Abstract
Most antidiabetic drugs treat disease symptoms rather than adipose tissue dysfunction as a key pathogenic cause in the metabolic syndrome and type 2 diabetes. Pharmacological targeting of adipose tissue through the nuclear receptor PPARg, as exemplified by glitazone treatments, mediates efficacious insulin sensitization. However, a better understanding of the context‐specific PPARg responses is required for the development of novel approaches with reduced side effects. Here, we identified the transcriptional cofactor Cited4 as a target and mediator of rosiglitazone in human and murine adipocyte progenitor cells, where it promoted specific sets of the rosiglitazone‐dependent transcriptional program. In mice, Cited4 was required for the proper induction of thermogenic expression by Rosi specifically in subcutaneous fat. This phenotype had high penetrance in females only and was not evident in beta‐adrenergically stimulated browning. Intriguingly, this specific defect was associated with reduced capacity for systemic thermogenesis and compromised insulin sensitization upon therapeutic rosiglitazone treatment in female but not male mice. Our findings on Cited4 function reveal novel unexpected aspects of the pharmacological targeting of PPARg.
Keywords: adipocyte progenitors, browning, Cited4, glitazones, insulin sensitivity
Subject Categories: Metabolism
Introduction
The functional status of adipose tissue has emerged as a key determinant for systemic metabolic homeostasis and disease. Obesity and in particular excess visceral fat are prominent risk factors for type 2 diabetes and cardiovascular disease (Cornier et al, 2008; Lee et al, 2013). The dissociation of obesity and metabolic dysfunction in the paradigms of lipodystrophy and metabolically healthy obesity indicates that it is not the quantity of fat per se but the impaired function which underlies the pathogenic process (Vegiopoulos et al, 2017). Inadequate lipid metabolism in adipocytes results in increased circulating and ectopically deposited lipids and consequently in lipotoxicity and malfunction of metabolism in multiple organs (Samuel & Shulman, 2012). Chronically, this contributes to the dysregulation of endocrine circuits, insulin resistance, and essentially to the development of type 2 diabetes. However, most of the current treatment options in type 2 diabetes and prediabetes target mainly the symptoms rather than insulin sensitivity and adipose tissue metabolism as causative factors (Soccio et al, 2014; Chatterjee et al, 2017).
The glitazone drugs of the thiazolidinedione (TZD) class, agonists of the peroxisome proliferator‐activated receptor gamma (PPARg), represent an exception in this regard. TZDs act as potent insulin sensitizers, and this action seems to be mediated predominantly by PPARg in adipose tissue (Soccio et al, 2014). Although the use of TZDs has strongly declined due to their side effects, PPARg remains a promising target in the prevention and treatment of type 2 diabetes as highlighted by ongoing basic and clinical research (Ahmadian et al, 2013; Soccio et al, 2014; Banks et al, 2015; Chatterjee et al, 2017). Thiazolidinediones have pleiotropic effects on adipose tissue, essentially resulting in improved uptake and metabolism of fatty acids and glucose as well as endocrine function (Rangwala & Lazar, 2004; Ye et al, 2004; Boden et al, 2005; Festuccia et al, 2009). Beyond their ability to enhance adipocyte formation and turnover, TZDs promote mitochondrial biogenesis and fatty acid oxidation in human and rodent white adipose tissue and increase its thermogenic potential (“browning”; Okuno et al, 1998; Fukui et al, 2000; Yamauchi et al, 2001; Wilson‐Fritch et al, 2004; Boden et al, 2005; Bogacka et al, 2005; Tang et al, 2011). Interestingly, the enrichment of pathways of mitochondrial oxidation and lipid metabolism in subcutaneous fat was recently shown to be the most prominent effect of the TZD rosiglitazone on the transcriptome across adipose tissues (Soccio et al, 2017). Increased capacity for adipose tissue thermogenesis is generally accepted to be protective against insulin resistance and dyslipidemia but to which extent the regulation of browning by TZDs mediates insulin sensitization remains unclear (Sidossis & Kajimura, 2015).
Thiazolidinediones are potent stimulators of adipocyte progenitor differentiation in human and murine cell culture and promote the formation of beige/brite thermogenic adipocytes (Digby et al, 1998; Elabd et al, 2009; Petrovic et al, 2010; Ohno et al, 2012; Ahmadian et al, 2013). In the adult organism, adipocyte progenitors mediate the recruitment of new white and beige adipocytes as it occurs upon tissue expansion, cold exposure, or TZD treatment (Tang et al, 2011; Hepler et al, 2017). Although the core transcriptional network driving adipogenesis downstream of PPARg activation is well established, the factors responsible for depot‐, sex‐, and stimulus‐specific recruitment of progenitors remain to be determined. Moreover, how the context‐dependent regulation of progenitors relates to tissue function and insulin sensitization is poorly understood.
In this study, we searched for novel mediators of PPARg activation in defined adipocyte progenitor cells and identified the transcriptional cofactor Cited4 (CREB‐binding protein/p300‐interacting transactivator with ED‐rich tail, [Braganca et al, 2002; Yahata et al, 2002)]. Little is known so far about the physiological function of Cited4, beyond its involvement in the regulation of cardiac hypertrophy (Bostrom et al, 2010; Bezzerides et al, 2016). We demonstrate that Cited4 promotes the transcriptional program induced by rosiglitazone in differentiating murine and human adipocyte progenitors and that Cited4 deficiency impairs TZD‐dependent but not β‐adrenergically stimulated browning specifically in subcutaneous fat. Remarkably, this defect also manifested upon therapeutic rosiglitazone treatment and was associated with reduced insulin sensitization in a sex‐specific manner.
Results
Cited4 is a target of rosiglitazone in murine and human adipocyte progenitors promoting beige differentiation and Ucp1 expression
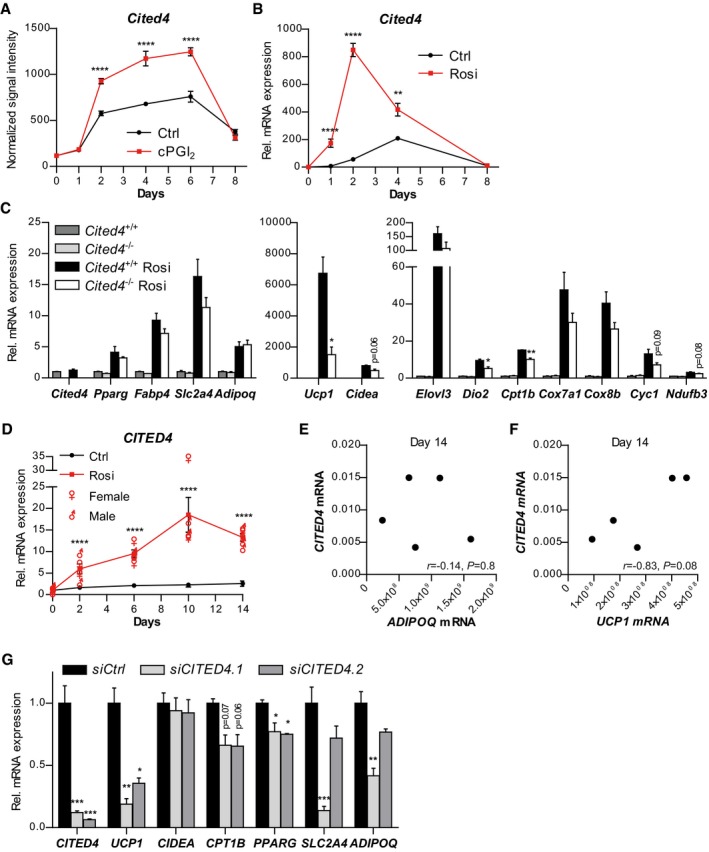
We have previously dissected the global transcriptional response of defined immuno‐selected Lin(Ter119/CD31/CD45)−Sca1+ adipocyte progenitors to carbaprostacyclin (cPGI2), the stable analogue of prostacyclin and PPARg agonist promoting beige adipocyte differentiation (Vegiopoulos et al, 2010; Bayindir et al, 2015; Ghandour et al, 2016; Babaei et al, 2017). To search for novel physiologically relevant factors mediating the effects of PPARg activation in progenitors, we mined time course expression profiles for cPGI2‐regulated genes (Bayindir et al, 2015). We identified Cited4 due to a robust but transient induction by cPGI2 during progenitor differentiation (Fig 1A). Treatment with rosiglitazone (Rosi) in place of cPGI2 potentiated the transient induction of Cited4 (Fig 1B) and this was recapitulated in the mesenchymal progenitor cell line C3H10T1/2 but not in the preadipocyte cell model 3T3‐L1 (Fig EV1A and B). In addition, pioglitazone, a TZD currently used as antidiabetic, transiently increased Cited4 mRNA expression in primary progenitor cells, albeit with overall lower potency compared to Rosi (Fig EV1C). These data demonstrate that Cited4 is a likely target of TZDs and PPARg in differentiating adipocyte progenitors.
Figure 1. Cited4 is a target of rosiglitazone in murine and human adipocyte progenitors promoting beige differentiation.
-
AmRNA expression in FACS‐isolated Lin(Ter119/CD31/CD45)−Sca1+ progenitor cells from female mouse subcutaneous fat, differentiated in the presence of 1 μM cPGI2 or vehicle for the indicated time, as determined by expression profiling (n = 3, E‐MTAB‐3693). ****P = 3 × 10−6 (Day 2), ****P = 4 × 10−7 (Day 4), ****P = 1 × 10−6 (Day 6) in 2 × 2 ANOVA with Bonferroni's posttests (cPGI2 vs. vehicle).
-
BmRNA expression in MACS‐isolated Lin−Sca1+ progenitor cells from female mouse subcutaneous fat, differentiated in the presence of 100 nM Rosi or vehicle for the indicated time, as determined by qRT–PCR (n = 4). ****P = 1 × 10−10 (Days 1 and 2), **P = 0.001, in 2 × 2 ANOVA with Bonferroni's posttests (Rosi vs. vehicle).
-
CmRNA expression in female Lin−Sca1+ cells, differentiated in the presence of 100 nM Rosi or vehicle for 8 days, as determined by qRT–PCR (n = 3). t‐test Cited4 −/− vs. Cited4 +/+ (Rosi) *P = 0.013 (Ucp1), **P = 0.004 (Cpt1b), *P = 0.026 (Dio2).
-
D–FmRNA expression in primary SVF cells from human subcutaneous fat, differentiated in the presence of 100 nM Rosi (D) or vehicle (D–F), as determined by qRT–PCR at the indicated time points (n = 5 patients). ♀/♂ represents individual data. (D) ****P = 3 × 10−5 (Day 2), ****P = 3 × 10−6 (Day 6), ****P = 4 × 10−9 (Day 10), ****P = 3 × 10−7 (Day 14), in 2 × 2 ANOVA with Bonferroni's posttests (Rosi vs. vehicle). (E, F) Pearson correlation coefficient (r) and P‐value are shown.
-
GmRNA expression in primary SVF cells from human female subcutaneous fat transfected with the indicated siRNA prior to differentiation in the presence of 100 nM Rosi for 9 days, as determined by qRT–PCR (n = 3). ***P = 0.0002 (CITED4), **P = 0.002 (UCP1), *P = 0.02 (UCP1), *P = 0.035/0.026 (PPARG), ***P = 0.0006 (SLC2A4), **P = 0.002 (ADIPOQ) in one‐way ANOVA with Tukey's posttests (vs. siCtrl).
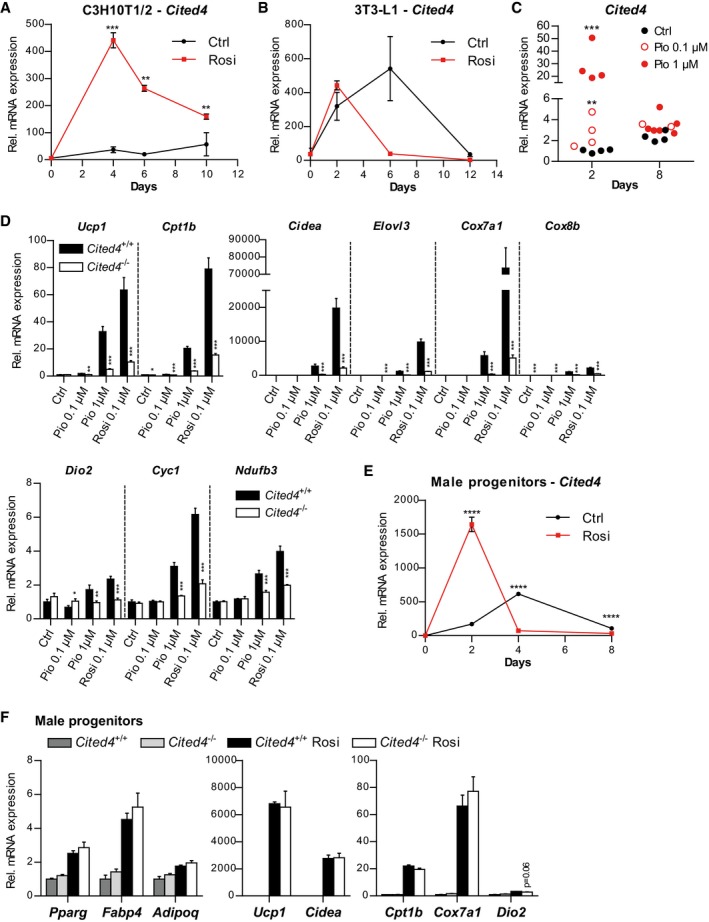
Figure EV1. Cited4 is a target of rosiglitazone in murine adipocyte progenitors promoting beige differentiation.
-
AmRNA expression in C3H10T1/2 cells, differentiated in the presence of 1 μM Rosi or vehicle for the indicated time, as determined by qRT–PCR (day 0, n = 3; day 4, n = 4; day 6, Ctrl, n = 2, Rosi, n = 4; day 10, n = 4). ***P = 0.001, **P = 0.007 (Day 6), **P = 0.005 (Day 10), in 2 × 2 ANOVA with Bonferroni's posttests (Rosi vs. vehicle).
-
BmRNA expression in 3T3‐L1 cells, differentiated in the presence of 1 μM Rosi or vehicle for the indicated time, as determined by qRT–PCR (n = 3). 2 × 2 ANOVA with Bonferroni's posttests, P > 0.05 (Rosi vs. vehicle).
-
CmRNA expression in female Lin−Sca1+ cells, differentiated in the presence of 0.1 or 1 μM pioglitazone (Pio) or vehicle for 8 days, as determined by qRT–PCR (n = 4 for Day 2, n = 4/2/6 for Day 8). ***P = 1*10−10, **P = 0.003, in 2 × 2 ANOVA with Bonferroni's posttests (Pio vs. vehicle).
-
DmRNA expression in female Lin−Sca1+ cells, differentiated in the presence of the indicated substances for 8 days, as determined by qRT–PCR (n = 3). **P = 0.0035, ***P = 4 × 10−10, ***P = 7 × 10−10 (Ucp1), *P = 0.036, ***P = 0.0002, ***P = 1 × 10−10, ***P = 1 × 10−10 (Cpt1b), ***P = 2 × 10−5 (Cidea), ***P = 1 × 10−6, ***P = 4 × 10−9, ***P = 1 × 10−8, (Elovl3), ***P = 9 × 10−8, ***P = 2 × 10−7 (Cox7a1), ***P = 0.0002, ***P = 1 × 10−8, ***P = 4 × 10−5, ***P = 0.0002 (Cox8b), *P = 0.032, **P = 0.006, ***P = 0.0007 (Dio2), ***P = 1 × 10−6, ***P = 2 × 10−8 (Cyc1), ***P = 8 × 10−5, ***P = 3 × 10−6 (Ndufb3) in 2 × 2 ANOVA with Holm–Sidak posttests (Cited4 −/− vs. Cited4 +/+).
-
EmRNA expression in male Lin−Sca1+ progenitor cells, differentiated in the presence of 100 nM Rosi or vehicle for the indicated time, as determined by qRT–PCR (n = 3). ****P = 1 × 10−10 (Days 2 and 4), ****P = 4 × 10−8 (Day 8) in 2 × 2 ANOVA with Bonferroni's posttests (Rosi vs. vehicle).
-
FmRNA expression in male Lin−Sca1+ cells, differentiated in the presence of 100 nM Rosi or vehicle for 8 days, as determined by qRT–PCR (n = 3, t‐test).
To determine whether the induction of Cited4 expression is of functional importance for Rosi‐stimulated progenitor differentiation and in particular for the oxidative/thermogenic adipocyte phenotype, we examined primary Lin−Sca1+ cells isolated from female Cited4 −/− knockout mice, lacking the complete Cited4 coding sequence and Cited4 protein (Appendix Fig S1A and B). Whereas only a trend of reduced mRNA expression of adipogenic marker genes could be detected in Cited4 −/− cells after 8 days of differentiation, we observed a significant reduction of key thermogenic marker genes, i.e., Ucp1, Cpt1b, and Dio2, with Ucp1 mRNA decreased by more than threefold (Fig 1C). The effects of Cited4 knockout on thermogenic markers were comparable in a direct comparison between pioglitazone and Rosi, at least at a higher pioglitazone dose, which was required for the effective stimulation of thermogenic expression (Fig EV1D). Intriguingly, there was no effect of the Cited4 knockout on the differentiation of progenitors from male mice despite the induction of Cited4 by Rosi, indicating a sex‐specific requirement (Fig EV1E and F).
We next sought to validate these findings in the human system. Indeed, Rosi treatment during the differentiation of stromal vascular fraction (SVF) cells, freshly isolated from subcutaneous fat, induced CITED4 expression by maximally 15‐fold independently of donor gender (Figs 1D and EV2A–C). Compared to mouse cells, maximal induction occurred late and declined only marginally at 14 days of differentiation. CITED4 upregulation paralleled the induction of the general differentiation marker ADIPOQ by Rosi (Fig EV2B). However, it is noteworthy that in the absence of Rosi, CITED4 expression tended to positively correlate with UCP1 rather than ADIPOQ mRNA (Fig 1E and F). Importantly, Rosi‐mediated UCP1 mRNA was markedly diminished in female cells upon CITED4 knockdown using independent siRNAs and this was accompanied by a milder but significant reduction in CPT1B and PPARG (Fig 1G), whereas SLC2A4 and ADIPOQ were only affected by one siRNA. In contrast to mouse cells, CITED4 knockdown resulted in reduced UCP1 levels in male cells (Fig EV2D). Overall, the Rosi‐dependent CITED4 expression and knockdown phenotype mirrored the murine data and indicate a conserved function of CITED4 in adipocyte progenitors despite differences possibly attributable to the strong dependency of human adipocyte differentiation on PPARg agonists.
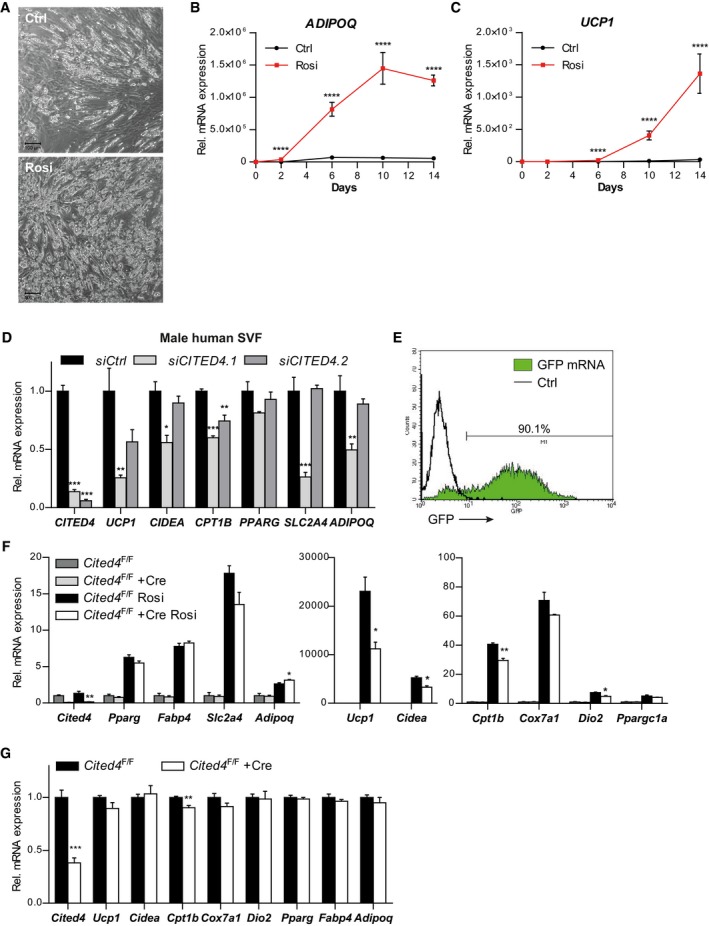
Figure EV2. Cited4 is a target of rosiglitazone in murine and human adipocyte progenitors promoting beige differentiation.
-
APhase contrast microscopy of primary SVF cells from human subcutaneous fat, differentiated in the presence of 100 nM Rosi or vehicle for 14 days (representative of n = 5 patients). Scale bar is 100 μm.
-
B, CmRNA expression in primary SVF cells from human subcutaneous fat, differentiated in the presence of 100 nM Rosi or vehicle, as determined by qRT–PCR (n = 5 patients). (B) ****P = 1 × 10−10 (Day 2), ****P = 5 × 10−8 (Day 6), ****P = 1 × 10−9 (Days 10 and 14), (C) ****P = 7 × 10−7 (Day 6), ****P = 1 × 10−9 (Days 10 and 14) in 2 × 2 ANOVA with Bonferroni's posttests (Rosi vs. vehicle).
-
DmRNA expression in primary SVF cells from human male subcutaneous fat transfected with the indicated siRNA prior to differentiation in the presence of 100 nM Rosi for 9 days, as determined by qRT–PCR (n = 3). ***P = 0.0002 (siCITED4.1 CITED4), ***P = 0.0003 (siCITED4.2 CITED4), **P = 0.005 (UCP1), *P = 0.01 (CIDEA), ***P = 0.0006 (CITED4.1 CPT1B), **P = 0.007 (CPT1B), ***P = 0.0006 (CITED4.1 SLC2A4),**P = 0.009 (ADIPOQ) in ANOVA with Tukey's posttests (vs. siCtrl).
-
EGFP fluorescence intensity distribution of Lin−Sca1+ progenitor cells 24 hours after transfection with GFP mRNA, determined by flow cytometry (compared to non‐transfected cells).
-
FmRNA expression in female Cited4 F/F Lin−Sca1+ progenitor cells transfected with Cre or control mRNA prior to differentiation in the presence of 100 nM Rosi or vehicle for 8 days, as determined by qRT–PCR (n = 3). t‐test Cre vs. Ctrl (Rosi), **P = 0.002 (Cited4), *P = 0.039 (Adipoq), *P = 0.015 (Ucp1), *P = 0.011 (Cidea), **P = 0.004 (Cpt1b), *P = 0.011 (Dio2).
-
GmRNA expression in female Cited4 F/F Lin−Sca1+ progenitor cells transfected with Cre or control mRNA 3 days after induction of differentiation in the presence of 100 nM Rosi or vehicle for 8 days, as determined by qRT–PCR (n = 4). t‐test Cre vs. Ctrl, ***P = 0.0005 (Cited4), **P = 0.009 (Cpt1b).
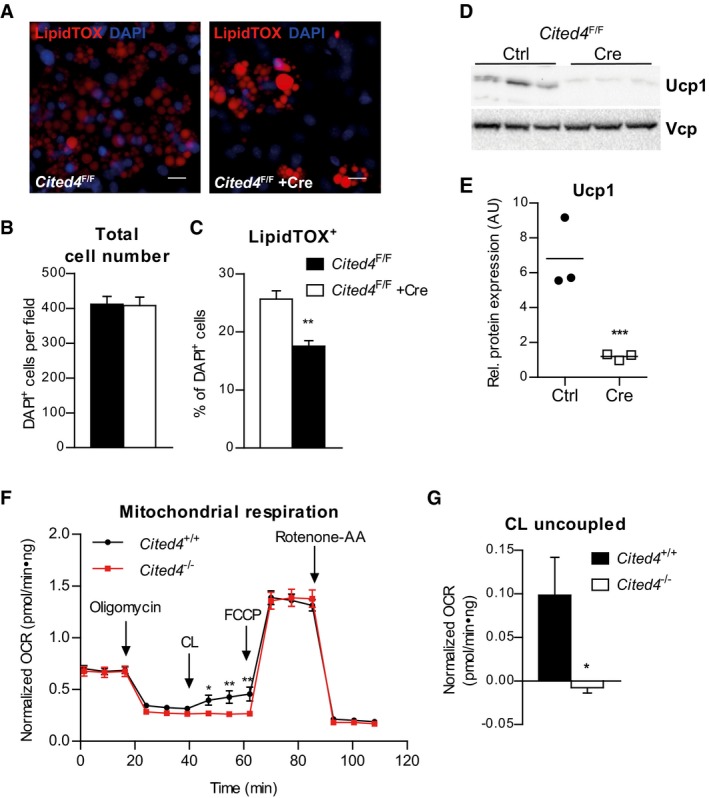
We went on to interrogate the murine phenotype and tested whether it was due to a cell‐autonomous function of Cited4 in progenitors. To this end, we transfected Lin−Sca1+ cells from Cited4 F/F mice with Cre recombinase prior to differentiation induction, which resulted in the efficient disruption of the floxed Cited4 alleles and loss of expression (Fig EV2E and F). In resemblance with the constitutive knockout, Cre‐transfected cells showed reduced expression of Ucp1, Cpt1b, and Dio2 upon differentiation, with no effect on general adipogenic markers (Fig EV2F). In contrast, transfection of Cited4 F/F cells with Cre after differentiation induction did not have any considerable effects on differentiation markers (Fig EV2G), suggesting that Cited4 exerts its essential function in immature progenitors. We further analyzed the consequences of Cited4 deficiency in immature cells for differentiation by immunofluorescence. Whereas there was no difference between genotypes in the total cell number, we observed a reduction in the number of LipidTOX+ adipocytes in Cited4 F/F + Cre cells by 1/3 (Fig 2A–C). This could be due to the preferential loss of beige adipocytes, given the lack of robust effects on general adipogenic markers. Accordingly, Western blotting revealed a robust reduction in Ucp1 protein in Cited4 F/F + Cre cells (Fig 2D and E). Finally, we assessed whether Cited4 knockout could affect mitochondrial respiration as a key function of beige adipocyte metabolism. Basal and maximal mitochondrial respiration was indistinguishable between genotypes arguing against reduced mitochondrial content or a general defect in mitochondrial oxidation (Fig 2F). Treatment with the β3‐adrenoreceptor agonist CL‐316,243 (CL), which stimulates Ucp1 activity and mitochondrial uncoupling, increased oxygen consumption in wild‐type cells. This was abolished in Cited4‐knockout cells, suggesting a specific defect in uncoupled respiration (Fig 2F and G).
Figure 2. Cited4 deficiency in progenitors affects Ucp1 protein expression and β3‐adrenoreceptor mediated uncoupled respiration.
-
A–CQuantitative fluorescence microscopy of LipidTOX‐ and DAPI‐stained female Cited4 F/F Lin−Sca1+ progenitor cells transfected with Cre or control mRNA prior to differentiation in the presence of 100 nM Rosi for 8 days (n = 5). **P = 0.002 in t‐test (Cre vs. Ctrl). Scale bar is 10 μm.
-
D, EUcp1 expression in female Cited4 F/F Lin−Sca1+ progenitor cells transfected with Cre or control mRNA prior to differentiation in the presence of 100 nM Rosi for 8 days, as determined by Western blot with VCP as loading control (n = 3). ***P = 0.0008 in t‐test (Cre vs. Ctrl).
-
FCellular respiration in female Lin−Sca1+ progenitor cells differentiated in the presence of 100 nM Rosi for 8 days. The extracellular oxygen consumption rate (OCR) was determined upon injection of the indicated substances and normalized to DNA content. CL: CL‐316243. Representative experiment (n = 9). *P = 0.041 (47 min), **P = 0.009 (55 min), **P = 0.003 (62 min) in 2 × 2 ANOVA with Holm–Sidak posttests (Cited4 −/− vs. Cited4 +/+).
-
GNormalized CL‐stimulated uncoupled respiration of cells in (F). Values represent the means of the three time points after CL injection after subtraction of the means of the oligomycin time points (n = 9). *P = 0.027 in t‐test (Cited4 −/− vs. Cited4 +/+).
Taken together, these findings argue for an essential function of Cited4 in the response of adipocyte progenitors to TZD stimulation and in particular in the Rosi‐mediated induction of Ucp1.
Cited4 promotes the transcriptional response to rosiglitazone in adipocyte progenitors
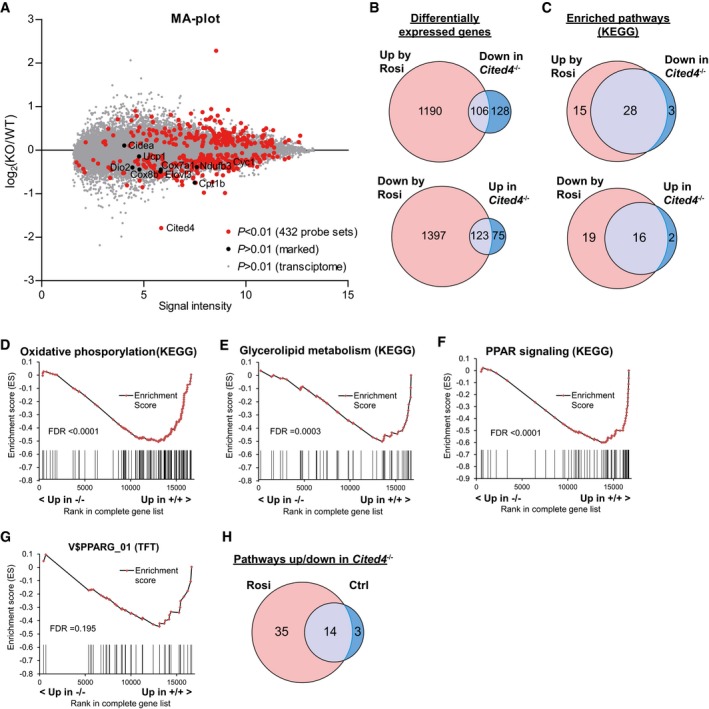
To address the transcriptional pathways regulated by Cited4 in adipocyte progenitors under Rosi stimulation, we performed expression profiling 2 days after differentiation induction at the peak of Rosi‐mediated Cited4 expression (Fig 1B). Ranking of the probe sets/genes by statistical significance resulted in 432 genes with P < 0.01 for differential expression between Cited4 −/− and Cited4 +/+ cells, with 234 down‐ and 198 upregulated genes in Cited4 −/− cells. Thermogenic and mitochondrial/oxidative marker genes clustered in the downregulated fraction (Fig 3A). However, besides Cited4 and Cyc1, these genes were either expressed at low levels at day 2 (Ucp1, Cidea, Dio2) or showed P > 0.01 for differential expression. Although a substantial proportion of the Cited4‐dependent genes (P < 0.01) were regulated by Rosi (P < 0.01 in Rosi vs. Ctrl in wild‐type cells), they represented only a minor fraction of the Rosi‐regulated transcriptome (Fig 3B). In contrast, our investigation at the level of biological pathways using gene set enrichment analysis (GSEA) showed a substantial overlap between KEGG pathways significantly induced by Rosi and pathways reduced by Cited4 knockout, and vice versa (Fig 3C). In accordance with the phenotype observed at the end of differentiation (Figs 1 and 2), several mitochondrial and lipid metabolism pathways were enriched in the gene fraction downregulated by Cited4 knockout (Fig 3D and E; Appendix Fig S2). In particular, the concerted reduction of genes for oxidative phosphorylation in Rosi‐treated Cited4 −/− cells suggests that Cited4 may promote the induction of oxidative/thermogenic genes at the onset of the differentiation process. In addition, the “PPAR signaling” gene set was among the Cited4‐dependent gene sets, and furthermore, PPARG was the 2nd highest ranking downregulated gene set in the GSEA for transcription factor motif enrichment (Fig 3F and G; Appendix Table S1). Overall, Rosi treatment doubled the number of pathways significantly affected by Cited4 knockout (Fig 3H). Taken together, the strong interaction between the Cited4‐ and Rosi/PPARg‐dependent transcriptional programs suggests that Cited4 has a specific role in immature progenitors to prime parts of the transcriptional response to Rosi treatment, particularly genes related to beige adipocyte function.
Figure 3. Cited4‐ and Rosi/PPARg‐dependent transcriptional programs overlap in differentiating adipocyte progenitors.
-
AMA‐plot of RNA expression profiles from female Lin−Sca1+ progenitors 2 days after induction of differentiation with 100 nM Rosi, displaying the log2‐ratio of Cited4 −/− to Cited4 +/+ intensities against the average log2‐intensities for all microarray probe sets (n = 3). t‐test with Welch's correction on Cited4 −/− vs. Cited4 +/+ (Rosi).
-
BComparison of the lists of genes significantly changed by Rosi in wild‐type cells (Cited4 +/+: Rosi vs. Ctrl, P < 0.01) or by Cited4‐knockout under Rosi treatment (Rosi: Cited4 −/− vs. Cited4 +/+, P < 0.01) in expression profiles from female Lin−Sca1+ progenitors 2 days after induction of differentiation with 100 nM Rosi or vehicle (n = 3, t‐test with Welch's correction).
-
CComparison of the lists of gene sets significantly enriched by Rosi in wild‐type cells (Cited4 +/+: Rosi vs. Ctrl, false discovery rate (FDR) <0.1) or by Cited4‐knockout under Rosi treatment (Rosi: Cited4 −/− vs. Cited4 +/+, FDR < 0.1) in GSEA (KEGG) (n = 3).
-
D–GEnrichment plots from GSEA with the KEGG (D–F) or TFT (G) gene set collection (Cited4 −/− vs. Cited4 +/+), performed on RNA expression profiles 2 days after induction of differentiation with 100 nM Rosi (n = 3). Vertical bars represent the individual genes of the gene set.
-
HComparison of the lists of gene sets affected by Cited4‐knockout under Rosi or under vehicle treatment (Cited4 −/− vs. Cited4 +/+, FDR < 0.1) in GSEA as in (C).
Cited4 is an essential regulator of thermogenic expression specific to rosiglitazone and subcutaneous fat
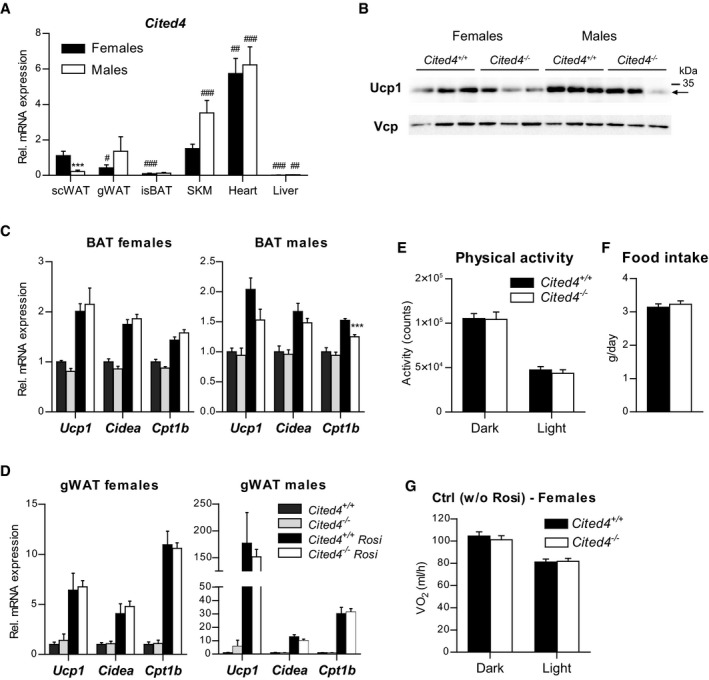
To determine the involvement of Cited4 in the Rosi response in vivo, we investigated the phenotype of Cited4 −/− mice. The highest Cited4 mRNA levels were detected in the heart of both female and male mice (Fig EV3A). In female mice, subcutaneous fat (scWAT) showed comparable levels to skeletal muscle and the highest expression among the fat depots examined including interscapular (BAT) and gonadal (gWAT) fat. Interestingly, Cited4 expression in scWAT was markedly elevated in female versus male mice, whereas no significant sex‐specific differences were observed in other tissues. Cited4 protein was detectable in the heart of wild type but not Cited4 −/− mice (Appendix Fig S1C). Protein expression was faintly detected in female wild‐type scWAT but was clearly observed in scWAT‐derived Lin−Sca1+ progenitor cells (Appendix Fig S1B and D). Examination of Cited4 −/− mice revealed no difference in body weight and body fat compared to wild type at the onset of adulthood (Appendix Fig S3A). Despite the relatively high expression levels, no physiologically relevant phenotypic effects were observed on heart weight, pulse, or blood pressure (Appendix Table S2).
Figure EV3. Cited4 deficiency specifically affects rosiglitazone‐mediated thermogenic expression in subcutaneous fat.
-
AmRNA expression in wild‐type mice without treatment, as determined by qRT–PCR (n = 6). scWAT: subcutaneous white adipose tissue; gWAT: gonadal WAT; isBAT: interscapular brown adipose tissue; SKM: gastrocnemius skeletal muscle. 2 × 2 ANOVA with Holm–Sidak posttests, ***P = 0.0004 (female vs. male), ### P = 0.0004, ### P = 7 × 10−6, ### P = 6 × 10−9, ### P = 3 × 10−10, (isBAT, SKM, heart, liver, respectively, vs. scWAT within indicated sex), # P = 0.032 (females: gWAT vs. scWAT), ## P = 0.006 (females: Heart vs. scWAT), ## P = 0.006 (males: Liver vs. scWAT).
-
BUcp1 expression in scWAT of mice fed a diet with 0.0075% Rosi or control diet for 2.5 weeks, determined by Western blot with VCP as loading control (n = 3, same samples as in Fig 4C and D).
-
CmRNA expression in BAT of mice treated as in (A), determined by qRT–PCR. t‐test Cited4 −/− vs. Cited4 +/+ (Rosi), females: n = 5/5/6/6, males: n = 5/4/5/5, ***P = 0.0004.
-
DmRNA expression in gWAT of mice treated as in (A), determined by qRT–PCR. t‐test Cited4 −/− vs. Cited4 +/+ (Rosi), females: n = 5/5/6/6, males: n = 5/4/5/5.
-
EThree‐day averages of total activity counts per day and mouse of female mice fed a diet with 0.0075% Rosi for 2.5 weeks, determined by Phenomaster (n = 9/10, t‐test).
-
FThree‐day averages of food intake per day and mouse of female mice shown in (E) (n = 9/10, t‐test).
-
GOxygen consumption rate of female mice fed control diet, determined by indirect calorimetry and adjusted for body weight by ANCOVA. Three‐day averages of VO2 were calculated for each mouse (n = 10). ANCOVA with Bonferroni's posttest (Cited4 −/− vs. Cited4 +/+).
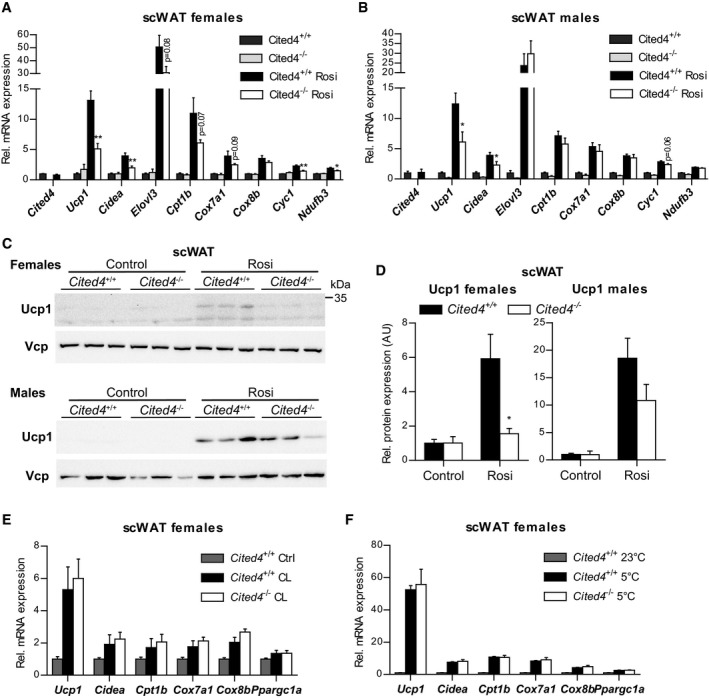
Upon 2.5 weeks of Rosi treatment, female mice did not show differential fat accumulation between genotypes, whereas male Cited4 −/− mice showed mildly increased weight in some fat depots but generally independent of Rosi (Appendix Fig S3B and C). Importantly, there were no differences in the size or number of adipocytes between genotypes, indicating that general adipogenesis and adipocyte turnover were not affected by Cited4 inactivation (Appendix Fig S3D–G). Cited4 expression in scWAT was not influenced by 2.5 weeks of Rosi treatment, which could be related to progenitor‐selective or an early transient induction (Fig 4A). Rosi induced thermogenic gene expression in subcutaneous fat of wild‐type mice but intriguingly, female Cited4−/− mice showed a 2.5‐fold reduction in Ucp1 expression (Fig 4A), which resulted in markedly compromised Ucp1 protein expression (Fig 4C and D). Further thermogenic markers were affected, including components of the oxidative phosphorylation system (Cyc1, Cox7a1, Ndufb3), mirroring the ex vivo progenitor phenotype. In male knockout mice, thermogenic gene expression was reduced but overall to a lesser extent compared to females (Fig 4B). Although Ucp1 mRNA was significantly lower in Cited4 −/− males, Ucp1 protein only showed a trend of reduction, which could be due to the weaker correlation between Ucp1 mRNA and protein in male compared to female mice (Figs 4C and D, and EV3B; Appendix Fig S4A). Examination of the interscapular (BAT) and gonadal (gWAT) fat depots did not yield any consistent effect of Cited4 inactivation on thermogenic gene expression (Fig EV3C and D). The question emerged whether Cited4 is generally essential for thermogenic expression and browning in scWAT. Neither a 10‐day treatment with the β3‐adrenoreceptor agonist CL‐316,243 (CL) nor a 2‐week exposure to 5°C resulted in any differences in thermogenic marker expression in scWAT (Fig 4E and F). In conclusion, the data suggest that Cited4 has an essential function in promoting thermogenic expression in scWAT specifically in response to TZD‐mediated PPARg activation and in a sex‐biased manner.
Figure 4. Cited4 deficiency specifically affects rosiglitazone‐mediated thermogenic expression in subcutaneous fat.
-
A, BmRNA expression in scWAT of mice fed a diet with 0.0075% Rosi or control diet for 2.5 weeks, determined by qRT–PCR. t‐test Cited4 −/− vs. Cited4 +/+ (Rosi), (A) n = 5/5/6/6, **P = 0.003 (Ucp1), **P = 0.008 (Cidea), **P = 0.007 (Cyc1), *P = 0.048 (Ndufb3), (B) n = 5/4/5/5, *P = 0.022 (Ucp1), *P = 0.037 (Cidea).
-
C, DUcp1 expression in scWAT of mice treated as in (A), as determined by Western blot with VCP as loading control. t‐test Cited4 −/− vs. Cited4 +/+ (Rosi), females: n = 5/5/6/6, *P = 0.011, males: n = 5/4/5/5.
-
EmRNA expression in scWAT of female mice treated with CL‐316,243 (CL) (1 mg/kg/day via Alzet minipumps) or vehicle for 10 days (n = 5/7/13, t‐test Cited4 −/− vs. Cited4 +/+ (CL)).
-
FmRNA expression in scWAT of female mice exposed to 5°C or 23°C for 2 weeks (n = 5/10/6, t‐test Cited4 −/− vs. Cited4 +/+ (5°C)).
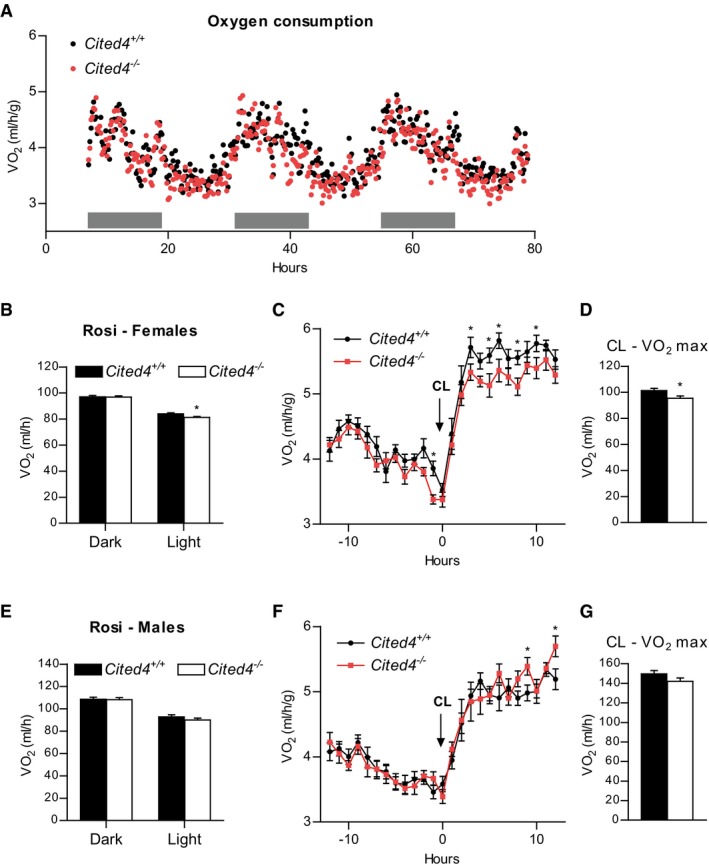
We next asked whether the compromised Rosi‐dependent thermogenic expression in scWAT of Cited4‐knockout mice could have consequences for systemic energy expenditure. Indirect calorimetry after 2.5 weeks of Rosi treatment revealed a moderate but significant reduction in the oxygen consumption of Cited4 −/− females in the light phase compared to wild types in the absence of differences in physical activity or food intake (Figs 5A and B, and EV3E and F). This effect was not observed in females on control diet (Fig EV3G). Importantly, a significant reduction could be detected in maximal oxygen consumption upon a single CL injection of Rosi‐treated mice, which specifically stimulates brown/beige adipocyte respiration and thermogenesis (Fig 5C and D). In contrast, Cited4 −/− males showed indistinguishable oxygen consumption under Rosi treatment, both in steady state and upon CL injection (Fig 5E–G). Taken together, the Cited4‐dependent regulation of thermogenic expression in scWAT is likely to be relevant for the systemic capacity for adipose tissue thermogenesis and therefore systemic metabolism in female but not male mice.
Figure 5. Cited4 deficiency affects energy expenditure under rosiglitazone treatment in female mice.
-
AOxygen consumption rate of female mice fed a diet with 0.0075% Rosi for 2.5 weeks, determined by indirect calorimetry and normalized to body weight (n = 9/10). Gray bars represent the dark phase.
-
BOxygen consumption rate of female mice shown in (A) adjusted for body weight by ANCOVA. Three‐day averages of VO2 were calculated for each mouse (n = 9/10). *P = 0.025 in ANCOVA with Bonferroni's posttest (Cited4 −/− vs. Cited4 +/+).
-
COxygen consumption rate of female mice fed a diet with 0.0075% Rosi for 3.5 weeks and injected with 1 mg/kg CL‐316,243 (CL) at the indicated time point, as determined by indirect calorimetry (n = 9/10). *P = 0.013 (−1 h), *P = 0.045 (3 h), *P = 0.015 (5 h), *P = 0.016 (6 h), *P = 0.019 (8 h), *P = 0.046 (10 h) in repeated‐measures 2 × 2 ANOVA with Holm–Sidak posttests (Cited4 −/− vs. Cited4 +/+).
-
DMaximal CL‐induced oxygen consumption rate of female mice shown in (C) adjusted for body weight by ANCOVA. Averages of VO2 throughout 3–8 h post‐CL injection were calculated for each mouse (n = 9/10). *P = 0.049 in ANCOVA with Bonferroni's posttest (Cited4 −/− vs. Cited4 +/+).
-
EOxygen consumption rate of male mice treated as in (A), determined as in (C) (n = 9/10). ANCOVA with Bonferroni's posttest (Cited4 −/− vs. Cited4 +/+).
-
FOxygen consumption rate of male mice treated as in (C), determined as in (C) (n = 9/10). *P = 0.044 (9 h), *P = 0.013 (12 h) in repeated‐measures 2 × 2 ANOVA with Holm–Sidak posttests (Cited4 −/− vs. Cited4 +/+).
-
GMaximal CL‐induced oxygen consumption rate of male mice shown in (F) and calculated as in (D) (n = 9/10). ANCOVA with Bonferroni's posttest (Cited4 −/− vs. Cited4 +/+).
Cited4 promotes thermogenic expression and insulin sensitization during therapeutic rosiglitazone treatment in females
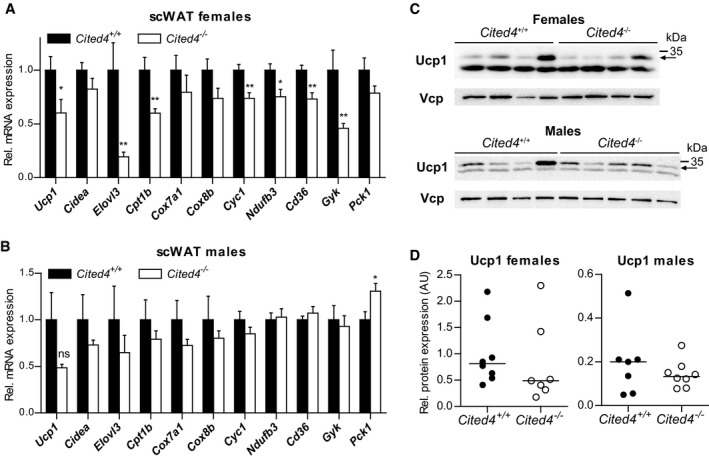
Adipose tissue and scWAT are considered to be key targets of TZDs for insulin sensitization (Soccio et al, 2014, 2017). We sought to determine whether Cited4 is involved in therapeutic Rosi‐mediated insulin sensitization and how this associates with the regulation of thermogenic capacity by Cited4 in scWAT. To this end, we fed mice with high‐fat diet (HFD) for 16–20 weeks including Rosi treatment in the last 5 weeks. HFD resulted in a 10–15 g gain of body fat mass by week 9 but there were no significant differences in fat accumulation between genotypes either before or after Rosi treatment (Appendix Fig S5A–C) and this was also reflected at the organ level (Appendix Fig S5D and E). Consistent with the phenotype of Rosi‐treated mice on normal diet (Fig 4A), key thermogenic and mitochondrial marker genes were expressed at lower levels in scWAT of female Cited4‐knockout mice, whereas the effects were not significant in male mice (Fig 6A and B). Ucp1 protein expression showed a trend of reduction in Cited4 knockout females but not in males (Fig 6C and D; Appendix Fig S5F). In addition, the expression of Cd36 and Gyk was affected by Cited4 inactivation in females, representing TZD target genes with key functions in lipid metabolism and thermogenic adipocyte function (Rangwala & Lazar, 2004).
Figure 6. Sex‐specific involvement of Cited4 in thermogenic expression upon therapeutic rosiglitazone treatment.
-
A, BmRNA expression in scWAT of mice fed a high‐fat diet (HFD) for 11 weeks followed by 5 weeks of HFD with 0.0075% Rosi (qRT–PCR). t‐test Cited4 −/− vs. Cited4 +/+, (A) n = 8/7, *P = 0.029 (Ucp1), **P = 0.009 (Elovl3), **P = 0.006 (Cpt1b), **P = 0.004 (Cyc1), *P = 0.016 (Ndufb3), **P = 0.003 (Cd36), **P = 0.009 (Gyk), (B) n = 7/8, *P = 0.026 (Pck1).
-
C, DUcp1 protein expression in scWAT of mice treated as in (A), determined by Western blot with VCP as loading control (n = 8/7 for females and n = 7/8 for males). Arrow indicates the band specific to Ucp1 (see Appendix Fig S5F ).
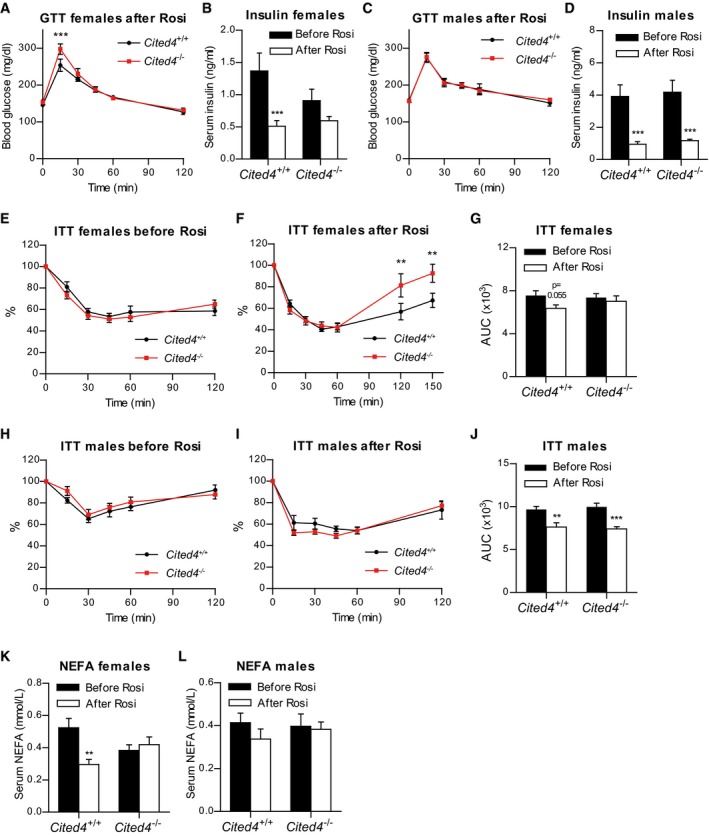
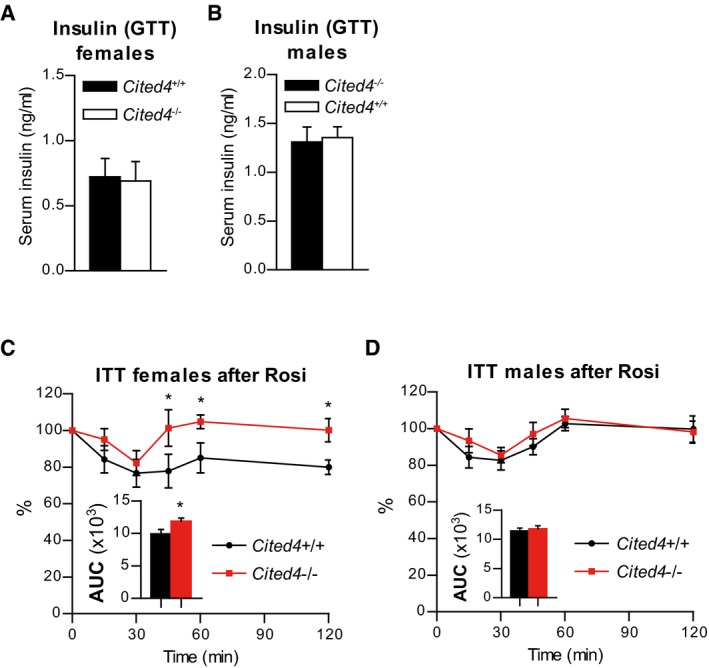
Glucose tolerance was mildly but significantly reduced in HFD‐fed Cited4 −/− females upon Rosi treatment but without a concomitant difference in serum insulin in response to glucose, excluding a gross pancreatic defect (Figs 7A and EV4A). In contrast, the beneficial effects of Rosi on fasting insulin were affected by Cited4 inactivation. Rosi treatment strongly reduced fasting insulin levels in wild type but not in Cited4 −/− females (Fig 7B). Post‐Rosi glucose tolerance and the insulin‐lowering effects of Rosi were intact in Cited4 −/− males (Figs 7C and D, and EV4B). To test whether the observed phenotypic differences in glucose tolerance and fasting insulin could be related to compromised insulin sensitization by Rosi in Cited4 knockout females, we performed insulin tolerance tests (ITT). Whereas insulin tolerance was not different between genotypes before Rosi treatment, Cited4 −/− mice had higher blood glucose levels in the rebound phase of the test (Fig 7E and F), which could be confirmed in an ITT with a lower insulin dose (Fig EV4C). Furthermore, the improvement in the overall response of wild‐type mice to insulin through Rosi treatment (AUC, P = 0.055) was not observed in Cited4 −/− mice (AUC, P = 0.568; Fig 7G). Consistently, there were no genotype effects detected in male mice undergoing the same tests (Figs 7H–J and EV4D). Finally, we observed that the reduction of circulating non‐esterified fatty acids (NEFA) in wild‐type females by Rosi did not occur in Cited4 −/− mice (Fig 7K and L), providing a possible link between lipid metabolism and insulin sensitization. In conclusion, Cited4 deficiency revealed an intriguing sex‐specific association between the enhancement of thermogenic capacity and insulin sensitization by therapeutic Rosi treatment.
Figure 7. Sex‐specific involvement of Cited4 in insulin sensitization upon therapeutic rosiglitazone treatment.
-
ABlood glucose during glucose tolerance test (GTT) on female mice after 11 weeks of HFD and 3 weeks of HFD + Rosi (n = 8). ***P = 0.001, in repeated‐measures 2 × 2 ANOVA with Holm–Sidak posttests (Cited4 −/− vs. Cited4 +/+).
-
BFasting serum insulin in female mice after 10 weeks of HFD (“Before Rosi”) or 11 weeks of HFD and 2.5 weeks of HFD+Rosi (“After Rosi”) (n = 8). ***P = 0.0009 in repeated‐measures 2 × 2 ANOVA with Holm–Sidak posttests (After Rosi vs. Before Rosi); P = 0.725 (Cited4 −/− vs. Cited4 +/+ Before Rosi).
-
CBlood glucose during GTT on male mice as in (A) (n = 5/8). Repeated‐measures 2 × 2 ANOVA with Holm–Sidak posttests (Cited4 −/− vs. Cited4 +/+).
-
DFasting serum insulin in male mice as in (C) (n = 7/7/8/8). ***P = 0.001 (Cited4 +/+), ***P = 0.0008 (Cited4 −/−) in repeated‐measures 2 × 2 ANOVA with Holm–Sidak posttests (After Rosi vs. Before Rosi).
-
E, FBlood glucose during insulin tolerance test (ITT) with 1 U insulin per kg body mass on female mice after 12 weeks of HFD (n = 8) (E) or 16 weeks of HFD and 4 weeks of HFD + Rosi (n = 7) (F), expressed as % of the 0‐time point value. **P = 0.003 in repeated‐measures 2 × 2 ANOVA with Holm–Sidak posttests (Cited4 −/− vs. Cited4 +/+).
-
GArea under the curve (AUC) of blood glucose during the ITT (0‐120 min) in (E,F). 2 × 2 ANOVA with Holm–Sidak posttests (After Rosi vs. Before Rosi).
-
H, IBlood glucose during insulin tolerance test (ITT) on male mice as in (E, F), n = 8 (H), n = 7/8 (I). Repeated‐measures 2 × 2 ANOVA with Holm–Sidak posttests (Cited4 −/− vs. Cited4 +/+).
-
JArea under the curve (AUC) of blood glucose during the ITT in (H, J). **P = 0.001, ***P = 0.00006 in 2 × 2 ANOVA with Holm–Sidak posttests (After Rosi vs. Before Rosi).
-
K, LFasting serum non‐esterified fatty acids (NEFA) in female (K) or male (L) mice after 10 weeks of HFD (“Before Rosi”) or 11 weeks of HFD and 2.5 weeks of HFD + Rosi (“After Rosi”), n = 7/8/8/8 (K), n = 6/6/6/8 (L). **P = 0.001 in 2 × 2 ANOVA with Holm–Sidak posttests (After Rosi vs. Before Rosi).
Figure EV4. Sex‐specific involvement of Cited4 insulin sensitization upon therapeutic rosiglitazone treatment.
-
A, BSerum insulin concentration 60 min after glucose injection in the GTT in mice after 11 weeks of HFD and 4 weeks of HFD + Rosi. t‐test Cited4 −/− vs. Cited4 +/+, (A) n = 8, (B) n = 7/8.
-
CBlood glucose during insulin tolerance test (ITT) with 1 U insulin per kg lean mass on female mice after 11 weeks of HFD and 4 weeks of HFD + Rosi, expressed as % of the 0‐time point value (n = 8). *P = 0.015 (45 min), *P = 0.039 (60 min), *P = 0.035 (120 min) in repeated‐measures 2 × 2 ANOVA with Holm–Sidak posttests; Inlet: AUC t‐test Cited4 −/− vs. Cited4 +/+ *P = 0.041.
-
DBlood glucose during ITT on male mice as in (C) (n = 7/8). Repeated‐measures 2 × 2 ANOVA with Holm–Sidak posttests; Inlet: AUC t‐test.
Discussion
Targeting adipose tissue and in particular specific functions of PPARg is a promising approach for the treatment of insulin resistance and type 2 diabetes but requires better understanding of the in vivo regulation of PPARg responses. Here, we report Cited4 as a context‐specific mediator of the TZD response. Cited4 was required for the proper induction of thermogenic expression specifically in scWAT of female mice, an effect which could be attributed to the regulation of the Rosi‐mediated transcriptional program in adipocyte progenitors. Intriguingly, this was accompanied by reduced capacity for thermogenesis in vivo as well as compromised therapeutic insulin sensitization. The transcriptional network around PPARg controlling adipogenesis and thermogenic adipocyte function has been characterized to great detail (Lefterova et al, 2014; Inagaki et al, 2016). However, the molecular factors linking these core processes to the sex‐, depot‐, and stimulus‐dependent differences in adipose tissue composition and metabolism are only beginning to be elucidated.
Extensive evidence suggests that adipose tissue is probably the major target of TZDs for insulin sensitization [reviewed in Ahmadian et al (2013), Soccio et al (2014)]. Given that compromised insulin sensitivity in Cited4 −/− mice on HFD was only observed after Rosi treatment, it appears plausible that the insulin‐sensitizing function of Cited4 occurs in adipose tissue. However, Cited4 was expressed at comparable levels in skeletal muscle, which is a target of TZDs responding with improved insulin sensitivity and mitochondrial oxidative potential (Kim et al, 2003; Schrauwen et al, 2006; Mensink et al, 2007; Liu et al, 2009). Although the conclusions from two mouse models with muscle‐specific Pparg inactivation are contradictory, skeletal muscle Cited4 could contribute to systemic insulin sensitization by Rosi (Hevener et al, 2003; Norris et al, 2003). Thus, it is not possible to exclude relevant Cited4 functions outside adipose tissue in the absence of adipocyte progenitor‐specific Cited4 inactivation. Nevertheless, the effects of Cited4 deficiency on energy expenditure are in support of the importance of adipose tissue Cited4 function. Cited4 −/− mice had reduced energy expenditure only under Rosi treatment and showed reduced maximal β3‐agonist‐stimulated oxygen consumption, which is generally attributed to adipose tissue thermogenesis. Furthermore, the effects on energy expenditure were absent in male Cited4 knockout mice, in which insulin sensitization was intact.
The associations of impaired scWAT function with effects on energy balance and insulin sensitivity in Rosi‐treated Cited4‐knockout mice are consistent with previous reports demonstrating the key role of subcutaneous fat for the therapeutic action of TZDs. This was evident in humans and rodents both at the level of gene expression and browning and at the level of tissue metabolism (Boden et al, 2005; Bogacka et al, 2005; Festuccia et al, 2009; Soccio et al, 2017). Obesity is associated with reduced capacity or compromised mitochondrial oxidation in adipose tissue and the ability of TZDs to restore these defects is likely to be important for insulin sensitization, possibly through the improvement of systemic lipid metabolism (Wilson‐Fritch et al, 2004; Rong et al, 2007; Soccio et al, 2017). To which extent the effects of TZDs on Ucp1 expression and uncoupled respiration are contributing to therapeutic action remains to be formally proven. Although TZDs do not induce thermogenesis per se, PPARg agonism has been shown to increase the capacity for adipose tissue thermogenesis (Sell et al, 2004). In Cited4‐knockout mice, the reduction in energy expenditure under Rosi treatment was modest, but of similar magnitude to mouse models with specific defects in scWAT browning (Cohen et al, 2014).
Cited4 deficiency did not affect thermogenic expression in scWAT under conditions of prolonged exposure to cold or β3‐adrenoreceptor agonist. This implies the existence of different modes of regulation for the induction of thermogenic expression in white fat depending on the stimulus. In line with this notion, the Farmer lab demonstrated the emergence of Ucp1‐expressing adipocytes with distinct nature upon β‐adrenergic versus Rosi/roscovitine stimulation (Wang et al, 2016). The observed differences may be due to different routes of recruitment of the thermogenic adipocytes, i.e., de novo differentiation from progenitor cells versus conversion or activation of pre‐existing adipocytes. In any case, we propose that Cited4 is involved in Rosi‐mediated beige adipocyte differentiation from progenitors, as concluded from its expression pattern and the differential phenotypes upon inactivation in immature versus committed cells. Is Cited4 important for white adipocyte formation? Although an effect on the number of lipid‐containing cells was observed ex vivo, Cited4 deficiency did not affect general adipogenic gene expression or the number of adipocytes upon Rosi treatment in vivo. Importantly, Cited4 knockout mice showed indistinguishable fat accumulation upon HFD or Rosi treatment, both potent inducers of adipogenesis. It is noteworthy though that in human SVF cells, mRNA expression of general adipogenic markers was affected to a small degree upon CITED4 knockdown. A further discordance between the human and mouse cell phenotypes was the sex‐independent reduction of UCP1 expression in human cells with CITED4 knockdown. How this applies to broader human populations and TZD‐treated patients remains to be determined, but it may relate to the strong dependency of human cells on PPARg agonists for adipocyte differentiation in culture.
In the mouse, Cited4 deficiency predominantly or exclusively affected female scWAT. Male Cited4 −/− mice showed decreases in thermogenic marker gene expression in the steady state and trends of reduction under Rosi treatment. However, the relevance of these effects is questionable since (i) Ucp1 protein could not be detected in scWAT in steady state and (ii) there was no association with reduced energy expenditure or insulin sensitivity under Rosi, in contrast to females. It is noteworthy that the inguinal scWAT depot examined here contains a mammary gland and therefore has high remodeling potential in females. In general, sex differences are characteristic for the subcutaneous fat depot including the differential regulation of progenitor cells (Fried et al, 2015; Jeffery et al, 2016), but little is known in relation to browning and the TZD response (Benz et al, 2012; Fried et al, 2015). Estrogen signaling has been implicated in the regulation of thermogenic adipose tissue expression with both central nervous system and local effects in adipocyte progenitor cells (Lapid et al, 2014; Martinez de Morentin et al, 2014). For instance, it was shown that the induction of Ucp1 by CL‐316,243 in mouse gWAT was compromised by experimental ovarian failure (Kim et al, 2016). Interestingly, the estrogen receptor is able to interfere with the activity of PPARg and thereby influence PPARg‐dependent metabolic processes (Foryst‐Ludwig et al, 2008; Benz et al, 2012). In this light, a functional or physical interaction of Cited4 with estrogen receptors is conceivable. Cited4 and both additional members of the Cited family, Cited1 and Cited2, have been shown to act as coactivators of the estrogen receptor (Yahata et al, 2001; Lau et al, 2013). Notably, Cited1 has been established as a beige adipocyte marker and is induced by Rosi during progenitor differentiation, which raises the possibility of functional compensation by Cited1 in models of Cited4 gene inactivation (Sharp et al, 2012). In any case, the molecular link of Cited4 to the PPARg/TZD transcriptional program could be mediated by direct modulation of PPARg or its cofactors, since this has been observed for Cited2 (Tien et al, 2004; Gonzalez et al, 2008; Sakai et al, 2012).
How Cited4 regulates the TZD response of scWAT sex‐specifically at the molecular level and what the implications are for sex‐dependent therapeutic efficacy of TZDs in patients remains to be determined. Uncovering how context‐specific differential regulation of distinct TZD responses is mediated by Cited4 may open up new possibilities for exploiting and personalizing PPARg modulation in type 2 diabetes or prediabetes.
Materials and Methods
Mice
Animal handling and experimentation were performed in accordance with the European Union directives and the German animal welfare act (Tierschutzgesetz). All procedures were approved by local authorities (Regierungspräsidium Karlsruhe). Seven‐ to twelve‐week‐old mice were housed in an environmentally controlled room at 22°C on 12‐h light/dark cycle and given ad libitum access to food and water. Unless otherwise indicated, mice were fed standard chow diet (Art. No. 3437, Provini Kliba AG, Kaiseraugst, Switzerland). Mouse strains used included C57BL/6N, NMRI (Charles River WIGA GmbH, Sulzfeld, Germany), Cited4 conditional knockout (Cited4 F/F), and Cited4 constitutive knockout (Cited4 −/−) mice. The Cited4 F/F line was generated by TaconicArtemis (now Taconic Biosciences, Cologne, Germany) and the GSF (now Helmholtz Zentrum München, Germany). Briefly, the mouse Cited4 ORF was subcloned using RP23 BAC library and recloned into the basic targeting vector harboring a neomycin selection cassette flanked by flp sites, a thymidine kinase selection cassette as well as two loxP sites enclosing the Cited4 ORF and the Neo cassette. Targeting was performed in C57BL/6N ES cells and confirmed by Southern blotting. Chimeric mice resulting from blastocyst injection were mated with C57BL/6N Tg(ACTB‐Flpe) mice to obtain transgenic founders with excised Neo cassette. Cited4 +/F mice were crossed to Gt(ROSA)26Sortm16(cre)Arte mice to obtain the Cited4 ‐ null allele. Genotyping was performed by PCR with the following primers: 5′‐AAGATCCAGGCAGCCCTAGC‐3′ (Oligo 1; for the detection of the floxed allele) or 5′‐TAACCACTGCCAAACGATGG‐3′ (Oligo 3; for the detection of knockout allele) (forward) and 5′‐CCAACTAGCTGAACCTATTCC‐3′ (Oligo 2; reverse). The WT allele generated a band at 359 bp and the floxed allele at 478 bp (Oligos 1/2), whereas the knockout allele was detected at 389 bp (Oligos 3/2; Appendix Fig S1A).
Rosiglitazone treatment was performed by feeding D12450B control diet or D12492 high‐fat diet (60% kcal from fat) each supplemented with 0.0075% rosiglitazone (Biomol/Cayman, Hamburg, Germany) resulting in an estimated daily dose of 8–9 mg/kg body weight. The diets incl. the non‐supplemented ones were manufactured by Research Diets, New Brunswick, NJ. CL‐316,243 (Tocris Bioscience, Bristol, UK) was administered by subcutaneously implanted ALZET® minipumps (DURECT Corporation, Cupertino, CA) at 1 mg/kg per day for 10 days. Body composition was analyzed by NMR using the EchoMRI analyzer (EchoMRI, Echo Medical Systems, Houston, TX). Oxygen consumption (indirect calorimetry), food intake, and locomotor activity measurements were performed with individually housed mice at 22°C in the PhenoMaster Home Cage System (TSE system, Bad Homburg, Germany). Intraperitoneal glucose tolerance test (GTT) was conducted by injecting the mice with 2 g glucose (Sigma‐Aldrich, Munich, Germany) per kg body weight after a 5‐h fast. Insulin tolerance test (ITT) was performed by injecting intraperitoneally 1 U insulin (HUMINSULIN®; Lilly, Bad Homburg, Germany) per kg body mass or lean mass as indicated after a 5‐h fast. Blood glucose was measured with an Accu‐Check glucometer (Roche Diagnostics, Mannheim, Germany). Non‐esterified fatty acids (NEFA) and insulin were determined in serum samples using the NEFA‐HR (2) (Wako Diagnostics, Neuss, Germany) and the ALPCO Insulin ELISA (BioCat, Heidelberg, Germany) kits, respectively.
Assessment of mouse cardiovascular function
Mice were acclimatized to the housing conditions for 4 weeks prior to analysis. The basal cardiovascular functions were assessed by measuring tail‐cuff blood pressure in conscious mice (at 9 weeks of age) using the MC4000 Blood Pressure Analysis Systems (Hatteras Instruments Inc., Cary, North Carolina, USA). Prewarmed metal platforms in metal boxes were used to restrain the animals. The tails were fixed through a tail‐cuff in a notch with an optical path containing an LED light and a photosensor. The blood pulse wave in the tail artery was determined as an optical pulse signal. The system software allowed automated detection of pulse, cuff inflation, and pressure evaluation. Following five initial inflation runs for acclimatization, 12 measurement runs were conducted per animal in one session. Runs with movement artifacts were not included in the analysis. The measurements were taken over four consecutive days (between 8:30 and 11:30 AM) after a day of training for the adaptation of animals to the protocol. Additionally, the heart weight was determined at 15 weeks of age along with body weight and tibia length.
Histology
Dissected tissue was rinsed in PBS, fixed in 4% paraformaldehyde at room temperature for 24 h, embedded in paraffin, cut into series of 5‐μm‐thick sections, and mounted onto glass slides. The section was deparaffinized (xylene), rehydrated (ethanol dilutions), and subjected to antigen retrieval in citrate buffer (pH 6.0) through boiling at 95°C for 20 min. The slides were blocked with 2% BSA in PBS, for 1 h at room temperature followed by overnight incubation at 4°C with rabbit α‐caveolin‐1 (Cav‐1) polyclonal antibody 1:400 in 2% BSA in PBS (Cell Signaling, Danvers, MA). Following washes with PBS, goat α‐rabbit IgG‐Alexa Fluor® 488 secondary antibody at 1:400 dilution in 2% BSA/PBS (Thermo Fisher Scientific, Rockford, IL) was applied for 60 min at room temperature. The sections were washed and mounted with ProLong® Gold Antifade Reagent (Cell Signaling, Danvers, MA) and coverslips. Three to five images per mouse were acquired at 100× magnification using the Zeiss Cell Observer with identical settings and acquisition times. For the calculation of cell surface, images were analyzed with a Fiji software algorithm (ImageJ). Cell mass was calculated from the median cell area per mouse assuming spherical cell shape and adipocyte density of 0.96 g/ml. The number of adipocytes per mg tissue was estimated as 1/(median cell mass) and multiplied by tissue mass to obtain the total number of adipocytes per depot.
SVF preparation from human and mouse adipose tissue
Biopsies from human abdominal subcutaneous fat were collected during bariatric surgery at the Department of Surgery, University Hospital Heidelberg, with approval by the Institutional Review Board of the Medical Faculty of the University of Heidelberg in accordance with the Declaration of Helsinki and its later amendments. Preoperative informed consent was obtained from all patients for the use of samples. Inguinal/gluteal subcutaneous adipose tissue was excised from 7‐ to 8‐week‐old mice. Murine or human fat biopsies were minced with scissors and digested in Hank's balanced salt solution (HBSS; Sigma‐Aldrich, Munich, Germany) containing 0.1 w.u./ml purified collagenase (LS005273; Worthington Biochemical, Lakewood, NJ), 2.4 U/ml purified neutral protease (LS02104; Worthington Biochemical), 4 mM CaCl2, and 0.05 mg/ml DNase I (1284932001; Roche Diagnostics, Grenzach‐Wyhlen, Germany) for 50–60 min at 37°C in a shaker at 70 rpm. The suspensions were strained through a 300‐μm mesh (4‐1411; Neolab, Heidelberg, Germany) and centrifuged at 145 g for 10 min at 20°C to separate the SVF and mature adipocytes. The mature adipocyte fraction was discarded, unless otherwise indicated, and SVF cells were washed in BSA buffer (0.5% BSA, 1 mM EDTA in D‐PBS) and collected by centrifuging at 300 g for 5 min at 20°C.
Isolation of primary adipose progenitors by MACS®
After obtaining the SVF, adipose progenitors were isolated as described previously (Bayindir et al, 2015; Babaei et al, 2017). In brief, the SVF cell pellet was resuspended in the appropriate volume of BSA buffer and preincubated with FcBlock (anti‐CD16/32; eBioscience, Frankfurt, Germany) for 10 minutes on ice. Cells were then stained with biotin‐conjugated lineage antibodies, namely Ter119 (TER‐119), CD31 (390), and CD45 (30‐F11) antibodies (eBioscience), for 30 min on ice to label erythrocytes, endothelial cells, and hematopoietic cells, respectively. After staining, cells were washed and incubated with Streptavidin MicroBeads (130‐048‐102, Miltenyi Biotec, Bergisch Gladbach, Germany) for magnetic separation with an OctoMACS Separator according to the manufacturer's instructions. Ter119−CD31–CD45– (Lin−) cells in the flow‐through were incubated with Anti‐Sca‐1 MicroBeads (130‐106‐641, Miltenyi Biotec) for magnetic separation following the manufacturer's protocol. The Lin−Sca1+ cells retained in the column were collected by removing the column from the magnet and plunging through with 1 ml BSA buffer. The cells were then centrifuged at 300 g for 5 min at 4°C for further processing.
Isolation of primary cell populations from mouse adipose tissue by FACS
After obtaining SVF single cell suspensions, different cell populations were sorted as described previously (Bayindir et al, 2015). Cells were preincubated with FcBlock (anti‐CD16/32; eBioscience) for 10 min on ice. Erythrocytes were depleted via magnetic separation with an OctoMACS Separator according to the manufacturer's instructions, following incubation with Anti‐Ter119 MicroBeads (130‐049‐901, Miltenyi Biotec) for 15 min on ice. The flow‐through was stained with CD45‐FITC (30‐F11, eBioscience), CD31‐eFluor450® (390, eBioscience), CD29‐PerCP‐eFluor® 710 (HMb1‐1, eBioscience), CD34‐Alexa Fluor® 647 (RAM34, BD Biosciences, Heidelberg, Germany), and Sca‐1‐Alexa Fluor® 700 (D7, eBioscience) for 30 min on ice. Cells were washed, sorted as Lin(CD31/CD45)−CD29+CD34+Sca1+ with a BD FACS Aria (BD Biosciences), and centrifuged at 300 g for 5 min at 4°C and resuspended either in culture medium with bFGF for culturing or in QIAzol® Lysis Reagent (Qiagen, Hilden, Germany) for RNA isolation. FCS files exported via BD FACSDiva™ software were analyzed with FlowJo Software (FlowJo, Ashland, OR).
Primary cell culture and adipogenic differentiation
Stromal vascular fraction cells derived from human fat biopsies were plated in culture medium (DMEM, 10% FCS, 1% penicillin/streptomycin [Life Technologies™, Darmstadt, Germany]) supplemented with 10 ng/ml recombinant human bFGF (R&D Systems) for up to three passages. Primary adipose progenitors isolated from murine inguinal subcutaneous depot by MACS® or FACS were seeded out on BIOCOAT laminin‐coated plates in culture medium supplemented with 10 ng/ml recombinant murine bFGF (R&D Systems) at a density of 1–3 × 104 cells/cm2. At confluency, adipogenic differentiation was induced with DMEM, 10% FCS (or 10% NCS for human SVF), 1% penicillin/streptomycin, 1 μg/ml insulin, 500 nM dexamethasone, 3 nM 3,3,5‐triiodo‐L‐thyronine (T3), 0.5 mM 3‐isobutyl‐1‐methylxanthine (IBMX; human cells only) [Sigma‐Aldrich]) for 2 days (or 3 days for human SVF). Induction was followed by DMEM supplemented with 5% FCS (or 5% NCS for human SVF), 1% penicillin/streptomycin, 1 μg/ml insulin and 3 nM T3) for up to 6 days (or 11 days for human SVF). Wherever indicated, cPGI2 (Biomol, Hamburg, Germany) was added to the medium at 1 μM for up to 8 days, rosiglitazone (Rosi; Biomol) was added to the medium at 100 nM for up to 5 days (mouse) or up to 14 days (human) and pioglitazone (Pio; Biomol) was added at the indicated concentration for up to 5 days. Corresponding concentrations of ethanol and DMSO served as control for cPGI2 and Rosi or Pio, respectively.
Human SVF cells were transfected at 60–70% confluency with Silencer® Select small interfering RNAs (siRNA) targeting CITED4 (s225785 and s46475; Life Technologies) or an equimolar amount of negative control (No. 1; 4390843; Life Technologies) using Lipofectamine® RNAiMAX transfection reagent (Life Technologies). Lipofectamine® RNAiMAX in serum‐free Opti‐MEM (Life Technologies) medium (20 μl/ml) was mixed with Opti‐MEM containing siRNA and incubated at room temperature (RT) for 5 min before transfer to the cultures with medium at 5 nM final concentration.
For the transfection of primary Lin−Sca1+ cells from Cited4 F/F mice with StemMACS™ Cre recombinase (130‐101‐113; Miltenyi Biotec) or StemMACS™ Nuclear eGFP messenger RNA (mRNA) (130‐101‐119; Miltenyi Biotec), a mixture of Lipofectamine® RNAiMAX transfection reagent (2 μl/ml) and Opti‐MEM medium was combined with Opti‐MEM containing the respective mRNA (500 ng/ml final concentration). After incubation at RT for 20 min, the transfection complexes were added dropwise to the cells for overnight incubation either before or 3 days after differentiation induction. To determine transfection efficiency, the cells were collected by trypsinization and analyzed for GFP expression by flow cytometry using a BD FACS Calibur (BD Biosciences).
C3H10T1/2 and 3T3‐L1 culture and adipogenic differentiation
C3H10T1/2 cells (Clone 8, ATCC® CCL‐226™) were propagated in DMEM containing 10% FCS and 1% penicillin/streptomycin until confluency. Adipogenic differentiation was induced by medium supplemented with 0.25 μM dexamethasone, 0.5 mM IBMX, 1 μg/ml insulin, and 3 nM T3 for 4 days, with a medium change every 2 days. Induction was followed by differentiation maturation medium (culture medium with 1 μg/ml insulin and 3 nM T3) for 6 days. 1 μM rosiglitazone or the corresponding concentration of DMSO was added to the medium throughout the differentiation. 3T3‐L1 preadipocytes (ATCC® CL‐173™) were cultured in low (1 g/l)‐glucose DMEM containing 10% FCS and 1% penicillin/streptomycin until confluency. Adipogenic differentiation was induced with high (4.5 g/l)‐glucose DMEM containing 10% FCS and 1% penicillin/streptomycin supplemented with 0.5 μM dexamethasone, 0.5 mM IBMX, 1 μg/ml insulin for 4 days. Following induction, cells were cultured in high (4.5 g/l)‐glucose DMEM with 10% FCS, 1% penicillin/streptomycin, 1 μg/ml insulin for an additional 6 days and further 2 days without insulin. 1 μM rosiglitazone or the corresponding concentration of DMSO was added to the medium up to day 6.
Cellular respiration assay (Seahorse)
Lin−Sca1+ cells isolated from the scWAT of female mice were seeded into XF96 V3‐PS cell culture microplate (Seahorse Bioscience, Copenhagen, Denmark), cultured, and differentiated as above. Insulin and T3 were omitted from the media at day 7 of differentiation. At day 8 of differentiation, Mito Stress Test was performed following the manufacturer's instructions. Briefly, the medium was replaced with prewarmed, unbuffered Seahorse Assay Medium (DMEM basal medium [Sigma Cat. No. D5030] supplemented with 25 mM glucose, 1 mM pyruvate, 1 mM glutamine, 15 mg/l phenol red, pH 7.4), and cells were incubated at 37°C in a CO2‐free incubator for at least 1 h. After calibration, respiration was measured in an XF96 Extracellular Flux Analyzer (Seahorse Bioscience). Cells were sequentially treated at the indicated time points with oligomycin (4 μM), CL‐316,243 (1 μM), trifluorocarbonylcyanide phenylhydrazone (FCCP, 2 μM), and rotenone‐antimycin A (rotenone‐AA, 1 μM each). Oxygen consumption rates were determined at the indicated time points and extracted by the Seahorse XF‐96 software. After completion of the assay, DNA content was determined using the CyQUANT Cell Proliferation Assay Kit (Thermo Fisher Scientific) according to the manufacturer's instructions. In brief, 200 μl of CyQUANT 1× lysis buffer with 1× CyQUANT GR dye was added per well while maintaining the plate on ice. The lysates were transferred to a flat bottom 96‐well plate along with a standard dilution series. Fluorescence was measured on a Mithras LB 940 plate reader (Berthold, Bad Wildbad, Germany) at 485/535 nm. DNA content was calculated using http://www.elisaanalysis.com/app and 5‐Parameter Logistic Regression. Oxygen consumption rates were normalized to total DNA content per well.
Lipid staining by LipidTOX™
Ex vivo differentiated adipocytes were washed and fixed with 4% paraformaldehyde solution (Thermo Fisher Scientific, Schwerte, Germany) for 15 min at RT. After washing with 1× PBS (Life Technologies), blocking was performed by incubating the cells in 5% BSA (Sigma‐Aldrich) in 1× PBS for 1 hour at RT. For LipidTOX™ (Life Technologies) and 4,6‐diamidino‐2‐phenylindole (DAPI; Sigma‐Aldrich) co‐staining, the staining solution was prepared with 1:200 LipidTOX™ Red Neutral Lipid stain and 0.5 μg/ml DAPI in 1× PBS. Cells were stained for minimum 30 min at RT in the dark prior to imaging.
A Cell Observer Z1 microscope and ZEN software (Carl Zeiss, Oberkochen, Germany) were used for fluorescence imaging. Forty images/group captured randomly across the wells and analyzed with an ImageJ (http://rsbweb.nih.gov/ij) algorithm. In brief, nuclei were segmented and counted and the LipidTOX™ signal intensity was quantified. The signal intensity was then used to count the LipidTOX+ cells by setting a threshold for the mean signal intensity for all samples. The number of LipidTOX+ cells was depicted as the percentage of DAPI+ nuclei per field.
RNA isolation, cDNA synthesis, and quantitative real‐time polymerase chain reaction
RNA was isolated from cells or snap‐frozen pulverized tissue with QIAzol® reagent and the RNeasy® Micro/Mini Kit (Qiagen) following the manufacturer's protocol. Complementary DNA (cDNA) synthesis was performed with 200–1,000 ng total RNA using QuantiTect® Reverse Transcription Kit (Qiagen). Quantitative real‐time polymerase chain reaction (qRT–PCR) was performed using TaqMan® Gene Expression Assays (Life Technologies) and TaqMan® Gene Expression Master Mix on a StepOnePlus™ Real‐Time PCR System (Life Technologies). Normalization to Tbp/TBP (TATA box binding protein) and calculation of relative expression values were performed with the (ΔΔC T) method. 18S ribosomal RNA was used instead of Tbp for normalization of Cited4 values in gene expression analysis comparing different tissues.
Microarray expression profiling
Expression profiling was performed with total RNA and the GeneChip® Mouse Gene 2.0 ST array platform (Affymetrix, High Wycombe, UK) by the DKFZ Genomics and Proteomics Core Facility according to the manufacturer's instructions. Gene‐level intensity values were calculated from the CEL files using robust multiarray average (RMA) normalization with the Affymetrix Expression Console and MoGene‐2_0‐st‐v1 library files and annotation files. Ranking of probe sets (gene level) for differential expression was based on P‐values obtained from t‐test with Welch's correction performed with TM4: MultiExperiment Viewer (MeV) software (Saeed et al, 2003). Gene set enrichment analysis (GSEA; Subramanian et al, 2005) was performed on the complete probe dataset using the c2.cp.kegg.v4.0.symbols.gmt and the c3.tft.v5.0.symbols.gmt gene set collections (Molecular Signatures Database, MSigDB; http://www.broadinstitute.org/gsea/msigdb/index.jsp). The parameters of the analysis were adjusted as follows: permutation type = phenotype (1,000 × ); enrichment statistic = weighted; metric for ranking genes = Signal2Noise; normalization mode = meandiv. Ranking of gene sets was by the false discovery rate (FDR).
Immunoblotting
For protein extraction, primary adipocytes were lysed in RIPA buffer (150 mM NaCl, 1% Triton™ X‐100, 50 mM Tris pH 8.0, 0.5% sodium deoxycholate, 0.1% SDS, 1× cOmplete™ protease inhibitor cocktail tablet [Roche]). After incubation on ice for 30 min, the lysates were centrifuged at 13,000 rpm, 4°C for 15 min. Snap‐frozen pulverized adipose tissue was lysed in 50 mM Tris pH 7.4, 1 mM EDTA pH 8, 1.5 mM MgCl2, 10 mM NaF, 2 mM Na3VO4, 1 mM DTT, 1× cOmplete™ protease inhibitor cocktail tablet, using a TissueLyzer. After 1 h on ice, the lysates were centrifuged shortly and the aqueous phase was supplemented with 150 mM NaCl, 0.5% deoxycholic acid, 1% NP‐40, 0.1% SDS, 1% glycerol and shaken at maximum speed at 4°C for 1 h. The lysates were cleared by centrifugation at 13,000 rpm, 4°C for 30 min. Protein concentration was determined using the Pierce® BCA Protein Assay Kit (Thermo Fisher Scientific). After SDS–PAGE and wet blotting, the nitrocellulose membranes were blocked either in 5% BSA or in 5% skim milk in TBS‐0.1% Tween‐20 for 1 h at RT. The membranes were incubated with antibodies detecting Ucp1 (1:1,000; PA1‐24894; Thermo Fisher Scientific), Cited4 (1:500; ab105797; Abcam), and Vcp (1:2,000; ab11433; Abcam) overnight at 4°C. Subsequently, they were incubated with the secondary antibodies goat anti‐rabbit IgG (H + L)‐HRP (1:4,000; 172‐1019; Bio‐Rad, Munich, Germany) or anti‐mouse IgG (H + L)‐HRP (1:5,000; 170‐6516; Bio‐Rad) for 1 h at RT. Following the enhanced chemiluminescence system reaction (ECL™ Western Blotting Detection Reagents; GE Healthcare, Solingen, Germany) protein bands were detected and quantified using the ChemiDoc™ XRS+ Molecular Imager® with ImageLab™ Software (Bio‐Rad).
Statistical analysis
For mouse experiments, a sample size calculation was performed by a statistician. For cell culture experiments in general, sample size was chosen for the detection of at least 30% effect, based on experiential estimation of variance. Recorded technical failures (prior to observation of results) and health deterioration of mice during experiments were applied as exclusion criteria for animals or samples. Mice or cell cultures were assigned to treatment groups before any information on individual random differences could be observed. Mice were assigned to groups based on genotype and sex and matched for age. Blinding was not performed. Data were plotted as mean ± standard error of the mean (SEM), unless stated otherwise. Statistical significance was determined using SigmaPlot 12.5 (Systat Software GmbH, Erkrath, Germany) and GraphPad Prism® 5.04 (GraphPad Software Inc., CA) statistical packages and Microsoft® Excel® (Microsoft Corp., WA). Expression data were log‐transformed for testing to approximate the normal distribution. Mostly, two‐sided t‐test was applied focusing on the difference Cited4‐knockout versus Cited4‐wild type under rosiglitazone treatment, with the control/vehicle groups presented descriptively. Two‐way ANOVA with two‐sided Holm–Sidak's or Bonferroni's post hoc tests was applied for time course experiments or two‐factorial designs as indicated. One‐way ANOVA with Tukey's posttests was used for designs with three groups. One‐way ANCOVA with two‐sided Bonferroni's posttests on body weight‐adjusted group means was applied to mouse oxygen consumption (VO2) data with body weight as covariate as described previously (Tschop et al, 2011). P < 0.05 was considered statistically significant.
Data availability
Microarray data have been deposited in the ArrayExpress database under accession number E‐MTAB‐6796.
Author contributions
Conceptualization, IB‐B, AV; Investigations, IB‐B, GW, SL, TS, MS, RB, LK, NS; Resources, JG, ATB, BPM‐S, PL; Software, DK; Funding Acquisition, IB‐B, LK, JLR, MHA, PL, SH, AV; Supervision, MHA, JLR, BPM‐S, MH, PL, SH, AV; Writing, IB‐B, AV.
Conflict of interest
The authors declare that they have no conflict of interest.
The paper explained.
Problem
Glitazones represent an exception among antidiabetic drugs in that they target excess substrate metabolism and insulin sensitivity as pathogenic causes in the metabolic syndrome and type 2 diabetes as opposed to treating disease symptoms. However, they are rarely prescribed due to their side effects. Understanding the mechanism of action of glitazones can help the exploitation of the nuclear receptor PPARg pathway as the main glitazone target to develop safer drugs.
Results
We found that the expression of the transcription cofactor gene Cited4 was upregulated by glitazone treatment in immature progenitor cells derived from adipose tissue, a major target of glitazones. Genetic inactivation of Cited4 resulted in defective differentiation of progenitors to beige adipocytes as reflected by the reduced induction of uncoupling protein 1 (Ucp1) and other thermogenic adipocyte markers by rosiglitazone in primary cultured human and murine cells as well as in adipose tissue in mice. This phenotype was more penetrant in females and was associated with reduced whole‐body energy expenditure, possibly due to lower capacity for adipose tissue thermogenesis in Cited4‐deficient mice. Furthermore, Cited4 inactivation resulted in compromised therapeutic insulin sensitization in female but not male mice when rosiglitazone was administered in the context of diet‐induced obesity.
Impact
Our study revealed unexpected sex‐, tissue‐, and context‐specific aspects of glitazone action which are relevant for the development and personalization of new therapeutic approaches targeting PPARg and adipose tissue metabolism in type 2 diabetes and prediabetes.
Supporting information
Appendix
Expanded View Figures PDF
Source Data for Expanded View and Appendix
Review Process File
Source Data for Figure 2
Source Data for Figure 4
Source Data for Figure 6
Acknowledgements
We thank Dr. Adam J Rose and Dr. Claus Kremoser for valuable discussions and know‐how, Dagmar Kindler, Patrick Matei, Jakob El Kholtei, and Anna Taranko for technical support, Dagmar Walter for technical know‐how, the DKFZ Genomics and Proteomics Core Facility, Steffen Schmitt and the DKFZ Imaging and Cytometry Core Facility, the DKFZ Center for Preclinical Research. This work was supported by the Human Frontier Science Program (RGY0082/2014), a stipend from the Helmholtz International Graduate School for Cancer Research to I.B.B, a Novo Nordisk postdoctoral fellowship in partnership with Karolinska Institutet to L.K., the German Federal Ministry of Education and Research (Infrafrontier grant 01KX1012), the Deutsche Forschungsgemeinschaft (HE 3260/8‐1), and the Brain Tumor Network (NGFNplus #01GS0883).
EMBO Mol Med (2018) 10: e8613
References
- Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM (2013) PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med 19: 557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei R, Bayindir‐Buchhalter I, Meln I, Vegiopoulos A (2017) Immuno‐magnetic isolation and thermogenic differentiation of white adipose tissue progenitor cells. Methods Mol Biol 1566: 37–48 [DOI] [PubMed] [Google Scholar]
- Banks AS, McAllister FE, Camporez JP, Zushin PJ, Jurczak MJ, Laznik‐Bogoslavski D, Shulman GI, Gygi SP, Spiegelman BM (2015) An ERK/Cdk5 axis controls the diabetogenic actions of PPARgamma. Nature 517: 391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayindir I, Babaeikelishomi R, Kocanova S, Sousa IS, Lerch S, Hardt O, Wild S, Bosio A, Bystricky K, Herzig S et al (2015) Transcriptional pathways in cPGI2‐induced adipocyte progenitor activation for browning. Front Endocrinol (Lausanne) 6: 129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz V, Kintscher U, Foryst‐Ludwig A (2012) Sex‐specific differences in Type 2 diabetes mellitus and dyslipidemia therapy: PPAR agonists. Handb Exp Pharmacol 214: 387–410. [DOI] [PubMed] [Google Scholar]
- Bezzerides VJ, Platt C, Lerchenmuller C, Paruchuri K, Oh NL, Xiao C, Cao Y, Mann N, Spiegelman BM, Rosenzweig A (2016) CITED4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight 1: e85904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Homko C, Mozzoli M, Showe LC, Nichols C, Cheung P (2005) Thiazolidinediones upregulate fatty acid uptake and oxidation in adipose tissue of diabetic patients. Diabetes 54: 880–885 [DOI] [PubMed] [Google Scholar]
- Bogacka I, Xie H, Bray GA, Smith SR (2005) Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo . Diabetes 54: 1392–1399 [DOI] [PubMed] [Google Scholar]
- Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakova D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A et al (2010) C/EBPbeta controls exercise‐induced cardiac growth and protects against pathological cardiac remodeling. Cell 143: 1072–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braganca J, Swingler T, Marques FI, Jones T, Eloranta JJ, Hurst HC, Shioda T, Bhattacharya S (2002) Human CREB‐binding protein/p300‐interacting transactivator with ED‐rich tail (CITED) 4, a new member of the CITED family, functions as a co‐activator for transcription factor AP‐2. J Biol Chem 277: 8559–8565 [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Khunti K, Davies MJ (2017) Type 2 diabetes. Lancet 389: 2239–2251 [DOI] [PubMed] [Google Scholar]
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ et al (2014) Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156: 304–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH (2008) The metabolic syndrome. Endocr Rev 29: 777–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby JE, Montague CT, Sewter CP, Sanders L, Wilkison WO, O'Rahilly S, Prins JB (1998) Thiazolidinedione exposure increases the expression of uncoupling protein 1 in cultured human preadipocytes. Diabetes 47: 138–141 [DOI] [PubMed] [Google Scholar]
- Elabd C, Chiellini C, Carmona M, Galitzky J, Cochet O, Petersen R, Penicaud L, Kristiansen K, Bouloumie A, Casteilla L et al (2009) Human multipotent adipose‐derived stem cells differentiate into functional brown adipocytes. Stem Cells 27: 2753–2760 [DOI] [PubMed] [Google Scholar]
- Festuccia WT, Blanchard PG, Turcotte V, Laplante M, Sariahmetoglu M, Brindley DN, Deshaies Y (2009) Depot‐specific effects of the PPARgamma agonist rosiglitazone on adipose tissue glucose uptake and metabolism. J Lipid Res 50: 1185–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foryst‐Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A et al (2008) Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross‐talk with PPARgamma. PLoS Genet 4: e1000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried SK, Lee MJ, Karastergiou K (2015) Shaping fat distribution: new insights into the molecular determinants of depot‐ and sex‐dependent adipose biology. Obesity (Silver Spring) 23: 1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y, Masui S, Osada S, Umesono K, Motojima K (2000) A new thiazolidinedione, NC‐2100, which is a weak PPAR‐gamma activator, exhibits potent antidiabetic effects and induces uncoupling protein 1 in white adipose tissue of KKAy obese mice. Diabetes 49: 759–767 [DOI] [PubMed] [Google Scholar]
- Ghandour RA, Giroud M, Vegiopoulos A, Herzig S, Ailhaud G, Amri EZ, Pisani DF (2016) IP‐receptor and PPARs trigger the conversion of human white to brite adipocyte induced by carbaprostacyclin. Biochim Biophys Acta 1861: 285–293 [DOI] [PubMed] [Google Scholar]
- Gonzalez YR, Zhang Y, Behzadpoor D, Cregan S, Bamforth S, Slack RS, Park DS (2008) CITED2 signals through peroxisome proliferator‐activated receptor‐gamma to regulate death of cortical neurons after DNA damage. J Neurosci 28: 5559–5569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler C, Vishvanath L, Gupta RK (2017) Sorting out adipocyte precursors and their role in physiology and disease. Genes Dev 31: 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J (2003) Muscle‐specific Pparg deletion causes insulin resistance. Nat Med 9: 1491–1497 [DOI] [PubMed] [Google Scholar]
- Inagaki T, Sakai J, Kajimura S (2016) Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol 17: 480–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery E, Wing A, Holtrup B, Sebo Z, Kaplan JL, Saavedra‐Pena R, Church CD, Colman L, Berry R, Rodeheffer MS (2016) The adipose tissue microenvironment regulates depot‐specific adipogenesis in obesity. Cell Metab 24: 142–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Gavrilova O, Chao L, Higashimori T, Choi H, Kim HJ, Yu C, Chen Y, Qu X et al (2003) Differential effects of rosiglitazone on skeletal muscle and liver insulin resistance in A‐ZIP/F‐1 fatless mice. Diabetes 52: 1311–1318 [DOI] [PubMed] [Google Scholar]
- Kim SN, Jung YS, Kwon HJ, Seong JK, Granneman JG, Lee YH (2016) Sex differences in sympathetic innervation and browning of white adipose tissue of mice. Biol Sex Differ 7: 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapid K, Lim A, Clegg DJ, Zeve D, Graff JM (2014) Oestrogen signalling in white adipose progenitor cells inhibits differentiation into brown adipose and smooth muscle cells. Nat Commun 5: 5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WM, Doucet M, Huang D, Weber KL, Kominsky SL (2013) CITED2 modulates estrogen receptor transcriptional activity in breast cancer cells. Biochem Biophys Res Commun 437: 261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Wu Y, Fried SK (2013) Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 34: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S (2014) PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol Metab 25: 293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chewchuk S, Lavigne C, Brule S, Pilon G, Houde V, Xu A, Marette A, Sweeney G (2009) Functional significance of skeletal muscle adiponectin production, changes in animal models of obesity and diabetes, and regulation by rosiglitazone treatment. Am J Physiol Endocrinol Metab 297: E657–664 [DOI] [PubMed] [Google Scholar]
- Martinez de Morentin PB, Gonzalez‐Garcia I, Martins L, Lage R, Fernandez‐Mallo D, Martinez‐Sanchez N, Ruiz‐Pino F, Liu J, Morgan DA, Pinilla L et al (2014) Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab 20: 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensink M, Hesselink MK, Russell AP, Schaart G, Sels JP, Schrauwen P (2007) Improved skeletal muscle oxidative enzyme activity and restoration of PGC‐1 alpha and PPAR beta/delta gene expression upon rosiglitazone treatment in obese patients with type 2 diabetes mellitus. Int J Obes (Lond) 31: 1302–1310 [DOI] [PubMed] [Google Scholar]
- Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, Hirshman MF, Rosen ED, Goodyear LJ, Gonzalez FJ et al (2003) Muscle‐specific PPARgamma‐deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest 112: 608–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Spiegelman BM, Kajimura S (2012) PPARgamma agonists induce a white‐to‐brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15: 395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H et al (1998) Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest 101: 1354–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J (2010) Chronic peroxisome proliferator‐activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1‐containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285: 7153–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala SM, Lazar MA (2004) Peroxisome proliferator‐activated receptor gamma in diabetes and metabolism. Trends Pharmacol Sci 25: 331–336 [DOI] [PubMed] [Google Scholar]
- Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, Okamoto Y, Mattie MD, Higashiyama H, Asano S et al (2007) Adipose mitochondrial biogenesis is suppressed in db/db and high‐fat diet‐fed mice and improved by rosiglitazone. Diabetes 56: 1751–1760 [DOI] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M et al (2003) TM4: a free, open‐source system for microarray data management and analysis. Biotechniques 34: 374–378 [DOI] [PubMed] [Google Scholar]
- Sakai M, Matsumoto M, Tujimura T, Yongheng C, Noguchi T, Inagaki K, Inoue H, Hosooka T, Takazawa K, Kido Y et al (2012) CITED2 links hormonal signaling to PGC‐1alpha acetylation in the regulation of gluconeogenesis. Nat Med 18: 612–617 [DOI] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148: 852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen P, Mensink M, Schaart G, Moonen‐Kornips E, Sels JP, Blaak EE, Russell AP, Hesselink MK (2006) Reduced skeletal muscle uncoupling protein‐3 content in prediabetic subjects and type 2 diabetic patients: restoration by rosiglitazone treatment. J Clin Endocrinol Metab 91: 1520–1525 [DOI] [PubMed] [Google Scholar]
- Sell H, Berger JP, Samson P, Castriota G, Lalonde J, Deshaies Y, Richard D (2004) Peroxisome proliferator‐activated receptor gamma agonism increases the capacity for sympathetically mediated thermogenesis in lean and ob/ob mice. Endocrinology 145: 3925–3934 [DOI] [PubMed] [Google Scholar]
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V et al (2012) Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 7: e49452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis L, Kajimura S (2015) Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest 125: 478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soccio RE, Chen ER, Lazar MA (2014) Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab 20: 573–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soccio RE, Li Z, Chen ER, Foong YH, Benson KK, Dispirito JR, Mullican SE, Emmett MJ, Briggs ER, Peed LC et al (2017) Targeting PPARgamma in the epigenome rescues genetic metabolic defects in mice. J Clin Invest 127: 1451–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES et al (2005) Gene set enrichment analysis: a knowledge‐based approach for interpreting genome‐wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zeve D, Seo J, Jo AY, Graff JM (2011) Thiazolidinediones regulate adipose lineage dynamics. Cell Metab 14: 116–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien ES, Davis JW, Vanden Heuvel JP (2004) Identification of the CREB‐binding protein/p300‐interacting protein CITED2 as a peroxisome proliferator‐activated receptor alpha coregulator. J Biol Chem 279: 24053–24063 [DOI] [PubMed] [Google Scholar]
- Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C et al (2011) A guide to analysis of mouse energy metabolism. Nat Methods 9: 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegiopoulos A, Muller‐Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nusing RM et al (2010) Cyclooxygenase‐2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 328: 1158–1161 [DOI] [PubMed] [Google Scholar]
- Vegiopoulos A, Rohm M, Herzig S (2017) Adipose tissue: between the extremes. EMBO J 36: 1999–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu L, Lin JZ, Aprahamian TR, Farmer SR (2016) Browning of white adipose tissue with roscovitine induces a distinct population of UCP1 + adipocytes. Cell Metab 24: 835–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson‐Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S (2004) Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest 114: 1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata T, Shao W, Endoh H, Hur J, Coser KR, Sun H, Ueda Y, Kato S, Isselbacher KJ, Brown M et al (2001) Selective coactivation of estrogen‐dependent transcription by CITED1 CBP/p300‐binding protein. Genes Dev 15: 2598–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata T, Takedatsu H, Dunwoodie SL, Braganca J, Swingler T, Withington SL, Hur J, Coser KR, Isselbacher KJ, Bhattacharya S et al (2002) Cloning of mouse Cited4, a member of the CITED family p300/CBP‐binding transcriptional coactivators: induced expression in mammary epithelial cells. Genomics 80: 601–613 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Murakami K, Motojima K, Komeda K, Ide T, Kubota N, Terauchi Y, Tobe K et al (2001) The mechanisms by which both heterozygous peroxisome proliferator‐activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem 276: 41245–41254 [DOI] [PubMed] [Google Scholar]
- Ye JM, Dzamko N, Cleasby ME, Hegarty BD, Furler SM, Cooney GJ, Kraegen EW (2004) Direct demonstration of lipid sequestration as a mechanism by which rosiglitazone prevents fatty‐acid‐induced insulin resistance in the rat: comparison with metformin. Diabetologia 47: 1306–1313 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Source Data for Expanded View and Appendix
Review Process File
Source Data for Figure 2
Source Data for Figure 4
Source Data for Figure 6
Data Availability Statement
Microarray data have been deposited in the ArrayExpress database under accession number E‐MTAB‐6796.


