Abstract
Objective
To examine survival of teenage women with pregnancies complicated by primary ovarian cancer.
Methods
This is a secondary analysis of a previously organized systematic literature review of primary ovarian cancer diagnosed during pregnancy. Cases eligible for analysis were patients whose age at cancer diagnosis and survival outcome were known (n=201). Pregnancy and oncologic outcome were then examined based on patient age.
Results
These were comprised of 95 (47.3%) epithelial ovarian cancers (EOCs), 82 (40.8%) malignant germ cell tumors (MGCTs), and 24 (11.9%) sex-cord stromal tumors (SCSTs). Teenage pregnancy was seen in 21 (10%) cases, and was highest among the SCST group compared to the other cancer types (EOC, 1.1%; MGCT, 14.6%; and SCST, 29.2%, p<0.001). Live birth rates, neonatal weight, full term delivery rates, and Cesarean section rates were similar between the teenage group and the non-teenage group (all, p>0.05); however, teenage pregnancy was significantly associated with an increased risk of serious maternal/neonatal adverse events (50% vs. 22.7%, p=0.013). On univariable analysis, teenage pregnancy was significantly associated with decreased ovarian cancer-specific survival (5-year rate: age ≥30, 79.6%; age 20–29, 87.2%; and age <20, 41.6%; p<0.001). On multivariable analysis controlling for calendar year, cancer type, cancer stage, and gestational age at ovarian cancer diagnosis, teenage pregnancy remained an independent prognostic factor for decreased ovarian cancer-specific survival compared to women aged ≥30 (adjusted-hazard ratio=4.71; 95% confidence interval=1.17–18.9; p=0.029).
Conclusion
Teenage women with pregnancies complicated by primary ovarian cancer may be at increased risk of poor survival from ovarian cancer.
Keywords: Pregnancy, Teenage, Ovarian Neoplasms, Survival, Review
INTRODUCTION
Approximately one in 1,000 pregnancies are complicated by malignancy, with the incidence of ovarian cancer 3–11 per 100,000 pregnancies [1]. Recent studies have shown that the average age at diagnosis of ovarian cancer during pregnancy was in the mid-20s to early-30s [1,2,3]. In general, age is an independent prognostic factor for survival of women with ovarian cancer, and older women are more likely to die from disease than their younger counterparts. However, little data is available on maternal outcomes of pregnancies in teen women complicated by malignancy.
Although teenage pregnancy in the US is decreasing, there is still a birth rate of 22.3 per 1,000 women [4]. Teenage pregnancy itself has not been shown to be associated with higher rates of maternal morbidity when compared to older counterparts [5]; however, multiple other measures of maternal and fetal well-being have been noted to be negatively affected by teenage pregnancy [6]. Teenagers often present late to care and have a variety of socioeconomic barriers to obtaining healthcare that can compromise outcomes in the rare cases of teenage pregnancy complicated by ovarian cancer.
The objective of the study was to examine the association of age and oncologic outcome of pregnant women with primary ovarian cancer.
MATERIALS AND METHODS
1. Data source
This is a secondary analysis of a previously organized dataset from a systematic literature review for pregnancy complicated by primary ovarian cancer, including epithelial ovarian cancer (EOC), sex-cord stromal tumor (SCST), and malignant germ cell tumor (MGCT) [1,2,3]. The previous dataset was compiled in PubMed and MEDLINE using relevant keywords limited to English. This review was Institutional Review Board-exempt, and literature searches were performed based on the Meta-analysis of Observational Studies in Epidemiology guidelines for systematic reviews [7].
2. Eligibility
Eligible articles included case reports or case series with adequate descriptions of patient age at ovarian cancer diagnosis and survival outcome. Cases describing ovarian cancer coincident with pregnancy with detailed descriptions (fetal, maternal, and tumor characteristics; interventions; pregnancy outcomes, and maternal survival) were included. References within included articles were also reviewed, and relevant articles that fit within the inclusion criteria were also extracted. Cases were excluded if they were reviews, case reports with suboptimal descriptions, or if the ovarian cancer diagnosis was made outside of pregnancy.
3. Clinical information
Among eligible cases, data extracted were 1) clinical demographics, including year, age at diagnosis, and gestational age (GA) at ovarian cancer diagnosis; 2) pregnancy outcomes including GA at delivery, route of delivery and birth weight; 3) tumor characteristics including histologic subtype, and ovarian cancer stage; and 4) survival outcome from ovarian cancer.
4. Definitions
Teenage pregnancy was defined as pregnancy with presentation to care at an age of less than 20 years. Serious adverse events (AEs) included tumor rupture/torsion, obstructed labor, placental abruption, fetal malformation among cases with in utero chemotherapy exposure, maternal death, and in utero fetal demise or neonatal death. If the exact GA was not given, “early” and “late” pregnancy were defined as first and third trimester, respectively. “Term” delivery was defined as at least 37 weeks GA. Cause-specific survival was defined as the time interval between the ovarian cancer diagnosis and the date of death from ovarian cancer. Cases without event at the last follow-up were censored. Ovarian cancer stage was re-classified based on the International Federation of Gynecology and Obstetrics 2009 staging system [8].
5.Statistical analysis
The primary objective of the analysis was to examine the association of age and oncologic outcome of pregnant women with primary ovarian cancer. The secondary objective of the analysis was to examine pregnancy outcomes of teenage gestations.
Distributions of continuous variables were assessed by the Kolmogorov-Smirnov test, and expressed with mean (±standard deviation) or median (interquartile range) as appropriate. Statistical significance of continuous variables was assessed with Student's t-test or Mann-Whitney U test as appropriate. Statistical differences in categorical variables were evaluated with χ2 test or Fisher's exact test.
The Kaplan-Meier method was used to construct survival curves, and the statistical significance between the curves was examined by log-rank test for univariate analysis. We estimated that pregnancy complicated by ovarian cancer is rare with a limited sample size for analysis. Thus, the association of patient age and cause-specific survival was adjusted for a priori survival factors on multivariate analysis. These factors included calendar year of publication, GA at ovarian cancer diagnosis, histology type, and cancer stage. A Cox proportional hazard regression model was used for multivariable analysis, and the magnitude of statistical significance was expressed with adjusted-hazard ratio (HR) and 95% confidence interval (CI). A p-value less than 0.05 was considered statistically significant (all, 2-sided hypothesis), and Statistical Package for Social Science software (SPSS), version 12.0 (SPSS Inc., Chicago, IL, USA) was used for all the analyses.
RESULTS
There were total 253 cases of pregnancy related to primary ovarian cancer from EOC (n=105), MGCT (n=102), and SCST (n=46). Among those, 10 cases with diagnosis in the postpartum period or prior to pregnancy were excluded. Among 243 cases that ovarian cancer was diagnosed during pregnancy, there were 14 cases without age that were excluded. Among 229 cases with known patient age, 28 cases with unknown survival status were excluded. The remaining 201 cases with known age and survival status comprised the study population.
Patient demographics are shown in Table 1. Teenage pregnancy was seen in 20 (10%; 95% CI=5.8–14.1) of 201 total cases. These were comprised of 95 (47.3%) EOC, 82 (40.8%) MGCT, and 24 (11.9%) SCST. Teenage pregnancy complicated by ovarian cancer was more common in older cases: 22.0% (prior to 1980), 12.5% (1980–1999), and 3.8% (2000 or later) (p<0.01).
Table 1. Patient demographics.
| Characteristic | Age <20 | Age ≥20 | p-value | |
|---|---|---|---|---|
| No. of patients | 20 (10.0) | 181 (90.0) | ||
| Calendar year | <0.01 | |||
| Before 1980 | 9 (45.0) | 32 (17.7) | ||
| 1980–1999 | 7 (35.0) | 49 (27.1) | ||
| 2000 or later | 4 (15.0) | 100 (55.2) | ||
| Histology | <0.01 | |||
| EOC | 1 (5.0) | 94 (51.9) | ||
| MGCT | 12 (60) | 70 (38.7) | ||
| SCST | 7 (35.0) | 17 (9.4) | ||
| Cancer stage | 0.26 | |||
| I | 12 (66.7) | 133 (73.5) | ||
| II | 3 (16.7) | 10 (5.5) | ||
| III | 3 (16.7) | 30 (16.6) | ||
| IV | 0 | 8 (4.4) | ||
| GA at diagnosis | 0.20 | |||
| 1st T | 4 (20) | 69 (38.5) | ||
| 2nd T | 7 (35) | 58 (32.4) | ||
| 3rd T | 9 (45) | 52 (29.1) | ||
| Live birth | 0.15 | |||
| No | 7 (35.0) | 36 (19.9) | ||
| Yes | 13 (65.0) | 145 (80.1) | ||
| Birth weight (g) | 2,164±1,074 | 2,522±831 | 0.21 | |
| SGA | 0.99 | |||
| No | 10 (83.3) | 130 (80.7) | ||
| Yes | 2 (16.7) | 31 (19.3) | ||
| Cesarean section | 0.35 | |||
| No | 12 (60) | 85 (47.0) | ||
| Yes | 8 (40) | 96 (53.0) | ||
| Full term birth | 0.34 | |||
| No | 14 (70) | 102 (56.4) | ||
| Yes | 6 (30) | 79 (43.6) | ||
| Serious AEs | 0.013 | |||
| No | 10 (50) | 140 (77.3) | ||
| Yes | 10 (50) | 79 (22.7) | ||
Number (%) per column or mean±standard deviation is shown. Fisher's exact test, χ2 test, or Student's t-test for p-values.
AE, adverse event; EOC, epithelial ovarian cancer; GA, gestational age; MGCT, malignant germ cell tumor; SCST, sex-cord stromal tumor; SD, standard deviation; T, trimester.
Tumor characteristics were analyzed. The SCST group had the highest proportion of teenage pregnancy among the 3 groups (EOC, 1.1%; MGCT, 14.6%; and SCST, 29.2%; p<0.01). However, MGCT was the most common histology type among 3 tumor types (60%) followed by SCST (35%) and EOC (5%). Most cases were diagnosed at early-stage (stage I, 66.7%; II, 16.7%; III, 16.7%; and IV, 0%), which was similar to the non-teenaged contingent (p=0.26). Nearly half of teenage women were diagnosed with ovarian cancer in the third trimester (45%) followed by the second (35%) and the first trimesters (20%). In contrast, women aged 20 years or greater showed a statistically insignificant trend (p=0.20) towards diagnosis earlier in pregnancy with only 29.1% of them receiving the diagnosis of primary ovarian cancer in the third trimester.
Pregnancy outcome was examined. Live birth, birth weight, preterm delivery, and Cesarean section rates were similar between the teenage group and the non-teenage group (all, p>0.05); however, teenage pregnancy was significantly associated with an increased risk of serious maternal or neonatal AEs (50% versus 22.7%, p=0.013). Amongst teenage women, live birth was noted in 13 (65.0%) women. Neonatal birth weight averaged 2,164 grams, and 2 (10.0%) infants were classified as small for GA. Half of the births (50.0%) were preterm. The mode of delivery was Cesarean section for 8 (40.0%) of the teenage women with primary ovarian cancer diagnosed in pregnancy.
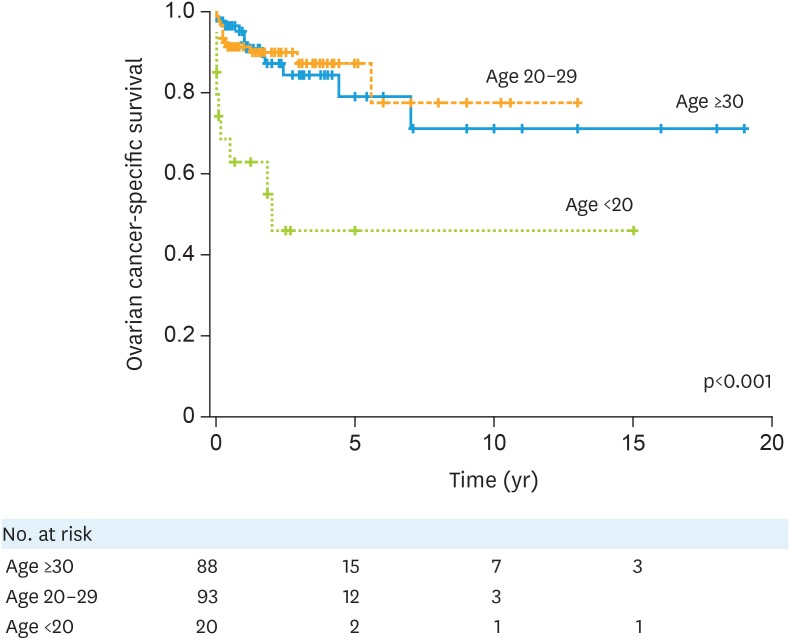
Survival analysis was also performed. Median follow-up time was 24 months, and there were 32 (15.9%) women who died of ovarian cancer in this cohort. On univariable analysis, teenage pregnancy was significantly associated with decreased ovarian cancer-specific survival (5-year rates: age ≥30, 79.6%; age 20–29, 87.2%; and age <20, 41.6%; p<0.001; Fig. 1). On multivariable analysis controlling for calendar year, ovarian cancer type, cancer stage, and GA, teenage pregnancy remained an independent prognostic factor for decreased ovarian cancer-specific survival compared to those aged ≥30 (adjusted-HR=5.31; 95% CI=1.29–21.8; p=0.021, Table 2). In comparison, women in their twenties had an ovarian cancer-specific survival similar to those who were in their thirties (adjusted-HR=0.60; 95% CI=0.22–1.63; p=0.32).
Fig. 1. Ovarian cancer-specific survival based on patient age at diagnosis. Log-rank test for p-value. Ovarian cancer-specific survival is shown based on patient age at the diagnosis.
Table 2. Multivariable analysis for ovarian cancer-specific survival.
| Characteristic | HR (95% CI) | p-value | |
|---|---|---|---|
| Age (yr) | |||
| ≥30 | 1 | ||
| 20–29 | 0.60 (0.22–1.63) | 0.32 | |
| <20 | 5.31 (1.29–21.8) | 0.021 | |
| Calendar year | |||
| Before 1980 | 1 | ||
| 1980–1999 | 0.27 (0.09–0.88) | 0.030 | |
| 2000 or later | 0.16 (0.06–0.47) | <0.01 | |
| Histology | |||
| EOC | 1 | ||
| MGCT | 0.72 (0.25–2.05) | 0.54 | |
| SCST | 0.37 (0.06–2.20) | 0.27 | |
| GA at diagnosis | |||
| 1st T | 1 | ||
| 2nd T | 0.77 (0.23–2.53) | 0.66 | |
| 3rd T | 1.15 (0.37–3.56) | 0.81 | |
| Cancer stage | |||
| I | 1 | ||
| II | 4.94 (1.04–23.5) | 0.044 | |
| III | 27.3 (8.75–85.0) | <0.01 | |
| IV | 173 (33.9–877) | <0.01 | |
A Cox proportional hazard regression model for analysis. All the listed variables were entered in the final model.
CI, confidence interval; EOC, epithelial ovarian cancer; GA, gestational age; HR, hazard ratio; MGCT, malignant germ cell tumor; SCST, sex-cord stromal tumor; T, trimester.
DISCUSSION
The findings of our study indicate that pregnancies complicated by primary ovarian cancer in teenage women are associated with worse cancer-specific survival than pregnancies with similar complications in their older counterparts. Primary ovarian cancer in teen pregnancy is extremely rare, and as such, this phenomenon is relatively understudied. Closely examining characteristics of these pregnancies allows for optimization of maternal and fetal outcomes.
The relationship of decreased ovarian cancer survival in teen women during pregnancy observed in our study contrasts with what is known in the general population: that older age is associated with worse oncologic outcome. It is hypothesized that increasing age causes decreased immune function against malignancy via immune-senescence [9]. A possible explanation for our findings relates to inadequate onco-immunity in teenage women, as the immune system generally peaks during middle age [9]. Furthermore, immuno-modulation inherent in pregnancy is well established and results in a variable maternal inflammatory environment, thus potentially altering the typical physiologic response to malignancy [10,11]. Inadequate onco-immunity, when combined with the alterations in the immune system during pregnancy, may contribute to decreased ovarian cancer survival [12,13].
In addition, other social factors surrounding teenage pregnancy may directly or indirectly influence the outcome of both pregnancy and ovarian cancer. Teenagers may not be compliant with treatment recommendations, and psychosocial factors associated with teen pregnancy, including a lack of family support, substance abuse, late presentation to care, and inadequate treatment resources may result in decreased ovarian cancer survival [14,15,16]. Furthermore, adolescent mothers are identified as having perinatal depression more frequently than their older counterparts, which can severely inhibit the mother's ability to care for herself and her child [17]. The barriers to care that are often present at baseline in teenage pregnancies can further exacerbate complications of a pregnancy with a coincident primary ovarian cancer.
Another possible reason for poor survival in teenage pregnancy is that those patients diagnosed before 1980s may not have received effective chemotherapeutic agents. As shown in Table 1, the majority of histology types in the teenage group were non-EOC. Effective chemotherapeutic regimens for such tumors, particularly MGCT, were not widely utilized until the mid-1980s to -1990 such as bleomycin, etoposide, and cisplatin (BEP) or cisplatin, vinblastine, bleomycin (PVB) [18,19]. This is reflected in the survival data (Table 2), and cases prior to this time were more likely to die from ovarian cancer whereas recent cases were not. This lack of effective chemotherapy treatment in the older cases is the most likely the reason for decreased survival in the teen group in this study. Moreover, prior analyses of pregnancy complicated by MGCT and SCST have shown that expectant management was not associated with decreased survival. It is likely that most of these cases were early-stage disease where chemotherapy was not indicated for treatment and thus not associated with survival.
It is estimated that over half of pregnant adolescents are exposed to either late or no prenatal care [16]. Our study did find a trend towards diagnosis later in pregnancy among the teenage group; however, it is unclear if this trend was due to late presentation to care or late recognition by the provider. In general, adolescent pregnancies at baseline are already at higher risk for poor outcomes including low birth weight and prematurity [15]. Our current study shows that pregnant teenagers are at increased risk for serious AEs, which often present later in pregnancy. Notably, our previous studies show serious AEs are common in pregnancies complicated by SCST and MGCT, and these events put the lives of both mother and fetus at risk [1,3].
Serious AEs captured included tumor rupture and hemoperitoneum, obstructed labor due to tumor size, ovarian torsion, recurrence during pregnancy, severe hypertension, rapid tumor progression, and fetal or maternal deaths. In this study, we found that half of the pregnant teenagers suffered from a serious AE during their pregnancy. While it is difficult to ascertain whether it is tumor, patient, or pregnancy characteristics that put teenage women at particular risk for adverse outcomes in these pregnancies, it is important to note the frequent occurrence of serious AEs and to monitor these pregnancies closely in order to avoid negative sequelae.
In regards to management of pregnancies in teenage women complicated by primary ovarian cancer, it is important to note that the majority of women in our cohort had live births. Pregnancy preservation is a reasonable option to consider, though management decisions must be informed by histology, GA at diagnosis, and stage of disease. Consistent with trends in non-pregnant patients, the most common tumor histologies in the teenage women in our cohort were MGCT and SCST. Depending on tumor characteristics at the time of diagnosis, both MGCT and SCST can be managed effectively with both definitive surgical management and chemotherapy during pregnancy [20,21].
The ideal timing of surgical management is gestational week 16–20, so recognition of a potential malignancy prior to this time is necessary to achieve an optimal outcome [22]. While radiation is contraindicated during pregnancy, the most common regimen for MGCT, the triple combination of BEP, is generally considered to be safe after 14 weeks of gestation, although long-term studies evaluating effects following in utero exposure to chemotherapies are lacking [21,23].
Strengths of the present study include the large sample size, which increases the ability to capture a rare disease entity. The systematic review format ensures a comprehensive analysis of all available literature on this rare subject, and the multivariable analysis performed functions to eliminate some of the confounders inherent in an observational review. Weaknesses of the study include the retrospective nature. Furthermore, any systematic review is compromised by publication bias in that cases without events are less likely to be published. While our analysis was designed to control for bias, there are confounders that are not able to be captured in this study format. For example, no information is available on health behaviors that could compromise pregnancy outcome, such as tobacco and drug use. Another limitation is that we had no objective measurement for immune system function. Finally, the sample size for teenage pregnancy remained small and sensitivity analysis for intra-group comparison for demographics and outcomes were not feasible.
In conclusion, our study suggests that teenage pregnancy complicated by primary ovarian cancer is associated with poor cancer-specific survival when compared to their older counterparts. It is necessary to consider malignancy in the differential for adnexal masses even in young women, as a low index of suspicion can lead to delays in care and worse outcomes. While we hypothesize a physiologic basis for this finding, emphasizing early care and ensuring adequate support throughout all stages of the pregnancy and puerperium could help to mitigate some of the socioeconomic factors that complicate the management of teenage pregnancy complicated by primary ovarian cancer.
Footnotes
Funding: This work was supported by Ensign Endowment for Gynecologic Cancer Research (K.M.).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: B.E., D.Z.M., M.E., K.S., K.M., G.B.
- Data curation: K.S., K.M.
- Formal analysis: M.K.
- Funding acquisition: M.K.
- Investigation: G.B., M.K.
- Software: M.K.
- Validation: M.K.
- Visualization: M.K.
- Writing - original draft: B.E., M.K.
- Writing - review & editing: D.Z.M., M.E., K.S., K.M., G.B.
References
- 1.Kodama M, Grubbs BH, Blake EA, Cahoon SS, Murakami R, Kimura T, et al. Feto-maternal outcomes of pregnancy complicated by ovarian malignant germ cell tumor: a systematic review of literature. Eur J Obstet Gynecol Reprod Biol. 2014;181:145–156. doi: 10.1016/j.ejogrb.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 2.Blake EA, Kodama M, Yunokawa M, Ross MS, Ueda Y, Grubbs BH, et al. Feto-maternal outcomes of pregnancy complicated by epithelial ovarian cancer: a systematic review of literature. Eur J Obstet Gynecol Reprod Biol. 2015;186:97–105. doi: 10.1016/j.ejogrb.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Blake EA, Carter CM, Kashani BN, Kodama M, Mabuchi S, Yoshino K, et al. Feto-maternal outcomes of pregnancy complicated by ovarian sex-cord stromal tumor: a systematic review of literature. Eur J Obstet Gynecol Reprod Biol. 2014;175:1–7. doi: 10.1016/j.ejogrb.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. About teen pregnancy [Internet] Atlanta, GA: Centers for Disease Control and Prevention; 2017. [cited 2017 Sep]. Available from: https://www.cdc.gov/teenpregnancy/about/index.htm. [Google Scholar]
- 5.Althabe F, Moore JL, Gibbons L, Berrueta M, Goudar SS, Chomba E, et al. Adverse maternal and perinatal outcomes in adolescent pregnancies: The Global Network's Maternal Newborn Health Registry study. Reprod Health. 2015;12(Suppl 2):S8. doi: 10.1186/1742-4755-12-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leftwich HK, Alves MV. Adolescent pregnancy. Pediatr Clin North Am. 2017;64:381–388. doi: 10.1016/j.pcl.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 8.FIGO Committee on Gynecologic Oncology Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. Int J Gynaecol Obstet. 2009;105:3–4. doi: 10.1016/j.ijgo.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Malaguarnera L, Cristaldi E, Malaguarnera M. The role of immunity in elderly cancer. Crit Rev Oncol Hematol. 2010;74:40–60. doi: 10.1016/j.critrevonc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brazão V, Kuehn CC, dos Santos CD, da Costa CM, Júnior JC, Carraro-Abrahão AA. Endocrine and immune system interactions during pregnancy. Immunobiology. 2015;220:42–47. doi: 10.1016/j.imbio.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Bonney EA. Immune regulation in pregnancy: a matter of perspective? Obstet Gynecol Clin North Am. 2016;43:679–698. doi: 10.1016/j.ogc.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soeters PB, Grimble RF. The conditional role of inflammation in pregnancy and cancer. Clin Nutr. 2013;32:460–465. doi: 10.1016/j.clnu.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Harden A, Brunton G, Fletcher A, Oakley A. Teenage pregnancy and social disadvantage: systematic review integrating controlled trials and qualitative studies. BMJ. 2009;339:b4254. doi: 10.1136/bmj.b4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azevedo WF, Diniz MB, Fonseca ES, Azevedo LM, Evangelista CB. Complications in adolescent pregnancy: systematic review of the literature. Einstein (Sao Paulo) 2015;13:618–626. doi: 10.1590/S1679-45082015RW3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiemann CM, Berenson AB, Pino LG, McCombs SL. Factors associated with adolescents' risk for late entry into prenatal care. Fam Plann Perspect. 1997;29:273–276. [PubMed] [Google Scholar]
- 17.Torres R, Goyal D, Burke-Aaronson AC, Gay CL, Lee KA. Patterns of symptoms of perinatal depression and stress in late adolescent and young adult mothers. J Obstet Gynecol Neonatal Nurs. 2017;46:814–823. doi: 10.1016/j.jogn.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gershenson DM, Kavanagh JJ, Copeland LJ, Del Junco G, Cangir A, Saul PB, et al. Treatment of malignant nondysgerminomatous germ cell tumors of the ovary with vinblastine, bleomycin, and cisplatin. Cancer. 1986;57:1731–1737. doi: 10.1002/1097-0142(19860501)57:9<1731::aid-cncr2820570904>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 19.Williams S, Blessing JA, Liao SY, Ball H, Hanjani P. Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: a trial of the Gynecologic Oncology Group. J Clin Oncol. 1994;12:701–706. doi: 10.1200/JCO.1994.12.4.701. [DOI] [PubMed] [Google Scholar]
- 20.Han SN, Verheecke M, Vandenbroucke T, Gziri MM, Van Calsteren K, Amant F. Management of gynecological cancers during pregnancy. Curr Oncol Rep. 2014;16:415. doi: 10.1007/s11912-014-0415-z. [DOI] [PubMed] [Google Scholar]
- 21.Amant F, Halaska MJ, Fumagalli M, Dahl Steffensen K, Lok C, Van Calsteren K, et al. Gynecologic cancers in pregnancy: guidelines of a second international consensus meeting. Int J Gynecol Cancer. 2014;24:394–403. doi: 10.1097/IGC.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 22.Amant F, Brepoels L, Halaska MJ, Gziri MM, Calsteren KV. Gynaecologic cancer complicating pregnancy: an overview. Best Pract Res Clin Obstet Gynaecol. 2010;24:61–79. doi: 10.1016/j.bpobgyn.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5:283–291. doi: 10.1016/S1470-2045(04)01466-4. [DOI] [PubMed] [Google Scholar]