Abstract
Background
Increased antiretroviral therapy (ART) availability has been associated with more patients developing cryptococcosis after ART initiation. Despite this changing epidemiology, data regarding cryptococcal meningitis in those already receiving ART are limited. We compared clinical presentations and outcomes among ART-naïve and ART-experienced Ugandans.
Methods
We prospectively enrolled 605 HIV-infected persons with first-episode cryptococcal meningitis from August 2013 to May 2017 who received amphotericin-based combination therapy. We classified participants by ART status and ART duration and compared groups for 2-week survival.
Results
Overall, 46% (281/605) of participants were receiving ART at presentation. Compared with those not receiving ART, those receiving ART had higher CD4 counts (P < .001) and lower cerebrospinal fluid fungal burdens (P < .001). Of those receiving ART, 56% (156/281) initiated ART within 6 months, and 18% (51/281) initiated ART within 14 days. Two-week mortality did not differ by ART status (27% in both ART-naïve and ART-experienced%; P > .99). However, 47% (24/51) of those receiving ART for ≤14 days died within 2 weeks, compared with 19% (20/105) of those receiving ART for 15–182 days and 26% (32/125) of those receiving ART for >6 months (P < .001). Among persons receiving ART for >6 months, 87% had HIV viral loads >1000 copies/mL.
Conclusions
Cryptococcosis after ART initiation is common in Africa. Patients initiating ART who unmask cryptococcal meningitis are at a high risk of death. Immune recovery in the setting of central nervous system infection is detrimental, and management of this population requires further study. Implementing pre-ART cryptococcal antigen screening is urgently needed to prevent cryptococcal meningitis after ART initiation.
Keywords: antiretroviral therapy, cryptococcal meningitis, cryptococcus, HIV, immune reconstitution inflammatory syndrome
Expanded access to antiretroviral therapy (ART) in Africa has corresponded to increasing numbers of patients presenting with opportunistic infections after initiating ART. Although cryptococcal meningitis occurring early after ART initiation can be largely prevented through pre-ART cryptococcal antigen (CrAg) screening, this practice has yet to be widely implemented [1, 2]. As a result, Cryptococcus remains the leading cause of adult meningitis in Africa, where it accounts for approximately 15% of AIDS-related mortality [3–5].
Despite the changing epidemiology of cryptococcal meningitis, most research studies to date have enrolled primarily ART-naïve subjects, and current guidelines focus on this population. Outcome data for persons developing cryptococcal meningitis while already receiving ART are lacking, with conflicting evidence on whether outcomes are improved in patients on ART compared with ART-naïve individuals [6–9]. Moreover, a “one size fits all” approach to understanding cryptococcal meningitis in ART-experienced persons may be overly simplistic. Persons with virologic failure and/or nonadherence may be immunologically similar to an ART-naïve population. However, the “unmasking” of existing subclinical, untreated cryptococcal infection shortly after ART initiation may be substantially different immunologically [10, 11].
Outcomes of unmasking cryptococcosis have not been well characterized. Early ART initiation after cryptococcal meningitis diagnosis in ART-naïve individuals is associated with higher mortality [12], but whether recent ART initiation before the diagnosis of cryptococcal meningitis is also associated with similarly poor outcomes is unknown. We compared the clinical presentation and outcomes in ART-naïve and ART-experienced Ugandans presenting with their first episode of cryptococcal meningitis.
METHODS
We prospectively enrolled 605 HIV-infected adults with first episode of cryptococcal meningitis who presented to 2 public referral hospitals in Uganda from August 2013 through May 2017 as part of an ongoing trial investigating the use of adjunctive sertraline for cryptococcal meningitis treatment (ASTRO-CM; ClinicalTrials.gov: NCT01802385). The first 149 participants were enrolled in an open-label dose-ranging phase of the study using sertraline at doses of 100–400 mg daily [13]. Starting in March 2015, participants were enrolled in a double-blind, 1:1 randomized clinical trial testing whether sertraline dosed initially at 400 mg daily has a survival benefit compared with placebo when receiving standard antifungal therapy [14].
Participants provided written informed consent at the time of cryptococcal diagnosis, which was made via cerebrospinal fluid (CSF) CrAg lateral flow assay (Immy Inc., Norman, OK). Clinical and laboratory data were collected over a 12–18-week period with approval from institutional review boards in Uganda and Minnesota.
Study Design
At enrollment, subjects were classified by whether they were receiving ART at initial presentation. Individuals were considered to be receiving ART if they had received any ART within 30 days of enrollment. Among those receiving ART, participants were grouped by ART duration into having initiated, reintroduced, or switched ART within (1) 14 days, (2) 15–182 days, or (3) >182 days (6 months) before meningitis diagnosis. ART duration was defined as time to presentation with meningitis from ART initiation in participants receiving uninterrupted ART (n = 233; 83% of those receiving ART), from time of ART reintroduction in those receiving ART but with a history of defaulting (n = 19; 7%), or from time of class switch in those with a previous history of virologic failure necessitating a change in ART regimen (n = 29; 10%). History of cryptococcal meningitis, ART status, and timing of ART initiation was ascertained through self-report and confirmed by review of medical records.
Participants received standard antifungal therapy plus placebo or adjunctive sertraline for 14 days, followed by a dose of 200 mg/d for the next 10–12 weeks before discontinuing sertraline or placebo in a tapered fashion. Standard antifungal therapy included amphotericin B (0.7–1.0 mg/kg/d) for up to 14 days and fluconazole (800 mg/d) for ~4 weeks, followed by fluconazole 400 mg/d for 8 weeks of consolidation therapy. Amphotericin was generally discontinued after 7 days if the baseline CSF culture was sterile at 7 days postcollection, with continuation of fluconazole and sertraline or placebo. Participants receiving ART at the time of presentation generally continued their ART regimen, though the decision to continue or suspend ART was left to provider discretion. Corticosteroid use for suspected immune reconstitution inflammatory syndrome or other indication was also left to provider discretion. Quantitative HIV-1 viral loads were obtained in those receiving ART for >6 months. For ART-naïve participants or those on failing regimens, ART was initiated or changed at 4–6 weeks.
Therapeutic lumbar punctures were routinely performed on day 3, day 7, at the end of amphotericin therapy, and as needed for control of intracranial pressure. Quantitative CSF cultures were performed with 5 serial 1:10 dilutions of 100 μL CSF, as described elsewhere [7, 15]. CSF culture sterility was defined as no growth of Cryptococcus after 10 days of incubation, with a limit of detection of 10 colony-forming units (CFU) per mL.
Study Outcomes and Analysis
We compared groups based on ART status and ART duration at meningitis diagnosis. The primary study end point was mortality at 2 weeks. Secondary end points included mortality at 10 weeks and CSF fungal clearance. CSF clearance rate over 2 weeks (early fungicidal activity [EFA]) by ART status and timing was calculated for all participants with at least 2 quantitative CSF cultures via linear regression models, as previously described [16, 17].
Baseline characteristics and outcomes were compared across ART groups, with Kruskal-Wallis tests for continuous variables and chi-square or Fisher exact tests, as appropriate, for categorical variables and outcomes. Unadjusted and adjusted Cox proportional hazards models and Kaplan-Meier curves were used to examine the association between ART groups and mortality. Adjusted models considered factors that may be confounded with early mortality, including baseline CSF sterility, Glasgow Coma Scale (GCS) score, CSF white blood cell count, and hemoglobin. Additional Cox proportional hazards models were used to check for possible interaction effects between time on ART and receipt of sertraline during the study. All analyses were conducted with SAS, version 9.3 (SAS Institute).
RESULTS
Study Population and Comparison by ART Status
Among 605 HIV-infected adults with first episode of cryptococcal meningitis, 46% (281/605) were receiving ART at presentation (Figure 1), having initiated ART a median (interquartile range) of 17 (3–104) weeks before cryptococcal meningitis diagnosis. Those receiving ART had higher CD4 counts, lower initial CSF fungal burdens, lower baseline hemoglobin levels, and were more likely to be receiving concurrent tuberculosis (TB) therapy than those not receiving ART at cryptococcal meningitis diagnosis (Table 1). A CD4 count of <100 cells/μL at the time of meningitis diagnosis was present in 89% of those receiving ART.
Figure 1.
Study cohort. The cohort included persons with first-episode cryptococcal meningitis, of whom 46% were receiving antiretroviral therapy (ART) at presentation. More than half of the patients receiving ART at cryptococcal meningitis diagnosis had initiated ART within 6 months of diagnosis, 18% of whom initiated ART within 14 days of cryptococcal meningitis diagnosis. Abbreviations: ART, antiretroviral therapy; CM, cryptococcal meningitis.
Table 1.
Clinical Presentation and Outcomes by ART Exposure Status
| No ART | Receiving ART | P Value | |
|---|---|---|---|
| No. per group | 324 | 281 | |
| Duration of ART, wk | -- | 17 (3, 104) | |
| Demographics | |||
| Age, y | 35 (30, 41) | 35 (30, 40) | .81 |
| Men | 202 (62) | 168 (60) | .56 |
| Receiving TB therapy | 16 (5) | 40 (14) | <.001 |
| Baseline clinical parameters | |||
| Glasgow Coma Scale < 15 | 139 (43) | 128 (46) | .57 |
| Weight, kg | 52 (48, 58) | 50 (44, 57) | .17 |
| Hemoglobin, g/dL | 11.9 (10.3, 13.2) | 11.2 (9.7, 12.6) | <.001 |
| Creatinine, mg/dL | 0.7 (0.6, 0.9) | 0.7 (0.6, 0.9) | .11 |
| CD4 count, cells/μL | 12 (6, 38) | 23 (7, 56) | <.001 |
| CD4 count < 100 cells/μL | 294 (95) | 240 (89) | <.01 |
| Baseline CSF parameters | |||
| Opening pressure > 25 cmH2O | 162 (57) | 133 (53) | .43 |
| CSF culture, log10 CFU/mL | 5.0 (4.0, 5.6) | 4.1 (2.1, 5.2) | <.001 |
| Sterile culture | 10 (3) | 38 (14) | <.001 |
| WBC < 5 cells/μL | 206 (66) | 163 (62) | .22 |
| Management and outcomes | |||
| Received corticosteroids | 33 (10) | 45 (16) | .04 |
| No. of LPs received | 3 (2, 4) | 3 (2, 4) | .20 |
| Attained CSF sterilitya | 136 (44) | 128 (54) | .04 |
| CSF clearance rateb | –0.34 (–0.48, –0.20) | –0.36 (–0.58, –0.23) | .21 |
| Mortality | |||
| Within 2 wk | 87 (27) | 76 (27) | >.99 |
| Within 10 wk | 139 (43) | 127 (45) | .62 |
Data are median (P25, P75) or No. (%). P value by Kruskal-Wallis test or Fisher exact test.
Abbreviations: ART, antiretroviral therapy; CFU, colony-forming units; CSF, cerebrospinal fluid; LP, lumbar puncture; TB, tuberculosis; WBC, white blood cell count.
aWithin 18 days of cryptococcal meningitis diagnosis; excludes those who started with sterile culture or died before day 14.
bLog10 CFU/mL CSF/d, calculated using a patient-specific linear regression model.
We did not observe a significant difference in CSF fungal clearance based on ART status. Those receiving ART were more likely to receive corticosteroids compared with ART-naïve participants (16% vs 10%; P = .04). Overall mortality was 27% (163/605) at 2 weeks and 44% (266/605) at 10 weeks. Mortality did not differ between ART-naïve and ART-experienced participants. Receipt of sertraline did not impact mortality, and there were no significant interactions between sertraline use and ART status or timing in subgroup analysis, as described elsewhere [13, 14].
The overall 2-week mortality did not differ for those who had positive vs sterile CSF cultures at cryptococcal meningitis (CM) diagnosis (27% in both groups). Although baseline CSF sterility was rare in ART-naïve individuals (3%; 10/324), it was associated with a 2-week mortality of 50% (5/10). Among those receiving ART, in contrast, baseline CSF sterility was more common (14%; 38/281), and 2-week mortality was 21% (8/38; mortality data not shown).
Clinical Characteristics and Outcomes by ART Duration
Among participants receiving ART at the time of enrollment, 56% (156/281) had initiated ART within 6 months and 18% (51/281) had initiated ART within 14 days (Table 2). Participants starting ART ≤6 months before diagnosis had higher CD4 counts and were more likely to present with CSF pleocytosis compared with those initiating ART >6 months before diagnosis. Baseline demographics and clinical characteristics were otherwise similar between ART duration groups of ≤14 days, 15–182 days (6 months), and >6 months.
Table 2.
Clinical Presentation and Outcomes by ART Duration
| ART Duration | ≤14 d | 15–182 d | >6 mo | P Value |
|---|---|---|---|---|
| No. per group | 51 | 105 | 125 | |
| Duration of ART, wk | 1 (1, 1) | 8 (4, 13) | 128 (63, 230) | |
| Demographics | ||||
| Age, y | 33 (28, 41) | 34 (30, 40) | 36 (30, 40) | .56 |
| Men | 31 (61) | 62 (59) | 75 (60) | .97 |
| Receiving TB therapy | 3 (6) | 17 (16) | 20 (16) | .15 |
| Baseline clinical parameters | ||||
| Glasgow Coma Scale < 15 | 26 (51) | 53 (50) | 49 (39) | .17 |
| Weight, kg | 48 (35, 56) | 48 (41, 53) | 53 (46, 64) | .18 |
| Hemoglobin, g/dL | 11.2 (10.3, 12.4) | 11.4 (9.7, 12.5) | 10.9 (9.2, 12.8) | .76 |
| Creatinine, mg/dL | 0.7 (0.6, 1.1) | 0.7 (0.5, 0.8) | 0.7 (0.5, 0.8) | .14 |
| CD4 count, cells/μL | 31 (9, 56) | 35 (15, 78) | 10 (5, 41) | <.001 |
| CD4 count <100 cells/μL | 41 (87) | 89 (86) | 110 (91) | .57 |
| Baseline CSF parameters | ||||
| Opening pressure > 25 cmH2O | 23 (51) | 48 (52) | 62 (55) | .90 |
| CSF culture, log10 CFU/mL | 4.6 (2.6, 5.3) | 3.0 (1.6, 4.8) | 4.1 (2.6, 5.3) | .08 |
| Sterile CSF culture | 5 (10) | 21 (20) | 12 (10) | .06 |
| WBC < 5 cells/μL | 27 (54) | 49 (51) | 87 (74) | <.001 |
| Management and outcomes | ||||
| Received corticosteroids | 14 (27) | 22 (21) | 9 (7) | <.001 |
| ART suspendeda | 13 (41) | 13 (14) | 24 (21) | .006 |
| No. of LPs received | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | .93 |
| Attained CSF sterilityb | 21 (48) | 53 (64) | 54 (49) | .07 |
| CSF clearance ratec | –0.36 (–0.85, –0.22) | –0.40 (–0.65, –0.24) | –0.30 (–0.47, –0.22) | .08 |
| Mortality | ||||
| Within 2 wk | 24 (47) | 20 (19) | 32 (26) | <.01 |
| Within 10 wk | 29 (57) | 45 (43) | 53 (42) | .19 |
Data are median (P25, P75) or No. (%). P value by Kruskal-Wallis test or Fisher exact test.
Abbreviations: ART, antiretroviral therapy; CFU, colony-forming units; CSF, cerebrospinal fluid; LP, lumbar puncture; TB, tuberculosis; WBC, white blood cell count.
aDefinition of ART suspension: ART discontinuation within 7 days of presentation, with intention to restart at 4–6 weeks.
bWithin 18 days of cryptococcal meningitis diagnosis; excludes those who started with sterile culture or died before day 14.
cLog10 CFU/mL CSF/d, calculated using patient-specific regression model.
Those receiving ART for 15–182 days tended to have lower baseline fungal burdens and better rates of CSF fungal clearance, though these differences were not significant (P = .08 for both comparisons). Corticosteroids were more commonly administered in participants receiving ART for ≤6 months (27% of cases receiving ART for ≤14 days and 21% of those receiving ART for 15–182 days, compared with 7% of those receiving ART for >6 months; P < .001), and ART was frequently suspended in those receiving ART for ≤14 days (41% of cases vs 14% and 21% for those on ART 15–182 days and >6 months, respectively; P < .01).
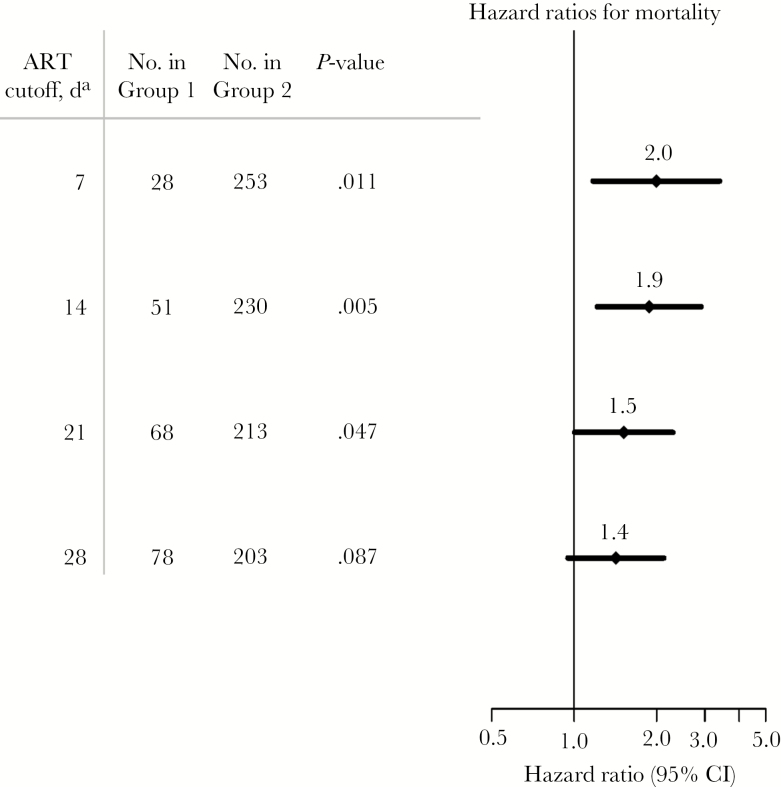
Two-week mortality was significantly higher in those receiving ART for ≤14 days (47%; 24/51) compared with those receiving ART for 15–22 days (19%; 20/105) and >6 months (26%; 32/125; P < .01). Differences in mortality occurred early during the first month of antifungal treatment, with those receiving ART for >14 days having similar mortality to ART-naïve participants (Figure 2). The hazard ratio (HR) for 2-week mortality comparing those on ART 1–14 days with those on ART >14 days was 1.9 (95% CI, 1.2–2.9; P < .01). Results were similar when the cutoff for days on ART was 7, 21, or 28 days (Figure 3). Additionally, in a model adjusted for baseline CSF sterility, GCS <15, CSF white blood cell count <5 cells/µL, and hemoglobin, the hazard ratio for 2-week mortality comparing those on ART 1–14 days with those on ART >14 days remained basically unchanged (HR, 1.8; 95% CI, 1.10–3.01; P = .02).
Figure 2.
Kaplan-Meier survival plot by antiretroviral therapy (ART) status and timing. Mortality was highest among 51 participants receiving ART for ≤14 days, compared with those receiving ART for a more extended period (n = 230) or those who were ART naïve (n = 324). The differences in mortality occurred over the initial ~3 weeks. Abbreviation: ART, antiretroviral therapy.
Figure 3.
Hazard ratio for mortality within 30 days by duration from antiretroviral therapy (ART) initiation to development of cryptococcal meningitis. Among participants receiving ART, individuals developing cryptococcal meningitis within 28 days of initiating ART had increased mortality. The figure plots the hazard ratio for mortality within 2 weeks as a function of ART duration from ART initiation to development of cryptococcal meningitis. Abbreviations: ART, antiretroviral therapy; CI, confidence interval.
Virologic Failure and Adherence in Late ART-Associated Cryptococcal Meningitis
Cryptococcal meningitis presenting after individuals had been receiving ART for >6 months was usually an indication of virologic failure. Plasma HIV-1 viral loads were obtained within 30 days of study enrollment for 36% (45/125) of those with an ART duration >6 months at diagnosis. Of those with viral load measurements, 91% (41/45) had detectable HIV virus (≥40 copies/mL), 87% (39/45) had >1000 copies/mL, and 47% (21/45) had >100 000 copies/mL.
In 110 participants on ART for >6 months who gave self-reports, adherence was >95% for 59 (54%) patients and 50%–95% for 51 (46%) patients. No participants reported <50% ART adherence. Among those on ART >6 months, follow-up HIV-1 viral loads were obtained after 6 weeks in 12 of the 41 individuals with detectable baseline HIV-1 RNA levels. Among these follow-up viral loads, no virologic suppression was observed despite ART adherence monitoring between measurements.
DISCUSSION
Individuals with cryptococcal meningitis in this cohort frequently presented after initiating ART, and persons initiating ART within 2 weeks before meningitis diagnosis had an almost 2-fold higher acute mortality. Few studies have compared the clinical presentation and outcomes of ART-naïve and ART-experienced individuals with cryptococcal meningitis, and this is the first study to specifically examine the timing of ART initiation in relation to the development of cryptococcal meningitis in those receiving ART. Several observations from this study should be highlighted. First, cryptococcal meningitis occurring in ART-experienced populations is now common in Africa [18]. In this cohort of adult patients in Uganda, nearly half of all subjects were already receiving ART at the time of diagnosis, paralleling trends in other recently published clinical trials [19–21].
Second, although participants receiving antecedent ART and those who were ART-naïve had similar survival overall, outcomes differed based on duration of ART relative to cryptococcal meningitis diagnosis. Most importantly, individuals who developed cryptococcal meningitis within 14 days of initiating ART appear to be at an increased risk for death. This finding is supported by a recent multicenter retrospective study that observed a shorter ART duration among those with unfavorable outcomes developing cryptococcal meningitis while already receiving ART (median, 2 months vs 19 months for those receiving ART and having favorable outcomes) [22]. Existing data comparing outcomes in Africa by baseline ART status consist of cohorts from Botswana [8] and South Africa [7, 9], which showed benefit and no benefit of ART, respectively. Conflicting outcomes from these studies might be explained by ART duration relative to diagnosis. Although these studies did not categorize participants by ART duration, the South African studies observed a median antecedent ART duration of 30 and 41 days. In contrast, the Botswana study reported a median time of 93 days. If the shorter duration of ART in the South African studies reflects a greater proportion of individuals developing cryptococcal meningitis shortly after initiating ART, then the benefits of ART observed in the Botswana study may have been mitigated by a higher mortality in those with recent ART initiation.
Our observation of increased mortality in those initiating ART shortly before developing cryptococcal meningitis is similar to other ART timing trials that have observed detrimental outcomes with early initiation of ART after the diagnosis of central nervous system (CNS) infection [12, 23–25]. In the case of cryptococcal meningitis, it therefore seems plausible to conclude that ART initiation in the context of active CNS infection is harmful, regardless of whether the diagnosis is made before or after starting ART. High early mortality could be driven by preexisting subclinical meningitis at the time of ART initiation, leading to an exaggerated aberrant inflammatory response in the CNS. Those receiving ART for slightly longer periods of time (15 days to 6 months), in contrast, may not yet have developed meningitis at the time of ART initiation. In this scenario, a more appropriate inflammatory response in the CNS might be expected once meningitis develops, resulting in the appearance of symptoms at lower fungal burdens and comparatively better outcomes. Per this model, the existence of subclinical meningitis at the time of ART initiation would be hazardous, whereas the progression from non-CNS cryptococcosis to meningitis after ART has already been initiated would not.
Finally, the development of cryptococcal meningitis in those receiving ART for >6 months is a strong predictor of HIV virologic failure. This underscores the importance of obtaining a thorough ART history to avoid restarting ART in nonadherent or defaulting patients. Virologic suppression was not attained in follow-up viral load measurements before ART regimen switch, suggesting HIV drug resistance rather than poor ART adherence or a bump in viral load secondary to acute illness. Thus, for individuals presenting with cryptococcal meningitis while on ART for >6 months, HIV drug resistance must be considered, with the need to switch to an alternative ART regimen after 4–6 weeks of antifungal therapy. Furthermore, as HIV virologic failure appears to be a risk factor for cryptococcal meningitis in patients already enrolled in ART programs, targeted CrAg screening of high-risk individuals with virologic failure could be useful for preventing cryptococcosis.
Findings from this study highlight 2 components of routine HIV care important for the prevention of ART-associated cryptococcosis. First, pre-ART CrAg screening could have identified CrAg-positive individuals requiring antifungal therapy beforeART initiation [10, 26]. Among individuals in this cohort who were diagnosed while receiving ART for ≤6 months, nearly 90% had a CD4 <100 cells/μL and would have been identified for preemptive antifungal therapy had pre-ART CrAg screening been universally implemented over the study period. Second, almost half of the individuals presenting with cryptococcal meningitis while on ART had been receiving ART for an extended duration and had virologic failure. Failing ART regimens would have been detected had universal viral load monitoring been available in Uganda over the study period.
We have characterized cases of cryptococcosis developing shortly after initiating ART as “unmasking” meningitis and have implied that this involves exaggerated inflammation due to restoration of cellular immune function consistent with a form of immune reconstitution inflammatory syndrome (IRIS) [11, 27]. This would be supported by the higher rates of baseline CSF cellular infiltrate observed in those initiating ART within ≤6 months of diagnosis. It is unclear if similar detrimental patterns observed with early ART initiation after cryptococcal meningitis diagnosis, such as increased macrophage activation or detrimental type 2 helper (Th2) CD4 T cell immune responses [28], also occur in cases of unmasking cryptococcal meningitis. Nonetheless, an association of mortality with the timing of ART suggests an immunologic mechanism leading to more acute illness [29], in contrast to the typical subacute course evolving over weeks in cases of cryptococcal meningitis in ART-naïve individuals [30].
More data are needed regarding the optimal management of unmasking cryptococcosis, and several important questions regarding potential therapeutic interventions exist. For example, do the risks inherent in abruptly stopping ART justify a possible benefit of ART discontinuation? It remains a matter of speculation as to whether interrupting ART would influence the mechanisms underlying the deleterious effects of ART. Given a presumed role of inflammation, another unanswered question is whether there could be a role for immunomodulating therapy in cases of unmasking cryptococcal meningitis. Although current guidelines suggest a potential benefit from glucocorticoids for paradoxical IRIS [31–33], there have not been controlled trials evaluating their use in unmasking IRIS. A recent large clinical trial was unable to demonstrate a benefit of adjunctive steroids in cryptococcal meningitis, even among individuals initiating ART within 3 months of diagnosis [19]. A limitation of this study is that both corticosteroid use and withholding ART were left to provider discretion and occurred more frequently in those who had recently initiated ART. Controlled trials should seek to better delineate the harms and benefits of these potential interventions in this subpopulation of patients.
Another limitation of this study was a modest sample size developing cryptococcal meningitis shortly after initiating ART. Although our sample size was small, we have observed growing numbers in this subpopulation and expect this trend to continue with the roll-out of a test-and-treat strategy geared toward HIV [34]. We hope this report promotes discourse regarding the management of these cases and emphasizes the urgency of implementing routine pre-ART CrAg screening. Future research should build upon our observations regarding the timing of ART, seek to provide validation and understanding, and help determine the optimal clinical approach for individuals developing cryptococcosis while already receiving ART.
In summary, this study demonstrated that cryptococcal meningitis now commonly occurs among ART-experienced persons and that developing cryptococcal meningitis within 14 days of initiating ART carries a high risk of mortality. These findings support urgent and widespread implementation of pre-ART CrAg screening to prevent cryptococcal meningitis after ART initiation. Further studies are needed to understand the immuno-pathologic mechanisms underlying the high mortality observed in those recently starting ART, with the goal of developing more optimal, customized, host-directed treatment strategies in the future.
Acknowledgments
ASTRO-CM Team members. Henry W. Nabeta, Jane Francis Ndyetukira, Cynthia Ahimbisibwe, Florence Kugonza, Carolyne Namuju, Alisat Sadiq, Alice Namudde, James Mwesigye, Tadeo Kiiza Kandole, Paul Kirumira, Michael Okirwoth, Andrew Akampurira, Tony Luggya, Julian Kaboggoza, Eva Laker, Leo Atwine, Davis Muganzi, Sruti S. Velamakanni, Bilal Jawed, Katelyn Pastick, Matthew Merry, Anna Stadelman, Andrew Flynn, A. Wendy Fujita, Liliane Mukaremera, Sarah M. Lofgren, Bozena M. Morawski, Kabanda Taseera, Kirsten Nielsen, Paul R. Bohjanen, and Andrew Kambugu.
Financial support. This work was supported by the United States Fogarty International Center (K01TW010268, R25TW009345), National Institute of Neurologic Diseases and Stroke (R01NS086312), National Institute of Allergy and Infectious Diseases (T32AI055433), United Kingdom Medical Research Council/Wellcome Trust/Department for International Development (MRC MR/M007413/1), and Grand Challenges Canada (S4-0296-01). This work was also supported in part by the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at the University of Minnesota. Andrew Flynn and A. Wendy Fujita are Doris Duke International Clinical Research Fellows. David Meya was supported in part by a DELTAS Africa Initiative grant (DEL-15-011) to THRiVE-2.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
ASTRO-CM study team:
Henry W Nabeta, Jane Francis Ndyetukira, Cynthia Ahimbisibwe, Florence Kugonza, Carolyne Namuju, Alisat Sadiq, Alice Namudde, James Mwesigye, Tadeo Kiiza Kandole, Paul Kirumira, Michael Okirwoth, Andrew Akampurira, Tony Luggya, Julian Kaboggoza, Eva Laker, Leo Atwine, Davis Muganzi, Sruti S Velamakanni, Bilal Jawed, Katelyn Pastick, Matthew Merry, Anna Stadelman, Andrew Flynn, A Wendy Fujita, Liliane Mukaremera, Sarah M Lofgren, Bozena M Morawski, Kabanda Taseera, Kirsten Nielsen, Paul R Bohjanen, and Andrew Kambugu
References
- 1. Mfinanga S, Chanda D, Kivuyo SL, et al. REMSTART trial team Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet 2015; 385:2173–82. [DOI] [PubMed] [Google Scholar]
- 2. Kaplan JE, Vallabhaneni S, Smith RM, et al. Cryptococcal antigen screening and early antifungal treatment to prevent cryptococcal meningitis: a review of the literature. J Acquir Immune Defic Syndr 2015; 68(Suppl 3):S331–9. [DOI] [PubMed] [Google Scholar]
- 3. Lawn SD, Harries AD, Anglaret X, et al. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS 2008; 22:1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jarvis JN, Meintjes G, Williams A, et al. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis 2010; 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lortholary O, Poizat G, Zeller V, et al. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS 2006; 20:2183–91. [DOI] [PubMed] [Google Scholar]
- 7. Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis 2007; 45:76–80. [DOI] [PubMed] [Google Scholar]
- 8. Bisson GP, Nthobatsong R, Thakur R, et al. The use of HAART is associated with decreased risk of death during initial treatment of cryptococcal meningitis in adults in Botswana. J Acquir Immune Defic Syndr 2008; 49:227–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jarvis JN, Meintjes G, Harrison TS. Outcomes of cryptococcal meningitis in antiretroviral naïve and experienced patients in South Africa. J Infect 2010; 60:496–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meya DB, Manabe YC, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis 2010; 51:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haddow LJ, Colebunders R, Meintjes G, et al. International Network for the Study of HIV-associated IRIS (INSHI) Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis 2010; 10:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boulware DR, Meya DB, Muzoora C, et al. COAT Trial Team Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rhein J, Morawski BM, Hullsiek KH, et al. ASTRO-CM Study Team Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 2016; 16:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rhein J, Huppler Hullsiek K, Tugume L, et al. Adjunctive sertraline in HIV-associated cryptococcal meningitis. Paper presented at: Conference on Retroviruses and Opportunistic Infections; 4–7 March, 2018; Boston, MA. [Google Scholar]
- 15. Dyal J, Akampurira A, Rhein J, et al. ASTRO-CM Trial Team Reproducibility of CSF quantitative culture methods for estimating rate of clearance in cryptococcal meningitis. Med Mycol 2016; 54:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bicanic T, Meintjes G, Rebe K, et al. Immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis: a prospective study. J Acquir Immune Defic Syndr 2009; 51:130–4. [DOI] [PubMed] [Google Scholar]
- 18. Flynn AG, Meya DB, Hullsiek KH, et al. Evolving failures in the delivery of human immunodeficiency virus care: lessons from a Ugandan meningitis cohort 2006–2016. Open Forum Infect Dis 2017; 4:ofx077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beardsley J, Wolbers M, Kibengo FM, et al. CryptoDex Investigators Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med 2016; 374:542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scriven JE, Lalloo DG, Meintjes G. Changing epidemiology of HIV-associated cryptococcosis in sub-Saharan Africa. Lancet Infect Dis 2016; 16:891–2. [DOI] [PubMed] [Google Scholar]
- 21. Molloy SF, Kanyama C, Heyderman RS, et al. ACTA Trial Study Team Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med 2018; 378:1004–17. [DOI] [PubMed] [Google Scholar]
- 22. Hakyemez IN, Erdem H, Beraud G, et al. Prediction of unfavorable outcomes in cryptococcal meningitis: results of the multicenter Infectious Diseases International Research Initiative (ID-IRI) cryptococcal meningitis study. Eur J Clin Microbiol Infect Dis. In press. [DOI] [PubMed] [Google Scholar]
- 23. Makadzange AT, Ndhlovu CE, Takarinda K, et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-saharan Africa. Clin Infect Dis 2010; 50:1532–8. [DOI] [PubMed] [Google Scholar]
- 24. Bisson GP, Molefi M, Bellamy S, et al. Early versus delayed antiretroviral therapy and cerebrospinal fluid fungal clearance in adults with HIV and cryptococcal meningitis. Clin Infect Dis 2013; 56:1165–73. [DOI] [PubMed] [Google Scholar]
- 25. Török ME, Yen NT, Chau TT, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)–associated tuberculous meningitis. Clin Infect Dis 2011; 52:1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mfinanga S, Chanda D, Kivuyo SL, et al. REMSTART trial team Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet 2015; 385:2173–82. [DOI] [PubMed] [Google Scholar]
- 27. French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS 2004; 18:1615–27. [DOI] [PubMed] [Google Scholar]
- 28. Scriven JE, Rhein J, Hullsiek KH, et al. COAT Team Early ART after cryptococcal meningitis is associated with cerebrospinal fluid pleocytosis and macrophage activation in a multisite randomized trial. J Infect Dis 2015; 212:769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woods ML 2nd, MacGinley R, Eisen DP, Allworth AM. HIV combination therapy: partial immune restitution unmasking latent cryptococcal infection. AIDS 1998; 12:1491–4. [DOI] [PubMed] [Google Scholar]
- 30. Kambugu A, Meya DB, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis 2008; 46:1694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sungkanuparph S, Jongwutiwes U, Kiertiburanakul S. Timing of cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in patients with AIDS and cryptococcal meningitis. J Acquir Immune Defic Syndr 2007; 45:595–6. [DOI] [PubMed] [Google Scholar]
- 33. Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010; 24:2381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abassi M, Rhein J, Meya DB, Boulware DR. Cryptococcal disease in the era of “test and treat”: is there cause for concern?Open Forum Infect Dis 2018; 5:ofx274. [DOI] [PMC free article] [PubMed] [Google Scholar]