Abstract
Sepsis, a life-threatening syndrome with uncontrolled inflammatory response, causes high morbidity and mortality worldwide. Currently, satisfactory treatments on sepsis are still lacking in clinic. Thus, new therapeutic strategies are urgently required. Recently, celastrol, a pentacyclic triterpene extracted from the traditional Chinese medicine Tripterygium Wilfordi plant, attracted great interest for its properties of anti-inflammation, anti-oxidative stress, and metabolism remodeling. However, the effect of celastrol on sepsis is still unclear. In this study, we investigated the effect of celastrol on lipopolysaccharides (LPS)-induced inflammation and organ injuries in mice. Following celastrol pretreatment, mice showed increased mortality rate and aggravated inflammation evidenced by further enhanced inflammatory markers of IL-6, IL-1β, TNF-α, IL-18, MCP-1, and ICAM-1 in circulation, liver, and kidney after LPS treatment. The serum levels of ALT, AST, and LDH were further increased in parallel with the deteriorated liver morphological damage (H&E) and oxidative stress in celastrol-treated mice, indicating an aggravated liver injury. In kidney, the expressions of tubular injury markers of kidney injury molecule-1 (KIM-1) and gelatinase-associated lipocalin (NGAL) were further upregulated along with higher levels of blood urea nitrogen (BUN), creatinine (Cr), and MDA in celastrol-treated mice. These findings not only indicated a detrimental role of celastrol therapy in LPS-induced inflammation and organ injuries but also suggested the restriction of celastrol usage in sepsis patients.
Keywords: LPS, celastrol, inflammation, liver injury, kidney injury
Introduction
Sepsis is a life-threatening syndrome characterized by enhanced inflammatory response accompanied by multiple organ dysfunction or failure [1]. Despite modern antimicrobials, the prevalence of sepsis and sepsis-related mortality remain high worldwide [2-4]. Thus, developing novel therapeutic strategies with powerful adjuvant therapy is urgently required to improve the survival outcome of septic patients.
For the development of the potential candidates of therapeutic drugs, natural medicines serve as the indispensable sources [5,6]. It has been estimated that more than half of the new drugs in the past three decades are natural compounds or their analogs [7]. Among them, celastrol, a pentacyclic triterpene extracted from the roots of Tripterygium Wilfordi plant, has attracted great interest for its potent roles in anti-inflammation, anti-oxidative stress, anti-cancer, and metabolism remodeling [8-10]. Recently, the anti-inflammatory effects of celastrol have been well demonstrated in animal disease models including obesity [11,12], arthritis [13,14], Alzheimer’s disease [15,16], asthma [17], inflammatory bowel disease [18] and systemic lupus erythematosus [19]. However, the role of celastrol in sepsis has not been reported.
In this study, we sought to investigate the therapeutic effects of celastrol on lipopolysaccharides (LPS)-induced sepsis and organ injuries in mice. Unexpectedly, we observed a pro-inflammatory role of celastrol in LPS-induced sepsis model. The findings from current study not only indicated multiple roles of celastrol under different disease conditions but also suggested the restriction of celastrol usage in sepsis patients in clinic.
Materials and methods
Chemicals and kits
LPS (Escherichia coli serotype 055:B5) and celastrol (purity ≥ 98%) were purchased from Sigma-Aldrich (St Louis, MO). ELISA kits of IL-6 (Cat#: DKW12-2060-096), IL-1β (Cat#: DKW12-2012-096) and TNF-α (Cat#: DKW12-2720-096) were from Dakewe Biotech (Beijing, China). MDA assay kit (Cat#: S0131) was bought from Beyotime Biotechnology (Shanghai, China).
Animals
Eight-week-old male C57BL/6 mice were obtained from Nanjing Medical University and were maintained in an air-conditioned room (22 ± 2°C) under a 12 h: 12 h light/dark cycle and allowed water and standard chow ad libitum. All animal experiment procedures were approved by the Nanjing Medical University Institutional Animal Care and Use Committee and carried out in accordance with the Declaration of Helsinki.
Establishment of LPS-induced sepsis model and celastrol treatment
For the test of celastrol toxicity, the mice were randomly divided into control group and celastrol group (N = 5 per group). The mice of celastrol group received intraperitoneal (i.p.) injection of celastrol (1.5 mg/kg/day) in saline for two consecutive days. Same volume of saline was administered to the control mice.
For the test of celastrol effects on LPS-induced sepsis, the mice were randomly divided into three groups (N = 13~15 per group): control group, LPS group, and LPS + celastrol group. The control mice and LPS group received single intraperitoneal (i.p.) injection of saline or LPS (10 mg/kg), while LPS + celastrol group was pretreated intraperitoneally with celastrol (1.5 mg/kg/day) for 24 hours before the challenge with LPS (10 mg/kg). After LPS treatment for another 24 hours, mice of all groups were sacrificed and the blood and tissues were collected for further analyses.
Serum biochemistry
Blood was collected from the inferior caval vein after anesthesia and serum samples were collected after centrifugation at 1,500 rpm for 10 min. BUN, Cr, ALT, AST, and LDH levels were evaluated using a serum biochemical autoanalyzer (Hitachi 7600 modular chemistry analyzer, Hitachi Ltd, USA).
Histopathological evaluation
Tissues were fixed in 4% paraformaldehyde (PFA) for 24 h at room temperature, dehydrated by grading ethanol and paraffin embedded. Subsequently, the liver and kidney tissues were cut into 5 μm sections and stained with Hematoxylin-eosin (H&E) and Periodic Acid Schiff (PAS), respectively. The images were captured with an Olympus BX51 microscopy (Olympus, Center Valley, PA).
Quantitative reverse transcriptase PCR (qRT-PCR)
Total tissue RNA extraction was performed using the RNAiso Plus reagent (TaKaRa Biotechnology Co., Ltd, Dalian, China) according to the manufacturer’s protocol. cDNA was generated from 1 μg total RNA using PrimeScriptTM Reverse Transcriptase (TaKaRa Biotechnology Co., Ltd, Dalian, China). Quantitative PCR was subsequently carried out using SYBR Green Master Mix (Vazyme, Nanjing, China) on a QuantStudio 3 Real-time PCR System (Applied Biosystems, Foster City, CA, USA). The primers used for PCR amplification were listed in Table 1. Cycling conditions were 95°C for 10 min, followed by 40 repeats of 95°C for 15 s and 60°C for 1 min. The mRNA was normalized to GAPDH and calculated using delta method from threshold cycle numbers.
Table 1.
The sequences of primers used in real-time PCR
| Name | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| GAPDH | GTCTTCACTACCATGGAGAAGG | TCATGGATGACCTTGGCCAG |
| IL-6 | GGCAATTCTGATTGTATG | GACTCTGGCTTTGTCTTT- |
| IL-18 | GACTCTTGCGTCAACTTCAAGG | CAGGCTGTCTTTTGTCAACGA |
| TNF-α | TCCCCAAAGGGATGAGAAG | CACTTGGTGGTTTGCTACGA |
| MCP-1 | GCTCTCTCTTCCTCCACCAC | ACAGCTTCTTTGGGACACCT |
| ICAM | CGCTTCCGCTACCATCAC | GGCGGCTCAGTATCTCCTC |
| KIM-1 | ATGAATCAGATTCAAGTCTTC | TCTGGTTTGTGAGTCCATGTG |
| NGAL | GCAGGTGGTACGTTGTGGG | CTCTTGTAGCTCATAGATGGTGC |
ELISA assay of IL-6, IL-1β, and TNF-α
Serum samples were diluted by 1:50 with enzyme immunoassay buffer. Concentrations of IL-6, IL-1β, and TNF-α were measured by ELISA kits (Dakewe Biotech, Beijing, China) in accordance with the manufacturer’s instructions.
Liver and kidney MDA assay
The MDA concentration in liver and kidney tissues were detected with a commercial MDA assay kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. Briefly, tissues were homogenized and centrifuged at 1600 g for 10 min at 4°C. The supernatants were mixed with thibabituric acid (100°C for 15 min) to form MDA-TBA adduct. The MDA levels were determined by measuring the absorbance of MDA-TBA adduct at 532 nm. Protein concentrations of the liver and kidney samples were assessed using a BCA protein assay kit (Beyotime Biotechnology, Shanghai, China, Cat#: P0012) to normalize the MDA level in each sample.
Statistical analysis
All data are represented as mean ± SEM. Statistical analysis was performed using ANOVA analysis followed by a Bonferroni posttest. P < 0.05 was considered statistically significant.
Results
Evaluation of celastrol toxicity in normal mice
Celastrol was previously reported to induce severe cardiotoxicity in zebrafish embryo [20]. Triptolide, another active component in Tripterygium wilfordii Hook and an analog of celastrol, was also reported to impose hepatotoxicity and nephrotoxicity, which restricted its clinical application [21]. Therefore, we assessed the safety of celastrol in mice. The dosage was chosen according to previous reports [22-24].
As shown by the data, serum biochemistry analysis of ALT, AST, LDH, and BUN showed no significant changes after celastrol treatment for 2 days in normal mice (Figure 1A-D). The mRNA levels of inflammatory cytokines such as TNF-α, IL-6, IL-18, MCP-1, and ICAM in liver and kidney were largely unaltered after celastrol therapy (Figure 1E and 1F). Together, these results demonstrated that i.p. injection of celastrol (1.5 mg/kg/day) for 2 consecutive days had no obvious toxicity in mice.
Figure 1.
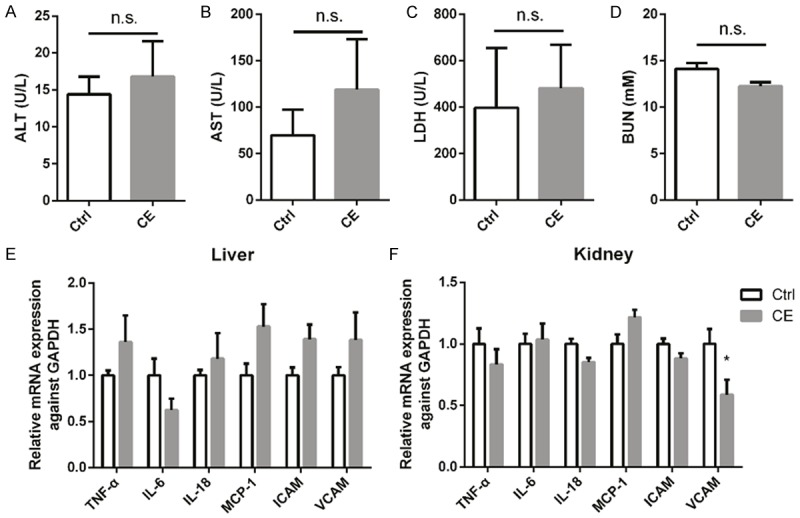
Evaluation of celastrol toxicity in normal mice. Serum levels of ALT (A), AST (B), LDH (C) and BUN (D) in mice received intraperitoneal (i.p.) injection of celastrol (CE) (1.5 mg/kg/day for two consecutive days) or saline. (E, F) mRNA levels of inflammatory cytokines TNF-α, IL-6, IL-18, MCP-1 and ICAM in livers and kidneys of mice treated with CE or vehicle. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni posttest. Data were expressed as mean ± SEM.
Celastrol aggravated LPS-induced mortality and systemic inflammation in mice
Next, we studied the effect of celastrol on LPS-induced sepsis. Strikingly, contrary to our expectation, pretreatment of celastrol decreased the survival rate of LPS-treated mice from 85.7% to 66.7% within 24 h LPS exposure (Figure 2A). Moreover, circulation levels of inflammatory cytokines of IL-6, IL-1β, and TNFα in LPS + celastrol group were further increased compared to LPS group (Figure 2B-D), demonstrating an enhanced systemic inflammation.
Figure 2.
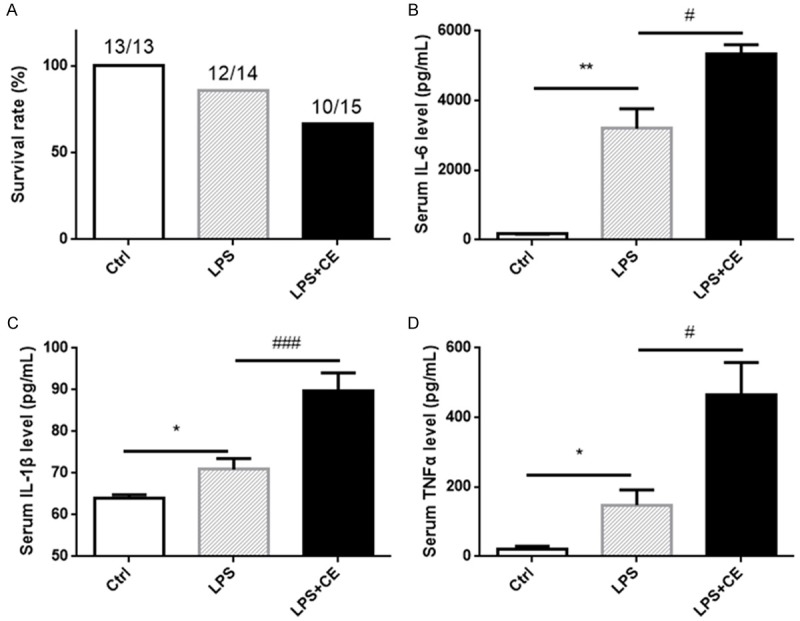
Celastrol increased LPS-induced mortality and systemic inflammation. (A) Survival rate of mice under indicated treatments. The control mice and LPS group received i.p. injection of saline or LPS (10 mg/kg). LPS + celastrol group was pretreated intraperitoneally with celastrol (1.5 mg/kg/day) for 24 h before the LPS challenge (10 mg/kg). Survival rate was estimated after LPS treatment for 24 h. (B-D) Serum level of IL-6 (B), IL-1β (C) and TNFα (D) were measured by ELISA kits. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni posttest. Data were expressed as mean ± SEM. **P < 0.01 and ***P < 0.001 compared to the Ctrl group; #P < 0.05 and ##P < 0.01 compared to the LPS group.
Celastrol aggravated LPS-induced acute liver injury
We then sought to investigate the organ injury in this experimental setting. As shown by the data, the serum levels of ALT, AST, and LDH were significantly elevated in LPS alone group, which was further enhanced in celastrol-treated mice (Figure 3A-C), suggesting an exacerbation of liver injury. Then liver histopathological changes were detected by hematoxylin-eosin (H&E) staining. Hepatic necrosis obviously occurred in LPS + celastrol group, while LPS alone group showed hepatocyte swelling and mild cellular necrosis (Figure 3D). As oxidative stress and inflammatory cytokines play critical roles in the development of organ injuries, we further examined oxidative stress marker malondialdehyde (MDA) and transcription levels of inflammatory cytokines of TNF-α, IL-6, IL-18, MCP-1 and ICAM. In parallel with the deterioration of liver function and morphological lesions, both oxidative stress and inflammatory response were further enhanced by celastrol in liver (Figure 4A and 4B). These data suggested a detrimental role of celastrol in LPS-induced liver injury in mice possibly through promoting the inflammation and oxidative stress.
Figure 3.
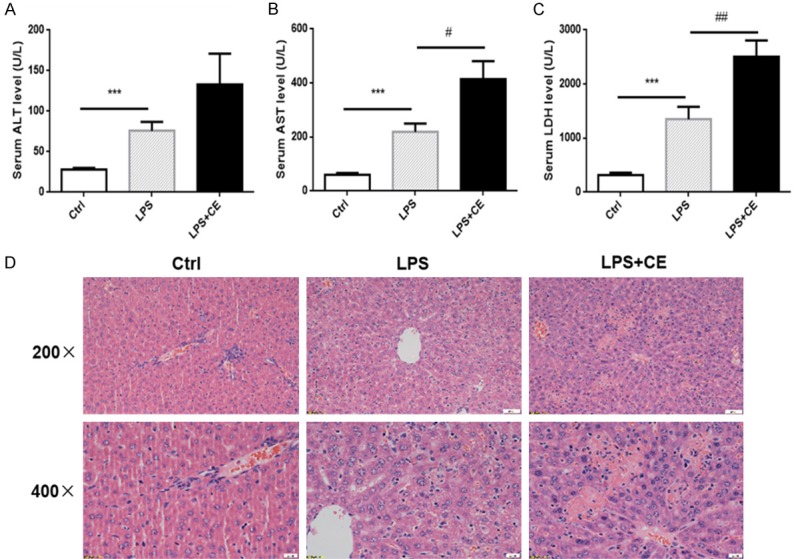
Celastrol treatment aggravated liver injury in LPS-treated mice. (A-C) Serum levels of ALT (A), AST (B) and LDH (C) in mice of corresponding groups. (D) H&E staining of liver sections. Upper panel: original magnification × 200, scale bar 50 μm; lower panal: original magnification × 400, scale bar 20 μm. Hepatic necrosis in LPS + celastrol group was more severe than that in LPS alone group. All statistical analyses were performed by one-way ANOVA, followed by a Bonferroni posttest. Data were expressed as mean ± SEM. ***P < 0.001 compared to the Ctrl group; #P < 0.05 compared to the LPS group.
Figure 4.
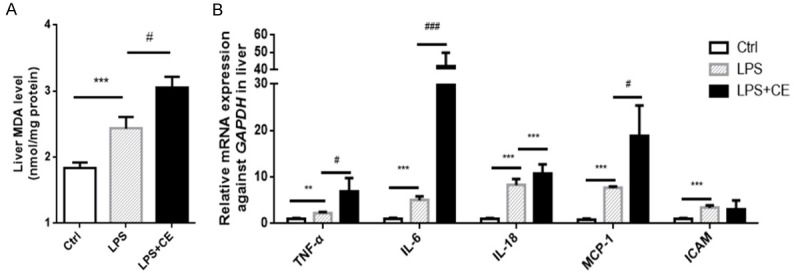
Celastrol enhanced the liver oxidative stress and inflammation in LPS-treated mice. A. Levels of an oxidative stress marker MDA in mouse livers under corresponding treatments. B. Relative mRNA expression of inflammatory cytokines of TNF-α, IL-6, IL-18, MCP-1 and ICAM in kidneys. All statistical analyses were performed by one-way ANOVA, followed by a Bonferroni posttest. Data were expressed as mean ± SEM. **P < 0.01 and ***P < 0.001 compared to the Ctrl group; #P < 0.05 and ###P < 0.001 compared to the LPS group.
Celastrol aggravated LPS-induced acute kidney injury
We further tested the renal function by measuring BUN and serum Cr levels. As shown by the data, BUN and serum Cr were increased in response to LPS challenge, which was further enhanced in celastrol-treated mice (Figure 5A and 5B). Besides BUN and Cr, neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) were found to be more reliable and sensitive as the markers of acute kidney injury [25,26]. Quantitative PCR results showed that NGAL and KIM-1 were robustly elevated (~1000 folds) in LPS group, which was further significantly increased by celastrol treatment (Figure 5C and 5D). Furthermore, Periodic acid-Schiff (PAS) staining indicated tubular structure damage as shown by the loss of brush border and tubular lumen dilation (Figure 5E), which was aggravated by celastrol therapy (Figure 5E). Finally, we also noticed increased oxidative stress and inflammatory response in the kidneys of celastrol-treated mice compared to LPS alone group (Figure 6A, 6B). These results suggested a detrimental effect of celastrol treatment on the kidneys of septic mice.
Figure 5.
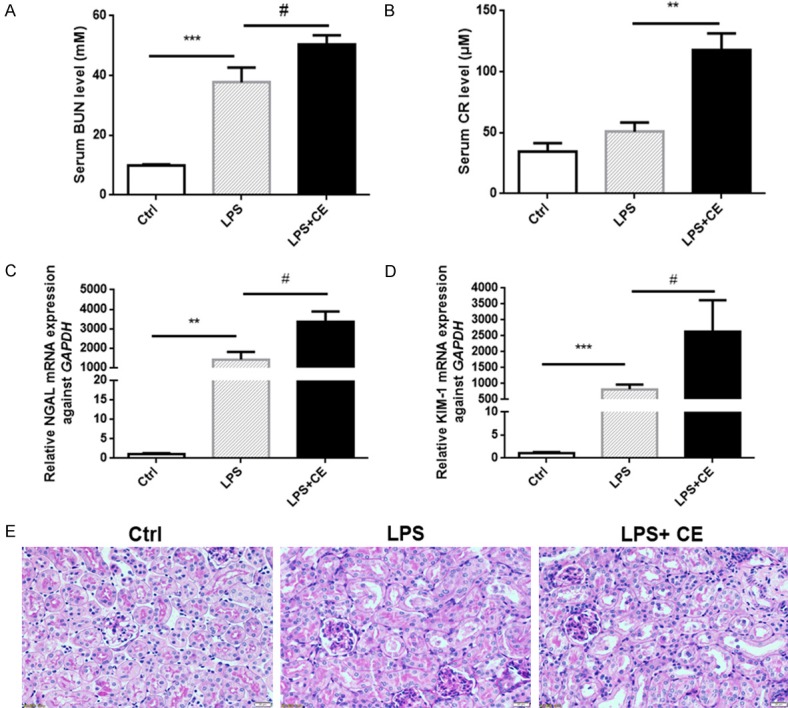
Celastrol treatment aggravated renal tubular injury in LPS-treated mice. (A, B) The serum levels of BUN and Cr in mice of different groups. (C, D) Relative mRNA expressions of tubular injury markers of NGAL (C) and KIM-1 (D) in kidneys. (E) PAS staining (400 ×) demonstrated higher degrees of the loss of tubular brush border and tubular dilatation in LPS + celastrol group compared to LPS alone group. All statistical analyses were performed by one-way ANOVA, followed by a Bonferroni posttest. Data were expressed as mean ± SEM. **P < 0.01 and ***P < 0.001 compared to the Ctrl group; #P < 0.05 compared to the LPS group.
Figure 6.
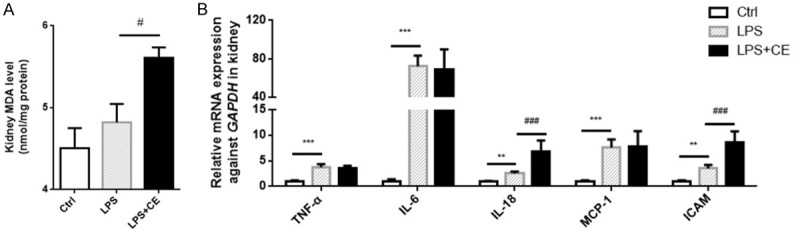
Celastrol enhanced the kidney oxidative stress and inflammation in LPS-treated mice. A. Levels of MDA in the kidneys of mice. B. Relative mRNA expressions of inflammatory cytokines of TNF-α, IL-6, IL-18, MCP-1 and ICAM in kidneys. All statistical analyses were performed by one-way ANOVA, followed by a Bonferroni posttest. Data were expressed as mean ± SEM. **P < 0.01 and ***P < 0.001 compared to the Ctrl group; #P < 0.05 and ###P < 0.001 compared to the LPS group.
Discussion
Identification of bioactive components from natural medicines provides the opportunity for modern pharmacology. Celastrol is a compound that was originally identified from traditional Chinese herbal medicine Tripterygium Wilfordi. Recently, the anti-inflammatory effects of celastrol have been demonstrated in various inflammatory disease models [27,28]. However, to our knowledge, the present study is the first research to evaluate the role of celastrol in LPS-induced sepsis in vivo.
We firstly chose the dose of celastrol according to previous literatures [22-24] and tested the safety of celastrol in normal mice. Results showed that treating mice with celastrol at a dose of 1.5 mg/kg/day for two consecutive days exhibited no obvious toxicity on mice. Based on this dose, we performed further experiments in LPS model. Contrary to previous concept, celastrol promoted LPS-induced inflammation and aggravated liver and kidney injuries in mice.
Referring to the literatures, the protective effect of celastrol against LPS induced inflammation is mainly based on in vitro studies [13,29-31]. However, our study demonstrated an interesting contrast between the in vivo and in vitro effects of celastrol in LPS-induced inflammation. However, the mechanism leading to this difference between the in vivo and in vitro studies is still unknown. Further studies are needed to figure out the molecular mechanisms of celastrol in promoting LPS-induced inflammation in vivo. Nevertheless, compared to the in vitro models using a clean cell line, animal disease models are definitely more complicated and have a better translational potential to the human diseases.
Sepsis also causes damages in multiple organs including kidney, liver, and heart. Our results showed that the organ injury-associated markers including BUN, Cr, KIM-1, NGAL, ALT, AST, and LDH were all significantly increased in LPS group, which was further elevated by celastrol treatment, indicating more severe injuries in kidney and liver. The liver histopathological changes detected by H&E staining also confirmed the aggravated liver injury. Increased levels of inflammatory cytokines and oxidative stress may account for the celastrol effect on the promoted organ injuries to some extent. These results differ from the protective effect of celastrol on ischemia-reperfusion-induced acute kidney injury. During the kidney ischemia-reperfusion, hypoxia condition within kidney results in epithelial cell necrosis and apoptosis and inflammatory cell infiltration [32]. The different pathogenic mechanisms between ischemia-reperfusion- and LPS-induced kidney injury may account for the different response to celastrol treatment. Certainly, as a multi-target drug, celastrol might play different roles under different conditions.
In summary, treatment with a safe dose of celastrol in normal mice deteriorated LPS-induced sepsis in mice. The increased systemic, liver, and kidney inflammation, as well as the organ injuries were obviously noticed in LPS plus celastrol group compared to the animals with LPS alone treatment. Our findings alerted the restriction of celastrol usage under a sepsis condition in clinic.
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (Nos. 81600557, 81600532, 81600352, 81670647, and 81570616), the National Key Research and Development Program (No. 2016YFC0906103) and the China Postdoctoral Science Foundation (No. 2018M632342).
Disclosure of conflict of interest
None.
Abbreviations
- LPS
Lipopolysaccharides
- NGAL
Neutrophil gelatinase-associated lipocalin
- KIM-1
Kidney injury molecule-1
- BUN
Blood urea nitrogen
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- LDH
Lactate dehydrogenase
- H&E
Hematoxylin-eosin
- PAS
Periodic acid-Schiff
- MCP-1
Monocyte Chemoattractant Protein-1
- ICAM-1
Intercellular adhesion molecule-1
- MDA
Malondialdehyde
- CE
Celastrol
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:2063. doi: 10.1056/NEJMc1312359. [DOI] [PubMed] [Google Scholar]
- 3.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 4.Jawad I, Luksic I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health. 2012;2:010404. doi: 10.7189/jogh.02.010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao H, Zheng X, Wang G. Insights into drug discovery from natural medicines using reverse pharmacokinetics. Trends Pharmacol Sci. 2014;35:168–177. doi: 10.1016/j.tips.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Lou HX. Strategies to diversify natural products for drug discovery. Med Res Rev. 2017:1–40. doi: 10.1002/med.21474. [DOI] [PubMed] [Google Scholar]
- 7.Li JW, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Shi C, Yang X, Yang M, Sun H, Wang C. Celastrol suppresses obesity process via increasing antioxidant capacity and improving lipid metabolism. Eur J Pharmacol. 2014;744:52–58. doi: 10.1016/j.ejphar.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Geng C, Liu X, Li M, Gao M, Liu X, Fang F, Chang Y. Celastrol ameliorates liver metabolic damage caused by a high-fat diet through Sirt1. Mol Metab. 2017;6:138–147. doi: 10.1016/j.molmet.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu M, Luo Q, Alitongbieke G, Chong S, Xu C, Xie L, Chen X, Zhang D, Zhou Y, Wang Z, Ye X, Cai L, Zhang F, Chen H, Jiang F, Fang H, Yang S, Liu J, Diaz-Meco MT, Su Y, Zhou H, Moscat J, Lin X, Zhang XK. Celastrol-induced Nur77 interaction with TRAF2 alleviates inflammation by promoting mitochondrial ubiquitination and autophagy. Mol Cell. 2017;66:141–153. e146. doi: 10.1016/j.molcel.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161:999–1011. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo D, Guo Y, Cheng Y, Zhao J, Wang Y, Rong J. Natural product celastrol suppressed macrophage M1 polarization against inflammation in diet-induced obese mice via regulating Nrf2/HO-1, MAP kinase and NF-kappaB pathways. Aging (Albany NY) 2017;9:2069–2082. doi: 10.18632/aging.101302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanjundaiah SM, Venkatesha SH, Yu H, Tong L, Stains JP, Moudgil KD. Celastrus and its bioactive celastrol protect against bone damage in autoimmune arthritis by modulating osteoimmune cross-talk. J Biol Chem. 2012;287:22216–22226. doi: 10.1074/jbc.M112.356816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cascao R, Vidal B, Jalmari Finnila MA, Lopes IP, Teixeira RL, Saarakkala S, Moita LF, Fonseca JE. Effect of celastrol on bone structure and mechanics in arthritic rats. RMD Open. 2017;3:e000438. doi: 10.1136/rmdopen-2017-000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paris D, Ganey NJ, Laporte V, Patel NS, Beaulieu-Abdelahad D, Bachmeier C, March A, Ait-Ghezala G, Mullan MJ. Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer’s disease. J Neuroinflammation. 2010;7:17. doi: 10.1186/1742-2094-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allison AC, Cacabelos R, Lombardi VR, Alvarez XA, Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–1357. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 17.Kim DY, Park JW, Jeoung D, Ro JY. Celastrol suppresses allergen-induced airway inflammation in a mouse allergic asthma model. Eur J Pharmacol. 2009;612:98–105. doi: 10.1016/j.ejphar.2009.03.078. [DOI] [PubMed] [Google Scholar]
- 18.Jia Z, Xu C, Shen J, Xia T, Yang J, He Y. The natural compound celastrol inhibits necroptosis and alleviates ulcerative colitis in mice. Int Immunopharmacol. 2015;29:552–559. doi: 10.1016/j.intimp.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Zhang YY, Huang XY, Sun YN, Jia YF, Li D. Beneficial effect of tripterine on systemic lupus erythematosus induced by active chromatin in BALB/c mice. Eur J Pharmacol. 2005;512:231–237. doi: 10.1016/j.ejphar.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Liu K, Wang X, He Q, Chen X. Toxic effects of celastrol on embryonic development of zebrafish (Danio rerio) Drug Chem Toxicol. 2011;34:61–65. doi: 10.3109/01480545.2010.494664. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Hu T, Tie C, Qu L, Zheng H, Zhang J. Quantitative proteomics and targeted fatty acids analysis reveal the damage of triptolide in liver and kidney. Proteomics. 2017;17:1700001. doi: 10.1002/pmic.201700001. [DOI] [PubMed] [Google Scholar]
- 22.Chu C, He W, Kuang Y, Ren K, Gou X. Celastrol protects kidney against ischemia-reperfusion-induced injury in rats. J Surg Res. 2014;186:398–407. doi: 10.1016/j.jss.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Koo TH, Yoon H, Jung HS, Jin HZ, Lee K, Hong YS, Lee JJ. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem Pharmacol. 2006;72:1311–1321. doi: 10.1016/j.bcp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Guo L, Luo S, Du Z, Zhou M, Li P, Fu Y, Sun X, Huang Y, Zhang Z. Targeted delivery of celastrol to mesangial cells is effective against mesangioproliferative glomerulonephritis. Nat Commun. 2017;8:878. doi: 10.1038/s41467-017-00834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury-pathophysiological basis and clinical performance. Acta Physiol (Oxf) 2017;219:554–572. doi: 10.1111/apha.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medić B, Rovcanin B, Vujovic KS, Obradovic D, Duric D, Prostran M. Evaluation of novel biomarkers of acute kidney injury: the possibilities and limitations. Curr Med Chem. 2016;23:1981–1997. doi: 10.2174/0929867323666160210130256. [DOI] [PubMed] [Google Scholar]
- 27.Cascao R, Fonseca JE, Moita LF. Celastrol: a spectrum of treatment opportunities in chronic diseases. Front Med (Lausanne) 2017;4:69. doi: 10.3389/fmed.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannaiyan R, Shanmugam MK, Sethi G. Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 2011;303:9–20. doi: 10.1016/j.canlet.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Kim DH, Shin EK, Kim YH, Lee BW, Jun JG, Park JH, Kim JK. Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii. Eur J Clin Invest. 2009;39:819–827. doi: 10.1111/j.1365-2362.2009.02186.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee JY, Lee BH, Kim ND, Lee JY. Celastrol blocks binding of lipopolysaccharides to a Toll-like receptor4/myeloid differentiation factor2 complex in a thiol-dependent manner. J Ethnopharmacol. 2015;172:254–260. doi: 10.1016/j.jep.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Xin W, Wang Q, Zhang D, Wang C. A new mechanism of inhibition of IL-1beta secretion by celastrol through the NLRP3 inflammasome pathway. Eur J Pharmacol. 2017;814:240–247. doi: 10.1016/j.ejphar.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]


