Abstract
Objective
Choice of hysterectomy and adjuvant treatment for International Federation of Gynecology and Obstetrics (FIGO) 2009 stage II endometrioid endometrial cancer (EEC) is still controversial. Aims of this study were to evaluate survival benefits and adverse effects of different hysterectomies with or without adjuvant radiotherapy (RT), and to identify prognostic factors.
Methods
The patients at 14 member hospitals of the Taiwanese Gynecologic Oncology Group from 1992 to 2013 were retrospectively investigated. Patients were divided into simple hysterectomy (SH) alone, SH with RT, radical hysterectomy (RH) alone, and RH with RT groups. Endpoints were recurrence-free survival (RFS), overall survival (OS), disease-specific survival (DSS), adverse effects and prognostic factors for survival.
Results
Total of 246 patients were enrolled. The 5-year RFS, OS, DSS and recurrence rates for the entire cohort were 89.5%, 94.3%, 96.2% and 10.2%, respectively. Patients receiving RH had more adverse effects including blood loss (p<0.001), recurrent urinary tract infections (p=0.013), and leg lymphedema (p=0.038). Age over 50-year (HR=9.2; 95% confidence interval [CI], 1.2–70.9) and grade 3 histology (HR=7.28; 95% CI, 1.45–36.6) were independent predictors of OS. Grade 3 histology was an independent predictor of RFS (HR=5.13; 95% CI, 1.38–19.1) and DSS (HR=5.97; 95% CI, 1.06–58.7). Patients receiving adjuvant RT had lower locoregional recurrence (p=0.046), but no impact on survival.
Conclusion
Different treatment modalities yield similar survival outcomes. Patients receiving SH with RT had lower locoregional recurrent with acceptable morbidity. Age and tumor grading remained significant predictors for survival among patients with FIGO 2009 stage II EEC.
Keywords: Uterine cancer, Endometrioid, General Surgery, Radiotherapy, Survival, Recurrence
INTRODUCTION
Endometrial cancer (EC) is one of the most common gynecologic cancers worldwide [1]. In Taiwan, the incidence of EC has increased rapidly in the past decade [2]. The most common histology is the endometrioid type, and the majority of tumors are confined to the uterine corpus. The 2009 International Federation of Gynecology and Obstetrics (FIGO) staging defined stage II disease as pathologic involvement of the uterine cervix stroma [3]. The presence of glandular involvement was not included in the definition of stage II disease because the prognosis of these patients was not worse than those with stage I. According to previous reports, 7%–12% of ECs are stage II [4,5,6].
Total hysterectomy, bilateral salpingo-oophorectomy (BSO), and bilateral pelvic and para-aortic lymph node dissection are recommended as staging surgery for EC [7]. The optimal type of hysterectomy for patients with stage II EC remains controversial, and treatment options range from simple (extrafascial) hysterectomy (SH) to radical hysterectomy (RH). RH has been recommended for stage II disease due to improvements in locoregional control and survival compared with SH [8,9]. However, other studies that evaluated the prognostic factors in patients with FIGO 2009 stage II did not find that the type of hysterectomy was a risk factor for survival or recurrence [10,11,12,13,14]. Therefore, the choice of hysterectomy for stage II EC is still controversial. Thus, we conducted a retrospective, multicenter study to evaluate survival benefits and adverse effects of different hysterectomies with or without adjuvant RT, and to identify prognostic factors among patients with FIGO stage II endometrioid endometrial cancer (EEC).
MATERIALS AND METHODS
1. Patients
The medical records of patients who underwent primary treatment between January 1992 and December 2013 at the member hospitals of the Taiwanese Gynecologic Oncology Group (TGOG) were retrospectively reviewed. The study protocol was approved by the Institutional Review Board of each hospital (approval number: 105-3816C). The inclusion criterion was post-operative pathology-proven FIGO 2009 stage II EEC. Patients with incomplete surgical staging (without BSO or lymphadenectomy), an inaccurate pathology report, incomplete medical records, or a lack of follow-up were excluded from the study. The detailed medical records were retrospectively evaluated until the end of the follow-up period (31 December 2014).
Demographic data, including age at the time of surgery, body mass index (BMI), parity, menopausal status, and history of medical diseases were obtained from the medical records. The staging and histologic grading criteria were determined post-operatively and were based on the FIGO 2009 staging system [3]. Surgical staging procedures consisted of washing cytology, SH or RH, BSO, and lymph node dissection (pelvic with or without para-aortic). The decision to give the patients adjuvant therapy was based on the extent of the disease, medical co-morbidities, and the institutional practices during that time. The clinical follow-up assessments of the disease consisted of pelvic physical examinations, vaginal cytology, determination of tumor markers, and imaging examinations when clinically indicated. Grading of adverse events was judged by the Common Terminology Criteria for Adverse Events (CTCAE) v4.0 [15]. Chronic adverse events were defined as repeated hospitalizations with the same diagnosis for more than 6 months after surgery. The stage of leg edema was defined according to the Fifth World Health Organization Expert Committee on Filariasis. Follow-up data, including sites of recurrence, date at the diagnosis of recurrence, and patient status at the last visit or the end of the follow-up period (31 December 2014).
The following pathologic factors were evaluated: FIGO grade (G); depth of myometrial invasion; lymphovascular space invasion (LVSI); size of the tumor; parametrium involvement; and washing cytology results. A pathology review was conducted at each institution.
2. Statistical methods
Recurrence-free survival (RFS) and locoregional recurrence free survival (LRFS) was calculated from the date of staging surgery to the date of diagnosis of recurrence or last contact for the recurrence-free patients. Locoregional recurrence was defined as the vaginal stump, pelvic or vaginal with pelvic recurrence. Overall survival (OS) was defined as the period from the date of surgery to the date of death or last contact. Disease-specific survival (DSS) was defined as the date of surgery to the date of death from EC or last contact.
The χ2 test was used to analyze categorical variables and the Student's t-test was used for continuous variables. The Kaplan-Meier method was used to calculate patient survival distribution. The significance of the survival distribution in each group was tested using the log-rank test. Cox proportional hazard analysis was used to evaluate the prognostic factors for survival. Multivariate analysis using Cox stepwise forward regression was conducted for the covariates in univariate analysis with a p-value <0.05, hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. A p-value <0.05 was considered to be statistically significant. All statistical calculations were performed using SPSS software for Windows version 20 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Characteristics of the patients
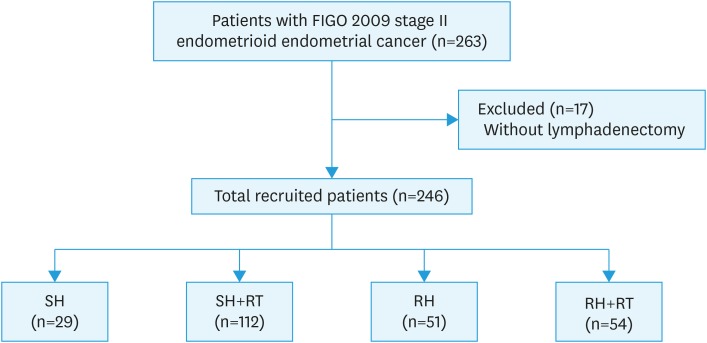
We identified 246 patients with stage II (FIGO 2009) EEC who were treated between 1992 and 2013 at the 14 TGOG member hospitals (Fig. 1). The median duration of follow-up was 78 months (range, 0.5–259 months). Table 1 shows the characteristics of the patients. The mean age was 53.1 years at the time EC was diagnosed. One hundred and forty-one (57.3%) of the patients underwent SHs and 105 patients (42.7%) underwent RHs. After surgery, 112 patients (79.4%) in the SH group and 54 patients (51.4%) in the RH group received adjuvant RT, while the other patients were managed with surveillance. Ten patients (4.1%) received vaginal brachytherapy only, 44 patients (18.1%) received pelvic radiotherapy (RT), and 111 patients (45.7%) received both vaginal and pelvic irradiation. Thirty patients (12.4%) received adjuvant chemotherapy (CT; 3 [10.7%] in the SH group, 12 [10.8%] in the SH + RT group, 8 [16.0%] in the RH group, and 7 [13.2%] in the RH + RT group). Twenty-five patients (10.2%) had tumor recurrence during the follow-up period, including locoregional recurrence 14 and distant recurrence in10 patients, while only one patient had both locoregional and distal recurrences. The pathologic characteristics are shown in Table 1. The clinic and pathologic characteristics of patients receiving different treatment modalities were showed in Supplementary Table 1. Patients with adjuvant RT after SH and RH had higher ratio of deep myometrium invasion and LVSI. Supplementary Table 2 showed the clinical and pathologic characteristics of patients receiving adjuvant RT or not. In adjuvant RT group, we found higher ratio of patients with deep myometrium invasion (RT vs. no RT: 56.1% vs. 23.8%, p<0.001), LVSI (RT vs. no RT: 27.9% vs. 10.0%, p=0.021) and SH (RT vs. no RT: 66.3% vs. 35.0%, p<0.001). Supplementary Table 3 showed the clinical and pathologic characteristics of patients receiving adjuvant CT or not. In adjuvant CT group, we found higher ratio of patients with grade 3 tumors (CT vs. no CT: 51.7% vs. 14.2%, p<0.001). Supplementary Table 4 showed the clinical and pathologic characteristics of patients receiving SH or RH. In SH group, we found higher ratio of patients with adjuvant RT (SH vs. RH: 78.0% vs. 53.3%, p<0.001) and lower ratio of chronic adverse effect (SH vs. RH: 15.2% vs. 26.7%, p=0.028).
Fig. 1. Flowchart of our retrospective study design.
FIGO, International Federation of Gynecology and Obstetrics; RH, radical hysterectomy; RT, radiotherapy; SH, simple hysterectomy.
Table 1. Patient characteristics (n=246).
| Variable | Values | |
|---|---|---|
| Age (yr) | 53.1±10.6 | |
| BMI (kg/m2) | 25.2±4.5 | |
| Nulliparity | 40 (16.3) | |
| Menopause | 124 (50.4) | |
| Medical diseases | 81 (32.9) | |
| Hypertension | 55 (22.4) | |
| Diabetes mellitus | 32 (13.0) | |
| Co-existing history of malignancy | 21 (8.5) | |
| Breast cancer | 3 (1.2) | |
| Colon cancer | 7 (2.8) | |
| Adjuvant CT | 30 (12.4) | |
| Treatment type | ||
| SH | 29 (11.8) | |
| SH + RT | 112 (45.5) | |
| RH | 51 (20.7) | |
| RH + RT | 54 (22.0) | |
| Adjuvant RT | 166 (67.5) | |
| Vagina only | 10 (4.1) | |
| Pelvis only | 44 (17.9) | |
| Pelvis and vagina | 111 (45.1) | |
| Lymphadenectomy | ||
| Pelvic only | 101 (41.1) | |
| Pelvic and para-aortic | 145 (58.9) | |
| Tumor FIGO grade | ||
| Grade 1 | 92 (37.4) | |
| Grade 2 | 105 (42.7) | |
| Grade 3 | 46 (18.7) | |
| Myometrium >1/2 | 111 (45.1) | |
| LVSI | 51 (20.7) | |
| PM invasion | 4 (1.6) | |
| Positive washing cytology | 6 (2.4) | |
| Recurrence | 25 (10.2) | |
| Recurrent sites | ||
| Locoregional | 14 | |
| Distant | 10 | |
| Mixed | 1 | |
| Cause of death | ||
| Disease-related | 8 (3.3) | |
| Non-disease related | 6 (2.4) | |
Continuous data are presented as the mean±standard deviation or number (%).
BMI, body mass index; CT, chemotherapy; FIGO, International Federation of Gynecology and Obstetrics; LVSI, lymphovascular space invasion; PM, parametrium; RH, radical hysterectomy; RT, radiotherapy; SH, simple hysterectomy.
2. Survival pattern of the patients receiving different treatments
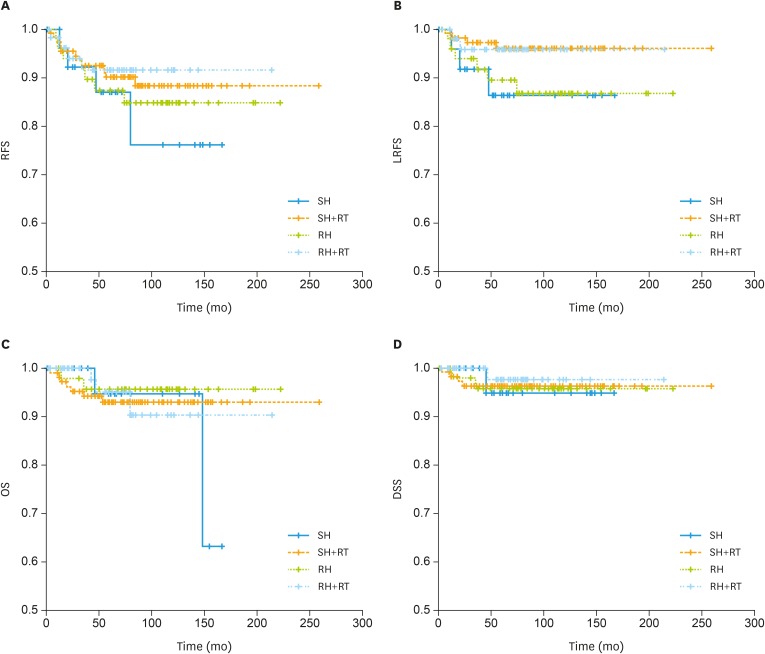
The 5-year RFS, OS, DSS, and LRFS, for the entire cohort were 89.5%, 94.3%, 96.2%, and 93.8%, respectively. RFS, LRFS, OS, and DSS curves of the patients receiving different treatment modalities are shown in Fig. 2, respectively. There were also no significant survival differences among the different treatment modalities.
Fig. 2. Survival curves for the patients receiving different treatments. (A) RFS, (B) LRFS, (C) OS, (D) DSS, according to the treatment modality in 246 patients with FIGO 2009 stage II EEC. (A) Five-year RFS rates for SH alone, SH with RT, RH alone, and RH with RT were 86.9%, 90.1%, 87.3%, and 91.5%, respectively (p=0.706). (B) Five-year LRFS rates for SH alone, SH with RT, RH alone, and RH with RT were 88.9%, 96.4%, 88.2%, and 93.9%, respectively (p=0.141). (C) Five-year OS rate for SH alone, SH with RT, RH alone, and RH with RT were 94.7%, 93.0%, 95.7%, and 95.1%, respectively (p=0.863). (D) Five-year DSS rates for SH alone, SH with RT, RH alone, and RH with RT were 94.7%, 96.2%, 95.7%, and 97.4%, respectively (p=0.938).
DSS, disease-specific survival; LRFS, locoregional recurrence free survival; OS, overall survival; RFS, recurrence-free survival; RH, radical hysterectomy; RT, radiotherapy; SH, simple hysterectomy.
3. Pattern of recurrence according to treatment
Twenty-five (10.2%) patients were diagnosed with tumor recurrences. There was a significant difference in the site of recurrence between the 4 groups (p=0.046; Table 2). The patients who received adjuvant RT had a lower locoregional recurrence rate (3.0% vs. 10.0%, p=0.021) regardless of the type of hysterectomy.
Table 2. Recurrence pattern according to different treatment groups.
| Treatment | No. | Recurrent site | Total | p-value | ||
|---|---|---|---|---|---|---|
| Locoregional | Distant | Mixed | ||||
| SH | 29 | 3 (10.3) | 0 | 0 | 3 (11.1) | 0.046 |
| SH + RT | 112 | 4 (3.6) | 7 (6.2) | 0 | 11 (9.8) | |
| RH | 51 | 6 (11.8) | 1 (2.0) | 0 | 7 (13.7) | |
| RH + RT | 54 | 1 (1.9) | 2 (3.7) | 1 (1.9) | 4 (7.5) | |
Data are presented as the number (%).
RH, radical hysterectomy; RT, radiotherapy; SH, simple hysterectomy.
4. Acute and chronic adverse effects according to different treatments
Adverse effects according to the treatment modality are shown in Table 3. The patients who received RH had significantly longer operative time and hospital stay, and more surgical blood loss. Of the chronic adverse effects, vesicovaginal fistulas and recurrent urinary tract infections were more frequently observed in the RH with or without RT groups (p=0.013). The patients who received SH only had the lowest rate of lower limb lymphedema (p=0.038). Lower limb lymphedema was most frequently observed in the RH with RT group.
Table 3. Acute and chronic adverse effects according to different treatment groups.
| Treatment | SH | SH + RT | RH | RH + RT | p-value | ||
|---|---|---|---|---|---|---|---|
| Acute | |||||||
| Operative time (min) | 194.1±77.2 | 223.2±84.0 | 286.4±106.3 | 245.9±80.7 | <0.001 | ||
| Blood loss (mL) | 282.9±149.8 | 361.0±355.5 | 1,003.2±860.1 | 904.2±1,105.2 | <0.001 | ||
| Hospital stay (day) | 7.6±1.6 | 9.3±6.6 | 12.1±6.5 | 12.4±7.8 | 0.001 | ||
| Chronic | |||||||
| Gastrointestinal | 0.660 | ||||||
| Ileus | 2 (6.9) | 8 (7.1) | 1 (2.0) | 2 (3.8) | |||
| Enterocutaneous fistula | 0 | 0 | 0 | 1 (1.9) | |||
| Rectovaginal fistula | 0 | 0 | 0 | 0 | |||
| Chronic colitis | 0 | 1 (0.9) | 0 | 0 | |||
| Urogenital | 0.013* | ||||||
| Vesico-vaginal fistula | 0 | 0 | 1 (2.0) | 0 | |||
| Uretero-vaginal fistula | 0 | 0 | 0 | 0 | |||
| Recurrent UTI | 1 (3.4) | 1 (0.9) | 5 (10.0) | 4 (7.7) | |||
| Leg edema | 0.038* | ||||||
| Stage 1 | 0 | 5 (4.5) | 2 (4.0) | 5 (9.6) | |||
| Stage 2 | 0 | 0 | 0 | 0 | |||
| Stage 3 | 0 | 5 (4.5) | 2 (4.0) | 6 (11.5) | |||
Continuous data are presented as the mean±standard deviation. Categorical data are presented as number (%). Stage of leg edema was defined according to the Fifth World Health Organization Expert Committee on Filariasis.
RH, radical hysterectomy; RT, radiotherapy; SH, simple hysterectomy; UIT, urinary tract infection.
*p-value <0.05.
5. Analysis of risk factors for recurrence and patient survival
The results of univariate (log-rank test) and multivariate (Cox proportional hazard) analyses of RFS, OS, DSS, and LRFS, are shown in Supplementary Tables 5, 6, 7, 8, respectively. Based on the multivariate analysis (Table 4), age more than 50-year-old (HR=9.22; 95% CI=1.20–70.9) and high tumor grade (HR=7.28; 95% CI=1.45–36.6) were significant poor predictors for OS. High tumor grade was the only significantly poor predictor for RFS (HR=5.13; 95% CI=1.38–19.1) and DSS (HR=5.97; 95% CI=1.06–58.7). High tumor grade (HR=5.57; 95% CI=1.58–19.5) and RT (HR=0.246; 95% CI=0.09–0.70) were significant predictors for locoregional recurrence. The types of surgery were not significant predictors for OS, RFS, DSS, or LRFS.
Table 4. The results of multivariate analysis for survival endpoints.
| Variable | OS | RFS | DSS | Locoregional recurrence | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI of HR | p-value | HR | 95% CI of HR | p-value | HR | 95% CI of HR | p-value | HR | 95% CI of HR | p-value | |
| Age (≥50 vs. <50 yr) | 9.22 | 1.20–70.90 | 0.033* | 2.72 | 0.82–9.04 | 0.103 | 9.93 | 0.58–82.30 | 0.125 | 1.62 | 0.36–7.32 | 0.533 |
| RT (yes vs. no) | 0.56 | 0.13–2.54 | 0.455 | 0.32 | 0.10–1.01 | 0.051 | 0.31 | 0.03–3.59 | 0.348 | 0.246 | 0.09–0.70 | 0.009* |
| Grade (2 vs. 1) | 2.30 | 0.46–11.50 | 0.309 | 1.78 | 0.47–6.77 | 0.367 | 1.42 | 0.13–16.00 | 0.754 | 1.08 | 0.27–4.37 | 0.909 |
| Grade (3 vs. 1) | 7.28 | 1.45–36.60 | 0.016* | 5.13 | 1.38–19.10 | 0.017* | 5.97 | 1.06–58.70 | 0.048* | 5.57 | 1.58–19.50 | 0.007* |
| Adjuvant CT (yes vs. no) | 1.33 | 0.28–6.22 | 0.721 | 1.50 | 0.51–4.37 | 0.459 | 2.44 | 0.30–19.70 | 0.405 | 0.27 | 0.02–3.29 | 0.302 |
| Depth of invasion (<1/2 vs. ≥1/2) | 5.10 | 0.90–28.80 | 0.065 | 2.78 | 0.99–7.75 | 0.051 | 2.04 | 0.40–10.40 | 0.391 | 3.32 | 0.67–16.40 | 0.141 |
| LVSI (yes vs. no) | 1.13 | 0.31–4.22 | 0.851 | 1.44 | 0.58–3.56 | 0.433 | 1.37 | 0.17–10.90 | 0.767 | 1.20 | 0.27–5.40 | 0.811 |
CI, confidence interval; CT, chemotherapy; DSS, disease-specific survival; HR, hazard ratio; LVSI, lymphovascular space invasion; OS, overall survival; RFS, recurrence-free survival; RT, radiotherapy.
*p-value <0.05.
DISCUSSION
This study demonstrated that the type of surgery was not associated with RFS, DSS, or OS for patients with FIGO 2009 stage II EEC regardless of whether or not they received RT. Adjuvant RT significantly improved the locoregional control rate, but not survivals. High tumor grade was an independent predictor of RFS, OS, DSS and LRFS. Age (≥50-years) was also an independent predictor of OS (Table 4).
In our series, the 5-year RFS, OS, and DSS rates for all of the patients were 89.5%, 94.3%, and 96.2%, respectively. The survival rates were better than previous reports [10,11,12,13,16,17]. A possible explanation for this finding is that we only included patients with FIGO 2009 stage II EEC with complete staging surgery; patients with high-risk histologic subtypes (carcinosarcoma, serous and clear cell carcinoma) were excluded from this study. Another interesting finding is that our patients were much younger than previously reported [7]. Age is known as a risk factor for survival [11,12,16], and the median age at diagnosis was 52 years (range, 26–86 years) in this study. This is not due to patient selection bias, because in Taiwan the median age at the time of diagnosis of uterine corpus cancer is 55 years [24], which is also younger than in a previous report [7]. In Mahdi et al.'s study [17], Asian women are younger than non-Hispanic white women at the initial presentation of endometrial adenocarcinoma. Further studies are required to investigate possible explanations for such racial differences.
Cohn et al. [18] and Sartori et al. [19] reported statistically significant improvements in 5-year survival in patients who underwent RHs; however, their results were not based on multivariate analysis. In addition, not all of the patients in the reports received adequate lymphadenectomies. Because cervical involvement is a risk factor for lymph node metastasis [20,21,22], these patients may have been assigned to a higher stage due to lymph node metastasis. In contrast, other investigators have not reported a survival benefit based on the type of surgical procedure performed [11,12,14,16]. In our multivariate model, RH had no significant beneficial effect on OS, RFS, DSS, or LRFS. RH alone does not increase survival in stage II EC, as in cervical cancer, because the tumor behavior is different for EC with cervical involvement compared to primary cervical cancer. A lower parametrium invasion [23] and higher pelvic lymph node metastasis had been reported in patients with EC with cervical involvement [20]. RH is mostly beneficial in women with gross cervical involvement pre-operatively [16].
After surgery for EC, external beam or vaginal brachytherapy is recommended for women with stage II disease [8]. A systematic review and meta-analysis assessed the benefits of adjuvant RT in women with early EC [25,26]. The investigators found that adjuvant RT reduced the risk of locoregional recurrence, but did not impact DSS or OS [13,16,19,25,26]. In our study, we also found that adjuvant RT improved locoregional control, but did no impact on RFS, OS, or DSS. Even, the patients with adjuvant RT had poor histologic factors as deeper myometrium invasion and LVSI (Supplementary Table 2).
There were no significant differences in major acute side effects including major organ injuries or death between the SH and RH groups in this study. RH required a significantly longer operative time, was associated with greater intra-operative blood loss, and led to longer hospital stay. Chronic side effects, including recurrent urinary tract infections, and leg edema occurred more frequently in the RH group. Our patients receiving RH and adjuvant RT had the highest rate of grade 3 leg edema (11.5%). Other investigators had reported that complications occurred more frequently in RH patients [10,11].
In the present study, histologic grade was an independent prognostic factor for OS, RFS, DSS, and locoregional recurrence (Supplementary Fig. 1), which is consistent with previous studies [9,12,13,16,27]. Both locoregional and distant recurrence rate were much higher in the patients with G3 tumors than in those with G1/G2 tumors (p=0.007, Supplementary Table 9). This finding suggests that adjuvant CT may play a role in these patients. Susumu et al. [28] reported a randomized control trial that compared pelvic RT and CT with cyclophosphamide, doxorubicin, and cisplatin in patients with stage IC-IIIC EC, and suggested a survival advantage with CT in the patients from the high-to-intermediate risk group (stage IC, >70 years of age, grade 3, stage II, or positive cytology with >50% myometrial invasion). However, Maggi et al. [29] failed to show any improvement in RFS or OS in patients with stage IC G3 or II G3 treated with adjuvant CT or RT. In addition, the Gynecologic Oncology Group (GOG) 249 trial did not show any benefit of adjuvant CT plus brachytherapy compared to external beam RT with respect to RFS and OS. In our series, of 46 patients with G3 EEC, six did not receive adjuvant treatment, four received adjuvant CT only, 24 received RT only and 12 received both of CT and RT. The results showed that only RT significantly improved locoregional control (p<0.001). We did not find any benefit in OS or RFS in the patients who received adjuvant CT. Taken together, the role of CT for stage II G3 EEC remains controversial.
The strengths of this study are that we only included patients with FIGO 2009 stage II EEC with complete staging surgery (SH or RH, BSO, and pelvic lymphadenectomy), patients from medical centers throughout Taiwan, the length of follow-up, comparisons of the survival with different treatment modalities, and the description of rates and recurrence patterns. In comparison, other studies have included stage II EC with or without pelvic lymphadenectomy [9,11,12,16,23]. This is also the largest series to date on women with FIGO 2009 stage II EEC with complete staging surgery.
The limitations of this study included the retrospective design and lack of central pathologic review. The retrospective nature of the current study and the potential for selection bias is the main limitation. We included patients who were treated over a long period of time (>20 years), and developments in RT technology were not considered in this study. In addition, the patients were treated at different academic institutions with different philosophies with regards to surgery and adjuvant treatments.
In conclusion, we demonstrated that different treatment modalities for FIGO stage II disease yielded similar survival outcomes. Adjuvant RT reduced locoregional recurrence, but did not have any impact on survival. If gross cervical involvement was found pre-operatively, RH may be beneficial. For small or subtle cervical lesions of patients with EC found by pelvic examination or imaging study before surgery, SH with RT appeared to be a suitable treatment strategy for FIGO 2009 stage II EEC due to acceptable morbidity and lower locoregional recurrence. Patients with poor prognostic factors including older age and high histologic grade (G3) may need aggressive adjuvant therapy such as RT or CT. Further well-designed studies are required to confirm our findings.
ACKNOWLEDGMENTS
We are grateful to Dr. How-Ran Guo of the Department of Environmental and Occupational Health, College of Medicine, National Cheng Kung University, for providing statistical consultation.
Footnotes
Presentation: The findings of the study had been presented at 2015 Asian Society of Gynecologic Oncology (ASGO) biennial meeting.
Funding: The work was supported by the research platform of the Taiwanese Gynecologic Oncology Group (TGOG), TR8-Taiwan Clinical Trial Consortium for Gynecologic Oncology V, supported by a grant from the National Science Council, Taiwan (NSC 103-2325-B-195.002). The work was also supported by the grant (CMRPG8E0841) from Kaohsiung Chang Gung Memorial Hospital.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: F.H.C., Y.M.S.
- Formal analysis: F.H.C.
- Methodology: F.H.C., C.J.R., C.M.Y., H.K.F., C.W.F., C.A.J., K.Y.M., C.Y.C., C.Y.Y., H.C.Y., K.C.Y., K.Y.Y., H.S.M., Y.M.S.
- Resources: F.H.C., C.J.R., C.M.Y., H.K.F., C.W.F., C.A.J., K.Y.M., C.Y.C., C.Y.Y., H.C.Y., K.C.Y., K.Y.Y., H.S.M., Y.M.S.
- Supervision: Y.M.S.
- Validation: Y.M.S.
- Writing - original draft: F.H.C.
- Writing - review & editing: C.J.R., C.M.Y., H.K.F., C.W.F., C.A.J., K.Y.M., C.Y.C., C.Y.Y., H.C.Y., K.C.Y., K.Y.Y., H.S.M., Y.M.S.
SUPPLEMENTARY MATERIALS
Clinic and pathologic characteristics of patients receiving different treatment modalities
Clinic and pathologic characteristics of patients with/without adjuvant RT
Clinic and pathologic characteristics of patients with/without adjuvant CT
Clinic and pathologic characteristics of patients with SH and RH
Risk factor association with RFS
Risk factor association with OS
Risk factor association with DSS
Risk factor association with LRFS
Recurrent pattern according to different histologic grade groups
Survival curves for the patients with different histologic grades.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chiang CJ, Chen YC, Chen CJ, You SL, Lai MS, Taiwan Cancer Registry Task Force Cancer trends in Taiwan. Jpn J Clin Oncol. 2010;40:897–904. doi: 10.1093/jjco/hyq057. [DOI] [PubMed] [Google Scholar]
- 3.Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Sorosky JI. Endometrial cancer. Obstet Gynecol. 2012;120:383–397. doi: 10.1097/AOG.0b013e3182605bf1. [DOI] [PubMed] [Google Scholar]
- 5.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 6.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 7.SGO Clinical Practice Endometrial Cancer Working Group. Endometrial cancer: a review and current management strategies: part I. Gynecol Oncol. 2014;134:385–392. doi: 10.1016/j.ygyno.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network (US) NCCN Clinical Practice Guidelines in Oncology. Uterine Neoplasms, version 2. 2016 [Internet] Fort Washington, PA: National Comprehensive Cancer Network; 2015. [Accessed 2016 September 16]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. [DOI] [PubMed] [Google Scholar]
- 9.Orezzoli JP, Sioletic S, Olawaiye A, Oliva E, del Carmen MG. Stage II endometrioid adenocarcinoma of the endometrium: clinical implications of cervical stromal invasion. Gynecol Oncol. 2009;113:316–323. doi: 10.1016/j.ygyno.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Lee TS, Kim JW, Kim DY, Kim YT, Lee KH, Kim BG, et al. Necessity of radical hysterectomy for endometrial cancer patients with cervical invasion. J Korean Med Sci. 2010;25:552–556. doi: 10.3346/jkms.2010.25.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takano M, Ochi H, Takei Y, Miyamoto M, Hasumi Y, Kaneta Y, et al. Surgery for endometrial cancers with suspected cervical involvement: is radical hysterectomy needed (a GOTIC study)? Br J Cancer. 2013;109:1760–1765. doi: 10.1038/bjc.2013.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elshaikh MA, Al-Wahab Z, Mahdi H, Albuquerque K, Mahan M, Kehoe SM, et al. Recurrence patterns and survival endpoints in women with stage II uterine endometrioid carcinoma: a multi-institution study. Gynecol Oncol. 2015;136:235–239. doi: 10.1016/j.ygyno.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Frandsen JE, Sause WT, Dodson MK, Soisson AP, Belnap TW, Gaffney DK. Survival analysis of endometrial cancer patients with cervical stromal involvement. J Gynecol Oncol. 2014;25:105–110. doi: 10.3802/jgo.2014.25.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozgul N, Boyraz G, Salman MC, Gultekin M, Yuce K, Ibrahimov A, et al. Oncological outcomes of stage II endometrial cancer: a retrospective analysis of 250 cases. Int J Gynecol Cancer. 2018;28:161–167. doi: 10.1097/IGC.0000000000001133. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute (US) Common terminology criteria for adverse events (CTCAE) Rev. ed. Bethesda: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2009. [Google Scholar]
- 16.Wright JD, Fiorelli J, Kansler AL, Burke WM, Schiff PB, Cohen CJ, et al. Optimizing the management of stage II endometrial cancer: the role of radical hysterectomy and radiation. Am J Obstet Gynecol. 2009;200:419.e1–419.e7. doi: 10.1016/j.ajog.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Mahdi H, Schlick CJ, Kowk LL, Moslemi-Kebria M, Michener C. Endometrial cancer in Asian and American Indian/Alaskan Native women: tumor characteristics, treatment and outcome compared to non-Hispanic white women. Gynecol Oncol. 2014;132:443–449. doi: 10.1016/j.ygyno.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Cohn DE, Woeste EM, Cacchio S, Zanagnolo VL, Havrilesky LJ, Mariani A, et al. Clinical and pathologic correlates in surgical stage II endometrial carcinoma. Obstet Gynecol. 2007;109:1062–1067. doi: 10.1097/01.AOG.0000260871.87607.25. [DOI] [PubMed] [Google Scholar]
- 19.Sartori E, Gadducci A, Landoni F, Lissoni A, Maggino T, Zola P, et al. Clinical behavior of 203 stage II endometrial cancer cases: the impact of primary surgical approach and of adjuvant radiation therapy. Int J Gynecol Cancer. 2001;11:430–437. doi: 10.1046/j.1525-1438.2001.01061.x. [DOI] [PubMed] [Google Scholar]
- 20.Solmaz U, Mat E, Dereli M, Turan V, Gungorduk K, Hasdemir P, et al. Lymphovascular space invasion and cervical stromal invasion are independent risk factors for nodal metastasis in endometrioid endometrial cancer. Aust N Z J Obstet Gynaecol. 2015;55:81–86. doi: 10.1111/ajo.12321. [DOI] [PubMed] [Google Scholar]
- 21.Boren T, Lea J, Kehoe S, Miller DS, Richardson D. Lymph node metastasis in endometrioid adenocarcinomas of the uterine corpus with occult cervical involvement. Gynecol Oncol. 2012;127:43–46. doi: 10.1016/j.ygyno.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Kwon JS, Qiu F, Saskin R, Carey MS. Are uterine risk factors more important than nodal status in predicting survival in endometrial cancer? Obstet Gynecol. 2009;114:736–743. doi: 10.1097/AOG.0b013e3181b96ec6. [DOI] [PubMed] [Google Scholar]
- 23.Phelippeau J, Koskas M. Impact of radical hysterectomy on survival in patients with stage 2 type1 endometrial carcinoma: a matched cohort study. Ann Surg Oncol. 2016;23:4361–4367. doi: 10.1245/s10434-016-5372-3. [DOI] [PubMed] [Google Scholar]
- 24.Taiwan Cancer Registry Task Force (TCRTF) Cancer registry annual report. Taipei: Department of Health, The Executive Yuan; 2013. [Google Scholar]
- 25.Kong A, Johnson N, Kitchener HC, Lawrie TA. Adjuvant radiotherapy for stage I endometrial cancer: an updated Cochrane systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1625–1634. doi: 10.1093/jnci/djs374. [DOI] [PubMed] [Google Scholar]
- 26.ASTEC/EN. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137–146. doi: 10.1016/S0140-6736(08)61767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong TW, Chang SJ, Paek J, Lee Y, Chun M, Ryu HS. Risk group criteria for tailoring adjuvant treatment in patients with endometrial cancer: a validation study of the Gynecologic Oncology Group criteria. J Gynecol Oncol. 2015;26:32–39. doi: 10.3802/jgo.2015.26.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226–233. doi: 10.1016/j.ygyno.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266–271. doi: 10.1038/sj.bjc.6603279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinic and pathologic characteristics of patients receiving different treatment modalities
Clinic and pathologic characteristics of patients with/without adjuvant RT
Clinic and pathologic characteristics of patients with/without adjuvant CT
Clinic and pathologic characteristics of patients with SH and RH
Risk factor association with RFS
Risk factor association with OS
Risk factor association with DSS
Risk factor association with LRFS
Recurrent pattern according to different histologic grade groups
Survival curves for the patients with different histologic grades.