Abstract
In order to fully harness the potential of immunotherapy with chimeric antigen receptor (CAR)-modified T cells, pre-clinical studies must be conducted in immunocompetent animal models that closely mimic the immunosuppressive malignant glioma (MG) microenvironment. Thus, the goal of this project was to study the in vivo fate of T cells expressing CARs specific for the MG antigen IL13Rα2 (IL13Rα2-CARs) in immunocompetent MG models. Murine T cells expressing IL13Rα2-CARs with a CD28.ζ (IL13Rα2-CAR.CD28.ζ) or truncated signaling domain (IL13Rα2-CAR.Δ) were generated by retroviral transduction, and their effector function was evaluated both in vitro and in vivo. IL13Rα2-CAR.CD28.ζ T cells’ specificity toward IL13Rα2 was confirmed through cytokine production and cytolytic activity. In vivo, a single intratumoral injection of IL13Rα2-CAR.CD28.ζ T cells significantly extended the survival of IL13Rα2-expressing GL261 and SMA560 glioma-bearing mice; long-term survivors were resistant to re-challenge with IL13Rα2-negative and IL13Rα2-positive tumors. IL13Rα2-CAR.CD28.ζ T cells proliferated, produced cytokines (IFNγ, TNF-α), and promoted a phenotypically pro-inflammatory glioma microenvironment by inducing a significant increase in the number of CD4+ and CD8+ T cells and CD8α+ dendritic cells and a decrease in Ly6G+ myeloid-derived suppressor cells (MDSCs). Our data underline the significance of CAR T cell studies in immunocompetent hosts and further validate IL13Rα2-CAR T cells as an efficacious therapeutic strategy for MG.
Keywords: CAR T cells, glioblastoma, syngeneic model, IL13Rα2, single-chain antibody, pro-inflammatory, solid tumor microenvironment, brain
This study demonstrates that IL13Rα2-targeted CAR-modified T cells have anti-tumor activity in immunocompetent glioma models. While CAR T cells reversed the immunosuppressive glioma microenvironment, their effector function was eventually eroded. These results provide the rationale to explore combinatorial therapies or additional genetic modifications to enhance the anti-glioma activity of CAR T cells.
Introduction
Malignant gliomas (MGs) are the most common and aggressive group of high-grade primary adult brain tumors. The current standard of care treatment, which includes surgical resection, radiation, and chemotherapy, extends the survival of patients to only 14.6 months.1 MGs are virtually incurable due to their heterogeneity, immune evasion, infiltrative nature, and protection by the blood-brain barrier (BBB).2, 3, 4 Thus, new therapeutic modalities directed at eliminating MGs are urgently needed. T cells engineered to express chimeric antigen receptors (CARs) have shown outstanding efficacy against hematological malignancies and offer great promise for the treatment of glioma.5, 6, 7 This immunotherapy harnesses the ability of T cells to kill malignant cells by recognizing tumor-associated antigens (TAAs), or antigens overexpressed by cancer cells. CAR T cells recognizing interleukin 13 receptor α2 (IL13Rα2),8, 9, 10, 11, 12 human epidermal growth factor receptor 2 (HER2),13, 14 epidermal growth factor variant III (EGFRvIII),15 and erythropoietin-producing hepatocellular carcinoma A2 (EphA2)16 have been evaluated already in pre-clinical models for glioblastoma or in phase I clinical trials.
CAR T cells targeting IL13Rα2 are emerging as an especially promising form of adoptive immunotherapy,10 as IL13Rα2 is almost exclusively expressed in cancer and not in normal cells (with an exception of the testis).17 In MG, the expression of IL13Rα2 is present in 50%–80% of cases.18, 19, 20 IL13Rα2 is also directly associated with an increased malignancy grade and aggressive mesenchymal type and is inversely correlated with patient survival.21 Furthermore, it has been shown that glioma-initiating cells express IL13Rα2.22 While IL13Rα2 was previously thought to be a decoy receptor, the crucial role of IL13Rα2 in cancer invasiveness continues to emerge,23, 24, 25, 26, 27 further validating IL13Rα2 as a target antigen.
One current IL13Rα2-targeted approach utilizes modified IL13 polypeptide variants known as zetakines for the CAR T cell-binding domain. Although zetakine CAR T cells demonstrate high affinity for IL13Rα2 binding, their interaction with the ubiquitously expressed IL13Rα18, 28 remains a concern due to potential off-target toxicity. Therefore, we engineered a single-chain variable antibody fragment (scFv47)29 derived from a well-characterized IL13Rα2-specific monoclonal antibody (mAb) developed in our laboratory.30 scFv47 selectively binds IL13Rα2, avoids binding IL13Rα1, and successfully directs fiber-modified adenovirus or CAR T cells to IL13Rα2-expressing glioma cells.9
CAR T cells directed with scFv47 to IL13Rα2 expressed in human glioma xenografts have demonstrated significant survival benefit in immune-deficient mice.9 While it validated the functionality of different IL13Rα2 CAR constructs and their therapeutic potentials, this study did not address the behavior of CAR T cells in the context of the immunosuppressive microenvironment, a hallmark of MGs.31 A recent phase I clinical trial of CAR T cells in patients with MGs suggested that the endogenous immune response significantly contributes to the therapeutic outcome of CAR T cell therapy, supporting the necessity of pre-clinical CAR T cell studies in syngeneic models of disease.10 Understanding how CAR T cells interact with the host immune system to influence the glioma microenvironment is thus critical for the development of successful CAR T cell therapies.
Here, we analyzed IL13Rα2-CAR T cell-mediated cytotoxicity, their persistence within the tumor, and their influence on the tumor microenvironment in a syngeneic model of glioblastoma. We show that IL13Rα2-CAR T cells can efficiently kill MGs in vitro and in vivo. Further, IL13Rα2-CAR T cells positively modulate the immune landscape of glioma-bearing mice, likely improving their anti-tumor activity.
Results
IL13Rα2-CAR T Cells Recognize and Kill IL13Rα2-Expressing Glioma Cells In Vitro
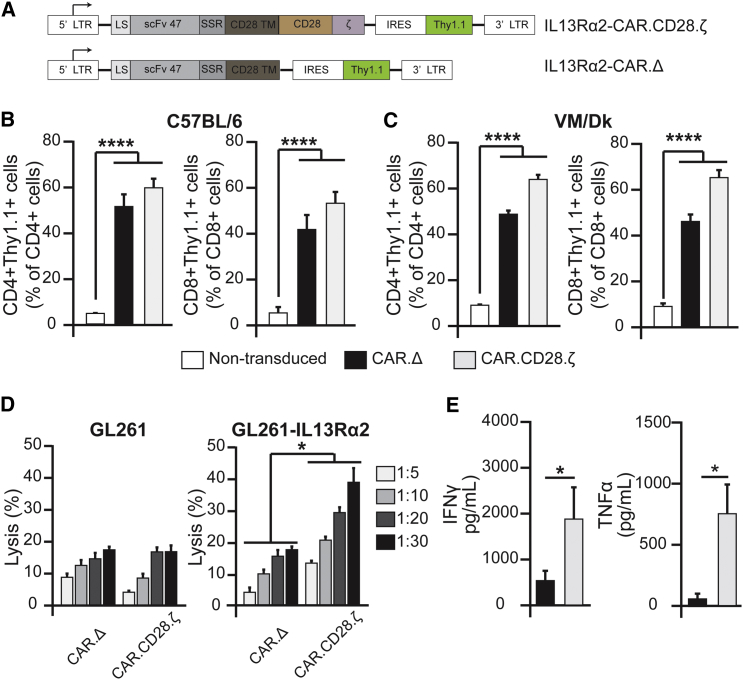
We have previously reported that mAb IL13Rα2 (clone 47) binds with a high affinity to human, but not murine, IL13Rα2.30 The single chain (scFv47)29 shares the epitope of the parental antibody and does not interact with murine IL13Rα2. In order to understand how murine T cells expressing IL13Rα2-CARs interact with the tumor microenvironment in an immune-competent glioma model, we generated IL13Rα2-CARs with a murine CD28 transmembrane and CD28.ζ endodomain (Figure 1A). An IL13Rα2-CAR with a truncated intracellular signaling domain, IL13Rα2- CAR.Δ, was generated as a control CAR. Activated CD3+ T cells were transduced with VSV-G-pseudotyped retroviral particles encoding the respective CAR and Thy1.1. Transduction efficiency was determined by staining T cells for Thy1.1 expression. On average, 40%–60% of CD3+CD4+ and 40%–65% of CD3+CD8+ T cells were genetically modified as judged by Thy1.1 expression (Figures 1B and 1C). To determine the functionality of IL13Rα2-CAR.CD28.ζ T cells, we performed cytotoxicity and co-culture assays. IL13Rα2-CAR.CD28.ζ T cells readily killed GL261 modified to express human IL13Rα2 (GL261-IL13Rα2; Figure S1A), but not parental GL261 cells in a standard 4-hr chromium-51 (51Cr) release assay. In contrast, IL13Rα2-CAR.Δ T cells killed neither GL261-IL13Rα2 nor GL261 (Figure 1D). IL13Rα2-CAR.CD28.ζ or IL13Rα2-CAR.Δ T cells were stimulated with IL13Rα2-expressing cells to determine cytokine production by ELISA. After 24 hr, production of IFNγ and TNF-α was significantly higher in IL13Rα2-CAR.CD28.ζ T cells than IL13Rα2-CAR.Δ T cells (Figure 1E), demonstrating antigen-specific CAR T cell activation.
Figure 1.
IL13Rα2-CAR.CD28.ζ T Cells Recognize and Kill GL261 Glioma Cells Expressing IL13Rα2
(A) IL13Rα2-CAR.CD28.ζ and IL13Rα2-CAR.Δ murine CAR constructs were designed as described in the Materials and Methods section. (B and C) Flow cytometry analysis of Thy1.1 expression in CD3+ T cells from C57BL/6 (B) and VM/Dk (C) mice was performed at day 5 post-viral-transduction (n ≥ 3,****p ≤ 0.0001, one-way ANOVA). (D) IL13Rα2-CAR.CD28.ζ and IL13Rα2-CAR.Δ T cells were tested for their cytotoxic activity against Gl261 and GL261-IL13Rα2 glioma cells in a standard 4-hr 51Cr release assay. IL13Rα2-CAR.CD28.ζ T cells killed GL261-IL13Rα2, but not GL261 glioma cells at all tested target:effector (T:E) ratios (1:5, 1:10, 1:20, 1:30) as compared to control IL13Rα2-CAR.Δ T cells (n ≥ 6, *p ≤ 0.05 for two-way ANOVA). (E) The IFNγ and TNF-α production were measured by ELISA after 24 hr with IL13α2-stumulation. (n ≥ 3, *p ≤ 0.05, Student’s t test). CAR abbreviations: CAR.Δ, IL13Rα2-CAR.Δ; CAR.CD28.ζ, IL13Rα2-CAR.CD28.ζ. Data are presented as mean ± SEM.
Generation of Immunocompetent Mouse Models of Glioblastoma Expressing Human IL13Rα2
In order to conduct our studies in an immunocompetent host, we developed two syngeneic MG mouse models in which murine GL261 and SMA560 glioma cell lines were modified to stably express human IL13Rα2. Flow cytometric analysis of IL13Rα2 expression on the cell surface revealed that 92% ± 0.3% of GL261 and 91% ± 0.9% of SMA560 cells were positive for IL13Rα2 in vitro (Figure S1A). Furthermore, analysis of excised tumor tissue confirmed that the expression of IL13Rα2 is maintained in vivo, albeit at lower levels than in in vitro (Figure S1B). Next, we determined the growth of GL261-IL13Rα2 and SMA560-IL13Rα2 tumors after intrancranial injection into their syngeneic hosts (C57BL/6 and VM/Dk mice, respectively). There were no differences observed in the survival of animals for either cell line in comparison to control parental cell lines, supporting the suitability of these models for our studies (Figure S1C). Collectively, these data indicate that both GL261-IL13Rα2 and SMA560-IL13Rα2 are viable and complementary syngeneic models of MG to study IL13Rα2-CAR T cells.
IL13Rα2-CAR T Cells Have Anti-glioma Activity in Two Immune-Competent Glioma Models
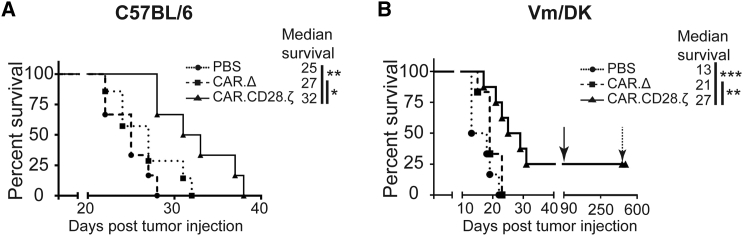
The anti-glioma activity of IL13Rα2-CAR.CD28.ζ T cells was evaluated in the GL261-IL13Rα2 and SMA560-IL13Rα2 immune-competent glioma models. On day 7 post-intracranial glioma cell injection, mice received an intratumoral (i.t.) injection of IL13Rα2-CAR.CD28.ζ or IL13Rα2-CAR.Δ T cells; PBS-injected mice served as controls. Mice that received IL13Rα2-CAR.CD28.ζ T cells had a significant survival benefit compared to PBS and IL13Rα2-CAR.Δ T cell-treated mice (Figures 2A and 2B). In addition, IL13Rα2-CAR.Δ T cell-treated mice had a survival advantage in comparison to PBS-treated mice, indicating that IL13Rα2-CAR.Δ T cells have limited therapeutic benefit. In the SMA560-IL13Rα2 model, 25% of mice survived long-term following IL13Rα2-CAR.CD28.ζ T cell therapy, while no mice survived in the GL261-IL13Rα2 model. Long-term survivors were re-challenged by injecting SMA560-IL13Rα2 cells into the contralateral brain and, after 11 months, injected again with SMA560 cells. While control animals succumbed to the disease, none of the re-challenged mice developed glioma, indicating the development of sustained long-term anti-glioma immunity.
Figure 2.
IL13Rα2-CAR.CD28.ζ T Cells Have Anti-glioma Activity in Mice Bearing IL13Rα2-Expressing Glioma
4.0 × 105 GL261-IL13Rα2 or 7.5 × 104 SMA560-IL13Rα2 glioma cells were intracranially injected into C57BL/6 and VM/Dk mice, respectively. Seven days later, animals were treated with a single intratumoral (i.t.) transplantation of PBS, 1.5 × 106 IL13Rα2-CAR.Δ T cells, or 1.5 × 106 IL13Rα2-CAR.CD28.ζ T cells. (A) IL13Rα2-CAR.CD28.ζ T cells significantly extended the survival of C57BL/6 animals from 25 to 32 days. (n ≥ 6–8, *p ≤ 0.05, **p ≤ 0.01, Mantel-Cox test). (B) IL13Rα2-CAR.CD28.ζ T cells significantly extended the survival of VM/Dk glioma-bearing mice as compared to PBS and control IL13Rα2-CAR.Δ T cells (n ≥ 6–8, *p ≤ 0.05, **p ≤ 0.01, Mantel-Cox test). Twenty-five percent of animals treated with IL13Rα2-CAR.CD28.ζ T cells survived for a prolonged time period and were re-challenged with 0.75 × 105 SMA560-IL13Rα2 glioma cells by an injection contralateral to the original tumor implantation hemisphere at day 90 as indicated by arrow. Eleven months later, animals were re-challenged again with 0.75 × 105 SMA560 cells as indicated by dashed arrow. While control animals (n = 4) injected in parallel with SMA560 cells succumbed to the disease (data not shown) within 3 weeks, none of the re-challenged animals developed tumors, suggesting the development of immunity against glioma (n ≥ 6–8, **p ≤ 0.01, ***p ≤ 0.001, Mantel-Cox test).
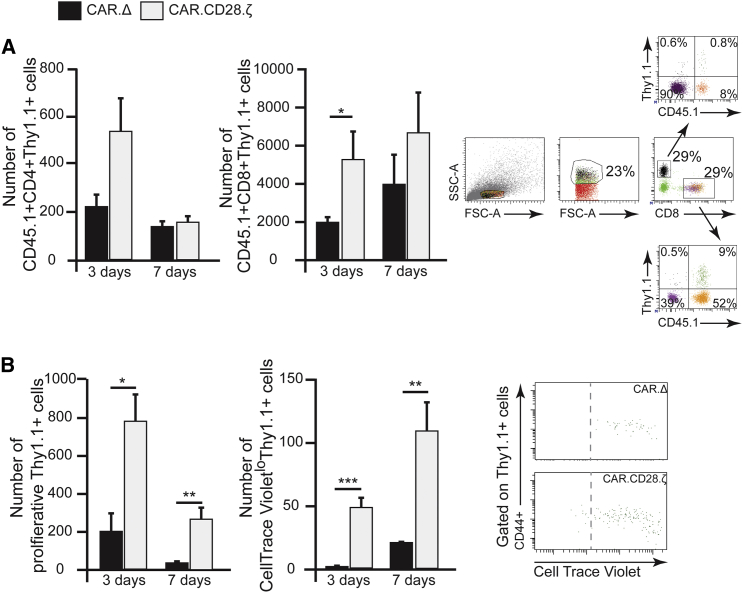
IL13Rα2-CAR T Cells Persist and Expand in IL13Rα2-Expressing GL261 Glioma-Bearing Mice
In order to clearly differentiate between adoptively transferred and host T cells and determine if IL13Rα2-CAR.CD28.ζ T cells persist in the glioma environment, we utilized CD3+CD45.1+ cells to generate IL13Rα2-CAR.CD28.ζ and control transduced T cells for subsequent analysis in CD45.2 C57BL/6 mice bearing GL261-IL13Rα2 glioma. We observed robust persistence of adoptively transferred CAR T cells at 3 and 7 days in the brain after i.t. injection of T cells, corresponding to 10 and 14 days of tumor development (Figure 3A). While the number of IL13Rα2-CAR.CD28.ζ T cells was significantly higher than control IL13Rα2-CAR.Δ CD3+CD8+ T cells at 3 days (respectively, 5.1 ± 2.7 × 103 versus 1.5 ±± 0.8 × 103) and at 7 days (respectively, 6.7 ± 4.0 × 103 versus 3.9 ± 3.1 × 103), there was no statistical difference determined in the persistence of CD3+CD4+ IL13Rα2-CAR.CD28.ζ and IL13Rα2-CAR.Δ T cells (Figure 3A). Furthermore, we were able to detect only a small number of CD3+CD8+, but not CD3+CD4+ CAR T cells in the brain of animals prior to euthanasia (Figure S2). Next, we determined if the persistence of IL13Rα2-CAR.CD28.ζ T cells was the result of antigen-dependent proliferation. Indeed, IL13Rα2-CAR.CD28.ζ CAR T cells demonstrated more robust proliferation than IL13Rα2-CAR.Δ T cells at 3 days post-i.t.-delivery (Figure 3B). By day 7, the vast majority of IL13Rα2-CAR.CD28.ζ T cells were in their highest proliferative state (Figure 3B). These data demonstrate that IL13Rα2-CAR.CD28.ζ T cells are capable of surviving and expanding in the immunosuppressive glioblastoma environment.
Figure 3.
IL13Rα2-CAR.CD28.ζ T Cells Persist and Proliferate in the Brains of Glioma-Bearing Mice
(A) In order to clearly distinguish between host and adoptively transferred cells, CD3+ T cells from CD45.1 mice were utilized to generate IL13Rα2-CAR.CD28.ζ and control IL13Rα2-CAR.Δ T cells and i.t. injected into CD45.2 mice bearing GL261-IL13Rα2 glioma. Gating strategy is presented in the right panel. Quantitative analysis of CD4+CD45.1+Thy1.1+ and CD8+CD45.1+Thy1.1+ T cells’ populations revealed a 2.5-fold higher number of CD8+ CARs at day 3, but not at day 7, in the brain of animals treated with therapeutic CAR T cells as compared to control CAR T cells (n ≥ 4, *p = 0.02, Student’s t test). A similar trend was observed for CD4+ CARs but did not reach significance. (B) For analysis of proliferation, CAR T cells were stained with CellTrace Violet prior to i.t. implantation. Consistent with a higher number of T cells treated with IL13Rα2-CAR.CD28.ζ, the total number of proliferating CD8+ cells was (1) four times higher in animals treated with IL13Rα2-CAR.CD28.ζ than those treated with IL13Rα2-CAR.Δ T cells at 3 days and (2) eight times higher at 7 days after i.t. injection. A more robust proliferation in IL13Rα2-CAR.CD28.ζ compared to IL13Rα2-CAR.Δ T cells is noticeable, and the vast majority of IL13Rα2-CAR.CD28.ζ T cells were in the highest proliferative state, as determined by Cell Trace Violet detection using flow cytometry (n ≥ 6, *p = 0.02, **p = 0.001, ***p < 0.001, Student’s t test). The right panel is an example of the gating strategy. Data are presented as mean ± SEM.
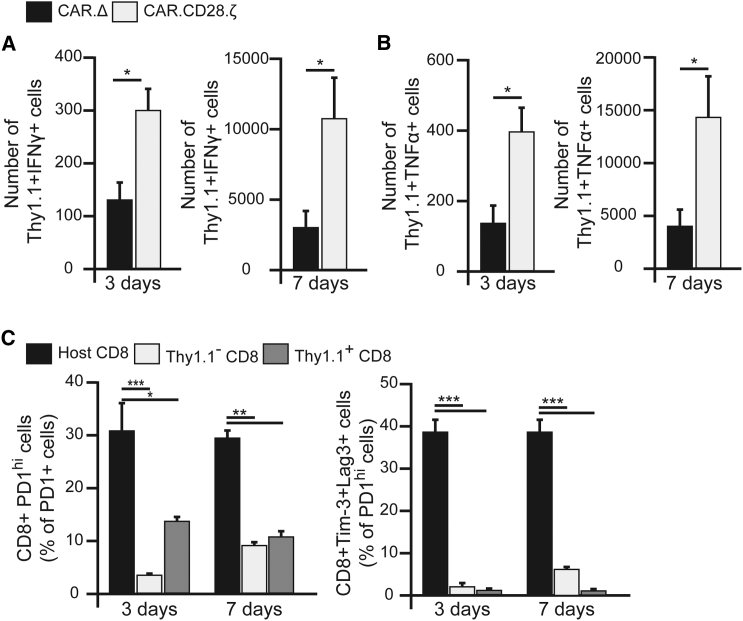
IL13Rα2-CAR CD8+ T Cells Produce Pro-inflammatory Cytokines in an Antigen-Dependent Fashion and Are Not Exhausted within the Glioma Environment
To determine the production of pro-inflammatory cytokines, IFNγ and TNF-α, by IL13Rα2-CAR.CD28.ζ T cells in vivo in response to antigenic stimulation, CAR T cells were isolated and analyzed at days 3 and 7 post-i.t.-injection in animals bearing GL261-IL13Rα2 tumors. A robust difference in cytokine production between therapeutic and control CAR T cells was observed at both time points (Figures 4A and 4B). Cytotoxic T cells characterized by the secretion of pro-inflammatory cytokines are likely to contribute an additional mechanism of CAR T cell anti-glioblastoma functionalities in vivo.
Figure 4.
IL13Rα2-CAR.CD28.ζ T Cells Produce Cytokines in the Brains of Mice Bearing GL261-IL13Rα2 Glioma and Show a Non-exhausted Phenotype
(A) Functional analysis of Th1.1+ T cells harvested from the brain of animals shows a significantly higher IFNγ production by IL13Rα2-CAR.CD28.ζ T cells at 3 and 7 days after i.t. injection than in control IL13Rα2-CAR.Δ T cells, as determined by flow cytometry (n ≥ 4, *p ≤ 0.05, Student’s t test). (B) Similarly, IL13Rα2-CAR.CD28.ζ T cells produced significantly more TNF-α compared to IL13Rα2-CAR.Δ T cells at 3 and 7 days (n ≥ 4, *p ≤ 0.05, Student’s t test). (C) IL13Rα2-CAR.CD28.ζ T cells significantly upregulate the expression of PD-1, as compared to IL13Rα2-CAR.Δ T cells within tumor. However, CAR T cells do not show an exhausted phenotype, as judged by a low expression of Tim-3 and LAG-3 expression in the PD-1hi population compared to the host’s CD8+ T cells (n ≥ 5, *p = 0.02, **p = 0.001, ***p < 0.001, Student’s t test). Data are presented as mean ± SEM.
Repeated antigenic stimulation has been shown to trigger exhaustion of CAR T cells, limiting their therapeutic activity. However, to date, it remains unknown whether CAR T cells become exhausted within gliomas in immunocompetent hosts. Thus, we analyzed the expression of the following exhaustion markers in CAR T cells at 3 and 7 days after i.t. injection: programmed cell death 1 (PD-1) receptor, lymphocyte-activation protein 3 (LAG-3), and T cell Ig- and mucin-domain-containing molecule–3 (Tim-3). At day 3, we found that only 11.2% ± 1.7% and 13.6% ± 4.3% were PD-1hi in the Thy1.1+ population of IL13Rα2-CAR.CD28.ζ and IL13Rα2-CAR.Δ T cells, respectively. Similarly, at day 7, 12.5% ± 1.7% and 16.1% ± 3.4% cells were PD-1hi in Thy1.1+ IL13Rα2-CAR.CD28.ζ and IL13Rα2-CAR.Δ T cells, respectively (Figure 4C). Since activated T cells naturally upregulate PD-1 expression, we further analyzed PD-1hi populations for the expression of LAG3 and Tim-3, which are well-known negative regulators of T cell activity and markers of exhaustion.32 In contrast to tumor-infiltrating host CD8+ T cells, of which 41.2% ± 4.8% of PD-1hi cells also expressed LAG3 and Tim-3, IL13Rα2-CAR.CD28.ζ T cells did not acquire an exhausted phenotype as judged by low LAG3 and Tim-3 expression (Figure 4C). In conjunction with the observed proliferation of CAR T cells within the tumor, these data further support the finding that CAR T cells can survive and expand in the immunosuppressive glioblastoma environment.
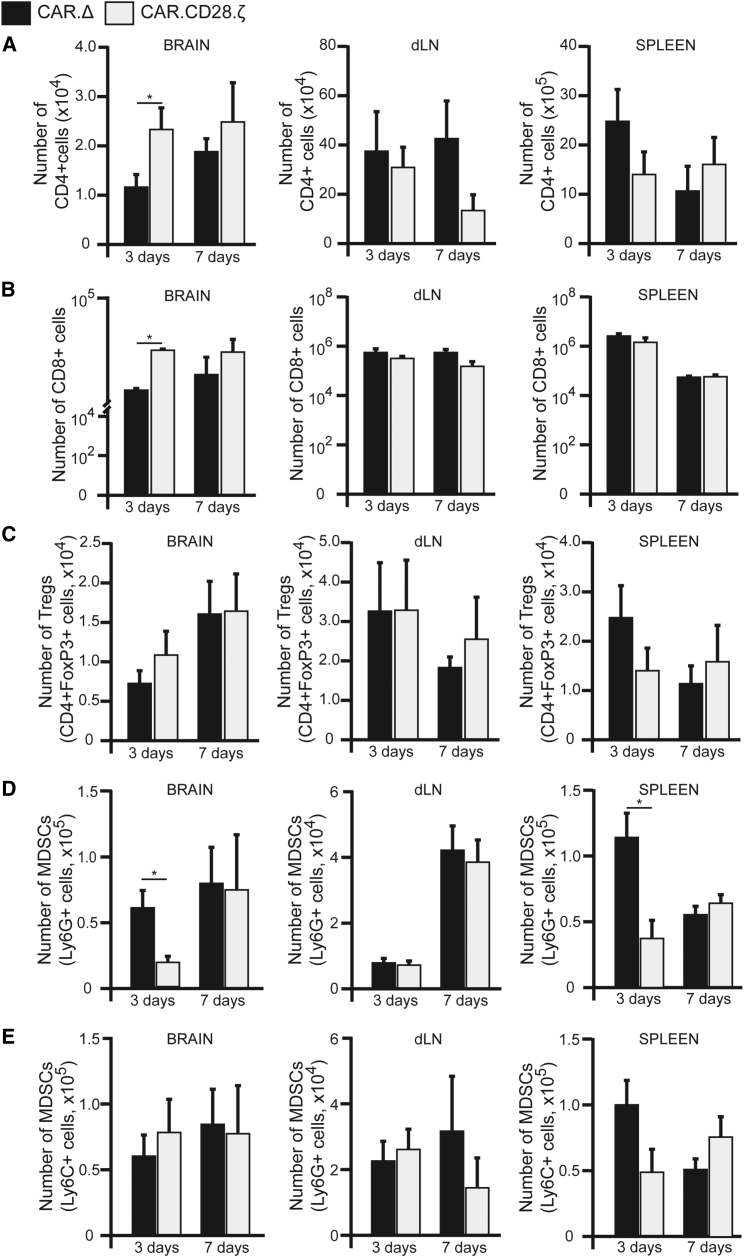
IL13Rα2-CAR T Cells Influence the Repertoire of Tumor-Infiltrating Immune Cells in the GL261-IL13Rα2 Model
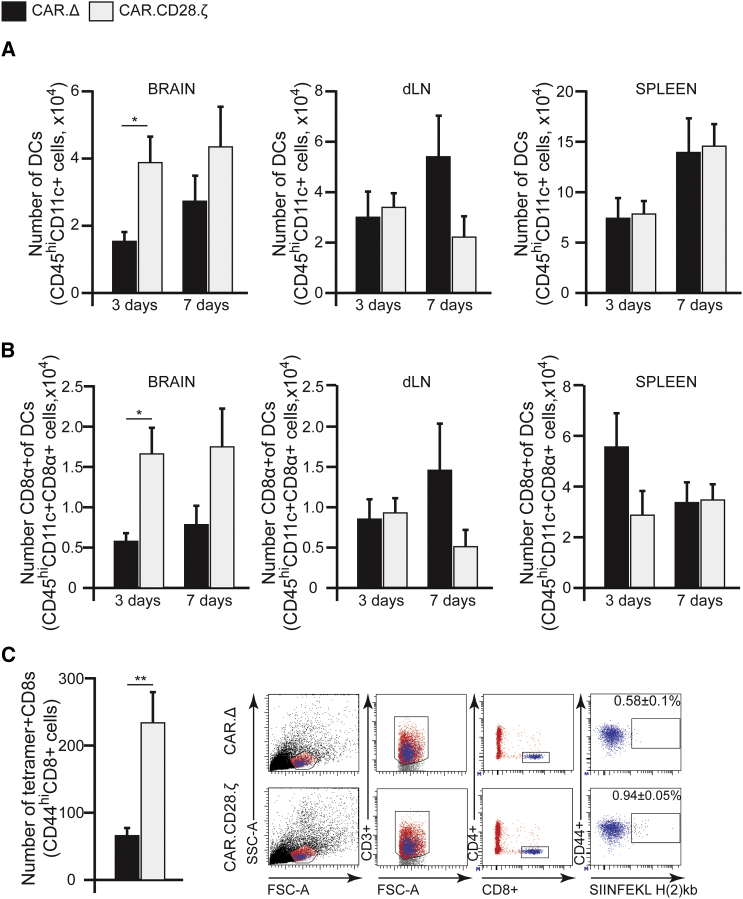
At present, it is largely unknown if CAR T cells can convert the immunosuppressive MG environment into an immunostimulatory one, resulting in activation of endogenous, glioma-specific immune responses. To investigate this, we analyzed tumor-infiltrating immune cells at days 3 and 7 following CAR T cell injection. We observed significant changes in the frequency of host CD4+ and CD8+ cells after CAR T cell treatment in the brain, but not in the draining lymph node (dLN) or spleen (Figures 5A and 5B). Treatment with IL13Rα2-CAR.CD28.ζ T cells did not change the frequency of the host’s regulatory T cells (Treg) or contribute to the pool of Tregs, as verified by analysis of FoxP3 expression in CD4+ host’s or CAR T cells (Figure 5C). However, treatment with IL13Rα2-CAR.CD28.ζ T cells resulted in a 2.3-fold decrease in Ly6G+ MDSCs in the brain and spleens at day 3, but not at day 7 (Figure 5D). There were no significant changes in the number of Ly6C+ MDSCs (Figure 5E), macrophages, or microglia (Figure S3) between groups of mice treated with control and IL13Rα2-CAR.CD28.ζ T cells. Intriguingly, we observed a 2.6-fold increase in CD45hiCD11c+ DCs in the brains of tumor-bearing mice treated with IL13Rα2-CAR.CD28.ζ T cells at day 3 compared to control CAR T cells (Figure 6A). Similarly, in these mice, we detected a 3.6-fold increase in the CD8α+ subset of DCs at day 3 (Figure 6B). The increased presence of DCs in the brain was maintained at 7 days post-injection of CAR T cells, with a noticeable decrease in DC numbers in dLN at day 7, but not in the spleen. To understand whether the increase in CD8α+ DCs lead to antigen presentation to CD8+ T cells, we analyzed the brains of mice bearing GL261-IL13Rα2 tumors engineered to overexpress the surrogate antigen ovalbumin (OVA) after control and IL13Rα2-CAR.CD28.ζ T cell treatment. We found a significant increase in the percentage and absolute number of tetramer+ CD8+ T cells for OVA (Figure 6C) in mice treated with therapeutic CAR T cells. Collectively, these data demonstrate that therapeutic CAR T cells not only directly kill IL13Rα2-expressing glioma cells but also influence the tumor immune landscape by decreasing the number of immunosuppressive Ly6G+ MDSCs and increasing the number of CD4+, CD8+, and CD8α+ DCs in the tumors. The increase in the number of tetramer+ CD8+ T cells for OVA, along with lack of tumor development upon re-challenge with both IL13Rα2-negative and IL13Rα2-positive SMA560 glioma cells, also suggests that animals can acquire an anti-tumor immunity in response to CAR T cell therapy.
Figure 5.
The Immune Landscape of Glioma-Bearing Mice after IL13Rα2-CAR.CD28.ζ T Cell Treatment
Analyses of host CD4+, CD8+, Treg, and MDSC subsets were performed at 3 and 7 days after i.t. injection of CAR T cells in animals bearing GL261-IL13Rα2 glioma. At 3 days, higher numbers of (A) CD4+ and (B) CD8+ cells were observed in the animals treated with IL13Rα2-CAR.CD28.ζ T cells when compared with animals treated with control IL13Rα2-CAR.Δ T cells (n ≥ 4, *p ≤ 0.05, Student’s t test). This difference was not statistically significant on day 7. There were no significant changes in these T cell compartments at either time point in analyzed dLN and spleen. (C) There was no change in the frequency of CD4+FoxP3+ regulatory T cells (Tregs) at any analyzed time point (n ≥ 4, *p ≤ 0.05, Student’s t test). (D) There was a significant drop in the number of Ly6G+ myeloid-derived suppressor cells (MDSCs) after 3 days in the brain parenchyma of animals treated with IL13Rα2-CAR.CD28.ζ T cells. This drop was associated with decrease presence of Ly6G+ MDSCs in the spleen at the same time point (n ≥ 4, *p ≤ 0.05, Student’s t test). (E) There was no change in the frequency of monocytic Ly6C+ MDSCs at any analyzed time point (n ≥ 4, *p ≤ 0.05 by Student’s t test). Data are presented as mean ± SEM.
Figure 6.
T Cells Modified with IL13Rα2-CAR.CD28.ζ Construct Mobilize Dendritic Cells to the Brain of Glioma-Bearing Mice
(A) Analysis showed a 3-fold higher number of DCs in the brain of mice treated with IL13Rα2-CAR.CD28.ζ T cells at day 3, as compared to control IL13Rα2-CAR.Δ T cells, but not in the dLNs or spleens (n ≥ 4, *p ≤ 0.05, Student’s t test). (B) Increased DC infiltration to the brain parenchyma was associated with an increased presence of the CD8α+ subset of DCs at day 3 (n ≥ 4, *p ≤ 0.05, Student’s t test). (C) Animals bearing GL261-IL13Rα2 glioma tumors also expressing a surrogate antigen, OVA (GL261-IL13Rα2-OVA), were analyzed for the presence of OVA-specific CD8+ cells on day 7 after i.t. transplantation of CAR T cells. There were significantly more OVA-reactive CD8+ cells present in the brains of animals treated with IL13Rα2-CAR.CD28.ζ T cells, as compared to IL13Rα2-CAR.Δ T cells-treated mice (n ≥ 5, **p ≤ 0.01, Student’s t test). The panel on the right is an example of the gating strategy. Data are presented as mean ± SEM.
Discussion
In the present work, we characterized the effects of IL13Rα2-CAR T cells in two immunocompetent models of glioblastoma. Our results demonstrated that, similarly to human CAR T cells, murine CAR T cells were activated upon engagement with IL13Rα2-expressing glioma cells, as judged by their cytolytic activity and the production of IFNγ and TNF-α in the presence of IL13Rα2-positive glioma cells. In vivo, IL13Rα2-CAR T cells had anti-glioma activity in two syngeneic glioma models and created a pro-inflammatory tumor microenvironment.
We show that IL13Rα2-CAR T cells retain the potential to produce IFNγ and TNF-α in vivo up to 7 days post-i.t.-injection in immunocompetent animal models. These cytokines have the potential to influence the tumor microenvironment by multiple mechanisms. For instance, IFNγ and TNF-α are known to promote anti-tumor immunity by upregulating the recruitment and maturation of antigen-presenting cells33, 34 and enhancing CD8+ T cell responses.35, 36, 37 We also observed an increased number of CD8α+ DCs in the brains of glioma-bearing mice treated with IL13Rα2-CAR.CD28.ζ CAR T cells. The CD8α-expressing subset of DCs has been shown to efficiently cross-present both cell-bound and soluble antigens in the MHC class I context and strongly promote CD8+ T cell responses.38 In our studies, surviving VM/Dk animals developed long-lasting immunity, as determined by resistance to glioma re-challenge. No long-term survivors were observed in C57BL/6 mice bearing GL261-IL13Rα2 tumors. Although the reason for observed differences between both models is not clear, these findings are in line with reports from Sampson et al.,39 who observed the development of a lasting immunity using CAR T cells directed against EGFRvIII in a syngeneic glioma mouse model. Thus, CAR T cells may stimulate the development of immune memory against glioma antigens.
Since the targeting of a single antigen invariably leads to escape variants, either through receptor downregulation or the selection of cells not expressing the targeted antigen(s), antigenic spread is important for the development of anti-glioma therapies.10, 13, 40 In order to determine if CARs indeed potentiate immune responses against non-targeted antigens via bystander effect and/or antigen spreading, we also treated GL261-IL13Rα2-OVA glioma-bearing mice with IL13Rα2-CAR.CD28.ζ or IL13Rα2-CAR.ΔT cells. We observed a significant increase in OVA-specific T cell response in the therapeutic, but not control CAR T cells group, demonstrating that the interaction of CARs with their cognate antigen potentiates immunity against other tumor-expressed antigens.
Antigen escape is one limitation of immunotherapeutic approaches for MGs.10, 12, 41 One way to overcome this obstacle is to engineer CAR T cells to target multiple TAAs. Indeed, several pre-clinical studies evaluating CAR T cells targeting two or three antigens (e.g., HER2, IL13Rα2, and EphA2) have demonstrated that it is feasible to prevent immune escape and also overcome heterogeneous TAA expression by MGs.13, 42, 43
The immunosuppressive microenvironment is a hallmark of many solid cancers, including MGs, and is highly influenced by MDSCs and Tregs.44, 45, 46, 47 Increased numbers of these cells positively correlates with poorer prognosis in patients with MGs and is one of the biggest obstacles to the success of immunotherapy. Our studies revealed that i.t. treatment with IL13Rα2-CAR.CD28.ζ T cells did not alter the number of tumor-infiltrating Tregs in mice, which is significant given the established inhibitory effects of Tregs on antigen presentation48 and the anti-tumor efficacy of adoptively transferred T cells.49, 50 Our study suggests that depleting Tregs39, 47, 51 prior to CAR T cell administration may enhance the efficacy of this therapy. Even though we did not observe sustained changes in the MDSC compartment, a decrease in presence of Ly6G+ MDSCs in the brain was associated with a significant decrease of these cells in the spleen at 3 but not 7 days following CAR T cell administration. Reducing the negative effects of MDSCs on antigen-presentation and T cell proliferation has been the subject of intense study in the context of glioma treatment.44, 52 In the framework of CAR T cell therapies, reducing granulocytic and monocytic MDSCs could contribute to additional anti-glioma efficacy. Treg infiltration of tumor tissue, along with an increase in expression of inhibitory molecules, was observed after a single infusion of CAR T cells targeting EGFRvIII in patient with MG.41 The data from this clinical study and our findings suggest that combinatorial therapies targeting the immunosuppressive MG environment has the potential to enhance the anti-glioma activity of CAR T cells.
Although less benefit to survival was seen in these models compared to in immunodeficient mice treated with the human version of these CAR T cells,9 our results are consistent with the survival benefit of EGFRvIII-CAR T cells in the SMA560-EGFRvIII glioma model.39 Despite lack of exhaustion, the limited proliferation and persistence of our CAR T cells likely decreased their efficacy in these models. Indeed, IL13Rα2-CAR T cell proliferation peaked in response to antigenic stimulation 3 days after i.t. injection. Furthermore, as in our xenograft glioma model, only a fraction of the injected CAR T cells survived, highlighting their limited persistence within the tumor environment.9 Several approaches are being developed to render CAR T cells resistant to the inhibitory tumor microenvironment. These include transgenic expression of cytokines, expression of so-called switch receptors that convert an inhibitory into a stimulatory signal, and/or silencing or knocking out inhibitory genes.53, 54, 55, 56, 57 For example, we recently have demonstrated that expression of IL15 in CAR T cells enhanced their effector function in vitro and improved their anti-glioma activity in xenograft models.12
Finally, we recognize that animal models of MGs utilized in our previous and current reports carry inherit limitations. Nevertheless, we were able to recapitulate the heterogeneity of IL13Rα2 expression normally present in human MG and demonstrated the therapeutic potential of IL13Rα2-CAR.CD28.ζ T cells in two complementary murine models of MGs. Results observed in these models complement our pre-clinical studies of human CAR T cells in a glioma xenograft model9 and a case report from a phase I clinical trial,10 demonstrating that IL13Rα2-targeted CAR T cells have significant therapeutic efficacy against MG. As seen here, these studies showed a significantly lower expression of the targeted antigen in recurrent tumor tissue, highlighting the importance of developing CAR T cell therapies targeting additional TAAs, a process already underway in our laboratories. However, in order to fully realize the potential of such an approach,13, 43 it has to be first thoroughly evaluated in immunocompetent models of disease such as the ones developed here.
In conclusion, our data from pre-clinical CAR T cell therapies in mouse models show great promise for immunotherapy for IL13Rα2-positive glioblastomas. We demonstrated that IL13Rα2-CAR T cells efficiently kill glioma cells both in vitro and in vivo. For the first time, we showed that CAR T cell therapy positively modulates the immune landscape by creating a pro-inflammatory microenvironment in glioma-bearing mice, likely enhancing the anti-tumor activity of these CAR T cells and warranting further future studies. All together, our work provides strong rationale for further development and rapid clinical translation of this highly promising and selective therapeutic strategy for MGs.
Materials and Methods
Detailed information is provided in the Supplemental Materials and Methods.
Cell Culture
293T (ATCC), murine parental GL261 (NIH), and SMA560 glioma cell lines (a generous gift from Dr. Sampson, J.H., Duke University), and their modified sublines, GL261-hIL13Rα2, GL261-hIL13Rα2-OVA, and SMA560-hIL13Rα2, were maintained in complete DMEM (Invitrogen, Grand Island, NY). T cells were maintained in RPMI 1640 (Invitrogen, Grand Island, NY). Cells were screened for mycoplasma with MycoAlert Mycoplasma Detection Kit (Lonza, Walkersville, MD) every 3–6 months.
Generation of Retroviral Chimeric Antigen Receptor Constructs
The codon-optimized cDNA encoding the CARs was synthesized by Thermo Fisher Scientific (Waltham, MA) and sub-cloned into a multiple cloning site (MCS) of the pRV2011(M) vector. The sequences of all final CAR constructs were verified by sequencing.
Generation of CAR T Cells
CD3+ T cells were isolated from the spleens of 6- to 8-week old C57BL/6 (syngeneic for GL261) or VM/Dk (syngeneic for SMA560) male or female mice using a Mouse T Cell Isolation Kit (eBioscience Affymetrix, San Diego, CA) according to the manufacturer’s directions. T cell cultures were transduced with replication-deficient retrovirus encoding for CARs for 3 consecutive days starting 24 hr after T cell stimulation. CAR and Thy1.1 marker gene expression was assessed on day 5 or 6 using flow cytometry.
Generation of Glioblastoma Cell Lines Expressing Human IL13Rα2 and/or OVA
GL261-hIL13Rα2 and SMA560-hIL13Rα2 were generated by transducing parental GL261 and SMA560 cell lines with a previously developed pEF6myc,his vector encoding human IL13Rα230 using Lipofectamine 2000 transfection reagent (Invitrogen, Grand Island, NY) according to the manufacturer’s specifications. Transfected cells were selected with 5 μg/mL and maintained with 2 μg/mL of blasticidin S HCl (Invitrogen, Grand Island, NY). GL261-hIL13Rα2 expressing OVA (GL261-hIL13Rα2-OVA) were produced by transducing GL261-hIL13Rα2 cells with pAc-Neo-OVA (Addgene, Cambridge, MA) using Lipofectamine 2000 transfection reagent. Transfected cells were selected with 400 μg/mL and maintained in 200 μg/mL of geneticin (G418, Invitrogen, Grand Island, NY) in complete culture medium.
Intracranial Implantation of Murine Glioma Cells and Treatment with CAR T Cells
CD45.1 and CD45.2 C57BL/6 mice were obtained from Jackson Laboratory. VM/Dk mice were bred in-house in accordance with a study-specific animal protocol approved by the Northwestern University Institutional Animal Care and Use Committee (IACUC). We utilized mixed-gender animals, 6–12 weeks of age, for glioma implantation. The following coordinates were used: 2 mm from bregma, 3 mm right of the cranial midline suture, and 3.5 mm depth below the dura. On day 7 after implantation of glioma cells, animals received an i.t. injection of 1.5 × 106 CAR T cells. Treated animals were randomly assigned to housing cages, separated by gender. Animals were monitored for survival according to Northwestern University IACUC-approved protocols.
Flow Cytometry
Flow cytometric analysis was carried out using the BD Fortessa flow cytometer (Becton Dickinson, Franklin Lakes, NJ) at the Robert H. Lurie Comprehensive Cancer Center Flow Cytometry Core Facility. Single-cell isolates were blocked with PBS supplemented with 2% fetal bovine serum (FBS) and mouse CD16/32 blocking reagent (BioLegend, San Diego, CA). Following the Fc blockade, flourochrome-conjugated antibodies (all from BioLegend, San Diego, CA, unless specified) were added and incubated on ice for 20 min. For detection of intracellular proteins, cells were fixed, permeabilized, and subsequently incubated with antibodies. Labeled cells were then washed and analyzed.
In Vitro and In Vivo Functional Assays
The cytotoxicity of CAR T cells against control and IL13Rα2-expressing glioma cells was determined in a standard 51Cr release assay with increasing ratios of the effector (CAR T cells) to target (glioma) cells. Antigen stimulation of CAR T cells was performed for the intracellular detection of cytokines by flow cytometry.
Statistical Analysis
All statistical analyses of collected data were performed with GraphPad 7 Software (Prism, La Jolla, CA). Student’s t test was used to compare the two groups. Significance, defined as p less than 0.05 in all statistical tests, was calculated with an unpaired Mann-Whitney test or an unpaired two-tailed Student’s t test, as indicated. Multiple groups were analyzed with either a one- or two-way ANOVA, followed by a Tukey’s multiple comparisons test. Kaplan-Meier plots were generated using GraphPad 7 Prism, and p values for curve comparisons were calculated using the log-rank method. All experiments, besides survival analyses, were carried out in at least triplicates. All survival studies were carried out at least twice. Data are presented as mean ± SEM.
Author Contributions
K.C.P., I.V.B., and J.M. designed the experiments. K.C.P., J.M., G.K., W.K.P., G.L., T.R.-C., Y.H., and S.G. generated data, developed protocols, and performed experiments. K.C.P. and M.W. performed statistical analyses. K.C.P., J.M., M.S.L., S.G., and I.V.B. wrote and reviewed the manuscript. All authors reviewed, edited, and commented on the manuscript.
Conflicts of Interest
M.S.L., I.V.B., and S.G. have patent applications in the field of T cell and gene therapy for cancer and/or IL13Rα2-targeted therapies.
Acknowledgments
This work was supported by NIH grants R21NS089802 and R21NS101150 and the James S. McDonnell Foundation. Authors are grateful to Aurora Lopez-Rosas for her excellent assistance in maintaining animal colonies. We are also grateful to the NIH tetramer facility for providing MHC I tetramers for our studies.
Footnotes
Supplemental Information includes Supplemental Materials and Methods and three figures and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.02.001.
Supplemental Information
References
- 1.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.van Tellingen O., Yetkin-Arik B., de Gooijer M.C., Wesseling P., Wurdinger T., de Vries H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Razavi S.M., Lee K.E., Jin B.E., Aujla P.S., Gholamin S., Li G. Immune evasion strategies of glioblastoma. Front. Surg. 2016;3:11. doi: 10.3389/fsurg.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osuka S., Van Meir E.G. Overcoming therapeutic resistance in glioblastoma: the way forward. J. Clin. Invest. 2017;127:415–426. doi: 10.1172/JCI89587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hombach A.A., Görgens A., Chmielewski M., Murke F., Kimpel J., Giebel B., Abken H. Superior therapeutic index in lymphoma therapy: CD30(+) CD34(+) hematopoietic stem cells resist a chimeric antigen receptor T-cell attack. Mol. Ther. 2016;24:1423–1434. doi: 10.1038/mt.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z., Wu Z., Liu Y., Han W. New development in CAR-T cell therapy. J. Hematol. Oncol. 2017;10:53. doi: 10.1186/s13045-017-0423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong S., Sengupta S., Tyler B., Bais A.J., Ma Q., Doucette S., Zhou J., Sahin A., Carter B.S., Brem H. Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells. Clin. Cancer Res. 2012;18:5949–5960. doi: 10.1158/1078-0432.CCR-12-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krenciute G., Krebs S., Torres D., Wu M.F., Liu H., Dotti G., Li X.N., Lesniak M.S., Balyasnikova I.V., Gottschalk S. Characterization and functional analysis of scFv-based chimeric antigen receptors to redirect T cells to IL13Rα2-positive glioma. Mol. Ther. 2016;24:354–363. doi: 10.1038/mt.2015.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown C.E., Alizadeh D., Starr R., Weng L., Wagner J.R., Naranjo A., Ostberg J.R., Blanchard M.S., Kilpatrick J., Simpson J. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown C.E., Badie B., Barish M.E., Weng L., Ostberg J.R., Chang W.C., Naranjo A., Starr R., Wagner J., Wright C. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015;21:4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krenciute G., Prinzing B.L., Yi Z., Wu M.F., Liu H., Dotti G., Balyasnikova I.V., Gottschalk S. Transgenic expression of IL15 improves antiglioma activity of IL13Ralpha2-CAR T cells but results in antigen-loss variants. Cancer Immunol. Res. 2017;5:571–581. doi: 10.1158/2326-6066.CIR-16-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegde M., Mukherjee M., Grada Z., Pignata A., Landi D., Navai S.A., Wakefield A., Fousek K., Bielamowicz K., Chow K.K. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Invest. 2016;126:3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed N., Brawley V., Hegde M., Bielamowicz K., Kalra M., Landi D., Robertson C., Gray T.L., Diouf O., Wakefield A. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3:1094–1101. doi: 10.1001/jamaoncol.2017.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao H., Choi B.D., Suryadevara C.M., Sanchez-Perez L., Yang S., De Leon G., Sayour E.J., McLendon R., Herndon J.E., 2nd, Healy P. EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma. PLoS ONE. 2014;9:e94281. doi: 10.1371/journal.pone.0094281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow K.K., Naik S., Kakarla S., Brawley V.S., Shaffer D.R., Yi Z., Rainusso N., Wu M.F., Liu H., Kew Y. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol. Ther. 2013;21:629–637. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debinski W., Slagle B., Gibo D.M., Powers S.K., Gillespie G.Y. Expression of a restrictive receptor for interleukin 13 is associated with glial transformation. J. Neurooncol. 2000;48:103–111. doi: 10.1023/a:1006446426611. [DOI] [PubMed] [Google Scholar]
- 18.Debinski W., Obiri N.I., Powers S.K., Pastan I., Puri R.K. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin. Cancer Res. 1995;1:1253–1258. [PubMed] [Google Scholar]
- 19.Joshi B.H., Plautz G.E., Puri R.K. Interleukin-13 receptor alpha chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res. 2000;60:1168–1172. [PubMed] [Google Scholar]
- 20.Jarboe J.S., Johnson K.R., Choi Y., Lonser R.R., Park J.K. Expression of interleukin-13 receptor alpha2 in glioblastoma multiforme: implications for targeted therapies. Cancer Res. 2007;67:7983–7986. doi: 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- 21.Brown C.E., Warden C.D., Starr R., Deng X., Badie B., Yuan Y.C., Forman S.J., Barish M.E. Glioma IL13Rα2 is associated with mesenchymal signature gene expression and poor patient prognosis. PLoS ONE. 2013;8:e77769. doi: 10.1371/journal.pone.0077769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown C.E., Starr R., Aguilar B., Shami A.F., Martinez C., D’Apuzzo M., Barish M.E., Forman S.J., Jensen M.C. Stem-like tumor-initiating cells isolated from IL13Rα2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T cells. Clin. Cancer Res. 2012;18:2199–2209. doi: 10.1158/1078-0432.CCR-11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartolomé R.A., García-Palmero I., Torres S., López-Lucendo M., Balyasnikova I.V., Casal J.I. IL13 receptor α2 signaling requires a scaffold protein, FAM120A, to activate the FAK and PI3K pathways in colon cancer metastasis. Cancer Res. 2015;75:2434–2444. doi: 10.1158/0008-5472.CAN-14-3650. [DOI] [PubMed] [Google Scholar]
- 24.Papageorgis P., Ozturk S., Lambert A.W., Neophytou C.M., Tzatsos A., Wong C.K., Thiagalingam S., Constantinou A.I. Targeting IL13Ralpha2 activates STAT6-TP63 pathway to suppress breast cancer lung metastasis. Breast Cancer Res. 2015;17:98. doi: 10.1186/s13058-015-0607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie M., Wu X.J., Zhang J.J., He C.S. IL-13 receptor α2 is a negative prognostic factor in human lung cancer and stimulates lung cancer growth in mice. Oncotarget. 2015;6:32902–32913. doi: 10.18632/oncotarget.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujisawa T., Joshi B., Nakajima A., Puri R.K. A novel role of interleukin-13 receptor alpha2 in pancreatic cancer invasion and metastasis. Cancer Res. 2009;69:8678–8685. doi: 10.1158/0008-5472.CAN-09-2100. [DOI] [PubMed] [Google Scholar]
- 27.Fujisawa T., Joshi B.H., Puri R.K. IL-13 regulates cancer invasion and metastasis through IL-13Rα2 via ERK/AP-1 pathway in mouse model of human ovarian cancer. Int. J. Cancer. 2012;131:344–356. doi: 10.1002/ijc.26366. [DOI] [PubMed] [Google Scholar]
- 28.Krebs S., Chow K.K., Yi Z., Rodriguez-Cruz T., Hegde M., Gerken C., Ahmed N., Gottschalk S. T cells redirected to interleukin-13Rα2 with interleukin-13 mutein--chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Rα1. Cytotherapy. 2014;16:1121–1131. doi: 10.1016/j.jcyt.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J.W., Young J.S., Solomaha E., Kanojia D., Lesniak M.S., Balyasnikova I.V. A novel single-chain antibody redirects adenovirus to IL13Rα2-expressing brain tumors. Sci. Rep. 2015;5:18133. doi: 10.1038/srep18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balyasnikova I.V., Wainwright D.A., Solomaha E., Lee G., Han Y., Thaci B., Lesniak M.S. Characterization and immunotherapeutic implications for a novel antibody targeting interleukin (IL)-13 receptor α2. J. Biol. Chem. 2012;287:30215–30227. doi: 10.1074/jbc.M112.370015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangani D., Weller M., Roth P. The network of immunosuppressive pathways in glioblastoma. Biochem. Pharmacol. 2017;130:1–9. doi: 10.1016/j.bcp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Jin H.T., Anderson A.C., Tan W.G., West E.E., Ha S.J., Araki K., Freeman G.J., Kuchroo V.K., Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker B.S., Rautela J., Hertzog P.J. Antitumour actions of interferons: implications for cancer therapy. Nat. Rev. Cancer. 2016;16:131–144. doi: 10.1038/nrc.2016.14. [DOI] [PubMed] [Google Scholar]
- 34.Schiavoni G., Mattei F., Gabriele L. Type I interferons as stimulators of DC-mediated cross-priming: impact on anti-tumor response. Front. Immunol. 2013;4:483. doi: 10.3389/fimmu.2013.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bubeník J. MHC class I down-regulation: tumour escape from immune surveillance? (review) Int. J. Oncol. 2004;25:487–491. [PubMed] [Google Scholar]
- 36.Hombach A.A., Holzinger A., Abken H. The weal and woe of costimulation in the adoptive therapy of cancer with chimeric antigen receptor (CAR)-redirected T cells. Curr. Mol. Med. 2013;13:1079–1088. doi: 10.2174/1566524011313070003. [DOI] [PubMed] [Google Scholar]
- 37.Barth R.J., Jr., Mulé J.J., Spiess P.J., Rosenberg S.A. Interferon gamma and tumor necrosis factor have a role in tumor regressions mediated by murine CD8+ tumor-infiltrating lymphocytes. J. Exp. Med. 1991;173:647–658. doi: 10.1084/jem.173.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shortman K., Heath W.R. The CD8+ dendritic cell subset. Immunol. Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 39.Sampson J.H., Choi B.D., Sanchez-Perez L., Suryadevara C.M., Snyder D.J., Flores C.T., Schmittling R.J., Nair S.K., Reap E.A., Norberg P.K. EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin. Cancer Res. 2014;20:972–984. doi: 10.1158/1078-0432.CCR-13-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sampson J.H., Heimberger A.B., Archer G.E., Aldape K.D., Friedman A.H., Friedman H.S., Gilbert M.R., Herndon J.E., 2nd, McLendon R.E., Mitchell D.A. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010;28:4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Rourke D.M., Nasrallah M.P., Desai A., Melenhorst J.J., Mansfield K., Morrissette J.J.D., Martinez-Lage M., Brem S., Maloney E., Shen A. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017;9:eaaa0984. doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bielamowicz K., Fousek K., Byrd T.T., Samaha H., Mukherjee M., Aware N., Wu M.F., Orange J.S., Sumazin P., Man T.K. Trivalent CAR T-cells overcome interpatient antigenic variability in glioblastoma. Neuro. Oncol. 2017 doi: 10.1093/neuonc/nox182. Published online September 16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hegde M., Corder A., Chow K.K., Mukherjee M., Ashoori A., Kew Y., Zhang Y.J., Baskin D.S., Merchant F.A., Brawley V.S. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol. Ther. 2013;21:2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamran N., Kadiyala P., Saxena M., Candolfi M., Li Y., Moreno-Ayala M.A., Raja N., Shah D., Lowenstein P.R., Castro M.G. Immunosuppressive myeloid cells’ blockade in the glioma microenvironment enhances the efficacy of immune-stimulatory gene therapy. Mol. Ther. 2017;25:232–248. doi: 10.1016/j.ymthe.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wainwright D.A., Balyasnikova I.V., Chang A.L., Ahmed A.U., Moon K.S., Auffinger B., Tobias A.L., Han Y., Lesniak M.S. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin. Cancer Res. 2012;18:6110–6121. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang A.L., Miska J., Wainwright D.A., Dey M., Rivetta C.V., Yu D., Kanojia D., Pituch K.C., Qiao J., Pytel P. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 2016;76:5671–5682. doi: 10.1158/0008-5472.CAN-16-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miska J., Rashidi A., Chang A.L., Muroski M.E., Han Y., Zhang L., Lesniak M.S. Anti-GITR therapy promotes immunity against malignant glioma in a murine model. Cancer Immunol. Immunother. 2016;65:1555–1567. doi: 10.1007/s00262-016-1912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 49.Antony P.A., Piccirillo C.A., Akpinarli A., Finkelstein S.E., Speiss P.J., Surman D.R., Palmer D.C., Chan C.C., Klebanoff C.A., Overwijk W.W. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 51.El Andaloussi A., Han Y., Lesniak M.S. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J. Neurosurg. 2006;105:430–437. doi: 10.3171/jns.2006.105.3.430. [DOI] [PubMed] [Google Scholar]
- 52.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dotti G., Gottschalk S., Savoldo B., Brenner M.K. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol. Rev. 2014;257:107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L., Morgan R.A., Beane J.D., Zheng Z., Dudley M.E., Kassim S.H., Nahvi A.V., Ngo L.T., Sherry R.M., Phan G.Q. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin. Cancer Res. 2015;21:2278–2288. doi: 10.1158/1078-0432.CCR-14-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu D., Song L., Wei J., Courtney A.N., Gao X., Marinova E., Guo L., Heczey A., Asgharzadeh S., Kim E. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J. Clin. Invest. 2012;122:2221–2233. doi: 10.1172/JCI59535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foster A.E., Dotti G., Lu A., Khalil M., Brenner M.K., Heslop H.E., Rooney C.M., Bollard C.M. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J. Immunother. 2008;31:500–505. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leen A.M., Sukumaran S., Watanabe N., Mohammed S., Keirnan J., Yanagisawa R., Anurathapan U., Rendon D., Heslop H.E., Rooney C.M. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol. Ther. 2014;22:1211–1220. doi: 10.1038/mt.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.