Abstract
Circulating immunoglobulin M (IgM) exists in a pentameric form, possessing a polyreactive nature that responds not only to foreign antigens but also to autoantigens; thus, it is involved in both beneficial and detrimental immune responses, including protection from infection and the progression of autoimmunity. On the other hand, IgM also behaves as a carrier of the apoptosis inhibitor of macrophage (AIM) protein, storing a large amount of the inactivated form of AIM in the blood through this association. Under different disease conditions, AIM can dissociate from IgM locally or systemically to exert its function, inducing the removal of various biological debris such as excess fat, bacteria, cancer cells or dead cell debris. Most typically, upon induction of acute kidney injury (AKI), IgM-free AIM is filtered by the glomerulus in the kidney, which stimulates the clearance of intraluminal dead cells debris at the obstructed proximal tubules, thereby facilitating the repair of kidney injury. Interestingly, cats exhibit a deficiency in AIM release from IgM, which may increase their susceptibility to renal failure. Conversely, association with AIM inhibits IgM binding to the Fcα/μ receptor on follicular dendritic cells at the splenic germinal center, thereby protecting the IgM immune complex from Fcα/μ receptor-mediated internalization, which supports IgM-dependent antigen presentation to B cells and stimulates high-affinity IgG antibody production. The regulation of AIM–IgM binding, resulting from the discovery of reciprocal actions between AIM and IgM, could lead to the development of novel therapies against different diseases.
Introduction
Various biological cascades respond to alerting signals against life-threatening events, such as tissue injury. In most cases, the production of effector molecules is rapidly upregulated via an increase in the transcription of mRNA for the relevant molecules. Conversely, in some cases, effectors that have already been generated and stored within cells are rapidly released in response to more acute events; this action is typically observed in immune cells secreting cytokines during infection or Langerhans β cells emitting insulin in response to hyperglycemia. However, to conserve the time and energy required for effector activation, it may be more efficient to maintain effectors on stand-by in the blood and to release them on demand in an active form against the target, similar to fighter jets being launched from a large aircraft carrier. Interestingly, we recently found that circulating immunoglobulin M (IgM) behaves as such a carrier and that its primary aircraft is the apoptosis inhibitor of macrophage (AIM) protein.
Characteristics of aim
AIM, also known as CD5-like antigen (CD5L), is a circulating protein initially identified as an apoptosis inhibitor that supports the survival of macrophages against different types of apoptosis-inducing stimuli.1 Serum AIM levels are relatively high (~5 μg/ml) in humans and mice.1,2,3 AIM belongs to the scavenger receptor cysteine-rich (SRCR) superfamily, which all share a highly conserved cysteine-rich domain of ~100 amino acids.4 The AIM protein sequence is well conserved between humans and mice, with 78% amino-acid homology, but exhibits variations in its glycosylation state.1,5,6 To the best of our knowledge, human and mouse AIM proteins are functionally equivalent.6 AIM is mainly produced by tissue macrophages, including liver Kupffer cells and peritoneal resident macrophages, and is transcriptionally regulated by nuclear receptor liver X receptor/retinoid X receptor heterodimers.7,8,9 Hamada et al. 10 reported that the transcription factor MafB is also involved in the regulation of AIM mRNA expression.10 We analyzed serum AIM levels in more than 20 000 healthy human individuals and found that AIM levels are high in young women (teens to 20s) and gradually decrease with increasing age until ~50 years of age, after which they are fairly steady. In contrast, AIM levels in men are fairly steady at all ages, similar to those found in women over 50 years of age.3
Aim associates with the igm pentamer in blood and is protected from urinary excretion
In 2002, Tissot et al. 11 first reported that the blood IgM immune complex (IC) contains AIM, which was determined using mass spectrometric analysis of co-precipitates with IgM. Indeed, when we tested mouse and human sera for AIM by immunoblotting under non-reducing conditions, although the molecular weight of AIM is 37 kDa in humans and 42 kDa in mice, the AIM signal was only observed at a position identical to that of the pentameric IgM (>500 kDa).2 Size fractionation of mouse serum also showed that both AIM and IgM were present in the same fractions, confirming that the majority of circulating AIM is associated with the IgM pentamer. We also determined that AIM binds to the Fc region of IgM2,12 (Figure 1a). Even the recombinant IgM-Fc, which lacks the variable region forming the pentamer, associates with AIM. Consistent with this, the association of AIM with different monoclonal IgM pentamers produced by B-cell hybridoma cells was achieved in vitro.2 However, it is not currently clear how AIM and the IgM pentamer associate with each other, mainly due to the difficulty of structural analysis of such a large complex. Additional efforts are required to elucidate this further.
Figure 1.
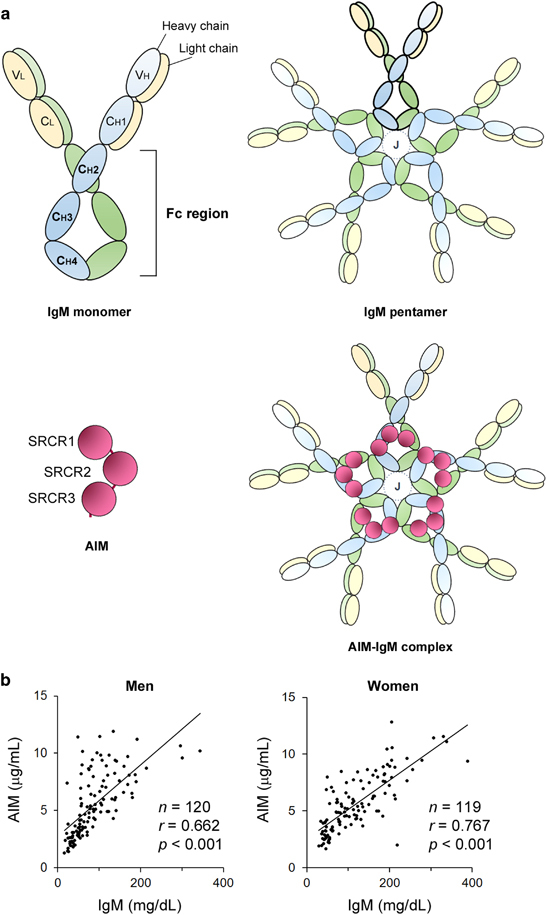
AIM binds to the Fc region of the IgM pentamer in blood. (a) Schematic structures for IgM and AIM. Like other immunoglobulins, such as IgG, the IgM monomer consists of two heavy chains (blue or green) and two light chains (yellow), each of which are covalently connected by disulfide bonds. The immunoglobulin monomer is divided into two regions: a variable region required for recognition of specific antigens and a constant region, which is identical among all the immunoglobulins with the same isotype. The fragment crystallizable (Fc) region is a tail part of the constant region including CH2-CH4 domains and is important for various immunological responses through binding to Fc receptors expressed on the surface of immune cells. Generally, IgM exists as a pentamer in blood. Each IgM monomer is linked by disulfide bonds, and a J chain indispensably joins to this pentameric association. AIM, which harbors three SRCR domains, binds to the Fc region of the IgM pentamer. Although five AIMs may theoretically bind to one IgM pentamer (illustrated as an AIM–IgM complex), it is hypothesized that one or two AIMs associate with an IgM pentamer in blood. (b) A strong correlation in serum levels of AIM and IgM in men (left, n=120, average age 49 years) and in women (right, n=119, average age 49 years) was observed. The data are modified from a previously published report.3
Based on the calculated molarities of blood AIM and IgM in wild-type mice, each IgM pentamer appears to harbor only one to two AIM molecules; the circulating IgM pentamer therefore has more space for AIM. This observation is substantiated by the fact that when mice were injected with ~10 μg of recombinant AIM (rAIM), most of the injected rAIM was bound to the IgM pentamer (Hiramoto and Miyazaki, unpublished results). Intriguingly, AIM cannot associate with the IgM pentamer in the absence of the J chain, which forms a complex with the IgM pentamer and possibly stabilizes the pentameric structure.2 Furthermore, the AIM–IgM pentamer association is abrogated in serum from mice deficient in the J chain.13 It is important to note that we confirmed that AIM does not bind directly to the J chain. It is possible that the J chain may change the structure of the IgM pentamer to expose the AIM binding site within the Fc.
Interestingly, association with IgM increases the stability of the AIM protein in blood. The association prevents the urinary excretion of AIM, and this response results in the accumulation of AIM in the blood to a relatively high concentration (~5 μg/ml). IgM-free AIM is easily filtered by the glomerulus due to its molecular size; therefore, serum AIM is largely lost in mice lacking circulating IgM, such as recombinase activating gene 1-deficient (RAG1 −/−) mice or secreted-type IgM-deficient (Δsμ) mice.14 The serum AIM level was also decreased in J chain-deficient mice, as AIM does not bind to the IgM pentamer in the absence of the J chain. It is noteworthy that the macrophage AIM mRNA level is comparable in various tissues in all types of mice. In line with these findings, serum AIM levels rapidly increased when a monoclonal mouse IgM was intravenously injected into Δsμ or RAG1 −/− mice.2,15 Consequently, a strong correlation between AIM and IgM levels in the blood has been found in both humans and mice2,3 (Figure 1b). In contrast, it is unlikely that AIM contributes to the protein stability of IgM, as wild-type and AIM-deficient (AIM −/−) mice showed comparable levels of blood IgM. Moreover, this correlation was not observed between AIM and IgG levels in the blood, in accordance with the fact that AIM does not bind to IgG.
Aim influences disease progression in an igm-free manner
Over the past decade, many research groups have clarified various AIM functions that essentially protect against different disease types. It is most noteworthy, however, that all the effects exhibited by AIM are achieved in the IgM-free form. When associated with the IgM pentamer, AIM is protected from renal excretion and is thus stabilized; however, it is inactivated (Figure 2).
Figure 2.
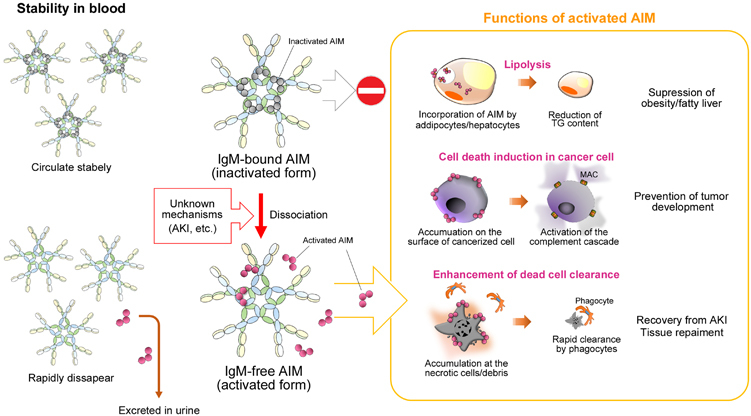
Scheme of two different states of AIM in the body: dissociation from IgM improves activity but reduces stability, and vice versa. Under physiologic conditions, AIM associates with the IgM pentamer in the blood, which prevents its urinary exclusion due to its large molecular size (>500 kDa); however, IgM-bound AIM is thought to be functionally inactivated. Once AIM is dissociated, such as during AKI or other specific circumstances, AIM demonstrates different functions, as depicted in the right panel. However, IgM-free AIM is easily filtered by the glomerular membrane into the urine, and rapidly disappears from the body. The precise mechanism that causes the dissociation of AIM from IgM is not yet known.
Unlike many other soluble proteins, AIM does not mediate signal transduction in target cells but is incorporated by several cell types through scavenger receptor-mediated endocytosis.16 In obese people, AIM is released locally or systemically in certain circumstances and endocytosed into adipocytes and hepatocytes through CD36, where it binds to and inactivates cytoplasmic fatty acid synthase.16,17 This leads to a reduction in lipid droplet-coating proteins, such as fat-specific protein 27 and perilipin, thereby decreasing triacylglycerol deposition within the cells.17 This AIM action prevents obesity and fatty liver progression (Figure 2). In AIM −/− mice fed a high-fat diet (HFD), body weight gain is significantly greater than that in wild-type mice, and AIM −/− mice show a remarkable increase in visceral adipose tissue mass.16 Conversely, this hyperobese phenotype is abrogated by the administration of rAIM to obese AIM −/− mice. Similarly, AIM −/− mice fed an HFD show more advanced liver steatosis than wild-type mice, with increased liver mass and liver triacylglycerol content.18 Note that IgM does not colocalize with AIM that is incorporated into the cytoplasm of adipocytes and hepatocytes, corroborating the fact that IgM-free AIM enters the target cells and functions within the cell. In humans, we have observed significant negative correlations between circulating AIM levels and body mass index, abdominal circumference and body fat percentage,3 demonstrating that circulating AIM dissociates from IgM and regulates cellular fat deposition, thereby preventing obesity and fatty liver.
Although AIM is incorporated into hepatocytes to decrease triacylglycerol deposition, once hepatocytes have undergone malignant transformation to hepatocellular carcinoma (HCC) cells, AIM is no longer endocytosed but instead accumulates on the cell surface18 (Figure 2). This phenotypic change in AIM is possibly due to defective endocytosis, which is a common characteristic of many different types of cancer cells.19,20 Thus, in HCC cells, the IgM-free AIM accumulates on the cell surface after AIM-CD36 binding with insufficient AIM cellular incorporation; hence, AIM distinguishes HCC cells from normal hepatocytes. Interestingly, cell surface AIM specifically stimulates HCC cell death via necrosis through specific activation of a complement cascade against cancer cells, thereby preventing tumor development. Indeed, most AIM −/− mice bear multiple HCC tumors when fed an HFD or a high-fructose diet for a year; in contrast, wild-type mice do not develop HCC tumors.18,21 Overall, AIM prevents HCC tumor development through the elimination of cancerous hepatocytes. In addition, Lozano’s group reported that AIM attaches to certain bacteria to induce coagulation, which aids effective bacterial eradication by the immune cells.22
From these findings, it can be hypothesized that AIM is stored in blood by binding to the IgM pentamer and is released on demand in an active form to remove ‘biological garbage’, for example, excess fat, cancer cells or bacteria, to maintain a healthy state and overall organismal homeostasis.23 Such garbage targeted by AIM also includes dead cell debris, and the adequate removal of such debris is important for maintaining tissue homeostasis (Figure 2). Several reports indicate that insufficient clearance of dead cells disturbs recovery from injury in various tissues, including the lung, heart, mammary gland and liver, primarily due to further inflammation caused by the debris.24,25,26,27,28 Thus, in the liver, to avoid secondary inflammation and to promote tissue recovery, the HCC cells killed by AIM need to be rapidly removed. In fact, the accumulation of AIM on the surface of necrotic HCC cells appears to serve this purpose. We have found that an AIM coating profoundly increases the preference of phagocytes, including macrophages, for necrotic cell debris. Accordingly, when we induced the expression of cell surface-bound AIM in Hepa1.6 mouse HCC cells after these cells were transplanted into the liver of AIM −/− mice, a massive increase in HCC cell necrosis caused by activation of the complement system was observed specifically in transplanted HCC cells, followed by a rapid clearance of dead HCC cell debris by infiltrating Kupffer macrophages.18 This AIM-stimulated clearance of dead cell debris also appears to be involved in injury recovery by different tissues, including the kidney. We recently found that the efficient release of AIM from the IgM pentamer is a critical response in the recovery from acute kidney injury (AKI), as impaired AIM-mediated cell debris clearance in the kidneys of AIM −/− mice dramatically impaired their recovery from kidney damage.
Pathology of aki
AKI is characterized by impaired kidney filtration function, caused by insults such as kidney ischemia/reperfusion (IR) injury), sepsis or nephrotoxins. AKI is associated with prolonged hospitalization and high mortality and, most importantly, predisposes patients to chronic kidney disease and end-stage renal disease.29,30,31,32,33 To date, although numerous therapeutic strategies have been proposed and tested for AKI, none have reached widespread clinical use. Renal tubular obstruction by debris from dead tubular epithelial cells is an important pathological characteristic often observed in AKI.34,35,36 Cell death in this tissue occurs due to apoptosis and necroptosis, `particularly in the proximal tubules at the corticomedullary junction; these dead cells detach from the tubular basement membrane physically obstructing the tubular lumen. These events reduce glomerular filtration and induce the production of inflammatory mediators by injured epithelial and infiltrating hematopoietic cells, further exacerbating tubular injury.35 Thus, sufficient removal of intraluminal debris is a key mechanism for restoring tubular structure and renal function after AKI.
Aim dissociates from igm and is excreted in the urine during aki
During AKI, AIM is systemically released from the IgM pentamer in the blood,37 resulting in urinary excretion of IgM-free AIM and allowing AIM to exert its ability to scavenge garbage. In sera from mice and humans with AKI, a substantial amount of IgM-free AIM (<40 kDa) was detected via immunoblotting. In contrast, healthy donors showed only a high molecular weight IgM-bound AIM (Figure 3a). In human AKI patients, serum levels for IgM-free AIM increase in correlation with serum creatinine (Cre) levels. In accordance, the strong correlation between whole AIM and IgM levels in blood, which is observed in healthy human individuals, was absent in AKI patients. The IgM-free AIM appeared in the urine of AKI mice and humans, as determined by ELISA (enzyme-linked immunosorbent assay). AIM is not detectable in urine samples from healthy mice and humans but increases markedly in urine samples from individuals with an AKI attack. During the recovery phase, urinary AIM levels subsequently decline in both mice and humans. Consequently, the AIM excreted in urine coats the intraluminal debris that obstructs the proximal tubules. Immunohistochemistry analysis of the kidneys of AKI mice showed prominent accumulation of AIM on dead cell debris within the proximal tubules (Figure 3b). Similarly, in the kidneys of human AKI individuals, marked AIM accumulation on intraluminal debris was observed (Figure 3b). IgM did not colocalize with AIM at the intraluminal debris in mice and humans with AKI, confirming that the AIM deposited on the debris was IgM-free. In addition, AIM accumulation was not detected at the intraluminal debris in Δsμ mice in contrast to wild-type mice. This observation strongly supports the need for AIM to be stored in blood, through association with circulating IgM pentamer, to respond to emergency situations.
Figure 3.
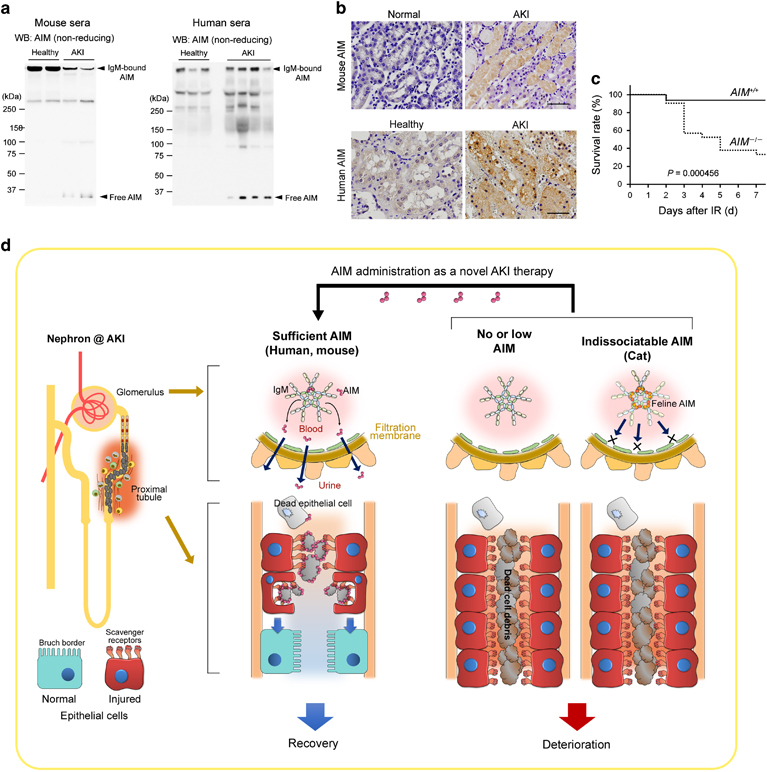
AIM in AKI. (a) Immunoblotting in non-reducing conditions for AIM involving sera from healthy and AKI-induced wild-type mice (n=2 each), as well as healthy individuals (n=3) and AKI patients (n=5). IgM-bound AIM and IgM-free AIM are indicated by arrows. IgM-free AIM is observed only in AKI conditions, and IgM-bound AIM is reduced with AKI in mice. (b) Immunohistostaining of the kidney specimens of healthy and AKI mice (upper panels), as well as a healthy individual and an AKI patient (lower panels) for AIM. Signals were visualized by horseradish peroxidase/3′-diaminobenzidine (HRP/DAB). Scale bars: 50 μm. Modified from a previously published report.2 (c) Survival of wild-type (AIM +/+) and AIM −/− mice after IR was analyzed using the Kaplan–Meier method. P: statistical significance between AIM +/+ and AIM −/− mice was calculated by using the log-rank test. Modified from our previous report.2 (d) Schema for the involvement of AIM in AKI pathogenesis. (Left) When sufficient IgM-free AIM dissociates from IgM pentamer during AKI (this usually occurs in wild-type mice and most humans), AIM is excreted in the urine, accumulating on the lumen-obstructing necrotic cell debris and thereby enhancing its clearance by injured proximal tubular epithelial cells (indicated by red cells), leading to the regeneration of epithelial cells (indicated by blue cells) and AKI recovery. (Middle) In the absence of AIM (for example, AIM-deficient mice), the debris removal is deficient. (Right) In cats, due to the strong binding of feline AIM to IgM, AIM is unable to dissociate from IgM during AKI, abolishing its excretion in urine; the intraluminal debris therefore cannot be removed efficiently. Intravenous rAIM (which is IgM-free) administration could be therapeutically applied for AKI treatment.
Abrogated intraluminar debris removal and impaired recovery from aki in aim-deficient mice
The role of AIM in the removal of intraluminal debris has been shown by comparative analysis of wild-type and AIM −/− mice after the induction of AKI by IR.37 On day 1 after IR, the structure of the proximal tubular cells was severely impaired in both wild-type and AIM −/− mice, and many proximal tubules located at the corticomedullary junction were obstructed with necrotic cell debris, which stained strongly with periodic acid-Schiff. In wild-type mice, the periodic acid-Schiff-positive intraluminal debris was reduced at day 3 post injury, whereas in AIM −/− mice, the clearance of debris was not apparent even at day 7. In parallel, there was no recovery of the brush border in the proximal tubules, a sign of healthy epithelial cells, which was apparent in wild-type mice at day 3. The differences between wild-type and AIM −/− mice were reflected more precisely in the acute tubular necrosis score, a standard method to evaluate the severity of kidney injury during AKI. In addition, as mentioned above, insufficient debris clearance caused subsequent inflammation in the AIM −/− kidney: considerably higher mRNA levels of various proinflammatory genes were sustained in the kidneys of AIM −/− mice post injury. This was followed by the progression of fibrosis in the kidneys; histologic analysis showed higher levels of fibrosis within the tubulointerstitial areas in AIM −/− mice than in wild-type mice. High mRNA levels of various fibrogenic genes were persistent in the kidneys of AIM −/− mice even at day 28 post injury.
Such a delay in the clearance of intraluminal debris, and the consequently, persistent inflammation and progressive fibrosis, resulted in impaired recovery from AKI in AIM −/− mice.37 When wild-type mice were subjected to IR, serum blood urea nitrogen and Cre levels, markers of renal dysfunction, peaked at day 1 and recovered thereafter. The general health status of the mice was lowest on day 1 and improved thereafter. The overall mortality of wild-type mice at day 7 was only 6.3% (Figure 3c). In contrast, in AIM −/− mice, although the serum blood urea nitrogen and Cre levels, as well as their general health status, were comparable to wild-type mice on day 1, these parameters continued to worsen after day 1 and remained elevated at day 7. Ultimately, after IR, more than 40% of AIM −/− mice died by day 3, and the mortality at day 7 was 67% (Figure 3c). The AIM −/− mice that survived were severely emaciated. Similar to AIM −/− mice, the increase in serum Cre and blood urea nitrogen levels, as well as animal mortality in response to IR, were also markedly greater in the circulating IgM-less Δsμ mice than in wild-type mice. Thus, the storage of sufficient AIM by the IgM pentamer in blood and its release and subsequent translocation of AIM in urine are required for efficient repair of AKI. The cellular and molecular mechanisms of how AIM accumulation induces the removal of intraluminal debris were clarified but are not described here (see Arai et al. 37). We would like to mention, however, that the administration of rAIM (in an IgM-free form) can facilitate the clearance of intraluminal debris and recovery from AKI in AIM −/− mice, as well as in wild-type mice that have undergone severe AKI but in which the level of endogenous AIM was not sufficient (Figure 3d).
Feline aim never dissociates from IgM
The importance of AIM release from IgM in facilitating recovery from AKI is more directly highlighted in cats. The number of pets is increasing worldwide due to the recently decreasing birth rate and increasingly greater age of the human population, and cats are the most popular pet in the vast majority of populations.38,39 Cats are profoundly more susceptible than other animals to, and more often die from, chronic kidney disease.40,41,42,43 However, the reason for their susceptibility to renal disease, which is one of the most pressing questions in veterinary medicine, has remained unclear. Based on the important involvement of AIM in the resolution of AKI described above, we evaluated feline AIM to assess whether it is related to the susceptibility of cats to renal disease.
In fact, before the start of this study, we had expected that cats might be genetically deficient for AIM (that is, AIM −/−); however, they do possess AIM. After feline AIM cDNA was isolated, feline AIM protein was produced, and several antibodies specifically recognizing feline AIM were generated, serum AIM levels were assessed by immunoblotting in cats of different strains.44 The average AIM level in cats was 21.2 μg/ml (in >30 cats), which is markedly higher than that in mice (2–3 μg/ml) and humans (~5 μg/ml). In blood, feline AIM associates with IgM pentamers at the Fc region, and serum levels of AIM and IgM are positively correlated, as in humans and mice.2,3
Surprisingly, however, when cats are subjected to IR for experimental induction of AKI,45 all IR cats showed no IgM-free AIM in the serum, in contrast to humans and mice in which a substantial level of IgM-free AIM was detected during AKI via immunoblotting. This was also the case when sera from several hospitalized cats with spontaneous AKI caused by various events, such as renal stones, were analyzed; no IgM-free AIM was observed in sera from these cats. In humans and mice, AIM is not detectable in urine samples from healthy individuals, and its levels increase during AKI.37 Consistent with the serum results above, AIM was not detectable in the urine of IR AKI cats, indicating no urinary AIM excretion during AKI. Furthermore, histological analysis demonstrated no AIM accumulation in the intraluminal debris of cats after IR, in contrast to humans and mice with AKI wherein AIM accumulation is observed in the debris. Thus, in cats, AIM does not dissociate from the IgM pentamer upon AKI induction, and is therefore not excreted in the urine.
To determine whether this inability to dissociate is due to feline AIM but not feline IgM, we generated transgenic mice expressing feline AIM under the regulation of the mouse AIM promoter on a mouse AIM −/− background, thereby felinizing AIM in mice. The transgenic feline AIM protein associated with mouse IgM pentamers, and the serum levels of feline AIM, in AIM-felinized mice, were comparable to those of murine AIM in wild-type mice (~2 μg/ml) when assessed by immunoblotting. As expected, IgM-free AIM was not detected after IR in AIM-felinized mice, as observed in cats. Consistent with this result, immunohistochemical analysis of the kidneys from IR AIM-felinized mice revealed that the intraluminal debris did not stain for AIM, in sharp contrast to the AIM-positive debris in wild-type mice, clearly demonstrating that AIM did not appear in urine after IR in AIM-felinized mice. Therefore, feline AIM has a defect that disturbs its release from IgM upon AKI induction.
A strong binding affinity of feline aim with IgM
We used surface plasmon resonance analysis to quantitatively compare the binding affinity constant of IgM-Fc pentamers in feline and murine AIM. The resulting dissociation–association rate (K D) was 5.97 × 10−9 M in feline AIM/IgM-Fc, whereas in mouse AIM/IgM-Fc, it was 5.82 × 10−6 m; thus, in cats, AIM associates with IgM ~1000-fold more strongly than in mice. The binding affinity constant between feline AIM and murine IgM-Fc pentamers was also high, comparable to that in feline AIM/feline IgM-Fc, further suggesting that the strong association is caused by feline AIM, not by feline IgM. The strong binding of feline AIM with IgM likely plays a role in the increasing serum AIM levels observed in cats.
Recently, we found that the third SRCR domain (SRCR3) is responsible for IgM binding, since its deletion abrogated the association with IgM in murine, human and feline AIM.15,44 Of note, three-dimensional mapping of the charge distribution of amino acids revealed that the SRCR3 domain of feline AIM possesses a specific cluster of positively charged amino acids on the surface of the molecule that is not observed in mouse and human AIM, and this feline AIM-specific cluster appeared to be involved in the increase in its binding affinity with IgM. Indeed, using a mutant feline AIM in which feline-specific arginine residues constituting the positively charged amino-acid cluster were substituted with the corresponding amino acids of a mouse sequence, the mutant feline AIM was found to exhibit decreased binding to the Fc pentamers, which was verified biochemically via immunoblotting.44 This finding highlights the crucial involvement of the positively charged amino acids of complement C1q in association with IgM-Fc.46 It is possible that additional parameters defining the binding affinity between AIM and IgM are present, as the binding potential decreased only partially in the mutant feline AIM. Further studies including structural assessments of the AIM/ IgM-Fc complex are required to clarify the complete nature of the AIM–IgM association.
Saving cats from kidney disease using aim
The apparent inability of feline AIM to dissociate from IgM appears to be an important cause of the susceptibility of cats to renal failure. When wild-type and AIM-felinized mice were subjected to an IR protocol to induce AKI, serum Cre levels peaked at day 1 and recovered thereafter in wild-type mice, and 75% of mice survived to day 5. In complete contrast, serum Cre levels in AIM-felinized mice increased to similar levels as in wild-type mice at day 1 after IR but continued to increase thereafter, resulting in the death of 100% of mice by day 3.44 By histology, on day 3 after IR, there was significantly less intraluminal debris at the proximal tubules located at the corticomedullary junction in wild-type mice than in AIM-felinized mice. Similarly, recovery of the brush border in the proximal tubules was apparent on the same day in wild-type mice but not in AIM-felinized mice. The overall acute tubular necrosis score at day 3 was significantly worse in AIM-felinized mice than in wild-type mice. Overall, as expected, AIM-felinized mice showed impaired recovery from AKI, similar to AIM −/− mice. Cats that underwent experimental IR (described above) exhibited poor recovery from AKI, as determined by the serum Cre levels and blood urea nitrogen.
As in AIM −/− mice, the administration of rAIM facilitated AKI recovery in AIM-felinized mice. We injected mouse rAIM intravenously into AIM-felinized mice on days 1, 2 and 3 after IR. The survival of rAIM-injected mice at day 3 was markedly improved to 80% from 0%.44 The serum Cre level in rAIM-injected felinized mice decreased by day 5, whereas in non-treated AIM-felinized mice, it continued to increase until the mice died. Using histology, the acute tubular necrosis score of rAIM-injected AIM-felinized mice was improved at day 5. We also confirmed using immunohistochemistry that the injected rAIM accumulated at the intraluminal debris. These observations strongly suggest the therapeutic potential of rAIM in cats (Figure 3d).
Aim retains the igm-ic on splenic follicular dendritic cells
Thus far, we have discussed the importance of the efficient take-off of the aircrafts (AIM) from the aircraft carrier (IgM) to protect against various diseases, particularly AKI. We will now turn to describing the potential influence of the aircrafts (AIM) on the aircraft carrier (IgM). It is well known that a large proportion of natural IgM is polyreactive, not only to foreign antigens but also to phylogenetically conserved autoantigens, and this is believed to be important for the progression of autoimmunity.47,48,49 The IgM-IC with an antigen, also containing complement component C3, is deposited on splenic follicular dendritic cells (FDCs) through the interaction between the activated C3 and the complement receptor (CD21/CD35) on FDCs50,51,52,53,54 where the antigen is presented to germinal center (GC) B cells, inducing the development of plasma cells that produce high-affinity IgGs.54 Therefore, increased IgM results in more chances to present autoantigens, thereby stimulating the autoantibody responses responsible for the progression of autoimmunity.
We asked whether the association of AIM might influence this process. Consistent with the association between AIM and the IgM pentamer, the accumulation of both AIM and IgM was specifically observed in FDCs within splenic GCs in mice2 (Figure 4a). When we injected rAIM, alone or in combination with IgM, into Δsμ mice, rAIM accumulation in FDCs was only observed when rAIM was injected in combination with IgM, suggesting that the AIM accumulation in FDCs is IgM-dependent.
Figure 4.

AIM retains IgM immune-complexes on splenic FDCs. (a) Immunohistostaining of specimens of WT mouse spleens for AIM (green), IgM (blue) and FDC (red). Nuclei were also stained by DAPI (4',6-diamidino-2-phenylindole) (light blue). FDC areas are indicated by white arrows. The data are modified from our previously published report.2 (b) Schema describing the role of AIM in retention of IgM-IC on the surface of FDCs. For the affinity maturation of GC B cells to plasma cells producing high-affinity IgGs, it is necessary that ICs are retained on the surface of FDCs for a sufficient period of time to present antigens to GC B cells. Although IgM-IC is retained by complement receptors (CRs), in the absence of AIM, it is internalized via Fcα/μR into FDCs and rapidly disappears from the surface, resulting in the failure of affinity maturation (right). When AIM is associated with IgM-IC, AIM prevents its internalization, supporting the retention of IgM-ICs on FDCs, which enables affinity maturation of GC B cells recognizing autoantigens that are presented by IgM-ICs (left).
Although the protein stability of IgM was not affected by the absence of AIM in blood, the AIM association influences the holding state of the IgM-IC at FDCs. We intravenously injected IgM alone or in association with rAIM (IgM/AIM) into Δsμ AIM −/− doubly deficient mice, and the presence of IgM at the FDC cell surface was tested kinetically. The IgM level at FDCs was profoundly increased when mice were injected with IgM/AIM compared with IgM alone. Similarly, when the 2,4,6-trinitrophenyl antigen, which was shown to be bound to IgM,55 was injected into wild-type and AIM −/− mice, the level of 2,4,6-trinitrophenyl antigen on FDCs was maintained longer in wild-type mice than in AIM −/− mice. Thus, the binding of AIM to IgM increases the retention of IgM-IC on FDCs.
Aim disturbs the binding between igm and the fcα/μ receptor
The Fcα/μR, the Fc receptor for both IgM and IgA,56 mainly found on FDCs,55,56 induces internalization of IgM, thereby reducing the retention of IgM on the cell surface.56 Indeed, Fcα/μR −/− mice demonstrate advanced antigen retention on FDCs when challenged with the 2,4,6-trinitrophenyl antigen.55 These facts led to the hypothesis that the association between AIM and IgM-Fc might affect the binding of IgM to the Fcα/μR at FDCs, which was found to be true. When HEK293T cells overexpressing Fcα/μR were incubated with a monoclonal IgM with or without rAIM association, the presence of AIM profoundly decreased the binding of IgM to Fcα/μR.2 Similarly, Fcα/μR-expressing HEK293T cells incubated with serum from wild-type mice showed reduced surface staining for IgM compared with cells incubated with AIM −/− mouse serum. Consistent with these binding results, internalization of IgM by HEK293T cells expressing Fcα/μR was markedly decreased by the rAIM association. Thus, AIM interferes with the binding of IgM to Fcα/μR, and its internalization through Fcα/μR. Such physical disturbance of the IgM/ Fcα/μR association by AIM was also observed in the activation of the complement cascade against HCC cells by AIM that accumulates on the HCC cell surface. On the cell surface, multiple regulators of complement activation (including CD55, complement regulator complement receptor 1-related gene/protein-y, complement factor H and CD59) protect cells from complement-dependent attack by suppression of C3 activation or formation of the membrane attack complex.57,58,59 Therefore, AIM interferes with the complement regulatory activity of the regulators of complement activation through direct association with regulators of complement activation, thereby triggering complement activation and leading to specific necrotic death of the HCC cells18 (Figure 2).
Obesity-associated increases in circulating autoantibodies and their prevention in AIM−/− MICE
It is possible, therefore, that the IgM-dependent antigen presentation to GC B cells at splenic FDCs is less efficient in AIM −/− mice than in wild-type mice due to a rapid internalization of IgM-IC via Fcα/μR (Figure 4b). This was demonstrated in the obesity-associated autoantibody production process. Accumulating etiological and clinical studies in humans have shown a strong correlation between obesity and autoimmune diseases. These are largely accompanied by increased levels of autoantibodies such as diabetes-associated antibodies against pancreatic β-cell antigens, including insulin, glutamic acid decarboxylase and protein tyrosine phosphatase-like protein (IA2); chronic thyroiditis-associated anti-thyroid peroxidase or anti-thyroglobulin antibody; or infertility-associated anti-sperm antibody.60,61,62,63,64 Winer et al. 65 also recently demonstrated the production of pathogenic IgG antibodies, including a unique profile of autoantibodies in obese humans and mice. Such antibody production was shown to be important in the acceleration of insulin resistance.
When mice are fed an HFD, the increased blood fatty acids stimulate the cell surface Toll-like receptor 4 on splenic marginal zone B cells, a major producer of natural IgM,66,67,68 and subsequently induce high amounts of polyclonal IgM production in an antigen-independent manner.69,70 Owing to its self-reactive nature, the augmentation of natural IgM stimulates IgG autoantibody production in obese mice. As expected, the serum from mice fed an HFD for 12 weeks contained significantly increased levels of IgG autoantibodies to more than 30 variable autoantigens related to DNA, U1RNP, histone, SSA/SSB and the cell matrix compared with lean mice when evaluated using a proteome microarray containing 70 autoantigens.71,72,73 In sharp contrast, serum from AIM −/− mice fed an HFD for the same period revealed markedly lower levels of IgG antibodies against most of the autoantigens to which wild-type mouse serum responded.2 Consistent with these observations, the number of long-lived plasma cells (intracellular-IgG+/B220−/IgM−/CD5−/Mac1−),74 which produce high-affinity IgGs, was decreased in the bone marrow of obese AIM −/− mice compared with obese wild-type mice.2 Thus, the absence of AIM abrogates the IgM-dependent maturation of high-affinity IgG-producing plasma B cells and thus prevents obesity-associated autoantibody production. It is well known that cats possess a number of autoantibodies and are therefore susceptible to various autoimmune diseases.75 It is possible that the extremely strong binding of feline AIM to IgM might be involved in the increased autoimmune nature of cats, which may be worth further assessment.
Future directions
In this review, we have illustrated the significance of the association of AIM with the IgM pentamer in blood in terms of disease protection, particularly in kidney disease. AIM harbors a number of unique characteristics. First, AIM is a circulating protein that can be delivered throughout the body. Second, AIM possesses a ‘sticky’ nature, which enables it to efficiently accumulate on the surface of widely varying ‘biological garbage’. This appears to be dependent on its cysteine-rich domains and is therefore a common feature shared by the members of the SRCR superfamily.4,76,77 Third, AIM is incorporated via endocytosis into cells through scavenger receptors, and does not mediate signal transduction, which is advantageous for the promotion of efficient garbage clearance. The storage of a relatively large amount of AIM in blood through binding to the IgM pentamer is likely beneficial for these AIM roles. The level of AIM in blood is high, as opposed to the levels of soluble signaling proteins, which are far lower.
To the best of our knowledge, the dissociation of AIM from IgM occurs during specific diseases; AKI causes the most prominent release. However, if we could release the stored AIM on a regular basis, it might flush away accumulating garbage that is still small enough not to cause any serious symptoms in different tissues, possibly preventing multiple chronic, progressive diseases,78 even aging. To achieve this, we need to clarify the release mechanism, which must occur along with the massive dissociation of AIM from the IgM pentamer. We speculate that materials that can effectively control the release of AIM from IgM may represent a novel therapeutic concept and tool for various diseases in the future, although significant work is necessary to achieve this goal. One could expect that forcing the dissociation of AIM from IgM might also prevent autoimmunity via abolishing autoantigen presentation at the splenic GCs. Thus, it is likely that humans may gain considerable benefit from AIM-releasing materials. For cats, regretfully, this might be not the case, as feline AIM did not dissociate even under AKI; the binding between AIM and IgM appears to be too robustly covalent. We may need to use AIM itself or seek alternative treatments for cats. We do, however, propose that there will be significant relationships between AIM and human immunopathology. We note, for example, that patients with primary biliary cholangitis (PBC) have elevated serum IgM. This is relatively unique in human autoimmunity. It is more common to have polyclonal B-cell activation with subsequent elevation of all serum immunoglobulins. In PBC, it is only serum IgM levels that are elevated. In addition, although the natural history of PBC may be measured in decades, there is a continued presence of IgM autoantibodies to mitochondria, and the levels of serum IgM remain elevated throughout the course of the disease, even in patients who undergo liver transplantation.79,80
Acknowledgements
This work was supported by AMED-CREST, Japan Agency for Medical Research Development (to TM), a MEXT Grant-in-Aid for Scientific Research (S) Grant number 16H06389 (to TM) and (B) Grant number 16H05313 (to SA).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Miyazaki T, Hirokami Y, Matsuhashi N, Takatsuka H, Naito M. Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J Exp Med. 1999;189:413–422. doi: 10.1084/jem.189.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai S, Maehara N, Iwamura Y, Honda S, Nakashima K, Kai T, et al. Obesity-associated autoantibody production requires AIM to retain IgM immune complex on follicular dendritic cells. Cell Rep. 2013;3:1187–1198. doi: 10.1016/j.celrep.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki T, Mori M, Arai S, Tateishi R, Abe M, Ban M, et al. Circulating AIM as an indicator of liver damage and hepatocellular carcinoma in humans. PLoS One. 2014;9:e109123. doi: 10.1371/journal.pone.0109123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Resnick D, Pearson A, Krieger M. The SRCR superfamily: a family reminiscent of the Ig superfamily. Trends Biochem Sci. 1994;19:5–8. doi: 10.1016/0968-0004(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 5.Gebe JA, Kiener PA, Ring HZ, Li X, Francke U, Aruffo A. Molecular cloning, mapping to human chromosome 1 q21-q23, and cell binding characteristics of Spalpha, a new member of the scavenger receptor cysteine-rich (SRCR) family of proteins. J Biol Chem. 1997;272:6151–6158. doi: 10.1074/jbc.272.10.6151. [DOI] [PubMed] [Google Scholar]
- 6.Mori M, Kimura H, Iwamura Y, Arai S, Miyazaki T. Modification of N-glycosylation modulates the secretion and lipolytic function of apoptosis inhibitor of macrophage (AIM) FEBS Lett. 2012;586:3569–3574. doi: 10.1016/j.febslet.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Valledor AF, Hsu LC, Ogawa S, Sawka-Verhelle D, Karin M, Glass CK. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc Natl Acad Sci USA. 2004;101:17813–17818. doi: 10.1073/pnas.0407749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, et al. A role of the apoptosis inhibitory factor AIM/Sp/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Hamada M, Nakamura M, Tran MT, Moriguchi T, Hong C, Ohsumi T, et al. MafB promotes atherosclerosis by inhibiting foam-cell apoptosis. Nat Commun. 2014;5:3147. doi: 10.1038/ncomms4147. [DOI] [PubMed] [Google Scholar]
- 11.Tissot JD, Sanchez JC, Vuadens F, Scherl A, Schifferli JA, Hochstrasser DF, et al. IgM are associated to Sp alpha (CD5 antigen-like) Electrophoresis. 2002;23:1203–1206. doi: 10.1002/1522-2683(200204)23:7/8<1203::AID-ELPS1203>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Kai T, Yamazaki T, Arai S, Miyazaki T. Stabilization and augmentation of circulating AIM in mice by synthesized IgM-Fc. PLoS One. 2014;9:e97037. doi: 10.1371/journal.pone.0097037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erlandsson L, Andersson K, Sigvardsson M, Lycke N, Leanderson T. Mice with an inactivated joining chain locus have perturbed IgM secretion. Eur J Immunol. 1998;28:2355–2365. doi: 10.1002/(SICI)1521-4141(199808)28:08<2355::AID-IMMU2355>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- 15.Yamazaki S, Sugisawa R, Hiramoto E, Takai R, Matsumoto A, Senda Y, et al. A proteolytic modification of AIM promotes its renal excretion. Sci Rep. 2016;6:38762. doi: 10.1038/srep38762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurokawa J, Arai S, Nakashima K, Nishijima A, Miyake K, Ose R, et al. AIM is endocytosed into adipocytes and decreases lipid droplets via inhibition of fatty acid synthase activity. Cell Metab. 2010;11:479–492. doi: 10.1016/j.cmet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Iwamura Y, Mori M, Nakashima K, Mikami T, Murayama K, Arai S, et al. Apoptosis inhibitor of macrophage (AIM) diminishes lipid droplet-coating proteins leading to lipolysis in adipocytes. Biochem Biophys Res Commun. 2012;422:476–481. doi: 10.1016/j.bbrc.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Maehara N, Arai S, Mori M, Iwamura Y, Kurokawa J, Kai T, et al. Circulating AIM prevents hepatocellular carcinoma through complement activation. Cell Rep. 2014;9:61–74. doi: 10.1016/j.celrep.2014.08.058. [DOI] [PubMed] [Google Scholar]
- 19.Polo S, Pece S, Di Fiore PP. Endocytosis and cancer. Curr Opin Cell Biol. 2004;16:156–161. doi: 10.1016/j.ceb.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–850. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- 21.Ozawa T, Maehara N, Kai T, Arai S, Miyazaki T. Dietary fructose-induced hepatocellular carcinoma development manifested in mice lacking apoptosis inhibitor of macrophage (AIM) Genes Cells. 2016;21:1320–1332. doi: 10.1111/gtc.12446. [DOI] [PubMed] [Google Scholar]
- 22.Vera J, Fenutría R, Cañadas O, Figueras M, Mota R, Sarrias MR, et al. The CD5 ectodomain interacts with conserved fungal cell wall components and protects from zymosan-induced septic shock-like syndrome. Proc Natl Acad Sci USA. 2009;106:1506–1511. doi: 10.1073/pnas.0805846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazaki T, Arai S. A defense system against multiple diseases via biological garbage clearance mediated by soluble scavenger proteins. Inflamm Regen. 2015;35:203–209. doi: 10.2492/inflammregen.35.203. [DOI] [Google Scholar]
- 24.Henson PM, Vandivier RW, Douglas IS. Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proc Am Thorac Soc. 2006;3:713–717. doi: 10.1513/pats.200605-104SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandahl M, Hunter DM, Strunk KE, Earp HS, Cook RS. Epithelial cell-directed efferocytosis in the post-partum mammary gland is necessary for tissue homeostasis and future lactation. BMC Dev Biol. 2010;10:122. doi: 10.1186/1471-213X-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, Borish L, et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2012;493:547–551. doi: 10.1038/nature11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res. 2013;113:1004–1012. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mochizuki A, Pace A, Rockwell CE, Roth KJ, Chow A, O'Brien KM, et al. Hepatic stellate cells orchestrate clearance of necrotic cells in a hypoxia-inducible factor-1α-dependent manner by modulating macrophage phenotype in mice. J Immunol. 2014;192:3847–3857. doi: 10.4049/jimmunol.1303195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009;76:1089–1097. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 30.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bedford M, Farmer C, Levin A, Ali T, Stevens P. Acute kidney injury and CKD: chicken or egg? Am J Kidney Dis. 2012;59:485–491. doi: 10.1053/j.ajkd.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest. 2014;124:2355–2363. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA. 2014;111:1527–1532. doi: 10.1073/pnas.1310653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arai S, Kitada K, Yamazaki T, Takai R, Zhang X, Tsugawa Y, et al. Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat Med. 2016;22:183–193. doi: 10.1038/nm.4012. [DOI] [PubMed] [Google Scholar]
- 38.The American Veterinary Medical Foundation. Total Pet Ownership and Pet Populations. US Pet Ownership and Demographics Sourcebook, Section 1, 2012, American Veterinary Medical Association: Schaumburg, IL, USA..
- 39.The European Pet Food Industry.Facts and Figures, 2014. Available at: http://www.fediaf.org/fileadmin/user_upload/Secretariat/facts_and_figures_2014.pdf.
- 40.Lulich JP, O’Brien TD, Osborne CA, Polzin DJ. Feline renal failure: questions, answers, questions. Compend Contin Educ Pract Vet. 1992;14:127–152. [Google Scholar]
- 41.Brown SA. Linking treatment to staging in chronic kidney disease. Consultations in Feline Internal Medicine (edit. By August JR) 2010;6:475–482. [Google Scholar]
- 42.White JD, Norris JM, Baral RM, Malik R. Naturally-occurring chronic renal disease in Australian cats: a prospective study of 184 cases. Aust Vet J. 2006;84:188–194. doi: 10.1111/j.1751-0813.2006.tb12796.x. [DOI] [PubMed] [Google Scholar]
- 43.White JD, Malik R, Norris JM. Feline chronic kidney disease: can we move from treatment to prevention? Vet J. 2011;190:317–322. doi: 10.1016/j.tvjl.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Sugisawa S, Hiramoto E, Matsuoka M, Iwai S, Takai R, Yamazaki T, et al. Impact of feline AIM on the susceptibility of cats to renal disease. Sci Rep. 2016;6:35251. doi: 10.1038/srep35251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmiedt CW, Brainard BM, Hinson W, Brown SA, Brown CA. Unilateral renal ischemia as a model of acute kidney injury and renal fibrosis in cats. Vet Pathol. 2016;53:87–101. doi: 10.1177/0300985815600500. [DOI] [PubMed] [Google Scholar]
- 46.Gadjeva MG, Rouseva MM, Zlatarova AS, Reid KB, Kishore U, Kojouharova MS. Interaction of human C1q with IgG and IgM: revisited. Biochemistry. 2008;47:13093–13102. doi: 10.1021/bi801131h. [DOI] [PubMed] [Google Scholar]
- 47.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 48.Hardy RR, Hayakawa K. CD5 B cells, a fetal B cell lineage. Adv Immunol. 1994;55:297–339. doi: 10.1016/S0065-2776(08)60512-X. [DOI] [PubMed] [Google Scholar]
- 49.Boes M. Role of natural and immune IgM antibodies in immune responses. Mol Immunol. 2000;37:1141–1149. doi: 10.1016/S0161-5890(01)00025-6. [DOI] [PubMed] [Google Scholar]
- 50.Pepys MB. Role of complement in the induction of immunological responses. Transplant Rev. 1976;32:93–120. doi: 10.1111/j.1600-065x.1976.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 51.Ahearn JM, Fearon DT. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21) Adv Immunol. 1989;46:183–219. doi: 10.1016/S0065-2776(08)60654-9. [DOI] [PubMed] [Google Scholar]
- 52.Heyman B. The immune complex: possible ways of regulating the antibody response. Immunol Today. 1990;11:310–313. doi: 10.1016/0167-5699(90)90126-T. [DOI] [PubMed] [Google Scholar]
- 53.Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- 54.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Honda S, Kurita N, Miyamoto A, Cho Y, Usui K, Takeshita K, et al. Enhanced humoral immune responses against T-independent antigens in Fc alpha/muR-deficient mice. Proc Natl Acad Sci USA. 2009;106:11230–11235. doi: 10.1073/pnas.0809917106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T, et al. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat Immunol. 2000;1:441–446. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 57.Kojima A, Iwata K, Seya T, Matsumoto M, Ariga H, Atkinson JP, et al. Membrane cofactor protein (CD46) protects cells predominantly from alternative complement pathway-mediated C3-fragment deposition and cytolysis. J Immunol. 1993;151:1519–1527. [PubMed] [Google Scholar]
- 58.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miwa T, Song WC. Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int Immunopharmacol. 2001;1:445–459. doi: 10.1016/S1567-5769(00)00043-6. [DOI] [PubMed] [Google Scholar]
- 60.Rosenbloom AL. Obesity, insulin resistance, beta-cell autoimmunity, and the changing clinical epidemiology of childhood diabetes. Diabetes Care. 2003;26:2954–2956. doi: 10.2337/diacare.26.10.2954. [DOI] [PubMed] [Google Scholar]
- 61.Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy. 2007;62:1205–1213. doi: 10.1111/j.1398-9995.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 62.Cambuli VM, Incani M, Cossu E, Congiu T, Scano F, Pilia S, et al. Prevalence of type 1 diabetes autoantibodies (GADA, IA2, and IAA) in overweight and obese children. Diabetes Care. 2010;33:820–822. doi: 10.2337/dc09-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marzullo P, Minocci A, Tagliaferri MA, Guzzaloni G, Di Blasio A, De Medici C, et al. Investigations of thyroid hormones and antibodies in obesity: leptin levels are associated with thyroid autoimmunity independent of bioanthropometric, hormonal, and weight-related determinants. J Clin Endocrinol Metab. 2010;95:3965–3972. doi: 10.1210/jc.2009-2798. [DOI] [PubMed] [Google Scholar]
- 64.Badaru A, Pihoker C. Type 2 diabetes in childhood: clinical characteristics and role of β-cell autoimmunity. Curr Diab Rep. 2012;12:75–81. doi: 10.1007/s11892-011-0247-2. [DOI] [PubMed] [Google Scholar]
- 65.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;217:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacLennan IC, Gray D, Kumararatne DS, Bazin H. The lymphocytes of splenic marginal zones: a distinct B-cell lineage. Immunol Today. 1982;3:305–307. doi: 10.1016/0167-5699(82)90032-9. [DOI] [PubMed] [Google Scholar]
- 67.Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunol Rev. 2004;197:192–205. doi: 10.1111/j.0105-2896.2004.0112.x. [DOI] [PubMed] [Google Scholar]
- 68.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu Rev Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 69.Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- 70.Meyer-Bahlburg A, Bandaranayake AD, Andrews SF, Rawlings DJ. Reduced c-myc expression levels limit follicular mature B cell cycling in response to TLR signals. J Immunol. 2009;182:4065–4075. doi: 10.4049/jimmunol.0802961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li QZ, Xie C, Wu T, Mackay M, Aranow C, Putterman C, et al. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J Clin Invest. 2005;115:3428–3439. doi: 10.1172/JCI23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol. 2007;147:60–70. doi: 10.1111/j.1365-2249.2006.03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li QZ, Zhou J, Lian Y, Zhang B, Branch VK, Carr-Johnson F, et al. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol. 2010;159:281–291. doi: 10.1111/j.1365-2249.2009.04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Underhill GH, Minges-Wols HA, Fornek JL, Witte PL, Kansas GS. IgG plasma cells display a unique spectrum of leukocyte adhesion and homing molecules. Blood. 2002;99:2905–2912. doi: 10.1182/blood.V99.8.2905. [DOI] [PubMed] [Google Scholar]
- 75.Halliwell RE. Autoimmune diseases in domestic animals. J Am Vet Med Assoc. 1982;181:1088–1096. [PubMed] [Google Scholar]
- 76.Martínez VG, Moestrup SK, Holmskov U, Mollenhauer J, Lozano F. The conserved scavenger receptor cysteine-rich superfamily in therapy and diagnosis. Pharmacol Rev. 2011;63:967–1000. doi: 10.1124/pr.111.004523. [DOI] [PubMed] [Google Scholar]
- 77.Sanjurjo L, Aran G, Roher N, Valledor AF, Sarrias MR. AIM/CD5L: a key protein in the control of immune homeostasis and inflammatory disease. J Leukoc Biol. 2015;98:173–184. doi: 10.1189/jlb.3RU0215-074R. [DOI] [PubMed] [Google Scholar]
- 78.Miyazaki T, Kurokawa J, Arai S. AIMing at metabolic syndrome—towards the development of novel therapies for metabolic diseases via apoptosis inhibitor of macrophage (AIM) Circ J. 2011;75:2522–2531. doi: 10.1253/circj.CJ-11-0891. [DOI] [PubMed] [Google Scholar]
- 79.Shuai Z, Wang J, Badamagunta M, Choi J, Yang G, Zhang W, et al. The fingerprint of antimitochondrial antibodies and the etiology of primary biliary cholangitis. Hepatology. 2017;65:1670–1682. doi: 10.1002/hep.29059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hisamoto S, Shimoda S, Harada K, Iwasaka S, Onohara S, Chong Y, et al. Hydrophobic bile acids suppress expression of AE2 in biliary epithelial cells and induce bile duct inflammation in primary biliary cholangitis. J Autoimmun. 2016;75:150–160. doi: 10.1016/j.jaut.2016.08.006. [DOI] [PubMed] [Google Scholar]


