Immune disorders are characterized by excessive immune cell activation and aberrant inflammatory responses. Cell signals have been noted to regulate immune cell functions and to contribute to immune diseases, for example, upon extracellular stimulation, cell receptor-mediated signals, including mammalian target of rapamycin/signal transducer and activator of transcription factor 31,2 and mitogen-activated protein kinase/nuclear factor-κB,3,4 induce the expression of proinflammatory cytokines and molecules, thereby resulting in immune cell activation and sustained inflammation, as seen in inflammatory bowel diseases and other immune disorders.
Beyond the active transcription of proinflammatory cytokines, posttranslational modifications have also been associated with inflammatory disorders.5 Posttranslational modification by the inflammasome promotes the maturation and activation of proinflammatory cytokines, interleukin (IL)-1β and IL-18 in particular, leading to the exacerbation of immune diseases.5 Recently, we noted that the inflammasome potentially mediates extracellular ATP/P2X7 receptor-driven dextran sulfate sodium (DSS) colitis,6 indicating the pivotal roles of the inflammasome in the regulation of immune responses in colitis.
Neddylation, another posttranslational modification, is mediated by the conjugation of neural-precursor-cell-expressed developmentally downregulated 8 (NEDD8) to the target proteins.7 NEDD8 is initially activated by NEDD8-activating enzyme and is subsequently transferred to the E2 NEDD8-conjugating enzyme. This enzyme then conjugates NEDD8 to the substrate proteins, inclusive of Cullin-1.7 Consequently, NEDD8 regulates conformational changes, by which it dominates the functions of the target proteins.7 Intriguingly, neddylation has been reported to interact with and subsequently to regulate inflammasome-dependent caspase-1 activation,8 suggestive of the putative roles of neddylation in the modulation of posttranslational gene expression. Meanwhile, the neddylation process is noted to regulate multiple immune cell functions,9,10 whereas its roles in the development of immune diseases remain to be elucidated.
To study the biofunctions of neddylation in immune disorders, we induced a DSS colitis model in BALB/c mice on day 1, according to the established protocol, with minor modifications,6 that was approved by the Institutional Animal Care Committee at the hospital. In parallel, the mice were treated intraperitoneally with MLN4924, a first-in-class NEDD8-activating enzyme inhibitor, starting on day 3. On day 8, we determined the neddylation levels in the colon tissues of the mice by western blotting and noted that, in the neddylated fraction, Cullin-1 levels were induced in the colonic mucosa in mice with DSS colitis (Figure 1a). Conversely, treatment with MLN4924 inhibited neddylation in the colon tissues of mice with colitis. The data indicate that neddylation occurs in the inflamed tissues of mice with DSS colitis and may be involved in the development of colitis.
Figure 1.
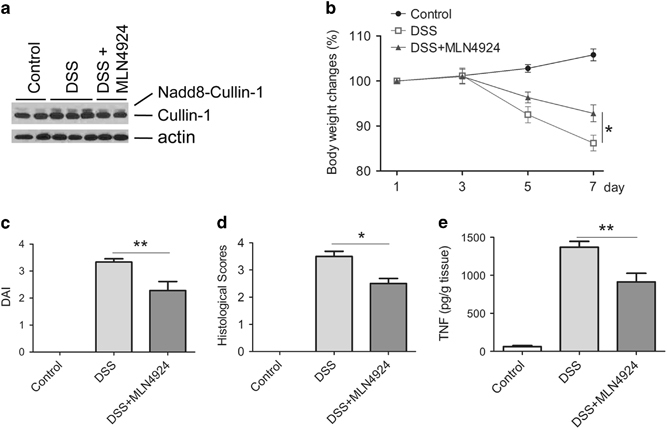
Inhibition of neddylation protects mice against dextran sulfate sodium (DSS) colitis. BALB/c mice were fed distilled water containing 5% DSS to induce a colitis model starting on day 1 of the experiment,6 and MLN4924 (15 mg/kg body weight) (DSS+MLN4924) or vehicle (DSS) was administered intraperitoneally to the mice daily from days 3 to 7. Control mice received distilled water only. (a) Proteins were extracted from colon tissues on day 8, and neddylation levels in the colon tissues of the mice were determined by western blotting. (b and c) Body weight changes and disease activity index (DAI) scores were recorded (n=9).6 (d) Hematoxylin and eosin staining was performed, and the histological scores in the colon tissues were graded on day 8 (n=9).6 (e) Tumor necrosis factor (TNF) levels in colonic homogenates of three groups of mice were determined by enzyme-linked immunosorbent assay (n=9). The data are expressed as the means±s.e.m. Significance was determined by a one-way analysis of variance using Tukey’s post hoc test (*P<0.05, **P<0.01).
Next, to further study the impacts of neddylation inhibition on DSS-induced colitis in mice, we determined multiple parameters of DSS colitis with or without MLN4924 treatment. DSS colitis in the mice was characterized by body weight loss, colitis symptoms, histological tissue damage and elevated proinflammatory cytokine production levels (Figures 1b–e). Nevertheless, the mice with MLN4924 treatment regained their body weight (Figure 1b) and had lower disease activity index scores (Figure 1c) and histological scores (Figure 1d) and reduced proinflammatory cytokine production levels (Figure 1e) in their colon tissues. These data indicate that inhibition of neddylation by MLN4924 significantly ameliorates DSS-induced colitis.
In summary, neddylation may have putative roles in regulating immune responses and may contribute to the pathogenesis of inflammatory diseases, including colitis. The regulation of posttranslational modifications, including inflammasomes and neddylation, represents a new strategy for human immune disease treatment.
Acknowledgements
This work was supported by the National Natural Science Foundation of China, Nos. 81770555, 81470828 and 81270472; the Principle Investigator Program of Jiangxi Province and the Natural Science Foundation of Jiangxi Province, No. 20142BAB205048 (all to A-PB).
Author contributions
A-PB designed the study; all authors conducted the experiments and collected the data; A-PB, X-DZ and YG performed data analysis and wrote and finalized the manuscript. All authors approved the final version for publication.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ratsimandresy RA, Indramohan M, Dorfleutner A, Stehlik C. The AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL-18/IL-22/STAT3 pathway. Cell Mol Immunol. 2017;14:127–142. doi: 10.1038/cmi.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai A, Moss A, Kokkotou E, Usheva A, Sun X, Cheifetz A, et al. CD39 and CD161 modulate Th17 responses in Crohn's disease. J Immunol. 2014;193:3366–3377. doi: 10.4049/jimmunol.1400346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai A, Kokkotou E, Zheng Y, Robson SC. Role of acid sphingomyelinase bioactivity in human CD4+ T-cell activation and immune responses. Cell Death Dis. 2015;6:e1828. doi: 10.1038/cddis.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai A, Moss A, Rothweiler S, Longhi MS, Wu Y, Junger WG, et al. NADH oxidase-dependent CD39 expression by CD8(+) T cells modulates interferon gamma responses via generation of adenosine. Nat Commun. 2015;6:8819. doi: 10.1038/ncomms9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Liu Z, Xiao TS. Post-translational regulation of inflammasomes. Cell Mol Immunol. 2017;14:65–79. doi: 10.1038/cmi.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan P, Liu X, Xiong Y, Ren Y, Chen J, Lu N, et al. Extracellular ATP mediates inflammatory responses in colitis via P2X7 receptor signaling. Sci Rep. 2016;6:19108. doi: 10.1038/srep19108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enchev RI, Schulman BA, Peter M. Protein neddylation: beyond cullin-RING ligases. Nat Rev Mol Cell Biol. 2015;16:30–44. doi: 10.1038/nrm3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segovia JA, Tsai SY, Chang TH, Shil NK, Weintraub ST, Short JD, et al. Nedd8 regulates inflammasome-dependent caspase-1 activation. Mol Cell Biol. 2015;35:582–597. doi: 10.1128/MCB.00775-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathewson N, Toubai T, Kapeles S, Sun Y, Oravecz-Wilson K, Tamaki H, et al. Neddylation plays an important role in the regulation of murine and human dendritic cell function. Blood. 2013;122:2062–2073. doi: 10.1182/blood-2013-02-486373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin HS, Liao L, Park Y, Liu YC. Neddylation pathway regulates T-cell function by targeting an adaptor protein Shc and a protein kinase Erk signaling. Proc Natl Acad Sci USA. 2013;110:624–629. doi: 10.1073/pnas.1213819110. [DOI] [PMC free article] [PubMed] [Google Scholar]


