Abstract
Tamoxifen is used to activate tamoxifen-dependent Cre recombinase (CreER) to generate time- and tissue-specific genetically mutant mice. However, tamoxifen is also an active estrogen analogue that binds with higher affinity to estrogen receptors and exhibits anti-apoptosis, anti-inflammation, and antifibrotic properties. Renal ischemia reperfusion (I/R) injury is characterized by increased apoptosis and inflammation, so optimal utility of tamoxifen-inducible CreER genetic systems in I/R model is important. The purpose of this study was to optimize the tamoxifen dose and evaluate its safety and tolerability in the development of mouse I/R injury. Seven-week-old C57/B6 mice were subjected to moderate reversible unilateral I/R and then injected intraperitoneally daily for 5 days with tamoxifen at doses of 50, 100, or 200 mg/kg/day. Regardless of the time of sacrifice, at 5 day or 28 day after I/R injury, there were no differences in pathological damage, apoptosis, inflammation, or the extent of fibrosis between untreated and treated mice from the time point of acute kidney injury (AKI) to subsequently chronic kidney disease. Data above indicated that tamoxifen with a dose among 0 to 200 mg/kg/day was safe and tolerable for mice, without influencing I/R induced kidney injury in mice. The results suggest that tamoxifen-inducible CreER genetic systems can be safely used in the mouse I/R model.
Keywords: Inflammation, tamoxifen, ischemia reperfusion injury, acute kidney injury, interstitial fibrosis
Introduction
The Cre-Lox recombination conditional gene knock-out technology is a site-specific recombinase method for gene deletions, insertions, translocations, and inversions at specific sites in the DNA of cells [1]. With the widespread use of conditional mutagenesis in biomedical research, Cre-Lox recombination has become a powerful platform, which spans many disciplines, for determining gene function in specific cell types and at specific developmental times.
The tamoxifen (TAM)-inducible Cre-ER (CreER) system has been fused to a mutated ligand-binding domain of the human estrogen receptor (ER) resulting in tamoxifen-dependent Cre recombinase that is activated by TAM [2]. When transgenic mice expressing CreER recombinase cross with mice containing a loxP-flanked sequence of interest, the offspring will carry the CreER-lox gene of interest. As CreER is restricted to the cytoplasm, which cannot gain access to the nucleus before exposure to TAM, the offspring show no gene mutant. However, after administration of TAM to the offspring, CreER in these mice is able to penetrate the nucleus and induce a targeted gene mutant at a chosen time and/or in a given tissue.
Previous research has shown that TAM is successfully in the treatment of fibrosclerotic disorders such as idiopathic retroperitoneal fibrosis [3], encapsulating peritoneal fibrosis [4], and fibrosing mediastinitis [5]. Tamoxifen is also effective in the treatment of desmoid tumors [6], suppressing human dermal fibroblast proliferation [7], excessive extracellular matrix (ECM) production in mesangial cells [8], the treatment of renal fibrosis in both the hypertensive nephrosclerosis model [9] and the unilateral ureteral obstruction (UUO) model [10], and attenuating acute liver failure by its anti-inflammation and anti-apoptosis properties [11]. However, little information has been focused on the safety and tolerability of TAM in the CreER transgenic mouse original background mice.
Renal ischemia reperfusion (I/R) is a very common occurrence during multifarious clinical and surgical settings. Characteristics of renal I/R include increased inflammation and apoptosis as well as vital potential risk factors for chronic kidney disease [12]. In this study, our goal was to estimate the value of different concentrations of TAM in mice with renal I/R injury and determine a suitable concentration for the application of TAM in CreER transgenic mice with I/R.
Materials and methods
Animal study
Male C57BL/6 mice (6-8 weeks old, 22-28 g) were purchased from Hua Fukang Experimental Animal Center (Beijing, China) and maintained under specific pathogen-free conditions. After a minimum of 7 days of acclimation, the mice were randomly divided into 4 groups with I/R-induced acute kidney injury (AKI): the sham group (Sham), in which mice underwent similar surgical procedures but without renal ischemia and reperfusion; the I/R with corn oil group (I/R + vehicle), in which mice were subjected to unilateral renal ischemia for 30 min and administered with corn oil; the I/R with 50 mg/kg of TAM group (I/R + 50 mg/kg); and the I/R with 100 mg/kg TAM group (I/R + 100 mg/kg). The I/R-induced chronic kidney disease (CKD) mice were also divided into four groups: the sham group (Sham), the I/R with corn oil group (I/R + vehicle), the I/R with 100 mg/kg TAM group (I/R + 100 mg/kg), and the I/R with 200 mg/kg TAM group (I/R + 200 mg/kg). The corn oil and TAM were injected intraperitoneally for five consecutive days after the unilateral I/R insult. Corn oil was bought from Sigma and TAM (>98.0% purity) was purchased from MedChemExpress.
This study was approved by Guidelines for Experimental Animal Ethical Committee of Huazhong University of Science and Technology. All procedures were approved and performed in accordance with the institutional guidelines for animal care.
Renal I/R model
Firstly, animals were anesthetized with a 1% sodium pentobarbital solution (6 mL/kg) by intraperitoneal injection and then placed in a prone position to maintain their body temperature at 36.8-37.2°C during surgery. After a unilateral dorsal incision to expose the kidneys, the renal artery was occluded using a microvascular clamp (RoBoz Surgical Instrument Company, Gaithersburg, MD, USA) for 30 min. Next, clamps were removed, and the animals were allowed to recover with free access to food and water. The mice subjected to unilateral I/R mice were sacrificed 5 days or 28 days after reperfusion, and kidneys were collected for further experiments.
Assessment of renal function
Blood samples were obtained by removing the eyeball 5 h after the last injection. To assess renal function, blood urea nitrogen (BUN) and serum creatinine (Cr) levels were assayed us-ing the QuantiChromTM Urea Assay Kit (DICT-500) and QuantiChromTM Creatinine Assay Kit (DIUR-500), respectively. The assay kits were purchased from BioAssay Systems (Hayward, CA, USA).
Histological analysis
Paraffin-embedded renal sections (4 μm) were subjected to periodic acid-Schiff and Massons staining as previously reported [29]. Tubulointerstitial fibrosis was analyzed according to the percentage of fibrosis in the tubulointerstitial area using the Image Pro Plus software (MediaCybernetics, Rockville, MD, USA) in more than 10 random fields.
Immunofluorescence
Paraffin-embedded renal sections (4 μm) were deparaffinized in xylene, rehydrated in graded alcohol, and blocked with 10% goat serum for 30 min. The slides were then incubated with antibodies against Kim-1 (R& Systems, USA), LTL (Vector Laboratories, USA), DBA (Vector Laboratories, USA), F4/80 (Abcam, UK), CD3 (Dako, USA), PCNA (Santa Cruz, USA), a-SMA (Abcam, USA), or PDGFR-β (Abcam, USA) at 4°C overnight and subsequently visualized using an F488- or 594-conjugated secondary antibody. The nuclei were counter-stained with DAPI. TUNEL was stained using a kit according to the manufacturer’s instructions (Roche, Switzerland). Color images were obtained under a Nikon fluorescence microscope (Nikon ECLIPSE TE2000-U, Tokyo, Japan).
Western blot
Fifty micrograms of proteins were separated by 12% SDS-PAGE and then electrophoretically transferred onto PVDF membranes. After blocking with 5% non-fat milk for 1 h at room temperature, the membranes were incubated with antibodies in optimal dilutions against Bax, Bcl2, caspase3, PDGFR-β, and α-SMA at 4°C overnight and then incubated with an HRP-conjugated secondary antibody. The target bands were detected using the ECL Plus Western blot kit. The density of the bands was quantified using Lab Works image J software.
Quantitative RT-PCR
Total RNA was extracted from renal tissues using Trizol reagent according to the manufacturer’s instructions (Invitrogen, USA). One microgram of RNA was reverse transcribed into first strand cDNA using the GoScript reverse transcription system (Promega, USA). Quantitative PCR was conducted using the SYBR mastermix (Qiagen, Germany) on the Roche light 480II. Relative mRNA expression levels were calculated using the 2-ΔΔCt method and were normalized to the expression levels of GAPDH. The primer sequences for mice were shown in Table 1.
Table 1.
Primer sequences for RT-PCR
| Genes | Forward and reverse primers |
|---|---|
| Mouse GAPDH | 5’-AGGTCGGTGTGAACGGATTTG-3’ |
| 5’-GGGGTCGTTGATGGCAACA-3’ | |
| Mouse Kim-1 | 5’-ACATATCGTGGAATCACAACGAC-3’ |
| 5’-ACTGCTCTTCTGATAGGTGACA-3’ | |
| Mouse NGAL | 5’-ATTTCCCAGAGTGAACTG-3’ |
| 5’-AATGTCACCTCCATCCTG-3’ | |
| Mouse α-SMA | 5’-TCGCTGTCAGGAACCCTGAGACG-3’ |
| 5’-ATCATCACCAGCGAAGCCGGC-3’ | |
| Mouse PDGFR-β | 5’-AGGAGTGATACCAGCTTTAGTCC-3’ |
| 5’-CCGAGCAGGTCAGAACAAAGG-3’ |
Statistical analyses
All data are expressed as the mean ± SEM. Excel or the Graphpad Prism 5 software (GraphPad Software, La Jolla, CA, USA) was used for statistical analyses. Statistical differences between groups were analyzed by one-way ANOVA followed by Tukey’s post-tests, and P<0.05 was considered significant.
Results
Tamoxifen treatment did not affect mice kidney size, renal function, pathological morphology, or injury after I/R at day 5
To assess the impact of TAM on AKI, mice were treated with TAM for 5 consecutive days at two different doses (50 and 100 mg/kg/day) after unilateral I/R injury (Figure 1A). Firstly, we detected the physical morphology of operated left kidneys. The gross kidney weights and left/right kidney weight ratios showed no difference between the TAM-treated groups and the vehicle-treated group at day 5 after I/R (Figure 1B and 1C), which demonstrated that treatment with TAM did not influence the kidney and body weight after renal I/R injury. Secondly, we tested renal function with blood urea nitrogen (BUN) and serum creatinine (Scr) by specific kits. These mice also displayed similar levels of BUN and Scr (Figure 1D), indicating that TAM did not cause a perceptible change in renal function at these given doses at day 5 after I/R injury.
Figure 1.
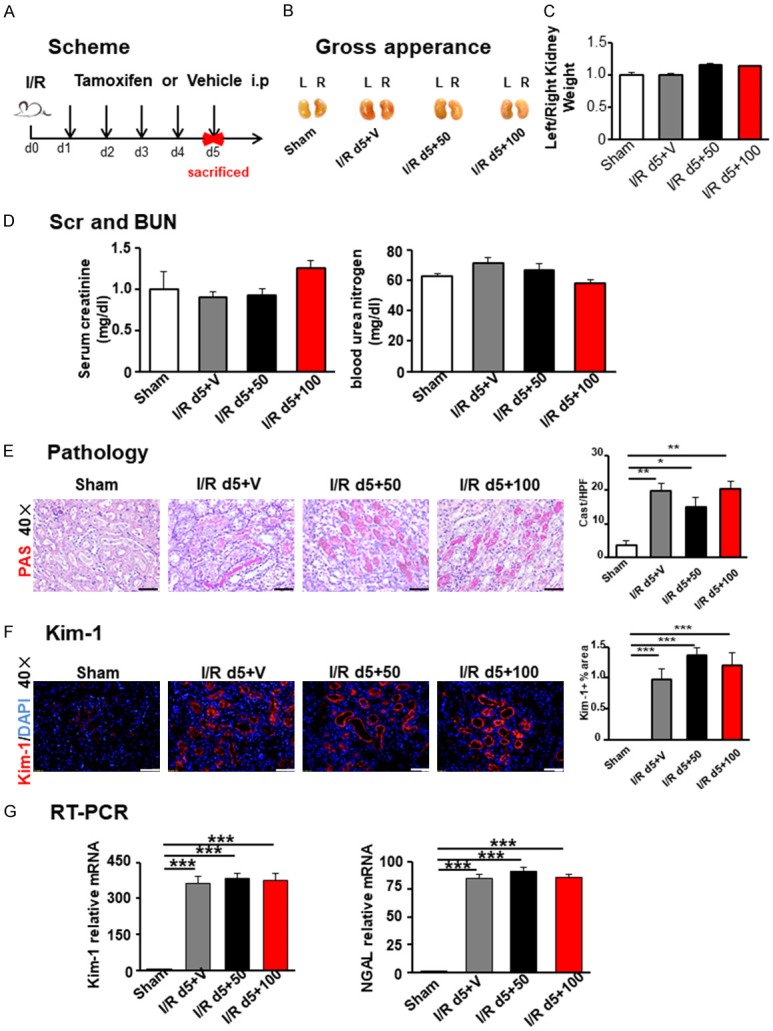
Tamoxifen treatment did not affect I/R-incited renal injury at day 5. A. Scheme of the experimental. B. Kidney gross appearance in each group. C. Statistical analysis of the ratio (left/right) of kidney weight. D. Scr and BUN levels among different groups. E. PAS staining. F. Kim-1 represented injured tubules. Semi-quantitative analysis of immunofluorescence staining of Kim-1 in the kidney, based on the positive area of Kim-1 in 10~15 random areas for all mice collected. Original magnification × 400. Scale bar =50 µm. G. Renal injury biomarkers KIM-1 and NGAL were detected by RT-PCR. Statistical differences between groups were analyzed by one-way ANOVA followed by Tukey’s post-tests. Values are mean ± SEM. (N=4/group. *P<0.05, **P<0.01, ***P<0.001).
A histological analysis at day 5 after I/R injury of kidneys from I/R + Vehicle and I/R + TAM groups compared with those from the sham operation group showed considerably increased cast formation, loss of the brush border, and widespread tubular necrosis at the site of renal corticomedullary junction. However, no differences in kidney histology were detected between groups with TAM administration and the vehicle-treated group (Figure 1E).
Kidney injury molecule 1 (Kim-1) has been identified as a biomarker for kidney injury [13-17]. We calculated the Kim-1 positive area in these kidneys. In accordance with the gross appearance and histological analysis described above, increased Kim-1 positive area was detected in the I/R group compared to the sham group, but there was no difference among I/R groups treated with TAM or vehicle (Figure 1F). RT-PCR for Kim-1 and NGAL, another kidney injury biomarker, were in line with this (Figure 1G). Lotus tetraglonolobus lectin (LTL, Figure 2A and 2D) and dolichos biflorusagglutinin (DBA, Figure 2B and 2E) were used to detect labeled normal proximal tubule and collecting duct, which also showed no difference among surgical groups treated with TAM or vehicle. Collectively, these results demonstrated that the injury induced by I/R did not change with TAM exposure at doses of 50 or 100 mg/kg.
Figure 2.
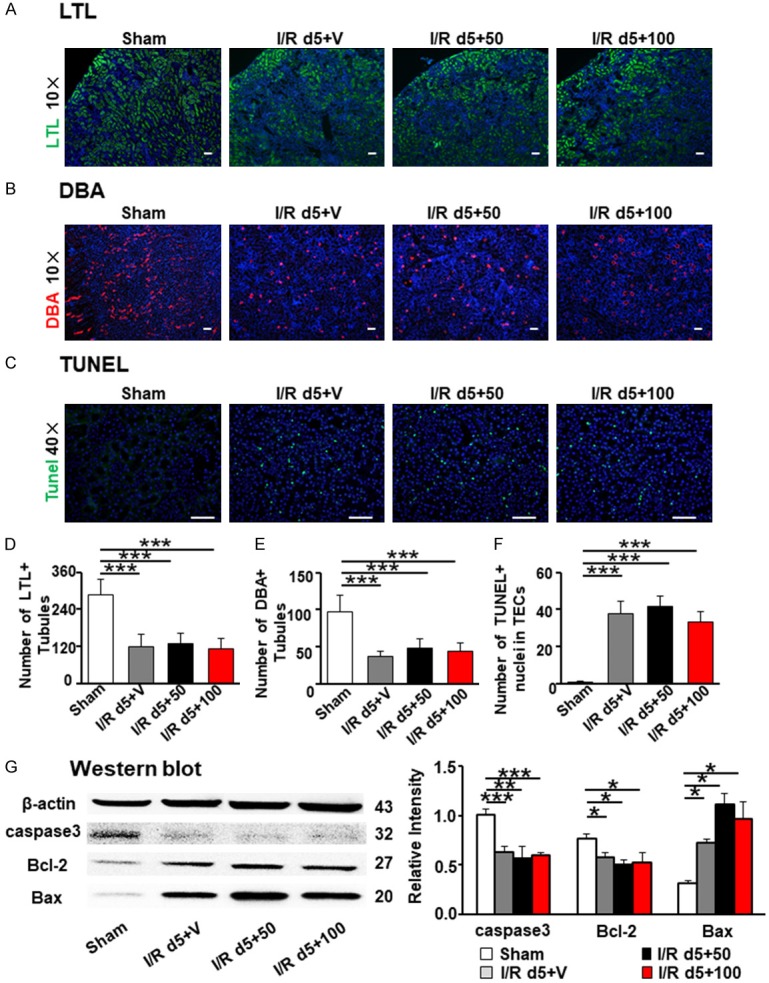
Tamoxifen treatment did not modify tubular injury and apoptosis in kidneys after I/R at day 5. (A) LTL: lotus tetraglonolobus lectin labeled normal proximal tubule. (B) DBA: dolichos biflorusagglutinin represented normal collecting duct. Original magnification × 100 (C) Apoptosis cells were stained by TUNEL. LTL (D), DBA (E) and TUNEL + cells (F) was calculated in 15 visual fields. (G) Expression of Caspase 3, Bax, and Bcl-2 were detected by western blot. Quantitation of Caspase 3, Bax, and Bcl-2 was normalized by β-actin. Statistical differences between groups were analyzed by one-way ANOVA followed by Tukey’s post-tests. Scale bar =50 µm. The data shown are mean ± SEM. (N=4/group. *P<0.05, **P<0.01, ***P<0.001).
Tamoxifen treatment did not modify renal apoptosis, inflammatory infiltration, or tubular epithelial proliferation in kidneys after I/R at day 5
Recent reports have inferred that TAM attenuates acute liver failure through its anti-apoptotic effect [11]. We performed TUNEL staining and evaluated the activity of the apoptosis-regulating genes Bax, Bcl2, and caspase-3. TUNEL-positive cells increased in all surgical groups, but no difference among surgical groups treated with TAM or vehicle (Figure 2C and 2F). In all I/R groups, mice had a lower expression of anti-apoptotic proteins Bcl2, and caspase-3, and an increased expression of apoptosis regulator Bax compared to the sham operation group. However, no difference was detected among I/R groups with TAM administration or vehicle alone (Figures 2G and S1). Therefore, apoptotic variations were not altered by TAM administration in renal I/R injury in mice.
Persistent tubulointerstitial inflammation after I/R has a vital impact on the advancement of AKI to CKD [18]. The most important inflammatory cells in this situation have been identified as macrophages [19]. Previous studies have demonstrated that TAM decreases the infiltration of inflammatory cells and suppresses inflammatory cytokines expression [10]. Therefore, we measured the infiltration of macrophages (F4/80 + cells, Figure 3A and 3D) and T cells (CD3 + cells, Figure 3B and 3E). Our results showed inflammatory cells increased in all I/R operation kidneys. However, the inflammatory infiltration was the same in all I/R groups both with and without TAM administration.
Figure 3.
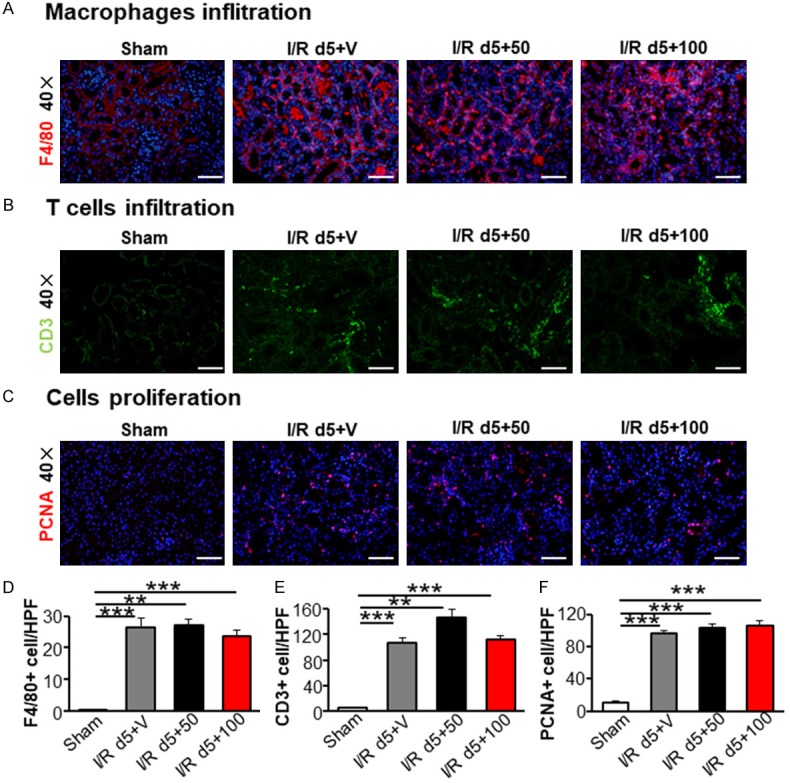
Tamoxifen treatment did not affect inflammatory infiltration and tubular epithelial cells proliferation in kidneys after I/R at day 5. Kidney sections were stained with F4/80 (A), CD3 (B) and PCNA (C). The number of macrophages (D), lymphocytes (E) and proliferating cells (F) were analyzed in 10~15 visual fields. Statistical differences between groups were analyzed by one-way ANOVA followed by Tukey’s post-tests. Scale bar =50 µm. The data shown are mean ± SEM. (N=4/group. *P<0.05, **P<0.01, ***P<0.001).
AKI is accompanied with regeneration and repair of renal tubular epithelial cells (TECs) [20,21]. The proliferating cell nuclear antigen (PCNA) is essential for replication in the nuclei of cells during the DNA synthesis phase of the cell cycle [22] and is a marker for the cell cycle and mitogenesis. We found I/R injury induced an increase in PCNA-positive nuclei in TECs (Figure 3C). However, no difference was detected in the groups with different doses of TAM treatment (Figure 3F). Taken together, these results show that TAM administration exhibited no anti-inflammatory or anti-proliferation activities in renal I/R-incited renal injury.
Tamoxifen treatment did not ameliorate kidney fibrosis after I/R at day 28
Renal fibrosis is the final pathway leading to end stage renal failure and characteristic feature of AKI-induced CKD [23-25], but the effect of TAM on renal I/R-induced fibrosis is little known. We increased the dose of TAM to evaluate the effect of TAM on I/R-induced fibrosis (Figure 4A). Kidney weight and size decreased in the I/R groups with different concentration of TAM (100 and 200 mg/kg) compared to those in the sham group on day 28 after I/R (Figure 4B and 4C). PAS, masson trichrome, and sirius red staining revealed an increase of cast formation, brush loss, collagen deposition, and fibrotic lesions in all I/R groups (Figure 4D-F), but no difference was detected in the groups with different doses of TAM treatment (Figure 4G-I). Western blot and immunofluorescence staining showed the rising expression of α-SMA and PDGFR-β in all I/R groups with TAM administration (Figures 5A, 5B and S2) compared to the sham operation group. However, no differences were found between TAM-treated groups and non-treated group. RT-PCR for α-SMA and PDGFR-β were in line with the results above (Figure 5D). Therefore, TAM treatment exhibited no effect on renal fibrosis after ischemic injury.
Figure 4.
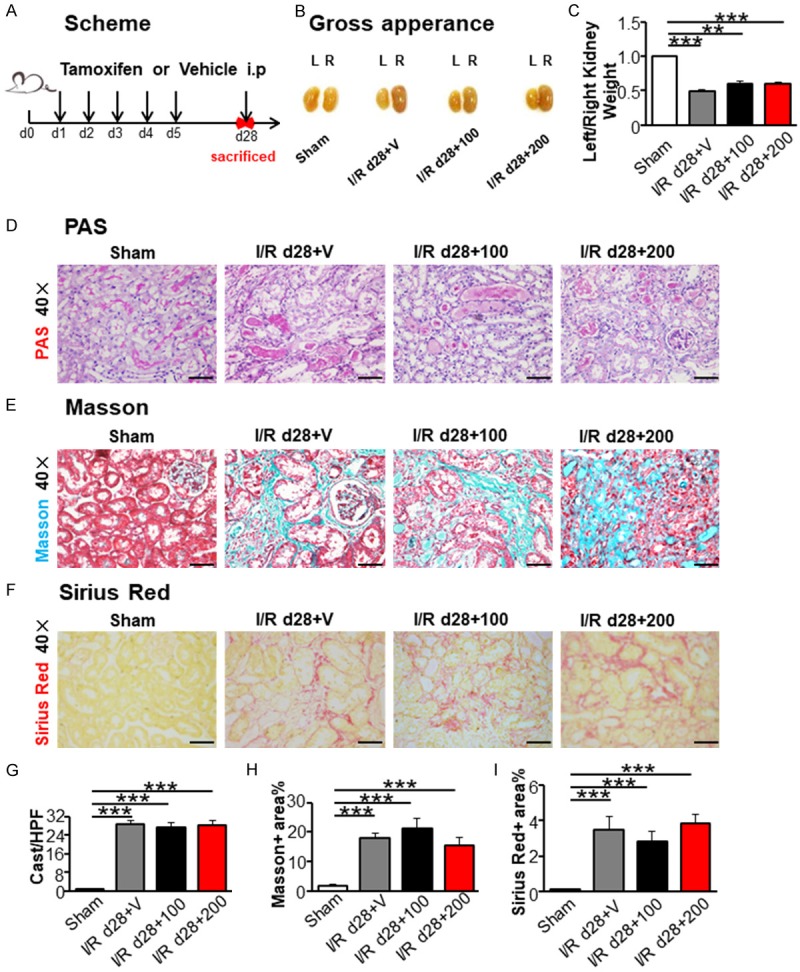
Tamoxifen treatment did not ameliorate renal injury induced by I/R at day 28. (A) Scheme of the experimental plan. (B) Kidney gross appearance from mice in each group. (C) The ratio (left/right) of kidney weight. (D) PAS staining. Masson trichrome staining (E) and Sirius red staining (F) representing interstitial collagen deposition, original magnification × 400. Graph (G-I) presented quantitative data of renal cast formation, Masson positive area and Sirius red positive area. Scale bar =50 µm. Statistical differences between groups were analyzed by one-way ANOVA followed by Tukey’s post-tests. Values were mean ± SEM. (N=4/group. *P<0.05, **P<0.01, ***P<0.001).
Figure 5.
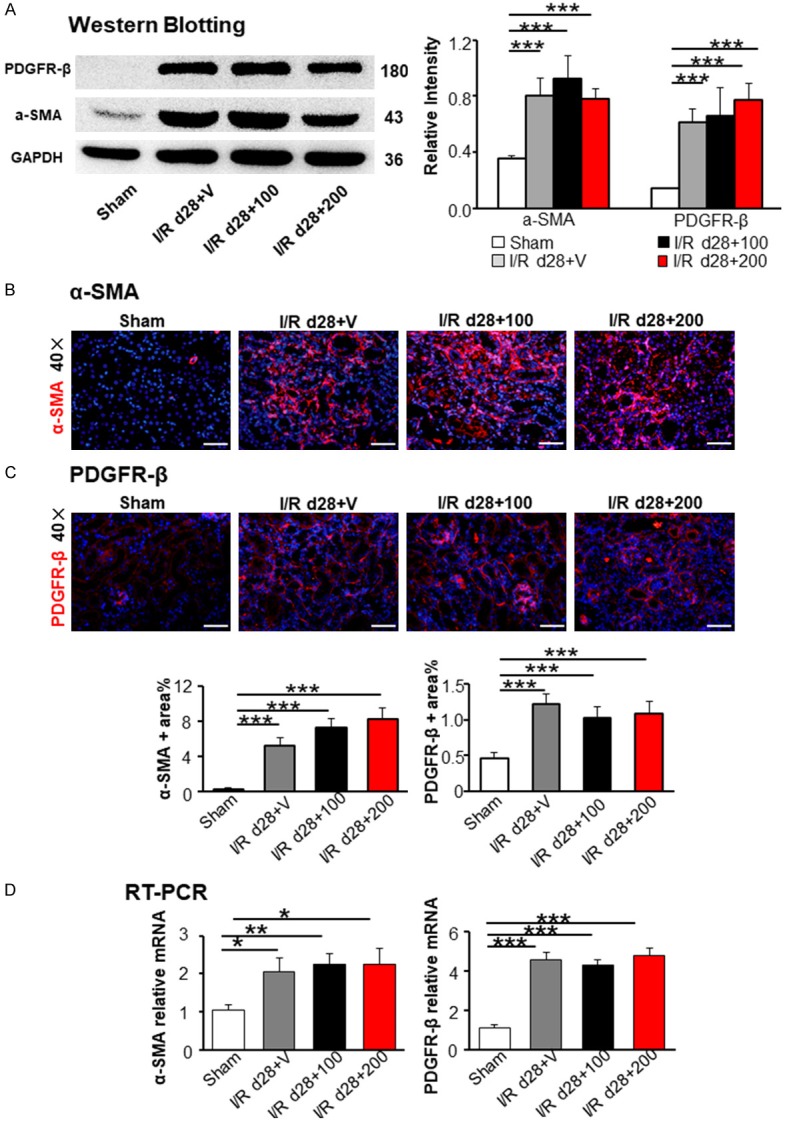
Tamoxifen treatment did not mitigate I/R-induced chronic renal fibrosis. (A) Western blot analysis of fibrotic markers α-SMA and PDGFR-β. Immunofluorescence staining presented the expression of α-SMA (B) and PDGFR-β (C) in the kidney. Semi-quantitative analysis of immunofluorescence staining of α-SMA and PDGFR-β in the kidney, based on the positive area of α-SMA and PDGFR-β in 10~15 random areas for all mice collected. (D) Renal fibrosis biomarkers α-SMA and PDGFR-β were detected by RT-PCR. Statistical differences between groups were analyzed by one-way ANOVA followed by Tukey’s post-tests. The data shown are mean ± SEM. (N=4/group; *P<0.05; **P<0.01; ***P<0.001).
Discussion
In this study, we showed that administration of TAM at doses among 0 to 200 mg/kg/day to mice following I/R-incited renal injury did not affect the development of apoptosis, inflammatory infiltration, proliferation, or interstitial fibrosis in kidney. These results provided a possible and referenced safe and tolerable dose of TAM in mice I/R model.
As previously presented, intraperitoneal administration of TAM to renal I/R-injured mice (daily administration for 5 days with a dose of either 50 or 100 mg/kg tamoxifen) showed no significant difference in the infiltration of macrophages and T cells, which was quite consistent to the reported result in hypertensive nephrosclerosis following TAM citrate treatment at 10 mg/kg [9].
However, our results presented appeared to contradict with a recent study by Zhang et al. [11], which showed that treatment with TAM attenuated acute liver failure by antagonizing apoptosis. It should be noted that TAM is metabolized in liver, thus some underlying mechanisms need further exploration.
Previously, TAM has been successfully used in various models of inflammation in which an anti-inflammatory activity of TAM was suspected. For example, subcutaneous administration of TAM in autoimmune NZB/W F1 mice decreases renal inflammation and alleviates renal injury [26]. The differences between this study of inflammation and our study are the TAM dose and administration scheme (22 mg/kg, every 2 weeks). In another study, oral gavage administration of a single dose of TAM (50 mg/kg) for 5 days before a UUO operation and continued TAM administration for 14 days postoperatively was shown to ameliorate UUO-induced macrophage infiltration [10]. Collectively, these studies have demonstrated that TAM, in comparable dosages yet by different administration routes, confers different effects in various inflammatory disease models.
In addition, a number of studies have shown antifibrotic effects of TAM treatment in different diseases in different models [3-5,8-10]. Similar to our findings, Falke et al. have shown that TAM does not attenuate fibrosis in male mice and confounded fibrosis studies in female mice [27]. All of the mice in our study were male, which might be the reason we have different results from other trials. Ischemia reperfusion injury is a reversible process with inflammation and oxidative damage. Therefore, the differences between our study and the data from other research studies might be explained by differences in animal model and TAM delivery system. These results imply further studies of renal fibrosis in different models of TAM-inducible CreER genetic systems should be conducted.
In conclusion, we provided evidences demonstrating the safety and tolerability of TAM in mice AKI and subsequently CKD induced by I/R-incited renal injury. It should be noted that this study examined the effect of TAM on I/R in male mice only. Female have been reported to gain better renal ischemia tolerance and kidney transplantation outcomes [28] and TAM is well-known as an active estrogen analogue, so further research should be focused on the female mice. Notwithstanding its limitation, this study confirmed that the application of TAM with the doses of 0, 50, 100, and 200 mg/kg is safe and tolerable for I/R-incited renal injury in mice, which suggesting the safety of TAM-inducible CreER genetic systems in the mouse I/R model.
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (grants 81570669, 81570615, 81770681, 81700597 and 91742204), Natural Sciences Foundation of Hubei Province of China (2016CFB627) and Wuhan health and family planning commission (2016whzqnyxgg02).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Orban PC, Chui D, Marth JD. Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992;89:6861–6865. doi: 10.1073/pnas.89.15.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci U S A. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Bommel EF, Pelkmans LG, van Damme H, Hendriksz TR. Long-term safety and efficacy of a tamoxifen-based treatment strategy for idiopathic retroperitoneal fibrosis. Eur J Intern Med. 2013;24:444–450. doi: 10.1016/j.ejim.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Korte MR, Fieren MW, Sampimon DE, Lingsma HF, Weimar W, Betjes MG. Tamoxifen is associated with lower mortality of encapsulating peritoneal sclerosis: results of the Dutch multicentre EPS study. Nephrol Dial Transplant. 2011;26:691–697. doi: 10.1093/ndt/gfq362. [DOI] [PubMed] [Google Scholar]
- 5.Savelli BA, Parshley M, Morganroth ML. Successful treatment of sclerosing cervicitis and fibrosing mediastinitis with tamoxifen. Chest. 1997;111:1137–1140. doi: 10.1378/chest.111.4.1137. [DOI] [PubMed] [Google Scholar]
- 6.Hansmann A, Adolph C, Vogel T, Unger A, Moeslein G. High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer. 2004;100:612–620. doi: 10.1002/cncr.11937. [DOI] [PubMed] [Google Scholar]
- 7.Ruffy MB, Kunnavatana SS, Koch RJ. Effects of tamoxifen on normal human dermal fibroblasts. Arch Facial Plast Surg. 2006;8:329–332. doi: 10.1001/archfaci.8.5.329. [DOI] [PubMed] [Google Scholar]
- 8.Neugarten J, Acharya A, Lei J, Silbiger S. Selective estrogen receptor modulators suppress mesangial cell collagen synthesis. Am J Physiol Renal Physiol. 2000;279:F309–318. doi: 10.1152/ajprenal.2000.279.2.F309. [DOI] [PubMed] [Google Scholar]
- 9.Delle H, Rocha JR, Cavaglieri RC, Vieira JM Jr, Malheiros DM, Noronha IL. Antifibrotic effect of tamoxifen in a model of progressive renal disease. J Am Soc Nephrol. 2012;23:37–48. doi: 10.1681/ASN.2011010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim D, Lee AS, Jung YJ, Yang KH, Lee S, Park SK, Kim W, Kang KP. Tamoxifen ameliorates renal tubulointerstitial fibrosis by modulation of estrogen receptor alpha-mediated transforming growth factor-beta1/Smad signaling pathway. Nephrol Dial Transplant. 2014;29:2043–2053. doi: 10.1093/ndt/gfu240. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P, Zhang M, Wan M, Huang X, Jiang Y, Xu S, Luo M. Tamoxifen attenuates Lipopolysaccharide/galactosamine-induced acute liver failure by antagonizing hepatic inflammation and apoptosis. Immunol Invest. 2017;46:284–294. doi: 10.1080/08820139.2016.1250219. [DOI] [PubMed] [Google Scholar]
- 12.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 13.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abulezz S. KIM-1 expression in kidney allograft biopsies: improving the gold standard. Kidney Int. 2008;73:522–523. doi: 10.1038/sj.ki.5002772. [DOI] [PubMed] [Google Scholar]
- 15.Rosen S, Heyman S. Concerns about KIM-1 as a urinary biomarker for acute tubular necrosis (ATN) Kidney Int. 2003;63:1955. doi: 10.1046/j.1523-1755.2003.00944.x. [DOI] [PubMed] [Google Scholar]
- 16.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 17.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209–217. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 18.Zuk A, Bonventre JV. Acute Kidney Injury. Annu Rev Med. 2016;67:293–307. doi: 10.1146/annurev-med-050214-013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baek JH, Zeng R, Weinmann-Menke J, Valerius MT, Wada Y, Ajay AK, Colonna M, Kelley VR. IL-34 mediates acute kidney injury and worsens subsequent chronic kidney disease. J Clin Invest. 2015;125:3198–3214. doi: 10.1172/JCI81166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Humphreys BD, Cantaluppi V, Portilla D, Singbartl K, Yang L, Rosner MH, Kellum JA, Ronco C. Targeting endogenous repair pathways after AKI. J Am Soc Nephrol. 2016;27:990–998. doi: 10.1681/ASN.2015030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonardi E, Girlando S, Serio G, Mauri FA, Perrone G, Scampini S, Dalla Palma P, Barbareschi M. PCNA and Ki67 expression in breast carcinoma: correlations with clinical and biological variables. J Clin Pathol. 1992;45:416–419. doi: 10.1136/jcp.45.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falke LL, Gholizadeh S, Goldschmeding R, Kok RJ, Nguyen TQ. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat Rev Nephrol. 2015;11:233–244. doi: 10.1038/nrneph.2014.246. [DOI] [PubMed] [Google Scholar]
- 24.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124:2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mack M, Yanagita M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 2015;87:297–307. doi: 10.1038/ki.2014.287. [DOI] [PubMed] [Google Scholar]
- 26.Wu WM, Lin BF, Su YC, Suen JL, Chiang BL. Tamoxifen decreases renal inflammation and alleviates disease severity in autoimmune NZB/W F1 mice. Scand J Immunol. 2000;52:393–400. doi: 10.1046/j.1365-3083.2000.00789.x. [DOI] [PubMed] [Google Scholar]
- 27.Falke LL, Broekhuizen R, Huitema A, Maarseveen E, Nguyen TQ, Goldschmeding R. Tamoxifen for induction of Cre-recombination may confound fibrosis studies in female mice. J Cell Commun Signal. 2017;11:205–211. doi: 10.1007/s12079-017-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aufhauser DD Jr, Wang Z, Murken DR, Bhatti TR, Wang Y, Ge G, Redfield RR 3rd, Abt PL, Wang L, Svoronos N, Thomasson A, Reese PP, Hancock WW, Levine MH. Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J Clin Invest. 2016;126:1968–1977. doi: 10.1172/JCI84712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang P, Zhang Y, Pang J, Zhang S, Yu Q, He L, Wagner KU, Zhou Z, Wang CY. Loss of Jak2 impairs endothelial function by attenuating Raf-1/MEK1/Sp-1 signaling along with altered eNOS activities. Am J Pathol. 2013;183:617–625. doi: 10.1016/j.ajpath.2013.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


