Abstract
Background/Aims
This study examined the accuracy of endoscopic evaluation for determining the Helicobacter pylori infection status in patients with mild atrophy who might not exhibit characteristic endoscopic findings.
Methods
Forty endoscopists determined the H. pylori infection status of 50 randomly presented H. pylori-positive and H. pylori-negative cases on the basis of a list of established findings.
Results
The median clinical endoscopy experience was 7 years (range, 1–35 years), including 22 board-certified endoscopists (55%) of the Japan Gastroenterological Endoscopy Society. The mean accuracy rate of endoscopic diagnosis was 67% and was unrelated to experience status (experienced vs. trainee: 69% vs. 65%, p=0.089) and total years of experience (R2 =0.022). The most frequently selected endoscopic findings were regular arrangement of collecting venules (59%), atrophy (45%), and red streak (22%), which had fair accuracy rates of 67%, 65%, and 73%, respectively. By contrast, the accuracy rates of nodularity (89%) and mucosal swelling (77%) were highest. The 20 endoscopists who more frequently identified these findings diagnosed H. pylori infection significantly more accurately than did the other endoscopists (71% vs. 64%, p=0.008).
Conclusions
Careful attention to nodularity and mucosal swelling in patients with mild atrophy may enhance diagnosis, enable prompt treatment, and avoid possible long-term carcinogenesis.
Keywords: Diagnostic accuracy, Endoscopic diagnosis, Helicobacter pylori, Mild atrophy
Introduction
As the association between Helicobacter pylori infection and the development of gastric cancer is well established [1], prompt H. pylori detection and eradication may prevent such disease [2,3]. Specifically, the treatment of H. pylori infection when gastric mucosal atrophy is mild may be more efficient in preventing gastric cancer because cancer risk increases with atrophy progression [4,5].
During endoscopic examination, assessment of H. pylori infection status, in addition to diseases such as gastric cancer and gastroduodenal ulcers, is important. Accordingly, studies have reported many endoscopic findings that correlate with infection status [6-8], but these studies did not make reference to the extent of gastric mucosal atrophy. Patients with endoscopically mild atrophy, that is, level C-0 or C-1 of the Kimura-Takemoto classification system [4,9], may lack the characteristic endoscopic findings of H. pylori infection and appear as uninfected cases. If clinicians misdiagnose these patients as H. pylori-negative, they may miss the optimal timing for eradication therapy.
This study assessed the accuracy of endoscopic diagnosis of H. pylori infection in patients with mild atrophy and sought to identify the key endoscopic features that contribute to an accurate diagnosis.
Materials and Methods
Study design
Forty endoscopists were enrolled from 8 institutions in Nagano prefecture. Blinded to other clinical findings, the participants were asked to judge the H. pylori infection status (H. pylori-positive or H. pylori-negative) of 50 randomly presented H. pylori-positive or H. pylori-negative cases on the basis of endoscopic images and, from a list of established endoscopic features, select multiple endoscopic findings used as the basis for judgment. Six endoscopic images of specific sites of the antrum, angulus, lesser and greater curvatures of the lower body, greater curvature of the upper body, and cardia of the stomach were given for each case (Fig. 1). All endoscopies were performed using an endoscope (GIF-Q260; Olympus, Tokyo, Japan) or high-vision endoscope (H260, H260Z, or H290; Olympus) with an electronic endoscopic system (Evis Lucera CV-260 SL or Elite CV-290; Olympus). The following 19 distinctive endoscopic findings identified in previous reports [6,7,10] were provided to the endoscopists for selection: atrophy, diffuse redness, foveolar-hyperplastic polyp, map-like redness, xanthoma, hematin, red streak, intestinal metaplasia, mucosal swelling, patchy redness, depressive erosion, enlarged fold, sticky mucus, fundic gland polyp, spotty redness, multiple white and flat elevated lesions, regular arrangement of collecting venules (RAC), nodularity, and raised erosion. Each finding was sufficiently explained before the trial to standardize the understanding of endoscopic findings among the participants. The study protocol was approved by the ethics committee of Shinshu University (approval No. 3412).
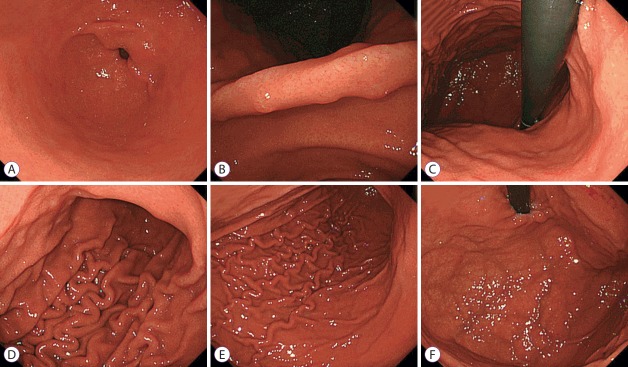
Fig. 1.
Helicobacter pylori-positive case (30-year-old man). (A) Antrum, (B) angulus, (C) lesser curvature of the lower body, (D) greater curvature of the lower body, (E) greater curvature of the upper body, and (F) cardia.
Patients
Among the patients who underwent endoscopic examination at Shinshu University Hospital between April 2010 and January 2016, 25 subjects with H. pylori infection who had C-0 or C-1 gastric mucosal atrophy according to the Kimura-Takemoto classification [4,9] as judged by 2 experienced board-certified endoscopists of the Japan Gastroenterological Endoscopy Society (TO and YI) were selected for the H. pylori-positive group. Atrophic mucosa was limited to the antrum in C-1, while atrophy was absent in C-0. Twenty-five age- and sex- matched H. pylori-negative patients were also selected from the same period. Patients who were taking proton pump inhibitors, nonsteroidal anti-inflammatory drugs, steroids, or antithrombogenic drugs were excluded from the study, as were those with gastric or duodenal ulcer, or scar from a history of gastric surgery.
Determination of H. pylori infection
H. pylori status was determined beforehand on the basis of histology, culture, and the presence of serum immunoglobulin G antibodies against H. pylori (E-plate; Eiken, Tokyo, Japan). H. pylori infection was judged as present if one or more of the tests showed a positive result. The H. pylori-negative patients in this study were defined as only H. pylori-uninfected. They were negative for infection in both histological and culture samples, and had neither atrophy nor intestinal metaplasia to exclude naturally eradicated subjects or cases of H. heilmannii-like organism infection.
Statistical analysis
Accuracy rate, positive predictive value (PPV), and negative predictive value (NPV) were calculated and analyzed statistically using the Mann-Whitney U test. Correlation coefficients and p-values were determined using the Pearson test. A p-value of less than 0.05 was accepted as statistically significant. All statistical analyses were performed using the StatFlex version 6.0 software (Artech, Osaka, Japan).
Results
Characteristics of the endoscopists and patients
The profiles of the endoscopists and patients are shown in Table 1. The median endoscopy experience was 7 years (range, 1–35 years). Of the endoscopists, 22 (55%) were board-certified fellows of the Japan Gastroenterological Endoscopy Society. The median age of the H. pylori-positive patients was 36 years (range, 15–72 years), and that of H. pylori-negative subjects was 35 years (range, 16–70 years). Each group was consisted of 9 men (36%).
Table 1.
Characteristics of Endoscopists and Patients
| Endoscopists (n=40) | ||
|---|---|---|
| Experience as an endoscopist, years, median (range) | 7 (1–35) | |
| Experienced endoscopists / Trainees, n (%) | 22 (55) / 18 (45) | |
| Patients (n=50) | H. pylori-positive (n=25) | H. pylori-negative (n=25) |
| Age, years, median (range) | 36 (15–72) | 35 (16–70) |
| Sex (male/female), n (%) | 9 (36) / 16 (64) | 9 (36) / 16 (64) |
H. pylori, Helicobacter pylori.
Accuracy rate of endoscopic diagnosis for H. pylori infection status
The overall mean accuracy rate of endoscopic diagnosis was 67.3%. The PPV and NPV were 73.7% and 67.2%, respectively. No statistical differences were found between the experienced and trainee endoscopists (overall: 68.9% vs. 65.3%, p=0.089; PPV: 75.4% vs. 71.5%, p=0.163; NPV: 69.1% vs. 64.9%, p=0.066; Table 2), nor a correlation between accuracy rate and level of endoscopy experience (R2=0.022; Fig. 2).
Table 2.
Accuracy Rate of Endoscopic Diagnosis for Helicobacter pylori Infection Status
| All endoscopists (n=40) | Experienced endoscopists (n=22) | trainees (n=18) | p-value | |
|---|---|---|---|---|
| Overall (%) | 67.3±8.3 | 68.9±8.5 | 65.3±7.6 | 0.089 |
| PPV (%) | 73.7±12.8 | 75.4±12.8 | 71.5±12.6 | 0.163 |
| NPV (%) | 67.2±10.0 | 69.1±10.2 | 64.9±9.4 | 0.066 |
Values are expressed as the mean±standard deviation.
PPV, positive predictive value; NPV, negative predictive value.
Fig. 2.
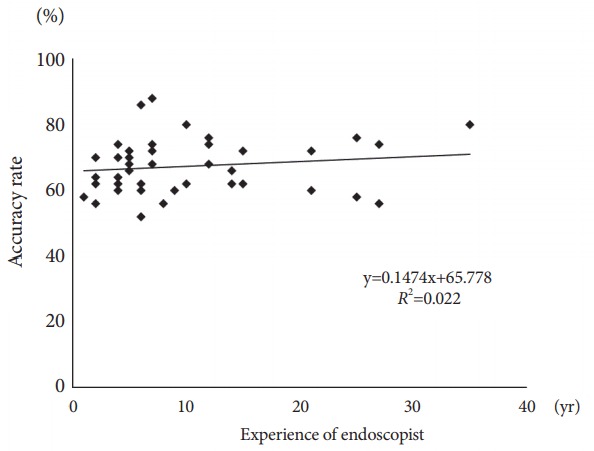
Correlation between accuracy rate and endoscopy experience.
Endoscopic findings
Of the 19 features provided, the most frequently selected endoscopic findings were RAC (59.0%), atrophy (45.1%), and red streak (21.7%), which had fair accuracy rates of 67.4%, 64.5%, and 73.0%, respectively. The accuracy rate of nodularity was highest (89.3%), followed by mucosal swelling (76.6%; Fig. 3 and Table 3). By using the mean number of selections of nodularity or mucosal swelling as threshold, the 20 endoscopists who selected at least one characteristic more frequently than the mean, that is, nodularity ≥5 times or mucosal swelling ≥6 times, had significantly higher accuracy rates than the other endoscopists (70.8% vs. 63.8%, p=0.008; Fig. 4).
Fig. 3.
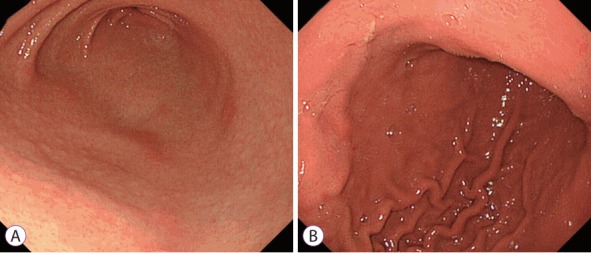
Endoscopic features. (A) Antrum with nodularity and (B) greater curvature of the lower body with mucosal swelling.
Table 3.
Endoscopic Findings Selected during Infection Status Judgment
| Endoscopic finding | Mean number of answers per endoscopist for 50 cases | Response rate (%) | Accuracy rate (%) |
|---|---|---|---|
| Atrophy | 22.6±14.4 | 45.1±28.8 | 64.5±13.3 |
| Diffuse redness | 4.6±7.5 | 9.3±15.1 | 74.1±32.4 |
| Foveolar-hyperplastic polyp | 0 | 0 | 0 |
| Map-like redness | 0.4±1.7 | 0.8±3.5 | 66.7±22.1 |
| Xanthoma | 0.3±1.0 | 0.6±1.9 | 41.7±32.4 |
| Hematin | 3.3±3.5 | 6.6±7.0 | 60.3±33.3 |
| Red streak | 10.8±10.0 | 21.7±20.0 | 73.0±21.8 |
| Intestinal metaplasia | 0 | 0 | 0 |
| Mucosal swelling | 5.1±7.1 | 10.3±14.2 | 76.6±24.1 |
| Patchy redness | 1.3±1.8 | 2.6±3.7 | 66.7±42.0 |
| Depressive erosion | 0.5±0.9 | 1.0±1.8 | 57.9±45.8 |
| Enlarged fold | 3.1±7.1 | 6.2±14.1 | 69.9±31.2 |
| Sticky mucus | 2.7±4.5 | 5.3±9.0 | 51.9±34.0 |
| Fundic gland polyp | 1.3±2.2 | 2.5±4.4 | 76.0±34.5 |
| Spotty redness | 2.9±5.1 | 5.9±10.2 | 70.1±35.2 |
| Multiple white and flat elevated lesions | 0 | 0 | 0 |
| Regular arrangement of collecting venules | 29.5±11.4 | 59.0±22.7 | 67.4±9.1 |
| Nodularity | 4.9±7.1 | 9.8±14.1 | 89.3±10.7 |
| Raised erosion | 1.1±2.0 | 2.2±4.1 | 59.1±33.4 |
Values are expressed as the mean±standard deviation.
Fig. 4.
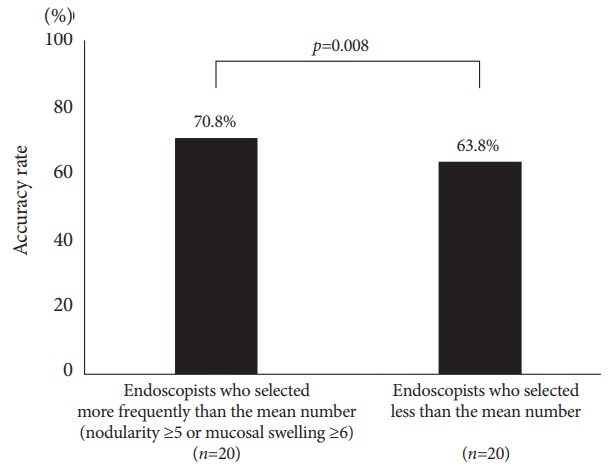
Accuracy rate according to the frequency of selecting nodularity or mucosal swelling.
Discussion
This study revealed that the accuracy of endoscopic diagnosis of H. pylori infection status in patients with mild atrophic gastritis was approximately 67%. Accurate endoscopic diagnosis was therefore difficult and did not depend on experienced status or years of endoscopy experience.
Many endoscopic findings associated with H. pylori infection status have been reported. Of these findings, RAC was described as an endoscopic feature of the H. pylori-negative normal stomach, with a diagnostic accuracy of >90% [8]. Atrophic change has also been proposed as a hallmark feature of H. pylori infection [7]. In the present study, although many endoscopists selected RAC and atrophy as the basis for their judgment, the precision of these findings was only fair for various reasons. For instance, RAC examination is performed in the entire gastric body, especially in the lesser curvature of the lower body, but the RAC-positive H. pylori-infected stomach with mild atrophy can present with inflammation localized at the antrum. In addition, because a slight difference in color between the normal antrum and corpus may exist in the absence of histological atrophy, endoscopists may mistake a normal mucosa to be an atrophic antral mucosa.
The accuracy rates of diagnosis of nodularity and mucosal swelling in this study were high. The generally accepted characteristic histological finding of nodularity is hyperplasia of lymphoid follicles [11]. This nodularity can be observed in the stomach of children much more frequently and may be characteristic of an early stage of H. pylori infection [12]. Although nodularity is also found in adults, atrophy develops during adolescence [13], and the intermediary period from nodularity to atrophy can be observed in adult patients with mild atrophy. Therefore, the nodularity found in adults may have different implications from those in children. Moreover, in nodularity, the follicle size is greater and follicles are positioned more superficially than in atrophic gastritis [14]. In the transition period to atrophy, because of changes in lymphoid follicle diameter and location, nodularity may not be outstanding in comparison with that in childhood. A previous report suggested that nodularity began to disappear from the antrum and became more localized with scattered type and other forms of mucosal atrophy [15]. Endoscopists should therefore examine carefully for characteristic nodularity in patients with mild atrophy, especially in the tangential direction to include the gastric angle, and anterior and posterior walls of the antrum.
Mucosal swelling is soft and thick, contains a convexo-concave mucosa endoscopically [6], and exhibits histological findings of inflammatory cell infiltration and edema of the mucosa [16]. These features have been reported to provide a high PPV in the identification of H. pylori infection in the gastric mucosa [6]. As mucosal swelling is generally easier to recognize at the gastric body than at the antrum [6], clinicians should inspect the mucosa of the gastric body cautiously. Hence, the diagnostic accuracy of endoscopists who paid closer attention to nodularity or mucosal swelling was significantly higher than that of other endoscopists. Careful evaluation of these findings will be useful for diagnosing H. pylori infection status correctly in patients with mild atrophy. As the endoscopists who more frequently selected both nodularity and mucosal swelling were insufficiently few (4), no statistical differences could be drawn (data not shown). Additional study with more participants is needed. The accuracy rates of diffuse redness, red streak, fundic gland polyp, and spotty redness were >70% in this investigation. During actual endoscopic examination, nodularity and mucosal swelling and the above-mentioned findings were evaluated for comprehensive judgment of H. pylori infection status. We herein reported on the 2 endoscopic findings that require more attention among all features. To improve diagnostic accuracy, one or both findings should be evaluated, although further study is necessary to confirm this.
As gastric mucosal atrophy with H. pylori infection progresses with age [17], patients with mild atrophy tend to be young; the median age of the H. pylori-positive group in the present study was 36 years. While the risk of differentiated gastric cancer is known to increase with the progression of atrophic change, undifferentiated cancers tend to be more frequent when mucosal atrophy is absent or mild and are prevalent in younger patients [4]. Eradication therapy of H. pylori at a younger age is therefore considered effective in preventing both differentiated and undifferentiated gastric cancers. Furthermore, the spread of H. pylori infection to the next generation can be halted by eradication during youth. To meaningfully reduce the occurrence of gastric cancer, detection of H. pylori-positive patients by close attention to the key endoscopic features will be essential among younger people whose H. pylori infection rate is low and atrophy is mild.
The present study has several limitations. First, the number of participating endoscopists and sample size were relatively small. As only 6 photographs were used for each analysis, some findings that may have been present could not be assessed. Additional validation studies are required to support our conclusions. Second, we evaluated endoscopic findings by using the conventional white-light endoscopy only. Chromoendoscopy with indigocarmine dye has been found to improve the diagnostic accuracy for nodularity and mucosal swelling [6], while magnifying endoscopy was shown to render evaluation of H. pylori status more accurate [18]. By addition of these approaches, diagnostic accuracy may be further improved. Third, H. pylori-eradicated cases were not included. Although gastric cancer can occur after eradication, carcinogenesis after eradication for mild atrophic gastritis is extremely rare. Therefore, detection of not H. pylori-eradicated cases but H. pylori-infected cases accurately is clinically important in patients with mild atrophy.
In conclusion, close attention to nodularity and mucosal swelling may improve the accuracy of endoscopic judgement of H. pylori infection status in mild atrophic gastritis. Early detection of younger patients with H. pylori infection will enable prompt eradication and possible prevention of gastric cancer.
Acknowledgments
We thank Trevor Ralph for his English editorial assistance.
Footnotes
Conflicts of Interest:The authors have no financial conflicts of interest.
REFERENCES
- 1.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 2.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 3.Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer. 2013;132:1272–1276. doi: 10.1002/ijc.27965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masuyama H, Yoshitake N, Sasai T, et al. Relationship between the degree of endoscopic atrophy of the gastric mucosa and carcinogenic risk. Digestion. 2015;91:30–36. doi: 10.1159/000368807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 6.Kato T, Yagi N, Kamada T, Shimbo T, Watanabe H, Ida K. Diagnosis of Helicobacter pylori infection in gastric mucosa by endoscopic features: a multicenter prospective study. Dig Endosc. 2013;25:508–518. doi: 10.1111/den.12031. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe K, Nagata N, Nakashima R, et al. Predictive findings for Helicobacter pylori-uninfected, -infected and -eradicated gastric mucosa: validation study. World J Gastroenterol. 2013;19:4374–4379. doi: 10.3748/wjg.v19.i27.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yagi K, Nakamura A, Sekine A. Characteristic endoscopic and magnified endoscopic findings in the normal stomach without Helicobacter pylori infection. J Gastroenterol Hepatol. 2002;17:39–45. doi: 10.1046/j.1440-1746.2002.02665.x. [DOI] [PubMed] [Google Scholar]
- 9.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87–97. [Google Scholar]
- 10.Kamada T, Haruma K, Inoue K, Shiotani A. [Helicobacter pylori infection and endoscopic gastritis -Kyoto classification of gastritis] Nihon Shokakibyo Gakkai Zasshi. 2015;112:982–993. doi: 10.11405/nisshoshi.112.982. [DOI] [PubMed] [Google Scholar]
- 11.Shiotani A, Kamada T, Kumamoto M, et al. Nodular gastritis in Japanese young adults: endoscopic and histological observations. J Gastroenterol. 2007;42:610–615. doi: 10.1007/s00535-007-2073-5. [DOI] [PubMed] [Google Scholar]
- 12.Kato S, Nishino Y, Ozawa K, et al. The prevalence of Helicobacter pylori in Japanese children with gastritis or peptic ulcer disease. J Gastroenterol. 2004;39:734–738. doi: 10.1007/s00535-004-1381-2. [DOI] [PubMed] [Google Scholar]
- 13.Ricuarte O, Gutierrez O, Cardona H, Kim JG, Graham DY, El-Zimaity HM. Atrophic gastritis in young children and adolescents. J Clin Pathol. 2005;58:1189–1193. doi: 10.1136/jcp.2005.026310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura T, Sakai Y, Hoshino H, Iwaya Y, Tanaka E, Kobayashi M. Superficially located enlarged lymphoid follicles characterise nodular gastritis. Pathology. 2015;47:38–44. doi: 10.1097/PAT.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura S, Yasuda M, Muguruma N, et al. Prevalence and characteristics of nodular gastritis in Japanese elderly. J Gastroenterol Hepatol. 2013;28:1154–1160. doi: 10.1111/jgh.12180. [DOI] [PubMed] [Google Scholar]
- 16.Nomura S, Terao S, Adachi K, et al. Endoscopic diagnosis of gastric mucosal activity and inflammation. Dig Endosc. 2013;25:136–146. doi: 10.1111/j.1443-1661.2012.01357.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohkuma K, Okada M, Murayama H, et al. Association of Helicobacter pylori infection with atrophic gastritis and intestinal metaplasia. J Gastroenterol Hepatol. 2000;15:1105–1112. doi: 10.1046/j.1440-1746.2000.02305.x. [DOI] [PubMed] [Google Scholar]
- 18.Yagi K, Aruga Y, Nakamura A, Sekine A. Regular arrangement of collecting venules (RAC): a characteristic endoscopic feature of Helicobacter pylori-negative normal stomach and its relationship with esophago-gastric adenocarcinoma. J Gastroenterol. 2005;40:443–452. doi: 10.1007/s00535-005-1605-0. [DOI] [PubMed] [Google Scholar]