Abstract
Accumulating evidence suggests that the neuropeptide oxytocin (OXT) can enhance empathy although it is unclear which specific behavioral and neural aspects are influenced, and whether the effects are modulated by culture, sex, and trait autism. Based on previous findings in Caucasian men, we hypothesized that a single intranasal dose of OXT would specifically enhance emotional empathy (EE) via modulatory effects on the amygdala in an Asian (Chinese) population and explored the modulatory role of sex and trait autism on the effects. We first conducted a double-blind, randomized between-subject design experiment using a modified version of the multifaceted empathy task to determine whether OXT’s facilitation of EE can be replicated in Chinese men (n = 60). To further explore neural mechanisms behind and potential sex differences, functional MRI and skin conductance measures were acquired in an independent experiment incorporating men and women (n = 72). OXT enhanced EE across experiments and sex, an effect that was accompanied by reduced amygdala activity and increased skin conductance responses. On the network level OXT enhanced functional coupling of the right amygdala with the insula and posterior cingulate cortex for positive valence stimuli but attenuated coupling for negative valence stimuli. The effect of OXT on amygdala functional connectivity with the insula was modulated by trait autism. Overall, our findings provide further support for the role of OXT in facilitating EE and demonstrate that effects are independent of culture and sex and involve modulatory effects on the amygdala and its interactions with other key empathy regions.
Keywords: amygdala, autism, cognitive empathy, culture, emotional empathy, oxytocin
Introduction
Empathy is a key social-cognitive capacity that facilitates interpersonal functioning by allowing us to recognize, understand, and respond appropriately to mental and affective states experienced by others (Decety and Jackson, 2004; Dziobek et al., 2008; Reniers et al., 2010). Impaired empathy is a core deficit in psychiatric disorders characterized by interpersonal dysfunctions, including autism (Dziobek et al., 2008), schizophrenia (Shamay-Tsoory et al., 2007; Lee et al., 2011; Rosenfeld et al., 2011), and personality disorders (Herpertz and Bertsch, 2014).
Empathy is a multidimensional construct, entailing cognitive processes of perspective-taking, to make inferences about others’ mental states (cognitive empathy, CE), as well as emotional processes reflecting a direct affective reaction involving understanding, sharing, and responding appropriately to others’ feelings (emotional empathy, EE) (Shamay-Tsoory et al., 2009; Shamay-Tsoory, 2011; Bernhardt and Singer, 2012). EE has been further divided into a direct component (direct emotional empathy, EED), referring to explicit emotional evaluation and empathic concern, and an indirect component (indirect emotional empathy, EEI), referring to a more general physiological arousal response to both person and context (Dziobek et al., 2008). Although the cognitive and emotional components of empathy represent partly dissociable systems (Shamay-Tsoory et al., 2009), integrative approaches propose that the experience of empathy evolves as a dynamic interplay between them requiring an explicit representation of the specific affective state of the other person, thereby making CE a prerequisite for EE (Decety and Jackson, 2004; Hillis, 2014). On the neural level the functional organization of empathy is partially mirrored in shared and separable anatomical representations (Lamm et al., 2007, 2011; Schulte-Ruther et al., 2007; Singer and Lamm, 2009; Bernhardt and Singer, 2012; Leigh et al., 2013), with the bilateral insula, posterior cingulate cortex (PCC), and anterior cingulate cortex (ACC) contributing to both (Fan et al., 2011), and the amygdala contributing to the emotional component of empathy (Cox et al., 2012; Leigh et al., 2013).
Converging evidence suggests that the hypothalamic neuropeptide oxytocin (OXT) facilitates empathy (Rosenfeld et al., 2011; Striepens et al., 2011; Riem, 2012). Genetic approaches have consistently revealed associations between individual variations in the OXT receptor gene and levels of trait empathy in Caucasian (Rodrigues et al., 2009; Smith et al., 2014) and Chinese populations (Wu et al., 2012), with more recent studies suggesting that the specific associations evolve in interaction with other factors, particularly culture (Luo et al., 2015b; Montag et al., 2017) and sex (Weisman et al., 2015). Studies investigating the behavioral effects of intranasal OXT administration on CE have reported enhanced accuracy in the reading the mind in the eyes test (RMET) (Domes et al., 2007b) and a paradigm requiring participants to infer the intensity of positive or negative emotions expressed by subjects portrayed in videos (Bartz et al., 2010). However, findings in the domain of CE have been variable, with OXT effects in the RMET being either restricted to difficult items (Feeser et al., 2015) or unable to be reproduced at all even when taking into account stimulus difficulty and valence (Radke and de Bruijn, 2015). Other studies have also reported that effects were more pronounced in individuals with poor baseline performance (Riem et al., 2014) or high trait autism (Bartz et al., 2010). Studies that aimed specifically at determining effects of OXT on EE focused on empathy for pain, an evolutionary conserved primary emotional component (Decety, 2011; Panksepp and Panksepp, 2013), and found no effect on pain empathy toward a partner (Singer et al., 2008), although an enhanced pain empathic response toward members of an out-group (Shamay-Tsoory et al., 2013). In contrast, another study in men using the multifaceted empathy test (MET) (Dziobek et al., 2008), which assesses both CE and EE, observed that OXT specifically enhanced both EED and EEI, but not CE (Hurlemann et al., 2010). This latter study additionally demonstrated selective EE deficits in amygdala lesion patients and therefore suggested that the amygdala may mediate the EE enhancing effects of OXT. Although several neuroimaging studies have demonstrated modulatory effects of intranasally administered OXT on the core neural components of the empathy network, including the insula, ACC, and amygdala and their functional interactions, across different task paradigms (Bakermans-Kranenburg and van IJzendoorn, 2013; Wigton et al., 2015; Herpertz and Bertsch, 2016), to date only two studies have directly explored the neural mechanisms underlying OXT’s empathy enhancing effects. The first reported that OXT increased activation in the superior temporal gyrus and insula during the RMET task (Riem et al., 2014), whereas the second reported reductions in left insula activity during pain empathic processing (Bos et al., 2015).
In summary, although the empathy enhancing effects of OXT are central to its proposed social-cognitive and therapeutic properties, it remains unclear whether it selectively enhances CE or EE, and which specific neural substrates are involved. To systematically address these questions, we employed two independent pharmacological between-subject placebo (PLC) controlled experiments in healthy Chinese individuals investigating the effects of intranasal OXT on CE and EE and the underlying neural basis of this effects during the MET (Dziobek et al., 2008).
Previous studies on the empathy enhancing potential of intranasal OXT are entirely based on observations in Caucasian populations. However, there is accumulating evidence from OXT-administration studies either employing comparable experimental protocols in Caucasian and Chinese subjects (Hurlemann et al., 2010; Hu et al., 2015) or examining moderating effects of key cultural orientation differences such as a collectivistic orientation (Pfundmair et al., 2014; Xu et al., 2017), suggesting culture-dependent social-cognitive effects of OXT. To this end, the first experiment aimed to replicate findings in a male Caucasian sample showing that OXT enhances EE but not CE (Hurlemann et al., 2010) in a male Chinese sample. In a second independent sample, male and female Chinese participants performed the same MET paradigm during functional magnetic resonance imaging (fMRI) to determine the neural substrates involved. Analyses on the neural level focused on the insula, amygdala, and ACC as core empathy regions (Lamm et al., 2007, 2011; Schulte-Ruther et al., 2007; Singer and Lamm, 2009; Bernhardt and Singer, 2012; Leigh et al., 2013). Given that the amygdala has been specifically (Cox et al., 2012; Leigh et al., 2013) and critically (Hurlemann et al., 2010) associated with emotional facets of empathy, we expected that OXT’s enhancement of EE would be accompanied by altered regional activity and network level connectivity of the amygdala. Previous studies reported increased as well as decreased amygdala activity and connectivity following OXT (Domes et al., 2007a; Striepens et al., 2012; Hu et al., 2015; Wigton et al., 2015; Tully et al., 2018) therefore no directed hypothesis with respect to OXT’s neural effect was formulated.
Based on a growing number of findings suggesting sex-dependent effects of OXT on social cognition (Chen et al., 2016; Gao et al., 2016; Luo et al., 2017), the second experiment additionally explored whether OXT differentially affects empathic processing in men and women. In line with a previous study reporting that sex does not affect OXT’s modulation of empathy (Shamay-Tsoory et al., 2013), we hypothesized that OXT facilitation of EE would generalize across sexes. Finally, in the context of increasing interest in the therapeutic application of OXT as a potential treatment to improve social cognitive deficits, including empathy, in autism spectrum disorders (Young and Barrett, 2015), and in line with previous studies in healthy subjects (Bartz et al., 2010; Scheele et al., 2014; Xu et al., 2015), the modulatory role of trait autism (assessed by the Autism Spectrum Quotient questionnaire, ASQ, Baron-Cohen et al., 2001) was explored.
Materials and Methods
Participants
To fully replicate the previous study on Caucasian participants (Hurlemann et al., 2010), only males were recruited in the first experiment but both males and females were enrolled in the second experiment to explore potential sex-dependent effects of OXT on empathy. Experiment 1 (Exp 1) included 60 participants (M ± SD, mean age = 22.42 ± 2.23 years, all male) and Experiment 2 (Exp 2) included an independent sample of 72 participants (34 females, mean age = 21.18 ± 1.95 years, 38 males, mean age = 22.61 ± 2.01 years). Both experiments incorporated a double-blind, between-participant design, with participants being randomly assigned to receive either OXT or PLC nasal-spray, resulting in n = 30 (Exp 1) and n = 36 (Exp 2, female = 17) participants treated with OXT. The experimental groups in both experiments were of comparable age (Exp 1, p = 0.53, T58 = 0.63; Exp 2, p = 0.66, T70 = -0.44), education (Exp 1, p = 0.66, T58 = 0.44; Exp 2, p = 0.63, T70 = -0.49) and, in Exp 2, of equivalent sex distribution (chi-square < 0.001, df = 1, p = 1). Exclusion criteria for all participants were past or current physical, neurological, or psychiatric disorders, regular or current use of medication or tobacco.
Participants were required to abstain from alcohol, caffeine, or nicotine for at least 12 h before the experiment. None of the females in Exp 2 were taking oral contraceptives or were tested during their menstrual period. Menstrual cycle phase was determined using validated procedures as described in Penton-Voak et al. (1999). The proportion of females estimated to be in their follicular or luteal phases did not differ significantly between the treatment groups (chi-square = 0.12, df = 1, p = 0.73). In Exp 1, one participant (in the OXT group) and in Exp 2, three participants (in the PLC group) failed to understand task instructions and were consequently excluded from all further analysis, leading to a total of n = 59 participants in Exp 1 and n = 69 participants in Exp 2.
Before the experiment, written informed consent was obtained from all participants. The study was approved by the local ethics committee of the University of Electronic Science and Technology of China and all procedures and the informed consent for study participation were in accordance with the latest revision of the declaration of Helsinki.
Experimental Protocol
To control for potential confounding variables, all participants initially completed the following questionnaires: Becks Depression Inventory (BDI; Beck et al., 1961), WLEIS-C Emotional Intelligence Scale (Wleis-C; Wong and Law, 2002), State Trait Anxiety Inventory (STAI; Spielberger et al., 1970), Empathy Quotient (EQ; Baron-Cohen and Wheelwright, 2004), and Positive and Negative Affect Scale (PANAS; Watson et al., 1988). To examine associations with trait autism, the ASQ questionnaire (Baron-Cohen et al., 2001) was administered. Intranasal treatment (OXT nasal spray, Sichuan Meike Pharmacy Co., Ltd., China, or PLC nasal spray with identical ingredients except OXT) was administered in line with recommendations for the intranasal administration of OXT in humans (Guastella et al., 2013) and 45 min before the start of the experimental paradigm. In Exp 1, three puffs per nostril (at 30 s intervals) were administered (24 IU) and in Exp 2, five puffs per nostril (40 IU). Both doses are in the typical range employed by other studies (Striepens et al., 2011; Guastella et al., 2013) with the rationale for increasing the dose in Exp 2 being to explore dose-dependent behavioral effects of OXT. In a previous study we found equivalent behavioral and neural effects of 24 and 48 IU OXT doses (Zhao et al., 2017). However, it should be noted that findings from some other studies investigating dose-dependent effects of intranasal OXT have suggested an inverted-U-shaped dose–response curve (Cardoso et al., 2013; Quintana et al., 2016, 2017; Spengler et al., 2017) and thus a stronger enhancement of EE with 24 IU relative to 40 IU is conceivable. In post experiment interviews, participants were unable to guess better than chance whether they had received the OXT nasal spray, confirming successful blinding.
Experimental Paradigm
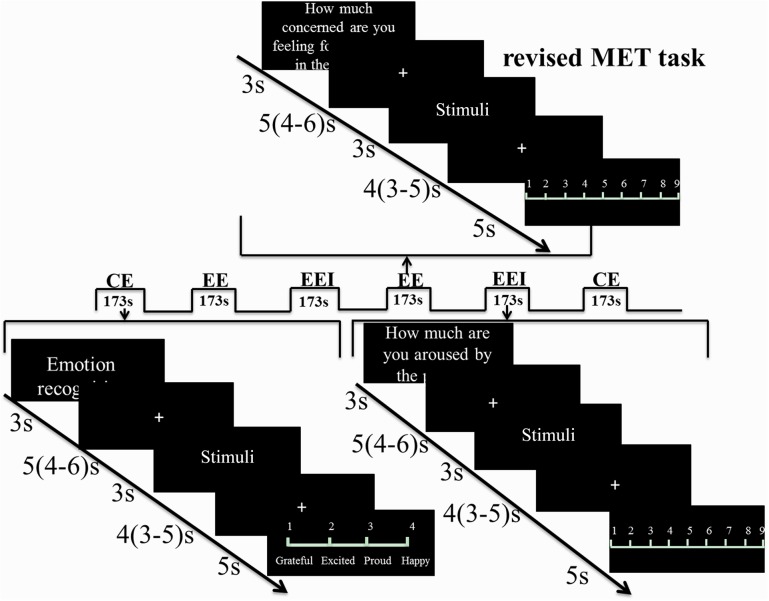
In line with a previous study on male Caucasian participants (Hurlemann et al., 2010), empathy was assessed using the MET (Dziobek et al., 2008; Hurlemann et al., 2010; Domes et al., 2013; Edele et al., 2013; Wingenfeld et al., 2014), which assesses both EE and CE components using ecologically valid photo-based stimuli of either negative or positive valence. To account for potential confounding effects of OXT on in-group versus out-group empathy (De Dreu and Kret, 2016) and a cultural empathy bias (Cao et al., 2015; Luo et al., 2015a), the original Caucasian MET stimuli were exchanged with corresponding pictures displaying Chinese protagonists. The Chinese stimuli were initially evaluated in an independent sample (Supplementary Materials) and the final set of Chinese stimuli (30 positive, 30 negative valence) closely resembled the Caucasian stimuli depicting daily life scenarios and conveying emotional mental states via facial expression, body posture, and contextual cues. To assess CE, participants were instructed to infer the emotional state of the protagonist in each scene and choose the corresponding answer from four options listed. The four options presented similar but distinct emotional states to ensure at least 70% accuracy for each stimulus picture. For EED, participants were required to rate how they felt for the protagonist in the depicted scene (1–9 scale, 1 = not at all, 9 = very strong), for EEI participants were required to rate how much they were aroused by the scene (1–9 scale, 1 = very calm, 9 = very aroused). Details of the paradigm are visually presented in Figure 1.
FIGURE 1.
Revised MET paradigm used in the fMRI experiment. Three blocks for CE, EED, and EEI were presented and in a balanced order. In each block, after 3 s of cue presentation, a jittered fixation (4–6 s) followed. Stimuli were shown for 3 s followed by another jittered fixation (3–5 s) and then a 5 s rating phase in order to separate the viewing and rating phases for fMRI analysis. Each block lasted for 173 s. There was a total of six runs with each run including three blocks, one block for CE, one for EED, and one for EEI.
The different components of empathy were presented in a mixed event/block-design. Following a 3 s instruction cue and a jittered inter-trial interval of 3.9 s (2.3–5.9 s), 10 stimuli per block were each presented for 3 s followed by either a choice of the emotion depicted for the CE condition (displayed for 4 s) or a rating scale (1–9) for the EED and EEI conditions (displayed for 5 s). Six blocks were presented for each condition, resulting in a total of 18 blocks. The order of blocks was counterbalanced across the experimental conditions, and the fMRI experiment was divided into six runs, each containing one block per empathy component. During fMRI (Exp 2) electrodermal activity was simultaneously acquired as an index of autonomic sympathetic activity (Stern et al., 2001) (technical details on the electrodermal data acquisition are provided in the Supplementary Materials). To allow baseline recovery of the electrodermal signal a mean inter-trial interval of 5 s (4–6 s) and a mean interval separating stimulus presentation and behavioral response of 4 s (3–5 s) was adopted for the fMRI experiment.
fMRI Data Acquisition
The fMRI data in Exp 2 were collected using a GE (General Electric Medical System, Milwaukee, WI, United States) 3.0T Discovery 750 MRI scanner. fMRI time series were acquired using a T2∗-weighted echo planar imaging pulse sequence (repetition time, 2000 ms; echo time, 30 ms; slices, 39; thickness, 3.4 mm; gap, 0.6 mm; field of view, 240 × 240 mm2; resolution, 64 × 64; flip angle, 90°). Additionally, a high resolution T1-weighted structural image was acquired using a 3D spoiled gradient recalled (SPGR) sequence (repetition time, 6 ms; echo time, 2 ms; flip angle 9°; field of view, 256 × 256 mm2; acquisition matrix, 256 × 256; thickness, 1 mm without gap) to exclude participants with apparent brain pathologies and to improve normalization of the fMRI data.
fMRI Data Processing
Functional magnetic resonance imaging data were analyzed using SPM12 (Wellcome Trust Center of Neuroimaging, University College London, London, United Kingdom). The first five volumes were discarded to allow T1 equilibration and images were realigned to the first image to correct for head motion. Tissue segmentation, bias-correction, and skull-stripping were done for the high-resolution structural images. The functional time series were co-registered with the skull-stripped anatomical scan and normalized to MNI space with a voxel size of 3 × 3 × 3 mm. Normalized images were then spatially smoothed using a Gaussian kernel with full-width at half-maximum (FWHM) of 8 mm. On the first level, event-related responses were modeled and subsequently convolved with the standard hemodynamic response function (HRF). The first level design matrix included valence- (positive, negative) and empathy type- (CE, EED, EEI) specific regressors for the viewing phases as main experimental conditions. In addition, regressors for the cue presentation, valence-, and empathy type-specific regressors for the rating phases, and for viewing and rating phases of incorrect trials as well as the six movement regressors were included. The experimental contrasts were next submitted to a second level random effects analysis.
To evaluate empathy-type specific main and interaction effects of treatment and valence, repeated-measured ANOVAs were employed in a flexible-factorial design. Based on our regional hypothesis and the core empathy network (Hurlemann et al., 2010; Bernhardt and Singer, 2012; Bakermans-Kranenburg and van IJzendoorn, 2013; Leigh et al., 2013; Hillis, 2014; Wigton et al., 2015; Shamay-Tsoory and Abu-Akel, 2016), the analyses focused on the bilateral amygdala, insula, and ACC which were structurally defined using 60% probability maps from the Harvard-Oxford (sub)cortical atlas. For the regionally focused analysis approach condition-specific parameter estimates were extracted from these regions of interest (ROI) using the Marsbar toolbox (Brett et al., 2002) and subjected to empathy type-specific ANOVAs with the between-participant factor treatment (OXT, PLC) and the within-participant factor valence (positive, negative) in SPSS (Statistical Package for the Social Sciences, Version 22). P-values for the post hoc tests of the ROI analysis were Bonferroni-corrected (P < 0.05). An exploratory voxel-wise whole-brain analysis in SPM that served to determine contributions of brain regions outside of the predefined network of interest was thresholded at P < 0.05, corrected using the family-wise error (FWE) approach.
To investigate the effects of OXT on the network level, a generalized form of psychophysiological interaction analysis (gPPI1; McLaren et al., 2012) was conducted using regions showing significant OXT effects in the BOLD level analysis as seeds and implementing an empathy-type specific voxel-wise whole-brain ANOVA approach including the between-participant factor treatment (OXT, PLC) and the within-participant factor valence (positive, negative) thresholded at P < 0.05, FWE-corrected at the cluster level. In line with recent recommendations for the control of false-positives in cluster-based correction approaches an initial cluster forming threshold of P < 0.001 was applied to data with a resolution of 3 × 3 × 3 mm (Eklund et al., 2016; Slotnick, 2017). Parameter estimates were extracted from the significant regions to disentangle the specific effects in post hoc comparisons. Finally, associations between neural indices and trait autism (ASQ scores) were conducted in SPSS using Pearson correlation analysis.
Results
In both experiments, there were no significant differences in trait and mood questionnaire scores between OXT and PLC treatment groups (Supplementary Table S1 for Exp 1, Supplementary Table S2 for Exp 2). In line with previous studies in Chinese populations (Melchers et al., 2015; Montag et al., 2017), no significant sex differences in ASQ and EQ scores were observed in Exp 2 (Supplementary Table S3).
Behavioral Results
Based on previous conceptualizations of empathy, proposing that CE is a prerequisite for EE (Decety and Jackson, 2004; Hillis, 2014), for EED and EEI measures only trials for which subjects successfully recognized the emotions displayed by the protagonist were analyzed [for a similar approach see Luo et al. (2015a)]. To this end, correctly recognized trials were initially determined based on the CE performance, with only correct trials subsequently entering the analyses for the EED and EEI facets.
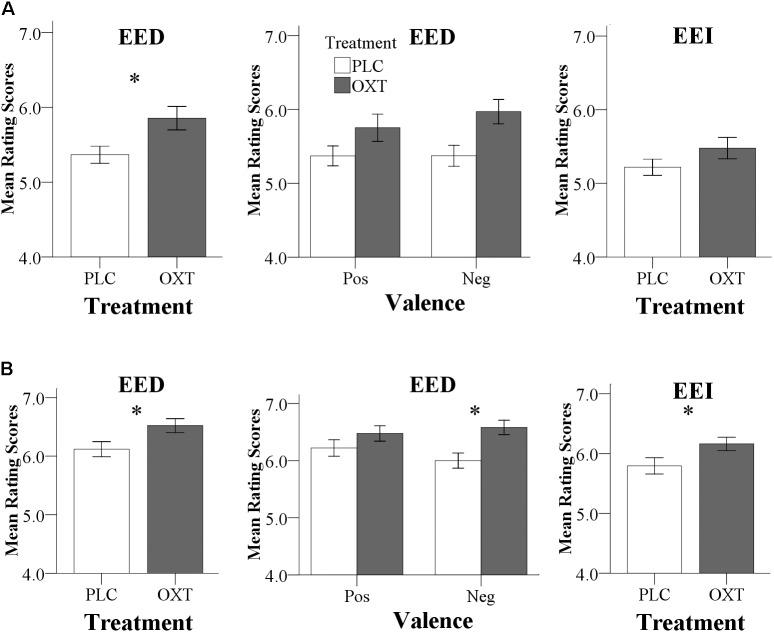
There were no significant differences in CE accuracy between the two treatment groups in both experiments (Exp 1, OXT, 77.53 ± 6.02%, PLC, 78.94 ± 5.78%, T57 = 0.92, P = 0.36; Exp 2, OXT, 80.10 ± 6.18%, PLC, 81.57 ± 5.83%, T67 = 1.02, P = 0.31). In Exp 1, there was a main effect of treatment [F(1,57) = 6.46, P = 0.01, = 0.10] for EED indicating that OXT generally enhanced EED (Figure 2A). There was no significant treatment × valence interaction [F(1,57) = 0.96, P = 0.33, = 0.02]. Analysis of EEI did not reveal a treatment main effect [F(1,57) = 2.19, P = 0.14, = 0.04] or valence × treatment interaction effect [F(1,57) = 3.16, P = 0.08, = 0.05].
FIGURE 2.
Behavioral results for Exp 1 (A) and Exp 2 (B). (A) In Exp 1 (24 IU administration), OXT significantly increased EED ratings. To allow for a better comparison with Exp 2, effects of OXT on positive and negative EED trials, as well as on EEI are also shown. (B) In Exp 2 (40 IU administration), OXT increased both EED and EEI ratings; an effect of OXT on negative EED drove the significant treatment by valence interaction. (∗P < 0.05).
Consistent with the findings for EED in Exp 1, Exp 2 also yielded a significant main effect of treatment on EED [F(1,67) = 5.81, P = 0.02, = 0.08] with higher ratings following OXT compared to PLC (Figure 2B). There was also a significant valence × treatment interaction [F(1,67) = 4.18, P = 0.05, = 0.06] with more pronounced effects of OXT on negative compared to positive valence stimuli [positive: F(1,67) = 1.68, P = 0.2, = 0.02; negative: F(1, 67) = 9.96, P = 0.002, = 0.13]. For EEI there was also a significant main effect of treatment [F(1,67) = 4.84, P = 0.03, = 0.07] but no treatment × valence interaction [F(1,67) = 2.02, P = 0.16, = 0.03). For CE, there were neither significant main effects nor interactions [F(1,65) = 0.80, P = 0.37, = 0.01). In Exp 2, no significant main or interaction effects involving sex were observed (all Ps > 0.18) arguing against sex-dependent effects of OXT on empathy.
Associations Between Behavior and Trait Autism
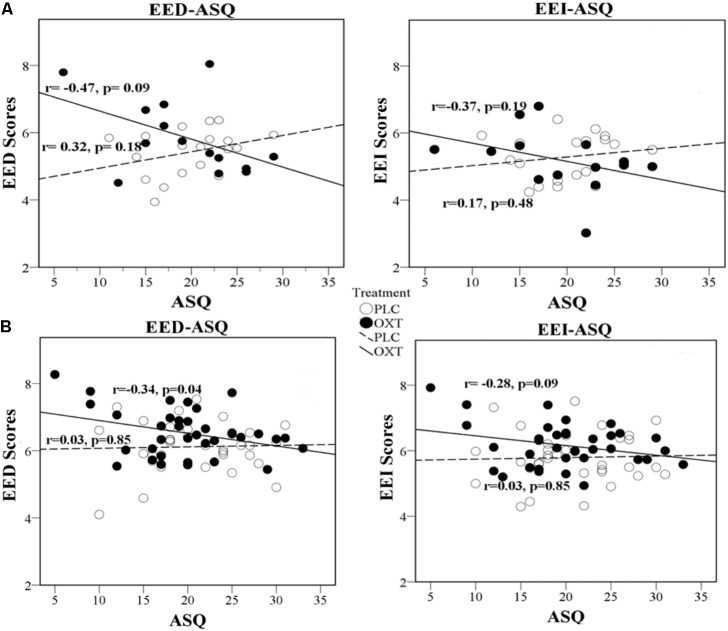
In Exp 1, there was a trend toward a negative correlation between the ASQ score and the total EED and EEI scores in the OXT group (ASQ Total: EED r = -0.47, P = 0.09; EEI r = -0.37, P = 0.19) but not the PLC group (EED r = 0.319, P = 0.18; EEI r = 0.17, P = 0.48). The correlation significantly differed between the PLC and OXT groups for EED (Fisher’s z = -2.13, P = 0.03) although not for EEI (Fisher’s z = -1.43, P = 0.15). In Exp 2, there was a similar pattern of correlation differences between EED and EEI scores and total ASQ scores, although these associations did not reach statistical significance other than for EED under OXT (EED – PLC r = 0.03, P = 0.85, OXT r = -0.34, P = 0.04, Fisher’s z = 1.53, P = 0.13; EEI – PLC r = 0.03, P = 0.85; OXT r = -0.28, P = 0.09, Fisher’s z = 1.29, P = 0.20). Regression plots are shown in Figure 3 and suggest that OXT is producing its main behavioral effects in participants with lower autism traits.
FIGURE 3.
Regression plots for ASQ score and EED and EEI ratings. Exp 1 (A) and Exp 2 (B) in OXT and PLC groups.
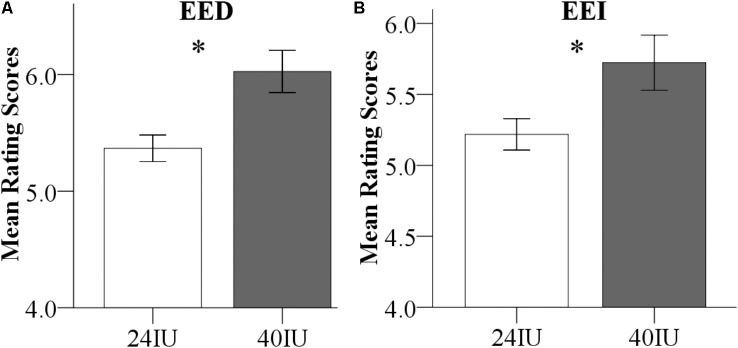
Dose-Dependent Effects Between Experiments 1 and 2 (24 vs. 40 IU)
Dose effects were explored by combining the data from male participants in Exp 1 (24 IU) and Exp 2 (40 IU). To initially explore potential effects of the different experimental environments (Exp 1, 24 IU, behavioral testing room; Exp 2, 40 IU, inside the MRI-scanner) on empathy per se, a first analysis focused on the PLC-treated subjects. A repeated ANOVA with environment (behavioral vs. MRI room) as a between-subject factor and valence as a within-subject factor revealed a significant environment main effect for both EE facets, indicating elevated EE ratings in the MRI room [main effects: EED: P = 0.003, F(1,47) = 10.19, = 0.18; EEI: P = 0.02, F(1,47) = 5.74, = 0.11, both interactions with valence > 0.38, non-significant, Figure 4], but no effects on CE [main effect: P = 0.15, F(1,47) = 2.19, = 0.05; interaction: P = 0.9, F(1,47) = 0.02, < 0.001]. These findings suggest that the MRI-environment per se increased EE, an effect possibly related to elevated levels of stress during the MRI assessments, which would be in line with a previous study reporting that stress-induction specifically increased EE, but not CE in the MET (Wolf et al., 2015). The environmental differences and potential interactions with OXT preclude the interpretation of dose-related differences between the experiments.
FIGURE 4.
(A) Direct emotional empathy (EED) and (B) indirect emotional empathy (EEI) ratings in the placebo (PLC) groups. Emotional empathy differences between Exp 1 and Exp 2 in the PLC-treated subjects. Examining the PLC-treated male subjects from the two experiments revealed that EE was significantly increased in the MRI environment (Exp 2) compared to the behavioral testing room (Exp 1).
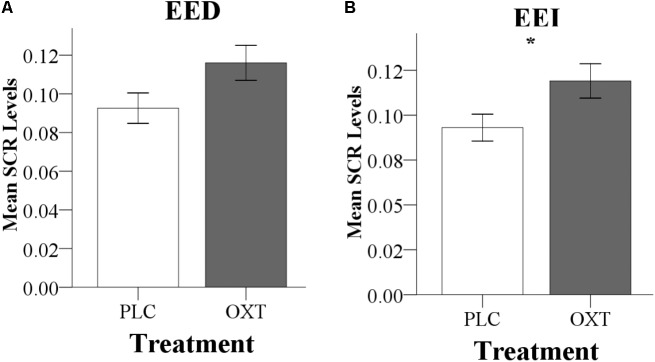
Oxytocin Effects on SCR
One participant was excluded from SCR analysis due to low skin impedance and thus a total of 68 participants from Exp 2 were included. Analyses of the SCR data paralleled the analyses of the empathy ratings, using ANOVAs with the between-subject factors treatment (OXT, PLC) and sex (male, female), and the within-subject factor valence. There was a marginal main effect of treatment on SCR during EED trials and significant during EEI trials [EED: F(1,66) = 3.77, P = 0.06, = 0.05; EEI: F(1,66) = 4.50, P = 0.04, = 0.06], but not during CE trials [F(1,66) = 2.14, P = 0.15, = 0.03]. This was due to SCR responses being increased in the OXT group during EE and EEI trials (Figure 5). There were no significant main effects of valence or treatment × valence or treatment × sex interactions for CE, EE, or EEI trials (all Ps > 0.2).
FIGURE 5.
Effects of oxytocin (OXT) on the SCR during (A) direct emotional empathy, EED, and (B) indirect emotional empathy, EEI. In Exp 2, OXT increased the SCR during both EED (P = 0.06) and EEI (P < 0.05). ∗Indicates P < 0.05.
Oxytocin Effects on Neural Activity
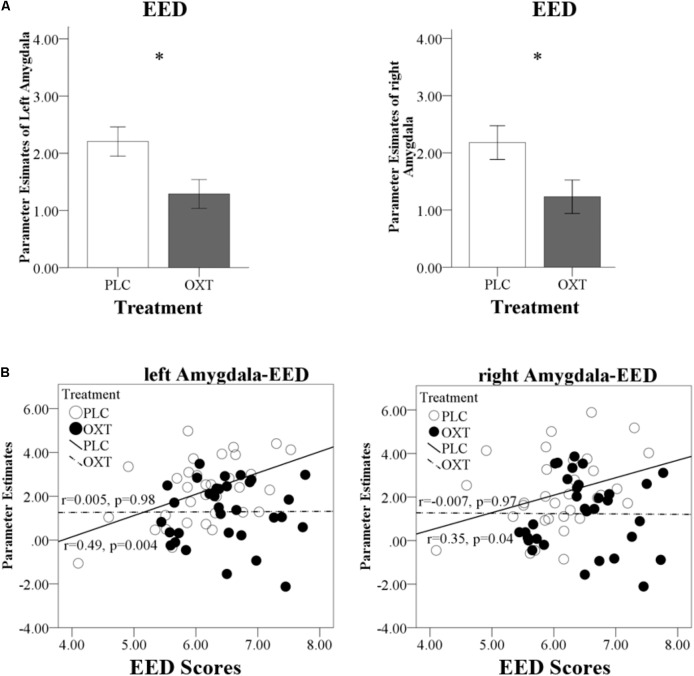
In view of the absence of sex-dependent effects in the behavioral analysis, and to increase the statistical power to determine OXT effects on the neural level, the data from male and female participants were pooled for the fMRI analyses. Four further participants were excluded from the fMRI analysis due to excessive head motion (head motion > 3 mm). The neural mechanisms underlying the behavioral effects of OXT were initially explored in the different priori ROIs (amygdala, insula, and ACC) using separate repeated measures ANOVAs for the three empathy (CE, EE, and EEI) conditions. Main treatment effects were only observed in the amygdala (Figure 6A) for EED [left amygdala: F(1,63) = 6.55, P = 0.01, = 0.09; right amygdala: F(1,63) = 5.18, P = 0.03, = 0.08]. There were no significant main effects for CE or EEI or any treatment × valence interactions for CE, EED, or EEI. An exploratory whole brain analysis revealed no regions that showed significant treatment-dependent changes under CE, EED, or EEI (all PFDR_corrected > 0.05) outside of the prior defined ROIs.
FIGURE 6.
Effects of OXT on bilateral amygdala responses. (A) Region of interest analysis results for left and right amygdala responses during EED trials. (B) Regression plots show correlations between left and right amygdala responses and EED scores in OXT and PLC groups. ∗P < 0.05.
Oxytocin Effects on Functional Connectivity
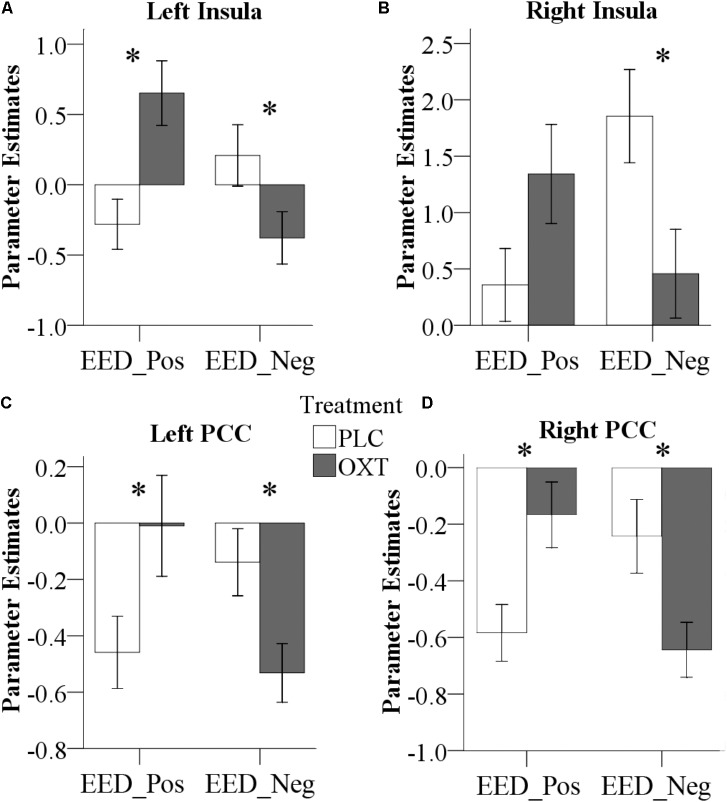
Repeated measures ANOVA models in SPM that included the between-subject factors treatment (OXT, PLC) and sex (male, female) and the within-subject factor valence (positive, negative) revealed a significant Treatment × Valence interaction effect for EED-associated functional coupling of the right amygdala with the bilateral insula and the bilateral PCC (left insula peak located at x/y/z, -33/6/-15, PFWE = 0.02, cluster size = 143 voxels; right insula peak located at 45/18/-12, PFWE = 0.03, cluster size = 121 voxels; left PCC peak located at -30/-33/30, PFWE = 0.003, cluster size = 213 voxels; right PCC peak located at 21/-36/33, PFWE = 0.01, cluster size = 149 voxels; coordinates given in MNI-space). Extraction of parameter estimates further revealed that OXT increased functional connectivity for positive valence stimuli whereas it decreased connectivity for negative valence ones [left insula: positive, F(1,61) = 10.52, P = 0.002, = 0.15, negative, F(1,61) = 3.86, P = 0.05, = 0.06; right insula: positive, F(1,61) = 3.34, P = 0.07, = 0.05, negative, F(1,61) = 5.53, P = 0.02, = 0.08; left PCC: positive, F(1,61) = 4.34, P = 0.04, = 0.07, negative, F(1,61) = 5.67, P = 0.02, = 0.09; right PCC: positive, F(1,61) = 8.00, P = 0.006, = 0.12, negative, F(1,61) = 5.48, P = 0.02, = 0.08] (Figure 7).
FIGURE 7.
Effects of OXT effect on right amygdala functional connectivity. Effects of treatment on functional connectivity of the right amygdala, indicating valence-dependent effects of OXT on the coupling of the right amygdala with the left (A) and right (B) insula, as well as the left (C) and right (D) PCC (∗P < 0.05) (Pos, Positive; Neg, Negative).
Associations Between Neural and SCR Effects of OXT and Behavioral and Autism Trait Scores
There was a significant positive correlation between EED scores and bilateral amygdala responses in the PLC group (left r = 0.49, P = 0.004; right r = 0.35, P = 0.04) which was absent in the OXT group (left r = 0.005, P = 0.98; right r = -0.007, P = 0.97). The correlation difference between the PLC and OXT groups was significant for the left (Fisher’s Z = 2.05, P = 0.04) but not the right (Fisher’s Z = 1.43, P = 0.15) amygdala (Figure 6B). There was no correlation between left or right amygdala responses with total ASQ scores.
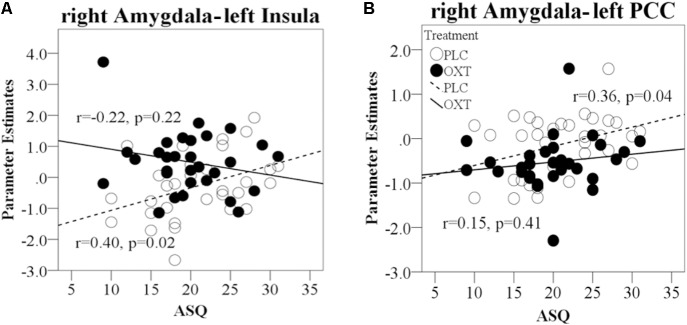
For the functional connections showing OXT effects for EED in terms of a treatment × valence interaction, coupling strength between the right amygdala and left insula during positive valence EED trials was positively correlated with the total ASQ in the PLC group (total ASQ – r = 0.40, P = 0.02) but not in the OXT group (total ASQ – r = -0.22, P = 0.22, Fisher’s Z = 2.50, P = 0.01; Figure 8A). OXT particularly appears to increase the strength of right amygdala functional connections with the insula in individuals with lower ASQ scores, although only for positive valence EED. The strength of link between the right amygdala and left PCC during negative valence EED trials was positively correlated with the total ASQ score in the PLC group but not the OXT, although the difference between the groups was not significant (total ASQ – PLC r = 0.36, P = 0.04; OXT r = 0.15, P = 0.41; Fisher’s Z = 0.85, P = 0.39; Figure 8B). There were no significant correlations between SCR values and ASQ scores during either EED or EEI trials (all Ps > 0.41).
FIGURE 8.
Regression plots for correlations of ASQ scores with amygdala responses and functional connectivity during EE trials in OXT and PLC groups. (A) Correlation between right amygdala–left PCC functional connectivity during negative EED trials and total ASQ score. (B) Correlation of right amygdala functional connectivity with left insula cortex during positive EE trials and total ASQ score. In all cases, significant positive correlations during PLC administration are absent in the OXT group.
Discussion
The present study confirmed in two independent samples that intranasal OXT specifically facilitates EE but not CE as assessed by the MET paradigm in Chinese participants, thereby replicating previous findings in Caucasian participants (Hurlemann et al., 2010). Our findings also demonstrated for the first time that the OXT-induced enhancement of EED is associated with decreased bilateral amygdala reactivity and enhanced functional coupling of the right amygdala with the insula and PCC for positive valence stimuli but attenuated coupling for negative valence stimuli. These behavioral and neural effects were not modulated by subject sex, suggesting a generalization across men and women. Finally, an exploratory analysis of associations with trait autism revealed that both behavioral and neural effects of OXT were modulated to some extent by trait autism scores.
Although many studies have reported cultural differences between Asian and Caucasian participants in the context of OXT receptor polymorphisms and empathy (Kim et al., 2010; Luo et al., 2015b; Jessica et al., 2016), we did not find any substantive difference with respect to the effects of intranasal OXT on empathy processing as assessed by MET. Thus, in both cultures, OXT enhanced EE but not CE (Hurlemann et al., 2010) for both valences, although in our second experiment we found stronger effects for negative valence stimuli. Effects of the scanning environment on EE ratings per se precluded the direct evaluation of dose–response effects between the two experiments; however, OXT specifically increased EE in both suggesting that its effects generalize across 24 and 40 IU doses, in line with our previous finding (Zhao et al., 2017). In general, the magnitude of the reported behavioral OXT effect on both EED and EEI reported in Caucasian participants was however somewhat stronger compared to both 24 and 40 IU doses administered in our study, although different MET stimuli were used.
In agreement with other studies, there were no sex-differences in EE, trait empathy (Wu et al., 2012) or trait autism (Kawamura et al., 2011; Montag et al., 2017) scores in our Chinese study cohort, whereas in Caucasian participants we found that females scored significantly higher than males for both positive and negative valence stimuli (Hurlemann et al., 2010). Thus, it is conceivable that in Caucasian females the effects of OXT in the MET might not be as pronounced as in males. The absence of an effect of OXT on CE in the MET contrasts with reports using other paradigms, notably the RMET (Domes et al., 2007b; Feeser et al., 2015). However, the robustness of these findings has been questioned by another study which failed to replicate them even when taking into account both item difficulty and valence (Radke and de Bruijn, 2015). Moreover, there are also notable differences between the MET and RMET with the images in the MET including more complex natural scenes and emotions conveyed by multiple cues (face, body posture, and context) whereas in the RMET emotions are only interpreted from pictures of eye regions and are also often more subtle. Thus, OXT can facilitate CE in some contexts, particularly with cues restricted to eyes, but not in others where multiple cues are present. Additionally, and in contrast to previous studies, we measured SCR responses during trials involving the three empathy components and OXT only increased the SCR in EE and not CE trials. Thus, OXT enhancement of EE is paralleled by increased physiological arousal not only in EEI trials (where participants are asked to score how aroused they are by the stimulus picture) but also in EED trials (where they are scoring the strength of their feelings toward to protagonist in the picture).
In line with the specific, and critical contribution of the amygdala to emotional, rather than cognitive aspects of empathy (Hurlemann et al., 2010), OXT’s enhancement of EE was accompanied by a reduction of associated amygdala activity. Exploratory analyses revealed that EED scores were positively associated with the magnitude of amygdala responses during positive valence trials in the PLC group, whereas this association was absent under OXT, possibly reflecting an enhancement of amygdala processing efficiency. While some previous studies found that OXT specifically reduced amygdala responses to negative emotional stimuli (Kirsch et al., 2005; Gamer et al., 2010), the suppression of EED-associated amygdala activity was observed irrespective of valence. A similar pattern of OXT-induced valence-independent suppression of amygdala activity has previously been suggested to reflect reduced uncertainty of a social stimulus which in turn motivates approach behavior (Domes et al., 2007a). In line with this interpretation, the valence-independent EED-associated amygdala suppression may reflect that OXT’s approach-facilitating properties (Arakawa et al., 2010) promote EE regardless of whether the emotions expressed by the protagonist are positive or negative, which is also in line with a rodent study reporting an overall reduction of amygdala EEG power following OXT (Sobota et al., 2015). Other studies have found that OXT’s modulation of amygdala responses dependent upon sex (Gao et al., 2016; Luo et al., 2017) and it is generally considered that the salience of cues as well as their context may play an important role in determining OXT’s effects (Shamay-Tsoory and Abu-Akel, 2016). In the present study, neither sex nor valence influenced amygdala reactivity. This possibly reflects the fact that both salience and context are broadly similar for EE responses in the two sexes.
Oxytocin also differentially altered the functional connectivity between the right amygdala and bilateral insula in a valence-dependent manner. In EED trials, the strength of the functional connectivity between the right amygdala and insula following OXT was significantly increased during positive valence stimuli but decreased during negative ones. A few previous studies have also reported OXT effects on functional connectivity between the insula and amygdala (Rilling et al., 2012; Striepens et al., 2012; Hu et al., 2015; Gao et al., 2016) and these two regions are key hubs of the brain salience network (Uddin, 2015). Thus, in the current context OXT may have acted to increase the salience of both positive and negative valence stimuli during EED trials by differentially altering the functional connectivity between the amygdala and insula. Rilling et al. (2012) have also previously suggested that the stronger the functional coupling between amygdala and insula, the more the amygdala is able to elicit subjective feeling states in response to salient social stimuli.
The effect of OXT on increasing functional connectivity between the right amygdala and bilateral PCC for positive valence stimuli and decreasing it for negative ones in EED trials may similarly reflect a modulatory influence on salience processing. A previous study has reported that OXT enhanced functional connectivity between amygdala and PCC during exposure to infant laughter (Riem et al., 2012), suggesting that it increased the incentive salience of infant laughter. In our current study, the consistent patterns of functional connectivity changes elicited by OXT for positive and negative valence stimuli for amygdala functional connectivity with the insula and PCC may indicate that these three regions comprise an integrated network mediating valence-dependent OXT effects.
Both the behavioral and neural effects of OXT were modified to some extent by trait autism scores, as measured by the ASQ. In both experiments OXT tended to produce a negative correlation between EE and ASQ scores, whereas this correlation was absent in the PLC group. However, this effect of OXT only achieved significance in Exp 1, which included only male participants, and indicates that increased EE scores were more evident in individuals with lower ASQ scores. For the neural associations, functional connectivity between the amygdala and insula was positively associated with total ASQ scores for positive valence EE trials in the PLC group, but this was absent in the OXT group. This indicates that OXT effects on functional connections between the right amygdala and left insula (for positive valence stimuli) were also strongest in individuals with lower ASQ scores. Thus overall, while both behavioral and neural OXT effects on EE were modified by ASQ scores, the extent to which these findings represent support for possible therapeutic use in ASD remains unclear. Indeed, a recent study on OXT enhancement of behavioral and neural responses to affective touch also reported stronger effects in individuals with lower ASQ scores (Scheele et al., 2014).
There are several limitations which should be acknowledged in the current study. Firstly, we were unable to directly compare behavioral and neural responses during the MET task in Caucasian as well Chinese participants, so we cannot totally exclude the possibility that some cultural differences in response to OXT during empathic processing may exist. Secondly, we only investigated effects using the MET paradigm and it is possible that OXT effects on CE as well as EE would have been found using other paradigms. Thirdly, no endogenous levels of OXT were assessed in the present study. Comparing the PLC-treated subjects between EXP 1 and EXP 2 suggests that the scanner environment per se may have had an influence on EE ratings, possibly due to elevated stress levels in the MRI environment. Given that previous studies reported endogenous OXT release in response to stress (Lang et al., 1983; Jong et al., 2015) we cannot completely rule out interactions between differential endogenous OXT levels in EXP 1 and EXP 2 and treatment. Lastly, the absence of sex-differences in OXT effects in the current study might have been contributed to by our Chinese male and female participants exhibiting similar EE scores, in contrast to Caucasian participants (Hurlemann et al., 2010), and also similar ASQ scores.
In summary, in the current study we have shown that in the MET paradigm, OXT enhances EE but not CE in Chinese participants, similar to Caucasian ones, and additionally that this occurs in female as well as male participants. Furthermore, we have shown for the first time that this EE effect of OXT is associated with decreased amygdala responses and differentially altered functional connectivity between the amygdala and insula and PCC for positive and negative valence stimuli. Finally, we have shown that both behavioral and neural effects of OXT are modified to some extent by trait autism scores, although behavioral and functional connectivity effects were strongest in individuals with lower scores.
Author Contributions
YG, RH, and KK designed the experiments. YG collected the data. YG, WZ, FZ, KK, and BB analyzed the data. YG, WZ, XM, SY, RH, BB, and KK interpreted the results. YG, BB, and KK wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This study was supported by the National Natural Science Foundation of China (NSFC) (Grant 31530032); Fundamental Research Funds for Central Universities (Grant ZYGX2015Z002) and the Science, Innovation and Technology Department of the Sichuan Province (Grant 2018JY0001).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2018.00512/full#supplementary-material
References
- Arakawa H., Arakawa K., Deak T. (2010). Oxytocin and vasopressin in the medial amygdala differentially modulate approach and avoidance behavior toward illness-related social odor. Neuroscience 171 1141–1151. 10.1016/j.neuroscience.2010.10.013 [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M. J., van IJzendoorn M. H. (2013). Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl. Psychiatry 3:e258. 10.1038/tp.2013.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S. (2004). The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J. Autism Dev. Disord. 34 163–175. 10.1023/b:jadd.0000022607.19833.00 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. (2001). The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 31 5–17. 10.1023/A:1005653411471 [DOI] [PubMed] [Google Scholar]
- Bartz J. A., Zaki J., Bolger N., Hollander E., Ludwig N. N., Kolevzon A., et al. (2010). Oxytocin selectively improves empathic accuracy. Psychol. Sci. 21 1426–1428. 10.1177/0956797610383439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. T., Ward C. H., Mendelson M., Mock J., Erbaugh J. (1961). An inventory for measuring depression. Arch. Gen. Psychiatry 4 561–571. 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- Bernhardt B. C., Singer T. (2012). The neural basis of empathy. Annu. Rev. Neurosci. 35 1–23. 10.1146/annurev-neuro-062111-150536 [DOI] [PubMed] [Google Scholar]
- Bos P. A., Montoya E. R., Hermans E. J., Keysers C., van Honk J. (2015). Oxytocin reduces neural activity in the pain circuitry when seeing pain in others. Neuroimage 113 217–224. 10.1016/j.neuroimage.2015.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J.-L., Valabregue R., Poline J.-B. (2002). Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage 16:S497. [Google Scholar]
- Cao Y., Contreras-Huerta L. S., McFadyen J., Cunnington R. (2015). Racial bias in neural response to others’ pain is reduced with other-race contact. Cortex 70 68–78. 10.1016/j.cortex.2015.02.010 [DOI] [PubMed] [Google Scholar]
- Cardoso C., Ellenbogen M. A., Orlando M. A., Bacon S. L., Joober R. (2013). Intranasal oxytocin attenuates the cortisol response to physical stress: a dose-response study. Psychoneuroendocrinology 38 399–407. 10.1016/j.psyneuen.2012.07.013 [DOI] [PubMed] [Google Scholar]
- Chen X., Hackett P. D., DeMarco A. C., Feng C., Stair S., Haroon E., et al. (2016). Effects of oxytocin and vasopressin on the neural response to unreciprocated cooperation within brain regions involved in stress and anxiety in men and women. Brain Imaging Behav. 10 581–593. 10.1007/s11682-015-9411-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. L., Uddin L. Q., Di Martino A., Castellanos F. X., Milham M. P., Kelly C. (2012). The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain’s intrinsic functional dynamics. Soc. Cogn. Affect. Neurosci. 7 727–737. 10.1093/scan/nsr051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu C. K., Kret M. E. (2016). Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biol. Psychiatry 79 165–173. 10.1016/j.biopsych.2015.03.020 [DOI] [PubMed] [Google Scholar]
- Decety J. (2011). The neuroevolution of empathy. Ann. N. Y. Acad. Sci. 1231 35–45. 10.1111/j.1749-6632.2011.06027.x [DOI] [PubMed] [Google Scholar]
- Decety J., Jackson P. L. (2004). The functional architecture of human empathy. Behav. Cogn. Neurosci. Rev. 3 71–100. 10.1177/1534582304267187 [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Glascher J., Buchel C., Braus D. F., Herpertz S. C. (2007a). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol. Psychiatry 62 1187–1190. 10.1016/j.biopsych.2007.03.025 [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Michel A., Berger C., Herpertz S. C. (2007b). Oxytocin improves “mind-reading” in humans. Biol. Psychiatry 61 731–733. [DOI] [PubMed] [Google Scholar]
- Domes G., Hollerbach P., Vohs K., Mokros A., Habermeyer E. (2013). Emotional empathy and psychopathy in offenders: an experimental study. J. Personal. Disord. 27 67–84. 10.1521/pedi.2013.27.1.67 [DOI] [PubMed] [Google Scholar]
- Dziobek I., Rogers K., Fleck S., Bahnemann M., Heekeren H. R., Wolf O. T., et al. (2008). Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the multifaceted empathy test (MET). J. Autism Dev. Disord. 38 464–473. 10.1007/s10803-007-0486-x [DOI] [PubMed] [Google Scholar]
- Edele A., Dziobek I., Keller M. (2013). Explaining altruistic sharing in the dictator game: the role of affective empathy, cognitive empathy, and justice sensitivity. Learn. Individ. Differ. 24 96–102. 10.1016/j.lindif.2012.12.020 [DOI] [Google Scholar]
- Eklund A., Nichols T. E., Knutsson H. (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U.S.A. 113 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Duncan N. W., de Greck M., Northoff G. (2011). Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci. Biobehav. Rev. 35 903–911. 10.1016/j.neubiorev.2010.10.009 [DOI] [PubMed] [Google Scholar]
- Feeser M., Fan Y., Weigand A., Hahn A., Gärtner M., Böker H., et al. (2015). Oxytocin improves mentalizing–pronounced effects for individuals with attenuated ability to empathize. Psychoneuroendocrinology 53 223–232. 10.1016/j.psyneuen.2014.12.015 [DOI] [PubMed] [Google Scholar]
- Gamer M., Zurowski B., Buchel C. (2010). Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc. Natl. Acad. Sci. U.S.A. 107 9400–9405. 10.1073/pnas.1000985107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Becker B., Luo L., Geng Y., Zhao W., Yin Y., et al. (2016). Oxytocin, the peptide that bonds the sexes also divides them. Proc. Natl. Acad. Sci. U.S.A. 113 7650–7654. 10.1073/pnas.1602620113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella A. J., Hickie I. B., McGuinness M. M., Otis M., Woods E. A., Disinger H. M., et al. (2013). Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology 38 612–625. 10.1016/j.psyneuen.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Herpertz S. C., Bertsch K. (2014). The social-cognitive basis of personality disorders. Curr. Opin. Psychiatry 27 73–77. 10.1097/YCO.0000000000000026 [DOI] [PubMed] [Google Scholar]
- Herpertz S. C., Bertsch K. (2016). Oxytocin effects on brain functioning in humans. Biol. Psychiatry 79 631–632. 10.1016/j.biopsych.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Hillis A. E. (2014). Inability to empathize: brain lesions that disrupt sharing and understanding another’s emotions. Brain 137 981–997. 10.1093/brain/awt317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. H., Qi S., Becker B., Luo L. Z., Gao S., Gong Q. Y., et al. (2015). Oxytocin selectively facilitates learning with social feedback and increases activity and functional connectivity in emotional memory and reward processing regions. Hum. Brain Mapp. 36 2132–2146. 10.1002/hbm.22760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R., Patin A., Onur O. A., Cohen M. X., Baumgartner T., Metzler S., et al. (2010). Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J. Neurosci. 30 4999–5007. 10.1523/JNEUROSCI.5538-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessica L. Y., Sasaki J. Y., Keiko I., Shinada M., Kim H. S. (2016). Gene-culture interaction influence of culture and oxytocin receptor gene polymorphism on loneliness. Cult. Brain 4 21–37. 10.1007/s40167 [DOI] [Google Scholar]
- Jong T. R., Menon R., Bludau A., Grund T., Biermeier V., Klampfl S. M., et al. (2015). Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: the Regensburg oxytocin challenge (ROC) study. Psychoneuroendocrinology 62 381–388. 10.1016/j.psyneuen.2015.08.027 [DOI] [PubMed] [Google Scholar]
- Kawamura Y., Liu X., Shimada T., Otowa T., Kakiuchi C., Akiyama T., et al. (2011). Association between oxytocin receptor gene polymorphisms and autistic traits as measured by the Autism-Spectrum Quotient in a non-clinical Japanese population. Asia Pac. Psychiatry 3 128–136. 10.1111/j.1758-5872.2011.00132.x [DOI] [Google Scholar]
- Kim H. S., Sherman D. K., Sasaki J. Y., Xu J., Chu T. Q., Ryu C., et al. (2010). Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proc. Natl. Acad. Sci. U.S.A. 107 15717–15721. 10.1073/pnas.1010830107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P., Esslinger C., Chen Q., Mier D., Lis S., Siddhanti S., et al. (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 25 11489–11493. 10.1523/JNEUROSCI.3984-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54 2492–2502. 10.1016/j.neuroimage.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Lamm C., Nusbaum H. C., Meltzoff A. N., Decety J. (2007). What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One 2:e1292 10.1371/journal.pone.0001292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. E., Heil J. W., Ganten D., Hermann K., Unger T., Rascher W. (1983). Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology 37 314–316. 10.1159/000123566 [DOI] [PubMed] [Google Scholar]
- Lee J., Zaki J., Harvey P. O., Ochsner K., Green M. F. (2011). Schizophrenia patients are impaired in empathic accuracy. Psychol. Med. 41 2297–2304. 10.1017/S0033291711000614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R., Oishi K., Hsu J., Lindquist M., Gottesman R. F., Jarso S., et al. (2013). Acute lesions that impair affective empathy. Brain 136 2539–2549. 10.1093/brain/awt177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Becker B., Geng Y., Zhao Z., Gao S., Zhao W., et al. (2017). Sex-dependent neural effect of oxytocin during subliminal processing of negative emotion faces. Neuroimage 162 127–137. 10.1016/j.neuroimage.2017.08.079 [DOI] [PubMed] [Google Scholar]
- Luo S. Y., Li B. F., Ma Y., Zhang W. X., Rao Y., Han S. H. (2015a). Oxytocin receptor gene and racial ingroup bias in empathy-related brain activity. Neuroimage 110 22–31. 10.1016/j.neuroimage.2015.01.042 [DOI] [PubMed] [Google Scholar]
- Luo S. Y., Ma Y. N., Liu Y., Li B. F., Wang C. B., Shi Z. H., et al. (2015b). Interaction between oxytocin receptor polymorphism and interdependent culture values on human empathy. Soc. Cogn. Affect. Neurosci. 10 1273–1281. 10.1093/scan/nsv019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren D. G., Ries M. L., Xu G., Johnson S. C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 61 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers M., Li M., Chen Y., Zhang W., Montag C. (2015). Low empathy is associated with problematic use of the Internet: empirical evidence from China and Germany. Asian J. Psychiatry 17 56–60. 10.1016/j.ajp.2015.06.019 [DOI] [PubMed] [Google Scholar]
- Montag C., Sindermann C., Melchers M., Jung S., Luo R., Becker B., et al. (2017). A functional polymorphism of the OXTR gene is associated with autistic traits in Caucasian and Asian populations. Am. J. Med. Genet. B Neuropsychiatr. Genet. 174 808–816. 10.1002/ajmg.b.32596 [DOI] [PubMed] [Google Scholar]
- Panksepp J., Panksepp J. B. (2013). Toward a cross-species understanding of empathy. Trends Neurosci. 36 489–496. 10.1016/j.tins.2013.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penton-Voak I. S., Perrett D. I., Castles D. L., Kobayashi T., Burt D. M., Murray L. K., et al. (1999). Menstrual cycle alters face preference. Nature 399 741–742. 10.1038/21557 [DOI] [PubMed] [Google Scholar]
- Pfundmair M., Aydin N., Frey D., Echterhoff G. (2014). The interplay of oxytocin and collectivistic orientation shields against negative effects of ostracism. J. Exp. Soc. Psychol. 55 246–251. 10.1016/j.jesp.2014.07.016 [DOI] [Google Scholar]
- Quintana D. S., Westlye L. T., Alnaes D., Rustan O. G., Kaufmann T., Smerud K. T., et al. (2016). Low dose intranasal oxytocin delivered with Breath Powered device dampens amygdala response to emotional stimuli: a peripheral effect-controlled within-subjects randomized dose-response fMRI trial. Psychoneuroendocrinology 69 180–188. 10.1016/j.psyneuen.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Quintana D. S., Westlye L. T., Hope S., Naerland T., Elvsashagen T., Dorum E., et al. (2017). Dose-dependent social-cognitive effects of intranasal oxytocin delivered with novel Breath Powered device in adults with autism spectrum disorder: a randomized placebo-controlled double-blind crossover trial. Transl. Psychiatry 7:e1136. 10.1038/tp.2017.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke S., de Bruijn E. R. (2015). Does oxytocin affect mind-reading? A replication study. Psychoneuroendocrinology 60 75–81. 10.1016/j.psyneuen.2015.06.006 [DOI] [PubMed] [Google Scholar]
- Reniers R., Corcoran R., Drake R., Shryane N., Vollm B. (2010). The QCAEP: a questionnaire of cognitive and affective empathy. J. Pers. Assess. 93 84–95. 10.1080/00223891.2010.528484 [DOI] [PubMed] [Google Scholar]
- Riem M. M., van IJzendoorn M. H., Tops M., Boksem M. A., Rombouts S. A., Bakermans-Kranenburg M. J. (2012). No laughing matter: intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology 37 1257–1266. 10.1038/npp.2011.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem M. M. E. (2012). Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol. Psychiatry 70 660–660. [DOI] [PubMed] [Google Scholar]
- Riem M. M. E., Bakermans-Kranenburg M. J., Voorthuis A., van IJzendoorn M. H. (2014). Oxytocin effects on mind-reading are moderated by experiences of maternal love withdrawal: an fMRI study. Prog. Neuro Psychopharmacol. Biol. Psychiatry 51 105–112. 10.1016/j.pnpbp.2014.01.014 [DOI] [PubMed] [Google Scholar]
- Rilling J. K., DeMarco A. C., Hackett P. D., Thompson R., Ditzen B., Patel R., et al. (2012). Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology 37 447–461. 10.1016/j.psyneuen.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues S. M., Saslow L. R., Garcia N., John O. P., Keltner D. (2009). Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc. Natl. Acad. Sci. U.S.A. 106 21437–21441. 10.1073/pnas.0909579106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld A. J., Lieberman J. A., Jarskog L. F. (2011). Oxytocin, dopamine, and the amygdala: a neurofunctional model of social cognitive deficits in schizophrenia. Schizophr. Bull. 37 1077–1087. 10.1093/schbul/sbq015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D., Kendrick K. M., Khouri C., Kretzer E., Schlapfer T. E., Stoffel-Wagner B., et al. (2014). An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology 39 2078–2085. 10.1038/npp.2014.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Ruther M., Markowitsch H. J., Fink G. R., Piefke M. (2007). Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. J. Cogn. Neurosci. 19 1354–1372. 10.1162/jocn.2007.19.8.1354 [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S. G. (2011). The neural bases for empathy. Neuroscientist 17 18–24. 10.1177/1073858410379268 [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S. G., Abu-Akel A. (2016). The social salience hypothesis of oxytocin. Biol. Psychiatry 79 194–202. 10.1016/j.biopsych.2015.07.020 [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S. G., Abu-Akel A., Palgi S., Sulieman R., Fischer-Shofty M., Levkovitz Y., et al. (2013). Giving peace a chance: oxytocin increases empathy to pain in the context of the Israeli-Palestinian conflict. Psychoneuroendocrinology 38 3139–3144. 10.1016/j.psyneuen.2013.09.015 [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S. G., Aharon-Peretz J., Levkovitz Y. (2007). The neuroanatomical basis of affective mentalizing in schizophrenia: comparison of patients with schizophrenia and patients with localized prefrontal lesions. Schizophr. Res. 90 274–283. 10.1016/j.schres.2006.09.020 [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S. G., Aharon-Peretz J., Perry D. (2009). Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 132(Pt 3), 617–627. 10.1093/brain/awn279 [DOI] [PubMed] [Google Scholar]
- Singer T., Lamm C. (2009). The social neuroscience of empathy. Ann. N. Y. Acad. Sci. 1156 81–96. 10.1111/j.1749-6632.2009.04418.x [DOI] [PubMed] [Google Scholar]
- Singer T., Snozzi R., Bird G., Petrovic P., Silani G., Heinrichs M., et al. (2008). Effects of oxytocin and prosocial behavior on brain responses to direct and vicariously experienced pain. Emotion 8 781–791. 10.1037/a0014195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick S. D. (2017). Cluster success: fMRI inferences for spatial extent have acceptable false-positive rates. Cogn. Neurosci. 8 150–155. 10.1080/17588928.2017.1319350 [DOI] [PubMed] [Google Scholar]
- Smith K. E., Porges E. C., Norman G. J., Connelly J. J., Decety J. (2014). Oxytocin receptor gene variation predicts empathic concern and autonomic arousal while perceiving harm to others. Soc. Neurosci. 9 1–9. 10.1080/17470919.2013.863223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobota R., Mihara T., Forrest A., Featherstone R. E., Siegel S. J. (2015). Oxytocin reduces amygdala activity, increases social interactions, and reduces anxiety-like behavior irrespective of NMDAR antagonism. Behav. Neurosci. 129 389–398. 10.1037/bne0000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler F. B., Schultz J., Scheele D., Essel M., Maier W., Heinrichs M., et al. (2017). Kinetics and dose dependency of intranasal oxytocin effects on amygdala reactivity. Biol. Psychiatry 82 885–894. 10.1016/j.biopsych.2017.04.015 [DOI] [PubMed] [Google Scholar]
- Spielberger C. D., Gorsuch R. L., Lushene R. E. (1970). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stern R. M., Ray W. J., Quigley K. S. (2001). Psychophysiological Recording. Oxford: Oxford University Press. [Google Scholar]
- Striepens N., Kendrick K. M., Maier W., Hurlemann R. (2011). Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front. Neuroendocrinol. 32 426–450. 10.1016/j.yfrne.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Striepens N., Scheele D., Kendrick K. M., Becker B., Schafer L., Schwalba K., et al. (2012). Oxytocin facilitates protective responses to aversive social stimuli in males. Proc. Natl. Acad. Sci. U.S.A. 109 18144–18149. 10.1073/pnas.1208852109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J., Gabay A. S., Brown D., Murphy D. G. M., Blackwood N. (2018). The effect of intranasal oxytocin on neural response to facial emotions in healthy adults as measured by functional MRI: a systematic review. Psychiatry Res. 272 17–29. 10.1016/j.pscychresns.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 16 55–61. 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L. A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Weisman O., Pelphrey K. A., Leckman J. F., Feldman R., Lu Y., Chong A., et al. (2015). The association between 2D:4D ratio and cognitive empathy is contingent on a common polymorphism in the oxytocin receptor gene (OXTR rs53576). Psychoneuroendocrinology 58 23–32. 10.1016/j.psyneuen.2015.04.007 [DOI] [PubMed] [Google Scholar]
- Wigton R., Radua J., Allen P., Averbeck B., Meyer-Lindenberg A., McGuire P., et al. (2015). Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. J. Psychiatry Neurosci. 40 E1–E22. 10.1503/jpn.130289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingenfeld K., Kuehl L. K., Janke K., Hinkelmann K., Dziobek I., Fleischer J., et al. (2014). Enhanced emotional empathy after mineralocorticoid receptor stimulation in women with borderline personality disorder and healthy women. Neuropsychopharmacology 39 1799–1804. 10.1038/npp.2014.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf O. T., Schulte J. M., Drimalla H., Hamacher-Dang T. C., Knoch D., Dziobek I. (2015). Enhanced emotional empathy after psychosocial stress in young healthy men. Stress 18 631–637. 10.3109/10253890.2015.1078787 [DOI] [PubMed] [Google Scholar]
- Wong C.-S., Law K. S. (2002). The effects of leader and follower emotional intelligence on performance and attitude: an exploratory study. Leadersh. Q. 13 243–274. 10.1016/S1048-9843(02)00099-1 [DOI] [Google Scholar]
- Wu N., Li Z., Su Y. J. (2012). The association between oxytocin receptor gene polymorphism (OXTR) and trait empathy. J. Affect. Disord. 138 468–472. 10.1016/j.jad.2012.01.009 [DOI] [PubMed] [Google Scholar]
- Xu L., Ma X., Zhao W., Luo L., Yao S., Kendrick K. M. (2015). Oxytocin enhances attentional bias for neutral and positive expression faces in individuals with higher autistic traits. Psychoneuroendocrinology 62 352–358. 10.1016/j.psyneuen.2015.09.002 [DOI] [PubMed] [Google Scholar]
- Xu X., Yao S., Xu L., Geng Y., Zhao W., Ma X., et al. (2017). Oxytocin biases men but not women to restore social connections with individuals who socially exclude them. Sci. Rep. 7:40589. 10.1038/srep40589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. J., Barrett C. E. (2015). Neuroscience. Can oxytocin treat autism? Science 347 825–826. 10.1126/science.aaa8120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Geng Y., Luo L., Zhao Z., Ma X., Xu L., et al. (2017). Oxytocin increases the perceived value of both self- and other-owned items and alters medial prefrontal cortex activity in an endowment task. Front. Hum. Neurosci. 11:272. 10.3389/fnhum.2017.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.