Abstract
AIM
To investigate the prognostic role of fibrinogen-to-albumin ratio (FAR) on patients with gallbladder cancer (GBC) in this study.
METHODS
One hundred and fifty-four GBC patients were retrospectively analyzed, who received potentially curative cholecystectomy in our institute from March 2005 to December 2017. Receiver operating characteristic curve (ROC curve) was used to determine the optimal cut-offs for these biomarkers. In addition, Kaplan-Meier survival analysis as well as multivariate analysis were applied for prognostic analyses.
RESULTS
ROC curve revealed that the optimal cut-off value for FAR was 0.08. FAR was significantly correlated with age (P = 0.045), jaundice (P < 0.001), differentiation (P = 0.002), resection margin status (P < 0.001), T stage (P < 0.001), TNM stage (P < 0.001), and CA199 (P < 0.001) as well as albumin levels (P < 0.001). Multivariate analysis indicated that the resection margin status [hazard ratio (HR): 2.343, 95% confidence interval (CI): 1.532-3.581, P < 0.001], TNM stage (P = 0.035), albumin level (HR = 0.595, 95%CI: 0.385-0.921, P = 0.020) and FAR (HR: 2.813, 95%CI: 1.765-4.484, P < 0.001) were independent prognostic factors in GBC patients.
CONCLUSION
An elevated preoperative FAR was significantly correlated with unfavorable overall survival in GBC patients, while an elevated preoperative albumin level was a protective prognostic factor for patients with GBC. The preoperative FAR could be used to predict the prognosis of GBC patients, which was easily accessible, cost-effective and noninvasive.
Keywords: Gallbladder cancer, Fibrinogen, Albumin, Fibrinogen-to-albumin ratio, Prognosis, Survival
Core tip: The vital prognostic significance of fibrinogen and serum albumin has been confirmed in diverse malignancies, presenting host hemostasis and nutrition, respectively. Moreover, elevated plasma fibrinogen and reduced serum albumin levels are significantly related to shortened survival of cancer patients. It is reported that fibrinogen-to-albumin ratio (FAR) is more potent in predicting cancer patient prognosis than elevated fibrinogen or reduced serum albumin level alone. Nevertheless, there has been no study on the prognostic role of FAR in gallbladder cancer (GBC). Herein, we defined an elevated preoperative FAR, featured by noninvasiveness, cost-effectiveness and easily-accessible, which was a potential prognostic indicator for GBC.
INTRODUCTION
Gallbladder cancer (GBC) is an uncommon malignancy among all types of cancer, but is the fifth most common gastrointestinal malignancy. Meanwhile, GBC is the most prevalent and aggressive cancer of the biliary tract[1-3]. Despite recent encouraging progress in the diagnosis and treatment of GBC, it is still a highly lethal disease, with overall 5-year survival rate under 5%[4]. Only surgical intervention renders probability of a long-term survival, however, most GBC patients generally present at late stage, with unresectable lesion. To be specific, fewer than 20% of cases are amenable to surgical treatment[5,6].
At present, a few clinicopathological parameters, such as clinical stage, performance status (PS), and pathological classification, have been demonstrated as independent survival predictors in patients harboring various types of common solid tumors[7]. Nevertheless, despite the wide application of high-resolution imaging systems, it is rather difficult to obtain accurate classification of clinical stage, and objective judgement of PS[8-10]. In addition, the pathological stage of tumor samples in these subjects is not as informative as that in untreated subjects[11]. In order to guarantee potent intense neoadjuvant therapy as well as regular follow-up in high-risk subjects, it is necessary to explore a simple and cost-effective predictor for the postoperative overall survival (OS) prior to surgery.
Accumulating evidence has demonstrated that nutritional deficiencies, hemostatic factors and systemic inflammatory response (SIR) are likely to be critically involved in the progression of human malignancies[12]. Fibrinogen plays an important regulatory role in both inflammation and cancer progression, including proliferation, angiogenesis as well as migration of tumor cells[13]. Serum albumin levels reflect the SIR of host and nutritional status[14-16]. Recently, accumulating researches have shown that both fibrinogen and serum albumin are important prognostic predictors in various cancers, and elevated plasma fibrinogen and lower serum albumin levels are significantly correlated with shorter survival in tumor patients[17-21].
From the results of the above studies, we can naturally hypothesize that the fibrinogen-to-albumin ratio (FAR) might be more powerful than elevated fibrinogen or lower serum albumin level in predicting the prognosis of patients with malignant tumors. In fact, Tan et al[22] have indicated that the preoperative FAR is an independent prognostic indicator for esophageal squamous-cell carcinoma (ESCC) patients, while Hwang et al[23] have indicated that the FAR is a more significant prognostic indicator than either indicator alone (elevated fibrinogen or lower serum albumin).
To our knowledge, there are no relevant studies concerning the prognostic significance of FAR in GBC patients. Herein, the study was designed to explore the prognostic roles of the preoperative FAR in GBC in terms of OS.
MATERIALS AND METHODS
Patients
Eligible patients were included in this study according to the following criteria: (1) patients with histological diagnosis of GBC; (2) GBC patients without other coexisting malignancies; (3) patients not undergoing other treatments before enrollment; (4) patients with complete clinical information and available follow-up data; and (5) patients aged > 18 years. The exclusion criteria were listed as follows: (1) patients with acute infection or chronic active inflammatory disease; (2) patients with collagen diseases, anemia and other diseases concerning the hematological system; (3) patients who received anticoagulant treatment or albumin transfusions before treatment; (4) patients with liver disease; and (5) patients with perioperative surgery-associated mortality. As a result, 154 GBC patients were retrospectively included and analyzed, who underwent potential curative resection at Peking Union Medical College Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS & PUMC) from January 2005 to May 2017.
Data collection
Baseline clinicopathological characteristics, including age, gender, comorbidities, ABO blood group, pathological classifications, tumor differentiation, resection margin status, maximal tumor diameter, TNM stage, and preoperative CA199, fibrinogen, and albumin levels. Patient age referred to the age at diagnosis of primary GBC. The eighth edition of the American Joint Committee on Cancer (AJCC-8th) TNM classification was utilized for TNM stage.
Ethical statement
The study was approved by the Medical Ethics Committee of Peking Union Medical College Hospital of CAMS & PUMC. All patients signed written informed consent. The study was carried out according to the ethical standard of the World Medical Association Declaration of Helsinki[24].
Fibrinogen and albumin measurements
Blood specimens were collected before breakfast within seven days before surgery, in order to assess the preoperative plasma fibrinogen and serum albumin concentrations. Afterwards, Datafai Fibrinogen (Sysmex Corporation, Kobe, Japan) and CA7000 analyzer (Sysmex Corporation, Kobe, Japan) were employed to assess fibrinogen level using the previously-mentioned Clauss method[25]. The normal reference values of plasma fibrinogen and serum albumin were 2-4 g/L and 35-51 g/L, respectively, according to relevant instructions.
FAR
FAR was defined by dividing the preoperative fibrinogen level by the preoperative serum albumin level.
Treatments and Follow up
All subjects received potential curative gallbladder resection at Peking Union Medical College Hospital of CAMS & PUMC. The extent of resection was classified as modified radical cholecystectomy or radical cholecystectomy and systemic therapy according to the extent of tumor invasion, which was identified by preoperative auxiliary examination results. Follow-up visits in our center were carried out every three months for the first two years, every six months for the third year and annually thereafter. The follow-up period was defined from the date of surgery to death or the last follow-up visit.
Statistical analysis
The continuous data with normal distribution were shown as the mean ± standard deviation (Kolmogorov-Smirnov test, P > 0.05), and those with abnormal distribution were expressed as the median (minimum-maximum). Frequencies and percentages were used for the categorical variables. Chi-square test or Fisher’s exact test was utilized to assess differences in baseline clinicopathological characteristics between groups. OS referred to the duration from the date of surgery to death or the last follow-up visit. The optimal cut-off values of fibrinogen, albumin and FAR were identified by the receiver operating characteristic (ROC) curve. Kaplan-Meier method was used to generate the survival curves, followed by analysis by log-rank test. Additionally, multivariate Cox proportional hazards model was used to further assess those significant factors indicated by univariate analysis. SPSS version 24.0 (IBM Corp., Armonk, NY, United States) was utilized for statistical analysis. A two-sided P < 0.05 was considered as statistical significance, and 95% confidence intervals (CIs) were calculated.
RESULTS
Patient characteristics
All the 154 GBC patients in this study were treated at Peking Union Medical Hospital from January 2005 to May 2017. The median follow-up period was 17 mo. In total, 103 subjects died during the follow-up period, with an estimated median OS of 14.5 mo (range: 0.5-153.0 mo). The 1- and 2-year survival rates were 55.8% and 35.7%, respectively. The clinical data of all patients who met all criteria were analyzed. Among these patients, the median age at diagnosis was 64 years old (range: 29-85 years old), of whom, 98 (63.6%) were > 60 years old. Ninety-one (59.1%) patients were female. One hundred fifty (97.4%) patients were pathologically diagnosed with adenocarcinoma, three (1.9%) with adenosquamous cell carcinoma and one (0.6%) with papillary carcinoma. Ninety-four (61.0%) patients were histologically diagnosed with moderately or well-differentiated disease. Fifty-eight (37.7%) patients harbored a positive resection margin. According to the TNM staging, most patients (59.7%) were classified as stage IIIA-IIIB. The detailed information of baseline characteristics of patients was shown in Table 1.
Table 1.
Baseline characteristics of 154 gallbladder cancer patients who underwent potential curative cholecystectomy n (%)
| Characteristic | Patients (n = 154) |
| Age (yr) | 64 (29-85) |
| ≤ 60 | 56 (36.4) |
| > 60 | 98 (63.6) |
| Sex | |
| Male | 63 (40.9) |
| Female | 91 (59.1) |
| Cholecystolithiasis | |
| Absent | 79 (51.3) |
| Present | 75 (48.7) |
| Diabetes | |
| Absent | 116 (75.3) |
| Present | 38 (24.7) |
| Jaundice | |
| Absent | 129 (83.8) |
| Present | 25 (8.9) |
| Blood groups | |
| A | 43 (27.9) |
| B | 56 (36.4) |
| AB | 9 (5.8) |
| O | 46 (29.9) |
| Pathological types | |
| Adenosquamous carcinoma | 3 (1.9) |
| Adenocarcinoma | 150 (97.4) |
| Papillocarcinoma | 1 (0.6) |
| Degree of differentiation | |
| Poor | 60 (39.0) |
| Moderate-well | 94 (61.0) |
| Resection margin status | |
| Negative | 96 (62.3) |
| Positive | 58 (37.7) |
| Maximum tumor diameter (cm) | 3 (0.2-13) |
| ≤ 2.45 | 68 (44.2) |
| > 2.45 | 86 (55.8) |
| T stage | |
| Tis-T1a | 10 (6.5) |
| T1b-T2b | 29 (18.8) |
| T3 | 103 (66.9) |
| T4 | 12 (7.8) |
| N stage | |
| 0 | 98 (63.6) |
| 1 | 47 (30.5) |
| 2 | 9 (5.8) |
| Distant metastasis | |
| Absent | 142 (92.2) |
| Present | 12 (7.8) |
| TNM stage | |
| 0-Istage | 16 (10.4) |
| IIA-IIB stage | 16 (10.4) |
| IIIA-IIIB stage | 92 (59.7) |
| IVA-IVB stage | 30 (19.5) |
| CA199 (U/mL) | 69.3 (0.6-10524) |
| ≤ 39 | 66 (42.9) |
| > 39 | 88 (57.1) |
| Fibrinogen concentration (g/L) | 3.54 (1.71-7.47) |
| ≤ 3.47 | 75 (48.7) |
| > 3.47 | 79 (51.3) |
| Albumin levels (g/L) | 41.0 (20.0-50.0) |
| ≤ 40.5 | 78 (50.6) |
| > 40.5 | 76 (49.4) |
| FAR | 0.09 (0.04-0.25) |
| ≤ 0.08 | 71 (46.1) |
| > 0.08 | 83 (53.9) |
FAR: Fibrinogen to albumin ratio.
The optimal cut-off value of the preoperative fibrinogen concentration, albumin level and FAR for survival analysis
The ROC curves of OS were generated to validate the optimal cut-off values for the preoperative fibrinogen concentration, albumin level and FAR (Figure 1). The median plasma fibrinogen concentration in all patients was 3.54 g/L (range: 1.71-7.47 g/L) (Table 1). As shown in Figure 1A, the area under the curve (AUC) was recorded as 0.735 (95%CI: 0.654-0.816), and the optimal cut-off value of preoperative fibrinogen concentration for OS was 3.47 g/L, with the highest sensitivity and specificity of 0.709 and 0.721, respectively. Based on this cut-off, there were 75 patients (48.7%) with a fibrinogen concentration ≤ 3.47 g/L, and 79 patients (51.3%) with a fibrinogen concentration > 3.47 g/L (Table 2).
Figure 1.
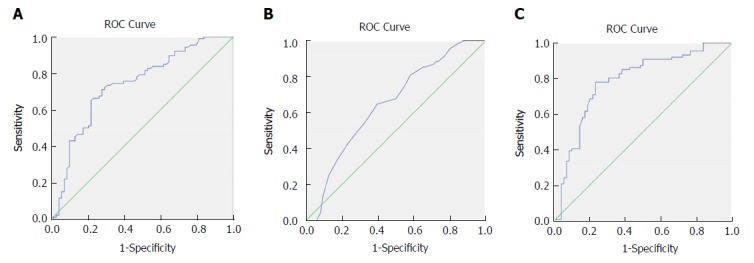
Receiver operating characteristics curve analysis based on fibrinogen (A), albumin (B), and fibrinogen to albumin ratio (C) for overall survival. A: The area under the ROC curve (AUC) indicates the diagnostic power of preoperative plasma fibrinogen concentration. In this model, the optimum cut-off point for fibrinogen concentration was 3.47 g/L, AUC was 0.735 (95%CI: 0.654-0.816), with a sensitivity of 0.709 and a specificity of 0.721 by the Youden index; B: The AUC indicates the diagnostic power of preoperative plasma albumin level. In this model, the optimum cut-off point for albumin level was 40.5 g/L, AUC was 0.648 (95%CI: 0.562-0.735), with a sensitivity of 0.647 and a specificity of 0.605 by the Youden index; C: The AUC indicates the diagnostic power of preoperative FAR. In this model, the optimum cut-off point for FAR was 0.08, AUC was 0.783 (95%CI: 0.707-0.859), with a sensitivity of 0.779 and a specificity of 0.765 by the Youden index. ROC: Receiver operating characteristics curve.
Table 2.
Correlation between fibrinogen concentration and clinicopathological characteristics in gallbladder cancer patients n (%)
| Characteristics |
Fibrinogen concentration |
P value | |
| ≤ 3.47 g/L (n = 75) | > 3.47 g/L (n = 79) | ||
| Age (yr) | |||
| ≤ 60 | 31 (20.1) | 25 (16.2) | 0.243 |
| > 60 | 44 (28.6) | 54 (35.1) | |
| Sex | |||
| Male | 33 (21.4) | 30 (19.5) | 0.513 |
| Female | 42 (27.3) | 49 (31.8) | |
| Cholecystolithiasis | |||
| Absent | 38 (24.7) | 41 (26.6) | 0.878 |
| Present | 37 (24.0) | 38 (24.7) | |
| Diabetes | |||
| Absent | 57 (37.0) | 59 (38.3) | 0.850 |
| Present | 18 (11.7) | 20 (13.0) | |
| Jaundice | |||
| Absent | 68 (44.2) | 61 (39.6) | 0.029 |
| Present | 7 (4.5) | 18 (11.7) | |
| Blood groups | |||
| A | 19 (12.3) | 24 (15.6) | 0.145 |
| B | 33 (21.4) | 23 (14.9) | |
| AB | 2 (1.3) | 7 (4.5) | |
| O | 21 (13.6) | 25 (16.2) | |
| Pathological types | |||
| Adenosquamous carcinoma | 0 (0) | 3 (1.9) | 0.142 |
| Adenocarcinoma | 75 (48.7) | 75 (48.7) | |
| Papillocarcinoma | 0 (0) | 1 (0.6) | |
| Degree of differentiation | |||
| Poor | 23 (14.9) | 37 (24.0) | 0.048 |
| Moderate-well | 52 (33.8) | 42 (27.3) | |
| Resection margin status | |||
| Negative | 56 (36.4) | 40 (26.4) | 0.003 |
| Positive | 19 (12.3) | 39 (25.3) | |
| Maximum tumor diameter (cm) | |||
| ≤ 2.45 | 34 (22.1) | 34 (22.1) | 0.871 |
| > 2.45 | 41 (26.6) | 45 (29.2) | |
| T stage | |||
| Tis-T1a | 8 (5.2) | 2 (1.3) | < 0.001 |
| T1b-T2b | 22 (14.3) | 7 (4.5) | |
| T3 | 43 (27.9) | 60 (39.0) | |
| T4 | 2 (1.3) | 10 (6.5) | |
| N stage | |||
| N0 | 50 (32.5) | 48 (31.2) | 0.748 |
| N1 | 21 (13.6) | 26 (16.9) | |
| N2 | 4 (2.6) | 5 (3.2) | |
| Distant metastasis | |||
| Absent | 69 (44.8) | 73 (47.4) | 0.925 |
| Present | 6 (3.9) | 6 (3.9) | |
| TNM stage | |||
| 0-Istage | 12 (7.8) | 4 (2.6) | 0.011 |
| IIA-IIB stage | 12 (7.8) | 4 (2.6) | |
| IIIA-IIIB stage | 39 (25.3) | 53 (34.4) | |
| IVA-IVB stage | 12 (7.8) | 18 (11.7) | |
| CA199 (U/mL) | |||
| ≤ 39 | 41 (26.6) | 25 (16.2) | 0.005 |
| > 39 | 34 (22.1) | 54 (35.1) | |
| Albumin levels (g/L) | |||
| ≤ 40.5 | 32 (20.8) | 44 (28.6) | 0.111 |
| > 40.5 | 43 (27.9) | 35 (22.7) | |
| FAR | |||
| ≤ 0.08 | 59 (38.3) | 12 (7.8) | < 0.001 |
| > 0.08 | 16 (10.4) | 67 (43.5) | |
FAR: Fibrinogen to albumin ratio.
The median serum albumin level in all patients was 41.0 g/L (range: 20.0-40.0 g/L) (Table 1). As shown in Figure 1B, the AUC was recorded as 0.648 (95%CI: 0.562-0.735), and the optimal cut-off value of the preoperative albumin level for OS was 40.5 g/L, with the highest sensitivity and specificity of 0.647 and 0.605, respectively. Based on this value, 76 patients (49.4%) had an albumin level ≤ 40.5 g/L, and 78 patients (50.6%) had an albumin level > 40.5 g/L (Table 3).
Table 3.
Correlation between albumin levels and clinicopathological characteristics in gallbladder cancer patients n (%)
| Characteristics |
Albumin levels |
P value | |
| ≤ 40.5g/L (n = 76) | > 40.5 g/L (n = 78) | ||
| Age (yr) | |||
| ≤ 60 | 22 (14.3) | 34 (22.1) | 0.067 |
| > 60 | 54 (35.1) | 44 (28.6) | |
| Sex | |||
| Male | 28 (18.2) | 35 (22.7) | 0.330 |
| Female | 48 (31.2) | 43 (27.9) | |
| Cholecystolithiasis | |||
| Absent | 34 (22.1) | 45 (29.2) | 0.147 |
| Present | 42 (27.3) | 33 (21.4) | |
| Diabetes | |||
| Absent | 53 (34.4) | 63 (40.9) | 0.136 |
| Present | 23 (14.9) | 15 (9.7) | |
| Jaundice | |||
| Absent | 54 (35.1) | 75 (48.7) | < 0.001 |
| Present | 22 (14.3) | 3 (1.9) | |
| Blood groups | |||
| A | 20 (13.0) | 23 (14.9) | 0.046 |
| B | 34 (22.1) | 22 (14.3) | |
| AB | 6 (7.9) | 3 (3.8) | |
| O | 16 (21.1) | 30 (19.5) | |
| Pathological types | |||
| Adenosquamous carcinoma | 0 (0) | 3 (1.9) | 0.137 |
| Adenocarcinoma | 75 (48.7) | 75 (48.7) | |
| Papillocarcinoma | 1 (0.6) | 0 (0.0) | |
| Degree of differentiation | |||
| Poor | 36 (23.4) | 24 (15.6) | 0.047 |
| Moderate-well | 40 (26.0) | 54 (35.1) | |
| Resection margin status | |||
| Negative | 39 (25.3) | 57 (37.0) | 0.008 |
| Positive | 37 (24.0) | 21 (13.6) | |
| Maximum tumor diameter (cm) | |||
| ≤ 2.45 | 36 (23.4) | 32 (20.8) | 0.516 |
| > 2.45 | 40 (26.0) | 46 (29.9) | |
| T stage | |||
| Tis-T1a | 2 (1.3) | 8 (5.2) | 0.021 |
| T1b-T2b | 9 (5.8) | 20 (13.0) | |
| T3 | 58 (37.7) | 45 (29.2) | |
| T4 | 7 (4.5) | 5 (3.2) | |
| N stage | |||
| N0 | 45 (29.2) | 53 (34.4) | 0.403 |
| N1 | 25 (16.2) | 22 (14.3) | |
| N2 | 6 (3.9) | 3 (1.9) | |
| Distant metastasis | |||
| Absent | 67 (43.5) | 75 (48.7) | 0.077 |
| Present | 9 (5.8) | 3 (1.9) | |
| TNM stage | |||
| 0-Istage | 3 (1.9) | 13 (8.4) | 0.007 |
| IIA-IIB stage | 6 (3.9) | 10 (6.5) | |
| IIIA-IIIB stage | 46 (29.9) | 46 (29.9) | |
| IVA-IVB stage | 21 (13.6) | 9 (5.8) | |
| CA199 (U/mL) | |||
| ≤ 39 | 24 (15.6) | 42 (27.3) | 0.006 |
| > 39 | 52 (33.8) | 36 (23.4) | |
| Fibrinogen concentration (g/L) | 0.111 | ||
| ≤ 3.47g/L | 32 (20.8) | 43 (27.9) | |
| > 3.47 g/L | 44 (28.6) | 35 (22.7) | |
| FAR | |||
| ≤ 0.08 | 21 (13.6) | 50 (32.5) | < 0.001 |
| > 0.08 | 55 (35.7) | 28 (18.2) | |
FAR: Fibrinogen to albumin ratio.
The median FAR in all patients was 0.09 (range: 0.04-0.25) (Table 1). As shown in Figure 1C, the AUC was recorded as 0.783 (95%CI: 0.707-0.859), and the optimal cut-off value of the preoperative FAR for OS was 0.08, with the highest sensitivity and specificity of 0.779 and 0.765, respectively. Based on this value, 71 patients (46.1%) harbored a FAR value ≤ 0.08, and 83 patients (53.9%) had a FAR value > 0.08 (Table 4).
Table 4.
Correlation between FAR and clinicopathological characteristics in gallbladder cancer patients n (%)
| Characteristics |
FAR |
P value | |
| ≤ 0.08 (n = 71) | > 0.08 (n = 83) | ||
| Age (yr) | |||
| ≤ 60 | 32 (20.8) | 24 (15.6) | 0.045 |
| > 60 | 39 (25.3) | 59 (38.3) | |
| Sex | |||
| Male | 30 (19.5) | 33 (21.4) | 0.870 |
| Female | 41 (26.6) | 50 (32.5) | |
| Cholecystolithiasis | |||
| Absent | 37 (24.0) | 42 (27.3) | 0.873 |
| Present | 34 (22.1) | 41 (26.6) | |
| Diabetes | |||
| Absent | 56 (36.4) | 60 (39.0) | 0.357 |
| Present | 15 (9.7) | 23 (14.9) | |
| Jaundice | |||
| Absent | 67 (43.5) | 62 (40.3) | < 0.001 |
| Present | 4 (2.6) | 21 (13.6) | |
| Blood groups | |||
| A | 22 (14.3) | 21 (13.6) | 0.148 |
| B | 28 (18.2) | 28 (18.2) | |
| AB | 1 (0.6) | 8 (5.2) | |
| O | 20 (13.0) | 26 (16.9) | |
| Pathological types | |||
| Adenosquamous carcinoma | 0 (0) | 3 (1.9) | 0.173 |
| Adenocarcinoma | 71 (46.1) | 79 (51.3) | |
| Papillocarcinoma | 0 (0) | 1 (0.6) | |
| Degree of differentiation | |||
| Poor | 18 (11.7) | 42 (27.3) | 0.002 |
| Moderate-well | 53 (34.4) | 41 (26.6) | |
| Resection margin status | |||
| Negative | 55 (35.7) | 41 (26.6) | < 0.001 |
| Positive | 16 (10.4) | 42 (27.3) | |
| Maximum tumor diameter (cm) | |||
| ≤ 2.45 | 37 (24.0) | 31 (20.1) | 0.075 |
| > 2.45 | 34 (22.1) | 52 (33.8) | |
| T stage | |||
| Tis-T1a | 8 (5.2) | 2 (1.3) | < 0.001 |
| T1b-T2b | 24 (15.6) | 5 (3.2) | |
| T3 | 36 (23.4) | 67 (43.5) | |
| T4 | 3 (1.9) | 9 (5.8) | |
| N stage | |||
| N0 | 48 (31.2) | 50 (32.5) | 0.623 |
| N1 | 19 (12.3) | 28 (18.2) | |
| N2 | 4 (2.6) | 5 (3.2) | |
| Distant metastasis | |||
| Absent | 68 (44.2) | 74 (48.1) | 0.145 |
| Present | 3 (1.9) | 9 (5.8) | |
| TNM stage | |||
| 0-Istage | 14 (9.1) | 2 (1.3) | < 0.001 |
| IIA-IIB stage | 14 (9.1) | 2 (1.3) | |
| IIIA-IIIB stage | 35 (22.7) | 57 (37.0) | |
| IVA-IVB stage | 8 (5.2) | 22 (14.3) | |
| CA199 (U/mL) | |||
| ≤ 39 | 43 (27.9) | 23 (14.9) | < 0.001 |
| > 39 | 28 (18.2) | 60 (39.0) | |
| Fibrinogen concentration (g/L) | |||
| ≤ 3.47g/L | 59 (38.3) | 16 (10.4) | < 0.001 |
| > 3.47 g/L | 12 (7.8) | 67 (43.5) | |
| Albumin levels (g/L) | |||
| ≤ 40.5g/L | 21 (13.6) | 55 (35.7) | < 0.001 |
| > 40.5 g/L | 50 (32.5) | 28 (18.2) | |
Correlations of the preoperative fibrinogen concentration, albumin level and FAR with clinicopathological factors
As shown in Table 2, based on the optimal cut-off value for the preoperative fibrinogen concentration, all patients could be divided into the low-value group (≤ 3.47 g/L) or the high-value group (> 3.47 g/L). Higher preoperative fibrinogen concentration was significantly correlated with jaundice (P = 0.003), degree of differentiation (P = 0.048), resection margin (P = 0.003), T stage (P < 0.001), TNM stage (P = 0.011), CA199 level (P = 0.005) as well as FAR (P < 0.001). However, there were no significant associations of the preoperative fibrinogen concentration with age, gender, cholecystolithiasis, diabetes, ABO blood group, pathological type, tumor size, N stage, distant metastasis or albumin level (P > 0.05). The survival curve stratified by the fibrinogen concentration indicated that GBC subjects with a fibrinogen concentration > 3.47 g/L had shorter OS than those with a fibrinogen concentration ≤ 3.47 g/L (Figure 2A).
Figure 2.
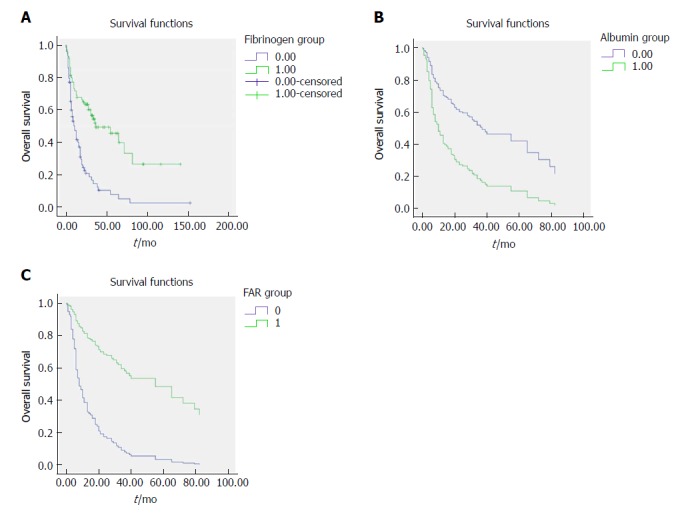
Survival curve according to the presence of preoperative fibrinogen concentration (A), albumin level (B), and fibrinogen to albumin ratio (C). A: Data compares fibrinogen concentration > 3.47 g/L vs ≤ 3.47 g/L group (P < 0.05). The number 1 for ≤ 3.47 g/L group, number 2 for > 3.47g/L group; B: Data compares albumin level > 40.5 g/L vs ≤ 40.5 g/L group (P < 0.05). The number 0 for albumin level > 40.5 g/L group, number 1 for albumin level ≤ 40.5 g/L group; C: Data compares FAR > 0.08 vs ≤ 0.08 group (P < 0.05). The number 1 for FAR > 0.08 group, number 0 for FAR ≤ 0.08 g/L group. FAR: Fibrinogen to albumin ratio.
As shown in Table 3, based on the optimal cut-off value for the preoperative albumin level, all patients could be categorized into the low-value group (≤ 40.5 g/L) or high-value group (> 40.5 g/L). Higher preoperative albumin levels were significantly associated with jaundice (P < 0.001), ABO blood group (P = 0.046), degree of differentiation (P = 0.047), resection margin status (P = 0.008), T stage (P = 0.021), TNM stage (P = 0.007), CA199 levels (P = 0.006) as well as FAR (P < 0.001). The survival curve stratified by the albumin level showed that GBC patients with an albumin level > 40.5 g/L had longer OS than those with an albumin level ≤ 40.5 g/L (Figure 2B).
As shown in Table 4, based on the optimal cut-off value for the preoperative FAR, all patients could be grouped into the low-value group (≤ 0.08) or high-value group (> 0.08). A higher preoperative FAR was significantly correlated with age (P = 0.045), jaundice (P < 0.001), degree of differentiation (P = 0.002), resection margin status (P < 0.001), T stage (P < 0.001), TNM stage (P < 0.001), CA199 level (P < 0.001) as well as albumin level (P < 0.001). The survival curve stratified by the FAR showed that GBC patients with a FAR > 0.08 harbored worse OS compared to those with a FAR ≤ 0.08 (Figure 2C).
Univariate and multivariate analysis results
Univariate and multivariate analyses for OS prediction in GBC patients are shown in Tables 5 and 6. In the univariate Cox analysis, jaundice (HR: 2.598, 95%CI: 1.644-4.106, P < 0.001), degree of differentiation (HR: 1.527, 95%CI: 1.031-2.261, P = 0.035), resection margin status (HR: 3.683, 95%CI: 2.468-5.496, P < 0.001), T stage (P < 0.001), N stage (P < 0.001), distant metastasis (HR: 2.550, 95%CI: 1.388-4.684, P = 0.003), TNM stage (P < 0.001), CA199 level (HR: 3.125, 95%CI: 2.010-4.858, P < 0.001), fibrinogen concentration (HR: 2.795, 95%CI: 1.853-4.214, P < 0.001), albumin level (HR: 0.391, 95%CI: 0.259-0.590,
Table 5.
Univariate analysis of overall survival in gallbladder cancer patients
| Characteristics | HR (95%CI) | P value |
| Age (yr) | 1.473 (0.973-2.230) | 0.067 |
| ≤ 60 | ||
| > 60 | ||
| Sex | 0.995 (0.670-1.477) | 0.981 |
| Male | ||
| Female | ||
| Cholecystolithiasis | 1.198 (0.814-1.764) | 0.360 |
| Absent | ||
| Present | ||
| Diabetes | 1.028 (0.651-1.623) | 0.906 |
| Absent | ||
| Present | ||
| Jaundice | 2.598 (1.644-4.106) | < 0.001 |
| Absent | ||
| Present | ||
| Blood groups | - | 0.113 |
| A | ||
| B | ||
| AB | ||
| O | ||
| Pathological types | - | 0.165 |
| Adenosquamous carcinoma | ||
| Adenocarcinoma | ||
| Papillocarcinoma | ||
| Degree of differentiation | 1.527 (1.031-2.261) | 0.035 |
| Poor | ||
| Moderate-well | ||
| Resection margin status | 3.683 (2.468-5.496) | < 0.001 |
| Negative | ||
| Positive | ||
| Maximum tumor diameter (cm) | 1.101 (0.744-1.630) | 0.631 |
| ≤ 2.45 | ||
| > 2.45 | ||
| T stage | - | < 0.001 |
| Tis-T1a | ||
| T1b-T2b | ||
| T3 | ||
| T4 | ||
| N stage | - | < 0.001 |
| 0 | ||
| 1 | ||
| 2 | ||
| Distant metastasis | 2.550 (1.388-4.684) | 0.003 |
| Absent | ||
| Present | ||
| TNM stage | - | < 0.001 |
| 0-Istage | ||
| IIA-IIB stage | ||
| IIIA-IIIB stage | ||
| IVA-IVB stage | ||
| CA199 (U/mL) | 3.125 (2.010-4.858) | < 0.001 |
| ≤ 39 | ||
| > 39 | ||
| Fibrinogen concentration (g/L) | 2.795 (1.853-4.214) | < 0.001 |
| ≤ 3.47 | ||
| > 3.47 | ||
| Albumin levels (g/L) | 0.391(0.259-0.590) | < 0.001 |
| ≤ 40.5 | ||
| > 40.5 | ||
| FAR | 4.626(2.987-7.165) | < 0.001 |
| ≤ 0.08 | ||
| > 0.08 |
FAR: Fibrinogen to albumin ratio; HR: Hazard ratio; CI: Confidence interval.
Table 6.
Multivariate analysis for overall survival in gallbladder cancer patients
| Characteristics | HR (95%CI) | Wald | P value |
| Resection margin status | 2.343 (1.532-3.581) | < 0.001 | |
| Negative | |||
| Positive | |||
| TNM stage | 8.595 | 0.035 | |
| IIA-IIB stage/0-1stage | 1.209 (0.287-5.095) | 0.067 | 0.796 |
| IIIA-IIIB stage/0-1 stage | 3.401 (1.033-11.202) | 4.051 | 0.044 |
| IVA-IVB stage/0-1 stage | 4.014 (1.142-14.107) | 4.696 | 0.030 |
| FAR | 2.813 (1.765-4.484) | < 0.001 | |
| ≤ 0.08 | |||
| > 0.08 | |||
| Albumin levels (g/L) | 0.595 (0.385-0.921) | 0.020 | |
| ≤ 40.5 | |||
| > 40.5 |
FAR: Fibrinogen to albumin ratio; HR: Hazard ratio; CI: Confidence interval.
P < 0.001) and FAR (HR: 4.626, 95%CI: 2.987-7.165, P < 0.001) were significant prognostic factors for OS in GBC patients (Table 5), whereas age, gender, cholecystolithiasis, diabetes, ABO blood group, pathological type and maximal tumor diameter were not significant predictors of OS (P > 0.05; Table 5). In the multivariate Cox regression analysis, resection margin status (HR: 2.343, 95%CI: 1.532-3.581, P < 0.001, TNM stage (P = 0.035), and FAR (HR: 2.813, 95%CI: 1.765-4.484, P < 0.001) were revealed as independent risk factors for poor OS in GBC patients, and the albumin level (HR: 0.595, 95%CI: 0.385-0.921, P = 0.020) was correlated with favorable OS in patients with GBC (Table 6).
Although the multivariate analysis showed that both FAR and albumin level were independent risk factors for the prognosis of GBC patients (Table 6), the AUC of FAR (0.783) was greater than that (0.648) of the albumin level (Figure 1B and C), indicating that the prognostic value of FAR was more powerful than that of the albumin level.
DISCUSSION
In this study, we demonstrate FAR is a significantly independent prognostic indicator for GBC. To our knowledge, it is the first research concerning the prognostic significance of FAR in patients with GBC. Although both FAR and serum albumin level were revealed to be significant prognostic indicators, the AUC of FAR was greater than that of the serum albumin level, and the P value of FAR was smaller than that of the serum albumin level.
In our study, a greater FAR was found to be correlated with a series of important clinicopathological indicators of GBC patients, such as resection margin status, TNM stage and albumin level, which were independent risk factors for OS in GBC, indicating that an elevated FAR might be associated with aggressiveness and systemic progression of GBC.
Relatively few previous studies have probed into the prognostic significance of FAR in patients with malignant tumors. To date, there are only two studies performed in the context of breast cancer[23] and ESCC[22]. In line with our findings, the optimal cut-off value of FAR for ESCC patients was also 0.08. However, the optimal cutoff value of FAR in breast cancer was 0.071, which is slightly lower than that in ESCC patients and in GBC subjects in our study. Together, the inconsistent findings indicate that the optimal FAR cut-off value varies in different malignancies. Although the exact cause and underlying mechanism of these differences remain unknown, they might be related to the different biological behaviors of different tumors and gender-associated hormone difference. Hence, more studies are needed to further verify these conclusions.
Inconsistent with these previous two studies, our study indicates that the preoperative albumin level is also an independent risk factor for OS in GBC patients, and an elevated albumin level is a favorable prognostic factor for GBC patients. Several studies have demonstrated that lower serum albumin levels could lead to deteriorated diseases and a greater risk of poor prognosis in patients with gastric cancer[26], ovarian cancer[27] and upper urinary tract urothelial carcinoma[12]. However, to our knowledge, it is the first study to assess the prognostic significance of preoperative serum albumin in GBC.
Accumulating studies have demonstrated the effect of activated coagulation with fibrinolysis, malnutrition and inflammation during carcinogenesis, cancer progression and metastasis[28-31]. Although the prognostic value of the preoperative FAR has been established in patients with malignant tumors[22,23], the real mechanisms underlying this association remains largely undefined. Our observations are supported by several previous experimental and clinical researches. As a P-globulin and pro-inflammatory protein, fibrinogen can be synthesized by malignant tumor cells apart from hepatic cells, which participates in extracellular matrix (ECM) formation[32-34]. Fibrinogen can promote tumor progression via regulation of tumor cell growth by binding to vascular endothelial growth factor (VEGF) as well as platelet-derived growth factor (PDGF)[33-35]. An experimental study has demonstrated that fibrinogen can induce epithelial-mesenchymal transition (EMT) to enhance the migration and invasion ability of tumor cells via modulation of the expression of vimentin and E-cadherin[36]. Another experimental study performed in fibrinogen-deficient mice indicates that fibrinogen-free internal environment can suppress the spread of tumor cells and the subsequent establishment of micro-metastases[37]. A previous study[38] also showed that fibrinogen could facilitate tumor cell metastasis by suppressing natural killer (NK) cell-mediated apoptosis. Fibrinogen has also been demonstrated to be critically involved in the tumorigenesis and tumor progression via aggravation of cell proliferation, suppression of apoptosis and stimulation of angiogenesis as well as hematogenous metastasis[33,34,39-41]. The albumin level can not only reflect the malnutrition status of host, but also implicate the existence of inflammation. Malnutrition is commonly detected in cancer patients, which might lead to multiple negative outcomes, including compromised immune function, insensitive therapeutic response as well as reduced OS[42]. As part of the SIR to tumor or from tumor itself, inflammatory mediators are secreted, including tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6 as well as acute-phase reactants. IL-6 has been suggested to modulate VEGF secretion from glioblastoma cells, and the latter can result in vascular permeability, contributing to declined serum albumin levels[43,44]. Proinflammatory cytokines, such as TNF-α, IL-1 and IL-6 can downregulate the hepatic synthesis of albumin[45-48]. Therefore, albumin level might be used to reflect tumor prognosis. Taken together, FAR could be considered as a prognostic factor for GBC patients.
There are certain limitations in this study. To begin with, this study was a retrospective, small-sample, single-center one, hence, there might be a selection bias. Secondly, due to the small sample size, we were unable to perform further subgroup analysis according to different models, such as the TNM stage model, treatment model and distant metastasis model. Thirdly, our findings lacked external verification, which requires further investigation. Fourth, although the cut-off values were calculated by ROC curves, they were based on a relatively small sample; as such, other cut-off values may be more accurate in the case of increased sample size. In this study, we mainly focused on the prognostic significance of the preoperative FAR, while changes in the postoperative FAR have not been studied; thus, the prognostic value of the postoperative FAR was not assessed. Therefore, more well-designed, prospective and large-sample multi-center studies are warranted to further verify the present conclusions.
In conclusion, the preoperative FAR is a significant and powerful negative prognostic indicator for OS in GBC patients, and the preoperative serum albumin level is a favorable prognostic factor for OS in GBC patients, and the predictive power of FAR is greater than that of the albumin level. As a simple, convenient and cost-effective indicator, FAR, defined as the fibrinogen-to-albumin ratio, could easily be applied in the clinical setting via routine preoperative laboratory tests to predict the prognosis of GBC patients. However, more related studies are warranted to validate these conclusions.
ARTICLE HIGHLIGHTS
Research background
Although gallbladder cancer (GBC) is a relatively rare hepato-biliary malignancy with a low incidence, it is generally insidious and progresses rapidly. Most GBC patients are diagnosed at an advanced stage, losing the chance of surgical intervention, which is considered to yield an optimal therapeutic effect. Despite the great advance in surgical techniques in recent years, the prognosis remains very poor. Therefore, it is urgent to explore a clinically simple, convenient and cost-effective prognostic indicator to detect and identify high-risk patients with GBC, on whom, appropriate surgical treatment can be performed as soon as possible. In recent years, a variety of studies have shown that the increased plasma fibrinogen concentration representing coagulation function of the body and the declined plasma albumin concentration indicating nutrient state of the body are independent risk factors for poor prognosis of malignant tumor patients. Integrating the results of studies on fibrinogen-to-albumin ratio (FAR) in the prognosis of patients with esophageal cancer and breast cancer, we naturally speculate that FAR might be significantly more effective than single elevated plasma fibrinogen concentration or reduced plasma albumin concentration in predicting the prognosis of GBC patients.
Research motivation
Hence, the present was mainly designed to determine and verify the role of high FAR in the prognosis of surgically-treated GBC patients. We aimed to detect a simple, convenient and cost-effective prognostic biomarker for GBC patients undergoing surgical treatment, which could facilitate the selection and identification of GBC patients suitable for surgical resection for clinical surgeons. Notably, this would be beneficial to both surgeons and GBC patients. Our findings would provide clinical evidence and research directions for other large-scale, multi-center randomized controlled trials in the future.
Research objectives
The main objective of our study was to determine whether high preoperative FAR was an independent risk factors for postoperative survival in GBC patients. As a result, we demonstrated that high preoperative plasma FAR value and low preoperative plasma albumin concentration were independent risk factors for poor post-operative prognosis of GBC patients. In addition, the prognostic effect of high preoperative FAR value was significantly stronger than the low preoperative plasma albumin concentration. Therefore, these above-described outcomes provided not only clinical direction for further clinical validation or relevant studies, but also clinical data for further researches concerning the underlying mechanisms.
Research methods
First, the present study was a clinical retrospective one. A prearranged EXCEL data collection table was utilized to collect and organize the various variables, including epidemiological data, clinicopathological characteristics, and research-related target data. Moreover, the receiver operating characteristic (ROC) curve was used to obtain the optimal cut-off values for fibrinogen, albumin, and FAR. Continuous variables in normal distribution were shown as mean ± SD, and continuous variables without normal distribution were expressed as medians (range: minimum-maximum). Categorical variables were expressed as percentages or frequencies. Variables from the EXCEL table were further imported into the SPSS 24.0 statistical software for statistical analysis. Of note, the statistical methods used in our study were different from those used in previous studies of survival analysis regarding the prognosis of cancer patients. To begin with, the ROC curve was used to identify the optimal cut-off value of fibrinogen, albumin, and FAR in this study, which was more reasonable and more scientific than the traditional methods, which used the mean value of the targeted or identified biomarkers based on previous studies. It was because the cut-off value identified by this method was significantly associated with the overall survival of the targeted population. Secondly, most of the previous studies on postoperative prognosis of GBC patients only focused on single index, such as plasma fibrinogen or plasma albumin. However, in this study, we used the plasma FAR, representing the division of high fibrinogen and low albumin, which contributed to the more significant prognostic effect of the index, and effectively inhibited the influence of confounding factors. Together, the method was more scientific and harbored higher statistical efficiency.
Research results
In this study, we demonstrated that high preoperative plasma FAR and low preoperative plasma albumin concentration were independent risk factors for poor postoperative outcome in GBC patients. To the best of our knowledge, this is the first study indicating that high preoperative plasma FAR is an unfavorable prognostic biomarker for GBC patients undergoing surgical intervention. Additionally, it also verifies the role of low preoperative plasma albumin in predicting the worse prognosis of GBC patients receiving surgery. Nevertheless, our study is a retrospective study but not a prospective study, which might lead to a systematic bias. Moreover, the sample size in our study is relatively small, and it is a single-center study, and these defects would attenuate the statistical effectiveness of our conclusions. Nevertheless, the study was conducted in China, which did not include GBC patients from other ethnic groups and countries, thereby affecting the clinical applicability and generalizability of the results. Therefore, more multiple-center, large-scale prospective studies enrolling GBC patients from different races and countries are necessary to further verify the conclusions of this study.
Research conclusions
At present, accumulating studies have confirmed that high preoperative plasma fibrinogen concentration and low preoperative plasma albumin concentration are independent risk factors for poor prognosis of GBC patients. In addition, some studies have further validated that high preoperative plasma FAR is an independent risk factor for poor prognosis of patients with esophageal cancer and breast cancer, and its predictive ability is significantly more potent than that of single biomarkers, such as high plasma fibrinogen and low plasma albumin. Therefore, we naturally speculated that FAR, representing the body’s coagulation function and the body’s nutritional status, might be an independent risk factor for predicting postoperative adverse outcomes of GBC patients, which has been confirmed in our study. Our study was the first to reveal the prognostic effect of FAR in GBC patients, and we also used the ROC curve as a novel method to identify the optimal cut-off value for the prognostic index studied. The potential mechanism for our conclusion might be indicated as follows: fibrinogen, as a coagulation factor, was associated with the growth, progression and metastasis of cancer cells, while albumin was correlated with the nutrient status and immune function of the body. Therefore, the high preoperative plasma fibrinogen and low preoperative albumin are both unfavorable prognostic factors for GBC patients. The FAR can enhance and magnify the prognostic effect of the single index such as fibrinogen and albumin. Collectively, our research provides a simple, convenient and cost-effective prognostic indicator to help clinicians to more efficiently screen and identify high-risk GBC patients in clinical practice, and to facilitate patients to adopt better surgical methods and optimal follow-up strategy in the future.
Research perspectives
In the present study, it is indicated that the plasma FAR, incorporating two biomarkers, harbors a significantly better prognostic impact on surgically-treated GBC patients compared to a single prognostic indicator, such as plasma albumin or plasma fibrinogen. In the future, more large-scale, multiple-center and prospective studies, including GBC patients from other races and countries, should be conducted to further investigate and verify the conclusion derived from our study. Additionally, more basic experiments exploring the potential mechanisms are also necessary in the future.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The publication of this manuscript has been reviewed and approved by the PUMCH Institutional Review Board.
Informed consent statement: All patients and their families signed informed consent statements before surgery, and the type of surgical procedure was performed according to the approved guidelines.
Conflict-of-interest statement: We declare that the authors have no conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started:April 20, 2018
First decision: June 6, 2018
Article in press: June 27, 2018
P- Reviewer: Okada S, Snyder J, Tatsuya O S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y
Contributor Information
Wei-Yu Xu, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Hao-Hai Zhang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Jian-Ping Xiong, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Xiao-Bo Yang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Yi Bai, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Jian-Zhen Lin, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Jun-Yu Long, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Yong-Chang Zheng, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Hai-Tao Zhao, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Xin-Ting Sang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China. sangxt@pumch.cn..
References
- 1.Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist. 2010;15:168–181. doi: 10.1634/theoncologist.2009-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paliogiannis P, Scognamillo F, Attene F, Marrosu A, Trignano E, Tedde L, Delogu D, Trignano M. Preneoplastic and neoplastic gallbladder lesions occasionally discovered after elective videocholecystectomy for benign disease. A single centre experience and literature review. Ann Ital Chir. 2013;84:281–285. [PubMed] [Google Scholar]
- 3.Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167–176. doi: 10.1016/s1470-2045(03)01021-0. [DOI] [PubMed] [Google Scholar]
- 4.Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, D’Angelica M, Dematteo RP, Blumgart LH, O’Reilly EM. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC) J Surg Oncol. 2008;98:485–489. doi: 10.1002/jso.21141. [DOI] [PubMed] [Google Scholar]
- 5.Lai EC, Lau WY. Aggressive surgical resection for carcinoma of gallbladder. ANZ J Surg. 2005;75:441–444. doi: 10.1111/j.1445-2197.2005.03401.x. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett DL, Fong Y, Fortner JG, Brennan MF, Blumgart LH. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224:639–646. doi: 10.1097/00000658-199611000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004;90:1704–1706. doi: 10.1038/sj.bjc.6601789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn HS, Lee HJ, Yoo MW, Kim SG, Im JP, Kim SH, Kim WH, Lee KU, Yang HK. Diagnostic accuracy of T and N stages with endoscopy, stomach protocol CT, and endoscopic ultrasonography in early gastric cancer. J Surg Oncol. 2009;99:20–27. doi: 10.1002/jso.21170. [DOI] [PubMed] [Google Scholar]
- 9.Lee HH, Lim CH, Park JM, Cho YK, Song KY, Jeon HM, Park CH. Low accuracy of endoscopic ultrasonography for detailed T staging in gastric cancer. World J Surg Oncol. 2012;10:190. doi: 10.1186/1477-7819-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ando M, Ando Y, Hasegawa Y, Shimokata K, Minami H, Wakai K, Ohno Y, Sakai S. Prognostic value of performance status assessed by patients themselves, nurses, and oncologists in advanced non-small cell lung cancer. Br J Cancer. 2001;85:1634–1639. doi: 10.1054/bjoc.2001.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajan R, Poniecka A, Smith TL, Yang Y, Frye D, Pusztai L, Fiterman DJ, Gal-Gombos E, Whitman G, Rouzier R, et al. Change in tumor cellularity of breast carcinoma after neoadjuvant chemotherapy as a variable in the pathologic assessment of response. Cancer. 2004;100:1365–1373. doi: 10.1002/cncr.20134. [DOI] [PubMed] [Google Scholar]
- 12.Ku JH, Kim M, Choi WS, Kwak C, Kim HH. Preoperative serum albumin as a prognostic factor in patients with upper urinary tract urothelial carcinoma. Int Braz J Urol. 2014;40:753–762. doi: 10.1590/S1677-5538.IBJU.2014.06.06. [DOI] [PubMed] [Google Scholar]
- 13.Perisanidis C, Psyrri A, Cohen EE, Engelmann J, Heinze G, Perisanidis B, Stift A, Filipits M, Kornek G, Nkenke E. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015;41:960–970. doi: 10.1016/j.ctrv.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Son HJ, Park JW, Chang HJ, Kim DY, Kim BC, Kim SY, Park SC, Choi HS, Oh JH. Preoperative plasma hyperfibrinogenemia is predictive of poor prognosis in patients with nonmetastatic colon cancer. Ann Surg Oncol. 2013;20:2908–2913. doi: 10.1245/s10434-013-2968-8. [DOI] [PubMed] [Google Scholar]
- 15.Bairey O, Shacham-Abulafia A, Shpilberg O, Gurion R. Serum albumin level at diagnosis of diffuse large B-cell lymphoma: an important simple prognostic factor. Hematol Oncol. 2016;34:184–192. doi: 10.1002/hon.2233. [DOI] [PubMed] [Google Scholar]
- 16.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Hyun JH, Kim DY, Yoo BC, Park JW, Kim SY, Chang HJ, Kim BC, Kim TH, Oh JH, et al. The role of fibrinogen as a predictor in preoperative chemoradiation for rectal cancer. Ann Surg Oncol. 2015;22:209–215. doi: 10.1245/s10434-014-3962-5. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Zhao M, Jin B, Yu P, Hu X, Teng Y, Zhang J, Luo Y, Zhang L, Zheng S, et al. Tumor response and survival in patients with advanced non-small-cell lung cancer: the predictive value of chemotherapy-induced changes in fibrinogen. BMC Cancer. 2012;12:330. doi: 10.1186/1471-2407-12-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pichler M, Hutterer GC, Stojakovic T, Mannweiler S, Pummer K, Zigeuner R. High plasma fibrinogen level represents an independent negative prognostic factor regarding cancer-specific, metastasis-free, as well as overall survival in a European cohort of non-metastatic renal cell carcinoma patients. Br J Cancer. 2013;109:1123–1129. doi: 10.1038/bjc.2013.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen J, Yang Y, Ye F, Huang X, Li S, Wang Q, Xie X. The preoperative plasma fibrinogen level is an independent prognostic factor for overall survival of breast cancer patients who underwent surgical treatment. Breast. 2015;24:745–750. doi: 10.1016/j.breast.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Borg N, Guilfoyle MR, Greenberg DC, Watts C, Thomson S. Serum albumin and survival in glioblastoma multiforme. J Neurooncol. 2011;105:77–81. doi: 10.1007/s11060-011-0562-0. [DOI] [PubMed] [Google Scholar]
- 22.Tan Z, Zhang M, Han Q, Wen J, Luo K, Lin P, Zhang L, Yang H, Fu J. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the Fibrinogen/Albumin Ratio. J Cancer. 2017;8:1025–1029. doi: 10.7150/jca.16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang KT, Chung JK, Roh EY, Kim J, Oh S, Kim YA, Rhu J, Kim S. Prognostic Influence of Preoperative Fibrinogen to Albumin Ratio for Breast Cancer. J Breast Cancer. 2017;20:254–263. doi: 10.4048/jbc.2017.20.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14–18. [PubMed] [Google Scholar]
- 25.CLAUSS A. [Rapid physiological coagulation method in determination of fibrinogen] Acta Haematol. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 26.Crumley AB, Stuart RC, McKernan M, McMillan DC. Is hypoalbuminemia an independent prognostic factor in patients with gastric cancer? World J Surg. 2010;34:2393–2398. doi: 10.1007/s00268-010-0641-y. [DOI] [PubMed] [Google Scholar]
- 27.Asher V, Lee J, Bali A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol. 2012;29:2005–2009. doi: 10.1007/s12032-011-0019-5. [DOI] [PubMed] [Google Scholar]
- 28.Unsal E, Atalay F, Atikcan S, Yilmaz A. Prognostic significance of hemostatic parameters in patients with lung cancer. Respir Med. 2004;98:93–98. doi: 10.1016/j.rmed.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 30.Sun KY, Xu JB, Chen SL, Yuan YJ, Wu H, Peng JJ, Chen CQ, Guo P, Hao YT, He YL. Novel immunological and nutritional-based prognostic index for gastric cancer. World J Gastroenterol. 2015;21:5961–5971. doi: 10.3748/wjg.v21.i19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 32.Simpson-Haidaris PJ, Rybarczyk B. Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein. Ann N Y Acad Sci. 2001;936:406–425. [PubMed] [Google Scholar]
- 33.Sahni A, Khorana AA, Baggs RB, Peng H, Francis CW. FGF-2 binding to fibrin(ogen) is required for augmented angiogenesis. Blood. 2006;107:126–131. doi: 10.1182/blood-2005-06-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahni A, Simpson-Haidaris PJ, Sahni SK, Vaday GG, Francis CW. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2) J Thromb Haemost. 2008;6:176–183. doi: 10.1111/j.1538-7836.2007.02808.x. [DOI] [PubMed] [Google Scholar]
- 35.Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology (Bethesda) 2010;25:85–101. doi: 10.1152/physiol.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shu YJ, Weng H, Bao RF, Wu XS, Ding Q, Cao Y, Wang XA, Zhang F, Xiang SS, Li HF, et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer. 2014;14:566. doi: 10.1186/1471-2407-14-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2002;62:6966–6972. [PubMed] [Google Scholar]
- 38.Zheng S, Shen J, Jiao Y, Liu Y, Zhang C, Wei M, Hao S, Zeng X. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009;100:859–865. doi: 10.1111/j.1349-7006.2009.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinbrecher KA, Horowitz NA, Blevins EA, Barney KA, Shaw MA, Harmel-Laws E, Finkelman FD, Flick MJ, Pinkerton MD, Talmage KE, et al. Colitis-associated cancer is dependent on the interplay between the hemostatic and inflammatory systems and supported by integrin alpha(M)beta(2) engagement of fibrinogen. Cancer Res. 2010;70:2634–2643. doi: 10.1158/0008-5472.CAN-09-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci USA. 2013;110:4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staton CA, Brown NJ, Lewis CE. The role of fibrinogen and related fragments in tumour angiogenesis and metastasis. Expert Opin Biol Ther. 2003;3:1105–1120. doi: 10.1517/14712598.3.7.1105. [DOI] [PubMed] [Google Scholar]
- 42.Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9 Suppl 2:S51–S63. doi: 10.1016/j.ejon.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledingham IM, Calman KC. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;1:781–784. doi: 10.1016/s0140-6736(85)91447-3. [DOI] [PubMed] [Google Scholar]
- 44.Loeffler S, Fayard B, Weis J, Weissenberger J. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int J Cancer. 2005;115:202–213. doi: 10.1002/ijc.20871. [DOI] [PubMed] [Google Scholar]
- 45.Rothschild MA, Oratz M, Schreiber SS. Serum albumin. Hepatology. 1988;8:385–401. doi: 10.1002/hep.1840080234. [DOI] [PubMed] [Google Scholar]
- 46.Deehan DJ, Heys SD, Simpson W, Herriot R, Broom J, Eremin O. Correlation of serum cytokine and acute phase reactant levels with alterations in weight and serum albumin in patients receiving immunotherapy with recombinant IL-2. Clin Exp Immunol. 1994;95:366–372. doi: 10.1111/j.1365-2249.1994.tb07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakashima J, Tachibana M, Ueno M, Miyajima A, Baba S, Murai M. Association between tumor necrosis factor in serum and cachexia in patients with prostate cancer. Clin Cancer Res. 1998;4:1743–1748. [PubMed] [Google Scholar]
- 48.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]


