Abstract
Objectives:
Periodontitis is an inflammation of periodontal tissues that is caused by the biofilm of periodontal pathogens. Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) is an opportunistic periodontopathogen that can be the cause of periodontal diseases via fimbriae as a virulence factor. In this study, we aimed to determine the expression level of A. actinomycetemcomitans rcpA gene as a virulence factor associated with biofilm formation after antimicrobial photodynamic therapy (aPDT) as a relatively new therapeutic modality.
Materials and Methods:
To determine sub-lethal doses of aPDT against A. actinomycetemcomitans ATCC 33384 strain, we used curcumin (CUR) as a photosensitizer at a final concentration of 40 μmol/ml, which was excited with a light-emitting diode (LED) at the wavelength of 450 nm. Quantitative real-time polymerase chain reaction (qRT-PCR) was then applied to monitor rcpA gene expression in A. actinomycetemcomitans.
Results:
10–40 μmol/ml of CUR caused a significant reduction in the growth of A. actinomycetemcomitans compared to control group (P<0.05). Also, the cell viability of A. actinomycetemcomitans was significantly decreased after more than four minutes of LED irradiation. Therefore, the sub-lethal dose of aPDT against A. actinomycetemcomitans was 5 μmol/ml of CUR with three minutes of LED irradiation at a fluency of 180–240 J/cm2, which reduced the expression of the rcpA gene by approximately 8.5-fold.
Conclusions:
aPDT with CUR leads to decreased cell survival and virulence of A. actinomycetemcomitans. Thus, CUR-aPDT can be used as an alternative approach for the successful treatment of periodontitis in vivo.
Keywords: Aggregatibacter actinomycetemcomitans, Biofilms, Curcumin, Periodontitis, Quantitative Real-Time Polymerase Chain Reaction
INTRODUCTION
Periodontitis is an infection-induced inflammatory disease caused by multispecies biofilm models of periodontal pathogens [1]. Biofilms are surface-attached microbial complex communities that are embedded in a matrix of extracellular polymeric substances [2]. Microbial biofilms are resistant to antimicrobial agents and can enhance the survival of the microbiota [3]. Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), an opportunistic periodontopathogen with various virulence factors, is able to resist the clearance attempts because of its protective extracellular matrix and due to the presence of resistant cells [4]. The tight-adherence (tad) gene locus in A. actinomycetemcomitans is necessary for surface attachment and biofilm formation. Different proteins encoded by the tad locus form A. actinomycetemcomitans fimbriae. Rough colony protein A (RcpA), a multimeric complex in the outer membrane of A. actinomycetemcomitans, forms a secreting channel that secretes the fimbriae components to outside the outer membrane [5]. The rcpA is a virulence factor that is known to be particularly important in A. actinomycetemcomitans isolates to form tight biofilms. It allows for interactions with host epithelial cells, which can lead to an up-regulation of biofilm-associated bacterial genes [6]. Several studies have suggested that antimicrobial photodynamic therapy (aPDT), also known as photochemotherapy and photoactivated disinfection [7], is a safe alternative strategy for periodontal therapy and can significantly change the cell survival of A. actinomycetemcomitans [8]. During aPDT, a photosensitizing chemical substance and a specific wavelength of light, used in conjunction with molecular oxygen, elicit cell death in microorganisms [7]. Recently, curcumin (CUR), a new class of photosensitizers, has been introduced for aPDT. CUR [1E,6E-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is a yellow dye (also known as the spice turmeric) isolated from Curcuma longa. Recent investigations have shown that CUR is a potent photoactivatable substance that exhibits a variety of pharmacological properties such as antimicrobial, anti-inflammatory, anticancer, and antitumor activities [9,10].
To date, no data are available on the effects of aPDT with CUR, as a photosensitizer, on the expression of A. actinomycetemcomitans virulence factors. In the current study, we report the results of the effects of CUR-mediated aPDT on the expression of rcpA gene in A. actinomycetemcomitans cells surviving the PDT.
MATERIALS AND METHODS
Bacterial strain and growth conditions:
A. actinomycetemcomitans ATCC 33384 strain, purchased from Institute of Microbiology, ETH Zurich, Switzerland, was cultured in microaerophilic conditions (<20% O2) for 48 hours at 37°C in a culture medium that was prepared by using brain heart infusion (BHI) agar (Merck KGaA, Darmstadt, Germany) to which the following compounds were added: 5% defibrinated sheep blood (Sigma-Aldrich Co., Ltd., Dorset, United Kingdom), 5 g/L of yeast extract (Merck KGaA, Darmstadt, Germany), 5 mg/L of hemin (Sigma-Aldrich Co., Ltd., Dorset, United Kingdom), and 1 mg/L of menadione (Sigma-Aldrich Co., Ltd., Dorset, United Kingdom). The strain was then inoculated into freshly prepared tubes containing BHI broth (Merck KGaA, Darmstadt, Germany), and the cell density was adjusted to 1.5×108 cells/ml as verified by a spectrophotometer (Eppendorf BioPhotometer, Hamburg, Germany) to measure the optical density at 600 nm (OD600) and to count the colonies.
Photosensitizing agent and light source:
CUR (Merck KGaA, Darmstadt, Germany), a photosensitizer, was prepared in 0.05% dimethyl sulfoxide (DMSO) at a final concentration of 40 μmol/ml. A light-emitting diode (LED, DY400-4, Denjoy Dental Co., Ltd., Shenzhen, China) at the wavelength of 450 nm with an output intensity of 1000–1400 mW/cm2 was used as a light source. The output powers were measured by a power meter (LaserPoint s.r.l, Milano, Italy) during the experiment.
Determination of sub-lethal doses of CUR, light source, and aPDT:
The sub-minimum inhibitory concentration (sMIC) of CUR against A. actinomycetemcomitans, which is defined as a sub-lethal dose, was determined based on a previous study [11]. Briefly, 100 μl of 2X BHI broth was added to the wells of a round-bottom 96-well microplate (TPP AG, Trasadingen, Switzerland), and 100 μl of CUR was poured into the wells of the first column and was diluted two-fold stepwise. Afterward, 100 μl of A. actinomycetemcomitans suspension with 1.5×106 colony-forming units (CFUs)/ml was added to each well. In this study, the wells containing only A. actinomycetemcomitans suspension and the wells containing BHI broth without A. actinomycetemcomitans suspension or CUR were used as positive and negative controls, respectively. The microplate was incubated in the dark for 5 minutes at room temperature (25±2°C) in microaerophilic conditions. Next, 10 μl from the contents of each well was cultured in an enriched BHI agar plate which its components were described above.
The plates were incubated for 48 hours at 37°C in microaerophilic conditions. FUs/ml were determined based on the Miles and Misra method [12]. The sub-lethal dose of LED irradiation time against A. actinomycetemcomitans was determined according to a previous study [11]. In this method, 300 μl of the free-floating A. actinomycetemcomitans in the planktonic suspension at the final concentration of 1.5×105 CFU/ml was placed inside the microplate’s wells.
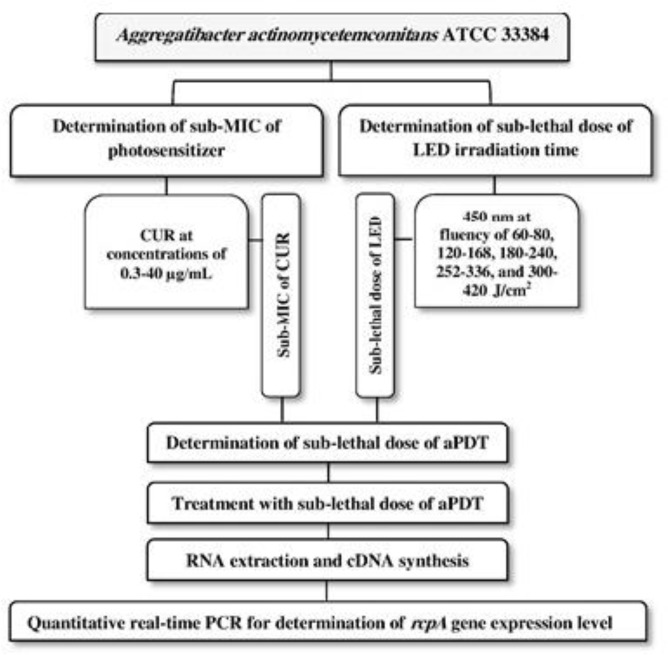
The LED in continuous mode was applied with an output power of 1000–1400 mW/cm2 for 1, 2, 3, 4, and 5 minutes with energy densities of 60-80, 120–168, 180–240, 252–336, and 300–420 J/cm2, respectively. The LED probe was fixed at a 1-mm distance above each well’s surface. A black paper was placed under the microplate to prevent beam reflection from the tabletop during LED irradiation. Calculations of A. actinomycetemcomitans CFUs/ml were performed according to the method mentioned above. Eventually, sub-lethal doses of aPDT were specified based on a previous study [13]. In brief, 100 μl of 2X BHI broth was added to the wells of the round-bottom 96-well microplate, and 100 μl of 2X MIC CUR was then added and serially diluted two-fold to 1.8 MIC. Finally, 100 μl of A. actinomycetemcomitans suspension with the concentration of 1.5×106 CFU/ml was poured into each well. The microplate was incubated for 5 minutes in the dark at room temperature in microaerophilic conditions before LED irradiation. The treated A. actinomycetemcomitans suspension in the well was exposed to the sub-lethal dose of LED irradiation time. Sub-lethal doses of aPDT were determined according to the methods mentioned previously [13]. The flowchart of the experimental steps is shown in Figure 1.
Fig. 1:
Flowchart of the experimental steps
Sub-lethal doses of aPDT against A. actinomycetemcomitans isolates:
After determining the sub-lethal doses of aPDT in the previous section, the A. actinomycetemcomitans isolate was exposed to the sub-lethal dose of CUR and to the sub-lethal dose of LED irradiation time. The untreated A. actinomycetemcomitans isolate which did not receive sub-lethal doses of aPDT was used as a control group.
RNA preparation and complementary DNA (cDNA) synthesis:
The total RNA of treated and untreated A. actinomycetemcomitans isolates was extracted by using an RNA Extraction Kit (GeneAll Hybrid-RTM RNA purification; GeneAll Biotechnology Co., Ltd., Korea) in accordance with the manufacturer’s recommendations. NanoDrop® ND-1000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 260 nm and 280 nm (A260/A280 ratio) was used to evaluate the quality of the total extracted RNA.
The total extracted RNA was treated by RNase-free DNase I (Thermo Fisher Scientific GmbH, Bremen, Germany) to eliminate the genomic DNA from RNA that could remain after the extraction. First-strand cDNA was then synthesized through random hexamer primed reactions by using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) according to the manufacturer’s protocol.
Quantitative real-time polymerase chain reaction (qRT-PCR) and gene expression analysis:
The primers for A. actinomycetemcomitans were determined based on rcpA and comparative 16S ribosomal RNA (16S rRNA) gene sequences by utilizing Primer3 Input version 0.4.0 online software (http://frodo.wi.mit.edu/primer3/).
Each nucleotide sequence was further evaluated by using the nucleotide algorithm of the Basic Local Alignment Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov/). Also, the primer specificity was determined by melting curve analysis. The qRT-PCR assays were performed in triplicate by the Line-GeneK Real-Time PCR Detection System (Bioer Technology Co., Ltd., Hangzhou, China). qRT-PCR process contained the following reaction components in a 20-μl reaction: 1 μl of cDNA (corresponding to the cDNA transcribed from approximately 10 ng of RNA), 10 μl of SYBR Premix Ex Taq II (2x) (Takara Bio Inc., Otsu, Japan), 1 μl of forward and 1μl of reverse primer, which are shown in Table 1, and 7 μl of sterile distilled water. The thermal cycling conditions for qRT-PCR (25-μl reaction volume) included an initial denaturation of 5 minutes at 95°C, followed by 35 cycles at 95°C for 10 seconds, at 59°C for 15 seconds, and at 72°C for 15 seconds.
Table 1.
Primer sequences used in the present study
| Gene | Primer | Sequences (5′– 3′)a | Tm (°C) | Amplicon Size (bp) |
|---|---|---|---|---|
| rcpA | F | TGGGCATTAACTGGAGCCAC | 60 | 72 |
| R | ATCCACCTCCGAAACCGAAG | |||
| 16S rRNA | F | AAGCACCGGCTAACTCCGT | 60 | 63 |
| R | TTCCGATTAACGCTCGCAC |
F=Forward primer, R=Reverse primer, Tm=Melting temperature, bp=Base pair
Nucleotides
Statistical analysis:
One-way analysis of variance (ANOVA) and Tukey’s post-hoc tests were performed to statistically analyze the data. The changes in the expression level of target gene were analyzed by using the method adopted by Livak and Schmittgen [14]. All the experiments were done in triplicate, and P-values lower than 0.05 were considered statistically significant.
RESULTS
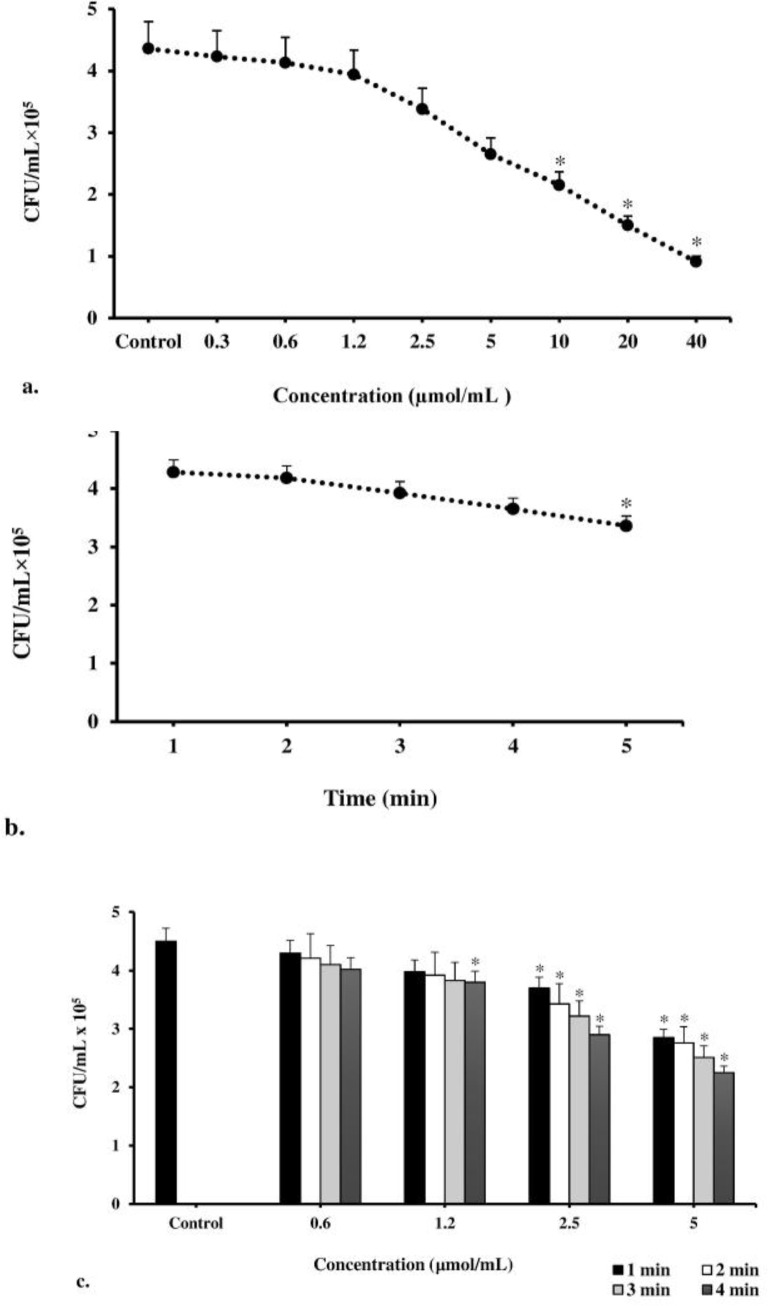
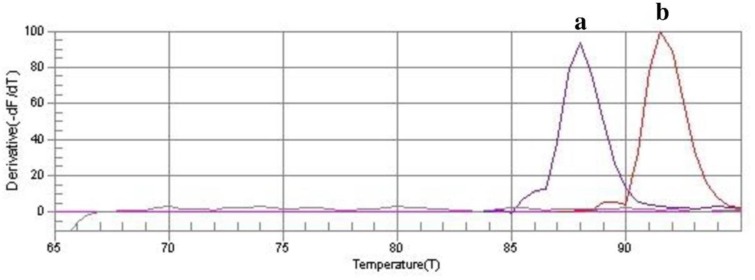
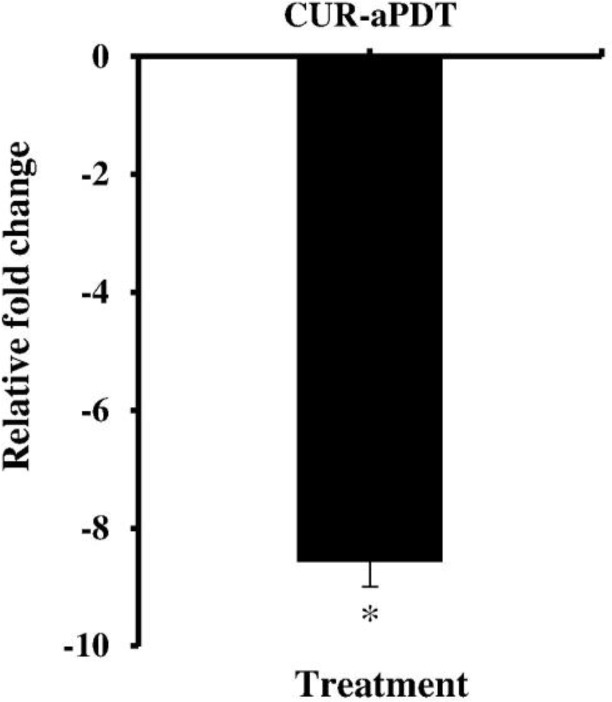
According to the results of our study, CUR at 10–40 μmol/ml concentrations significantly reduced the cell survival of A. actinomycetemcomitans isolates from 50.5% to 79.1% compared to control group (untreated bacteria; P<0.05, Fig. 2a), whereas there was no statistically significant reduction when the concentration of CUR was increased from 0.3 to 5 μmol/ml. Therefore, the MIC and maximal sub-MIC doses of CUR against A. actinomycetemcomitans were found to be 5 and 2.5 μmol/ml, respectively. According to Figure 2b, our assays revealed that the cell viability of A. actinomycetemcomitans isolates was significantly reduced (30.8%) after 5 minutes of LED exposure with 300–420 J/cm2 energy density. There was no decrease in the count of A. actinomycetemcomitans cells in the other LED radiation times (P>0.05). Therefore, the sub-lethal dose of LED irradiation time was four minutes with an output light energy of 252–336 J/cm2. According to the results presented in Figure 2c, the maximal CUR-mediated sub-lethal dose of aPDT which exhibited a non-significant reduction in A. actinomycetemcomitans cell count was 1.2 μmol/ml of CUR plus three minutes of irradiation time at a fluency of 180–240 J/cm2. The results of each primer’s melting curve in Figure 3 show that the presence of a single curve for each primer implies the formation of a single product, which shows the specificity of the primers for target genes. As shown in Figure 4, maximal sub-lethal doses of aPDT presented a significant 8.5-fold reduction of rcpA in A. actinomycetemcomitans cells and can be effective in reducing the biofilm formation of A. actinomycetemcomitans isolates.
Fig. 2:
Minimum inhibitory concentration (MIC) of (a) Curcumin (CUR), (b) light-emitting diode (LED), and (c) antimicrobial photodynamic therapy (aPDT) against Aggregatibacter actinomycetemcomitans. *Significantly different from the control (P<0.05)
Fig. 3:
The melting curve profiles generated by real-time amplification to assess potential primer-dimer artifacts (or non-specific PCR product). a.16S rRNA, b.rcpA
Fig. 4:
Effect of the sub-lethal dose of antimicrobial photodynamic therapy (aPDT) on the expression ratio of rcpA gene in Aggregatibacter actinomycetemcomitans. *Significantly different from the control (P<0.05)
DISCUSSION
Periodontal infection is a polymicrobial infection where hundreds of different microorganism species are present in a periodontal lesion [15]. A. actinomycetemcomitans is an opportunistic periodontopathogen that can firmly attach to dental surfaces and subgingival crevicular epithelial cells to form subgingival biofilms [16,17].
A. actinomycetemcomitans demonstrates a great genetic diversity in its ability as a periodontopathogen to express different virulence factors [18]. Fimbriae are important in the initial adhesion of A. actinomycetemcomitans to dental surfaces and in biofilm formation. It has been reported that the tad locus contains 14 genes, including rcpA, that are found tandemly located downstream of the fimbriae. Overall, rcpA appears to play a critical role in the colonization of A. actinomycetemcomitans and in the formation of biofilms [5].
Interestingly, the microbiota in the biofilm phase are up to 1000-fold more tolerant and/or resistant to antimicrobial agents compared to the planktonic phase [19]. Also, the transformation efficiency rates can be 10- to 600-fold higher in biofilms than in planktonic cells [20].
A. actinomycetemcomitans can create persistent infections in periodontal regions and can promote resistance to antimicrobial agents and periodontal treatment via biofilm formation [19]. Therefore, the development of a new antimicrobial approach with fewer complications is necessary when there is a possibility of acquired resistance to antimicrobial elements by A. actinomycetemcomitans.
Previous studies have shown that aPDT, as a noninvasive therapeutic modality, is a new promising strategy to eliminate pathogenic microbiota [21–23]. During aPDT, a pharmacologically inert chromophore called a photosensitizer is used with a low-power laser of an appropriate wavelength. The photosensitizer is stimulated through exposure to light. In the presence of oxygen, the excited photosensitizer can release reactive nitrogen species (RNS) and oxygen species (ROS), such as superoxide, hydroxyl radicals, and hydrogen peroxide, which are harmful to cell membrane integrity and can cause biological death [21].
The antibacterial and antibiofilm effects of aPDT with different photosensitizers have been previously assessed. Haag et al [24] evaluated the in-vitro antimicrobial efficacy of aPDT against periodontopathogenic bacteria and reported that aPDT with methylene blue (MB) resulted in a significant reduction of the surviving bacteria. The results of their study showed that the range of log10-reduction was about 39% to 100% with MB-aPDT against A. actinomycetemcomitans [24]. On interpreting the results of previous studies, it becomes obvious that aPDT could reduce the load of periodontopathogenic bacteria in cases of chronic periodontitis, aggressive periodontitis, and peri-implantitis [25].
On the other hand, Eick et al [26] found that toluidine blue O (TBO) and a diode laser (625–635 nm) were effective in reducing the viability of A. actinomycetemcomitans.
CUR is a hydrophobic photosensitizer that is soluble in DMSO, acetone, ethanol, and oils, and it has an absorption spectrum in the ultraviolet UV)/blue wavelength range of 300–500 nm with a maximum absorption at 430 nm [27,28].
Najafi et al [29] conducted a study to investigate the effect of aPDT with CUR against A. actinomycetemcomitans and demonstrated that aPDT with 5 mg/ml of CUR decreased the A. actinomycetemcomitans CFUs/ml by approximately 65% in comparison with control group. Araújo et al [30] reported the photodynamic effects of CUR against cariogenic pathogens. A reduction of up to 99.99% in the viability of Streptococcus mutans was observed when 1.5 g/L of CUR was used, whereas the reduction of the cell viability of Lactobacillus acidophilus was considerably lower (37.6%) after CUR-aPDT [30].
The results of the present study revealed that CUR at the concentrations above 5 μmol/ml can significantly reduce the A. actinomycetemcomitans CFUs/ml. To the best of our knowledge, this is the first experiment that has determined the effects of aPDT with CUR on the expression level of rcpA gene in A. actinomycetemcomitans. Other studies have shown that aPDT can significantly reduce the pathogenicity of other microorganisms [31,32]. Based on the results obtained from the current experiment, A. actinomycetemcomitans rcpA gene was down-regulated 8.5-fold, which can reduce the A. actinomycetemcomitans strain’s ability to form biofilms.
One of the limitations of this study was in-vitro analysis without in-vivo assays. Further studies should be conducted on CUR-aPDT. Also, in-vivo evaluations are needed to confirm the clinical relevance of these results. In addition, CUR needs to be further improved for clinical use.
CONCLUSION
The results of the present study indicate that CUR-mediated aPDT shows antibacterial potential against A. actinomycetemcomitans and can repress the expression level of rcpA which is a gene involved in biofilm formation. It can be concluded that CUR-aPDT is a useful alternative strategy for periodontal therapy.
ACKNOWLEDGMENTS
This research has been supported by Tehran University of Medical Sciences (Grant No# 95-01-97-31614).
REFERENCES
- 1-.Khan SA, Kong EF, Meiller TF, Jabra-Rizk MA. Periodontal Diseases: Bug Induced, Host Promoted. PLoS Pathog. 2015. July 30;11(7):e1004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2-.Kommerein N, Stumpp SN, Müsken M, Ehlert N, Winkel A, Häussler S, et al. An oral multispecies biofilm model for high content screening applications. PLoS One. 2017. March 15;12(3):e0173973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3-.Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol. 2017. September;15:740–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4-.Åberg CH, Kelk P, Johansson A. Aggregatibacter actinomycetemcomitans: virulence of its leukotoxin and association with aggressive periodontitis. Virulence. 2015;6(3):188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5-.Gholizadeh P, Pormohammad A, Eslami H, Shokouhi B, Fakhrzadeh V, Kafil HS. Oral pathogenesis of Aggregatibacter actinomycetemcomitans. Microb Pathog. 2017. December;113:303–311. [DOI] [PubMed] [Google Scholar]
- 6-.Zijnge V, Kieselbach T, Oscarsson J. Proteomics of protein secretion by Aggregatibacter actinomycetemcomitans. PLoS One. 2012. July;7(7):e41662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7-.Pourhajibagher M, Chiniforush N, Shahabi S, Sobhani S, Monzavi MM, Monzavi A, et al. Monitoring gene expression of rcpA from Aggregatibacter actinomycetemcomitans versus antimicrobial photodynamic therapy by relative quantitative real-time PCR. Photodiagnosis Photodyn Ther. 2017. September;19:51–55. [DOI] [PubMed] [Google Scholar]
- 8-.Fekrazad R, Khoei F, Bahador A, Hakimiha N. Photo-activated elimination of Aggregatibacter actinomycetemcomitans in planktonic culture: Comparison of photodynamic therapy versus photothermal therapy method. Photodiagnosis Photodyn Ther. 2017. September;19:28–32. [DOI] [PubMed] [Google Scholar]
- 9-.Vera-Ramirez L, Pérez-Lopez P, Varela-Lopez A, Ramirez-Tortosa M, Battino M, Quiles JL. Curcumin and liver disease. Biofactors. 2013. Jan-Feb;39(1):88–100. [DOI] [PubMed] [Google Scholar]
- 10-.Wright LE, Frye JB, Gorti B, Timmermann BN, Funk JL. Bioactivity of turmeric-derived curcuminoids and related metabolites in breast cancer. Curr Pharm Des. 2013;19(34):6218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11-.Pourhajibagher M, Monzavi A, Chiniforush N, Monzavi MM, Sobhani S, Shahabi S, et al. Real-time quantitative reverse transcription-PCR analysis of expression stability of Aggregatibacter actinomycetemcomitans fimbria-associated gene in response to photodynamic therapy. Photodiagnosis Photodyn Ther. 2017. June;18:78–82. [DOI] [PubMed] [Google Scholar]
- 12-.Miles AA, Misra SS, Irwin JO. The estimation of the bactericidal power of the blood. J Hyg (Lond). 1938. November;38(6):732–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13-.Pourhajibagher M, Chiniforush N, Shahabi S, Ghorbanzadeh R, Bahador A. Sub-lethal doses of photodynamic therapy affect biofilm formation ability and metabolic activity of Enterococcus faecalis. Photodiagnosis Photodyn Ther. 2016. September;15:159–66. [DOI] [PubMed] [Google Scholar]
- 14-.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001. December;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 15-.Rivera MF, Lee JY, Aneja M, Goswami V, Liu L, Velsko IM, et al. Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoE(null) mice. PLoS One. 2013. February;8(2):e57178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16-.Nunes AC, Longo PL, Mayer MP. Influence of Aae Autotransporter Protein on Adhesion and Biofilm Formation by Aggregatibacter actinomycetemcomitans. Braz Dent J. 2016. May-Jun;27(3):255–60. [DOI] [PubMed] [Google Scholar]
- 17-.Kuboniwa M, Lamont RJ. Subgingival biofilm formation. Periodontol 2000. 2010. February;52(1):38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18-.Kittichotirat W, Bumgarner RE, Chen C. Evolutionary Divergence of Aggregatibacter actinomycetemcomitans. J Dent Res. 2016. January;95(1):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19-.Berlanga M, Guerrero R. Living together in biofilms: the microbial cell factory and its biotechnological implications. Microb Cell Fact. 2016. October;15(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20-.Li YH, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. Natural genetic transformation of Streptococcus mutans growing in biofilms. J Bacteriol. 2001. February;183(3):897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21-.Rosa RAD, Santini MF, Figueiredo JAP, Visioli F, Pereira JR, Vivan RR, et al. Effectiveness of photodynamic therapy associated with irrigants over two biofilm models. Photodiagnosis Photodyn Ther. 2017. December;20:169–174. [DOI] [PubMed] [Google Scholar]
- 22-.Jeong S, Lee J, Im BN, Park H, Na K. Combined photodynamic and antibiotic therapy for skin disorder via lipase-sensitive liposomes with enhanced antimicrobial performance. Biomaterials. 2017. October;141:243–250. [DOI] [PubMed] [Google Scholar]
- 23-.Barin LM, Barcelos RCS, Vey LT, Pillusky FM, Palma VM, Kantorski KZ, et al. Role of the adjunctive antimicrobial photodynamic therapy to periodontal treatment at plasmatic oxidative stress and vascular behavior. J Photochem Photobiol B. 2017. August;173:538–544. [DOI] [PubMed] [Google Scholar]
- 24-.Haag PA, Steiger-Ronay V, Schmidlin PR. The in vitro antimicrobial efficacy of PDT against periodontopathogenic bacteria. Int J Mol Sci. 2015. November 13;16(11):27327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25-.Malik R, Manocha A, Suresh DK. Photodynamic therapy--a strategic review. Indian J Dent Res. 2010. Apr-Jun;21(2):285–91. [DOI] [PubMed] [Google Scholar]
- 26-.Eick S, Markauskaite G, Nietzsche S, Laugisch O, Salvi GE, Sculean A. Effect of photoactivated disinfection with a light-emitting diode on bacterial species and biofilms associated with periodontitis and peri-implantitis. Photodiagnosis Photodyn Ther. 2013. May;10(2):156–67. [DOI] [PubMed] [Google Scholar]
- 27-.Pourhajibagher M, Chiniforush N, Parker S, Shahabi S, Ghorbanzadeh R, Kharazifard MJ, et al. Evaluation of antimicrobial photodynamic therapy with indocyanine green and curcumin on human gingival fibroblast cells: An in vitro photocytotoxicity investigation. Photodiagnosis Photodyn Ther. 2016. September;15:13–8. [DOI] [PubMed] [Google Scholar]
- 28-.Baltazar LM, Ray A, Santos DA, Cisalpino PS, Friedman AJ, Nosanchuk JD. Antimicrobial photodynamic therapy: an effective alternative approach to control fungal infections. Front Microbiol. 2015. March 13;6:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29-.Najafi S, Khayamzadeh M, Paknejad M, Poursepanj G, Kharazi Fard MJ, Bahador A. An in vitro comparison of antimicrobial effects of curcumin-based photodynamic therapy and chlorhexidine, on Aggregatibacter actinomycetemcomitans. J Lasers Med Sci. 2016. Winter;7(1):21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30-.Araújo NC, Fontana CR, Bagnato VS, Gerbi ME. Photodynamic effects of curcumin against cariogenic pathogens. Photomed Laser Surg. 2012. July;30(7):393–9. [DOI] [PubMed] [Google Scholar]
- 31-.Nastri L, Donnarumma G, Porzio C, De Gregorio V, Tufano MA, Caruso F, et al. Effects of toluidine blue-mediated photodynamic therapy on periopathogens and periodontal biofilm: in vitro evaluation. Int J Immunopathol Pharmacol. 2010. Oct-Dec;23(4):1125–32. [DOI] [PubMed] [Google Scholar]
- 32-.Decker EM, Bartha V, Kopunic A, von Ohle C. Antimicrobial efficiency of mouthrinses versus and in combination with different photodynamic therapies on periodontal pathogens in an experimental study. J Periodontal Res. 2017. April;52(2):162–175. [DOI] [PubMed] [Google Scholar]