (0.58–0.73) | <0.001 | |
| Marital status | |||||||
| Single | 1.00 | 1.00 | 1.00 | ||||
| Others | 1.06 (0.96–1.16) | 0.26 | 1.08 (0.99–1.18) | 0.09 | 0.98 (0.87–1.09) | 0.66 | |
| Unknown | 1.04 (0.84–1.30) | 0.70 | 0.97 (0.79–1.19) | 0.78 | 0.68 (0.51–0.90) | 0.008 | |
| Cancer stage | |||||||
| I | 1.00 | 1.00 | 1.00 | ||||
| II | 0.88 (0.80–0.96) | 0.003 | 0.87 (0.80–0.95) | 0.001 | 0.92 (0.83–1.03) | 0.13 | |
| Histology type | |||||||
| Serous | 1.00 | 1.00 | 1.00 | ||||
| Endometrioid | 1.14 (1.01–1.28) | 0.037 | 1.08 (0.99–1.18) | 0.14 | 1.03 (0.90–1.19) | 0.67 | |
| Clear cell | 1.15 (1.05–1.27) | 0.004 | 0.97 (0.79–1.19) | 0.007 | 1.06 (0.94–1.19) | 0.36 | |
| Mucinous | 0.95 (0.84–1.07) | 0.42 | 0.87 (0.80–0.95) | 0.035 | 0.84 (0.72–0.98) | 0.026 | |
| Grade | |||||||
| 1 | 1.00 | 1.00 | 1.00 | ||||
| 2 | 0.95 (0.85–1.06) | 0.35 | 0.96 (0.87–1.07) | 0.45 | 0.97 (0.85–1.10) | 0.62 | |
| 3 | 1.07 (0.96–1.21) | 0.22 | 1.05 (0.94–1.17) | 0.39 | 1.07 (0.93–1.23) | 0.35 | |
| Unknown | 0.95 (0.84–1.08) | 0.45 | 0.97 (0.86–1.09) | 0.61 | 1.02 (0.87–1.19) | 0.82 | |
| Tumor size (cm) | |||||||
| ≤10 | 1.00 | 1.00 | 1.00 | ||||
| >10 | 0.96 (0.88–1.05) | 0.38 | 0.98 (0.90–1.06) | 0.6 | 1.04 (0.94–1.15) | 0.47 | |
| Unknown | 0.95 (0.86–1.04) | 0.28 | 0.96 (0.87–1.05) | 0.34 | 1.03 (0.91–1.17) | 0.62 | |
| Hosmer-Lemeshow | p=0.31 | p=0.52 | p=0.12 | ||||
Binary logistic regression models for p-values (adequate vs. inadequate pelvic lymphadenectomy). All the covariates listed were entered in the final models. Significant p-values are emboldened.
CGR, Collaborative Group Report criteria for bladder cancer; CI, confidence interval; GOG, Gynecologic Oncology Group; Mayo, Mayo Clinic criteria for endometrial cancer; OR, odds ratio.
The median follow-up of the censored cases was 7.1 years. There were 3,699 (17.2%) women who died of ovarian cancer in this study population. On univariable analysis, the extent of lymphadenectomy was significantly associated with cause-specific survival with women who had adequate lymphadenectomy having the highest 10-year cause-specific survival rate followed by those who had inadequate lymphadenectomy and those who did not undergo lymphadenectomy in the 3 criteria: the GOG criteria, 84.1%, 79.4%, and 70.1% (p<0.001); the CGR criteria, 84.9%, 80.3%, and 70.1% (p<0.001); and the Mayo criteria, 84.6%, 81.9%, and 70.1% (p<0.001), respectively.
On multivariable analysis (Table 3), adequate lymphadenectomy was independently associated with improved cause-specific survival compared to inadequate lymphadenectomy: adjusted-HR=0.75, 95% CI=0.68–0.83 per the GOG criteria (p<0.001); adjusted-HR=0.77, 95% CI=0.70–0.85 per the CGR criteria (p<0.001); and adjusted-HR=0.85, 95% CI=0.74–0.97 per the Mayo criteria (p=0.018). When compared to inadequate lymphadenectomy, absence of any lymphadenectomy was independently associated with increased ovarian cancer mortality by 45%–66% (all, p<0.001).
Table 3. Multivariable analysis for cause-specific survival.
| Characteristic | GOG criteria | CGR criteria | Mayo criteria | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age (yr) | |||||||
| <40 | 1.00 | 1.00 | 1.00 | ||||
| 40–49 | 1.42 (1.21–1.68) | <0.001 | 1.42 (1.21–1.68) | <0.001 | 1.42 (1.20–1.68) | <0.001 | |
| 50–59 | 1.82 (1.55–2.13) | <0.001 | 1.82 (1.55–2.13) | <0.001 | 1.81 (1.55–2.12) | <0.001 | |
| 60–69 | 2.10 (1.79–2.47) | <0.001 | 2.10 (1.79–2.47) | <0.001 | 2.10 (1.79–2.47) | <0.001 | |
| ≥70 | 3.81 (3.26–4.46) | <0.001 | 3.82 (3.26–4.47) | <0.001 | 3.83 (3.27–4.49) | <0.001 | |
| Race | |||||||
| White | 1.00 | 1.00 | 1.00 | ||||
| Black | 1.39 (1.22–1.58) | <0.001 | 1.39 (1.22–1.59) | <0.001 | 1.40 (1.23–1.60) | <0.001 | |
| Hispanic | 0.92 (0.81–1.04) | 0.17 | 0.92 (0.81–1.04) | 0.17 | 0.92 (0.81–1.05) | 0.20 | |
| Asian | 0.91 (0.80–1.04) | 0.18 | 0.91 (0.80–1.04) | 0.18 | 0.92 (0.80–1.05) | 0.21 | |
| Others | 0.84 (0.62–1.15) | 0.27 | 0.85 (0.62–1.16) | 0.30 | 0.85 (0.62–1.16) | 0.31 | |
| Year | |||||||
| Before 1990 | 1.00 | 1.00 | 1.00 | ||||
| 1990–1999 | 0.98 (0.85–1.14) | 0.80 | 0.98 (0.84–1.14) | 0.78 | 0.98 (0.84–1.13) | 0.74 | |
| 2000–2009 | 0.94 (0.81–1.09) | 0.39 | 0.93 (0.80–1.08) | 0.35 | 0.92 (0.79–1.07) | 0.29 | |
| 2010 or later | 0.84 (0.69–1.02) | 0.078 | 0.84 (0.69–1.01) | 0.07 | 0.82 (0.68–0.99) | 0.043 | |
| Registry | |||||||
| West | 1.00 | 1.00 | 1.00 | ||||
| Central | 0.96 (0.88–1.04) | 0.29 | 0.95 (0.87–1.04) | 0.26 | 0.96 (0.88–1.04) | 0.32 | |
| East | 0.94 (0.86–1.02) | 0.12 | 0.94 (0.86–1.02) | 0.12 | 0.94 (0.87–1.02) | 0.14 | |
| Marital status | |||||||
| Single | 1.00 | 1.00 | 1.00 | ||||
| Others | 0.89 (0.82–0.98) | 0.013 | 0.90 (0.82–0.98) | 0.015 | 0.89 (0.82–0.98) | 0.014 | |
| Unknown | 0.92 (0.75–1.12) | 0.39 | 0.91 (0.74–1.11) | 0.35 | 0.91 (0.74–1.11) | 0.34 | |
| Cancer stage | |||||||
| I | 1.00 | 1.00 | 1.00 | ||||
| II | 2.21 (2.06–2.37) | <0.001 | 2.21 (2.06–2.37) | <0.001 | 2.21 (2.07–2.37) | <0.001 | |
| Histology type | |||||||
| Serous | 1.00 | 1.00 | 1.00 | ||||
| Endometrioid | 0.94 (0.85–1.04) | 0.24 | 0.94 (0.85–1.04) | 0.23 | 0.93 (0.84–1.04) | 0.19 | |
| Clear cell | 0.65 (0.60–0.71) | <0.001 | 0.65 (0.60–0.71) | <0.001 | 0.65 (0.59–0.71) | <0.001 | |
| Mucinous | 0.74 (0.67–0.83) | <0.001 | 0.74 (0.66–0.83) | <0.001 | 0.74 (0.66–0.83) | <0.001 | |
| Grade | |||||||
| 1 | 1.00 | 1.00 | 1.00 | ||||
| 2 | 1.60 (1.41–1.81) | <0.001 | 1.60 (1.41–1.81) | <0.001 | 1.60 (1.41–1.82) | <0.001 | |
| 3 | 2.36 (2.09–2.67) | <0.001 | 2.36 (2.08–2.67) | <0.001 | 2.36 (2.08–2.67) | <0.001 | |
| Unknown | 1.93 (1.70–2.19) | <0.001 | 1.93 (1.70–2.20) | <0.001 | 1.94 (1.70–2.20) | <0.001 | |
| Tumor size (cm) | |||||||
| ≤10 | 1.00 | 1.00 | 1.00 | ||||
| >10 | 1.23 (1.13–1.34) | <0.001 | 1.23 (1.13–1.34) | <0.001 | 1.23 (1.13–1.34) | <0.001 | |
| Unknown | 1.41 (1.30–1.52) | <0.001 | 1.41 (1.30–1.52) | <0.001 | 1.41 (1.30–1.53) | <0.001 | |
| Lymphadenectomy | |||||||
| Inadequate | 1.00 | 1.00 | 1.00 | ||||
| Adequate | 0.75 (0.68–0.83) | <0.001 | 0.77 (0.70–0.85) | <0.001 | 0.85 (0.74–0.97) | 0.018 | |
| Unstaged | 1.45 (1.34–1.58) | <0.001 | 1.53 (1.42–1.66) | <0.001 | 1.66 (1.54–1.78) | <0.001 | |
Cox proportional hazard regression models for p-values. All the covariates listed were entered in the final models. Significant p-values are emboldened.
CGR, Collaborative Group Report criteria for bladder cancer; CI, confidence interval; GOG, Gynecologic Oncology Group; HR, hazard ratio; Mayo, Mayo Clinic criteria for endometrial cancer.
When the impact of lymphadenectomy on survival was examined by patient age (Supplementary Table 1), absolute differences in 5-year survival rates between unstaged, inadequately staged, and adequately staged cases were increased in elderly women compared to non-elderly women. Unstaged elderly women had the poorest survival among the groups.
Clinico-pathological demographics across the 4 histology types are shown in Supplementary Table 2. Women with serous histology were significantly older compared to other histology types (p<0.001). Performance of pelvic lymphadenectomy was examined based on histology (Table 4). Across the 4 histologic types examined, women with endometrioid histology were more likely to undergo lymphadenectomy (71.4%) followed by clear cell carcinomas (67.8%). Conversely, only approximately half of women with serous (53.7%) or mucinous (54.4%) histologic types underwent lymphadenectomy (p<0.001). Among women who underwent pelvic lymphadenectomy, women with endometrioid or clear cell histology had the highest number of nodes sampled (median 12, IQR=14) whereas those who had mucinous had the lowest (median 10, IQR=13) (p<0.001). Women with endometrioid histology type were more likely to undergo adequate lymphadenectomy where as those with mucinous type were the least likely per the 3 criteria examined (all, p<0.001).
Table 4. Characteristics of lymphadenectomy based on histology types.
| Characteristic | Serous | Endometrioid | Clear cell | Mucinous | p-value | ||
|---|---|---|---|---|---|---|---|
| Pelvic lymphadenectomy | |||||||
| Not performed | 3,456 (46.3) | 888 (28.6) | 2,220 (32.2) | 1,856 (45.6) | <0.001 | ||
| Performed | 4,010 (53.7) | 2,214 (71.4) | 4,683 (67.8) | 2,210 (54.4) | |||
| Median sampled | 11 (IQR 14) | 12 (IQR 14) | 12 (IQR 14) | 10 (IQR 13) | <0.001 | ||
| Lymphadenectomy performance | |||||||
| GOG criteria | <0.001 | ||||||
| Inadequate | 1,489 (37.1) | 713 (32.2) | 1,563 (33.4) | 863 (39.0) | |||
| Adequate | 2,521 (62.9) | 1,501 (67.8) | 3,120 (66.6) | 1,347 (61.0) | |||
| CGR criteria | <0.001 | ||||||
| Inadequate | 2,134 (53.2) | 1,075 (48.6) | 2,305 (49.2) | 1,254 (56.7) | |||
| Adequate | 1,876 (46.8) | 1,139 (51.4) | 2,378 (50.8) | 956 (43.3) | |||
| Mayo criteria | <0.001 | ||||||
| Inadequate | 3,267 (81.5) | 1,753 (79.2) | 3,751 (80.1) | 1,858 (94.1) | |||
| Adequate | 743 (18.5) | 461 (20.8) | 932 (19.9) | 352 (15.9) | |||
Number (%) or median (IQR) is shown. The χ2 test or Kruskal-Wallis H test for p-values. Significant p-values are emboldened.
CGR, Collaborative Group Report criteria for bladder cancer; GOG, Gynecologic Oncology Group; IQR, interquartile range.
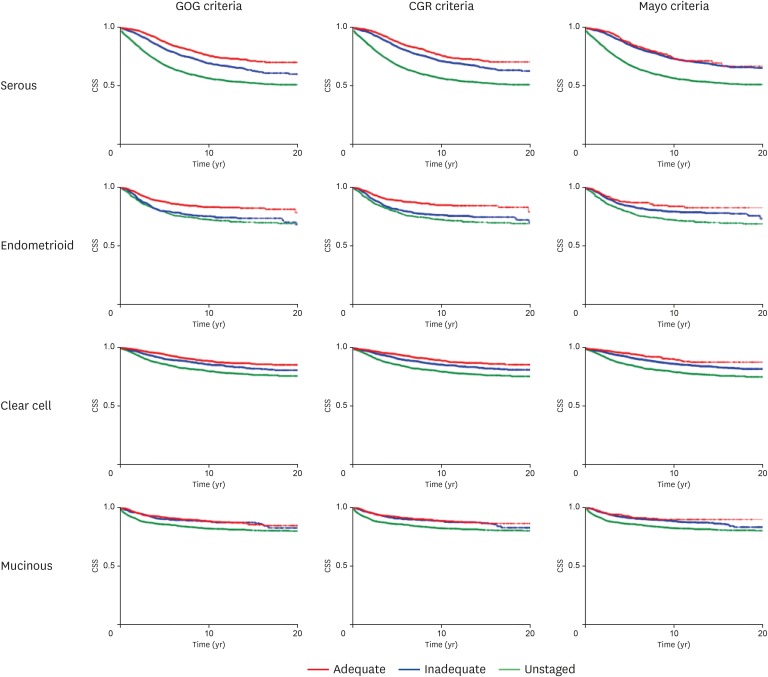
An association between adequacy of pelvic lymphadenectomy and cause-specific survival was examined for each histologic type (Table 5 and Fig. 2). Compared to women who had inadequate lymphadenectomy, women who had adequate lymphadenectomy had improved cause-specific survival for serous (the GOG criteria, HR=0.67; and the CGR criteria, HR=0.73), endometrioid (the GOG criteria, HR=0.61; and the CGR criteria, HR=0.59), and clear cell (the GOG criteria, HR=0.67; the CGR criteria, HR=0.73) tumor types (all, p<0.001). Per the Mayo criteria, adequate lymphadenectomy was associated with improved cause-specific survival in clear cell type (HR=0.66; p=0.004) but not in serous and endometrioid types (both, p>0.05). In contrast, for women with mucinous histology, adequate pelvic lymphadenectomy was not associated with improved cause-specific survival compared to inadequate pelvic lymphadenectomy per the 3 criteria (all, p>0.05).
Table 5. Histology type-specific cause-specific survival based on lymphadenectomy performance.
| Histology type | Nodal extent | GOG criteria | CGR criteria | Mayo criteria | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10-yr (%) | HR (95% CI) | p-value | 10-yr (%) | HR (95% CI) | p-value | 10-yr (%) | HR (95% CI) | p-value | ||
| Serous | Inadequate | 68.5 | 1.00 | 71.4 | 1.00 | 73.5 | 1.00 | |||
| Adequate | 76.4 | 0.67 (0.58–0.78) | <0.001 | 76.5 | 0.73 (0.63–0.85) | <0.001 | 73.9 | 0.86 (0.70–1.06) | 0.17 | |
| Unstaged | 56.5 | 1.69 (1.50–1.90) | <0.001 | 56.5 | 1.86 (1.67–2.07) | <0.001 | 56.5 | 2.06 (1.87–2.27) | <0.001 | |
| Endometrioid | Inadequate | 75.3 | 1.00 | 76.4 | 1.00 | 79.6 | 1.00 | |||
| Adequate | 83.2 | 0.61 (0.49–0.76) | <0.001 | 84.9 | 0.59 (0.47–0.73) | <0.001 | 84.2 | 0.76 (0.56–1.02) | 0.07 | |
| Unstaged | 72.5 | 1.11 (0.90–1.38) | 0.33 | 72.5 | 1.19 (0.98–1.45) | 0.08 | 72.5 | 1.43 (1.19–1.71) | <0.001 | |
| Clear cell | Inadequate | 85.6 | 1.00 | 85.8 | 1.00 | 86.8 | 1.00 | |||
| Adequate | 88.5 | 0.73 (0.60–0.88) | 0.001 | 89.3 | 0.71 (0.58–0.86) | <0.001 | 90.5 | 0.66 (0.50–0.88) | 0.004 | |
| Unstaged | 79.8 | 1.45 (1.22–1.74) | <0.001 | 79.8 | 1.52 (1.29–1.78) | <0.001 | 79.8 | 1.65 (1.43–1.91) | <0.001 | |
| Mucinous | Inadequate | 88.6 | 1.00 | 88.6 | 1.00 | 89.8 | 1.00 | |||
| Adequate | 88.7 | 0.91 (0.69–1.20) | 0.51 | 88.8 | 0.90 (0.68–1.19) | 0.46 | 83.6 | 0.80 (0.53–1.22) | 0.31 | |
| Unstaged | 82.5 | 1.54 (1.21–1.96) | 0.001 | 82.5 | 1.56 (1.26–1.93) | <0.001 | 82.5 | 1.58 (1.30–1.91) | <0.001 | |
Unadjusted Cox proportional hazard regression test for p-values. Significant p-values are emboldened.
CGR, Collaborative Group Report criteria for bladder cancer; CI, confidence interval; GOG, Gynecologic Oncology Group; HR, hazard ratio; 10-yr (%), 10-year cause-specific survival rate.
Fig. 2. Histology type-specific cause-specific survival. Cause-specific survival curves are shown for serous, endometrioid, clear cell, and mucinous ovarian carcinomas based on the 3 criteria (GOG, CGR for bladder cancer, and Mayo Clinic criteria for endometrial cancer).
CGR, Collaborative Group Report; CSS, cause-specific survival; GOG, Gynecologic Oncology Group.
Women who did not undergo pelvic lymphadenectomy had decreased cause-specific survival compared to those who had inadequate lymphadenectomy in serous (HR ranges=1.69–2.06), clear cell (HR ranges=1.11–1.43), and mucinous histology types (HR ranges=1.54–1.58) per the 3 criteria (all, p<0.001). For endometrioid tumors, women who had inadequate pelvic lymphadenectomy had ovarian cancer mortality rates similar to those who did not undergo pelvic lymphadenectomy per the GOG criteria and the CGR criteria (both, p>0.05) but not per the Mayo criteria (p<0.001).
DISCUSSION
This study noted that proportion of women with early-stage ovarian cancer who underwent adequate lymphadenectomy has been significantly increased over time and that women with early-stage ovarian cancer who had adequate lymphadenectomy had improved survival compared to those who had inadequate lymphadenectomy. The beneficial effects of lymphadenectomy were however not seen in mucinous type.
The benefit of an adequate lymphadenectomy likely stems from the greater chance of diagnosing advanced-stage disease when a greater number of nodes are removed by identifying the occult metastasis (stage-shifting). This may be particularly important as many women with early-stage ovarian cancer would not undergo chemotherapy (e.g., stage IA–B grade 1 disease) or would be administered fewer cycles of chemotherapy than those with advanced-stage neoplasms [3,6].
The survival benefit of adequate lymphadenectomy decreased slightly when a higher nodal cut-point was utilized (cause-specific mortality: 25% reduction with cutoffs of 8–12 nodes vs. 15% reduction with >22 nodes). This may imply that at least 8–12 nodal resection is sufficient enough to identify one nodal metastasis in early-stage ovarian cancer. This statistic is somehow similar to a recent nation-wide cohort study reporting that ≥10 nodes are recommended to be removed for early-stage ovarian cancer [31].
Contrary, this recommended nodal resection number is fewer compared to endometrioid endometrial cancer where 21–25 [32] or >22 nodes [11] are suggested to remove to detect one nodal metastasis. This difference likely represents that nodal spread in early-stage ovarian cancer is more frequent (14.2% per 14 studies) [33] than endometrioid endometrial cancer (4.4%–6.0%) [32,34], requiring less nodal resection to identify one metastasis in this disease spectrum.
When lymphadenectomy was examined for each histologic subtype, we found that adequate lymphadenectomy was associated with a survival benefit for serous, endometrioid, and clear cell tumors. For endometrioid tumors, inadequate lymphadenectomy was associated with a similar survival as non-lymphadenectomy. Thus, maximizing efforts to perform adequate lymphadenectomy is paramount in endometrioid tumors. In contrast, in mucinous type there was no survival benefit associated with performance of lymphadenectomy. Mucinous ovarian carcinoma has a substantially lower incidence of nodal metastasis [35], likely blunting the impact of nodal count on survival and accounting for low rates of performance of lymphadenectomy in the mucinous histology group. Lymphadenectomy was also performed less frequently in the serous histology group. Women with serous histology were found to be significantly older, and age was an independent clinical factor associated with decreased utilization of lymphadenectomy (Table 2).
The proportion of women who underwent adequate lymphadenectomy increased during the study period. This finding is consistent with a prior analysis of this database in that extent of lymphadenectomy in ovarian cancer has been improving [32]. Because their study was conducted more than a decade ago and analyzed mainly cases in 1990s, our study demonstrates that the trend toward removal of more nodal tissue has continued. It is unknown why surgical performance of pelvic lymphadenectomy has improved over time. It is speculated that increasing surgeon awareness of the importance of surgical staging for apparent early-stage epithelial ovarian cancer and improvements in preoperative assessment to differentiate benign vs. malignant ovarian tumors with appropriate referral to gynecologic oncologists may likely responsible for this trend.
Strengths of this study are that we analyzed the largest population-based tumor registry in the United States. This study examined multiple criteria for adequate lymphadenectomy demonstrating the consistency in the results that served as internal validations. In addition, histology-specific analysis provides useful information in the management of early-stage ovarian cancer.
We also recognize a number of important limitations. There was undoubtedly selection-bias in the decision to perform lymphadenectomy as well as for the extent of resection. Preferential performance of the procedure in lower risk women may have influenced our findings. Similarly, we lack information on use of postoperative chemotherapy, medical comorbidities particularly obesity, surgeon type, quality of care, and hospital volume and type, factors that have all been previously associated with survival for ovarian cancer [36,37,38]. These factors may affect not only survival, but also the extent of lymphadenectomy. This database also does not have information for sequelae of lymphadenectomy, such as lymphedema, or surgical complications related to lymphadenectomy [39]. Therefore, composite endpoint assessment including survival, postoperative complications, and quality of life were not able to be assessed in this study.
Moreover, we lack central pathologic review and there was not a standardized protocol for pathologic nodal assessment. Prior studies have demonstrated significant variability in node count based on the protocol for pathologic evaluation of submitted tissue [40,41,42]. Similarly, central pathology review was not performed to distinguish high-grade tumors from low-grade tumors with serous histology. However, low-grade serous ovarian carcinoma is a rare tumor, and in the serous histology group, less than 5% were described as grade 1 [21]. Thus, most of the serous tumors in this study are likely high-grade. Last, this study only examined resected lymph node number, and extents of anatomical sites, laterality, or anatomical boundary of resected areas were not evaluable. Thus, it is unknown if the lymphadenectomy was performed only in the limited area of nodal bed or in the hemi-pelvis. Likewise, we lack information on performance of para-aortic lymphadenectomy and other staging procedures.
These data have important implications for women with apparent early-stage ovarian cancer. First, this study endorses the importance of adequate lymphadenectomy to assess the lymphatic metastasis particularly in cases in which adjuvant therapy may be omitted or altered. Second, these findings suggest that the extent of lymphadenectomy may be tailored based on histology. In this scenario women with mucinous tumors may not require an extensive nodal assessment whereas a more extensive nodal evaluation is of greater importance for other epithelial tumors. Importantly, adequate lymphadenectomy in early-stage ovarian cancer needs to be defined particularly based on histology types as our study suggests that nodal spreading patterns may possibly differ across the histology types.
Footnotes
Presentation: Part of the study was presented at 49th Annual Meeting on Women's Cancer, New Orleans, LA, March 24–27, 2018.
Funding: This work was supported by grants from Ensign Endowment for Gynecologic Cancer Research (K.M.).
Conflict of Interest: Dr. Wright has served as a consultant for Clovis Oncology and Tesaro. The other authors have nothing to disclose.
- Conceptualization: M.K.
- Data curation: M.H.
- Formal analysis: M.K., M.H.
- Funding acquisition: M.K., R.L.D.
- Investigation: M.K., M.H., M.A., M.R.S., G.G.E., G.B.S., R.L.D., W.J.D.
- Methodology: M.K.
- Project administration: M.K.
- Resources: M.H., M.K.
- Software: M.H., M.K.
- Supervision: M.K., W.J.D.
- Validation: M.H., M.K.
- Visualization: M.H., M.K.
- Writing - original draft: M.K.
- Writing - review & editing: M.K., M.H., M.A., M.R.S., G.G.E., G.B.S., R.L.D., W.J.D.
SUPPLEMENTARY MATERIALS
Survival outcome based on age and lymphadenectomy performance
Clinico-pathological demographics per histology types
References
- 1.National Cancer Institute (US) Cancer statistics [Internet] Bethesda, MD: National Cancer Institute; [cited 2017 Dec 2]. Available from: https://seer.cancer.gov/faststats/selections.php?#Output. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (US) NCCN Clinical Practice Guidelines in Oncology. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer [Internet] Fort Washington, PA: National Comprehensive Cancer Network; [cited 2017 Dec 2]. Available from: https://nccn.org. [Google Scholar]
- 4.Nick AM, Coleman RL, Ramirez PT, Sood AK. A framework for a personalized surgical approach to ovarian cancer. Nat Rev Clin Oncol. 2015;12:239–245. doi: 10.1038/nrclinonc.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang SJ, Bristow RE. Evolution of surgical treatment paradigms for advanced-stage ovarian cancer: redefining ‘optimal’ residual disease. Gynecol Oncol. 2012;125:483–492. doi: 10.1016/j.ygyno.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Matsuo K, Sood AK, Gershenson DM. Management of early-stage ovarian cancer. In: Bristow RE, Karlan BY, Chi DS, editors. Surgery for ovarian cancer. 3rd ed. Boca Raton, FL: CRC Press; 2015. pp. 67–104. [Google Scholar]
- 7.Prat J. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Chan J, Fuh K, Shin J, Cheung M, Powell C, Chen LM, et al. The treatment and outcomes of early-stage epithelial ovarian cancer: have we made any progress? Br J Cancer. 2008;98:1191–1196. doi: 10.1038/sj.bjc.6604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouzier R, Bergzoll C, Brun JL, Dubernard G, Selle F, Uzan S, et al. The role of lymph node resection in ovarian cancer: analysis of the Surveillance, Epidemiology, and End Results (SEER) database. BJOG. 2010;117:1451–1458. doi: 10.1111/j.1471-0528.2010.02633.x. [DOI] [PubMed] [Google Scholar]
- 10.Herr H, Lee C, Chang S, Lerner S. Standardization of radical cystectomy and pelvic lymph node dissection for bladder cancer: a collaborative group report. J Urol. 2004;171:1823–1828. doi: 10.1097/01.ju.0000120289.78049.0e. [DOI] [PubMed] [Google Scholar]
- 11.Bakkum-Gamez JN, Mariani A, Dowdy SC, Weaver AL, McGree ME, Cliby WA, et al. The impact of surgical guidelines and periodic quality assessment on the staging of endometrial cancer. Gynecol Oncol. 2011;123:58–64. doi: 10.1016/j.ygyno.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Whitney CW, Spirtos N. Gynecologic Oncology Group surgical procedures manual. Philadelphia, PA: Gynecologic Oncology Group; 2009. [Google Scholar]
- 13.National Cancer Institute (US) Title [Internet] Bethesda, MD: National Cancer Institute; [cited 2017 Dec 2]. Available from: https://seer.cancer.gov/ [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute (US) ICD-O-3 coding materials [Internet] Bethesda, MD: National Cancer Institute; [cited 2017 Dec 2]. Available from: https://seer.cancer.gov/icd-o-3/ [Google Scholar]
- 16.Matsuo K, Machida H, Stone RL, Soliman PT, Thaker PH, Roman LD, et al. Risk of subsequent ovarian cancer after ovarian conservation in young women with stage I endometrioid endometrial cancer. Obstet Gynecol. 2017;130:403–410. doi: 10.1097/AOG.0000000000002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuo K, Machida H, Horowitz MP, Shahzad MM, Guntupalli SR, Roman LD, et al. Risk of metachronous ovarian cancer after ovarian conservation in young women with stage I cervical cancer. Am J Obstet Gynecol. 2017;217:580.e1–580.10. doi: 10.1016/j.ajog.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 19.Centers for Disease Control and Prevention (US) National death index [Internet] Atlanta, GA: Centers for Disease Control and Prevention; [cited 2017 Dec 2]. Available from: https://www.cdc.gov/nchs/ndi/ [Google Scholar]
- 20.National Cancer Institute (US) Joinpoint trend analysis software [Internet] Bethesda, MD: National Cancer Institute; [cited 2017 Dec 2]. Available from: http://surveillance.cancer.gov/joinpoint. [Google Scholar]
- 21.Matsuo K, Machida H, Grubbs BH, Sood AK, Gershenson DM. Trends of low-grade serous ovarian carcinoma in the United States. J Gynecol Oncol. 2018;29:e15. doi: 10.3802/jgo.2018.29.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo K, Ross MS, Machida H, Blake EA, Roman LD. Trends of uterine carcinosarcoma in the United States. J Gynecol Oncol. 2018;29:e22. doi: 10.3802/jgo.2018.29.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 24.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;16:965–980. doi: 10.1002/(sici)1097-0258(19970515)16:9<965::aid-sim509>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Vaughan S, Coward JI, Bast RC, Jr, Berchuck A, Berek JS, Brenton JD, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver KE, Brady WE, Birrer M, Gershenson DM, Fleming G, Copeland LJ, et al. An evaluation of progression free survival and overall survival of ovarian cancer patients with clear cell carcinoma versus serous carcinoma treated with platinum therapy: an NRG Oncology/Gynecologic Oncology Group experience. Gynecol Oncol. 2017;147:243–249. doi: 10.1016/j.ygyno.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuo K, Hasegawa K, Yoshino K, Murakami R, Hisamatsu T, Stone RL, et al. Venous thromboembolism, interleukin-6 and survival outcomes in patients with advanced ovarian clear cell carcinoma. Eur J Cancer. 2015;51:1978–1988. doi: 10.1016/j.ejca.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuo K, Nishimura M, Bottsford-Miller JN, Huang J, Komurov K, Armaiz-Pena GN, et al. Targeting SRC in mucinous ovarian carcinoma. Clin Cancer Res. 2011;17:5367–5378. doi: 10.1158/1078-0432.CCR-10-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiavone MB, Herzog TJ, Lewin SN, Deutsch I, Sun X, Burke WM, et al. Natural history and outcome of mucinous carcinoma of the ovary. Am J Obstet Gynecol. 2011;205:480.e1–480.e8. doi: 10.1016/j.ajog.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 30.Mansfield ER, Helms BP. Detecting multicollinearity. Am Stat. 1982;36:158–160. [Google Scholar]
- 31.Kleppe M, van der Aa MA, Van Gorp T, Slangen BF, Kruitwagen RF. The impact of lymph node dissection and adjuvant chemotherapy on survival: a nationwide cohort study of patients with clinical early-stage ovarian cancer. Eur J Cancer. 2016;66:83–90. doi: 10.1016/j.ejca.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Chan JK, Urban R, Cheung MK, Shin JY, Husain A, Teng NN, et al. Lymphadenectomy in endometrioid uterine cancer staging: how many lymph nodes are enough? A study of 11,443 patients. Cancer. 2007;109:2454–2460. doi: 10.1002/cncr.22727. [DOI] [PubMed] [Google Scholar]
- 33.Kleppe M, Wang T, Van Gorp T, Slangen BF, Kruse AJ, Kruitwagen RF. Lymph node metastasis in stages I and II ovarian cancer: a review. Gynecol Oncol. 2011;123:610–614. doi: 10.1016/j.ygyno.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Creasman WT, Ali S, Mutch DG, Zaino RJ, Powell MA, Mannel RS, et al. Surgical-pathological findings in type 1 and 2 endometrial cancer: an NRG Oncology/Gynecologic Oncology Group study on GOG-210 protocol. Gynecol Oncol. 2017;145:519–525. doi: 10.1016/j.ygyno.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmeler KM, Tao X, Frumovitz M, Deavers MT, Sun CC, Sood AK, et al. Prevalence of lymph node metastasis in primary mucinous carcinoma of the ovary. Obstet Gynecol. 2010;116:269–273. doi: 10.1097/AOG.0b013e3181e7961d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan JK, Kapp DS, Shin JY, Husain A, Teng NN, Berek JS, et al. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstet Gynecol. 2007;109:1342–1350. doi: 10.1097/01.AOG.0000265207.27755.28. [DOI] [PubMed] [Google Scholar]
- 37.Bristow RE, Palis BE, Chi DS, Cliby WA. The National Cancer Database report on advanced-stage epithelial ovarian cancer: impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol. 2010;118:262–267. doi: 10.1016/j.ygyno.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Wright JD, Chen L, Hou JY, Burke WM, Tergas AI, Ananth CV, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130:545–553. doi: 10.1097/AOG.0000000000002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim MC, Lee JS, Nam BH, Seo SS, Kang S, Park SY. Lower extremity edema in patients with early ovarian cancer. J Ovarian Res. 2014;7:28. doi: 10.1186/1757-2215-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santoso JT, Azadi A, Wan J, Handorf C, Coleman RL, Tillmanns TD. Lymph node counts in uterine cancer: a randomized double blind trial. Gynecol Oncol. 2009;113:159–162. doi: 10.1016/j.ygyno.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Cormier B, Sauthier P, Lussier C, Zang G, Mayrand MH. Determinants of lymph node count in endometrial cancer surgical staging. Int J Gynecol Cancer. 2012;22:1361–1366. doi: 10.1097/IGC.0b013e318269e29d. [DOI] [PubMed] [Google Scholar]
- 42.Abbassi-Ghadi N, Boshier PR, Goldin R, Hanna GB. Techniques to increase lymph node harvest from gastrointestinal cancer specimens: a systematic review and meta-analysis. Histopathology. 2012;61:531–542. doi: 10.1111/j.1365-2559.2012.04357.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survival outcome based on age and lymphadenectomy performance
Clinico-pathological demographics per histology types