Abstract
Objective
To determine the effect of surgeon experience on intraoperative, postoperative and long-term outcomes among patients undergoing pelvic exenteration for gynecologic cancer.
Methods
This was a retrospective analysis of all women who underwent exenteration for a gynecologic malignancy at MD Anderson Cancer Center, between January 1993 and June 2013. A logistic regression was used to model the relationship between surgeon experience (measured as the number of exenteration cases performed by the surgeon prior to a given exenteration) and operative outcomes and postoperative complications. Cox proportional hazards regression was used to model survival outcomes.
Results
A total of 167 exenterations were performed by 19 surgeons for cervix (78, 46.7%), vaginal (43, 25.8%), uterine (24, 14.4%), vulvar (14, 8.4%) and other cancer (8, 4.7%). The most common procedure was total pelvic exenteration (69.4%), incontinent urinary diversion (63.5%) and vertical rectus abdominis musculocutaneous reconstruction (42.5%). Surgical experience was associated with decreased estimated blood loss (p<0.001), intraoperative transfusion (p=0.009) and a shorter length of stay (p=0.03). No difference was noted in the postoperative complication rate (p=0.12–0.95). More surgeon experience was not associated with overall or disease specific survival: OS (hazard ratio [HR]=1.02; 95% confidence interval [CI]=0.97–1.06; p=0.46) and DSS (HR=1.01; 95% CI=0.97–1.04; p=0.66), respectively.
Conclusion
Patients undergoing exenteration by more experienced surgeons had improvement in intraoperative factors such as estimated blood loss, transfusion rates and length of stay. No difference was seen in postoperative complication rates, overall or disease specific survival.
Keywords: Pelvic Exenteration, Surgeon Volume, Pregnancy Outcomes, Gynecologic Neoplasms
INTRODUCTION
Over the past 2 decades, compelling evidence from a number of different specialties has demonstrated that surgeons' experience and hospital qualifications play a significant role in surgical outcomes. This paradigm suggests that high-volume surgeons, at high-volume hospitals deliver superior outcomes in terms of fewer surgical complications, decreased operative mortality and improved long-term survival for a range of high risk operative procedures [1,2,3,4]. Among gynecologic oncology procedures including ovarian cancer tumor reduction, laparoscopic radical hysterectomy, and abdominal or laparoscopic hysterectomy for endometrial cancer, the effect of surgeon volume on outcome is less clear [5,6,7,8].
Pelvic exenteration is a complex surgical procedure first described in the 1940s [9]. Substantial improvement in surgical techniques, perioperative care and reconstructive modalities have occurred, resulting in decreased perioperative mortality from 20 to less than 5%, and an improvement in 5-year survival to 20%–73% [10,11,12]. Postoperative morbidity remains a concern, however, with complication rates as high as 60%–95% [12,13]. Given the limited number of pelvic exenterations done each year, it is now a procedure that is performed relatively infrequently even among subspecialists at high-volume centers.
The primary objective of our study was to determine the effect of surgeon experience on peri-operative outcome among women who underwent pelvic exenteration at a large tertiary referral center. Our secondary objective was to assess differences in recurrence-free survival (RFS), overall survival (OS), and disease-specific survival (DSS). This information could help determine if the development of centers of excellence should be considered for these complex procedures.
MATERIALS AND METHODS
Following approval by the University of Texas, MD Anderson Cancer Center Institutional Review Board (approval number: PA12-0794), a retrospective review of all women who underwent anterior, posterior or total pelvic exenteration in the Department of Gynecologic Oncology & Reproductive Medicine, for any indication, from January 1993 to June 2013 was performed. Only patients with follow up of at least 12 months from surgery were included except when death occurred prior to 12 months. Demographic data, operative reports, pathology reports, and clinical outcomes were abstracted from medical records.
Surgeon experience was quantified in the total number of exenterations performed prior to a given exenteration based on the hospital's billing data. Because the total number of exenterations per surgeon were relatively low, we also quantified surgeon experience by the total number of surgical cases as well as procedural units performed by the primary surgeon prior to the exenteration. While the total number of surgical cases gave us broad indication of surgeon experience, procedural units allowed us to account for the complexity of each procedure performed by a given surgeon. For example, a tumor reductive surgery requiring extensive lysis of adhesion, 2 bowel resections, appendectomy and bilateral ureterolysis would count as one case, but 5 procedural units. Using these different methods allowed us to account for the increase in a surgeon's experience over time as the study spanned a 21-year period. To account for multi-surgeon or multi-disciplinary surgical teams, only the primary surgeon for each component of the exenteration or reconstruction was considered, and procedures performed by surgeons of subspecialties other than gynecologic oncology (i.e., plastic surgery) were excluded from the analysis.
Descriptive statistics were used to summarize the patient characteristics including age, body mass index (BMI), race, cancer diagnosis, tumor characteristics, previous surgery, medical comorbidities, and prior cancer therapy. Operative data included type of exenteration, duration of surgery (DOS), estimated blood loss (EBL), intraoperative complications, transfusion rates, and final pathology. Length of stay (LOS), as well as short-term (<60 days) and long-term (≥60 days) postoperative complications, were also assessed. Postoperative complications were graded according to the Clavien-Dindo Classification of Surgical Complications scale 1 to 5, which defines the severity of each complication according to the treatment required [14]. For patients who had more than one complication, the highest-grade complication was recorded. Grade 1 complications included any deviation from the normal postoperative course, grade 2 required pharmacological treatment, grade 3 required surgical, endoscopic or radiologic intervention, grade 4 required intensive care, and grade 5 indicated death of the patient.
For the statistical analysis, Spearman's rank correlation (corr) was used to estimate the relationship between surgeon experience at the time of exenteration and both operative and post-operative findings. Logistic regression was used to model the relationship between surgeon's experience and events of interest (i.e., complications and readmission rates). Cox proportional hazards regression was used to model OS and DSS as a function of surgeon experience [15].
RESULTS
Between 1993 and 2013, a total of 167 pelvic exenteration procedures were performed by 19 surgeons at our institution and were included in this analysis. Demographic characteristics are summarized in Table 1. Median age at the time of surgery was 55 years (30.4–85.9). Median BMI was 28 kg/m2 (15.2–50.9). The primary diagnoses were cervix (78, 46.7%), vaginal (43, 25.8%), uterine (24, 14.4%), vulvar (14, 8.4%), and other cancer (8, 4.7%). Most of these cancers were squamous in histology (84, 50.3%) followed by adenocarcinoma (52, 31.1%). The most common indication for the exenteration was recurrent disease (125, 74.9%), followed by persistent disease (26, 15.6%), and primary disease (16, 9.6%). A majority of patients underwent total pelvic exenteration (116, 69.4%), incontinent urinary diversion (101, 60.4%). When reconstruction was done, the most common procedure performed was vertical rectus abdominis musculocutaneous (VRAM) reconstruction (71, 42.5%). Only 2 of the urinary diversions were performed by urology and 18/111 (16%) of flaps were performed by plastic surgery. Negative margins were achieved in 85.6% of the cases.
Table 1. Patient characteristics (n=167).
| Patient characteristics | Values | ||
|---|---|---|---|
| Age (yr) | 54.8 (12.4)/55.2 (30.4–85.9) | ||
| BMI (kg/m2) | 29.0 (7.5)/28.0 (15.2–50.9) | ||
| Race | |||
| White | 124 (74.3) | ||
| Hispanic | 32 (19.2) | ||
| Black | 9 (5.4) | ||
| Asian | 2 (1.2) | ||
| Comorbidities | |||
| Smoker (current or history) | 62 (37.2) | ||
| Hypertension | 43 (25.7) | ||
| Diabetes | 23 (13.8) | ||
| Cardiac | 13 (7.8) | ||
| Pulmonary | 12 (7.2) | ||
| Type of exenteration | |||
| Total | 116 (69.4) | ||
| Anterior | 33 (19.8) | ||
| Posterior | 18 (10.8) | ||
| Urinary diversion | |||
| Continent | 48 (28.7) | ||
| Miami pouch | 39 (23.4) | ||
| Indiana pouch | 4 (2.4) | ||
| Incontinent | 101 (60.5) | ||
| Ileal | 88 (52.7) | ||
| Descending colon | 15 (9.0) | ||
| Vaginal repair | |||
| Gracilis flaps | 44 (26.4) | ||
| VRAM | 71 (42.5) | ||
| Omental/skin graft | 4 (2.4) | ||
| Primary closure | 22 (13.2) | ||
Data shown are number (%) or mean (SD)/median (range).
BMI, body mass index; SD, standard deviation; VRAM, vertical rectus abdominis musculocutaneous.
Among the 19 surgeons who performed at least one exenteration procedure, the median number of exenterations was 6 (range 1–29). Median surgeon experience at the time of exenteration was 476 cases (5–1,949). Median EBL was 1,800 mL (range, 280–13,000). Median DOS was 546 minutes (range, 252–930). Median intraoperative units of blood transfused were 3 units (range, 0–21). Median LOS was 15 days (range, 5–69). When intraoperative outcomes were compared with surgeon experience (defined as number of exenterations cases performed at the time of exenteration), more experience was associated with less EBL (corr −0.255, p<0.001), less administration of intraoperative blood products (corr −0.200, p=0.009) and a shorter LOS (corr −0.182, p=0.031). There was no difference in margin status based on surgeon experience (p=0.69). When the number of total cases or procedural units was used as a surrogate for surgeon's experience, similar findings were noted.
At least one postoperative complication occurred in 95% of patients. Table 2 shows the most common postoperative complications that occurred within 60 days of surgery. These complications were mostly grade 2 in severity (31.1%) and included wound separation (32.9%), urostomy complication (22.2%), abscess (15.6%), flap complication (10.8%), bowel obstruction (9.6%), and sepsis (8.4%). Readmission rate within 30 days was 24%. Reasons for readmission were pyelonephritis (28.5%), sepsis or urosepsis (22.8%), small bowel obstruction (14.2%), anastomostic leak (14.2%), hydronephrosis, wound infection or abscess (8.5% each), mental status changes (5.7%), and DVT (2.8%). There was no association between surgeon experience and postoperative complications within 60 days of surgery (p=0.12–0.95; p=0.13–0.94; and p=0.23–1.0, respectively)
Table 2. Postoperative complications within 60 days.
| Postoperative complications | No. (%) |
|---|---|
| Wound separation | 55 (32.9) |
| Urostomy complication | 37 (22.2) |
| Abscess | 26 (15.6) |
| Flap complication | 18 (10.8) |
| Bowel obstruction | 16 (9.6) |
| Sepsis | 14 (8.4) |
| Pneumonia | 13 (7.8) |
| Ureteral complication | 10 (6.0) |
| Fistula | 8 (4.8) |
| Colostomy complication | 8 (4.8) |
| Deep vein thrombosis, pulmonary embolism | 5 (3.0) |
| Acute kidney injury | 5 (3.0) |
| Readmission within 30 days | 40 (24.0) |
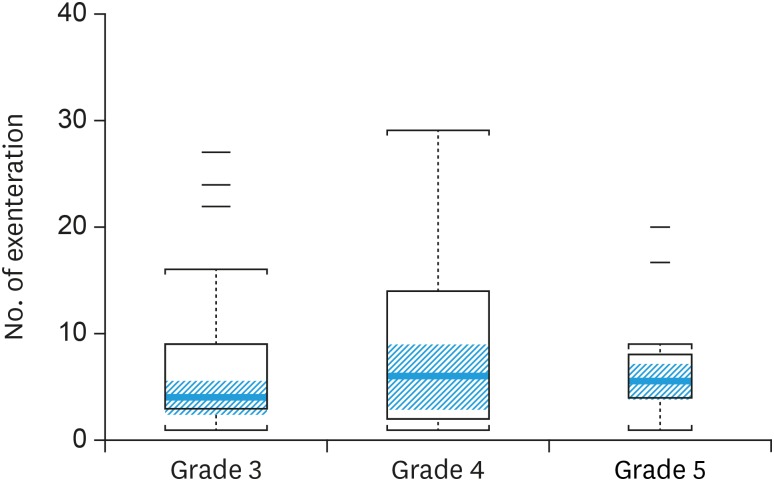
Postoperative complications were also categorized by grade according to Clavien-Dindo Classification (Table 3). There was 1 death within 60 days of surgery. There was no difference in median surgeon experience when compared across grades (p=0.43). Fig. 1 is a box-plot of surgeon experience and grade 3–5 complications. There was no significant difference in median experience among the grades (p=0.57).
Table 3. Complication grade and in relation to surgeon's experience*.
| Complication grade | No. | Minimum | Median | Range | p-value |
|---|---|---|---|---|---|
| 0 | 7 | 1 | 7 | 1–15 | 0.432 |
| 1 | 7 | 1 | 9 | 1–22 | |
| 2 | 52 | 1 | 5 | 1–28 | |
| 3 | 37 | 1 | 4 | 1–27 | |
| 4 | 38 | 1 | 6 | 1–29 | |
| 5 | 14 | 1 | 5.5 | 1–20 |
*Measured as median number of exenterations performed at the time of the exenteration.
Fig. 1. Complication grades 3–5 in relation to median number of exenteration. The orange line across the box represents the median. The top of the box is the 75th percentile, and the bottom of the box is the 25th percentile. The orange shaded region is the 95% confidence interval for the median. The staples mark the nearest value not beyond 1.5 × the interquartile range. The short dashes indicate outliers. Widths of boxes are proportional to the sample size (p=0.57).
Median follow up time was 32.4 months (range, 1.4–235.1). At the time of analysis, 89 patients had recurred (53.2%). The majority of recurrences were distant (54, 60.6%), whereas pelvic recurrences occurred in 30 patients (33.7%). Sixty-six patients (39.5%) had died of disease. Surgeon experience per number of exenterations performed was not associated with OS (hazard ratio [HR]=1.02, 95% confidence interval [CI]=0.97–1.06; p=0.46), RFS (HR=1.03; 95% CI=0.99–1.08; p=0.10) or DSS (HR=1.01; 95% CI=0.97–1.04; p=0.66). We also evaluated all of the outcomes by total number of cases and by procedural units to account for the complexity of the procedures contributing to surgeon experience and the results were similar to the number of exenterations performed (data not shown).
DISCUSSION
In patients who underwent a pelvic exenteration for a gynecologic cancer, we found that increasing surgeon experience was associated with decreased duration of the procedure, decreased EBL and less frequent blood transfusion. We did not, however, find a difference in postoperative complications or OS based on surgeon volume. This is the first study to evaluate surgeon experience in the context of pelvic exenteration for a gynecologic cancer.
A number of studies have looked at the relationship between surgeon volume and other procedures in gynecologic oncology and the findings are variable. When performing abdominal radical hysterectomy for early cervix cancer, high-volume surgeons had fewer postoperative hemorrhages, gastrointestinal complications, lower transfusion requirements, shorter LOS, and lower intensive care unit (ICU) admission [8]. Likewise, Worley et al. [16], evaluated the impact of surgeon volume and surgical complexity on more immediate perioperative outcomes among patients undergoing gynecologic laparoscopy. He reported higher overall complication rates, and a longer length of hospital stay for low-volume versus high-volume surgeons. Similar to our current study, Worley et al. [16] did not find an association between surgeon's experience and long-term complications, readmission rates or mortality.
While our study did show an improvement in immediate peri-operative outcomes, others' studies have shown conflicting results. Wright et al. [5] reported on 6,015 patients who underwent abdominal hysterectomy for endometrial cancer. After adjustment for case-mix variables and hospital volume, perioperative surgical complications (odds ratio [OR]=0.57; 95% CI=0.38–0.85), medical complications (OR=0.57; 95% CI=0.37–0.88), and ICU utilization (OR=0.47; 95% CI=0.28–0.80) were lower in patients treated by high-volume surgeons. Other factors such as operative injury, transfusion, LOS, or readmission were not associated with surgeon volume.
In ovarian cancer tumor reductive surgery, a complex yet more commonly performed procedure, the impact of surgeon and hospital volume varies between studies. Bristow et al. [17] showed that ovarian cancer surgery performed by a high-volume surgeon was associated with a 69% reduction in the risk of in-hospital death. In addition, care at a high-volume hospital increased the likelihood of optimal cytoreduction and resulted in lower cost for care. A population-based cohort study of 2,952 patients undergoing ovarian cancer resection, however, failed to demonstrate hospital- or surgeon-specific procedure volumes as strong predictors of OS [6]. Similarly, data evaluating outcomes of laparoscopic hysterectomy for endometrial cancer demonstrated that neither physician nor hospital volume had a significant effect on intraoperative complications, surgical-site or medical complications, transfusion rates or perioperative mortality (p>0.05 for all) in 4,137 cases collected from a nationwide inpatient database [18].
Among studies looking at surgical volume and outcome, the effect of volume appears to be most pronounced for high risk cardiovascular and oncologic procedures [19]. Liu et al. [20] examined the influence of the surgeon volume in a nationwide population study of over 61,000 patients with colorectal cancer, in which, 5-year mortality rate was significantly improved with increasing surgeon volume. Similar results were noted in surgical treatment of patients with other gastrointestinal and oral cancers [20,21,22,23]. While these studies done in cancer patients have shown differences in perioperative mortality and OS, our data did not suggest this in patients who underwent pelvic exenteration. It is possible that due to the high complexity of the procedure and relatively low survival rates, that surgeon experience may have less impact on patient outcome.
Perhaps our hospital characteristics played a more important role than the individual's surgeon experience. In a study by Birkmeyer et al. [3], the relative importance of surgeon volume and hospital volume on mortality varied according to the procedure. The mortality for lung cancer resection for example, depended more on the hospital volume rather than the surgeon's volume. Thus, it is possible that after an exenteration, hospital based services such as the intensive care units, blood bank, pain management services and nursing care may have a relatively higher influence on the outcomes than the technical skills of the surgeon.
The main strength of this study was the inclusion of a large number of patients undergoing a relatively uncommon, complex oncological procedure associated with high morbidity and mortality. Our statistical model allowed us to account for the change in a surgeon's experience over time. We measured surgeon experience in number of exenterations performed at the time of the exenteration. We also looked at surgeon's experience in number of surgical cases as well as number of procedural units performed at the time of exenteration. For example, a surgeon performing his/her second exenteration after having done 500 complex oncologic cases has higher experience than someone who is doing his/her second exenteration after only 100 cases. In addition, we were able to obtain detailed clinical information with a longitudinal follow up for more than 12 months, which would have been difficult to obtain had we used a restrictive or public database. The limitations include its retrospective nature, with the potential for underreporting of events, complications, and diagnoses. It also spanned 21 years, during which our surgical techniques changed and our quality of care in the preoperative and postoperative period likely improved. All procedures involved fellow participation. It is possible that this could dampen the effect of surgeon experience on outcome as the fellows were all early on their learning curve and may have performed a majority of the procedure under supervision. Finally, it is difficult to determine a “high-volume” surgeon in the setting of such an uncommon procedure. The median number of exenterations was 6 with a range of 1–29, therefore, no single surgeon had performed a large number of exenterations.
In conclusion, intraoperative outcomes among patients undergoing pelvic exenteration appear to improve with increasing surgeon experience. Both OS and the rate of postoperative complications, which were high, did not appear to be influenced by surgeons' experience. Additional studies are needed to determine the impact of hospital volume on the outcome patients undergoing these complex procedures. Consideration of a focused group of surgeons within our large volume center may have further impact on intraoperative and perhaps survival outcomes.
Footnotes
Presentation: This work was presented as poster at the Society of Gynecologic Oncology Annual Meeting in 2013.
Funding: This work was supported in part by Cancer Center Support Grant (NCI grant P30 CA016672). National Institutes of Health K12 Calabresis Scholar Award (K12 CA 0088084) was granted to SN.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: S.P.T.
- Formal analysis: M.M.F.
- Investigation: N.A.M.
- Methodology: J.R.J., M.M.F., S.P.T.
- Resources: N.A.M.
- Software: M.M.F.
- Validation: M.M.F.
- Visualization: J.R.J., W.S.N., S.P.T.
- Writing - original draft: J.R.J.
- Writing - review & editing: J.R.J., N.A.M., W.S.N., R.P.T., F.M., S.P.T.
References
- 1.Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–188. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 2.Begg CB, Riedel ER, Bach PB, Kattan MW, Schrag D, Warren JL, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–1144. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 4.Schrag D, Cramer LD, Bach PB, Cohen AM, Warren JL, Begg CB. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284:3028–3035. doi: 10.1001/jama.284.23.3028. [DOI] [PubMed] [Google Scholar]
- 5.Wright JD, Lewin SN, Deutsch I, Burke WM, Sun X, Herzog TJ. Effect of surgical volume on morbidity and mortality of abdominal hysterectomy for endometrial cancer. Obstet Gynecol. 2011;117:1051–1059. doi: 10.1097/AOG.0b013e31821647a0. [DOI] [PubMed] [Google Scholar]
- 6.Wright JD, Herzog TJ, Siddiq Z, Arend R, Neugut AI, Burke WM, et al. Failure to rescue as a source of variation in hospital mortality for ovarian cancer. J Clin Oncol. 2012;30:3976–3982. doi: 10.1200/JCO.2012.43.2906. [DOI] [PubMed] [Google Scholar]
- 7.Schrag D, Earle C, Xu F, Panageas KS, Yabroff KR, Bristow RE, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. J Natl Cancer Inst. 2006;98:163–171. doi: 10.1093/jnci/djj018. [DOI] [PubMed] [Google Scholar]
- 8.Wright JD, Lewin SN, Deutsch I, Burke WM, Sun X, Herzog TJ. The influence of surgical volume on morbidity and mortality of radical hysterectomy for cervical cancer. Am J Obstet Gynecol. 2011;205:225.e1–225.e7. doi: 10.1016/j.ajog.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer. 1948;1:177–183. doi: 10.1002/1097-0142(194807)1:2<177::aid-cncr2820010203>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Khoury-Collado F, Einstein MH, Bochner BH, Alektiar KM, Sonoda Y, Abu-Rustum NR, et al. Pelvic exenteration with curative intent for recurrent uterine malignancies. Gynecol Oncol. 2012;124:42–47. doi: 10.1016/j.ygyno.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Westin SN, Rallapalli V, Fellman B, Urbauer DL, Pal N, Frumovitz MM, et al. Overall survival after pelvic exenteration for gynecologic malignancy. Gynecol Oncol. 2014;134:546–551. doi: 10.1016/j.ygyno.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur M, Joniau S, D'Hoore A, Vergote I. Indications, techniques and outcomes for pelvic exenteration in gynecological malignancy. Curr Opin Oncol. 2014;26:514–520. doi: 10.1097/CCO.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 13.Benn T, Brooks RA, Zhang Q, Powell MA, Thaker PH, Mutch DG, et al. Pelvic exenteration in gynecologic oncology: a single institution study over 20 years. Gynecol Oncol. 2011;122:14–18. doi: 10.1016/j.ygyno.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeves GK, Cox DR, Darby SC, Whitley E. Some aspects of measurement error in explanatory variables for continuous and binary regression models. Stat Med. 1998;17:2157–2177. doi: 10.1002/(sici)1097-0258(19981015)17:19<2157::aid-sim916>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.Worley MJ, Jr, Anwandter C, Sun CC, dos Reis R, Nick AM, Frumovitz M, et al. Impact of surgeon volume on patient safety in laparoscopic gynecologic surgery. Gynecol Oncol. 2012;125:241–244. doi: 10.1016/j.ygyno.2011.12.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bristow RE, Zahurak ML, Diaz-Montes TP, Giuntoli RL, Armstrong DK. Impact of surgeon and hospital ovarian cancer surgical case volume on in-hospital mortality and related short-term outcomes. Gynecol Oncol. 2009;115:334–338. doi: 10.1016/j.ygyno.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Wright JD, Hershman DL, Burke WM, Lu YS, Neugut AI, Lewin SN, et al. Influence of surgical volume on outcome for laparoscopic hysterectomy for endometrial cancer. Ann Surg Oncol. 2012;19:948–958. doi: 10.1245/s10434-011-2090-8. [DOI] [PubMed] [Google Scholar]
- 19.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 20.Liu CJ, Chou YJ, Teng CJ, Lin CC, Lee YT, Hu YW, et al. Association of surgeon volume and hospital volume with the outcome of patients receiving definitive surgery for colorectal cancer: a nationwide population-based study. Cancer. 2015;121:2782–2790. doi: 10.1002/cncr.29356. [DOI] [PubMed] [Google Scholar]
- 21.Lin CC, Lin HC. Effects of surgeon and hospital volume on 5-year survival rates following oral cancer resections: the experience of an Asian country. Surgery. 2008;143:343–351. doi: 10.1016/j.surg.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Killeen SD, O'Sullivan MJ, Coffey JC, Kirwan WO, Redmond HP. Provider volume and outcomes for oncological procedures. Br J Surg. 2005;92:389–402. doi: 10.1002/bjs.4954. [DOI] [PubMed] [Google Scholar]
- 23.Schrag D, Panageas KS, Riedel E, Hsieh L, Bach PB, Guillem JG, et al. Surgeon volume compared to hospital volume as a predictor of outcome following primary colon cancer resection. J Surg Oncol. 2003;83:68–78. doi: 10.1002/jso.10244. [DOI] [PubMed] [Google Scholar]