Abstract
Gas chromatography/mass spectrometry (GC/MS) has been used for steroid analysis since the 1960s. The advent of protective derivatization, capillary columns, and inexpensive electron ionization bench-top single quadrupole soon made it the method of choice for studying disorders of steroid synthesis and metabolism. However, the lengthy sample workup prevented GC/MS from becoming routine for steroid hormone measurement, which was dominated by radioimmunoassay. It was the emergence of liquid chromatography/tandem MS (LC/MS/MS) that sparked a renewed interest in GC/MS for the multicomponent analysis of steroids. GC/MS is excellent at providing an integrated picture of a person's steroid metabolome, or steroidome, as we term it. We review the recent work on newly described disorders and discuss the technical advances such as GC coupling to triple quadrupole and ion trap analyzers, two-dimensional GC/MS, and alternative ionization and detection systems such as atmospheric pressure chemical ionization (APCI) and time of flight. We believe that no novel GC/MS-based technique has the power of GC(electron ionization)/MS/MS as a “discovery tool,” although APCI might provide ultimate sensitivity, which might be required in tissue steroidomics. Finally, we discuss the role of LC/MS/MS in steroidomics. This remains a challenge but offers shorter analysis times and advantages in the detection and discovery of steroids with a known structure. We describe recent advances in LC/MS steroidomics of hydrolyzed and intact steroid conjugates and suggest the technique is catching up with GC/MS in this area. However, in the end, both techniques will likely remain complementary and both should be available in advanced analytical laboratories.
Keywords: GC/MS, LC/MS/MS, metabolomics, steroid profiles, steroidomics, urine steroids
We review recent work on newly described disorders and discuss technical advances on GC/MS. We also discuss recent advances in LC/MS steroidomics of hydrolyzed and intact steroid conjugates.
The separation, characterization, and quantification of metabolic profiles of steroids of clinical interest in biological specimens have a long history, beginning with paper and thin-layer chromatography [1, 2]. In the present report, we used the advent of gas chromatography (GC) coupled to electron impact (EI) mass spectrometry (GC/MS) as the starting point. Three early developments ~50 years ago made current metabolic profiling possible: First, the development of GC in 1960 [3]; second, the combination of GC with mass spectrometry (MS) through Ryhage’s molecular separator invention [4]; and, finally, derivatization methodologies that increased the volatility and stability of steroids. Soon computers were added, and the introduction of capillary columns maximized separation [5]. By 1975, the currently preferred general method for multicomponent steroid analysis had been established. Major column, instrument, and data system improvements have occurred in the intervening years. To this day, metabolomics researchers follow the method described ∼30 years ago [6, 7].
Traditionally, multicomponent steroid analysis has been termed steroid profiling; however, in the current fashion of “omics” usage, it is an “analyte specific” part of metabolomics. Although the term “steroid metabolomics” might be more correct, other terms have been used. Professor Jan Sjövall [8], one of the original GC/MS developers, already suggested in 2004 applying the term “steroidomics” to work on steroid profiling in earlier decades. Further simplification to “sterome” (steromics) has also been suggested [9]; however, we believe that abbreviation oversimplifies. In the present minireview, we will use Sjövall’s suggestion, steroidomics.
From an analytical viewpoint, metabolomics can be divided into targeted and untargeted analyses [10]. The term “untargeted metabolome” is associated with the evaluation of the biological material in an open way, meaning that no restrictions in terms of the nature of the monitored molecules are established. Steroidomics would loosely be “targeted,” because it focuses on one particular family within the global metabolome. What we discuss is a further division, untargeted and targeted steroidomics. Targeting approaches are less comprehensive but make accurate and precise quantitative information easier to obtain. It requires an a priori knowledge about the relevant metabolites in the studied samples. Thus, steroidomics normally refers to those targeted metabolomics approaches focused on steroid related pathways.
Whether targeted or untargeted, the sample extracted and prepared for MS analysis should contain close to all steroids present in the medium (e.g., urine, serum, tissue). Sample treatment has traditionally been a critical factor for steroidomics. First, an extraction method must be used that can recover all the desired components. For GC/MS, if the steroids are present in conjugated forms (sulfates and glucuronides), a hydrolysis step must be included to remove the conjugate moiety. Finally, for GC/MS, chemical modification of steroids (derivatization) is necessary to improve steroid volatility and stability and encourage specific fragmentations with EI ionization. In a GC/MS spectrum, a molecular ion (equivalent to molecular weight) and pattern of fragment ions often provides sufficient information to characterize a component, especially if an authentic standard is also available.
When the derivatized steroid mixture is formed, it can be subjected to untargeted or targeted analysis. Untargeted steroidomics is achieved with the instrument in “scan mode,” in which the full mass spectra are repetitively collected throughout the GC run. After the run, the identity of all peaks can be ascertained from their mass spectra. This form of analysis lacks sensitivity for identifying and quantifying minor, but important, components, and targeted analysis is often preferred for routine quantitation. In the latter mode, the instrument switches between chosen specific ions of the compounds of interest, which, when quantified, results in a multifold increase in sensitivity. Clearly, a priori knowledge of important components is required for targeted analysis. This technique is termed selected-ion-monitoring (SIM). In our studies [Institute of Metabolism and Systems Research (IMSR), Birmingham, UK], we always run samples using both targeted SIM and scanning. Routine sensitive quantitative analysis is achieved by SIM; however, any questions arising from the SIM analysis can often be resolved by extracting further data from the scanned run. Furthermore, steroids not requested in the SIM run can be sought in the scanned run.
Despite the extensive workup required, GC/MS remains the reference standard for comprehensive steroidomics. The separating ability is unchallenged, and an existing extensive knowledge of EI fragmentation of steroids has allowed characterization of unexpected or novel compounds. We consider it the best “discovery tool” for unusual or unknown metabolomes [11]. However, we also concede some challenges, in particular, the existence of both non–enzyme hydrolysable and derivatization-resistant metabolites. These situations are definitely ones for which LC/MS/MS has advantage [12].
Which types of steroid quantifications can be considered steroidomics? Most likely, the independent quantification of a few steroids (i.e., a panel) should not be considered steroidomics. It need not require the untargeted analysis of the steroid content in the sample [13] but should include any comprehensive study of the steroid profile of a fluid or tissue extract, whether the study is conducted in a targeted or untargeted manner. What might be the principal or goal of steroidomics is that the extract analyzed contains close to complete representation of the total steroid content of the biological medium studied and allow for monitoring activity of the biosynthetic pathway or system in question.
Steroidomics has importance in many fields, including clinical and diagnostic studies (e.g., characterization of inborn errors of metabolism), cancer, Cushing disorder, sports doping analysis, and in vivo or in vitro toxicology assays. Our objective for the present review was to report GC/MS steroidomic advances in this millennium. However, we also discuss to what extent LC/MS/MS is competing with, or replacing, GC/MS in this field.
1. Methods
For the present review, different searches for clinical and analytical chemistry studies were performed in the human-curated scientific database PubMed and focused on this millennium (the latest search was performed in April 2018). We used key words such as urinary steroids, GC/MS, GC/MS/MS, LC/MS/MS, congenital adrenal hyperplasia (CAH), steroid metabolism, and steroid profile analysis. We also reviewed the publication history of known prominent investigators in the field and reviewed the references they cited. Table 1 summarizes a selection of pertinent reviews.
Table 1.
Reviews Pertinent to Steroid Metabolomics
| Title | Author, Year | Reference |
|---|---|---|
| Profiling steroid hormones and urinary steroids | Shackleton, 1986 | 6 |
| Tandem mass spectrometry in the study of fatty acids, bile acids, and steroids | Griffiths, 2003 | 14 |
| Fifty years with bile acids and steroids in health and disease | Sjövall, 2004 | 8 |
| Steroid analysis | Griffiths et al., 2005 | 15 |
| GC-MS steroid profiling: diagnosis of disorders affecting steroid synthesis and metabolism | Shackleton et al., 2006 | 16 |
| Mass spectrometry in sports drug testing: Structure characterization and analytical assays | Thevis et al., 2007 | 17 |
| Clinical steroid mass spectrometry: a 45-year history culminating in HPLC-MS/MS becoming an essential tool for patient diagnosis | Shackleton, 2010 | 18 |
| Liquid chromatography-tandem mass spectrometry applications in endocrinology | Kushnir et al., 2010 | 19 |
| Steroid profiling by gas chromatography-mass spectrometry and high performance liquid chromatography-mass spectrometry for adrenal diseases | McDonald et al., 2011 | 20 |
| Role of a disordered steroid metabolome in the elucidation of sterol and steroid biosynthesis | Shackleton, 2012 | 21 |
| Mass spectrometry theory and application to adrenal diseases | Wooding et al., 2013 | 22 |
| Urinary steroid profiling | Taylor, 2013 | 23 |
| Introduction to gas chromatography-mass spectrometry | Sánchez-Guijo et al., 2013 | 24 |
| LC-MS/MS in clinical laboratories | Kushnir et al. 2013 | 25 |
| Mass spectrometry for steroids | Honor 2014 | 26 |
| Analytical strategies based on mass spectrometric techniques for the study of steroid metabolism | Gomez et al. 2014 | 27 |
| Derivatization of steroids in biological samples for GC-MS and LC-MS analyses | Marcos et al. 2015 | 28 |
| Adrenal steroidogenesis and congenital adrenal hyperplasia | Turcu et al., 2015 | 29 |
| Bringing GC-MS profiling of steroids into clinical applications | Choi et al., 2015 | 30 |
| Liquid chromatography tandem mass spectrometry in the clinical laboratory | Adaway et al., 2015 | 31 |
| Chemical derivatization for enhancing sensitivity during LC/ESI-MS/MS quantification of steroids in biological samples: a review | Higashi et al. 2016 | 32 |
| Steroid LC-MS has come of age | Wudy et al., 2016 | 33 |
| LC-MS/MS analysis of steroids in the clinical laboratory | Keevil, 2016 | 34 |
| Current LC-MS methods and procedures applied to the identification of new steroid metabolites | Marcos et al., 2016 | 12 |
| The evolution of methods for urinary steroid metabolomics in clinical investigations particularly in childhood | Honor et al., 2018 | 35 |
| The utility of ultra-high performance supercritical fluid chromatography-tandem mass spectrometry (UHPSFC-MS/MS) for clinically relevant steroid analysis | Storbeck et al., 2018 | 36 |
| The art of measuring steroids: principles and practice of current hormonal steroid analysis | Wudy et al., 2018 | 37 |
| Targeting human urinary metabolome by LC-MS/MS: a review | Rodríguez-Morató et al., 2018 | 38 |
Abbreviation: HPLC, high-performance liquid chromatography.
2. Results
A. Classical GC/MS Methods
The basic principles of steroid identification by GC(EI)/MS were covered in the reviews by Griffiths et al. [14, 15]. These contain tabulated data of the influence of individual derivatized steroid moieties on the fragment losses and fragment ions produced, largely restricted to trimethylsilyl (TMS) and methyloxime-trimethylsilyl (MO-TMS) derivatives.
A-1. Determination of targeted steroidome by classical GC/MS methods
It is probable that the number of components in the human urinary steroidome at a level >1 µg/day number in the 100s, and the vast majority of these will appear in the extract obtained after Helix pomatia (HP) sulfatase/β-glucuronidase hydrolysis and will be derivatized as an MO-TMS derivative. Complete evaluation of the steroidome to include the minor components by scanning GC/MS with a single quadrupole instrument is challenging from a sensitivity perspective, and a targeted steroidome of important components quantified by SIM will be more practical. For humans, such a targeted profile should contain representative metabolites of all major hormones and their precursors (e.g., androgens, 3β-hydroxy-Δ5 steroids, pregnanes, corticosteroid metabolites, and mineralocorticoids, such as aldosterone).
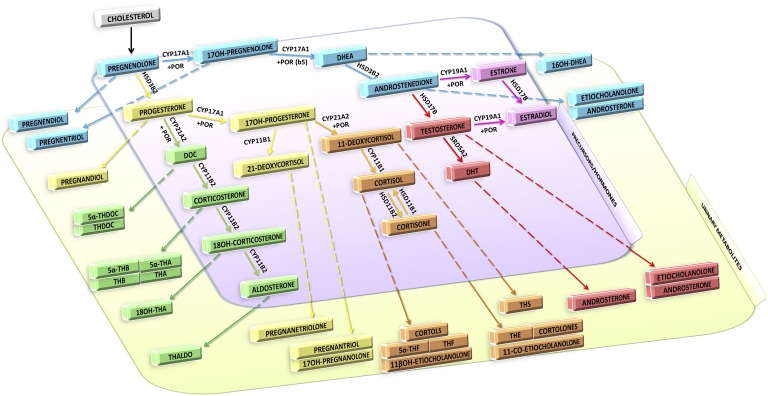
Figure 1 illustrates the mature (child and adult), but simplified, adrenal biosynthetic pathway in humans, showing enzymes, intermediates, products, and metabolites, with the latter representing the targeted steroidome used by us at IMSR for most clinical investigations. In addition, many of our studies focused on neonates who excrete novel metabolites that must be added to the profile. Other studies by us focused on pregnancy and the prenatal diagnosis of low-pregnancy-estriol disorders, which also require analysis of specific metabolites.
Figure 1.
Biosynthesis of steroid hormones and prominent urinary metabolites. This targeted steroidome is the principal one used for adults and children by the IMSR at the University of Birmingham, United Kingdom. Cortols and cortolones are familiar terms. 3α5α17HP, 3α,17α-dihydroxy-5α-pregnan-20-one; 5αTHA, 5α-tetrahydro-11-dehydrocorticosterone; 5αTHB, 5α-tetrahydrocorticosterone; 5αTHDOC, 5α tetrahydrodeoxycorticosterone; 5αTHF, 5α-tetrahydrocortisol; 5PD, 5-pregnene-3β,20α-diol; 5PT, 5-pregnene-3β,17α,20α-triol; 6βOHF, 6β-OH-cortisol; 11-oxo-Et, 11-oxoetiocholanolone; 11β-OH-An, 11β-OH-androsterone; 11β-OH-Et, 11β-OH-etiocholanolone; 16αDHEA, 16α-OH-DHEA; 17HP, 17-OH-pregnanolone; Andro, androsterone; DHEA, dehydroepiandrosterone; Etio, etiocholanolone; PD, pregnanediol; PT, 5β-pregnane-3α,17α,20α-triol; PTONE, 5β-pregnane-3α,17α,20α-triol-11-one; THA, tetrahydro-11-dehydrocorticosterone; THAldo, tetrahydroaldosterone; THB, tetrahydrocorticosterone; THDOC, tetrahydrodeoxycorticosterone; THE, tetrahydrocortisone; THF, tetrahydrocortisol.
In addition to clinical studies, we have been involved in testing for misuse of endogenous and exogenous steroids (anabolic agents and corticosteroids) in sport. The doping control steroidomes have expanded greatly over recent years, with a focus on the development of the “biological passport” and a search for unique metabolites of misused steroids with a long half-life.
The well-known classical method based on solid-phase extraction, enzymatic hydrolysis, TMS or MO-TMS derivatization, and analysis by GC/MS in single quadrupole instruments is still the foundation of most steroidomic studies.
Our own studies in the use of targeted GC/MS for the diagnosis disorders of steroid biosynthesis and metabolism have been largely described by Shackleton [7], Shackleton and Marcos [16], and Krone et al. [11]. The latter report described the importance of diagnostic precursor metabolite/product metabolite ratios in disorder recognition. These are important because, in many cases, only spot/random urine samples can be obtained from patients, especially infants.
A-2. Reporting steroidomic data
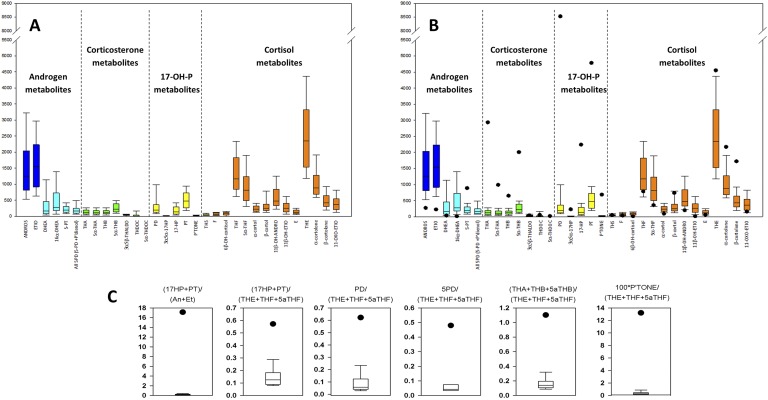
Evaluating and presenting metabolomics data remain a challenge. In the drug metabolism and doping fields, this is not generally a difficulty, because the data are usually reviewed by other experts in steroid metabolism. However, in clinical metabolomics, evaluation and presentation are a problem. Clinicians are used to data for a small selection of hormonal analytes, not the large amount of data for complex unfamiliar structures presented in metabolomics. Classical tables with a list of normal and pathological ranges have become inadequate. A simple graphical display of the normative data superimposed with the results from a patient are an improvement (Fig. 2A and 2B), as are diagnostic precursor metabolite/product metabolite ratios, which target altered individual enzyme activities typically found in steroidopathies, such as forms of CAH, cancer, or Cushing disease. An example from our own studies on P450 oxidoreductase deficiency (PORD) is shown in Fig. 2C. The leftmost panel reflects 17,20-lyase activity in a patient, with the numerator the sum of 17-OH-pregnanolone plus pregnanetriol excretions (metabolites of 17-OH-progesterone) and the denominator, androsterone plus etiocholanolone (metabolites of androstenedione and testosterone). The high values would suggest attenuated lyase activity, an important feature of PORD. The remaining panels show the ratios of other diagnostic steroids relative to the sum of the three main cortisol metabolites (tetrahydrocortisone plus tetrahydrocortisol plus 5α-tetrahydrocortisol; Fig. 1).
Figure 2.
Quantitative excretion and precursor/product ratios in diagnosis of a steroid disorder. (A) Normative quantitative data for 32 components of the targeted steroidome depicted in Fig. 1. (B) Superimposed values for an adult patient with PORD illustrating decreased androgen excretion and elevated excretions of the metabolites of corticosterone and 17-OH-progesterone. (C) Precursor metabolite/product metabolite ratios distinguishing the PORD condition. For example, the leftmost panel shows the ratio of 17-OH-progesterone metabolites over testosterone and androstenedione metabolites. High values in PORD are indicative of PORD-related 17,20-lyase deficiency. The remaining ratios are all distinctive for the condition; 17-OH-progesterone and corticosterone metabolites over total of three major cortisol metabolites [tetrahydrocortisone (THE) plus tetrahydrocortisol (THF) plus 5α-tetrahydrocortisol (5αTHF)]. Black dots indicate results for a patient, with and normative data shown below. Adapted under Creative Commons CC BY 3.0 license from Krone N, Hughes BA, Lavery GG, et al. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J Steroid Biochem Mol Biol 2010; 121(3-5):496-504 [11].
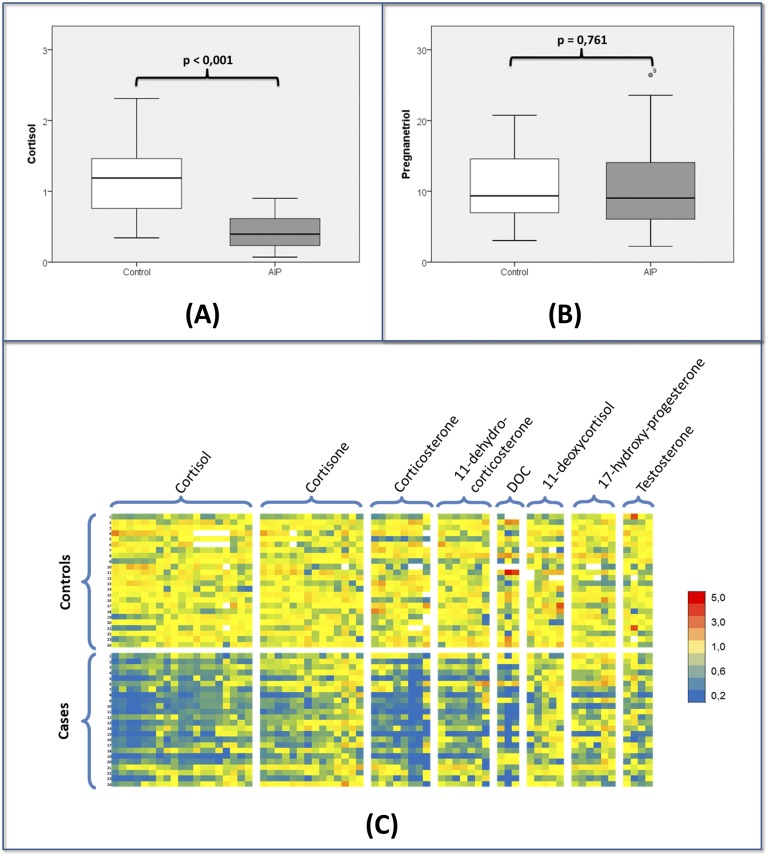
Complex graphical displays such as heat maps have been increasingly used to analyze steroidomic data [39, 40]. Heat maps allow for the visual interpretation of a large amount of related data. As an example, Pozo et al. [41] used heat maps in an LC/MS/MS study of steroid excretion in acute intermittent porphyria, discussed in “C-2. Steroidomics and recently described disorders of synthesis and metabolism” (Fig. 3). Focusing on individual metabolites, a decrease in cortisol (Fig. 3A) was observed, but no variations were found in pregnanetriol (Fig. 3B). The use of heat maps clearly showed that affected individuals excreted lower concentrations, not only of cortisol, but also of 13 of its metabolites (Fig. 3C). Additionally, the ratios between the main metabolites of 17-hydroxyprogesterone and cortisol showed a positive correlation with heme-precursors [41]. Arlt et al. [42] are developing machine learning techniques to improve the diagnostic accuracy of automated data interpretation and have reported on such methods for the diagnosis of adrenal cancer. Eisenhofer et al. [43] included advanced computation and graphical presentation in the diagnosis of Cushing disease using LC/MS/MS analysis of a 15-steroid panel of plasma steroids, largely 3-oxo-Δ4 steroids.
Figure 3.
LC/MS/MS steroidome in acute intermittent porphyria (AIP). (A) Boxplot of urinary cortisol. (B) Boxplot of urinary pregnanetriol showing normal values in affected patients. (C) Heat map showing the changes observed for all 55 detected metabolites. In the heat map, every row corresponds to a patient and every column corresponds to one steroid metabolite. Yellow tones indicate no variation from the mean of the controls. Blue tones represent a decrease in concentration, and red tones symbolize increased levels. The heat map shows that patients with AIP show a general decrease, not only for cortisol, but also for most corticosteroid metabolites. Normal values were generally seen for pregnanetriol and the other is 17-OH-progesterone metabolites (mostly showing yellow tones).
A-3. New, alternate, and improved sample preparation approaches
Sample extraction.
Regarding sample preparation, despite the large variety of new materials, solid-phase extraction protocols based on silica-based nonpolar C18 remains the most widely used method of extraction for conjugates and free and deconjugated steroids. Other types of sorbents have been tested for the profiling of steroids, such as pH-durable copolymeric sorbents with combinations of hydrophilic and lipophilic groups [30].
Hydrolysis.
Although chemical hydrolysis has been described to release steroids from their conjugates, the use of commercial bacterial or mollusk enzymes has been the most applied strategy. Preparations of the digestive juice of the snail HP have been most widely used, because they contain both β-glucuronidase and arylsulfatase and thus are effective for studying the whole urinary steroidome. The use of HP preparations under certain conditions can yield byproducts mainly associated with the hydroxysteroid dehydrogenase/Δ5-4 isomerase and isomerase activities [44]. These activities can be reduced by the addition of ascorbate [45]. In contrast, β-glucuronidase from Escherichia coli does not yield byproducts, without the addition of any antioxidant and thus is popular if only glucuronides are to be monitored (e.g., when studying the androgen component of the steroidome as a part of doping studies) [46]. All enzyme preparations have their weaknesses: HP cannot hydrolyze 17α- or 20α-sulfates, and our studies of testosterone metabolism have shown that glucuronides of 6β-hydroxyandrosterone and 6β-hydroxyetiocholanolone (important steroids in proving testosterone misuse) are not hydrolyzed by β-glucuronidase, necessitating a harsh chemical lysis [47].
Derivatization.
Derivatization methods for steroids for both GC and LC were recently reviewed by Marcos and Pozo [28]. Many derivatization methods have been used over the years for GC/MS steroidomics. However, the most useful has been the combination of methyloxime formation of carbonyl groups and TMS ether formation of hydroxyl groups, first introduced in the late 1960s, and now the most widely used technique. The classical two-step protocol consists of first converting ketones in methyloximes by reaction with a 2% solution of methoxyamine in pyridine, (60min at 55°C), followed by the reaction of hydroxyls with trimethylsilylimidazole (16 hours at 100°C, conveniently overnight). Recently, it was shown that microwave ovens can be used to speed up the second step of this long derivatization process, accomplishing adequate reaction yields within a few minutes [48]. However, this powerful reagent is involatile and cannot be injected directly into the GC/MS; thus, extraction is necessary after derivatization.
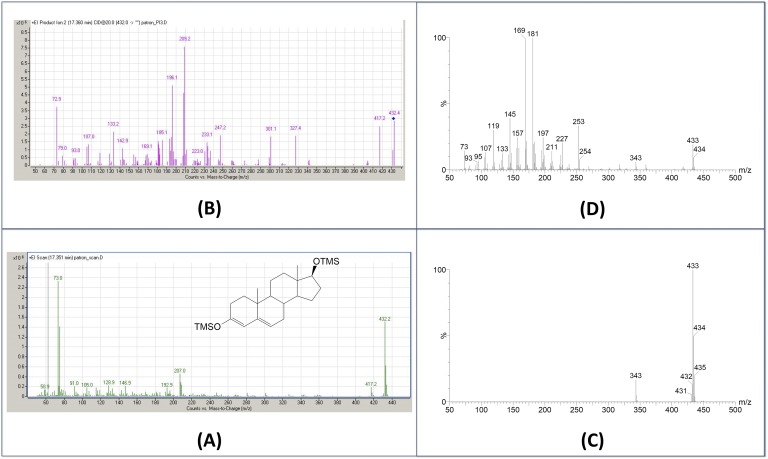
Trimethylsilylation alone is also used if carbonyl groups are silylated with a shift of unconjugation to ring structure (Fig. 4), the derivative then being referred to as an enol-TMS ether. This is used mostly in androgen studies and less in whole steroidome analysis in which complex side-chain derivatization can be challenging. The specific oxime fragmentation (M-31) useful for identifying carbonyl-containing steroids is also lost, a distinct disadvantage. Total silylation is achieved with N-methyl-N-trimethylsilyl-trifluoroacetamide/NH4I reagent, which has advantage of being volatile and injectable without purification.
Figure 4.
Testosterone analysis with different ionization techniques. (A) Full scan EI-MS spectrum of testosterone-enol-TMS, and (B) full-scan APCI-MS spectrum. Note the different molecular ion species formed: [M·]+ in EI and [M+H]+ in APCI. (C) Product ion scan of m/z 432 using EI as ionization source, and (D) product ion spectra of m/z 433 using APCI ionization. Different fragmentation results from the species selected as precursor. The near exclusive formation of one ion for targeting as precursor accounts for the high sensitivity of APCI.
For some specific investigations, novel derivatization protocols have been applied, such as the formation of pentafluorophenyldimethylsilyl-trimethylsilyl derivatives for androgens in urine, which allows for limits of quantification of 50 pg/mL [49]. Some derivatization reagents can be also used as extractive agents. This is the case in ethoxycarbonylation, which was used for estrogen determinations in hair [50]. More recently, it has been tested to quantify 15 estrogens, 6 androgens, and 2 progestins in breast tissue samples [51].
A-4. Instrument advances
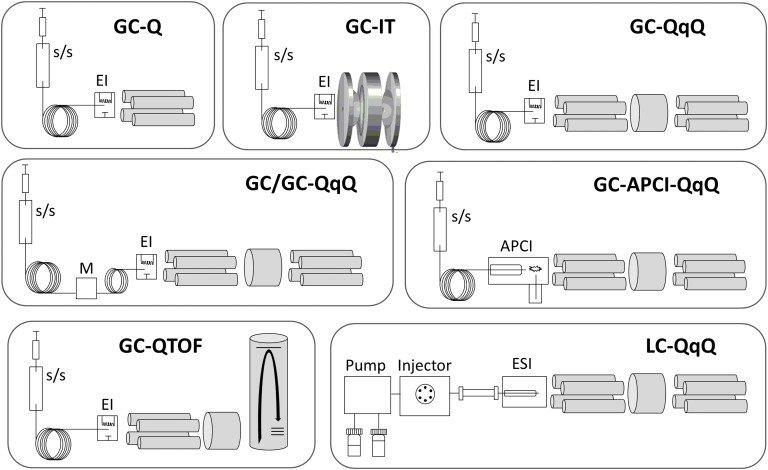
Advancing from the basic single quadrupole GC/MS, the past 2 decades have seen the development of advanced GC and LC combinations, and Figure 5 illustrates the instrumental configurations discussed in this section.
Figure 5.
Mass spectrometric configurations used in steroidomics. (Top) GC coupled to single quadrupole (GC/Q), ion trap (GC/ITMS), and triple quadrupole (GC/QqQ) analyzers, primarily used with split/splitless (s/s) injectors and EI ionization. (Middle, Left) Combination of two GC columns to improve separation. Timed portions of first separation collected in a modulator (M) and transferred to second column of different stationary phase and separation ability. (Middle, Right) GC coupled to an APCI source for more sensitive analysis. (Bottom, Left) GC coupled to a quadrupole TOF analyzer for higher mass resolution analyses. (Bottom, Right) Classic LC triple quadrupole instrument, most commonly used with ESI.
Atmospheric pressure chemical ionization.
In addition to EI ionization, the introduction of APCI interfaces for the soft ionization of steroids has also been evaluated and seems to provide exceptional sensitivity [52]. By coupling the new interface to a quadrupole time of flight (TOF) analyzer, the ionization of 60 anabolic steroids was investigated. For silylated steroids, the presence of water in the source as a modifier promoted the formation of principally [M+H]+, [M+H-TMSOH]+, and [M+H-2·TMSOH]+ ions. The formed ions preserve the intact steroid skeleton and are excellent precursors in MS/MS-based methods. The relationship between ionization behavior and structure has been established, which has led to the development and validation of a selected reaction monitoring (SRM) method for detection of 16 exogenous anabolic steroids. After TMS derivatization, urine extracts were analyzed with a triple quadrupole instrument (GC-APCI-MS/MS), reaching limits of detection <0.5 ng/mL for most steroids. Figure 4 shows the full mass spectrum and the product ion scan spectrums of testosterone obtained with both EI and APCI sources. The results were compared using a familiar method based on GC-EI-MS/MS. EI analysis was found to be slightly more reproducible; however, the lower limits of detection were found for the APCI interface [53].
More recently, Hennig et al. [54] used both GC-EI-MS/MS and GC-APCI-MS/MS to analyze the steroidome of breast adipose tissue. Although the methods investigated allow for the analysis of 27 androgens and progestogen metabolites, only APCI could reach the sensitivity levels required for estrogens, which account for only 1% of extracted steroids. The sensitivity for estrogen achieved the limit of quantitation of 0.05 to 0.1 ng/g of lipids in matrix (100 to 200 fg on column for standards).
This method has not been used to examine the complete urine steroid metabolome of MO-TMS ethers. Because ultimate sensitivity is rarely required, the technique is unlikely to offer advantages compared with GC/MS/MS.
GC-ion trap.
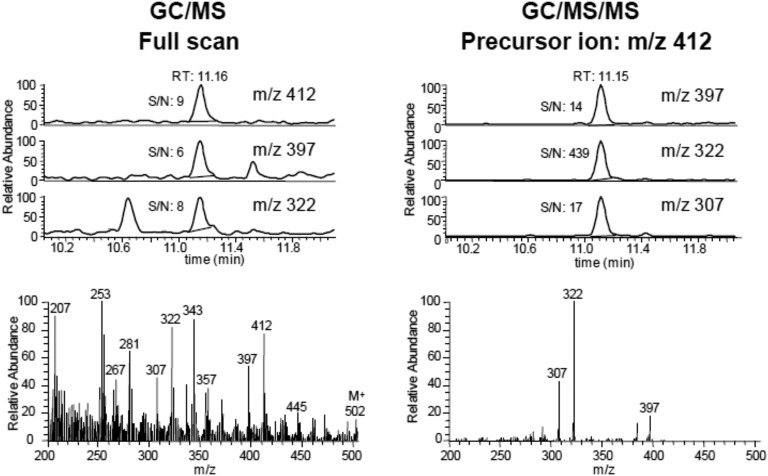
Ion traps were introduced ~2 decades ago. They consist of three hyperbolic electrodes, a ring electrode halfway between two end cap electrodes. The GC eluant enters the trap, and the separated components are ionized by EI. By applying direct current and radio frequencies, ions can be trapped in the space bounded by them. Ions can be kept in stable orbits inside the trap and further fragmented and/or ejected. Ion traps have the advantage of being capable of multiple MS stages (MSn). They can produce mass spectra with greater abundances of higher m/z ions, but they have limitations in the low mass range. Unlike GC/triple quadrupole, ion traps are not capable of conducting precursor ion scanning experiments. Our ion trap instrument offered great improvement in sensitivity compared with a single quadrupole instrument, and we used it successfully to measure minor, but essential, diagnostic steroids in serum from individuals with low-pregnancy estriol at risk of carrying a Smith-Lemli-Opitz syndrome (7-dehydrosterol reductase deficiency) fetus. As an example of specificity improvement, Fig. 6 shows the full scan and product ion MS/MS spectrum for 8-dehydroestriol, a diagnostic steroid for fetal Smith-Lemli-Opitz syndrome [55]. When using the full-scan mode, the detection of the most abundant ions (m/z 412, 397, 322) and the visual appearance of the full spectrum will be compromised by the high background. In contrast, acquisition in the MS/MS mode (precursor ion, m/z 412) provides a clean spectrum and better single/noise ratio, especially for the transition product ion m/z 322. However, in recent years, GC-ion traps (with either external or internal ion sources) have been largely replaced by GC/triple quadrupole MS instruments.
Figure 6.
Serum 8-dehydroestriol by GC/ITMS. (Left) Chromatograms and mass spectrum obtained in scan mode. (Right) Same sample analyzed by isolating and fragmenting m/z 412 in the ion trap (product-ion-scan). Note improved specificity and increased sensitivity (high signal/noise ratio). Steroid important in prenatal diagnosis of Smith-Lemli-Opitz syndrome.
GC triple quadrupole MS.
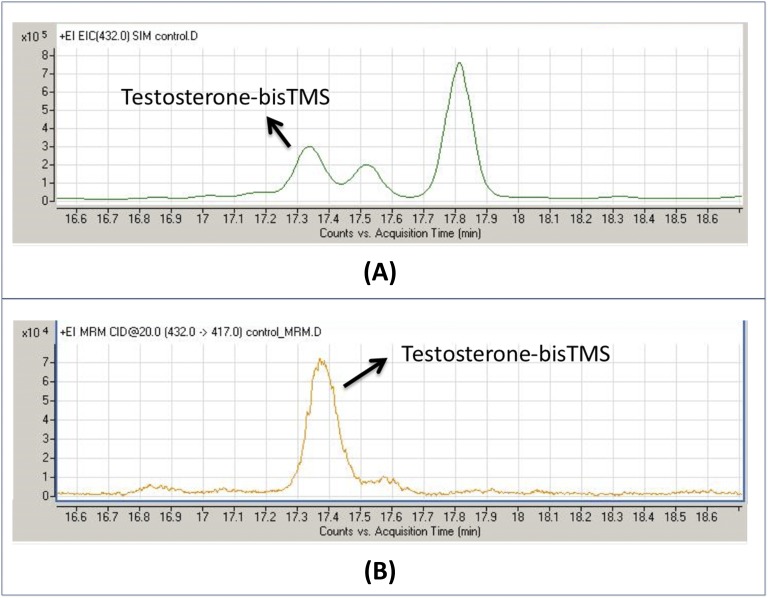
GC coupled to triple quadrupoles have been increasingly used to conduct metabolic studies, because they provide greater sensitivity, ≤20-fold in the study reported by Homer et al. [56], and thus offer major improvement in steroidomic analysis of minor components. In Fig. 7, we compare the chromatograms for urinary testosterone analysis using single quadrupole in SIM mode and tripe quadrupole in SRM mode. The urine extract was prepared by classical enzymatic hydrolysis and enol-TMS derivatization. GC/MS/MS analysis (SRM 432-417 transition) showed improved specificity (one peak observed) and a 20-fold increase in sensitivity.
Figure 7.
GC-MS/MS sensitivity enhancement. Determination of urinary testosterone after hydrolysis and enol-TMS derivatization. (A) GC/MS determination with a single quadrupole in SIM mode of the molecular ion m/z 432 and (B) GC/MS/MS determination using the transition 432 → 417. Note the enhanced specificity (only one peak shown) and 20-fold sensitivity increase.
To the best of our knowledge, the most comprehensive steroidomic project has been the identification of steroid metabolites in urine from neonates with 21-hydroxylase defect undertaken by Christakoudi et al. [39]. In a series of detailed studies [39, 57], they characterized and identified scores of steroids excreted in urine by neonates affected by 21-hydroxylase deficiency. The steroid metabolism at this period of life is relatively well known; however, multiple minor metabolites with unusual hydroxylation (e.g., 2α-, 4β-) and 6- and 7-carbonyl groups were detected in some metabolites, some of which might aid in early confirmation of the disorder. Through the process, a description of the fragmentation of the MO-TMS derivatives for all these steroids was accomplished. Such a comprehensive study would not be possible using LC/MS/MS and would be challenging for the sensitivity issues using single quadrupole GC/MS.
de Jong et al. [58] recently used GC/MS/MS for urinary targeted steroidomics and determined the reference intervals for most of the steroid metabolites commonly measured (those shown in Fig. 1 and others) for males and females. Although theirs was a well-conducted study, little information was included that could not have been obtained using a single quadrupole instrument. GC/MS/MS was also used for measurement of a select panel of 22 estrogens, androgens, and simple pregnanes by Robles et al. [59] using enol-TMS derivatives. They presented excellent high sensitivity data.
To the best of our knowledge, a comprehensive description and listing of the fragmentation of endogenous steroids under variable conditions in the collision cell of triple quadrupoles has not been prepared or reported. When achieved, this will simplify steroid identification.
TOF and quadrupole TOF.
TOF mass spectrometry is based on the measurement of the time taken by ions to reach a detector at a known distance after applying a known acceleration. That time depends on the m/z. The intrinsic characteristics of TOF analyzers allow for obtaining accurate mass measurements and high resolution. The use of TOF in bioanalysis has substantially increased in recent years mainly because of its high sensitivity when working in scan mode and the selectivity provided by the high resolution. TOF analyzers are able to accurately determine the m/z value of an ion (to several decimal places); thus, co-eluting compounds (interferences) with the same nominal m/z but with different empirical formula than the analyte can be distinguished by having a different accurate mass. High MS resolution is less useful in endogenous steroidomics than in general metabolomics, because many of the interfering species are also steroids and have the same empirical formula and thus the same accurate mass. Thus, few studies have reported on the GC/TOF determination of endogenous steroids. This platform seems to be more useful for the detection of exogenous steroids in the doping control field. Thus, a high-resolution full scan EI GC coupled to quadrupole TOF mass spectrometry approach has been developed to screen anabolic androgenic steroids in human urine samples [60].
Two-Dimensional GC quadrupole MS.
Zhang et al. [61] investigated the power of two-dimensional GC (GC/GC), coupled to either a TOF MS or quadrupole MS. The use of columns with different separation qualities can greatly increase the number of peaks separated compared with conventional GC/MS. Two-dimensional chromatography was optimized with 15 acetylated steroids. A GC/GC quadrupole MS in mass scanning mode was investigated with EI and positive chemical ionization (PCI), using CH4 and NH3 as reagent gases. Compared with EI, PCI-NH3 produced more abundant molecular ions and high-mass, structure-specific ions for steroid acetates. Eleven endogenous target steroid acetates were identified in normal urine based on their two retention times and EI and PCI-NH3 mass spectra. Nine of these endogenous target steroid acetates were identified in patients with CAH. The difference between the urinary steroid profiles from normal individuals and those from patients with CAH can easily be visually distinguished by their GC/GC quadrupole MS chromatograms. Various mass spectra of the targeted endogenous steroids were compared. PCI-NH3 mass spectra were the most useful for unambiguous molecular weight determination and for establishing the number of hydroxyls by the losses of one or more acetate groups. However, Zhang et al. [61] concluded that despite the unique abilities afforded by GC/GC, it is unlikely to improve on routine human steroidome evaluation because analyte separation has not been a substantial problem. The greatest use of this approach will probably be in unraveling doping control drug metabolites.
B. GC/MS Steroidomics in Disease
B-1. Steroidomics and recently described disorders of synthesis and metabolism
By this millennium, most of the disorders of steroid synthesis and metabolism had been described. However, another few have been recently characterized, and diagnostic protocols have been developed with the help of GC/MS. One of importance was PORD (a sixth form of CAH), which has a unique steroidome showing aspects of the 21- and 17-hydroxylase forms of CAH. The GC/MS steroidome of this disorder was first described in the 1980s [62], but it was 2004 before the cause of the disorder was understood and the metabolome described [63–65]. Figure 2 shows the quantitative results from an adult patient with the disorder and graphical results for the diagnostic ratios. Our group (Professor Arlt, IMSR, Birmingham, UK), as well as documenting the steroidome of the condition in many patients, through study of a pregnancy with an affected fetus, proposed the existence of an alternative pathway to active androgens synthesis in pregnancy, the cause of fetal virilization of affected females [63, 66].
Other disorders affecting steroid metabolism have also been described for the first time in the past 2 decades. The serum GC/MS steroidome of patients with Smith-Lemli-Opitz syndrome (7-dehydrosterol reductase deficiency) was described in the 1990s [67], allowing serum 7-and 8-dehydrocholesterol to be established as diagnostic parameters of the disorder. However, it was not until years later that the effect on adrenal steroids, fetal, newborn, and adult, was described [68, 69]. Many components of the steroidome were “conventional” metabolites but with additional B-ring unsaturation. Two metabolites, 7-dehydropregnanetriol and 8-dehydroestriol, were established as midterm urinary biomarkers of the condition [69].
Another disorder defined recently by its steroidome is cortisone reductase deficiency (CRD), in which the excretion of tetrahydrocortisone and cortolones (11-carbonyl-containing steroids) is increased relative to the tetrahydrocortisols and cortols (11β-hydroxylated steroids). The first patients studied genetically did not have inactivating mutations in 11β-hydroxysteroid, and in 2013, Lavery et al. [70] showed that mutations in the enzyme H6PDH (hexose-6-phosphate dehydrogenase; encoded by H6PD) caused the disorder. H6PDH generates cofactor NADPH (reduced form nicotinamide adenine dinucleotide phosphate) for 11β-HSD1. The condition was then renamed apparent CRD. Later, patients with true CRD (11HSDB1 deficiency) were identified. From the few patients studied, it appeared that apparent CRD and CRD can be distinguished by GC/MS analysis; however, more data are desirable.
The GC/MS metabolomic diagnosis of disorders of sexual differentiation in newborns has resulted in many studies of the normal neonate and affected patients. Caulfield et al. [71] focused on the first week of life and proposed diagnostic ratios for four forms of CAH (21-, 17α-, and 11β-hydroxylase deficiencies and hydroxysteroid dehydrogenase/Δ5-4 isomerase deficiencies). Homma et al. [72] measured 50 steroids excreted by Japanese neonates. Further normative data of 67 components of the neonatal metabolome (to 1 year of life) has been reported by Dhayat et al. [73], who also reported studies discussing the influence of the alternative androgen pathway in young infants [74]. Normative data (obtained by GC/MS/MS) for the full age range from newborn to adult was reported by de Jong et al. [58]. All these investigators essentially used the common MO-TMS derivative method.
B-2. Steroidomics in other steroid-related disorders
The study of the steroidome has also proved to be important in cancer diagnostics. Arlt et al. [62] used a targeted steroidome to distinguish patients with adrenocortical carcinoma (ACC) from those with incidentalomas such as adrenocortical adenoma. They measured 32 urinary steroids in 102 patients with diagnosed ACC and 45 with ACC and used machine learning to identify the most discriminatory analytes to distinguish ACC from adrenocortical adenoma. Steroid excretion data were subjected to generalized matrix learning vector quantization to identify the most discriminative steroids. Using the nine most discriminatory analytes gave a sensitivity and specificity of 88%. The most useful biomarker was tetrahydro-11-deoxycortisol, closely followed by elevated 3β-hydroxy-Δ5 steroid such as pregnanediol and pregnanetriol. This group followed up the study by monitoring the effect of a common ACC therapeutic drug, mitotane, on steroid production and excretion. The steroidome showed markedly reduced excretions of all 5α-reduced steroids and stimulation CYP3A4, shown through the increased excretion of 6β-hydroxycortisol. The changed metabolism explains the finding of glucocorticoid insufficiency in many treated patients and the inefficiency of testosterone replacement in mitotane-treated men [75].
Kotłowska et al. [76], studying Cushing disease, reported increased urinary levels of cortisol metabolites, androgens, and pregnanetriol and decreased level of tetrahydrocortisone. These data were similar to those reported in the 1990s [77]; however, they used heat maps to illustrate their data. GC/MS steroidomics also gives a convenient tool to investigate possible steroid relationships in additional disorders. Thus, the steroid profile of patients with hair growth problems (androgen alopecia, effluvium), psychiatric problems (major depression, anorexia nervosa, and bulimia), and osteoporosis have been recently characterized [78]. Additionally, GC/MS steroidomics has allowed for establishing reduced activities of steroid-related enzymes in several pathologies. For example, reduced activities for 11β-HSD type 1 and 5α-reductase have been found in boys with obesity and acute intermittent porphyria, respectively [79, 80]. Interesting studies by Idkowiak et al. [81] have studied the changes in steroid metabolism in patients with steroid sulfatase deficiency at puberty, and the same group reported that the urinary profile of young daughters of patients with polycystic ovary syndrome showed increased 5α-reductase activity [82].
3. LC/MS/MS in Steroidomics: Competitor or Complementary?
Despite all the methodologic and technical advances related to GC/MS, during the past 18 years, the greatest advances in steroid analysis have been associated with LC/MS. We believe LC/MS/MS in steroidomics is both a competitor and complementary to GC/MS. In the early 2000s, LC/MS/MS using triple quadrupole instruments was quickly proved to be a sensitive, specific, and rapid technique for quantifying hormonal 3-oxo-Δ4 steroids and precursors from the polarity of androstenedione to cortisol. The specificity of the technique lay in the selection of a precursor ion in the first quadrupole, its collision-induced dissociation in the collision cell, and the subsequent selection of a product ion in the third quadrupole. Product ions shared between the 3-oxo-Δ4 steroids were demonstrated, easing the development of multiple reaction monitoring (MRM) methods for the quantitation of steroid panels useful for clinical diagnosis. MRM represents the quantitation of the transition from precursor ion to product ion and is thus a measure of the quantity of the analyte (represented by the precursor ion). Enthusiasm resulted about LC/MS/MS replacing radioimmunoassays for the accurate measurement of circulating hormonal steroids and precursors, and this has largely occurred. In addition, the use of LC/MS/MS has allowed for the detection of important levels of novel androgens such as 11β-hydroxyandrostenedione in serum, spurring a renewed interest in the activity and importance of 11-oxygenated androgens [83].
One example of the measurement of largely 3-oxo-Δ4 steroids at the borderline of a “steroid panel” and metabolome was the measurement of 15 plasma steroids by Eisenhofer et al. [43] for the diagnosis of Cushing syndrome. Using sophisticated statistical analysis and presentation, they showed that of 222 candidate patients, 138 had no disease, 51 had adrenal Cushing disease, 12 had ectopic ACTH secretion, and 13 had other adrenal disease.
The ionization of steroids without the 3-oxo-Δ4 unconjugated ring system by electrospray (ESI) sources has proved to be more challenging [84]; thus, broad steroidomic application of the technique has been slow to develop. We estimate that in the urinary metabolome <5% of steroids have the intact 3-oxo-4-ene group, with the remainder being conjugates of primarily 3α,5α, 3α,5β, 3β,5α, and 3β-OH-Δ5 steroids. All these metabolites have poor ionization, resulting in insensitive measurement and poorly understood collision cell fragmentation [85]. In an earlier report [11], it was proposed that GC/MS makes a good “discovery tool” for investigating novel metabolomes, a task that LC/MS/MS finds more challenging because of the more limited database regarding the connection between fragmentation and structural information. However, with known metabolomes, such as urinary steroids, it should be straightforward to mimic established GC/MS profiles of hydrolyzed conjugates because almost all reference steroids are commercially available.
We, and others, have been developing LC/MS methods for hydrolyzed steroid metabolomes. Marcos et al. [85] investigated the ESI mass spectrometric properties of 67 C21 steroids (free steroids and hydrolyzed glucuronides) to include cortisol, deoxycorticosterone, corticosterone, and a multitude of their precursors and metabolic products, far more intermediates than are normally targeted in steroid profiles. Using the high-performance LC conditions reported, all the steroids could be eluted within 20 minutes, about one-half that required for GC/MS, with further time saving achieved because no derivatization was used. Agreeing with early observations, they found that 3-oxo-Δ4 steroids gave the simplest spectra, with [M+H]+ dominating, and a high sensitivity of measurement with MRM. Reduction of 3-oxo-Δ4 steroids to 5α- and 5β- metabolites decreases the formation of [M+H]+, making the ionization of reduced steroids difficult. The addition of ammonium-containing additives into the mobile phase shifted the dominant precursor ion in ESI to [M+NH4]+, with typically a 10-fold sensitivity loss. The addition of a further hydroxyl tends to shift the molecular ions to other precursor ions such as [M+H-H2O]+ or [M+H-2H2O]+. Importantly, 6β-hydroxylation gave prominent molecular ions at [M+H+MeOH]+. In LC/MS/MS, the structurally dependent varied forms of dominant precursor ions is a challenge for method development but also an opportunity to improve the performance of a specific steroid if the reference compound is available. GC/MS has always maintained its advantage of having single and uniform molecular ions M+ and probably only a maximum total ion current relative sensitivity factor of two between all steroid structures. This is certainly not the case in LC/MS, in which a 20-fold sensitivity difference is not uncommon between different steroids. We found that matrix effects typical of LC/MS were common and must be carefully evaluated and corrected for. Such effects refer to the suppression or enhancement of the ionization of one analyte produced by co-eluting matrix components. Matrix effects are minimal in GC/MS, in which the injected solutions are crystal clear and baselines flat, although co-eluting compounds and contaminants must be noted. The LC/MS method was compared in terms of quantitation with established GC/MS methods and performed well.
Using the LC/MS method, Pozo et al. [41] performed an extensive study of steroid metabolism in acute intermittent porphyria, targeting 55 free and hydrolyzed glucuronide-conjugated steroids using LC/MS/MS. The principal result (summarized in Fig. 3) suggested an adrenal imbalance in patients with acute intermittent porphyria, resulting in lower cortisol production. However, the method [85] did not include the more challenging hydrolyzed sulfates, because of the poor sensitivity of 3β-hydroxy-Δ5 steroids, although that can be improved by derivatization [28]. LC/MS/MS can clearly compete in the human steroidomic field when built on the broad existing knowledge of steroid synthesis and catabolism. Removing the derivatization step essential in GC/MS results in substantial time-saving simplification.
Estrogen analysis by LC/MS/MS remains challenging. However, Wang et al. [86] reported the results of an ultrasensitive analysis of six unconjugated and conjugated estrogens in human serum from men and postmenopausal women. The quantification used a new derivatization procedure, which formed analytes as preionized N-methyl pyridinium-3-sulfonyl derivatives. This group has recently reported an excellent review on estrogen steroidomics by LC/MS/MS [87].
A. Sample Introduction
In recent years, novel forms of sample introduction have been shown to improve performance. Thus, the use of turbulent flow chromatography coupled online to a LC/MS/MS system has been reported to improve the sensitivity in the determination of serum steroids [88]. In contrast, the use of differential ion mobility spectrometry has been reported to improve specificity for some steroids in serum [89].
B. LC/MS/MS of Intact Steroid Conjugates
A major time-consuming step in steroidomics is the enzymatic hydrolysis of the steroid conjugates. MS not only can analyze intact conjugates, but also prefers them because of the presence of an ionic center, typically glucuronic or sulfuric acid in steroid glucuronides and sulfates, respectively. In the 1980s, one of us (C.S.) demonstrated the effectiveness of intact conjugate MS to study steroid sulfates and glucuronides, at that time using a fast atom bombardment–related technique [90]. This was soon replaced by LC/thermospray MS and ESI MS, and the latter ionization technique is the method of choice today.
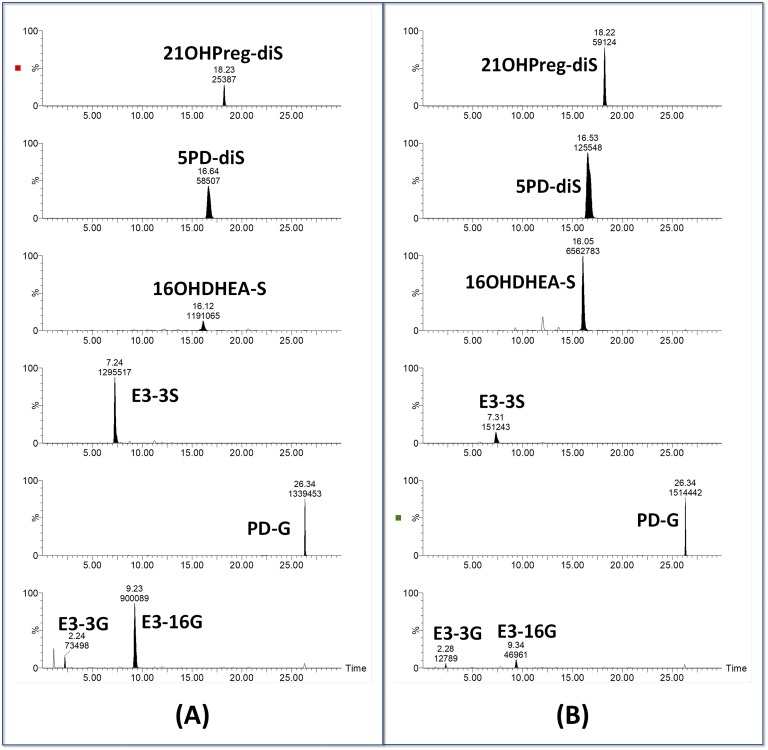
Fabregat et al. [91] performed very detailed studies of C19 steroid glucuronides in negative and positive ion mode and documented consistent patterns of prominent ions formed by neutral loss or precursor ion scans. Ke et al. [92] also reported on the measurement of intact glucuronides in serum. Intact steroid sulfates and disulfates are also readily analyzed by negative ion ESI; one example is of the serum sulfate metabolome reported by Sánchez-Guijo et al. [93], and another is the open detection method for steroid disulfates developed by McLeod et al. [94]. We have recently reported a preliminary study of diagnostic urinary metabolomics of intact conjugates such as steroid monosulfates and glucuronides and the understudied disulfate metabolites [95]. Figure 8 shows examples of the direct determination of steroid conjugates using LC/MS/MS and their potential usefulness for steroid sulfatase deficiency diagnosis.
Figure 8.
Urinary steroid conjugate analysis by LC/MS/MS. Although examples of monosulfate, glucuronide, and disulfate analytes are shown. This was an open analysis; data on all urinary steroid conjugates were collected. Analyte selection and analysis occurs after run completion. Shown are conjugates in urine collected from (A) a normal pregnancy and (B) a pregnancy with a steroid sulfatase deficiency–affected fetus. Abbreviations and transitions selected: E3-3G, estriol-3-glucuronide (463 → 287); E3-16G, estriol-16-glucuronide (463 → 287); PD-G, pregnanediol-glucuronide (495 → 75); E3-3S, estriol-3-sulfate (367 → 287); 16OHDHEA-S, 16-hydroxy-DHEA-sulfate (383 → 97); 5PD-diS, pregn-5-ene-3β,20α-diol-disulfate (238 → 379); 21OHPreg-diS, 21-hydroxypregnenolone disulfate (245 → 393). Note the increase of 21OHPreg-diS, 5PD-dis, and 16OHDHEA-S and the decrease in the E3 metabolites in the steroid sulfatase deficiency–affected pregnancy.
Thus, although a comprehensive LC/MS/MS method for the evaluation of the intact conjugate steroidome is not yet available, it seems that in the near future it will be achievable. However, some roadblocks remain, including the lack of authentic reference materials and labeled internal standards. Several laboratories are making advances in the synthesis of these prized standards [96].
C. A New Player in Separation, Ultra-High Performance Supercritical Fluid Chromatography/MS/MS
In addition to GC and LC, a third form of chromatography is proving itself for steroid and steroid conjugate separation: supercritical fluid chromatography (SFC). SFC-MS has been used for steroid analysis for 30 years [97]; however, its impact has been limited. Storbeck et al. [36] reported a recent excellent review on the subject. The principles of SFC are similar to those of LC; however, SFC uses supercritical carbon dioxide as the mobile phase. Thus, the entire chromatographic flow path must be pressurized. SFC has been likened to a process having the combined properties of the power of a liquid to dissolve materials with the chromatographic interactions and kinetics of a gas and, thus, combining the advantages of LC and GC. The elution sequence of steroids is different from what we are familiar with GC or LC (using common columns and mobile phases). Polarity has less influence, but hydrogen bonding capacity of analytes is critical.
More recently, the use of columns packed with fully porous sub–2-µm and sub–3-µm superficially porous particles has led to the development of commercial ultra-high performance SFC/MS/MS systems, which, in combination with MS/MS, is proving to be an excellent option for the analysis of steroids in biological matrices. Several variables need to be optimized to establish a particular separation. In addition to the selection of the column, gradient, and flow rate, other specific parameters must be adjusted [98]. Ultra-high performance SFC/MS/MS has been recently applied to the analysis of steroids in doping control studies and clinical investigations [98, 99].
4. Conclusion
By request of the editors, we have focused on GC/MS, applauding its power in providing a detailed fingerprint of steroid production. The structural information rendered by a GC/MS mass spectrum of a derivatized steroid is unparalleled, allowing the technique to be readily used in studies of novel metabolomes, such as in clinical disorders, doping studies, and laboratory animals. Although GC/MS (with a single quadrupole) is a mature technique, many advances have occurred during the past 2 decades that can improve steroid characterization and sensitivity, in particular, GC/MS/MS.
We suggest that LC/MS/MS might have caught up with GC/MS in quantifying the well-known human urine steroidome using less labor-intensive methods. Whether it can detect, with the same accuracy, small differences in complex evolving metabolomes, such as in the human neonatal period or patients with cancer remains to be seen. LC/MS remains more suited to targeted steroidomics and is very unsuited to fine-detailed untargeted steroidomics such as illustrated by the studies by Christakoudi et al. [39].
We are close to analyzing the urinary steroidome without the necessity for including two major sample workup steps: the conjugate hydrolysis and chemical derivatization needed for GC/MS. Direct determination of intact conjugates will also assist in evaluating the role of conjugating enzymes in disease. Intact conjugate analysis will be, at the very least, a sophisticated screening technique, even if in certain cases, GC/MS remains invaluable for validation.
Acknowledgments
Financial Support: This work was supported by a grant from the Instituto de Salud Carlos III FEDER (grant PI14/00147 to O.P.J.) and a grant from the Spanish Health National System (grant CPII16/00027 to O.P.J.). C.S. acknowledges the support of Professor Wiebke Arlt and the Institute of Metabolism and Systems Research.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACC
adrenocortical carcinoma
- APCI
atmospheric pressure chemical ionization
- CAH
congenital adrenal hyperplasia
- CRD
cortisone reductase deficiency
- EI
electron ionization
- ESI
electrospray ionization
- GC/MS
gas chromatography/mass spectrometry
- GC/GC/MS
two-dimensional gas chromatography/mass spectrometry
- HP
Helix pomatia
- IMSR
Institute for Metabolism and Systems Research
- LC/MS/MS
liquid chromatography/tandem mass spectrometry
- MO
methyloxime
- MRM
multiple reaction monitoring
- PCI
positive chemical ionization
- PORD
P450 oxidoreductase deficiency
- SFC
supercritical fluid chromatography
- SIM
selected-ion-monitoring
- SRM
selected reaction monitoring
- TOF
time of flight
- TMS
trimethylsilyl
References and Notes
- 1. Bush IE. The Chromatography of Steroids. New York: Elsevier; 1961. [Google Scholar]
- 2. Shackleton CH, Charro-Salgado AL, Mitchell FL. Urinary neutral steroid profile analysis in adults and infants. Clin Chim Acta. 1968;21(1):105–118. [DOI] [PubMed] [Google Scholar]
- 3. Vandenheuvel WJ, Horning EC. Gas chromatography of adrenal cortical steroid hormones. Biochem Biophys Res Commun. 1960;3(4):356–360. [DOI] [PubMed] [Google Scholar]
- 4. Eneroth P, Hellstroem K, Ryhage R. Identification and quantification of neutral fecal steroids by gas-liquid chromatography and mass spectrometry: studies of human excretion during two dietary regimens. J Lipid Res. 1964;5:245–262. [PubMed] [Google Scholar]
- 5. Völlmin JA. High resolution gas chromatography of urinary steroids on glass capillary columns. Clin Chim Acta. 1971;34(2):207–214. [DOI] [PubMed] [Google Scholar]
- 6. Shackleton CH. Profiling steroid hormones and urinary steroids. J Chromatogr A. 1986;379:91–156. [DOI] [PubMed] [Google Scholar]
- 7. Shackleton CH. Mass spectrometry in the diagnosis of steroid-related disorders and in hypertension research. J Steroid Biochem Mol Biol. 1993;45(1-3):127–140. [DOI] [PubMed] [Google Scholar]
- 8. Sjövall J. Fifty years with bile acids and steroids in health and disease. Lipids. 2004;39(8):703–722. [DOI] [PubMed] [Google Scholar]
- 9. Ceglarek U, Shackleton C, Stanczyk FZ, Adamski J. Steroid profiling and analytics: going towards sterome. J Steroid Biochem Mol Biol. 2010;121(3-5):479–480. [DOI] [PubMed] [Google Scholar]
- 10. Patti GJ, Yanes O, Siuzdak G. Innovation: metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 2012;13(4):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CH. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J Steroid Biochem Mol Biol. 2010;121(3-5):496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marcos J, Pozo OJ. Current LC-MS methods and procedures applied to the identification of new steroid metabolites. J Steroid Biochem Mol Biol. 2016;162:41–56. [DOI] [PubMed] [Google Scholar]
- 13. Jeanneret F, Tonoli D, Rossier MF, Saugy M, Boccard J, Rudaz S. Evaluation of steroidomics by liquid chromatography hyphenated to mass spectrometry as a powerful analytical strategy for measuring human steroid perturbations. J Chromatogr A. 2016;1430:97–112. [DOI] [PubMed] [Google Scholar]
- 14. Griffiths WJ. Tandem mass spectrometry in the study of fatty acids, bile acids, and steroids. Mass Spectrom Rev. 2003;22(2):81–152. [DOI] [PubMed] [Google Scholar]
- 15. Griffiths WJ, Shackleton CH, Sjövall J. Steroid analysis In: Capriolli RM, ed. The Encylopedia of Mass Spectrometry. Vol. 5 Oxford, UK: Elsevier; 2005:447–472. [Google Scholar]
- 16. Shackleton CHL, Marcos P. GC/MS Steroid profiling: Diagnosis of disorders affecting steroid synthesis and metabolism In: Gross M, Caprioli R, eds. The Encyclopedia of Mass Spectrometry. Vol. 8 Amsterdam, Netherlands: Elsevier; 2006:789–813. [Google Scholar]
- 17. Thevis M, Schänzer W. Mass spectrometry in sports drug testing: structure characterization and analytical assays. Mass Spectrom Rev. 2007;26(1):79–107. [DOI] [PubMed] [Google Scholar]
- 18. Shackleton C. Clinical steroid mass spectrometry: a 45-year history culminating in HPLC-MS/MS becoming an essential tool for patient diagnosis. J Steroid Biochem Mol Biol. 2010;121(3-5):481–490. [DOI] [PubMed] [Google Scholar]
- 19. Kushnir MM, Rockwood AL, Bergquist J. Liquid chromatography-tandem mass spectrometry applications in endocrinology. Mass Spectrom Rev. 2010;29(3):480–502. [DOI] [PubMed] [Google Scholar]
- 20. McDonald JG, Matthew S, Auchus RJ. Steroid profiling by gas chromatography-mass spectrometry and high performance liquid chromatography-mass spectrometry for adrenal diseases. Horm Cancer. 2011;2(6):324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shackleton CH. Role of a disordered steroid metabolome in the elucidation of sterol and steroid biosynthesis. Lipids. 2012;47(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wooding KM, Auchus RJ. Mass spectrometry theory and application to adrenal diseases. Mol Cell Endocrinol. 2013;371(1-2):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor NF. Urinary steroid profiling. Methods Mol Biol. 2013;1065:259–276. [DOI] [PubMed] [Google Scholar]
- 24. Sánchez-Guijo A, Hartmann MF, Wudy SA. Introduction to gas chromatography-mass spectrometry. Methods Mol Biol. 2013;1065:27–44. [DOI] [PubMed] [Google Scholar]
- 25. Kushnir MM, Rockwood AL, Bergquist J. LC-MS/MS in clinical laboratories. Bioanalysis. 2013;5(1):5–6. [DOI] [PubMed] [Google Scholar]
- 26. Honour JW. Mass spectrometry for steroids. Ann Clin Biochem. 2014;51(Pt 3):309–311. [DOI] [PubMed] [Google Scholar]
- 27. Gomez C, Fabregat A, Pozo ÓJ, Marcos J, Segura J, Ventura R. Analytical strategies based on mass spectrometric techniques for the study of steroid metabolism. Trends Analyt Chem. 2014;53:106–116. [Google Scholar]
- 28. Marcos J, Pozo OJ. Derivatization of steroids in biological samples for GC-MS and LC-MS analyses. Bioanalysis. 2015;7(19):2515–2536. [DOI] [PubMed] [Google Scholar]
- 29. Turcu AF, Auchus RJ. Adrenal steroidogenesis and congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 2015;44(2):275–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi MH, Chung BC. Bringing GC-MS profiling of steroids into clinical applications. Mass Spectrom Rev. 2015;34(2):219–236. [DOI] [PubMed] [Google Scholar]
- 31. Adaway JE, Keevil BG, Owen LJ. Liquid chromatography tandem mass spectrometry in the clinical laboratory. Ann Clin Biochem. 2015;52(Pt 1):18–38. [DOI] [PubMed] [Google Scholar]
- 32. Higashi T, Ogawa S. Chemical derivatization for enhancing sensitivity during LC/ESI-MS/MS quantification of steroids in biological samples: a review. J Steroid Biochem Mol Biol. 2016;162:57–69. [DOI] [PubMed] [Google Scholar]
- 33. Wudy SA, Choi MH. Steroid LC-MS has come of age. J Steroid Biochem Mol Biol. 2016;162:1–3. [DOI] [PubMed] [Google Scholar]
- 34. Keevil BG. LC-MS/MS analysis of steroids in the clinical laboratory. Clin Biochem. 2016;49(13-14):989–997. [DOI] [PubMed] [Google Scholar]
- 35. Honour JW, Conway E, Hodkinson R, Lam F. The evolution of methods for urinary steroid metabolomics in clinical investigations particularly in childhood. J Steroid Biochem Mol Biol. 2018;181:28–51. [DOI] [PubMed] [Google Scholar]
- 36. Storbeck KH, Gilligan L, Jenkinson C, Baranowski ES, Quanson JL, Arlt W, Taylor AE. The utility of ultra-high performance supercritical fluid chromatography-tandem mass spectrometry (UHPSFC-MS/MS) for clinically relevant steroid analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1085:36–41. [DOI] [PubMed] [Google Scholar]
- 37. Wudy SA, Schuler G, Sánchez-Guijo A, Hartmann MF. The art of measuring steroids: principles and practice of current hormonal steroid analysis. J Steroid Biochem Mol Biol. 2018;179:88–103. [DOI] [PubMed] [Google Scholar]
- 38. Rodríguez-Morató J, Pozo OJ, Marcos J. Targeting human urinary metabolome by LC-MS/MS: a review. Bioanalysis. 2018;10(7):489–516. [DOI] [PubMed] [Google Scholar]
- 39. Christakoudi S, Cowan DA, Christakudis G, Taylor NF. 21-Hydroxylase −deficiency in the neonate—trends in steroid anabolism and catabolism during the first weeks of life. J Steroid Biochem Mol Biol. 2013;138:334–347. [DOI] [PubMed] [Google Scholar]
- 40. Moon JY, Jung HJ, Moon MH, Chung BC, Choi MH. Heat-map visualization of gas chromatography-mass spectrometry based quantitative signatures on steroid metabolism. J Am Soc Mass Spectrom. 2009;20(9):1626–1637. [DOI] [PubMed] [Google Scholar]
- 41. Pozo OJ, Marcos J, Fabregat A, Ventura R, Casals G, Aguilera P, Segura J, To-Figueras J. Adrenal hormonal imbalance in acute intermittent porphyria patients: results of a case control study. Orphanet J Rare Dis. 2014;9(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arlt W, Biehl M, Taylor AE, Hahner S, Libé R, Hughes BA, Schneider P, Smith DJ, Stiekema H, Krone N, Porfiri E, Opocher G, Bertherat J, Mantero F, Allolio B, Terzolo M, Nightingale P, Shackleton CH, Bertagna X, Fassnacht M, Stewart PM. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab. 2011;96(12):3775–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eisenhofer G, Masjkur J, Peitzsch M, Di Dalmazi G, Bidlingmaier M, Grüber M, Fazel J, Osswald A, Beuschlein F, Reincke M. Plasma steroid metabolome profiling for diagnosis and subtyping patients with Cushing syndrome. Clin Chem. 2018;64(3):586–596. [DOI] [PubMed] [Google Scholar]
- 44. Gomes RL, Meredith W, Snape CE, Sephton MA. Analysis of conjugated steroid androgens: deconjugation, derivatisation and associated issues. J Pharm Biomed Anal. 2009;49(5):1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Christakoudi S, Cowan DA, Taylor NF. Sodium ascorbate improves yield of urinary steroids during hydrolysis with Helix pomatia juice. Steroids. 2008;73(3):309–319. [DOI] [PubMed] [Google Scholar]
- 46. Mareck U, Geyer H, Opfermann G, Thevis M, Schänzer W. Factors influencing the steroid profile in doping control analysis. J Mass Spectrom. 2008;43(7):877–891. [DOI] [PubMed] [Google Scholar]
- 47. Kotronoulas A, Marcos J, Segura J, Ventura R, Joglar J, Pozo OJ. Ultra high performance liquid chromatography tandem mass spectrometric detection of glucuronides resistant to enzymatic hydrolysis: implications to doping control analysis. Anal Chim Acta. 2015;895:35–44. [DOI] [PubMed] [Google Scholar]
- 48. Casals G, Marcos J, Pozo OJ, Alcaraz J, Martínez de Osaba MJ, Jiménez W. Microwave-assisted derivatization: application to steroid profiling by gas chromatography/mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;960:8–13. [DOI] [PubMed] [Google Scholar]
- 49. Lee SH, Choi MH, Lee WY, Chung BC. Isotope-dilution mass spectrometry for quantification of urinary active androgens separated by gas chromatography. Mass Spec Lett. 2010;1(1):29–32. [Google Scholar]
- 50. Choi MH, Kim KR, Chung BC. Determination of estrone and 17 β-estradiol in human hair by gas chromatography-mass spectrometry. Analyst (Lond). 2000;125(4):711–714. [DOI] [PubMed] [Google Scholar]
- 51. Moon JY, McNamara KM, Lee JJ, Chung BC, Sasano H, Choi MH. Improved detectability of sex steroids from frozen sections of breast cancer tissue using GC-triple quadrupole-MS. J Steroid Biochem Mol Biol. 2018;178:185–192. [DOI] [PubMed] [Google Scholar]
- 52. Raro M, Portolés T, Sancho JV, Pitarch E, Hernández F, Marcos J, Ventura R, Gómez C, Segura J, Pozo OJ. Mass spectrometric behavior of anabolic androgenic steroids using gas chromatography coupled to atmospheric pressure chemical ionization source. Part I: ionization. J Mass Spectrom. 2014;49(6):509–521. [DOI] [PubMed] [Google Scholar]
- 53. Raro M, Portolés T, Pitarch E, Sancho JV, Hernández F, Garrostas L, Marcos J, Ventura R, Segura J, Pozo OJ. Potential of atmospheric pressure chemical ionization source in gas chromatography tandem mass spectrometry for the screening of urinary exogenous androgenic anabolic steroids. Anal Chim Acta. 2016;906:128–138. [DOI] [PubMed] [Google Scholar]
- 54. Hennig K, Antignac JP, Bichon E, Morvan ML, Miran I, Delaloge S, Feunteun J, Le Bizec B. Steroid hormone profiling in human breast adipose tissue using semi-automated purification and highly sensitive determination of estrogens by GC-APCI-MS/MS. Anal Bioanal Chem. 2018;410(1):259–275. [DOI] [PubMed] [Google Scholar]
- 55. Shackleton CH, Marcos J, Palomaki GE, Craig WY, Kelley RI, Kratz LE, Haddow JE. Dehydrosteroid measurements in maternal urine or serum for the prenatal diagnosis of Smith-Lemli-Opitz syndrome (SLOS). Am J Med Genet A. 2007;143A(18):2129–2136. [DOI] [PubMed] [Google Scholar]
- 56. Homer N, Kothiya S, Rutter A, Walker BR, Andrew R. Gas chromatography tandem mass spectrometry offers advantages for urinary steroids analysis. Anal Biochem. 2017;538:34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Christakoudi S, Cowan DA, Taylor NF. Steroids excreted in urine by neonates with 21-hydroxylase deficiency: characterization, using GC-MS and GC-MS/MS, of the D-ring and side chain structure of pregnanes and pregnenes. Steroids. 2010;75(1):34–52. [DOI] [PubMed] [Google Scholar]
- 58. de Jong WHA, Buitenwerf E, Pranger AT, Riphagen IJ, Wolffenbuttel BHR, Kerstens MN, Kema IP. Determination of reference intervals for urinary steroid profiling using a newly validated GC-MS/MS method. Clin Chem Lab Med. 2017;56(1):103–112. [DOI] [PubMed] [Google Scholar]
- 59. Robles J, Marcos J, Renau N, Garrostas L, Segura J, Ventura R, Barceló B, Barceló A, Pozo OJ. Quantifying endogenous androgens, estrogens, pregnenolone and progesterone metabolites in human urine by gas chromatography tandem mass spectrometry. Talanta. 2017;169:20–29. [DOI] [PubMed] [Google Scholar]
- 60. Abushareeda W, Lyris E, Kraiem S, Wahaibi AA, Alyazidi S, Dbes N, Lommen A, Nielen M, Horvatovich PL, Alsayrafi M, Georgakopoulos C. Gas chromatographic quadrupole time-of-flight full scan high resolution mass spectrometric screening of human urine in antidoping analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1063:74–83. [DOI] [PubMed] [Google Scholar]
- 61. Zhang Y, Tobias HJ, Auchus RJ, Brenna JT. Comprehensive 2-dimensional gas chromatography fast quadrupole mass spectrometry (GC × GC-qMS) for urinary steroid profiling: mass spectral characteristics with chemical ionization. Drug Test Anal. 2011;3(11-12):857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Peterson RE, Imperato-McGinley J, Gautier T, Shackleton C. Male pseudohermaphroditism due to multiple defects in steroid-biosynthetic microsomal mixed-function oxidases: a new variant of congenital adrenal hyperplasia. N Engl J Med. 1985;313(19):1182–1191. [DOI] [PubMed] [Google Scholar]
- 63. Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, Chalder SM, Borucka-Mankiewicz M, Hauffa BP, Malunowicz EM, Stewart PM, Shackleton CH. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004;363(9427):2128–2135. [DOI] [PubMed] [Google Scholar]
- 64. Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, Jabs EW, Mendonça BB, Fujieda K, Miller WL. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet. 2004;36(3):228–230. [DOI] [PubMed] [Google Scholar]
- 65. Shackleton C, Marcos J, Malunowicz EM, Szarras-Czapnik M, Jira P, Taylor NF, Murphy N, Crushell E, Gottschalk M, Hauffa B, Cragun DL, Hopkin RJ, Adachi M, Arlt W. Biochemical diagnosis of Antley-Bixler syndrome by steroid analysis. Am J Med Genet A. 2004;128A(3):223–231. [DOI] [PubMed] [Google Scholar]
- 66. Shackleton C, Marcos J, Arlt W, Hauffa BP. Prenatal diagnosis of P450 oxidoreductase deficiency (ORD): a disorder causing low pregnancy estriol, maternal and fetal virilization, and the Antley-Bixler syndrome phenotype. Am J Med Genet A. 2004;129A(2):105–112. [DOI] [PubMed] [Google Scholar]
- 67. Kelley RI. Diagnosis of Smith-Lemli-Opitz syndrome by gas chromatography/mass spectrometry of 7-dehydrocholesterol in plasma, amniotic fluid and cultured skin fibroblasts. Clin Chim Acta. 1995;236(1):45–58. [DOI] [PubMed] [Google Scholar]
- 68. Shackleton CH, Roitman E, Kelley R. Neonatal urinary steroids in Smith-Lemli-Opitz syndrome associated with 7-dehydrocholesterol reductase deficiency. Steroids. 1999;64(7):481–490. [DOI] [PubMed] [Google Scholar]
- 69. Craig WY, Haddow JE, Palomaki GE, Kelley RI, Kratz LE, Shackleton CH, Marcos J, Stephen Tint G, MacRae AR, Nowaczyk MJ, Kloza EM, Irons MB, Roberson M. Identifying Smith-Lemli-Opitz syndrome in conjunction with prenatal screening for Down syndrome. Prenat Diagn. 2006;26(9):842–849. [DOI] [PubMed] [Google Scholar]
- 70. Lavery GG, Idkowiak J, Sherlock M, Bujalska I, Ride JP, Saqib K, Hartmann MF, Hughes B, Wudy SA, De Schepper J, Arlt W, Krone N, Shackleton CH, Walker EA, Stewart PM. Novel H6PDH mutations in two girls with premature adrenarche: “apparent” and “true” CRD can be differentiated by urinary steroid profiling. Eur J Endocrinol. 2013;168(2):K19–K26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Caulfield MP, Lynn T, Gottschalk ME, Jones KL, Taylor NF, Malunowicz EM, Shackleton CH, Reitz RE, Fisher DA. The diagnosis of congenital adrenal hyperplasia in the newborn by gas chromatography/mass spectrometry analysis of random urine specimens. J Clin Endocrinol Metab. 2002;87(8):3682–3690. [DOI] [PubMed] [Google Scholar]
- 72. Homma K, Hasegawa T, Masumoto M, Takeshita E, Watanabe K, Chiba H, Kurosawa T, Takahashi T, Matsuo N. Reference values for urinary steroids in Japanese newborn infants: gas chromatography/mass spectrometry in selected ion monitoring. Endocr J. 2003;50(6):783–792. [DOI] [PubMed] [Google Scholar]
- 73. Dhayat NA, Frey AC, Frey BM, d’Uscio CH, Vogt B, Rousson V, Dick B, Flück CE. Estimation of reference curves for the urinary steroid metabolome in the first year of life in healthy children: tracing the complexity of human postnatal steroidogenesis. J Steroid Biochem Mol Biol. 2015;154:226–236. [DOI] [PubMed] [Google Scholar]
- 74. Dhayat NA, Dick B, Frey BM, d'Uscio CH, Vogt B, Flück CE. Androgen biosynthesis during minipuberty favors the backdoor pathway over the classic pathway: Insights into enzyme activities and steroid fluxes in healthy infants during the first year of life from the urinary steroid metabolome. J Steroid Biochem Mol Biol.2017;165(Pt B):312–322. [DOI] [PubMed] [Google Scholar]
- 75. Chortis V, Taylor AE, Schneider P, Tomlinson JW, Hughes BA, O’Neil DM, Libé R, Allolio B, Bertagna X, Bertherat J, Beuschlein F, Fassnacht M, Karavitaki N, Mannelli M, Mantero F, Opocher G, Porfiri E, Quinkler M, Sherlock M, Terzolo M, Nightingale P, Shackleton CH, Stewart PM, Hahner S, Arlt W. Mitotane therapy in adrenocortical cancer induces CYP3A4 and inhibits 5α-reductase, explaining the need for personalized glucocorticoid and androgen replacement. J Clin Endocrinol Metab. 2013;98(1):161–171. [DOI] [PubMed] [Google Scholar]
- 76. Kotłowska A, Puzyn T, Sworczak K, Stepnowski P, Szefer P. Metabolomic biomarkers in urine of Cushing’s syndrome patients. Int J Mol Sci. 2017;18(2):E294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stewart PM, Walker BR, Holder G, O’Halloran D, Shackleton CH. 11 Beta-hydroxysteroid dehydrogenase activity in Cushing’s syndrome: explaining the mineralocorticoid excess state of the ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1995;80(12):3617–3620. [DOI] [PubMed] [Google Scholar]
- 78. Poór V, Bufa A, Bíró I, Telegdy E, Tényi T, Gáti A, Osváth P, Wilhelm F, Juricskay S. Urinary steroid measurements in some endocrine and psychiatric diseases. Curr Med Chem. 2005;12(11):1339–1342. [DOI] [PubMed] [Google Scholar]
- 79. Wiegand S, Richardt A, Remer T, Wudy SA, Tomlinson JW, Hughes B, Grüters A, Stewart PM, Strasburger CJ, Quinkler M. Reduced 11beta-hydroxysteroid dehydrogenase type 1 activity in obese boys. Eur J Endocrinol. 2007;157(3):319–324. [DOI] [PubMed] [Google Scholar]
- 80. Casals G, Marcos J, Pozo ÓJ, Aguilera P, Herrero C, To-Figueras J. Gas chromatography-mass spectrometry profiling of steroids in urine of patients with acute intermittent porphyria. Clin Biochem. 2013;46(9):819–824. [DOI] [PubMed] [Google Scholar]
- 81. Idkowiak J, Taylor AE, Subtil S, O’Neil DM, Vijzelaar R, Dias RP, Amin R, Barrett TG, Shackleton CH, Kirk JM, Moss C, Arlt W. Steroid sulfatase deficiency and androgen activation before and after puberty. J Clin Endocrinol Metab. 2016;101(6):2545–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Torchen LC, Idkowiak J, Fogel NR, O’Neil DM, Shackleton CH, Arlt W, Dunaif A. Evidence for increased 5α-reductase activity during early childhood in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2016;101(5):2069–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bloem LM, Storbeck KH, Swart P, du Toit T, Schloms L, Swart AC. Advances in the analytical methodologies: profiling steroids in familiar pathways—challenging dogmas. J Steroid Biochem Mol Biol. 2015;153:80–92. [DOI] [PubMed] [Google Scholar]
- 84. Pozo OJ, Van Eenoo P, Deventer K, Delbeke FT. Ionization of anabolic steroids by adduct formation in liquid chromatography electrospray mass spectrometry. J Mass Spectrom. 2007;42(4):497–516. [DOI] [PubMed] [Google Scholar]
- 85. Marcos J, Renau N, Casals G, Segura J, Ventura R, Pozo OJ, Delbeke FT. Investigation of endogenous corticosteroids profiles in human urine based on liquid chromatography tandem mass spectrometry. Anal Chim Acta. 2014;812:92–104. [DOI] [PubMed] [Google Scholar]
- 86. Wang Q, Rangiah K, Mesaros C, Snyder NW, Vachani A, Song H, Blair IA. Ultrasensitive quantification of serum estrogens in postmenopausal women and older men by liquid chromatography-tandem mass spectrometry. Steroids. 2015;96:140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang Q, Mesaros C, Blair IA. Ultra-high sensitivity analysis of estrogens for special populations in serum and plasma by liquid chromatography-mass spectrometry: assay considerations and suggested practices. J Steroid Biochem Mol Biol. 2016;162:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Søeborg T, Frederiksen H, Johannsen TH, Andersson AM, Juul A. Isotope-dilution TurboFlow-LC-MS/MS method for simultaneous quantification of ten steroid metabolites in serum. Clin Chim Acta. 2017;468:180–186. [DOI] [PubMed] [Google Scholar]
- 89. Ray JA, Kushnir MM, Yost RA, Rockwood AL, Wayne Meikle A. Performance enhancement in the measurement of 5 endogenous steroids by LC-MS/MS combined with differential ion mobility spectrometry. Clin Chim Acta. 2015;438:330–336. [DOI] [PubMed] [Google Scholar]
- 90. Shackleton CH. Inborn errors of steroid biosynthesis: detection by a new mass-spectrometric method. Clin Chem. 1983;29(2):246–249. [PubMed] [Google Scholar]
- 91. Fabregat A, Pozo OJ, Marcos J, Segura J, Ventura R. Use of LC-MS/MS for the open detection of steroid metabolites conjugated with glucuronic acid. Anal Chem. 2013;85(10):5005–5014. [DOI] [PubMed] [Google Scholar]
- 92. Ke Y, Gonthier R, Isabelle M, Bertin J, Simard JN, Dury AY, Labrie F. A rapid and sensitive UPLC-MS/MS method for the simultaneous quantification of serum androsterone glucuronide, etiocholanolone glucuronide, and androstan-3α, 17β diol 17-glucuronide in postmenopausal women. J Steroid Biochem Mol Biol. 2015;149:146–152. [DOI] [PubMed] [Google Scholar]
- 93. Sánchez-Guijo A, Oji V, Hartmann MF, Traupe H, Wudy SA. Simultaneous quantification of cholesterol sulfate, androgen sulfates, and progestagen sulfates in human serum by LC-MS/MS. J Lipid Res. 2015;56(9):1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. McLeod MD, Waller CC, Esquivel A, Balcells G, Ventura R, Segura J, Pozo ÓJ. Constant ion loss method for the untargeted detection of bis-sulfate metabolites. Anal Chem. 2017;89(3):1602–1609. [DOI] [PubMed] [Google Scholar]
- 95. Pozo OJ, Marcos J, Khymenets O, Pranata A, Fitzgerald C, McLeod MD, Shackleton C. Steroid sulfation pathways targeted by disulfates determination. Application to prenatal diagnosis [published online ahead of print February 19, 2018]. J Mol Endocrinol. 2018 doi: 10.1530/JME-17-0286. [DOI] [PubMed]
- 96. Waller CC, McLeod MD. A simple method for the small scale synthesis and solid-phase extraction purification of steroid sulfates. Steroids. 2014;92:74–80. [DOI] [PubMed] [Google Scholar]
- 97. Tuomola M, Hakala M, Manninen P. Determination of androstenone in pig fat using packed column supercritical fluid chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl. 1998;719(1-2):25–30. [DOI] [PubMed] [Google Scholar]
- 98. Doué M, Dervilly-Pinel G, Pouponneau K, Monteau F, Le Bizec B. Analysis of glucuronide and sulfate steroids in urine by ultra-high-performance supercritical-fluid chromatography hyphenated tandem mass spectrometry. Anal Bioanal Chem. 2015;407(15):4473–4484. [DOI] [PubMed] [Google Scholar]
- 99. Desfontaine V, Nováková L, Ponzetto F, Nicoli R, Saugy M, Veuthey JL, Guillarme D. Liquid chromatography and supercritical fluid chromatography as alternative techniques to gas chromatography for the rapid screening of anabolic agents in urine. J Chromatogr A. 2016;1451:145–155. [DOI] [PubMed] [Google Scholar]