Abstract
Mesenchymal stem cells (MSCs) represent a valuable cell source in regenerative medicine, and large numbers of MSCs can be isolated from the amnion noninvasively. Sclerosing cholangitis is a chronic cholestatic disease and characterized by progressive biliary destruction leading to cirrhosis. Many factors are involved in the development of sclerosing cholangitis; however, effective medical therapy is not established. We investigated the effects of human amnion-derived MSCs (hAMSCs) and conditioned medium (CM) obtained from hAMSC cultures in rats with sclerosing cholangitis. Sclerosing cholangitis was induced via the intragastric administration of 100 mg/kg alpha-naphthylisothiocyanate (ANIT) twice weekly for 4 weeks. One million hAMSCs or 200 μL of CM were intravenously administered on days 15 and 22. Rats were sacrificed on day 29 and evaluated via histological, immunohistochemical, and mRNA expression analyses. hAMSC transplantation and CM administration significantly improved the histological score. In addition, these two interventions significantly improved biliary hyperplasia, peribiliary fibrosis, and inflammation in Glisson’s sheath. Accordingly, CK19, MMP-9, and TNF-α, and MCP-1 expression in the liver was also decreased by hAMSC and CM administration. In conclusion, hAMSC and CM administration ameliorated biliary hyperplasia, peribiliary fibrosis, and inflammation in a rat model of sclerosing cholangitis. hAMSCs and CM may represent new modalities for treating sclerosing cholangitis.
Keywords: Mesenchymal stem cells, sclerosing cholangitis, amnion, alpha-naphthylisothiocyanate, regenerative medicine
Introduction
Sclerosing cholangitis is a chronic cholestatic disease, characterized by inflammation, obliterative fibrosis of bile ducts, stricture formation and progressive biliary destruction leading to cirrhosis [1]. Primary sclerosing cholangitis (PSC), the most common form, is an idiopathic sclerosing cholangitis resulting in liver fibrosis, cirrhosis, and eventually liver failure [2]. There is no effective medical therapy for PSC, and patients with progressive PSC require liver transplantation [3]. In addition, immune disorders, ischemia, infections, parasites, infiltrative processes and metastasis can cause secondary sclerosing cholangitis. However, the effective medical therapy for sclerosing cholangitis has not been established [1]. Therefore, a new strategy to delay or prevent disease progression of sclerosing cholangitis is urgently required.
Cell therapy with mesenchymal stem cells (MSCs) is expected as a new therapeutic strategy. MSCs are multipotent cells that can differentiate into various lineages, including bone, cartilage, or fat, and reside in a variety of tissues [4]. Because of their ability to regulate several inflammatory responses, MSCs have been investigated in a variety of animal models of inflammatory disorders [5-7], and bone marrow (BM)- and adipose tissue-derived MSCs have been the major cell sources for clinical trials [8]. The fetal membrane (FM) comprises the amnion and chorion, which envelop the developing fetus. Although the human FM is generally discarded as medical waste after delivery, fetal tissues have been identified as rich sources of MSCs [9,10]. In small animal experiments, we demonstrated that the intravenous administration of human amnion-derived MSCs (hAMSCs) alleviated inflammatory diseases, such as liver cirrhosis [11], acute and chronic pancreatitis [12], inflammatory bowel diseases [13,14], and radiation proctitis [15]. In addition, we observed that enema using conditioned medium (CM) obtained from hAMSCs alleviated chemically induced colitis in rats [14] and that oral CM administration prevented stricture formation after esophageal submucosal dissection in pigs [16].
There are not well-characterized animal models with sclerosing cholangitis. Alpha-naphthylisothiocyanate (ANIT) is a toxin that targets intrahepatic bile ducts, and it is used to generate animal models of sclerosing cholangitis because the pathology of ANIT-induced cholangitis resembles that of human PSC in terms of onion-skin-type-like peribiliary fibrosis and biliary-type liver fibrosis [17,18]. In the acute setting, ANIT is metabolized by hepatocytes and, following conjugation with glutathione, secreted via multidrug resistance-associated protein 2 into bile, through which it exerts its toxic effects on cholangiocytes [19,20]. This results in a chronic, low-level cytotoxic challenge to intrahepatic cholangiocytes, causing the release of bile acids and eliciting cholangiocellular proliferation, inflammation, and peribiliary fibrosis [21-25].
We investigated whether intravenous administration of hAMSCs and CM obtained from hAMSC cultures improves ANIT-induced portal inflammation and peribiliary fibrosis in rats.
Materials and methods
Animals
The experimental protocol was approved by the Animal Care and Use Committees of Hokkaido University. Six-week-old male Sprague-Dawley rats were procured from Japan SLC (Hamamatsu, Japan). Three rats were housed per cage in a temperature-controlled room (24°C) on a 12-h/12-h light/dark cycle. All rats had ad libitum access to standard pellets throughout the study including the 5-day acclimatization period.
Induction of sclerosing cholangitis
A 1.79-mm-diameter tube was inserted into the stomach from the mouth. Cholangitis was induced via the intragastric administration of 100 mg/kg ANIT (Sigma-Aldrich, St. Louis, MO, USA) in 500 μL of olive oil (Wako Pure Chemical Industries, Osaka, Japan) twice weekly for 4 weeks. In the control group, rats were administrated olive oil alone (Figure 1).
Figure 1.
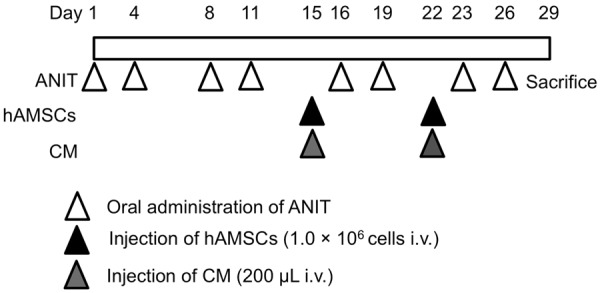
Experimental protocol for creating an alpha-naphthylisothiocyanate (ANIT)-induced sclerosing cholangitis model. Rats received 100 mg/kg ANIT twice weekly for 4 weeks. One million human amnion-derived mesenchymal stem cells (hAMSCs) or 200 μL of conditioned medium (CM) were intravenously administered on days 15 and 22. All rats were sacrificed on day 29.
hAMSC isolation and expansion
The Medical Ethical Committee of Hokkaido University Faculty of Medicine and Graduate School of Medicine, Sapporo, Japan approved this work, and a pregnant woman provided written informed consent. A FM was obtained during cesarean delivery, and the amnion was manually peeled from the chorion. hAMSCs were isolated and expanded via digestion with Brightase-C (Nippi, Tokyo, Japan) and dispase (Wako Pure Chemical Industries). Subsequently, hAMSCs (1 × 106 cells) were seeded in uncoated plastic dishes with minimal essential medium alpha (MEMα) (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Life Technologies), 100 U/mL penicillin, and 100 µg/mL streptomycin (Wako Pure Chemical Industries). Cell cultures were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. After 3-4 days in culture, nonadherent cells were removed, and adherent cells were maintained in culture until they reached 80% confluence. Passage was performed using 0.5% trypsin-ethylenediaminetetraacetic acid (Life Technologies).
We previously confirmed that cultured hAMSCs are multipotent and that they express surface markers such as CD44, CD73, CD90, and CD105 but not CD34, CD11b, CD19, CD45, and HLA-DR [16], reflecting characteristic findings of MSCs [26].
Preparation of CM from hAMSC cultures
hAMSCs were cultured in 15-cm dishes until reaching a subconfluent state. After washing with phosphate-buffered saline (PBS, Life Technologies) thrice, cells were further cultured with serum-free MEMα for 48 h. Next, CM was collected and centrifuged at 400 × g for 5 min. The supernatant was stored at -80°C until use.
Transplantation of hAMSCs or CM
In the ANIT+hAMSCs group (N = 10), one million hAMSCs suspended in 200 μL of PBS were intravenously injected through the penile vein on days 15 and 22 under anesthesia with intraperitoneal pentobarbital (50 mg/kg, Kyoritsu Seiyaku, Tokyo, Japan). Similarly, 200 μL of CM were injected into animals in the ANIT+CM group (N = 10), and 200 μL of PBS were injected into animals in the control (N = 6) and ANIT groups (N = 10).
Histological examination
All rats were sacrificed on day 29. The abdomens of rats were opened under anesthesia with 50 mg/kg intraperitoneal pentobarbital. In each animal, the left lobe of the liver was removed, fixed in 40 g/L formaldehyde saline, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin (H&E; Wako Pure Chemical Industries) and Sirius Red (Wako Pure Chemical Industries). A pathologist certified by the Japanese Society of Pathology (M.I.) blindly evaluated 10 random sections of the liver in each rat. Biliary hyperplasia, fibroblast proliferation, and biliary neutrophilic infiltration were scored as described previously [27,28] as follows: 0, no evidence of abnormality; 1, minimal; 2, mild; 3, moderate; and 4, severe. The number of necrotic lesions in H&E-stained sections was quantitatively measured as described previously [29]. To quantify collagen deposits, 10 random fields (× 100) of Sirius Red-stained sections from each rat were photographed, and red-stained areas were measured using a digital image analyzer (WinROOF; Mitani Co., Fukui, Japan) as described previously [29].
Immunohistochemical examination
Tissue sections were stained with anti-rat cytokeratin 19 (CK19) antibody (dilution 1:100; Proteintech, Chicago, IL, USA) to assess biliary hyperplasia. To assess activated hepatic stellate cells, tissue sections were stained with anti-rat α-smooth muscle actin (α-SMA) antibody (dilution 1:800; Thermo Scientific, Waltham, MA, USA). To assess peribiliary collagen deposition, tissue sections were stained with anti-rat type I collagen antibody (dilution 1:100,000; LSL, Tokyo, Japan). To assess the infiltration of Kupffer cells in Glisson’s sheath, tissue sections were stained with anti-rat CD68 antibody (dilution, 1:50; AbD Serotec, Kidlington, UK). Ten random fields in sections from each rat were photographed for CK19 (× 100), α-SMA (× 100), and type I collagen staining (× 100). Data were presented as a percentage of the total positive area for each type of staining using a digital image analyzer (WinROOF) as described previously [25,29-31]. Ten randomly selected areas (× 400) of each rat at the periportal area were photographed, and the number of CD68-positive cells was counted as described previously [32,33].
RNA isolation and quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNAs of the rat liver were extracted using an RNeasy Mini Kit (Qiagen, Hilden, Germany) with elimination of genomic DNA. One microgram of total RNA was reverse-transcribed into cDNA using a QuantiTect Reverse Transcription Kit (Qiagen). PCR amplification was performed using a 25-μL reaction mixture containing 1 μL of cDNA and 12.5 μL of Platinum SYBR Green PCR Mix (Life Technologies). β-actin mRNA amplified from the same samples served as an internal control. After initial denaturation at 95°C for 2 min, a two-step cycle procedure was used (denaturation at 95°C for 15 s and annealing and extension at 60°C for 1 min) for 40 cycles in a 7700 Sequence Detector (Applied Biosystems, Foster City, CA, USA). Gene expression levels were determined via the comparative threshold cycle (ddCt) method using β-actin as an internal control [34]. Data were analyzed using Sequence Detection Systems software (Applied Biosystems). Primer sequences are shown in Table 1.
Table 1.
Primer sequences for quantitative reverse transcription-polymerase chain reaction
| Gene | Primer sequence |
|---|---|
| CK19 | F: TATCTGGATCTGCGTAGTGTGG |
| R: ATACAAAACCAAACTGGGGATG | |
| α-SMA | F: CGGGCTTTGCTGGTGATG |
| R: CCCTCGATGGATGGGAAA | |
| TGF-β | F: CCCCTGGAAAGGGCTCAACAC |
| R: TCCAACCCAGGTCCTTCCTAAAGTC | |
| Type I collagen | F: GATGGCTGCACGAGTCACAC |
| R: ATTGGGATGGAGGGAGTTTA | |
| MMP-2 | F: CACCACCGAGGACTATGACC |
| R: TGTTGCCCAGGAAAGTGAAG | |
| MMP-9 | F: TGGAACTCACACAACGTCTTTCA |
| R: TCACCCGGTTGTGGAAACTC | |
| TIMP-1 | F: TCCTCTTGTTGCTATCATTGATAGCTT |
| R: CGCTGGTATAAGGTGGTCTCGAT | |
| TNF-α | F: AGAACTCCAGCGGTGTCT |
| R: GAGCCCATTTGGGAACTTCT | |
| MCP-1 | F: ATGCAGTTAATGCCCCACTC |
| R: TTCCTTATTGGGGTCAGCAC | |
| β-actin | F: CCAACCGTGAAAAGATGACC |
| R: ACCAGAGGCATACAGGGACA |
CK, cytokeratin; SMA, smooth muscle actin; TGF, transforming growth factor; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase; TNF, tumor necrosis factor; MCP, monocyte chemotactic protein.
Statistical analysis
Data are shown as the mean ± SEM. Parameters among the groups were compared via one-way ANOVA followed by Tukey’s test. Differences were considered statistically significant at probability (P) levels less than 0.05.
Results
Effects of hAMSC transplantation and CM administration on histological parameters in ANIT-treated rats
We first examined histological parameters via H&E staining. Marked biliary hyperplasia, fibroblast proliferation, and necrotic lesions were observed in the ANIT group (Figure 2A). Histological scoring demonstrated that hAMSC transplantation and CM administration significantly suppressed biliary hyperplasia (Figure 2B). However, fibroblast proliferation was not decreased by hAMSC or CM treatment. Biliary neutrophilic infiltration was not significantly increased by ANIT, and no change was observed following hAMSC transplantation or CM administration. The number of necrotic lesions was significantly decreased by hAMSC transplantation (Figure 2C).
Figure 2.
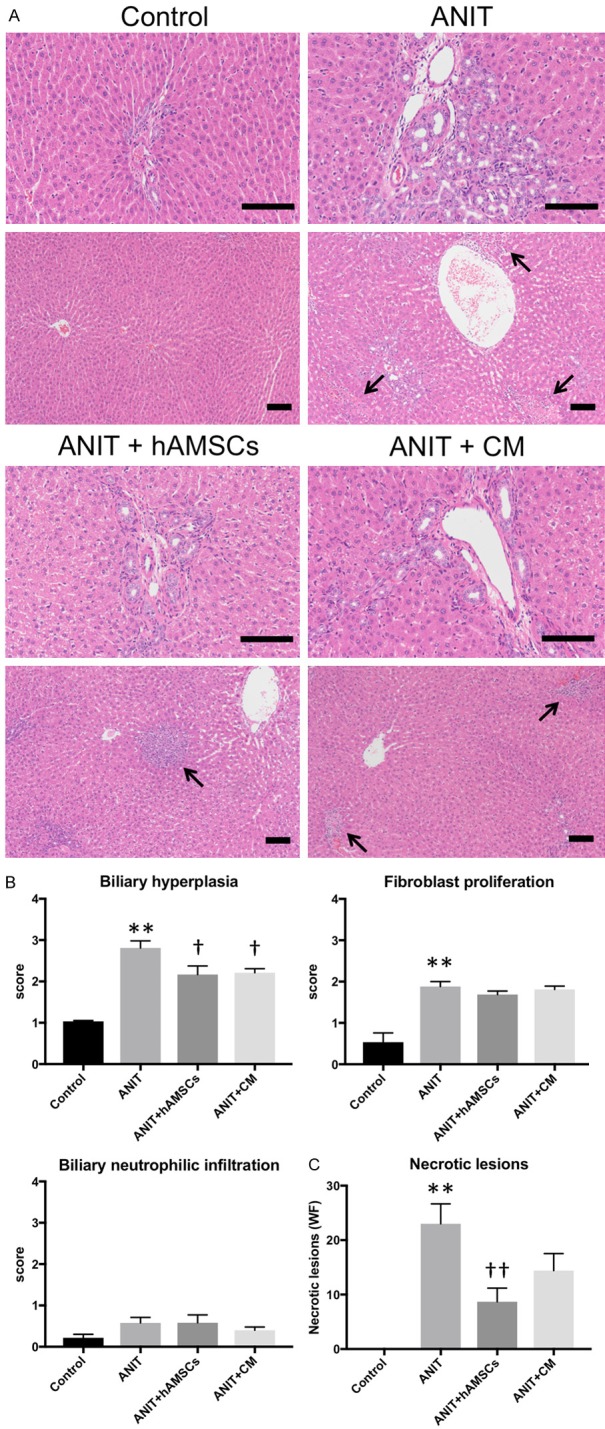
Effect of human amnion-derived mesenchymal stem cells (hAMSCs) and conditioned medium (CM) obtained from hAMSCs on histological parameters. A. Hematoxylin and eosin staining. Glisson’s sheath (upper) and necrotic lesions (lower, arrows) are shown. Scale bars, 100 μm. B. Liver histology scores regarding biliary hyperplasia, fibroblast proliferation and neutrophilic infiltration in Glisson’s sheath in 10 sections per sample in each high-power field. C. Number of necrosis lesions in whole field (WF). Values are expressed as the mean ± SEM (N = 6-10 animals/group). **P < 0.01 vs. the control group. †P < 0.05 and ††P < 0.01 vs. the alpha-naphthylisothiocyanate (ANIT) group.
Effects of hAMSC transplantation and CM administration on biliary hyperplasia in ANIT-treated rats
To confirm the results of histological findings, we performed immunohistological examination. The expression of CK19 was significantly increased in the ANIT group, an effect that was significantly decreased by hAMSC transplantation and CM administration (Figure 3A). Consistently, CK19 mRNA expression in the liver was significantly increased in ANIT group, and hAMSC transplantation and CM administration significantly decreased its expression (Figure 3B). These results suggest that hAMSC transplantation and CM administration suppress ANIT-induced biliary hyperplasia.
Figure 3.
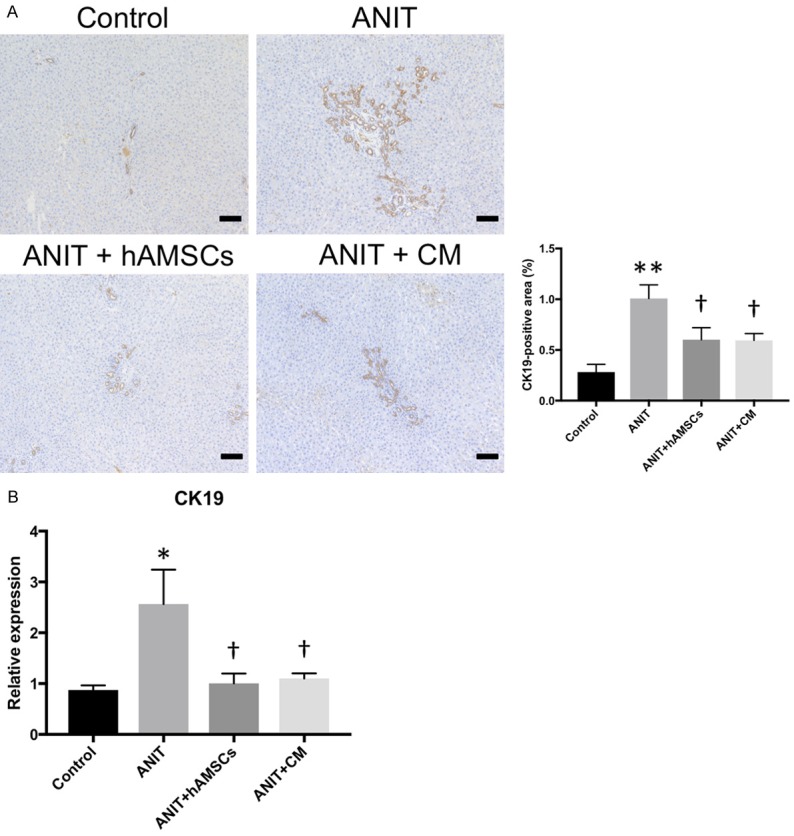
Effects of human amnion-derived mesenchymal stem cells (hAMSCs) and conditioned medium (CM) obtained from hAMSCs on biliary hyperplasia. A. Cytokeratin 19 (CK19) expression. B. Quantitative reverse transcription-polymerase chain reaction for CK19. The stained areas were measured from 10 sections per sample in each low-power field. Scale bars, 100 μm. Values are expressed as the mean ± SEM (N = 6-10 animals/group). *P < 0.05 and **P < 0.01 vs. the control group. †P < 0.05 vs. the alpha-naphthylisothiocyanate (ANIT) group.
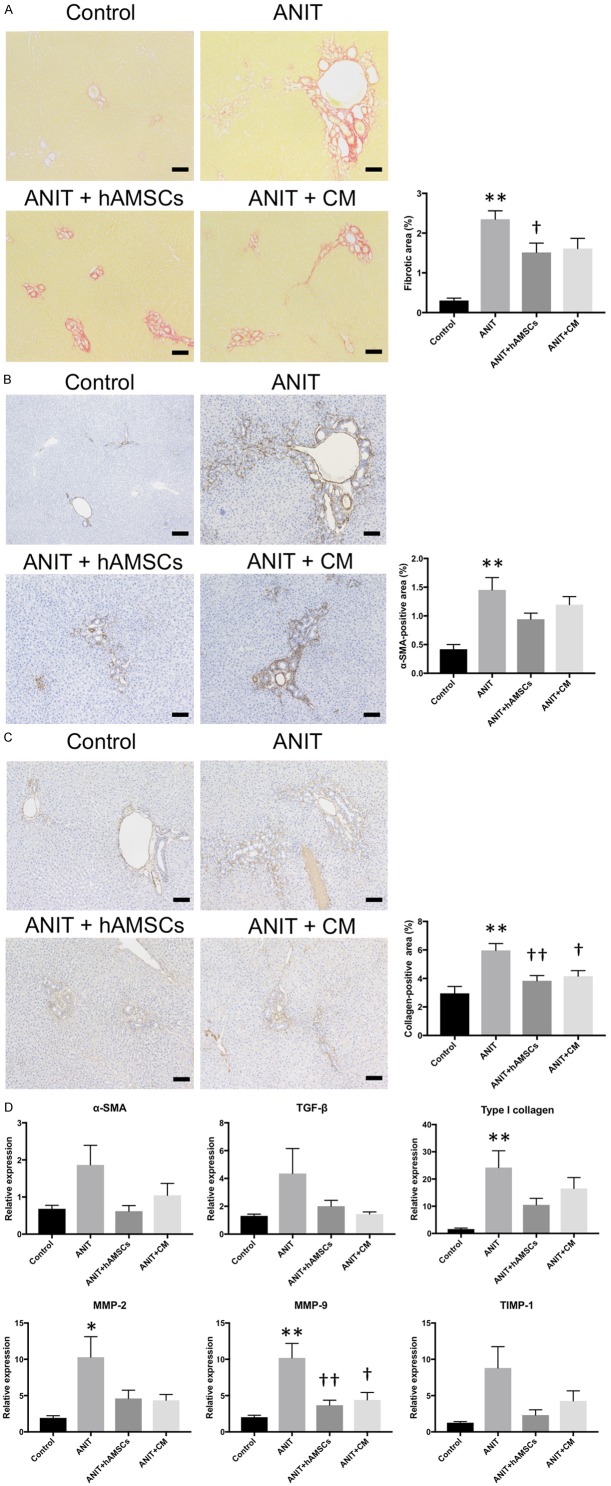
Effects of hAMSC transplantation and CM administration on peribiliary fibrosis in ANIT-treated rats
We next investigated peribiliary fibrosis in the liver. Sirius Red staining demonstrated that peribiliary fibrosis was induced by ANIT; meanwhile, hAMSC transplantation significantly reduced fiber accumulation, and CM administration tended to reduce fiber accumulation (Figure 4A). Immunohistological examination demonstrated that the expression of α-SMA, a maker for activated hepatic stellate cells, was significantly increased in the ANIT group, and hAMSC transplantation and CM administration tended to reduce α-SMA expression, albeit without significance (Figure 4B). Type I collagen expression was significantly increased by ANIT and significantly decreased by hAMSC transplantation and CM administration (Figure 4C). Quantitative PCR demonstrated that the mRNA expression of type I collagen, MMP-2, and MMP-9 was significantly increased by ANIT exposure, and α-SMA, TGF-β, and TIMP-1 expression tended to be increased (Figure 4D). hAMSC transplantation and CM administration significantly decreased the mRNA levels of MMP-9 and tended to decrease those of α-SMA, TGF-β, type I collagen, MMP-2, and TIMP-1.
Figure 4.
Effects of human amnion-derived mesenchymal stem cell (hAMSCs) and conditioned medium (CM) obtained from hAMSCs on fibrosis, collagen deposition, and fibrosis-related marker expression. A. Sirius Red staining. B. α-smooth muscle actin (SMA) expression. C. Type I collagen expression. D. Quantitative reverse transcription-polymerase chain reaction. Stained areas were measured from 10 sections per sample in each low-power field. Scale bars, 100 μm. Values are expressed as the mean ± SEM (N = 6-10 animals/group). *P < 0.05 and **P < 0.01 vs. the control group. †P < 0.05 and ††P < 0.01 vs. the alpha-naphthylisothiocyanate (ANIT) group.
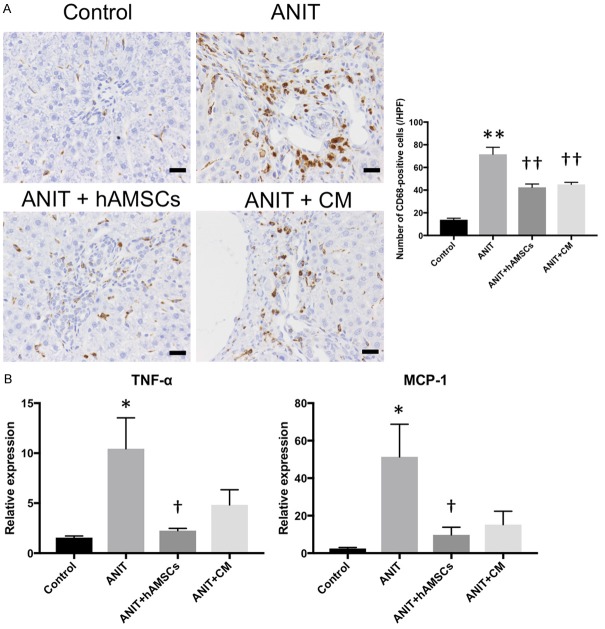
Effects of hAMSC transplantation and CM administration on inflammation in Glisson’s sheath in ANIT-treated rats
We finally investigated the inflammatory reaction in the peribiliary area. Immunohistological examination demonstrated that the number of CD68-positive Kupffer cells was significantly increased in the ANIT group, and hAMSC transplantation and CM administration significantly reduced the number of CD68-positive Kupffer cells (Figure 5A). TNF-α and MCP-1 mRNA expression was significantly increased by ANIT treatment. Their expression was significantly decreased by hAMSC transplantation, and it tended to be decreased by CM administration (Figure 5B). These results suggest that the inflammatory reaction induced by ANIT was suppressed by hAMSC transplantation and CM administration.
Figure 5.
Effects of human amnion-derived mesenchymal stem cell (hAMSCs) and conditioned medium (CM) obtained from hAMSCs on the infiltration of inflammatory cells and inflammatory mediators. A. CD68 expression. B. Quantitative reverse transcription-polymerase chain reaction. The number of positive cells was counted in 10 sections per sample in each high-power field. Scale bars, 20 μm. Values are expressed as the mean ± SEM (N = 6-10 animals/group). *P < 0.05 and **P < 0.01 vs. the control group. †P < 0.05 and ††P < 0.01 vs. the alpha-naphthylisothiocyanate (ANIT) group.
Discussion
This was the first study to investigate the therapeutic potential of hAMSCs and CM in rats with ANIT-induced sclerosing cholangitis, which resembles sclerosing cholangitis in humans. We found that both hAMSC transplantation and CM injection significantly improved biliary hyperplasia, peribiliary fibrosis, and inflammation in Glisson’s sheath.
Biliary hyperplasia is a characteristic change in the livers of ANIT-exposed rats [21-24]; however, the mechanisms of biliary hyperplasia are not entirely understood. Bile duct ligation (BDL) is one model of cholestasis, and biliary hyperplasia is also caused by BDL due to cholestatic liver injury similarly as observed for ANIT exposure [35,36]. In addition, hepatic neutrophil accumulation and necrosis have been observed in both ANIT- and BDL-induced liver injury [37]. These studies supported the hypothesis that biliary hyperplasia is related to inflammation and necrosis. However, the connections of biliary hyperplasia and neutrophil inflammation with necrosis in ANIT-induced sclerosing cholangitis have not been clarified [38]. The report suggested that inflammation was partially involved in biliary hyperplasia. Therefore, it is possible that hAMSCs and CM reduced peribiliary fibrosis in part through suppressing inflammation and the consequent biliary hyperplasia.
In the present study, hAMSC and CM administration reduced peribiliary fibrosis in rats with ANIT-induced sclerosing cholangitis. Although there was no difference in the fibroblast proliferation score between the ANIT group and the treatment groups, this can be explained by several factors. First, the histological assessment of fibroblast proliferation using H&E staining was based on subjective scoring. Faiola et al. reported modest differences in the degree of injury based on subjective scoring [27]. Only subjective differences of > 0.2 units were interpreted as significant changes in their report. Second, assessments of fibroblast proliferation using H&E staining in previous reports [27,28] focused on several bile ducts opposed to all intrahepatic bile ducts. Meanwhile, it has been reported that various humoral factors including anti-fibrotic factors are secreted by cultured hAMSCs, such as hepatocyte growth factor, vascular endothelial growth factor, insulin-like growth factor 1, and basic fibroblast growth factor [39,40]. In addition, MSCs secrete MMP, which can mediate fibrolytic effects [41]. In the present study, the mRNA expression of TIMP-1, which is a marker of fibrosis [42], was decreased in the ANIT+hAMSCs and ANIT+CM groups. Similarly, MMP-2 and MMP-9 mRNA expression was also decreased in these groups. Therefore, it appeared that hAMSCs and CM influenced inflammation rather than the fibrinolytic system in rats with ANIT-induced sclerosing cholangitis.
The present study demonstrated that hAMSCs and CM reduced the infiltration of Kupffer cells into Glisson’s sheath. In small animals with ANIT-induced sclerosing cholangitis, neutrophils invaded the liver within 12 h after ANIT treatment and remained abundant in the liver for at least 24 h, and their numbers decreased within a few days [23]. In addition, it has been reported that the number of Kupffer cells was increased at the highest treatment level 3 days after ANIT injection [33]. In the present study, there was no significant difference in biliary neutrophil infiltration among the groups (myeloperoxidase staining, data not shown); however, it is possible that neutrophils had already disappeared at the time of sacrifice.
It has been reported that transplanted human adipose tissue-derived MSCs accumulated in bile ducts in mice with liver injury [43]. Therefore, we hypothesized that hAMSCs engrafted in bile ducts affected bile injury. To confirm the existence of transplanted hAMSCs, we stained the tissues with anti-human Ku80 antibody, however, there were no human Ku80-positive cells observed in the liver (data not shown). hAMSCs might have already disappeared from the liver, or they potentially engrafted in other organs, such as the lungs [15]. Conversely, van Poll et al. demonstrated that the injection of CM from human MSCs prevented the release of liver injury biomarkers in a rat model of fulminant hepatic failure (FHF) [44]. CM injection significantly reduced the maximum serum levels of liver enzymes 36 h after the induction of FHF; however, no significant differences were observed at any other time points. This suggests that CM only has a short-term anti-inflammatory effect. Therefore, our data indicate that hAMSC transplantation was more effective than CM injection at the same frequency of administration. However, MSCs have been reported to frequently congregate within the pulmonary circulation after intravenous administration in small animals and likely lead to thromboembolism [45,46]. The most suitable dose and frequency of CM injection remain to be clarified.
Few studies have focused on the transplantation of MSCs in models of acute and chronic cholangitis. The efficacy of BM-derived MSCs in rat models of primary biliary cholangitis (PBC) was investigated, demonstrating that BM-MSCs slightly reduced serum liver enzyme levels and ameliorated monocyte infiltration in bile ducts [47]. In human studies, transplantation of umbilical cord-derived MSCs into patients with PBC significantly reduced the serum levels of liver enzymes at 12 months; however, the exact mechanism of this effect remains unknown [48,49].
The amnion is an attractive cell source for MSCs because large quantities of hAMSCs can be obtained from human FMs without invasive procedures [14]. Furthermore, a first-in-human clinical trial of FM-MSC transplantation in nine patients with steroid-refractory acute graft-versus-host disease (GVHD) demonstrated that FM-MSCs appeared safe for intravenous infusion, and the overall response rate in patients with severe refractory acute GVHD was similar to that observed in patients administered BM-MSCs [50]. This is encouraging because the collection of AMSCs involves less invasive procedures than those used to obtain MSCs from BM or adipose tissue from donors.
This study has several limitations. First, we did not investigate the dose-dependency and optimum frequencies of hAMSC and CM administration. Second, although we used hAMSCs and CM obtained from a single donor, the existence of individual variability of hAMSCs and CM should be clarified. Third, as large bile ducts are not injured by ANIT, we could evaluate only intrahepatic bile ducts. Finally, because we used a rat model of sclerosing cholangitis in this study, it is unclear whether our results can be extrapolated to human sclerosing cholangitis, such as PSC.
In conclusion, hAMSC transplantation and CM administration ameliorated biliary hyperplasia, peribiliary fibrosis, and inflammation in Glisson’s sheath in a rat model of sclerosing cholangitis. Because FM has been considered medical waste and it can be obtained noninvasively, hAMSCs and CM obtained from hAMSC cultures may represent highly relevant therapeutic modalities for treating sclerosing cholangitis.
Acknowledgements
This study was supported by a Grant-in-Aid (B) from the Japan Society for the Promotion of Science (JSPS, 16H05282). The authors thank Dr. Takahiro Yamada for performing cesarean deliveries, Dr. Kenichi Yamahara for culturing and expanding hAMSCs, and Hidetaka Hosono for technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology. 2006;44:1063–1074. doi: 10.1002/hep.21405. [DOI] [PubMed] [Google Scholar]
- 2.Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med. 2016;375:1161–1170. doi: 10.1056/NEJMra1506330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–536. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 6.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 7.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Wang LT, Ting CH, Yen ML, Liu KJ, Sytwu HK, Wu KK, Yen BL. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci. 2016;23:76. doi: 10.1186/s12929-016-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 10.Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M, Lanzoni G, Cantoni S, Cavallini C, Bianchi F, Tazzari PL, Pasquinelli G, Foroni L, Ventura C, Grossi A, Bagnara GP. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11. doi: 10.1186/1471-213X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubo K, Ohnishi S, Hosono H, Fukai M, Kameya A, Higashi R, Yamada T, Onishi R, Yamahara K, Takeda H, Sakamoto N. Human amnion-derived mesenchymal stem cell transplantation ameliorates liver fibrosis in rats. Transplant Direct. 2015;1:e16. doi: 10.1097/TXD.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawakubo K, Ohnishi S, Fujita H, Kuwatani M, Onishi R, Masamune A, Takeda H, Sakamoto N. Effect of fetal membrane-derived mesenchymal stem cell transplantation in rats with acute and chronic pancreatitis. Pancreas. 2016;45:707–713. doi: 10.1097/MPA.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 13.Onishi R, Ohnishi S, Higashi R, Watari M, Yamahara K, Okubo N, Nakagawa K, Katsurada T, Suda G, Natsuizaka M, Takeda H, Sakamoto N. Human amnion-derived mesenchymal stem cell transplantation ameliorates dextran sulfate sodium-induced severe colitis in rats. Cell Transplant. 2015;24:2601–2614. doi: 10.3727/096368915X687570. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto S, Ohnishi S, Onishi R, Tsuchiya I, Hosono H, Katsurada T, Yamahara K, Takeda H, Sakamoto N. Therapeutic effects of human amnion-derived mesenchymal stem cell transplantation and conditioned medium enema in rats with trinitrobenzene sulfonic acid-induced colitis. Am J Transl Res. 2017;9:940–952. [PMC free article] [PubMed] [Google Scholar]
- 15.Ono M, Ohnishi S, Honda M, Ishikawa M, Hosono H, Onishi R, Nakagawa K, Takeda H, Sakamoto N. Effects of human amnion-derived mesenchymal stromal cell transplantation in rats with radiation proctitis. Cytotherapy. 2015;17:1545–1559. doi: 10.1016/j.jcyt.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Mizushima T, Ohnishi S, Hosono H, Yamahara K, Tsuda M, Shimizu Y, Kato M, Asaka M, Sakamoto N. Oral administration of conditioned medium obtained from mesenchymal stem cell culture prevents subsequent stricture formation after esophageal submucosal dissection in pigs. Gastrointest Endosc. 2017;86:542–552. e541. doi: 10.1016/j.gie.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Fickert P, Pollheimer MJ, Beuers U, Lackner C, Hirschfield G, Housset C, Keitel V, Schramm C, Marschall HU, Karlsen TH, Melum E, Kaser A, Eksteen B, Strazzabosco M, Manns M, Trauner M International PSC Study Group (IPSCSG) Characterization of animal models for primary sclerosing cholangitis (PSC) J Hepatol. 2014;60:1290–1303. doi: 10.1016/j.jhep.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollheimer MJ, Fickert P. Animal models in primary biliary cirrhosis and primary sclerosing cholangitis. Clin Rev Allergy Immunol. 2015;48:207–217. doi: 10.1007/s12016-014-8442-y. [DOI] [PubMed] [Google Scholar]
- 19.Becker BA, Plaa GL. The nature of alpha-naphthylisothiocyanate-induced cholestasis. Toxicol Appl Pharmacol. 1965;7:680–685. doi: 10.1016/0041-008x(65)90125-0. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich CG, Ottenhoff R, de Waart DR, Oude Elferink RP. Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology. 2001;167:73–81. doi: 10.1016/s0300-483x(01)00459-0. [DOI] [PubMed] [Google Scholar]
- 21.Tjandra K, Sharkey KA, Swain MG. Progressive development of a Th1-type hepatic cytokine profile in rats with experimental cholangitis. Hepatology. 2000;31:280–290. doi: 10.1002/hep.510310204. [DOI] [PubMed] [Google Scholar]
- 22.Lesage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, Phinizy JL, Francis H, Alpini G. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G182–190. doi: 10.1152/ajpgi.2001.281.1.G182. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Lee G, Wang H, Vierling JM, Maher JJ. Limited role for CXC chemokines in the pathogenesis of alpha-naphthylisothiocyanate-induced liver injury. Am J Physiol Gastrointest Liver Physiol. 2004;287:G734–741. doi: 10.1152/ajpgi.00300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moritoki Y, Ueno Y, Kanno N, Yamagiwa Y, Fukushima K, Gershwin ME, Shimosegawa T. Lack of evidence that bone marrow cells contribute to cholangiocyte repopulation during experimental cholestatic ductal hyperplasia. Liver Int. 2006;26:457–466. doi: 10.1111/j.1478-3231.2006.01250.x. [DOI] [PubMed] [Google Scholar]
- 25.Luyendyk JP, Kassel KM, Allen K, Guo GL, Li G, Cantor GH, Copple BL. Fibrinogen deficiency increases liver injury and early growth response-1 (Egr-1) expression in a model of chronic xenobiotic-induced cholestasis. Am J Pathol. 2011;178:1117–1125. doi: 10.1016/j.ajpath.2010.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 27.Faiola B, Peterson RA, Kimbrough CL, Jordan HL, Cullen JM. Acute ANIT toxicity in male IL-10 knockout and wild-type mice. Toxicol Pathol. 2010;38:745–755. doi: 10.1177/0192623310374970. [DOI] [PubMed] [Google Scholar]
- 28.He H, Mennone A, Boyer JL, Cai SY. Combination of retinoic acid and ursodeoxycholic acid attenuates liver injury in bile duct-ligated rats and human hepatic cells. Hepatology. 2011;53:548–557. doi: 10.1002/hep.24047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi N, Kopec AK, O’Brien KM, Towery KL, Cline-Fedewa H, Williams KJ, Copple BL, Flick MJ, Luyendyk JP. Coagulation-driven platelet activation reduces cholestatic liver injury and fibrosis in mice. J Thromb Haemost. 2015;13:57–71. doi: 10.1111/jth.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soroka CJ, Mennone A, Hagey LR, Ballatori N, Boyer JL. Mouse organic solute transporter alpha deficiency enhances renal excretion of bile acids and attenuates cholestasis. Hepatology. 2010;51:181–190. doi: 10.1002/hep.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai SY, Mennone A, Soroka CJ, Boyer JL. All-trans-retinoic acid improves cholestasis in alpha-naphthylisothiocyanate-treated rats and Mdr2-/- mice. J Pharmacol Exp Ther. 2014;349:94–98. doi: 10.1124/jpet.113.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim ND, Moon JO, Slitt AL, Copple BL. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006;90:586–595. doi: 10.1093/toxsci/kfj111. [DOI] [PubMed] [Google Scholar]
- 33.Golbar HM, Izawa T, Yano R, Ichikawa C, Sawamoto O, Kuwamura M, Lamarre J, Yamate J. Immunohistochemical characterization of macrophages and myofibroblasts in alpha-Naphthylisothiocyanate (ANIT)--induced bile duct injury and subsequent fibrogenesis in rats. Toxicol Pathol. 2011;39:795–808. doi: 10.1177/0192623311413790. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Arias M, Sauer-Lehnen S, Treptau J, Janoschek N, Theuerkauf I, Buettner R, Gressner AM, Weiskirchen R. Adenoviral expression of a transforming growth factor-beta1 antisense mRNA is effective in preventing liver fibrosis in bile-duct ligated rats. BMC Gastroenterol. 2003;3:29. doi: 10.1186/1471-230X-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bridle KR, Popa C, Morgan ML, Sobbe AL, Clouston AD, Fletcher LM, Crawford DH. Rapamycin inhibits hepatic fibrosis in rats by attenuating multiple profibrogenic pathways. Liver Transpl. 2009;15:1315–1324. doi: 10.1002/lt.21804. [DOI] [PubMed] [Google Scholar]
- 37.Kodali P, Wu P, Lahiji PA, Brown EJ, Maher JJ. ANIT toxicity toward mouse hepatocytes in vivo is mediated primarily by neutrophils via CD18. Am J Physiol Gastrointest Liver Physiol. 2006;291:G355–363. doi: 10.1152/ajpgi.00458.2005. [DOI] [PubMed] [Google Scholar]
- 38.Joshi N, Ray JL, Kopec AK, Luyendyk JP. Dose-dependent effects of alpha-naphthylisothiocyanate disconnect biliary fibrosis from hepatocellular necrosis. J Biochem Mol Toxicol. 2017;31:1–7. doi: 10.1002/jbt.21834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HG, Choi OH. Neovascularization in a mouse model via stem cells derived from human fetal amniotic membranes. Heart Vessels. 2011;26:196–205. doi: 10.1007/s00380-010-0064-6. [DOI] [PubMed] [Google Scholar]
- 40.Danieli P, Malpasso G, Ciuffreda MC, Cervio E, Calvillo L, Copes F, Pisano F, Mura M, Kleijn L, de Boer RA, Viarengo G, Rosti V, Spinillo A, Roccio M, Gnecchi M. Conditioned medium from human amniotic mesenchymal stromal cells limits infarct size and enhances angiogenesis. Stem Cells Transl Med. 2015;4:448–458. doi: 10.5966/sctm.2014-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, Watanabe T, Okano H, Matsuzaki Y, Shiota G, Okazaki I. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45:213–222. doi: 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- 42.Yoshiji H, Kuriyama S, Miyamoto Y, Thorgeirsson UP, Gomez DE, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Tsujinoue H, Nakatani T, Thorgeirsson SS, Fukui H. Tissue inhibitor of metalloproteinases-1 promotes liver fibrosis development in a transgenic mouse model. Hepatology. 2000;32:1248–1254. doi: 10.1053/jhep.2000.20521. [DOI] [PubMed] [Google Scholar]
- 43.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, Kawamata M, Kato T, Okochi H, Ochiya T. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells. 2008;26:2705–2712. doi: 10.1634/stemcells.2008-0034. [DOI] [PubMed] [Google Scholar]
- 44.van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 45.Furlani D, Ugurlucan M, Ong L, Bieback K, Pittermann E, Westien I, Wang W, Yerebakan C, Li W, Gaebel R, Li RK, Vollmar B, Steinhoff G, Ma N. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77:370–376. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 47.Wang D, Zhang H, Liang J, Gu Z, Ma X, Huang J, Lin J, Hou Y, Lu L, Sun L. Effect of allogeneic bone marrow-derived mesenchymal stem cells transplantation in a polyI: C-induced primary biliary cirrhosis mouse model. Clin Exp Med. 2011;11:25–32. doi: 10.1007/s10238-010-0105-6. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Li J, Liu H, Li Y, Fu J, Sun Y, Xu R, Lin H, Wang S, Lv S, Chen L, Zou Z, Li B, Shi M, Zhang Z, Wang FS. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28(Suppl 1):85–92. doi: 10.1111/jgh.12029. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Han Q, Chen H, Wang K, Shan GL, Kong F, Yang YJ, Li YZ, Zhang X, Dong F, Wang Q, Xu D, Hu ZJ, Wang SH, Keating A, Bi YL, Zhang FC, Zhao RC. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev. 2014;23:2482–2489. doi: 10.1089/scd.2013.0500. [DOI] [PubMed] [Google Scholar]
- 50.Ringden O, Erkers T, Nava S, Uzunel M, Iwarsson E, Conrad R, Westgren M, Mattsson J, Kaipe H. Fetal membrane cells for treatment of steroid-refractory acute graft-versus-host disease. Stem Cells. 2013;31:592–601. doi: 10.1002/stem.1314. [DOI] [PubMed] [Google Scholar]