Abstract
The dopaminergic system has been shown to have substantial effects on the etiology of attention-deficit hyperactivity disorder (ADHD). However, while some studies found a significant direct effect, others did not. In this context, social behavior might play an important role as a factor that is related both to the dopaminergic system and ADHD. In a large epidemiological sample of adolescents (N = 462; 16–17 years), we assessed the level of ADHD symptoms using the Strengths and Difficulties Questionnaire, social behavior using the Social Responsiveness Scale, and the allelic distribution of the dopaminergic catechol-O-methyltransferase (COMT) Val158Met polymorphism. We found a significant association between COMT and social impairment, insofar as Met-allele carriers showed increased levels of social impairment. Moreover, social impairment significantly determined an association between COMT and ADHD (explained variance: 19.09%). This effect did not significantly differ between males and females. COMT and social impairment might interactively affect ADHD symptomatology, and could thus represent significant gene-phenotypic risk factors for ADHD symptomatology. This might have interesting implications for prevention and intervention strategies with a focus on social behavior in genetically at-risk individuals.
Keywords: ADHD, COMT, social impairment, adolescence, moderation
Introduction
Attention-deficit hyperactivity disorder (American Psychiatric Association [APA], 2013), characterized by inattention, impulsivity, and/or hyperactivity, is one of the most common neurodevelopmental disorders in childhood. In clinical, epidemiological, and behavioral genetic studies impairments in social behavior have frequently been found in ADHD patients (Thapar et al., 2005a; Hoza, 2007; Caspi et al., 2008; McQuade and Hoza, 2008; Andrade and Tannock, 2013; Bunford et al., 2015). Although social impairment has not been considered a core feature of the disorder, it is an important secondary characteristic of ADHD that has implications in real-world functioning (Hoza, 2007; McQuade and Hoza, 2008; Andrade and Tannock, 2014; Bunford et al., 2015) and is identified as a marker for the heterogeneity of the disorder (Caspi et al., 2008). The most pervasive and persistent impairments in children with ADHD are difficulties in peer interactions and experiences of peer rejections (Nixon, 2001; Hoza et al., 2005; Hoza, 2007; Ronk et al., 2011; Janssens et al., 2017). As a consequence, ADHD children might not have enough opportunities to practice social interactions, which can in turn increase socially immature behavior and lead to fewer prosocial skills (Dodge et al., 2003; Hoza, 2007; Tseng et al., 2014).
There is evidence that impairment in social behavior in ADHD is partly determined by common genetic factors (Nadder et al., 2002) for example the catechol O-methyltransferase gene (COMT) (Thapar et al., 2005b; Caspi et al., 2008). The COMT gene is located on chromosome 22q11.2 (Winqvist et al., 1992) and codes for an enzyme involved in one of the major degradative pathways of the catecholaminergic neurotransmitters. One common single-nucleotide polymorphism (SNP) which is due to a guanine to adenine transition at codon 158 and results in a valine-to-methionine substitution leads to a three- to fourfold difference in enzyme activity and as a consequence to a higher dopaminergic state (Lachman et al., 1996). Previous research has demonstrated direct associations between this COMT polymorphism and hyperactivity as well as inattention symptoms, traits, and behavior. However, so far, results have been heterogenous. Some studies reported a significant effect of the Val allele (Akutagava-Martins et al., 2016), which was found to be more frequent in children with ADHD compared to healthy control individuals (Qian et al., 2003; Song et al., 2009) or to be related to inferior frontal cortex response to failed inhibitory behavior (White et al., 2014). Others reported a significant effect of the Met allele being preferentially transmitted to ADHD (Qian et al., 2003). The discrepant findings may be partly explained by the use of different analytic approaches in the aforementioned studies.
Interestingly, has not only ADHD been associated with changes in the dopaminergic system, but the regulation of social behavior in general has also been shown to be determined by dopaminergic functioning (Montag et al., 2008; Yacubian and Buchel, 2009; Mier et al., 2010; Skuse and Gallagher, 2011). With respect to COMT, carriers of the Val allele, and thus individuals with enhanced COMT enzyme activity, showed an increase in social cooperative behavior and a stronger response to social interactions and experiences compared to Met/Met-allele carriers (Reuter and Hennig, 2005; Walter et al., 2011). Moreover, dopaminergic augmentation via COMT inhibition was found to be associated with increased egalitarian tendencies (Saez et al., 2015).
So far, research has mostly focused on direct associations between ADHD and social problems, almost not considering potential mediating effects. Results of a large epidemiological study in healthy children (Langley et al., 2010) suggest social impairment as an intermediate phenotype explaining the association between COMT and antisocial behavior in ADHD. In line with prior findings (Caspi et al., 2008) impaired social understanding mediated the link between COMT and impaired social behavior in children with higher scores of ADHD (Langley et al., 2010). Less efficient processing of the prefrontal cortex (PFC) and a resulting impairment in executive functioning as well as emotional dysregulation were discussed by the authors as the assumed mechanisms underlying this interaction (Caspi et al., 2008). Recently, van Goozen et al. (2016) reported a significant indirect effect of the COMT Val allele on aggressive behavior in ADHD patients who were mediated by social/emotional mechanisms, but not by deficits in executive functioning. They specifically identified impaired fear learning and fear empathy as critical risk mechanisms in this context.
Due to the inconsistent findings in the literature, further studies are needed to gain a deeper understanding of the underlying mechanisms and effects of the association between COMT and ADHD, and to unravel the possible role of additional factors that might bear any influence of COMT on ADHD symptomatology. The investigation of gene–phenotype interactions in this context may add to previous findings and could help to identify vulnerable phenotypes for ADHD symptomatology. Although social behavior has been identified as one critical factor in ADHD, and it is also associated with the dopaminergic system, we have little information on the interaction between dopaminergic genetic predispositions, social impairment, and ADHD symptomatology.
Because of the continuous nature of ADHD symptoms and impairments (Chen et al., 2008; Larsson et al., 2012) we examined the effects of the COMT gene Val158Met polymorphism on the degree of ADHD symptoms and on social behavior, as well as their interactions in a large epidemiological sample of adolescents.
Materials and Methods
Subjects and Recruitment
The subjects of the present study were part of the European Imaging Genetics (IMAGEN) study (Schumann et al., 2010), a study in a large population-based sample of adolescents. Participants were recruited via school visits, flyers, and residents’ registration offices in Germany, the United Kingdom, Ireland, and France. The present study used data from N = 462 adolescents (242 female) at the age of 16–17 years. Data from the sample assessed in France were not analyzed (N = 96), because no French validation was available for one of our measures, the Social Responsiveness Scale (SRS) (see below for details) (Supplementary Figure S1).
Exclusion criteria for participation in the study were: any mental disorder as defined by the Development and Well-Being Assessment (DAWBA) (Goodman et al., 2000), IQ < 80, alcohol use disorder, serious medical conditions, and previous head trauma with unconsciousness. The study was approved by the local ethics committees and was conducted in accordance with the Declaration of Helsinki. After explaining the study to the adolescents and their legal guardians, written informed consent was obtained.
Measures
Deoxyribonucleic Acid (DNA) Extraction and Genotyping
Deoxyribonucleic acid was extracted from venous blood samples. To ensure high quality and sufficient quantity, DNA extraction was performed by a semi-automated process. All samples were part of a genome-wide genotyping of ∼600,000 autosomal SNPs within the IMAGEN study using the Illumina Quad 610 chips (Illumina, San Diego, CA, United States).
The genotype frequencies of COMT Val158Met in the sample were as follows: Val/Val: n = 93, Val/Met: n = 243, and Met/Met: n = 126. The genotype distribution did not differ according to sex (χ2 = 3.324, df = 2, p = 0.190) or site (χ2 = 9.800, df = 12, p = 0.654).
ADHD Symptoms
To assess ADHD symptom strength, we used the hyperactivity scale from the Strengths and Difficulties Questionnaire (SDQ) (Goodman, 1997), a screening instrument used to detect behavioral and psychosocial problems in children aged 4–17 years. The SDQ is a well-validated instrument, which includes five dimensions: emotional symptoms, conduct problems, hyperactivity/inattention problems, peer problems, and prosocial behavior, which can either be used as continuous variables (scores 0–10) or as categories (for detailed information: www.sdqinfo.org). For the present analyses, we only used the hyperactivity/inattention problem scale, with the total hyperactivity score as dimensional variable [sum of the five hyperactivity items (self-ratings)] (see Tables 1, 2).
Table 1.
Distribution of ADHD symptoms in the current sample.
| SDQ-scores for hyperactivity/ | Frequency | Original 3-band |
|---|---|---|
| inattention problem scale | categorization | |
| 0 | 43 | |
| 1 | 48 | |
| 2 | 76 | Normal N = 382 |
| 3 | 86 | |
| 4 | 79 | |
| 5 | 50 | |
| 6 | 35 | Borderline N = 35 |
| 7 | 26 | |
| 8 | 12 | Abnormal N = 45 |
| 9 | 7 | |
| Total | 462 | |
Table 2.
Sample characteristics for the whole group and separately for males and females.
| Males (N = 220) | Females (N = 242) | Total (N = 462) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | 16.39 | (0.43) | 16.43 | (0.44) | 16.41 | (0.43) | |||
| ADHD symptom strength | 3.09 | (2.17) | 3.67 | (2.10) | 3.40 | (2.15) | |||
| Range of ADHD symptom scores | 0–9 | 0–9 | |||||||
| Social impairment | 24.78 | (15.94) | 24.51 | (16.45) | 24.64 | (16.20) | |||
| Range of social impairment scores | 1–93 | 0–100 | |||||||
Data are reported as means (standard deviation).
Social Behavior/Impairment
To measure social behavior/impairment, we used the SRS (Constantino and Gruber, 2005), a widely used, well-validated scale rated by parents/teachers for use in 4–18-year-olds [for details, see Bölte et al. (2008) for the German adaptation and Constantino and Gruber (2005) for the English original version]. The 65 items focus on behavior during the past 6 months and assess engagement in reciprocal social interactions, understanding of emotional and social cues, and motivation to engage with others. Subscales include social awareness, social information processing, capacity for reciprocal social communication, social anxiety, and autistic mannerisms. The total score of social impairment was used as a continuous variable.
To assess the two questionnaires, we used the Psytools software (Delosis Ltd., London, United Kingdom) via its Internet-based platform.
Statistical Analysis
All analyses were conducted using the Predictive Analytic Software (PASW, SPSS Inc., Chicago, IL, United States) for Windows, version 24.
Effects of COMT on ADHD symptoms and social behavior: To test the direct effect of the COMT Val158Met polymorphism on ADHD symptoms and social impairment, we conducted a univariate analysis of covariance (ANCOVA), with ADHD symptoms or social impairment as dependent variables and COMT as between-subject factor with three levels (Val/Val, Val/Met, and Met/Met), adjusting for sex and conduct problems as potential confounding variables. Data were also corrected for multiple comparisons.
Interaction of COMT, social behavior, and ADHD symptoms: In a subsequent moderator analysis (Preacher and Hayes, 2008), we tested the impact of social impairment as a moderator of any effect of COMT on ADHD symptoms using the COMT Val158Met genotype as independent variable (predictor) and ADHD symptoms as dependent variable (outcome) (Baron and Kenny, 1986). Sex and conduct problems were again used as covariates. Using this analysis, associations between the predictor and the outcome, the predictor and the moderator, and the outcome and the moderator are investigated, and it is assumed that the associations between the predictor and the outcome significantly depend on a third variable, the moderator [evaluated via the Sobel (1982) test]. This analysis is based on a priori hypotheses and was thus also performed in the case of non-significant effects of COMT on ADHD symptoms (e.g., according to Shrout and Bolger, 2002).
For all analyses, p-values < 0.05 are reported.
Results
Impact of COMT Genotype on ADHD Symptoms and Social Impairment
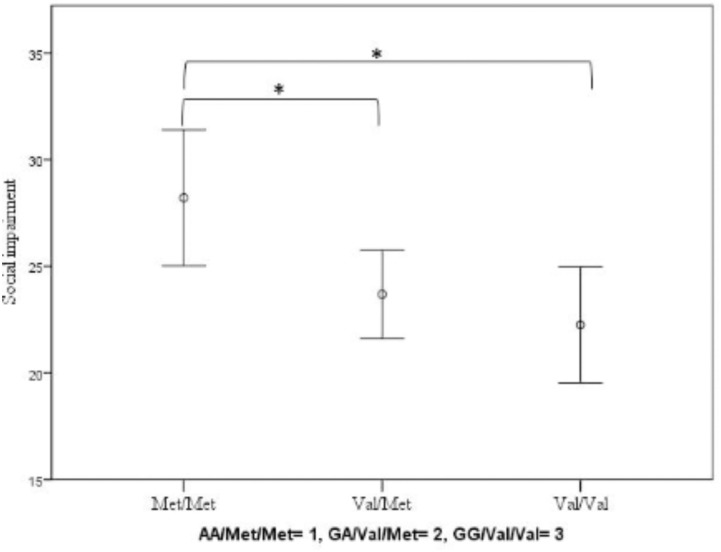
We found no significant effect of COMT on ADHD (F(2.460) = 1.537; p = 0.216; partial eta-squared = 0.007; covariates: sex: F(1.460) = 16.248; p < 0.001; partial eta-squared = 0.034; conduct problems: F(1.460) = 94.844; p < 0.001; partial eta-squared = 0.172). However, COMT did have a significant impact on the level of social impairment (F(2.460) = 4.376; p = 0.013; partial eta-squared = 0.019; covariates: sex: F(1.460) = 0.026; p = 0.873; partial eta-squared = 0.000; conduct problems: F(1.460) = 22.941; p < 0.001; partial eta-squared = 0.048): Homozygote Met-allele carriers showed increased levels of social impairment compared to Val-allele carriers (Figure 1).
FIGURE 1.
Impact of COMT Val158Met genotype on social impairment measured by the SRS. Means and error bars ±2 standard errors (SE) are shown. Significant ∗p < 0.05.
Interaction of COMT, ADHD Symptoms, and Social Impairment
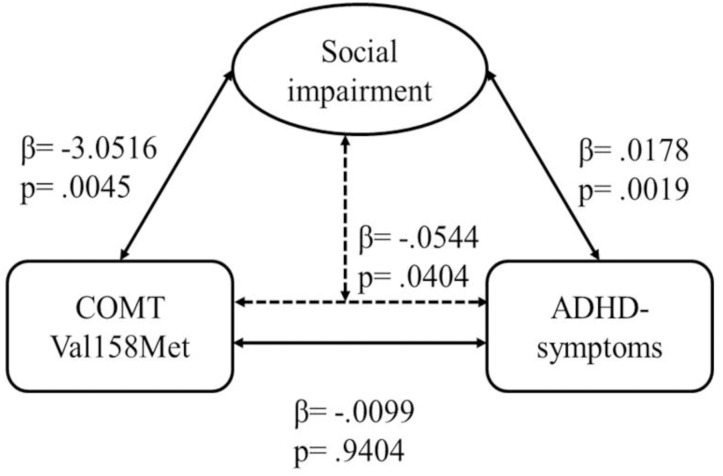
The regression model with COMT as predictor, ADHD symptoms as dependent variable, and social impairment as moderator was significant [F = 29.834; p < 0.001; explained variance of ADHD symptoms: R2 = 19.09%; female: 13.55% (p < 0.001); male: 22.32% (p < 0.001)]. There was a direct effect of COMT on social impairment (β = -3.0516; t = -2.8557; p = 0.0045), and of social impairment on ADHD symptom strength (β = 0.0178; t = 3.1175; p = 0.0019), but no effect of COMT on ADHD symptoms (β = -0.0099; t = -0.0748; p = 0.9404) (Figure 2). However, we found an indirect effect of COMT on ADHD moderated by social impairment (β = -0.0544; Z = -2.0492; p = 0.0404), and significant effects of the two covariates (sex: β = 0.7060; t = 3.9237; p = 0.0001; conduct problems: β = 0.5757; t = 8.9919; p < 0.0001).
FIGURE 2.
Moderation analysis for effects of COMT Val158Met genotype on ADHD symptom strength via social impairment. The association between COMT and ADHD symptom strength was significant when the measure of social impairment was included in the model; Sobel test: p = 0.0404.
Discussion
The dopaminergic system has substantial effects on ADHD etiology. However, so far, studies have yielded inconsistent results, with some finding a significant association and others failing to do so. These discrepant findings may be attributable to a significant effect of further factors such as social behavior, which might modulate the direct association between COMT and ADHD, and thus represent a significant moderator driving the influence of the dopaminergic system on ADHD symptomatology. In the present study, we found no significant association between the dopaminergic COMT polymorphism and ADHD symptoms, although this might depend on social impairment: The level of social impairment served as a moderator of the association between COMT and the levels of ADHD symptoms. Moreover, COMT was further directly significantly associated with social impairment.
While previous studies found positive associations of both the Val and the Met allele with ADHD symptomatology (Qian et al., 2003; Song et al., 2009); several meta-analyses reported no significant association between ADHD and Val158Met (Cheuk and Wong, 2006; Gizer et al., 2009; Lee and Song, 2015; Bonvicini et al., 2016), which is in line with our findings. Moreover, studies have yielded mixed results with respect to sex: Some reported effects of COMT Val158Met on ADHD and related symptoms or traits only in boys, while others observed the opposite results. In the present study, we did not observe any significant difference between male and female participants. This indicates that social impairment is an important general facet of ADHD that is strongly triggered by the catecholaminergic system, but not so much by sex-related biological or social constraints.
In our study, we observed a significant effect of the Met/Met genotype on social impairment, insofar as Met/Met carriers showed increased levels of impairment. Thus, higher synaptic dopamine levels following neurotransmitter release may increase social impairment and related problematic behavior. Dopaminergic systems are related to neural networks that support attentional control, salience detection, and self-referential cognition, and are associated with high levels of intrinsic motivation and reward (e.g., Gangi et al., 2016; Di Domenico and Ryan, 2017). Moreover, the Met compared to the Val/Val allele genotype has been shown to be related to increased trait anxiety (Montag et al., 2008) and higher loss-aversion behavior (Schmack et al., 2008). Such behavioral changes in, for example, responsiveness to punishment have also been found in ADHD (Tsang et al., 2015; Furukawa et al., 2017; Ruf et al., 2017). COMT and its associated functional consequences may thus serve as critical neurobiological determinants for ADHD-related risk and problem behavior. By contrast, compared to the Met genotype, the Val genotype was associated with positive emotionality and extraversion (Reuter and Hennig, 2005), and may thus serve a protective function in reducing ADHD symptomatology and related problems.
Beyond ADHD, the role of COMT is investigated in conjunction with many other mental disorders (Taylor, 2018). Moreover, social impairment even if it is immanent for ADHD (Ros and Graziano, 2018), it is not specific for ADHD, but also present in many other mental disorders (American Psychiatric Association [APA], 2013; WHO, 2016). Therefore, one further possible explanation for the association between COMT, social impairment, and ADHD might be that the association is not specific for ADHD but associated with a possible general impact resulting from mental disorders. This would be in line with the RDoC approach which aims in understanding the nature of mental health/illness not in diagnostic categories but in terms of dysfunction in general psychological systems.
Previous studies have reported that not only children with ADHD but also individuals with ADHD symptoms at subthreshold diagnostic levels have problems with peers (Hoza, 2007; McQuade and Hoza, 2008) and more often experience peer rejection, friendship problems, and peer neglect (Hodgens et al., 2000; Bagwell et al., 2001; Diamantopoulou et al., 2007; Willcutt et al., 2012). Moreover, symptoms of ADHD such as intrusiveness and salience are suggested to have an important impact on peer functioning (Pope et al., 1989; Diamantopoulou et al., 2007; Andrade and Tannock, 2013, 2014), and predicted social problems at a 2-year follow-up period (Humphreys et al., 2016). The dopaminergic system may play a critical role in this context, as it was also shown to be related to social learning (e.g., Diaconescu et al., 2017) and social motivation (Gunaydin and Deisseroth, 2014). Our present results add to these findings by demonstrating that social impairment serves as a significant moderator of ADHD symptoms in individuals who are genetically at risk through higher dopaminergic functioning. In genetically high-risk individuals, adequate behavioral assistance and guidance with respect to social relationships may thus be an important factor in order to improve ADHD symptomatology. This could be realized, for instance, through interventions to target the interpretation of affective cues to assist processes of social decision-making (Humphreys et al., 2016).
As one limitation of the present study we have to mention that our sample sizes (N = 462) is rather small for a genetic association study; thus, we were not able to further subdivide the sample to perform a replication or sex-related analyses. It could be speculated that a direct effect between COMT and ADHD – against conclusion from three meta-analyses (Cheuk and Wong, 2006; Gizer et al., 2009; Lee and Song, 2015) – could be detected in a larger sample with sufficient power. Therefore, results must be independently replicated in at least on other clinical as well as non-clinical sample and until then should be viewed as tentative. One could also criticize that we focused in our analysis on only one genetic variant and did not include other SNPs reported to be of relevance in ADHD. We based our hypothesis on former reports concerning the influence of COMT on antisocial behavior in ADHD patients (Thapar et al., 2005a; Caspi et al., 2008) and possible mediating effects (Langley et al., 2010; van Goozen et al., 2016) and thus chose to only investigate COMT effects in this context. However, it would also be of interest to include other dopaminergic genes or genes that have been identified by GWAS in ADHD samples in further investigations using larger clinical or non-clinical samples. Moreover, finding has to be treated with caution due to a possible overestimation of the genetic effect (Lohmueller et al., 2003) based on the used SNP analysis. In sum, a significant interrelation between COMT, ADHD symptoms, and social impairment in an epidemiological sample of adolescents was observed. This indicates significant gene–environment risk factors for ADHD symptomatology also at subthreshold levels, and could inform strategies to prevent or manage social problems in daily life in genetically at-risk individuals.
Author Contributions
AB, CBü, HG, PG, AH, J-LM, TP, MR, MS, HW, RW, GS, TB, and HF study design. SM, FN, SHo, SHe, CBa, SV-K, UB, EQ, JUF, and HL data assessment and recruitment. SM, FN, JOF, SD, VF, BI, M-LM, DP, LP, and TB data management and analyses. SM, FN, SHo, and TB paper writing.
Conflict of Interest Statement
TB has served as an advisor or consultant to Bristol-Myers Squibb, Desitin Arzneimittel, Eli Lilly, Medice, Novartis, Pfizer, Shire, UCB, and Vifor Pharma; he has received conference attendance support, conference support, or speaker’s fees from Eli Lilly, Janssen McNeil, Medice, Novartis, Shire, and UCB; and he is involved in clinical trials conducted by Eli Lilly, Novartis, and Shire; the present work is unrelated to these relationships. SM received conference attendance support from Shire. SHo, SHe, SV-K, AB, UB, CB, EQ, SD, HF, VF, HG, PG, AH, BI, J-LM, M-LM, DP, TP, LP, JF, MS, HW, RW, GS, and FN report no financial relationships with commercial interests.
Footnotes
Funding. This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (reinforcement-related behavior in normal brain function and psychopathology) (LSHM-CT-2007-037286); the FP7 projects IMAGEMEND (602450; IMAging GEnetics for MENtal Disorders), AGGRESSOTYPE (602805), and MATRICS (603016); the Innovative Medicine Initiative Project EU-AIMS (115300-2); the Medical Research Council Grants “Developmental pathways into adolescent substance abuse” (93558) and Consortium on Vulnerability to Externalizing Disorders and Addictions [c-VEDA] (MR/N000390/1), the Swedish funding agencies VR, FORTE, and FORMAS, the Medical Research Council and the Wellcome Trust (Behavioral and Clinical Neuroscience Institute, University of Cambridge), the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministerium für Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-1, SM 80/7-2, SFB 940/1), the National Institutes of Health, United States (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centers of Excellence.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2018.00284/full#supplementary-material
Differences between French data and data from the other IMAGEN centers were also found in the subscales social cognition, social communication, social motivation, and social awareness. Excluded French data: N = 96.
References
- Akutagava-Martins G. C., Rohde L. A., Hutz M. H. (2016). Genetics of attention-deficit/hyperactivity disorder: an update. Expert Rev. Neurother. 16 145–156. 10.1586/14737175.2016.1130626 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association [APA] (2013). Diagnostic and Statistical Manual of Mental Disorders DSM-5 5th Edn. Arlington: American Psychiatric Association; 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Andrade B. F., Tannock R. (2013). The direct effects of inattention and hyperactivity/impulsivity on peer problems and mediating roles of prosocial and conduct problem behaviors in a community sample of children. J. Atten. Disord. 17 670–680. 10.1177/1087054712437580 [DOI] [PubMed] [Google Scholar]
- Andrade B. F., Tannock R. (2014). Sustained impact of inattention and hyperactivity-impulsivity on peer problems: mediating roles of prosocial skills and conduct problems in a community sample of children. Child Psychiatry Hum. Dev. 45 318–328. 10.1007/s10578-013-0402-x [DOI] [PubMed] [Google Scholar]
- Bagwell C. L., Molina B. S., Pelham W. E., Jr., Hoza B. (2001). Attention-deficit hyperactivity disorder and problems in peer relations: predictions from childhood to adolescence. J. Am. Acad. Child Adolesc. Psychiatry 40 1285–1292. 10.1097/00004583-200111000-00008 [DOI] [PubMed] [Google Scholar]
- Baron R. M., Kenny D. A. (1986). The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 51 1173–1182. 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- Bölte S., Poustka F., Constantino J. N. (2008). Assessing autistic traits: cross-cultural validation of the social responsiveness scale (SRS). Autism Res. 1 354–363. 10.1002/aur.49 [DOI] [PubMed] [Google Scholar]
- Bonvicini C., Faraone S. V., Scassellati C. (2016). Attention-deficit hyperactivity disorder in adults: a systematic review and meta-analysis of genetic, pharmacogenetic and biochemical studies. Mol. Psychiatry 21 872–884. 10.1038/mp.2016.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunford N., Evans S. W., Becker S. P., Langberg J. M. (2015). Attention-deficit/hyperactivity disorder and social skills in youth: a moderated mediation model of emotion dysregulation and depression. J. Abnorm. Child Psychol. 43 283–296. 10.1007/s10802-014-9909-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Langley K., Milne B., Moffitt T. E., O’Donovan M., Owen M. J., et al. (2008). A replicated molecular genetic basis for subtyping antisocial behavior in children with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 65 203–210. 10.1001/archgenpsychiatry.2007.24 [DOI] [PubMed] [Google Scholar]
- Chen W., Zhou K., Sham P., Franke B., Kuntsi J., Campbell D., et al. (2008). DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B 1450–1460. 10.1002/ajmg.b.30672 [DOI] [PubMed] [Google Scholar]
- Cheuk D. K., Wong V. (2006). Meta-analysis of association between a catechol-O-methyltransferase gene polymorphism and attention deficit hyperactivity disorder. Behav. Genet. 36 651–659. 10.1007/s10519-006-9076-5 [DOI] [PubMed] [Google Scholar]
- Constantino J. N., Gruber C. P. (2005). Social Responsiveness Scale (SRS). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Di Domenico S. I., Ryan R. M. (2017). The emerging neuroscience of intrinsic motivation: a new frontier in self-determination research. Front. Hum. Neurosci. 11:145. 10.3389/fnhum.2017.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaconescu A. O., Mathys C., Weber L. A. E., Kasper L., Mauer J., Stephan K. E. (2017). Hierarchical prediction errors in midbrain and septum during social learning. Soc. Cogn. Affect. Neurosci. 12 618–634. 10.1093/scan/nsw171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantopoulou S., Rydell A. M., Thorell L. B., Bohlin G. (2007). Impact of executive functioning and symptoms of attention deficit hyperactivity disorder on children’s peer relations and school performance. Dev. Neuropsychol. 32 521–542. 10.1080/87565640701360981 [DOI] [PubMed] [Google Scholar]
- Dodge K. A., Lansford J. E., Burks V. S., Bates J. E., Pettit G. S., Fontaine R., et al. (2003). Peer rejection and social information-processing factors in the development of aggressive behavior problems in children. Child Dev. 74 374–393. 10.1111/1467-8624.7402004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa E., Alsop B., Sowerby P., Jensen S., Tripp G. (2017). Evidence for increased behavioral control by punishment in children with attention-deficit hyperactivity disorder. J. Child Psychol. Psychiatry 58 248–257. 10.1111/jcpp.12635 [DOI] [PubMed] [Google Scholar]
- Gangi D. N., Messinger D. S., Martin E. R., Cuccaro M. L. (2016). Dopaminergic variants in siblings at high risk for autism: associations with initiating joint attention. Autism Res. 9 1142–1150. 10.1002/aur.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizer I. R., Ficks C., Waldman I. D. (2009). Candidate gene studies of ADHD: a meta-analytic review. Hum. Genet. 126 51–90. 10.1007/s00439-009-0694-x [DOI] [PubMed] [Google Scholar]
- Goodman R. (1997). The strengths and difficulties questionnaire: a research note. J. Child Psychol. Psychiatry 38 581–586. 10.1111/j.1469-7610.1997.tb01545.x [DOI] [PubMed] [Google Scholar]
- Goodman R., Ford T., Richards H., Gatward R., Meltzer H. (2000). The development and well-being assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J. Child Psychol. Psychiatry 41 645–655. 10.1111/j.1469-7610.2000.tb02345.x [DOI] [PubMed] [Google Scholar]
- Gunaydin L. A., Deisseroth K. (2014). Dopaminergic dynamics contributing to social behavior. Cold Spring Harb. Symp. Quant. Biol. 79 221–227. 10.1101/sqb.2014.79.024711 [DOI] [PubMed] [Google Scholar]
- Hodgens J. B., Cole J., Boldizar J. (2000). Peer-based differences among boys with ADHD. J. Clin. Child Psychol. 29 443–452. 10.1207/S15374424JCCP2903-15 [DOI] [PubMed] [Google Scholar]
- Hoza B. (2007). Peer functioning in children with ADHD. J. Pediatr. Psychol. 32 655–663. 10.1093/jpepsy/jsm024 [DOI] [PubMed] [Google Scholar]
- Hoza B., Mrug S., Gerdes A. C., Hinshaw S. P., Bukowski W. M., Gold J. A., et al. (2005). What aspects of peer relationships are impaired in children with attention-deficit/hyperactivity disorder? J. Consult. Clin. Psychol. 73 411–423. 10.1037/0022-006X.73.3.411 [DOI] [PubMed] [Google Scholar]
- Humphreys K. L., Galan C. A., Tottenham N., Lee S. S. (2016). Impaired social decision-making mediates the association between ADHD and social problems. J. Abnorm. Child Psychol. 44 1023–1032. 10.1007/s10802-015-0095-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens A., Van Den Noortgate W., Goossens L., Verschueren K., Colpin H., Claes S., et al. (2017). Adolescent externalizing behaviour, psychological control, and peer rejection: transactional links and dopaminergic moderation. Br. J. Dev. Psychol. 35 420–438. 10.1111/bjdp.12184 [DOI] [PubMed] [Google Scholar]
- Lachman H. M., Papolos D. F., Saito T., Yu Y. M., Szumlanski C. L., Weinshilboum R. M. (1996). Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6 243–250. 10.1097/00008571-199606000-00007 [DOI] [PubMed] [Google Scholar]
- Langley K., Heron J., O’Donovan M. C., Owen M. J., Thapar A. (2010). Genotype link with extreme antisocial behavior: the contribution of cognitive pathways. Arch. Gen. Psychiatry 67 1317–1323. 10.1001/archgenpsychiatry.2010.163 [DOI] [PubMed] [Google Scholar]
- Larsson H., Anckarsater H., Rastam M., Chang Z., Lichtenstein P. (2012). Childhood attention-deficit hyperactivity disorder as an extreme of a continuous trait: a quantitative genetic study of 8,500 twin pairs. J. Child Psychol. Psychiatry 53 73–80. 10.1111/j.1469-7610.2011.02467.x [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Song G. G. (2015). BDNF 196 G/A and COMT Val158Met polymorphisms and susceptibility to ADHD: a meta-analysis. J. Atten. Disord. 22 872–877. 10.1177/1087054715570389 [DOI] [PubMed] [Google Scholar]
- Lohmueller K. E., Pearce C. L., Pike M., Lander E. S., Hirschhorn J. N. (2003). Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat. Genet. 33 177–182. 10.1038/ng1071 [DOI] [PubMed] [Google Scholar]
- McQuade J. D., Hoza B. (2008). Peer problems in attention deficit hyperactivity disorder: current status and future directions. Dev. Disabil. Res. Rev. 14 320–324. 10.1002/ddrr.35 [DOI] [PubMed] [Google Scholar]
- Mier D., Kirsch P., Meyer-Lindenberg A. (2010). Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol. Psychiatry 15 918–927. 10.1038/mp.2009.36 [DOI] [PubMed] [Google Scholar]
- Montag C., Buckholtz J. W., Hartmann P., Merz M., Burk C., Hennig J., et al. (2008). COMT genetic variation affects fear processing: psychophysiological evidence. Behav. Neurosci. 122 901–909. 10.1037/0735-7044.122.4.901 [DOI] [PubMed] [Google Scholar]
- Nadder T. S., Rutter M., Silberg J. L., Maes H. H., Eaves L. J. (2002). Genetic effects on the variation and covariation of attention deficit-hyperactivity disorder (ADHD) and oppositional-defiant disorder/conduct disorder (Odd/CD) symptomatologies across informant and occasion of measurement. Psychol. Med. 32 39–53. 10.1017/S0033291701004792 [DOI] [PubMed] [Google Scholar]
- Nixon E. (2001). The social competence of children with attention deficit hyperactivity disorder: a review of the literature. Child Adolsec. Ment. Health 6 172–180. 10.1111/1475-3588.00342 [DOI] [Google Scholar]
- Pope A. W., Bierman K. L., Mumma G. H. (1989). Relations between hyperactive and aggressive behavior and peer relations at three elementary grade levels. J. Abnorm. Child Psychol. 17 253–267. 10.1007/BF00917397 [DOI] [PubMed] [Google Scholar]
- Preacher K. J., Hayes A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 40 879–891. 10.3758/BRM.40.3.879 [DOI] [PubMed] [Google Scholar]
- Qian Q., Wang Y., Zhou R., Li J., Wang B., Glatt S., et al. (2003). Family-based and case-control association studies of catechol-O-methyltransferase in attention deficit hyperactivity disorder suggest genetic sexual dimorphism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 118B 103–109. 10.1002/ajmg.b.10064 [DOI] [PubMed] [Google Scholar]
- Reuter M., Hennig J. (2005). Association of the functional catechol-O-methyltransferase VAL158MET polymorphism with the personality trait of extraversion. Neuroreport 16 1135–1138. 10.1097/00001756-200507130-00020 [DOI] [PubMed] [Google Scholar]
- Ronk M. J., Hund A. M., Landau S. (2011). Assessment of social competence of boys with attention-deficit/hyperactivity disorder: problematic peer entry, host responses, and evaluations. J. Abnorm. Child Psychol. 39 829–840. 10.1007/s10802-011-9497-3 [DOI] [PubMed] [Google Scholar]
- Ros R., Graziano P. A. (2018). Social functioning in children with or at risk for attention deficit/hyperactivity disorder: a meta-analytic review. J. Clin. Child Adolesc. Psychol. 47 213–235. 10.1080/15374416.2016.1266644 [DOI] [PubMed] [Google Scholar]
- Ruf B. M., Bessette K. L., Pearlson G. D., Stevens M. C. (2017). Effect of trait anxiety on cognitive test performance in adolescents with and without attention-deficit/hyperactivity disorder. J. Clin. Exp. Neuropsychol. 39 434–448. 10.1080/13803395.2016.1232373 [DOI] [PubMed] [Google Scholar]
- Saez I., Zhu L., Set E., Kayser A., Hsu M. (2015). Dopamine modulates egalitarian behavior in humans. Curr. Biol. 25 912–919. 10.1016/j.cub.2015.01.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmack K., Schlagenhauf F., Sterzer P., Wrase J., Beck A., Dembler T., et al. (2008). Catechol-O-methyltransferase val158met genotype influences neural processing of reward anticipation. Neuroimage 42 1631–1638. 10.1016/j.neuroimage.2008.06.019 [DOI] [PubMed] [Google Scholar]
- Schumann G., Loth E., Banaschewski T., Barbot A., Barker G., Büchel C., et al. (2010). The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol. Psychiatry 15 1128–1139. 10.1038/mp.2010.4 [DOI] [PubMed] [Google Scholar]
- Shrout P. E., Bolger N. (2002). Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol. Methods 7 422–445. 10.1037/1082-989X.7.4.422 [DOI] [PubMed] [Google Scholar]
- Skuse D. H., Gallagher L. (2011). Genetic influences on social cognition. Pediatr. Res. 69(5 Pt 2) 85R–91R. 10.1203/PDR.0b013e318212f562 [DOI] [PubMed] [Google Scholar]
- Sobel M. (1982). “Asymptotic confidence intervals for indirect effects in structural equation models,” in Sociological Methodology ed. Leinhart S. (Washington, DC: American Sociological Association; ) 290–312. 10.2307/270723 [DOI] [Google Scholar]
- Song E. Y., Paik K. C., Kim H. W., Lim M. H. (2009). Association between catechol-O-methyltransferase gene polymorphism and attention-deficit hyperactivity disorder in Korean population. Genet. Test. Mol. Biomarkers 13 233–236. 10.1089/gtmb.2008.0110 [DOI] [PubMed] [Google Scholar]
- Taylor S. (2018). Association between COMT Val158Met and psychiatric disorders: a comprehensive meta-analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 177 199–210. 10.1002/ajmg.b.32556 [DOI] [PubMed] [Google Scholar]
- Thapar A., Langley K., Fowler T., Rice F., Turic D., Whittinger N., et al. (2005a). Catechol O-methyltransferase gene variant and birth weight predict early-onset antisocial behavior in children with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 62 1275–1278. 10.1001/archpsyc.62.11.1275 [DOI] [PubMed] [Google Scholar]
- Thapar A., O’Donovan M., Owen M. J. (2005b). The genetics of attention deficit hyperactivity disorder. Hum. Mol. Genet. 2 R275–R282. 10.1093/hmg/ddi263 [DOI] [PubMed] [Google Scholar]
- Tsang T. W., Kohn M. R., Efron D., Clarke S. D., Clark C. R., Lamb C., et al. (2015). Anxiety in young people with ADHD: clinical and self-report outcomes. J. Atten. Disord. 19 18–26. 10.1177/1087054712446830 [DOI] [PubMed] [Google Scholar]
- Tseng W. L., Kawabata Y., Gau S. S., Crick N. R. (2014). Symptoms of attention-deficit/hyperactivity disorder and peer functioning: a transactional model of development. J. Abnorm. Child Psychol. 42 1353–1365. 10.1007/s10802-014-9883-8 [DOI] [PubMed] [Google Scholar]
- van Goozen S. H., Langley K., Northover C., Hubble K., Rubia K., Schepman K., et al. (2016). Identifying mechanisms that underlie links between COMT genotype and aggression in male adolescents with ADHD. J. Child Psychol. Psychiatry 57 472–480. 10.1111/jcpp.12464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter N. T., Markett S. A., Montag C., Reuter M. (2011). A genetic contribution to cooperation: dopamine-relevant genes are associated with social facilitation. Soc. Neurosci. 6 289–301. 10.1080/17470919.2010.527169 [DOI] [PubMed] [Google Scholar]
- White T. P., Loth E., Rubia K., Krabbendam L., Whelan R., Banaschewski T., et al. (2014). Sex Differences in COMT polymorphism effects on prefrontal inhibitory control in adolescence. Neuropsychopharmacology 39 2560–2569. 10.1038/npp.2014.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2016). International Statistical Classification of Diseases and Related Health Problems. Geneva: WHO. [Google Scholar]
- Willcutt E. G., Nigg J. T., Pennington B. F., Solanto M. V., Rohde L. A., Tannock R., et al. (2012). Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J. Abnorm. Psychol. 121 991–1010. 10.1037/a0027347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winqvist R., Lundstrom K., Salminen M., Laatikainen M., Ulmanen I. (1992). The human catechol-O-methyltransferase (COMT) gene maps to band q11.2 of chromosome 22 and shows a frequent RFLP with BglI. Cytogenet. Cell Genet. 59 253–257. 10.1159/000133262 [DOI] [PubMed] [Google Scholar]
- Yacubian J., Buchel C. (2009). The genetic basis of individual differences in reward processing and the link to addictive behavior and social cognition. Neuroscience 164 55–71. 10.1016/j.neuroscience.2009.05.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differences between French data and data from the other IMAGEN centers were also found in the subscales social cognition, social communication, social motivation, and social awareness. Excluded French data: N = 96.