Abstract
Aseptic loosening is mainly caused by wear debris generated by friction that can increase the expression of receptor activation of nuclear factor (NF)-κB (RANKL). RANKL has been shown to support the differentiation and maturation of osteoclasts. Although autophagy is a key metabolic pathway for maintaining the metabolic homeostasis of cells, no study has determined whether autophagy induced by Al2O3 particles is involved in the pathogenesis of aseptic loosening. The aim of this study was to evaluate RANKL levels in patients experiencing aseptic loosening after total hip arthroplasty (THA) and hip osteoarthritis (hOA) and to consequently clarify the relationship between RANKL and LC3II expression. We determined the levels of RANKL and autophagy in fibroblasts treated with Al2O3 particles in vitro while using shBECN-1 interference lentivirus vectors to block the autophagy pathway and BECN-1 overexpression lentivirus vectors to promote autophagy. We established a novel rat model of femoral head replacement and analyzed the effects of Al2O3 particles on autophagy levels and RANKL expression in synovial tissues in vivo. The RANKL levels in the revision total hip arthroplasty (rTHA) group were higher than those in the hOA group. In patients with rTHA with a ceramic interface, LC3II expression was high, whereas RANKL expression was low. The in vitro results showed that Al2O3 particles promoted fibroblast autophagy in a time- and dose-dependent manner and that RANKL expression was negatively correlated with autophagy. The in vivo results further confirmed these findings. Al2O3 particles induced fibroblast autophagy, which reduced RANKL expression. Decreasing the autophagy level promoted osteolysis and aseptic prosthetic loosening, whereas increasing the autophagy level reversed this trend.
Introduction
THA is one of the most effective procedures for treating severe trauma, rheumatoid arthritis, osteoarthritis and other end-stage joint diseases1–3. Although complications rarely arise after arthroplasty, periprosthetic osteolysis and subsequent aseptic prosthetic loosening are the most common complications that limit the longevity of prostheses4–6.
Wear debris that separate from the surface of prostheses is a primary contributor to aseptic loosening7,8. Current bearing surface materials for THA have four main combinations including metal-on-polyethylene (MoP), ceramic-on-polyethylene, ceramic-on-ceramic (CoC), and metal-on-metal (MoM), of which MoP implants have been the most widely used for over 40 years. MoP bearing surfaces were once considered the gold standard for THA. However, there have always been concerns about polyethylene wear debris-induced aseptic loosening and osteolysis9–12. MoM resurfacings have caused several adverse reactions, such as pseudotumor development, hypersensitivity reactions, and toxicity from metal ions; MoM prostheses were recalled in 2010 due to significantly greater revision rates compared with all other conventional prostheses13,14. CoC surface materials also have a long history. As early as April 1970, Dr. Pierre Boutin first introduced ceramic bearings into the field of artificial joints15. However, early CoC prostheses had a higher revision rate due to poor designs, inadequate material properties, and imperfect surgical techniques. Therefore, the following complications related to CoC prosthesis have been observed: ceramic fracture, rupture of the prosthesis during operation, liner chipping on insertion, liner canting or dissociation, edge loading, squeaking and aseptic loosening of the prosthesis. Aseptic loosening of CoC bearings during 1970s and 1980s were mainly due to the method of fixation of the components rather than a biological reaction to wear debris15–18. Studies in vitro have shown fewer macrophage reactions and decreased cytokine secretion with exposure to ceramic particles in comparison to titanium particles16,19. Histological staining has identified ceramic wear particles within individual macrophages in peri-prosthetic tissues, and these particles did not generate serious foreign body granulomas15.
Nevertheless, the effects of Al2O3 particles on aseptic loosening remain unclear. Research has shown that Al2O3 particles can induce autophagy formation20. Our prior studies also support this conclusion; moreover, we have determined that Al2O3 particles can increase the level of autophagy more than Cobalt-Chromium alloy particles. Therefore, we hypothesized that Al2O3 particles can induce RANKL expression, resulting in osteoclast formation. Additionally, Al2O3 particle-induced autophagy reduces osteolysis by negative feedback regulation of RANKL expression in fibroblasts. Nevertheless, the effects of Al2O3 particles on aseptic loosening remain unclear, particularly with regard to the regulation of autophagy and osteoclasts. Thus, we determined the RANKL and autophagy levels in fibroblasts challenged with Al2O3 particles in vitro. We also established a novel rat model of femoral head replacement to analyze the effects of Al2O3 particles on autophagy and RANKL levels in vivo.
The expression of osteoclasts is reportedly increased in the pseudomembrane of the hips after revision arthroplasty21. The RANKL, which is a member of the tumor necrosis factor family, facilitates the differentiation and maturation of osteoclasts. The macrophage colony-stimulating factor (M-CSF) is another essential cytokine for osteoclast differentiation and maturation22–25. Fibroblasts and macrophages are the primary sources of RANKL. However, no study has clarified how RANKL is regulated in fibroblasts challenged with Al2O3 particles in patients experiencing aseptic loosening.
Numerous studies have recently demonstrated that autophagy is a dynamic process responsible for the turnover of cellular organelles and long-lived proteins, suggesting that autophagy plays a crucial role in cellular homeostasis and adaptation to adverse environments20,26–28. Previous studies have demonstrated that certain nanomaterials induce autophagy to degrade materials that are perceived by cells as foreign or aberrant20,29,30. The metal wear particles retrieved from patients experiencing aseptic loosening are reportedly as small as 50 nm (ranging from 6 to 834 nm)31. Currently, the relationship between aseptic loosening induced by Al2O3 wear debris and autophagy remains unclear.
In the present study, we hypothesized that wear-debris-induced autophagy in fibroblasts is responsible for Al2O3-particle-induced aseptic loosening. Autophagy inhibits the activation of osteoclasts and reduces osteolysis and aseptic loosening. We evaluated our hypothesis by means of synovial samples collected from patients with rTHA and hOA, fibroblasts and a novel rat model of femoral head replacement.
Results
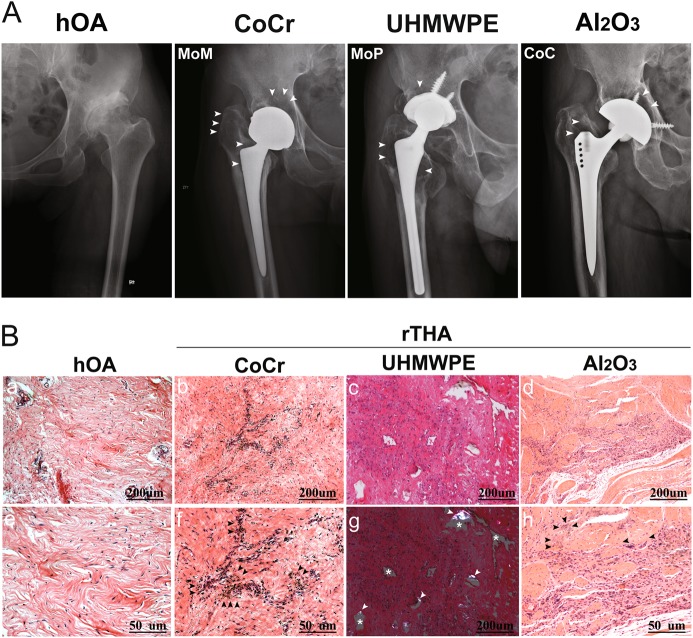
Imaging and histomorphological manifestations of aseptic loosening in patients with THA of MoM, MoP, and CoC-bearing surfaces
Four groups were formed, namely, hOA (control), CoCr, UHMWPE and Al2O3. For the rTHA group, CoCr, UHMWPE, and Al2O3 were the wear debris of the MoM, MoP, and CoC interfaces, respectively. The range of the osteolytic areas (white arrow) was determined with anterior and posterior X-ray images. (Fig. 1a). The Al2O3 group showed a smaller osteolytic area than the CoCr and UHMWPE groups. H&E staining revealed that inflammatory cells infiltrated into the synovial tissue of patients with hOA (Fig. 1b, panels a, e). The rTHA group had more inflammatory cells than the hOA group. Moreover, CoCr (Fig. 1b, panels b, f), UHMWPE (Fig. 1b, panels c, g), and Al2O3 (Fig. 1b, panels d, h) wear debris were observed in the corresponding groups. However, the Al2O3 group had fewer synovial fibroblasts and mononuclear macrophages than the CoCr and UHMWPE groups, suggesting that a less intense inflammatory response occurred in the Al2O3 group (Fig. 1b).
Fig. 1. Imaging and histomorphological manifestations of prosthesis loosening with different frictional interfaces.
a Anterior and posterior X-ray images demonstrating a wide range of osteolytic areas (white arrow) around the proximal femur and the acetabular cup at the MoM, MoP, and CoC interfaces in hOA patients. b (a, e) H&E staining showed that inflammatory cells infiltrated into the synovial tissue of patients with hOA. b, f H&E staining of the synovial tissue of the hip joint of patients with rTHA (metal-on-metal interface, MoM) showed a large number of black particles of uniform size (black arrow). c H&E staining of the synovial tissues from the hip joints of patients with rTHA (metal-on-polyethylene interface, MoP) showed that the uneven area of the blank (white asterisk) was surrounded by increased synovial fibroblasts and aggregated mononuclear macrophages in the synovium from patients with rTHA. (g) UHMWPE particles were observed as double refractions by polarized light micrographs (white arrow). d, h Gray or black micro- and nanosized scattered ceramic particles were observed in the synovium with fewer inflammatory cells (black arrow) from patients with rTHA (ceramic-on-ceramic interface, CoC). a, b, c, d, g Scale bar: 200 μm; (e, f, h) Scale bar: 50 μm. Revision total hip arthroplasty (rTHA), hip osteoarthritis (hOA); CoCr, UHMWPE, and Al2O3 refer to the wear debris of MoM, MoP, and CoC, respectively
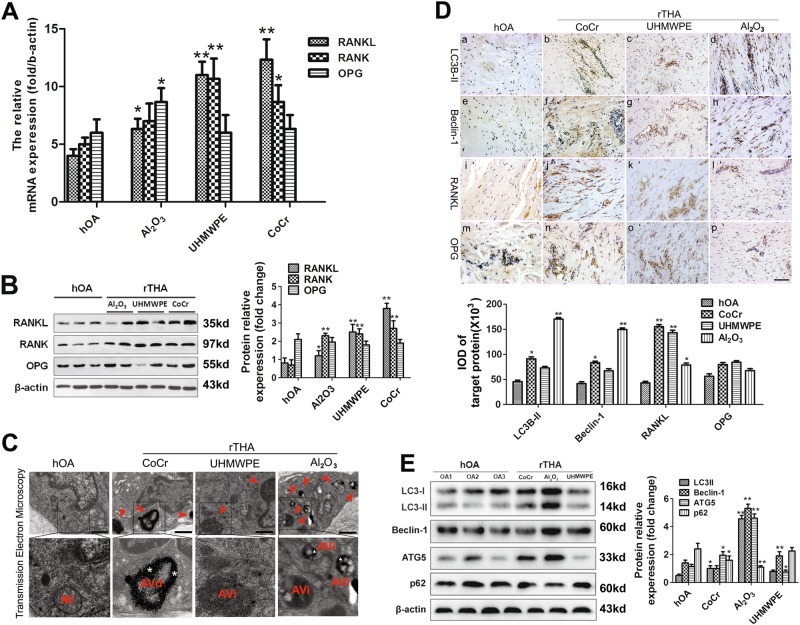
Upregulation of autophagy induced by wear debris with downregulation of RANKL expression in the synovial tissue of patients with rTHA
RT-PCR and Western blot were performed to assess the levels of RANKL, RANK, and OPG. The levels in the rTHA group were higher than those in the hOA group. In addition, the protein level of RANKL in the Al2O3 group was lower than that in the CoCr and UHMWPE groups (P < 0.05) (Figs. 2a, b). A transmission electron microscope was used to detect autophagy in the synovial tissue of patients experiencing loosening of prostheses with different friction interfaces. The CoC interface group showed different stages of autophagy formation. Dark black Al2O3 particles were wrapped in autophagosomes. The typical double-layer structure was observed in the high-power field, and its quantity and volume were markedly higher than that in the MoM and MoP groups (Fig. 2c). The protein expression of RANKL, OPG, Beclin1, and LC3B-II in the synovial tissue of patients experiencing prosthetic loosening was detected by immunohistochemical staining. The results indicated that the expression of RANKL was negatively correlated with that of Beclin-1 and LC3B-II (Fig. 2d). Western blot analysis was used to detect the expression of autophagy-related proteins, such as LC3-I, LC3-II, Beclin-1, ATG-5, and p62, in the synovial tissue of each group. The Al2O3 group had a significantly higher expression of autophagy-related proteins than the control, CoCr and UHMWPE groups (Fig. 2e)
Fig. 2. Autophagy induced by wear debris and expression of RANKL, RANK, OPG in the synovial tissue of patients with rTHA.
a–b The RNA and protein expression levels of RANKL, RANK, and OPG in the synovial tissue of patients with different frictional prosthesis loosening. The right side shows the quantitative results of Western blot analysis. c Detection of autophagy in the synovial tissues of patients experiencing loosening of prosthetics with different friction interfaces, as revealed by transmission electron microscopy (red arrow). Scale bar: 500 nm. d The expression of RANKL, OPG, Beclin1, and LC3B-II in the synovial tissues of patients experiencing prosthetic loosening, as detected by immunohistochemical staining. Scale bar: 100 μm. e Western blot analysis was used to detect the expression of autophagy-related proteins in the synovial tissue of each group. The data are expressed as the means ± SD of three independent experiments. [n = 3; * compared with the hOA group (*P < 0.05; **P < 0.01).] (AVi initial autophagic vacuoles, AVd degrading autophagic vacuoles, MI mitochondria)
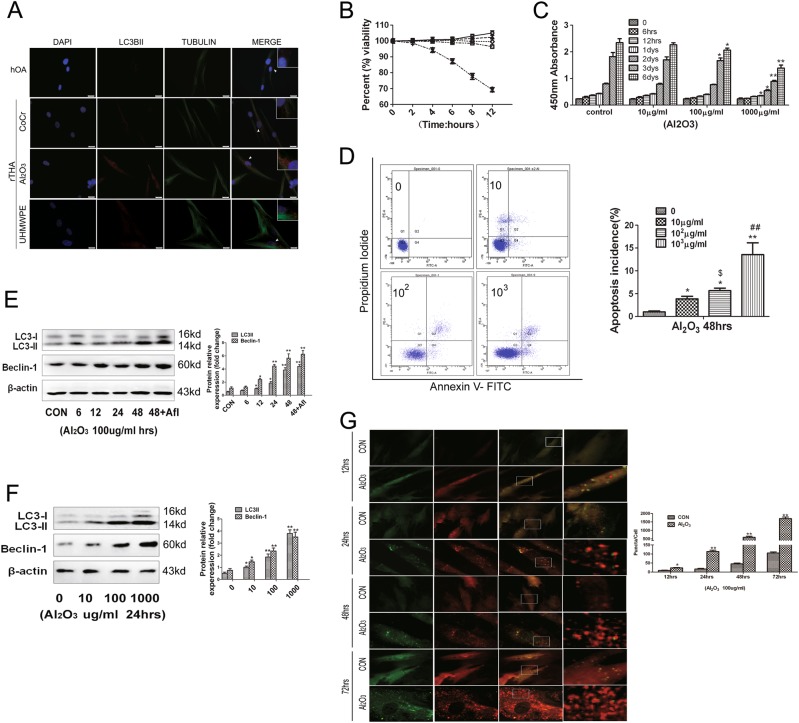
Al2O3 particles induced autophagy of FLSs
The fluorescence intensity level indicating the amount of the LC3B-II protein was observed by immunofluorescence staining. The Al2O3 group showed a higher fluorescence intensity than the other experimental groups (Fig. 3a). LDH cytotoxicity testing revealed that the groups treated with Al2O3 concentrations of 10, 100, and 1000 μg/mL had no significant difference from the control group within 12 h (Fig. 3b). CCK-8 was used to analyze the proliferation of FLSs by Al2O3, and the results showed a significant difference between the concentration at 100 μg/mL Al2O3 and the control group within 3 d. The difference became more marked at 1000 μg/mL on the following day. Therefore, 100 μg/mL Al2O3 was used in subsequent experiments (Fig. 3c). The effects of Al2O3 nanoparticles on the apoptosis of FLSs were detected by flow cytometry. The results indicated that the number of apoptotic cells was low at a concentration of 100 μg/ml, and the number of apoptotic cells increased significantly when the concentration was more than 1000 μg/mL (Fig. 3d). Western blot analysis suggested that the autophagy level of fibroblasts treated with 100 μg/mL Al2O3 particles increased in a time-dependent manner (Fig. 3e). In addition, the Western blot results demonstrated that the effect of Al2O3 nanoparticles on the autophagy level in fibroblasts increased in a dose-dependent manner (Fig. 3f). MRFP-GFP-LC3 autophagy fluorescent double-labeled dynamic observation revealed that the effects of concentration of 100 μg/mL Al2O3 nanoparticles on fibroblast autophagy levels was time-dependent within 72 h (Fig. 3g).
Fig. 3. Al2O3 particles induced autophagy in FLSs.
a The fluorescence intensity indicating LC3B-II protein levels was observed by immunofluorescence staining: nucleus (DAPI, blue), LC3B-II (red), and Tubulin (green). Scale bar: 25 μm. b Lactate dehydrogenase cytotoxicity assay was used to determine the toxicity of Al2O3 nanoparticles in fibroblasts. c Effects of CCK-8 on the proliferation of fibroblast-like synovial cells by Al2O3 nanoparticles. d Effects of Al2O3 nanoparticles on the apoptosis of fibroblast-like synovial cells by flow cytometry. e Western blot analysis showed that the autophagy level of fibroblasts loaded with 100 μg/mL Al2O3 nanoparticles was increased in a time-dependent manner. f Western blot analysis showed that the effect of Al2O3 nanoparticles at 0, 10, 100, and 1000 μg/ml on the autophagy level in fibroblasts was increased in a dose-dependent manner through 24 h. g mRFP-GFP-LC3 autophagy fluorescent double-labeled dynamic observation of the effects of a 100 μg/mL concentration of Al2O3 nanoparticles on fibroblast autophagy levels; autophagy increased in a time-dependent manner through 72 h. The data are expressed as the means ± SD of three independent experiments. Scale bar: 10 μm. [n = 3; * compared with the control group (*P < 0.05; **P < 0.01) $ compared with the control group 10 μg/mL ($P < 0.05), # compared with the control group 10 μg/mL (#P < 0.05; ##P < 0.01)]
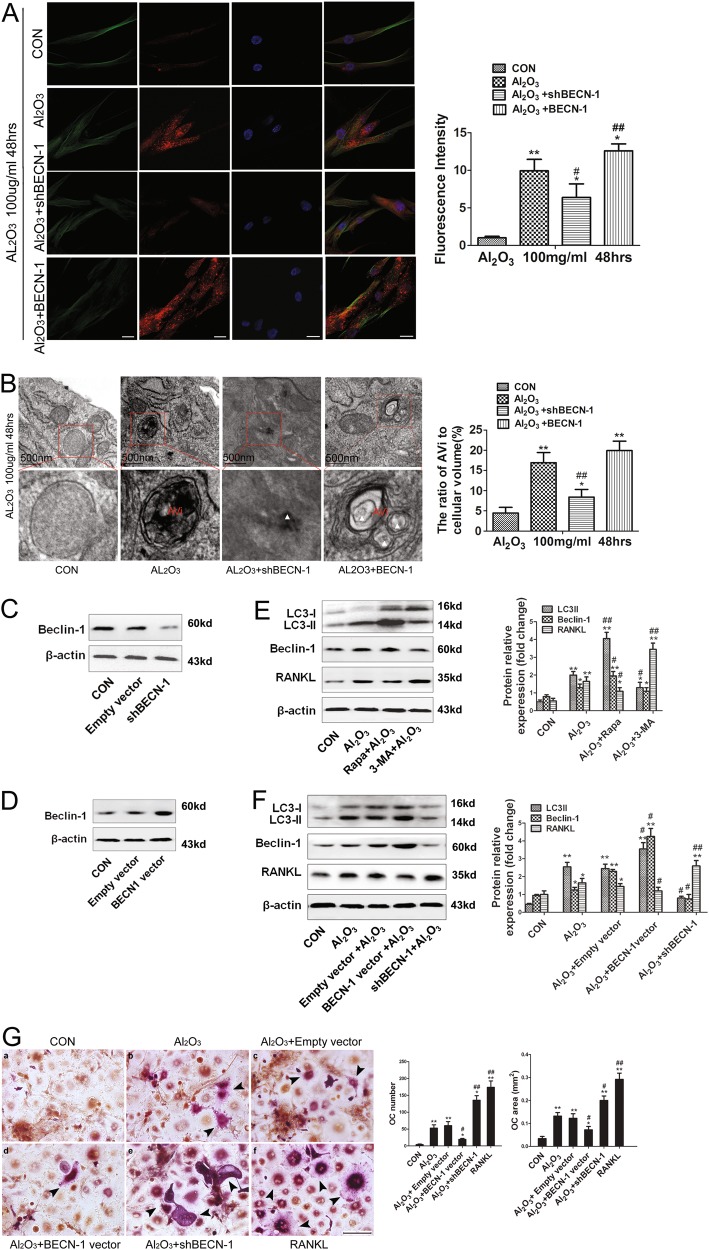
Autophagy induced by Al2O3 nanoparticles attenuated the expression of RANKL in FLSs
The samples were divided into four groups: a control group, an Al2O3 nanoparticle group, an Al2O3 nanoparticle + BECN-1 group, and an Al2O3 nanoparticle + shBECN-1 group. The fluorescence intensity of the LC3B-II protein was observed by immunofluorescence staining. The FLSs were incubated with 100 μg/mL Al2O3 nanoparticles for 48 h. The results showed that the autophagy level increased in the Al2O3 nanoparticles compared with control group, while it decreased in the Al2O3 + shBECN-1 group. This trend was reversed in the Al2O3 + BECN-1 group (Fig. 4a). Transmission electron microscopy revealed the formation of autophagosomes in the synovial tissue of fibrous synovial cells. The number of autophagosomes in the Al2O3 nanoparticle group were significantly higher than those in the control and Al2O3 nanoparticles + shBECN-1 groups (Fig. 4b). Western blot analysis confirmed the effectiveness of the shBECN-1 and BECN-1 lentiviral vectors. Compared with the control and empty vector groups, the shBECN-1 group showed a lower protein level, whereas the BECN-1 lentiviral vector group showed a higher protein level (Figs. 4c, d). Histograms showed that the autophagy levels in fibroblasts were inversely proportional to their intracellular RANKL expression. [autophagy inhibitors (3-methyladenine, 3-MA); autophagic inducers (rapamycin, RAPA)] (Figs. 4e, f). TRAP staining was used to observe the effects of exocrine RANKL on the number and area of osteoclast formation when fibroblast autophagy levels were changed. Compared with the other experimental groups, the shBECN-1 group showed a greater number and a larger area of osteoclast formation, whereas the BECN-1 lentiviral vector group displayed the opposite trend (Fig. 4g).
Fig. 4. Autophagy induced by Al2O3 particles mediated the expression of RANKL in FLSs.
a The fluorescence intensity indicating the level of LC3B-II protein was observed by immunofluorescence staining. The fibroblast-like synoviocytes were incubated with 100 μg/ml Al2O3 nanoparticles for 48 h; Nucleus (DAPI, blue), LC3B-II (red), and Tubulin (green). The experimental groups were as follows: control group, Al2O3 nanoparticle group, Al2O3 nanoparticle + BECN-1 group, and Al2O3 nanoparticle + shBECN-1 group. Scale bar: 25 μm. b The formation of autophagosomes in synovial tissues of fibrous synovial cells was observed by transmission electron microscopy, Scale bar: 500 nm; c–d Western blot analysis confirmed the effectiveness of shBECN-1 and BECN-1 lentiviral vectors; e–f Western blot analysis was used to detect the effect on the expression of RANKL of the level of autophagy in fibroblasts induced by Al2O3 nanoparticles, Autophagy Inhibitors, (3-Methyladenine, 3-MA), and Autophagy Inducers (Rapamycin, RAPA); g TRAP staining was used to observe the effects of exocrine RANKL on the number and area of osteoclast formation when fibroblast autophagy levels were changed. CM refers to conditioned medium (80% culture medium containing 25 ng/ml M-CSF), RANKL concentrations were 50 ng/ml, Al2O3 nanoparticle concentrations were 100 μg/ml. The multinucleated giant cells (black arrows) stained with purple were TRAP+ cells. Data are expressed as the means ± SD of three independent experiments. Scale bar: 50 μm. [* compared with Control group (*P < 0.05; **P < 0.01). # compared with Empty vector (#P < 0.05; ##P < 0.01)]
A rat model of biologic femoral head replacement
CAD drawings of the femoral head prosthesis in rats are shown in Fig. 5a. A rat model of biologic femoral head replacement is presented in Figs. 5b, c (panel a) shows the surgical approach, (panel b) illustrates the rat biologic femoral head replacement, and (panel c) presents the AP & LAT X-ray performance of rat femoral head replacement one month after surgery. The results suggested that the prosthesis was positioned well. The morphology of the synovial tissue around the prosthesis was measured 4 weeks after the biologic femoral head replacement in rats. The synovial tissue around the prosthesis was stained with H&E. The results revealed that gray Al2O3 nanoparticles (white arrows) were scattered and that the number of FLSs was greater than that of the control group (Fig. 5d, panels a, b). Scanning electron microscopy of the synovial tissue around the prosthesis showed Al2O3 nanoparticles on the surface of the synovial tissue (Fig. 5d, panels c, d).
Fig. 5. A rat model of biological femoral head replacement.
a CAD drawings of the femoral head prosthesis strategy in rats; b The schematic and physical objects of the femoral head prosthesis. c Establishing a rat model of biologic femoral head replacement. a Surgical approach, (b) Rat biological femoral head replacement, (c, d) AP & LAT X-ray performance of rat biological femoral head replacement one month after surgery. d 4 weeks after biologic femoral head replacement in rats, the morphology of the synovium around the prosthesis was detected. a, b The synovial tissue around the prosthesis was stained with H&E, showing that gray nanoparticles (white arrows) were scattered. c, d The synovial tissue around the prosthesis was examined by scanning electron microscopy, showing the surface of Al2O3 nanoparticles. Data are expressed as the means ± SD of three independent experiments. (a, Scale bar: 100 μm; c, Scale bar: 50 μm.)
Autophagy mediated periprosthetic osteolysis by regulating osteoclastogenesis in the rat model of biologic femoral head replacement
Compared with Al2O3 nanoparticle group, the Al2O3 nanoparticle + BECN-1 group had thinner synovial tissues, as detected by Masson’s trichrome staining; these tissues were thicker in the Al2O3 nanoparticle + shBECN-1 group (Fig. 6a). The fluorescence intensity indicating the relative amount of LC3B-II protein was observed by immunofluorescence staining. The Al2O3 group and the Al2O3 nanoparticle + BECN-1 group showed a higher fluorescence intensity than the Al2O3 nanoparticle + shBECN-1 group (Fig. 6b). Transmission electron microscopy showed more autophagosomes were induced in the Al2O3 nanoparticle group and the Al2O3 nanoparticle + BECN-1 group, whereas the Al2O3 nanoparticle + shBECN-1 group showed a lower expression (Fig. 6c). Immunohistochemical staining implied that CD68 expression was affected by the autophagy level in the synovial tissue surrounding the prosthesis. Compared with the Al2O3 nanoparticle group, the Al2O3 nanoparticle + shBECN-1 group exhibited greater CD68 expression, whereas the Al2O3 nanoparticle + BECN-1 group showed reduced expression. This result suggested that, compared with Al2O3 nanoparticle group, the Al2O3 nanoparticle + BECN-1 group had fewer macrophages whereas the Al2O3 nanoparticle + shBECN-1 group had more (Fig. 6d). TRAP staining was used to observe the effects of autophagy levels on the number and area of osteoclasts in the synovial tissue surrounding the prosthesis. The results showed that the autophagy level was inversely proportional to the number and area of osteoclasts in the synovial tissue surrounding the prosthesis (Fig. 6e). Schematic representation of the involved mechanisms illustrating the protective role of autophagy induced by Al2O3 nanoparticles in preventing aseptic loosening (Fig. 6f).
Fig. 6. Autophagy mediated periprosthetic osteolysis in the rat model of biological femoral head replacement.
a Masson’s trichrome staining showed the effects of autophagy levels on synovial tissue thickness around the prosthesis (IM: interface membrane). Scale bar: 50 μm. b The expression of LC3B-II in the synovial tissue of patients experiencing prosthetic loosening, as detected by immunohistochemical staining. Scale bar: 50 μm. c The synovial tissue around the prosthesis was observed by transmission electron microscopy, showing that Al2O3 nanoparticles within fibroblast-like synovial cells induced the formation of different stages of autophagy. Scale bar: 500 nm. d Immunohistochemical staining showed that CD68 expression was affected by the level of autophagy in the synovial tissue surrounding the prosthesis. Scale bar: 50 μm. e TRAP staining to observe the effects of autophagy levels in the synovial tissues around the prosthesis on the number and area of osteoclast formation. Scale bar: 50 μm. f Schematic representation of relevant mechanisms indicating the protective role of autophagy induced by Al2O3 nanoparticles in preventing aseptic loosening. Data are expressed as the means ± SD of three independent experiments. [* compared with Control group (*P < 0.05; **P < 0.01). # compared with Al2O3 + Empty vector group (#P < 0.05; ##P < 0.01)]
Discussion
Aseptic loosening is the second-most serious joint surgery complication, after periprosthetic osteolysis and is the most significant limitation of THA32. The pathogenesis of aseptic loosening of joint prostheses primarily includes interface membranes regarded as foreign bodies by the patient’s body. These debris particles from the prosthesis initiate a reaction that plays a major role in periprosthetic osteolysis and aseptic loosening33. Furthermore, wear particles enter into the periprosthetic tissue and are phagocytosed by macrophages, consequently inducing various important events, including cytokine production and osteoclast activation34. However, fibroblasts, which constitute 70% of the cells in the pseudosynovial membrane, have received much less research attention than macrophages, which constitute only 15% of the cells in this membrane. Fibroblasts secrete RANKL and promote osteoclast formation when stimulated by titanium particles35. Several studies have highlighted the importance of RANKL in osteolysis7,36,37.
To date, limited information has been reported regarding the relationship between autophagy, RANKL, and osteolysis, particularly under conditions where Al2O3 particles surround the hip joints. To gain insight into the mechanism underlying the progress of aseptic loosening caused by Al2O3 particles, the effect of Al2O3 particles on autophagy must be investigated. Then, the relationship between autophagy and osteoclasts can be assessed to reveal the role of RANKL in osteolysis and aseptic loosening.
To ascertain whether Al2O3 particles induced autophagy, we collected serum, synovial fluid, and synovial tissue samples from patients with hOA and rTHA. We then identified fibroblasts and determined the levels of autophagy related-genes, such as LC3II, BECN-1, ATG5, and P62. Higher expression levels of autophagy related-genes were found in the rTHA group compared to the hOA group. A similar trend was observed when the CoC group was compared with the MoM and MoP groups. Numerous studies have reported that autophagy regulates RANKL-induced osteoclast38–40, which is consistent with our findings. Higher autophagy levels were observed in the Al2O3 group, and these levels increased in a time and dose-dependent manner. In contrast, a smaller number and area of osteoclasts was observed in the CoCr and UHMWPE groups. When shBECN-1 interference and BECN-1 overexpression lentiviral vectors were used to block the autophagy pathway and promote autophagy, respectively, the RANKL-induced osteoclast formation showed an opposite trend. We established in vivo a novel rat model of hip replacement and determined the effects of Al2O3 particles on autophagy levels and RANKL expression in the synovial tissue. We assessed the effects of autophagy on RANKL-induced osteoclasts when autophagy was blocked or increased. Accordingly, the in vivo findings were entirely consistent with our in vitro results.
With regard to the relationship between RANKL and osteolysis followed by aseptic loosening, many studies have verified that functional osteoclasts induced by proinflammatory cytokines result in local osteolysis41–43. We identified the same trend in the present study.
Compared with the control group, Al2O3 particles increased the level of autophagy in the synovial tissue and reduced the RANKL expression. The number and area of osteoclasts in the bone–prosthesis interface tissue increased when the autophagy pathway was blocked. This phenomenon is summarized in our schematic representation of the mechanisms.
Overall, our findings demonstrated that RANKL expression in the serum, synovial fluid, and synovial tissues of rTHA patients was higher than in the hOA group and that Al2O3 particles induced fibroblast autophagy to reduce RANKL expression. Decreasing the autophagy level promoted osteolysis and the aseptic loosening of prosthesis, whereas increasing the autophagy level inhibited this trend. Our findings suggest that, to some extent, ceramic prostheses performed better than mental and polyethylene prostheses, particularly with regards to osteolysis and aseptic loosening.
In summary, we report the first in vivo study using a femoral head replacement model in rats to characterize particle-induced osteolysis. The results of this study demonstrate that nanosized Al2O3 particle-induced autophagy attenuates osteolysis in aseptic loosening by negative feedback, reducing the RANKL expression in fibroblasts. This finding indicates that autophagy may be a therapeutic candidate for the prevention and treatment of wear debris-induced osteolysis by inhibiting osteoclast formation and activation. In addition, COC-bearing surfaces may be good choices for younger, more active patients who will need to use their prosthetic joints more over the long term.
Materials and methods
Reagents
Rapamycin (R117), Bafilomycin A1 (B1793), and 3-MA (M9281) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s media (DMEM): nutrient mixture F-12 (12400–024) and fetal calf serum (FCS) (10099–141) were obtained from Gibco (Grand Island, NY, USA). RIPA lysis buffer (P0013C) and protease inhibitor cocktail for general use (P1005) were acquired from Beyotime (Haimen, China). Recombinant M-CSF (400–23) and Recombinant RANKL (400–30) were obtained from PeproTech (Rocky Hill, NJ, USA). Beclin1-shRNA (LV-Beclin1-shRNA), Beclin1 (LV-Beclin1), and control (LV-control) lentiviral vector constructs were generated and produced by GenePharma (Shanghai, China). Polyclonal antibodies against beta-actin (β-actin), CD68, beclin1, LC3II/I, LC3B-II, ATG5, P62, RANKL, OPG, and Vimentin were purchased from Novus Biologicals (Littleton, CO, USA).
Particle preparation
The aluminum particles, which were purchased from Sigma Aldrich, had a mean diameter of less than 50 nm. The samples were soaked separately in ddH2O, anhydrous alcohol, and 70% alcohol for 24 h and then washed with ddH2O three times. Then, the particles were autoclaved for 30 min at 121 °C and 102.9 kPa. Bacterial endotoxin levels (<0.03 EU/mL) were not detected via limulus amoebocyte lysate assay (Sigma-Aldrich, USA). The particles were suspended in phosphate-buffered saline (PBS) at a concentration of 100 mg/mL and refrigerated as stock solutions at 4 °C. For the in vitro and vivo experiments, the particles were further diluted in DMEM/F-12 medium or PBS to attain various concentrations ranging from 10 µg/mL to 1000 µg/mL and ultrasonicated for 20 min prior to their exposure to the cells.
Specimens and patients
Synovial tissues were collected from the hip joints of patients experiencing aseptic loosening after rTHA. Control specimens were obtained from patients with hOA who underwent primary THA operations. The surgeries were performed from 2013 to 2015 in the orthopedic department of Xinhua Hospital, which was affiliated with the Shanghai Jiao Tong University School of Medicine. This study was approved by the ethics committee of Xinhua Hospital. The samples obtained during the operations were prepared only for testing purposes and with signed informed consent from the patients.
Cell cultures and treatments
FLSs were isolated from the synovium of the hip joint of patients with aseptic loosening and hOA during their surgeries. The adipose tissues were removed. Then, the synovial specimens were cut into 1–2 mm3 pieces and repeatedly washed with PBS. The pieces were digested in DMEM-F12 containing 0.25% trypsinethylenediaminetetraacetic acid (catalog number 25300–054; Gibco/Life Technologies, USA) for 1 h and incubated in a digestion mixture containing 0.1% collagenase I (1 mg/mL; Sigma-Aldrich, USA) for 3 h at 37 °C. The suspension was centrifuged at 200xg for 5 min and cultured in DMEM/F-12 medium with 10% FCS and 1% penicillin–streptomycin at 37 °C under 95% air humidity and 5% CO2. The primary human FLSs were obtained and passaged in a six-well plate at a density of 4 × 105 cells. Cells of low passage (P3–P6) were used for further studies according to our previously reported method44.
Lactate dehydrogenase assay
The FLSs were plated in 96-well culture plates at a density of 6 × 103 cells/well and incubated with various concentrations of aluminum particles (0, 10, 100, and 1,000 µg/mL) and puromycin (1 µg/mL) for 12 h. Lactate dehydrogenase (LDH) activity was measured by using the LDH Release Assay Kit (catalog number C0016; Beyotime, China) in accordance with the manufacturer’s instructions.
CCK-8 assay for cell viability
The cells were seeded in 96-well plates at a density of 6 × 103 cells/well and co-cultured with various concentrations of aluminum particles (0, 10, 100, and 1,000 µg/mL) for 0, 6 h, 12 h, 1, 2, 3, and 6 d. Each specified well was then supplemented with 2 µL of 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5 -(2,4-disulfo-phenyl)- 2Htetrazolium (WST-8) using a Cell Counting Kit-8 (CCK-8; catalog number C0037; Beyotime, China). The cells were incubated at 37 °C for 2 h. The absorbance of the optical densities at each time point was determined at 450 nm by using a microplate spectrophotometer (Thermo Scientific, Multiskan FC, Waltham, MA, USA).
Flow cytometry analysis
The FLSs were seeded into six-well plates at a density of 1 × 106 cells/well and cultured with various concentrations of aluminum particles (0, 10, 100, and 1000 µg/mL) for 24 h. After treatment, the cells were stained using the Annexin V-FITC/PI apoptosis detection kit (Invitrogen, USA) in accordance with to the manufacturer’s instructions. The samples were then analyzed by flow cytometry (Beckman Coulter, Miami, FL, USA).
Cell interfering experiment
LV-Beclin1-shRNA, LV-Beclin1, and nontargeting LV-control constructs were generated and produced by GenePharma (Shanghai, China). The FLSs were seeded into 12-well plates at a density of 1 × 105 cells/well overnight. The complete medium was mixed with Polybrene (Sigma-Aldrich, USA) at a final concentration of 5 µg/mL. The lentiviral vector stock was adjusted to 40 MOI in 500 µL of culture medium. After being cultured overnight, the medium was replaced with fresh medium, and the cells were further cultured for 3–5 d. The positive, stable transfectants were selected using puromycin at a concentration of 2 µg/mL and expanded for further analysis.
Histology and immunohistochemistry analysis
The synovial tissues of the hip joint, which were obtained during rTHA and THA operations, were fixed with 4% paraformaldehyde for 24 h. After 4, 8, and 12 weeks, the femurs and hips from each group of Sprague-Dawley (SD) rats were fixed and decalcified for 8 weeks at 4 °C. The tissues were embedded in paraffin and were cut in the coronal or sagittal plane centered over the area of particle erosion. Some of the sections were stained with H&E or Masson’s trichrome, and other sections were immunostained with the respective antibodies of interest. Negative controls were obtained without a specific antibody. The specimens were then observed and imaged under a light microscope (Olympus BX-FLA, Japan).
Immunofluorescence staining
The FLSs were seeded into 24-well plates at a density of 6 × 103 cells/well with sterile glass cover slips. Aluminum particles were added into the well at a concentration of 100 µg/mL for 48 h. After being incubated according to the study design, the cells were fixed with 4% paraformaldehyde for 15 min, blocked with 5% bovine serum albumin (BSA) for 15 min at room temperature, and incubated with Map1-LC3B2 anti-body (NBP1-99115; Novus, USA) in 5% BSA (dilution 1:50) at 4 °C overnight. The treated cells were washed with PBS three times and incubated with a secondary antibody (dilution 1:200), Alexa Fluor 555 donkey anti-mouse IgG (catalog number A0460; Beyotime, China) or Alexa Fluor 488 goat anti-rabbit IgG (catalog number A0423; Beyotime, China), for 30 min at 37 °C. The nuclei were stained with DAPI. The cells were imaged under fluorescence microscopy at 400 × magnification (Olympus BX-FLA, Japan)45.
AD-mRFP-GFP-LC3 for autophagy detection
The FLSs were seeded into 12-well plates at a density of 1 × 105 cells/well overnight. The MRFP-GFP-LC3 adenoviral vector stock, which was purchased from HanBio Technology Co. Ltd. (Shanghai, China), was adjusted to 50 MOI in 500 µL of culture medium. Then, the fibroblasts were incubated with adenoviruses for 2 h at 37 °C, and the culture medium was replaced with fresh medium containing 100 µg/mL aluminum particles. The cells were further cultured for 3 d. Autophagy was observed under fluorescence microscopy (Olympus BX-FLA, Japan) at 12, 24, 48, and 72 h, and the autophagic flux was evaluated by counting the number of GFP and mRFP puncta.
TRAP staining
PBMCs were isolated from the peripheral blood of healthy male subjects and cultured in α-MEM/FBS supplemented with 25 ng/mL M-CSF and various 20%-conditioned media (CM) for 14–21 d. The culture medium and the supplemented factors were exchanged every 3 d for various CM. The CM were obtained from cultured FLSs under various conditions, including no aluminum particles + no LV-vector particles (Group A, negative control), aluminum particles (Group B, 100 µg/mL), aluminum particles + control LV-vector particles (Group C, 100 µg/mL), aluminum particles + LV-Beclin1 (Group D, 100 µg/mL), aluminum particles + LV-Beclin1-shRNA (Group E, 100 µg/mL), and aluminum particles + RANKL (Group F, 50 ng/mL, positive control). The cells and the sections of femoral samples were stained with tartrate-resistant acidic phosphatase (NO. 387, Sigma-Aldrich, USA) in accordance with the manufacturer’s instructions. The sections were then stained with or without hematoxylin46. TRAP-positive cells exhibiting three or more nuclei were considered osteoclasts and examined by light microscopy. These TRAP-positive cells (middle panel) revealed osteoclastic resorption pits on the bone disks.
Real-time polymerase chain reaction
The mRNA was extracted from the cells and hip synovial tissues of patients using TRIzol reagent (Invitrogen, USA) in accordance with the manufacturer’s instructions. The gene expression level was determined by real-time polymerase chain reaction (RT-PCR) with SYBR Premix Ex Taq (Takara, Japan) and an ABI Prism 7500 sequence detection system (Applied Biosystems, USA). Actb/β-actin primers were used as internal controls. The primer sequence is listed in Table 1. The fold-change in the gene expression with respect to the control was calculated by the 2-△△CT method.
Table 1.
Primer sequences used for real-time PCR for human samples
| Gene | Sequence (sense, antisense: 5′–3′) |
|---|---|
| MAP1LC3B | AGCAGCATCCAACCAAAATC |
| CCGTTCACCAACAGGAAGAA | |
| BECN1 | TCCACAGAAAGTGCCAACAG |
| GTCAAAAAGGTCCCCAGTGA | |
| ATG5 | GCCATCAATCGGAAACTCAT |
| CAGCCACAGGACGAAACAG | |
| SQSTM1(p62) | TAGGAACCCGCTACAAGTGC |
| GAGAAGCCCTCAGACAGGTG | |
| RANKL | GATGAAAGGAGGAAGCACCA |
| TAAGGAGGGGTTGGAGACCT | |
| RANK | GCCTTTCTCTTGGCATCATC |
| TGCTTTTCCCCAGTATTTGC | |
| OPG | CAGGCACTTGAGGCTTTCA |
| TTGGGGTTTATTGGAGGAGA |
Western blot analysis
The cells and tissues were treated according to the study design and harvested at the specified time points. The samples were sonicated and lysed in RIPA lysis buffer with a protein inhibitor cocktail for 30 min on ice and centrifuged at 12,000xg for 10 min at 4 °C. Then, the total protein was collected, measured, and denatured. Approximately 60 µg of the total protein was subjected to SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with TBS (50 mM Tris, 200 mM NaCl, 0.2% Tween-20) and 5% nonfat milk powder for 2 h. The membranes were then incubated with the primary antibody overnight at 4 °C, followed by horseradish peroxidase (HRP)-conjugated anti-rabbit IgG or HRP-conjugated anti-mouse IgG antibodies (Beyotime, China) for 1 h. The membranes were detected for enhanced chemiluminescence (MultiSciences Biotech, China), and the resulting autoradiograms were quantified by densitometry with ImageJ software47.
TEM and scanning electron microscopy (SEM)
Synovial tissues were collected from rTHA, THA, and femoral head replacement models in rats treated with aluminum particles for 8 weeks. The obtained synovial tissues were cut into 1–2 mm3 pieces. The FLSs were seeded in six-well plates at a density of 1 × 106 cells/well and treated with aluminum particles (100 μg/mL) for 48 h. Then, the tissues and the cells were fixed with 2.5% glutaraldehyde in 0.1 M sodium dihydrogen phosphate (pH 7.4) for 2 h and with 1% osmium tetroxide for 1 h at 4 °C, and then stained with 2% uranyl acetate in water for 1 h in the dark. After being hydrated with increasing concentrations of ethanol, the samples were embedded in epoxy resin and cut into 80 nm-thick sections. The sections were stained with uranyl acetate and lead citrate, and then observed with a transmission electron microscope (Philips CM, the Netherlands)45,47. The synovial samples were directly used after excision for SEM (QUANTA-250, Philips, Netherlands).
Uncemented femoral components of rats
Ten 16-week-old SD rats weighing 400–450 g were scanned by a high-resolution microCT (SkyScan1172; SkyScan, Belgium) at a resolution of 10 µm. The parameters of the acetabulum, the femoral head, and the proximal femoral medullary cavity were measured and analyzed with a CT Analyzer (Version: 1.15.4.0). The diagrams of the uncemented femoral component were designed with CAD2014 software (version: 19.1.18.0) on the basis of the characteristics of the proximal femoral anatomical morphology. Double Medical Co., Ltd. (Xiamen, China) fabricated the femoral components, which consisted of a 15 mm and 130° neck shaft angle titanium alloy femoral prosthesis and a 3.8 mm cobalt–chromium–molybdenum alloy femoral head.
Femoral head replacement model in rats
Sixteen-week-old SD rats weighing 400–450 g were used in our experiment. The animals were randomly divided into four groups: group A, femoral head replacement/ 50 µL PBS (control group); group B, femoral head replacement/aluminum particles (50 µL, 10 µg/mL); group C, femoral head replacement/aluminum particles (25 µL, 20 µg/mL)/Beclin1 (25 µL); and group D, femoral head replacement/aluminum particles (25 µL, 20 µg/mL)/LV-Beclin1-shRNA (25 µL). Groups A–D received the specified PBS, aluminum particles, and lentivirus vectors every 2 weeks through intra-articular hip injection until the animals were sacrificed.
The rats were anesthetized intraperitoneally with 1% sodium pentobarbital (50 mg/kg, body weight) and placed in prone position. The hibateral hind limb and half of the lower back of each animal was shaved and sterilized. A 2-cm-long vertical incision centered over the femoral head was made. The subcutaneous fascia was isolated, and the gluteus superficialis muscle was split in the direction of its muscle fibers by using vascular forceps. Then, the gluteus medius and the biceps femoris muscle were exposed and dissected along the muscle gap. The sciatic nerve was exposed and passed on the internal obturator muscle, which covered the posterior hip joint capsule. The capsule was opened with a T-shaped incision to expose the femoral head. The round ligament of the femur was cut, and the femoral head was dislocated. The head was removed. The piriformis fossa, which was located directly over the medullary canal, was the proper entry point. The medullary canal was gradually enlarged with a dentistry burr. Then, a cavity was drilled to a depth of 10 mm with a reamer, and the uncemented femoral component was placed in an approximately 20° anteversion. Finally, the hip stability, the leg length, and the soft tissue tension were assessed. The capsule was repaired, and the remaining tissue was sutured in layers. Buprenorphine was given for postoperative analgesia at a dose of 0.01–0.05 mg/kg (IP, SQ, q12 h), and cefazolin was administered at a dose of 20–40 mg/kg (IM, SQ, q daily) to prevent infection. For in vivo fluorescent labeling, six mice from each group were intraperitoneally injected with calcein (20 mg/kg body weight; Sigma-Aldrich, C0875) at day 12 and 2 days before being sacrificed.
Statistical analysis
The results are presented as the mean ± standard error of at least three independent replications. Statistical analyses were performed using SPSS 16 statistical software (SPSS, Chicago, IL, USA). Multiple comparisons of data among the groups were analyzed by one-way ANOVA, followed by least significant difference testing (Fisher’s exact test). Comparisons between two groups were analyzed by Student’s t-test. A P-value of less than 0.05 was considered statistically significant.
Electronic supplementary material
Acknowledgements
The National Natural Science Foundation of Youth in China (No. 81601866). Three-year planning for promoting clinical skills and innovation in municipal hospitals by develop-center of Shanghai Shenkang Hospital(16CR2036B)
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by G.M. Fimia
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaoling Zhang, Email: xlzhang@sibs.ac.cn.
Lei Shen, Email: shenlei@xinhuamed.com.cn.
Xiaodong Chen, Email: chenxiaodong@xinhuamed.com.cn.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41419-018-0862-9).
References
- 1.Abu-Amer Y, Darwech I, Clohisy JC. Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis Res. Ther. 2007;9(Suppl 1):S6. doi: 10.1186/ar2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao F, et al. Geraniin suppresses RANKL-induced osteoclastogenesis in vitro and ameliorates wear particle-induced osteolysis in mouse model. Exp. Cell Res. 2015;330:91–101. doi: 10.1016/j.yexcr.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Tozun IR, Ozden VE, Dikmen G, Beksac B. Mid-term result of ceramic bearings in total hip arthroplasty. Int Orthop. 2014;38:2027–2031. doi: 10.1007/s00264-014-2387-x. [DOI] [PubMed] [Google Scholar]
- 4.Gallo J, Vaculova J, Goodman SB, Konttinen YT, Thyssen JP. Contributions of human tissue analysis to understanding the mechanisms of loosening and osteolysis in total hip replacement. Acta Biomater. 2014;10:2354–2366. doi: 10.1016/j.actbio.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Y, Jia T, Wooley PH, Yang SY. Current research in the pathogenesis of aseptic implant loosening associated with particulate wear debris. Acta Orthop. Belg. 2013;79:1–9. [PubMed] [Google Scholar]
- 6.Yoo JJ, et al. Revision total hip arthroplasty using an alumina-on-alumina bearing surface in patients with osteolysis. J. Arthroplast. 2013;28:132–138. doi: 10.1016/j.arth.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, et al. The fibroblast expression of RANKL in CoCrMo-particle-induced osteolysis is mediated by ER stress and XBP1s. Acta Biomater. 2015;24:352–360. doi: 10.1016/j.actbio.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Deng Z, et al. SIRT1 protects osteoblasts against particle-induced inflammatory responses and apoptosis in aseptic prosthesis loosening. Acta Biomater. 2017;49:541–554. doi: 10.1016/j.actbio.2016.11.051. [DOI] [PubMed] [Google Scholar]
- 9.Tsertsvadze A, et al. Total hip replacement for the treatment of end stage arthritis of the hip: a systematic review and meta-analysis. PLoS ONE. 2014;9:e99804. doi: 10.1371/journal.pone.0099804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epinette JA, Manley MT. No differences found in bearing related hip survivorship at 10-12 years follow-up between patients with ceramic on highly cross-linked polyethylene bearings compared to patients with ceramic on ceramic bearings. J. Arthroplast. 2014;29:1369–1372. doi: 10.1016/j.arth.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 11.López-López JA, et al. Choice of implant combinations in total hip replacement: systematic review and network meta-analysis. BMJ. 2017;359:j4651. doi: 10.1136/bmj.j4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajpura A, Kendoff D, Board TN. The current state of bearing surfaces in total hip replacement. Bone Jt. J. 2014;96-B:147–156. doi: 10.1302/0301-620X.96B2.31920. [DOI] [PubMed] [Google Scholar]
- 13.Voleti PB, Baldwin KD, Lee GC. Metal-on-metal vs conventional total hip arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J. Arthroplast. 2012;27:1844–1849. doi: 10.1016/j.arth.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 14.de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL acetabular system and the ASR hip resurfacing system: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J. Bone Jt. Surg. Am. 2011;93:2287–2293. doi: 10.2106/JBJS.J.01727. [DOI] [PubMed] [Google Scholar]
- 15.Jeffers JR, Walter WL. Ceramic-on-ceramic bearings in hip arthroplasty: state of the art and the future. J. Bone Jt. Surg. Br. 2012;94:735–745. doi: 10.1302/0301-620X.94B6.28801. [DOI] [PubMed] [Google Scholar]
- 16.Ding Y, Qin CQ, Fu YR, Xu J, Huang DS. In vitro comparison of the biological activity of alumina ceramic and titanium particles associated with aseptic loosening. Biomed. Mater. 2012;7:045019. doi: 10.1088/1748-6041/7/4/045019. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz SM, Lau E, Baykal D, Springer BD. Outcomes of ceramic bearings after primary total hip arthroplasty in the medicare population. J. Arthroplast. 2017;32:743–749. doi: 10.1016/j.arth.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 18.Rambani R, et al. Revision total hip arthroplasty for fractured ceramic bearings: a review of best practices for revision cases. J. Arthroplast. 2017;32:1959–1964. doi: 10.1016/j.arth.2016.12.050. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YF, Zheng YF, Qin L. A comprehensive biological evaluation of ceramic nanoparticles as wear debris. Nanomedicine. 2011;7:975–982. doi: 10.1016/j.nano.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Li Y, Jiao J, Hu HM. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat. Nanotechnol. 2011;6:645–650. doi: 10.1038/nnano.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakai H, et al. Fibroblasts from the inner granulation tissue of the pseudocapsule in hips at revision arthroplasty induce osteoclast differentiation, as do stromal cells. Ann. Rheum. Dis. 2002;61:103–109. doi: 10.1136/ard.61.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross FP, Teitelbaum SL. alphavbeta3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol. Rev. 2005;208:88–105. doi: 10.1111/j.0105-2896.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 23.Chakraborty S, Kloos B, Harre U, Schett G, Kubatzky KF. Pasteurella multocida Toxin Triggers RANKL-Independent Osteoclastogenesis. Front Immunol. 2017;8:185. doi: 10.3389/fimmu.2017.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zauli G, et al. TNF-related apoptosis-inducing ligand (TRAIL) blocks osteoclastic differentiation induced by RANKL plus M-CSF. Blood. 2004;104:2044–2050. doi: 10.1182/blood-2004-03-1196. [DOI] [PubMed] [Google Scholar]
- 25.Kauther MD, et al. RANKL-associated suppression of particle-induced osteolysis in an aged model of Calcitonin and alpha-CGRP deficiency. Biomaterials. 2013;34:2911–2919. doi: 10.1016/j.biomaterials.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 26.Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 2015;16:461–472. doi: 10.1038/nrm4024. [DOI] [PubMed] [Google Scholar]
- 27.Cadwell K. Crosstalk between autophagy and inflammatory signalling pathways: balancing defence and homeostasis. Nat. Rev. Immunol. 2016;16:661–675. doi: 10.1038/nri.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calzolai L, Franchini F, Gilliland D, Rossi F. Protein–nanoparticle interaction: identification of the ubiquitin–gold nanoparticle interaction site. Nano Lett. 2010;10:3101–3105. doi: 10.1021/nl101746v. [DOI] [PubMed] [Google Scholar]
- 30.Peynshaert K, et al. Exploiting intrinsic nanoparticle toxicity: the pros and cons of nanoparticle-induced autophagy in biomedical research. Chem. Rev. 2014;114:7581–7609. doi: 10.1021/cr400372p. [DOI] [PubMed] [Google Scholar]
- 31.Doorn PF, et al. Metal wear particle characterization from metal on metal total hip replacements: transmission electron microscopy study of periprosthetic tissues and isolated particles. J. Biomed. Mater. Res. 1998;42:103–111. doi: 10.1002/(SICI)1097-4636(199810)42:1<103::AID-JBM13>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 32.Tsutsumi R, et al. Differential effects of biologic versus bisphosphonate inhibition of wear debris-induced osteolysis assessed by longitudinal micro-CT. J. Orthop. Res. 2008;26:1340–1346. doi: 10.1002/jor.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pap G, et al. Development and characteristics of a synovial-like interface membrane around cemented tibial hemiarthroplasties in a novel rat model of aseptic prosthesis loosening. Arthritis Rheum. 2001;44:956–963. doi: 10.1002/1529-0131(200104)44:4<956::AID-ANR153>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Catelas I, et al. Induction of macrophage apoptosis by ceramic and polyethylene particles in vitro. Biomaterials. 1999;20:625–630. doi: 10.1016/S0142-9612(98)00214-2. [DOI] [PubMed] [Google Scholar]
- 35.Wei X, et al. Fibroblasts express RANKL and support osteoclastogenesis in a COX-2-dependent manner after stimulation with titanium particles. J. Bone Miner. Res. 2005;20:1136–1148. doi: 10.1359/JBMR.050206. [DOI] [PubMed] [Google Scholar]
- 36.Alexander MY. RANKL links arterial calcification with osteolysis. Circ. Res. 2009;104:1032–1034. doi: 10.1161/CIRCRESAHA.109.198010. [DOI] [PubMed] [Google Scholar]
- 37.Lynch CC, et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. 2005;7:485–496. doi: 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Li RF, et al. The adaptor protein p62 is involved in RANKL-induced autophagy and osteoclastogenesis. J. Histochem. Cytochem. 2014;62:879–888. doi: 10.1369/0022155414551367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung YH, et al. Beclin-1 is required for RANKL-induced osteoclast differentiation. J. Cell. Physiol. 2014;229:1963–1971. doi: 10.1002/jcp.24646. [DOI] [PubMed] [Google Scholar]
- 40.Wu DJ, et al. Autophagy-linked FYVE containing protein WDFY3 interacts with TRAF6 and modulates RANKL-induced osteoclastogenesis. J. Autoimmun. 2016;73:73–84. doi: 10.1016/j.jaut.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu, X. et al. Alexidine dihydrochloride attenuates osteoclast formation and bone resorption and protects against LPS-induced osteolysis. J. Bone Miner. Res. 31, 560–572 (2015). [DOI] [PubMed]
- 42.Zhai Z, et al. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-kappaB and NFATc1 signaling. Biomaterials. 2014;35:6299–6310. doi: 10.1016/j.biomaterials.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 43.Ouyang Z, et al. Hypericin suppresses osteoclast formation and wear particle-induced osteolysis via modulating ERK signalling pathway. Biochem. Pharmacol. 2014;90:276–287. doi: 10.1016/j.bcp.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, et al. Increasing expression of substance P and calcitonin gene-related peptide in synovial tissue and fluid contribute to the progress of arthritis in developmental dysplasia of the hip. Arthritis Res. Ther. 2015;17:4. doi: 10.1186/s13075-014-0513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang YH, et al. Estradiol inhibits osteoblast apoptosis via promotion of autophagy through the ER-ERK-mTOR pathway. Apoptosis. 2013;18:1363–1375. doi: 10.1007/s10495-013-0867-x. [DOI] [PubMed] [Google Scholar]
- 46.Dickerson TJ, et al. Rheumatoid and pyrophosphate arthritis synovial fibroblasts induce osteoclastogenesis independently of RANKL, TNF and IL-6. J. Autoimmun. 2012;39:369–376. doi: 10.1016/j.jaut.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen, C., Yan, J., Erkocak, O. F., Zheng, X. F. & Chen, X. D. Nitric oxide inhibits autophagy via suppression of JNK in meniscal cells. Rheumatology. 53, 1022–1033 (2014). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.