Abstract
Multiple sclerosis therapies include interferons, glatiramer, and multiple immunosuppressive drugs. Discerning infectious risks of immunosuppressive drugs requires understanding their mechanisms of action and analyzing interventional studies and postmarketing observational data. Though identical immunosuppressive therapies are sometimes used in non-neurologic conditions, infectious risks may differ in this population. Screening for and treatment of latent tuberculosis (TB) infection should be prioritized for patients receiving alemtuzumab; ocrelizumab is likely not associated with an increased risk of TB. Hepatitis B virus (HBV) reactivation can be devastating for patients treated with ocrelizumab and alemtuzumab, whereas the small molecule oral agents do not likely pose substantial risk of HBV. Progressive multifocal leukoencephalopathy is a particular concern with natalizumab. Alemtuzumab, and possibly natalizumab and fingolimod, risks herpes virus reactivation and may warrant prophylaxis. Unusual opportunistic infections have been described. Vaccination is an important tool in preventing infections, though vaccine timing and contraindications can be complex.
Keywords: immunosuppression, multiple sclerosis, opportunistic infections
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) responsible for substantial morbidity and mortality. Because of the importance of humoral and cell-mediated immunity in the pathophysiology of MS, nearly all therapies involve modulation of the immune system with interferons, glatiramer acetate, and immunosuppressive medications. As of January 2018, 7 immunosuppressive medications have been approved by the US Food and Drug Administration (FDA) to treat MS, including 3 in the past 5 years (Table 1). These drugs include monoclonal antibodies (natalizumab, alemtuzumab, and ocrelizumab), a chemotherapeutic agent (mitoxantrone), and small-molecule oral agents (fingolimod, dimethyl fumarate, and teriflunomide; daclizumab, an anti-CD25 monoclonal antibody, was withdrawn in March of 2018) [1]. Siponimod performed well in a phase III clinical trial and may be FDA-approved in the future [2]. The drugs have diverse mechanisms of action, including alteration of lymphocyte trafficking (natalizumab and fingolimod), lymphocyte depletion (alemtuzumab and ocrelizumab), and disruption of lymphocyte replication (mitoxantrone and teriflunomide). Dimethyl fumarate acts via unknown mechanisms, though it clearly causes lymphocytopenia.
Table 1. .
Drugs for Treatment of Multiple Sclerosis, Excluding Interferons, Glatiramer Acetate, and Drugs Withdrawn, Including Details of Approval by the US Food and Drug Administration
| Generic Name | Approved Indications | Year Approved for MS | Mechanism of Action |
|---|---|---|---|
| Monoclonal antibodies | |||
| Natalizumab | RRMS | 2004 | Anti-integrin antibody |
| Alemtuzumab | RRMS | 2014 | Anti-CD52 antibody |
| Ocrelizumab | RRMS, PPMS | 2017 | Anti-CD20 antibody |
| Chemotherapeutic agents | |||
| Mitoxantrone | RRMS, SPMS | 2000 | Topoisomerase inhibitor |
| Small-molecule oral agents | |||
| Fingolimod | RRMS | 2010 | Sphingosine-1-phosphate receptor modulator |
| Dimethyl fumarate |
RRMS | 2013 | Unknown |
| Teriflunomide | RRMS | 2012 | Dihydroorotate dehydrogenase inhibitor |
Abbreviations: MS, multiple sclerosis; PPMS, primary progressive multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis.
As with all immunosuppressive medications, those used in the treatment of MS carry risks of opportunistic infections (OIs), including multifocal leukoencephalopathy (PML) in natalizumab-treated patients [3]. In this article, we propose an approach to risk-stratifying potential infectious complications of MS therapies while highlighting particular infections of concern and proposing strategies for screening and prophylaxis (Table 2). Interferons and glatiramer acetate are not known to cause OIs, and corticosteroids are rarely used for prolonged therapy and have well-established infectious risks, so these drugs will not be extensively discussed in this paper. Rituximab, another anti-CD20 monoclonal antibody, is sometimes used off-label for the treatment of MS. Although a complete discussion of rituximab is beyond the scope of this paper, the safety of this agent has been discussed elsewhere and is likely similar to that of ocrelizumab, with low rates of OIs except for hepatitis B virus (HBV) reactivation [4–6].
Table 2. .
Recommendations for Screening and Prophylaxis by Druga
| Drug | LTBI Screening | Acyclovir Prophylaxisa | PML Screening and Monitoring | HBV Risk | Other |
|---|---|---|---|---|---|
| Natalizumab | Considerb | Considerc | Yes; see Table 4 | Universal screening; see Table 3 | Universal screening for HCV and HIV |
| Alemtuzumab | Yes | Yesd | No | Universal screening; see Table 3 | Universal screening for HCV and HIV |
| Ocrelizumab | Noe | Nof | No | Universal screening; see Table 3 | Universal screening for HCV and HIV |
| Mitoxantrone | Considerb | Nof | No | Universal screening; see Table 3 | Universal screening for HCV and HIV |
| Fingolimod | Considerb | Considerg | No | Universal screening; see Table 3 | Universal screening for HCV and HIV |
| Dimethyl fumarate | Considerb | Nof | No | Universal screening; see Table 3 | Universal screening for HCV and HIV |
| Teriflunomide | Yes | Nof | No | Universal screening; see Table 3 | Universal screening for HCV and HIV |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; LTBI, latent tuberculosis infection; PML, progressive multifocal leukoencephalopathy.
aDosed at 200–400 mg by mouth twice daily assuming normal renal function.
bConsider screening for latent tuberculosis in patients from endemic countries or otherwise at high risk.
cConsider on a case-by-case basis, for example, in patients with prior immunosuppression or with frequent oral or genital herpes simplex recurrences.
dContinue from start of alemtuzumab until CD4+ ≥200 cells/µL and until at least 2 months after alemtuzumab is administered.
eScreening is not indicated unless the patient meets some other criteria for screening (such as injection drug use or recent immigration from a country of high tuberculosis endemicity).
fAs with any other patient, chronic suppressive therapy with acyclovir should be considered in those with frequent oral or genital herpes simplex recurrences.
gConsider when co-administered with corticosteroids (except 3–5 days of high-dose corticosteroid treatment without tapering) or in patients with frequent oral or genital herpes simplex recurrences.
ASSESSING INFECTIOUS RISKS OF MULTIPLE SCLEROSIS THERAPIES
Patients with MS undergoing immunosuppression may be at risk of reactivation of latent pathogens, worsening of asymptomatic chronic infections, and contracting de novo infections. Prevention is preferable to treatment, reducing both infectious morbidity and mortality, as well as interruptions to MS therapy. Simultaneously, unnecessary screening, particularly using tests with poor sensitivity and specificity, risks false-negative and false-positive results, which can result in either unfounded reassurance or delayed treatment for MS, adverse drug reactions (ADRs) from unnecessary anti-infective therapy, and other harms. Therefore, preventive approaches should be tailored to individual patient and treatment risk factors.
Precise explication of the infectious risks associated with immunosuppressive therapies for MS is sometimes complicated by factors such as low frequency and delayed presentations. MS clinical trial data may be misleading because of variable screening and diagnostic approaches, whereas extrapolation from trials of the medications for treatment of non-neurologic diseases may be misleading because of the presence of additional risk factors in these other patients.
TUBERCULOSIS
Active tuberculous disease in adults usually results from reactivation of latent foci of bacteria previously under immunologic control. The MS drugs potentially most likely to be associated with activation or progression of this disease are those affecting cell-mediated immunity, whereas those targeting only humoral immunity such as CD20 antagonists, are expected to have little impact on TB reactivation. Regardless of the immunosuppressive medication, testing for and treating latent tuberculosis infection (LTBI) is recommended for those at high risk of TB reactivation, such as recent immigrants from countries of high TB incidence and those with diabetes mellitus or chronic renal failure [7]. Still, given the poor performance characteristics of tests for LTBI and significant risks of harm associated with LTBI treatment, particularly hepatotoxicity, screening and subsequent treatment should only be performed for patients who are likely to benefit. As with other patients being evaluated for LTBI, a focused history and physical exam, along with a chest radiograph, are needed to clinically rule out active infection before initiating treatment. Therapy generally involves 4 months of rifampin, 9 months of isoniazid, or 3 months of weekly isoniazid and rifapentine administered via directly observed therapy. Monthly assessments with a health care provider to assess adherence and adverse drug reactions (particularly hepatotoxicity) are standard. Other details regarding the diagnosis and treatment of LTBI have been extensively described and will not be recapitulated here [7].
Alemtuzumab results in prolonged profound lymphocytopenia affecting both humoral and cell-mediated immunity. Nonetheless, TB occurred in only 2 of the >900 individuals randomized to alemtuzumab in 2 pivotal large-scale phase III clinical trials. (At the same time, none of the nearly 400 control patients treated with interferon developed TB [8, 9].) Notably, these TB cases occurred despite widespread screening for LTBI in these trials, including mandated universal screening in the CARE-MS I trial and recommended screening in TB-endemic areas in the CARE-MS II trial (personal communications with Jeffrey Cohen, MD on 10/20/2017 and Alasdair Coles, BM BCh, PhD on 10/21/2017). Additionally, alemtuzumab is associated with high rates of TB among patients with hematologic malignancies, though these patients are at increased risk at baseline given their underlying malignancies [10]. For all these reasons, screening for LTBI is likely indicated before treatment with alemtuzumab for MS.
Teriflunomide, a dihydroorotate dehydrogenase inhibitor that impairs pyrimidine synthesis and thereby affects lymphocyte proliferation, could also conceivably affect the risk of TB reactivation. In fact, 3 cases of TB were reported among the >2000 teriflunomide-treated patients, though LTBI screening was not mandated in most of these studies [11–14]. Due to these cases, LTBI screening is recommended in teriflunomide’s FDA-approved product label and is appropriate [15].
Ocrelizumab is likely not associated with a significant risk of TB reactivation given its specificity for CD20, resulting in B-cell depletion without affecting cell-mediated immunity. Three large clinical trials of ocrelizumab without required LTBI screening (though LTBI screening may have been conducted at some sites according to country-specific guidelines) found no cases of TB [16, 17]. Similarly, another monoclonal antibody targeting CD20, rituximab, is associated with a remarkably low risk of TB reactivation in patients treated with this agent for rheumatologic disease, likely no more than placebo [5]. Screening for LTBI before use of anti-CD20 monoclonal antibodies is not routine when these agents are used for other indications, and MS patients without other indications for LTBI screening should not be tested before starting ocrelizumab.
The risks of TB reactivation in patients treated with natalizumab, fingolimod, dimethyl fumarate, and mitoxantrone are likely to be intermediate between those of anti-CD20 monoclonal antibodies and either alemtuzumab or teriflunomide. Natalizumab, an alpha-4 integrin antagonist, prevents lymphocyte migration across the blood–brain barrier (as well as into some other organs), so it could plausibly affect immune control over TB, as it does over JC virus. CNS herpes virus infections have been reported with natalizumab, consistent with impaired cellular immunity [18]. TB did not, however, occur in natalizumab clinical trials (though trial screening protocols have not been reported), and cases have not been widely reported in postmarketing experience [3, 19, 20]. Fingolimod, a sphingosine-1-phosphate receptor modulator, similarly could plausibly risk TB reactivation given the drug’s effect of sequestering lymphocytes in lymphoid tissue. Additionally, herpes virus and other opportunistic infections associated with impaired cell-mediated immunity have occurred in fingolimod-treated patients [21–23]. Still, no cases of TB were reported in the major clinical trials of fingolimod, despite the lack of mandated LTBI screening in at least 1 of the trials [24–26]. Finally, although dimethyl fumarate causes lymphocytopenia via unknown mechanisms, cases of TB or other concerning opportunistic infections have not been reported in clinical trials, despite a lack of required LTBI screening [27, 28]. For these 3 drugs, LTBI screening can be considered for patients at high epidemiologic risk for infection or with other risk factors for reactivation. Sparse data exist on the risk of TB reactivation among patients treated for MS with mitoxantrone, a chemotherapeutic agent evaluated in a small phase III clinical trial in the 1990s, during which no patients developed tuberculosis [29]. Nonetheless, the drug’s immunosuppressive properties and lack of robust safety data regarding reactivation of infections suggest consideration of LTBI screening.
HEPATITIS B VIRUS INFECTION
Unlike tests and treatment for LTBI, tests for HBV infection are generally highly sensitive and specific, and pharmacotherapy is substantially more benign, both of which affect the risk–benefit analysis of screening and treatment or prophylaxis. The most well-established strategy for patients at high risk for HBV reactivation or HBV flares involves prophylaxis with antiviral drugs, which are effective and safe [30]. Alternatively, a preemptive strategy of monitoring of HBV DNA with polymerase chain reaction (PCR), typically every 3 months, followed by initiation of antiviral medication if needed, can be considered, though it has less supporting evidence [30]. Both prophylaxis and preemptive approaches are typically recommended during and for 6 months after immunosuppression, except in patients treated with CD20 monoclonal antibodies, who are at risk for up to 12 months after the last dose.
Anti-CD20 monoclonal antibodies such as ocrelizumab pose a uniquely high risk of HBV-associated hepatitis and liver failure, sometimes associated with death [6, 30]. Alemtuzumab therapy, which also causes profound B-lymphocyte (along with T-lymphocyte) lymphocytopenia, similarly risks severe disease due to HBV infection [10]. Patients with serologic evidence of HBV infection were excluded from these trials, and no cases of HBV infection were reported. (The ocrelizumab trials allowed patients with detectable anti-HBc as long as they had negative HBsAg and HBV DNA at baseline and as long as HBV DNA remained undetectable with testing every 12 weeks [16, 17].)
The risks of HBV activation in patients treated with fingolimod, dimethyl fumarate, and teriflunomide have not been well established but are likely low. In at least 1 major clinical trial of fingolimod, screening for viral hepatitis was not routinely performed, and no cases of severe HBV infection were identified [24]. In the 2 phase III trials for dimethyl fumarate, patients were screened for HBsAg and excluded if found to be positive, though no screening was done for anti-HBc and there have not been reports of severe liver disease [27, 28]. In at least 3 of the 4 major teriflunomide trials, HBV screening was not performed universally, and no HBV cases were reported [11–14]. Leflunomide, the parent compound of teriflunomide, has been widely used for rheumatologic conditions and is not associated with high rates of HBV reactivation [31].
The risks of HBV reactivation or flare with natalizumab and mitoxantrone are similarly difficult to characterize but may fall between the known high risks of anti-CD20 monoclonal antibodies and alemtuzumab and the likely lower risks of the small molecule oral agents. Although integrins seem to affect lymphocyte trafficking in the liver, the implications for HBV control are unclear. No cases of HBV infection in major clinical trials have been reported, though screening protocols are not available [3, 19]. At least 1 postmarketing case of HBV infection was reported, but the serologic markers reported in the study do not distinguish between primary infection and reactivation [32]. The risk of HBV reactivation in patients receiving mitoxantrone is unknown, as screening protocols were not described in the landmark clinical trial of the drug [29]. The chemically related and more commonly used anthracycline class of drugs is associated with relatively high rates of HBV reactivation and flares [33].
Given known or potential risk for HBV flares or reactivation among patients being treated with immunosuppressant agents for MS, all patients should be screened for the presence of HBV infection with HBsAg and anti-HBc. Any patient receiving ocrelizumab with any marker of HBV infection past or present should undergo antiviral prophylaxis during and for 12 months after cessation of immunosuppressive therapy. Management of other patients is less well established but should reflect the risk of HBV reactivation (based on the presence of HBsAg and the agent used) and patient and provider preferences (Table 3) [30]. These recommendations are similar to consensus guidelines, though they allow for a preemptive approach in lieu of prophylaxis, particularly when the risk of HBV reactivation is either not well established or known to be low [30].
Table 3. .
Recommendations for Approach to Patients With Serologic Markers of HBV Infection by Drug
| Drug | Risk of HBV Reactivation or Flare | HBsAg (+) | HBsAg (-) Anti-HBc (+) |
Duration of Preemptive or Prophylactic Management |
|---|---|---|---|---|
| Natalizumab | Moderate | Prophylaxis | Prophylaxis or preemptive | During and for 6 mo after therapy |
| Alemtuzumab | High | Prophylaxis | Prophylaxis or preemptive | During and for 6 mo after therapy |
| Ocrelizumab | Very high | Prophylaxis | Prophylaxis | During and for 12 mo after therapy |
| Mitoxantrone | Moderate | Prophylaxis | Prophylaxis or preemptive | During and for 6 mo after therapy |
| Fingolimod | Low | Prophylaxis or preemptive | Preemptive or periodic LFT monitoring | During and for 6 mo after therapy |
| Dimethyl fumarate | Low | Prophylaxis or preemptive | Preemptive or periodic LFT monitoring | During and for 6 mo after therapy |
| Teriflunomide | Low | Prophylaxis or preemptive | Preemptive or periodic LFT monitoring | During and for 6 mo after therapy |
Abbreviations: anti-HBc, hepatitis B core antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; LFT, liver function test.
Progressive Multifocal Leukoencephalopathy
JC virus (JCV), the etiologic agent of PML, is a ubiquitous double-stranded DNA virus with a seroprevalence of >50% in most studied populations [34]. The mechanism of transmission is unknown, though the virus is most prevalent in genitourinary tissues [34]. Cell-mediated immunity prevents clinically significant reactivation of JCV in immunocompetent individuals, and lack of immunological control allows infection of oligodendrocytes and astrocytes and results in demyelination mostly of the subcortical white matter or white matter of the cerebellar hemispheres or peduncles [34].
As of late 2017, more than 650 cases of PML have been reported in association with natalizumab therapy, with an approximate incidence of 4.2 cases per 1000 treated patients [35]. In contrast, fingolimod and dimethyl fumarate have each been associated with fewer than 10 cases of PML, with most patients having previously received natalizumab [36]. Lymphocytopenia associated with these drugs has been inconsistently reported as a risk factor for PML, and monitoring the absolute lymphocyte count has emerged as standard practice despite a lack of strong evidence supporting this approach [36]. In MS patients, therapies other than natalizumab, fingolimod, and dimethyl fumarate have not been associated with development of PML [36]. Although most affected patients survive, disability is common [37].
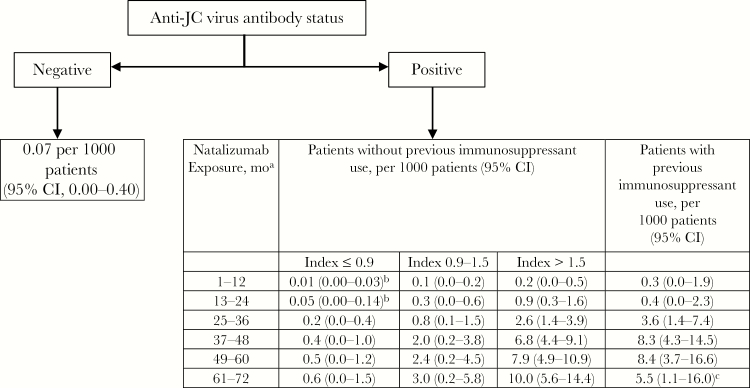
Risk factors for natalizumab-associated PML include prior immunosuppression, prolonged treatment duration with natalizumab (particularly >24 months), and presence of anti-JCV antibodies (which are >98% sensitive in predicting development of PML but very nonspecific) [35, 38]. Seroconversion with development of newly detected anti-JCV antibodies occurs in up to 10% of natalizumab-treated patients per year but likely does not reflect new infection and does not seem to confer any additional risk of PML beyond that associated with known seropositive status [35]. Seroreversion also occurs and is also of unclear significance, though these patients are generally assumed to have a risk of PML similar to those who remain with detectable antibodies [35]. More recently, a quantitative anti-JCV index, reflecting an antibody titer normalized to standard control serum, has been shown to be more predictive of PML than the qualitative antibody value. Actuarial tables have been generated in an attempt to further risk-stratify patients receiving natalizumab based on the above variables (Figure 1) [35, 38].
Figure 1. .
Updated progressive multifocal leukoencephalopathy (PML) risk estimate based on natalizumab exposure, previous immunosuppressant use, and anti–JC virus (JCV) antibody index. Reprinted with permission from Elsevier from Lancet Neurology: Ho PR, Koendgen H, Campbell N, Haddock B, Richman S, Chang I. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol 2017; 16(11):925–933. Conditional probability of developing PML using the life table method in each year of treatment is presented, with multiple imputation used to account for missing data in the pooled cohort (n = 21 696). PML risk estimates were calculated using a life table method in the pooled cohort of anti-JCV antibody-positive patients who participated in the STRATIFY-2 [41], TOP [42], TYGRIS [43], and STRATA [44] clinical studies. aData beyond 6 years of treatment are scarce. bAlthough estimates below 0.1 per 1000 patients were rounded up to 0.1 per 1000 patients for regulatory documents and management guidelines, these estimates are shown with greater precision in this article. cVariability might be due to small sample size. Abbreviation: CI, confidence interval.
Because of the lack of effective treatment of natalizumab-associated PML (other than discontinuation of natalizumab and, potentially, plasmapheresis), avoidance or discontinuation of natalizumab when benefits outweigh risks is critical (Figure 1) [37, 39, 40]. Anti-JCV antibodies should typically be tested at baseline before treatment with natalizumab, 12 months after treatment is initiated, and every 6 months thereafter, except if the antibody index is greater than 1.5, in which case the risk is sufficiently high that further testing would not change management [39, 40]. Because radiographic evidence of PML precedes PML-associated neurologic deficits, serial brain magnetic resonance imaging (MRI) scans are recommended to detect early radiographic manifestations of PML that would prompt natalizumab discontinuation [39, 40]. Our overall approach is highlighted in Table 4. Monitoring should continue for 6 months after natalizumab therapy ceases [39].
Table 4. .
Recommendations for PML Prevention Among Patients Receiving Natalizumab
| Anti-JCV Antibody Index ≤0.9 or Seronegative | Anti-JCV Antibody Index >0.9 and <1.5 | Anti-JCV Antibody Index ≥1.5 or Prior Immunosuppression and on Natalizumab for ≥2 y (Regardless of Serostatus) | |
|---|---|---|---|
| Repeat anti-JCV antibody testing | At 12 mo and every 6 mo | At 12 mo and every 6 mo | Not indicated |
| Repeat MRI | Annually | At 12 and 18 mo and every 6 mo thereafter | At 12 and 18 mo and every 3–4 mo thereafter |
| Other notes | All patients should have baseline anti-JCV antibody testing and brain MRI Consider abbreviated protocols consisting of axial FLAIR and DWI sequences |
||
Abbreviations: DWI, diffusion weighted imaging; FLAIR, fluid-attenuated inversion recovery; JCV, JC virus; MRI, magnetic resonance imaging; PML, progressive multifocal leukoencephalopathy.
The risks of PML with fingolimod and dimethyl fumarate are low enough that routine screening for antibodies to JCV or monitoring for PML are of uncertain benefit and not routinely performed. Alemtuzumab and anti-CD20 monoclonal antibodies have been associated with PML in non-MS patients, mostly those with hematologic malignancies, but no cases of PML have been published among those undergoing treatment for MS, a discrepancy that likely reflects additional immune impairments of this more vulnerable patient population [36].
HERPES VIRUSES
The risk of reactivation of latent herpesvirus infection is increased by immunosuppressive therapy (particularly therapy that affects cellular immunity) and by physiologic stress and other factors. Herpes simplex virus (HSV) 1 and 2, varicella zoster virus (VZV), and cytomegalovirus (CMV) are the most common causes of herpes virus infections requiring treatment.
When compared with interferon treatment, alemtuzumab administration was associated with remarkably high rates of HSV infections, sometimes severe enough to require hospitalization, with some VZV infections noted as well [8, 9]. As a consequence, clinical trial protocols were amended to institute acyclovir 200 mg twice daily during alemtuzumab therapy and for 28 days thereafter [8, 9]. The FDA-approved product label recommends prophylaxis with acyclovir from the start of treatment until CD4+ lymphocytes recover to at least 200 cells/µL, with a minimum duration of prophylaxis of 2 months even if CD4+ lymphocytopenia resolves earlier [45]. Although a lower dose of acyclovir was used in clinical trials, acyclovir is typically given at 400 mg twice daily for prophylaxis, and this dose could be considered in alemtuzumab-treated patients as well. In contrast to its frequent occurrence in hematology patients, CMV disease rarely occurs in MS patients treated with alemtuzumab [46, 47]. Because of the infrequency of CMV disease in these patients, preemptive monitoring with PCR, as is done in hematology patients, is not warranted in MS patients receiving alemtuzumab.
Given its effects on lymphocyte trafficking, fingolimod could theoretically increase the risk of herpes virus infections. Two fatal herpes virus infections occurred in patients receiving fingolimod in a pivotal clinical trial, including a nonimmune patient with primary varicella infection after exposure to an infected child and a patient with HSV encephalitis [24]. Another fatal case was reported involving VZV reactivation [21]. Both fatal VZV cases, however, involved patients concomitantly receiving corticosteroids [21]. Subsequent analysis confirmed a higher risk of total (but not serious) VZV infections in patients treated with fingolimod compared with placebo, and rates were overall low [21]. As with all other patients, those not immune to VZV should be vaccinated before immunosuppression unless a contraindication exists [21]. Given the overall low risk of herpes virus infections, particularly serious infections, routine prophylaxis with acyclovir is not recommended, though it could be considered when the drug is co-administered with corticosteroids (except 3–5 days of high-dose corticosteroid treatment without tapering) or in patients with frequent oral or genital herpes simplex recurrences [21].
Although fingolimod may prevent immune surveillance for herpes viruses given its mechanism of action, natalizumab may be expected to impair immune surveillance in the CNS. Initial studies of fingolimod did not report frequent or serious herpes virus infections, though postmarketing data have yielded dozens of cases of CNS herpes virus infections with fingolimod, mostly CNS HSV infection and some CNS VZV infections [18]. Although some of these patients experienced death or disability, most recovered [18]. Infections occurred in patients with and without prior immunosuppression and seemingly irrespective of the length of natalizumab therapy [18]. Current product labeling does not advise routine acyclovir prophylaxis, and the risks and benefits of routine prophylaxis for this uncommon but potentially devastating complication of natalizumab therapy are uncertain; acyclovir prophylaxis, however, could be considered in patients with prior immunosuppression or with frequent oral or genital herpes simplex recurrences.
Ocrelizumab does seem to increase risk of herpes virus infections, though nearly all infections were mild to moderate and there is no need for routine antiviral prophylaxis in these patients [16, 17]. Other anti-MS therapies, including teriflunomide, dimethyl fumarate, and mitoxantrone, are not clearly associated with increased risk of herpes virus infection frequency or severity, though scattered case reports exist [48].
OTHER INFECTIONS OF CONCERN
Given their relative prevalence and implications for immunosuppressive therapy, most clinical trials for MS drugs screened participants for HIV and hepatitis C virus (HCV) infections, practices that are reasonable to continue in clinical practice.
OIs typically associated with HIV infection other than those already discussed have been only rarely reported in association with MS therapies—mostly medications that affect lymphocyte trafficking, such as natalizumab and fingolimod, or those resulting in depletion of T lymphocytes, such as alemtuzumab. CNS, cutaneous, and disseminated cryptococcosis have been rarely reported with fingolimod use, and to a lesser extent natalizumab use [23]. Individual cases of Kaposi sarcoma, cutaneous histoplasmosis, and CNS toxoplasmosis have also been reported in non-HIV-infected patients with MS receiving fingolimod [22, 49, 50]. Similarly, rare cases of Pneumocystis pneumonia, nocardiosis, and listeriosis have been reported with alemtuzumab in MS patients [46, 47, 51]. Routine screening or prophylaxis is not indicated in this population, though clinicians should be alert to the possibility of these unusual OIs.
Several anti-MS therapies, including alemtuzumab, ocrelizumab, and mitoxantrone, are associated with a statistically significant increased overall risk of infection, mostly mild or moderate nasopharyngitis, upper respiratory tract infections, and urinary tract infections, though no particular preventive approach is indicated [8, 9, 16, 17, 29]. Despite OIs associated with fingolimod and natalizumab, no increased risk in overall infections was seen in clinical trials [3, 19, 24–26]. Teriflunomide was not associated with an increased risk in overall infections, and clinical studies on dimethyl fumarate found conflicting results on the risk of infections [11–14, 27, 28].
VACCINATION
Though varicella vaccination is particularly emphasized as a preventive health care intervention for susceptible patients before fingolimod administration, all patients being treated for MS should receive vaccinations based on age, sex, and other risk factors. Emphasis should also be placed on seasonal influenza vaccination. Unless or until data emerge supporting the safety of live-attenuated vaccine administration in patients treated with immunosuppressive therapies for MS, these vaccines should be avoided [52]. A minimum duration of 4 weeks is typically recommended between administration of a live-attenuated vaccine and induction of immunosuppression, though some product labels of MS drugs advise a 6-week waiting period [45, 53, 54]. Live-attenuated vaccines are similarly contraindicated during therapy and for a variable period after therapy ends, typically ranging from 2 to 6 months, depending in part on the pharmacokinetic qualities of the drug [15, 55].
Inactivated, subunit, and toxoid vaccines do not risk the same safety concerns as live-attenuated vaccines, but immunologic responses may be attenuated or absent in the context of immunosuppression [53]. (Although novel adjuvants, including AS01B in the recombinant zoster vaccine, could theoretically exacerbate MS, this concern has not proven true so far in this or other immune-mediated conditions and should not preclude use of vaccines, though ongoing postmarketing surveillance is needed.) Patients treated with alemtuzumab and ocrelizumab (inferring from recommendations for rituximab-treated patients) are unlikely to respond to vaccination during therapy and for up to 6 months after therapy ceases [53]. Mitoxantrone, like other chemotherapeutic agents, may impair vaccine responses for up to 3 months [53]. Data support the immunogenicity (though sometimes reduced compared with placebo-treated patients) of vaccines given to patients treated with natalizumab, fingolimod, dimethyl fumarate, and teriflunomide, so inactivated, subunit, and toxoid vaccines can likely be given without regard for these MS therapies [52, 56–58].
CONCLUSIONS
Numerous immunosuppressant therapies are joining interferons and glatiramer as treatments for MS. Overall, these agents are safe, have favorable risk–benefit profiles, and can dramatically improve the quality of life for patients with a potentially disabling neurologic illness. Still, we must anticipate and respond to iatrogenic complications to minimize harm and ensure that these drugs continue to benefit patients. Although some of these agents have predictable or well-established effects on infectious risk, the infectious risks associated with others are unclear. Imputing infectious risks from studies of these drugs in other populations may lead to overestimation of these risks in this population, most of whom do not have underlying immunologic deficits or exposure to overlapping immunosuppressive therapies. At the same time, the sequential therapies some patients are administered may confound attribution of OI risk to particular drugs. Still, methodical approaches can yield rational estimates of infectious risks and guide preventive and preemptive management strategies.
Acknowledgments
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Brooks M. MS drug Daclizumab (zinbryta) pulled from the market. Medscape Medical News; 2 March 2018. Available at: http://www.medscape.com/viewarticle/893352. Accessed 6 March 2018. [Google Scholar]
- 2. Kappos L, Bar-Or A, Cree BAC, et al. ; EXPAND Clinical Investigators Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 2018; 391:1263–73. [DOI] [PubMed] [Google Scholar]
- 3. Rudick RA, Stuart WH, Calabresi PA, et al. ; SENTINEL Investigators. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis N Engl J Med 2006; 354:911–23. [DOI] [PubMed] [Google Scholar]
- 4. Hawker K, O’Connor P, Freedman MS, et al. ; OLYMPUS trial group Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 2009; 66:460–71. [DOI] [PubMed] [Google Scholar]
- 5. Liao TL, Lin CH, Chen YM, et al. Different risk of tuberculosis and efficacy of isoniazid prophylaxis in rheumatoid arthritis patients with biologic therapy: a nationwide retrospective cohort study in Taiwan. PLoS One 2016; 11:e0153217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pei SN, Chen CH, Lee CM, et al. Reactivation of hepatitis B virus following rituximab-based regimens: a serious complication in both HBsAg-positive and HBsAg-negative patients. Ann Hematol 2010; 89:255–62. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. Division of Tuberculosis Elimination. Latent Tuberculosis Infection: A Guide for Primary Health Care Providers, Treatment of Latent TB Infection. Centers for Disease Control and Prevention; 2016. Available at: http://www.cdc.gov/tb/publications/ltbi/treatment.htm. Accessed 2 December 2017. [Google Scholar]
- 8. Cohen JA, Coles AJ, Arnold DL, et al. ; CARE-MS I investigators Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012; 380:1819–28. [DOI] [PubMed] [Google Scholar]
- 9. Coles AJ, Twyman CL, Arnold DL, et al. ; CARE-MS II investigators Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012; 380:1829–39. [DOI] [PubMed] [Google Scholar]
- 10. Kim SJ, Moon JH, Kim H, et al. Non-bacterial infections in Asian patients treated with alemtuzumab: a retrospective study of the Asian Lymphoma Study Group. Leuk Lymphoma 2012; 53:1515–24. [DOI] [PubMed] [Google Scholar]
- 11. O’Connor P, Wolinsky JS, Confavreux C, et al. ; TEMSO Trial Group Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011; 365:1293–303. [DOI] [PubMed] [Google Scholar]
- 12. Confavreux C, O’Connor P, Comi G, et al. ; TOWER Trial Group Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13:247–56. [DOI] [PubMed] [Google Scholar]
- 13. Miller AE, Wolinsky JS, Kappos L, et al. ; TOPIC Study Group Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13:977–86. [DOI] [PubMed] [Google Scholar]
- 14. Vermersch P, Czlonkowska A, Grimaldi LM, et al. ; TENERE Trial Group Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler 2014; 20:705–16. [DOI] [PubMed] [Google Scholar]
- 15. Aubagio [package insert]. Cambridge, MA: Genzyme; 2016. [Google Scholar]
- 16. Hauser SL, Bar-Or A, Comi G, et al. ; OPERA I and OPERA II Clinical Investigators. Ocrelizumab versus interferon Beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376:221–34. [DOI] [PubMed] [Google Scholar]
- 17. Montalban X, Hauser SL, Kappos L, et al. ; ORATORIO Clinical Investigators. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017; 376:209–20. [DOI] [PubMed] [Google Scholar]
- 18. Fine AJ, Sorbello A, Kortepeter C, Scarazzini L. Central nervous system herpes simplex and varicella zoster virus infections in natalizumab-treated patients. Clin Infect Dis 2013; 57:849–52. [DOI] [PubMed] [Google Scholar]
- 19. Polman CH, O’Connor PW, Havrdova E, et al. ; AFFIRM Investigators A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354:899–910. [DOI] [PubMed] [Google Scholar]
- 20. Mulero P, Caminero AB, Neri Crespo MJ, et al. Latent tuberculosis seems not to reactivate in multiple sclerosis patients on natalizumab. J Neuroimmunol 2012; 243:103–5. [DOI] [PubMed] [Google Scholar]
- 21. Arvin AM, Wolinsky JS, Kappos L, et al. Varicella-zoster virus infections in patients treated with fingolimod: risk assessment and consensus recommendations for management. JAMA Neurol 2015; 72:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tully T, Barkley A, Silber E. Kaposi sarcoma in a patient with relapsing-remitting multiple sclerosis receiving fingolimod. Neurology 2015; 84:1999–2001. [DOI] [PubMed] [Google Scholar]
- 23. Grebenciucova E, Reder AT, Bernard JT. Immunologic mechanisms of fingolimod and the role of immunosenescence in the risk of cryptococcal infection: a case report and review of literature. Mult Scler Relat Disord 2016; 9:158–62. [DOI] [PubMed] [Google Scholar]
- 24. Cohen JA, Barkhof F, Comi G, et al. ; TRANSFORMS Study Group Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362:402–15. [DOI] [PubMed] [Google Scholar]
- 25. Kappos L, Radue EW, O’Connor P, et al. ; FREEDOMS Study Group A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362:387–401. [DOI] [PubMed] [Google Scholar]
- 26. Lublin F, Miller DH, Freedman MS, et al. ; INFORMS study investigators Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2016; 387:1075–84. [DOI] [PubMed] [Google Scholar]
- 27. Fox RJ, Miller DH, Phillips JT, et al. ; CONFIRM Study Investigators Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367:1087–97. [DOI] [PubMed] [Google Scholar]
- 28. Gold R, Kappos L, Arnold DL, et al. ; DEFINE Study Investigators Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367:1098–107. [DOI] [PubMed] [Google Scholar]
- 29. Hartung HP, Gonsette R, König N, et al. ; Mitoxantrone in Multiple Sclerosis Study Group (MIMS) Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet 2002; 360:2018–25. [DOI] [PubMed] [Google Scholar]
- 30. Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT; American Gastroenterological Association Institute American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015; 148:215–9; quiz e16–7. [DOI] [PubMed] [Google Scholar]
- 31. Fukuda W, Hanyu T, Katayama M, et al. Incidence of hepatitis B virus reactivation in patients with resolved infection on immunosuppressive therapy for rheumatic disease: a multicentre, prospective, observational study in Japan. Ann Rheum Dis 2017; 76:1051–6. [DOI] [PubMed] [Google Scholar]
- 32. Hillen ME, Cook SD, Samanta A, et al. Fatal acute liver failure with hepatitis B virus infection during nataluzimab treatment in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2015; 2:e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Voican CS, Mir O, Loulergue P, et al. Hepatitis B virus reactivation in patients with solid tumors receiving systemic anticancer treatment. Ann Oncol 2016; 27:2172–84. [DOI] [PubMed] [Google Scholar]
- 34. Brew BJ, Davies NW, Cinque P, et al. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol 2010; 6:667–79. [DOI] [PubMed] [Google Scholar]
- 35. Schwab N, Schneider-Hohendorf T, Melzer N, et al. Natalizumab-associated PML: challenges with incidence, resulting risk, and risk stratification. Neurology 2017; 88:1197–205. [DOI] [PubMed] [Google Scholar]
- 36. Berger JR. Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord 2017; 12:59–63. [DOI] [PubMed] [Google Scholar]
- 37. Landi D, De Rossi N, Zagaglia S, et al. ; Italian PML Study Group No evidence of beneficial effects of plasmapheresis in natalizumab-associated PML. Neurology 2017; 88:1144–52. [DOI] [PubMed] [Google Scholar]
- 38. Ho PR, Koendgen H, Campbell N, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol 2017; 16:925–33. [DOI] [PubMed] [Google Scholar]
- 39. Tysabri [package insert]. Cambridge, MA: Biogen; 2017. [Google Scholar]
- 40. McGuigan C, Craner M, Guadagno J, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry 2016; 87:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Campagnolo D, Ho P-R, Patel R, et al. Four-year longitudinal index stability data from STRATIFY-2 support the clinical utility of index for risk stratification of natalizumab-associated progressive multifocal leukoencephalopathy. Mannheim, Germany:Congress of the German Society for Neurology; 2016; IP 468. [Google Scholar]
- 42. Butzkueven H, Kappos L, Pellegrini F, et al. Efficacy and safety of natalizumab inmultiple sclerosis: interim observational programme results. J Neurol Neurosurg Psychiatry 2014; 85:1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Foley J, Carrillo-Infante C, Wenten M, et al. Long-term safety of natalizumab treatment in multiple sclerosis (MS) in clinical practice: results from the tysabri global observational program in safety (TYGRIS). London, UK: European Committee for Treatment and Research in Multiple Sclerosis Congress; 2016; P1229. [Google Scholar]
- 44. O’Connor P, Goodman A, Kappos L, et al. Long-term safety and effectiveness of natalizumab redosing and treatment in the STRATA MS Study. Neurology 2014; 83:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lemtrada [package insert]. Cambridge, MA: Genzyme; 2017. [Google Scholar]
- 46. Yann K, Jackson F, Sharaf N, et al. Acute respiratory distress syndrome following alemtuzumab therapy for relapsing multiple sclerosis. Mult Scler Relat Disord 2017; 14:1–3. [DOI] [PubMed] [Google Scholar]
- 47. Brownlee WJ, Chataway J. Opportunistic infections after alemtuzumab: new cases of norcardial infection and cytomegalovirus syndrome. Mult Scler 2017; 23:876–7. [DOI] [PubMed] [Google Scholar]
- 48. Ma BB, Ostrow LW, Newsome SD. Disseminated zoster with paresis in a multiple sclerosis patient treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm 2016; 3:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Veillet-Lemay GM, Sawchuk MA, Kanigsberg ND. Primary cutaneous histoplasma capsulatum infection in a patient treated with fingolimod: a case report. J Cutan Med Surg 2017; 21:553–5. [DOI] [PubMed] [Google Scholar]
- 50. Enriquez-Marulanda A, Valderrama-Chaparro J, Parrado L, et al. Cerebral toxoplasmosis in an MS patient receiving Fingolimod. Mult Scler Relat Disord 2017; 18:106–8. [DOI] [PubMed] [Google Scholar]
- 51. Coles AJ, Compston DA, Selmaj KW, et al. ; CAMMS223 Trial Investigators Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008; 359:1786–801. [DOI] [PubMed] [Google Scholar]
- 52. von Hehn C, Howard J, Liu S, et al. Immune response to vaccines is maintained in patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm 2018; 5:e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rubin LG, Levin MJ, Ljungman P, et al. ; Infectious Diseases Society of America 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44–100. [DOI] [PubMed] [Google Scholar]
- 54. Ocrevus [package insert]. South San Francisco, CA: Genentech; 2017. [Google Scholar]
- 55. Gilenya [package insert]. East Hanover, NJ: Novartis Pharmaceuticals; 2017. [Google Scholar]
- 56. Kaufman M, Pardo G, Rossman H, et al. Natalizumab treatment shows no clinically meaningful effects on immunization responses in patients with relapsing-remitting multiple sclerosis. J Neurol Sci 2014; 341:22–7. [DOI] [PubMed] [Google Scholar]
- 57. Kappos L, Mehling M, Arroyo R, et al. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology 2015; 84:872–9. [DOI] [PubMed] [Google Scholar]
- 58. Bar-Or A, Freedman MS, Kremenchutzky M, et al. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology 2013; 81:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]