Abstract
Objective
To determine and compare treatment outcomes between cobalt-60 (Co-60) and iridium-192 (Ir-192) high dose rate (HDR) brachytherapy in stage IB2–IIIB cervical cancer patients at Department of Radiology, Faculty of Medicine Vajira Hospital, Navamindrahiraj University.
Methods
A retrospective cohort study of patients diagnosed with cervical cancer and treated with radiotherapy at the Department of Radiation Oncology, Faculty of Medicine Vajira Hospital between 2004 and 2014. Survival rate was analyzed by Kaplan-Meier method and were compared between groups with log-rank test. Multivariate analysis was performed using Cox proportional hazards model.
Results
A total of 480 patients with cervical cancer and treated with radiotherapy were included, 274 patients for Ir-192 group and 206 patients for Co-60 group. The 2- and 5-year disease-free survival rate in Ir-192 group were 80.4% and 73.1% and in Co-60 group were 82.5% and 74.7%, respectively (p=0.365). Overall survival rates at 2 and 5 years were 89.4% and 77% of the Ir-192 group, and 91.6% and 81.9% in the Co-60 group, respectively (p=0.238). The complications were primarily grade 1 or 2. Grade 3 and 4 complications were found in 13 of 274 and 7 of 206 in Ir-192 and Co-60 groups, respectively (p=0.232). Grade and clinical stage of cancer significantly affected the survival outcome.
Conclusion
Cervical cancer patients who were treated with HDR Co-60 brachytherapy were comparable in survival and toxicity outcomes of those with HDR Ir-192 brachytherapy. Co-60 source has lots of economic advantages over Ir-192 and hence suitable for low resource radiotherapy setting.
Keywords: Uterine Cervical Neoplasms, Brachytherapy, Cobalt-60, Iridium-192, Outcomes
INTRODUCTION
Cervical cancer is a major world health problem for women. It is the 9th most common cancer worldwide. The global yearly incidence of cervical cancer for 2015 was 526,000; the annual death rate was 239,000 [1]. In Thailand, it is the second most common cancer and most common leading cause of cancer death among women with an estimated incidence rate of 18.1 per 100,000 women [2].
The important prognostic factors for cervical cancer are tumor volume, clinical staging, histology, age and treatment modality [3,4,5,6,7,8,9,10,11,12,13,14,15]. Cervical cancer is a highly curable disease. Intracavitary brachytherapy (ICBT) with external beam radiotherapy (EBRT) is an essential component in the curative treatment of cervical cancer and has a high therapeutic index by delivering a high dose for the tumor and lower dose to adjacent organs, resulting in an increase in local control and survival without an in increase in toxicity [16].
High dose rate (HDR) brachytherapy is now accepted for cervical cancer treatment. The specific advantage over low dose rate (LDR) brachytherapy is its convenience and applicability in outpatient basis. In the past, the production of small sources for HDR was possible only for iridium-192 (Ir-192) sources. This resulted in a widespread use of Ir-192 sources in HDR brachytherapy. However, the Ir-192 HDR system is costly due to its short half-life, only 74 days, requiring extensive source change at 3-months intervals.
Cobalt-60 (Co-60) source in HDR brachytherapy is not popular because the size of earlier version of Co-60 source was larger than Ir-192 [17]. Currently, miniaturized Co-60 source is available with geometrical dimensions identical to those of Ir-192 sources. The equivalence to Ir-192 sources has been demonstrated in relation to physical data, source construction, and dose distribution of a single source, besides clinically applied more complex dose distributions [18,19,20,21,22,23,24,25].
New system using a miniaturized Co-60 source is getting very popular [18,19,20,21,22,23,24,25,26,27,28,29]. This is due to the fact that Co-60 sources attain the advantage of a longer half-life (5.3 years), requiring source replacement approximately every 5 years. This represents a significant advantage from Co-60 source by having a reduction of resource sparing transport of radioactive source into countries, change and disposal of the sources, quality assurance regulatory requirements, and additional workload [30]. Co-60 then offers logistical and economical save.
In carcinoma of the cervix, the response to radiotherapy is clearly dose-dependent; as the dose increases, so does the probability of tumor control. However, the risk of damage and late complications in normal tissues also increase with the dose usage. The higher energy of 1.25 MeV of Co-60 compared with 0.397 MeV of Ir-192 raises several concerns about efficacy, toxicity and quality of life of the patients who are treated with Co-60 source in HDR brachytherapy when compared with Ir-192 source.
The objective of this study is to determine and compare treatment outcomes between Co-60 and Ir-192 HDR brachytherapy in stage IB2–IIIB cervical cancer patients treated at Radiation Oncology Unit of Vajira Hospital, Navamindrahiraj University in term of disease-free survival (DFS), survival response rate and complication to radiation therapy. The results of this study are the initial guide to improve efficacy, reduce toxicity and enhance quality of life.
MATERIALS AND METHODS
The authors retrospectively studied the data of 480 patients who were diagnosed with cervical cancer and treated with radiotherapy at the Department of Radiation Oncology, Faculty of Medicine Vajira Hospital, Navamindrahiraj University between January 2004 and December 2014 were selected. The numbers of 274 patients were treated with Ir-192 and 206 patients were treated with Co-60 source in HDR brachytherapy. Prior to commencing the study, Vajira Hospital's Institutional Ethical Committee clearance was sought and obtained. Data were accumulate also obtained from patient's medical records.
1. Eligibility criteria
Criteria of inclusion for study were patients who had histologic diagnosis of International Federation of Gynecology and Obstetrics (FIGO) stage IB2–IIIB cervical cancer and treated with radiation therapy alone or concurrent chemoradiation therapy completely. Patients were excluded if they had histopathology of neuroendocrine or clear cell, history of cancer in other organs, uncontrolled medical illness e.g., chronic renal failure or human immunodeficiency virus infection.
2. Treatment modality
All patients were treated with a combination of external and intracavitary irradiation. Total dose was calculated based on equivalent total dose in 2 Gy fraction (EQD2) of external beam + EQD2Gy HDR brachytherapy = Total dose to point A (80–90 Gy) and delivered to the point A. Whole pelvis was treated to a total dose of 45 Gy in 5 weeks. Patients were treated once a day, 5 days a week with a daily fraction size of 1.8–2 Gy. A midline block might be used at the discretion of the treating radiation oncologist, using either parallel-opposed antero-posterior or four field box technique. The parametrium and pelvic side walls were boosted up to 54–60 Gy according to the staging. HDR brachytherapy might start as early in the third week. When HDR brachytherapy began, at least one insertion was performed per week with no external beam therapy given on the day of the insertion. If most of the external beam radiation had been given, then 2 insertions per week could be done and separated by at least 72 hours in order to complete all treatments within 8 weeks. The usage of 3 to 5 fractions of 6.5–7.5 Gy each to point A depended on tumor volume. The point A, bladder point and rectal point was defined in accordance with ICRU 38.
3. Assessment and treatment outcomes
All patients were followed-up to receive pelvic examination every 3 months during the first 2 years then every 6 months until death. Tumor response was determined from pelvic examination and confirmed by biopsy at 3 months after completion of treatment. The primary outcome was 2-year and 5-year overall survival (OS). The secondary outcomes were 2- and 5-year DFS, response rate and complication rate. OS was obtained from the 1st date of treatment to the date of death from all causes or the last follow-up. DFS was calculated from the 1st date of treatment until date of disease progression or recurrence. Patients who lost a follow-up, DFS data were right-censored at the time of the last follow-up. Acute complication for gastrointestinal (GI) and genitourinary (GU) systems were graded by the radiation oncologist during a course of treatment until 6 months after the completion of therapy. Late complications were graded after 6 months of treatment. All toxicities were recorded following RTOG/EORTC toxicity criteria [31].
4. Statistical methods
All data were analyzed using SPSS statistical analysis for Windows (version 22.0; IBM Corp., Armonk, NY, USA). Data were compared by χ2 test and Fisher's exact test. DFS and OS were analyzed by Kaplan-Meier method and were compared between groups with log-rank test. Multivariate analysis was performed using Cox proportional hazards model. All p-values were 2 sided, and p-value less than 0.05 was considered statistically significant.
RESULTS
The total number of cervical cancer patients eligible for inclusion in the present study was 274 for Ir-192 group and 206 for Co-60 group. Baseline patient characteristics are shown in Table 1. The patient's characteristics between these 2 arms were comparable. The median follow-up time for Ir-192 and Co-60 treated patients was 74 and 36 months, respectively. The mean age at the time of diagnosis was 53.98 year in Ir-192 group and 53.41 year in Co-60 group.
Table 1. Patient and tumor characteristics.
| Characteristics | Ir-192 | Co-60 | p-value | |
|---|---|---|---|---|
| All | 274 (57.1) | 206 (42.9) | ||
| Age (yr) | 54.11 (20–85) | 53.41 (27–87) | 0.167 | |
| Histology | 0.823 | |||
| Squamous cell carcinoma | 223 (81.4) | 163 (79.1) | ||
| Adenocarcinoma | 46 (16.8) | 39 (18.9) | ||
| Adenosquamous | 5 (1.8) | 4 (1.9) | ||
| Tumor grade | 0.103 | |||
| Well differentiated | 64 (23.4) | 51 (24.8) | ||
| Moderate differentiated | 153 (55.8) | 128 (62.1) | ||
| Poorly differentiated | 57 (20.8) | 27 (13.1) | ||
| Tumor size (cm) | 4.57 (1–10) | 4.82 (0.5–9) | 0.086 | |
| ≤4 | 132 (48.2) | 83 (40.3) | ||
| >4 | 142 (51.8) | 123 (59.7) | ||
| FIGO stage | 0.429 | |||
| IB2 | 5 (1.8) | 10 (4.8) | ||
| IIA | 8 (2.9) | 7 (3.4) | ||
| IIB | 147 (53.6) | 99 (48.1) | ||
| IIIA | 7 (2.6) | 5 (2.4) | ||
| IIIB | 107 (39.1) | 85 (41.3) | ||
Values are presented as number of patients (%) or mean (range).
Co-60, cobalt-60; FIGO, Federation of Gynecology and Obstetrics; Ir-192, iridium-192.
Treatment modality data which 480 cervix cancer patients were received is shown in Table 2. The median treatment time was 55 days and 56 days in the Ir-192 and Co-60 groups, respectively. The mean total dose at point A EQD2Gy was 89.62 (75.00–105.58) Gy and 91.04 (84.20–102.69) Gy in the Ir-192 and Co-60 groups, respectively.
Table 2. Treatment characteristics.
| Characteristics | Ir-192 | Co-60 | p-value | |
|---|---|---|---|---|
| Concurrent chemotherapy | 0.063 | |||
| No | 44 (16.1) | 21 (10.2) | ||
| Yes | 230 (83.9) | 185 (89.8) | ||
| Mean total EBRT dose (Gy) | 54.39 (43.00–56.00) | 54.51 (52–60) | 0.342 | |
| HDR mean total point A EQD2 (Gy) | 35.24 (24.00–49.58) | 36.53 (30.96–44.69) | ||
| Mean total dose EQD2 (Gy) | 89.62 (75.00–105.58) | 91.04 (84.20–102.69) | ||
| Mean bladder point EQD2 (Gy) | 20.71 (7.62–35.31) | 22.39 (8–36.46) | ||
| Mean rectal point EQD2 (Gy) | 22.82 (11.78–37.69) | 23.12 (11.84–38.80) | ||
| Median RT duration (day) | 55 (33–98) | 56 (34–96) | ||
Values are presented as number of patients (%) or mean or median (range).
Co-60, cobalt-60; EBRT, external beam radiotherapy; EQD2, equivalent total dose in 2 Gy fraction; HDR, high dose rate; Ir-192, iridium-192; RT, radiotherapy.
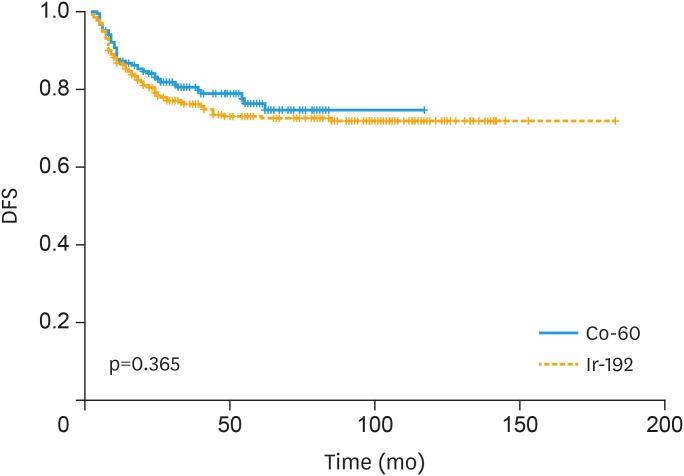
In the Ir-192 group, the percentage of tumor recurrence was not significantly different with the Co-60 group (p=0.215). The relapse pattern of both groups are summarized in Table 3. The 2- and 5-year DFS rate in Ir-192 group were 80.4% and 73.1% and in Co-60 group were 82.5% and 74.7%, respectively (p=0.365; Fig. 1).
Table 3. Treatment outcomes.
| Characteristics | Ir-192 | Co-60 | p-value | |
|---|---|---|---|---|
| Complete response | 0.895 | |||
| Yes | 271 (98.9) | 204 (99.0) | ||
| No | 3 (1.1) | 2 (1.0) | ||
| Recurrence | 0.215 | |||
| No | 202 (73.7) | 161 (78.2) | ||
| Loco-regional | 14 (5.1) | 7 (3.4) | ||
| Systemic | 48 (17.5) | 36 (17.5) | ||
| Combined loco-regional and systemic | 10 (3.6) | 2 (1.0) | ||
Values are presented as number of patients (%).
Co-60, cobalt-60; Ir-192, iridium-192.
Fig. 1. DFS curve stratified by type of brachytherapy.
Co-60, cobalt-60; DFS, disease-free survival; Ir-192, iridium-192.
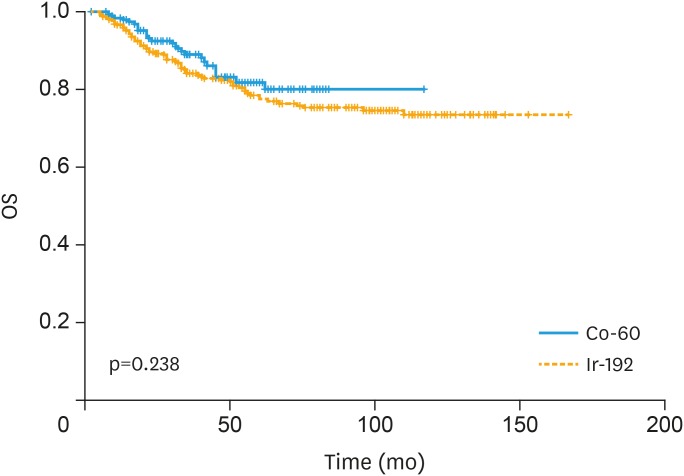
The survival rates are shown in Fig. 2. OS rates at 2 and 5 years were 89.4% and 77% of the Ir-192 group, and 91.6% and 81.9% in the Co-60 group, respectively (p=0.238). No statistically significant differences were found in OS, recurrent rate and DFS between the Ir-192 and Co-60 groups.
Fig. 2. Overall survival curve stratified by type of brachytherapy.
Co-60, cobalt-60; Ir-192, iridium-192; OS, overall survival.
Radiation related complications are shown in Table 4. The radiation toxicities were similar in both groups ranging from 1% to 14.6%. The complications were primarily grade 1 or 2. Acute GI toxicity was most commonly recorded in 34 (12.4%) patients having grade 1, while 1 (0.4%) had grade 2 GI toxicity in Ir-192 group and in 11(12%) patients having grade 1, while no patients had grade 2 toxicity in Co-60 group, respectively. Grade 3 and 4 complications were found in 13 of 274 and 7 of 206 in Ir-192 and Co-60 groups, respectively (p=0.23). GI toxicity was also the most common late reaction experienced by 3.7% and 2.4% of patients in Ir-192 and Co-60 groups, respectively.
Table 4. Radiation related complications.
| Characteristics | Ir-192 | Co-60 | p-value | |
|---|---|---|---|---|
| Acute toxicity | ||||
| GI grade 1 | 34 (12.4) | 11 (5.3) | 0.21 | |
| GI grade 2 | 1 (0.4) | 0 (0) | ||
| GU grade 1 | 9 (3.3) | 0 (0) | 0.009 | |
| Late toxicity | ||||
| GI grade 1 | 40 (14.6) | 17 (8.3) | 0.31 | |
| GI grade 2 | 34 (12.4) | 15 (7.3) | ||
| GI grade 3 | 9 (3.3) | 5 (2.4) | ||
| GI grade 4 | 1 (0.4) | 0 (0) | ||
| GU grade 1 | 18 (6.6) | 6 (2.9) | 0.156 | |
| GU grade 2 | 11 (4.0) | 4 (1.9) | ||
| GU grade 3 | 3 (1.1) | 2 (1.0) | ||
Values are presented as number of patients (%).
Co-60, cobalt-60; GI, gastrointestinal; GU, genitourinary; Ir-192, iridium-192.
The univariate and multivariate analysis showed the same results (Table 5). The histopathologic grade and clinical stage were factors which statistically influenced survival rate significantly in both Ir-192 group and Co-60 group (p<0.001).
Table 5. Factors affected treatment outcomes: univariate and multivariate analysis.
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Pathological result (SCC vs. ADC) | 1.19 | 0.70–2.00 | 0.528 | 1.15 | 0.67–1.98 | 0.620 |
| Histologic grade (I–II vs. III) | 1.79 | 1.43–2.24 | <0.001 | 1.51 | 1.19–1.91 | <0.001 |
| Preteatment Hb level (<10 vs. ≥10) | 0.66 | 0.39–1.11 | 0.115 | 0.84 | 0.50–1.44 | 0.531 |
| Stage (I–II vs. III) | 1.82 | 1.46–2.27 | <0.001 | 1.72 | 1.35–2.18 | <0.001 |
| Treatment modality (RT vs. CCRT) | 0.85 | 0.47–1.53 | 0.582 | 0.91 | 0.50–1.65 | 0.746 |
| TTT (≤56 days vs. >56 days) | 1.33 | 0.87–2.05 | 0.194 | 1.22 | 0.78–1.90 | 0.380 |
| HDR brachytherapy source (Ir-192 vs. Co-60) | 0.78 | 0.49–1.23 | 0.286 | 0.94 | 0.58–1.51 | 0.798 |
ADC, adenocarcinoma; CCRT, concurrent chemoradiotherapy; CI, confidence interval; Co-60, cobalt-60; Hb, hemoglobin; HR, hazard ratio; Ir-192, iridium-192; RT, radiotherapy; SCC, squamous cell carcinoma; TTT, total treatment time.
DISCUSSION
EBRT combined with brachytherapy is the standard treatment modality of most patients with cervical cancer. Since early 1960s, Henschke et al. and O'connell et al. introduced HDR remote after loading brachytherapy [32,33]. Nowadays, this technique has been accepted as a standard method of delivering brachytherapy. Ir-192 HDR brachytherapy has been widely used for a long time; miniaturized Co-60 HDR brachytherapy has been introduced in the past few years and is getting popular owing to its long half-life which is a lot more economical and attractive for low resource setting. Its equivalence to Ir-192 sources has been demonstrated in relation to physical data, source construction, and dose distribution of a single source and clinically applied more complex dose distributions [18,19,20,21,22,23,24,25,26,27,28,29]. Several researches published their experience using Co-60 HDR brachytherapy in cervical cancer treatment with favorable result [29,30,34]. However, to our knowledge, this is the first study which has revealed the results of curative effects and complication for a period of 5 years for cervical cancer patients treated with Ir-192 or Co-60 HDR brachytherapy combined with EBRT.
In the present study, no statistically significant differences in 5-year DFS rate and 5-year OS rate were found in Ir-192 group and Co-60 group, the 5-year DFS and 5-year OS of patients in Ir-192 group and Co-60 group were 73.1%, 74.7%, 77%, and 81.9%, respectively, in line with other studies [35]. Five-year OS rate for stage I, II, and III of patients in Ir-192 group were 100%, 83.8%, and 66.4%, respectively, and in Co-60 group were 100%, 88.8%, and 65.9%, respectively. The survival outcome was favorable compared with 5-year OS for stage I, II, and III of 80%–93%, 58%–63% and 32%–35%, respectively as reported by the American Joint Committee on Cancer [36].
However, the higher energy of 1.25 MeV of Co-60 compared with 0.6 MeV for Ir-192 raises concerns of possible increase in toxicity to patients. Combined teletherapy along with HDR Co-60 brachytherapy for the cervical cancer demonstrated a slightly higher incidence of grade 2 radiation proctitis [37]. In contrast, our results have shown that acute-late GI and GU toxicity within 5 years from HDR brachytherapy with Co-60 radionuclide source in treatment of cervical cancer was low and comparable with Ir-192 HDR source.
Significant cost saving may be achieved with Co-60 since source replacements are required every 5–6 years compared with Ir-192 where new sources are needed every 3–4 months. Equipment down-time and physics support that time is also reduced by around 40% with Co-60 in comparison with Ir-192. The estimate cost of 20 Ir-192 sources for 5 years is Thai Baht (THB) 7,000,000. Although the cost for single Co-60 source is THB 3,000,000. The cost saving for 5-year period with Co-60 source is approximately THB 4,000,000 [38]. The infrequent source changes may allow greater access to brachytherapy which would be most impactful for optimal care of cervical cancer patients. HDR Co-60 brachytherapy may be most beneficial in low-and middle-incomes countries with limited resources.
On the other hand, source change to Co-60 has to increase shielding because the energy of Co-60 source is higher compared with Ir-192. In future studies, shielding cost should be considered in cost-effective analysis.
Cervical cancer patients who were treated with Co-60 HDR brachytherapy were comparable in survival and toxicity outcomes with Ir-192 HDR brachytherapy. Co-60 source has lots of economic advantages over Ir-192 and hence suitable for low resource radiotherapy setting.
ACKNOWLEDGMENTS
The authors would like to thank the Faculty of Medicine Vajira Hospital, Navamindrahiraj University for the all work support.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: T.T., R.K.
- Data curation: T.T., R.K.
- Formal analysis: R.K.
- Funding acquisition: T.T.
- Investigation: T.T., R.K.
- Methodology: T.T., R.K.
- Project administration: T.T., R.K.
- Resources: T.T.
- Supervision: T.T.
- Validation: T.T.
- Visualization: T.T., R.K.
- Writing - original draft: T.T., R.K.
- Writing - review & editing: T.T., R.K.
References
- 1.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015. A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–554. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization/Institut Català d'Oncologia. Summary Report on HPV and Cervical cancer statistics in Thailand. Barcelona: Institut Català d'Oncologia; 2010. pp. 1–61. [Google Scholar]
- 4.Petereit DG, Eifel PJ, Gilian M, Thomas GM. Cervical cancer. In: Gunderson LL, Tepper JE, editors. Clinical radiation oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. pp. 886–907. [Google Scholar]
- 5.Perez CA, Kavanagh BD. Uterine cervix. In: Perez CA, Brady LW, Halperin EC, Schmidt-Ullrich RR, editors. Principles and practice of radiation and oncology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 1800–1916. [Google Scholar]
- 6.Eifel PJ, Berek JS, Markman MA. Carcinoma of the cervix. In: Devita VT, Hellman S, Rosenberg SA, editors. Principles and practice of oncology. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 1295–1320. [Google Scholar]
- 7.Chi DS, Perez CA, Lanciano RM, Kavanagh J. Cervical cancer. In: Pazdur R, Coia LR, Hoskins WJ, Wagman LD, editors. Cancer management: a multidisciplinary approach. 10th ed. Lawrence, KS: CMP Healthcare Media LLC; 2007-2008. pp. 441–470. [Google Scholar]
- 8.Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:352–357. doi: 10.1016/0090-8258(90)90072-s. [DOI] [PubMed] [Google Scholar]
- 9.Zaino RJ, Ward S, Delgado G, Bundy B, Gore H, Fetter G, et al. Histopathologic predictors of the behavior of surgically treated stage IB squamous cell carcinoma of the cervix. A Gynecologic Oncology Group study. Cancer. 1992;69:1750–1758. doi: 10.1002/1097-0142(19920401)69:7<1750::aid-cncr2820690717>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Burghardt E, Baltzer J, Tulusan AH, Haas J. Results of surgical treatment of 1028 cervical cancers studied with volumetry. Cancer. 1992;70:648–655. doi: 10.1002/1097-0142(19920801)70:3<648::aid-cncr2820700318>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Stehman FB, Bundy BN, DiSaia PJ, Keys HM, Larson JE, Fowler WC. Carcinoma of the cervix treated with radiation therapy. I. A multi-variate analysis of prognostic variables in the Gynecologic Oncology Group. Cancer. 1991;67:2776–2785. doi: 10.1002/1097-0142(19910601)67:11<2776::aid-cncr2820671111>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Fagundes H, Perez CA, Grigsby PW, Lockett MA. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1992;24:197–204. doi: 10.1016/0360-3016(92)90671-4. [DOI] [PubMed] [Google Scholar]
- 13.Monk BJ, Tian C, Rose PG, Lanciano R. Which clinical/pathologic factors matter in the era of chemoradiation as treatment for locally advanced cervical carcinoma? Analysis of two Gynecologic Oncology Group (GOG) trials. Gynecol Oncol. 2007;105:427–433. doi: 10.1016/j.ygyno.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steren A, Nguyen HN, Averette HE, Estape R, Angioli R, Donato DM, et al. Radical hysterectomy for stage IB adenocarcinoma of the cervix: the University of Miami experience. Gynecol Oncol. 1993;48:355–359. doi: 10.1006/gyno.1993.1062. [DOI] [PubMed] [Google Scholar]
- 15.Gallup DG, Harper RH, Stock RJ. Poor prognosis in patients with adenosquamous cell carcinoma of the cervix. Obstet Gynecol. 1985;65:416–422. [PubMed] [Google Scholar]
- 16.Lanciano RM, Won M, Coia LR, Hanks GE. Pretreatment and treatment factors associated with improved outcome in squamous cell carcinoma of the uterine cervix: a final report of the 1973 and 1978 patterns of care studies. Int J Radiat Oncol Biol Phys. 1991;20:667–676. doi: 10.1016/0360-3016(91)90007-q. [DOI] [PubMed] [Google Scholar]
- 17.Thomadsen B, Das RK. Physics and dosimetry of high-dose-rate brachytherapy. In: Chao KS, Perez CA, Brady LW, editors. Radiation oncology: management decisions. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 89–94. [Google Scholar]
- 18.Ballester F, Granero D, Pérez-Calatayud J, Casal E, Agramunt S, Cases R. Monte Carlo dosimetric study of the BEBIG Co-60 HDR source. Phys Med Biol. 2005;50:N309–N316. doi: 10.1088/0031-9155/50/21/N03. [DOI] [PubMed] [Google Scholar]
- 19.Mosalaei A, Mohammadianpanah M, Omidvari S, Ahmadloo N. High-dose rate brachytherapy in the treatment of carcinoma of uterine cervix: twenty-year experience with cobalt after-loading system. Int J Gynecol Cancer. 2006;16:1101–1105. doi: 10.1111/j.1525-1438.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 20.Bocharova I. The history of brachytherapy in Russia: comparison of Co-60 vs. Ir-192 sources. J Contemp Brachytherapy. 2011;3:48–49. [Google Scholar]
- 21.Strohmaier S, Zwierzchowski G. Comparison of 60Co and 192Ir sources in HDR brachytherapy. J Contemp Brachytherapy. 2011;3:199–208. doi: 10.5114/jcb.2011.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venselaar JL, van der Giessen PH, Dries WJ. Measurement and calculation of the dose at large distances from brachytherapy sources: Cs-137, Ir-192, and Co-60. Med Phys. 1996;23:537–543. doi: 10.1118/1.597811. [DOI] [PubMed] [Google Scholar]
- 23.Richter J, Baier K, Flentje M. Comparison of 60cobalt and 192iridium sources in high dose rate afterloading brachytherapy. Strahlenther Onkol. 2008;184:187–192. doi: 10.1007/s00066-008-1684-y. [DOI] [PubMed] [Google Scholar]
- 24.Park DW, Kim YS, Park SH, Choi EK, Ahn SD, Lee SW, et al. A comparison of dose distributions of HDR intracavitary brachytherapy using different sources and treatment planning systems. Appl Radiat Isot. 2009;67:1426–1431. doi: 10.1016/j.apradiso.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 25.Granero D, Pérez-Calatayud J, Ballester F. Technical note: dosimetric study of a new Co-60 source used in brachytherapy. Med Phys. 2007;34:3485–3488. doi: 10.1118/1.2759602. [DOI] [PubMed] [Google Scholar]
- 26.Islam MA, Akramuzzaman MM, Zakaria GA. Dosimetric comparison between the microSelectron HDR (192)Ir v2 source and the BEBIG (60)Co source for HDR brachytherapy using the EGSnrc Monte Carlo transport code. J Med Phys. 2012;37:219–225. doi: 10.4103/0971-6203.103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baltas D, Lymperopoulou G, Zamboglou N. On the use of HDR 60Co source with the MammoSite® radiation therapy system. Med Phys. 2008;35:5263–5268. doi: 10.1118/1.3002312. [DOI] [PubMed] [Google Scholar]
- 28.Richter J, Baier K, Flentje M. The use of 60Co-sources for afterloading alternate to 192Ir-sources; IFMBE Proceedings of World Congress on Medical Physics and Biomedical Engineering Seoul Korea; 2006 Aug 27–Sep 1; Seoul, Korea. New York, NY: Springer; 2007. pp. 1726–1730. [Google Scholar]
- 29.Salminen EK, Kiel K, Ibbott GS, Joiner MC, Rosenblatt E, Zubizarreta E, et al. International conference on advances in radiation oncology (ICARO): outcomes of an IAEA meeting. Radiat Oncol. 2011;6:11. doi: 10.1186/1748-717X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ntekim A, Adenipekun A, Akinlade B, Campbell O. High dose rate brachytherapy in the treatment of cervical cancer: preliminary experience with cobalt 60 radionuclide source-a prospective study. Clin Med Insights Oncol. 2010;4:89–94. doi: 10.4137/cmo.s5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 32.Henschke UK, Hilaris BS, Mahan GD. Remote afterloading with intracavitary applicators. Radiology. 1964;83:344. doi: 10.1148/83.2.344. [DOI] [PubMed] [Google Scholar]
- 33.O'Connell D, Howard N, Joslin CA, Ramsey NW, Liversage WE. A new remotely controlled unit for the treatment of uterine carcinoma. Lancet. 1965;2:570. doi: 10.1016/s0140-6736(65)90871-8. [DOI] [PubMed] [Google Scholar]
- 34.Pesee M, Krusun S, Padoongcharoen P. High dose rate cobalt-60 afterloading intracavitary therapy of uterine cervical carcinomas in Srinagarind Hospital - analysis of complications. Asian Pac J Cancer Prev. 2010;11:491–494. [PubMed] [Google Scholar]
- 35.Lertsanguansinchai P, Lertbutsayanukul C, Shotelersuk K, Khorprasert C, Rojpornpradit P, Chottetanaprasith T, et al. Phase III randomized trial comparing LDR and HDR brachytherapy in treatment of cervical carcinoma. Int J Radiat Oncol Biol Phys. 2004;59:1424–1431. doi: 10.1016/j.ijrobp.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 36.American Joint Committee on Cancer. Cervix uteri. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York, NY: Springer; 2010. pp. 395–402. [Google Scholar]
- 37.Pesee M, Krusun S, Padoongcharoen P. High dose rate cobalt-60 afterloading intracavitary therapy for cervical carcinoma in Srinagarind Hospital - analysis of survival. Asian Pac J Cancer Prev. 2010;11:1469–1471. [PubMed] [Google Scholar]
- 38.Nikam DS, Jagtap AS, Vinothraj R. Resolving the brachytherapy challenges with government funded hospital. Indian J Cancer. 2016;53:132–134. doi: 10.4103/0019-509X.180856. [DOI] [PubMed] [Google Scholar]