Abstract
Nucleic acid binding polymers (NABPs) have been extensively used as vehicles for DNA and RNA delivery. More recently, we discovered that a subset of these NABPs can also serve as anti-inflammatory agents by capturing pro-inflammatory extracellular nucleic acids and associated protein complexes that promote activation of toll-like receptors (TLRs) in diseases such as lupus erythematosus. Nucleic-acid-mediated TLR signaling also facilitates tumor progression and metastasis in several cancers, including pancreatic cancer (PC). In addition, extracellular DNA and RNA circulate on or within lipid microvesicles, such as microparticles or exosomes, which also promote metastasis by inducing pro-tumorigenic signaling in cancer cells and pre-conditioning secondary sites for metastatic establishment. Here, we explore the use of an NABP, the 3rd generation polyamidoamine dendrimer (PAMAM-G3), as an anti-metastatic agent. We show that PAMAM-G3 not only inhibits nucleic-acid-mediated activation of TLRs and invasion of PC tumor cells in vitro, but can also directly bind extracellular microvesicles to neutralize their pro-invasive effects as well. Moreover, we demonstrate that PAMAM-G3 dramatically reduces liver metastases in a syngeneic murine model of PC. Our findings identify a promising therapeutic application of NABPs for combating metastatic disease in PC and potentially other malignancies.
Keywords: nucleic acid binding polymer, metastasis, damage-associated molecular patterns
Naqvi et al. present the concept of using a specialized polymer to limit pancreatic cancer (PC) metastasis by binding and neutralizing circulating nucleic acids and microvesicles that upregulate pro-inflammatory and pro-invasive pathways. This polymer-based strategy may offer a multifaceted approach for inhibiting metastatic progression in PC and other cancer types.
Introduction
Nucleic acid binding polymers (NABPs) have been widely utilized as vehicles for DNA and RNA delivery.1 Recently, our laboratory discovered that a subset of such polymers can also serve as anti-inflammatory agents that capture pro-inflammatory extracellular nucleic acids and neutralize their activation of the nucleic-acid-sensing toll-like family of receptors (TLRs).2 Although TLRs evolved to provide innate immunity against infection by recognizing pathogen associated molecular patterns (PAMPs), they can also be activated by endogenous damage associated molecular patterns (DAMPs), such as nucleic acids and nucleic-acid-protein complexes released by dead and dying cells.3, 4 Excessive activation of TLRs contributes to disease progression in several autoimmune or inflammatory disorders.4 We previously demonstrated that certain NABPs, such as the 3rd generation polyamidoamine dendrimer (PAMAM-G3), can bind pro-inflammatory extracellular nucleic acids and nucleic-acid-protein complexes and prevent subsequent TLR activation2 and improve outcomes in animal models of lupus, acute liver failure, and influenza infection2, 5 without evidence of systemic toxicity.
An additional pathological role for inappropriate TLR activation in tumor progression and metastasis has now been established. For example, TLR signaling in lung cancer leads to increased angiogenesis and tumor cell invasion.6 TLR agonists also promote increased tumor invasion in vitro in breast cancer and metastasis in vivo in pancreatic and colorectal cancer.7, 8, 9 Moreover, circulating levels of innate TLR agonists, including cell-free nucleic acids and associated complexes, are elevated in a multitude of cancers and can further increase following chemotherapy, radiation, and surgery.9, 10, 11, 12 These endogenous factors can circulate alone or on/within lipid microvesicles, such as microparticles or exosomes, to induce pro-tumorigenic signaling in cancer cells and the tumor microenvironment and pre-condition secondary sites for metastatic establishment.6, 7, 8, 13, 14, 15, 16, 17
Recent work has highlighted specific contributions of TLR activation mediated by circulating nucleic acid DAMPs to disease progression in pancreatic cancer (PC),7, 13, 15 which has the worst prognosis of all major cancers due in part to its aggressive, metastatic nature.18, 19 Surgical resection is the only potentially curative treatment option. However, most patients who undergo resection ultimately suffer recurrence with distant metastatic disease,20 and the median survival of patients with metastatic disease is measured in months, even with aggressive chemotherapy.21 The grave outcomes for PC patients justify the pursuit of more innovative therapeutic strategies.22, 23 Based on prior efficacy in non-cancerous disease models, we explored the ability of PAMAM-G3 to neutralize the downstream TLR-mediated and pro-invasive effects of extracellular nucleic acids and nucleic-acid-containing DAMPs in PC.
Results
Nucleic-Acid-Containing DAMPs Are Elevated in PC Patients with Advanced Disease and Post-treatment
We first quantified levels of cell-free DNA (cfDNA) and associated DAMPs such as nucleosomes in the sera of PC patients. We found that PC patients with early stage (radiographically localized) disease have mildly elevated cfDNA levels compared to healthy volunteers, whereas patients with advanced stage or metastatic disease have dramatically higher cfDNA levels (Figure 1A). In order to further analyze the pattern of cfDNA release in patients with early stage disease during treatment, we collected sera at four time points: baseline (before any treatment), 4–6 weeks after the end of preoperative (neoadjuvant) chemoradiation therapy (CRT), intraoperatively during surgical resection, and 1 week postoperatively. We found that serum cfDNA and nucleosome levels were increased in response to CRT in our PC patient population, regardless of clinical response to therapy (Figures 1A and S1). Moreover, these markers were further elevated in the PC patients intraoperatively and to even a greater degree postoperatively (Figure S2).
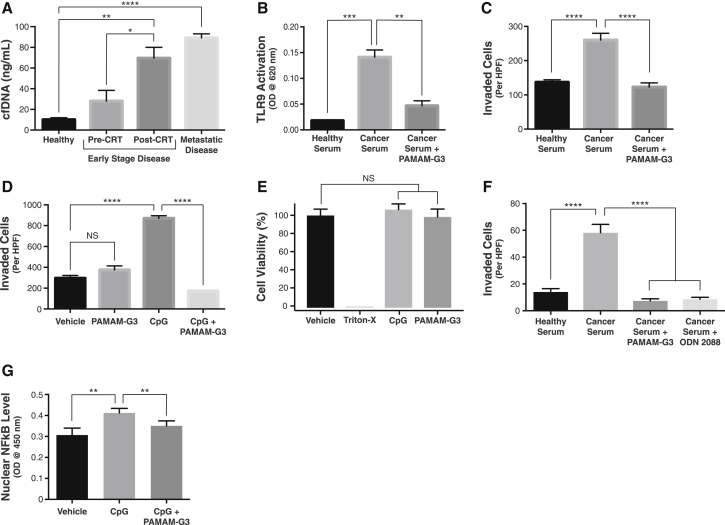
Figure 1.
PAMAM-G3 Inhibits TLR-9-Activating, Pro-invasive DAMPs in Pancreatic Cancer
(A) Serum cfDNA levels in healthy individuals, PC patients with localized, early-stage disease before and after CRT, and PC patients with known metastatic disease (n = 8 for all groups). (B) Activation of TLR-9-specific reporter cells by either healthy human sera or PC patient sera in the absence or presence of PAMAM-G3 (20 μg/mL). (C) Invasion of Panc1 PC cells in a transwell-Matrigel assay after addition of either healthy human sera or PC patient sera in the absence or presence of PAMAM-G3 (20 μg/mL). (D) Invasion of Panc1 cells after treatment with vehicle (media) or the TLR-9-specific agonist CpG ODN 2006 (5 μM) in the absence or presence of PAMAM-G3 (20 μg/mL). Effect of PAMAM-G3 alone on Panc1 cell invasion is also shown. (E) Cell viability as measured by Cell-titer Glo luminescence assay was determined after incubation of Panc1 PC cells with vehicle (media), CpG ODN (5 μM), PAMAM-G3 (20 μg/mL), or 1% Triton X-100 for 24 hr. (F) Invasion of KPC4580P PC cells in a transwell-Matrigel assay after addition of either healthy human sera, PC patient sera, or PC patient sera in the presence of PAMAM-G3 (20 μg/mL) or the TLR 9 inhibitor ODN 2088. (G) Effect of vehicle (media) or CpG ODN 2006 (5 μM) treatment, alone or in combination with PAMAM-G3 (20 μg/mL), on nuclear translocation of NF-κB in BxPC3 PC cells. Bar graphs denote mean ± SEM. TLR 9 activation, PC cell invasion and viability, and NF-κB translocation experiments were repeated at least three times, and figures depict a single representative experiment. HPF, high powered field; RLU, relative light units. ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; and NS, not significant, by two-tailed t test.
NABP Inhibits TLR 9 Activating and Pro-invasive DAMPs from PC Patients
To determine whether PC patient sera contains DAMPs such as cfDNA and nucleic-acid-protein complexes that may stimulate the TLR family of receptors, we quantified activation of TLRs 9 and 4 in response to patient sera. Although TLR 9 recognizes unmethylated, CpG motif-bearing cfDNA species, TLR 4 can be activated by nucleosomes, and both of these types of DAMPs are known to circulate at elevated levels in other cancer and inflammatory disease states.17, 24, 25, 26, 27 We observed that sera from PC patients induced significant activation of TLRs 9 and 4 as compared to normal sera (Figures 1B and S3). Addition of PAMAM-G3 significantly reduced stimulation of TLR 9 induced by the PC patient sera but not TLR 4 (Figures 1B and S3). These results suggest that nucleic acid DAMPs, such as cfDNA, present in PC patient sera induce TLR 9 activation in a manner that can be abrogated by PAMAM-G3.
Based on prior reports demonstrating that cfDNA/TLR 9 agonists can promote cancer cell invasion and metastasis,8, 9, 15, 28, 29, 30 we examined whether PC patient sera induces invasion of the Panc1 human PC cell line, which is known to express TLR 9, in a transwell-Matrigel assay.31 Addition of PC patient sera, but not normal human sera, to Panc1 cells significantly increased their invasion, and this effect could be mitigated by administration of PAMAM-G3 (Figure 1C). To support the notion that the pro-invasive phenotype induced by PC patient sera may be nucleic acid/TLR 9 mediated, we confirmed that the TLR-9-specific agonist CpG oligonucleotide (ODN) 2006 also induced invasion of Panc1 cells, which was in turn inhibited by PAMAM-G3 (Figure 1D). Importantly, treatment with PAMAM-G3 by itself did not affect tumor cell invasion. In addition, neither CpG ODN nor PAMAM-G3 treatment alone influenced PC cell proliferation (Figures 1E and S4). Similar results were obtained with other human (BxPC3 and MiaPaCa2) or murine (KPC4580P) PC cell lines, all of which were known or found to express TLR 9 (Figure S5).
To further investigate whether the pro-invasive activity induced by PC patient sera is TLR 9 dependent, we performed similar PC cell invasion assays in the presence of the TLR-9-specific oligonucleotide inhibitor ODN 2088.32 The TLR 9 inhibitor not only inhibited CpG ODN-mediated invasion of PC cells (Figure S6), it also inhibited cell invasion upon stimulation by PC patient sera as effectively as PAMAM-G3 (Figure 1F). These results are consistent with the notion that the neutralizing effects of PAMAM-G3 on PC patient sera-induced invasion are TLR 9 dependent.
To better understand the intracellular signaling mediating this TLR-9-mediated pro-invasive effect, we analyzed the activation of nuclear factor κB (NF-κB), a transcription factor and master regulator of the immune system that also contributes to tumor progression and metastasis in PC.33 Upregulation of NF-κB-dependent transcriptional pathways is classically observed upon pro-inflammatory TLR activation.34 Addition of CpG ODN to human BxPC3 and murine KPC4580P cells caused an increase in nuclear translocation of NF-κB, whereas co-treatment with PAMAM-G3 maintained nuclear NF-κB levels similarly to baseline (Figures 1F and S7A), suggesting that downstream activation of NF-κB in this manner may be responsible for the pro-invasive phenotype induced by TLR 9 ligation. Activation of other common TLR-dependent signaling pathways, such as the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), Src, or STAT3 pathways,35, 36, 37, 38, 39, 40 was not observed in response to CpG treatment in these PC tumor cell lines (Figure S7B).
NABP Binds and Neutralizes Pro-invasive PC Microvesicles
Extracellular nucleic acids and nucleic acid complexes also circulate in or on lipid vesicles, including microparticles (MPs) and exosomes, and these vesicles are also known to potentiate tumor cell invasion and metastasis.13, 16, 17, 41, 42 To determine if circulating microvesicles could also induce a similar pro-invasive effect, we isolated MPs from PC patient sera by differential centrifugation and tested their ability to stimulate Panc1 cell invasion. Both the vesicle-containing and vesicle-free fractions of PC patient sera contained notable cfDNA levels, confirming that cfDNA in PC patient sera is present in both vesicle-bound and unbound forms (Figure S8). Both serum fractions significantly promoted Panc1 cell invasion, effects that were in turn blocked by treatment with PAMAM-G3 (Figure 2A). These results indicate that PAMAM-G3 can neutralize pro-invasive nucleic-acid-containing DAMPs in PC patient sera in the form of free nucleic acids or nucleic-acid-protein complexes as well as microvesicles.
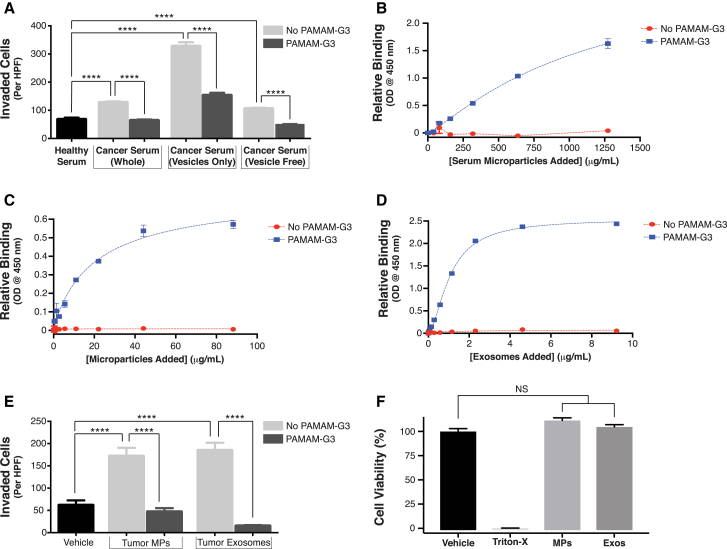
Figure 2.
PAMAM-G3 Binds and Inhibits Pro-invasive Microvesicles Isolated from Both Pancreatic Cancer Patient Sera and Cell Lines
(A) PC patient sera were separated by centrifugation into MP-containing and MP-free fractions (supernatant). Quantification of Panc1 cell invasion in a transwell-Matrigel assay after addition of healthy human serum, whole PC patient serum, and each serum fraction in the absence or presence of PAMAM-G3 (20 μg/mL). (B–D) Concentration-dependent binding of MPs isolated from PC patient sera (B) as well as MPs (C) and exosomes (D) secreted by the KPC4580P PC cell line to assay plates coated without or with PAMAM-G3 (0.2 μg/mL), quantified using a custom-designed ELISA as described in the Materials and Methods section. (E) Invasion of KPC4580P cells after addition of KPC4580P-derived MPs (100 ng/μL) and exosomes (50 ng/μL), alone or in combination with PAMAM-G3 (20 μg/mL) or media (vehicle) in a transwell-Matrigel assay. (F) Cell viability as measured by Cell-titer Glo luminescence assay was determined after incubation of KPC4580P cells with vehicle (media), KPC4580P-derived MPs (100 ng/μL) or exosomes (exos, 50 ng/μL), or 1% Triton X-100 for 24 hr. Bar graphs depict mean ± SEM; all experiments were repeated at least three times, and figures depict a single representative experiment. HPF, high powered field; RLU, relative light units. ****p < 0.0001 by two-tailed t test.
To our knowledge, a role for NABPs like PAMAM-G3 in modulating the activity of pro-invasive microvesicles has not been described before. To further investigate this observation, we assessed whether PAMAM-G3 can directly bind MPs isolated from PC patient sera. Assay plates pre-coated with PAMAM-G3 were incubated with varying concentrations of MPs, and microvesicle binding was quantified with a primary antibody recognizing a surface epitope. We found that PAMAM-G3 can directly bind MPs isolated from patient sera (Figure 2B) in a concentration-dependent manner. Analogously, we also demonstrated that PAMAM-G3 can bind tumor-derived MPs (Figure 2C) and exosomes (Figure 2D) harvested from cultured PC cells (KPC4580P) as well. Quantification of the zeta potential of MPs and exosomes secreted by KPC4580P cells showed that they bear a net electronegative surface charge, consistent with the presence of surface nucleic acids, as has been demonstrated for other cell-derived microvesicles,16 as well as binding by PAMAM-G3 (Figure S9).
Based on prior studies showing that tumor-derived microvesicles can promote autocrine invasion of tumor cells in other cancer types,41 we evaluated whether PC-derived microvesicles can also induce a similar pro-invasive phenotype and whether an NABP could mitigate this effect. In transwell-Matrigel invasion assays, addition of either MPs or exosomes (Figure 2E) harvested from cultured KPC4580P cells strongly induced tumor cell invasion in an autocrine fashion, and these effects were again significantly inhibited with PAMAM-G3 treatment. Neither addition of MPs nor exosomes alone influenced PC cell proliferation (Figure 2F). Thus, our findings suggest that PAMAM-G3 can neutralize the in vitro pro-invasive effects of both TLR-9-activating nucleic acid DAMPs and circulating microvesicles.
NABP Treatment Prevents Liver Metastasis and Reduces Levels of Pro-invasive DAMPs
Next, we sought to evaluate the therapeutic potential of PAMAM-G3 in a syngeneic immunocompetent murine model of PC metastasis. Established genetically engineered murine models (GEMM) of pancreatic adenocarcinoma such as the KPC model are poorly suited for studying experimental anti-metastatic therapies due to the limited occurrence of liver metastasis (∼30%) and highly variable tumor growth in those mice.43, 44 For this reason, we established a syngeneic immunocompetent murine model of PC metastasis using the murine KPC4580P cell line, which is derived from the KPC GEMM model of PC45 and bears the added benefit of constitutive luciferase expression, enabling quantitative measurement of tumor burden (Figure S10). In this model, C57BL/6 mice surgically implanted with 105 KPC4580P cells in their spleens reliably develop fulminant liver metastasis within 3 to 4 weeks. Because orthotopic implantation of PC tumor cells directly into the pancreas often does not yield consistent liver metastasis in murine models, as was also observed for the KPC4580P cell line, other investigators have used splenic injection to facilitate reproducible and timely liver metastasis.43 Although this splenic implantation model of PC liver metastasis is limited by the fact that it only captures the final metastatic steps of tumor cell extravasation and colonization,43 our in vitro studies demonstrating that PAMAM-G3 inhibits DAMP-induced invasion of PC tumor cells suggest that this model is suitable for investigating the polymer’s effect on in vivo liver metastasis.
Groups of 25 mice were treated twice weekly with intraperitoneal injections of PAMAM-G3 (20 mg/kg; 10-fold below the maximum tolerated dose as described elsewhere) or saline vehicle starting 48 hr after tumor cell implantation. Primary (spleen) and metastatic (liver) tumor burden were quantified by measuring tumor-specific ex vivo organ bioluminescence and weight after 3 weeks. As shown in Figures 3A and 3B, PAMAM-G3 treatment had no effect on primary tumor burden in the spleen. Remarkably, however, PAMAM-G3 treatment reduced liver metastatic burden by >90% (p < 0.0001) as measured by tumor cell bioluminescence (Figure 3A). Even more strikingly, the liver weights of PAMAM-G3-treated mice were normal and statistically equivalent to the weights of livers from healthy, age-matched tumor-free mice, whereas the liver weights of tumor-bearing mice not treated with the NABP increased by >70% (p < 0.001) (Figure 3B, left panel). In addition, the gross appearance of the livers from PAMAM-G3-treated mice was a stark contrast to the appearance of the livers from mice that did not receive the NABP, which were littered with metastatic disease (Figure 3B, right panel).
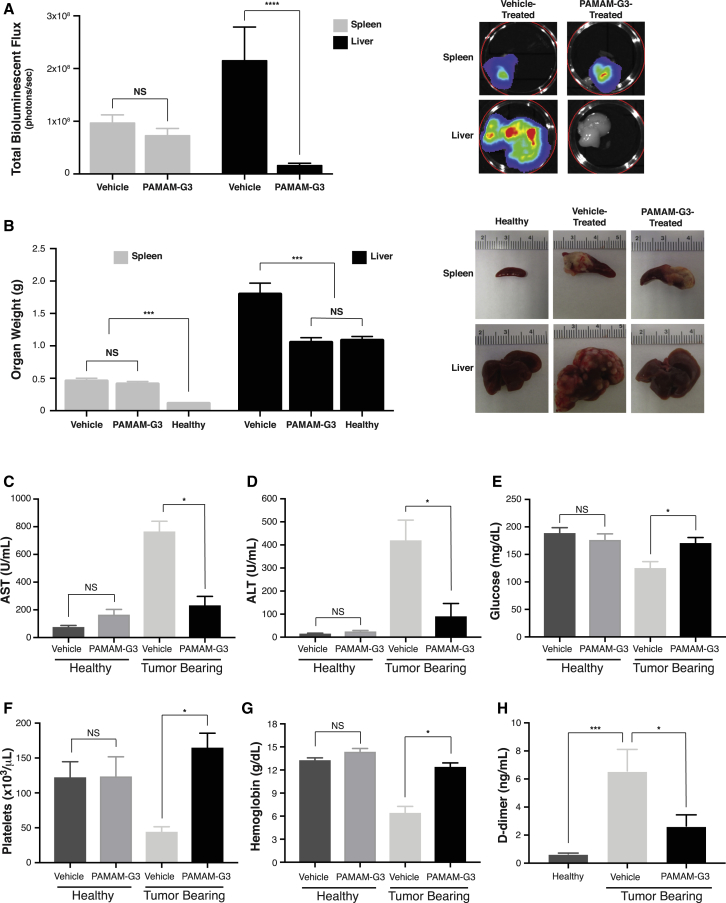
Figure 3.
PAMAM-G3 Inhibits In Vivo Liver Metastasis without Affecting Primary Tumor Growth in a Syngeneic Murine Model of Pancreatic Cancer
(A and B) Quantification of primary (spleen) and metastatic (liver) tumor burden in vehicle (saline) or PAMAM-G3-treated KPC4580P tumor-bearing mice, as measured by both total organ bioluminescence (A, left panel) and gross organ weight (B, left panel). Figures depict cumulative data obtained from three individual tumor treatment studies for a total of n = 25 mice per group (vehicle or PAMAM-G3 treated). Representative images illustrate bioluminescent intensity (A, right panel) and gross appearance (B, right panel) of spleen and liver organs from mice treated with or without PAMAM-G3. Representative photographs and mean gross weights of spleen and liver organs from healthy C57BL6 control mice (n = 5) are also displayed. (C–H) Quantification of plasma AST (C), ALT (D), glucose (E), platelet (F), hemoglobin (G), and D-dimer (H) levels from tumor-bearing mice treated with vehicle or PAMAM-G3. AST, ALT, glucose, platelet, and hemoglobin levels measured in healthy tumor-free mice treated with vehicle (n = 10) or PAMAM-G3 (n = 9) are also illustrated. Bar graphs depict mean ± SEM. ****p < 0.0001; ***p < 0.001; *p < 0.05; and NS, not significant, by Kruskal-Wallis test.
Because polycationic NABPs like PAMAM-G3 are primarily cleared by the liver,46 we measured plasma levels of the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzymes, which serve as biochemical markers of parenchymal damage (Figures 3C and 3D). We also looked for evidence of hepatic toxicity using two cohorts of mice that were healthy (tumor free), age-matched controls and treated with the same PAMAM-G3 or vehicle dosing scheme (20 mg/kg, twice weekly) and for the same duration (3 weeks) as the tumor-bearing mice. As shown in Figures 3C and 3D, vehicle-treated tumor-bearing mice displayed massive elevations in both AST and ALT compared to the vehicle-treated healthy controls, reflecting the large degree of hepatic dysfunction due to extensive tumor burden. In contrast, AST and ALT levels in tumor-bearing mice treated with PAMAM-G3 were significantly lower than their vehicle-treated counterparts and only mildly elevated compared to the healthy vehicle-treated controls. Importantly, no statistically significant increase in AST or ALT levels was detected in the cohort of healthy tumor-free mice given the same PAMAM-G3 regimen compared to those treated with vehicle, suggesting that PAMAM-G3 administration at the particular dosing scheme employed is not associated with liver injury. Likewise, considering that NABPs are secondarily cleared by the kidney,46 no differences in renal function as measured by plasma creatinine and blood urea nitrogen (BUN) levels were observed between these two groups, further highlighting that PAMAM-G3 treatment demonstrated no evidence of clearance organ toxicity (Figures S11A and S11B).
Tumor-bearing mice treated with PAMAM-G3 also displayed improvements in other clinical measures that reflect systemic illness compared to those treated with vehicle. For example, although the tumor-bearing vehicle-treated group showed evidence of the hypoglycemia that is often clinically associated with hepatic failure due to extensive tumor burden, plasma glucose levels in the tumor-bearing PAMAM-G3-treated group were statistically unchanged from the healthy vehicle-treated controls (Figure 3E). Furthermore, platelet counts and hemoglobin levels were significantly decreased in the vehicle-treated tumor-bearing mice compared to the tumor-free controls, whereas levels of D-dimer (a fibrin degradation product) were significantly elevated (Figures 3F–3H). Taken together, these results are suggestive of acute clinical illness in the vehicle-treated tumor-bearing mice, leading to a disseminated intravascular coagulation (DIC)-like phenotype, which is consistent with the extensive tumor burden observed in these mice and the fact that PC cell lines are associated with high expression of the procoagulant tissue factor.47 In contrast, the PAMAM-G3-treated tumor-bearing mice showed no evidence of thrombocytopenia or anemia compared to the healthy controls and only mildly elevated D-dimer levels. Beyond these systemic markers, the PAMAM-G3-treated PC mice were also visibly healthier than mice not given the NABP in qualitative measures, exhibiting normal activity and posture as well as positive response to stimulation. Moreover, no significant differences were observed between the healthy tumor-free mice treated with vehicle or PAMAM-G3 in regards to glucose, platelet, hemoglobin, albumin, or white blood cell levels (Figures 3E– 3G, S11C, and S11D), further underscoring the absence of any observed toxicity associated with PAMAM-G3 administration at the employed dosages.
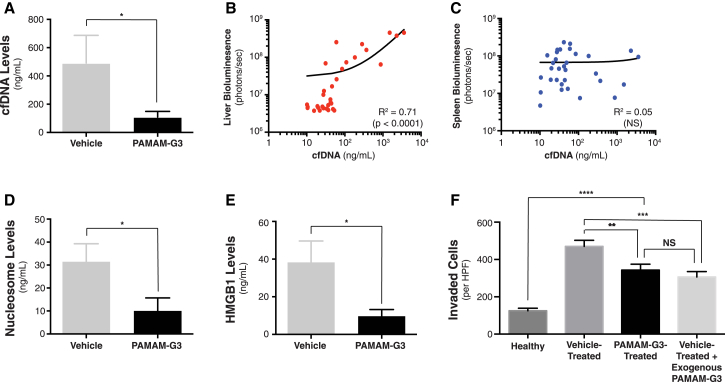
Mice treated with PAMAM-G3 also showed significantly reduced levels of plasma cfDNA (Figure 4A), and cfDNA levels in all mice correlated with metastatic tumor burden (Figure 4B, R2 = 0.71), but not primary tumor burden (Figure 4C, R2 = 0.05). Moreover, mice treated with the NABP also displayed significantly lower levels of circulating nucleosomes and high mobility group box 1 (HMGB1) protein, which both represent pro-inflammatory nucleic acid associated DAMPs that have been implicated in promoting tumor invasion or metastasis3, 11, 28, 48 (Figures 4D and 4E). Importantly, pooled sera collected from vehicle-treated tumor-bearing mice triggered significantly more potent invasion of KPC4580P cells in vitro in comparison to sera isolated from mice treated with PAMAM-G3 or from healthy C57BL/6 controls (Figure 4F). Furthermore, exogenous addition of PAMAM-G3 to sera from vehicle-treated mice abrogated this pro-invasive effect on KPC4580P cells, reducing invasion levels similarly to those observed in the presence of sera from tumor-bearing mice treated with PAMAM-G3, but still higher than that seen with healthy mouse sera (Figure 4F). These findings support a proposed mechanism, wherein systemic administration of PAMAM-G3 in tumor-bearing mice may aid in neutralizing circulating factors, such as extracellular nucleic acids, nucleic-acid-protein complexes, and microvesicles, which promote tumor invasion and metastasis.
Figure 4.
PAMAM-G3 Reduces Circulating Levels of Pro-invasive DAMPs in a Syngeneic Murine Model of Pancreatic Cancer
(A) Quantification of plasma cfDNA levels in KPC4580P tumor-bearing mice treated with vehicle or PAMAM-G3. Figure depicts cumulative data obtained from three individual tumor treatment studies for a total of n = 25 mice per group (vehicle or PAMAM-G3 treated). (B and C) Correlation between cfDNA levels and tumor burden in the liver (B) or spleen (C) in all mice. Data points in (B) and (C) reflect individual mice in the study. (D and E) Measurement of plasma nucleosome (D) and HMGB1 (E) levels in mice treated with or without PAMAM-G3. (F) In vitro transwell-Matrigel invasion of KPC4580P cells in the presence of pooled sera isolated from either healthy C57BL6 mice, PAMAM-G3-treated tumor-bearing mice, or vehicle-treated tumor-bearing mice, with or without exogenous PAMAM-G3 (20 μg/mL addition). Bar graphs depict mean ± SEM. ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; and NS, not significant, by two-tailed t test (A–F). cfDNA raw data in (A) were log-transformed in order to satisfy the normality requirement for parametric analysis by two-tailed t test.
Discussion
Here, we present the concept of using an NABP (PAMAM-G3) as a potential therapeutic to inhibit metastatic progression in PC. Our data indicate that cfDNA present in PC patient sera is a potent activator of TLR 9, which is expressed by PC cells and whose activation in the tumor stroma has been shown to potentiate PC metastasis in vivo.7 The activation of TLR 9 leads to PC cell invasion in vitro, a critical step in tumor progression and metastasis. We observed that activation of TLR 9 by CpG ODN led to increased nuclear translocation of NF-κB, which has been associated with the expression of numerous pro-metastatic genes.33 All of these pro-metastatic effects of TLR 9 activation are abrogated by PAMAM-G3 treatment in vitro. However, multiple redundant TLRs as well as non-TLR receptors exist that are activated by cfDNA, cfRNA, and associated proteins.4 By directly neutralizing nucleic-acid-containing DAMPs upstream of these pathways, PAMAM-G3 can potentially inhibit this network.2
Our group has previously investigated the mechanism by which PAMAM-G3 neutralizes the downstream effects of TLR 9 agonists such as CpG ODN.2 We observed that PAMAM-G3 inhibits the functional activity of B-type CpG ODN by both decreasing its cellular uptake and re-localizing the fraction that is taken up by cells from the endosomal compartment to the nucleus, thereby preventing its subsequent interaction with TLR 9.2 We expect that these multifaceted actions underlie the ability of PAMAM-G3 to inhibit nucleic acid DAMP-induced PC cell invasion in vitro and PC metastasis in vivo, as observed in the present study.
Extracellular nucleic acids and nucleic acid complexes also circulate in or on lipid microvesicles. These particles can potentiate metastasis by acting on tumor cells directly as well as pre-conditioning the pre-metastatic site for tumor implantation.13, 14, 41 Our data show that these particles have a net electronegative charge, presumably due to the presence of negatively charged nucleic acids on the vesicle surface as other studies have demonstrated,16 allowing for direct binding by an NABP-like PAMAM-G3. Furthermore, we show that PAMAM-G3 can neutralize the pro-invasive effects of microparticles and exosomes on tumor cells in culture. Thus, NABPs also represent a useful tool for studying and modulating the pro-metastatic effects of microvesicles.
The inhibitory effects of NABPs on PC in vitro motivated us to study them in a syngeneic immunocompetent murine model of PC metastasis. By utilizing the bioluminescent KPC4580P cell line derived from the clinically relevant KPC genetically engineered murine model of pancreatic adenocarcinoma, this model captures the critical genetic hallmarks of human PC disease. Likewise, splenic implantation of the PC cells facilitates a robust and reliable metastatic time course, making the model well-suited for assessing the efficacy of a potential anti-metastatic therapeutic strategy.44 We found that PAMAM-G3-treated mice had dramatically less metastatic disease in the liver than vehicle-control-treated mice. The therapeutic benefit of PAMAM-G3 administration was not only reflected by the quantifiable reduction in tumor burden, but also by substantial improvements in plasma indicators of liver damage and other clinical markers of illness. However, PAMAM-G3 therapy did not significantly affect primary tumor growth in the spleen, suggesting that its effect targets the metastatic process and not proliferation, consistent with our in vitro observations. Despite the limitation that our model does not fully recapitulate the natural course of PC metastasis on account of splenic implantation of the PC cells, our model successfully captures the final metastatic steps of tumor cell extravasation and colonization. In this light, we believe that NABPs may represent an innovative anti-metastatic therapy. Our observations are also consistent with studies demonstrating that extracellular DNA and DNA-protein complexes promote cancer progression9, 15, 28, 29, 49, 50, 51 and that DNase I treatment can reduce metastasis in other tumor models.9, 15, 29 However, our findings also suggest that using an NABP to target circulating nucleic acids and associated complexes that facilitate metastasis may offer the added and important benefit of neutralizing pro-metastatic contributions of tumor-derived microvesicles, which may potentiate its robust anti-metastatic therapeutic response in vivo.15, 29
PC is a devastating disease, largely due to its aggressively metastatic nature. Neoadjuvant (preoperative) and adjuvant (postoperative) therapy with chemotherapy and with or without radiation therapy are often given to patients with localized disease, with the goal of eradicating micrometastatic disease.52 However, even with aggressive preoperative and postoperative therapy, most patients ultimately develop and succumb to metastatic disease. Studies have also demonstrated that tumor manipulation during surgical resection may result in the release of circulating tumor cells in colorectal and lung cancers.9, 53 Our data suggest that two of the treatments we use for treatment of localized disease (chemoradiation therapy and surgical resection) may lead to increased levels of circulating DAMPs that, in turn, promote metastatic disease. Unfortunately, the treatments we are using to try to cure patients with PC may predispose them to distant metastasis. NABPs could be combined with standard treatment regimens to inhibit the effects of treatment-induced DAMPs. For example, NABP treatment could be initiated with neoadjuvant therapy and/or prior to surgical resection, potentially decreasing the risk of distant metastasis and increasing the proportion of patients who are cured. The anti-thrombotic effects of NABPs that we have previously observed could be an important secondary benefit in this population at high-risk for thrombotic complications.54, 55 Despite many improvements in the treatment of PC, we have made little impact on the cure rate. Thus, NABPs represent an inventive approach to modulate the tumor microenvironment and potentially reduce metastasis in patients with localized PC. Moreover, because certain NABPs have already been evaluated clinically as vehicles for small interfering RNA (siRNA) delivery in cancer patients, with minimal evidence of toxicity, a precedent exists for safely translating these agents to the bedside, albeit not without pre-assembling the NABP into a nucleic-acid-containing complex.56 Thus, given the major unmet need for therapies that limit metastasis, particularly in PC, we believe that clinical development of this NABP-based approach is feasible.
Although concerns regarding the safety and clinical use of NABPs like PAMAM-G3 have been raised,46 the work presented here, together with prior studies by our group and others in the literature, may serve to alleviate some of these reservations. For example, although it is known that polycationic dendrimers such as PAMAM-G3 are primarily cleared by the liver and kidney and can be toxic to these organs,46 the toxicity of these polymers is dosage dependent and the maximum tolerated in vivo doses of PAMAM-G3 in prior murine studies were reportedly up to 1,000 mg/kg, 50 times higher than the efficacious dose used in our current study and previous studies in other disease models.5, 46, 57 Thus, the dosages of PAMAM-G3 needed to inhibit metastatic spread in the presented model of PC are well within the limits of the maximally tolerated dose. Furthermore, herein we show that healthy non-tumor-bearing mice treated with the same PAMAM-G3 dosing scheme displayed no laboratory abnormalities in hepatic or renal function or other common clinical markers over the duration of the study, suggesting that the NABP was well-tolerated. These results highlight that an NABP-based therapeutic strategy can be safely administered at doses that may prove clinically efficacious.
Although the results of the study provide an encouraging proof of concept demonstrating the potential value of an NABP as an agent for combating metastatic disease in a cancer model, it will be informative to explore the generalized therapeutic utility of NABPs in other inflammatory cancer types, including breast cancer or soft tissue sarcoma. Moreover, future work should also aim to exploit the binding properties of an NABP-like PAMAM-G3 that enable it to capture an array of circulating bioactive molecules. Characterizing the diversity and composition of these extracellular DAMPs or microvesicles will aid in developing a more thorough systems-biology-driven understanding of tumor progression and metastasis in vivo. Because an NABP-based strategy inhibits multiple pro-metastatic pathways, elucidating a complete mechanistic understanding of the agent’s in vivo actions is challenging, but this property may in fact represent a therapeutic advantage, given the ease with which tumors evolve drug resistance to treatments that target only a single pathway. In addition, although we selected a well-characterized NABP for these initial studies, we recognize that PAMAM-G3 and other established NABPs were not originally identified on the basis of their ability to bind and neutralize extracellular nucleic acids and microvesicles that play a pathological role in cancer. Thus, we believe the findings of this study present an opportunity for exploiting existing polymer screening libraries58, 59, 60 to identify novel NABPs that may have enhanced efficacy for treating cancer.
Materials and Methods
Patient Blood Collection
With approval from the Duke University Institutional Review Board, blood was collected from patients with PC after presentation with localized (early stage) or advanced disease and—in those patients with early stage disease—at various time points in their treatment course, including before and after neoadjuvant (preoperative) chemoradiation therapy, intraoperatively during surgical resection immediately post-Kocher maneuver, and 1 week postoperatively. Neoadjuvant chemoradiation therapy consisted of 45–50 Gy of external beam radiation therapy delivered over approximately 5 weeks with concurrent oral capecitabine as a radiation sensitizer.61 Patients with advanced disease had undergone a variety of therapies, including chemotherapy and/or radiation therapy.
Reagents
Generation 3.0 polyamidoamine dendrimer solution (PAMAM-G3) was purchased from Sigma Aldrich (Cat. # 412422). The TLR agonists CpG ODN 2006, CpG ODN 1826, ODN 2088, Poly I:C, lipopolysaccharide (LPS), and R848 were purchased from Invivogen.
PC Cell Lines
Human BxPC3, Panc1, MiaPaCa2, and AsPc1 PC cell lines were purchased from ATCC. The luciferase-expressing KPC4580P murine PC cell line, derived from the KPC genetic mouse model of pancreatic ductal adenocarcinoma (a gift of Jen Jen Yeh, MD), was generated as described in the Supplemental Materials and Methods, along with information regarding growth media for all cell lines used.
Quantification of Cell-free DNA, Nucleosome, and HMGB1 Levels
Total DNA was isolated from sera using the DNA Blood Mini Kit (QIAGEN) and quantified using the PicoGreen Staining Kit (Life Technologies). Nucleosome levels were quantified using the Cell Death ELISA Plus Kit (Roche). HMGB1 levels were quantified using an ELISA kit (Chondrex).
TLR Activation Assays
HEK-Blue TLR 4 and 9 reporter cell lines were purchased from Invivogen, and activation in response to control agonists or human sera was determined according to the manufacturer’s instructions. These cells stably co-express a TLR gene and an NF-κB-inducible SEAP (secreted embryonic alkaline phosphatase) reporter gene that can be monitored using SEAP detection media. Additional details are provided in the Supplemental Materials and Methods. Briefly, these cells were plated in 96-well plates at a density of 100,000 cells per well and treated for 18–24 hours with either (1) media alone, (2) a control agonist for each given TLR (LPS [1 μg/mL] for TLR 4 and CpG ODN [5 μM] for TLR 9), (3) cancer patient sera (3.5 μL), (4) normal human sera (3.5 μL), (5) media + PAMAM-G3 (20 μg/mL), (6) control agonist + PAMAM-G3 (20 μg/mL), (7) cancer patient sera (3.5 μL) + PAMAM-G3 (20 μg/mL), or (8) normal patient sera (3.5 μL) + PAMAM-G3 in a final volume of 100 μL. The cell supernatant was subsequently collected and mixed with Quantiblue (Invivogen) at a 60:40 v:v ratio and incubated for 5 hr at 37°C, after which time absorbance at 650 nm was measured using a Spectramax i3 plate reader (Molecular Devices).
Microparticle and Exosome Isolation from Cultured PC Cell Lines and PC Patient Sera
Further details are provided in the Supplemental Materials and Methods. Briefly, KPC-4580P cells were cultured to confluence, at which time media was replaced with complete medium supplemented with exosome-depleted FBS. After 72 hr, the supernatant was collected and centrifuged at 4,000 × g for 5 min to deplete cell debris. The residual supernatant was centrifuged at 20,000 × g for 20 min at 4°C to pellet MPs. The supernatant was removed and saved for exosome isolation. The MP pellet was washed once with PBS (−/−) before collection and resuspension in PBS. The supernatant from the initial MP spin was centrifuged at 100,000 × g for 2 hr at 4°C to pellet exosomes. After discarding the supernatant, the exosome pellet was washed once with PBS before collection and resuspension in PBS. The protein concentration of the MPs and exosomes was quantified via Pierce BCA assay (Thermo Fisher Scientific). To isolate MPs from human sera, sera was similarly centrifuged at 20,000 × g for 20 min at 4°C, washed, and resuspended in PBS.
Transwell-Matrigel Invasion Chamber Assays
Transwell-Matrigel invasion chambers were purchased from Corning and assays were performed according to the manufacturer’s instructions. Briefly, the invasion chambers were first incubated with serum-free media in the top and bottom chambers at 37°C to allow the Matrigel to solidify and the two chambers to equilibrate in preparation for the addition of PC cells and various agonists and/or inhibitors, such as CpG ODN, PC-cell-line-derived MPs and exosomes, PAMAM-G3, or ODN 2088. The human (BxPC3, MiaPaca2, PANC-1, and AsPC-1) or mouse (KPC-4580P) PC cell lines were trypsinized, resuspended, and diluted in serum-free media. After aspiration of media from both chambers, cells were plated in the top chamber at a density of 50,000 cells/well (500-μL final volume) in the presence of serum-free media alone or various combinations of agonists and/or PAMAM-G3 (20 μg/mL) or ODN 2088 (100 μM). Agonists tested included CpG ODN (5 μM), PC-cell-line-derived MPs or exosomes (isolated as described above), PC patient sera or normal human sera (50 μL), or mouse sera (45 μL). 750 μL of complete media was added to the bottom chambers. The plated invasion chambers were incubated at 37°C for 24 hr, after which time media was aspirated from the top chambers. The top chambers were then removed and treated with 10% formaldehyde to fix the invaded cells on the bottom surface of the chamber, washed with PBS, and stained with crystal violet solution (5% w/v crystal violet, 25% v/v methanol). The chambers were then washed thoroughly in deionized water, and membranes were imaged using an inverted light microscope (Olympus IX50) at 4X magnification. 10 random images of each membrane were taken using an eyepiece camera (Dino-Eye AM7023B). The cells in the images were then counted using an ImageJ algorithm unique for each cell line (http://imagej.net/Particle_Analysis). Additional details are provided in the Supplemental Materials and Methods.
Analysis of Nuclear NF-κB Translocation
KPC-4580P cells (2 × 106 cells per plate) and BxPC3 cells (2 × 105 cells per plate) were seeded in 35-mm plates overnight. The cells were then treated with serum-free media alone, CpG ODN (1826 or 2006) (5 μM), or CpG (5 μM) and PAMAM-G3 (20 μg/mL) for 1 hr. Cell nuclei were then isolated using a nuclear isolation kit (Active Motif) using the manufacturer’s guidelines. The protein content of each nuclear extract was quantified and standardized using a Protein Quantification Kit (Active Motif). The nuclear extract was then used to quantify nuclear translocation of NF-κB via ELISA. For the murine nuclear extracts, nuclear translocation of p50 NF-κB was quantified using the Pierce p50 Transcription Factor Assay Kit (Thermo Fisher Scientific) using the manufacturer’s instructions. For the human nuclear extracts, nuclear translocation of p65 NF-κB was quantified using the p65 TransAM ELISA (Active Motif).
Analysis of Cell Viability
In order to replicate conditions used for the invasion chamber assays, Panc1 or KPC4580P cells were seeded overnight in 96-well assay plates at a density of 50,000 cells/well. Panc1 cells were then treated with CpG ODN 2006 (5 μM), PAMAM-G3 (20 μg/mL), 1% Triton X-100, or media (12 wells per treatment group). After 24 hr, cell viability was measured according to the Cell Titer Glo Luminescent Cell Viability Assay (Promega) according to the manufacturer’s instructions. KPC4580P cells were treated with CpG ODN 1826 (5 μM), KPC4580P-derived MPs (100 ng/μL), KPC4580P-derived exosomes (50 ng/μL), PAMAM-G3 (20 μg/mL), or media (4 wells per treatment group). After 24 hr, cell viability was measured according to the Cell Titer Glo Luminescent Cell Viability Assay (Promega) according to the manufacturer’s instructions.
ELISA for Detection of PAMAM-G3 Binding to MPs
The binding of MPs and exosomes to PAMAM-G3 was quantified using a custom-designed ELISA assay and is described in detail in the Supplemental Materials and Methods. MPs and exosomes were isolated from KPC4580P PC cell cultures as described above. 96-well high-binding, flat-bottom plates (Corning) were coated overnight at 4°C with PAMAM-G3 diluted into PBS (−/−) at varying concentrations. After the wells were washed with PBS, incubated for 2 hr at room temperature (RT) with blocking buffer (PBS plus 5% BSA and 0.05% Tween 20), and washed again with PBS, the wells were incubated with varying concentrations of MPs or exosomes for 2 hr at RT. After a subsequent washing step, the wells were then incubated with either the polyclonal goat immunoglobulin G (IgG) against murine tissue factor (AF3178, R&D Systems) for MP detection or the monoclonal mouse IgG antibody against phosphatidylserine (05-719, Millipore) for exosome detection, followed by washing and detection using a horseradish-peroxidase-conjugated secondary antibody, and finally quantification of absorbance at 450 nm after TMB substrate addition. The binding of PC patient-derived MPs to PAMAM was quantified analogously.
Syngeneic Murine Model of PC Metastasis
All animal experiments were performed in compliance with Duke Institutional Animal Care and Use Committee (IACUC) protocols. Briefly, for the metastasis studies, KPC4580P luciferase expressing, murine PC cells (described above) were surgically implanted into the spleens of 12-week-old female C57BL6 mice. Starting on day 2 after tumor cell implantation, mice were treated twice per week with either intraperitoneal PAMAM-G3 (20 mg/kg) or saline for 3 weeks or until humane endpoints were reached, at which time the mice were sacrificed. Liver and spleen were harvested from each mouse for assessment of gross organ weight and ex vivo quantification of bioluminescent intensity (IVIS Kinetic). Just prior to sacrifice, caudal vena cava blood was collected from each mouse for analysis of blood cell counts (Heska HemaTrue blood analyzer), plasma cfDNA (described above), nucleosome levels (described above), HMGB1 levels (described above), or chemistry (performed by Duke DLAR Veterinary Diagnostic Laboratory) or for serum invasion assays (described above). Detailed description of the tumor cell implantation procedure is provided in the Supplemental Materials and Methods. For PAMAM-G3 toxicity studies, 12-week-old female C57BL6 mice were treated twice per week with either intraperitoneal PAMAM-G3 (20 mg/kg) or saline for 3 weeks, at which time the mice were sacrificed. Just prior to sacrifice, submandibular blood was collected from each mouse for blood cell count analysis or plasma chemistry analysis as described above.
Statistical Analysis
All data were plotted and analyzed using GraphPad Prism or JMP software. Data were all tested for normality prior to statistical analyses. If normally distributed, differences between groups were compared using a two-tailed Student’s t test. If the data were not normally distributed, as in the case of the in vivo organ bioluminescence and gross weight data, statistical analysis between groups was performed using the Kruskal Wallis test.
Author Contributions
I.N., R.G., R.R.W., and B.A.S. designed the experiments. I.N., R.G., J.E.M., A.M., R.E.R., and D.C.R. performed the experiments. S.G.H. generated the KPC4580P cell line. I.N., R.G., D.S.P., J.L., R.R.W., and B.A.S. interpreted the data. I.N., R.G., R.R.W., and B.A.S. wrote the manuscript.
Conflicts of Interest
Duke University has applied for a patent on this strategy to limit cancer progression.
Acknowledgments
This work was supported in part by the Elsa Pardee Foundation (to R.R.W.), Department of Defense (DoD) Breast Cancer Research Program (BCRP) award W81WH-16-1-0513 (to B.A.S.), and NIH grants F30HL127977 (to R.G.), T32 GM007171 (to R.G. and I.N.), R01HL065222 (to B.A.S.), and U19AI067798 (to B.A.S.). We also acknowledge Duke Cancer Institute grant P30-CA014236 for support of the in vivo imaging facility. We would also like to acknowledge Jen Jen Yeh, MD, for her help with acquiring the KPC4580P cell line, the Duke DLAR Veterinary Diagnostic Lab for assistance with murine plasma chemistry analysis, Duke Cancer Institute grant P30-CA014236 for support of the Duke Optical Molecular Imaging and Analysis facility, and George Pitoc, Eda Holl, and Smita Nair for useful discussion.
Footnotes
Supplemental Information includes Supplemental Materials and Methods and eleven figures and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.02.018.
Contributor Information
Rebekah R. White, Email: rewhite@ucsd.edu.
Bruce A. Sullenger, Email: bruce.sullenger@duke.edu.
Supplemental Information
References
- 1.Morille M., Passirani C., Vonarbourg A., Clavreul A., Benoit J.-P.P. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2008;29:3477–3496. doi: 10.1016/j.biomaterials.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 2.Lee J., Sohn J.W., Zhang Y., Leong K.W., Pisetsky D., Sullenger B.A. Nucleic acid-binding polymers as anti-inflammatory agents. Proc. Natl. Acad. Sci. USA. 2011;108:14055–14060. doi: 10.1073/pnas.1105777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pisetsky D.S. The origin and properties of extracellular DNA: from PAMP to DAMP. Clin. Immunol. 2012;144:32–40. doi: 10.1016/j.clim.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawasaki T., Kawai T., Akira S. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol. Rev. 2011;243:61–73. doi: 10.1111/j.1600-065X.2011.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holl E.K., Shumansky K.L., Borst L.B., Burnette A.D., Sample C.J., Ramsburg E.A., Sullenger B.A. Scavenging nucleic acid debris to combat autoimmunity and infectious disease. Proc. Natl. Acad. Sci. USA. 2016;113:9728–9733. doi: 10.1073/pnas.1607011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harmey J.H., Bucana C.D., Lu W., Byrne A.M., McDonnell S., Lynch C., Bouchier-Hayes D., Dong Z. Lipopolysaccharide-induced metastatic growth is associated with increased angiogenesis, vascular permeability and tumor cell invasion. Int. J. Cancer. 2002;101:415–422. doi: 10.1002/ijc.10632. [DOI] [PubMed] [Google Scholar]
- 7.Zambirinis C.P., Levie E., Nguy S., Avanzi A., Barilla R., Xu Y., Seifert L., Daley D., Greco S.H., Deutsch M. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J. Exp. Med. 2015;212:2077–2094. doi: 10.1084/jem.20142162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilvesaro J.M., Merrell M.A., Li L., Wakchoure S., Graves D., Brooks S., Rahko E., Jukkola-Vuorinen A., Vuopala K.S., Harris K.W., Selander K.S. Toll-like receptor 9 mediates CpG oligonucleotide-induced cellular invasion. Mol. Cancer Res. 2008;6:1534–1543. doi: 10.1158/1541-7786.MCR-07-2005. [DOI] [PubMed] [Google Scholar]
- 9.Tohme S., Yazdani H.O., Al-Khafaji A.B., Chidi A.P., Loughran P., Mowen K., Wang Y., Simmons R.L., Huang H., Tsung A. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76:1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarzenbach H., Hoon D.S.B., Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer. 2011;11:426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 11.Holdenrieder S., Nagel D., Schalhorn A., Heinemann V., Wilkowski R., von Pawel J., Raith H., Feldmann K., Kremer A.E., Müller S. Clinical relevance of circulating nucleosomes in cancer. Ann. N Y Acad. Sci. 2008;1137:180–189. doi: 10.1196/annals.1448.012. [DOI] [PubMed] [Google Scholar]
- 12.Diaz L.A., Jr., Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J. Clin. Oncol. 2014;32:579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K., Becker A., Hoshino A., Mark M.T., Molina H. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janowska-Wieczorek A., Wysoczynski M., Kijowski J., Marquez-Curtis L., Machalinski B., Ratajczak J., Ratajczak M.Z. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int. J. Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 15.Wen F., Shen A., Choi A., Gerner E.W., Shi J. Extracellular DNA in pancreatic cancer promotes cell invasion and metastasis. Cancer Res. 2013;73:4256–4266. doi: 10.1158/0008-5472.CAN-12-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ullal A.J., Reich C.F., 3rd, Clowse M., Criscione-Schreiber L.G., Tochacek M., Monestier M., Pisetsky D.S. Microparticles as antigenic targets of antibodies to DNA and nucleosomes in systemic lupus erythematosus. J. Autoimmun. 2011;36:173–180. doi: 10.1016/j.jaut.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Thierry A.R., El Messaoudi S., Gahan P.B., Anker P., Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35:347–376. doi: 10.1007/s10555-016-9629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varghese A.M., Lowery M.A., Yu K.H., O’Reilly E.M. Current management and future directions in metastatic pancreatic adenocarcinoma. Cancer. 2016;122:3765–3775. doi: 10.1002/cncr.30342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 20.Oettle H., Neuhaus P., Hochhaus A., Hartmann J.T., Gellert K., Ridwelski K., Niedergethmann M., Zülke C., Fahlke J., Arning M.B. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 21.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuveson D.A., Neoptolemos J.P. Understanding metastasis in pancreatic cancer: a call for new clinical approaches. Cell. 2012;148:21–23. doi: 10.1016/j.cell.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Varadhachary G.R., Wolff R.A. Current and evolving therapies for metastatic pancreatic cancer: are we stuck with cytotoxic chemotherapy? J. Oncol. Pract. 2016;12:797–805. doi: 10.1200/JOP.2016.015586. [DOI] [PubMed] [Google Scholar]
- 24.Marsman G., Zeerleder S., Luken B.M. Extracellular histones, cell-free DNA, or nucleosomes: differences in immunostimulation. Cell Death Dis. 2016;7:e2518. doi: 10.1038/cddis.2016.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger R., Fiegl H., Goebel G., Obexer P., Ausserlechner M., Doppler W., Hauser-Kronberger C., Reitsamer R., Egle D., Reimer D. Toll-like receptor 9 expression in breast and ovarian cancer is associated with poorly differentiated tumors. Cancer Sci. 2010;101:1059–1066. doi: 10.1111/j.1349-7006.2010.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian J., Avalos A.M., Mao S.Y., Chen B., Senthil K., Wu H., Parroche P., Drabic S., Golenbock D., Sirois C. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 27.Yuan Y., Yang M., Wang K., Sun J., Song L., Diao X., Jiang Z., Cheng G., Wang X. Excessive activation of the TLR9/TGF-β1/PDGF-B pathway in the peripheral blood of patients with systemic lupus erythematosus. Arthritis Res. Ther. 2017;19:70. doi: 10.1186/s13075-017-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Yan W., Tohme S., Chen M., Fu Y., Tian D., Lotze M., Tang D., Tsung A. Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9. J. Hepatol. 2015;63:114–121. doi: 10.1016/j.jhep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J., Wysocki R.W., Amoozgar Z., Maiorino L., Fein M.R., Jorns J., Schott A.F., Kinugasa-Katayama Y., Lee Y., Won N.H. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016;8:361ra138. doi: 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merrell M.A., Ilvesaro J.M., Lehtonen N., Sorsa T., Gehrs B., Rosenthal E., Chen D., Shackley B., Harris K.W., Selander K.S. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol. Cancer Res. 2006;4:437–447. doi: 10.1158/1541-7786.MCR-06-0007. [DOI] [PubMed] [Google Scholar]
- 31.Wu H.Q., Wang B., Zhu S.K., Tian Y., Zhang J.H., Wu H.S. Effects of CPG ODN on biological behavior of PANC-1 and expression of TLR9 in pancreatic cancer. World J. Gastroenterol. 2011;17:996–1003. doi: 10.3748/wjg.v17.i8.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stunz L.L., Lenert P., Peckham D., Yi A.K., Haxhinasto S., Chang M., Krieg A.M., Ashman R.F. Inhibitory oligonucleotides specifically block effects of stimulatory CpG oligonucleotides in B cells. Eur. J. Immunol. 2002;32:1212–1222. doi: 10.1002/1521-4141(200205)32:5<1212::AID-IMMU1212>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 33.Prabhu L., Mundade R., Korc M., Loehrer P.J., Lu T. Critical role of NF-κB in pancreatic cancer. Oncotarget. 2014;5:10969–10975. doi: 10.18632/oncotarget.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawai T., Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Rakoff-Nahoum S., Medzhitov R. Toll-like receptors and cancer. Nat. Rev. Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 36.Kawai T., Akira S. Toll-like receptor downstream signaling. Arthritis Res. Ther. 2005;7:12–19. doi: 10.1186/ar1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grivennikov S.I., Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lowell C.A. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb. Perspect. Biol. 2011;3:3. doi: 10.1101/cshperspect.a002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner E.F., Nebreda A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 41.Menck K., Scharf C., Bleckmann A., Dyck L., Rost U., Wenzel D., Dhople V.M., Siam L., Pukrop T., Binder C., Klemm F. Tumor-derived microvesicles mediate human breast cancer invasion through differentially glycosylated EMMPRIN. J. Mol. Cell Biol. 2015;7:143–153. doi: 10.1093/jmcb/mju047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janowska-Wieczorek A., Marquez-Curtis L.A., Wysoczynski M., Ratajczak M.Z. Enhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cells. Transfusion. 2006;46:1199–1209. doi: 10.1111/j.1537-2995.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 43.Soares K.C., Foley K., Olino K., Leubner A., Mayo S.C., Jain A., Jaffee E., Schulick R.D., Yoshimura K., Edil B., Zheng L. A preclinical murine model of hepatic metastases. J. Vis. Exp. 2014:51677. doi: 10.3791/51677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponz-Sarvise M., Tuveson D.A., Yu K.H. Mouse models of pancreatic ductal adenocarcinoma. Hematol. Oncol. Clin. North Am. 2015;29:609–617. doi: 10.1016/j.hoc.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Hingorani S.R., Wang L., Multani A.S., Combs C., Deramaudt T.B., Hruban R.H., Rustgi A.K., Chang S., Tuveson D.A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 46.Sadekar S., Ghandehari H. Transepithelial transport and toxicity of PAMAM dendrimers: implications for oral drug delivery. Adv. Drug Deliv. Rev. 2012;64:571–588. doi: 10.1016/j.addr.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J.G., Geddings J.E., Aleman M.M., Cardenas J.C., Chantrathammachart P., Williams J.C., Kirchhofer D., Bogdanov V.Y., Bach R.R., Rak J. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119:5543–5552. doi: 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu L.X., Yan L., Yang W., Wu F.Q., Ling Y., Chen S.Z., Tang L., Tan Y.X., Cao D., Wu M.C. Platelets promote tumour metastasis via interaction between TLR4 and tumour cell-released high-mobility group box1 protein. Nat. Commun. 2014;5:5256. doi: 10.1038/ncomms6256. [DOI] [PubMed] [Google Scholar]
- 49.Demers M., Wagner D.D. NETosis: a new factor in tumor progression and cancer-associated thrombosis. Semin. Thromb. Hemost. 2014;40:277–283. doi: 10.1055/s-0034-1370765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cedervall J., Zhang Y., Olsson A.-K. Tumor-induced NETosis as a risk factor for metastasis and organ failure. Cancer Res. 2016;76:4311–4315. doi: 10.1158/0008-5472.CAN-15-3051. [DOI] [PubMed] [Google Scholar]
- 51.Erpenbeck L., Schön M.P. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene. 2016;36:2483–2490. doi: 10.1038/onc.2016.406. [DOI] [PubMed] [Google Scholar]
- 52.Khorana A.A., Mangu P.B., Berlin J., Engebretson A., Hong T.S., Maitra A., Mohile S.G., Mumber M., Schulick R., Shapiro M. Potentially curable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2016;34:2541–2556. doi: 10.1200/JCO.2016.67.5553. [DOI] [PubMed] [Google Scholar]
- 53.Sawabata N., Okumura M., Utsumi T., Inoue M., Shiono H., Minami M., Nishida T., Sawa Y. Circulating tumor cells in peripheral blood caused by surgical manipulation of non-small-cell lung cancer: pilot study using an immunocytology method. Gen. Thorac. Cardiovasc. Surg. 2007;55:189–192. doi: 10.1007/s11748-007-0101-2. [DOI] [PubMed] [Google Scholar]
- 54.Jain S., Pitoc G.A., Holl E.K., Zhang Y., Borst L., Leong K.W., Lee J., Sullenger B.A. Nucleic acid scavengers inhibit thrombosis without increasing bleeding. Proc. Natl. Acad. Sci. USA. 2012;109:12938–12943. doi: 10.1073/pnas.1204928109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein P.D., Beemath A., Meyers F.A., Skaf E., Sanchez J., Olson R.E. Incidence of venous thromboembolism in patients hospitalized with cancer. Am. J. Med. 2006;119:60–68. doi: 10.1016/j.amjmed.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 56.Zuckerman J.E., Davis M.E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 2015;14:843–856. doi: 10.1038/nrd4685. [DOI] [PubMed] [Google Scholar]
- 57.Holl E.K., Shumansky K.L., Pitoc G., Ramsburg E., Sullenger B.A. Nucleic acid scavenging polymers inhibit extracellular DNA-mediated innate immune activation without inhibiting anti-viral responses. PLoS One. 2013;8:e69413. doi: 10.1371/journal.pone.0069413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Celiz A.D., Smith J.G.W., Patel A.K., Hook A.L., Rajamohan D., George V.T., Flatt L., Patel M.J., Epa V.C., Singh T. Discovery of a novel polymer for human pluripotent stem cell expansion and multilineage differentiation. Adv. Mater. 2015;27:4006–4012. doi: 10.1002/adma.201501351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Celiz A.D., Smith J.G.W., Patel A.K., Langer R., Anderson D.G., Barrett D.A., Young L.E., Davies M.C., Denning C., Alexander M.R. Chemically diverse polymer microarrays and high throughput surface characterisation: a method for discovery of materials for stem cell culture†Electronic supplementary information (ESI) available. See DOI: 10.1039/c4bm00054dClick here for additional data file. Biomater. Sci. 2014;2:1604–1611. doi: 10.1039/c4bm00054d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng H., Kastrup C.J., Ramanathan R., Siegwart D.J., Ma M., Bogatyrev S.R., Xu Q., Whitehead K.A., Langer R., Anderson D.G. Nanoparticulate cellular patches for cell-mediated tumoritropic delivery. ACS Nano. 2010;4:625–631. doi: 10.1021/nn901319y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papalezova K.T., Tyler D.S., Blazer D.G., 3rd, Clary B.M., Czito B.G., Hurwitz H.I., Uronis H.E., Pappas T.N., Willett C.G., White R.R. Does preoperative therapy optimize outcomes in patients with resectable pancreatic cancer? J. Surg. Oncol. 2012;106:111–118. doi: 10.1002/jso.23044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.