Abstract
Health related quality of life (HRQOL) is a key priority for patients with ovarian cancer as there is significant morbidity associated with the disease and the treatment. It is therefore essential to include measures of HRQOL and patient reported outcomes (PROs) in all clinical trials and ideally report them in the initial manuscript. The results of these analyses help interpret the primary trial endpoints which are typically progression free survival and overall survival from the perspective of the patients, but can also assist with regulatory approval of new drugs and inform future patients regarding the potential benefits and downsides of the treatment as well as help support clinical recommendations. Including PROs in clinical trials allows patient-defined clinical benefits to be assessed in parallel to traditional survival outcomes to provide a more holistic overview and aid in the interpretation of the trial results. Given the importance of these instruments in clinical trials, greater effort is required to improve the appropriate inclusion, quality of analyses and reporting of PROs. It is also essential that all clinicians understand the intricacies of the selection, implementation and interpretation of these measures of HRQOL and PRO's and how important their contribution is to clinical trials as well as clinical practice. This review is a practical guide for clinicians to gain a better understanding of PROs and how they can be incorporated into ovarian cancer trials.
Keywords: Ovarian Neoplasms, Quality of Life, Patient Reported Outcomes, Endpoints
INTRODUCTION
Health related quality of life (HRQOL) is of key importance to patients with advanced cancer independent of tumor type and is particularly relevant to patients with ovarian cancer due to the significant morbidity associated with both the disease and the treatment. HRQOL and other patient reported outcomes (PROs) are important measures which are included in clinical trials and help interpret the primary trial endpoints of progression free survival (PFS) and overall survival (OS) from the perspective of the patient experience. In addition, they can assist with regulatory approvals of new drugs and ultimately also be used to inform future patients about the specific treatment including the impact on PRO's and help guide decision making (Table 1). The Gynaecological Cancer InterGroup (GCIG) unanimously acknowledged the importance of measuring PROs in clinical trials for patients with epithelial ovarian cancer [3]. It is therefore important that all practicing clinicians understand the intricacies of the selection, implementation, analyses and interpretation of PROs in clinical trials. This review will address the critical elements of trial design and inclusion and reporting of PRO's in ovarian cancer trials.
Table 1. Key definitions.
| Health related quality of life (HRQOL): “HRQOL is defined as the subjective assessment of the impact of disease and treatment across the physical, psychological, social and somatic domains of functioning and well-being” Revicki et al., 2000, p888 [1] |
| Patient-reported outcome (PRO): “A PRO is any report of the status of a patient's health condition that comes directly from the patient, without interpretation of the patient's response by a clinician or anyone else. The outcome can be measured in absolute terms (e.g., severity of a symptom, sign, or state of a disease) or as a change from a previous measure. In clinical trials, a PRO instrument can be used to measure the effect of a medical intervention on one or more concepts (i.e., the thing being measured, such as a symptom or group of symptoms, effects on a particular function or group of functions, or a group of symptoms or functions shown to measure the severity of a health condition).” Food and Drug Administration, 2009, p6 [2] |
WHY INCLUDE PROS?
Incorporating the patient's experience of treatment in clinical trials is essential to inform clinical decision making and guide health policy [4]. Regulatory agencies such as the Food and Drug Administration (FDA) and European Medicine Agency (EMA) now recognize the importance of PRO measures in the evaluation of treatments [5,6,7]. Furthermore, the FDA recommends assessment of disease- and treatment-specific issues which are directly related to the disease and its treatment beyond global HRQOL and other multidimensional patient-reported constructs [7]. Both European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology (ASCO) have acknowledged the importance of defining the clinical benefit to patients of new therapies, and have proposed standardized approaches to evaluate the results of clinical trials by either using scores to evaluate the Magnitude of Clinical Benefit (MCB; by ESMO) or the Net Health Benefit (NHB; by ASCO) which include survival endpoints in addition to toxicity and HRQOL. The ESMO MCB scores based on OS and PFS are upgraded if there is evidence to indicate an improved HRQOL or delayed deterioration in HRQOL or a substantial reduction in adverse events. The ESMO and ASCO recommendations underscore the importance of including PRO endpoints in clinical trials.
WHAT MEASURE SHOULD BE USED?
Multiple well validated PRO measures exist including generic and ovarian cancer specific measures. The PRO measure that is best suited to address the specific PRO hypotheses and the PRO endpoints should be selected for inclusion in the clinical trial [3,8]. The two most commonly used HRQOL instruments in epithelial ovarian cancer trials are the generic core quality of life questionnaire (QLQ-C30) from the European Organization for Research and Treatment of Cancer (EORTC) and the Functional Assessment of Cancer Therapy - General (FACT-G) from the Functional Assessment of Chronic Illness Therapy (FACIT) measurement symptom [3,9]. These questionnaires are modular systems which allow for additional disease site and/or treatment specific modules to be included (for example QLQ-OV28 and FACT-O/FOSI) [10,11,12].
There is no best HRQOL questionnaire. Measure selection must be matched to well-defined and clinically-motivated PRO hypotheses and endpoints that are determined a priori during the design of the trial. Further, the measure should have sound validity, including content validity, which is the degree to which the content of the measure reflects the construct being measured [13]. The measure should also produce a score for the key domains. For example, if social domains are of interest for the study, an investigator may consider different aspects to this construct. If the impact on the family and social activities is of interest, then QLQ-C30 may be a better choice as it produces score for social functioning based on items assessing the impact of disease and treatment on family and social activities [14]. In contrast, if relationships and support is a more central focus then FACT-G may be a better alternative, as the Social/Family Wellbeing scale focuses more on these aspects [14]. Independent of what measure is used, it is important that the PRO endpoint and measure reflects key disease symptoms, treatment side effects and physical function in the context of the specific trial, in order to provide a more sensitive measure of patient benefit [6]. The choice of measure may be very different in trials of 1st line therapy, maintenance therapy or palliative therapy in patients with platinum resistant ovarian cancer where the endpoints vary considerably.
Donovan and colleagues identified key HRQOL domains of interest in patients with ovarian cancer [15]. These included specific abdominal symptoms, weight issues and sexual functioning, but few trials have reported these specific domains [15]. In a recent review of published phase III trials in ovarian cancer, the majority of trials (86%) reported global HRQOL, despite most of these trials using PRO measures which produced scores for Donovan's recommended domains [16]. More recently, the GCIG established a working group to develop a fit for purpose instrument - Measure of Ovarian cancer Symptoms and Treatment concerns (MOST) to be used to measure the potential benefit of palliative chemotherapy in clinical trials in patients with recurrent ovarian cancer. MOST has 24 items and 5 multi-item scales: abdominal symptoms (MOST-Abdo), disease or treatment-related symptoms (MOST-DorT), chemotherapy-related symptoms (MOST-Chemo), psychological symptoms (MOST-Psych), and MOST-Well-being [17]. It was designed to be flexible so it can be adapted and modified to reflect the target population, treatments and PRO hypotheses. As MOST focuses on patient reported symptoms and adverse effects, it will be used in combination with other PRO measures like EORTC or FACT depending on the research question and PRO hypotheses [17].
Measurement of PROs adds valuable information regarding adverse effects of treatment from the patients experience over and above conventional reporting of toxicity by clinicians using the National Cancer Institute (NCI) common terminology criteria for adverse events (CTCAE) criteria. For example, the severity of neuropathic symptoms can vary significantly from a patient and clinician's perspective [18,19]. In International Collaboration on Ovarian Neoplasms 7 (ICON7) trial, significant discordance was seen between clinician and patient reporting of peripheral neuropathy [19]. Moderate to severe (grade 2 or 3) clinician-reported neuropathy was documented in 28% of patients [19]. In contrast, 67% of patients reported ‘quite a bit’ or ‘very much’ tingling or numbness in the QLQ-OV28 questionnaire [19]. Hoskins and colleagues found similar discrepancies when they investigated doublet therapy with carboplatin and paclitaxel versus sequential therapy with cisplatin and topotecan followed by carboplatin and paclitaxel in ovarian cancer [20]. More self-reported neurotoxicity was described at cycle 3 and 5 than shown by the toxicity data. These results highlight important questions as to whether patient- or clinician-reported neuropathy is more clinically relevant. Arguably, PROs should take precedence as it is the patients who are experiencing the symptoms.
A patient-reported version of the common terminology criteria for adverse events (PRO-CTCAE) has been developed as an electronic based system for patients to report their adverse events in an attempt to improve the accuracy and precision of grading of this class of adverse events. This aims to provide a patient voice to trial reporting [21]. PRO-CTCAE was developed by a multidisciplinary team of methodologists, clinicians, informatics experts, patients and regulators and is being integrated into clinical trials through the NCI [22]. PRO-CTCAE is now being translated into other languages using best-practice methods, including Japanese [23]. We expect that inclusion of PRO-CTCAE in trials will provide valuable data and improve patient satisfaction by facilitating better communication and responsiveness to patients concerns.
A major barrier to the interpretation of PRO measures is reliably converting subjective scores to objective measures of outcome and defining what is clinically meaningful and understood by clinicians. The definition for a minimal clinically important difference (MCID) for the EORTC QLQ-C30 was originally defined as a 10 point change although this is not universally accepted and a more recent study recommended that a MCID of 15 points should be used [24]. In an analysis of 12 articles, group differences in mean scale scores were reliably classified as trivial, small, moderate or large [25]. In a systematic review of HRQOL in trials in epithelial ovarian cancer, the pre-specified significant change ranged from 5–15 [16]. It is critical this is considered when determining the necessary sample size if the PRO is the primary endpoint and again when interpreting the final results as positive or negative. The MCID to be used needs to be predefined in the trial statistical analysis plan and clinically relevant.
WHEN SHOULD WE MEASURE PROS?
The timing and duration of PRO assessments needs to be tailored to the population of patients in the trial and the PRO hypotheses. In patients treated with palliative intent, the domains of interest will differ significantly from those selected in asymptomatic patients treated in maintenance studies or patients treated with curative intent where survivorship and late toxicities assume more importance to patients (Table 2). The duration of assessment needs to reflect this and collecting information on late effects may be important in some trials. For example, in colorectal cancer survivors neuropathy-related symptoms were still reported 2 to 11 years post-therapy, and continued to have a negative influence on their HRQOL [26]. In ovarian cancer the duration of assessment in first line trials has been shown to average only six months post completion of therapy (range 0–24 months) which arguably is not long enough to document longer term impact [16]. Friedlander and colleagues recently demonstrated that almost 25% of patients report moderate to severe symptoms of peripheral neuropathy 2 years after 1st line chemotherapy, with an additional 25% experiencing mild symptoms [27]. Longer follow up is critical to better understand the impact on HRQOL and to understand the true extent of toxicities like neuropathy.
Table 2. Context specific PRO endpoints in clinical trials in ovarian cancer.
| Context | PRO endpoints of interest | |
|---|---|---|
| First-line treatment | ||
| Good prognostic group | • Late toxicity | |
| • Survivorship issues | ||
| • HRQOL, Q-TWIST, PRO-CTCAE, compliance with treatment, patient preference | ||
| Poor prognostic group | • Delay time to deterioration of HRQOL or specific symptoms | |
| • Q-TWIST | ||
| • Time to second-line therapy | ||
| • HRQOL at and after progression | ||
| • Toxicity | ||
| • Patient preference | ||
| Relapse | ||
| Late relapse without symptoms | • Time to deterioration of HRQOL or specific symptoms | |
| • HRQOL at and after progression | ||
| • Toxicity | ||
| Late relapse with symptoms | • Proportion of patients with symptom improvement/deterioration | |
| • HRQOL at and after progression | ||
| • Duration of control of symptoms | ||
| Early relapse | • Proportion of patients with symptom improvement/deterioration | |
| • Duration of control of symptoms | ||
| • Time to deterioration of HRQOL or specific symptoms | ||
| • HRQOL at and after progression | ||
| • Proportion of patients treated within 4 weeks of death | ||
Reprinted from Joly et al. [3] with permission by Elsevier.
HRQOL, health related quality of life; PRO, patient-reported outcome; PRO-CTCAE, patient reported outcomes version of the common terminology criteria for adverse events; Q-TWIST,quality-adjusted time without symptoms of disease or toxicity of treatment.
Timing of assessments is dependent on whether the HRQOL domain of interest is deemed to be acute versus persistent. In the upfront treatment of ovarian cancer, two studies investigating dose-dense chemotherapy approached this very differently [28,29,30]. While both identified neuropathy as their domain of interest, they chose different longitudinal measures. Pignata and colleagues employed a weekly PRO assessment during treatment to investigate the acute toxicities of this regimen and were therefore able to demonstrate that the FACT-O/Trial Outcome Index (TOI) worsened with each cycle of 3 weekly treatment but remained stable with weekly treatment [29]. There was more grade 2 or greater sensory neuropathy with 3 weekly (17%) vs. 6% with the weekly regimen. The PFS was the same with both regimens supporting a weekly regimen in selected patients [29]. In contrast, Harano and colleagues assessed PROs after 3 and 6 cycles and 12 months post-therapy to assess persistent toxicity which does not give the same degree of information regarding tolerability as the Pignata study [30]. They reported that 7% of patients had grade 3/4 sensory neuropathy which was similar in the weekly vs. 3 weekly regimen. Neuropathy has been reported to be the principal toxicity that interferes with self-care, mobility and HRQOL [31].
Pujade-Lauraine and colleagues and Stockler and colleagues investigated palliative chemotherapy with/without bevacizumab in patients with platinum resistant ovarian cancer and also assessed the impact of treatment on control of cancer-related symptoms [32,33]. They focused specifically on gastrointestinal symptoms which are the predominant symptoms in patients with platinum resistant ovarian cancer. The PRO hypothesis was that more patients receiving bevacizumab and chemotherapy than chemotherapy alone would achieve at least a 15% (>15-point) absolute improvement on the QLQ-OV28 abdominal/gastrointestinal symptom subscale (items 31–36) at week 8/9 [32,33]. They were able to demonstrate a significant improvement of gastrointestinal symptoms in conjunction with a PFS benefit and bevacizumab was subsequently approved for patients with platinum resistant ovarian cancer.
With the increasing number of trials investigating the role of maintenance therapy following chemotherapy, integration of appropriate PROs into these trials is essential to interpret the potential benefits of maintenance therapy to patients beyond a prolongation in PFS based on Response Evaluation Criteria in Solid Tumors (RECIST). A recent randomized phase II study (Study 19) investigating olaparib (a PARP inhibitor) as maintenance therapy in patients with platinum sensitive recurrent ovarian cancer following response to chemotherapy demonstrated a significant improvement in PFS with olaparib which did not translate to an OS advantage [34,35]. Prolonged maintenance therapy could potentially result in a sustained detrimental impact on HRQOL due to the side effects of a chronic treatment. This needs to be counterbalanced against the symptoms associated with earlier progression and further chemotherapy in patients randomized to placebo. In Study 19 the FACT-O questionnaire was completed at baseline and every 28 days until progression, and additional measures included the FACT/National Comprehensive Cancer Network (NCCN) Ovarian Symptom Index (FOSI) and the TOI. TOI of the FACT-O was the primary PRO outcome measure. In Study 19 maintenance olaparib had no adverse impact on HRQOL [36].
At the time of the development of Study 19, there were limited studies on the HRQOL in patients receiving maintenance treatment and there were no specifically agreed PRO endpoints in this setting. Building on results of Study 19, the SOLO2 study investigated the role of maintenance olaparib in a randomized phase III trial in patients with platinum sensitive ovarian cancer and an underlying BRCA mutation [37,38]. In contrast to Study 19 (Table 3), there was a more intensive evaluation of HRQOL and PROs including a number of additional patient-centered endpoints to help interpret the results of the trial. Of key importance they demonstrated no appreciable detrimental impact on HRQOL, using FACT-O TOI, but also found that olaparib was associated with a greater quality adjusted PFS (mean, 14.0 vs. 7.3 months) and a longer duration of time without symptoms of disease or toxicity (TWIST) (13.5 vs. 7.2 months) for patients on olaparib compared to placebo and a significantly longer time to first and second subsequent treatment supporting maintenance therapy with olparib [38].
Table 3. Comparison of QOL measures integrated in Study 19 and SOLO2.
| Context | Study 19 [34,35,36] | SOLO2 [37,38] |
|---|---|---|
| Trial design | Randomised phase II comparing maintenance olaparib with placebo in patients with platinum sensitive relapsed ovarian cancer | Randomised phase III trial comparing maintenance olaparib with placebo in patients with platinum sensitive ovarian cancer and an underlying BRCA mutation |
| Primary endpoint | PFS | PFS |
| HRQOL measures | Assessed improvement and deterioration rates and time to deterioration in TOI and FOSI | Assessed Change from baseline in FACT-O TOI score during the first 12 months |
| Assessed duration of ‘good quality of life’ by TWIST and quality-adjusted PFS (a single measure of PFS and HRQOL outcomes) |
FACT-O, Functional Assessment of Cancer Therapy - Ovarian; FOSI, Functional Assessment of Cancer Therapy/National Comprehensive Cancer Network Ovarian Symptom Index; HRQOL, health related quality of life; PFS, progression free survival; QOL, quality of life; TOI, Trial Outcome Index; TWIST, time without symptoms of disease or toxicity.
HOW CAN INVESTIGATORS INVOLVED IN CLINICAL TRIALS HELP ENSURE THE PRO DATA ARE MEANINGFUL?
Recent reports have critically evaluated the methodology of PRO studies within ovarian cancer trials and suggest that more work is needed to improve the standard of PRO research [8,9,10,16,39]. A study of 26 phase III ovarian cancer trials determined that most protocols included basic elements, such as the name of the PRO measure being used and the corresponding assessment schedule, however critical items were often omitted [39]. Only 58% provided a rationale, 31% described a PRO objective and 19% included a PRO hypothesis [39]. Studies addressed an average of 28% of recommended and applicable PRO protocol items. Items relating to the administration of PRO measures were least likely to be addressed, which raises concerns for quality assurance, as without clear administration instructions it is difficult for site staff to implement study procedures in a consistent and uniform manner.
Without attention to these details, it is difficult to determine the relevance of the PRO endpoint's and the PRO study's design. The recently-published Standard Protocol Items: Recommendations for Interventional Trials-PRO Extension (SPIRIT-PRO) lists 16 recommended items for inclusion in trial protocols with primary or key secondary PRO endpoints [40]. These consensus-based guidelines address all aspects of the protocol, including describing a research question and evidence-based rationale, defining objectives and hypotheses as relevant to specific PRO domains, justifying the chosen PRO measure, specifying the PRO assessment schedule with acceptable windows for each assessment, specifying strategies for minimizing missing PRO data and specifying whether and how PRO data will be monitored for clinical purposes during the trial [40]. Thoughtful adherence to these guidelines should improve the design of PRO research protocols and improve data quality.
However, good study implementation is also crucial to achieving high quality data. Missing PRO data (missed PRO assessments) is arguably the biggest implementation problem experienced in PRO studies because PRO data is time-sensitive and once missed, cannot be retrieved or reliably estimated. Missing PRO data is often indicative of worsening illness, meaning that available data may be from patients who do better on treatment and may not provide an accurate picture of the impact of treatment if those patients with missing PRO data are not considered in the interpretation. Thus, it is crucial to minimize rates of avoidable missing PRO data, and to implement strategies to assist in handling unavoidable missing data [41].
A recent systematic review outlined methods for preventing, handling and reporting missing PRO data to minimize any potential for biased interpretation [41]. In addition to developing a strong trial protocol and clearly reporting missing data rates and causes, the review highlighted the importance of ongoing quality assurance strategies, for example, maintaining excellent communication between trial coordinating offices and site staff regarding PRO compliance, educating patients about the importance of PRO assessments, and utilizing new technology, such as electronic data capture, which enables reminders to be sent to both research nurses and patients when PRO assessments are due, and real-time monitoring of compliance rates [41]. Future trials for ovarian cancer therapies should implement these approaches. Ultimately it is up to the clinicians and research nurses running the trial to focus on the importance of PROs and try and ensure all the data is collected.
Finally, transparent, complete and timely reporting will ensure that high-quality PRO data can inform clinical practice and policy. International consensus guidelines for the reporting of PRO endpoints, namely, the Consolidated Standards of Reporting Trials (CONSORT)-PRO Extension, was published in 2013 [42]. These guidelines should be used in conjunction with the CONSORT 2010 statement [43]. CONSORT-PRO recommends that the PRO endpoint be described in the study abstract, that a PRO hypotheses with relevant domains is described, approaches for handling missing PRO data are described and that PRO results and limitations are discussed in the context of other trial outcomes [42].
Reviews of ovarian cancer trial publications have highlighted that reporting is often incomplete according to CONSORT-PRO criteria [9,16]. Mercieca-Bebber and colleagues reviewed 36 phase III ovarian cancer randomized controlled trials and determined that on average only 48% of the CONSORT-PRO checklist was addressed [9]. The reporting of missing data was particularly poor [9]. Furthermore, PRO findings from 3 of 36 (8%) randomized controlled trials were not reported. This rate is based on randomized controlled trials that specified a PRO endpoint in the publication and should therefore be considered a conservative estimate of the true rate of non-reporting of PRO endpoints in ovarian cancer randomized controlled trials [39]. Wilson and colleagues reported similar results [16]. Adhering to the guidelines for protocol development, PRO administration, quality assurance, minimizing missing PRO data and transparent reporting described above will ensure that PRO data collected in clinical trials will be of more value to the research and clinical community and will contribute to our understanding of patient outcomes.
WHERE CAN WE IMPROVE
Early consideration during the design phase of the clinical trial of how PRO data could be informative and help interpret the primary outcome measure of a trial such as PFS or OS, is essential to the success of the trial. Quality-adjusted time without symptoms of disease or toxicity of treatment (Q-TWIST) and the time until definitive deterioration (TUDD) have been identified as potentially important endpoints to include to assess benefit, in trials in ovarian cancer [3]. These endpoints have not been used in ovarian cancer trials with the exception of Q-TWIST in SOLO2 and not at all in trials in patients with platinum resistant ovarian cancer where the aim of treatment is control of symptoms and delaying progression and arguably would be important endpoints in this setting [37,38].
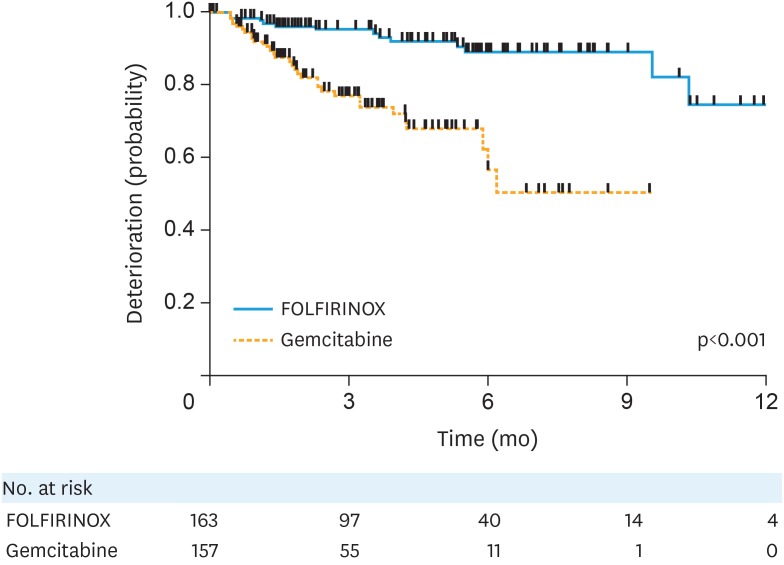
There are good precedents in other cancers and in particular in pancreatic cancer, where the assessment of the TUDD in QLQ-C30 scale has been used to clearly document the palliative benefit of chemotherapy. For example, TUDD supported the use of the triplet combination of fluorouracil, oxaliplatin and irinotecan despite greater toxicity in comparison to gemcitabine alone [44]. In conjunction with a PFS benefit, the investigators reported a longer time until a 10-point and 20-point deterioration in global score of QLQ-C30 in the triplet arm, supporting the value of the more toxic chemotherapy regimen (Fig. 1) [44]. This is an excellent example of how well considered PRO endpoints can help interpret the results of clinical trials and change the standard of care.
Fig. 1.
Time until definitive deterioration in pancreatic cancer [44]. Kaplan Meier curve demonstrating TUDD more than 20 points for European Organisation for the research and treatment of cancer quality of life questionnaire C30 global health status/quality of life. Reprinted from Gourgou-Bourgade et al. [44] with permission by © 2013 American Society of Clinical Oncology.
FOLFORINOX, oxaliplatin, irinotecan, fluorouracil, leucovorin; TUDD, time until definitive deterioration.
More recently, the KEYNOTE-045 study comparing pembrolizumab with chemotherapy in advanced urothelial cancer integrated HRQOL measures to compliment the survival endpoints [45]. Clinical benefit of pembrolizumab was supported by two prespecified endpoints: 1) time to deterioration and 2) mean change from baseline to week 15 in EORTC QLQ-C30. Importantly, they specified a 10-point change as clinically relevant [46]. Pembrolizumab was associated with a longer mean time to deterioration (3.5 vs. 2.3 months; p=0.004) [46]. Mean change from baseline was 0.96 for the patients treated with pembrolizumab and −8.36 with chemotherapy (mean difference, 9.05; p<0.001) [46]. Additionally, reasons for non-completion were documented and compliance and completion rates were published at each time point. This study highlights the value of integrating disease relevant PROs with survival endpoints and also the importance of acknowledging compliance and dealing with missing data.
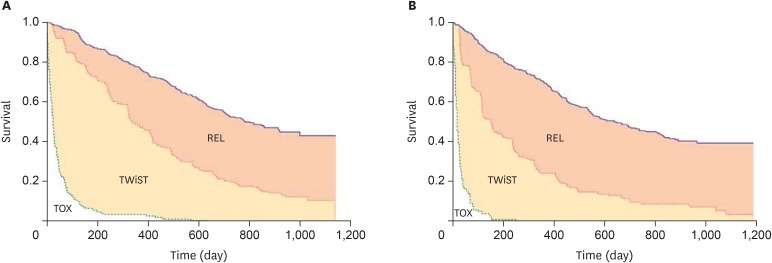
In renal cancer, sunitinib demonstrated a benefit in PFS (11 vs. 5 months) in comparison to interferon alone but with more adverse events reported [47,48]. To better estimate patient benefit, Q-TWIST was included to measure patient benefit [48]. This measure simultaneously compares time with toxicities and clinical outcomes to evaluate the trade-off between adverse events and benefit over the entire survival time. It separates survival time into three periods: 1) toxicity period with grade 3 or higher adverse events after randomization and before disease progression or censoring; 2) time without symptoms or toxicities (Twist) representing the time without toxicities or symptoms before disease progression; and 3) relapse, representing the time following disease progression and ending with death or censoring (Fig. 2). The median time spent without symptoms of disease progression or toxicity of treatment was 151 days longer for patients treated with sunitinib compared to those treated with interferon [48]. The difference in Q-TWIST ranged from a maximum of 177 to a minimum of 55 [48]. This measure has also supported the use of interferon in myeloma and ipilimumab in melanoma [49,50].
Fig. 2.
Q-TWIST assessment in patients with renal cancer receiving sunitinib versus IFN-α [48]. (A) Kaplan-Meier curves for OS (blue) and PFS (red) for the sunitinib arm with toxicity (green) for patients who experienced any treatment-related grade 3 or 4 toxicity. (B) Kaplan-Meier curves for OS (blue) and PFS (red) for the IFN-α arm with toxicity (green) for patients who experienced any treatment-related grade 3 or 4 toxicity. Q-TWIST separates survival time into three periods: 1) toxicity period with grade 3 or higher adverse events after randomisation and before disease progression or censoring (TOX); 2) TWIST representing the time without toxicities or symptoms before disease progression; and 3) relapse, representing the time following disease progression and ending with death or censoring (REL). Reprinted from Patil et al. [48] with permission by © 2012 Springer Nature.
IFN, interferon; OS, overall survival; PFS, progression free survival; TWIST, time without symptoms of disease or toxicity.
We have seen progress in the inclusion of PROs in a growing number of trials in ovarian cancer, and more recently also with the relevance of the additional measures used [16]. The 5th Ovarian Cancer Consensus Conference recommended that future trials should include additional patient-centered benefits beyond HRQOL alone such as the duration of time without symptoms of disease or treatment toxicity and quality adjusted PFS [3]. SOLO2 is an example of the change in approach to inclusion of patient-centered endpoints in ovarian cancer trials, and there are now many more trials that are paying a lot more attention to PRO endpoints early in the planning and design phase [37,38].
Lack of inclusion of appropriate PROs in clinical trials has led to many missed opportunities in the past [8]. For example, a randomized phase II trial comparing olaparib to pegylated liposomal doxorubicin in women with BRCA-related recurrent ovarian cancer found no significant differences in survival end points, but it is likely that clinically relevant differences in HRQOL between the two very different treatments may have been missed due to the measures used [51]. There were no significant differences between groups for the FACT-O symptom index or Trial Outcome Index mean scores. A higher improvement rate was noted for olaparib for the total FACT-O score (odds ratio=7.23; p=0.039), but this was not discussed [8]. If this study was designed today, the PRO measures and PRO endpoints would be very different. Patient preferences (daily oral tablets versus intravenous monthly chemotherapy), patient-reported toxicity, patient-reported experience (inconvenience of treatment), and TUDD may have resulted in a different conclusion to this study [8]. This again supports the importance of early involvement of researchers with expertise in these areas, to prevent the missed opportunities in such trials.
The 5th Ovarian Cancer Consensus Conference in Tokyo 2015 also encouraged the reporting of the proportion of patients with recurrent ovarian cancer who experience a symptomatic benefit from treatment, and the duration of that benefit [3,52]. Reporting findings in this format is more clinically meaningful than mean changes. To date, less than 5% of phase III studies in ovarian cancer have reported the duration of benefit of palliative chemotherapy [16]. The Aide et Recherche en Cancérologie Digestive group (ARCAD) are currently developing “PRO-RECIST” criteria to use as co-primary endpoints in trials in patients with recurrent colon cancer and pancreatic cancer and it makes sense that the ovarian research community reach out to ARCAD and learns from their experience as the same principles will apply in recurrent ovarian cancer. Similar to a waterfall plot, the cumulative distribution of PRO changes from baseline could provide a powerful graphical display to illustrate the proportion of patients who experience every magnitude of change in a specific measure at a time point of interest and would allow for comparison between trial arms [8]. A RECIST-like approach to reporting PRO may help provide more robustness to the interpretation of these measures as well as reinforce greater rigour with collection of PRO data and avoiding missing data.
CONCLUSION
PROs are important measures of the benefits and harms of treatment from the perspective of the patient, which help to reinforce survival benefits (both PFS and OS), assist regulatory decisions and help guide decision making for patients with ovarian cancer. The value of including PROs in ovarian cancer clinical trials has been recognized by key stakeholders: patients, clinicians, and regulatory agencies. It is fundamental that the PROs selected for inclusion in clinical trials reflect disease- and treatment-specific issues and correlate with patient experience. Clinicians need to understand the intricacies of the choice, implementation and interpretation of these measures. Given the importance of these instruments in assessing benefits, it is critical we continue to implement practices to improve the quality of analyses and reporting of PROs. Increasing rigour is required and ongoing investment from the GCIG symptom benefit group and collaboration with other similar groups such as the ARCAD will help us achieve this. The potential knowledge gained from closer attention to PRO hypotheses, the inclusion of appropriate PRO endpoints and high-quality design, implementation, interpretation and reporting is huge and can significantly impact patient outcomes.
Footnotes
Funding: RMB is supported by an NHMRC Early Career Fellowship (APP1138100) and MF is supported by an NHMRC Program (grant ID: APP1092856).
Conflict of Interest: M K Wilson has received travel support from MSD and Roche. M Friedlander has received honoraria for being part of the advisory board for Astra Zeneca and MSD. R Mercieca-Bebber has no conflicts.
- Conceptualization: W.M.K., M.R., F.M.
- Writing - original draft: W.M.K., M.R., F.M.
- Writing - review & editing: W.M.K., M.R., F.M.
References
- 1.Revicki DA, Osoba D, Fairclough D, Barofsky I, Berzon R, Leidy NK, et al. Recommendations on health-related quality of life research to support labeling and promotional claims in the United States. Qual Life Res. 2000;9:887–900. doi: 10.1023/a:1008996223999. [DOI] [PubMed] [Google Scholar]
- 2.Food and Drug Administration (US) Guidance for industry: patient-reported outcome measures: use in medical product development to support labelling claims [Internet] Silver Spring, MA: Food and Drug Administration; 2009. [cited year month day]. Available from: http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf. [Google Scholar]
- 3.Joly F, Hilpert F, Okamoto A, Stuart G, Ochiai K, Friedlander M, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer. 2017;78:133–138. doi: 10.1016/j.ejca.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Basch E, Abernethy AP, Mullins CD, Reeve BB, Smith ML, Coons SJ, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30:4249–4255. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 5.Bottomley A, Jones D, Claassens L. Patient-reported outcomes: assessment and current perspectives of the guidelines of the Food and Drug Administration and the reflection paper of the European Medicines Agency. Eur J Cancer. 2009;45:347–353. doi: 10.1016/j.ejca.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal GM, Kluetz PG, Schneider J, Goldberg KB, McKee AE, Pazdur R. Oncology drug approvals: evaluating endpoints and evidence in an era of breakthrough therapies. Oncologist. 2017;22:762–767. doi: 10.1634/theoncologist.2017-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kluetz PG, Papadopoulos EJ, Johnson LL, Donoghue M, Kwitkowski VE, Chen WH, et al. Focusing on core patient-reported outcomes in cancer clinical trials-response. Clin Cancer Res. 2016;22:5618. doi: 10.1158/1078-0432.CCR-16-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedlander M, Mercieca-Bebber RL, King MT. Patient-reported outcomes (PRO) in ovarian cancer clinical trials-lost opportunities and lessons learned. Ann Oncol. 2016;27(Suppl 1):i66–i71. doi: 10.1093/annonc/mdw080. [DOI] [PubMed] [Google Scholar]
- 9.Mercieca-Bebber R, Friedlander M, Calvert M, Stockler M, Kyte D, Kok PS, et al. A systematic evaluation of compliance and reporting of patient-reported outcome endpoints in ovarian cancer randomised controlled trials: implications for generalisability and clinical practice. J Patient Rep Outcomes. 2017;1:5. doi: 10.1186/s41687-017-0008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedlander ML, King MT. Patient-reported outcomes in ovarian cancer clinical trials. Ann Oncol. 2013;24(Suppl 10):x64–x68. doi: 10.1093/annonc/mdt474. [DOI] [PubMed] [Google Scholar]
- 11.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, Webster K, Cella D, Hu S, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol. 2001;19:1809–1817. doi: 10.1200/JCO.2001.19.6.1809. [DOI] [PubMed] [Google Scholar]
- 12.Greimel E, Bottomley A, Cull A, Waldenstrom AC, Arraras J, Chauvenet L, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-OV28) in assessing the quality of life of patients with ovarian cancer. Eur J Cancer. 2003;39:1402–1408. doi: 10.1016/s0959-8049(03)00307-1. [DOI] [PubMed] [Google Scholar]
- 13.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63:737–745. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Luckett T, King MT, Butow PN, Oguchi M, Rankin N, Price MA, et al. Choosing between the EORTC QLQ-C30 and FACT-G for measuring health-related quality of life in cancer clinical research: issues, evidence and recommendations. Ann Oncol. 2011;22:2179–2190. doi: 10.1093/annonc/mdq721. [DOI] [PubMed] [Google Scholar]
- 15.Donovan KA, Donovan HS, Cella D, Gaines ME, Penson RT, Plaxe SC, et al. Recommended patient-reported core set of symptoms and quality-of-life domains to measure in ovarian cancer treatment trials. J Natl Cancer Inst. 2014;106:dju128. doi: 10.1093/jnci/dju128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson MK, Friedlander ML, Joly F, Oza AM. A systematic review of health-related quality of life reporting in ovarian cancer phase III clinical trials: room to improve. Oncologist. 2018;23:203–213. doi: 10.1634/theoncologist.2017-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King MT, Stockler MR, O'Connell RL, Buizen L, Joly F, Lanceley A, et al. Measuring what matters MOST: validation of the Measure of Ovarian Symptoms and Treatment, a patient-reported outcome measure of symptom burden and impact of chemotherapy in recurrent ovarian cancer. Qual Life Res. 2018;27:59–74. doi: 10.1007/s11136-017-1729-8. [DOI] [PubMed] [Google Scholar]
- 18.Bennett BK, Park SB, Lin CS, Friedlander ML, Kiernan MC, Goldstein D. Impact of oxaliplatin-induced neuropathy: a patient perspective. Support Care Cancer. 2012;20:2959–2967. doi: 10.1007/s00520-012-1428-5. [DOI] [PubMed] [Google Scholar]
- 19.Park SB, Kwok JB, Asher R, Lee CK, Beale P, Selle F, et al. Clinical and genetic predictors of paclitaxel neurotoxicity based on patient- versus clinician-reported incidence and severity of neurotoxicity in the ICON7 trial. Ann Oncol. 2017;28:2733–2740. doi: 10.1093/annonc/mdx491. [DOI] [PubMed] [Google Scholar]
- 20.Hoskins P, Vergote I, Cervantes A, Tu D, Stuart G, Zola P, et al. Advanced ovarian cancer: phase III randomized study of sequential cisplatin-topotecan and carboplatin-paclitaxel vs carboplatin-paclitaxel. J Natl Cancer Inst. 2010;102:1547–1556. doi: 10.1093/jnci/djq362. [DOI] [PubMed] [Google Scholar]
- 21.Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362:865–869. doi: 10.1056/NEJMp0911494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Natl Cancer Inst. 2014;106:dju244. doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyaji T, Iioka Y, Kuroda Y, Yamamoto D, Iwase S, Goto Y, et al. Japanese translation and linguistic validation of the US National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Patient Rep Outcomes. 2017;1:8. doi: 10.1186/s41687-017-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joly F, Vardy J, Pintilie M, Tannock IF. Quality of life and/or symptom control in randomized clinical trials for patients with advanced cancer. Ann Oncol. 2007;18:1935–1942. doi: 10.1093/annonc/mdm121. [DOI] [PubMed] [Google Scholar]
- 25.Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2011;29:89–96. doi: 10.1200/JCO.2010.28.0107. [DOI] [PubMed] [Google Scholar]
- 26.Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol. 2013;31:2699–2707. doi: 10.1200/JCO.2013.49.1514. [DOI] [PubMed] [Google Scholar]
- 27.Friedlander M, King M, Nagle C, Obermair A, Grant PT, deFazio A, et al. Getting the most out of follow-up: a prospective study using the Measure of Ovarian Symptoms and Treatment concerns (MOST) symptom index to evaluate and track adverse effects (AEs) and detect symptoms of recurrence in patients with ovarian cancer (OC) following first line chemotherapy (1LT); Poster session presented at 2018 ASCO Annual Meeting, Abstract 10062; 2018 Jun 1–5; Chicago, IL. Alexandria, VA: American Society of Clinical Oncology; 2018. [Google Scholar]
- 28.Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 29.Pignata S, Scambia G, Katsaros D, Gallo C, Pujade-Lauraine E, De Placido S, et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014;15:396–405. doi: 10.1016/S1470-2045(14)70049-X. [DOI] [PubMed] [Google Scholar]
- 30.Harano K, Terauchi F, Katsumata N, Takahashi F, Yasuda M, Takakura S, et al. Quality-of-life outcomes from a randomized phase III trial of dose-dense weekly paclitaxel and carboplatin compared with conventional paclitaxel and carboplatin as a first-line treatment for stage II–IV ovarian cancer: Japanese Gynecologic Oncology Group Trial (JGOG3016) Ann Oncol. 2014;25:251–257. doi: 10.1093/annonc/mdt527. [DOI] [PubMed] [Google Scholar]
- 31.Greimel ER, Bjelic-Radisic V, Pfisterer J, Hilpert F, Daghofer F, du Bois A, et al. Randomized study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group comparing quality of life in patients with ovarian cancer treated with cisplatin/paclitaxel versus carboplatin/paclitaxel. J Clin Oncol. 2006;24:579–586. doi: 10.1200/JCO.2005.02.4067. [DOI] [PubMed] [Google Scholar]
- 32.Stockler MR, Hilpert F, Friedlander M, King MT, Wenzel L, Lee CK, et al. Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J Clin Oncol. 2014;32:1309–1316. doi: 10.1200/JCO.2013.51.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 34.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 35.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 36.Ledermann JA, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Quality of life during olaparib maintenance therapy in platinum-sensitive relapsed serous ovarian cancer. Br J Cancer. 2016;115:1313–1320. doi: 10.1038/bjc.2016.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 38.Friedlander M, Gebski V, Gibbs E, Bloomfield R, Hilpert F, Wenzel LB, et al. Health-related quality of life (HRQOL) and patient-centered outcomes with maintenance olaparib compared with placebo following chemotherapy in patients with germline (g) BRCA-mutated (m) platinum-sensitive relapsed serous ovarian cancer (PSR SOC): SOLO2 phase III trial. J Clin Oncol. 2017;35:Abstr 5507 [Google Scholar]
- 39.Mercieca-Bebber R, Friedlander M, Kok PS, Calvert M, Kyte D, Stockler M, et al. The patient-reported outcome content of international ovarian cancer randomised controlled trial protocols. Qual Life Res. 2016;25:2457–2465. doi: 10.1007/s11136-016-1339-x. [DOI] [PubMed] [Google Scholar]
- 40.Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan AW, King MT, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO Extension. JAMA. 2018;319:483–494. doi: 10.1001/jama.2017.21903. [DOI] [PubMed] [Google Scholar]
- 41.Mercieca-Bebber R, Palmer MJ, Brundage M, Calvert M, Stockler MR, King MT. Design, implementation and reporting strategies to reduce the instance and impact of missing patient-reported outcome (PRO) data: a systematic review. BMJ Open. 2016;6:e010938. doi: 10.1136/bmjopen-2015-010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calvert M, Brundage M, Jacobsen PB, Schünemann HJ, Efficace F. The CONSORT patient-reported outcome (PRO) extension: implications for clinical trials and practice. Health Qual Life Outcomes. 2013;11:184. doi: 10.1186/1477-7525-11-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulz KF, Altman DG, Moher D, CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, Ychou M, Bouché O, Guimbaud R, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31:23–29. doi: 10.1200/JCO.2012.44.4869. [DOI] [PubMed] [Google Scholar]
- 45.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaughn DJ, Bellmunt J, Fradet Y, Lee JL, Fong L, Vogelzang NJ, et al. Health-related quality-of-life analysis from KEYNOTE-045: a phase III study of pembrolizumab versus chemotherapy for previously treated advanced urothelial cancer. J Clin Oncol. 2018;36:1579–1587. doi: 10.1200/JCO.2017.76.9562. [DOI] [PubMed] [Google Scholar]
- 47.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 48.Patil S, Figlin RA, Hutson TE, Michaelson MD, Negrier S, Kim ST, et al. Q-TWIST analysis to estimate overall benefit for patients with metastatic renal cell carcinoma treated in a phase III trial of sunitinib vs interferon-α. Br J Cancer. 2012;106:1587–1590. doi: 10.1038/bjc.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sherrill B, Wang J, Kotapati S, Chin K. Q-TWIST analysis comparing ipilimumab/dacarbazine vs placebo/dacarbazine for patients with stage III/IV melanoma. Br J Cancer. 2013;109:8–13. doi: 10.1038/bjc.2013.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zee B, Cole B, Li T, Browman G, James K, Johnston D, et al. Quality-adjusted time without symptoms or toxicity analysis of interferon maintenance in multiple myeloma. J Clin Oncol. 1998;16:2834–2839. doi: 10.1200/JCO.1998.16.8.2834. [DOI] [PubMed] [Google Scholar]
- 51.Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30:372–379. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 52.Wilson MK, Pujade-Lauraine E, Aoki D, Mirza MR, Lorusso D, Oza AM, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recurrent disease. Ann Oncol. 2017;28:727–732. doi: 10.1093/annonc/mdw663. [DOI] [PMC free article] [PubMed] [Google Scholar]