Abstract
Objective: The use of human epidermal growth factor receptor-2 (HER2) as a biomarker for gastric cancer (GC) has greatly helped some patients receive benefit from HER2-targeted therapy. However, the correlation between HER2 and other biochemical markers is unclear. The aim of this study was to examine the relationship between HER2 and lactate dehydrogenase A (LDHA) in GC tissues and GC cells. Methods: The correlation between clinicopathological features and HER2 was analyzed in 179 cases of GC. The expression of HER2 and LDHA was examined by immunohistochemical staining in 12 pairs of GC tissues and by western blotting in seven pairs of fresh GC tissues and adjacent normal tissues. Wound healing, transwell migration assay, quantitative real-time reverse-transcription polymerase chain reaction (RT-PCR), and LDH activity assays were performed with GC cells. Results: HER2 expression and serum LDH levels were closely correlated (P = 0.027) in 179 GC patient cases. Immunohistochemical staining demonstrated a positive correlation between HER2 and LDHA in 12 pairs of GC tissues (P = 0.0308). Knocking down LDHA suppressed cell migration and invasion in GC cells. In addition, HER2 positively regulated hypoxia-inducible factor-1α (HIF-1α) and LDHA. Furthermore, the expressions of HER2, HIF-1α, and LDHA were consistent in 5/7 pairs of fresh GC tissues and adjacent normal tissues as well as in GC cell lines. Conclusions: The HER2-HIF-1α-LDHA axis may serve as the basis for new methods and strategies for the treatment of GC.
Keywords: Gastric cancer, HER2, LDHA, HIF-1α, migration
Introduction
Gastric cancer (GC) is one of the most common malignancies in the world [1,2]. The latest cancer data show that GC morbidity and mortality have already risen to the second place for malignant tumors in China [3]. Patients have no obvious symptoms and signs in the early stages of GC, so the first diagnosis in the majority of patients is advanced stage GC with unresectable metastasis [4]. Chemotherapy and targeted treatment of advanced GC has had little progress in recent years, and the median overall survival (OS) has remained the same at approximately 1 year. Therefore, elucidation of the molecular mechanisms of GC and clinical applications for inhibiting metastasis are the current hot research topics for GC.
Overexpression of human epidermal growth factor receptor-2 (HER2), a member of the ErbB receptor tyrosine kinase family, suggests a high degree of malignancy, rapid disease progress, resistance to radiotherapy and chemotherapy, shortened disease-free survival, and decreased OS. Furthermore, HER2 overexpression is seen in 3.7-20.2% of all patients with GC. Research of anti-HER2 targeted therapies has been encouraging in patients with breast cancer. Studies concerning HER2 and GC have shown that cisplatin resistance in GC cells is associated with HER2 upregulation-induced epithelial-mesenchymal transition [5]. Although HER2 overexpression as an independent prognostic factor for GC remains controversial [6-8], it can be combined with other biomarkers such as JWA (an ADP ribosylation factor like GTPase 6 interacting protein 5), c-MET (a receptor tyrosine kinase that binds with hepatocyte growth factor), and Lauren classification to determine the prognosis of GC [9-11]. Trastuzumab, a monoclonal antibody of HER2, is the first targeted drug showing survival benefit in patients with advanced GC [12].
The ability of malignant tumor cells to increase glycolysis under aerobic conditions is called the Warburg effect [13]. Lactate dehydrogenase A (LDHA) catalyzes the conversion of pyruvate to lactic acid, which is a key step in glycolysis. Hypoxia-inducible factor-1α (HIF-1α) can regulate LDHA by transcription. LDHA is elevated in many types of cancer and is reported to be associated with tumor proliferation, growth, invasion, and metastasis. Inhibition of LDHA can limit the energy supply in cancer cells [14-19], thereby reducing metastasis and invasion of cancer cells. LDHA is overexpressed in most patients with GC, and many studies have suggested that LDHA is a prognostic factor [20,21] and a potential target for treatment in GC [22].
We found that HER2 and LDHA are closely correlated with GC, but the regulatory relationship between HER2 and LDHA in GC has not yet been reported. The aim of this study was to identify the molecular mechanism and clinical significance of LDHA in HER2-mediated progression of GC.
Materials and methods
Cell lines and culture conditions
The AGS and NCI-N87 gastric cell lines (American Type Culture Collection, Manassas, VA, USA) as well as the HGC-27, SGC-7901, BGC-823 and GES-1 gastric cell lines (Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai, China) were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FBS; Gibco, Grand Island, NY, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cultures were maintained at 37°C and 5% CO2 in a humidified atmosphere.
Patients and specimens
A total of 179 GC patients who underwent HER2 testing by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) in the First Affiliated Hospital of Nanjing Medical University from January 2011 to July 2013 were included in the study. The correlations between HER2 and clinicopathological features were retrospectively analyzed. HER2 positivity was defined as IHC 3+ or IHC 2+/FISH+. The exclusion criteria included patients with previous history of acute myocardial infarction, pulmonary embolism, chronic viral hepatitis, cirrhosis, and liver and hematological malignancies. The detailed medical history of each patient was collected from patient records. The data were completed and checked by two persons. In total, 12 pairs of HER2-positive and HER2-negative GC samples that were confirmed by HER2 immunohistochemical staining were obtained from the First Affiliated Hospital of Nanjing Medical University. The immunoreactive score (IRS) was calculated for the LDHA staining analysis by multiplying the staining intensity by the percentage of GC cells as described previously [23]. In addition, seven pairs of fresh histology-confirmed GC tissues and adjacent normal gastric mucosa tissues were obtained from the Yixing City People’s Hospital. Western blotting was employed for determining HER2, HIF-1α, and LDHA expressions. This study was approved by the Research Ethics Committee of the corresponding hospitals.
Plasmids, small interfering RNA, and reagents
The HER2 wild-type (HER2 WT) and its control plasmid vector were purchased from Addgene (Cambridge, MA, USA), and the target gene fragment was sequenced. The HER2 small hairpin RNAs (shRNAs) were synthesized (GeneChem, Shanghai, China). The LDHA small interfering RNA (h) [siRNA (h)]: sc-43893 was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). The HER2 inhibitor CP724714 was purchased from Selleck Chemicals (Houston, TX, USA). The plasmid DNA or siRNA was transiently transfected into cells with Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol.
Cell Counting Kit-8 assay
Cell count was determined using a cell counting kit (CCK8) purchased from Dojindo Laboratories (Kamimashiki-gun, Japan). After adding CCK8 reagent (1:10 ratio) for 2 h, test kit operation steps were followed and the cell absorbance value at 450 nm was assessed.
Western blotting and antibodies
Western blotting was performed as previously described [24]. The antibodies used include monoclonal anti-β-tubulin (1:1000, Beyotime Biotechnology, Jiangsu, Beijing, China), monoclonal anti-JWA (1:500, contract produced by AbMax Biotechnology Company, Ltd., Beijing, China), polyclonal anti-HER2 (1:1000, Santa Cruz), monoclonal anti-HER2 (1:250, Abcam, Cambridge, MA, USA), polyclonal anti-LDHA (1:500, Abgent, San Diego, CA, USA), and polyclonal anti-HIF-1α (1:200, Cayman Chemical, Ann Arbor, MI, USA).
Wound healing assay
The GC cells in a 6-well plate were carefully scratched using 200-μl sterile pipette tips, and cell debris was discarded. Images were taken at 0 and 24 h and analyzed using ImageJ2x software (Rawak Software, Inc., Dresden, Germany).
Transwell assay
The migration assay used 1×105 cells that were seeded onto the non-coated upper chamber. Matrigel-coated transwell inserts with 8.0-μm filters (Corning Inc., Corning, NY, USA) were used for the invasion assay. After culturing for 24 h, cells were fixed by methanol and stained with 0.4% trypan blue staining solution. The migrated cell population was evaluated using ImageJ2x software (Rawak Software, Inc.).
Quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) assay
Total RNA was extracted from cell cultures using the Trizol reagent (Gibco) according to the manufacturer’s instructions. Approximately 1 μg of RNA was used for the reverse transcription reaction with Oligo dT (18T) (Life Technologies, Carlsbad, CA, USA). The cDNA was amplified with the following primers: 5’-GCCGGTGCTGAGTATGTC-3’ (forward) and 5’-CTTCTGGGTGGCAGTGAT-3’ (reverse) for GAPDH; 5’-TGTGACTGCCTGTCCCTACAA-3’ (forward) and 5’-CCAGACCATAGCACACTCGG-3’ (reverse) for HER2; 5’-GAACGTCGAAAAGAAAAGTCTCG-3’ (forward) and 5’-CCTTATCAAGATGCGAACTCACA-3’ (reverse) for HIF-1α; and 5’-ATGGCAACTCTAAAGGATCAGC-3’ (forward) and 5’-CCAACCCCAACAACTGTAATCT-3’ (reverse) for LDHA. The following thermal cycling conditions were used: denaturation at 94°C for 5 min, followed by 36 cycles of denaturation at 94°C for 35 s, annealing at 56°C for 30 s, and then extension at 72°C for 35 s.
LDH activity assay
LDH activity was detected using the LDH Cytotoxicity Assay Kit according to the manufacturer’s protocol (Beyotime).
Statistical analysis
The statistical analyses were performed using STATA 14.0 and GraphPad 6.0 software (GraphPad Software Inc., La Jolla, CA, USA). The data between two groups were analyzed with the Student’s t test. The Spearman test was used to investigate the correlation between HER2 and LDHA. A value of P < 0.05 was chosen to indicate a statistically significant difference.
Results
Correlation between clinicopathological features and HER2 expression in 179 GC patients
The correlations between clinicopathological features and HER2 expression in 179 GC patients are shown in Table 1. The positive rate of HER2 was 16.2% (29/179) overall, 20.3% (13/64) in gastroesophageal junction cancer, and 10.6% (5/47) in gastric antrum cancer. The positive rates of HER2 expression in poorly, moderately, and well-differentiated GC were 10.3% (10/97), 22.2% (18/81), and 100% (1/1), respectively. The correlation between HER2 expression and histological differentiation of GC was statistically significant (P = 0.007; Fisher’s exact test). Interestingly, we found the correlation between HER2 expression and serum LDH level in GC patients was statistically significant (P = 0.027; Fisher’s exact test). Moreover, serum LDH was positively correlated with the tumor maximum diameter (P = 0.035; Fisher’s exact test) and distant metastasis of GC (P < 0.001; Fisher’s exact test).
Table 1.
Comparison of clinicopathological features between HER2-negative and HER2-positive groups and serum LDH-normal and serum LDH-elevated groups in 179 cases
| Variable | Total (n = 179) | HER2 [n (%)] | Serum LDH [n (%)] | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Negative (n = 150) | Positive (n = 29) | P a | Normal (n = 163) | Elevated (n = 16) | P a | ||
| Gender | 0.821 | 0.774 | |||||
| Male | 129 | 107 (82.9) | 22 (17.1) | 118 (91.5) | 11 (8.5) | ||
| Female | 50 | 43 (86.0) | 7 (14.0) | 45 (90.0) | 5 (10.0) | ||
| Age | 0.422 | 0.431 | |||||
| < 60 | 99 | 85 (85.9) | 14 (14.1) | 92 (92.9) | 7 (7.1) | ||
| ≥ 60 | 80 | 65 (81.2) | 15 (18.8) | 71 (88.8) | 9 (11.2) | ||
| Tumor diameter | 0.229 | 0.035 | |||||
| < 5 cm | 92 | 74 (80.4) | 18 (19.6) | 88 (95.7) | 4 (4.3) | ||
| ≥ 5 cm | 87 | 76 (87.4) | 11 (12.6) | 75 (86.2) | 12 (13.8) | ||
| Location | 0.437 | 0.461 | |||||
| Junctionb | 64 | 51 (79.7) | 13 (20.3) | 61 (95.3) | 3 (4.7) | ||
| Body | 55 | 45 (81.8) | 10 (18.2) | 48 (87.3) | 7 (12.7) | ||
| Antrum | 47 | 42 (89.4) | 5 (10.6) | 42 (89.4) | 5 (10.6) | ||
| Diffuse | 13 | 12 (92.3) | 1 (7.7) | 12 (92.3) | 1 (7.7) | ||
| Differentiation | 0.007 | 0.461 | |||||
| Poor | 97 | 87 (89.7) | 10 (10.3) | 86 (88.7) | 11 (11.3) | ||
| Moderate | 81 | 63 (77.8) | 18 (22.2) | 76 (93.8) | 5 (6.2) | ||
| Well | 1 | 0 (0.0) | 1 (100.0) | 1 (100.0) | 0 (0.0) | ||
| Depth of invasion | 0.587 | 0.079 | |||||
| T1+T2 | 30 | 24 (80.0) | 6 (20.0) | 30 (100.0) | 0 (0.0) | ||
| T3+T4 | 149 | 126 (84.6) | 23 (15.4) | 134 (89.9) | 15 (10.1) | ||
| Lymph node metastasis | 1.000 | 0.472 | |||||
| N0 | 28 | 24 (85.7) | 4 (14.3) | 27 (96.4) | 1 (3.6) | ||
| N1-3 | 151 | 126 (83.4) | 25 (16.6) | 137 (90.7) | 14 (9.3) | ||
| Distant metastases | 0.177 | < 0.001 | |||||
| M0 | 130 | 112 (86.2) | 18 (13.8) | 127 (97.7) | 3 (2.3) | ||
| M1 | 49 | 38 (77.6) | 11 (22.4) | 36 (73.5) | 13 (26.5) | ||
| TNM stage | 0.818 | 0.120 | |||||
| I+II | 46 | 38 (82.6) | 8 (17.4) | 45 (97.8) | 1 (2.2) | ||
| III+IV | 133 | 112 (84.2) | 21 (15.8) | 119 (89.5) | 14 (10.5) | ||
| Serum LDH | 0.027 | ||||||
| Normal | 163 | 140 (85.9) | 23 (14.1) | ||||
| Elevated | 16 | 10 (62.5) | 6 (37.5) | ||||
Two-sided Fisher’s exact test;
Gastroesophageal junction.
High expression of LDHA in HER2 positive GC
In order to verify the relationship between HER2 and LDHA in GC tissues shown in Table 1, we examined LDHA in paraffin-embedded histopathological specimens of GC using immunohistochemical staining. The results show that the expression of LDHA in HER2 positive group was significantly higher than that in HER2 negative group (16.7%; P = 0.0308, Spearman rank test; Figure 1A). Typical immunohistochemical staining results for HER2 and LDHA in six patients (cases 1-3: HER2 and LDHA both weak; cases 4-6: HER2 and LDHA both strong) are shown in Figure 1B.
Figure 1.
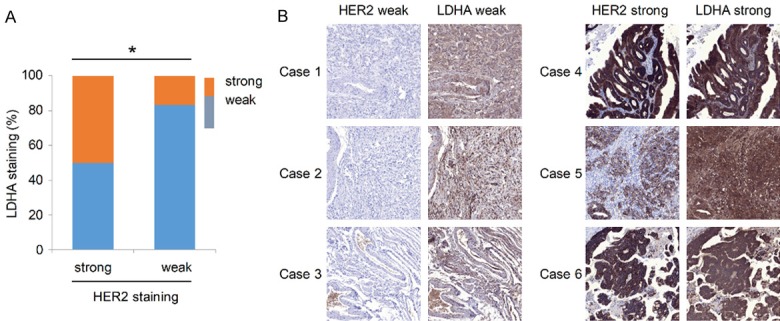
HER2 and LDHA expressions in GC tissues. A. LDHA and HER2 expressions in 12 pairs of paraffin-embedded GC tissues. Key: (*) P < 0.05 (Spearman rank test). B. Typical immunohistochemical staining of HER2 and LDHA in six patients (cases 1-3: HER2 and LDHA both weak; cases 4-6: HER2 and LDHA both strong).
HER2 expression in GC cell lines
In order to choose the appropriate cell lines as experimental models, the expression of HER2 protein was detected in metastatic GC cell lines (HGC-27, SGC-7901, NCI-N87) and primary GC cell lines (BGC-823, AGS). The results (Figure 2A) show that the expression of HER2 protein was higher in metastatic GC cell than in primary GC cell lines. The HER2 expression level was the highest in NCI-N87 among metastatic GC cell lines (Figure 2A and 2B). Immunofluorescence assay results show a positive correlation between LDHA expression and HER2 expression in GC cell lines (Figure 2C). The results show that the expression of LDHA was the highest in HER2-positive NCI-N87 cells, moderate in HGC-27 cells, and lowest in HER2-negative SGC-7901 cells.
Figure 2.
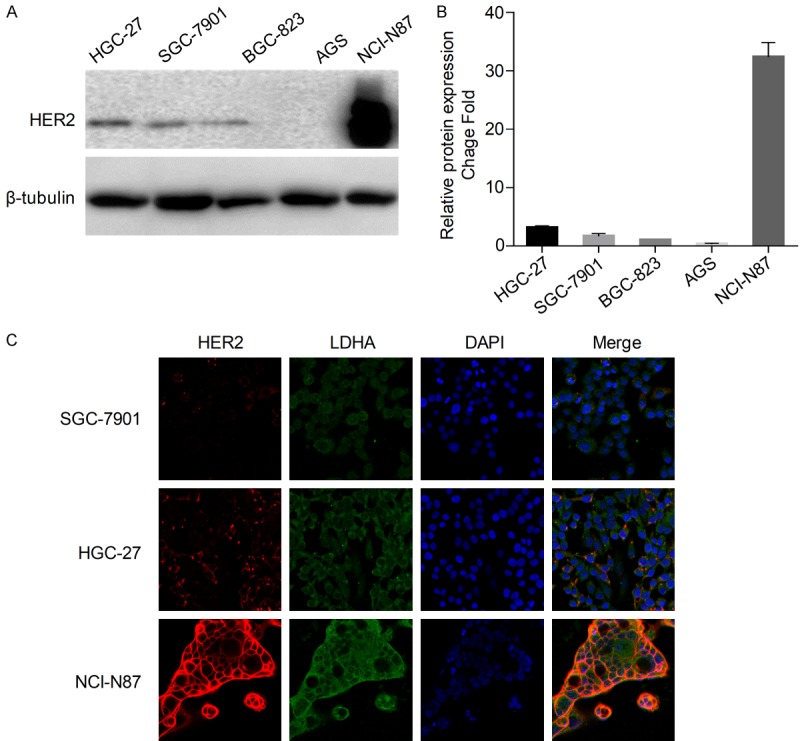
HER2 expression in GC cell lines. A. Detection of HER2 and β-tubulin by western blot analysis of cells from three metastatic GC cell lines (HGC-27, SGC-7901, NCI-N87) and two primary GC cell lines (BGC-823, AGS). B. HER2 bands were normalized to β-tubulin. The data are expressed as the means ± SD from three independent experiments. C. Immunofluorescence imaging of HER2 (red), LDHA (green), and nucleus labeled as DAPI (blue), and the co-localization of the three signals (merge) in SGC-7901, HGC-27, and NCI-N87.
Down-regulation of LDHA inhibits cell invasion and migration in GC cells
Three LDHA siRNAs were verified in the normal gastric epithelium cell line GES-1. The results of qRT-PCR showed that LDHA mRNA levels were significantly decreased after siRNA transfection. In addition, si-LDHA-1 had the best interference effect (P < 0.001, Student’s t test; Figure S1), so it was used for subsequent experiments.
In order to determine the effect of LDHA knockdown on the proliferation of GC cells, we transfected the LDHA siRNA separately into HGC-27 and SGC-7901 cell lines. The proliferation ability of the following groups was detected using a CCK8 assay. The cell proliferation curves of the LDHA siRNA transfected group and the vector control group were similar in both HGC-27 and SGC-7901 cell lines (Figure S2). Thus, the inhibitory effect of LDHA on proliferation of the two GC cell lines was not observed.
Western blotting analysis showed that LDHA protein levels were significantly down-regulated in HGC-27, SGC-7901, and NCI-N87 cell lines following transfection with LDHA siRNA (Figure 3A, 3E and 3I). The wound healing assay revealed that LDHA knockdown inhibited the migration ability of SGC-7901 and HGC-27 cells compared with the control group (Figure 3B and 3F). In addition, the numbers of invasion and migration cells of SGC-7901 and HGC27 were significantly decreased (P < 0.001, Student’s t test) in the siRNA interference group (Figure 3C, 3D, 3G and 3H). The number of migration cells of NCI-N87 was also significantly decreased compared with the control group (Figure 3J and 3K).
Figure 3.
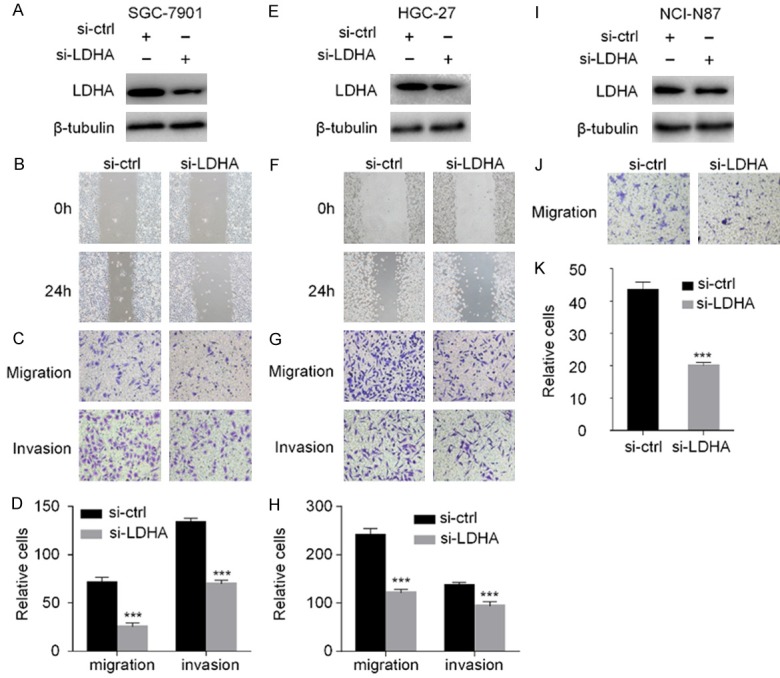
Silencing of LDHA inhibits cell invasion and migration. SGC-7901, HGC-27, and NCI-N87 cells were transfected with LDHA siRNA for 48 h (A, E, I). The expression of LDHA in the three cell lines was detected by western blot analysis (B, F). The wound healing assay was carried out at 0 and 24 h to observe the migration ability of the HGC-27 and SGC-7901 after down-regulation of LDHA (C, G, J). Transwell migration and invasion experiments with SGC-7901, HGC-27, and NCI-N87 were performed and microscopic images (200× magnification) were acquired (D, H, K). Differences of the cell migration and invasion between the RNA interference group and the control group are shown. Each group of cells was counted by five fields of view. The data are expressed as the means ± SD from three independent experiments. The data were analyzed by a two-tailed Student’s t test. ***: P < 0.001.
HER2 regulates HIF-1α and LDHA expression
After transfection with HER2 WT or HER2 shRNA into HGC-27 and SGC-7901 cells, western blotting showed that HER2 expression was consistent with the expression of HIF-1α and LDHA (Figures 4 and S3). Because NCI-N87 is an HER2-positive cell line, we only used HER2 shRNA to downregulate HER2 expression, which down-regulated the protein levels of HIF-1α and LDHA (Figures 4 and S3).
Figure 4.
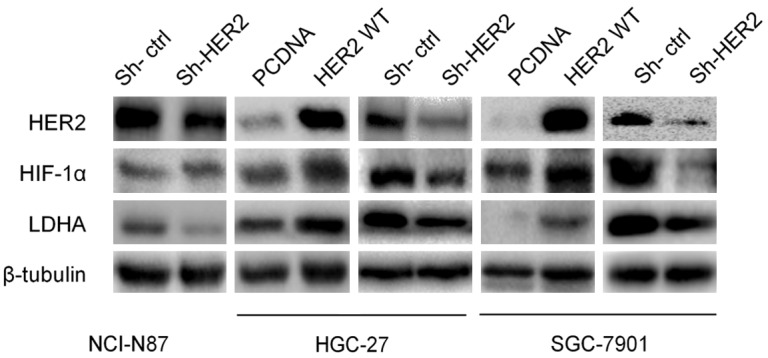
HER2 regulates HIF-1α and LDHA expressions. NCI-N87 was transfected with HER2 shRNA plasmid and control for 48 h. HER2 WT, HER2 shRNA, and control plasmids were respectively transfected into HGC-27 and SGC-7901. Western blot analysis was used to detect HER2, HIF-1α, and LDHA expressions.
HER2 and LDHA regulate the activity of LDH in GC cells
After transfection with HER2 WT plasmids into HGC-27 and SGC-7901 cells, the activity of LDH was increased compared with that of control group (P < 0.05, Student’s t test). Also, the activity of LDH in HGC-27 and SGC-7901 cells was decreased by LDHA siRNA (Figure 5A and 5B). The activity of LDH in NCI-N87 cells was also decreased by LDHA siRNA compared with that of control group (P < 0.05, Student’s t test). After the HER2 activity inhibitor CP724714 (2 μM) was used to treat NCI-N87 cells for 24 h, the activity of LDH was significantly decreased compared with that of control group (P < 0.001, Student’s t test; Figure 5C). CP724714 inhibited HER2 phosphorylated protein expression (Figure 5D) and significantly reduced the number of migration cells of NCI-N87 compared with that of the control group (P < 0.01, Student’s t test) (Figure 5E and 5F).
Figure 5.
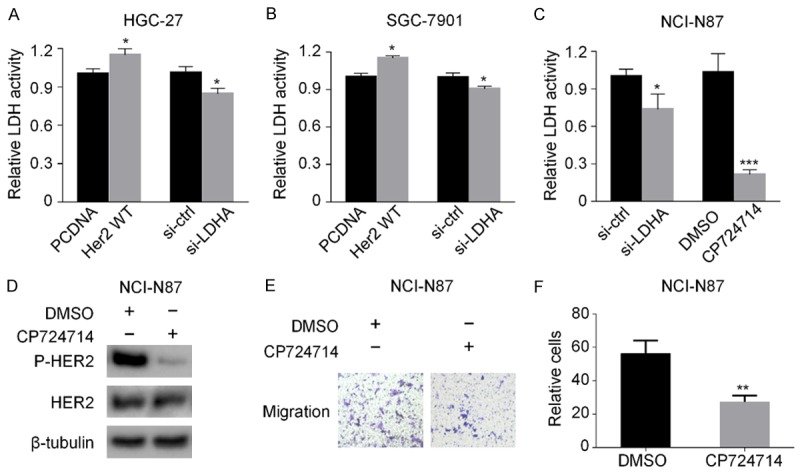
HER2 and LDHA regulate the activity of LDH in GC cells. A, B. HGC-27 and SGC-7901 cells were transfected with HER2 WT or LDHA siRNA, respectively, for 48 h. Then the activity of LDH was detected. C. NCI-N87 cells were transfected with LDHA siRNA or treated with HER2 tyrosine kinase inhibitor CP724714. Then the activity of LDH was detected. D. NCI-N87 cells were treated with CP724714. Then the expressions of HER2 and phosphorylated HER2 were determined by western blot analysis. E. NCI-N87 cells were treated with CP724714, the transwell migration experiments were performed, and microscopic images were acquired (200× magnification). F. The difference of the migrated NCI-N87 cells between the DMSO group and the CP724714 group. The data are expressed as the means ± SD from three independent experiments. The data were analyzed by a two-tailed Student’s t test. Key: (*) P < 0.05, (**) P < 0.01, (***) P < 0.001.
Expressions of HER2, HIF-1α, and LDHA in GC are consistent and significantly higher than those in normal tissues
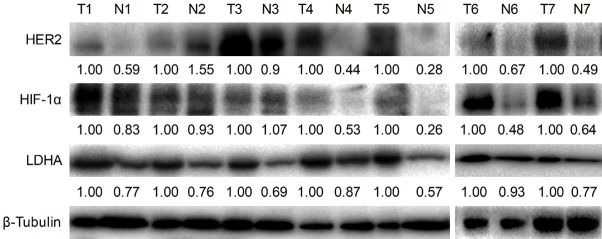
We used seven pairs of fresh GC tissues and the adjacent normal tissues (Yixing City People’s Hospital) to examine HER2 and LDHA levels by western blotting. The results show that the expression of LDHA was significantly increased (100%) in GC tissues compared with adjacent normal tissues, the expressions of HER2 and HIF-1α were significantly higher in cancer tissues than those in adjacent tissues in 6/7 (85.7%) pairs of tissues, and the expressions levels of HER2, HIF-1α, and LDHA were consistent in 5/7 (71.4%) pairs of tissues (Figures 6 and S3).
Figure 6.
HER2, HIF-1α. and LDHA expressions in seven pairs of GC tissues and adjacent normal tissues. Western blot analysis was used to detect HER2, HIF-1α, and LDHA expressions in the seven pairs of GC tissues and adjacent normal tissues. Key: (N) paired non-cancerous gastric tissues, (T) gastric cancerous tissues.
Discussion
The mortality rate of patients with GC in China has risen to the second place among malignant tumors [3]. GC has a high degree of malignancy and heterogeneity with characteristics of “three highs and three lows” (high incidence, high recurrence rate, and high mortality, and low early diagnosis rate, low cure rate, and low 5-year survival rate). Radical surgery is the only potential curative treatment. Because the early symptoms and signs of GC are often not obvious, approximately 70% of GC patients are diagnosed with advanced GC at first diagnosis. Systemic chemotherapy is usually used to treat these patients, yet the median OS remains only 10 to 12 months [25]. Therefore, elucidation of the symptoms and mechanisms of the occurrence and development of GC as well as treatment methods is urgently needed and has important translational research significance.
In recent years, with the rapid development of molecular biology and gene detection technology, predictive molecular markers and targeted therapy drugs have achieved some encouraging results. As an example, activation of the HER2 downstream signaling pathway can promote cell proliferation, differentiation, migration, and anti-apoptosis, thus leading to cancer. Indeed, HER2 positivity is not only a poor prognostic factor but also a target for treatment in breast cancer. HER2-targeted therapy has shown great progress in breast cancer patients [26,27]. Until recently, HER2 overexpression has not been successfully identified as an independent prognostic factor in GC, but more and more studies suggest that HER2 overexpression is associated with the prognosis of GC and can promote tumor progression. Trastuzumab, a monoclonal antibody that interferes with the activation of HER2, is the first drug that has been used for HER2-targeted therapy in advanced GC patients. The results of ToGA (Trastuzumab for Gastric Cancer; an open-label, international, phase 3, randomized controlled trial undertaken in 122 centers in 24 countries) show that HER2 positivity is associated with histological type and primary site of GC. The ToGA trial assessed trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastroesophageal junction cancer. The results show that the median OS was 13.8 months for the combination therapy vs. 11.1 months for chemotherapy alone, indicating that trastuzumab significantly prolonged the survival of HER2-positive advanced GC patients.
Although these results are encouraging, HER2-targeted drug resistance is a common clinical treatment problem in GC patients. Activation of HER2 together with HER3 dimerization or c-MET pathway crosstalk has been reported to lead to activation of the downstream signaling PI3K pathway [28]. The gene changes lead to abnormal activation of HER downstream signaling pathways, epithelial-mesenchymal transformation signal [29], heterogeneity of GC [30,31], and so on. Therefore, it is of significant importance to improve of the therapeutic effect of GC, overcome HER2-targeted drug resistance, and find other innovative treatment drugs.
In the 179 cases in this study, the overall HER2 positive rate was 16.2% and the HER2 positive rate of gastroesophageal junction cancer was higher than that of gastric antrum cancer. The positive expression of HER2 in GC was associated with the degree of histological differentiation. We also found a correlation between HER2 positive expression and elevated serum LDH in GC. In addition, the elevated serum LDH was positively correlated with maximum tumor diameter and distant metastasis.
There are five active LDH isozymes in human tissues and each is composed of two major subunits; namely, M and H (or A and B) homologous or heterotetrameric metabolites. The M and H subunits are regulated by LDHA and LDHB genes, respectively. The M/A subunit is mainly from skeletal muscle, whereas the H/B subunit is mainly from the heart muscle. The five active LDH isoenzymes are LDH1 (4H), LDH2 (3H, 1M), LDH3 (2H, 2M), LDH4 (1H, 3M), and LDH5 (4M). Among these isoenzymes, the higher the M/A subunit content, the more efficient the LDH isoenzyme becomes in catalyzing pyruvate into lactic acid. In contrast, excess of the H/B subunit, especially LDH1 (LDHB), favors the transformation of pyruvate into the tricarboxylic acid cycle (Krebs cycle) to acetyl coenzyme A. LDHA is involved in cell metabolism in normal tissue cells, which regulates cell cycle and functions as a transcription factor. In addition, LDHA is closely correlated with malignancy. The elevated serum LDH is mainly due to LDHA overexpression in tumor cells [32]. LDHA is highly expressed in malignant tumors and can be used as a biomarker for many malignancies, including lymphoma, prostate cancer, renal cell carcinoma, and melanoma [33]. LDHA abnormal upregulation and LDHB downregulation are common features of malignant tumors that promote metabolic switching to aerobic glycolysis and produce lactate as a byproduct. LDHA plays an important role in malignant tumor cells by producing hypoxia and an acidic microenvironment, promoting tumor cell growth, survival, invasion, migration, metastasis, and angiogenesis, and leading to immune escape of tumor cells [33].
HER2 can promote cell glycolysis and growth by up-regulating LDHA via heat shock factor-1 (HSF-1) in breast cancer [34]. Overexpression of HER2 in non-small cell lung cancer (NSCLC) can lead to elevated LDH in the pleural effusion [35]. In this study, we are the first to report that HER2 was not only positively correlated with elevated serum LDH in GC, but also positively correlated with LDHA expression in 12 pairs of GC tissues and GC cells. In addition, HER2, HIF-1α, and LDHA were detected in seven pairs of fresh GC tissues and corresponding adjacent normal tissues. LDHA expression was significantly higher in GC tissues than in the adjacent tissues. Moreover, the expressions of HER2, HIF-1α, and LDHA were positively correlated in GC tissues and GC cell lines. Laughner et al. reported that HER2 signaling increased the synthesis rate of HIF-1α [36]. Moreover, HIF-1 is reported to transcriptionally activate LDHA gene expression [37]. These studies are consistent with our findings that HER2, HIF-1α, and LDHA expression levels are positively correlated and HER2 plays a regulatory role in promoting the progression of GC via the HIF-1α-LDHA signaling pathway.
In our study of the role of LDHA in migration and invasion of GC cells, the wound healing assay confirmed that LDHA knockdown inhibited the migration ability of the HGC-27 and SGC-7901 cell lines. Transwell assays also confirmed that LDHA down-regulation exhibited an inhibitory effect on migration and invasion of the two cell lines. Transwell migration experiments confirmed that cell migration in the NCI-N87 cell line was significantly reduced after interfering with LDHA. Zhang et al. [13] found that lentivirus-mediated LDHA siRNA can inhibit the migration of an intestinal type of GC. These results suggest that LDHA plays an important role in the migration of GC cells. It is speculated that HER2 may promote the expression of LDHA through the HIF-1α-LDHA signaling pathway, thereby leading to the progression and metastasis of GC. In addition, we found that LDHA siRNA directly reduced LDH activity, whereas HER2 can indirectly regulate LDH activity. Knowing LDH plays an important role in the local microenvironment suitable for the growth and survival of malignant tumors [38], targeted treatment of HER2 and LDHA can not only inhibit the migration of tumor cells but also improve the microenvironment to inhibit tumor progression.
Our recent studies show that JWA negatively regulates HER2 expression and can be a biomarker for lapatinib sensitivity in GC [9,39]. We speculate that the JWA-HER2-HIF-1α-LDHA signaling pathway may play a specific role in the regulation of GC progression. Further prospective studies with a large number of GC patients are warranted to elucidate the specific role and mechanism of the JWA-HER2-HIF-1α-LDHA pathway in the development, progression, and metastasis of GC.
HER2-targeted drugs have been successfully utilized in clinical application. Other targets, like LDHA, are still in research and exploration stages and have generated great interest in recent years. Zhang et al. [13] suggest that inhibition of LDHA by lentivirus-mediated siRNA suppresses intestinal-type GC tumorigenicity through the downregulation of the Oct4 protein. In addition to LDHA siRNA, more than 900 types of plant extracts and traditional Chinese medicines have been found to be LDHA inhibitors in recent years (e.g., oxamate, FX11, galloflavin, gossypol, quinoline 3-sulfonamides, N-hydroxyindole-based inhibitors, spatholobus suberectus, etc.) [40-45]. However, these LDHA inhibitors tend to act on multiple target genes, which can cause other side effects, or may require intolerable high doses. Therefore, no suitable LDHA inhibitors have been used clinically. Recent studies of the LDHA inhibitors are more concerned with inhibiting activity of LDH enzyme than inhibiting LDHA protein expression. LDHA inhibitors, if successfully applied in the clinical setting, will play an important role in the synergistic effect based on radiotherapy and chemotherapy [46].
In summary, GC is not only a genetic disease, but also a metabolic disease with biological energy disorders. In addition to the many identified oncogene functions of HER2 in cancer development and progression, it is worth considering that HER2 also contributes to the regulation of glycolysis in GC cells.
Acknowledgements
We thank the staff members in the Jiangsu Key Lab of Cancer Biomarkers, Prevention and Treatment at Nanjing Medical University for their suggestions and assistance. This work was supported by the National Natural Science Foundation of China [grants 81602071, 81672896, and 81520108027], the Wu Jieping Medical Foundation [grant 320.6750.14289], and the Priority Academic Program Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine) [grant JX10231802].
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham SC, Schulick RD. Palliative management of gastric cancer. Surg Oncol. 2007;16:267–275. doi: 10.1016/j.suronc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Huang D, Duan H, Huang H, Tong X, Han Y, Ru G, Qu L, Shou C, Zhao Z. Cisplatin resistance in GC cells is associated with HER2 upregulation-induced epithelial-mesenchymal transition. Sci Rep. 2016;6:20502. doi: 10.1038/srep20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Xu P, Qiu H, Liu J, Chen S, Xu D, Li W, Zhan Y, Li Y, Chen Y, Zhou Z, Sun X. Clinical utility of HER2 assessed by immunohistochemistry in patients undergoing curative resection for GC. Onco Targets Ther. 2016;9:949–958. doi: 10.2147/OTT.S100979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen GS, Zhao JD, Zhao JH, Ma XF, Du F, Kan J, Ji FX, Ma F, Zheng FC, Wang ZY, Xu BH. Association of HER2 status with prognosis in GC patients undergoing R0 resection: a large-scale multicenter study in China. World J Gastroenterol. 2016;22:5406–5414. doi: 10.3748/wjg.v22.i23.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Junior PN, Neto RA, Forones NM. Her2 Expression as a prognostic factor in metastatic GC. Arq Gastroenterol. 2016;53:62–67. doi: 10.1590/S0004-28032016000200003. [DOI] [PubMed] [Google Scholar]
- 9.Qian J, Zhu W, Wang K, Ma L, Xu J, Xu T, Røe OD, Li A, Zhou J, Shu Y. JWA loss promotes cell migration and cytoskeletal rearrangement by affecting HER2 expression and identifies a high-risk subgroup of HER2-positive gastric carcinoma patients. Oncotarget. 2016;7:36865–36884. doi: 10.18632/oncotarget.9211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuse N, Kuboki Y, Kuwata T, Nishina T, Kadowaki S, Shinozaki E, Machida N, Yuki S, Ooki A, Kajiura S, Kimura T, Yamanaka T, Shitara K, Nagatsuma AK, Yoshino T, Ochiai A, Ohtsu A. Prognostic impact of HER2, EGFR, and c-MET status on OS of advanced GC patients. Gastric Cancer. 2015;19:183–191. doi: 10.1007/s10120-015-0471-6. [DOI] [PubMed] [Google Scholar]
- 11.Qiu M, Zhou Y, Zhang X, Wang Z, Wang F, Shao J, Lu J, Jin Y, Wei X, Zhang D, Wang F, Li Y, Yang D, Xu R. Lauren classification combined with HER2 status is a better prognostic factor in Chinese GC patients. BMC Cancer. 2014;14:823. doi: 10.1186/1471-2407-14-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Zhang X, Wang X, Gan L, Yu G, Chen Y, Liu K, Li P, Pan J, Wang J, Wang X. Inhibition of LDH-A by lentivirus-mediated small interfering RNA suppresses intestinal-type GC tumorigenicity through the downregulation of Oct4. Cancer Lett. 2012;321:45–54. doi: 10.1016/j.canlet.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Xie H, Hanai J, Ren JG, Kats L, Burgess K, Bhargava P, Signoretti S, Billiard J, Duffy KJ, Grant A, Wang X, Lorkiewicz PK, Schatzman S, Bousamra M 2nd, Lane AN, Higashi RM, Fan TW, Pandolfi PP, Sukhatme VP, Seth P. Targeting lactate dehydrogenase--a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussien R, Brooks GA. Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol Genomics. 2010;43:255–264. doi: 10.1152/physiolgenomics.00177.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koukourakis MI, Giatromanolaki A, Panteliadou M, Pouliliou SE, Chondrou PS, Mavropoulou S, Sivridis E. Lactate dehydrogenase 5 isoenzyme overexpression defines resistance of prostate cancer to radiotherapy. Br J Cancer. 2014;110:2217–2223. doi: 10.1038/bjc.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Trarbach T, Folprecht G, Shi MM, Lebwohl D, Jalava T, Laurent D, Meinhardt G, Harris AL. Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (Vatalanib) antiangiogenic therapy. Clin Cancer Res. 2011;17:4892–4900. doi: 10.1158/1078-0432.CCR-10-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rong Y, Wu W, Ni X, Kuang T, Jin D, Wang D, Lou W. Lactate dehydrogenase A is overexpressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumour Biol. 2013;34:1523–1530. doi: 10.1007/s13277-013-0679-1. [DOI] [PubMed] [Google Scholar]
- 19.Kayser G, Kassem A, Sienel W, Schulte-Uentrop L, Mattern D, Aumann K, Stickeler E, Werner M, Passlick B, zur Hausen A. Lactate-dehydrogenase 5 is overexpressed in non-small cell lung cancer and correlates with the expression of the transketolase-like protein 1. Diagn Pathol. 2010;5:22. doi: 10.1186/1746-1596-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo AWI, Sun X, Sun Z, Zhu Z, Guan H, Zhang J, Zhang Y, Xu H, Sun M. Clinicopathological significance and prognostic value of lactate dehydrogenase a expression in GC patients. PLoS One. 2014;9:e91068. doi: 10.1371/journal.pone.0091068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HS, Lee HE, Yang HK, Kim WH. High lactate dehydrogenase 5 expression correlates with high tumoral and stromal vascular endothelial growth factor expression in GC. Pathobiology. 2014;81:78–85. doi: 10.1159/000357017. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Yang Z, Chen Z, Chen R, Zhao D, Zhou Y, Qiao L. Effects of the suppression of lactate dehydrogenase A on the growth and invasion of human GC cells. Oncol Rep. 2015;33:157–162. doi: 10.3892/or.2014.3600. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Z, Han F, Yang S, Wu J, Zhan W. Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in GC cells: involvement of the Akt-mTOR signaling pathway. Cancer Lett. 2015;358:17–26. doi: 10.1016/j.canlet.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Wu X, Zhang J, Chen Y, Xu J, Xia X, He S, Qiang F, Li A, Shu Y, Roe OD, Li G, Zhou JW. CHIP functions as a novel suppressor of tumour angiogenesis with prognostic significance in human GC. Gut. 2013;62:496–508. doi: 10.1136/gutjnl-2011-301522. [DOI] [PubMed] [Google Scholar]
- 25.Digklia A, Wagner AD. Advanced gastric cancer: current treatment land scape and future perspectives. World J Gastroenterol. 2016;22:2403–2414. doi: 10.3748/wjg.v22.i8.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 27.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 28.Shimoyama S. Unraveling trastuzumab and lapatinib inefficiency in GC. Future steps (Review) Mol Clin Oncol. 2014;2:175–181. doi: 10.3892/mco.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HP, Han SW, Song SH, Jeong EG, Lee MY, Hwang D, Im SA, Bang YJ, Kim TY. Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive GC. Oncogene. 2014;33:3334–3341. doi: 10.1038/onc.2013.285. [DOI] [PubMed] [Google Scholar]
- 30.Barros-Silva JD, Leitão D, Afonso L, Vieira J, Dinis-Ribeiro M, Fragoso M, Bento MJ, Santos L, Ferreira P, Rêgo S, Brandão C, Carneiro F, Lopes C, Schmitt F, Teixeira MR. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer. 2009;100:487–493. doi: 10.1038/sj.bjc.6604885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong SJ, Jin CJ, Rha SY, Chung HC. Growth inhibitory effects of trastuzumab and chemotherapeutic drugs in GC cell lines. Cancer Lett. 2004;214:215–224. doi: 10.1016/j.canlet.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Scott EN, Meinhardt G, Jacques C, Laurent D, Thomas AL. Vatalanib: the clinical development of a tyrosine kinase inhibitor of angiogenesis in solid tumours. Expert Opin Investig Drugs. 2007;16:367–379. doi: 10.1517/13543784.16.3.367. [DOI] [PubMed] [Google Scholar]
- 33.Valvona CJ, Fillmore HL, Nunn PB, Pilkington GJ. The regulation and function of lactate dehydrogenase a: therapeutic potential in brain tumor. Brain Pathol. 2016;26:3–17. doi: 10.1111/bpa.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao YH, Zhou M, Liu H, Ding Y, Khong HT, Yu D, Fodstad O, Tan M. Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene. 2009;28:3689–3701. doi: 10.1038/onc.2009.229. [DOI] [PubMed] [Google Scholar]
- 35.Ziaian B, Saberi A, Ghayyoumi MA, Safaei A, Ghaderi A, Mojtahedi Z. Association of high LDH and low glucose levels in pleural space with HER2 expression in non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15:1617–1620. doi: 10.7314/apjcp.2014.15.4.1617. [DOI] [PubMed] [Google Scholar]
- 36.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 38.Ma L, Qiu J, Zhang Y, Qiu T, Wang B, Chen W, Li X, Sun J, Wang K, Li X, Gu Y, Shu Y, Chen X. Prognostic factors for operable biliary tract cancer: serum levels of lactate dehydrogenase, a strong association with survival. Onco Targets Ther. 2018;11:2533–2543. doi: 10.2147/OTT.S150502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma L, Zhu W, Wang Q, Yang F, Qian J, Xu T, Wang S, Zhou J, Shu Y. JWA down-regulates HER2 expression via c-Cbl and induces lapatinib resistance in human GC cells. Oncotarget. 2016;7:71790–71801. doi: 10.18632/oncotarget.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Billiard J, Dennison JB, Briand J, Annan RS, Chai D, Colón M, Dodson CS, Gilbert SA, Greshock J, Jing J, Lu H, McSurdy-Freed JE, Orband-Miller LA, Mills GB, Quinn CJ, Schneck JL, Scott GF, Shaw AN, Waitt GM, Wooster RF, Duffy KJ. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 2013;1:19. doi: 10.1186/2049-3002-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Granchi C, Roy S, De Simone A, Salvetti I, Tuccinardi T, Martinelli A, Macchia M, Lanza M, Betti L, Giannaccini G, Lucacchini A, Giovannetti E, Sciarrillo R, Peters GJ, Minutolo F. N-Hydroxyindole-based inhibitors of lactate dehydrogenase against cancer cell proliferation. Eur J Med Chem. 2011;46:5398–5407. doi: 10.1016/j.ejmech.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Wang D, Han S, Wang N, Mo F, Loo TY, Shen J, Huang H, Chen J. Bioactivity-guided identification and cell signaling technology to delineate the lactate dehydrogenase A inhibition effects of Spatholobus suberectus on breast cancer. PLoS One. 2013;8:e56631. doi: 10.1371/journal.pone.0056631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao D, Zou SW, Liu Y, Zhou X, Mo Y, Wang P, Xu YH, Dong B, Xiong Y, Lei QY, Guan KL. Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell. 2013;23:464–476. doi: 10.1016/j.ccr.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Granchi C, Roy S, Giacomelli C, Macchia M, Tuccinardi T, Martinelli A, Lanza M, Betti L, Giannaccini G, Lucacchini A, Funel N, León LG, Giovannetti E, Peters GJ, Palchaudhuri R, Calvaresi EC, Hergenrother PJ, Minutolo F. Discovery of N-hydroxyindole-based inhibitors of human lactate dehydrogenase isoform A (LDH-A) as starvation agents against cancer cells. J Med Chem. 2011;54:1599–1612. doi: 10.1021/jm101007q. [DOI] [PubMed] [Google Scholar]
- 45.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maftouh M, Avan A, Sciarrillo R, Granchi C, Leon LG, Rani R, Funel N, Smid K, Honeywell R, Boggi U, Minutolo F, Peters GJ, Giovannetti E. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer. 2014;110:172–182. doi: 10.1038/bjc.2013.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.