Abstract
Sessile serrated adenoma/polyps (SSA/Ps) are early precursor lesions in the serrated neoplasia pathway, which results in colorectal carcinomas with BRAF mutations, methylation for DNA repair genes, a CpG island methylator phenotype, and high levels of microsatellite instability. Some of these lesions can rapidly become dysplastic or invasive carcinomas that exhibit high lymphatic invasion and lymph node metastasis potentials. Detecting serrated lesions, including SSA/Ps with and without dysplasia/carcinoma, is critical, but SSA/Ps can be difficult to detect, are inconsistently identified by endoscopists and pathologists, and are often incompletely resected. Therefore, SSA/Ps are considered to be major contributors to “interval cancers”. If colonoscopists can identify the specific endoscopic characteristics of SSA/Ps, their detection and the effectiveness of colonoscopy may improve. Here, the endoscopic features of SSA/Ps with and without dysplasia/carcinoma, including the characteristics determined using magnifying endoscopy, are reviewed in the context of previous reports. Endoscopically, these subtle polyps are like hyperplastic polyps, because they are slightly elevated and pale. Unlike hyperplastic polyps, SSA/Ps are usually larger than 5 mm, frequently covered by a thin layer called the ‘‘mucus cap’’, and are more commonly located in the proximal colon. Magnifying narrow-band imaging findings, which include dark spots inside the crypts and varicose microvascular vessels, in addition to the type II-open pit patterns detected using magnifying chromoendoscopy, effectively differentiate SSA/Ps from hyperplastic polyps. The lesions’ endoscopic characteristics, which include their (semi)pedunculated morphologies, double elevations, central depressions, and reddishness, and the use of magnifying endoscopy, might help to detect dysplasia/carcinoma within SSA/Ps. Greater awareness may promote further research into improving the detection, identification, and complete resection rates of SSA/Ps with and without dysplasia/carcinoma and reduce the interval cancer rates.
Keywords: Sessile serrated adenoma/polyp, Invasive carcinoma arising from sessile serrated adenoma/polyp, Serrated neoplasia pathway, Endoscopic diagnosis, Sessile serrated adenoma/polyp with cytological dysplasia
Core tip: The endoscopic features of sessile serrated adenoma/polyps (SSA/Ps) with and without dysplasia/carcinoma are reviewed. Conventional endoscopic characteristics, including a proximal location, a slightly elevated morphology, a pale color, and a mucus cap, are useful for diagnosing SSA/Ps. Magnifying narrow-band imaging, which detects dark spots inside the crypts and varicose microvascular vessels, and magnifying chromoendoscopy, which identifies the type II-open pit pattern, are also effective for differentiating between SSA/Ps and hyperplastic polyps. Furthermore, the lesions’ endoscopic characteristics, which include their (semi)pedunculated morphologies, double elevations, central depressions, and reddishness, and the use of magnifying endoscopy, might help to detect dysplasia/carcinoma within SSA/Ps.
INTRODUCTION
Colorectal serrated lesions were called “hyperplastic polyps”, and they were not considered to be malignant[1,2]. Torlakovic et al[3] described abnormal proliferations in colorectal serrated polyps that resembled hyperplastic polyps superficially, but could be distinguished histologically based on their abnormal architectural features, and they introduced the term “sessile serrated adenoma”. Currently, these polyps are categorized as sessile serrated adenoma/polyp (SSA/P) in accordance with the World Health Organization’s recommendations[4]. The typical histology of an SSA/P in a representative case is shown in Figure 1.
Figure 1.
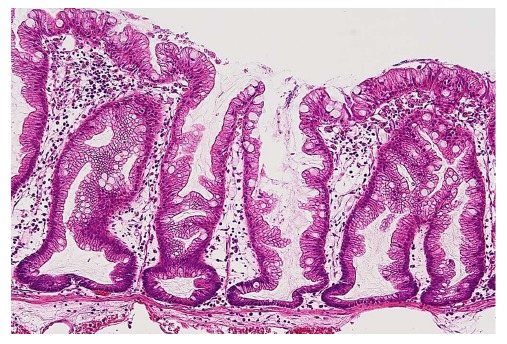
Typical histology of a sessile serrated adenoma/polyp. Crypts with a serrated architecture include those that are irregularly dilated, branch irregularly, and are horizontally arranged (basal).
SSA/Ps are early precursor lesions in the serrated neoplasia pathway, which results in colorectal carcinomas with high levels of microsatellite instability[5-7]. Recent studies have shown associations between SSA/Ps with and without dysplasia or carcinoma and the methylation or loss of protein expression for DNA repair genes, including MLH1[3,6,8-12], a CpG island methylator phenotype[5,6,8,10], BRAF mutations[5,6,8-17], and a lack of genetic alterations in CTNNB1, which is the gene that codes for β-catenin protein[17]. This pathway is thought to be distinct from the conventional adenoma-carcinoma pathway in which adenomas progress to invasive colorectal carcinomas as a result of a series of genetic alterations, including adenomatous polyposis coli (APC) and KRAS mutations[6,8,13,14,18,19].
Some researchers[20-22] have suggested that some serrated lesions might progress rapidly to dysplasia or invasive carcinomas. Furthermore, we reported that the submucosal invasive carcinomas that arose in SSA/Ps exhibited higher potentials for lymphatic invasion and lymph node metastasis than their conventional counterparts that arose from tubular adenomas[23]. Therefore, the detection of serrated lesions, including SSA/Ps with and without dysplasia, is critical. However, SSA/Ps can be difficult to detect, are inconsistently identified by endoscopists and pathologists, and are often incompletely resected[24-27]. Therefore, SSA/Ps are major contributors to the failure of colonoscopy to prevent proximal colonic cancer[28-30], and they account for 5%-7% of the colorectal cancers that occur in the interval between a complete colonoscopy and surveillance, that is, “interval cancer”[31-33]. The identification of the specific endoscopic characteristics of SSA/Ps by colonoscopists may improve their detection and, eventually, may enhance the effectiveness of colonoscopy. Some studies have investigated the endoscopic features of SSA/Ps without dysplasia[34-38], and we clarified the endoscopic characteristics of SSA/Ps that had advanced histology[39].
Here, the endoscopic features of SSA/Ps with and without dysplasia or carcinoma are reviewed in the context of previous reports, including the features detected using magnifying endoscopy.
DIAGNOSIS OF SSA/P USING CONVENTIONAL WHITE-LIGHT ENDOSCOPY
Generally, hyperplastic polyps are traditionally considered non-neoplastic, but SSA/Ps have malignant potential to progress to invasive carcinomas. Therefore, differentiating an SSA/P from a hyperplastic polyp is clinically important to determine the necessity of an endoscopic resection or to provide support for a recommendation of a surveillance interval[40,41]. Typical hyperplastic polyps are highly prevalent, diminutive sessile polyps that are most commonly located in the sigmoid colon and rectum, and identifying them endoscopically is not particularly difficult[42]. SSA/Ps are subtle polyps, and their endoscopic findings are similar to those associated with hyperplastic polyps, which include a slightly elevated morphology and a pale color. However, in contrast to hyperplastic polyps, SSA/Ps are usually larger than 5 mm, frequently covered by a thin layer called a ‘‘mucus cap’’[4,34,43,44], and are more commonly located in the proximal colon[14,45]. Conversely, although SSA/Ps are difficult to detect because of their slightly elevated morphology, adhesion of mucus in the proximal colon can be one of the most useful clues for SSA/P detection. Additionally, using white-light endoscopy, Hazewinkel et al[37] described the presence of indistinct borders and a cloud-like surface, and showed that these were independently predictive endoscopic characteristics that were associated with the histology of SSA/Ps. Figure 2 shows representative endoscopic images of SSA/Ps.
Figure 2.
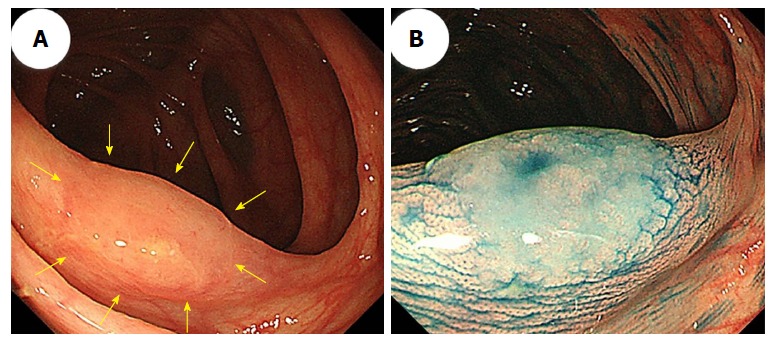
A sessile serrated adenoma/polyp in the transverse colon that measured 13 mm. A: An image from conventional colonoscopy showing the lesion’s location (arrows); B: An image from chromoendoscopy following indigo carmine dye spraying.
ENDOSCOPIC DIAGNOSIS OF SSA/P USING NARROW-BAND IMAGING
Difficulties distinguishing between an SSA/P and a hyperplastic polyp are commonly encountered. Many authors have used image-enhanced endoscopy to characterize polyps[46], which involves the use of innovative optical technologies, such as narrow-band imaging (NBI)[47-50]. Bile appears as a bright red fluid using NBI. When a tenacious mucus cap covers SSA/Ps, the mucus cap is clearly viewed using NBI. Therefore, NBI enhances the visibility of SSA/Ps that have mucus caps, which are usually an intense red color[34] (Figure 3A and B).
Figure 3.
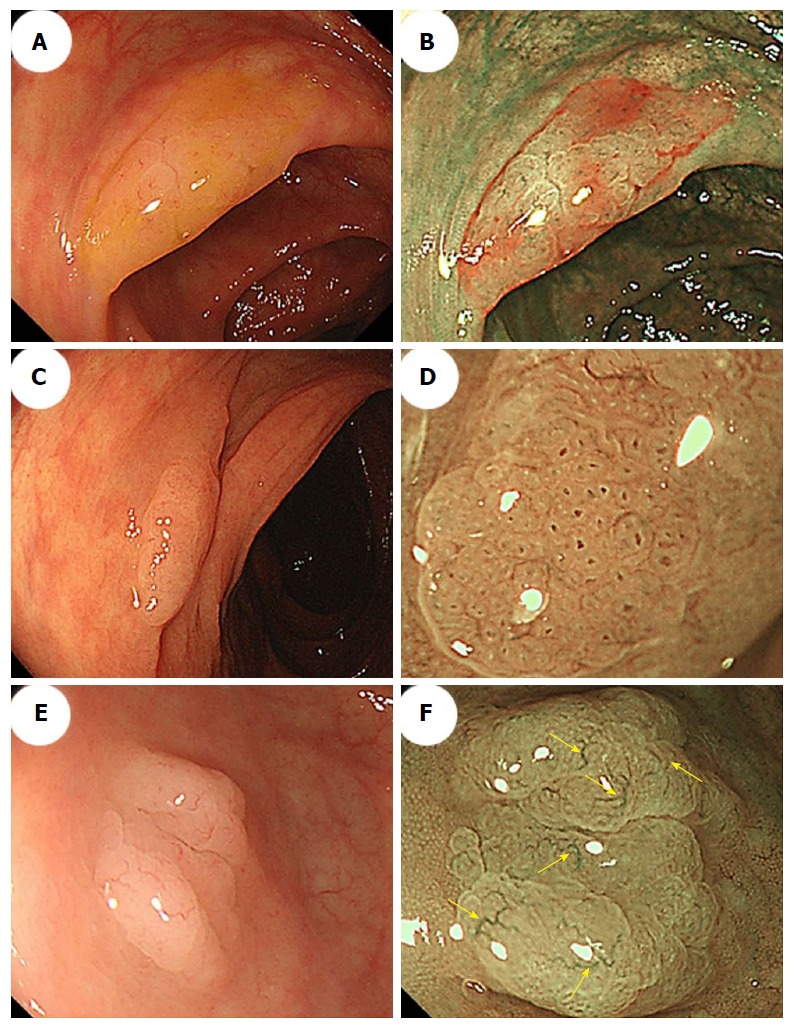
Morphologic characteristics of sessile serrated adenoma/polyps. A: Conventional endoscopy revealed a flat-elevated lesion with a 20-mm diameter that was covered with a mucus cap in the transverse colon. B: Narrow-band imaging (NBI) showed that the SSA/P in (A) was covered with a mucus cap that appeared intensely red. C: Conventional endoscopy showed a flat-elevated lesion with a 14-mm diameter in the ascending colon. D: Magnifying NBI of the SSA/P in (C) revealed dark spots inside the crypts in part of the lesion. E: A conventional endoscopic image shows a flat-elevated pale colored lesion with a 10-mm diameter in the cecum. F: Magnifying NBI of the SSA/P in (E) revealed varicose microvascular vessels (arrows) in part of the lesion. SSA/P: Sessile serrated adenoma/polyp.
Furthermore, NBI often reveals small dark dots inside the openings to the crypts of SSA/Ps[37]; these are thought to indicate crypt dilations, which are a key histological feature of SSA/Ps. The presence of these dark spots inside the crypts might help endoscopists to differentiate between premalignant SSA/Ps and hyperplastic polyps during colonoscopy[37,38] (Figure 3C and D). Hazewinkel et al[37] have reported that in white-light endoscopy, indistinctive borders and cloud-like surface are two independent predictive characteristics of SSA/P, while in NBI, it is possible to discern an irregular shape and dark spots inside the crypts. The sensitivities, specificities, and overall accuracies determined using white-light endoscopy were 75%, 79%, and 77%, respectively, and those determined using NBI were 89%, 96%, and 93%, respectively[37].
Magnifying NBI can enhance the visibility of the microvessels on a lesion’s surface. Yamada et al[51] conducted a multivariate analysis, demonstrated that dilated and branching vessels, defined as thickened capillary vessels with branching that is observed on the surface, had a 2.3-fold odds ratio among SSA/Ps compared with hyperplastic polyps. They stated that when dilated and branching vessels, a proximal location, and a tumor size of ≥ 10 mm were combined, the positive predictive value exceeded 90%. Additionally, Uraoka et al[52] reported that the presence of varicose microvascular vessels, which were found using magnifying NBI, was useful for differentiating between SSA/Ps and hyperplastic polyps. Unlike the blood vessels around the glands of the superficial mucosal layer such as dilated and branching vessels, varicose microvascular vessels are characterized by the observation of blood vessels running throughout the deep mucosal layer. The presence of varicose microvascular vessels had a significantly higher specificity (88%) for predicting a diagnosis of SSA/P (Figure 3E and F).
DIAGNOSIS OF SSA/P USING MAGNIFYING CHROMOENDOSCOPY
Magnifying chromoendoscopy, which uses indigo carmine or crystal violet staining, follows careful conventional endoscopic examinations. Kudo et al[53,54] proposed a classification of colorectal lesions’ pit patterns that is associated with the lesions’ histologic characteristics. As previously explained[54-56], magnifying colonoscopy is useful for differentiating between neoplastic and nonneoplastic lesions, and for assessing early colorectal cancers’ depths of invasion. Both hyperplastic polyps and SSA/Ps have type II pit patterns. Recently, the type II-open pit pattern has been described as a hallmark of SSA/Ps (sensitivity: 66%; specificity: 97%)[35]. Like the small dark dots detected using NBI, a type II-open pit pattern detected using magnifying chromoendoscopy is thought to indicate crypt dilation, which is one of the major histological features of SSA/Ps (Figure 4).
Figure 4.
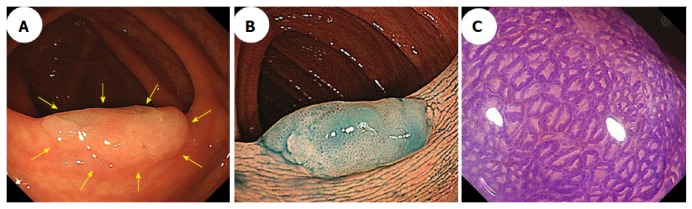
Conventional colonoscopic image (A) and a chromoendoscopic image (B) following indigo carmine dye spraying show an 18-mm sessile serrated adenoma/polyp with a mucus cap that was in the transverse colon (arrows). C: Magnifying chromoendoscopy using crystal violet staining identified a type II-open pit pattern in the lesion.
Distinct endoscopic characteristics between SSA/Ps and hyperplastic polyps are summarized in Table 1.
Table 1.
Distinct endoscopic characteristics between sessile serrated adenoma/polyps and hyperplastic polyps
| SSA/Ps | Hyperplastic polyps | |
| Conventional endoscopic features | ||
| Location | Proximal | Distal |
| Size of tumor | > 5 mm | ≤ 5 mm |
| Color | Pale | Pale |
| Morphology | Flat elevated | Flat elevated |
| Mucus cap | Yes | No |
| Endoscopic features by using NBI | Irregular shape | - |
| Small dark dots | ||
| Dilated and branching vessels | ||
| Varicose microvascular vessels | ||
| Magnifying chromoendoscopic features | Type II-open pit pattern | Type II pit pattern |
SSA/P: Sessile serrated adenoma/polyp; NBI: Narrow-band imaging.
ENDOSCOPIC DETECTION OF SSA/P
The detection of SSA/Ps requires careful colonoscopy. As stated above, because most SSA/Ps are slightly flat-elevated and have subtle mucosal features, SSA/Ps are difficult to detect with endoscopy, and could easily be missed. Therefore, bowel preparation must be excellent. Potential SSA/Ps are initially considered at long view and investigated at close-up view. At long view, the presence of SSA/P is suspected when there is a patch that appears nodular, reddish, covered with mucus, and/or circled by fine debris. Then such a lesion must be approached and the mucosa washed. Finally, at close-up view, using white light and under NBI, the surface pattern and vessels are examined.
Recently, some studies[57,58] have shown that image-enhanced endoscopy such as NBI might increase the detection of serrated lesions in the proximal colon, although the results did not reach significance. Therefore, image-enhanced endoscopy currently cannot be recommended as a detection tool for SSA/P. Additional studies assessing SSA/P detection rates with image-enhanced endoscopy are needed.
ENDOSCOPIC DIAGNOSIS OF SSA/P WITH DYSPLASIA/CARCINOMA
SSA/Ps with advanced histology, including cytologic dysplasia or minimally invasive carcinomas, are rare. Indeed, a previous study’s findings showed that the frequencies of cytologic dysplasia and invasive carcinomas among SSA/P lesions were 14% and 1.0%, respectively[59]. The findings from another study showed that three (0.7%) high-grade dysplasias and one (0.2%) submucosal invasive carcinoma were detected among 430 SSA/Ps[60]. Therefore, only a few studies have investigated the endoscopic characteristics of SSA/Ps with dysplasia or carcinoma in detail[39,61,62]. We demonstrated that SSA/Ps without dysplasia (354 of 414; 86%) and SSA/Ps with dysplasia or carcinomas (40 of 48; 83%) were frequently located in the proximal colon[39]. Furthermore, we showed a stepwise increase in the median size of the SSA/Ps that accompanied their dysplastic progression, specifically, from a 10-mm SSA/P that did not have dysplasia to a 12-mm SSA/P with cytologic dysplasia and a 19-mm SSA/P with an invasive carcinoma, but 19 of 48 (39.6%) SSA/Ps with dysplasia or carcinomas measured ≤ 10 mm[39]. The findings from a study by Goldstein[20] showed that the median size of eight SSA/Ps with focal invasive adenocarcinomas or high-grade dysplasia was 8.5 mm (range: 6-12 mm). Another study’s findings[63] showed that among eight SSA/Ps with intramucosal carcinomas, submucosal carcinomas, or advanced carcinomas, the largest diameter was ≤ 10 mm. Therefore, SSA/P with dysplasia/carcinoma must be attended to even if the lesion measures 10 mm or less.
Macroscopically, a mucus cap was found in almost all of the SSA/P lesions, including the SSA/Ps with and without dysplasia or carcinoma, in our study[39], suggesting that a mucus cap may be one of the strongest markers of an SSA/P. Additionally, (semi)pedunculated morphologies, double elevations, central depressions, and reddishness were found more frequently in SSA/Ps with dysplasia (17.1%, 63.4%, 9.8%, and 39.0%, respectively) or carcinoma (28.6%, 57.1%, 28.6%, and reddishness 85.7%, respectively) than the frequencies at which these features were found in SSA/Ps without dysplasia (4.6%, 4.6%, 3.9%, and 3.4%, respectively). The presence of at least one of these four markers had a high sensitivity (91.7%) for the identification of dysplasia or a carcinoma within an SSA/P; the specificity was 85.3%. These findings suggested that the endoscopic characteristics, including a (semi)pedunculated morphology, a double elevation, a central depression, and reddishness, may be useful for accurately diagnosing the presence of advanced histology within an SSA/P.
Magnifying chromoendoscopy is also useful for detecting components associated with dysplasia or a carcinoma within an SSA/P. We found that a type II-open pit pattern was present in SSA/Ps without dysplasia and in SSA/Ps with dysplasia or carcinomas, which indicates that a type II-open pit pattern may be strongly suggestive of the presence of SSA/P components[39]. Furthermore, the type II pit pattern only was detected in all of the cases who had SSA/Ps without dysplasia, whereas type II and other pit patterns, including mixtures of IIIL, IV, VI, or VN, were found in most of the SSA/Ps with dysplasia or carcinoma. Moreover, all of the cases who had SSA/Ps with invasive carcinomas had the VI or VN pit patterns (invasive patterns), which were consistent with the depths of invasion. Accordingly, determining the pit patterns using magnifying endoscopy can effectively assess the depth of invasion of early colorectal cancers that arise from SSA/Ps. Figures 5 and 6 show representative endoscopic images of SSA/Ps with dysplasia or carcinoma.
Figure 5.
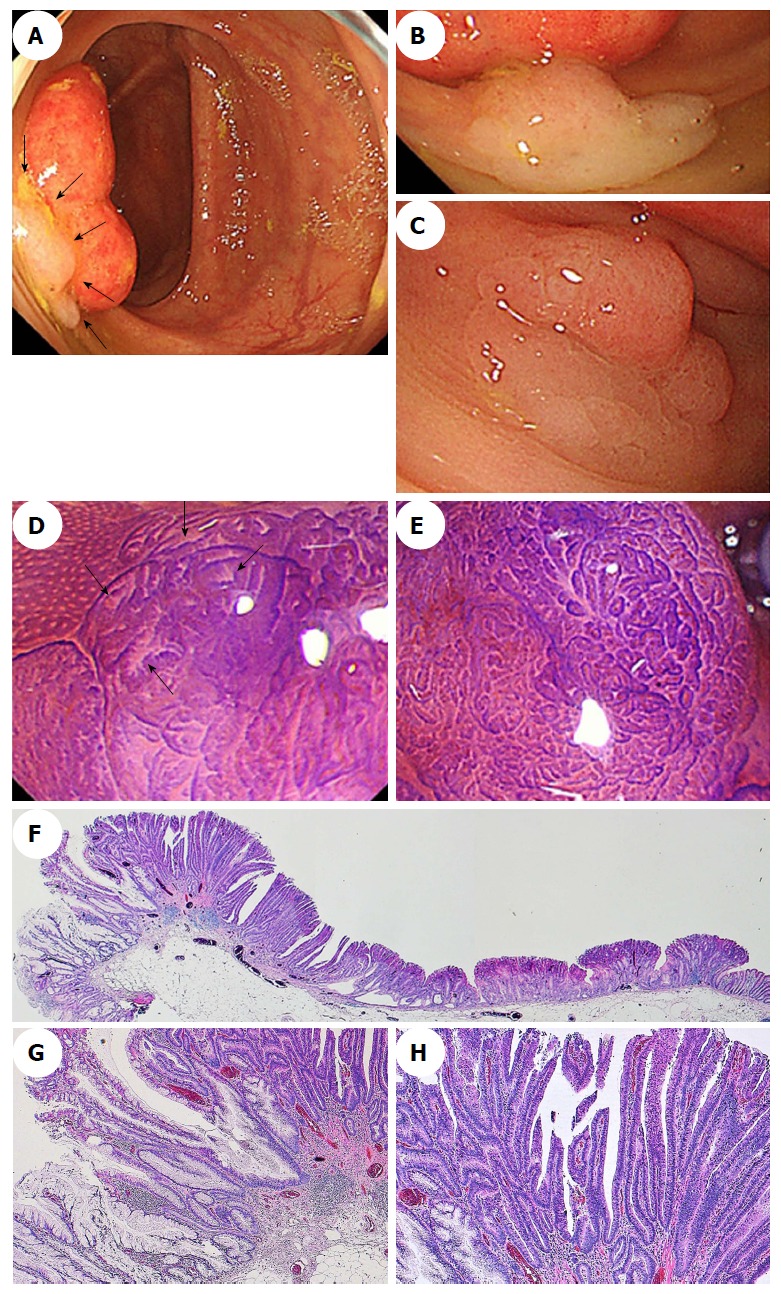
Endoscopic images of a sessile serrated adenoma/polyp with high-grade cytologic dysplasia in a representative case. A-C: A conventional endoscopic view using white-light imaging. A: An endoscopic image shows a pale-color, flat-elevated lesion covered with mucus at the ascending colon (arrows). B: The lesion is covered with mucus cap. C: After washing the target lesion to sufficiently remove mucus, a flat-elevated lesion that had a 13-mm diameter and a dome-shaped double elevation can be clearly seen. The dome-shaped area is slightly red-colored. D and E: Magnifying chromoendoscopic views using crystal violet staining. D: A type II-open pit pattern is partly evident in the edge of the lesion (arrows). E: Type VI-mild pit pattern consisting of areas with irregular pits can be observed at the dome-shaped area. We endoscopically diagnosed the lesion as an SSA/P with cytologic dysplasia, and achieved an en bloc resection by performing an endoscopic mucosal resection. F-H: Histopathologic findings with hematoxylin-eosin staining of the resected specimen. G: Crypts with a serrated architecture exhibit irregularly dilated crypts, irregularly branching crypts, and horizontally arranged basal crypts, corresponding to SSA/P. H: A high-power view shows conventional adenomatous high-grade dysplasia with cytological atypia and architectural dysplasia in the dome-shaped area. The lesion was pathologically consistent with an SSA/P with high-grade cytologic dysplasia. SSA/P: Sessile serrated adenoma/polyp.
Figure 6.
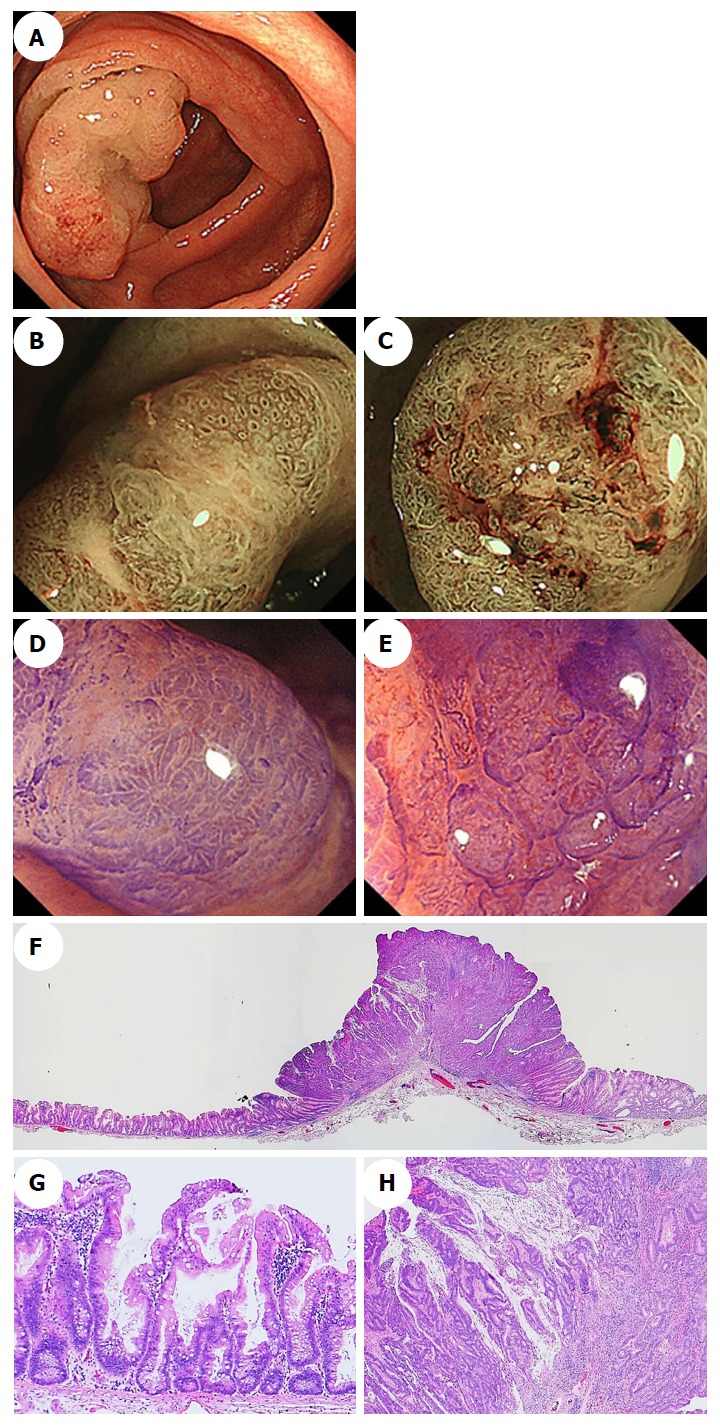
Endoscopic images of a sessile serrated adenoma/polyp with an invasive carcinoma in a representative case. A: A conventional endoscopic image captured using white-light imaging shows a red 55-mm semipedunculated lesion in the ascending colon. B and C: Magnifying narrow-band imaging revealed dark spots inside the crypts on an edge of the lesion and irregular vessel patterns over a large part of the lesion, respectively. D and E: Magnifying chromoendoscopy using crystal violet staining; D: A high-powered view of the marginal zone, the dilated openings of the crypts have a type II-open pit pattern; E: A high-powered view of the middle region in which a type VI-severe pit pattern is evident. We endoscopically diagnosed the lesion as a carcinoma associated with an SSA/P, and achieved an en bloc resection by performing an endoscopic submucosal dissection. F-H: Histopathologic findings with hematoxylin-eosin staining of the resected specimen; G: Crypts with a serrated architecture exhibiting irregularly dilated crypts and irregularly branching crypts, corresponding to SSA/P; H: Well to moderately differentiated adenocarcinomas invade the submucosa with extracellular mucin production. The lesion was pathologically consistent with an invasive submucosal adenocarcinoma associated with an SSA/P. SSA/P: Sessile serrated adenoma/polyp.
Finally, there is one important point that must be kept in mind when observing SSA/Ps using colonoscopy. Most SSA/Ps were covered with rich mucus, and subtle endoscopic findings were difficult to detect when sticky mucus was present. After washing the target lesion to sufficiently remove mucus, endoscopic findings such as (semi)pedunculated morphology, double elevation, central depression, and reddishness should be assessed, and pit pattern analysis must be performed.
CONCLUSION
Conventional endoscopic characteristics, including a proximal location, a slightly elevated morphology, a pale color, and a mucus cap, are useful for diagnosing SSA/Ps. Magnifying endoscopy with NBI, which detects dark spots inside the crypts and varicose microvascular vessels, and magnifying chromoendoscopy, which identifies the type II-open pit pattern, are also effective for differentiating between SSA/Ps and hyperplastic polyps. Furthermore, a lesion’s endoscopic characteristics, for example, a (semi)pedunculated morphology, a double elevation, a central depression, and reddishness, in addition to the use of magnifying endoscopy, might be useful for identifying dysplasia or a carcinoma within an SSA/P. Greater awareness may promote further research into improving the detection, recognition, and complete resection rates of SSA/Ps with and without dysplasia or carcinoma and reduce the interval cancer rates.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare no conflicts of interest.
Peer-review started: April 3, 2018
First decision: May 30, 2018
Article in press: June 28, 2018
P- Reviewer: Lee EJ, Tsuji Y, Yamada A S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
Contributor Information
Takashi Murakami, Department of Gastroenterology, Juntendo University School of Medicine, Tokyo 113-8421, Japan. t-murakm@juntendo.ac.jp.
Naoto Sakamoto, Department of Gastroenterology, Juntendo University School of Medicine, Tokyo 113-8421, Japan.
Akihito Nagahara, Department of Gastroenterology, Juntendo University School of Medicine, Tokyo 113-8421, Japan.
References
- 1.Lane N. The precursor tissue of ordinary large bowel cancer. Cancer Res. 1976;36:2669–2672. [PubMed] [Google Scholar]
- 2.Jørgensen H, Mogensen AM, Svendsen LB. Hyperplastic polyposis of the large bowel. Three cases and a review of the literature. Scand J Gastroenterol. 1996;31:825–830. doi: 10.3109/00365529609010360. [DOI] [PubMed] [Google Scholar]
- 3.Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Snover DC, Ahnen DJ, Burt RW. Serrated polyps of the colon and rectum and serrated polyposis. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, eds, et al., editors. WHO classification of tumours of the digestive system. Lyon: IARC Press;; 2010. pp. 160–165. [Google Scholar]
- 5.Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, Barker MA, Arnold S, McGivern A, Matsubara N, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–1144. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien MJ, Yang S, Mack C, Xu H, Huang CS, Mulcahy E, Amorosino M, Farraye FA. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30:1491–1501. doi: 10.1097/01.pas.0000213313.36306.85. [DOI] [PubMed] [Google Scholar]
- 7.Patil DT, Shadrach BL, Rybicki LA, Leach BH, Pai RK. Proximal colon cancers and the serrated pathway: a systematic analysis of precursor histology and BRAF mutation status. Mod Pathol. 2012;25:1423–1431. doi: 10.1038/modpathol.2012.98. [DOI] [PubMed] [Google Scholar]
- 8.Kim YH, Kakar S, Cun L, Deng G, Kim YS. Distinct CpG island methylation profiles and BRAF mutation status in serrated and adenomatous colorectal polyps. Int J Cancer. 2008;123:2587–2593. doi: 10.1002/ijc.23840. [DOI] [PubMed] [Google Scholar]
- 9.Sandmeier D, Benhattar J, Martin P, Bouzourene H. Serrated polyps of the large intestine: a molecular study comparing sessile serrated adenomas and hyperplastic polyps. Histopathology. 2009;55:206–213. doi: 10.1111/j.1365-2559.2009.03356.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim KM, Lee EJ, Ha S, Kang SY, Jang KT, Park CK, Kim JY, Kim YH, Chang DK, Odze RD. Molecular features of colorectal hyperplastic polyps and sessile serrated adenoma/polyps from Korea. Am J Surg Pathol. 2011;35:1274–1286. doi: 10.1097/PAS.0b013e318224cd2e. [DOI] [PubMed] [Google Scholar]
- 11.Dhir M, Yachida S, Van Neste L, Glöckner SC, Jeschke J, Pappou EP, Montgomery EA, Herman JG, Baylin SB, Iacobuzio-Donahue C, et al. Sessile serrated adenomas and classical adenomas: an epigenetic perspective on premalignant neoplastic lesions of the gastrointestinal tract. Int J Cancer. 2011;129:1889–1898. doi: 10.1002/ijc.25847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami T, Mitomi H, Saito T, Takahashi M, Sakamoto N, Fukui N, Yao T, Watanabe S. Distinct WNT/β-catenin signaling activation in the serrated neoplasia pathway and the adenoma-carcinoma sequence of the colorectum. Mod Pathol. 2015;28:146–158. doi: 10.1038/modpathol.2014.41. [DOI] [PubMed] [Google Scholar]
- 13.Jass JR, Baker K, Zlobec I, Higuchi T, Barker M, Buchanan D, Young J. Advanced colorectal polyps with the molecular and morphological features of serrated polyps and adenomas: concept of a ‘fusion’ pathway to colorectal cancer. Histopathology. 2006;49:121–131. doi: 10.1111/j.1365-2559.2006.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, Pike T, Simms LA, Young J, James M, Montgomery GW, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400–1407. doi: 10.1053/j.gastro.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Carr NJ, Mahajan H, Tan KL, Hawkins NJ, Ward RL. Serrated and non-serrated polyps of the colorectum: their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J Clin Pathol. 2009;62:516–518. doi: 10.1136/jcp.2008.061960. [DOI] [PubMed] [Google Scholar]
- 16.Fujita K, Yamamoto H, Matsumoto T, Hirahashi M, Gushima M, Kishimoto J, Nishiyama K, Taguchi T, Yao T, Oda Y. Sessile serrated adenoma with early neoplastic progression: a clinicopathologic and molecular study. Am J Surg Pathol. 2011;35:295–304. doi: 10.1097/PAS.0b013e318205df36. [DOI] [PubMed] [Google Scholar]
- 17.Yachida S, Mudali S, Martin SA, Montgomery EA, Iacobuzio-Donahue CA. Beta-catenin nuclear labeling is a common feature of sessile serrated adenomas and correlates with early neoplastic progression after BRAF activation. Am J Surg Pathol. 2009;33:1823–1832. doi: 10.1097/PAS.0b013e3181b6da19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 19.Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1:229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein NS. Small colonic microsatellite unstable adenocarcinomas and high-grade epithelial dysplasias in sessile serrated adenoma polypectomy specimens: a study of eight cases. Am J Clin Pathol. 2006;125:132–145. [PubMed] [Google Scholar]
- 21.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–386. doi: 10.1111/his.12055. [DOI] [PubMed] [Google Scholar]
- 23.Murakami T, Mitomi H, Yao T, Saito T, Shibuya T, Sakamoto N, Osada T, Watanabe S. Distinct histopathological characteristics in colorectal submucosal invasive carcinoma arising in sessile serrated adenoma/polyp and conventional tubular adenoma. Virchows Arch. 2018;472:383–393. doi: 10.1007/s00428-017-2234-8. [DOI] [PubMed] [Google Scholar]
- 24.Hetzel JT, Huang CS, Coukos JA, Omstead K, Cerda SR, Yang S, O’Brien MJ, Farraye FA. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105:2656–2664. doi: 10.1038/ajg.2010.315. [DOI] [PubMed] [Google Scholar]
- 25.Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–46. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74–80.e1. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 27.de Wijkerslooth TR, Stoop EM, Bossuyt PM, Tytgat KM, Dees J, Mathus-Vliegen EM, Kuipers EJ, Fockens P, van Leerdam ME, Dekker E. Differences in proximal serrated polyp detection among endoscopists are associated with variability in withdrawal time. Gastrointest Endosc. 2013;77:617–623. doi: 10.1016/j.gie.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 29.Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 30.Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128–1137. doi: 10.1053/j.gastro.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 31.Sawhney MS, Farrar WD, Gudiseva S, Nelson DB, Lederle FA, Rector TS, Bond JH. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131:1700–1705. doi: 10.1053/j.gastro.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Arain MA, Sawhney M, Sheikh S, Anway R, Thyagarajan B, Bond JH, Shaukat A. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010;105:1189–1195. doi: 10.1038/ajg.2009.699. [DOI] [PubMed] [Google Scholar]
- 33.Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer. 2012;118:3044–3052. doi: 10.1002/cncr.26602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tadepalli US, Feihel D, Miller KM, Itzkowitz SH, Freedman JS, Kornacki S, Cohen LB, Bamji ND, Bodian CA, Aisenberg J. A morphologic analysis of sessile serrated polyps observed during routine colonoscopy (with video) Gastrointest Endosc. 2011;74:1360–1368. doi: 10.1016/j.gie.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Kimura T, Yamamoto E, Yamano HO, Suzuki H, Kamimae S, Nojima M, Sawada T, Ashida M, Yoshikawa K, Takagi R, et al. A novel pit pattern identifies the precursor of colorectal cancer derived from sessile serrated adenoma. Am J Gastroenterol. 2012;107:460–469. doi: 10.1038/ajg.2011.457. [DOI] [PubMed] [Google Scholar]
- 36.Ishigooka S, Nomoto M, Obinata N, Oishi Y, Sato Y, Nakatsu S, Suzuki M, Ikeda Y, Maehata T, Kimura T, et al. Evaluation of magnifying colonoscopy in the diagnosis of serrated polyps. World J Gastroenterol. 2012;18:4308–4316. doi: 10.3748/wjg.v18.i32.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hazewinkel Y, López-Cerón M, East JE, Rastogi A, Pellisé M, Nakajima T, van Eeden S, Tytgat KM, Fockens P, Dekker E. Endoscopic features of sessile serrated adenomas: validation by international experts using high-resolution white-light endoscopy and narrow-band imaging. Gastrointest Endosc. 2013;77:916–924. doi: 10.1016/j.gie.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 38.Yamashina T, Takeuchi Y, Uedo N, Aoi K, Matsuura N, Nagai K, Matsui F, Ito T, Fujii M, Yamamoto S, et al. Diagnostic features of sessile serrated adenoma/polyps on magnifying narrow band imaging: a prospective study of diagnostic accuracy. J Gastroenterol Hepatol. 2015;30:117–123. doi: 10.1111/jgh.12688. [DOI] [PubMed] [Google Scholar]
- 39.Murakami T, Sakamoto N, Ritsuno H, Shibuya T, Osada T, Mitomi H, Yao T, Watanabe S. Distinct endoscopic characteristics of sessile serrated adenoma/polyp with and without dysplasia/carcinoma. Gastrointest Endosc. 2017;85:590–600. doi: 10.1016/j.gie.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–1329; quiz 1314, 1330. doi: 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Morson BC. Precancerous lesions of the colon and rectum. Classification and controversial issues. JAMA. 1962;179:316–321. doi: 10.1001/jama.1962.03050050006002. [DOI] [PubMed] [Google Scholar]
- 43.Boparai KS, van den Broek FJ, van Eeden S, Fockens P, Dekker E. Hyperplastic polyposis syndrome: a pilot study for the differentiation of polyps by using high-resolution endoscopy, autofluorescence imaging, and narrow-band imaging. Gastrointest Endosc. 2009;70:947–955. doi: 10.1016/j.gie.2009.03.1172. [DOI] [PubMed] [Google Scholar]
- 44.Gurudu SR, Heigh RI, De Petris G, Heigh EG, Leighton JA, Pasha SF, Malagon IB, Das A. Sessile serrated adenomas: demographic, endoscopic and pathological characteristics. World J Gastroenterol. 2010;16:3402–3405. doi: 10.3748/wjg.v16.i27.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology. 2005;47:32–40. doi: 10.1111/j.1365-2559.2005.02180.x. [DOI] [PubMed] [Google Scholar]
- 46.Tajiri H, Niwa H. Proposal for a consensus terminology in endoscopy: how should different endoscopic imaging techniques be grouped and defined? Endoscopy. 2008;40:775–778. doi: 10.1055/s-2008-1077507. [DOI] [PubMed] [Google Scholar]
- 47.Sano Y, Horimatsu T, Fu KI, Katagiri A, Muto M, Ishikawa H. Magnifying observation of microvascular architecture of colorectal lesions using a narrow band imaging system. Dig Endosc. 2006;18:S44–S51. [Google Scholar]
- 48.Chiu HM, Chang CY, Chen CC, Lee YC, Wu MS, Lin JT, Shun CT, Wang HP. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut. 2007;56:373–379. doi: 10.1136/gut.2006.099614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uraoka T, Sano Y, Saito Y, Saito H, Matsuda T, Yamamoto K. Narrow-band imaging for improving colorectal adenoma detection: appropriate system function settings are required. Gut. 2009;58:604–605. doi: 10.1136/gut.2008.157164. [DOI] [PubMed] [Google Scholar]
- 50.Nakao Y, Saito S, Ohya T, Aihara H, Arihiro S, Kato T, Ikegami M, Tajiri H. Endoscopic features of colorectal serrated lesions using image-enhanced endoscopy with pathological analysis. Eur J Gastroenterol Hepatol. 2013;25:981–988. doi: 10.1097/MEG.0b013e3283614b2b. [DOI] [PubMed] [Google Scholar]
- 51.Yamada M, Sakamoto T, Otake Y, Nakajima T, Kuchiba A, Taniguchi H, Sekine S, Kushima R, Ramberan H, Parra-Blanco A, et al. Investigating endoscopic features of sessile serrated adenomas/polyps by using narrow-band imaging with optical magnification. Gastrointest Endosc. 2015;82:108–117. doi: 10.1016/j.gie.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 52.Uraoka T, Higashi R, Horii J, Harada K, Hori K, Okada H, Mizuno M, Tomoda J, Ohara N, Tanaka T, et al. Prospective evaluation of endoscopic criteria characteristic of sessile serrated adenomas/polyps. J Gastroenterol. 2015;50:555–563. doi: 10.1007/s00535-014-0999-y. [DOI] [PubMed] [Google Scholar]
- 53.Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, Yagyuu A. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880–885. doi: 10.1136/jcp.47.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8–14. doi: 10.1016/s0016-5107(96)70222-5. [DOI] [PubMed] [Google Scholar]
- 55.Kudo S, Kashida H, Tamura T, Kogure E, Imai Y, Yamano H, Hart AR. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg. 2000;24:1081–1090. doi: 10.1007/s002680010154. [DOI] [PubMed] [Google Scholar]
- 56.Kudo S, Rubio CA, Teixeira CR, Kashida H, Kogure E. Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy. 2001;33:367–373. doi: 10.1055/s-2004-826104. [DOI] [PubMed] [Google Scholar]
- 57.Parikh ND, Chaptini L, Njei B, Laine L. Diagnosis of sessile serrated adenomas/polyps with image-enhanced endoscopy: a systematic review and meta-analysis. Endoscopy. 2016;48:731–739. doi: 10.1055/s-0042-107592. [DOI] [PubMed] [Google Scholar]
- 58.Rex DK, Clodfelter R, Rahmani F, Fatima H, James-Stevenson TN, Tang JC, Kim HN, McHenry L, Kahi CJ, Rogers NA, et al. Narrow-band imaging versus white light for the detection of proximal colon serrated lesions: a randomized, controlled trial. Gastrointest Endosc. 2016;83:166–171. doi: 10.1016/j.gie.2015.03.1915. [DOI] [PubMed] [Google Scholar]
- 59.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 2010;63:681–686. doi: 10.1136/jcp.2010.075507. [DOI] [PubMed] [Google Scholar]
- 60.Chino A, Yamamoto N, Kato Y, Morishige K, Ishikawa H, Kishihara T, Fujisaki J, Ishikawa Y, Tamegai Y, Igarashi M. The frequency of early colorectal cancer derived from sessile serrated adenoma/polyps among 1858 serrated polyps from a single institution. Int J Colorectal Dis. 2016;31:343–349. doi: 10.1007/s00384-015-2416-2. [DOI] [PubMed] [Google Scholar]
- 61.Bouwens MW, van Herwaarden YJ, Winkens B, Rondagh EJ, de Ridder R, Riedl RG, Driessen A, Dekker E, Masclee AA, Sanduleanu S. Endoscopic characterization of sessile serrated adenomas/polyps with and without dysplasia. Endoscopy. 2014;46:225–235. doi: 10.1055/s-0034-1364936. [DOI] [PubMed] [Google Scholar]
- 62.Burgess NG, Pellise M, Nanda KS, Hourigan LF, Zanati SA, Brown GJ, Singh R, Williams SJ, Raftopoulos SC, Ormonde D, et al. Clinical and endoscopic predictors of cytological dysplasia or cancer in a prospective multicentre study of large sessile serrated adenomas/polyps. Gut. 2016;65:437–446. doi: 10.1136/gutjnl-2014-308603. [DOI] [PubMed] [Google Scholar]
- 63.Ban S, Mitomi H, Horiguchi H, Sato H, Shimizu M. Adenocarcinoma arising in small sessile serrated adenoma/polyp (SSA/P) of the colon: clinicopathological study of eight lesions. Pathol Int. 2014;64:123–132. doi: 10.1111/pin.12147. [DOI] [PubMed] [Google Scholar]


