Abstract
Body weight regain often causes failure of obesity therapies while the underlying mechanism remains largely unknown. In this study, we report that immune cells, especially CD4+ T cells, mediate the ‘memory’ of previous obese status. In a weight gain-loss-regain model, we found that C57BL/6J mice with an obesity history showed a much faster rate of body weight regain. This obesity memory could last for at least 2 months after previously obese mice were kept at the same body weight as non-obese mice. Surprisingly, such obesity memory was abrogated by dexamethasone treatment, whereas immunodeficient Rag1−/− and H2A−/− mice failed to establish such memory. Rag1−/− mice repossessed the obesity memory when immune cells or CD4+ T cells isolated from previously obese mice were transferred. Furthermore, depletion of CD4+ T cells led to obesity memory ablation. Taken together, we conclude that CD4+ T cells mediate obesity memory and promote weight regain.
Introduction
Obesity is a major public health problem that is causally associated with many diseases, including type II diabetes, hepatic steatosis, cardiovascular diseases and systemic chronic inflammation.1,2,3,4 Anti-obesity drugs, as well as lifestyle interventions, have been developed for the weight loss of obese patients. Unfortunately, although many of these treatments are effective at the beginning of intervention, it is difficult to maintain a 10% body weight reduction in the first year and most patients typically regain 30–50% of their initial weight during the next 2–3 years.5,6,7 Some patients even obtain a heavier body weight with severe adiposity after treatment.8 Clinical studies have confirmed that weight regain emerges regardless of different therapy types, including lifestyle intervention, pharmacotherapy and bariatric surgeries.9,10,11 Moreover, body weight regain can occur not only after therapy termination but also during the treatment.12 It is often accompanied by a recurrence of obesity-related comorbidities.13 Overall, there seems to be a strong tendency for individuals who experienced obesity to regain lost weight and fat.14
The underlying mechanism for weight regain is largely unknown. A commonly assumed perspective is that weight regain is caused by metabolic adaptions, such as increased appetite and reduced energy expenditure.15 Previous studies have demonstrated that weight loss leads to a significant increase in orexigenic hormones, including ghrelin and glucagon-like peptide-1 (GLP-1),16,17,18 and a decrease in anorexigenic hormones, including peptide YY (PYY), cholecystokinin (CCK) and leptin.19 Weight loss also reduces total energy expenditure, thus favoring weight regain.20 However, weight regain was correlated with higher leptin and lower ghrelin levels in human patients.21 Appetite sensation and resting metabolic rate also remained unaffected after weight reduction.22
Chronic systemic inflammation is associated with obesity onset. Immune cells, especially T lymphocytes, have a crucial role in obesity-related inflammation.23 Activated CD4+ T cells were shown to accumulate in adipose tissue before inflammatory macrophage infiltration, which contributed to inflammatory cell activation.24 Recent studies have also indicated that the IL-33/ST2 axis was crucial for the proliferation and differentiation of visceral adipose tissue-resident regulatory T cells (Tregs, a subset of CD4+ T cells), whereas the increased Tregs could restrain the obesity-induced inflammation in adipose tissue and thereby improve obesity-associated metabolic parameters.25,26
Intriguingly, accumulating evidence has also highlighted the potential roles of chronic systemic inflammation in weight regain. Increases in leukocytes and neutrophils in circulation and HAM56 (human alveolar macrophage) in adipose tissue were utilized to predict unsuccessful weight reduction and rapid weight regain.27 Weight cycling induced T lymphocyte accumulation in epididymal adipose tissue.28 CD4+ effector T cell-mediated macrophages were shown to be recruited and retained in the adipose tissues of previously obese mice, despite normalized body weight.29,30 Meanwhile, when subjected to short-term fasting followed by refeeding or long-term weight loss intervention, inflammatory mediators including tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) were elevated in epididymal adipose tissue.15,31 These results suggest a potential link between chronic systemic inflammation and weight regain. However, the underlying mechanism and biological significance remain unclear.
In this study, we provide evidence that mice with obesity experience displayed much faster body weight regain, which was not due to physiological adaptions. This obesity memory had a long-lasting effect, and it was mainly mediated by CD4+ T cells from previously obese mice.
Materials and methods
Animals and diets
Mice were provided by the Model Animal Research Center (MARC) of Nanjing University (Nanjing, Jiangsu Province, China) and housed in a specific pathogen-free (SPF) and Association for Assessment and Accreditation of Laboratory Animal Care International (AAALACI) accredited animal facility. All experiments were performed according to the animal protocol approved by the MARC Animal Care and Use Committee. For most studies, 8-week-old mice were separated into two groups, an obesity history group (MOH) and a control group (CTR). The MOH group was fed a high fat diet (HFD, 60% energy from fat, Research Diets, New Brunswick, NJ, USA) for 1 month (weight gain period). Then, the MOH mice underwent calorie restriction to lose weight. It took about 3 weeks for the MOH mice to reach the same body weight as the even-aged CTR group (weight loss period). During the weight gain and loss period, the CTR group was fed a regular chow diet (RD, 6% energy from fat, Xietong Company, Nanjing, China). For weight maintenance, the MOH mice were kept at the same body weight as the CTR cohort after weight loss. Afterwards, both the MOH and the CTR groups were fed a HFD or RD ad libitum to induce weight regain.
Body weight and body composition measurement
Body weight was measured once or twice per week at 1600 hours. The body weight increment ratio was calculated by dividing the weight gain during a certain period (weight regain or HFD feeding periods) by the body weight at the beginning of this period. For body composition measurements, mice were anesthetized with 2.5% avertin (0.5 mg/10 g body weight) after overnight fasting (16 h). Lean mass, fat mass and bone mineral density (BMD) values were assessed with a dual-energy X-ray absorptiometry (DEXA) system (PIXImus 2, GE lunar, Chicago, IL, USA). Meanwhile, mouth to anus length was measured. For tissue weight measurements, mice were killed and tissues were isolated and weighed immediately.
Blood glucose, GTT and ITT
Blood was collected from mouse tail tips. The fasting (overnight) blood glucose and postprandial blood glucose levels were tested using a glucocard blood glucose test meter (GT-1640, ARKRAY, Kyoto, Japan). Glucose tolerance tests (GTT) and insulin tolerance tests (ITT) were carried out after fasting for 8- and 6-hour periods, from 0800 to 1600 hours or 1400 hours, respectively. Mice were intraperitoneally injected with 2 g/kg body weight of d-(+)-glucose (G6152, Sigma, Virginia Beach, VA, USA) for the GTT and with 0.5 IU/kg body weight of insulin (Novo Nordisk, Tianjing, China) for the ITT. Blood glucose levels were detected at 0, 15, 30, 45, 60, 90 and 120 min after glucose/insulin injection.
Blood cell counting and blood biochemistry
Anticoagulant-treated whole blood was collected from the posterior orbital venous plexus of the mice with a 15 g/l EDTA·2 K solution. Blood cell counting was performed immediately using a Hemavet 950FS Hematology Analyzer (Drew Scientific, Miami Lakes, FL, USA). For blood biochemical analysis, blood was collected from the venous sinus of the optical orbit and clotted by standing on ice for 2 h. Sera were isolated by centrifugation at 3000g at 4 °C for 20 min and stored at −20 and −80 °C for short- and long-term storage, respectively. Cholesterol (CHOL), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C) and low-density lipoprotein-cholesterol (LDL-C) in the sera were detected with a 7600 Clinical Analyzer (Hitachi High-Technologies, Tokyo, Japan).
Comprehensive Lab Animal Monitoring System
Metabolic parameters of the mice were acquired with a computer-controlled open-circuit monitoring system (Oxymax indirect calorimetry system, Oxymax/CLAMS, Columbus Instruments, Columbus, OH, USA). The mice were housed individually in metabolic chambers with free access to water and a group-associated diet for at least 1 day of acclimation and 3 days of monitoring. Parameters including O2 consumption, CO2 production and daily food intake were detected and locomotor activities were measured on the X axis through infrared beams. Respiratory exchange ratio (RER) and heat production levels were calculated from the O2 consumption, CO2 production and daily food intake values.
Gene expression analysis
Total RNA was extracted from inguinal and epididymal adipose tissue and used for quantitative real-time PCR (qPCR) and microarray assays (Agilent Technologies, Capitalbio, Beijing, China). Data were analyzed using the chipster platform and normalized with the quantile normalization method. The accession number for the microarray data reported is GEO: GSE76944. The primers used for qPCR are listed in Supplementary Table S1.
Immunosuppressant treatment
Before weight loss, obese mice were intraperitoneally injected with 5 mg dexamethasone sodium phosphate/kg body weight (Longship, Pizhou, China) per day for three consecutive days. Saline was injected into CTR mice as a control.
Flow cytometry
Epididymal adipose tissue was isolated and digested in collagenase II (2 mg/ml, Worthington Biochemical, Lakewood, NJ, USA) for 30 min in a 37 °C water bath. After the adipose fraction and erythrocytes were removed, the cells were incubated with antibodies (0.2 mg/ml for each) at 4 °C for 30 min. CD4-Cy5.5, CD8a-APC, TCRβ-PE, TCRβ-APC, IFNγ-FITC and IL-17a-PE antibodies (Biolegend, San Diego, CA, USA) and CD4-FITC and CD8a-PE (Ebiosciences, San Diego, CA, USA) were used. To detect the T helper 1 (Th1) and T helper 17 (Th17) cells, associated cells were pre-stimulated with Phorbol-12-myristate-13-acetate (PMA) and ionomycine at 37 °C for 30 min and then incubated with the corresponding antibodies. To detect the CD4+ T cell depletion efficiency, erythrocyte-removed blood cells were incubated with CD4-FITC and CD8a-PE antibodies. The stained cells were analyzed by flow cytometry (FACS Calibur, Becton Dickinson, Franklin Lakes, NJ, USA), and the data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
CD4+ T cell depletion
After weight loss, mice were tail vein injected with 100 μg anti-CD4 antibody (GK 1.5, Ebioscience) once (dMOH, CD4+ T cell-deficient MOH). IgG2bκ isotype antibody (eB149/10H5, Ebioscience) was used as a negative control (sMOH, CD4+ T cell-sufficient MOH). CTR mice were injected with saline. Two weeks after injection, the mice were fed HFD. The depletion efficiency was measured by flow cytometry.
Adoptive transfer
EGFP transgenic mice (EGFP, C57BL/6J background) were used as donors to examine the transfer efficiency, as their splenocytes are EGFP-positive. The splenocytes of C57BL/6J mice were harvested before weight regain or after weight maintenance. A total of 2 × 107 cells were transferred into each recipient recombination-activating gene 1 knockout mouse (Rag1−/−) via the tail vein. After 2 weeks, the recipient mice were fed HFD to induce weight regain.
Statistics
Data were statistically analyzed using unpaired two-tailed Student’s t-tests or two-way ANOVA followed by the Bonferroni post hoc test. All data are expressed as the mean±s.d. A P-value <0.05 was considered statistically significant.
Results
Mice with an obesity history display faster weight regain after weight loss
We designed a weight gain-loss-regain model with C57BL/6J mice (Figure 1a). Mice with an obesity history (MOH) were fed a HFD for 1 month and then subjected to weight loss for about 3 weeks, whereas control mice (CTR) were fed a RD at the same time. Then, both groups were fed with the HFD to induce diet-induced-obesity (DIO). During this regain period, the MOH mice gained significantly more weight than the CTR cohort (Figures 1b and e), indicating a rapid obesity relapse. No difference was detected between the two cohorts in body length, BMD, skeletal content and lean mass (Supplementary Figures 1a–c). The extra weight gain in the MOH mice was attributed to greater fat accumulation (Supplementary Figure 1c and d). The MOH mice consumed more oxygen during the daytime and generated a normal amount of carbon dioxide (Supplementary Figures 1e and f). The RER shift from 0.8 to 0.7 suggested that MOH mice were more inclined to acquire energy from fat oxidation after weight regain (Supplementary Figure 1g). MOH mice yielded more heat than CTR mice, whereas both cohorts displayed similar levels of physical activity and food intake (Supplementary Figures 1h–j). In addition, MOH mice showed increased fasting and postprandial blood glucose levels and decreased insulin sensitivity (Supplementary Figures 1k and l). Notably, MOH mice still displayed a higher weight gain rate even when the HFD was replaced with the RD during the weight regain period (Figures 1c and f). These results suggest that after experiencing obesity, the mice retained a strong obesogenic tendency, which was independent of food type.
Figure 1.
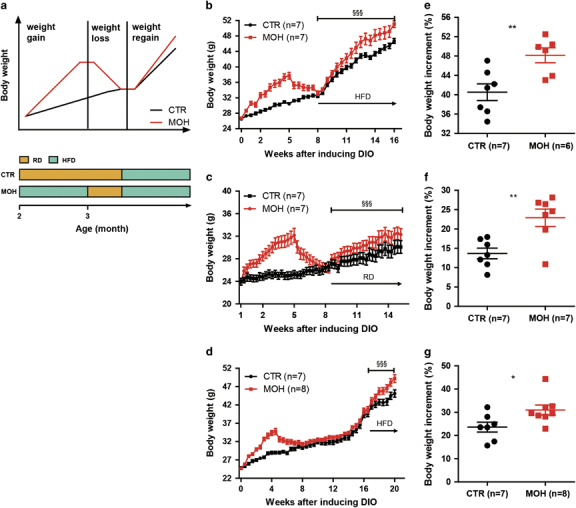
Weight gain-loss-regain model and long-term obesity memory. The DIO model in C57BL/6J mice was established as per the simulative graph and timeline (a). Body weight curves (b–d) and body weight increment ratios during the weight regain period (e–g) were recorded to display obesity memory. Weight regain was induced with a HFD (b, e and d, g) and a RD (d, f). The data are shown as means±s.d. *P<0.05 and **P<0.01, unpaired Student’s t-test. §§§ P<0.001, two-way ANOVA followed by the Bonferroni post hoc test. CTR, control group; DIO, diet-induced-obesity; HFD, high fat diet; MOH, obesity history group; RD, regular diet.
To determine how long this obesogenic tendency persists, MOH mice were given a mild food restriction to maintain body weight at a similar level to that of the RD-fed CTR mice for 2 months. Then, both cohorts were subjected to HFD feeding. We observed a similar phenomenon as before, with a faster weight regain in the MOH mice (Figures 1d and g). This result indicated that the obesogenic tendency in previously obese mice was a long-lasting ‘obesity memory’.
Obesity memory is independent of hyperphagia, reduced thermogenesis or decreased metabolic rate
Previous studies have suggested that increased appetite, reduced thermogenesis and decreased metabolic rate accompanying DIO contributed to weight regain.32 To examine whether metabolic abnormalities occurred in previously obese mice, we measured their body composition and metabolic parameters. In contrast to previous reports, we found that MOH mice after weight loss had not only the same body weight but also similar body length, BMD, skeletal content, lean mass and fat mass values as those in the CTR cohort (Figures 2a–e). Identical fasting and postprandial blood glucose levels and insulin sensitivity were also observed between the MOH and the CTR mice (Figures 2f and g). O2 consumption, CO2 production and RER remained normal in MOH mice (Figures 2h–j). Furthermore, physical activity, heat production and food intake were equivalent between the two cohorts (Figures 2k–m). These results suggest that the MOH mice following weight loss displayed unaffected glucose metabolism and energy homeostasis.
Figure 2.
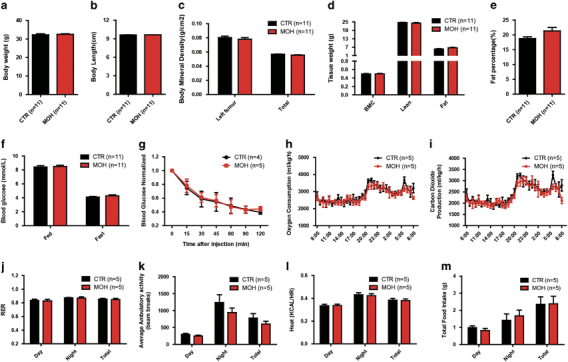
Metabolic parameters of C57BL/6J mice before weight regain. Body weight (a), body length (b), overall and local bone mineral density (c), bone mineral content, lean mass and fat mass (d) and body fat percentage (e) values were measured to determine body compositions, especially fat content, of the MOH and CTR mice. The fasting and postprandial blood glucose levels (f) and insulin sensitivity (g) were detected to determine glucose metabolism in both cohorts. O2 consumption (h), CO2 production (i), RER (j), ambulatory activity (k), heat production (l) and food intake (m) were detected to examine energy expenditure. Data are shown as means±s.d. No difference was detected in this figure. CTR, control group; MOH, obesity history group; RER, respiratory exchange ratio.
Immune cells are essential for obesity memory
We noticed that the spleens and thymuses were swollen in the MOH mice compared with the CTR mice after weight regain, suggesting that the immune system might be involved in this obesity memory (Supplementary Figure 1m). To further confirm this observation, we performed a microarray assay using inguinal adipose tissue from mice experiencing weight regain and confirmed the results via qPCR. Interestingly, immune-related signaling pathways, including T cell receptor (TCR), toll-like receptor (TLR) and Jak-STAT pathways, were significantly upregulated in MOH mice (Supplementary Figures 2a–e), suggesting an activation status. We further detected these alterations in both gonadal and inguinal adipose tissues from mice experiencing weight loss and found increased pro-inflammatory gene expression, including interferon γ (IFNγ) (Supplementary Figures 2f and g). Moreover, T cells, including CD4+, CD8+ T cells and Tregs, were significantly increased in adipose tissue, whereas their numbers remained unchanged in the spleens of MOH mice after weight regain (Supplementary Figures 3a and b). Before weight regain, the T cell numbers in the adipose tissue and spleens of MOH mice were higher than those in the CTR mice even though their body weights were identical (Supplementary Figures 3c and d). Circulating white blood cells, including lymphocytes, monocytes and granulocytes (for example, eosinophils, neutrophils and basophils), were also increased before weight regain, suggesting a systemic immune activation status in these mice (Supplementary Figures 3e–i).
To further study the relationship between immune activation and obesity memory, we established a weight gain-loss-regain model using Rag1−/− mice, which lack mature T and B cells.33 Intriguingly, the MOH Rag1−/− mice displayed no previous obesity memory as they showed similar weight gain rates during the weight regain period to those of the CTR Rag1−/− mice (Figures 3a and b). Consistently, the MOH Rag1−/− mice exhibited a similar body composition, as well as blood glucose and insulin sensitivities to those of the CTR Rag1−/− mice after both weight regain and weight loss (Supplementary Figures 4a–f and 5a–f). MOH Rag1−/− mice also showed similar energy metabolism as the CTR cohort after weight loss (Supplementary Figures 5g–m). In addition, we suppressed systemic immunologic functions in C57BL/6J mice by injecting dexamethasone (dexa), a common immunosuppressant that inhibits T cells and pro-inflammatory cytokines,34 into mice after weight gain. Interestingly, these immuno-suppressed MOH mice were unable to establish obesity memory (Figures 3c and d). These results demonstrate that immune activation serves as a precondition for acquiring or implementing obesity memory.
Figure 3.

Immunodeficiency leads to failure in establishing obesity memory. Immunodeficient Rag1−/− mice and C57BL/6J mice that were treated with dexamethasone (dexa) were subjected to a weight gain-loss-regain cycle. Body weight curve (a, c) and body weight increment ratio values during the weight regain period (b, d) were recorded to examine obesity memory in Rag1−/− mice (a, b) and dexa-treated C57BL/6J mice (c, d), respectively. Adoptive transfer of splenocytes was performed to determine the role of immune cells in memorizing obesity. The body weight curves of donor C57BL/6J mice (e, h) and recipient Rag1−/− mice (f, i) and the body weight increment ratios of recipients during HFD feeding (g, j) were recorded after weight loss (e–g) and after 1 month of weight maintenance (h–j), respectively. The cell collection time is indicated by a black arrow. Data are shown as means±s.d. ***P<0.001, unpaired Student’s t-test. §§§ P<0.001, two-way ANOVA followed by the Bonferroni post hoc test. HFD, high fat diet; Ns, no significance.
To test whether immune cells from MOH are sufficient to transfer the stored ‘obesity memory’ to naive mice, we collected splenocytes from MOH and CTR mice after weight loss (Figure 3e) and transferred them into Rag1−/− mice. We found that MOH Rag1−/− recipients that received MOH mouse immune cells displayed significantly faster weight gain after being fed HFD (Figures 3f and g). Considering that obesity memory is a long-lasting effect, we collected splenocytes after 1 month of body weight maintenance (Figure 3h) and performed the same adoptive transfer. The transfer of the obesity memory to Rag1−/− recipient mice was achieved using the MOH splenocytes (Figures 3i and j). Therefore, we conclude that immune cells are responsible for the obesity memory.
CD4+ T cells contribute to the obesity memory
To examine whether T cells contributed to the weight regain, TCRβ−/− mice, which are deficient in both CD4+ and CD8+ T cells,35 were subjected to the weight gain-loss-regain cycle. We found that MOH TCRβ−/− mice were unable to establish obesity memory and gained similar body weights to those of the CTR cohort during the regain period (Supplementary Figures 6a and b). Correspondingly, MOH TCRβ−/− mice showed normal glucose metabolism and insulin sensitivity after weight loss (Supplementary Figures 6c–e). These results suggest that T cells are crucial for establishing obesity memory.
To determine which subset of T cells is necessary for obesity memory, adipose tissue-resident T cells were isolated from C57BL/6J mice that had experienced obesity and different categories of T cells were counted. We found that CD4+ effector T lymphocytes, including Th1 and Th17 cells, were noticeably increased in epididymal adipose tissue of MOH C57BL/6J mice (Supplementary Figure 7), suggesting a vital role of CD4+ T cells. Therefore, H2A−/− mice, which lack CD4+ T cells, were subjected to the weight gain-loss-regain model. DIO H2A−/− mice failed to establish obesity memory and displayed similar weight regain rates to those of CTR H2A−/− mice (Figures 4a and b). These results suggest that T cells, and particularly CD4+ T cells, have an important role in establishing obesity memory.
Figure 4.
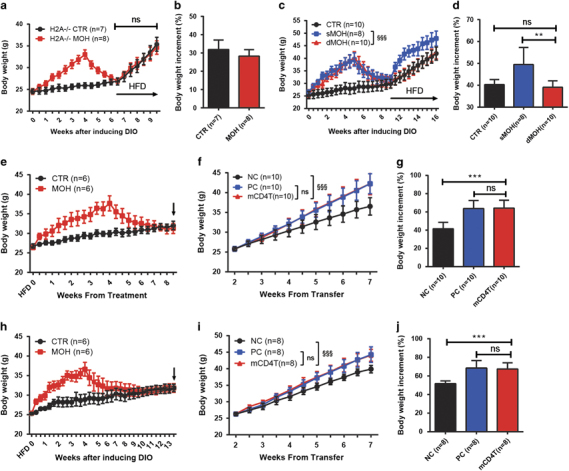
Role of CD4+ T cells in establishing and storing obesity memory. CD4+T cell-deficient H2A−/− mice were subjected to the weight gain-loss-regain cycle. Body weight curve (a) and body weight increment ratio values during the weight regain period (b) were recorded to examine obesity memory in H2A−/− mice. MOH C57BL/6J mice were injected with an anti-CD4 antibody (dMOH) or corresponding isotype IgG (sMOH) to determine the role of CD4+ T cells in obesity memory. CTR C57BL/6J mice were injected with saline. The injection time is indicated with a black arrow. Body weight curve (c) and body weight increment ratio values during the weight regain period (d) were recorded to examine obesity memory. Adoptive transfer of CD4+ T cells was conducted to confirm their role in memorizing obesity. The body weight curves of donor C57BL/6J (e, h) and recipient Rag1−/− mice (f, i) and the body weight increment ratios of recipients during the HFD feeding period (g, j) were recorded after weight loss (e–g) and after 1 month of weight maintenance (h–j), respectively. The cell collection time is indicated by a black arrow. Data are shown as means±s.d. *P<0.05, **P<0.01 and ***P<0.001, unpaired Student’s t-test. §§§ P<0.001, two-way ANOVA followed by the Bonferroni post hoc test. CTR, control group; dMOH, CD4+ T cell-deficient MOH group; HFD, high fat diet; mCD4T, CD4+ T cells carrying obesity memory group; MOH, obesity history group; NC, negative control group; Ns, no significance; PC, positive control group; sMOH, CD4+ T cell-sufficient MOH group.
To further confirm the requirement of CD4+ T cells in obesity memory, CD4+ T cells were depleted in C57BL/6J mice after weight loss by administration of an anti-CD4 antibody that did not affect CD8+ T cells and B cells (Supplementary Figures 8a and b). Compared with the CD4+ T cell-sufficient MOH (sMOH, injected with an isotype IgG2bκ antibody) and CTR mice (injected with saline), the obesity memory in the CD4+ T cell-deficient MOH mice (dMOH, injected with anti-CD4 antibody) was completely eliminated (Figures 4c and d), suggesting that CD4+ T cells are indispensable for obesity memory. To further explore the role of CD4+ T cells, we collected splenocytes from CTR and MOH mice after weight loss and isolated CD4+ T cells with magnetic beads (Figure 4e). Then, CD4+ T cells from MOH mice and CD4+ T cell-removed splenocytes from CTR mice were mixed and transferred into naive Rag1−/− recipient mice (mCD4T, CD4+ T cells carrying obesity memory). Thus, this splenocyte mixture contained CD4+ T cells that might carry obesity memory and other splenocytes that did not carry obesity memory. Rag1−/− mice transferred with total splenocytes of CTR and MOH mice were used as negative controls (NC) and positive controls (PC), respectively. After 2 weeks of transfer, Rag1−/− recipients were fed with HFD to induce obesity. Interestingly, the body weight increment ratio of the mCD4T cohort was similar to that of the PC mice, and it was significantly greater than that of the NC cohort (Figures 4f and g). In addition, we isolated CD4+ T cells from donors that experienced 1 month of weight maintenance (Figure 4h) and conducted the transfer experiment again. Remarkably, a higher rate of weight gain was observed in mCD4T recipients when compared with the NC counterparts (Figures 4i and j). These results demonstrate that CD4+ T cells are the key contributors to the storage and transfer of obesity memory.
Discussion
Obesity has become one of the most serious health problems globally, and the obese and overweight populations are growing rapidly.36 It is difficult to reduce weight, while maintaining a long-term reduced weight is also a huge challenge.9,37 Physiological adaptions have been regarded as the potential cause of weight regain.37,38 However, we showed that no significant metabolic alterations were observed after weight loss and that they were not the major reason for obesity relapse. Instead, faster weight regains were related to dysregulated immune activation, which is consistent with the observation that enhanced inflammation is induced by weight fluctuation.39,40,41,42 We observed that circulating and adipose tissue-resident T cells were present at higher levels in the weight-lost mice when compared with the weight-matched normal mice. Mice with severe immune deficiency failed to develop obesity memory. Furthermore, we found that obesity memory was stored in immune cells and could be adoptively transferred to immune-deficient mice.
It is known that obesity triggers chronic inflammation in white adipose tissue, represented by accumulated effector lymphocytes, including CD4+ T cells.43 Fat-produced leptin can induce CD4+ T cell proliferation and activation showing increased expression of early and late activation markers.44 Here, we show that CD4+ T cells are also related to obesity memory. CD4+ effector T cells, including Th1 and Th17 cells, accumulated in adipose tissue before weight regain. CD4+ T cell-deficient H2A−/− mice were unable to establish obesity memory. Depletion of CD4+ T cells prevented obesity memory development, suggesting that obesity memory depends on CD4+ effector T cells. Surprisingly, we also observed that the weight regain was accelerated when we introduced CD4+ T cells of previously obese mice into Rag1−/− mice. These results suggest that CD4+ T cells have a vital role in establishing obesity memory.
It is difficult to identify the specific antigens that trigger the activation of these obesity memory-related CD4+ T cells. Although it was reported that fatty acids could promote the proliferation of memory-like CD4+ T cells, it is still unknown whether they are able to directly activate CD4+ T cells and trigger obesity relapse.45 Meanwhile, there are many different subsets of CD4+ T cells that have been associated with obesity and inflammation in adipose tissue. Th1-polarized cells were reported to be associated with inflammation and insulin resistance in obesity.46 IFNγ, a prototypical Th1 cytokine, was increased in obese adipose tissue and promotes obesity-related inflammation.47 Th1 responses could be promoted by M1 macrophage-mediated NKT cell activation, which therefore exacerbated obesity and chronic inflammation.48 Th17 cells, another pro-inflammatory T cell subtype, infiltrated obese adipose tissue, and Th17 cytokines promoted TNF-α production in obese and metabolically unhealthy individuals.49,50 Although our data show that Th1 and Th17 were increased after weight loss, their functions in obesity memory remain unknown. On the other hand, Tregs, known as an important immune-suppressive CD4+ T cell subset, are abundant in the adipose tissue of normal mice but are reduced in proportion in obese mice.51 Depletion of Tregs leads to adipose tissue inflammation and metabolic abnormalities.52,53 In particular, visceral adipose tissue-resident Tregs have been reported as a crucial suppressor of obesity-associated inflammation.25,26 Further studies are required to identify the role of Tregs in obesity memory.
Previous studies suggest that many cytokines are associated with obesity-related inflammation and weight regain. Pro-inflammatory cytokines and chemokines, including IFNγ, IL-6 and IL-1β, can be induced by leptin.44 Monocyte chemoattractant protein-1 (MCP-1), which was increased in HFD-induced obesity, remained at higher levels after weight loss and during weight regain.41 High TNF-α levels in white adipose tissue were detected in weight-loss patients.30 Weight regain could be predicted by higher CRP and IL-1β levels, whereas successful weight maintenance was associated with lower levels of these proteins.31 IL-25, a member of the IL-17 family, reduced body weight gain and alleviated lipid accumulation in adipose tissue via stimulating M2 macrophage polarization.54 Here, we also observed elevated IFNγ expression in adipose tissue before and after weight regain. The above-mentioned inflammatory cytokines and their functions in obesity memory require further study.
Physical activity seems to be the most beneficial approach for promoting long-term weight loss and preventing weight regain.55 It was recently reported that strengthening physical activity promotes immune system hypofunction.56 Although upregulation of inflammatory molecules, including hsCRP, IL-1, TNF-α and TNF receptor, could be reversed through dieting, physical exercise was shown to be essential to keep these inflammatory molecules at low levels.56,57 Our data show that immune cell-induced inflammatory status facilitates obesity relapse, which may also implicate the efficacy of dieting-plus-exercise treatment.
In conclusion, we provide new insight into the mechanism of weight regain. Obesity can be memorized by immune cells and stored in CD4+ T cells, resulting in unavoidable weight regain. With further investigation into the specific antigens and CD4+ T lymphocyte subtypes involved in obesity memory, we may develop more efficient anti-obesity strategies.
Electronic supplementary material
Acknowledgements
We thank Dr Jiong Chen for manuscript editing assistance; Dr Dejing Pan for technical suggestions; Dr Yun Wang for immunosuppression experiment advice; and Dr Jianghuai Liu, Dr Demin Wang, Dr Quan Zhao and Dr Ying Xu for their experimental design suggestions and advice. This work was supported by the National Natural Science Foundation of China (Grant 31301217) and the Ministry of Science and Technology of China (Grants 2015BAI08B02 and 2014BAI02B01).
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website 10.1038/cmi.2017.36
References
- 1.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 2.Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22:s176–s185. [PubMed] [Google Scholar]
- 3.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 4.Woo Baidal JA, Lavine JE. The intersection of nonalcoholic fatty liver disease and obesity. Sci Transl Med. 2016;8:323rv321. doi: 10.1126/scitranslmed.aad8390. [DOI] [PubMed] [Google Scholar]
- 5.Kraschnewski JL, Boan J, Esposito J, Sherwood NE, Lehman EB, Kephart DK, et al. Long-term weight loss maintenance in the United States. Int J Obes. 2010;34:1644–1654. doi: 10.1038/ijo.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 7.Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity. 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Look ARG. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity. 2014;22:5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langeveld M, DeVries JH. The long-term effect of energy restricted diets for treating obesity. Obesity. 2015;23:1529–1538. doi: 10.1002/oby.21146. [DOI] [PubMed] [Google Scholar]
- 10.Gursoy A, Erdogan MF, Cin MO, Cesur M, Baskal N. Comparison of orlistat and sibutramine in an obesity management program: efficacy, compliance, and weight regain after noncompliance. Eat Weight Disord. 2006;11:e127–e132. doi: 10.1007/BF03327578. [DOI] [PubMed] [Google Scholar]
- 11.Odom J, Zalesin KC, Washington TL, Miller WW, Hakmeh B, Zaremba DL, et al. Behavioral predictors of weight regain after bariatric surgery. Obes Surg. 2010;20:349–356. doi: 10.1007/s11695-009-9895-6. [DOI] [PubMed] [Google Scholar]
- 12.Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 13.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 14.Dietz WH, Goodwin NJ, Hill JO, Pi-Sunyer FX, Rolls B, Stern J, et al. Long-term pharmacotherapy in the management of obesity. National Task Force on the prevention and treatment of obesity. JAMA. 1996;276:1907–1915. doi: 10.1001/jama.1996.03540230057036. [DOI] [PubMed] [Google Scholar]
- 15.Kliewer KL, Ke JY, Lee HY, Stout MB, Cole RM, Samuel VT, et al. Short-term food restriction followed by controlled refeeding promotes gorging behavior, enhances fat deposition, and diminishes insulin sensitivity in mice. J Nutr Biochem. 2015;26:721–728. doi: 10.1016/j.jnutbio.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 17.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 18.de Luis DA, Gonzalez Sagrado M, Conde R, Aller R, Izaola O. Decreased basal levels of glucagon-like peptide-1 after weight loss in obese subjects. Ann Nutr Metab. 2007;51:134–138. doi: 10.1159/000103273. [DOI] [PubMed] [Google Scholar]
- 19.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 20.Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307:2627–2634. doi: 10.1001/jama.2012.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crujeiras AB, Goyenechea E, Abete I, Lage M, Carreira MC, Martinez JA, et al. Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J Clin Endocrinol Metab. 2010;95:5037–5044. doi: 10.1210/jc.2009-2566. [DOI] [PubMed] [Google Scholar]
- 22.Chaput JP, Pelletier C, Despres JP, Lemieux S, Tremblay A. Metabolic and behavioral vulnerability related to weight regain in reduced-obese men might be prevented by an adequate diet-exercise intervention. Appetite. 2007;49:691–695. doi: 10.1016/j.appet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin T, Liu LF, Lamendola C, Shen L, Morton J, Rivas H, et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34:2637–2643. doi: 10.1161/ATVBAHA.114.304636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 25.Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- 26.Hu ZQ, Zhao WH. The IL-33/ST2 axis is specifically required for development of adipose tissue-resident regulatory T cells. Cell Mol Immunol. 2015;12:521–524. doi: 10.1038/cmi.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong LC, Wuillemin PH, Bastard JP, Sokolovska N, Gougis S, Fellahi S, et al. Insulin resistance and inflammation predict kinetic body weight changes in response to dietary weight loss and maintenance in overweight and obese subjects by using a Bayesian network approach. Am J Clin Nutr. 2013;98:1385–1394. doi: 10.3945/ajcn.113.058099. [DOI] [PubMed] [Google Scholar]
- 28.Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes. 2013;62:3180–3188. doi: 10.2337/db12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitz J, Evers N, Awazawa M, Nicholls HT, Bronneke HS, Dietrich A, et al. Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Mol Metab. 2016;5:328–339. doi: 10.1016/j.molmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambeba EJ, Styn MA, Kuller LH, Brooks MM, Evans RW, Burke LE. Longitudinal effects of weight loss and regain on cytokine concentration of obese adults. Metabolism. 2013;62:1218–1222. doi: 10.1016/j.metabol.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology’s response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301:R581–R600. doi: 10.1152/ajpregu.00755.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-G. [DOI] [PubMed] [Google Scholar]
- 34.Diehl R, Ferrara F, Muller C, Dreyer AY, McLeod DD, Fricke S, et al. Immunosuppression for in vivo research: state-of-the-art protocols and experimental approaches. Cell Mol Immunol. 2017;14:146–179. doi: 10.1038/cmi.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 36.Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord. 2004;28((Suppl 3)):S2–S9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- 37.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 38.Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes. 2015;39:1188–1196. doi: 10.1038/ijo.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blomain ES, Dirhan DA, Valentino MA, Kim GW, Waldman SA. Mechanisms of weight regain following weight loss. ISRN Obes. 2013;2013:210524. doi: 10.1155/2013/210524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thaiss CA, Itav S, Rothschild D, Meijer M, Levy M, Moresi C, et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016;540:544–551. doi: 10.1038/nature20796. [DOI] [PubMed] [Google Scholar]
- 41.Barbosa-da-Silva S, Fraulob-Aquino JC, Lopes JR, Mandarim-de-Lacerda CA, Aguila MB. Weight cycling enhances adipose tissue inflammatory responses in male mice. PLoS ONE. 2012;7:e39837. doi: 10.1371/journal.pone.0039837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strohacker K, McFarlin BK. Influence of obesity, physical inactivity, and weight cycling on chronic inflammation. Front Biosci (Elite Ed) 2010;2:98–104. doi: 10.2741/e70. [DOI] [PubMed] [Google Scholar]
- 43.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vadacca M, Margiotta DP, Navarini L, Afeltra A. Leptin in immuno-rheumatological diseases. Cell Mol Immunol. 2011;8:203–212. doi: 10.1038/cmi.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mauro C, Smith J, Cucchi D, Coe D, Fu H, Bonacina F, et al. Obesity-induced metabolic stress leads to biased effector memory CD4+ T cell differentiation via PI3K p110delta-Akt-mediated signals. Cell Metab. 2017;25:593–609. doi: 10.1016/j.cmet.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity. 2010;18:1918–1925. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Xue R, Zhu S, Fu S, Chen Z, Zhou R, et al. M2-specific reduction of CD1d switches NKT cell-mediated immune responses and triggers metaflammation in adipose tissue. Cell Mol Immunol. 2017;14:1–12. doi: 10.1038/cmi.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pandolfi JB, Ferraro AA, Sananez I, Gancedo MC, Baz P, Billordo LA, et al. ATP-induced inflammation drives tissue-resident Th17 cells in metabolically unhealthy obesity. J Immunol. 2016;196:3287–3296. doi: 10.4049/jimmunol.1502506. [DOI] [PubMed] [Google Scholar]
- 50.Ip B, Cilfone NA, Belkina AC, DeFuria J, Jagannathan-Bogdan M, Zhu M, et al. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFalpha production. Obesity. 2016;24:102–112. doi: 10.1002/oby.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou X, Tang J, Cao H, Fan H, Li B. Tissue resident regulatory T cells: novel therapeutic targets for human disease. Cell Mol Immunol. 2015;12:543–552. doi: 10.1038/cmi.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X, Wu Y, Wang L. Fat-resident Tregs: an emerging guard protecting from obesity-associated metabolic disorders. Obes Rev. 2013;14:568–578. doi: 10.1111/obr.12033. [DOI] [PubMed] [Google Scholar]
- 53.Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, et al. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60:2954–2962. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng J, Li L, Ou Z, Li Q, Gong B, Zhao Z et al. IL-25 stimulates M2 macrophage polarization and thereby promotes mitochondrial respiratory capacity and lipolysis in adipose tissues against obesity. Cell Mol Immunol 2017. [DOI] [PMC free article] [PubMed]
- 55.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 56.Snel M, van Diepen JA, Stijnen T, Pijl H, Romijn JA, Meinders AE, et al. Immediate and long-term effects of addition of exercise to a 16-week very low calorie diet on low-grade inflammation in obese, insulin-dependent type 2 diabetic patients. Food Chem Toxicol. 2011;49:3104–3111. doi: 10.1016/j.fct.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 57.You T, Berman DM, Ryan AS, Nicklas BJ. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89:1739–1746. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


