Abstract
Anti-viral immunity presents a major hurdle for systemically administered oncolytic viruses (OV). Intratumoral OV therapy has a potential to overcome this problem through activation of anti-tumor immune response, with local and abscopal effects. However, the effects of anti-viral immunity in such a setting are still not well defined. Using Newcastle Disease Virus (NDV) as a model, we explore the effects of pre-existing anti-viral immunity on therapeutic efficacy in syngeneic mouse tumor models. Unexpectedly, we find that while pre-existing immunity to NDV limits its replication in tumors, tumor clearance, abscopal anti-tumor immune effects, and survival are not compromised and, on the contrary, are superior in NDV-immunized mice. These findings demonstrate that pre-existing immunity to NDV may increase its therapeutic efficacy through potentiation of systemic anti-tumor immunity, which provides clinical rationale for repeated therapeutic dosing and prompts investigation of such effects with other OVs.
Keywords: Newcastle Disease Virus, oncolytic virus, pre-existing immunity, cancer immunotherapy, immuno-oncology, tumor immunology, NDV
Pre-existing anti-viral immunity presents a challenge for systemically administered oncolytic viruses, but its effects on the intratumoral oncolytic immunotherapy are not well defined. Ricca and colleagues find that pre-existing immunity to intratumoral oncolytic Newcastle Disease Virus does not reduce, but rather potentiates its therapeutic efficacy through enhanced anti-tumor immune response.
Introduction
Oncolytic viruses (OVs) selectively infect, replicate within, and lyse cancer cells by exploiting cellular defects inherent to oncogenesis.1, 2, 3 One of the perceived limitations of OVs is their inability to sufficiently replicate and lyse tumors in the setting of neutralizing anti-viral antibodies, which would preclude treatment of patients with pre-existing immunity to the OV or repetitive dosing with OV after initial treatment.2, 4, 5, 6
With improved understanding of the mechanisms of action of OVs came the recognition that in addition to directly lysing cancer cells, OVs carry a potential to activate systemic anti-tumor immune response, which likely plays a major role in the efficacy of such agents. Increasingly, OVs are used as in situ vaccines rather than tumor debulking agents, best exemplified by talimogene laherparepvec (T-vec), the first OV-based therapy to obtain FDA approval for intratumoral treatment of advanced melanoma.1, 3, 7, 8, 9 With recognition of the immunotherapeutic mechanism of action of OVs, the role of anti-viral immunity has become less clear. We have previously demonstrated that intratumoral administration of Newcastle Disease Virus (NDV) leads to activation of systemic anti-tumor immune response with local and distant (abscopal) immune effects and potentiation of systemic immune checkpoint blockade, which is apparent even in models that do not strongly support NDV replication.10, 11 In prior clinical studies with systemically administered NDV, repetitive dosing with the virus led to durable responses in several patients.12, 13 In particular, one patient with metastatic cervical cancer first developed a response after 10 months on therapy and went on to have a durable complete response after therapy completion,12, 13 highlighting the potential role of the immune system in the observed therapeutic activity. In a recent study by Ribas et al.14 using combination of intratumoral T-vec with pembrolizumab, there were patients that exhibited responses that were delayed more than would be expected with PD-1 blockade alone, including a patient that exhibited a close to a 200% increase in tumor growth at 24 weeks of treatment, which was subsequently followed by a marked tumor regression with eventual complete response.
These findings question the potential negative impact of anti-OV immunity, especially in the setting of intratumoral therapy, where minimal virus replication could be sufficient to activate anti-tumor immune response. To formally explore this question, here we use NDV as a model to examine the effects of pre-existing anti-viral immunity on therapeutic efficacy. Unexpectedly, we find that pre-existing immunity does not reduce but rather potentiates the therapeutic efficacy of intratumorally administered NDV, which is mediated through enhanced anti-tumor immune response. These findings highlight that antiviral immunity in the setting of oncolytic immunotherapy may drive therapeutic efficacy, providing rationale for treatment of patients with prior exposure to OV, repetitive therapeutic dosing, and possibly a design of clinical trials utilizing anti-viral immunization prior to local OV treatment.
Results
Anti-tumor Efficacy of NDV Is Dependent on the Adaptive Immune Response
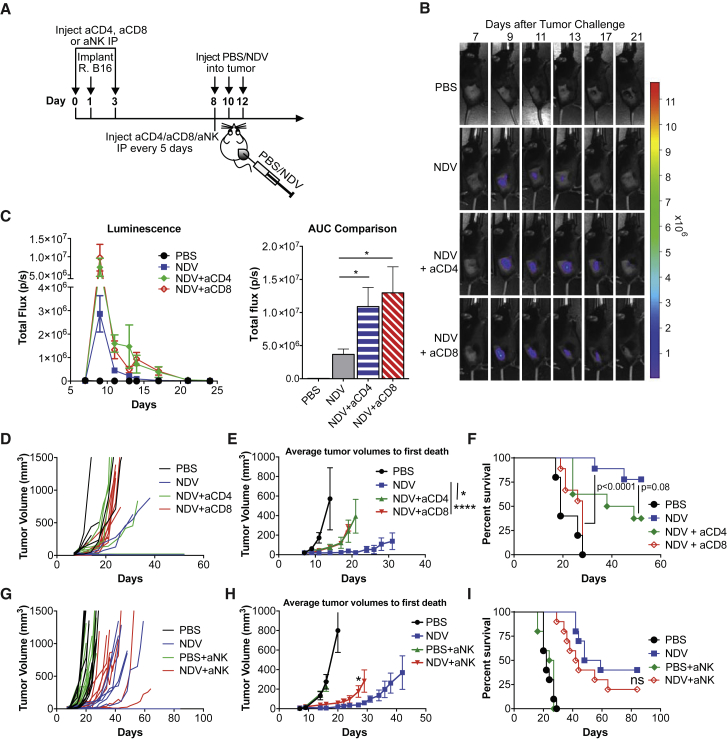
For our studies, we used the non-pathogenic NDV LaSota strain, which has a poor ability to undergo multi-cycle replication in the absence of efficient proteolytic cleavage of its fusion (F) protein. Despite the limited replication, this strain has an ability to infect mouse and human tumor cells both in vitro and in vivo and has a capacity to express a therapeutic or an imaging transgene.10, 15, 16 With intratumoral injection in B16-F10 tumor model, NDV treatment can lead to complete tumor rejection in a subset of animals.11 To evaluate the role of the immune system in NDV-mediated tumor rejection, B16-F10 tumor-bearing mice were treated with three intratumoral injections of NDV-expressing firefly luciferase (NDV-Fluc) in the presence of CD4 or CD8-depleting antibodies (Figures 1A and S1). While CD4 and CD8 depletion led to enhanced and prolonged luciferase signal indicative of improved viral persistence (Figures 1B and 1C), there was a significant reduction in anti-tumor efficacy with CD8 depletion and to a lesser extent with CD4 depletion (Figures 1D–1F). Depletion of NK cells in a similar setting also resulted in an accelerated early tumor outgrowth (Figures 1G and 1H), although this did not lead to a statistically significant survival detriment (Figure 1I). These findings suggest that while NK cells may play a role in early tumor control with NDV therapy, the long-term anti-tumor effect is primarily dependent on CD8+ cells. The role of CD4+ cells in this setting remains less clear, and the marginal decrease in anti-tumor efficacy with CD4 depletion suggests that NDV-induced anti-tumor immunity may be in part CD4 dependent. Overall, these results indicate that NDV-mediated inflammation and possibly tumor-specific immune response, rather than direct virus-mediated lysis, are the primary mechanisms driving the anti-tumor efficacy in the setting of intratumoral therapy with this virus. We proceeded to evaluate whether in the setting of pre-existing anti-viral immunity, which would limit viral persistence, intratumoral NDV therapy would still be sufficient to induce tumor rejection.
Figure 1.
Therapeutic Efficacy of Intratumoral NDV Is Dependent on Adaptive Immunity
(A) Treatment scheme. Tumors were established by implantation of 1 × 105 (B–F) or 2 × 105 B16-F10 cells in the right flank. (B) Representative luminescence images from animals treated with NDV-fluc. (C) Quantification of average luminescence from the tumor sites at the indicated time points. Left, average luminescence. Right, area under curve (AUC) calculated from the curves on the left. (D) Individual tumor growth curves. (E) Average tumor volumes followed until first death in each treatment group. Indicated statistical comparisons were performed using t test using the average tumor volumes on the last day of measurement before the first death. (F) Overall survival with CD4 or CD8 depletion. (G) Individual tumor growth curves with NK cell depletion. (H) Average tumor volumes followed until first death in each treatment group. (I) Overall survival with NK depletion. Data in (A)–(F) and (G)–(I) each represent one of two independent experiments with n = 5–10 per group. Mean ± SEM is shown. ns, not significant; *p < 0.05; ****p < 0.0001.
Prior Immunity to NDV Potentiates Its Therapeutic Efficacy with Intratumoral Administration
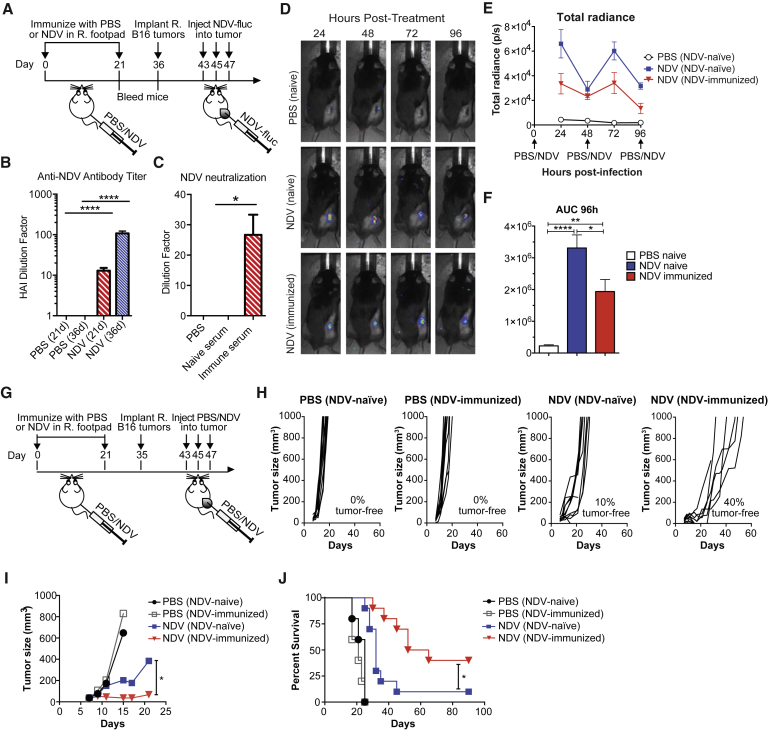
To study the effects of pre-existing anti-NDV immunity on anti-tumor immune and therapeutic response, animals were immunized with NDV using the prime-boost schedule outlined in Figure 2A. As expected, immunization of mice with NDV led to development of neutralizing antibodies to NDV, as determined by hemagglutination inhibition (HI) assay, which assesses for the ability of the serum to neutralize virus binding to red blood cells (Figure 2B). In this assay, the titer of 1:8 is considered to be protective against NDV infection, as reported in the vaccination literature.17, 18, 19 Serum from immunized animals was confirmed to inhibit NDV infection in a neutralization assay (Figure 2C). To track the effect of immunization on the viral replication in vivo, NDV-naive and NDV-immunized animals bearing B16-F10 melanoma tumors were treated intratumorally with recombinant NDV-Fluc reporter (Figure 2A). Prior immunization with NDV diminished but did not completely abolish viral infection in tumor, as evidenced by reduced luminescence signal with each administration (Figures 2D–2F). To determine whether the observed reduction of NDV replication within the tumor of NDV-immunized mice affects therapeutic efficacy, NDV-naive and NDV-immunized animals bearing B16-F10 melanoma were treated intratumorally with NDV and followed for survival (Figure 2G). Treated NDV-naive mice exhibited delayed tumor growth and an increase in long-term survival over the two control groups (Figures 2H–2J). Surprisingly, prior immunization with NDV did not diminish its therapeutic efficacy but rather led to superior anti-tumor effects and prolonged animal survival when compared to the treated NDV-naive mice (Figures 2H–2J). To ensure the observed results were not limited to one tumor model, the experiments were repeated with animals bearing MB49 bladder carcinoma, yielding similar results (Figure S2).
Figure 2.
Prior Immunity to NDV Potentiates Its Therapeutic Efficacy with Intratumoral Administration
(A) Prime-boost immunization scheme. Tumors were established by implantation of 2 × 105 B16-F10 cells in the right flank. (B) Anti-NDV antibody serum titers determined by hemagglutination inhibition (HI) assay at 21 and 36 days after immunization. The y axis indicates the dilution factor at which HI is no longer seen. (C) Neutralization of NDV by day 21 serum, as determined by infectivity of A549 cells. The y axis indicates the dilution factor at which NDV neutralization is no longer seen. (D) Representative luminescence images from animals treated with NDV-fluc. (E) Quantification of average luminescence from the tumor sites 24, 48, 72, and 96 hr after initial treatment. (F) AUC calculated from the data in (D). (G) Survival experiment treatment scheme. Tumors were established by implantation of 2 × 105 B16-F10 cells in the right flank. (H and I) Growth of individual injected tumors (H) and average B16-F10 tumor growth until first death in each group (I). (J) Overall survival of the treated B16-F10 tumor-bearing mice. Data for (D)–(F) represent one of two experiments with n = 5 per group. Data for (G)–(J) show representative results from one of four experiments with n = 10 per group. Mean ± SEM is shown. ns, not significant; *p < 0.05; **p < 0.01; ****p < 0.0001. R, right.
Pre-existing Immunity to NDV Potentiates Local and Systemic Anti-tumor Immune Responses
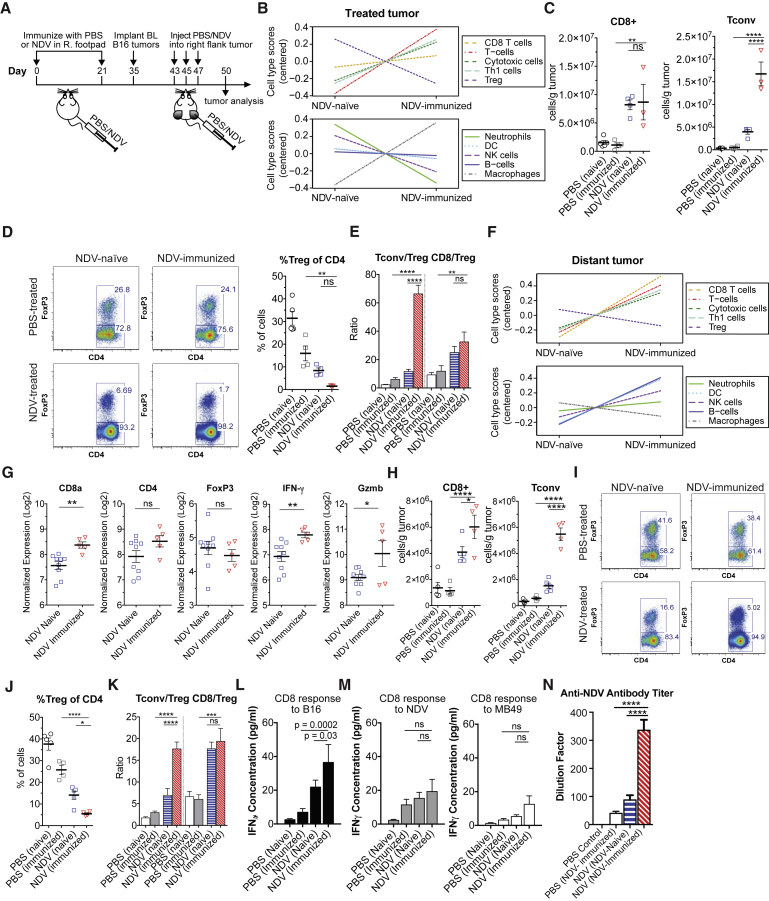
Since immunization with NDV was associated with diminished NDV viral replication in tumors, therapeutic enhancement in the NDV-immune animals was unlikely to be caused by direct virus-mediated lysis, implicating the potential role of the immune system in the observed therapeutic efficacy. To distinguish between the effects of local inflammatory response to the virus and systemic anti-tumor immune response, we used a bilateral flank B16-F10 melanoma model with NDV administered to a single flank tumor (Figure 3A). In this model, NDV replication is restricted to the treated tumor, and we previously were unable to detect the virus in distant tumors (Figure S5).11 Following the treatment with NDV, bilateral tumors were collected and processed for gene expression analysis using NanoString PanCancer immune profiling gene panel and for flow cytometry. We initially focused on the NDV-injected tumors to characterize the responses directly elicited by NDV infection. Gene-expression-based deconvolution analyses measuring relative abundance of specific immune cell populations demonstrated relative increase in most immune cell populations in both NDV-naive and previously immunized mice after intratumoral NDV treatment (Figure S3A). The comparison between treated NDV-naive and treated NDV-immunized animals revealed a more prominent increase in the cell populations related to Th1 response and a decrease in regulatory T cells (Figures 3B, S3B, and S3C). Flow cytometry analyses confirmed a prominent increase in CD4+FoxP3− conventional T cells (Tcon) and a relative decrease in the number of CD4+FoxP3+ regulatory T cells (Treg) in the treated NDV-immunized group, compared to the treated NDV-naive controls (Figures 3C and 3D). There was no apparent increase in the tumor-infiltrating CD8+ lymphocytes (Figures 3C and S3B). The decrease in Tregs resulted in significant enhancement in Tcon/Treg ratios in the NDV-immunized animals (Figure 3E). With understanding that the enhanced T cell infiltration in the treated tumors were likely to represent anti-viral rather than anti-tumor response, we next turned our attention to the distant tumors from the same animals, where the T cells are less likely to be influenced by the presence of virus antigens. Similar to the results in virus-injected tumors, cell type deconvolution analyses revealed a prominent increase of cell populations related to Th1 and cytotoxic T cell response in both NDV-naive and NDV-immunized mice treated with NDV when compared to the respective untreated control animals (Figure S4A). Additionally, a relative global increase of immune-related genes was observed in the tumors of treated NDV-immunized mice compared to those of treated NDV-naive mice (Figure S4B). Further comparison between NDV-immunized and NDV-naive tumors of NDV-treated mice demonstrated relative increase in cell type gene scores of most immune cell populations in the NDV-immunized group, with most prominent increases in CD8, Th1, and NK cells, as well as a concomitant decrease in Tregs (Figures 3F and S4C), with specific increases in genes related to CD8 and Th1 response (Figure 3G). In concordance with gene expression analyses, flow cytometry analyses confirmed a prominent increase in CD8+ T cells and Tcons and a relative decrease in Tregs in the treated NDV-immunized group compared to those of the treated NDV-naive mice (Figures 3H–3J), leading to enhanced Tcon to Treg ratios in the NDV-immunized group (Figure 3K).
Figure 3.
Pre-existing Immunity to NDV Potentiates Local, Abscopal, and Systemic Anti-tumor Immune Responses
(A) Treatment scheme. Tumors were established by implantation of 2 × 105 B16-F10 cells in the right and left flanks. (B) Relative cell type gene signature scores from the NDV-treated tumors of NDV-naive and NDV-immunized mice (all n = 5). (C) Absolute number of tumor-infiltrating CD8+ and CD4+FoxP3− (Tcon) cells per gram of tumor calculated from flow cytometry of treated tumors. (D) Representative flow cytometry plots of percentages of Tcon and Treg cells (left) and average percentages of Treg cells (right). (E) Calculated CD8+:Treg and Tcon:Treg ratios in the treated tumors. (F) Relative cell type gene signature scores from the distant tumors of NDV-naive (n = 9) and NDV-immunized (n = 5) mice treated with NDV. (G) Relative expression of genes related to T cell response in the distant tumors of NDV-treated NDV-naive and NDV-treated NDV-immunized mice. (H) Absolute number of tumor-infiltrating CD8+ and Tcon cells per gram of tumor calculated from flow cytometry of distant tumors. (I and J) Representative flow cytometry plots with percentages of Tcon and Treg cells (I) and average percentages of Treg cells calculated from flow cytometry in distant tumors (J). (K) Calculated CD8+:Treg and Tcon:Treg ratios. (L and M) Production of IFNγ in response to re-stimulation of splenic CD8+ lymphocytes with (L) B16-F10 cells or (M) with MB49 cells infected with NDV (left) and non-infected MB49 cells (right). (N) Anti-NDV antibody serum titers (day 50) determined by HI. Data from (B), (F), and (G) represent results from one experiment. Data from (C)–(E) and (H)–(J) represent results from one of three independent experiments with n = 3–5 animals per group. Data from (K) represent combined results from two independent experiments with 8–14 animals per group. Data from (N) represent results from one of three independent experiments with n = 8–10 per group. Mean ± SEM is shown. ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. R, right; BL, bilateral.
Previous studies with reovirus have demonstrated that pre-existing immunity to the virus could improve its systemic targeting through uptake by myeloid cells.20, 21 To determine whether pre-immunization increased NDV trafficking to the distant tumors, bilateral flank tumor-bearing mice were treated intratumorally with NDV-Fluc. There were no detectable luciferase signal (Figure S5A) and no detectable NDV RNA at 24 and 72 hr post-infection in the distant tumors in any of the groups (Figure S5B), suggesting that the observed enhancement in abscopal inflammatory effect with pre-immunization was not mediated by improved virus trafficking to these sites.
The enhanced abscopal immune effects with increased CD8+ T cell infiltration imply that pre-existing immunity to NDV may in fact potentiate systemic cytotoxic anti-tumor immune response following intratumoral NDV treatment. To test this, splenic CD8+ cells isolated from the treated B16-F10 tumor-bearing mice were co-cultured with non-infected irradiated B16-F10 cells or irradiated NDV-infected MB49 bladder carcinoma cells to measure tumor-specific and virus-specific immune response, respectively, using IFNγ release as the outcome measure. Enhanced IFNγ production was observed in response to stimulation with B16-F10 cells in both NDV-naive and NDV-immunized animals treated with NDV, with the highest IFNγ concentrations observed in the NDV-immunized group (Figure 3L). Supporting these findings, in the immunized group there was enhanced IFNγ production by splenic CD8+ lymphocytes in response to stimulation with B16-F10 lysates using enzyme-linked immunospot (ELISPOT), with trend toward improved responses to some melanoma-associated peptides (Figure S6). In contrast, there was no significant difference in IFNγ response to NDV-infected MB49 cells between the NDV-treated NDV-naive and NDV-treated NDV-immunized animals (Figure 3M), although the NDV-immunized animals demonstrated significant increase in anti-NDV antibody titers (Figure 3N). Interestingly, there was also a small increase of IFNγ secretion in response to stimulation with non-infected MB49 cells (Figure 3M), suggesting part of the immune response could be non-specific secondary to persistent activation of CD8+ cells, or implying a potential cross-reactivity of CD8 cells with MB49 antigens.
Therapeutic Effect Is Dependent on CD8 but Not CD4 Lymphocytes
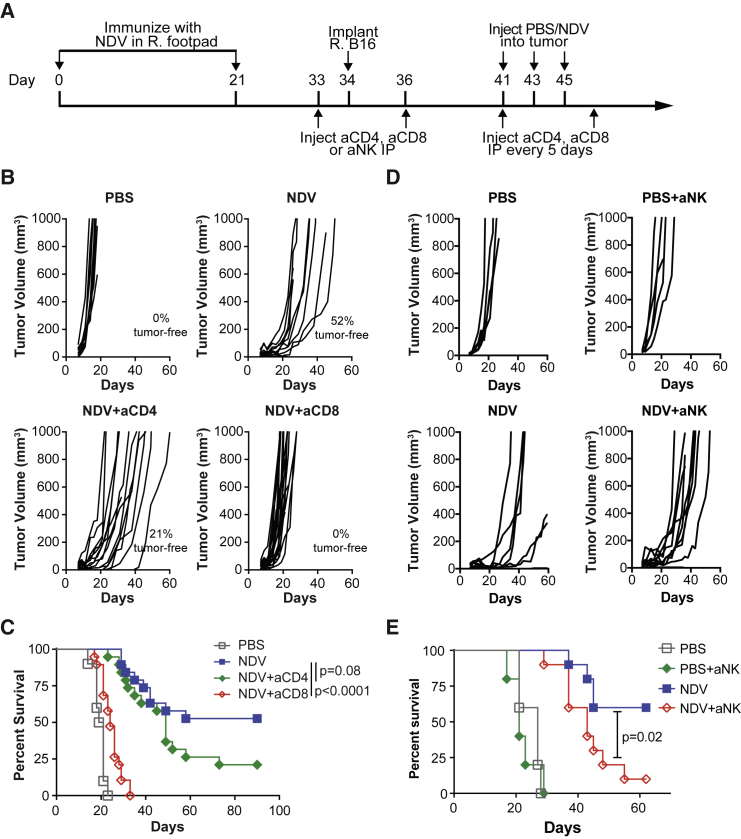
These findings highlighted the potential role of CD8-mediated tumor-specific immune response in the NDV-immunized animals. To formally test this, we proceeded to determine whether CD8 and/or CD4 cells were required for the observed anti-tumor effect. NDV-immunized animals were treated with depleting antibodies to CD8, CD4, or NK cells and with intratumoral NDV as above (Figure 4A). Treatment with anti-CD8 antibodies resulted in complete abrogation of NDV-mediated anti-tumor effect, and the animals quickly succumbed to the tumors (Figures 4B and 4C). These findings suggest that CD8 cells are indispensible for the observed therapeutic efficacy of NDV in a setting of pre-immunization. Interestingly, despite the significant expansion of CD4+ lymphocytes seen in the virus-injected and distant tumors in NDV-immunized mice, depletion of CD4+ lymphocytes had only a modest effect on the therapeutic efficacy (Figures 4B and 4C), which was similar to our prior observations with NDV-based immunotherapy11 and to our observations in the naive setting (Figure 1). It is possible that the expansion of Tcon may represent primarily anti-viral rather than anti-tumor helper T cells, which is further supported by significant enhancement of NDV-specific antibody response in serum (Figure 3N). Furthermore, depletion of CD4 cells results in removal of both effector and regulatory T cell subsets, making it difficult to delineate the true contribution of the conventional CD4 T cells in this setting. We next turned our attention to the role of NK cells. While the role of NK cells in the setting of treatment of naive mice was marginal (Figure 1G and 1H), depletion of NK cells in the setting of prior immunization with NDV significantly decreased therapeutic efficacy (Figures 4D and 4E), albeit to a lower extent than that seen with CD8 depletion. Furthermore, while the majority of the animals treated with NK-depleting antibody eventually succumbed to tumors, they still exhibited tumor growth delay and prolonged survival, when compared to untreated or CD8-depleted animals. These findings suggest that the NK-depleted animals are still able to control the tumor in CD8-dependent manner, albeit with suboptimal efficacy. It is thus possible that robust memory responses to NDV infection generate a more efficient inflammatory response involving NK cells, which likely play a role in the early tumor clearance and possibly a more effective recruitment and/or activation of CD8 cells, which in turn mediate long-term tumor control.
Figure 4.
Therapeutic Efficacy of NDV Is Dependent on CD8+ Cells and NK Cells but Not CD4+ Lymphocytes
Mice immunized and treated with NDV were depleted for CD4 or CD8 lymphocytes (B and C) or NK cells (D and E). (A) Treatment scheme. Tumors were established by implantation of 2 × 105 B16-F10 cells in the right flank. (B) Individual tumor growth curves after CD4 or CD8 depletion. (C) Overall survival after CD4 or CD8 depletion. (D) Individual tumor growth curves after NK depletion. (E) Overall survival after NK depletion. (B and C) Data represent cumulative results from two experiments with n = 10 (PBS), n = 19 (NDV + aCD4), and n = 19 (NDV + aCD8). (D and E) Data represent results of one experiment with n = 5 (PBS and PBS + aNK groups) and n = 10 (NDV and NDV + aNK groups). R, right; IP; intraperitoneal.
Treatment with NDV Is Effective in a Setting of Recurrent Tumor in NDV-Immune Mice
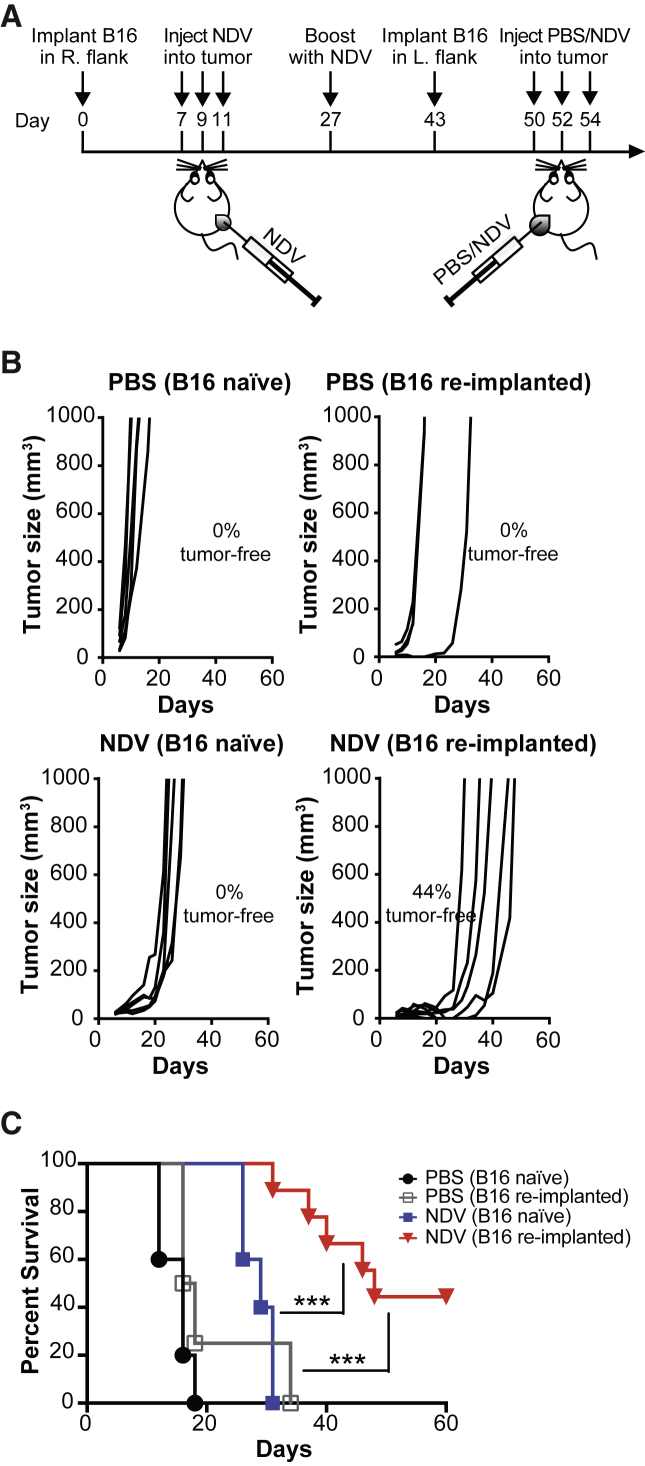
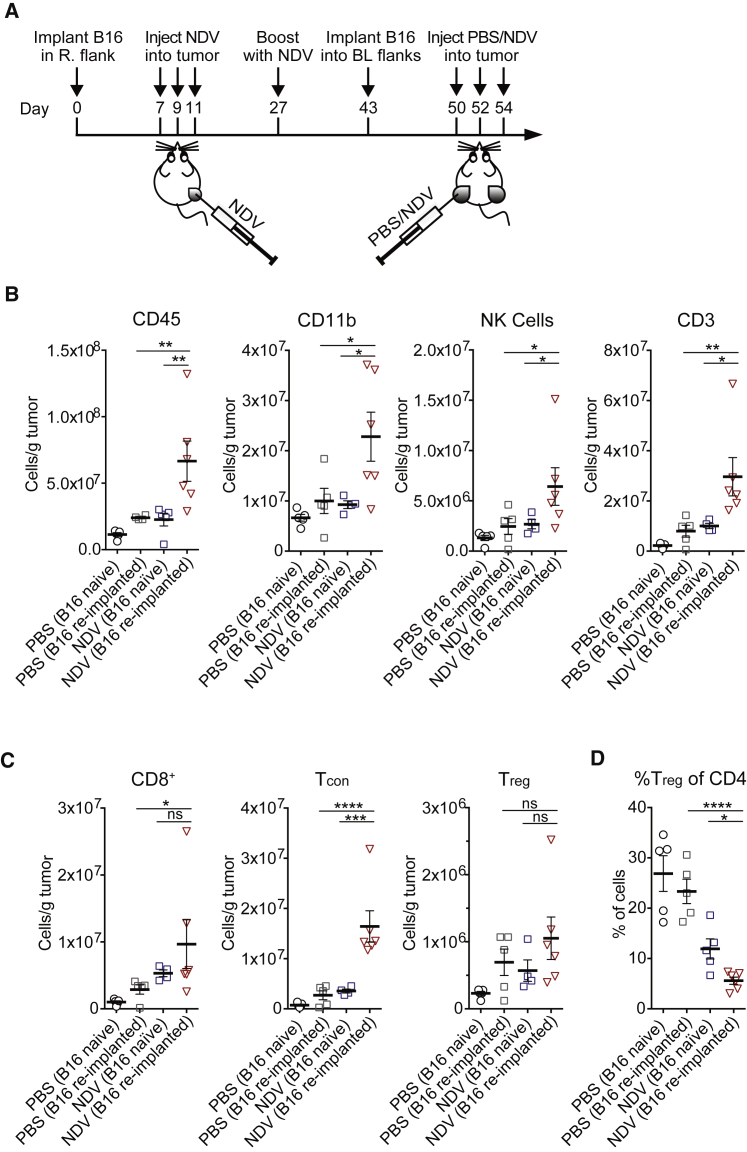
To determine whether therapeutic efficacy of NDV would hold true in the setting of NDV immunity developed during anti-cancer therapy, animals that completely cleared B16-F10 tumors with frontline NDV therapy were re-implanted with a lethal dose of B16-F10 cells in the opposite flank (Figure 5A). In this setting, approximately 70% of animals developed tumors, implying incomplete protection with frontline NDV therapy against such tumor challenge. Re-treatment of the animals with NDV led to significant tumor growth delay and long-term animal survival when compared to treated NDV-naive and untreated animals (Figures 5B and 5C). As expected, all of the animals developed high anti-NDV antibody titers (Figure S7). To characterize the abscopal immune effects in this setting, an analogous experiment was performed using the bilateral B16 tumor model (Figure 6A). Analysis of tumor-infiltrating leukocytes from the distant tumors demonstrated increase in both innate (CD11b, NK) and adaptive (CD8, CD4) immune cells (Figures 6B and 6C), with a decrease of relative percent Tregs (Figure 6D). These findings imply that the development of anti-NDV immunity during cancer treatment does not preclude and may in fact augment its therapeutic efficacy upon re-treatment for recurrent tumor.
Figure 5.
Efficacy of NDV in a Setting of Recurrent Tumor in the Presence of Anti-NDV Immunity
(A) Treatment scheme. Primary tumors were established by implantation of 1 × 105 B16-F10 cells in the right flank. Recurrent tumors were modeled by implantation of 4 × 105 B16-F10 cells in the left flank. (B) Individual tumor growth. (C) Overall survival. Data represent one of two independent experiments with n = 5 (B16-naive PBS), n = 4 (B16 re-implanted PBS), n = 5 (B16 naive NDV), and n = 9 (B16 re-implanted NDV) mice per group. ***p < 0.001.
Figure 6.
In a Setting of Recurrent Tumor, Prior Immunity to NDV Potentiates Immune Infiltration in Distant Tumors
(A) Treatment scheme in the bilateral flank tumor model. Primary tumors were established by implantation of 1 × 105 B16-F10 cells in the right flank. Recurrent tumors were modeled by implantation of 4 × 105 B16-F10 cells in the bilateral flanks. (B) Absolute number of tumor-infiltrating CD45+, CD11b+, NK, and CD3+ cells in distant tumors, calculated from flow cytometry. (C) Absolute number of tumor-infiltrating CD8+, Tcon, and Treg cells per gram of tumor in distant tumors, calculated from flow cytometry. (D) Relative percentages of tumor-infiltrating Tregs out of CD4+ cells. Data represent one of two independent experiments with n = 5, n = 5, n = 5, and n = 6, as above. Mean ± SEM is shown. ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. R, right; L, left; BL, bilateral.
Discussion
To date, clinical efficacy of systemically administered OVs has been limited, with neutralizing anti-viral antibodies presenting a major challenge for the delivery and replication of such agents at the tumor site.2, 5, 6, 22, 23 Moreover, systemic administration of OVs, while logistically attractive, requires large therapeutic doses to ensure for adequate delivery to the tumor site and carries obvious safety implications, including systemic inflammatory response and off-target toxicity.5, 6
To this end, several studies have now demonstrated that intratumoral OV therapy can lead to abscopal therapeutic effects and control of distant tumors not directly affected by the virus. Trials using intratumorally administered OVs are currently ongoing, with T-vec already FDA-approved for metastatic melanoma and several additional agents demonstrating the ability to regress both treated and distant tumors (Andtbacka et al., 2014, J. Clin. Oncol., abstract).9 With an assumption that neutralizing antibodies are detrimental even in the setting of intratumoral therapy, many of the ongoing clinical trials follow the schedule of frequent OV dosing upfront, followed by a less-frequent maintenance schedule. The question remains, however, whether pre-existing or newly developed anti-viral immunity could adversely impact the efficacy of intratumoral approaches.
In the current study, we used NDV as a model to determine the impact of pre-existing immunity to the virus on its therapeutic efficacy with intratumoral administration. Contrary to our expectations, pre-existing immunity to the virus did not negatively impact the viral anti-tumor efficacy, and in fact the anti-tumor efficacy was superior to that observed in NDV-naive mice. Moreover, NDV was effective in a model of “recurrent” tumor that previously responded to NDV, resulting in long-term tumor control and significant enhancement in abscopal immune effects. While this model is unlikely to fully recapitulate the biology of a recurrent tumor and its immune escape mechanisms, such as loss of tumor antigens and establishment of additional immunosuppressive pathways, it nevertheless suggests that the virus could still incite an inflammatory response that could lead to both local and systemic tumor control.
While the current study suggests that prior exposure to an OV does not diminish its therapeutic efficacy in the setting of intratumoral administration, neutralizing antibodies likely still present a challenge with systemic virus administration. Since NDV LaSota strain has only a modest replicative capacity, it exhibits poor delivery to systemic sites11 and thus pre-existing immunity to the virus would likely be detrimental if the virus were used systemically. Interestingly, in prior studies with systemically administered reovirus, pre-existing neutralizing antibodies to reovirus also enhanced viral therapeutic efficacy, which was thought to be mediated by enhanced rather than diminished viral delivery to tumors by myeloid cells.20, 21 The mechanisms underlying the therapeutic enhancement by pre-existing antiviral immunity with intratumoral administration remain to be elucidated. In the treated tumors, it is possible that the robust memory response to the virus could mediate a strong inflammatory response with recruitment of NK cells and additional innate and adaptive immune cells mediating bystander effect against the neighboring cells.24, 25 The enhanced local inflammatory response generated by the virus could also potentially result in epitope spreading and a systemic immune response against a broader range of tumor antigens, as was reported by Woller and colleagues26 with oncolytic adenovirus. It is also possible that limited viral replication in the setting of pre-existing immunity may be beneficial for anti-tumor immunity by limiting anti-viral adaptive immune responses, which may otherwise act in an immunodominant fashion.27 Indeed, prior studies with other viruses indicate that immune-mediated anti-tumor activity of OVs appears to be independent of lytic efficacy.28, 29 Finally, we cannot fully rule out that the immune activity found by pre-immunization could be against a post-translational modification (e.g., glycosylation) that is shared between the virus and the tumor. We consider this to be an unlikely possibility, given that the virus is produced in embryonated chicken eggs and the finding that pre-immunized untreated animals did not exhibit evidence of improved tumor control or tumor-specific CD8 activity.
In summary, our studies indicate that with intratumoral therapy, pre-existing immunity to NDV does not prevent but may potentiate its therapeutic efficacy through induction of a more robust anti-tumor immune response. These findings are not necessarily generalizable to all OVs, as the viruses differ in their mechanisms in activation of innate immune response and reliance on CD8, CD4, NK, and antibody responses for viral clearance, which may offset the balance between the anti-viral and anti-tumor immunity. Clinical studies using oncolytic T-vec employed an initially low dosing of the virus to seroconvert the patients to improve tolerance, followed by subsequent intrapatient dose escalation.9 Based on our findings, one may speculate that this strategy may have actually augmented rather than inhibited therapeutic efficacy. These findings carry obvious clinical implications, suggesting that pre-existing immunity to an OV should not preclude therapy with intratumoral administration and provide justification for treatment in the setting of prior anti-OV immunity and possibly repeated intratumoral therapeutic dosing, as each administration may drive a stronger tumor inflammatory response and anti-tumor immunity.
Materials and Methods
Mice
C57BL/6J mice were purchased from Jackson Laboratory. All mice were maintained in microisolator cages and treated in accordance with the NIH and American Association of Laboratory Animal Care regulations. Animals that had evidence of non-specific dermatitis prior to initiation of treatment were excluded to remove the potential confounding variable that could influence the results.
Cell Lines
The murine cancer cell line for melanoma (B16-F10) was originally obtained from I. Fidler (MD Anderson Cancer Center); the cell line for bladder cancer (MB49) was originally obtained from Dr. James Allison (MD Anderson Cancer Center). B16-F10 and MB49 cells were maintained in RPMI medium supplemented with 7.5% fetal calf serum and penicillin with streptomycin. A549 cells were obtained from American Type Culture Collection (ATCC) and maintained in DMEM medium supplemented with 10% fetal calf serum and penicillin with streptomycin. All of the cell lines have been tested and were found to be negative for mycoplasma contamination.
Antibodies
Therapeutic anti-CD8+ (clone 2.43), anti-CD4+ (clone GK1.5), and anti-NK1.1 (clone PK136), antibodies were produced by BioXcell. Antibodies used for flow cytometry were purchased from the following sources (dilutions are indicated in parentheses): eBioscience (CD45.2 Alexa Fluor 700, cat. 56-0454 [1:200]; CD3 PE-Cy7, cat. 25-0031 [1:200]; CD4 ef450, cat. 48-0041 [1:200]; CD4 APC-efluor780, cat. 47-0041 [1:400]; CD8 PerCP-efluor710, cat. 46-0083 [1:200]; CD11b APC-efluor 780, cat. 47-0112 [1:600]; NK 1.1 PE, cat. 12-5941 [1:200]; FoxP3 APC, cat. 17-5773 [1:200]); and Invitrogen (Granzyme B PE-Texas red, cat. GRB17 [1:125]).
Viruses
Recombinant lentogenic NDV LaSota strain was generated from cDNA and used for all non-luminescence experiments. To generate NDV virus expressing firefly luciferase, a DNA fragment encoding the firefly luciferase flanked by the appropriate NDV-specific RNA transcriptional signals was inserted into the SacII site created between the P and M genes of pT7NDV/LS. Generation of recombinant NDV has been described previously.30 All generated viruses were propagated in embryonated 9-day-old specific pathogen-free chicken eggs. Virus titers were determined by serial dilution and infection of A549 cells, followed by immunofluorescent detection of viral antigens.
Bioluminescence Imaging
Mice were imaged every 24 hr over a 96-hr period starting on day 44, 24 hr post-infection. Mice were injected retro-orbitally with 50 μL of 40 mg/mL D-luciferin (Caliper Life Sciences). Gray-scale photographic images and bioluminescence color images were superimposed using The Living Image, version 4.0 (Caliper Life Sciences) software overlay. A region of interest (ROI) was manually selected over the tumor and the area of the ROI was kept constant.
Hemagglutination Inhibition Assay
Post-immunization sera were collected at day 21 (after single immunization), day 36 (after boost), and day 50 (after intratumoral treatment). Isolated sera were stored at −80°C. Hemmaglutination inhibition assay was performed following the World Health Organization protocol.31
NDV Neutralization Assay
The assay for NDV infectivity neutralization was adopted from the influenza literature32 and was modified by detection by fluorescent microscopy. Sera from control or NDV-immunized mice were serially diluted in PBS and incubated for 1 hr at 37°C with NDV encoding GFP (NDV-GFP) at a final concentration of 1 × 104 plaque-forming units (pfu)/mL in a 40 μL volume. Following the incubation, 20 μL of the virus/serum mixture was used to infect A549 cells plated in 96-well plates, at a final MOI of 0.005 in DMEM supplemented with 10% fetal calf serum. Twenty-four hours post-infection, presence or absence of GFP signal was determined for each well, and the highest dilution resulting in infection inhibition was determined for each sample.
Tumor Implantation Survival Experiments
All mouse procedures and experiments for this study were approved by the Memorial Sloan Kettering Cancer Center Institutional Animal Care and Use Committee. Treatment schedules and cell doses were established for each tumor model to achieve 10% to 30% tumor clearance by NDV. For pre-existing immunity survival experiments, mice were immunized with 5 × 106 pfu NDV diluted in PBS in a total volume of 50 μL or PBS alone as a control in the right hind footpad, and at day 20–21 mice were boosted with NDV or PBS into the right hind footpad with the same concentration. Cell implantation quantities were experiment specific and are indicated in the figure legends. Tumors were measured on a regular basis, and the animals were euthanized for signs of distress or when the tumor volume reached 1,000 mm3. For depletion of immune cells, mice were injected intraperitoneally with 500 μg of monoclonal antibodies to CD8+ or CD4+ 1 day before and 2 days after tumor implantation, followed by intraperitoneal injection with 250 μg of antibodies every 5 days throughout the remainder of the experiment. For tumor re-implantation experiments, mice were implanted intradermally with 1 × 105 B16-F10 cells into the right flank. On days 7, 9, and 11, mice were treated intratumorally with 100 μL NDV (1 × 107 pfu). Mice that cleared their tumors were boosted on day 27 with NDV, and 4 × 105 B16-F10 cells were implanted on day 43 into the left flank, for survival experiments, and bilateral flanks, for measurement of abscopal effects in the tumor microenvironment. Mice were then treated intratumorally on days 50, 52, and 54 with 100 μL PBS or NDV (1 × 107 pfu).
Isolation of Tumor-Infiltrating Lymphocytes
For experiments that utilized flow cytometry analysis, mice were euthanized on day 50, and bilateral tumors were removed using forceps and a scalpel and then weighed. Tumors from each group were minced with scissors prior to incubation with 1.67 Wünsch U/mL Liberase and 0.2 mg/mL DNase for 30 min at 37°C shaking at 225 rpm. Tumors were further homogenized by filtering through a 70-μm nylon filter, and the homogenized samples were transferred to 15 mL centrifuge tubes. Cell suspensions were washed once with complete RPMI, resuspended in blocking buffer (fluorescence-activated cell sorting [FACS] buffer with 1:500 2.4G2 antibody), and transferred to a V-bottom 96-well plate to be stained for flow cytometry.
Flow Cytometry
Cells isolated from tumors were processed for surface labeling with appropriate antibodies. Fixable viability dye eFluor506 (eBioscience) was used to separate dead and live cells. Cells were further permeabilized using FoxP3 fixation and permeabilization kit (eBioscience) and stained for Ki-67, FoxP3, Granzyme B, and CD3. Data were acquired using the LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (Treestar).
IFNγ Release Assay, Analysis of Cytokine Production, and IFNγ Enzyme-Linked Immunospot Assay
For measurement of cytokine production, mice were euthanized on day 50 and spleens were dissected, passed through a 70-μm nylon filter, subjected to red blood cell (rbc) lysis, and enriched for CD8+ T cells using a Miltenyi CD8a (Ly-2) purification kit. Isolated CD8+ cells were counted and co-cultured for 48 hr in 24-well plates with irradiated B16-F10, MB49, or MB49 cells infected with NDV (MOI = 2) at a 10:1 effector to target ratio (5 × 105:5 × 104) in the presence of 20U/mL murine IL-2 (R&D). After 48 hr of restimulation, supernatants were collected from each well and analyzed for interferon γ (IFNγ) concentration using the mouse IFNγ simplex kit in conjugation with the ProcartaPlex mouse basic kit (eBioscience) and detected with a Magpix (Luminex) machine and analyzed with Luminex xPONENT software. For ELISPOT assay, 1 × 105 isolated CD8+ T cells were stimulated with irradiated B16-F10 cells or with CD11b+ splenic antigen presenting cells (APCs) loaded with the indicated peptides at 1:1 ratio. IFNγ-producing CD8 cells were quantified using a Mouse IFNγ ELISPOT Set (BD PharMingen, cat. 551083) according to the manufacturer’s instructions. Spots were quantified using Immunospot Analyzer.
NanoString Gene Expression Analyses
Immunization and tumor implantation were performed according to the schedule described above. On day 50, the animals were euthanized, and the tumors were excised, placed in TRIzol reagent (Invitrogen), and homogenized. The samples were flash-frozen in dry ice and ethanol and stored at −80°C. RNA was later purified from TRIzol using the Direct-zol RNA MiniPrep kit (Zymo Research). Isolated RNA was hybridized with the NanoString nCounter PanCancer Immune Profiling mouse panel codeset and quantified using the nCounter Digital Analyzer at the MSKCC Genomics Core Facility. Raw data were processed and normalized with NanoString’s nSolver Analysis Software using the Advanced Analysis module, and GSVA scores for cell types and immune response categories were generated in the R Statistical Environment using gene signatures provided by the NanoString panel. Heatmaps representing these scores were generated using the gplots/heatmap.2 package.
Real-Time PCR
To detect NDV-specific RNA within a tumor, a set of primers and a TaqMan probe specific for NDV NP transcript was generated (forward, AGGAGATAATGCGCCGTACA; reverse, TGCTCATAAAGTCCCTGGCA; probe, TGCGCCTGCCGAGAATGCACA). Real-time PCR was prepared with 1 μL cDNA according to the manufacturer’s instructions. All amplifications were done using the ABI 7500 Real Time PCR system (Applied Biosystems). Gene expression levels were normalized to Gapdh (ΔCt = Ct NDV-NP − Ct Gapdh) and reported as relative mRNA expression (2−(ΔCt)).
Statistics
The overall study design included a sequence of controlled laboratory experiments as described above. In all experiments, animals were randomly assigned to experimental groups. For survival experiments, 5 to 15 mice per group were used. The experiments were replicated two to four times as noted, and the final analysis included either pooled data or representative data where indicated. Data were analyzed by two-tailed unpaired Student’s t test (for comparisons of two groups) and ANOVA (for comparison of multiple groups). Data for survival were analyzed by log-rank (Mantel-Cox) test. Two-sided p < 0.05 was considered statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). The numbers of animals included in the study are discussed in each figure and in parts of the Materials and Methods section. All outliers were included in the analyses. In the survival experiments, animals that died early secondary to other reasons (e.g., anesthesia) were not included in the analyses (one animal in anti-CD4 group in Figure 1). In the tumor lymphocyte isolation experiments, animals that completely cleared the tumors were not included.
Author Contributions
J.M.R. designed and performed the experiments, analyzed data, and prepared the manuscript; T.W., A.O., and C.L. assisted in performing experiments and manuscript preparation; L.M. performed bioinformatics and generated heatmaps; T.M. and J.D.W. assisted in experimental design, data interpretation, and manuscript preparation; D.Z. designed and performed the experiments, analyzed data, and prepared the manuscript.
Conflicts of Interest
D.Z. and J.D.W. are inventors on a patent concerning the uses of recombinant Newcastle Disease Virus for cancer therapy.
Acknowledgments
This work was supported by the NIH (CA056821 to J.D.W.). The laboratory of J.D.W. receives funding from the Ludwig Institute for Cancer Research, and Swim Across America. D.Z. is the Bart A. Kamen Fellow of the Damon Runyon Cancer Research Foundation and received additional funding from the Ovarian Cancer Research Foundation, Bladder Cancer Awareness Network, MSKCC Cycle for Survival, and the Department of Defense Ovarian Cancer Research Academy (OC150111). D.Z., T.M., and J.D.W. are members of the Parker Institute for Cancer Immunotherapy, which supports the MSKCC Cancer Immunotherapy Program. The MSKCC Genomics Core Facility is supported by the NCI Core grant P30 CA008748.
Footnotes
Supplemental Information includes seven figures and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.01.019.
Supplemental Information
References
- 1.Kaufman H.L., Kohlhapp F.J., Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prestwich R.J., Errington F., Diaz R.M., Pandha H.S., Harrington K.J., Melcher A.A., Vile R.G. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum. Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Yu D.C., Charlton D., Henderson D.R. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposals for human therapy. Hum. Gene Ther. 2000;11:1553–1567. doi: 10.1089/10430340050083289. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson M.S., Lemoine N.R., Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv. Virol. 2012;2012:805629. doi: 10.1155/2012/805629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong H.H., Lemoine N.R., Wang Y. Oncolytic viruses for cancer therapy: overcoming the obstacles. Viruses. 2010;2:78–106. doi: 10.3390/v2010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamarin D., Wolchok J.D. Potentiation of immunomodulatory antibody therapy with oncolytic viruses for treatment of cancer. Mol. Ther. Oncolytics. 2014;1:14004. doi: 10.1038/mto.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koks C.A., Garg A.D., Ehrhardt M., Riva M., Vandenberk L., Boon L., De Vleeschouwer S., Agostinis P., Graf N., Van Gool S.W. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int. J. Cancer. 2015;136:E313–E325. doi: 10.1002/ijc.29202. [DOI] [PubMed] [Google Scholar]
- 9.Andtbacka R.H., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 10.Zamarin D., Holmgaard R.B., Ricca J., Plitt T., Palese P., Sharma P., Merghoub T., Wolchok J.D., Allison J.P. Intratumoral modulation of the inducible co-stimulator ICOS by recombinant oncolytic virus promotes systemic anti-tumour immunity. Nat. Commun. 2017;8:14340. doi: 10.1038/ncomms14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamarin D., Holmgaard R.B., Subudhi S.K., Park J.S., Mansour M., Palese P., Merghoub T., Wolchok J.D., Allison J.P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014;6:226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotte S.J., Lorence R.M., Hirte H.W., Polawski S.R., Bamat M.K., O’Neil J.D., Roberts M.S., Groene W.S., Major P.P. An optimized clinical regimen for the oncolytic virus PV701. Clin. Cancer Res. 2007;13:977–985. doi: 10.1158/1078-0432.CCR-06-1817. [DOI] [PubMed] [Google Scholar]
- 13.Zamarin D., Palese P. Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiol. 2012;7:347–367. doi: 10.2217/fmb.12.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O., Olszanski A.J., Malvehy J., Cebon J., Fernandez E. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170:1109–1119e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamarin D., Vigil A., Kelly K., García-Sastre A., Fong Y. Genetically engineered Newcastle disease virus for malignant melanoma therapy. Gene Ther. 2009;16:796–804. doi: 10.1038/gt.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zamarin D., Martínez-Sobrido L., Kelly K., Mansour M., Sheng G., Vigil A., García-Sastre A., Palese P., Fong Y. Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses. Mol. Ther. 2009;17:697–706. doi: 10.1038/mt.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadman H.F., Kelly P.J., de Angelis N.D., Rohde C., Collins N., Zulu T. Comparison of enzyme-linked immunosorbent assay and haemagglutination inhibition test for the detection of antibodies against Newcastle disease virus in ostriches (Struthio camelus) Avian Pathol. 1997;26:357–363. doi: 10.1080/03079459708419218. [DOI] [PubMed] [Google Scholar]
- 18.Czifra G., Nilsson M., Alexander D.J., Manvell R., Kecskemeti S., Engstrom B.E. Detection of PMV-1 specific antibodies with a monoclonal antibody blocking enzyme-linked immunosorbent assay. Avian Pathol. 1996;25:691–703. doi: 10.1080/03079459608419175. [DOI] [PubMed] [Google Scholar]
- 19.Brown J., Resurreccion R.S., Dickson T.G. The relationship between the hemagglutination-inhibition test and the enzyme-linked immunosorbent assay for the detection of antibody to Newcastle disease. Avian Dis. 1990;34:585–587. [PubMed] [Google Scholar]
- 20.Ilett E., Kottke T., Donnelly O., Thompson J., Willmon C., Diaz R., Zaidi S., Coffey M., Selby P., Harrington K. Cytokine conditioning enhances systemic delivery and therapy of an oncolytic virus. Mol. Ther. 2014;22:1851–1863. doi: 10.1038/mt.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adair R.A., Roulstone V., Scott K.J., Morgan R., Nuovo G.J., Fuller M., Beirne D., West E.J., Jennings V.A., Rose A. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci. Transl. Med. 2012;4:138ra77. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaiss A.K., Machado H.B., Herschman H.R. The influence of innate and pre-existing immunity on adenovirus therapy. J. Cell. Biochem. 2009;108:778–790. doi: 10.1002/jcb.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiao J., Wang H., Kottke T., White C., Twigger K., Diaz R.M., Thompson J., Selby P., de Bono J., Melcher A. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin. Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarahian M., Watzl C., Fournier P., Arnold A., Djandji D., Zahedi S., Cerwenka A., Paschen A., Schirrmacher V., Momburg F. Activation of natural killer cells by newcastle disease virus hemagglutinin-neuraminidase. J. Virol. 2009;83:8108–8121. doi: 10.1128/JVI.00211-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schirrmacher V., Bai L., Umansky V., Yu L., Xing Y., Qian Z. Newcastle disease virus activates macrophages for anti-tumor activity. Int. J. Oncol. 2000;16:363–373. [PubMed] [Google Scholar]
- 26.Woller N., Gürlevik E., Fleischmann-Mundt B., Schumacher A., Knocke S., Kloos A.M., Saborowski M., Geffers R., Manns M.P., Wirth T.C. Viral infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T-cell responses. Mol. Ther. 2015;23:1630–1640. doi: 10.1038/mt.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridle B.W., Stephenson K.B., Boudreau J.E., Koshy S., Kazdhan N., Pullenayegum E., Brunelliere J., Bramson J.L., Lichty B.D., Wan Y. Potentiating cancer immunotherapy using an oncolytic virus. Mol. Ther. 2010;18:1430–1439. doi: 10.1038/mt.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galivo F., Diaz R.M., Wongthida P., Thompson J., Kottke T., Barber G., Melcher A., Vile R. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2010;17:158–170. doi: 10.1038/gt.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prestwich R.J., Ilett E.J., Errington F., Diaz R.M., Steele L.P., Kottke T., Thompson J., Galivo F., Harrington K.J., Pandha H.S. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin. Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakaya T., Cros J., Park M.S., Nakaya Y., Zheng H., Sagrera A., Villar E., García-Sastre A., Palese P. Recombinant Newcastle disease virus as a vaccine vector. J. Virol. 2001;75:11868–11873. doi: 10.1128/JVI.75.23.11868-11873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO Collaborating Center for Reference and Research on Influenza, Chinese National Influenza Center, and National Institute for Viral Disease Control and Prevention, China CDC (2013). Serological detection of avian influenza A(H7N9) virus infections by modified horse red blood cells haemagglutination-inhibition assay. World Health Organization, http://www.who.int/influenza/gisrs_laboratory/cnic_serological_diagnosis_hai_a_h7n9_20131220.pdf.
- 32.Lin Y., Gu Y., McCauley J.W. Optimization of a quantitative micro-neutralization assay. J. Vis. Exp. 2016. 2016:54897. doi: 10.3791/54897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.