Abstract
Increased resistance to antimicrobials in clinically important bacteria has been widely reported. The major mechanism causing multidrug resistance (MDR) is mediated by efflux pumps, proteins located in the cytoplasmic membrane to exclude antimicrobial drug. Some efflux pumps recognize and expel a variety of unrelated antimicrobial agents, while other efflux pumps can expel only one specific class of antibiotics. Previously, we have reported that xylose decreases the efflux-mediated antimicrobial resistance in Salmonella typhimurium, Pseudomonas aeruginosa, and Acinetobacter baumannii in vitro. In this work, we assessed the effectiveness of combining xylose with antibiotics to kill resistant Acinetobacter baumannii and Klebsiella pneumoniae in a murine model of skin infection. Skin infections were established by seeding 109 bacteria onto eroded skin of mice. Mice treated with the antibiotic alone or with a mixture of glucose and antibiotics or xylose and antibiotics were compared to a control group that was infected but received no further treatment. We observed that the mixtures xylose-tetracycline and xylose-chloramphenicol produced a decrease of at least 10 times viable Acinetobacter baumannii and Klebsiella pneumoniae recovered from infected skin, compared with mice treated with the antibiotic alone. Our results show that xylose improves the antibiotic activity of tetracycline and chloramphenicol against efflux-mediated resistance Acinetobacter baumannii and Klebsiella pneumoniae, in a murine model of skin infection. We envision these combined formulations as an efficient treatment of skin infections with bacteria presenting efflux-mediated resistance, in both humans and animals.
1. Introduction
Skin infections are one of the most common infections [1]. Breaks in the skin, such as leg ulcers and surgical or traumatic wounds, constitute a perfect environment for infections by a broad range of bacteria [2]. Most skin infections are caused by Gram-positive bacteria, commonly Staphylococcus aureus and group A β-haemolytic Streptococcus [1]. However, Gram-negative bacteria such as Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae may also cause skin infections [2]. The incidence of skin infections has increased due to ageing of the general population, increased number of critically ill patients, increased number of immunocompromised patients, and recent emergence of multidrug-resistant pathogens [3]. Multidrug resistance (MDR) is defined as the resistant phenotype to antibiotics belonging to two or more classes of antibiotics and represents a serious problem in healthcare settings [4, 5]. Drug-resistant bacteria are responsible for more than 30,000 deaths per year in the UK and Europe, and it is estimated that 23,000 people in the United States die from pathogens that are not responsive to treatments with current antibiotic therapies [6].
Bacteria exhibit different strategies to resist antibiotics. One of the most important mechanisms, considered a major contributor to the emergence of MDR pathogens, is the antibiotic efflux achieved by efflux pumps [7]. Efflux pumps are proteins located in the inner membrane of Gram-negative bacteria and in the cytoplasmic membrane of Gram-positive bacteria [7]. The continuous onset of MDR in bacterial strains limits the clinical efficacy of most available antibiotics. Therefore, there is an urgent need to introduce novel antimicrobial molecules that may be active by themselves or potentiate current available antibiotics [8].
In a previous in vitro study, we found that xylose decreases the efflux-mediated antimicrobial resistance in S. typhimurium, P. aeruginosa, and A. baumannii. Although the mechanism behind sensitization remains elusive, it has been speculated that either competitions for limited space in the inner membrane or interference with the translocon systems may affect translocation of efflux pumps into membrane, thereby affecting efflux-mediated resistance [9]. Because the in vitro potentiation of actively expelled antimicrobials was fairly significant in the presence of xylose, we ought to find whether this potentiation can be reproduced in vivo. Therefore, in this work, we assessed the effectiveness of combining xylose with antibiotics in vivo. Our results show that xylose increases the antibiotic activity of tetracycline and chloramphenicol against efflux-dependent resistant A. baumannii and K. pneumoniae, in a model of skin infection in mice.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
Clinical strains of A. baumannii and K. pneumoniae were collected from different healthcare facilities throughout Santiago, Chile, and collected at Servicio de Laboratorios Clínicos, Escuela de Medicina, Pontificia Universidad Católica de Chile in Santiago, between 2014 and 2015. The A. baumannii strains were isolated from tracheal secretions from patients with respiratory infection. The K. pneumoniae strains were isolated from urine of patients with urinary infection. Strains were grown in LB broth at 37°C with aeration. Solid media (LB agar) included Bacto agar (15 g/L).
2.2. Antimicrobial Susceptibility Test
We used a modification of the disc diffusion assay previously described [9, 10]. Briefly, cultures were grown for 16 h in LB broth; bacteria were washed three times and resuspended in PBS. 106 cells were spread on M9 plates supplemented with glucose or xylose (2 mg/mL) [9]. When required, the medium was supplemented with 12.5 μM carbonyl cyanide-m-chlorophenylhydrazone (CCCP), an indirect efflux pumps inhibitor that acts by impairing the proton transport. Ten microliters of tetracycline 10 μg/mL or chloramphenicol 20 μg/mL were spotted in a sterile filter paper disc, placed on the center of the plate. The diameters of the inhibition haloes, obtained after an overnight culture at 37°C, were reported as the average of two measurements.
The MICs for each bacterial strain were determined by microdilution in liquid medium as recommended by the CLSI [11], with modifications. Briefly, bacteria were grown overnight in LB medium, washed 3 times with PBS, diluted in fresh M9 glucose medium or M9 xylose medium, and aliquoted (100 μL) into the wells of sterile microtitre plates (106 CFU/mL in each well). The lowest concentration of antimicrobial agents that inhibited growth (measured as the optical density at 640 nm) by at least 50%, relative to growth in the absence of antimicrobial agents, was defined as the MIC. When required, the medium was supplemented with 12.5 μM CCCP.
2.3. Skin Infections
Female BALB/c mice (7–8 weeks old) were anesthetized with a mixture of 1 : 3 of xylazine 2% and ketamine 115 mg/mL intraperitoneally injected (1 µL per g of weight). Followed by this, 1 cm2 of skin was shaved, on the back of each animal, to produce a slight irritation. The area was immediately infected with 20 µL of a PBS-bacterial suspension containing 1 × 109 CFU/mL of A. baumannii or K. pneumoniae. Littermate mice were distributed into four groups: the control group, which was only infected but received no further treatment; the antibiotic-treated group, which was treated topically with 20 µL of antibiotics (tetracycline 2.5 μg/mL when A. baumannii was used or chloramphenicol 2.5 μg/mL when K. pneumoniae was used); and the glucose-antibiotic group and the xylose-antibiotic group, which were treated topically with a mixture of 20 µL of 2.5 μg/mL antibiotic (tetracycline or chloramphenicol) plus 20 µL of sugar (glucose 2% or xylose 2%). The treatments started 4 h after infection and were repeated every 12 h. Three days after infection, mice were euthanized to dissect 1 cm2 of the infected areas, which were homogenized in 0.5 mL of PBS to obtain the CFU by plating onto LB agar. Bacterial recovered from the infected skin were confirmed as A. baumannii or K. pneumoniae by using API10s test. Experiments with animals were performed according to Protocol 012-2013 approved by the Bioethics Committee at Universidad Andrés Bello and in accordance with the NIH guide for the care and use of laboratory animal.
3. Results
3.1. The Presence of Xylose Decreased the Efflux-Pump-Mediated Antimicrobial Resistance in A. baumannii and K. pneumoniae
In a previous study, we determined that xylose, as the sole carbon source, decreases efflux-mediated resistance of Gram-negative bacteria to different antibiotics in vitro [9]. Since A. baumannii and K. pneumoniae are frequently resistant to tetracycline and chloramphenicol by mechanisms involving efflux pumps [12, 13], in this study we used these antibiotics to test the effect of xylose. In the in vitro experiments, we observed that 60% (9/15) of the A. baumannii strains tested increased their susceptibility to tetracycline when xylose was the sole carbon source (Supplementary Table S1). Similarly, 77% (10/13) of tested K. pneumoniae strains were more susceptible to chloramphenicol when xylose was the sole carbon source (Supplementary Table S1). To study how xylose may affect efflux-dependent resistance, we choose two strains of A. baumannii (34702 and 34280) and two strains of K. pneumoniae (28296 and 28341) to perform the in vitro and in vivo experiments. In Table 1, we show that A. baumannii 34702 and K. pneumoniae 28296 exhibited efflux-dependent resistance (EDR) to tetracycline or chloramphenicol, respectively, as assessed by the increased susceptibility in presence of the efflux pump inhibitor CCCP [9, 14]. It has been reported that resistance in A. baumannii to tetracycline is attained by expelling the drug mainly through the AedC, CraA, AdeB, AdeG, and AdeJ efflux pumps [12,15–17]. Similarly, in K. pneumoniae, the resistance to chloramphenicol is attained by expelling the drug mainly through the AcrB efflux pump [18]. By contrast, A. baumannii 34280 and K. pneumoniae 28341 showed efflux-independent resistance (EIR) to tetracycline or chloramphenicol, respectively, since the presence of CCCP exerted no effect in the growth inhibition haloes. Furthermore, the presence of xylose as the sole carbon source increased the antibiotic susceptibility only in the EDR strains (Table 1). To quantitatively confirm our results, we performed MIC assay for each bacterial strain. As observed in Table 1, the MIC for tetracycline in A. baumannii 34702 was 2 μg/mL in presence of xylose, compared with 64 μg/mL in the presence of glucose; therefore, the same strain was 32 times more susceptible to tetracycline in presence of xylose. In the same way, the results observed for K. pneumoniae 28296 showed that the MIC for chloramphenicol was 2 μg/mL in the presence of xylose, compared with 32 μg/mL in the presence of glucose; therefore, the same strain was 16 times more susceptible to chloramphenicol in the presence of xylose. These results confirm that both strains exhibited efflux-dependent resistance (EDR) to tetracycline or chloramphenicol, respectively.
Table 1.
Susceptibility profile of A. baumannii and K. pneumoniae strains used in a model of skin infection in mice.
| Strains | Resistance | Antibiotics | Inhibition halo (mm) | MIC (μg/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| Glu | Xyl | Glu + CCCP | Glu | Xyl | Glu + CCCP | |||
| A. baumannii 34702 | EDR | Tet | 45 | 50 | 52 | 64 | 2 | 32 |
| A. baumannii 34280 | EIR | Tet | 45 | 45 | 44 | 64 | 64 | 64 |
| K. pneumoniae 28296 | EDR | Cam | 39 | 49 | 48 | 32 | 2 | 16 |
| K. pneumoniae 28341 | EIR | Cam | 13 | 15 | 15 | 8 | 8 | 8 |
As a control, we performed a growth curve for A. baumannii 34702 and K. pneumoniae 28296 with glucose or xylose as the sole carbon source to determine the bacterial growth rate. The results in Figure S1 (in supplementary data) show that the growth rate of A. baumannii 34702 in xylose is slightly lower than that in the presence of glucose, as the sole carbon source (in logarithmic phase: A. baumannii + Glu: 0.18 h−1 and A. baumannii + Xyl: 0.17 h−1). A similar result was observed in K. pneumoniae 28296 (in logarithmic phase: K. pneumoniae + Glu: 0.19 h−1 and K. pneumoniae + Xyl: 0.16 h−1) (Figure S2 in supplementary data). Notwithstanding the lower growth rate in minimal medium plus xylose, the growth inhibition haloes were evident for both A. baumannii and K. pneumoniae in the presence of either tetracycline or chloramphenicol. In addition, the inhibition haloes measured after 12 h remained unmodified when measured after 24, 48, or 72 h (Tables S2 and S3, included in supplementary data). Thus, we conclude that xylose affects resistance to tetracycline or chloramphenicol independently from its effects on growth rates. These results strongly suggest that both strains exhibit efflux-dependent resistance (EDR) to tetracycline or chloramphenicol, respectively.
As expected, A. baumannii 34280 and K. pneumoniae 28341, both showing efflux-independent resistance (EIR) to tetracycline or chloramphenicol, presented no changes in the MIC, whether cultured in the presence of xylose or glucose as the sole carbon source (Table 1). In addition, the presence of CCCP exerted no effect on the MIC (Table 1, glucose versus glucose + CCCP). In our experiments, we used CCCP at concentrations ranging from 4 to 10 times less than used in previous reports [19, 20]. Furthermore, we assessed the growth of A. baumannii 34702 and K. pneumoniae 28296 in presence or absence of CCCP (12.5 μM). The results in Figures S1 and S2 (supplementary data) show that CCCP does not affect the growth rate of A. baumannii 34702 (in logarithmic phase: A. baumannii + Glu: 0.18 h−1 and A. baumannii + Glu + CCCP: 0.33 h−1) or K. pneumoniae 28296 (in logarithmic phase: K. pneumoniae + Glu: 0.19 h−1 and K. pneumoniae + Glu + CCCP: 0.14 h−1).
Altogether, these in vitro experiments show that xylose potentiated the antibiotic activity of tetracycline and chloramphenicol in EDR strains of A. baumannii and K. pneumoniae.
3.2. Xylose Potentiates the Antibiotic Activity of Tetracycline and Chloramphenicol against EDR Strains of A. baumannii and K. pneumoniae in a Murine Model of Skin Infection
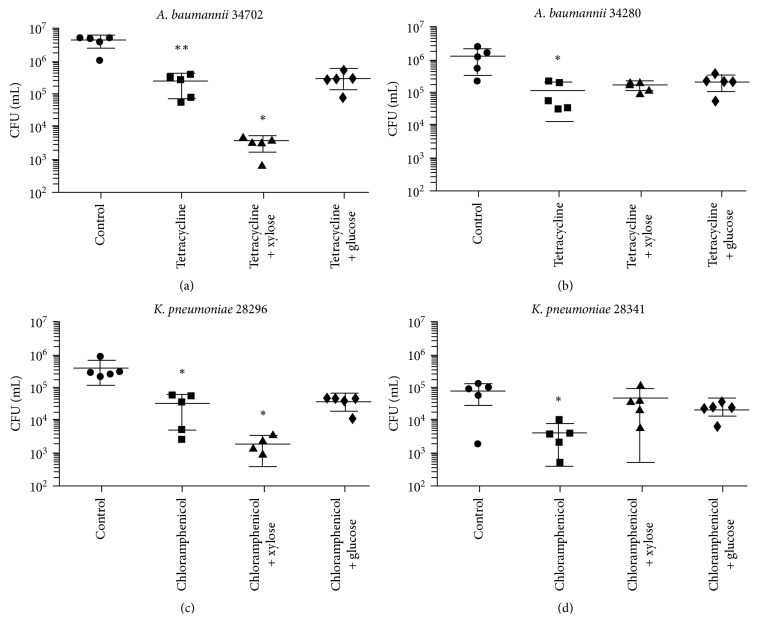
To determine whether xylose can potentiate antibiotic activity, not only in vitro but also in vivo, a murine model of skin infection was chosen using the same strains and antibiotics described in Table 1. Skin infections were established by seeding A. baumannii or K. pneumoniae on the skin of mice, previously shaved to generate lesions, as previously described in method sections and in references [18]. Bacteria recovered from the infected skin and treated with the antibiotics or with a mixture of glucose and antibiotics or xylose and antibiotics were compared with a control group that was infected but received no further treatment. As shown in Figure 1(a), we recovered 10 times less CFU of A. baumannii 34702 (EDR strain) from tetracycline-treated mice than from the untreated group. In addition, we observed that the mixture of xylose and tetracycline was the most efficient treatment, decreasing the recovery of A. baumannii 34702 100 times compared with the untreated control mice and 10 times compared with the tetracycline-treated mice. Control group of infected mice treated with xylose alone and mice treated with a mixture of glucose and tetracycline showed no differences in the bacterial count, compared to untreated control group and antibiotic-treated group, respectively. Importantly, the addition of xylose did not improve the effect of tetracycline in mice infected with A. baumannii 34280, an EIR strain, as expected from our in vitro assays (Figure 1(b)).
Figure 1.
Xylose potentiates antibiotic activity of efflux-dependent resistant bacteria in a murine model of skin infection. The ability of xylose to increase the susceptibility of clinical A. baumannii and K. pneumomiae strains to tetracycline and chloramphenicol, respectively, was determined in vivo. Bacteria were used to infect skin lesion in mice, prior to treating mice with the antibiotic alone or with a mixture of the antibiotic and xylose. As control, a group of mice was treated with antibiotic and glucose, while another was left untreated. We tested the EDR strain A. baumannii 34702 (a) and the EIR strain A. baumannii 34280 (b) with tetracycline; and the EDR strain K. pneumomiae 28296 (c) and the EIR strain K. pneumomiae 28341 (d) with chloramphenicol. Results are expressed as CFU/mL of homogenized tissue. Experiments were repeated at least 3 times. ∗ p < 0.05 and ∗∗ p < 0.01 according to Student's t-test.
We obtained similar results with K. pneumoniae, where xylose clearly improved the effect of chloramphenicol only in K. pneumoniae 28296 (300 times compared to the recovered CFU from untreated mice and 30 times compared to the antibiotic-treated group), an EDR strain, but not in K. pneumoniae 28341, an EIR strain (Figures 1(c) and 1(d)). A control group of infected mice treated with xylose alone and mice treated with a mixture of glucose and chloramphenicol showed no differences in the bacterial count. Again, these results are consistent with those obtained in vitro (Table 1).
As a control, we grew A. baumannii 34702 and K. pneumoniae 28296 in the presence of different concentrations of xylose. Our observations did not show impaired growth or antibacterial effect of this sugar alone against bacteria (Table S4 in supplementary data).
4. Discussion
The data presented show that xylose can be used to potentiate the antibiotic activity of tetracycline and chloramphenicol in vivo in strains presenting EDR. For this study, we have chosen tetracycline and chloramphenicol, antibiotics which resistance is commonly achieved through efflux pumps. The antibiotic tetracycline is still used against both Gram-positive and Gram-negative bacteria. Although tetracycline is not the first choice against skin infections because it can cause phototoxic reactions [21], it is useful in several other types of infections, including rickettsial, chlamydial and periodontal infections, and atypical pneumonias [22]. Considering that tetracycline resistance easily arises because of horizontal gene transfer encoding efflux systems, the results described in this manuscript may have a major impact on the treatment of tetracycline-resistant bacteria.
Chloramphenicol presents excellent in vitro activity against most anaerobes, which are a major cause of skin infections [23]. Therefore, the evidence presented here suggests that adding xylose to antibiotics from different families is useful for increasing the antimicrobial effect of antibiotics that show prevalence for resistance through active efflux. In a previous work, we hypothesized that it is possible to alter the efflux pump-mediated antibiotic resistance by overproducing unrelated inner membrane proteins, such as carbohydrate permeases. An easy way to increase the production of permeases is to culture bacteria with a non-PTS carbohydrate as the sole carbon source, such as xylose. Indeed, xylose was the best non-PTS sugar at potentiating antibiotic effects, compared to galactose or arabinose [9]. In addition, xylose was chosen because it is apparently innocuous, and its pharmacokinetic/pharmacodynamics parameters have been extensively studied. Indeed, xylose is administered systemically at high doses to assess intestinal absorption in human.
In a next step, we will incorporate various excipients to the mixture of antibiotic and xylose to enhance the effect as part of a topic formulation. Importantly, and beside our predictions, wound exudates, which may contain glucose, a sugar that exerts catabolic repression over xylose metabolism (Table S5 in supplementary data) apparently did not inhibit the in vivo effects of xylose regarding antibiotic sensitization.
5. Conclusion
In summary, the data we presented here demonstrate that xylose potentiates the in vivo antibiotics effect of tetracycline and chloramphenicol in bacteria that actively expel these antibiotics. Such effect was previously demonstrated in vitro for tetracycline and chloramphenicol, both actively expelled antibiotics. In addition, we show evidence that xylose is effective in potentiating antibiotic activity of tetracycline and chloramphenicol besides glucose present on wound exudates. These results are not only a proof of principles but also ground to expand the use of xylose with two purposes: uncovering EDR mechanisms and potentiating susceptibility of bacteria showing EDR, to antibiotics tested in this study and others.
Acknowledgments
This work was funded by FONDECYT (Fondo Nacional de Desarrollo Científico y Tecnológico, Government of Chile) through Grant no. 1151393 to Guido C. Mora, Grant no. 11150588 to Alejandro A. Hidalgo, and UNAB Regular DI-4-17/RG to Nicolás A.Villagra. The authors thank Mr. Víctor Ahumada and David Pezoa DVM, Ph.D., for assisting with animal procedures.
Abbreviations
- MDR:
Multidrug resistant
- CCCP:
Carbonyl cyanide-m-chlorophenylhydrazone
- MIC:
Minimal inhibitory concentration
- M9:
Minimal medium 9
- CFU:
Colony formation unit
- EDR:
Efflux-dependent resistance
- IDR:
Efflux-independent resistance.
Data Availability
All data generated during or analyzed during this study are included in this published article and supplementary figures and tables.
Disclosure
An early version of this work was presented as an abstract at 27th ECCMID Congress, 2017.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Alejandro A. Hidalgo was responsible for conception, design, analysis of data, and critical revision of the article. Ángel J. Arias was responsible for acquisition of data. Juan A. Fuentes and Patricia García performed critical revision and drafting of the article. Guido C. Mora was responsible for analysis of data and critical revision and drafting of the article. Nicolás A. Villagra was responsible for conception, design, analysis of data, and writing the manuscript.
Supplementary Materials
Table S1: A. baumannii and K. pneumoniae strains used in this study, and their susceptibility to tetracycline and chloramphenicol in the presence of glucose or xylose. Table S2: inhibition haloes of A. baumannii 34702 and K. pneumoniae 28296 measured in millimeter after 12 hours of incubation. Table S3: inhibition haloes of A. baumannii 34702 and K. pneumoniae 28296 measured in millimeter after 72 hours of incubation. Table S4: susceptibility profile of A. baumannii 34702 and K. pneumoniae 28296 strains with different concentrations of xylose. Table S5: susceptibility profile of A. baumannii and K. pneumoniae strains in the presence of glucose, xylose, and a mixture of glucose and xylose. Figure S1: growth curves for A. baumannii 34702 in the presence of glucose or xylose, as the sole carbón source, and glucose plus CCCP. Figure S2: growth curves for K. pneumoniae 28296 in the presence of glucose or xylose, as the sole carbón source, and glucose plus CCCP.
References
- 1.Badal R. E., Bouchillon S. K., Lob S. H., Hackel M. A., Hawser S. P., Hoban D. J. Etiology, extended-spectrum beta-lactamase rates and antimicrobial susceptibility of Gram-negative bacilli causing intra-abdominal infections in patients in general pediatric and pediatric intensive care units–global data from the study for monitoring antimicrobial resistance trends 2008 to 2010. Pediatric Infectious Disease Journal. 2013;32(6):636–640. doi: 10.1097/INF.0b013e3182886377. [DOI] [PubMed] [Google Scholar]
- 2.Dryden M. S. Skin and soft tissue infection: microbiology and epidemiology. International Journal of Antimicrobial Agents. 2009;34(1):S2–S7. doi: 10.1016/S0924-8579(09)70541-2. [DOI] [PubMed] [Google Scholar]
- 3.Unal S. Treatment options for skin and soft tissue infections: ‘oldies but goldies’. International Journal of Antimicrobial Agents. 2009;34(1):S20–S23. doi: 10.1016/S0924-8579(09)70545-X. [DOI] [PubMed] [Google Scholar]
- 4.Kwa A. L., Low J. G., Lee E., Kurup A., Chee H. L., Tam V. H. The impact of multidrug resistance on the outcomes of critically ill patients with Gram-negative bacterial pneumonia. Diagnostic Microbiology and Infectious Disease. 2007;58(1):99–104. doi: 10.1016/j.diagmicrobio.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Alekshun M. N., Levy S. B. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128(6):1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Centers of Diseases Control and Prevention. 2016. http://www.cdc.gov/features/AntibioticResistanceThreats/index.html. [Google Scholar]
- 7.Sun J., Den Z., Yan A. Bacterial multidrug efflux pumps: mechanisms, physiology and pharmacological exploitations. Biochemical and Biophysical Research Communications. 2014;453(2):254–267. doi: 10.1016/j.bbrc.2014.05.090. [DOI] [PubMed] [Google Scholar]
- 8.Penesyan A., Gillings M., Paulsen I. Antibiotic discovery: combatting bacterial resistance in cells and in biofilm communities. Molecules. 2015;20(4):5286–5298. doi: 10.3390/molecules20045286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villagra N. A., Fuentes J. A., Jofré M. R., Hidalgo A. A., Garcia P., Mora G. C. The carbon source influences the efflux pump-mediated antimicrobial resistance in clinically important Gram-negative bacteria. Journal of Antimicrobial Chemotherapy. 2012;67(4):921–927. doi: 10.1093/jac/dkr573. [DOI] [PubMed] [Google Scholar]
- 10.Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology. 1966;45(5):493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically—Seventh Edition: Approved Standard M7–A7. Wayne, PA, USA: CLSI; 2006. [Google Scholar]
- 12.Bialek-Davenet S., Lavigne J. P., Guyot K., et al. Differential contribution of AcrAB and OqxAB efflux pumps to multidrug resistance and virulence in Klebsiella pneumoniae . Journal of Antimicrobial Chemotherapy. 2015;70(1):81–88. doi: 10.1093/jac/dku340. [DOI] [PubMed] [Google Scholar]
- 13.Roca I., Marti S., Espinal P., Martinez P., Gilbert I., Vila J. CraA, a major facilitator superfamily efflux pump associated with chloramphenicol resistance in Acinetobacter baumannii . Antimicrobial Agents and Chemotherapy. 2009;53(9):4013–4014. doi: 10.1128/aac.00584-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ardebili A., Talebi M., Azimi L., Rastegar Lari A. Effect of efflux pump inhibitor carbonyl cyanide 3-chlorophenylhydrazone on the minimum inhibitory concentration of ciprofloxacin in Acinetobacter baumannii clinical isolates. Jundishapur Journal of Microbiology. 2014;7(1) doi: 10.5812/jjm.8691.e86911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohler T., Michea-Hamzehpour M., Epp S. F., Pachere J. C. Carbapenem activities against Pseudomonas aeruginosa: respective contributions of OprD and efflux systems. Antimicrobial Agents and Chemotherapy. 1999;43(2):424–427. doi: 10.1128/aac.43.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnet S., Courvalin P., Lambert T. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrobial Agents and Chemotherapy. 2001;45(12):3375–3380. doi: 10.1128/aac.45.12.3375-3380.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasdemir U. O., Chevalier J., Nordmann P., Pages J. M. Detection and prevalence of active drug efflux mechanism in various multidrug-resistant Klebsiella pneumoniae strains from Turkey. Journal of Clinical Microbiology. 2004;42(6):2701–2706. doi: 10.1128/jcm.42.6.2701-2706.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kugelberg E., Norström T., Petersen T., Duvold T., Andersson D. I., Hughes D. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes . Antimicrobial Agents and Chemotherapy. 2005;49(8):3435–3441. doi: 10.1128/aac.49.8.3435-3441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pournaras S., Maniati M., Spanakis N., et al. Spread of efflux pump- overexpressing, non-metallo-b-lactamase-producing, meropenem- resistant but ceftazidime-susceptible Pseudomonas aeruginosa in a region with blaVIM endemicity. Journal of Antimicrobial Chemotherapy. 2005;56(4):761–764. doi: 10.1093/jac/dki296. [DOI] [PubMed] [Google Scholar]
- 20.Ni W., Li Y., Guan J., et al. Effects of efflux pump inhibitors on colistin resistance in multidrug-resistant gram-negative bacteria. Antimicrobial Agents and Chemotherapy. 2016;60(5):3215–3218. doi: 10.1128/aac.00248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rok J., Buszman E., Delijewski M., Otreba M., Beberok A., Wrzesniok D. Effect of tetracycline and UV radiation on melanization and antioxidant status of melanocytes. Journal of Photochemistry and Photobiology B: Biology. 2015;148:168–173. doi: 10.1016/j.jphotobiol.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Bahrami F., Morris D., Pourgholami M. Tetracyclines: drugs with huge therapeutic potential. Mini-Reviews in Medicinal Chemistry. 2012;12(1):44–52. doi: 10.2174/138955712798868977. [DOI] [PubMed] [Google Scholar]
- 23.Brook I., Wexler H. M., Goldstein E. J. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clinical Microbiology Reviews. 2013;26(3):526–546. doi: 10.1128/CMR.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: A. baumannii and K. pneumoniae strains used in this study, and their susceptibility to tetracycline and chloramphenicol in the presence of glucose or xylose. Table S2: inhibition haloes of A. baumannii 34702 and K. pneumoniae 28296 measured in millimeter after 12 hours of incubation. Table S3: inhibition haloes of A. baumannii 34702 and K. pneumoniae 28296 measured in millimeter after 72 hours of incubation. Table S4: susceptibility profile of A. baumannii 34702 and K. pneumoniae 28296 strains with different concentrations of xylose. Table S5: susceptibility profile of A. baumannii and K. pneumoniae strains in the presence of glucose, xylose, and a mixture of glucose and xylose. Figure S1: growth curves for A. baumannii 34702 in the presence of glucose or xylose, as the sole carbón source, and glucose plus CCCP. Figure S2: growth curves for K. pneumoniae 28296 in the presence of glucose or xylose, as the sole carbón source, and glucose plus CCCP.
Data Availability Statement
All data generated during or analyzed during this study are included in this published article and supplementary figures and tables.