Abstract
Avian pathogenic Escherichia coli (APEC) causes airsacculitis, polyserositis, septicemia, and other mainly extraintestinal diseases in chickens, ducks, geese, pigeons, and other avian species, and is responsible for great economic losses in the avian industry. The autoinducer 2 (AI‐2) quorum sensing system is widely present in many species of gram‐negative and gram‐positive bacteria and has been proposed to be involved in interspecies communication. In clinical APEC strains, whether or not AI‐2 affects the expression of antibiotic‐related genes has not been reported. In this study, we have reported that exogenous AI‐2 increase the susceptibility of APEC strains to trimethoprim‐sulfamethoxazole (SXT) in a folate synthesis‐dependent pathway but not in the LsrR‐dependent manner. Our results further explained that exogenous AI‐2 can down regulate the transcription of the folate synthetase encoding genes folA and folC, and the folate synthesis‐related genes luxS, metE, and metH. Gel shift assays confirmed that LsrR, the AI‐2 receptor, did not bind to the promoters of folA and folC, suggesting that exogenous AI‐2 might influence folate metabolism by a feedback inhibition effect but not in the LsrR‐dependent pathway. This study might provide further information in the search for potential drug targets for prophylaxis of avian colibacillosis and for auxiliary antibiotics in the treatment of avian colibacillosis.
Keywords: autoinducer 2, avian pathogenic Escherichia coli, folate synthesis, trimethoprim‐sulfamethoxazole
1. INTRODUCTION
Avian pathogenic Escherichia coli (APEC) is known to possess specific virulence attributes associated with colibacillosis in birds, and it is responsible for severe economic losses for the poultry industry worldwide (Dhomoulin & Fairbrother, 1999; Gross, 1994; Zhao et al., 2005). Avian colibacillosis can cause a variety of severe systemic and localized extraintestinal infections, with a complex syndrome characterized by multiple organ lesions like airsacculitis, pericarditis, perihepatitis, peritonitis, salpingitis, osteomyelitis, polyserositis, septicemia, and yolk sac infection (Dziva & Stevens, 2008; Ewers, Janssen, Kiessling, Philipp, & Wieler, 2004; Schouler et al., 2012; Wang et al., 2016). In the past few years, both the morbidity and mortality of APEC infections have increased rapidly and have become a major problem in the poultry industry (Altekruse et al., 2002; Blanco, Blanco, Mora, & Blanco, 1997). It has necessitated the use of antimicrobial chemotherapy to prevent and control outbreaks of APEC infections (Aggad, Ammar, Hammoudi, & Kihal, 2010; Watts, Salmon, Yancey, Nersessian, & Kounev, 1993).
A fixed‐dose combination of trimethoprim‐sulfamethoxazole (SXT) has been widely used as a broad‐spectrum antimicrobial agent since the 1960s (Church, Fitzgerald, Walker, Gibb, & Prendergast, 2015; Mcguinness, 1969). The rationale for the combination of trimethoprim with sulfamethoxazole is that each component blocks a different step in the folate biosynthetic pathway (Wormser, Keusch, & Heel, 1982). Sulfamethoxazole, a sulfonamide drug, is a structural analog of para‐aminobenzoic acid and competitively inhibits the synthesis of the intermediary dihydrofolic acid from its precursors, and trimethoprim is a structural analog of the pteridine portion of dihydrofolic acid that competitively inhibits dihydrofolate reductase and, consequently, decreases the production of the physiologically active tetrahydrofolic acid from dihydrofolic acid (Action, 2003; Church et al., 2015; Epstein, Amodio‐Groton, & Sadick, 1997). Although each agent alone is bacteriostatic, their blockade of two sequential enzymes results in bactericidal activity when combined (Action, 2003; Church et al., 2015). The synergy between trimethoprim and sulfamethoxazole together provides effective inhibition of enzymes involved in the bacterial synthesis of tetrahydrofolic acid, which is a necessary cofactor in bacterial nucleic acid synthesis (Grim, Rapp, Martin, & Evans, 2005; Sköld, 2011; Wormser et al., 1982). It has been reported that continuous intravenous SXT is a potential therapy to treat E. coli meningitis in rabbits and that the pharmacokinetic characteristics of sulfamethoxazole and trimethoprim given in combination to poultry produces a synergistic level for both antimicrobials and thus is considered to be a useful combination in the management of various avian diseases (Mylotte, Bates, Sergeant, Matson, & Jr, 1981; Queralt & Castells, 1985). The impact of SXT on E. coli in broiler intestinal or digestive tracts has already been reported (Dheilly et al., 2012). Furthermore, SXT has also been used in the treatment of bovine respiratory tract infections and feline and canine urinary tract infections caused by E. coli (Boothe, Smaha, Carpenter, Shaheen, & Hatchcock, 2012; Morrissey et al., 2016).
Quorum sensing (QS) is a process in which bacteria use chemical molecules as a signaling language to change behavior to adapt to specific environments (Bassler, 1999, 2002; Miller & Bassler, 2001). It has been reported that QS is a regulator of cellular processes such as bioluminescence production, virulence gene expression, cell division, biofilm formation, motility, metabolism, recombinant protein production, and the responsiveness to antibiotics (Ahmed, Petersen, & Scheie, 2007, 2009). Autoinducer 2 (AI‐2), a signal molecule, is produced to mediate both intra and interspecies communication and has the potential to influence both gene regulation and bacterial fitness. AI‐2 signals derive from spontaneous rearrangement of (4S)‐4, 5‐dihydroxy‐2, 3‐pentanedione (DPD) and are synthesized by a highly conserved AI synthase, LuxS, which is present in a variety of gram‐negative and gram‐positive bacteria species. In addition, AI‐2 supports cell population‐dependent behavior by interacting with central metabolism through the intracellular activated methyl cycle, which is related to the synthesis of tetrahydrofolic acid (Action, 2003; Bushby, 1973; Fuqua, Winans, & Greenberg, 1994; Vendeville, Winzer, Heurlier, Tang, & Hardie, 2005). It has been reported that AI‐2 increases the susceptibility in Streptococcus anginosus to ampicillin and erythromycin and decreases the susceptibility in Staphylococcus aureus to vancomycin, and AI‐2 is also involved in increased biofilm formation in Streptococcus intermedius (Ahmed et al., 2009; Xue, Zhao, & Sun, 2013). Our previous study confirmed that exogenous AI‐2 increased the antibiotic (such as ampicillin, oxacillin, and penicillin‐G) resistance of a clinical E. coli strain isolated from a dairy cow with mastitis by upregulating the expression of TEM‐type enzyme in an LsrR (the AI‐2 receptor)‐dependent manner (Xue et al., 2016). However, whether or not AI‐2 affects the expression of SXT susceptibility genes in APEC strains has not been reported.
In this study, we performed real‐time reverse transcription (RT)‐PCR experiments and digoxigenin (DIG) gel shift assays to evaluate how AI‐2 affects the SXT susceptibility of APEC. In this study, we reported that exogenous AI‐2 increses the susceptibility of APEC strains to trimethoprim‐sulfamethoxazole (SXT) in a folate synthesis‐dependent pathway but not in the LsrR‐dependent manner. Our results showed that exogenous AI‐2 can downregulate the transcription of the folate synthetase encoding genes folA and folC, and the folate synthesis‐related genes luxS, metE, and metH. Gel shift assays confirmed that LsrR, the AI‐2 receptor, did not bind to the promoters of folA and folC, suggesting that AI‐2 might influence folate metabolism by a feedback inhibition effect but not in the LsrR‐dependent pathway.
2. MATERIALS AND METHODS
2.1. Bacterial strains and culture conditions
The four E. coli strains used in this study were isolated from poultry, such as chickens, ducks, and pigeons (Table 1). These E. coli strains were stored at −80°C. Before each experiment, all strains were first cultured on Luria broth (LB) agar plates which contained 10 g/L Bacto tryptone (Oxoid, Basingstoke, UK), 5 g/L yeast extract (Oxoid, Basingstoke, UK), 10 g/L NaCl (Sangon, Shanghai, China), and 20 g/L agar power (Sangon, Shanghai, China) for 16 hr at 37°C in air supplied with 5% CO2. Colonies were then cultivated overnight in 2 ml of Mueller‐Hinton (MH) broth (Oxoid, Basingstoke, UK), named the first overnight cultures, and subsequently E. coli from the first overnight growth was diluted to an optical density at 600 nm (OD600) of approximately 0.03 in fresh MH broth for the following experiments. Cultures of all E. coli strains were grown at 37°C with shaking at 200 rpm. All the APEC strains used in this study were listed in Table 1.
Table 1.
Strains used in this study and their susceptibility testing results
| Strains | The host source | SXT MIC breakpoint (μg/ml) | MIC values (μg/ml) | |
|---|---|---|---|---|
| Susceptible | Resistant | |||
| APEC 17 | Chicken | ≤2/38 | ≥4/76 | 0.5/9.5 |
| APEC 19 | Pigeon | ≤2/38 | ≥4/76 | 2/38 |
| APEC 29 | Duck | ≤2/38 | ≥4/76 | 2/38 |
| APEC 40 | Pigeon | ≤2/38 | ≥4/76 | 2/38 |
2.2. Antimicrobial activity assay
The overnight cultures were inoculated into fresh MH broth and diluted to a final concentration of 0.03 by optical density at 600 nm, and 200 μl dilutions were dispensed into 96‐well plates (Costar, Corning, Steuben, NY, USA) containing SXT or the same volume of fresh MH medium with antibiotic‐free for control group, whereas the test group was with adding a final concentration of 39 μmol/L AI‐2. Every group was four technical replicates. The plates were incubated at 37°C for 16 hr, and then 10‐fold serial dilutions of cultures were performed by successive transfer (0.1 ml) through seven microfuge tubes containing 0.9 ml of MH. Next, 100 μl dilutions were dropped on Luria‐Bertani agar plates. After culturing for 16 hr at 37°C, viable colonies were counted and compared between the control and test groups via their colony‐forming units on LB agar plates. The survival rates of the control groups with or without exposure to SXT (without AI‐2) were designated as 100%. Experiments were repeated three times with four parallels.
2.3. Total RNA isolation, cDNA generation, and real‐time PCR processing
Overnight cultures of the four E. coli strains were diluted 1:100 in MH broth with or without SXT and, when necessary, AI‐2 was added for a final concentration of 39 μmol/L. The cultures were grown to the middle or late exponential phase at 37°C with shaking. Cells were collected and resuspended in RNase‐free water, and subsequently, total RNA was extracted from cells using the Trizol reagent (Ambion, Austin, TX), and residual DNA was removed using DNaseI (TaKaRa, Dalian, China). Real‐time RT‐PCR was performed using the PrimeScript 1st Strand cDNA synthesis kit, SYBR Premix Ex Taq (TaKaRa, Dalian, China), and a StepOne real‐time PCR system (Applied Biosystems, Carlsbad, CA). Differences in gene expression were calculated by ∆∆C t (where C t = cycle threshold) method, using the 16s rDNA gene as the housekeeping gene, normalized by subtracting the C t value of 16s cDNA from target cDNA. All of the real‐time RT‐PCR assays were repeated at least three times with similar results. The primers used in this study are listed in Table 2, and the PCR amplification efficiency was controlled between 1.93 and 2.09.
Table 2.
Oligonucleotide primers used in this study
| Primer name | Oligonucleotide (5′‐3′) |
|---|---|
| g‐folA‐f | ATGATCAGTCTGATTGCGGCGTTAG |
| g‐folA‐r | TTACCGCCGCTCCAGAATCTCAAAG |
| g‐folC‐f | ATGATTATCAAACGCACTCCTCAAG |
| g‐folC‐r | TTACTTGCCACCGCTTCTCCTCGCG |
| g‐metE‐f | TGACAATATTGAATCACACCCTC |
| g‐metE‐r | ATTGTTCACCTTTCACTTTCCCC |
| g‐metH‐f | GTGAGCAGCAAAGTGGAACAACT |
| g‐metH‐r | TTTGGTGGACTCTCGATAAGCCG |
| rt‐16s‐f | TTTGAGTTCCCGGCC |
| rt‐16s‐r | CGGCCGCAAGGTTAA |
| rt‐lsrR‐f | CGGATCGCGTGGTTTT |
| rt‐lsrR‐r | TCAACATATGCGCCGC |
| rt‐folA‐f | GTAACGTGGGTGAAGTCGGT |
| rt‐folA‐r | TCAGCATCGTGGAATTCGCT |
| rt‐folC‐f | TGAAACCAGCGCCATTTGTG |
| rt‐folC‐r | CTGCTTGAACAGCCACAACG |
| rt‐luxS‐f | TCACAGTCGATCATACCCGG |
| rt‐luxS‐r | CTTCTTTGTTCGGCACGCAG |
| rt‐metE‐f | CCTGCGTCGCGAGCTGAAAA |
| rt‐metE‐r | GTTGATCCCAGTGACGAGCA |
| rt‐metH‐f | TGGAACAACTGCGTGCGCAG |
| rt‐metH‐r | AGTCGGCAAAGCGTTCACCA |
| p‐folA‐f | TAAAGAGTGACGTAAATC |
| p‐folA‐r | TGAGATTTCCCGATAAAA |
| p‐folC‐f | AATCTGCCAGCGCCGAAT |
| p‐folC‐r | GGTATCCGCTGATTCGTT |
2.4. Gel shift assay
The transcriptional regulator LsrR protein was overexpressed and purified according to the methods described previously (Xue, Zhao, Sun, Zhou, & Sun, 2009). Two DNA fragments, p‐folA (191 bp) containing folA promoter and p‐folC (150 bp) containing folC promoter, were amplified by PCR from chromosomes of four APEC strains using two pairs of primers (p‐folA‐f, p‐folA‐r, p‐folC‐f, and p‐folC‐r) and pfu DNA polymerase (Promega, Madison, USA). The PCR products were purified by agarose gel electrophoresis. A digoxigenin (DIG) gel shift kit (Roche, Indianapolis, IN) was used for labeling of DNA fragments and detection of signals according to the manufacturer's instructions. Binding reactions were performed by incubating the labeled DNA fragments with various amounts of purified LsrR protein or the unlabeled DNA fragments at 25°C for 20 min in 4 μl of Binding buffer (5×). After incubation, 5 μl of loading buffer (5×) with bromophenol blue was added to the mixtures, which were then were electrophoresed in a 4% native polyacrylamide gel in 0.5× Tris‐borate EDTA buffer (45 mmol/L Tris‐borate and 1 mmol/L EDTA; pH 8.0). The band shifts were detected and analyzed according to the manufacturer's instructions.
2.5. Statistical analysis
The data were analyzed using the statistical software SPSS (ver. 19.0, IBM Corp., Armonk, NY) by a one‐way ANOVA method; the test results were shown as mean ± SD. The paired t test was used for statistical comparisons between groups. The level of statistical significance was set at a p‐value of ≤.05.
3. RESULTS
3.1. Exogenous AI‐2 increased the SXT susceptibility in APEC
To examine the susceptibility of 31 E. coli isolates (21, 3, 1, and 6 from chickens, ducks, geese, and pigeons with avian colibacillosis, respectively) to SXT, antimicrobial susceptibility testing was performed using the broth dilution technique. The minimal inhibitory concentrations (MIC) (Table 1) were determined and classified according to the guidelines of the Clinical Laboratory Standard Institute (CLSI). Strains APEC 17, APEC 19, APEC 29, and APEC 40 are representative of antibiotic susceptibility in all of the 31 APEC strains and were selected as the experimental subjects to perform the following test in this work. In addition, the SXT concentrations of strains APEC 17, APEC 19, APEC 29, and APEC 40 were selected (100/1900, 20/380, 20/380, and 2/38 ng/ml, respectively), as effective experimental concentrations inhibited the growth of the four APEC strains but according to their MIC values these were not considered to be lethal concentrations. The growth curves of four APEC strains were measured with the addition of exogenous AI‐2 in the absence or present of SXT to determine the effect of AI‐2 on cells growth of four APEC strains. These results illustrated that AI‐2 did not affect the growth of APEC in the absence of SXT (Figure S1), however, in the presence of SXT, addition of exogenous AI‐2 increased the susceptibility of APEC 17, APEC 19, APEC 29, and APEC 40 to SXT (Figure S2).
Antibacterial assays were performed to examine the changes in SXT susceptibility of APEC 17, APEC 19, APEC 29, and APEC 40 with the addition of exogenous AI‐2. This result showed that the survival rates of the test groups with AI‐2 were similar to that of the control groups (Figure 1a); however, decreased survival rates were observed in the test groups with exposure to SXT and AI‐2 compared to the control groups with SXT alone. The survival rates of APEC 17, APEC 19, APEC 29, and APEC 40 with SXT and AI‐2 were almost two times, 344 times, 19 times, and 30 times, respectively, lower than that of the control group with SXT alone (Figure 1b). These results indicated that with the addition of exogenous AI‐2, susceptibility of APEC 17, APEC 19, APEC 29, and APEC 40 to SXT was increased, suggesting that, AI‐2 might play an important role in the regulation of SXT susceptibility in these APEC isolates.
Figure 1.
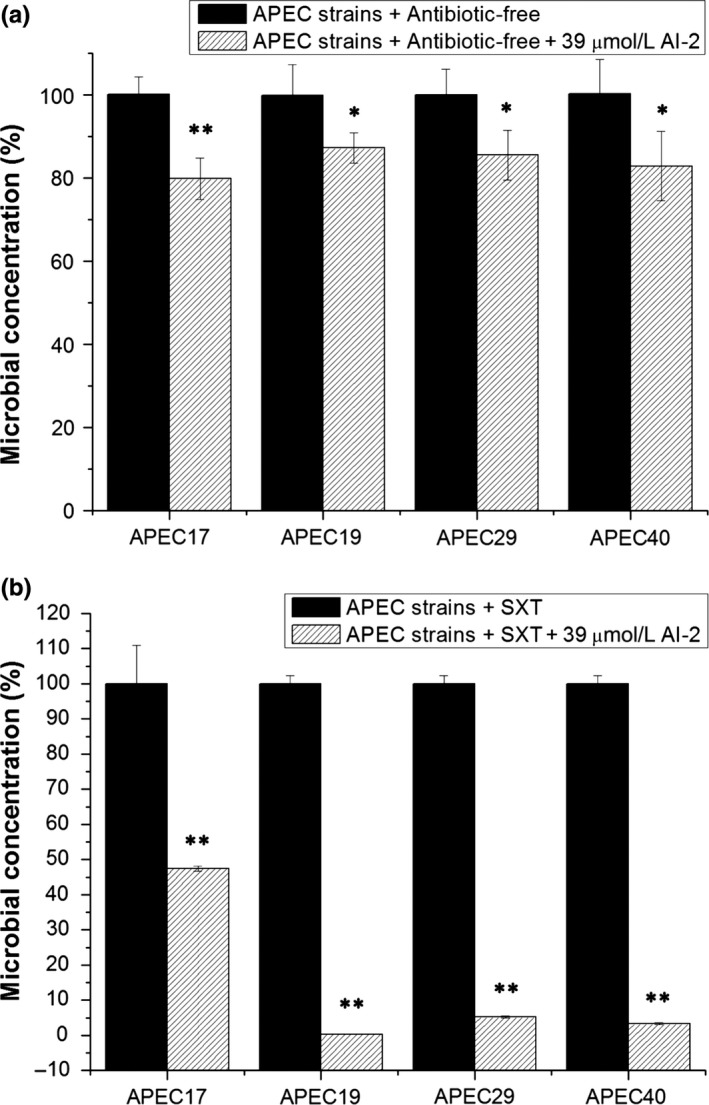
Colony‐forming units assays of the four APEC strains in the absence or presence of SXT. Colony‐forming units assays of APEC 17, APEC 19, APEC 29, APEC 40, and APEC 17, APEC 19, APEC 29, APEC 40 cultured with 39 μmol/L AI‐2 and with or without SXT: (a) without SXT, (b) with SXT: APEC 17 with 0.25/4.75 μg/ml, APEC 19 with 1/19 μg/ml, APEC 29 with 1/19 μg/ml, APEC 40 with 1/19 μg/ml. The survival rate of the control group without the addition of AI‐2 was designated 100%. The colony counts of the test group cultured with AI‐2 and SXT were compared to that of the control group (without AI‐2). Error bars indicate standard deviations. The results represent a mean of three independent experiments; **p < .01, *p < .05, indicating a difference between SXT alone and SXT + AI‐2
3.2. Exogenous AI‐2 increased SXT susceptibility by downregulating the folate synthetase encoding genes folA and folC
To investigate the mechanism of how AI‐2 affects SXT susceptibility in APEC, the transcript levels of genes associated with SXT susceptibility and AI‐2 QS were assessed by performing real‐time RT‐PCR experiments. It has already been reported that SXT could lead to bacterial death by affecting the folate metabolic pathway and folA and folC are two important encoding genes in folate metabolism. The transcript levels of folA, folC, and lsrR, which encode dihydrofolate reductase (DHFR), dihydrofolate synthetase (DHFS), and the receptor of AI‐2, respectively, were measured in the four APEC strains under different conditions. As shown in Figure 2, the transcript levels of lsrR were increased 2.0‐fold, 2.2‐fold, 3.1‐fold, and 2.2‐fold by the addition of exogenous AI‐2 in the absence of SXT in APEC 17, APEC 19, APEC 29, and APEC 40, respectively. Accordingly, the transcript levels of folA were decreased 2.9‐fold, 2.4‐fold, 2.3‐fold, and 2.2‐fold, and the transcript levels of folC were decreased 1.9‐fold, 1.9‐fold, 2.1‐fold, and 3.2‐fold in APEC 17, APEC 19, APEC 29, and APEC 40, respectively. Furthermore, in the presence of SXT, as shown in Figure 3, the addition of AI‐2 resulted in 1.8‐fold, 1.6‐fold, 2.1‐fold, and 1.9‐fold increases in the transcription of lsrR, 3.7‐fold, 2.6‐fold, 3.3‐fold, and 2.1‐fold decreases in the transcription of folA, and 1.9‐fold, 1.8‐fold, 3.2‐fold, and 2.0‐fold decreases in the transcription of folC in APEC 17, APEC 19, APEC 29, and APEC 40, respectively. These results suggested that exogenous AI‐2 might downregulate the transcription of folA and folC and influence folate metabolism in an LsrR‐dependent pathway.
Figure 2.
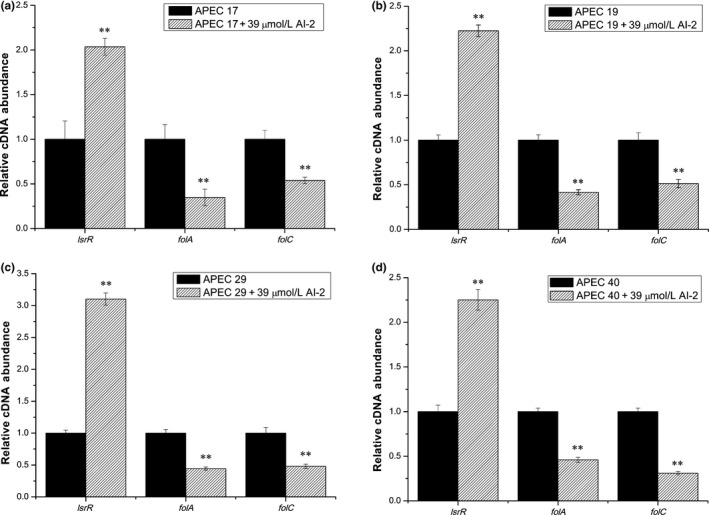
Comparative measurement of transcription of the folate synthetase encoding genes and the receptor encoding gene of AI‐2 in APEC strains without SXT. Comparative measurement of transcription (cDNA abundance) of folA, folC, and lsrR in APEC 17, APEC 19, APEC 29, and APEC 40. Relative transcript levels of folA, folC, and lsrR were tested by real‐time reverse transcription‐PCR in APEC 17, APEC 19, APEC 29, APEC 40, and APEC 17, APEC 19, APEC 29, APEC 40 cultured with 39 μmol/L AI‐2 in the absence of SXT: (a) APEC 17, (b) APEC 19, (c) APEC 29, and (d) APEC 40. Error bars indicate standard deviations. The results represent a mean of three independent experiments; **p < .01, indicating a difference between antibiotic‐free and AI‐2
Figure 3.
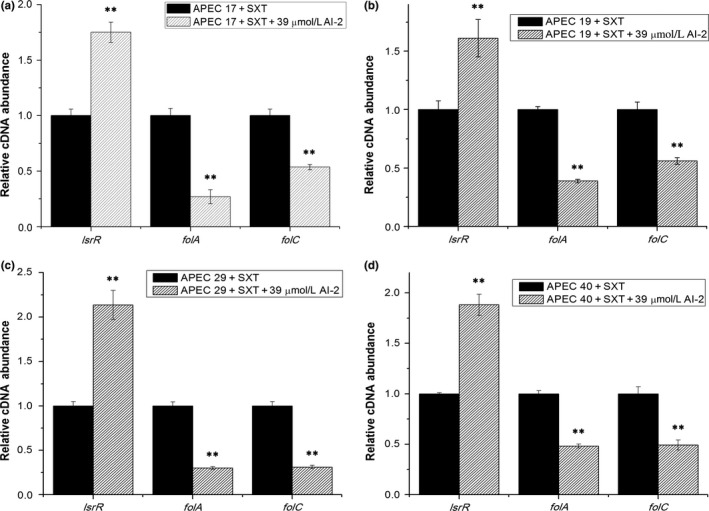
Comparative measurement of transcription of the folate synthetase encoding genes and the receptor encoding gene of AI‐2 in APEC strains with SXT. Comparative measurement of transcription (cDNA abundance) of folA, folC, and lsrR in APEC 17, APEC 19, APEC 29, and APEC 40. Relative transcript levels of folA, folC, and lsrR were tested by real‐time reverse transcription‐PCR in APEC 17, APEC 19, APEC 29, APEC 40, and APEC 17, APEC 19, APEC 29, APEC 40 cultured with 39 μmol/L AI‐2 in the presence of SXT: (a) APEC 17 with 50/950 ng/ml, (b) APEC 19 with 20/380 ng/ml, (c) APEC 29 with 20/380 ng/ml, and (d) APEC 40 with 20/380 ng/ml. Error bars indicate standard deviations. The results represent a mean of three independent experiments; **p < .01, indicating a difference between SXT and SXT+AI‐2
3.3. LsrR did not bind to the promoters of folA and folC
It has been reported that LsrR contains a helix‐turn‐helix (HTH) DNA binding domain and is a QS regulator that can regulate the transcription of a variety of genes by binding to their promoters. On the basis of these findings, we hypothesized that exogenous AI‐2 might activate LsrR, which then inhibits the transcription of folA and folC by directly binding to their promoter regions. To determine whether or not LsrR can bind to the promoters of folA and folC, we performed DIG‐gel shift assays. The results showed that LsrR can not bind to the promoters of folA and folC (Figure 4), indicating that AI‐2 might influence folate metabolism in an LsrR‐independent pathway.
Figure 4.
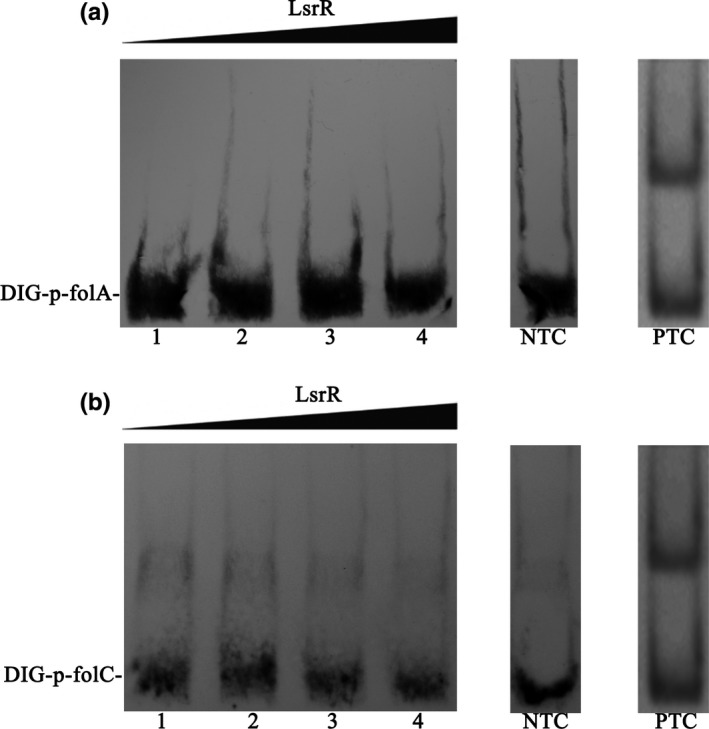
The binding ability of LsrR to the folA and folC promoters were determined by gel‐shift assays. Increasing amounts of LsrR were incubated with DIG‐labeled folA and folC promoters (DIG‐p‐folA and DIG‐p‐folC). In each panel, from lane 1 to 5, the amounts of LsrR were 0, 0.5, 1, 2, and 2 pmol, respectively; 200 fmol of DIG‐labeled probes was added to all lanes. NTC, negative control, except for the labeled probes, 1 pmol of unlabeled probes was incubated with the LsrR protein as the competitive control. PTC, positive control, the binding ability of LsrR to the TEM promoter. (a) the folA promoter and (b) the folC promoter
3.4. Exogenous AI‐2 influenced folate metabolism by downregulating the transcription of the folate synthesis‐related genes luxS, metE, and metH in APEC
It has been reported that luxS (encodes S‐ribosylhomocysteinase), metE (encodes B12‐dependent homocysteine‐5‐methyltetrahydrofolate methyltransferase, MTR), and metH (encodes methionine synthase, MS) are related to the folate synthesis pathway, and AI‐2 is one of the products of LuxS (Figure 5). We speculated that exogenous AI‐2 might inhibit the transcription of luxS by a feedback inhibition effect, and then decrease the amount of homocysteine in the APEC strains, and finally, influence the transcription of other relative genes in folate metabolism. To prove this hypothesis, the transcript levels of luxS, metH, and metE were measured in the four APEC strains under different conditions. As shown in Figure 6, the transcript levels of luxS were decreased 2.0‐fold, 2.4‐fold, 2.0‐fold, and 1.3‐fold, the transcript levels of metE were decreased 3.9‐fold, 1.7‐fold, 1.6‐fold, and 1.4‐fold, and the transcript levels of metH were decreased 2.9‐fold, 1.4‐fold, 4.9‐fold, and 1.4‐fold by the addition of exogenous AI‐2 in the absence of SXT in APEC 17, APEC 19, APEC 29, and APEC 40, respectively. Furthermore, in the presence of SXT, as shown in Figure 7, the addition of AI‐2 resulted in 2.9‐fold, 5.5‐fold, 7.1‐fold, and 1.7‐fold decreases in transcription of luxS, 2.6‐fold, 7.2‐fold, 6.6‐fold, and 1.9‐fold decreases in transcription of metE, and 1.8‐fold, 5.1‐fold, 16.1‐fold, and 2.1‐fold decreases in transcription of metH in APEC 17, APEC 19, APEC 29, and APEC 40, respectively. These results confirmed that exogenous AI‐2 can affect folate synthesis by downregulating the transcription of luxS, metE, metH, folA, and folC, which then affects the SXT susceptibility in APEC strains by a folate synthesis‐associated pathway.
Figure 5.
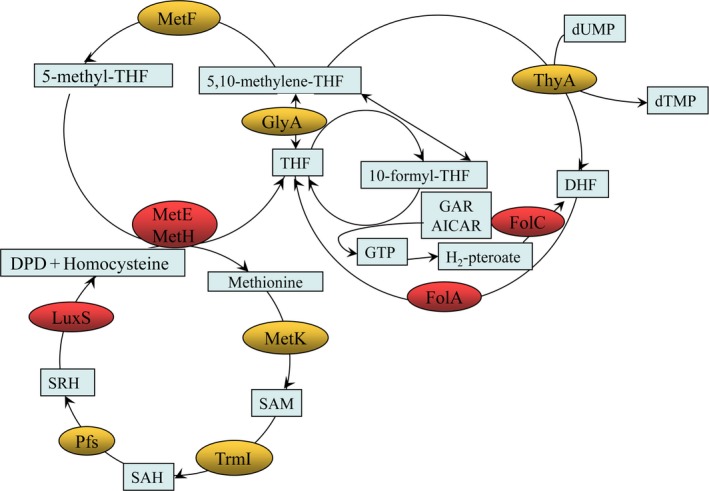
The folate synthesis‐associate pathways. DHF, dihydrofolate; FolC, dihydrofolate synthase; FolA, dihydrofolate reductase; THF, tetrahydrofolate; SAH, S‐adenosylhomocysteine; SAM, S‐adenosylmethionine; SRH, S‐ribosylhomocysteine; GlyA, serine hydroxymethyltransferase; MetF, 5,10‐methylenetetrahydrofolate reductase; AICAR, 5‐aminoimidazole‐4‐carboxamine ribonucleotide; dTMP, deoxythymidine monophosphate; dUMP, deoxyuridine monophosphate; GAR, glycinamide ribonucleotide; GTP, guanosine triphosphate; ThyA, thymidylate synthase; MetE, methionine synthase; MetH, B12‐dependent homocysteine‐5‐methyltetrahydrofolate methyltransferase; MetK, S‐ adenosylmethionine synthase; TrmI, SAM‐dependent methylase; Pfs, S‐adenosylhomocysteine nucleosidase; LuxS, S‐ribosylhomocysteinase; DPD, 4,5‐dihydroxy‐2,3‐pentanedione, the precursor of AI‐2
Figure 6.
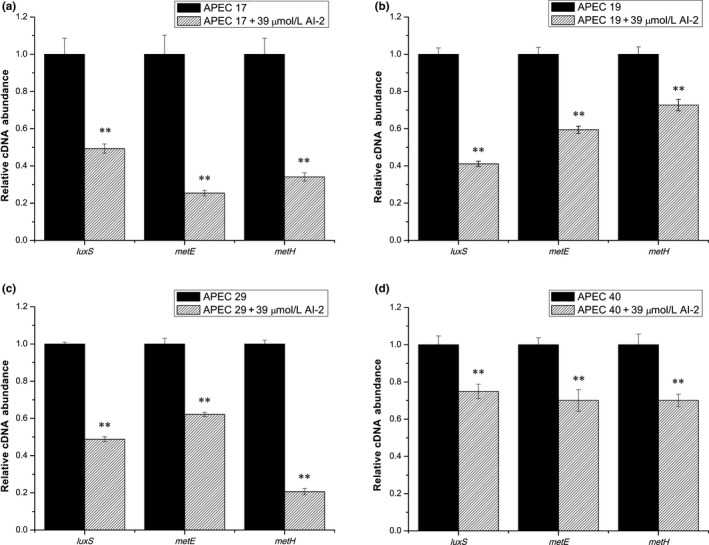
Comparative measurement of transcription of the folate synthesis‐related genes in APEC strains without SXT. Comparative measurement of transcription (cDNA abundance) of luxS, metE, and metH in APEC 17, APEC 19, APEC 29, and APEC 40. Relative transcript levels of luxS, metE, and metH were tested by real‐time reverse transcription‐PCR in APEC 17, APEC 19, APEC 29, APEC 40, and APEC 17, APEC 19, APEC 29, APEC 40 cultured with 39 μmol/L AI‐2 in the absence of SXT: (a) APEC 17, (b) APEC 19, (c) APEC 29, and (d) APEC 40. Error bars indicate standard deviations. The results represent a mean of three independent experiments; **p < .01, indicating a difference between antibiotic‐free and AI‐2
Figure 7.
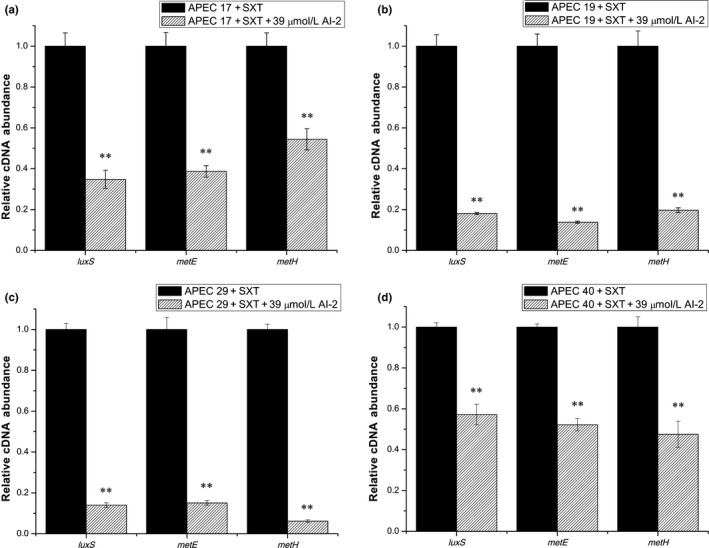
Comparative measurement of transcription of the folate synthesis‐related genes in APEC strains with SXT. Comparative measurement of transcription (cDNA abundance) of luxS, metE, and metH in APEC 17, APEC 19, APEC 29, and APEC 40. Relative transcript levels of luxS, metE, and metH were tested by real‐time reverse transcription‐PCR in APEC 17, APEC 19, APEC 29, APEC 40, and APEC 17, APEC 19, APEC 29, APEC 40 cultured with 39 μmol/L AI‐2 in the presence of SXT: (a) APEC 17 with 50/950 ng/ml, (b) APEC 19 with 20/380 ng/ml, (c) APEC 29 with 20/380 ng/ml, and (d) APEC 40 with 20/380 ng/ml. Error bars indicate standard deviations. The results represent a mean of three independent experiments; **p < .01, indicating a difference between SXT and SXT + AI‐2
4. DISCUSSION
In recent years, the QS system has emerged as a topic of great interest due to its involvement in various biochemical processes. Previous studies reported that AI‐2 is an important QS signal molecule in gram‐negative and ‐positive bacteria, including E. coli, suggesting that AI‐2 could play an important role in the physiological activity or regulation of E. coli (Ahmed et al., 2009; Vendeville et al., 2005; Xue et al., 2009). In E. coli model strains, the functional mechanisms of AI‐2 have been well studied (Wang, Li, March, Valdes, & Bentley, 2005; Xue et al., 2009). However, these studies did not involve the antibiotic resistance‐ or susceptibility‐associated genes. Our previous study demonstrated that exogenous AI‐2 increased the antibiotic (such as ampicillin, oxacillin, and penicillin‐G) resistance of a clinical E. coli strain isolated from a dairy cow with mastitis by upregulating the expression of resistance gene TEM, and the regulation was directly related to LsrR which is the receptor of AI‐2 (Xue et al., 2016). However, in avian pathogenic E. coli, whether or not AI‐2 affects antibiotic‐related genes and pathogenic genes has not been reported. In this study, we found that, in the presence of SXT, exogenous AI‐2 decreased the physiological activity of APEC, and increased the susceptibility of APEC to SXT by an LsrR‐independent pathway. Therefore, we have confirmed that exogenous AI‐2 affects SXT susceptibility in APEC strains by a folate synthesis‐related pathway, indicating that the combined use of AI‐2 and SXT can minimize the dose of SXT in the treatment.
Previous studies on AI‐2 did not explain its metabolic effect on APEC. This study indicated that AI‐2 increases the SXT susceptibility of APEC in a folate‐dependent manner. The addition of exogenous pre‐AI‐2 molecule DPD triggers a product feedback inhibition effect, which then decreases the expression of luxS and the amount of the other products of LuxS, such as homocysteine (Figure 5). Homocysteine is the substrate of MetE and MetH, which are important enzymes in the folate synthesis pathway. The substrate inhibition effect caused by the decrease in homocysteine downregulates the expression of metE and metH, and then results in a decrease in the intermediate metabolite tetrahydrofolate (THF), which is an important substrate used to synthesize purine and pyrimidine. In THF metabolism, purine and pyrimidine are two important intermediate metabolites used to synthesize THF again, and folA and folC are two important folate synthetase encoding genes. In the absence of SXT, AI‐2 down‐regulated the transcript levels of folate synthetase encoding genes folA and folC through the folate cycle pathway only, and did not affect the survival rates of the test groups (with AI‐2) compared to the control groups. However, in the presence of SXT, exogenous AI‐2 strengthened the growth inhibition of SXT on the APEC strains by downregulating the transcript levels of folate‐associated genes. This study might provide further information in the search for potential drug targets for prophylaxis of avian colibacillosis and for auxiliary antibiotics in the treatment of avian colibacillosis.
CONFLICT OF INTEREST
The authors report no conflicts of interest in this work.
Supporting information
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grants 31672571 and 31371324) and the Foundation for Graduate Innovation of Anhui Agricultural University.
Yu L, Li W, Zhang M, et al. Autoinducer2 affects trimethoprim‐sulfamethoxazole susceptibility in avian pathogenic Escherichia coli dependent on the folate synthesis‐associate pathway. MicrobiologyOpen. 2018;7:e582 10.1002/mbo3.582
Contributor Information
Fei Shang, Email: shf@ahau.edu.cn.
Ting Xue, Email: xuet@ahau.edu.cn.
REFERENCES
- Action, M. O. F. (2003). Trimethoprim‐sulfamethoxazole revisited. Archives of Internal Medicine, 163(4), 402–410. [DOI] [PubMed] [Google Scholar]
- Aggad, H. , Ammar, Y. A. , Hammoudi, A. , & Kihal, M. (2010). Antimicrobial resistance of Escherichia coli isolated from chickens with colibacillosis. Global Veterinaria, 4(3), 303–306. [Google Scholar]
- Ahmed, N. A. , Petersen, F. C. , & Scheie, A. A. (2007). AI‐2 quorum sensing affects antibiotic susceptibility in Streptococcus anginosus . Journal of antimicrobial chemotherapy, 60(1), 49–53. 10.1093/jac/dkm124 [DOI] [PubMed] [Google Scholar]
- Ahmed, N. A. , Petersen, F. C. , & Scheie, A. A. (2009). AI‐2/LuxS is involved in increased biofilm formation by Streptococcus intermedius in the presence of antibiotics. Antimicrobial Agents & Chemotherapy, 53(10), 4258–4263. 10.1128/AAC.00546-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altekruse, S. F. , Elvinger, F. , Lee, K. Y. , Tollefson, L. K. , Pierson, F. W. , Eifert, J. , & Sriranganathan, N. (2002). Antimicrobial susceptibilities of Escherichia coli strains from a turkey operation. Journal of the American Veterinary Medical Association, 221(3), 411–416. 10.2460/javma.2002.221.411 [DOI] [PubMed] [Google Scholar]
- Bassler, B. L. (1999). How bacteria talk to each other: Regulation of gene expression by quorum sensing. Current Opinion in Microbiology, 2(6), 582–587. 10.1016/S1369-5274(99)00025-9 [DOI] [PubMed] [Google Scholar]
- Bassler, B. L. (2002). Small talk. Cell‐to‐cell communication in bacteria. Cell, 109(4), 421–424. 10.1016/S0092-8674(02)00749-3 [DOI] [PubMed] [Google Scholar]
- Blanco, J. E. , Blanco, M. , Mora, A. , & Blanco, J. (1997). Prevalence of bacterial resistance to quinolones and other antimicrobials among avian Escherichia coli strains isolated from septicemic and healthy chickens in Spain. Journal of clinical microbiology, 35(8), 2184–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothe, D. , Smaha, T. , Carpenter, D. M. , Shaheen, B. , & Hatchcock, T. (2012). Antimicrobial resistance and pharmacodynamics of canine and feline pathogenic E. coli in the United States. Journal of the American Animal Hospital Association, 48(6), 379–389. 10.5326/JAAHA-MS-5805 [DOI] [PubMed] [Google Scholar]
- Bushby, S. R. M. (1973). Trimethoprim‐sulfamethoxazole: In vitro microbiological aspects. Journal of Infectious Diseases, 128(3), 442–462. 10.1093/infdis/128.Supplement_3.S442 [DOI] [PubMed] [Google Scholar]
- Church, J. A. , Fitzgerald, F. , Walker, A. S. , Gibb, D. M. , & Prendergast, A. J. (2015). The expanding role of co‐trimoxazole in developing countries. Lancet Infectious Diseases, 15(3), 327–339. 10.1016/S1473-3099(14)71011-4 [DOI] [PubMed] [Google Scholar]
- Dheilly, A. , Le, D. L. , Mourand, G. , Bouder, A. , Jouy, E. , & Kempf, I. (2012). Resistance gene transfer during treatments for experimental avian colibacillosis. Antimicrobial agents and chemotherapy, 56(1), 189–196. 10.1128/AAC.05617-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhomoulin, M. , & Fairbrother, J. M. (1999). Avian pathogenic Escherichia coli (APEC). Veterinary Research, 30(2–3), 299–316. [PubMed] [Google Scholar]
- Dziva, F. , & Stevens, M. P. (2008). Colibacillosis in poultry: Unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathology, 37(4), 355–366. 10.1080/03079450802216652 [DOI] [PubMed] [Google Scholar]
- Epstein, M. E. , Amodio‐Groton, M. , & Sadick, N. S. (1997). Antimicrobial agents for the dermatologist. II. Macrolides, fluoroquinolones, rifamycins, tetracyclines, trimethoprim‐sulfamethoxazole, and clindamycin. Journal of the American Academy of Dermatology, 37(3), 365–384. 10.1016/S0190-9622(97)70135-X [DOI] [PubMed] [Google Scholar]
- Ewers, C. , Janssen, T. , Kiessling, S. , Philipp, H. C. , & Wieler, L. H. (2004). Molecular epidemiology of avian pathogenic Escherichia coli (APEC) isolated from colisepticemia in poultry. Veterinary Microbiology, 104(1–2), 91–101. 10.1016/j.vetmic.2004.09.008 [DOI] [PubMed] [Google Scholar]
- Fuqua, W. C. , Winans, S. C. , & Greenberg, E. P. (1994). Quorum sensing in bacteria: The LuxR‐LuxI family of cell density‐responsive transcriptional regulators. Journal of Bacteriology, 176(2), 269–275. 10.1128/jb.176.2.269-275.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim, S. A. , Rapp, R. P. , Martin, C. A. , & Evans, M. E. (2005). Trimethoprim‐sulfamethoxazole as a viable treatment option for infections caused by methicillin‐resistant Staphylococcus aureus . Pharmacotherapy the Journal of Human Pharmacology & Drug Therapy, 25(2), 253–264. 10.1592/phco.25.2.253.56956 [DOI] [PubMed] [Google Scholar]
- Gross, W. G. (1994). Diseases due to Escherichia coli in poultry In Gyles C. L. (Ed.), Escherichia coli in domestic animals and humans (pp. 234–260). Wallingford, Oxon, United Kingdom: CAB International. [Google Scholar]
- Mcguinness, B. W. (1969). A combination of trimethoprim and sulphamethoxazole in upper respiratory infection. Postgraduate Medical Journal, 45(24), 99–101. [PubMed] [Google Scholar]
- Miller, M. B. , & Bassler, B. L. (2001). Quorum sensing in bacteria. Annual review of microbiology, 55(1), 165–199. 10.1146/annurev.micro.55.1.165 [DOI] [PubMed] [Google Scholar]
- Morrissey, I. , Moyaert, H. , de Jong, A. , El, G. F. , Klein, U. , Ludwig, C. , … Youala, M. (2016). Antimicrobial susceptibility monitoring of bacterial pathogens isolated from respiratory tract infections in dogs and cats across Europe: ComPath results. Veterinary Microbiology, 191(2016), 44–51. 10.1016/j.vetmic.2016.05.020 [DOI] [PubMed] [Google Scholar]
- Mylotte, J. M. , Bates, T. R. , Sergeant, K. A. , Matson, R. E. , & Jr, B. T. (1981). Trimethoprim‐sulfamethoxazole therapy of experimental Escherichia coli meningitis in rabbits. Antimicrobial Agents & Chemotherapy, 20(1), 81–87. 10.1128/AAC.20.1.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queralt, J. , & Castells, I. (1985). Pharmacokinetics of Sulfamethoxazole and trimethoprim association in hens. Poultry Science, 64(12), 2362–2367. 10.3382/ps.0642362 [DOI] [PubMed] [Google Scholar]
- Schouler, C. , Schaeffer, B. , Brée, A. , Mora, A. , Dahbi, G. , Biet, F. , … Moulinschouleur, M. (2012). Diagnostic Strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. Journal of clinical microbiology, 50(5), 1673–1678. 10.1128/JCM.05057-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld, O. (2011). Sulfonamides and trimethoprim In Hoboken N. J. (Ed.), Antibiotics and Antibiotic Resistance (pp. 612–615). USA, John: Wiley & Sons Inc; 10.1002/9781118075609 [DOI] [Google Scholar]
- Vendeville, A. , Winzer, K. , Heurlier, K. , Tang, C. M. , & Hardie, K. R. (2005). Making ‘sense’ of metabolism: autoinducer‐2, LuxS and pathogenic bacteria. Nature Reviews Microbiology, 3(5), 383–396. 10.1038/nrmicro1146 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Li, J. , March, J. C. , Valdes, J. J. , & Bentley, W. E. (2005). luxS‐dependent gene regulation in Escherichia coli K‐12 revealed by genomic expression profiling. Journal of Bacteriology, 187(24), 8350–8360. 10.1128/JB.187.24.8350-8360.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Wei, L. , Wang, B. , Zhang, R. , Liu, C. , Bi, D. , … Chen, T. (2016). Complete genome sequence and characterization of avian pathogenic Escherichia coli field isolate ACN001. Standards in Genomic Sciences, 11(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, J. L. , Salmon, S. A. , Yancey Jr, R. J. , Nersessian, B. , & Kounev, Z. V. (1993). Minimum inhibitory concentrations of bacteria isolated from septicemia and airsacculitis in ducks. Journal of Veterinary Diagnostic Investigation Official Publication of the American Association of Veterinary Laboratory Diagnosticians Inc, 5(4), 625–628. 10.1177/104063879300500423 [DOI] [PubMed] [Google Scholar]
- Wormser, G. P. , Keusch, G. T. , & Heel, R. C. (1982). Co‐trimoxazole (trimethoprim‐sulfamethoxazole): An updated review of its antibacterial activity and clinical efficacy. Drugs, 24(6), 459–518. 10.2165/00003495-198224060-00002 [DOI] [PubMed] [Google Scholar]
- Xue, T. , Yu, L. , Shang, F. , Li, W. , Zhang, M. , Ni, J. , & Chen, X. (2016). Short communication: The role of autoinducer 2 (AI‐2) on antibiotic resistance regulation in an Escherichia coli strain isolated from a dairy cow with mastitis. Journal of Dairy Science, 99(6), 4693–4698. 10.3168/jds.2015-10543 [DOI] [PubMed] [Google Scholar]
- Xue, T. , Zhao, L. , & Sun, B. (2013). LuxS/AI‐2 system is involved in antibiotic susceptibility and autolysis; in Staphylococcus aureus NCTC 8325. International Journal of Antimicrobial Agents, 41(1), 85–89. 10.1016/j.ijantimicag.2012.08.016 [DOI] [PubMed] [Google Scholar]
- Xue, T. , Zhao, L. , Sun, H. , Zhou, X. , & Sun, B. (2009). LsrR‐binding site recognition and regulatory characteristics in Escherichia coli AI‐2 quorum sensing. Cell Research, 19(11), 1258–1268. 10.1038/cr.2009.91 [DOI] [PubMed] [Google Scholar]
- Zhao, S. , Maurer, J. J. , Hubert, S. , De Villena, J. F. , McDermott, P. F. , Meng, J. , … White, D. G. (2005). Antimicrobial susceptibility and molecular characterization of avian pathogenic Escherichia coli isolates. Veterinary Microbiology, 107(3–4), 215–224. 10.1016/j.vetmic.2005.01.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


