Abstract
The optimal first-line treatment for primary Ewing sarcoma (ES) of the spine is unclear, especially when the patients present with acute neurological deficits. This study aimed to retrospectively analyze the effect of first-line treatment with surgery or chemotherapy on neurological and survival outcomes of ES of the spine. 39 patients treated between January 2005 and December 2016 were included in the present analysis. 29 (74.4%) presented with symptomatic spinal cord compression at diagnosis. 21 patients were submitted to primary surgery followed by chemotherapy and local radiotherapy, while 18 patients received induction chemotherapy before surgery and/or local radiotherapy. Neurological deficit before and after treatment, event-free survival and overall survival were analyzed. The results indicated that chemotherapy as the first-line treatment could achieve similar results as primary surgery in preserving neurological function, even in case of major neurological deficits. Compared with primary surgery, induction chemotherapy contributed to a higher rate of en bloc resection with a microscopic negative margin (R0) of primary tumor (72.7% vs. 28.6%, P < 0.05). Multivariate Cox regression analyses revealed that initial chemotherapy was a favorable independent prognostic factor of event-free survival (hazard ratio, 0.215; 95% confidence interval, 0.077-0.596; P = 0.003) and overall survival (hazard ratio, 0.288, 95% confidence interval, 0.098-0.852; P = 0.024). In conclusion, our study suggests that first-line treatment of ES of the spine should be induction chemotherapy, even in case of major neurological deficits.
Keywords: Ewing sarcoma, spine, induction chemotherapy, surgery, prognosis
Introduction
Ewing sarcoma (ES) is the second most common primary sarcoma of bone in children and adolescents [1]. The most common primary sites of involvement of ES are the extremities and pelvis [2-4]. The outcome of ES has improved with introduction of multi-agent chemotherapy and multidisciplinary management. Survival has been reported to approach 65-80% in localized ES [5-7], although overall survival (OS) remains dismal for patients with metastatic disease [8].
Generally, treatment of ES consists of three to six cycles of induction chemotherapy after diagnosis by biopsy, followed by surgery and/or local radiotherapy and then maintenance chemotherapy [5,9,10]. However, primary ES of the spine has special characteristics. First, there is no consensus on the first-line treatment for ES, especially when the patients present with acute neurological deficits. Primary decompressive surgery has often been preferred to prevent major neurological sequelae [11-13]. However, a few recent studies reported that adequate and quick decompression of neural structures could be achieved by initial chemotherapy because of the high chemosensitivity of the tumor [14]. Second, there is no consensus on the optimal form of local treatment for ES. Surgery and radiotherapy have their limits and complications. Wide resection is often not feasible because of adjacent critical structures, while radiotherapy is limited by tolerance of the spinal cord [13]. Finally, primary ES of the spine is an exceedingly rare entity, which accounts for 3.5-6% of all primary sites [3,15]. Most studies on primary ES of the spine were limited to case reports or small series treated homogeneously in a single institution [11,16-20]. This fact makes performing large series studies difficult for comparing outcomes of different treatment strategies.
In this retrospective study, we reviewed our experience in treating ES of the spine initially with surgery or chemotherapy. This study aimed to analyze treatment-related factors that affected neurological and survival outcomes of this rare disease.
Materials and methods
Patients
This study was approved by the local ethics committee. Because of the retrospective nature of the study, informed consent was waived. Between January 2005 and December 2016, 39 consecutive patients with primary ES of the spine were treated by the same multidisciplinary team in our center (Table 1). All of the patients had histopathologically diagnosed ES of the spine based on the results of core needle biopsy or open surgery. Diagnosis of ES required the presence of a small blue round-cell tumor and positive immunohistochemical staining for CD99 (MIC2). Additionally, evaluation for translocation t (11; 22) (q24; q12) was performed in 15 patients in the present study. Staging procedures consisted of a thorough clinical examination, bone marrow biopsy and imaging studies. Imaging studies included X-rays, multiplanar reconstruction computed tomography and magnetic resonance imaging (MRI) of the involved vertebrae, a chest computed tomography scan, and a bone scan or positron emission tomography.
Table 1.
Baseline characteristics of the patients based on first-line treatment
| Characteristics | Primary surgery (n = 21) | Initial CT (n = 18) | P value |
|---|---|---|---|
| Gender | 0.726 | ||
| Male | 15 | 14 | |
| Female | 6 | 4 | |
| Age at diagnosis | 0.497 | ||
| < 18 y | 7 | 4 | |
| ≥ 18 y | 14 | 14 | |
| Spinal site | 0.750 | ||
| Sacral | 7 | 7 | |
| Nonsacral | 14 | 11 | |
| Number of pathological vertebra | 0.523 | ||
| 1 | 10 | 11 | |
| ≥ 2 | 11 | 7 | |
| Stage | 0.723 | ||
| Localized | 16 | 12 | |
| Metastatic | 5 | 6 | |
| Diameter of primary tumor | 1.000 | ||
| < 8 cm | 13 | 11 | |
| ≥ 8 cm | 8 | 7 | |
| Site of metastasis | 1.000 | ||
| Lung only | 3 | 3 | |
| Lung/bone/bone marrow | 2 | 3 | |
| Spinal cord compression | 0.141 | ||
| Yes | 18 | 11 | |
| No | 3 | 7 |
Unless otherwise stated, data shown are numbers of patients. CT, chemotherapy.
Treatments
All of the patients were submitted to multi-agent chemotherapy for a total of 48 weeks. Among the 18 patients who underwent induction chemotherapy, a median of 4 cycles (range, 3-6 cycles) were administered. The chemotherapy regimen included vincristine (1.4 mg/m2 on day 1, maximum of 2 mg), doxorubicin (75 mg/m2 on day 1), and cyclophosphamide (1.2 g/m2 on day 1) alternating with etoposide (100 mg/m2/day, days 1-5) and ifosfamide (1.8 g/m2/day, days 1-5) every 3 weeks. Doxorubicin was replaced by actinomycin D (1.25 mg/m2) after reaching a cumulative dose of 450 mg/m2. As a routine, sustained grade 3-4 neutropenia for > 3 days or neutropenic fever, and grade 4 thrombocytopenia were indications of dose reduction in the present study. Granulocyte colony-stimulating factor prophylaxis was recommended to avoid new episodes of neutropenia and delay of subsequent courses.
The timing and form of local therapy were determined on an individual basis depending on the severity of spinal cord compression, the resectability of the primary tumor, the presence of metastases, and the patients’ preferences. All of the patients received intensity modulated radiotherapy, except for one patient who received proton therapy (not at our institute) for local control. For patients with prior surgery, radiotherapy was started within 9-12 weeks after surgery. For patients with no prior surgery, definitive radiotherapy was performed after induction chemotherapy. The median dose on the operative bed was 45 Gy (1.8-2.0 Gy/day) in cases of resection with microscopic negative margins (R0). In cases of resection with microscopic positive margins (R1) or gross residual disease (R2) or exclusive radiotherapy, patients received radiotherapy at a median dose of 50 Gy (40-60 Gy), with 1.8-2 Gy given per day, 5 days a week, over 5-6 weeks (Figure 1).
Figure 1.
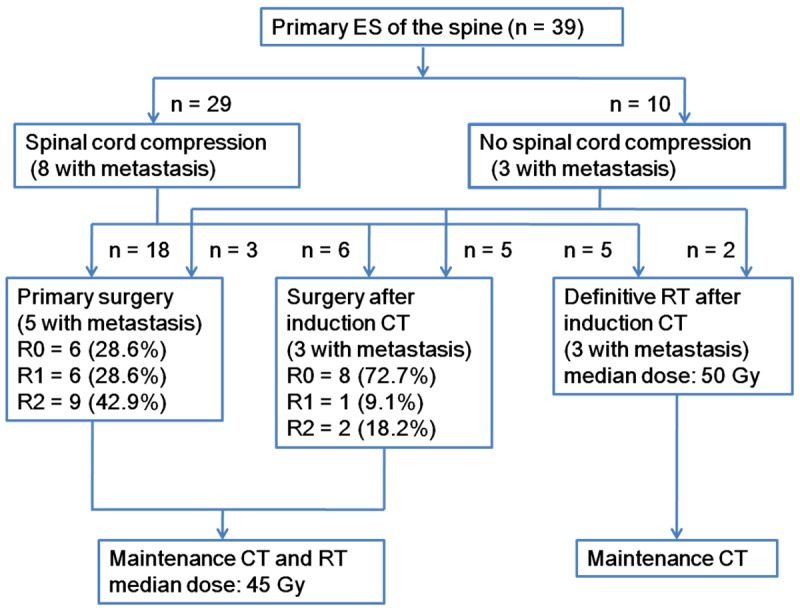
Flow chart of the treatment. Unless otherwise stated, data shown are numbers of patients; ES, Ewing sarcoma; R0, microscopic negative margin; R1, microscopic positive margin; R2, gross residual disease; CT, chemotherapy; RT, radiotherapy.
Data collection
A database was designed to retrospectively collect data on baseline clinicopathological features, treatment modalities, and neurological and survival outcomes. For evaluating neurological function, the Frankel grade was used, with class A representing complete paralysis, class B representing sensory function only below the injury level, class C representing incomplete motor function below injury level, class D representing fair to good motor function below injury level, and class E representing normal motor and sensory function [21]. Event-free survival (EFS) was defined as the time from diagnosis to disease recurrence, progression, second malignancy, death from any cause, or last contact. OS was defined as the interval between diagnosis and death from any cause or last follow-up. Clinical responses were classified as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to response evaluation criteria in solid tumors for the soft-tissue component of the primary lesion as well as non-osseous metastases [22]. The data were collected and checked by two of the authors from clinical charts and questionnaire responses. Missing data were collected from patients or their family members by telephone.
Statistical analysis
All analyses were performed using commercially available software (Statistical Package for the Social Sciences, version 18.0; SPSS, Inc., Chicago, IL). A P value < 0.05 was considered significant. Continuous variables are shown as mean ± SD. Qualitative variables are expressed as absolute and relative frequencies. The X2 or Fisher’s exact test was used to compare proportions. Kaplan-Meier survival curves were used to estimate the proportion surviving and the log-rank test was used to compare differences among subgroups. To identify independent prognostic factors, univariable and multivariable analyses were performed using Cox regression models. If variables were significant at the 0.1 level in univariate analysis, they were included in multiple regression.
Results
Patient characteristics
This study consisted of 39 patients (29 males, 10 females), with a mean age of 25.3 ± 9.1 years (range, 5-48 years) at diagnosis. Eleven (28.2%) patients were < 18 years of age, whereas 28 (71.8%) patients were ≥ 18 years. The primary tumor site was cervical in six patients, thoracic in ten, lumbar in nine, and sacral in 14. The diameter of the primary lesion was < 8 cm in 24 patients and ≥ 8 cm in 15 patients. Symptomatic spinal cord compression was present in 29 (74.4%) patients. Among all 39 patients, 28 (71.8%) presented with localized disease, eleven (28.2%) presented with metastatic disease (6 with lung metastases, 2 with bone metastases, 2 with both lung and bone metastases, and 1 with simultaneous bone and bone marrow metastases) at diagnosis (Table 1). Primary surgery was performed in 21 (53.8%) patients before diagnosis and chemotherapy. Eleven of the remaining 18 patients underwent surgery of the primary tumors after induction chemotherapy. Overall, 32 (82.1%) patients underwent surgery for local control, seven (17.9%) patients received definitive radiotherapy for local control.
Clinical outcomes
Among the 21 patients who underwent primary surgery, en bloc resection was performed in 12 patients; six patients had R0 resection and six had R1 resection. The remaining nine patients underwent R2 resection. For the eleven patients who underwent surgery after induction chemotherapy, en bloc resection was performed in nine patients (8 R0, 1 R1) and two patients underwent R2 resection. The histological response to induction chemotherapy was good (necrosis of ≥ 90% of the resected specimen) in eight (72.7%) patients. The R0 resection rate was significantly lower in patients who had primary surgery than in those who had surgery after induction chemotherapy (28.6% vs. 72.7%, P < 0.05). The clinical response in all 18 patients who underwent induction chemotherapy included nine CRs, seven PRs and two SDs. The data in 6 patients with metastatic diseases included three CRs, two PRs and one SD. The overall response rates were similar for patients with and without metastasis (91.7% vs. 83.3%, P = 1.000). The clinical and histological responses to induction chemotherapy were not submitted to subgroup analyses because of the small number of available patients.
The median follow-up of all of the 39 patients was 42 months (range, 12-145 months). Local relapses occurred in eleven patients; seven of them were in patients with initial spinal cord compression and four of the patients had received induction chemotherapy as first-line therapy. Among the eleven patients with local relapses, six presented with simultaneous metastatic relapses (including metastatic progression) and five had delayed metastatic relapses. Ten patients experienced isolated metastatic relapse as a first oncological event. Local and metastatic relapses occurred within a median interval of 22 months (range, 6-62 months). Additionally, one patient who suffered from ES at T5 presented with a second malignancy (synovial sarcoma) at C6-T1 (not within the radiation field) 3 years after completion of treatment. She then received standard therapy of synovial sarcoma and survived continuously for 109 months after diagnosis of ES. Overall, the 5-year EFS was 37.0%. For subgroup analysis, EFS was lower in patients who were submitted to primary surgery than in patients who had initial chemotherapy (log-rank test, P = 0.011; Figure 2). A total of 20 patients died of disease progression during the follow-up period. There was no treatment-related death occurred, leading to a 5-year OS of 44.0%. Subgroup analysis indicated that OS was higher in patients who had induction chemotherapy than in patients who were submitted to primary surgery (log-rank test, P = 0.032; Figure 3).
Figure 2.
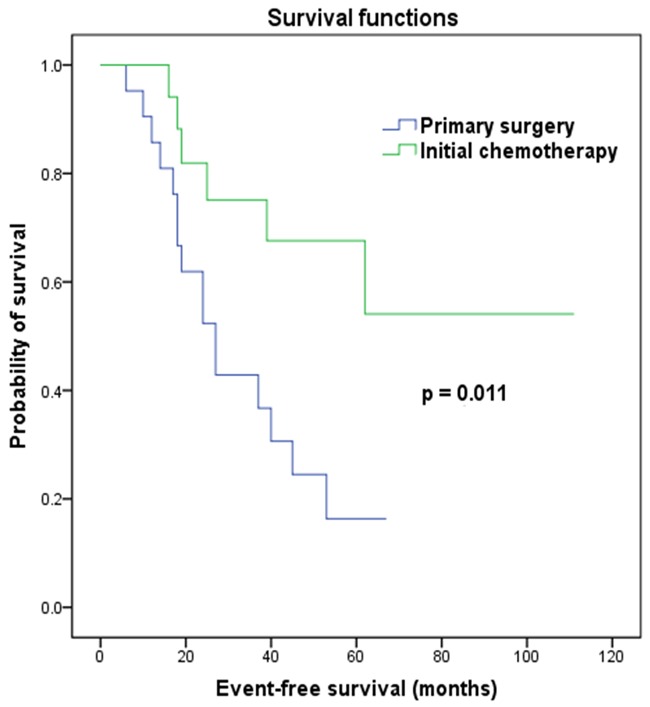
Kaplan-Meier survival curves for event-free survival according to first-line treatment.
Figure 3.
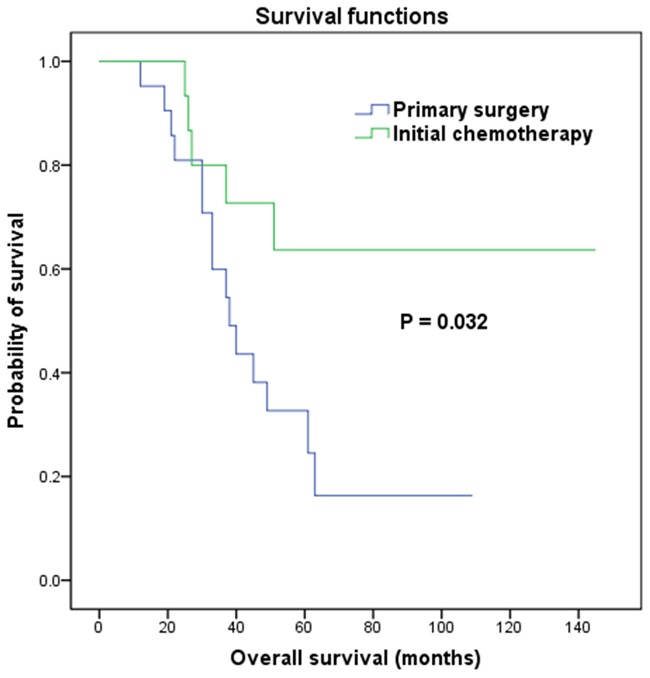
Kaplan-Meier survival curves for overall survival according to first-line treatment.
Neurological outcomes
The neurological deficits in the 29 patients with spinal cord compression consisted of different degrees of paraparesis, paraplegia, and/or cauda equina syndrome. After local and systematic treatments, all of the 29 patients experienced substantial improvement of neurological function as expressed in Frankel grade at 1 year and at the last follow-up, irrespective of the type of first-line treatment (Figure 4). At diagnosis, 13 (72.2%) of the 18 patients who had primary surgery and seven (63.6%) of the eleven patients who had initial chemotherapy had Frankel grades A and B injuries, respectively (P = 0.694). Despite local recurrences that occurred in seven patients, the neurological function of most patients in the two subgroups improved to Frankel grades D and E at the last follow-up (88.9% vs. 81.8%, P = 0.622).
Figure 4.
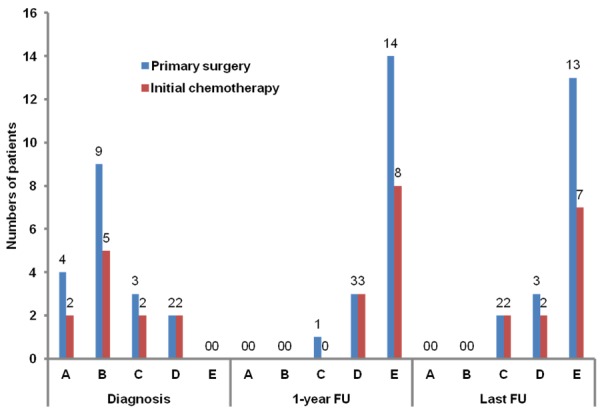
Frankel grade improvement in patients with spinal cord compression. Data shown are numbers of patients; FU, follow-up.
Safety
Drug toxicities to the hematologic system, liver, kidneys, heart, bladder and mucosa were monitored during chemotherapy. Grade 3-4 neutropenia was reported in 22 patients (56.4%), grade 3-4 thrombocytopenia in 10 patients (25.6%). Toxicity-related dose reduction occurred in 14 patients (35.9%), but no deaths due to toxic effects were observed. Toxic effects according to first-line treatment are reported in Table 2, there were no differences between the two treatment groups. The four patients younger than 16 years in the present study experienced a higher incidence of grade 3-4 chemotherapy induced nausea and vomiting compared to the whole group (100% vs. 25.6%, P < 0.05).
Table 2.
Chemotherapy toxicity according to first-line treatment
| Toxicity and grade | Primary surgery (n = 21) | Initial CT (n = 18) | P value |
|---|---|---|---|
| Hematological toxicitie | |||
| Neutropenia | |||
| All | 20 (95.2%) | 18 (100%) | 1.000 |
| 3-4 | 11 (52.4%) | 11 (61.1%) | 0.748 |
| Anemia | |||
| All | 9 (42.9%) | 6 (33.3%) | 0.742 |
| 3 | 2 (9.5%) | 1 (5.6%) | 1.000 |
| Thrombocytopenia | |||
| All | 10 (47.6%) | 10 (55.6%) | 0.751 |
| 3-4 | 5 (23.8%) | 5 (27.8%) | 1.000 |
| Nausea and vomiting | |||
| All | 16 (76.2%) | 12 (66.7%) | 0.723 |
| 3-4 | 6 (28.6%) | 4 (22.2%) | 0.726 |
| Hepatic dysfunction | |||
| All | 3 (14.3%) | 2 (11.1%) | 1.000 |
| 3-4 | 1 (4.8%) | 1 (5.6%) | 1.000 |
| Renal dysfunction | |||
| 1-2 | 1 (4.8%) | 0 (0%) | 1.000 |
| Alopecia | |||
| 1-2 | 19 (90.5%) | 18 (100%) | 0.490 |
| Mucositis | |||
| All | 4 (19.0%) | 4 (22.2%) | 1.000 |
| 3-4 | 2 (9.5%) | 2 (11.1%) | 1.000 |
| Cardiac toxicities | |||
| Arrhythmia | |||
| 1-2 | 3 (14.3%) | 4 (22.2%) | 0.682 |
| Myocardial ischemia | |||
| 1-2 | 2 (9.5%) | 3 (16.7%) | 0.647 |
| Hemorrhagic cystitis | |||
| 1-2 | 0 (0%) | 1 (5.6%) | 0.462 |
| Dose reduction | |||
| All | 8 (38.1%) | 6 (33.3%) | 1.000 |
Categorical data are presented as numbers (percentages). CT, chemotherapy.
In addition, patients in the primary surgery group rehabilitated more quickly postoperatively compared to patients in the initial chemotherapy group, the mean interval between surgery and the first cycle of maintenance chemotherapy were 16 days and 21 days, respectively (P < 0.05).
Analysis of prognostic factors of survival
Univariable analysis showed that initial chemotherapy (P = 0.016) was significantly associated with improved EFS. This factor along with localized disease at the time of diagnosis (P = 0.076) and R0 resection of the primary tumor (P = 0.089) were submitted to multivariable analysis. The results showed that both initial chemotherapy (P = 0.003) and localized disease at the time of diagnosis (P = 0.004) were favorable independent prognostic factors of EFS (Table 3). As far as OS was concerned, univariable analysis showed that localized disease at the time of diagnosis (P = 0.005), initial chemotherapy (P = 0.042) and R0 resection of the primary tumor (P = 0.044) were significantly associated with improved OS. Multivariable analysis showed that these three factors remained significant predictors of OS. Initial chemotherapy was associated with improved OS after adjustment for the stage of disease and R0 resection of the primary tumor (P = 0.024, Table 4). Stratified analyses of overall survival were performed based on different status of spinal cord compression. The results indicated that initial chemotherapy didn’t improve overall survival in our patients with no neurological deficits (log-rank test, P = 0.460). However, initial chemotherapy showed a nonsignificant trend in improving the prognosis of our patients with spinal cord compression (log-rank test, P = 0.054).
Table 3.
Univariable and multivariable Cox proportional hazard regression analyses of event-free survival
| Factor | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender | 0.710 | |||
| Male | Reference | |||
| Female | 1.187 (0.482-2.921) | |||
| Age at diagnosis | 0.565 | |||
| < 18 y | Reference | |||
| ≥ 18 y | 0.768 (0.313-1.887) | |||
| Spinal site | 0.663 | |||
| Sacral | Reference | |||
| Nonsacral | 0.830 (0.359-1.917) | |||
| Number of pathological vertebra | 0.486 | |||
| 1 | Reference | |||
| ≥ 2 | 1.348 (0.582-3.126) | |||
| Stage | 0.076 | 0.004 | ||
| Localized | Reference | Reference | ||
| Metastatic | 2.332 (0.915-5.943) | 4.846 (1.655-14.192) | ||
| Diameter of primary tumor | 0.528 | |||
| < 8 cm | Reference | |||
| ≥ 8 cm | 1.315 (0.561-3.082) | |||
| Spinal cord compression | 0.408 | |||
| No | Reference | |||
| Yes | 1.525 (0.561-4.146) | |||
| First-line treatment | 0.016 | 0.003 | ||
| Surgery | Reference | Reference | ||
| Chemotherapy | 0.310 (0.119-0.807) | 0.215 (0.077-0.596) | ||
| Local treatment | 0.475 | |||
| Radiotherapy | Reference | |||
| Surgery + radiotherapy | 1.562 (0.460-5.299) | |||
| En bloc resection of primary tumor | 0.892 | |||
| No | Reference | |||
| Yes | 0.943 (0.403-2.204) | |||
| R0 resection of primary tumor | 0.089 | 0.056 | ||
| No | Reference | Reference | ||
| Yes | 0.451 (0.173-1.180) | 0.375 (0.137-1.026) | ||
HR, hazard ratio; CI, confidence interval; R0 resection, resection with a microscopic negative margin.
Table 4.
Univariable and multivariable Cox proportional hazard regression analyses of overall survival
| Factor | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender | 0.959 | |||
| Male | Reference | |||
| Female | 1.026 (0.393-2.675) | |||
| Age at diagnosis | 0.880 | |||
| < 18 y | Reference | |||
| ≥ 18 y | 1.081 (0.392-2.982) | |||
| Spinal site | 0.672 | |||
| Sacral | Reference | |||
| Nonsacral | 0.827 (0.343-1.995) | |||
| Number of pathological vertebra | 0.566 | |||
| 1 | Reference | |||
| ≥ 2 | 1.294 (0.537-3.114) | |||
| Stage | 0.005 | 0.000 | ||
| Localized | Reference | Reference | ||
| Metastatic | 4.211 (1.557-11.393) | 8.610 (2.738-27.078) | ||
| Diameter of primary tumor | 0.470 | |||
| < 8 cm | Reference | |||
| ≥ 8 cm | 1.393 (0.567-3.420) | |||
| Spinal cord compression | 0.683 | |||
| No | Reference | |||
| Yes | 1.235 (0.448-3.406) | |||
| First-line treatment | 0.042 | 0.024 | ||
| Surgery | Reference | Reference | ||
| Chemotherapy | 0.348 (0.126-0.961) | 0.288 (0.098-0.852) | ||
| Local treatment | 0.695 | |||
| Radiotherapy | Reference | |||
| Surgery + radiotherapy | 1.279 (0.374-4.376) | |||
| En bloc resection of primary tumor | 0.467 | |||
| No | Reference | |||
| Yes | 0.721 (0.299-1.739) | |||
| R0 resection of primary tumor | 0.044 | 0.047 | ||
| No | Reference | Reference | ||
| Yes | 0.321 (0.106-0.969) | 0.308 (0.096-0.986) | ||
HR, hazard ratio; CI, confidence interval; R0 resection, resection with a microscopic negative margin.
Discussion
Whether the same treatment strategy of ES outside of the spine can be used for ES originating in the spine is controversial, especially when the patients present with major neurological deficits [8,23]. The timing of local treatment for ES of the spine is critical because spinal cord compression is a common emergency due to epidural tumor extension [13,14,24,25]. In a French retrospective study, 79% of the 75 patients with ES of the spine presented with symptomatic neurological compression [13]. In a series of 33 patients, reported by Venkateswaran et al., neurological deficits were determined in 94% of all patients [25]. Mirzaei et al. reported that all of the 15 treated patients had varying degrees of neurological deficits [14]. The degree of neurological recovery is often fixed at 48-72 h after spinal cord injury [26]. Prompt decompression is urgently required because delay in treatment can result in irreversible outcomes [27]. In such circumstances, immediate surgical decompression is often preferred [11-13,19,28]. In our study population, 29 (74.4%) patients presented with symptomatic spinal cord compression at diagnosis. Primary surgery was performed in 21 patients for functional and curative purpose (en bloc resection in 12 patients, intralesional excision in 9 patients). As expected, all of the 18 patients with symptomatic spinal cord compression experienced substantial improvement of neurological function after primary surgery. Interestingly, the eleven patients with symptomatic spinal cord compression who received initial chemotherapy also had an acceptable recovery from neurological deficits. No serious neurological sequelae were determined during the follow-up period, despite seven patients initially presenting with Frankel grades A and B injury. Similar effects of chemotherapy on neurological preservation in ES of the spine were reported by other authors [14]. The most likely explanation for this effect of chemotherapy is that the high chemosensitivity of the tumor leads to shrinkage of the tumor, and then decreases compression of the spinal cord in a timely manner.
Although neurological function is an important endpoint for measuring the success of treatment of primary spinal ES, survival is a more important issue that needs to be addressed. Surgery as the first-line treatment of ES of the spine is controversial because it may induce local tumor spilling and ultimately compromise survival [15]. Induction chemotherapy can eradicate micrometastases and induce shrinkage of the primary tumor, which contribute to effective local and systemic control [14,29]. Unfortunately, no formal comparison has been made between these two types of first-line treatments in ES of the spine because of the rarity of this disease. In the present study, all of the patients were treated at the same institution by the same multidisciplinary team, and data concerning neurological and survival outcomes were available for all patients. Therefore, we were able to compare between these two types of first-line treatments. We found that patients who had initial chemotherapy had significantly better EFS and OS compared with patients who underwent primary surgery. Furthermore, induction chemotherapy contributed to a higher rate of R0 resection, which was a favorable prognostic factor of OS in the present cohort.
The 5-year OS and EFS of our complete series were 44.0% and 37.0%, respectively, which are less than those of other recent reports [2,13,15]. Notably, these previous studies only included ES of the mobile spine, but not sacral ES, and their patients were younger than those in our study. Sacral origin and older age are unfavorable prognostic factors of ES [30-33]. Additionally, patients with metastatic diseases are always associated with poorer survival compared with patients with localized ES. In the present study, the 5-year OS was 53.0% in 28 patients with localized diseases, which is comparable with that of other reports; Bacci et al. found a 5-year OS of 42.0% in a series of 43 cases [34] and Marco et al. found 46.0% in a series of 13 cases [4]. Interestingly, our data indicate that primary surgery is detrimental to the prognosis of the present cohort. Similarly, a recent systematic review suggested that initial chemotherapy offered significant improvement in local control and long-term survival for spinal ES [35]. On the basis of these findings, the authors recommend performing surgery after induction chemotherapy instead of before chemotherapy to facilitate R0 resection and decrease surgical morbidity and risks.
Two important issues should be emphasized. First, induction chemotherapy in patients who are neurologically at risk should start immediately after establishment of a timely histopathological diagnosis. In the case where a definitive diagnosis is delayed (e.g., a soft tissue mass within the vertebral canal without any bony lesion), emergency surgery should be considered as first-line treatment. This is because any delay in intervention would compromise effectiveness in alleviating a neurological deficit [14]. Second, to achieve an R0 margin at the greatest extent, en bloc resection should be performed by orthopedic oncologists with specific expertise in the management of spinal tumors. Spinal reconstruction and stabilization are recommended to prevent subsequent development of spinal deformity and neurological demise.
This study has several limitations. First, owing to the small sample size and the retrospective study design, selection bias was unavoidable. However, the baseline characteristics of the patients were similar in the two subgroups submitted to primary surgery and initial chemotherapy. Second, all patients, including those who had R0 resection, received local radiotherapy in the present study. This situation makes analysing the role of en bloc resection alone for local control of ES of the spine difficult. However, considering that most en bloc resection around the spinal canal was marginal, but not wide resection, we suggest that combining resection with additional radiotherapy is prudent [36,37]. Prospective studies to evaluate the role of adjuvant radiotherapy in patients who undergo marginal resection of the primary spinal ES should be performed in the future.
In conclusion, our study shows that chemotherapy as the first-line treatment of ES of the spine can achieve similar results as primary surgery in preserving neurological function, even in case of major neurological deficits. Initial chemotherapy is associated with better EFS and OS compared with primary surgery. Furthermore, induction chemotherapy contributes to a higher rate of en bloc resection with an R0 margin, which is an independent prognostic factor of improved OS. Our findings suggest that the treatment strategy could be the same for ES located at the spine and at other sites. R0 resection following induction chemotherapy is strongly recommended when technically achievable.
Acknowledgements
This study received funding from the National Natural Science Foundation of China (81503396) and Shanghai Xinglinxinxing Program (ZY3-RCPY-2-2035). We thank Ellen Knapp, PhD, from Liwen Bianji, Edanz Group China for editing the English text of a draft of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Gupta AA, Pappo A, Saunders N, Hopyan S, Ferguson P, Wunder J, O’Sullivan B, Catton C, Greenberg M, Blackstein M. Clinical outcome of children and adults with localized Ewing sarcoma: impact of chemotherapy dose and timing of local therapy. Cancer. 2010;116:3189–94. doi: 10.1002/cncr.25144. [DOI] [PubMed] [Google Scholar]
- 2.Berger M, Fagioli F, Abate M, Riccardi R, Prete A, Cozza R, Bertulli R, Podda M, Ferrari S, Luksch R. Unusual sites of Ewing sarcoma (ES): a retrospective multicenter 30-year experience of the Italian association of pediatric hematology and oncology (AIEOP) and Italian sarcoma group (ISG) Eur J Cancer. 2013;49:3658–65. doi: 10.1016/j.ejca.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein M, Kovar H, Paulussen M, Randall RL, Schuck A, Teot LA, Juergens H. Ewing’s sarcoma family of tumors: current management. Oncologist. 2006;11:503–19. doi: 10.1634/theoncologist.11-5-503. [DOI] [PubMed] [Google Scholar]
- 4.Marco RA, Gentry JB, Rhines LD, Lewis VO, Wolinski JP, Jaffe N, Gokaslan ZL. Ewing’s sarcoma of the mobile spine. Spine (Phila Pa 1976) 2005;30:769–73. doi: 10.1097/01.brs.0000157755.17502.d6. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari S, Palmerini E, Alberghini M, Staals E, Mercuri M, Barbieri E, Longhi A, Cantero L, Cesari M, Abate M, Balladelli A, Picci P, Bacci G. Vincristine, doxorubicin, cyclophosfamide, actinomycin D, ifosfamide, and etoposide in adult and pediatric patients with nonmetastatic Ewing sarcoma. Final results of a monoinstitutional study. Tumori. 2010;96:213–8. doi: 10.1177/030089161009600205. [DOI] [PubMed] [Google Scholar]
- 6.Marina NM, Pappo AS, Parham DM, Cain AM, Rao BN, Poquette CA, Pratt CB, Greenwald C, Meyer WH. Chemotherapy dose-intensification for pediatric patients with Ewing’s family of tumors and desmoplastic small round-cell tumors: a feasibility study at St. Jude Children’s Research Hospital. J. Clin. Oncol. 1999;17:180–90. doi: 10.1200/JCO.1999.17.1.180. [DOI] [PubMed] [Google Scholar]
- 7.Biswas B, Bakhshi S. Management of Ewing sarcoma family of tumors: current scenario and unmet need. World J Orthop. 2016;7:527–38. doi: 10.5312/wjo.v7.i9.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaspar N, Hawkins DS, Dirksen U, Lewis IJ, Ferrari S, Le Deley MC, Kovar H, Grimer R, Whelan J, Claude L, Delattre O, Paulussen M, Picci P, Sundby Hall K, van den Berg H, Ladenstein R, Michon J, Hjorth L, Judson I, Luksch R, Bernstein ML, Marec-Berard P, Brennan B, Craft AW, Womer RB, Juergens H, Oberlin O. Ewing sarcoma: current management and future approaches through collaboration. J. Clin. Oncol. 2015;33:3036–46. doi: 10.1200/JCO.2014.59.5256. [DOI] [PubMed] [Google Scholar]
- 9.Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, Marcus K, Sailer S, Healey JH, Dormans JP, Weiss AR. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. J. Clin. Oncol. 2012;30:4148–54. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H, Ge Y, Guo L, Huang L. Potential approaches to the treatment of Ewing’s sarcoma. Oncotarget. 2017;8:5523–39. doi: 10.18632/oncotarget.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopalakrishnan CV, Shrivastava A, Easwer HV, Nair S. Primary Ewing’s sarcoma of the spine presenting as acute paraplegia. J Pediatr Neurosci. 2012;7:64–6. doi: 10.4103/1817-1745.97630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharafuddin MJ, Haddad FS, Hitchon PW, Haddad SF, el-Khoury GY. Treatment options in primary Ewing’s sarcoma of the spine: report of seven cases and review of the literature. Neurosurgery. 1992;30:610–8. discussion 618-9. [PubMed] [Google Scholar]
- 13.Vogin G, Helfre S, Glorion C, Mosseri V, Mascard E, Oberlin O, Gaspar N. Local control and sequelae in localised Ewing tumours of the spine: a French retrospective study. Eur J Cancer. 2013;49:1314–23. doi: 10.1016/j.ejca.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Mirzaei L, Kaal SE, Schreuder HW, Bartels RH. The neurological compromised spine due to Ewing sarcoma. What first: surgery or chemotherapy? Therapy, survival, and neurological outcome of 15 cases with primary Ewing sarcoma of the vertebral column. Neurosurgery. 2015;77:718–724. doi: 10.1227/NEU.0000000000000903. discussion 724-715. [DOI] [PubMed] [Google Scholar]
- 15.Boriani S, Amendola L, Corghi A, Cappuccio M, Bandiera S, Ferrari S, Picci R, Difiore M, Gasbarrini A. Ewing’s sarcoma of the mobile spine. Eur Rev Med Pharmacol Sci. 2011;15:831–9. [PubMed] [Google Scholar]
- 16.Sewell MD, Tan KA, Quraishi NA, Preda C, Varga PP, Williams R. Systematic review of En Bloc resection in the management of Ewing’s sarcoma of the mobile spine with respect to local control and disease-free survival. Medicine (Baltimore) 2015;94:e1019. doi: 10.1097/MD.0000000000001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sade R, Cakir M, Ogul H, Yuce I, Kantarci M. Primary extraosseous Ewing sarcoma of the lumbar spine presenting with left leg weakness. Spine J. 2015;15:1488–9. doi: 10.1016/j.spinee.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Vogin G, Biston MC, Marchesi V, Amessis M, De Marzi L, Lacroix F, Leroy A, Gassa F, Zefkili S, Helfre S. [Localized Ewing sarcoma of the spine: a preliminary dose-escalation study comparing innovative radiation techniques in a single patient] . Cancer Radiother. 2013;17:26–33. doi: 10.1016/j.canrad.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Choi SW, Shin SJ, Nam KW, Seo KB, Kim GM. Primary Ewing sarcoma of lumbar spine in an 8-year-old boy: a case report. J Pediatr Orthop B. 2012;21:322–4. doi: 10.1097/BPB.0b013e328351b907. [DOI] [PubMed] [Google Scholar]
- 20.Karikari IO, Mehta AI, Nimjee S, Hodges TR, Tibaleka J, Montgomery C, Simpson L, Cummings TJ, Bagley CA. Primary intradural extraosseous Ewing sarcoma of the spine: case report and literature review. Neurosurgery. 2011;69:E995–9. doi: 10.1227/NEU.0b013e318223b7c7. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Zhen Y, Wu C, Li H, Yang Y, Shen Z, Zhao H, Yao Y. CT fluoroscopy-guided percutaneous osteoplasty for the treatment of osteolytic lung cancer bone metastases to the spine and pelvis. J Vasc Interv Radiol. 2012;23:1135–42. doi: 10.1016/j.jvir.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Ladenstein R, Potschger U, Le Deley MC, Whelan J, Paulussen M, Oberlin O, van den Berg H, Dirksen U, Hjorth L, Michon J, Lewis I, Craft A, Jurgens H. Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. J. Clin. Oncol. 2010;28:3284–91. doi: 10.1200/JCO.2009.22.9864. [DOI] [PubMed] [Google Scholar]
- 23.Grubb MR, Currier BL, Pritchard DJ, Ebersold MJ. Primary Ewing’s sarcoma of the spine. Spine (Phila Pa 1976) 1994;19:309–13. doi: 10.1097/00007632-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Samartzis D, Marco RA, Benjamin R, Vaporciyan A, Rhines LD. Multilevel en bloc spondylectomy and chest wall excision via a simultaneous anterior and posterior approach for Ewing sarcoma. Spine (Phila Pa 1976) 2005;30:831–7. doi: 10.1097/01.brs.0000158226.49729.6c. [DOI] [PubMed] [Google Scholar]
- 25.Venkateswaran L, Rodriguez-Galindo C, Merchant TE, Poquette CA, Rao BN, Pappo AS. Primary Ewing tumor of the vertebrae: clinical characteristics, prognostic factors, and outcome. Med Pediatr Oncol. 2001;37:30–5. doi: 10.1002/mpo.1159. [DOI] [PubMed] [Google Scholar]
- 26.Wuermser LA, Ho CH, Chiodo AE, Priebe MM, Kirshblum SC, Scelza WM. Spinal cord injury medicine. 2. Acute care management of traumatic and nontraumatic injury. Arch Phys Med Rehabil. 2007;88:S55–61. doi: 10.1016/j.apmr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 27.O’Phelan KH, Bunney EB, Weingart SD, Smith WS. Emergency neurological life support: spinal cord compression (SCC) Neurocrit Care. 2012;17(Suppl 1):S96–101. doi: 10.1007/s12028-012-9756-3. [DOI] [PubMed] [Google Scholar]
- 28.Dini LI, Mendonca R, Gallo P. Primary Ewings sarcoma of the spine: case report. Arq Neuropsiquiatr. 2006;64:654–9. doi: 10.1590/s0004-282x2006000400026. [DOI] [PubMed] [Google Scholar]
- 29.Subbiah V, Anderson P, Lazar AJ, Burdett E, Raymond K, Ludwig JA. Ewing’s sarcoma: standard and experimental treatment options. Curr Treat Options Oncol. 2009;10:126–40. doi: 10.1007/s11864-009-0104-6. [DOI] [PubMed] [Google Scholar]
- 30.Pilepich MV, Vietti TJ, Nesbit ME, Tefft M, Kissane J, Burgert O, Prichard D, Gehan EA. Ewing’s sarcoma of the vertebral column. Int J Radiat Oncol Biol Phys. 1981;7:27–31. doi: 10.1016/0360-3016(81)90056-0. [DOI] [PubMed] [Google Scholar]
- 31.Arshi A, Sharim J, Park DY, Park HY, Yazdanshenas H, Bernthal NM, Shamie AN. Prognostic determinants and treatment outcomes analysis of osteosarcoma and Ewing sarcoma of the spine. Spine J. 2017;17:645–55. doi: 10.1016/j.spinee.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nesbit ME Jr, Gehan EA, Burgert EO Jr, Vietti TJ, Cangir A, Tefft M, Evans R, Thomas P, Askin FB, Kissane JM, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: a long-term follow-up of the first intergroup study. J. Clin. Oncol. 1990;8:1664–74. doi: 10.1200/JCO.1990.8.10.1664. [DOI] [PubMed] [Google Scholar]
- 33.Uyeturk U, Helvaci K, Demirci A, Sonmez OU, Turker I, Afsar CU, Budakoglu B, Arslan UY, Oksuzoglu OB, Zengin N. Clinical outcomes and prognostic factors of adult’s Ewing sarcoma family of tumors: single center experience. Contemp Oncol (Pozn) 2016;20:141–6. doi: 10.5114/wo.2016.58487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacci G, Boriani S, Balladelli A, Barbieri E, Longhi A, Alberghini M, Scotlandi K, Forni C, Pollastri P, Vanel D, Mercuri M. Treatment of nonmetastatic Ewing’s sarcoma family tumors of the spine and sacrum: the experience from a single institution. Eur Spine J. 2009;18:1091–5. doi: 10.1007/s00586-009-0921-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sciubba DM, Okuno SH, Dekutoski MB, Gokaslan ZL. Ewing and osteogenic sarcoma: evidence for multidisciplinary management. Spine (Phila Pa 1976) 2009;34:S58–68. doi: 10.1097/BRS.0b013e3181ba6436. [DOI] [PubMed] [Google Scholar]
- 36.Indelicato DJ, Keole SR, Shahlaee AH, Morris CG, Gibbs CP Jr, Scarborough MT, Pincus DW, Marcus RB Jr. Spinal and paraspinal Ewing tumors. Int J Radiat Oncol Biol Phys. 2010;76:1463–71. doi: 10.1016/j.ijrobp.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 37.Schuck A, Ahrens S, von Schorlemer I, Kuhlen M, Paulussen M, Hunold A, Gosheger G, Winkelmann W, Dunst J, Willich N, Jurgens H. Radiotherapy in Ewing tumors of the vertebrae: treatment results and local relapse analysis of the CESS 81/86 and EICESS 92 trials. Int J Radiat Oncol Biol Phys. 2005;63:1562–7. doi: 10.1016/j.ijrobp.2005.05.036. [DOI] [PubMed] [Google Scholar]


