Abstract
γδ T cells, a subgroup of T cells based on the γδ TCR, when compared with conventional T cells (αβ T cells), make up a very small proportion of T cells. However, its various subgroups are widely distributed in different parts of the human body and are attractive effectors for infectious disease immunity. γδ T cells are activated and expanded by nonpeptidic antigens (P-Ags), major histocompatibility complex (MHC) molecules, and lipids which are associated with different kinds of pathogen infections. Activation and proliferation of γδ T cells play a significant role in diverse infectious diseases induced by viruses, bacteria, and parasites and exert their potential effector function to effectively eliminate infection. It is well known that many types of infectious diseases are detrimental to human life and health and give rise to high incidence of illnesses and death rate all over the world. To date, there is no comprehensive understanding of the correlation between γδ T cells and infectious diseases. In this review, we will focus on the various subgroups of γδ T cells (mainly Vδ1 T cells and Vδ2 T cells) which can induce multiple immune responses or effective functions to fight against common pathogen infections, such as Mycobacterium tuberculosis, Listeria monocytogenes, influenza viruses, HIV, EBV, and HBV. Hopefully, the gamma-delta T cell study will provide a novel effective way to treat infectious diseases.
1. Introduction
Infectious diseases are mainly caused by pathogen infection (including viruses, bacteria, and parasites). Many types of infectious diseases are detrimental to human life and health and give rise to high incidence of illnesses and death rate all over the world [1]. Dual infection by different types of viruses and a secondary infection is a common clinical phenomenon, which threatens the health of human beings [2–4]. At the beginning, major focus has been put on pathogens instead of host immune response [5]. But pathogens develop chemical resistance which causes a decrease in curative effect [6, 7]. Therefore, more and more researchers are focusing on conventional T cells and their subpopulations with different phenotypes [8–11]. However, the study on the function and immune response of unconventional T cells (γδ T cells) is still neither enough nor systematic. In this review, we will introduce the direct and indirect effector function and immunity of γδ T cells in detail in a variety of pathogen infections in the hope to provide more information for clinical treatment based on the better understanding of the function of different subsets of gamma-delta T cells.
γδ T cells, a subgroup of T cells based on the different T cell receptor (TCR), when compared with conventional T cells (αβ T cells), make up a very small proportion of T cells. They are widely distributed in different parts of the human body [12]. γδ T cells are mainly divided into three subgroups according to the expression of γ (including 2/3/4/5/8/9) and δ (including 1/2/3/5) chains: Vδ1 T cells, Vδ2 T cells, and Vδ3 T cells [13]. Specifically, Vδ1 gene is paired with different Vγ elements (including Vγ2/3/4/5/8), Vδ2 gene is paired with Vγ9 chain, and Vδ3 gene is associated with Vγ2 or Vγ3 [14]. The distribution and function of different subgroups of γδ T cells are strikingly different.
Vδ1 T cells are mostly found in the mucosal epithelium and are in connection with infection of many pathogens [15], such as Listeria monocytogenes, human immunodeficiency virus (HIV), and cytomegalovirus (CMV) [16–21]. Vδ2 T cells are primarily enriched in circulating blood. Vδ2 T cells are uniquely matched with Vγ gene usage of Vγ9 (termed Vγ9Vδ2) and they make up the majority of γδ T cells in the peripheral blood [22, 23]. Vδ2 T cells also exhibit their effective immune response to bacteria and viruses (like mycobacteria, influenza viruses, and Epstein–Barr virus) like Vδ1 T cells [24–27]. Vδ2 T cells based on expressing CD27 and CD45RA are segmented into four different functional subsets with respective characteristic: CD45RA+CD27+ (naïve), CD45RA−CD27+ (central memory without effector function which are rich in lymph nodes), CD45RA−CD27− (effector memory), and CD45RA+CD27− (terminal differentiation which massively appears in the inflammatory site) [28, 29]. They play a significant role via their effector functions and memory responses during infections [28]. The natural killer cell receptor (NKG2D) and Toll-like receptors (TLRs) are also expressed on the surface of both Vδ1 T cells and Vδ2 T cells to exert their effector function during infections even in tumor immunity [30–32]. In contrast with Vδ1 T cells and Vδ2 T cells, Vδ3 T cells, the smallest subset of γδ T cells, are abundant in the liver and are mainly involved in the process of chronic viral infections [33, 34].
In addition, γδ T cells are categorized into a suite of multiple functional populations as follows: IFN-γ-producing γδ T cells, IL-17A-producing γδ T cells, and antigen-presenting γδ T cells. They indirectly promote immune response against pathogen infection by γδ T cells themselves or other immune cells (like CD8+ T cell and B cells) [35–37].
Murine γδ T cells also have various subsets on the basis of characteristic Vγ usage (including1/2/3/4/5/6/7): Vγ1 combined with Vδ6.3, Vγ5 paired with Vδ1, Vγ6 paired with Vδ1, and Vγ7 paired with three diverse Vδ elements (including Vδ4/5/6) [38]. Interestingly, human Vδ1 cells are the primary subtypes found at mucosal surfaces and share certain characteristics with murine γδ intraepithelial lymphocytes (which are associated with Vγ7) [39]. On the contrary, Vγ9Vδ2 T cells are restricted to certain species and are found only in humans and higher primates [39].
2. γδ T Cells Recognize Antigens
αβ T cells which depend on antigen presentation and restrictive major histocompatibility complex (MHC) molecules recognize antigens. γδ T cells, however, can recognize various types of antigens (including nonpeptide antigens and stress-induced ligands) without restrictive MHC molecules [40]. Mounting evidence indicates that γδ T cells exert their protective function in elimination of pathogens and tissue repair via producing cytokines, chemokines, and lytic enzymes, cytotoxic and noncytolytic antiviral activities, and so on [41].
Based on the diverse subtypes, γδ T cells could recognize different types of antigens. Vδ1 T cells could recognize MHC class I chain-related antigens A and B (MICA/B) and stress-induced molecules frequently expressed on epithelial cell in a γδ TCR-dependent manner [40, 42–44]. Activated Vδ1 T cells could exert their effector function against bacterial infection and kill infected cells by production of interleukins and interferons [45]. Interestingly, MICA/B expressed on cancer cell are recognized by both Vδ1 T cells and Vδ2 T cells but in a NKG2D-dependent manner [46, 47]. In addition, Vδ1 T cells respond to MICA-related UL16-binding proteins (ULBPs) based on their ability to combine with human cytomegalovirus (HCMV) glycoprotein UL16 in the same manner [48, 49]. ULBPs are a family of MHC class I-related human cell surface molecules and ligands of NKG2D which play a key role in regulation of innate and adaptive immune responses [50, 51]. Lipids and glycolipid which are relevant to various bacteria (like mycobacteria) are required for the presentation of MHC-related class Ib molecules which are expressed on antigen-presenting cells (APCs), and thus, the bacteria-derived antigens can be recognized by Vδ1 T cells [52–55].
Vδ2 T cells, in particular, are activated by low molecular weight nonpeptidic antigens (also called phosphoantigens (P-Ags)) which are produced by transformed cells or cells infected by pathogens (such as viruses and bacteria) [56, 57]. IPP (isopentenyl pyrophosphate) and HMBPP ((E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate) are the most prominent ones. In general, P-Ags associated with infected or transformed cells are produced by way of the mevalonate pathway (like IPP) when compared with the microbes in the isoprenoid pathway (like HMBPP) [58, 59]. In other words, P-Ags generated by diverse cells and different metabolic pathways are different to each other. For example, HMBPP primarily comes from Mycobacterium tuberculosis, Listeria monocytogenes, and so on [57]. Some clinical medicines can alter the intracellular level of P-Ags to some degree. Nitrogen-containing bisphosphonates (N-BPs) and statins (a kind of anticholesterol drugs) are the most common medicines to increase or decrease the P-Ag level via inhibiting the P-Ag-relevant enzyme [60]. The level of P-Ags also has an obvious trend of increase during stress and infection. Antigen presentation to Vδ2 T cells is independent of restrictions of MHC molecules [60]. Early studies suggested that Vδ2 T cells recognize P-Ags by presentation of CD1d which is expressed on APCs (such as dendritic cells) and monocytes [52, 61]. Butyrophilin 3A1 (BTN3A1) is involved in the process of presenting P-Ags [62]. BTN3A1 binds with P-Ags by its B30.2 domain, and finally, P-Ags are recognized by Vδ2 TCR [63, 64]. Besides, BTN3A1 combined with P-Ags also plays an import role in the process of activation of Vδ2 T cells following N-BP treatment [63]. Like αβ T cells, the activation and proliferation of Vδ2 T cell also need the second signals which depend on costimulators including CD40-CD40L, CD28-B7.1/7.2, CD137 (4-1BB), and CD2 [65, 66]. Toll-like receptors, as the most common pathogen recognition receptors, have the capacity to recognize infectious pathogen-associated molecule patterns [32]. Activated Vδ2 T cells and Vδ1 T cells could activate the expression of Toll-like receptors in reverse [32]. After activation, Vδ2 T cells exert their potential effector functions in the following ways: producing cytokines, chemokines, and lytic enzymes; performing cytotoxic and noncytolytic antiviral activities; inducing maturation of dendritic cells (DCs); providing B cell help; and presenting antigens to CD4+ and CD8+ T cells (Figure 1).
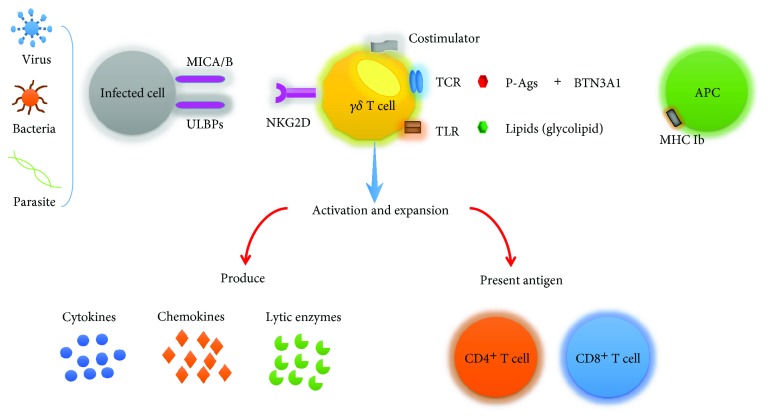
Figure 1.
γδ T cells recognize antigens. Diverse subtypes of γδ T cells could recognize different types of antigens. γδ T cells (both Vδ1 and Vδ2) could recognize stress-induced molecules MICA/B and ULBPs which are expressed in cancer and transformed and infected cells in a NKG2D-dependent manner. Vδ1 T cells could recognize bacteria-derived antigens (including lipids and glycolipid) via MHC-related class Ib molecules which are expressed on antigen-presenting cells. Vδ2 T cells recognize phosphoantigens via forming tight complexes following binding with BTN3A1, and in the context of costimulators, Vδ2 T cells are activated and expanded. Vδ3 T cells can be activated by CD1d which may combine with glycolipid and kill CD1d target cells. Activated Vδ2 T cells and Vδ1 T cells could activate the expression of Toll-like receptors which have the capacity to recognize infectious pathogen-associated molecule patterns. Activation and proliferation of γδ T cells exert their potential effector functions via producing cytokines, chemokines, and lytic enzymes, performing cytotoxic and noncytolytic antiviral activities, presenting antigens to CD4+ and CD8+ T cells, inducing maturation of dendritic cells (DCs), providing B cell help, and so on.
Vδ3 T cells can be activated by CD1d which may combine with glycolipid and kill CD1d target cells and release different kinds of cytokines (includingTh1, Th2, and Th17) and even promote maturation of DC into APCs [33].
3. Function of γδ T Cells in Infectious Diseases
In early report, researchers pay more attention on αβ T cells' protective immunity during infectious diseases. But there is no systematic understanding on γδ T cells' direct or indirect protective ability to fight against pathogens. This review will summarize the diverse functions of γδ T cells in various infectious diseases.
3.1. Bacteria
3.1.1. Mycobacterium tuberculosis (MTB)
γδ T cells play a significant role in MTB infection. Interestingly, Vγ9Vδ2 T cells which exist in humans and the vast majority of nonhuman primates carry huge weight in mycobacterial infections [67]. On the contrary, Vδ1 T cells seem to be more relevant to other infectious diseases, such as HIV diseases [68].
Vγ9Vδ2 T cells recognize HMBPP via forming tight complexes following binding with BTN3A1 during MTB infection. In the presence of costimulators, Vγ9Vδ2 T cells are subsequently activated and expanded [69]. Recently, a number of studies show that phosphoantigen HMBPP and many cytokines participate not only in expansion but also in recall-like expansion and effector functions of Vγ9Vδ2 T cells after MTB infection [24]. Compared with CD4+ T cells, HMBPP-activated Vγ9Vδ2 T cells produce a speck of IL-2 which contributes to the proliferation of unconventional T cells. It has been demonstrated in cynomolgus monkeys that low-dose IL-2 could synergize with nitrogen-containing bisphosphonate or pyrophosphomonoester drugs to expand Vγ9Vδ2 T cells [70]. Similarly, in nonhuman primate models, HMBPP together with IL-2 maximizes its stimulating effect [71]. Besides, T cell growth cytokines (like IL-15 and IL-21) and Th17-related cytokines are also involved in the above process [24]. After Vγ9Vδ2 T cells are activated and proliferated, they take part in the process to fight against MTB. In early years, Gercken et al. [72] have already proven that the mononuclear phagocytes as accessory cells infected by MTB could activate γδ T cells and rest upon costimulators to show a number of functions, especially secretion of cytokine and expression of cytolytic effectors. Generally, MTB phosphoantigen-activated γδ T cell produces TNF-α and IFN-γ to enhance the protective responses to MTB [73]. Meanwhile, cytolytic effector function based on granulysin and perforin is essential for γδ T cell to defend against the MTB infections. There is direct evidence that γδ T cell inhibits and even kills the intracellular MTB by granulysin and perforin with bactericidal ability in macaque models [74]. In addition to the above anti-MTB effects of γδ T cell, it is newly discovered that activated γδ T cell may stimulate the maturation of DCs to modulate other cells (like CD4 T helper cells and B cells) to enhance immune response to MTB [75–77]. Phenotype differentiation of Vγ9Vδ2 T cells also help to strengthen the effective function of αβ T cells to fight against MTB, like promoting CD4+ and CD8+αβ T cells to secrete TNF-α and IFN-γ to kill MTB [78]. Research evidence also suggests that memory response of Vγ9Vδ2 T cells may be based on its phenotype differentiation, and further research is needed to unveil the exact mechanism [25].
Overall, the immune response of HMBPP-activated Vγ9Vδ2 T cells to fight against MTB is dependent on secretion of cytokine, expression of cytolytic effector function, and maturation of DCs.
3.1.2. Listeria monocytogenes
Listeria monocytogenes (L. monocytogenes) is an intracellular bacterium and exists in food (like meat and other dairy products). It can cause a wide range of foodborne diseases in both animals and human [79]. L. monocytogenes can cross the blood-brain barrier, intestinal barrier, or feto-placental barrier and lead to serious infectious illness and death in different populations [80].
IL-17A is mainly produced by γδ T cells during L. monocytogenes infection to promote innate and adaptive immune responses, and it promotes host function of effective elimination of infection by producing cytokines and CXC chemokines [81–84]. Herein, the proliferation and accumulation of neutrophils depending on cytokines and CXC chemokines induced by IL-17A are involved in cross-priming to CD8+ T cells during L. monocytogenes infection [85]. In the early infective stage in the liver of mouse models, IL-17A produced by γδ T cells enhances the antibacterial activity of nonphagocytic cells infected by L. monocytogenes, which is involved in promoting antimicrobial peptide mouse β-defensin (mBD) gene expression [86]. Besides, the IL-17A-producing γδ T cells which are activated rapidly following L. monocytogenes infection mediate its antibacterial immune response via IL-23 production by pathogen-activated macrophages/DCs during the early phase of infection [86]. Moreover, in the IL-17A−/− mouse model, following L. monocytogenes infection, the bacterial burden in the spleen and liver was significantly higher than that of control mice within the stipulated time [87, 88]. Therefore, it can be concluded that IL-17A plays a significant role in the innate immune response to L. monocytogenes. Subsequently, IL-17A has been proven to be indispensable in cytotoxic T cell response against primary L. monocytogenes infection. It can also promote the expansion of cytotoxic T cell (CD8+ T cell). Collectively, innate IL-17A produced mainly by γδ T cells could induce the proliferation of cytotoxic T cell and play their effective cytotoxic T cell response to eliminate L. monocytogenes [87, 88].
IL-17A also plays a crucial role in controlling intestinal pathogens during secondary L. monocytogenes infection. In the mouse model infected with the internalin A mutant recombinant strain of L. monocytogenes (which simulate human intestinal invasion conditions), Vγ4+ memory γδ T cells are confirmed as resident memory (Trm) population in the mesenteric lymph nodes (MLNs) [18]. γδ Trms exert effective elimination of bacteria by early IL-17A secretion to mediate the process in which γδ Trms contain the bacteria within granulomas in the liver and form large clusters with myeloid cells (including neutrophil) at the sites of L. monocytogenes replication foci [18].
3.2. Viruses
3.2.1. Influenza Virus
Due to annual cocirculation and rapid spreading, influenza viruses lead to a large amount of global morbidity and mortality. Influenza viruses widely spread not only from children to the elderly but also to the diverse crowds [89]. Influenza viruses could be divided into the following categories: influenza A viruses (IVA), influenza B viruses (IVB), and influenza C viruses (IVC). IVA show a much more severe infection when compared with IVB and IVC viruses. IVA are derived from swine and avian species and can infect the human respiratory tract through several ways of virus transmission. Recently, researchers are increasingly focusing on the establishment of mouse models following avian influenza H5N1 infection to explore a nicely controlled mechanism of influenza virus infection by gamma-delta T cells [90].
Innate immunity acts as a frontline defense to eliminate virus by interferon and at the same time enhance the adaptive immune response [91]. Phosphoantigen-activated γδ T cells secret substances associated with killing cells infected by influenza viruses to fight against viruses, such as perforin, granzyme B, and granulysin [92, 93]. In humanized mouse models, phosphoantigen treatment significantly decreased weight loss and mortality associated with IVA infection and could control human IVA infection possibly via the selective activation and expansion of human Vδ2 T cells. Thus, phosphoantigen-activated γδ T cells have a significant ability to clear human and avian influenza viruses [90]. In addition, γδ T cells also assist in strengthening the activity of APCs by providing significant signal molecules. After that, APCs play their antigen-presenting role to present influenza antigens to acquire T cells (like CD8+ T cells and CD4+ T cells) and influenza viruses will finally be cleared by these antigen-specific T cell responses. Moreover, phosphoantigen-activated and expanded γδ T cells also induce the expression of CCR1 [94]. CCRs are inflammatory chemokine receptors that promote the ability of elimination of viruses [92, 93].
The number of activated and proliferating γδ T cells, however, varies from person to person after influenza vaccination. Studies compared the number of activated and proliferating γδ T cells between young and elderly healthy human measured by flow cytometry following vaccination. It has been discovered that elderly individuals have lower number and slower kinetics changes of activated and proliferating γδ T cells than young men. It can be concluded from the study that age serves as an important factor to affect the efficiency of T cell response and may make vaccination have a severe drop-off in effectiveness [95].
Besides phosphoantigen and age, type I IFNs and other cytokines could also influence γδ T cell immune response against influenza infection [96, 97]. In the mouse model infected with IVA, researchers exposed IVA-infected mice to smoke or air. Mice exposed to chronic cigarette smoke recovered poorly from primary influenza A pneumonia but recruited γδ T cells to the lungs that predominantly expressed IL-17A. Depletion of IL-17A significantly increased T-bet expression in γδ T cells and improved recovery from acute IVA infection [97]. Collectively, cytokines and phosphoantigen play a crucial part in γδ T cell-mediated antiviral immune response during influenza virus infection.
3.2.2. Human Immunodeficiency Virus (HIV)
HIV infection is different from other viral infections that it does not depend on any one γδ T cell subset alone but need two primary subsets of γδ T cells to participate together [98]. The percentage of two subsets of γδ T cells, however, can be changed or reversed during HIV infections [99]. Vδ1 and Vδ2 T cells in good proportion would play a key role in HIV infections. It has been reported that increasing Vδ1 during HIV infection correlated with the proliferation of CD8+ T cells [100]. Recently, researchers found that the changes in γδ T cell and CD8+ T cell in primary and chronic stages of HIV infection (PHI and CHI) are different. Specifically, in untreated chronic HIV infection (UT-CHI), researchers found a positive correlation between γδ T cell frequency and CD8+ T cell activation. In contrast, in primary HIV Infection (PHI) patients, a negative correlation was found [101]. In addition to Vδ1 and CD8+ T cells, there is a correlation between Vδ2 T cells and CD4+ T cell and they are inversely associated with viral loads [102]. Moreover, inversion of the Vδ2-to-Vδ1 ratio was detected before the inversion of the CD4-to-CD8 ratio, which suggests that the abnormal percentage of Vδ1 and Vδ2 T cells also affected the CD4+ to CD8+ T cell ratio [103]. Recent studies highlight that the CD4/CD8 ratio may serve as a better biomarker for assessing disease progression and HIV's immune suppression in HIV-infected population [104]. It is also supported by another finding that there is a significant relationship between early levels of soluble biomarkers and exhausted CD4/activated CD8 T cells via systematic analysis of correlation between soluble inflammatory biomarker expression and CD4/CD8 T cells at the different stages of HIV infection (including PHI, CHI, and UT-CHI) in HIV-infected Mozambican adults [105]. The lopsided proportion of Vδ1 and Vδ2 T cells causes a negative response against HIV with inhibited cytotoxicity of γδ T cells to kill HIV-infected cells, inhibited secretion of proinflammatory cytokines which is associated with antiviral ability, inhibited ability to block coreceptors for HIV entry, inhibited activation of innate and acquired immunity, and imbalance between cell activation and killing [106, 107]. Thus, dysfunction of γδ T cells leads to HIV immune evasion and finally causes chronic infection [98] (Figure 2). Recently, it was reported that in acute HIV-1 infection, the phenomenon of the lopsided proportion of Vδ1 and Vδ2 T cells can be reversed by syphilis coinfection.
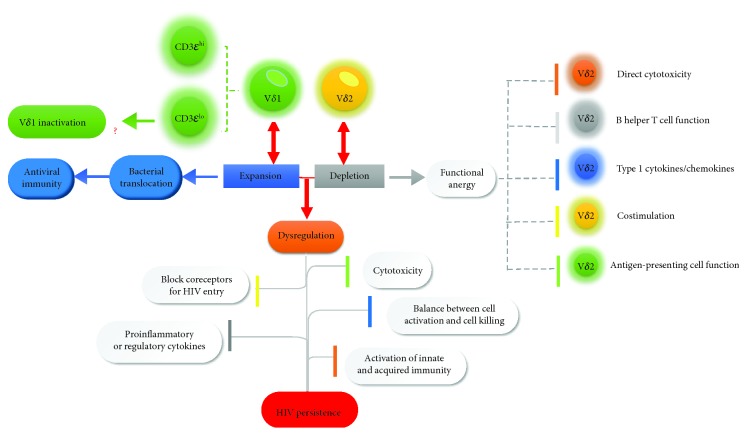
Figure 2.
Dysregulation of γδ T cells during human immunodeficiency virus (HIV) infection. Expansion of Vδ1 T cells during HIV infections was associated with microbial translocation which has relevance to immune activation and exhibited its antiviral immune response. Recently, Vδ1 T cells are segmented into two subsets: CD3εlo Vδ1 T cells and CD3εhi Vδ1 T cells, and CD3εlo Vδ1 T cells may at least partially induce Vδ1 T cell inactivation based on its lower responsiveness to antigenic stimulation. However, the number and function of Vδ2 T cells are depleted during HIV infection. Depletion of Vδ2 T cells leads to inefficient immune response to HIV with inhibited direct cytotoxicity, B helper T cell function, type 1 cytokine or chemokine secretion, antigen-presenting cell function, and costimulation of NK cells. The lopsided proportion of Vδ1 and Vδ2 T cells causes a negative response against HIV infection with inhibited cytotoxicity, coreceptor for HIV entry, proinflammatory or regulatory cytokine release, activation of innate and acquired immunity, and imbalance between cell activation and killing. Thus, dysfunction of γδ T cells leads to HIV immune evasion and finally causes chronic infection.
The effects of both Vδ1 and Vδ2 T cells to defend against HIV have been identified in past years [19]. Expansion of Vδ1 T cells was associated with microbial translocation which has relevance to immune activation [108]. Recently, researchers found that HIV-infected patients have a higher percentage (but not absolute numbers) of Vδ1 T cells [109]. Interestingly, according to the expression of the ε chain of the CD3 protein which is used for TCR signaling, Vδ1 T cells can be segmented into two subsets: CD3εlo Vδ1 T cells and CD3εhi Vδ1 T cells [109]. CD3εlo and CD3εhi T cells have diverse phenotypes and functions. CD3εlo cells frequently express terminally differentiated (TD) cells, exhausted phenotypes, and programmed death-1 (PD-1) and fail to produce IL-17, suggesting that CD3εlo Vδ1 T cells have a lower responsiveness to antigenic stimulation than CD3εhi Vδ1 T cells [109]. This study indicates that HIV may partially induce Vδ1 T cell inactivation and inhibit their effector functions to control virus during HIV infection. Vδ2 T cells exhibited their functions in multiple ways when compared with Vδ1 T cells. Phosphoantigen-activated Vδ2 T cells have direct cytotoxicity for HIV-infected cells even for tumor cells and exhibit B helper T cell function [110–112]. Besides, activated Vδ2 T cells have immune response by producing type 1 cytokines or chemokines including IFN-γ, TNF-α, RANTS, and MIP [106, 113, 114]. In the context of diverse kinds of chemokines (especially β-chemokine), Vδ2 T cells can inhibit coreceptors for HIV entry [110, 115]. Vδ2 T cells, in addition to being immune cells, are also confirmed as APCs [116]. Interestingly, antigen-stimulated γδ T cells costimulate NK cells and increase NK cell killing of autologous DC (editing) which is impaired in HIV+ patients [117]. Interaction between DC and γδ T cells also plays a key role in immune response to pathogen infections and virus-induced immune evasion [118]. Especially, in HIV-1 infection, exposure of DC to HIV-1 leads to its dysfunction but inversely stimulates γδ T cell proliferation and IFN-γ secretion via CCR5-mediated mechanism and plays a crucial role in controlling of HIV-1 replication, virus dissemination within DC via CCL4-mediated mechanism, and HIV-1 transfer to susceptible CD4+ T cells [119].
Effector function of Vδ2 T cells and Vδ1 T cells at different stages of HIV infection, namely, PHI and CHI, is remarkably different. Vδ2 T cells are reported as potential regulatory T cells (Tregs) and play a crucial role in controlling immune activation by anti-inflammatory cytokine secretion during P-HIV [101]. Compared with C-HIV, both mucosal Vδ2 T cells and Vδ1 T cells exert more effective antiviral response in P-HIV [115].
Above all, Vδ2 T cells act as a bridge between innate and acquired immunity to eliminate HIV. However, study shows that the number and function of Vδ2 T cells are depleted during HIV infection [120]. Depletion of Vδ2 T cells is caused by activation of the p38-caspase pathway via combination of HIV and CC chemokine receptor (CCR5) and integrin a4β7 [121]. There is no doubt that the depletion of Vδ2 T cells leads to the inefficient immune response to HIV.
Though the majority of Vδ2 T cells are decreased in HIV infection, activated CD16+ Vγ9Vδ2 T cells as a subset of Vγ9Vδ2 T cells (based on expression of Fc receptor for IgG, also called CD16) have the capacity to induce antibody-dependent cell-mediated cytotoxicity (ADCC) and exert their antiviral functions in HIV type 1 disease [122]. In an earlier report, Vδ2 T cells expanded by zoledronate (one kind of bisphosphonates) and IL-2 are capable of enhancing ADCC cytotoxic effectors in HIV patients [107].
3.2.3. Epstein–Barr Virus (EBV)
EBV, a virus related to transformation of B cell, could cause severe infections in individuals and more likely cause diseases including acute infectious mononucleosis, chronic active EBV infection, Burkitt lymphoma, and tumor (nasopharyngeal carcinoma) [123–125]. There were initial reports that cytotoxic lymphocytes have important influence on anti-EBV action, such as adaptive CD8+ T cell responses [126, 127]. Recently, it has been reported that innate cytotoxic lymphocyte participates in EBV infections [128]. NK cells and Vγ9Vδ2 T cells also exert their cytotoxic lymphocyte function against EBV infection [128]. Furthermore, latent EBV infection shows much a more significant increase in the expansion of both natural killer cells and Vγ9Vδ2 T cells when compared with lytic EBV infection [129]. Expanded Vγ9Vδ2 T cells interact with P-Ag which is produced by the mevalonate pathway by TCR of Vγ9Vδ2 T and BTN3A1 in EBV-infected individuals [129, 130]. In acute infectious mononucleosis, the expression of γδ TCR and the number of γδ T cells were increased analyzed by whole transcriptome profiling [27]. Overexpression of HSP60, HSP70, HSP90, and ULBPs, as protein ligands, can strengthen the recognition and effective cytotoxicity function of γδ T cells against virus-infected cells or malignant host cells [131, 132]. Human MutS homologue (including hMSH2/3/6), which is one kind of protein for DNA mismatch repair and also as a stress-induced protein ligand, is overexpressed in B lymphoblastic cells. This improves the recognition and effective cytotoxicity function of γδ T cells as well as protein ligands [133]. Besides, EBNA1 as nuclear antigen (also called latency I) is expressed on EBV-infected memory B cells and is indispensable for replication of viral genome. It can be recognized by Vγ9Vδ2 T cells and leads to Vγ9Vδ2 T cell expansion [128, 134]. Finally, activated Vγ9Vδ2 T cells could fight against EBV latency. In addition, activated Vγ9Vδ2 T cells which are based on FasL and TRAIL may exert effective elimination function of EBV-transformed lymphoblastoid cell lines [128]. Indeed, P-Ag-stimulated Vγ9Vδ2 T cells were able to prevent outgrowth of adoptively transferred EBV-transformed lymphoblastoid cell lines in vivo [135]. And adoptive transfer of Vγ9Vδ2 T cells could prevent tumorigenesis in mice in which EBV-associated lymphoma formation was induced by EBV infection [136]. In summary, Vγ9Vδ2 T cells combined with other cytotoxic innate lymphocyte subsets (NK T cells) can target various stages of EBV infection.
3.2.4. Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV)
HBV and HCV are involved in liver damage and can lead to viral hepatitis and even liver cancer [137, 138]. The liver is rich with multiple innate immune cells (like natural killer cells and γδ T cells) and plays an important role in innate immunity in the various stages of liver diseases [139–141]. Hepatic γδ T cells occupy a small proportion in total liver lymphocytes [139]. At the beginning, the number of Vδ2 T cells, which account for a considerable proportion of γδ T cells in the liver, tends to decline accompanied by disease progression [142, 143]. Nevertheless, Vδ1 T cells are expanded in liver diseases (especially acute-on-chronic liver failure infected by hepatitis B virus) when compared with Vδ2 T cells and defense against liver damage by producing increased cytotoxicity and inflammatory cytokine [144]. Researchers recently revealed that the frequency of γδ T cell subsets (both Vδ1 and Vδ2) has increased in HBV-infected patients without symptoms. In HBV-infected patients, increased effector memory Vδ2 T cells play a protective role by producing interferon-γ [145]. But in chronic HCV-infected patients, activation and differentiation of Vδ2 T cells exert cytotoxicity via acquisition and expression of cytotoxic natural killer-like phenotype to eradicate the virus instead of producing interferon-γ [146]. Interestingly, γδ T cells could strengthen TNF-α production (induce IFN-γ expression) and CD107a expression (a functional marker for cytotoxicity) with antiviral drug interferon-α treatment. In other words, interferon-α can enhance cytotoxic function of γδ T cells in chronic HBV infection [147]. Moreover, peripheral Vδ2 T cells activated by nonpeptidic antigens (such as pyrophosphomonoesters) can inhibit the replication of HCV via noncytolytic antiviral ability [148]. In contrast, it has been reported that in HBV-infected immunocompetent mice, γδ T cells mediated CD8+ T cell exhaustion by mobilizing myeloid-derived suppressor cell (MDSC) infiltration to the liver in HBV-induced tolerance [149].
3.3. Parasite
3.3.1. Plasmodium
Malaria caused by Plasmodium occurs in tropical and subtropical regions and endangers the physical health. An earlier report demonstrated that conventional T cells (CD4+ and CD8+ T cells) exhibit a protective role in the elimination of Plasmodium falciparum [150]. Accumulating findings indicate that γδ T cells play a key role in defending against Plasmodium infection. γδ T cells are found increased during Plasmodium infection [151]. In γδ T cell depletion mice, the level of protective antibody (IgG2a) which eradicates the malaria parasite exhibits an apparent decline when compared with control [152]. In mouse models without sufficient γδ T cell, it was discovered that, in the context of agonistic anti-CD40 antibody, γδ T cells are involved in controlling Plasmodium berghei XAT (PbXAT). Afterwards, DCs can be activated by unconventional T cells by means of CD40 ligand expression, and whereafter, helper T lymphocyte 1 cells exert their effector response defending against Plasmodium via Th1 differentiation during PbXAT infection [152–154]. In addition, cytokines such as IL-12 and TNF are also crucial for controlling Plasmodium infection and decrease the risk of fever, clinical malaria, and parasitemia [155]. IL-12 and IL-18 are essential for expression of TIM3 (T cell immunoglobulin domain and mucin domain 3), one member of the TIM protein family, in γδ T cell, which could offer clinical malaria important opportunities for risk reduction [156]. Especially, IL-17A, which is largely produced by γδ T cells, could slow down the course of diverse pathogen infections. According to the report, IL-17A-producing γδ T cells in combination with monocytes are involved in the early process of fighting against parasites [157]. Some cytokines and chemokines (such as TNF and MIP-1β/1α) which increase the risks of severe malaria, however, are derived from γδ T cell [158]. Collectively, cytokines and chemokines have dual effects on Plasmodium infections.
Different subgroups of γδ T cell play various roles in controlling Plasmodium infections. Vγ9Vδ2T cells activated by P. falciparum antigens produce cytotoxic granules to kill merozoites and control parasite density during the blood stage of infection [159]. The proportion of Vδ2+γδ T cells increased in previously naïve adults following malaria infection. But children with repeated malaria were associated with reduced percentages of Vδ2+γδ T cells and cytokine secretion and increased expression of immunoregulatory genes. Taken together, the loss and dysfunction of Vδ2+γδ T cells in children with repeated malaria may lead to clinical tolerance of the parasite [160]. Moreover, the diminished Vδ2+γδ T cell proinflammatory cytokine production in this situation was associated with expression of the immunoregulatory markers TIM3 and CD57. Higher Vδ2+γδ T cell proinflammatory cytokine production was associated with protection from subsequent P. falciparum infection [161]. Recently, it was discovered that both reduction and dysfunction of Vδ2+γδ T cells promote the expression of CD16 which causes Vδ2+γδ T cells to exhibit inefficient recognition of nonpeptidic antigens [162]. Vγ1+γδ T cells are also important for defense against Plasmodium infection. During early Plasmodium berghei XAT (PbXAT) infection stage, expanding Vγ1+γδ T cells promotes CD40 ligand expression and IFN-γ secretion. CD40 ligand- (CD40L-) CD40 signaling activates DCs to induce protective immunity. It was manifested that the Vγ1+γδ T cell response is dependent on IFN-γ-activated DCs [163]. Nonetheless, at the late stage, the IFN-γ positivity of Vγ1+γδ T cells is reduced due to γδ T cell dysfunction. Indeed, Vγ1+γδ T cells promote inhibitory receptor expression, such as PD-1, LAG-3, and TIM3 at the late stage [163].
4. Possible γδ T Cell-Based Clinical Application
Bisphosphonates (also called aminobisphosphonates (ABP)) are commonly used to activate Vγ9Vδ2 T cells via accumulating and elevating the level of cellular IPP and its metabolites [164]. Pamidronate (PAM) and zoledronate (Zol) are bisphosphonates that can inhibit the IPP-metabolizing enzyme farnesyl diphosphate synthase (FDPS) which is a key enzyme of the mevalonate pathway [165, 166]. PAM is considered as an economical and practical way to activate Vγ9Vδ2 T cells [167]. In humanized mouse models, it is reported that PAM reduces disease severity and mortality and controls lung inflammation and viral replication after human influenza virus infection [168]. Zol is broadly exploited to enhance adoptive cancer immunotherapy and stimulate effector γδ T cells with antitumor activity [169, 170]. However, ABP as an anti-infection agent have certain limitations in clinical use. Intravenous infusion of ABP gives rise to immune-mediated diseases (such as persistent autoimmune syndromes) because of TNF-α and IFN-γ release by Vγ9δ2 T cells which will induce inflammatory response or acute clinical response [171]. ABP affect oral absorption and inhibit bone resorption and even lead to bone side effects in cancer treatment [172]. Tetrakis-pivaloyloxymethyl 2-(thiazole-2-ylamino) ethylidene-1,1-bisphosphonate (PTA) as a synthetic bisphosphonate prodrug can also inhibit FDPS. It can get inside the cells where it is converted into acid enzymes with activity by intracellular esterases [173]. PTA could activate the expansion of peripheral blood Vγ9δ2 T cells which are separated from cancer patients (prostate and breast cancer) [174]. Compared with Zol, PTA activates γδ T cell expansion more effectively and produces more cytokines (TNF-α and IFN-γ) [173].
Besides P-Ag-induced activation of γδ T cells, BTN3A-specific monoclonal antibody (mAb) 20.1 can also activate Vγ9Vδ2 TCR by CDR3 of Vγ9 and Vδ2 chain responsiveness to mAb 20.1 [175]. Meanwhile, mAb 20.1 can interfere with the P-Ags-response [175]. Thus, BTN3A-specific antibody may be useful agents against pathogen infections.
Adoptive transfer of γδ T cells by intravenous infusion is the most common way for the clinical trials of patients [176, 177]. Adoptive transfer therapy is confirmed as a safe way without requiring preconditioning to expand Vγ9Vδ2 T cells and has been reported in many studies [178, 179]. Researchers recently pay more attention to not only the safety but also the clinical effects of in vitro expanded γδ T cells in multiple ways including DNA copy number and negative conversion rate of HbeAg during active HBV infections (https://www.clinicaltrials.gov/). In nonhuman primate models infected by Mycobacterium tuberculosis, adoptive transfer of Vγ9Vδ2 T cells has no or reduced tuberculosis dissemination when compared with control [180]. Vγ9Vδ2 T cells by adoptive transfer therapy display central/effector memory and exert their effector function defense against MTB infections via secreting anti-M. tuberculosis cytokines and inhibiting intracellular bacteria [180]. Adoptive transfer therapy based on γδ T cells is also applicable for treatment of a range of cancers including renal cancer, breast and cervical cancer, and non-small-cell lung cancer [181, 182]. Interestingly, it is more vulnerable to accomplish successfully adoptive transfer of γδ T cells following ABP treatment [183].
An earlier study reports that low-dose IL-2 could synergize with nitrogen-containing bisphosphonate or pyrophosphomonoester drugs to expand Vγ9Vδ2 T cells [71]. Phosphoantigens combined with IL-2 are an efficient method to activate and expand Vγ9Vδ2 T cells both in vitro and in vivo [74, 184]. Expression of NO synthase (NOS2) exerts profound influence on γδ T cell properties, including IL-2 secretion, its expansion, and glycolysis metabolism. Recently, there is a report that IL-2 is not completely necessary for Vγ9Vδ2 T cells in adoptive immunotherapy [174]. IL-18 represents a new potential treatment for HIV-positive individuals since it activates Vγ9Vδ2 T cell responses to phosphoantigen [185].
Broadly speaking, γδ T cell-based clinical application has both advantages and limits in controlling and even eliminating pathogen infections. γδ T cells have the following extraordinary advantages: firstly, γδ T cell-based clinical application emphasizes the importance of host immune response instead of pathogens themselves. Secondly, γδ T cells rapidly gather at the site of infection and exert effective function of elimination of pathogens. Thirdly, γδ T cells play multiple roles in controlling infection on the basis of different subsets of γδ T cells with different functions and γδ T cells act as functionally diversified cells such as APC and potential regulatory T cells. Fourthly, though γδ T cells make up a very small proportion of T cells in the human body, they can be directly activated by phosphoantigens or indirectly activated by drugs that induce IPP accumulation or monoclonal antibody, both of which are economical and practical. Fifthly, there is a relatively safe way for the clinical trials of patients: adoptive transfer of γδ T cells by intravenous infusion. However, current application of conventional therapy also has certain limitations in clinical use. It has been reported that phosphoantigen reapplication may lead effector cells to an incapable, exhausted, and even dead condition [186]. Irrational drug use like overdoses may lead to autoimmune diseases. Moreover, activated γδ T cells by drugs like ABP release many proinflammatory cytokines and may also give rise to immune-mediated diseases such as persistent autoimmune syndromes. Therefore, it is important to confirm both the safety and the dose of clinical medication in the future and γδ T cell-based immune therapy still needs further discussion and research.
Above all, though the mentioned potential therapeutic methods have some limitations, it put forward ideas and methods for further clinical research. To achieve an effective and safe treatment on infected patients, no doubt, we need a broader and deeper understanding of effector function of different subgroups of human γδ T cells.
5. Summary
Since the diverse subpopulations of γδ T cells possess different biological characteristics, they play different roles in various infectious illnesses induced by bacteria, viruses, and parasites. Different kinds of antigens associated with various pathogen infections including nonpeptidic antigens (P-Ags), MHC molecules, and lipids could be directly or indirectly recognized by γδ T cells. Some γδ T cells are immediately activated, while some γδ T cells also need a second signal costimulation. The activation and expansion of γδ T cells exert their effector function during pathogen infections. Growing evidence suggests that γδ T cells act as a link to connection innate with adaptive immunity. It is intriguing to find that γδ T cells can also work as APC to present pathogen infection-associated antigen to CD4+ and CD8+ T cells. In addition, γδ T cells exert their protective function in the elimination of pathogens and tissue repair via producing cytokines, chemokines, and lytic enzymes and cytotoxic and noncytolytic antiviral activities. γδ T cells can also promote DC maturation and provide B cell help to produce antibody. Collectively, γδ T cells play a significant role in the elimination of pathogens. In view of the promising implications of γδ T cells to treat infectious diseases in preclinical studies, it is hoped that γδ T cells will provide a potentially effective new way to treat infectious diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant nos. 81503093, 81602166, and 81672444) and the joint funds of Southwest Medical University and Luzhou, China (2016LZXNYD-T01, 2017LZXNYD-Z05, and 2017LZXNYD-J09).
Contributor Information
Zhangang Xiao, Email: xzg555898@hotmail.com.
Jing Shen, Email: crystal_stray@126.com.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
Yueshui Zhao and Ling Lin contributed equally to this manuscript.
References
- 1.Román G. C., Spencer P. S., Schoenberg B. S., et al. Tropical spastic paraparesis: HTLV-I antibodies in patients from the Seychelles. New England Journal of Medicine. 1987;316(1):51–52. doi: 10.1056/NEJM198701013160114. [DOI] [PubMed] [Google Scholar]
- 2.Robinson K. M., Ramanan K., Clay M. E., et al. The inflammasome potentiates influenza/Staphylococcus aureus superinfection in mice. JCI Insight. 2018;3(7) doi: 10.1172/jci.insight.97470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzales Zamora J. A. Dual infection of the central nervous system caused by Cryptococcus and Toxoplasma in a patient with AIDS: a case report and literature review. Acta Clinica Belgica. 2018:1–5. doi: 10.1080/17843286.2018.1457761. [DOI] [PubMed] [Google Scholar]
- 4.Hebberecht L., Vancoillie L., Schauvliege M., et al. Frequency of occurrence of HIV-1 dual infection in a Belgian MSM population. PLoS One. 2018;13(4, article e0195679) doi: 10.1371/journal.pone.0195679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann S. H. E. Robert Koch, the nobel prize, and the ongoing threat of tuberculosis. New England Journal of Medicine. 2005;353(23):2423–2426. doi: 10.1056/NEJMp058131. [DOI] [PubMed] [Google Scholar]
- 6.Soothill G., Hu Y., Coates A. Can we prevent antimicrobial resistance by using antimicrobials better? Pathogens. 2013;2(2):422–435. doi: 10.3390/pathogens2020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thabit A. K., Crandon J. L., Nicolau D. P. Antimicrobial resistance: impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opinion on Pharmacotherapy. 2015;16(2):159–177. doi: 10.1517/14656566.2015.993381. [DOI] [PubMed] [Google Scholar]
- 8.Vernal R., Garcia-Sanz J. A. Th17 and Treg cells, two new lymphocyte subpopulations with a key role in the immune response against infection. Infectious Disorders Drug Targets. 2008;8(4):207–220. doi: 10.2174/187152608786734197. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay P. K., Roederer M. Immunophenotyping of T cell subpopulations in HIV disease. Current Protocols in Immunology. 2005;65(1):12.12.1–12.12.15. doi: 10.1002/0471142735.im1212s65. [DOI] [PubMed] [Google Scholar]
- 10.Belkaid Y., Rouse B. T. Natural regulatory T cells in infectious disease. Nature Immunology. 2005;6(4):353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 11.Rouse B. T., Sarangi P. P., Suvas S. Regulatory T cells in virus infections. Immunological Reviews. 2006;212(1):272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 12.Caccamo N., Dieli F., Wesch D., Jomaa H., Eberl M. Sex-specific phenotypical and functional differences in peripheral human Vγ9/Vδ2 T cells. Journal of Leukocyte Biology. 2006;79(4):663–666. doi: 10.1189/jlb.1105640. [DOI] [PubMed] [Google Scholar]
- 13.Wesch D., Hinz T., Kabelitz D. Analysis of the TCR Vgamma repertoire in healthy donors and HIV-1-infected individuals. International Immunology. 1998;10(8):1067–1075. doi: 10.1093/intimm/10.8.1067. [DOI] [PubMed] [Google Scholar]
- 14.Wu D., Wu P., Qiu F., Wei Q., Huang J. Human γδT-cell subsets and their involvement in tumor immunity. Cellular & Molecular Immunology. 2017;14(3):245–253. doi: 10.1038/cmi.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabelitz D., Glatzel A., Wesch D. Antigen recognition by human γδT lymphocytes. International Archives of Allergy and Immunology. 2000;122(1):1–7. doi: 10.1159/000024353. [DOI] [PubMed] [Google Scholar]
- 16.Regan T., MacSharry J., Brint E. Tracing innate immune defences along the path of Listeria monocytogenes infection. Immunology and Cell Biology. 2014;92(7):563–569. doi: 10.1038/icb.2014.27. [DOI] [PubMed] [Google Scholar]
- 17.Xu S., Han Y., Xu X., Bao Y., Zhang M., Cao X. IL-17A-producing gammadeltaT cells promote CTL responses against Listeria monocytogenes infection by enhancing dendritic cell cross-presentation. Journal of Immunology. 2010;185(10):5879–5887. doi: 10.4049/jimmunol.1001763. [DOI] [PubMed] [Google Scholar]
- 18.Romagnoli P. A., Sheridan B. S., Pham Q. M., Lefrancois L., Khanna K. M. IL-17A–producing resident memory γδ T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(30):8502–8507. doi: 10.1073/pnas.1600713113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodara V. L., Parodi L. M., Chavez D., Smith L. M., Lanford R., Giavedoni L. D. Characterization of γδT cells in naïve and HIV-infected chimpanzees and their responses to T-cell activators in vitro. Journal of Medical Primatology. 2014;43(4):258–271. doi: 10.1111/jmp.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H., Li D. J., Jin L. P. γδT cells and related diseases. American Journal of Reproductive Immunology. 2016;75(6):609–618. doi: 10.1111/aji.12495. [DOI] [PubMed] [Google Scholar]
- 21.Kallemeijn M. J., Boots A. M. H., van der Klift M. Y., et al. Ageing and latent CMV infection impact on maturation, differentiation and exhaustion profiles of T-cell receptor gammadelta T-cells. Scientific Reports. 2017;7(1):p. 5509. doi: 10.1038/s41598-017-05849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinz T., Wesch D., Halary F., et al. Identification of the complete expressed human TCR V gamma repertoire by flow cytometry. International Immunology. 1997;9(8):1065–1072. doi: 10.1093/intimm/9.8.1065. [DOI] [PubMed] [Google Scholar]
- 23.Rakasz E., MacDougall A. V., Zayas M. T., et al. γδT cell receptor repertoire in blood and colonic mucosa of rhesus macaques. Journal of Medical Primatology. 2000;29(6):387–396. doi: 10.1111/j.1600-0684.2000.290602.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z. W. Protective immune responses of major Vγ2Vδ2 T-cell subset in M. tuberculosis infection. Current Opinion in Immunology. 2016;42:105–112. doi: 10.1016/j.coi.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meraviglia S., El Daker S., Dieli F., Martini F., Martino A. γδT cells cross-link innate and adaptive immunity in Mycobacterium tuberculosis infection. Clinical & Developmental Immunology. 2011;2011, article 587315:11. doi: 10.1155/2011/587315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldeck D., Theeten H., Hassouneh F., et al. Frequencies of peripheral immune cells in older adults following seasonal influenza vaccination with an adjuvanted vaccine. Vaccine. 2017;35(34):4330–4338. doi: 10.1016/j.vaccine.2017.06.082. [DOI] [PubMed] [Google Scholar]
- 27.Zhong H., Hu X., Janowski A. B., et al. Whole transcriptome profiling reveals major cell types in the cellular immune response against acute and chronic active Epstein-Barr virus infection. Scientific Reports. 2017;7(1):p. 17775. doi: 10.1038/s41598-017-18195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dieli F., Poccia F., Lipp M., et al. Differentiation of effector/memory Vδ2 T cells and migratory routes in lymph nodes or inflammatory sites. The Journal of Experimental Medicine. 2003;198(3):391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gioia C., Agrati C., Casetti R., et al. Lack of CD27−CD45RA−Vγ9Vδ2+ T cell effectors in immunocompromised hosts and during active pulmonary tuberculosis. Journal of Immunology. 2002;168(3):1484–1489. doi: 10.4049/jimmunol.168.3.1484. [DOI] [PubMed] [Google Scholar]
- 30.Pietschmann K., Beetz S., Welte S., et al. Toll-like receptor expression and function in subsets of human γδ T lymphocytes. Scandinavian Journal of Immunology. 2009;70(3):245–255. doi: 10.1111/j.1365-3083.2009.02290.x. [DOI] [PubMed] [Google Scholar]
- 31.Rincon-Orozco B., Kunzmann V., Wrobel P., Kabelitz D., Steinle A., Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. Journal of Immunology. 2005;175(4):2144–2151. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 32.Wesch D., Peters C., Oberg H. H., Pietschmann K., Kabelitz D. Modulation of γδ T cell responses by TLR ligands. Cellular and Molecular Life Sciences. 2011;68(14):2357–2370. doi: 10.1007/s00018-011-0699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangan B. A., Dunne M. R., O'Reilly V. P., et al. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vδ3 T cells. Journal of Immunology. 2013;191(1):30–34. doi: 10.4049/jimmunol.1300121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y. L., Ding Y. P., Tanaka Y., et al. γδ T cells and their potential for immunotherapy. International Journal of Biological Sciences. 2014;10(2):119–135. doi: 10.7150/ijbs.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesch D., Glatzel A., Kabelitz D. Differentiation of resting human peripheral blood gamma delta T cells toward Th1- or Th2-phenotype. Cellular Immunology. 2001;212(2):110–117. doi: 10.1006/cimm.2001.1850. [DOI] [PubMed] [Google Scholar]
- 36.Moser E. K., Sun J., Kim T. S., Braciale T. J. IL-21R signaling suppresses IL-17+ gamma delta T cell responses and production of IL-17 related cytokines in the lung at steady state and after influenza A virus infection. PLoS One. 2015;10(4, article e0120169) doi: 10.1371/journal.pone.0120169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moser B., Eberl M. γδ T-APCs: a novel tool for immunotherapy? Cellular and Molecular Life Sciences. 2011;68(14):2443–2452. doi: 10.1007/s00018-011-0706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munoz-Ruiz M., Sumaria N., Pennington D. J., Silva-Santos B. Thymic determinants of γδ T cell differentiation. Trends in Immunology. 2017;38(5):336–344. doi: 10.1016/j.it.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Pang D. J., Neves J. F., Sumaria N., Pennington D. J. Understanding the complexity of γδ T-cell subsets in mouse and human. Immunology. 2012;136(3):283–290. doi: 10.1111/j.1365-2567.2012.03582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groh V., Steinle A., Bauer S., Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279(5357):1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 41.Vantourout P., Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nature Reviews Immunology. 2013;13(2):88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu B., Pizarro J. C., Holmes M. A., et al. Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(6):2414–2419. doi: 10.1073/pnas.1015433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groh V., Rhinehart R., Secrist H., Bauer S., Grabstein K. H., Spies T. Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MICA and MICB. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(12):6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Born W. K., Kemal Aydintug M., O'Brien R. L. Diversity of γδ T-cell antigens. Cellular & Molecular Immunology. 2013;10(1):13–20. doi: 10.1038/cmi.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kapp J. A., Kapp L. M., McKenna K. C., Lake J. P. γδ T-cell clones from intestinal intraepithelial lymphocytes inhibit development of CTL responses ex vivo. Immunology. 2004;111(2):155–164. doi: 10.1111/j.0019-2805.2003.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poggi A., Venturino C., Catellani S., et al. Vδ1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated bytrans-retinoic acid. Cancer Research. 2004;64(24):9172–9179. doi: 10.1158/0008-5472.CAN-04-2417. [DOI] [PubMed] [Google Scholar]
- 47.Catellani S., Poggi A., Bruzzone A., et al. Expansion of Vdelta1 T lymphocytes producing IL-4 in low-grade non-Hodgkin lymphomas expressing UL-16-binding proteins. Blood. 2007;109(5):2078–2085. doi: 10.1182/blood-2006-06-028985. [DOI] [PubMed] [Google Scholar]
- 48.Sutherland C. L., Chalupny N. J., Cosman D. The UL16-binding proteins, a novel family of MHC class I-related ligands for NKG2D, activate natural killer cell functions. Immunological Reviews. 2001;181(1):185–192. doi: 10.1034/j.1600-065X.2001.1810115.x. [DOI] [PubMed] [Google Scholar]
- 49.Bauman Y., Drayman N., Ben-Nun-Shaul O., et al. Downregulation of the stress-induced ligand ULBP1 following SV40 infection confers viral evasion from NK cell cytotoxicity. Oncotarget. 2016;7(13):15369–15381. doi: 10.18632/oncotarget.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutherland C. L., Rabinovich B., Chalupny N. J., Brawand P., Miller R., Cosman D. ULBPs, human ligands of the NKG2D receptor, stimulate tumor immunity with enhancement by IL-15. Blood. 2006;108(4):1313–1319. doi: 10.1182/blood-2005-11-011320. [DOI] [PubMed] [Google Scholar]
- 51.Vivier E., Tomasello E., Paul P. Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition? Current Opinion in Immunology. 2002;14(3):306–311. doi: 10.1016/S0952-7915(02)00337-0. [DOI] [PubMed] [Google Scholar]
- 52.Agea E., Russano A., Bistoni O., et al. Human CD1-restricted T cell recognition of lipids from pollens. The Journal of Experimental Medicine. 2005;202(2):295–308. doi: 10.1084/jem.20050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uldrich A. P., Le Nours J., Pellicci D. G., et al. CD1d-lipid antigen recognition by the γδ TCR. Nature Immunology. 2013;14(11):1137–1145. doi: 10.1038/ni.2713. [DOI] [PubMed] [Google Scholar]
- 54.Luoma A. M., Castro C. D., Mayassi T., et al. Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity. 2013;39(6):1032–1042. doi: 10.1016/j.immuni.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poggi A., Zocchi M. R. γδ T lymphocytes as a first line of immune defense: old and new ways of antigen recognition and implications for cancer immunotherapy. Frontiers in Immunology. 2014;5:p. 575. doi: 10.3389/fimmu.2014.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka Y., Morita C. T., Tanaka Y., Nieves E., Brenner M. B., Bloom B. R. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375(6527):155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 57.Eberl M., Hintz M., Reichenberg A., Kollas A. K., Wiesner J., Jomaa H. Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Letters. 2003;544(1–3):4–10. doi: 10.1016/S0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 58.Hintz M., Reichenberg A., Altincicek B., et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Letters. 2001;509(2):317–322. doi: 10.1016/S0014-5793(01)03191-X. [DOI] [PubMed] [Google Scholar]
- 59.Gober H. J., Kistowska M., Angman L., Jeno P., Mori L., De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. The Journal of Experimental Medicine. 2003;197(2):163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kabelitz D. Critical role of butyrophilin 3A1 in presenting prenyl pyrophosphate antigens to human γδ T cells. Cellular & Molecular Immunology. 2014;11(2):117–119. doi: 10.1038/cmi.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dieude M., Striegl H., Tyznik A. J., et al. Cardiolipin binds to CD1d and stimulates CD1d-restricted γδ T cells in the normal murine repertoire. Journal of Immunology. 2011;186(8):4771–4781. doi: 10.4049/jimmunol.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esser C. A fat story—antigen presentation by butyrophilin 3A1 to γδ T cells. Cellular & Molecular Immunology. 2014;11(1):5–7. doi: 10.1038/cmi.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harly C., Guillaume Y., Nedellec S., et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood. 2012;120(11):2269–2279. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allison T. J., Winter C. C., Fournie J. J., Bonneville M., Garboczi D. N. Structure of a human gammadelta T-cell antigen receptor. Nature. 2001;411(6839):820–824. doi: 10.1038/35081115. [DOI] [PubMed] [Google Scholar]
- 65.Ribot J. C., deBarros A., Silva-Santos B. Searching for “signal 2”: costimulation requirements of γδ T cells. Cellular and Molecular Life Sciences. 2011;68(14):2345–2355. doi: 10.1007/s00018-011-0698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Witherden D. A., Havran W. L. Molecular aspects of epithelial γδ T cell regulation. Trends in Immunology. 2011;32(6):265–271. doi: 10.1016/j.it.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harly C., Peigne C. M., Scotet E. Molecules and mechanisms implicated in the peculiar antigenic activation process of human Vγ9Vδ2 T cells. Frontiers in Immunology. 2014;5:p. 657. doi: 10.3389/fimmu.2014.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martini F., Urso R., Gioia C., et al. γδ T-cell anergy in human immunodeficiency virus-infected persons with opportunistic infections and recovery after highly active antiretroviral therapy. Immunology. 2000;100(4):481–486. doi: 10.1046/j.1365-2567.2000.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vavassori S., Kumar A., Wan G. S., et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nature Immunology. 2013;14(9):908–916. doi: 10.1038/ni.2665. [DOI] [PubMed] [Google Scholar]
- 70.Casetti R., Perretta G., Taglioni A., et al. Drug-induced expansion and differentiation of Vγ9Vδ2 T cells in vivo: the role of exogenous IL-2. Journal of Immunology. 2005;175(3):1593–1598. doi: 10.4049/jimmunol.175.3.1593. [DOI] [PubMed] [Google Scholar]
- 71.Sicard H., Ingoure S., Luciani B., et al. In vivo immunomanipulation of Vγ9Vδ2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. Journal of Immunology. 2005;175(8):5471–5480. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- 72.Gercken J., Pryjma J., Ernst M., Flad H. D. Defective antigen presentation by Mycobacterium tuberculosis-infected monocytes. Infection and Immunity. 1994;62(8):3472–3478. doi: 10.1128/iai.62.8.3472-3478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casetti R., Martino A. The plasticity of γδ T cells: innate immunity, antigen presentation and new immunotherapy. Cellular & Molecular Immunology. 2008;5(3):161–170. doi: 10.1038/cmi.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen C. Y., Yao S., Huang D., et al. Phosphoantigen/IL2 expansion and differentiation of Vγ2Vδ2 T cells increase resistance to tuberculosis in nonhuman primates. PLoS Pathogens. 2013;9(8, article e1003501) doi: 10.1371/journal.ppat.1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petrasca A., Doherty D. G. Human Vδ2+γδ T cells differentially induce maturation, cytokine production, and alloreactive T cell stimulation by dendritic cells and B cells. Frontiers in Immunology. 2014;5 doi: 10.3389/fimmu.2014.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caccamo N., Battistini L., Bonneville M., et al. CXCR5 identifies a subset of Vγ9Vδ2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. Journal of Immunology. 2006;177(8):5290–5295. doi: 10.4049/jimmunol.177.8.5290. [DOI] [PubMed] [Google Scholar]
- 77.Eberl M., Roberts G. W., Meuter S., Williams J. D., Topley N., Moser B. A rapid crosstalk of human gammadelta T cells and monocytes drives the acute inflammation in bacterial infections. PLoS Pathogens. 2009;5(2, article e1000308) doi: 10.1371/journal.ppat.1000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ali Z., Yan L., Plagman N., et al. γδ T cell immune manipulation during chronic phase of simian HIV infection confers immunological benefits. Journal of Immunology. 2009;183(8):5407–5417. doi: 10.4049/jimmunol.0901760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vazquez-Boland J. A., Kuhn M., Berche P., et al. Listeria pathogenesis and molecular virulence determinants. Clinical Microbiology Reviews. 2001;14(3):584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramaswamy V., Cresence V. M., Rejitha J. S., et al. Listeria-review of epidemiology and pathogenesis. Journal of Microbiology Immunology and Infection. 2007;40(1):4–13. [PubMed] [Google Scholar]
- 81.Nakae S., Komiyama Y., Nambu A., et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17(3):375–387. doi: 10.1016/S1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 82.Park H., Li Z., Yang X. O., et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunology. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khader S. A., Gaffen S. L., Kolls J. K. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunology. 2009;2(5):403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu S., Cao X. Interleukin-17 and its expanding biological functions. Cellular & Molecular Immunology. 2010;7(3):164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tvinnereim A. R., Hamilton S. E., Harty J. T. Neutrophil involvement in cross-priming CD8+ T cell responses to bacterial antigens. Journal of Immunology. 2004;173(3):1994–2002. doi: 10.4049/jimmunol.173.3.1994. [DOI] [PubMed] [Google Scholar]
- 86.Hamada S., Umemura M., Shiono T., et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. Journal of Immunology. 2008;181(5):3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hall C., Thrower S., Lim L., Davison A. N. Purification of oestradiol receptor by chromatography on oligo(dT)-cellulose. Biochemical Society Transactions. 1976;4(4):766–769. doi: 10.1042/bst0040766. [DOI] [PubMed] [Google Scholar]
- 88.Bezzerri V., Borgatti M., Finotti A., Tamanini A., Gambari R., Cabrini G. Mapping the transcriptional machinery of the IL-8 gene in human bronchial epithelial cells. Journal of Immunology. 2011;187(11):6069–6081. doi: 10.4049/jimmunol.1100821. [DOI] [PubMed] [Google Scholar]
- 89.Nussing S., Sant S., Koutsakos M., Subbarao K., Nguyen T. H. O., Kedzierska K. Innate and adaptive T cells in influenza disease. Frontiers of Medicine. 2018;12(1):34–47. doi: 10.1007/s11684-017-0606-8. [DOI] [PubMed] [Google Scholar]
- 90.Tu W. W., Lau Y. L., Peiris J. S. Use of humanised mice to study antiviral activity of human γδ-T cells against influenza A viruses. Hong Kong Medical Journal. 2014;20(Supplement 6):4–6. [PubMed] [Google Scholar]
- 91.Randall R. E., Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. The Journal of General Virology. 2008;89(1):1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 92.Kabelitz D., Lettau M., Janssen O. Immunosurveillance by human γδ T lymphocytes: the emerging role of butyrophilins. F1000Research. 2017;6 doi: 10.12688/f1000research.11057.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bonneville M., Scotet E. Human Vgamma9Vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Current Opinion in Immunology. 2006;18(5):539–546. doi: 10.1016/j.coi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Qin G., Liu Y., Zheng J., et al. Type 1 responses of human Vγ9Vδ2 T cells to influenza A viruses. Journal of Virology. 2011;85(19):10109–10116. doi: 10.1128/JVI.05341-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stervbo U., Pohlmann D., Baron U., et al. Age dependent differences in the kinetics of γδ T cells after influenza vaccination. PLoS One. 2017;12(7, article e0181161) doi: 10.1371/journal.pone.0181161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen M., Hong M. J., Sun H., et al. Essential role for autophagy in the maintenance of immunological memory against influenza infection. Nature Medicine. 2014;20(5):503–510. doi: 10.1038/nm.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hong M. J., Gu B. H., Madison M. C., et al. Protective role of γδ T cells in cigarette smoke and influenza infection. Mucosal Immunology. 2017;11(3):894–908. doi: 10.1038/mi.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pauza C. D., Poonia B., Li H., Cairo C., Chaudhry S. γδ T cells in HIV disease: past, present, and future. Frontiers in Immunology. 2014;5:p. 687. doi: 10.3389/fimmu.2014.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Paoli P., Gennari D., Martelli P., et al. A subset of gamma delta lymphocytes is increased during HIV-1 infection. Clinical and Experimental Immunology. 1991;83(2):187–191. doi: 10.1111/j.1365-2249.1991.tb05612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Margolick J. B., Scott E. R., Odaka N., Saah A. J. Flow cytometric analysis of gamma delta T cells and natural killer cells in HIV-1 infection. Clinical Immunology and Immunopathology. 1991;58(1):126–138. doi: 10.1016/0090-1229(91)90154-3. [DOI] [PubMed] [Google Scholar]
- 101.Bhatnagar N., Girard P.-M., Lopez-Gonzalez M., et al. Potential role of Vδ2+γδ T cells in regulation of immune activation in primary HIV infection. Frontiers in Immunology. 2017;8:p. 1189. doi: 10.3389/fimmu.2017.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li H., Peng H., Ma P., et al. Association between Vγ2Vδ2 T cells and disease progression after infection with closely related strains of HIV in China. Clinical Infectious Diseases. 2008;46(9):1466–1472. doi: 10.1086/587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Maria A., Ferrazin A., Ferrini S., Ciccone E., Terragna A., Moretta L. Selective increase of a subset of T cell receptor γδ T lymphocytes in the peripheral blood of patients with human immunodeficiency virus type 1 infection. The Journal of Infectious Diseases. 1992;165(5):917–919. doi: 10.1093/infdis/165.5.917. [DOI] [PubMed] [Google Scholar]
- 104.McBride J. A., Striker R. Imbalance in the game of T cells: what can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathogens. 2017;13(11, article e1006624) doi: 10.1371/journal.ppat.1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pastor L., Urrea V., Carrillo J., et al. Dynamics of CD4 and CD8 T-cell subsets and inflammatory biomarkers during early and chronic HIV infection in Mozambican adults. Frontiers in Immunology. 2017;8 doi: 10.3389/fimmu.2017.01925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Biswas P., Ferrarini M., Mantelli B., et al. Double-edged effect of Vγ9/Vδ2 T lymphocytes on viral expression in an in vitro model of HIV-1/mycobacteria co-infection. European Journal of Immunology. 2003;33(1):252–263. doi: 10.1002/immu.200390028. [DOI] [PubMed] [Google Scholar]
- 107.Poonia B., Pauza C. D. Gamma delta T cells from HIV+ donors can be expanded in vitro by zoledronate/interleukin-2 to become cytotoxic effectors for antibody-dependent cellular cytotoxicity. Cytotherapy. 2012;14(2):173–181. doi: 10.3109/14653249.2011.623693. [DOI] [PubMed] [Google Scholar]
- 108.Harris L. D., Klatt N. R., Vinton C., et al. Mechanisms underlying γδ T-cell subset perturbations in SIV-infected Asian rhesus macaques. Blood. 2010;116(20):4148–4157. doi: 10.1182/blood-2010-05-283549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dunne P. J., Maher C. O., Freeley M., et al. CD3ε expression defines functionally distinct subsets of Vδ1 T cells in patients with human immunodeficiency virus infection. Frontiers in Immunology. 2018;9:p. 940. doi: 10.3389/fimmu.2018.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Poccia F., Battistini L., Cipriani B., et al. Phosphoantigen-reactive Vgamma9Vdelta2 T lymphocytes suppress in vitro human immunodeficiency virus type 1 replication by cell-released antiviral factors including CC chemokines. The Journal of Infectious Diseases. 1999;180(3):858–861. doi: 10.1086/314925. [DOI] [PubMed] [Google Scholar]
- 111.Maniar A., Zhang X., Lin W., et al. Human γδ T lymphocytes induce robust NK cell–mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116(10):1726–1733. doi: 10.1182/blood-2009-07-234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Caccamo N., Todaro M., La Manna M. P., Sireci G., Stassi G., Dieli F. IL-21 regulates the differentiation of a human γδ T cell subset equipped with B cell helper activity. PLoS One. 2012;7(7, article e41940) doi: 10.1371/journal.pone.0041940. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Boismenu R., Feng L., Xia Y. Y., Chang J. C., Havran W. L. Chemokine expression by intraepithelial gamma delta T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. Journal of Immunology. 1996;157(3):985–992. [PubMed] [Google Scholar]
- 114.Wallace M., Bartz S. R., Chang W. L., Mackenzie D. A., Pauza C. D., Malkovsky M. γδ T lymphocyte responses to HIV. Clinical and Experimental Immunology. 1996;103(2):177–184. doi: 10.1046/j.1365-2249.1996.d01-625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cimini E., Agrati C., D’Offizi G., et al. Primary and chronic HIV infection differently modulates mucosal Vδ1 and Vδ2 T-cells differentiation profile and effector functions. PLoS One. 2015;10(6, article e0129771) doi: 10.1371/journal.pone.0129771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brandes M., Willimann K., Moser B. Professional antigen-presentation function by human gammadelta T cells. Science. 2005;309(5732):264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 117.Cairo C., Surendran N., Harris K. M., et al. Vγ2Vδ2 T cell costimulation increases NK cell killing of monocyte-derived dendritic cells. Immunology. 2014;144(3):422–430. doi: 10.1111/imm.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scotet E., Nedellec S., Devilder M. C., Allain S., Bonneville M. Bridging innate and adaptive immunity through γδ T-dendritic cell crosstalk. Frontiers in Bioscience : a Journal and Virtual Library. 2008;13:6872–6885. doi: 10.2741/3195. [DOI] [PubMed] [Google Scholar]
- 119.Cardone M., Ikeda K. N., Varano B., Gessani S., Conti L. HIV-1-induced impairment of dendritic cell cross talk with γδ T lymphocytes. Journal of Virology. 2015;89(9):4798–4808. doi: 10.1128/JVI.03681-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Strbo N., Alcaide M. L., Romero L., et al. Loss of intra-epithelial endocervical gamma delta (GD) 1 T cells in HIV-infected women. American Journal of Reproductive Immunology. 2016;75(2):134–145. doi: 10.1111/aji.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li H., Pauza C. D. HIV envelope-mediated, CCR5/α4β7-dependent killing of CD4-negative γδ T cells which are lost during progression to AIDS. Blood. 2011;118(22):5824–5831. doi: 10.1182/blood-2011-05-356535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.He X., Liang H., Hong K., et al. The potential role of CD16+ Vγ2Vδ2 T cell-mediated antibody-dependent cell-mediated cytotoxicity in control of HIV type 1 disease. AIDS Research and Human Retroviruses. 2013;29(12):1562–1570. doi: 10.1089/aid.2013.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cohen J. I. Optimal treatment for chronic active Epstein-Barr virus disease. Pediatric Transplantation. 2009;13(4):393–396. doi: 10.1111/j.1399-3046.2008.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luzuriaga K., Sullivan J. L. Infectious mononucleosis. The New England Journal of Medicine. 2010;362(21):1993–2000. doi: 10.1056/NEJMcp1001116. [DOI] [PubMed] [Google Scholar]
- 125.Yap L. F., Velapasamy S., Lee H. M., et al. Down-regulation of LPA receptor 5 contributes to aberrant LPA signalling in EBV-associated nasopharyngeal carcinoma. The Journal of Pathology. 2015;235(3):456–465. doi: 10.1002/path.4460. [DOI] [PubMed] [Google Scholar]
- 126.Callan M. F. C., Tan L., Annels N., et al. Direct visualization of antigen-specific CD8+T cells during the primary immune response to Epstein-Barr virus in vivo. The Journal of Experimental Medicine. 1998;187(9):1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Taylor G. S., Long H. M., Brooks J. M., Rickinson A. B., Hislop A. D. The immunology of Epstein-Barr virus-induced disease. Annual Review of Immunology. 2015;33(1):787–821. doi: 10.1146/annurev-immunol-032414-112326. [DOI] [PubMed] [Google Scholar]
- 128.Munz C. Epstein-Barr virus-specific immune control by innate lymphocytes. Frontiers in Immunology. 2017;8:p. 1658. doi: 10.3389/fimmu.2017.01658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Djaoud Z., Guethlein L. A., Horowitz A., et al. Two alternate strategies for innate immunity to Epstein-Barr virus: one using NK cells and the other NK cells and γδ T cells. The Journal of Experimental Medicine. 2017;214(6):1827–1841. doi: 10.1084/jem.20161017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chitadze G., Oberg H. H., Wesch D., Kabelitz D. The ambiguous role of γδ T lymphocytes in antitumor immunity. Trends in Immunology. 2017;38(9):668–678. doi: 10.1016/j.it.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 131.Kong Y., Cao W., Xi X., Ma C., Cui L., He W. The NKG2D ligand ULBP4 binds to TCRγ9/δ2 and induces cytotoxicity to tumor cells through both TCRγδ and NKG2D. Blood. 2009;114(2):310–317. doi: 10.1182/blood-2008-12-196287. [DOI] [PubMed] [Google Scholar]
- 132.Wang Y., Aïssi-Rothe L., Virion J. M., et al. Combination of Epstein-Barr virus nuclear antigen 1, 3 and lytic antigen BZLF1 peptide pools allows fast and efficient stimulation of Epstein-Barr virus-specific T cells for adoptive immunotherapy. Cytotherapy. 2014;16(1):122–134. doi: 10.1016/j.jcyt.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 133.Dai Y. M., Liu H. Y., Liu Y. F., Zhang Y., He W. EBV transformation induces overexpression of hMSH2/3/6 on B lymphocytes and enhances γδT-cell-mediated cytotoxicity via TCR and NKG2D. Immunology. 2018 doi: 10.1111/imm.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hochberg D., Middeldorp J. M., Catalina M., Sullivan J. L., Luzuriaga K., Thorley-Lawson D. A. Demonstration of the Burkitt’s lymphoma Epstein-Barr virus phenotype in dividing latently infected memory cells in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(1):239–244. doi: 10.1073/pnas.2237267100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiang Z., Liu Y., Zheng J., et al. Targeted activation of human Vγ9Vδ2-T cells controls Epstein-Barr virus-induced B cell lymphoproliferative disease. Cancer Cell. 2014;26(4):565–576. doi: 10.1016/j.ccr.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 136.Zumwalde N. A., Sharma A., Xu X., et al. Adoptively transferred Vγ9Vδ2 T cells show potent antitumor effects in a preclinical B cell lymphomagenesis model. JCI Insight. 2017;2(13) doi: 10.1172/jci.insight.93179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tsoulfas G., Goulis I., Giakoustidis D., et al. Hepatitis C and liver transplantation. Hippokratia. 2009;13(4):211–215. [PMC free article] [PubMed] [Google Scholar]
- 138.Ott J. J., Stevens G. A., Groeger J., Wiersma S. T. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 139.Gao B., Jeong W. I., Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2008;47(2):729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 140.Bandyopadhyay K., Marrero I., Kumar V. NKT cell subsets as key participants in liver physiology and pathology. Cellular & Molecular Immunology. 2016;13(3):337–346. doi: 10.1038/cmi.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Peng H., Wisse E., Tian Z. Liver natural killer cells: subsets and roles in liver immunity. Cellular & Molecular Immunology. 2016;13(3):328–336. doi: 10.1038/cmi.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen M., Zhang D., Zhen W., et al. Characteristics of circulating T cell receptor γδ T cells from individuals chronically infected with hepatitis B virus (HBV): an association between V(delta)2 subtype and chronic HBV infection. The Journal of Infectious Diseases. 2008;198(11):1643–1650. doi: 10.1086/593065. [DOI] [PubMed] [Google Scholar]
- 143.Kenna T., Golden-Mason L., Norris S., Hegarty J. E., O'Farrelly C. Distinct subpopulations of γδ T cells are present in normal and tumor-bearing human liver. Clinical Immunology. 2004;113(1):56–63. doi: 10.1016/j.clim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 144.Chen M., Hu P., Peng H., et al. Enhanced peripheral γδT cells cytotoxicity potential in patients with HBV-associated acute-on-chronic liver failure might contribute to the disease progression. Journal of Clinical Immunology. 2012;32(4):877–885. doi: 10.1007/s10875-012-9678-z. [DOI] [PubMed] [Google Scholar]
- 145.Conroy M. J., Mac Nicholas R., Taylor M., et al. Increased frequencies of circulating IFN-γ-producing Vδ1+ and Vδ2+γδ T cells in patients with asymptomatic persistent hepatitis B virus infection. Viral Immunology. 2015;28(4):201–208. doi: 10.1089/vim.2014.0133. [DOI] [PubMed] [Google Scholar]
- 146.Yin W., Tong S., Zhang Q., et al. Functional dichotomy of Vδ2 γδ T cells in chronic hepatitis C virus infections: role in cytotoxicity but not for IFN-γ production. Scientific Reports. 2016;6(1, article 26296) doi: 10.1038/srep26296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen M., Hu P., Ling N., et al. Enhanced functions of peripheral γδ T cells in chronic hepatitis B infection during interferon α treatment in vivo and in vitro. PLoS One. 2015;10(3, article e0120086) doi: 10.1371/journal.pone.0120086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Agrati C., Alonzi T., De Santis R., et al. Activation of Vγ9Vδ2 T cells by non-peptidic antigens induces the inhibition of subgenomic HCV replication. International Immunology. 2006;18(1):11–18. doi: 10.1093/intimm/dxh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kong X., Sun R., Chen Y., Wei H., Tian Z. γδT cells drive myeloid-derived suppressor cell–mediated CD8+ T cell exhaustion in hepatitis B virus–induced immunotolerance. Journal of Immunology. 2014;193(4):1645–1653. doi: 10.4049/jimmunol.1303432. [DOI] [PubMed] [Google Scholar]
- 150.Dieli F., Troye-Blomberg M., Farouk S. E., Sireci G., Salerno A. Biology of γδ T cells in tuberculosis and malaria. Current Molecular Medicine. 2001;1(4):437–446. doi: 10.2174/1566524013363627. [DOI] [PubMed] [Google Scholar]
- 151.Nakazawa S., Brown A. E., Maeno Y., Smith C. D., Aikawa M. Malaria-induced increase of splenic γδ T cells in humans, monkeys, and mice. Experimental Parasitology. 1994;79(3):391–398. doi: 10.1006/expr.1994.1101. [DOI] [PubMed] [Google Scholar]
- 152.Kobayashi F., Niikura M., Waki S., et al. Plasmodium berghei XAT: contribution of γδ T cells to host defense against infection with blood-stage nonlethal malaria parasite. Experimental Parasitology. 2007;117(4):368–375. doi: 10.1016/j.exppara.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 153.Inoue S.-I., Niikura M., Takeo S., et al. Enhancement of dendritic cell activation via CD40 ligand-expressing γδ T cells is responsible for protective immunity to Plasmodium parasites. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(30):12129–12134. doi: 10.1073/pnas.1204480109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Inoue S.-I., Niikura M., Inoue M., et al. The protective effect of CD40 ligand-CD40 signalling is limited during the early phase of Plasmodium infection. FEBS Letters. 2014;588(13):2147–2153. doi: 10.1016/j.febslet.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 155.Dodoo D., Omer F. M., Todd J., Akanmori B. D., Koram K. A., Riley E. M. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. The Journal of Infectious Diseases. 2002;185(7):971–979. doi: 10.1086/339408. [DOI] [PubMed] [Google Scholar]
- 156.Schofield L., Ioannidis L. J., Karl S., et al. Synergistic effect of IL-12 and IL-18 induces TIM3 regulation of γδ T cell function and decreases the risk of clinical malaria in children living in Papua New Guinea. BMC Medicine. 2017;15(1):p. 114. doi: 10.1186/s12916-017-0883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sheel M., Beattie L., Frame T. C. M., et al. IL-17A–producing γδ T cells suppress early control of parasite growth by monocytes in the liver. The Journal of Immunology. 2015;195(12):5707–5717. doi: 10.4049/jimmunol.1501046. [DOI] [PubMed] [Google Scholar]
- 158.Stanisic D. I., Cutts J., Eriksson E., et al. γδ T cells and CD14+ monocytes are predominant cellular sources of cytokines and chemokines associated with severe malaria. The Journal of Infectious Diseases. 2014;210(2):295–305. doi: 10.1093/infdis/jiu083. [DOI] [PubMed] [Google Scholar]
- 159.Costa G., Loizon S., Guenot M., et al. Control of Plasmodium falciparum erythrocytic cycle: γδ T cells target the red blood cell–invasive merozoites. Blood. 2011;118(26):6952–6962. doi: 10.1182/blood-2011-08-376111. [DOI] [PubMed] [Google Scholar]
- 160.Jagannathan P., Kim C. C., Greenhouse B., et al. Loss and dysfunction of Vdelta2+γδ T cells are associated with clinical tolerance to malaria. Science Translational Medicine. 2014;6(251, article 251ra117) doi: 10.1126/scitranslmed.3009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jagannathan P., Lutwama F., Boyle M. J., et al. Vδ2+ T cell response to malaria correlates with protection from infection but is attenuated with repeated exposure. Scientific Reports. 2017;7(1, article 11487) doi: 10.1038/s41598-017-10624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Farrington L. A., Jagannathan P., McIntyre T. I., et al. Frequent malaria drives progressive Vδ2 T-cell loss, dysfunction, and CD16 up-regulation during early childhood. The Journal of Infectious Diseases. 2016;213(9):1483–1490. doi: 10.1093/infdis/jiv600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Inoue S. I., Niikura M., Asahi H., Iwakura Y., Kawakami Y., Kobayashi F. Preferentially expanding Vγ1+γδ T cells are associated with protective immunity against Plasmodium infection in mice. European Journal of Immunology. 2017;47(4):685–691. doi: 10.1002/eji.201646699. [DOI] [PubMed] [Google Scholar]
- 164.Mönkkönen H., Auriola S., Lehenkari P., et al. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. British Journal of Pharmacology. 2006;147(4):437–445. doi: 10.1038/sj.bjp.0706628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Thompson K., Rojas-Navea J., Rogers M. J. Alkylamines cause Vγ9Vδ2 T-cell activation and proliferation by inhibiting the mevalonate pathway. Blood. 2006;107(2):651–654. doi: 10.1182/blood-2005-03-1025. [DOI] [PubMed] [Google Scholar]
- 166.Thompson K., Rogers M. J. Statins prevent bisphosphonate-induced γ,δ-T-cell proliferation and activation in vitro. Journal of Bone and Mineral Research. 2004;19(2):278–288. doi: 10.1359/JBMR.0301230. [DOI] [PubMed] [Google Scholar]
- 167.Xi Y., Miao T., Wan L., et al. Amplification efficency and optimization of culture conditions of γδ T cells in peripheral blood by different phosphate compounds. Xi bao yu fen zi mian yi xue za zhi = Chinese Journal of Cellular and Molecular Immunology. 2014;30(8):868–871. [PubMed] [Google Scholar]
- 168.Tu W., Zheng J., Liu Y., et al. The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a γδ T cell population in humanized mice. The Journal of Experimental Medicine. 2011;208(7):1511–1522. doi: 10.1084/jem.20110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zocchi M. R., Costa D., Venè R., et al. Zoledronate can induce colorectal cancer microenvironment expressing BTN3A1 to stimulate effector γδ T cells with antitumor activity. OncoImmunology. 2017;6(3, article e1278099) doi: 10.1080/2162402X.2016.1278099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nada M. H., Wang H., Workalemahu G., Tanaka Y., Morita C. T. Enhancing adoptive cancer immunotherapy with Vγ2Vδ2 T cells through pulse zoledronate stimulation. Journal for Immunotherapy of Cancer. 2017;5(1):p. 9. doi: 10.1186/s40425-017-0209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Markovits N., Loebstein R., Bank I. Immune-mediated syndromes following intravenous bisphosphonate therapy. Inflammopharmacology. 2017;25(6):665–671. doi: 10.1007/s10787-017-0365-9. [DOI] [PubMed] [Google Scholar]
- 172.Matsumoto K., Hayashi K., Murata-Hirai K., et al. Targeting cancer cells with a bisphosphonate prodrug. ChemMedChem. 2016;11(24):2656–2663. doi: 10.1002/cmdc.201600465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tanaka Y., Iwasaki M., Murata-Hirai K., et al. Anti-tumor activity and immunotherapeutic potential of a bisphosphonate prodrug. Scientific Reports. 2017;7(1):p. 5987. doi: 10.1038/s41598-017-05553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tanaka Y., Murata-Hirai K., Iwasaki M., et al. Expansion of human γδ T cells for adoptive immunotherapy using a bisphosphonate prodrug. Cancer Science. 2018;109(3):587–599. doi: 10.1111/cas.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Starick L., Riano F., Karunakaran M. M., et al. Butyrophilin 3A (BTN3A, CD277)-specific antibody 20.1 differentially activates Vγ9Vδ2 TCR clonotypes and interferes with phosphoantigen activation. European Journal of Immunology. 2017;47(6):982–992. doi: 10.1002/eji.201646818. [DOI] [PubMed] [Google Scholar]
- 176.Fisher J. P. H., Heuijerjans J., Yan M., Gustafsson K., Anderson J. γδ T cells for cancer immunotherapy. Oncoimmunology. 2014;3(1, article e27572) doi: 10.4161/onci.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wilhelm M., Smetak M., Schaefer-Eckart K., et al. Successful adoptive transfer and in vivo expansion of haploidentical γδ T cells. Journal of Translational Medicine. 2014;12(1):p. 45. doi: 10.1186/1479-5876-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kobayashi H., Tanaka Y., Yagi J., et al. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunology, Immunotherapy : CII. 2007;56(4):469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Izumi T., Kondo M., Takahashi T., et al. Ex vivo characterization of γδ T-cell repertoire in patients after adoptive transfer of Vγ9Vδ2 T cells expressing the interleukin-2 receptor β-chain and the common γ-chain. Cytotherapy. 2013;15(4):481–491. doi: 10.1016/j.jcyt.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 180.Qaqish A., Huang D., Chen C. Y., et al. Adoptive transfer of phosphoantigen-specific γδ T cell subset attenuates Mycobacterium tuberculosis infection in nonhuman primates. The Journal of Immunology. 2017;198(12):4753–4763. doi: 10.4049/jimmunol.1602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nicol A. J., Tokuyama H., Mattarollo S. R., et al. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. British Journal of Cancer. 2011;105(6):778–786. doi: 10.1038/bjc.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kakimi K., Matsushita H., Murakawa T., Nakajima J. γδ T cell therapy for the treatment of non-small cell lung cancer. Translational Lung Cancer Research. 2014;3(1):23–33. doi: 10.3978/j.issn.2218-6751.2013.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kobayashi H., Tanaka Y., Yagi J., Minato N., Tanabe K. Phase I/II study of adoptive transfer of γδ T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunology, Immunotherapy. 2011;60(8):1075–1084. doi: 10.1007/s00262-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sato K., Kondo M., Sakuta K., et al. Impact of culture medium on the expansion of T cells for immunotherapy. Cytotherapy. 2009;11(7):936–946. doi: 10.3109/14653240903219114. [DOI] [PubMed] [Google Scholar]
- 185.Murday A. S., Chaudhry S., Pauza C. D. Interleukin-18 activates Vγ9Vδ2+ T cells from HIV-positive individuals: recovering the response to phosphoantigen. Immunology. 2017;151(4):385–394. doi: 10.1111/imm.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zou C., Zhao P., Xiao Z., Han X., Fu F., Fu L. γδ T cells in cancer immunotherapy. Oncotarget. 2017;8(5):8900–8909. doi: 10.18632/oncotarget.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]