As the interface between the gut lumen and the body, the intestinal epithelial layer is continuously exposed to large numbers of microbial and dietary antigens. Consequently, intestinal homeostasis requires sophisticated immune networks that effectively clear pathogenic microbes while maintaining tolerance to harmless antigens. To achieve this balance, intraepithelial lymphocytes (IELs) that are scattered among intestinal epithelial cells carry out numerous important functions.1
Most IELs are a heterogeneous population of T lymphocytes, including both TCRαβ+ and TCRγδ+ IELs. Unlike T cells in other tissues, most TCRαβ+ IELs in the small intestine belong to the CD8+ subset.2 Moreover, a sizeable fraction of these TCRαβ+IELs are CD4−CD8α+CD8β− cells,3 which are referred to as CD8αα IELs based on their expression of the CD8αα homodimer.
CD8αα IELs are distinct from conventional T cells in several aspects. For example, CD8αα IELs exhibit a self-reactive TCR repertoire, lack expression of some surface markers that are typically expressed by conventional T cells, and express natural killer (NK) cell receptors such as NK1.1 and Ly49 molecules.1 Moreover, similar to a recently identified ILC1 subset, CD8αα IELs require the transcription factor T-bet for development,4,5 and constitutively express CD69, a molecule associated with cell activation and tissue residency.6
Although the phenotype and function of CD8αα IELs have been well characterized, the origin and development of CD8αα IELs have not been fully investigated. Early studies suggest that CD8αα IELs may develop locally in the intestine;1,7 however, compelling evidence from recent studies supports a thymic origin for CD8αα IELs.8,9,10 It has been increasingly appreciated that CD8αα IELs develop from CD4 and CD8 double-negative (DN) thymocytes through strong recognition of thymic self-ligands.8,9,10 More recently, a study published in Nature Immunology by Ruscher et al.11 described two distinct populations of CD8αα IEL precursors (IELps) in the thymus.
As previous studies have found that IELps are concentrated in thymic TCRβ+CD5+CD4−CD8− cells,8,9 Ruscher et al.11 initially excluded invariant NKT cells using CD1d tetramers and CD25+ regulatory T cells, and then gated TCRβ+CD5+CD4−CD8− cells for further analysis.11 The authors showed that these thymic DN T cells partially express the mature markers CD122 and the MHC class I molecule H-2Kb, and form two distinct populations, which are termed ‘type A’ IELps and ‘type B’ IELps, respectively, based on T-bet and PD-1 expression. Type A IELps are PD-1 positive but do not express T-bet.11 By contrast, type B IELps are PD-1−T-bet+ and express NK1.1 and IFN-γ, which are not expressed by type A IELps.11 Using spanning-tree analysis, the authors found that type A IELps are located in the thymic CD4 single-positive (SP) cell branch and that type B IELps emerged near the CD4 and CD8 double-positive (DP) progenitor group. Moreover, only type B IELps express the activation and memory marker CD44 and the chemokine receptor CXCR3, while both cell types are negative for CCR7.11 Additionally, type A IELps are α4β7+ and type B IELps are CD103+, suggesting that they have the potential to migrate to the gut.
Subsequently, Ruscher et al. focused on the development potential of these IELps. After adoptive transfer of type A or type B IELps into Rag2−/− mice, both IELp populations can give rise almost exclusively to CD8αα IELs in the intestine. To compare their maturation state, Ruscher et al.11 used Rag2GFP mice and found that, in contrast to the lack of Rag2 expression in type B IELps, type A IELps and CD4SP thymocytes were Rag2 positive, suggesting that type A IELps are ‘younger’ than type B IELps. Consistent with this hypothesis, the number of type A IELps decreased with age, while that of type B IELps increased.11 These findings raised the question of whether type B IELps develop from type A IELps. To this end, the authors performed ‘time-stamp’ experiments using Cd4creERT2× Rosa26floxedSTOPTdT mice, in which cells expressing CD4 and their progeny could be labeled with tdTomato at the time of exposure to tamoxifen. Surprisingly, while the frequency of labeled type A IELps peaked on day 5 and dramatically decreased 10 days after tamoxifen injection, type B IELps were not labeled in this period.11 Therefore, it is likely that type A IELps exit directly from the thymus without differentiation into type B IELps. Moreover, the differences in MHC restriction and TCR use between type A IELps and type B IELps further suggests that these IELps may represent two developmentally separate subsets.11
Differential expression of homing molecules between these IELp populations led to the investigation of their location within the thymus and migration properties. Using immunofluorescence-based quantitative histocytometry, Ruscher et al. found that 70% of type A IELps are located in the cortex, while 80% of type B IELps were found in the medulla (Figure 1). Further analysis shows that the G-protein-coupled receptor S1PR1 and its related transcription factor KLF2, which are required for lymphocyte egress from tissues,12,13 are preferentially expressed by type A IELps. Consistent with this finding, type A IELps rather than type B IELps are the main population migrating from the thymus to the periphery, which is supported by the enrichment of type A IELps among the recent thymic emigrants (RTEs), and the emigration of type A IELps is dependent on S1PR1.11
Figure 1.
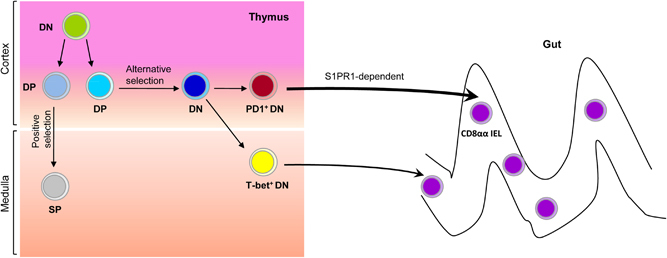
The origin and development of intestinal CD8αα IELs. In the thymus, CD4 and CD8 double-negative (DN) thymocytes give rise to CD4 and CD8 double-positive (DP) thymocytes. Through positive selection, DP thymocytes further differentiate into CD8αβ+or CD4+ single-positive (SP) cells. DP thymocytes can also undergo alternative selection and downregulate the expression of CD4 and CD8, becoming the precursors of CD8αα IELs. There are two distinct subsets of CD8αα IEL precursors (IELps) in the thymus: one subset is PD1+T-bet− (type A IELps), which localizes to the thymic cortex and efficiently emigrates in an S1PR1-dependent manner, while the other is PD1−T-bet+ (type B IELps), which localizes to the thymic medulla and is less migration-competent.
This new study by Ruscher et al. highlights that the two distinct populations of thymic mature DN T cells have the potential to selectively develop into CD8αα IELs. Despite their similarities in developmental potential, the two subtypes of IELps identified in this study are different in maturation status, thymic location, migration efficiency, and antigen-receptor specificities. However, it remains unclear whether these distinct IELps generate phenotypically and/or functionally different CD8αα IEL subsets. Moreover, while the migration of type A IELps in an S1PR1-dependent manner has been demonstrated, the mechanism underlying type B IELps egress from the thymus has not been investigated. Overall, exploring the origin and characteristics of IELps provides new insights into the heterogeneity of IELs and also helps to understand the functionality of different IEL subsets.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camerini V, Panwala C, Kronenberg M. Regional specialization of the mucosal immune system. Intraepithelial lymphocytes of the large intestine have a different phenotype and function than those of the small intestine. J Immunol. 1993;151:1765–1776. [PubMed] [Google Scholar]
- 3.Sheridan BS, Lefrancois L. Intraepithelial lymphocytes: to serve and protect. Curr Gastroenterol Rep. 2010;12:513–521. doi: 10.1007/s11894-010-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klose CS, Blatz K, d'Hargues Y, Hernandez PP, Kofoed-Nielsen M, Ripka JF, et al. The transcription factor T-bet is induced by IL-15 and thymic agonist selection and controls CD8alphaalpha(+) intraepithelial lymphocyte development. Immunity. 2014;41:230–243. doi: 10.1016/j.immuni.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Reis BS, Hoytema van Konijnenburg DP, Grivennikov SI, Mucida D. Transcription factor T-bet regulates intraepithelial lymphocyte functional maturation. Immunity. 2014;41:244–256. doi: 10.1016/j.immuni.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang HC, Zhou Q, Dragoo J, Klein JR. Most murine CD8+ intestinal intraepithelial lymphocytes are partially but not fully activated T cells. J Immunol. 2002;169:4717–4722. doi: 10.4049/jimmunol.169.9.4717. [DOI] [PubMed] [Google Scholar]
- 7.Rocha B. The extrathymic T-cell differentiation in the murine gut. Immunol Rev. 2007;215:166–177. doi: 10.1111/j.1600-065X.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 8.Gangadharan D, Lambolez F, Attinger A, Wang-Zhu Y, Sullivan BA, Cheroutre H. Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity. 2006;25:631–641. doi: 10.1016/j.immuni.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 9.McDonald BD, Bunker JJ, Ishizuka IE, Jabri B, Bendelac A. Elevated T cell receptor signaling identifies a thymic precursor to the TCRalphabeta(+)CD4(−)CD8beta(−) intraepithelial lymphocyte lineage. Immunity. 2014;41:219–229. doi: 10.1016/j.immuni.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayans S, Stepniak D, Palida S, Larange A, Dreux J, Arlian B, et al. alphabetaT cell receptors expressed by CD4(−)CD8alphabeta(−) intraepithelial T cells drive their fate into a unique lineage with unusual MHC reactivities. Immunity. 2014;41:207–218. doi: 10.1016/j.immuni.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruscher R, Kummer RL, Lee YJ, Jameson SC, Hogquist KA. CD8alphaalpha intraepithelial lymphocytes arise from two main thymic precursors. Nat Immunol. 2017;18:771–779. doi: 10.1038/ni.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 13.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]


