Abstract
Stroke is the fifth leading cause of death and the most frequent cause of disability worldwide. Currently, stroke diagnosis is based on neuroimaging; therefore, the lack of a rapid tool to diagnose stroke is still a major concern. In addition, therapeutic approaches to combat ischemic stroke are still scarce, since the only approved therapies are directed toward restoring blood flow to the affected brain area. However, due to the reduced time window during which these therapies are effective, few patients benefit from them; therefore, alternative treatments are urgently needed to reduce stroke brain damage in order to improve patients’ outcome. The inflammatory response triggered after the ischemic event plays an important role in the progression of stroke; consequently, the study of inflammatory molecules in the acute phase of stroke has attracted increasing interest in recent decades. Here, we provide an overview of the inflammatory processes occurring during ischemic stroke, as well as the potential for these inflammatory molecules to become stroke biomarkers and the possibility that these candidates will become interesting neuroprotective therapeutic targets to be blocked or stimulated in order to modulate inflammation after stroke.
Keywords: biomarkers, cerebrovascular disease, inflammation, neuroprotection, stroke, therapeutic targets
Introduction
Stroke is caused by the disruption of the blood flow into a brain region and the consequent oxygen and nutrient deprivation, resulting in cell death and severe brain damage. It is the fifth most common cause of death and the most frequent cause of permanent disability in adults worldwide. Ischemic stroke, which is caused by the obstruction of a blood vessel by a thrombus, represents 87% of all strokes.1
After years of in-depth research and despite advances in the understanding of stroke pathophysiology, the therapeutic options for acute stroke remain very scarce. Currently, the only approved therapy for treating acute ischemic stroke is the administration of intravenous recombinant tissue–plasminogen activator (rt-PA) together with endovascular mechanical thrombectomy to remove the thrombus. However, rt-PA treatment carries a significant risk of secondary bleeding.2 Furthermore, it has a reduced time window of action, given that it is effective only when administered within 4.5 h after the onset of symptoms.3 This limitation reduces the number of stroke patients who can be treated to approximately 15%.4 Although mechanical thrombectomy has recently demonstrated its efficacy even 24 h after the onset of the cerebrovascular event,5 neuroprotective therapies are sought in order to rescue the compromised tissue in the peri-infarct zone of the brain. Although different neuroprotective therapies have shown good results in experimental models, none of them has achieved good results at the clinical level. For that reason, alternative therapies are urgently needed to combat stroke. In addition, stroke diagnosis is currently based on medical history, neurological exploration and neuroimaging; therefore, there are no rapid diagnostic tools, such as a blood test, available in clinics. Such a test would help accelerate stroke treatment and optimize patients’ management to ameliorate stroke outcomes.
Given the complexity of stroke, different mechanisms are thought to be involved in its pathophysiology. In fact, increasing evidence shows that inflammation plays an important role in the progression of stroke.6 For that reason, therapeutic targeting of postischemic inflammation in acute stroke has gained interest as a potential neuroprotective strategy. Here, we provide an overview of the inflammatory processes occurring during ischemic stroke, the potential for these inflammatory molecules as stroke biomarkers and the possibility that these biomarkers will become important therapeutic targets to be blocked or stimulated in order to modulate inflammation after stroke. To that end, studies in which pharmacological agents are specifically directed to modulate inflammatory molecules in the context of stroke will be reviewed.
In our attempt to focus on translational evidence as much as possible, studies including knockout animals for inflammation-related genes are considered beyond the scope of this review. In addition, studies indirectly modulating inflammatory molecules will not be considered either, given that we want to describe the specific role of each individual protein in the pathophysiology of ischemic stroke.
Postischemic stroke inflammation
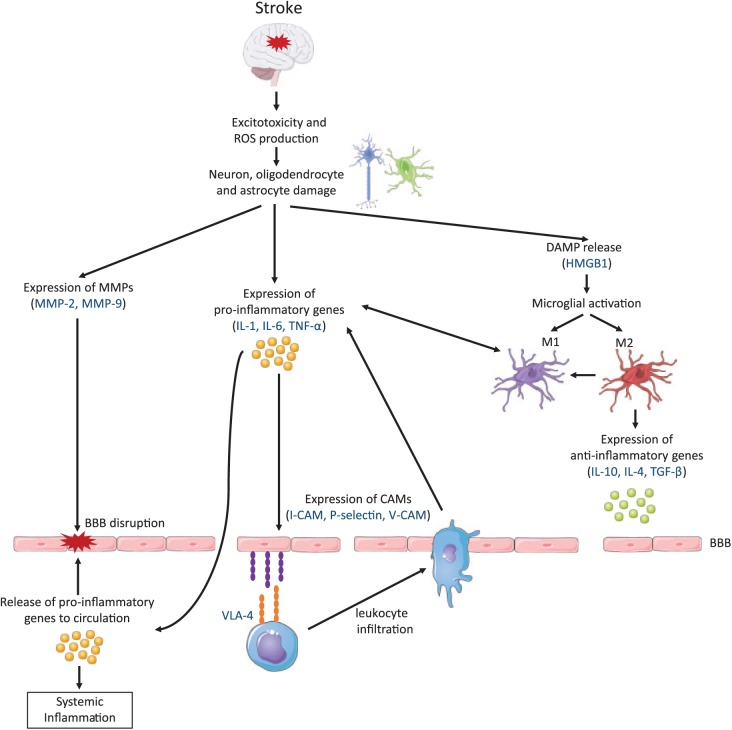
After the ischemic event, the blockage of blood flow produces a reduction of glucose and oxygen availability, which leads to an impaired adenosine triphosphate production and ultimately causes a bioenergetics failure.7 In addition, an ionic imbalance occurs within minutes after the arterial occlusion, generating a reduced reuptake of glutamate and subsequently leading to excitotoxicity and apoptosis. At the same time, catabolic enzymes are activated, producing an increase in the generation of reactive oxygen species. This fact triggers the expression of pro-inflammatory genes, such as cytokines and chemokines, by the injured brain cells.8 Consequently, the expression of cell adhesion molecules on the endothelial cell surface is induced, including P-selectin, E-selectin and intercellular adhesion molecule-1 (ICAM-1). These molecules mediate the infiltration of leukocytes into the brain parenchyma and facilitate the clearance of debris in the infarct area. Moreover, endothelial cells increase the expression of chemokines in order to guide leukocytes to the site of injury.9 However, there is evidence showing that beside their beneficial role, infiltrating immune cells also impair the ischemic brain by producing cytotoxic mediators that can extend the inflammatory response and increase brain damage.10
In addition, dying brain cells secrete damage-associated molecular patterns (DAMPs) that are recognized by microglia and cause their activation. Thus, both resident and infiltrated immune cells produce a burst of pro-inflammatory molecules within the infarct area.11 This fact couples with an increase in matrix metalloproteinase (MMP) production, which mediates the destruction of the basal lamina, increasing blood–brain barrier (BBB) permeability and facilitating the entrance of additional peripheral immune cells into the affected brain area.12
Furthermore, the complement cascade, a major constituent of innate immunity, has been shown to play an important role in stroke. The activation of the complement system is mediated by an increased expression of complement cascade receptors and activators (C1q and C3) by damaged cells in the site of injury. Its activation generates opsins (C3b, C4b and C5b), which enhance the phagocytic response, as well as inflammatory mediators and a membrane attack complex (C5b, C6, C7, C8 and C9) that has cytolytic activity.13 In addition, complement system activation has been implicated in adhesion molecule upregulation, chemotaxis and leukocyte activation.14
Acute stroke not only causes a local inflammatory response in the ischemic brain but also triggers a systemic immune response. After the ischemic event, there is a major release of pro-inflammatory mediators into the systemic circulation, causing an overactivation of peripheral immune cells. This excessive activation leads to an exhaustion of mature leukocytes, provoking the recruitment of immature leukocytes that are unable to respond properly to brain damage.15 In fact, the recruitment and expansion of this immature subpopulation ultimately lead to lymphocytopenia, contributing to a significant immunodepression that predisposes patients to poststroke infections and influence stroke outcome.16 Furthermore, the disproportionate concentration of pro-inflammatory mediators can stimulate the activation of the hypothalamic–pituitary–adrenal axis and the sympathetic nervous system, with the consequent release of catecholamines and glucocorticoids. All this leads to the inhibition of pro-inflammatory pathways and the stimulation of anti-inflammatory mechanisms through the release of interleukins and growth factors. In addition, this immunosuppression helps prevent autoimmune reactions against the central nervous system (CNS) antigens that are present in the bloodstream due to BBB leakage.17
Finally, in the brain, the immune system also works to terminate the inflammatory response at later stages. To that end, infiltrating macrophages and activated microglia are known to phagocytose dead cells and debris. Moreover, they promote the production of anti-inflammatory molecules that contribute to the suppression of the immune response, and at the same time, inhibit the expression of adhesion molecules and the production of pro-inflammatory cytokines.6 In addition, both macrophages and microglial cells release neuroprotective factors required for the recovery of the ischemic brain injury that promote neurogenesis and angiogenesis, among other processes.18 Figure 1 summarizes the postischemic inflammatory cascade, highlighting key proteins that play a relevant role in the process.
Figure 1.
The inflammatory cascade following a stroke.
BBB, blood–brain barrier; CAM, cellular adhesion molecule; DAMP, damage-associated molecular pattern; HMGB1, high-mobility group box 1; I-CAM, intercellular adhesion molecule; IL, interleukin; MMP, matrix metalloproteinase; ROS, reactive oxygen species; TGF, transforming growth factor; TNF, tumor necrosis factor; V-CAM, vascular cell adhesion molecule; VLA, very late antigen.
Biomarkers in stroke
Biomarkers are defined as characteristics that can be objectively measured and evaluated as indicators of normal biological processes, pathogenic processes, or pharmacologic responses to therapeutic interventions.19 Clinical biomarkers can be detectable molecules from different biological fluids such as blood, urine, or saliva. Ideally, biomarkers should have high specificity and sensitivity for the indicated process, and their measurements should be accurate, reproducible, and easily interpretable by clinicians. In terms of clinical application, stroke biomarkers are considered relevant tools for different phases, including both the diagnosis and the prognosis of stroke. These biomarkers should be representative of the brain injury processes triggered by stroke. As previously described, BBB disruption favors the release of brain antigens into the peripheral circulation, making these molecules promising stroke-associated biomarkers that could reflect in the circulatory system those pathological processes occurring in brain after the ischemic event. In addition, both local and peripheral inflammatory response can produce systemic indicators that might serve as stroke biomarkers.
Different inflammation-related proteins have been found altered after ischemic stroke and have shown a potential role in the detection and modulation of inflammation, making them potential biomarkers or therapeutic targets for stroke management. Those being studied for both roles will be reviewed in the following sections. Table 1 summarizes the main studies performed for each of the proteins described in this review.
Table 1.
Main studies performed on inflammatory-related proteins having an important role in stroke pathophysiology.
| Protein | Role as biomarker |
Experimental studies |
Human clinical trials |
||||
|---|---|---|---|---|---|---|---|
| Type | Blood levels | Intervention | Rodent model | Infarct volume changes | Design | Results | |
| IL-1β/IL-1Ra | Diagnosis | Higher levels of IL-1β in IS than in controls within 24 h after
the event.20–24 No differences in IL-1β levels between IS
and controls at 12 or 72 h after the event.25,26
Higher levels of IL-1Ra in IS than in controls25–27 |
IL-1Ra/IL-1β administration | tMCAO mouse | IL-1Ra ↓28,29
IL-1β ↑28 |
34 IS: 17 received intravenous IL-1Ra over 3 days and 17 placebo.30,31 | Drug is safe and well tolerated; it produced a reduction of the
inflammatory response (white cell counts and pro-inflammatory
proteins) and reversed peripheral
immunosuppression; improved 3-month outcome |
| tMCAO rat | IL-1Ra ↓32
IL-1β ↑33 |
80 IS: 39 receiving subcutaneous IL-1Ra for 3 days and 41 receiving placebo39 | Drug reduces plasma inflammatory markers; no improvement in 3-month outcome | ||||
| pMCAO rat | IL-1Ra ↓34,35–38
IL-1β ↑34 |
||||||
| VCAM-1/VLA-4 | Diagnosis/prognosis | Higher levels of VCAM-1 in IS than controls;23,24,40–43
association between higher VCAM-1 levels and worse outcome44,45 |
Anti-VLA-4 administration | tMCAO mouse | No change46,47
↓46,48,49 |
161 IS: 79 received intravenous natalizumab and 82 placebo50 | Natalizumab did not reduce infarct volume; natalizumab-treated group had better functional outcome than placebo group |
| pMCAO mouse | No change48
↓47 |
270 participants receiving Natalizumab in a low or a high dose, or placebo | Ongoing [ClinicalTrials.gov identifier: NCT02730455] | ||||
| tMCAo rat | ↓51,52 | ||||||
| ICAM-1 | Diagnosis/prognosis | Higher levels of ICAM-1 in IS than controls within 24 h after
the event,21,23,24,53,54 although
another study found no difference;40
one small study showed lower levels of ICAM-1 in IS than in controls;55 association between higher ICAM-1 levels and larger infarct size and worse long-term outcome,21 although no association also found40,56 |
Anti-ICAM-1 administration | tMCAO rat | ↓57–59 | 32 IS: several dose ranges of enlimomab within first 24 hours + four daily doses60 | 160 mg on day 1 + 40 mg/day for 4 days produce the desired blood levels (⩾10 µg/ml) without increasing adverse effects |
| pMCAO rat | No change58 | 625 IS: 317 received enlimomab and 308 placebo61 | Enlimomab-treated group had worsening of neurological functions, increased mortality and adverse drug reactions compared with placebo group | ||||
| TNF-α | Diagnosis/prognosis | Higher levels of TNF-α in IS than in controls;21,23,24,62–65
smaller studies showed no differences in TNF-α between IS and controls;25,26,66,67 associations between higher TNF-α levels and poor outcome20,62 and larger lesion size65,68 are not clear enough26,69,70 |
anti-TNF/TNF-bp administration | tMCAO mouse | Anti-TNF ↓71 | ||
| pMCAO mouse | TNF-bp ↓72,73 | ||||||
| tMCAO rat | Anti-TNF ↓74,75 | ||||||
| pMCAO rat | Anti-TNF ↓76
TNF-bp ↓77 |
||||||
| TGF-β | Diagnosis/prognosis | Controversial results: lower,78,79 equal80,81 or higher82 levels of TGF-β in IS than controls; association between high late TGF-β levels and large infarct volume and stroke severity81 |
TGF-β/TGF-β antagonist administration | tMCAO mouse | TGF-β ↓83 | ||
| pMCAO mouse | TGF-β ↓84 | ||||||
| tMCAO rat | TGF-β antagonist ↑85 | ||||||
| MMP-9/MMP-2 | Diagnosis/prognosis | Higher levels of MMP-9 in IS than controls86–93 and stroke
mimics;94–96
no differences between stroke patients (ischemic and hemorrhagic) and controls;97 association between higher MMP-9 levels and worse outcome and larger infarct volumes;21,68, 86,89,93,98–106 association between higher MMP-9 levels and hemorrhagic transformation following rt-PA administration;92,107–110 lower levels of MMP-2 in IS than controls;93,102 smaller study showed no differences92 |
Anti-MMP-9 + anti-MMP-2 administration (SB-3CT) | tMCAO mouse | ↓111 | ||
| pMCAO mouse | ↓112 | ||||||
| IL-6 | Diagnosis/prognosis | Higher levels of IL-6 in IS patients compared with
controls20,21,
23,24,26,62,66,67,113–120 and
stroke mimics;94
association between higher IL-6 levels and poor functional outcome, infections44,121 and larger infarct volumes122,123 |
IL-6 administration | pMCAO mouse | No change124 | ||
| pMCAO rat | ↓125 | ||||||
| IL-10 | Diagnosis/prognosis | Lower78,115,117,126,127or equal24,128IL-10 levels when comparing IS and
controls; no association between IL-10 and infarct volume70,129 or stroke outcome;70, 126,130,131 association between higher levels of IL-10 and infections132 |
IL-10 administration | pMCAO rat | ↓133–135 | ||
| IL-4 | Diagnosis/prognosis | Higher levels of IL-4 in IS than controls;116
no association between IL-4 and neurological worsening69 |
IL-4 administration | tMCAO mouse | ↓136
No change137 |
||
| P-selectin | Diagnosis | Higher levels of P-selectin in IS than in controls23,24,138–140 | Anti-P-selectin administration | tMCAO mouse | ↓141 | ||
| tMCAO rat | No change142 | ||||||
| pMCAO rat | ↓143,144 | ||||||
| HMGB1 | Diagnosis/prognosis | Higher levels of HMGB1 in IS than in controls;86,145–147
association between higher HMGB1 levels and poor outcome,86,148 although another study found no association147 |
HMGB/anti-HMGB1 administration | tMCAO rat | HMGB1 ↑;149
anti-HMGB1 ↓149 |
||
| ANXA1 | Diagnosis | No differences in ANXA1 levels between IS and controls or stroke mimics.150 | ANXA1/anti-ANXA1 | pMCAO rat | ANXA1 ↓;151
anti-ANXA1 ↑151 |
||
Inflammatory-related proteins are sorted from the most to the least studied.
ANXA1, annexin A1; HMGB1, high-mobility group box 1; ICAM-1, intercellular adhesion molecule-1; IL, interleukin; IL-1Ra, interleukin-1 receptor antagonist; IL-1β, interleukin-1 beta; IS, ischemic stroke patients; MMP, matrix metalloproteinase; pMCAO, permanent middle artery occlusion; rt-PA, recombinant tissue–plasminogen activator; SB-3CT, methylthiirane; TGF-β, transforming growth factor beta; tMCAO, transient middle cerebral artery occlusion; TNF-α, tumor necrosis factor alpha; TNF-bp, tumor-necrosis-factor-binding protein; VCAM-1, vascular cell adhesion molecule-1; VLA-4, very late antigen-4.
Main inflammatory players in stroke
Cytokines
Cytokines are small proteins that through extracellular signaling regulate different biological functions such as innate and acquired immunity, inflammation, proliferation and repair. Cytokines have both pro- and anti-inflammatory properties and play an important role in the progression of the stroke-associated inflammation.152 After the ischemic event, cytokines are upregulated in the brain, expressed by both cells of the immune system and resident brain cells such as neurons and microglia.153
Pro-inflammatory cytokines
Interleukin-1 (IL-1) is produced in the CNS by microglia, astrocytes, endothelium and neurons. It has two different forms, one intracellular (IL-1α) and one secreted (IL-1β). Both forms act through the IL-1 type I receptor (IL-1RI) which is expressed by immune and endothelial cells. This receptor is competitively blocked by the naturally occurring IL-1 receptor antagonist (IL-1Ra), also secreted by endothelial and immune cells and binds to the receptor without inducing any effect.154 In reference to the plausible role of the IL-1 family as stroke biomarkers, there is some controversy. Distinct studies revealed that ischemic stroke patients have higher circulating levels of IL-1β within 24 h after the ischemic event when compared with controls, as well as when compared with patients with other neurological conditions such as Alzheimer disease and Parkinson disease.20–24 However, two other studies have reported that IL-1β levels in serum or plasma are not higher in stroke patients when compared with healthy controls, at 12 h25 and 72 h after the onset of symptoms.26 These contradictory results observed among studies might be explained, in part, by the differences in the time of evaluation of the biomarker or the sample size of the stroke patient cohorts (up to 120 for those studies reporting higher levels in stroke patients versus less than 50 patients in those reporting no differences). In addition, it has been shown that plasma levels of IL-1Ra are higher in patients than in controls within the first 24 h and even at 72 h after the ischemic event.25–27 However, these elevated levels of IL-1Ra may not be sufficiently high to prevent IL-1 activation of receptors in target cells.27 Several studies have investigated the therapeutic effect of the modulation of this pathway. Various studies have shown that IL-1β administration increases the infarct size in mice after transient middle cerebral artery occlusion (tMCAO)28 and rats after permanent (pMCAO) or transient ischemia.33,34 On the other hand, the administration of recombinant human IL-1Ra greatly reduces (up to 60%) the infarct volume after MCAO in both mice28,29 and rats. 32,34–38 In line with this, there are studies showing an improved functional outcome after the administration of IL-1Ra.155,156 The results obtained in these studies indicate a deleterious role of IL-1 and a plausible mechanism of neuroprotective action through the blockage of this pathway. This has led to the development of clinical trials testing the therapeutic potential of recombinant human IL-1Ra (anakinra) in stroke. In a phase II randomized clinical trial, 34 ischemic stroke patients within 6 h of the onset of symptoms received anakinra (100 mg loading dose over 60 s, followed by a 2 mg/kg/h infusion over 72 h) or placebo, intravenously. This study showed that the drug was safe and well tolerated and that patients receiving anakinra showed a reduced inflammatory response, with lower levels of neutrophils and total white cell counts as well as C-reactive protein (CRP) and IL-6 blood levels. Moreover, although no differences were observed in infarct volumes, 3-month clinical outcome was better in the treated group than in the placebo group.30 This could be related to the reversal of the peripheral immunosuppression, given that the production of cytokines such as tumor necrosis factor alpha (TNF-α), IL-6 and IL-10 was reduced in the anakinra-treated group.31 Very recently, a new phase II trial has explored the subcutaneous administration of IL-1Ra, which increased the half-life of the drug and facilitated its administration. In this study, 39 ischemic stroke patients received a total of six doses of anakinra (100 mg administered twice a day for 3 days) and 49 patients received placebo. The results confirmed that IL-1Ra administration significantly reduced plasma inflammatory markers associated with worse outcome, such as IL-6. However, patients receiving IL-1Ra subcutaneously did not show an improvement on the 3-month outcome when compared with the placebo group.39 Therefore, although the overall results seem promising, further studies need to be done before its translation to practice. Moreover, it would be interesting to evaluate the efficacy of anakinra on reducing the infarct volume to better understand the role of IL-1 in the stroke pathophysiology.
Interleukin-6 (IL-6) is a pro-inflammatory cytokine that acts as a messenger molecule. It is secreted by different cells including microglia, astrocytes, leukocytes and endothelial cells in response to cerebral injury.157 Several studies show that there is an increase in IL-6 circulating levels in stroke patients early after the ischemic event in comparison with controls20,21,23,24,26,57, 62,66,113–120 and even in front of stroke-mimicking conditions such as syncope, seizures, and primary headache disorders.94 Moreover, high IL-6 levels in blood 24 h after the ischemic event are associated with poor long-term functional outcome44,121 and with large infarct volumes.122,123 In addition, it has been shown that an acute increase of IL-6 levels in ischemic stroke patients is associated with poststroke infections within the first week after the event.121 However, while higher circulating levels of IL-6 are related to morbidity, there is also evidence that IL-6 can have neuroprotective functions after ischemic stroke.158 For example, it has been described that IL-6 produced by injured brain cells promotes poststroke angiogenesis.159 For that reason, some studies have explored the neuroprotective role of IL-6 in experimental stroke. One study performed using a pMCAO mouse model showed that IL-6 administration right after reperfusion did not reduce infarct volume but improved functional outcome.124 Similarly, another independent study performed in a transient tMCAO mouse model showed an improved functional outcome after IL-6 administration.160 Loddick and colleagues showed that the intracerebroventricular injection of recombinant human IL-6 protein reduced infarct size in rats.125 In addition, IL-6 prevented learning disability and hippocampal neuron loss when administered in gerbils.161 All these findings could be indicating different effects of IL-6 between central and systemic inflammation, as well as at different times poststroke. Thus, IL-6 seems to be a good biomarker for stroke diagnosis and prognosis but further studies need to be done to better evaluate its therapeutic effect. Moreover, a blockade of this pathway might also be of interest in order to further evaluate the dual role of this protein.
TNF-α is another important mediator implicated in the pathophysiology of stroke. TNF-α has two forms: a transmembrane form that regulates local inflammation by cell-to-cell interactions, and a soluble form generated by TNF-α-converting enzyme (TACE).162 The soluble form acts at the systemic level amplifying the phagocytic and cytotoxic action of macrophages and enhancing the expression of other cytokines, such as IL-6 and IL-1. In reference to TNF-α blood levels after ischemic stroke, there are contrasting reports. Some studies have reported that circulating TNF-α levels do not change after the ischemic event within the first 24 h,25,26,66,67 whereas others reported an increase in the TNF-α blood levels after stroke in comparison with controls.21,23,24,62–64,163 However, the lack of differences reported in some studies could be, partly due to a reduced sample size. Specifically, studies reporting no differences include fewer patients (from 19 to 34 ischemic stroke patients) than the ones showing increased TNF-α levels (from 23 to 131 ischemic stroke patients, including 4 studies with more than 100 patients). Moreover, the role of TNF-α as a biomarker for stroke prognosis has also been studied, and contradictory results have also been obtained. Some studies have found an association between higher TNF-α levels and poor outcome20,62 or larger lesion size.65,68 However, some other studies have not found these associations.26,69,70 Thus, further studies need to be done before any conclusions can be drawn about the use of TNF-α as a stroke biomarker. In reference to the experimental models, TNF-α is probably one of the most extensively studied cytokines in the field of stroke research. In a tMCAO mouse model, Yang and colleagues showed that intracerebroventricular injection of an antibody against TNF-α after the brain artery occlusion significantly decreased the infarct volume.71 Moreover, three independent studies showed that intraperitoneal, intravenous and intracerebroventricular administration of anti-TNF-α in rats decreased the infarct volume using tMCAO74,75 and pMCAO models.76 In line with this, other studies showed that either intracranial, intraperitoneal, intravenous or even topic administration of TNF-binding protein (TNF-bp, which binds to and inhibits TNF-α) decreased the infarct volume following pMCAO in mice72,73 and when administered intravenously in rats.77 Finally, Wang and colleagues proved that the administration of a TACE inhibitor reduced the infarct volume in rats.164 Thus, all these studies seem to indicate that TNF-α has a detrimental role in stroke, since its inhibition seems to be a promising tool for stroke treatment. To corroborate the findings reported in experimental models, it might be interesting to perform a clinical trial in ischemic stroke patients to evaluate the therapeutic potential of inhibiting TNF-α.
Anti-inflammatory cytokines
Interleukin-4 (IL-4) is a cytokine produced mainly by leukocytes. Its signaling contributes to a potent anti-inflammatory response through the inhibition of pro-inflammatory cytokines and chemokines, among other functions.165 Although it is poorly studied as a stroke biomarker, Kim and colleagues found that acute ischemic stroke patients had higher levels of IL-4 in serum than controls,116 while similar IL-4 levels were found in ischemic stroke patients with or without neurological worsening.130 In reference to animal models, functional and cognitive improvement was shown following continuous (starting 6 h after ischemia and lasting for one week) IL-4 administration in a tMCAO mouse model, but without differences in infarct volume when compared with the control group.137 In contrast, others have found that IL-4 administration decreased infarct volume and improved the behavioral performance and neurological recovery 14 days after stroke in a tMCAO mouse model.136 Finally, IL-4 administration in rat tMCAO did not reduce the number of degenerating neurons and produced an increase in the number of macrophages/microglia in the infarct zone.166
Interleukin-10 (IL-10) is an anti-inflammatory cytokine expressed mainly by monocytes in response to brain injury. It has different immunomodulatory functions during the inflammatory response, being particularly important during the resolution phase. In fact, IL-10 reduces the activation of T cells, macrophages and monocytes, as well as attenuating the synthesis of pro-inflammatory cytokines. Moreover, it also reduces leukocyte adhesion and extravasation through the endothelium.167 Various studies have measured the circulating levels of this protein after ischemic stroke at different time points. Two studies have reported that patients have lower levels of IL-10 than controls within 12 h after the onset of symptoms.115,117 However, an independent study reported no differences between patients and controls at this same time point.24 Moreover, it has also been reported that circulating levels of this protein remain decreased at 48 h,126 72 h,127 and even more than 28 days after the ischemic event.78 However, a lack of differences in IL-10 circulating levels between patients and controls 72 h after the ischemic event has also been reported.128 Therefore, the majority of studies seem to indicate a plausible role of IL-10 as a biomarker for stroke diagnosis, but more studies need to be performed. In addition to this, two independent studies revealed that circulating levels of IL-10 were not associated with the initial infarct volume.70,129 Moreover, some studies have explored the potential of IL-10 as an outcome predictor, although with some controversy as well. One study found that lower IL-10 levels 24 h after the ischemic event were associated with neurological worsening at 48 h.130 However, another found that high circulating levels of IL-10 at 48 h after the event were independently associated with severe neurological deficits and predicted 3-month adverse clinical outcome.126 Another study revealed that those patients who survived after the ischemic event, had decreased levels of IL-10 at 24 h but increased levels of IL-10 at 72 h and at 6 days compared with those patients who died.131 However, Sahan and colleagues showed no significant association between prognosis and IL-10 levels.70 In addition to this, some studies showed that patients who developed infections within the first week after the ischemic event had higher IL-10 levels within the first 24 h.132 Regarding these results, further studies need to be carried before any practical conclusion can be drawn. In reference to experimental studies, two independent studies demonstrated that intracerebroventricular and intravenous administration of IL-10 reduces the infarct volume after pMCAO in rats.133,134 Moreover, Ooboshi and colleagues reported that postischemic gene transfer of IL-10 into the lateral ventricle reduced the infarct size in a pMCAO rat model.135 Hence, all experimental studies seem to indicate that the administration of IL-10 has a neuroprotective effect, but its application as a new stroke therapy needs to be further studied before its translation to the clinical setting.
Transforming growth factor beta (TGF-β) is produced by microglia, macrophages and astrocytes after ischemia and it regulates a variety of functions, such as cell growth and differentiation, immune function, and apoptosis.168 In reference to the differences observed in the circulating levels of this cytokine between ischemic stroke patients and controls, there are contradictory results. Two independent studies reported decreased levels of TGF-β in patients after acute ischemic stroke in comparison with controls,78,79 while two other studies found no differences.80,81 Moreover, another study reported that TGF-β levels were significantly elevated 24 h after the ischemic event.82 In addition, Stanzani and colleagues reported that higher TGF-β levels on the fourth day were associated with larger infarct volumes and an increased severity of ischemic stroke.81 However, all studies carried out so far have had limited sample sizes, therefore, larger studies need to be undertaken. In addition, various studies have explored the modulation of TGF-β as a potential treatment in experimental stroke. Ma and colleagues reported that the intranasal administration of TGF-β produced a significant reduction of the infarct volume in a tMCAO mouse model.83 Moreover, another study showed that the intracerebroventricular administration of TGF-β decreased the lesion size after pMCAO in mice.84 A different approach was attempted by Ruocco and colleagues, who showed that the administration of a TGF-β antagonist increased the infarct volume in a rat tMCAO model,85 thus demonstrating the beneficial role of TGF-β in the ischemic brain.
Cell adhesion molecules
Cell adhesion molecules (CAMs) play a key role in the trafficking and recruitment of leukocytes to activated endothelia in acute ischemic stroke. In fact, after the ischemic event, there is an increase in CAM expression on the cerebral endothelium. During the progression of inflammation, soluble isoforms of CAMs are shed from the cell surface and released into the bloodstream. CAMs are divided into three groups: the immunoglobulin gene superfamily (ICAM-1 and 2, VCAM-1, PECAM-1 and MAdCAM-1), the selectins (P-, E- and L-selectin), and the integrins (CD11, CD18, CD29 and CD49).9 Of these, only those CAMs described in the following sections have been studied as both biomarkers and therapeutic targets for stroke.
ICAM-1 is one of the most studied CAMs in the context of ischemic stroke. In reference to changes in the ICAM-1 plasma levels that occur after ischemic stroke, there are contradictory results. Various independent studies reported higher levels of this protein in ischemic stroke patients compared with controls within 24 h after the ischemic event.21,23,24,53,54 However, another study reported no difference in ICAM-1 levels when comparing patients and controls at the same time point.40 In addition, reduced levels of this protein at 72 h after the onset of ischemic stroke symptoms were also reported; however, this study included only 14 stroke patients, therefore, these results might not be as robust as those of the other studies, which all included more than 100 ischemic stroke patients.55 Controversies also exist in reference to ICAM-1 levels and stroke outcome. While two studies reported no association between ICAM-1 levels and stroke severity within the first week after the event and neither with functional outcome at 10 days or 3 months,40,56 Sotgiu and colleagues found a significant association with higher admission ICAM-1 levels and worse 3-month outcome, as well as with larger infarct size.21 In addition, various studies have evaluated the therapeutic potential of anti-ICAM-1 antibody in experimental stroke. Three independent studies reported a reduction of the infarct size after intravenous or intraperitoneal administration of anti-ICAM-1 in a tMCAO rat model.57–59 However, this treatment seems not to be able to reduce the infarct size after a pMCAO in rats.58 In addition to this, the inhibition of ICAM-1 as a potential stroke therapy has also been studied at the clinical level. Enlimomab is a murine monoclonal antibody that binds to ICAM-1, inhibiting neutrophil adhesion and migration through the brain endothelium. It was tested for safety, pharmacokinetics and biological activity in 32 stroke patients. The aim of this trial was to identify the dosing regimen that would achieve therapeutic serum levels (above 10 µg/ml), which were based on an in vitro study. To that end, a loading dose of enlimomab (ranging between 60 and 160 mg of antibody) was administered within 24 h after the onset of symptoms followed by four daily maintenance doses (ranged between 20 and 80 mg/day). The results obtained revealed that a regimen of initial 160 mg followed by a maintenance dose of 40 mg/day produced the desired blood levels of enlimomab and was well tolerated given that it did not increase the risk of adverse events in stroke patients.60 Once the safe dosage of the treatment was established, the Enlimomab Acute Stroke Trial (EAST) tested the efficacy of this compound in a total of 625 patients with ischemic stroke (317 received enlimomab and 308 placebo). Unfortunately, the enlimomab-treated group of patients had worsening of neurological functions, an increased mortality and adverse drug reactions (infections and fever) compared with the placebo group, showing the inefficacy of this therapeutic drug in stroke patients.61 The negative results obtained in this trial have been attributed to a detrimental immunoactivation due to the administration of a mouse antibody. In fact, Furuya and colleagues reproduced this design in an experimental study in which murine antibodies to ICAM-1 were serially administered to rats. They found that serial administration of the antibody-sensitized rats to produce antimouse antibodies, covered up any potential benefit from the inhibition of leukocyte infiltration to the site of injury.169
Another important CAM in the stroke pathophysiology is vascular cell adhesion molecule-1 (VCAM-1), also known as CD106. VCAM-1 is a protein that is expressed on the surface of endothelial cells and mediates cell-to-cell recognition and adhesion, as well as the activation and subsequent passage of leukocytes into the inflamed region.65 The major ligand of VCAM-1 is very late antigen-4 (VLA-4), which is an integrin constitutively expressed on the membrane of leukocytes. In reference to the plasma levels of these proteins, the circulating levels of VLA-4 in ischemic stroke patients have not been studied for the moment. In addition, several distinct studies have found that soluble VCAM-1 levels in circulation are higher after stroke than in stroke-free controls.23,24,41–43 Nonetheless, it seems that these increased VCAM-1 levels are found only in thromboembolic stroke patients and not in those with lacunar strokes.40 In addition, one study showed that higher levels of VCAM-1 at admission were associated with the risk of recurrence or death within 1 year after the ischemic event.45 Similarly, an association was found between higher VCAM-1 levels 2–3 weeks after the event and a worse 3-month outcome in ischemic stroke patients.44 The interaction between VLA-4 and VCAM-1 is crucial for the transmigration of leukocytes to the site of injury; for that reason, the inhibition of this interaction by specific antibodies seems to be a promising strategy to combat stroke.48 In fact, several experimental studies have analyzed the effect of VCAM-1/VLA-4 axis blockade in stroke animal models. Although not all studies obtained the same results, the vast majority have reported beneficial effects of this blockade. In 2001, two independent studies reported for the first time that VLA-4 blockade by antibodies reduced the infarct size and improved the neurological function after a transient occlusion in rats.51,52 However, the results obtained in mouse models are not as consistent. VLA-4 blockade produced a reduced infarct size and improved neurological function only when the antibody was administered after 30–45 min of occlusion in a tMCAO mouse model,46,48,49 and not after 60 min of tMCAO46 or after pMCAO.48 The discrepancies obtained between the studies are probably due to important differences in the design and methodology. To find more robust results, a group of researchers from six different centers performed a multicenter and randomized preclinical trial to explore the efficacy of VLA-4 blockade in two distinct mouse models of stroke. The results of this study revealed that the treatment with anti-VLA-4 significantly reduced infarct volume after distal pMCAO, which produced small cortical infarctions. However, after 60 min of tMCAO, the procedure that produces larger lesions, the blockade of VLA-4 did not reduce infarct size, suggesting that the efficacy of this treatment depends on infarct severity and localization.47 Even with this information in mind, the efficacy of VLA-4 blockade has also been studied in stroke patients. Natalizumab is a recombinant immunoglobulin G4 monoclonal antibody that binds to the alpha subunit of the VLA-4 integrin to block the leukocyte transmigration. Last year, the ACTION trial, a double-blind phase II study exploring the safety and efficacy of natalizumab in reducing the infarct volume in acute ischemic stroke was concluded. A total of 161 stroke patients were randomly assigned to 300 mg intravenous natalizumab (n = 79) or placebo (n = 82). It revealed that a single dose of natalizumab administered within the first 9 h after the onset of symptoms was not able to reduce infarct volume. However, functional outcome at 30 and 90 days was improved in patients receiving natalizumab.50 An ongoing ACTION 2 trial [ClinicalTrials.gov identifier: NCT02730455] with 270 participants is currently testing the efficacy of this treatment using a high (600 mg) or a low dose (300 mg), given that the ACTION trial revealed that patients with higher serum natalizumab concentrations showed better clinical outcomes. The results from the ACTION 2 trial are still not available, although a press release on February 2018 from the company behind the study points to the failure of the trial since the primary and secondary efficacy endpoints were not met.
P-selectin is an adhesion molecule expressed on the surface of activated endothelial cells and platelets that mediates the rolling of leukocytes.138 In reference to changes in the circulating levels of this protein after ischemic stroke, various studies have reported that ischemic stroke patients have higher levels of P-selectin than controls.23,24,138–140 Therapies targeting P-selectin have also been shown as effective in various experimental studies. Connolly and colleagues demonstrated for the first time that the blockade of P-selectin after tMCAO in mice reduced the infarct volume and improved the functional outcome.141 Similarly, a reduction of the infarct volume was also reported after anti-P-selectin administration following pMCAO in rats.143,144 However, Goussev and colleagues found that the administration of the anti-P-selectin antibody upon reperfusion after tMCAO in rat did not reduce the infarct size.142 Thus, further studies need to be performed in translational experimental models in order to confirm the potential role of this protein as a therapeutic target, but the results obtained for the moment do seem promising.
Damage-associated molecular patterns
DAMPs are endogenous molecules released from injured cells that can initiate or maintain the inflammatory response. A well-known DAMP implicated in ischemic brain injury is a protein named high-mobility group box 1 (HMGB1), a nuclear protein that functions as a nucleosome stabilizer and transcription factor. In addition to this, HMGB1 is able to activate endothelial cells, increasing VCAM-1, ICAM-1 and E-selectin expression, and consequently, allowing leukocyte extravasation. Moreover, HMGB1 can act itself as a pro-inflammatory cytokine because it is secreted by activated immune cells and can mediate the systemic inflammatory response.15 In reference to the circulating levels of this protein in ischemic stroke patients, distinct studies have reported an elevation of HMGB1 after stroke in comparison with controls. 86,145–147 Moreover, increased plasma levels of HMGB1 have been described as associated with a poor functional outcome within 1 month or 1 year after stroke.86,148 However, Schulze and colleagues reported no association between HMGB1 levels and outcome or infarct size.147 Experimental studies on a tMCAO rat model have reported that the intracerebroventricular injection of HMGB1 increased the severity of infarction, whereas the administration of anti-HMGB1 reduced the infarct size, improved the neurological function149 and decreased the edematous area.170 Although the results obtained seem promising, more experimental studies using different animal models are still needed to confirm the findings reported.
Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are zinc-binding proteolytic enzymes that remodel the extracellular matrix. In addition to this, MMPs are also involved in different biological processes such as the cleavage of cell receptors, the release of death signals, the activation of inflammatory mediators, cell proliferation and apoptosis.12 MMPs are secreted by neurons, glial and endothelial cells, as well as by infiltrating leukocytes as inactive enzymes; thus, they must be cleaved by other proteases to become active.171 The most studied MMPs in the field of ischemic stroke are MMP-9 and MMP-2, which are gelatinases that specifically degrade type IV collagen, laminin, and fibronectin, the major components of the basal lamina around cerebral blood vessels.172 In fact, a huge amount of studies have reported increased circulating levels of MMP-9 in ischemic stroke patients within 24 h after the event in comparison with controls86–93 and even stroke mimics.94–96 However, these differences in MMP-9 plasma levels were not observed in one study comparing controls with stroke patients (including both the ischemic and hemorrhagic stroke subtypes).97 In reference to MMP-2, two independent studies have reported decreased levels of this protein in ischemic stroke patients when compared with controls.93,102 However, the lack of differences between patients and controls has also been reported.92 In addition to this, higher MMP-9 levels have also been associated with larger infarct volumes and worse 3-month outcomes, including mortality.21,68, 86,89,93,98,99,102–106 Furthermore, distinct studies have reported that increased levels of MMP-9 at admission (before rt-PA administration) are associated with hemorrhagic transformation following the thrombolytic treatment.92,107–110 For that reason, MMP-9 levels at admission could work as a good biomarker to predict this secondary complication. In reference to experimental models of ischemic stroke targeting MMPs, a highly selective inhibitor that targets both MMP-2 and MMP-9 [(4-phenoxyphenylsulfonyl) methylthiirane (SB-3CT)] has been tested. Gu and colleagues explored whether the administration of SB-3CT after transient ischemia in mice could be neuroprotective. They found that the blockade of MMP-2 and MMP-9 produces a decrease in the infarct volume in comparison with vehicle-treated controls and ameliorates the functional outcome.111 Moreover, the same results were obtained when administering SB-3CT after pMCAO in mice.112 Thus, all experimental studies performed so far seem promising, although further studies need to be undertaken before any conclusions can be drawn. Moreover, no one has deeply studied the effect of specifically blocking MMP-9 or MMP-2, which may be interesting in terms of revealing the specific role of each protein in stroke pathophysiology.
Annexins
Annexins are a family of proteins involved in vesicle transport, membrane scaffolding, apoptosis, proliferation, differentiation and inflammation. Annexin A1 (ANXA1), previously known as lipocortin 1, is the only annexin that has been studied in the context of stroke as a possible biomarker and therapeutic target. There is an increasing evidence of the role of ANXA1 in different anti-inflammatory processes, such as the regulation of macrophage phagocytosis and neutrophil migration through the inhibition of neutrophil adhesion to the endothelium.173 One study exploring a small cohort of patients has reported no differences in the ANXA1 plasma levels between ischemic stroke patients, controls and patients with stroke-mimicking conditions.150 In addition, studies in animal models of cerebral ischemia found that the intracerebral administration of ANXA1 reduced the infarct size after pMCAO in rats, whereas the inhibition of ANXA1 by antibodies produced larger lesions in the same model.151 Hence, only one study has explored the effect of modulating this protein in experimental models; thus, it is necessary to perform more studies in order to confirm these promising results. Moreover, it would be interesting to explore the potential role of this protein as a stroke biomarker in large cohorts of patients.
Limitations and future steps
As described earlier, the understanding and modulation of the postischemic inflammatory response is gaining interest among scientists in the field of stroke. In recent years, several studies have explored the potential of inflammatory molecules to become stroke biomarkers or therapeutic targets. Although the majority of the results obtained seem promising, there also exist contradictory results and lack of robustness in certain cases. However, it is important to bear in mind that ischemic stroke is a complex disease in which several biological processes are altered, and different molecules may interact. Moreover, the inflammatory response triggered after the ischemic event shows dynamic behavior; thus, the implicated molecules may have different roles depending on the moment they act. For that reason, studies exploring the role of some inflammatory proteins as stroke biomarkers may not be as robust as expected, mainly due to the differences in the time of measurement of the proteins among exploratory studies, and the limited sample size of several studies. In addition to this, almost all studies assaying the potential usage of inflammatory proteins as biomarkers for stroke diagnosis are comparing circulating levels of these proteins between ischemic stroke patients and healthy controls; this experimental design is far from reality and does not ensure the efficacy of these biomarkers in stroke diagnosis. Studies exploring acutely the differences in circulating concentrations of these proteins between strokes and stroke-mimicking conditions such as seizures, headaches or brain tumors, seem thus necessary, as the inflammatory response is also altered in some of these mimics, who are the real confounders in stroke diagnosis.
In addition, the pharmacological modulation of the inflammatory response seems to be a promising strategy for stroke treatment as reported in several experimental studies. However, the existence of different ischemic stroke models and differences in experimental designs, including different administration times and protocols, may generate opposite results among studies that are also influenced by the dynamic nature of the inflammatory processes following stroke. To make feasible the translation of these treatments to the clinical setting, it seems necessary to adjust the experimental designs to a situation closer to the pathophysiology and timing occurring in humans. Moreover, the application of the current guidelines for preclinical studies in stroke would add great value to the search for new therapies.174,175
The results summarized in this review underlined a plausible use of inflammation-related proteins as blood biomarkers, therapeutic targets and even surrogate biomarkers of the efficacy of therapeutic strategies directed against the same target molecule. This will definitely provide a more personalized management of stroke patients. For that reason, a better characterization and understanding of the stroke pathophysiological processes such as inflammation is necessary to overcome the current limitations, as well as a deeper understanding of the importance of precise times in the diagnosis and treatment response in stroke.
The current context of stroke, in which reperfusion therapies are the only approved treatments, seems ideal for the exploration of new biomarkers and therapeutic targets, such as those described here. In an ideal scenario, some of these inflammatory-related molecules might be measured by future point-of-care devices in order to rapidly diagnose ischemic stroke. This might help increasing the number of patients who can benefit from the actual reperfusion therapies. Moreover, since many stroke patients do not respond properly to these therapies, the pharmacological modulation of inflammatory-related molecules as a complementary therapeutic tool could be an interesting approach to improve stroke patients’ management and consequently, their outcome.
Conclusion
Despite improvements in ischemic stroke management, appropriate diagnostic tools and therapies beyond vascular reperfusion are still missing. There is an urgent need for pharmacological agents to reduce the ischemic damage. Inflammation is a key process involved in the pathophysiology of stroke and its modulation seems a promising strategy for neuroprotection. Various studies have demonstrated that inflammatory molecules could work as biomarkers for stroke diagnosis or prognosis. Moreover, numerous animal model studies have shown that pharmacological modulation of these inflammatory molecules can reduce infarct size and improve functional outcome. However, the translation from bench to bedside has not been yet accomplished. To that end, a better understanding of the postischemic inflammatory response would increase the chances to find new diagnostic and therapeutic strategies.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Neurovascular Research Laboratory acknowledges funding for this project through a PI15/00354 grant from Fondo de Investigaciones Sanitarias of the Instituto de Salud Carlos III (cofinanced by the European Regional Development Fund, FEDER). The Neurovascular Research Laboratory also takes part in the Spanish stroke research network INVICTUS+ (RD16/0019). L Ramiro is supported by a predoctoral fellowship grant from the Instituto de Salud Carlos III (IFI17/00012), and A Simats is supported by a predoctoral fellowship grant from AGAUR (2015 FI_B00952).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Laura Ramiro  https://orcid.org/0000-0002-4121-4609
https://orcid.org/0000-0002-4121-4609
Contributor Information
Laura Ramiro, Neurovascular Research Laboratory, Vall d’Hebron Institute of Research, Universitat Autònoma de Barcelona, Barcelona, Spain.
Alba Simats, Neurovascular Research Laboratory, Vall d’Hebron Institute of Research, Universitat Autònoma de Barcelona, Barcelona, Spain.
Teresa García-Berrocoso, Neurovascular Research Laboratory, Vall d’Hebron Institute of Research, Universitat Autònoma de Barcelona, Barcelona, Spain.
Joan Montaner, Neurovascular Research Laboratory, Vall d’Hebron Institute of Research, Pg. Vall d’Hebron 119–129, Hospital Universitari Vall d’Hebron, 08035 Barcelona, Spain.
References
- 1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018; 137: e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Lo EH, Dalkara T, Moskowitz MA. Neurological diseases: mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 2003; 4: 399–414. [DOI] [PubMed] [Google Scholar]
- 3. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 4. Urra X, Abilleira S, Dorado L, et al. Mechanical thrombectomy in and outside the REVASCAT trial: insights from a concurrent population-based stroke registry. Stroke 2015; 46: 3437–3442. [DOI] [PubMed] [Google Scholar]
- 5. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2017; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 6. Vidale S, Consoli A, Arnaboldi M, et al. Postischemic inflammation in acute stroke. J Clin Neurol 2017; 13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hertz L. Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology 2008; 55: 289–309. [DOI] [PubMed] [Google Scholar]
- 8. Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 2009; 7: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mizuma A, Yenari MA. Anti-inflammatory targets for the treatment of reperfusion injury in stroke. Front Neurol 2017; 8: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simats A, García-Berrocoso T, Montaner J. Neuroinflammatory biomarkers: from stroke diagnosis and prognosis to therapy. Biochim Biophys Acta - Mol Basis Dis 2016; 1862: 411–424. [DOI] [PubMed] [Google Scholar]
- 11. Lee Y, Lee SR, Choi SS, et al. Therapeutically targeting neuroinflammation and microglia after acute ischemic stroke. Biomed Res Int 2014; 2014: 297241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morancho A, Rosell A, García-Bonilla L, et al. Metalloproteinase and stroke infarct size: role for anti-inflammatory treatment? Ann N Y Acad Sci 2010; 1207: 123–133. [DOI] [PubMed] [Google Scholar]
- 13. Orsini F, De Blasio D, Zangari R, et al. Versatility of the complement system in neuroinflammation, neurodegeneration and brain homeostasis. Front Cell Neurosci 2014; 8: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D’Ambrosio AL, Pinsky DJ, Connolly ES. The role of the complement cascade in ischemia/reperfusion injury: implications for neuroprotection. Mol Med 2001; 7: 367–382. [PMC free article] [PubMed] [Google Scholar]
- 15. Liesz A, Dalpke A, Mracsko E, et al. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci 2015; 35: 583–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liesz A, Hu X, Kleinschnitz C, et al. The functional role of regulatory lymphocytes in stroke: facts and controversies. Stroke 2016; 46: 1422–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esmaeili A, Dadkhahfar S, Fadakar K, et al. Post-stroke immunodeficiency: effects of sensitization and tolerization to brain antigens. Int Rev Immunol 2012; 31: 396–409. [DOI] [PubMed] [Google Scholar]
- 18. Shichita T, Ito M, Yoshimura A. Post-ischemic inflammation regulates neural damage and protection. Front Cell Neurosci 2014; 8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Atkinson AJ, Colburn WA, DeGruttola VG, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69: 89–95. [DOI] [PubMed] [Google Scholar]
- 20. Mazzotta G, Sarchielli P, Caso V, et al. Different cytokine levels in thrombolysis patients as predictors for clinical outcome. Eur J Neurol 2004; 11: 377–381. [DOI] [PubMed] [Google Scholar]
- 21. Sotgiu S, Zanda B, Marchetti B, et al. Inflammatory biomarkers in blood of patients with acute brain ischemia. Eur J Neurol 2006; 13: 505–513. [DOI] [PubMed] [Google Scholar]
- 22. Wytrykowska A, Prosba-Mackiewicz M, Nyka WM. IL-1β, TNF-α, and IL-6 levels in gingival fluid and serum of patients with ischemic stroke. J Oral Sci 2016; 58: 509–513. [DOI] [PubMed] [Google Scholar]
- 23. Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Plasma levels of inflammatory and thrombotic/fibrinolytic markers in acute ischemic strokes: relationship with TOAST subtype, outcome and infarct site. J Neuroimmunol 2009; 215: 84–89. [DOI] [PubMed] [Google Scholar]
- 24. Licata G, Tuttolomondo A, Di Raimondo D, et al. Immuno-inflammatory activation in acute cardio-embolic strokes in comparison with other subtypes of ischaemic stroke. Thromb Haemost 2009; 101: 929–937. [PubMed] [Google Scholar]
- 25. Emsley HCA, Smith CJ, Gavin CM, et al. Clinical outcome following acute ischaemic stroke relates to both activation and autoregulatory inhibition of cytokine production. BMC Neurol 2007; 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ormstad H, Aass HCD, Lund-Sørensen N, et al. Serum levels of cytokines and C-reactive protein in acute ischemic stroke patients, and their relationship to stroke lateralization, type, and infarct volume. J Neurol 2011; 258: 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beamer NB, Coull VBM, Clark WM, et al. Interleukin-6 and interleukin-1 antagonist in acute stroke. Ann Neurol 1995; 37: 800–805. [DOI] [PubMed] [Google Scholar]
- 28. Touzani O, Boutin H, LeFeuvre R, et al. Interleukin-1 influences ischemic brain damage in the mouse independently of the interleukin-1 type I receptor. J Neurosci 2002; 22: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boutin H, LeFeuvre R a, Horai R, et al. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci 2001; 21: 5528–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Emsley HCA, Smith CJ, Georgiou RF, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry 2005; 76: 1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith CJ, Emsley HC, Udeh CT, et al. Interleukin-1 receptor antagonist reverses stroke-associated peripheral immune suppression. Cytokine 2012; 58: 384–389. [DOI] [PubMed] [Google Scholar]
- 32. Mulcahy NJ, Ross J, Rothwell NJ, et al. Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br J Pharmacol 2003; 140: 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamasaki Y, Matsuura N, Shozuhara H, et al. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke 1995; 26: 676–681. [DOI] [PubMed] [Google Scholar]
- 34. Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischemia in the rat. J Cereb blood flow Metab 1996; 16: 932–940. [DOI] [PubMed] [Google Scholar]
- 35. Relton JK, Martin D, Thompson RC, et al. Peripheral administration of interleukin-1 receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Exp Neurol 1996; 138: 206–213. [DOI] [PubMed] [Google Scholar]
- 36. Relton JK, Rothwell NJ. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull 1992; 29: 243–246. [DOI] [PubMed] [Google Scholar]
- 37. Pradillo JM, Denes A, Greenhalgh AD, et al. Delayed administration of interleukin-1 receptor antagonist reduces ischemic brain damage and inflammation in comorbid rats. J Cereb Blood Flow Metab 2012; 32: 1810–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stroemer RP, Rothwell NJ. Cortical protection by localized striatal injectino of IL-1ra following cerebral ischemia in the rat. J Cereb Blood Flow Metab 1997; 17: 597–604. [DOI] [PubMed] [Google Scholar]
- 39. Smith CJ, Hulme S, Vail A, et al. SCIL-STROKE (Subcutaneous Interleukin-1 Receptor Antagonist in Ischemic Stroke). Stroke. Epub ahead of print March 22, 2018. DOI: 10.1161/STROKEAHA.118.020750. [DOI] [PubMed] [Google Scholar]
- 40. Supanc V, Biloglav Z, Kes VB, et al. Role of cell adhesion molecules in acute ischemic stroke. Ann Saudi Med 2011; 31: 365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brondani R, Rieder CRM, Valente D, et al. Levels of vascular cell adhesion molecule-1 and endothelin-1 in ischemic stroke: a longitudinal prospective study. Clin Biochem 2007; 40: 282–284. [DOI] [PubMed] [Google Scholar]
- 42. Blann A, Kumar P, Krupinski J, et al. Soluble intercelluar adhesion molecule-1, E-selectin, vascular cell adhesion molecule-1 and von Willebrand factor in stroke. Blood Coagul fibrinolysis 1999; 10: 277–284. [DOI] [PubMed] [Google Scholar]
- 43. Fassbender K, Mossner R, Motsch L, et al. Circulating selectin- and immunoglobulin-type adhesion molecules in acute ischemic stroke. Stroke 1995; 26: 1361–1364. [DOI] [PubMed] [Google Scholar]
- 44. Richard S, Lagerstedt L, Burkhard PR, et al. E-selectin and vascular cell adhesion molecule-1 as biomarkers of 3-month outcome in cerebrovascular diseases. J Inflamm 2015; 12: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Castillo J, Álvarez-Sabín J, Martínez-Vila E, et al. Inflammation markers and prediction of post-stroke vascular disease recurrence: the MITICO study. J Neurol 2009; 256: 217–224. [DOI] [PubMed] [Google Scholar]
- 46. Liesz A, Zhou W, Mracskó É, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain 2011; 134: 704–720. [DOI] [PubMed] [Google Scholar]
- 47. Llovera G, Hofmann K, Roth S, et al. Results of a preclinical randomized controlled multicenter trial (pRCT): anti-CD49d treatment for acute brain ischemia. Sci Transl Med 2015; 7: 299ra121. [DOI] [PubMed] [Google Scholar]
- 48. Langhauser F, Kraft P, Göb E, et al. Blocking of α4 integrin does not protect from acute ischemic stroke in mice. Stroke 2014; 45: 1799–1806. [DOI] [PubMed] [Google Scholar]
- 49. Neumann J, Riek-Burchardt M, Herz J, et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol 2015; 129: 259–277. [DOI] [PubMed] [Google Scholar]
- 50. Elkins J, Veltkamp R, Montaner J, et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol 2017; 16: 217–226. [DOI] [PubMed] [Google Scholar]
- 51. Becker K, Kindrick D, Relton J, et al. Antibody to the α4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke 2001; 32: 206–211. [DOI] [PubMed] [Google Scholar]
- 52. Relton JK, Sloan KE, Frew EM, et al. Inhibition of α4 integrin protects against transient focal cerebral ischemia in normotensive and hypertensive rats. Stroke 2001; 4: 199–206. [DOI] [PubMed] [Google Scholar]
- 53. Rallidis LS, Zolindaki MG, Vikelis M, et al. Elevated soluble intercellular adhesion molecule-1 levels are associated with poor short-term prognosis in middle-aged patients with acute ischaemic stroke. Int J Cardiol 2009; 132: 216–220. [DOI] [PubMed] [Google Scholar]
- 54. Shyu KG, Chang H, Lin CC. Serum levels of intercellular adhesion molecule-1 and E-selectin in patients with acute ischaemic stroke. J Neurol 1997; 244: 90–93. [DOI] [PubMed] [Google Scholar]
- 55. Clark WM, Coull BM, Briley DP, et al. Circulating intercellular adhesion molecule-1 levels and neutrophil adhesion in stroke. J Neuroimmunol 1993; 44: 123–125. [DOI] [PubMed] [Google Scholar]
- 56. Orion D, Schwammenthal Y, Reshef T, et al. Interleukin-6 and soluble intercellular adhesion molecule-1 in acute brain ischaemia. Eur J Neurol 2008; 15: 323–328. [DOI] [PubMed] [Google Scholar]
- 57. Cao J, Shi X, Li W, et al. Protective effect of anti-intercellular adhesion molecule-1 antibody on global cerebral ischemia/reperfusion injury in the rat. Biosci Trends 2009; 3: 48–52. [PubMed] [Google Scholar]
- 58. Zhang RL, Chopp M, Jiang N, et al. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke 1995; 26: 1438–43. [DOI] [PubMed] [Google Scholar]
- 59. Matsuo Y, Onodera H, Shiga Y, et al. Role of cell adhesion molecules in brain injury after transient middle cerebral artery occlusion in the rat. Brain Res 1994; 656: 344–352. [DOI] [PubMed] [Google Scholar]
- 60. Schneider D, Berrouschot J, Brandt T, et al. Safety, pharmacokinetics and biological activity of enlimomab (anti-ICAM-1 antibody): an open-label, dose escalation study in patients hospitalized for acute stroke. Eur Neurol 1998; 40: 78–83. [DOI] [PubMed] [Google Scholar]
- 61. Enlimomab Acute Stroke Trial Investigators. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology 2001; 57: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 62. Castellanos M, Castillo J, García MM, et al. Inflammation-mediated damage in progressing. Stroke 2002; 33: 982–987. [DOI] [PubMed] [Google Scholar]
- 63. Intiso D, Zarrelli MM, Lagioia G, et al. Tumor necrosis factor alpha serum levels and inflammatory response in acute ischemic stroke patients. Neurol Sci 2004; 24: 390–396. [DOI] [PubMed] [Google Scholar]
- 64. Bokhari FA, Shakoori TA, Butt A, et al. TNF-alpha: a risk factor for ischemic stroke. J Ayub Med Coll Abbottabad 2014; 26: 111–114. [PubMed] [Google Scholar]
- 65. Zaremba J, Losy J. Adhesion molecules of immunoglobulin gene superfamily in stroke. Folia Morphol (Warsz) 2002; 61: 1–6. [PubMed] [Google Scholar]
- 66. Ferrarese C, Mascarucci P, Zoia C, et al. Increased cytokine release from peripheral blood cells after acute stroke. J Cereb Blood Flow Metab 1999; 19: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 67. Emsley HCA, Smith CJ, Gavin CM, et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol 2003; 139: 93–101. [DOI] [PubMed] [Google Scholar]
- 68. Montaner J, Rovira A, Molina CA, et al. Plasmatic level of neuroinflammatory markers predict the extent of diffusion-weighted image lesions in hyperacute stroke. J Cereb Blood Flow Metab 2003; 23: 1403–1407. [DOI] [PubMed] [Google Scholar]
- 69. Vila N, Castillo J, Dávalos A, et al. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 2000; 31: 2325–2329. [DOI] [PubMed] [Google Scholar]
- 70. Sahan M, Sebe A, Acikalin A, et al. Acute-phase reactants and cytokines in ischemic stroke: do they have any relationship with short-term mortality? Eur Rev Med Pharmacol Sci 2013; 17: 2773–2777. [PubMed] [Google Scholar]
- 71. Yang GY, Gong C, Qin Z, et al. Inhibition of TNFalpha attenuates infarct volume and ICAM-1 expression in ischemic mouse brain. Neuroreport 1998; 9: 2131–2134. [DOI] [PubMed] [Google Scholar]
- 72. Nawashiro H, Martin D, Hallenbeck JM. Neuroprotective effects of TNF binding protein in focal cerebral ischemia. Brain Res 1997; 778: 265–271. [DOI] [PubMed] [Google Scholar]
- 73. Nawashiro H, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor and amelioration of brain infarction in mice. J Cereb Blood Flow Metab 1997; 17: 229–232. [DOI] [PubMed] [Google Scholar]
- 74. Hosomi N, Ban CR, Naya T, et al. Tumor necrosis factor-alpha neutralization reduced cerebral edema through inhibition of matrix metalloproteinase production after transient focal cerebral ischemia. J Cereb Blood Flow Metab 2005; 25: 959–67. [DOI] [PubMed] [Google Scholar]
- 75. Arango-Dávila CA, Vera A, Londoño AC, et al. Soluble or soluble/membrane TNF-α inhibitors protect the brain from focal ischemic injury in rats. Int J Neurosci 2015; 125: 936–940. [DOI] [PubMed] [Google Scholar]
- 76. Meistrell ME, Botchkina GI, Wang H, et al. Tumor necrosis factor is a brain damaging cytokine in cerebral ischemia. Shock 1997; 8: 341–348. [PubMed] [Google Scholar]
- 77. Dawson DA, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor-alpha reduces focal cerebral ischemic injury in the spontaneously hypertensive rat. Neurosci Lett 1996; 218: 41–44. [DOI] [PubMed] [Google Scholar]
- 78. Hu Y, Zheng Y, Wu Y, et al. Imbalance between IL-17A-producing cells and regulatory T cells during ischemic stroke. Mediators Inflamm 2014; 2014: 813045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim JS, Yoon SS, Kim YH, et al. Serial measurement of interleukin-6, transforming growth factor-beta, and S-100 protein in patients with acute stroke. Stroke 1996; 27: 1553–1557. [DOI] [PubMed] [Google Scholar]
- 80. Slevin M, Krupinski J, Slowik A, et al. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke 2000; 32: 275–278. [DOI] [PubMed] [Google Scholar]
- 81. Stanzani L, Zoia C, Sala G, et al. Nerve growth factor and transforming growth factor-β serum levels in acute stroke patients: possible involvement of neurotrophins in cerebrovascular disease. Cerebrovasc Dis 2001; 12: 240–244. [DOI] [PubMed] [Google Scholar]
- 82. Yan J, Greer JM, McCombe PA. Prolonged elevation of cytokine levels after human acute ischaemic stroke with evidence of individual variability. J Neuroimmunol 2012; 246: 78–84. [DOI] [PubMed] [Google Scholar]
- 83. Ma M, Ma Y, Yi X, et al. Intranasal delivery of transforming growth factor-beta1 in mice after stroke reduces infarct volume and increases neurogenesis in the subventricular zone. BMC Neurosci 2008; 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Henrich-Noack P, Prehn JH, Krieglstein J. Neuroprotective effects of TGF-beta 1. J Neural Transm Suppl 1994; 43: 33–45. [PubMed] [Google Scholar]
- 85. Ruocco A, Nicole O, Docagne F, et al. A transforming growth factor-β antagonist unmasks the neuroprotective role of this endogenous cytokine in excitotoxic and ischemic brain injury. J Cereb Blood Flow Metab 1999; 19: 1345–1353. [DOI] [PubMed] [Google Scholar]
- 86. Sapojnikova N, Kartvelishvili T, Asatiani N, et al. Correlation between MMP-9 and extracellular cytokine HMGB1 in prediction of human ischemic stroke outcome. Biochim Biophys Acta - Mol Basis Dis 2014; 1842: 1379–1384. [DOI] [PubMed] [Google Scholar]
- 87. Castellanos M, Leira R, Serena J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke 2003; 34: 40–45. [PubMed] [Google Scholar]
- 88. Reynolds MA, Kirchick HJ, Dahlen JR, et al. Early biomarkers of stroke. Clin Chem 2003; 49: 1733–1739. [DOI] [PubMed] [Google Scholar]
- 89. Lucivero V, Prontera M, Mezzapesa DM, et al. Different roles of matrix metalloproteinases-2 and -9 after human ischaemic stroke. Neurol Sci 2007; 28: 165–170. [DOI] [PubMed] [Google Scholar]
- 90. Kim MH, Kang SY, Kim MC, et al. Plasma biomarkers in the diagnosis of acute ischemic stroke. Ann Clin Lab Sci 2010; 40: 336–341. [PubMed] [Google Scholar]
- 91. Sarfo FS, Owusu D, Adamu S, et al. Plasma glial fibrillary acidic protein, copeptin, and matrix metalloproteinase-9 concentrations among west African stroke subjects compared with stroke-free controls. J Stroke Cerebrovasc Dis 2018; 27: 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Montaner J, Alvarez-Sabín J, Molina CA, et al. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke 2001; 32: 2762–7. [DOI] [PubMed] [Google Scholar]
- 93. Kreisel SH, Stroick M, Griebe M, et al. True effects or bias? MMP-2 and MMP-9 serum concentrations after acute stroke. Cerebrovasc Dis 2016; 42: 352–360. [DOI] [PubMed] [Google Scholar]
- 94. An SA, Kim J, Kim OJ, et al. Limited clinical value of multiple blood markers in the diagnosis of ischemic stroke. Clin Biochem 2013; 46: 710–715. [DOI] [PubMed] [Google Scholar]
- 95. Lynch JR, Blessing R, White WD, et al. Novel diagnostic test for acute stroke. Stroke 2004; 35: 57–63. [DOI] [PubMed] [Google Scholar]
- 96. Glickman SW, Phillips S, Anstrom KJ, et al. Discriminative capacity of biomarkers for acute stroke in the emergency department. J Emerg Med 2011; 41: 333–339. [DOI] [PubMed] [Google Scholar]
- 97. Vanni S, Polidori G, Pepe G, et al. Use of biomarkers in triage of patients with suspected stroke. J Emerg Med 2011; 40: 499–505. [DOI] [PubMed] [Google Scholar]
- 98. Rodríguez-Yáñez M, Castellanos M, Blanco M, et al. New-onset hypertension and inflammatory response/poor outcome in acute ischemic stroke. Neurology 2006; 67: 1973–1978. [DOI] [PubMed] [Google Scholar]
- 99. Chan CPY, Jiang HL, Leung LY, et al. Multiple atherosclerosis-related biomarkers associated with short- and long-term mortality after stroke. Clin Biochem 2012; 45: 1308–1315. [DOI] [PubMed] [Google Scholar]
- 100. Zhong C, Yang J, Xu T, et al. Serum matrix metalloproteinase-9 levels and prognosis of acute ischemic stroke. Neurology 2017; 89: 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Barbieri A, Giuliani E, Carone C, et al. Clinical severity of ischemic stroke and neural damage biomarkers in the acute setting: the STROke MArkers (STROMA) study. Minerva Anestesiol 2013; 79: 750–757. [PubMed] [Google Scholar]
- 102. Vukasovic I, Tesija-Kuna A, Topic E, et al. Matrix metalloproteinases and their inhibitors in different acute stroke subtypes. Clin Chem Lab Med 2006; 44: 428–434. [DOI] [PubMed] [Google Scholar]
- 103. Montaner J, Alvarez-Sabín J, Molina C, et al. Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke 2001; 32: 1759–66. [DOI] [PubMed] [Google Scholar]
- 104. Horstmann S, Kalb P, Koziol J, et al. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: influence of different therapies. Stroke 2003; 34: 2165–2170. [DOI] [PubMed] [Google Scholar]
- 105. Rosell A, Alvarez-Sabín J, Arenillas JF, et al. A matrix metalloproteinase protein array reveals a strong relation between MMP-9 and MMP-13 with diffusion-weighted image lesion increase in human stroke. Stroke 2005; 36: 1415–1420. [DOI] [PubMed] [Google Scholar]
- 106. Ning M, Furie KL, Koroshetz WJ, et al. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology 2006; 66: 1550–1555. [DOI] [PubMed] [Google Scholar]
- 107. Heo JH, Kim SH, Lee KY, et al. Increase in plasma matrix metalloproteinase-9 in acute stroke patients with thrombolysis failure. Stroke 2003; 34: e48–e50. [DOI] [PubMed] [Google Scholar]
- 108. Montaner J, Molina CA, Monasterio J, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation 2003; 107: 598–603. [DOI] [PubMed] [Google Scholar]
- 109. Montaner J, Fernández-Cadenas I, Molina CA, et al. Safety profile of tissue plasminogen activator treatment among stroke patients carrying a common polymorphism (C-1562T) in the promoter region of the matrix metalloproteinase-9 gene. Stroke 2003; 34: 2851–2855. [DOI] [PubMed] [Google Scholar]
- 110. Castellanos M, Sobrino T, Millán M, et al. Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke: a multicenter confirmatory study. Stroke 2007; 38: 1855–1859. [DOI] [PubMed] [Google Scholar]
- 111. Gu Z, Cui J, Brown S, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci 2005; 25: 6401–6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cui J, Chen S, Zhang C, et al. Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia. Mol Neurodegener 2012; 7: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Song JW, Song KS, Choi JR, et al. Plasma level of IL-6 and its relationship to procoagulant and fibrinolytic markers in acute ischemic stroke. Yonsei Med J 2006; 47: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Worthmann H, Tryc AB, Goldbecker A, et al. The temporal profile of inflammatory markers and mediators in blood after acute ischemic stroke differs depending on stroke outcome. Cerebrovasc Dis 2010; 30: 85–92. [DOI] [PubMed] [Google Scholar]
- 115. Perini F, Morra M, Alecci M, et al. Temporal profile of serum anti-inflammatory and pro-inflammatory interleukins in acute ischemic stroke patients. Neurol Sci 2001; 22: 289–296. [DOI] [PubMed] [Google Scholar]
- 116. Kim HM, Shin HY, Jeong HJ, et al. Reduced IL-2 but elevated IL-4, IL-6, and IgE serum levels in patients with cerebral infarction during the acute stage. J Mol Neurosci 2000; 14: 191–196. [DOI] [PubMed] [Google Scholar]
- 117. Basic Kes V, Simundic AM, Nikolac N, et al. Pro-inflammatory and anti-inflammatory cytokines in acute ischemic stroke and their relation to early neurological deficit and stroke outcome. Clin Biochem 2008; 41: 1330–1334. [DOI] [PubMed] [Google Scholar]
- 118. Fassbender K, Rossol S, Kammer T, et al. Proinflammatory cytokines in serum of patients with acute cerebral ischemia: kinetics of secretion and relation to the extent of brain damage and outcome of disease. J Neurol Sci 1994; 122: 135–139. [DOI] [PubMed] [Google Scholar]
- 119. Waje-Andreassen U, Kråkenes J, Ulvestad E, et al. IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol Scand 2005; 111: 360–365. [DOI] [PubMed] [Google Scholar]
- 120. Tarkowski E, Rosengren L, Blomstrand C, et al. Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke 1995; 26: 1393–1398. [DOI] [PubMed] [Google Scholar]
- 121. Bustamante A, Sobrino T, Giralt D, et al. Prognostic value of blood interleukin-6 in the prediction of functional outcome after stroke: a systematic review and meta-analysis. J Neuroimmunol 2014; 274: 215–224. [DOI] [PubMed] [Google Scholar]
- 122. Smith CJ, Emsley HCA, Gavin CM, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol 2004; 4: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Martínez-Sánchez P, Gutiérrez-Fernández M, Fuentes B, et al. Biochemical and inflammatory biomarkers in ischemic stroke: translational study between humans and two experimental rat models. J Transl Med 2014; 12: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Grønhøj MH, Clausen BH, Fenger CD, et al. Beneficial potential of intravenously administered IL-6 in improving outcome after murine experimental stroke. Brain Behav Immun 2017; 65: 296–311. [DOI] [PubMed] [Google Scholar]
- 125. Loddick SA, Turnbull A V, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab 1998; 18: 176–179. [DOI] [PubMed] [Google Scholar]
- 126. Chang LT, Yuen CM, Liou CW, et al. Link between interleukin-10 level and outcome after ischemic stroke. Neuroimmunomodulation 2010; 17: 223–228. [DOI] [PubMed] [Google Scholar]
- 127. Singh HV, Pandey A, Shrivastava AK, et al. Prognostic value of neuron specific enolase and IL-10 in ischemic stroke and its correlation with degree of neurological deficit. Clin Chim Acta 2013; 419: 136–138. [DOI] [PubMed] [Google Scholar]
- 128. Protti GG, Gagliardi RJ, Forte WCN, et al. Interleukin-10 may protect against progressing injury during the acute phase of ischemic stroke. Arq Neuropsiquiatr 2013; 71: 846–851. [DOI] [PubMed] [Google Scholar]
- 129. Arponen O, Muuronen A, Taina M, et al. Acute phase IL-10 plasma concentration associates with the high risk sources of cardiogenic stroke. PLoS One 2015; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Vila N, Castillo J, Dávalos A, et al. Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke 2003; 34: 671–675. [DOI] [PubMed] [Google Scholar]
- 131. Nayak AR, Kashyap RS, Purohit HJ, et al. Evaluation of the inflammatory response in sera from acute ischemic stroke patients by measurement of IL-2 and IL-10. Inflamm Res 2009; 58: 687–691. [DOI] [PubMed] [Google Scholar]
- 132. Bustamante A, Simats A, Vilar-Bergua A, et al. Blood/brain biomarkers of inflammation after stroke and their association with outcome: from c-reactive protein to damage-associated molecular patterns. Neurotherapeutics 2016; 13: 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Spera PA, Ellison JA, Feuerstein GZ, et al. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett 1998; 251: 189–192. [DOI] [PubMed] [Google Scholar]
- 134. Liesz A, Bauer A, Hoheisel JD, et al. Intracerebral interleukin-10 injection modulates post-ischemic neuroinflammation: an experimental microarray study. Neurosci Lett 2014; 579: 18–23. [DOI] [PubMed] [Google Scholar]
- 135. Ooboshi H, Ibayashi S, Shichita T, et al. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation 2005; 111: 913–919. [DOI] [PubMed] [Google Scholar]
- 136. Zhao X, Wang H, Sun G, et al. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J Neurosci 2015; 35: 11281–11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Liu X, Liu J, Zhao S, et al. Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke 2016; 47: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kozuka K, Kohriyama T, Ikeda J, et al. Endothelial markers and adhesion molecules in acute ischemic stroke-sequential change and differences in stroke subtype. Atherosclerosis 2002; 161: 161–168. [DOI] [PubMed] [Google Scholar]
- 139. Nadar SK, Lip GYH, Blann AD. Platelet morphology, soluble P selectin and platelet P-selectin in acute ischaemic stroke. The West Birmingham stroke project. Thromb Haemost 2004; 92: 1342–1348. [DOI] [PubMed] [Google Scholar]
- 140. Cherian P, Hankey GJ, Eikelboom JW, et al. Endothelial and platelet activation in acute ischemic stroke and its etiological subtypes. Stroke 2003; 34: 2132–2137. [DOI] [PubMed] [Google Scholar]
- 141. Connolly ES, Winfree CJ, Prestigiacomo CJ, et al. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circ Res 1997; 81: 304–310. [DOI] [PubMed] [Google Scholar]
- 142. Goussev AV, Zhang Z, Anderson DC, et al. P-selectin antibody reduces hemorrhage and infarct volume resulting from MCA occlusion in the rat. J Neurol Sci 1998; 161: 16–22. [DOI] [PubMed] [Google Scholar]
- 143. Suzuki H, Abe K, Tojo SJ, et al. Reduction of ischemic brain injury by anti-P-selectin monoclonal antibody after permanent middle cerebral artery occlusion in rat. Neurol Res 1999; 21: 2692–76. [DOI] [PubMed] [Google Scholar]
- 144. Suzuki H, Hayashi T, Tojo SJ, et al. Anti-P-selectin antibody attenuates rat brain ischemic injury. Neurosci Lett 1999; 265: 163–166. [DOI] [PubMed] [Google Scholar]
- 145. Goldstein RS, Gallowitsch-Puerta M, Yang L, et al. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock 2006; 25: 571–574. [DOI] [PubMed] [Google Scholar]
- 146. Vogelgesang A, May VEL, Grunwald U, et al. Functional status of peripheral blood T-cells in ischemic stroke patients. PLoS One 2010; 5: e8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Schulze J, Zierath D, Tanzi P, et al. Severe stroke induces long-lasting alterations of high-mobility group Box 1. Stroke 2013; 44: 246–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Huang JM, Hu J, Chen N, et al. Relationship between plasma high-mobility group box-1 levels and clinical outcomes of ischemic stroke. J Crit Care 2013; 28: 792–797. [DOI] [PubMed] [Google Scholar]
- 149. Liu K, Mori S, Takahashi HK, et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J 2007; 21: 3904–3916. [DOI] [PubMed] [Google Scholar]
- 150. Llombart V, García-Berrocoso T, Bech-Serra JJ, et al. Characterization of secretomes from a human blood brain barrier endothelial cells in-vitro model after ischemia by stable isotope labeling with aminoacids in cell culture (SILAC). J Proteomics 2016; 133: 100–112. [DOI] [PubMed] [Google Scholar]
- 151. Relton JK, Strijbos PJ, O’Shaughnessy CT, et al. Lipocortin-1 is an endogenous inhibitor of ischemic damage in the rat brain. J Exp Med 1991; 174: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin 2007; 45: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Barone FC, Feuerstein GZ. Inflammatory mediators and stroke : new opportunities for novel therapeutics. J Cereb blood flow Metab 1999; 19: 819–834. [DOI] [PubMed] [Google Scholar]
- 154. Sobowale OA, Parry-Jones AR, Smith CJ, et al. Interleukin-1 in stroke: from bench to bedside. Stroke 2016; 47: 2160–2167. [DOI] [PubMed] [Google Scholar]
- 155. Garcia JH, Liu KF, Relton JK. Interleukin-1 receptor antagonist decreases the number of necrotic neurons in rats with middle cerebral artery occlusion. Am J Pathol 1995; 147: 1477–1486. [PMC free article] [PubMed] [Google Scholar]
- 156. Pradillo JM, Murray KN, Coutts GA, et al. Reparative effects of interleukin-1 receptor antagonist in young and aged/co-morbid rodents after cerebral ischemia. Brain Behav Immun 2017; 61: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Clark WM, Lutsep HL. Potential of anticytokine therapies in central nervous system ischaemia. Expert Opin Biol Ther 2001; 1: 227–237. [DOI] [PubMed] [Google Scholar]
- 158. Herrmann O, Tarabin V, Suzuki S, et al. Regulation of body temperature and neuroprotection by endogenous interleukin-6 in cerebral ischemia. J Cereb Blood Flow Metab 2003; 23: 406–415. [DOI] [PubMed] [Google Scholar]
- 159. Gertz K, Kronenberg G, Kälin RE, et al. Essential role of interleukin-6 in post-stroke angiogenesis. Brain 2012; 135: 1964–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Meng C, Zhang JC, Shi RL, et al. Inhibition of interleukin-6 abolishes the promoting effects of pair housing on post-stroke neurogenesis. Neuroscience 2015; 307: 160–170. [DOI] [PubMed] [Google Scholar]
- 161. Matsuda S, Wen TC, Morita F, et al. Interleukin-6 prevents ischemia-induced learning disability and neuronal and synaptic loss in gerbils. Neurosci Lett 1996; 204: 109–112. [DOI] [PubMed] [Google Scholar]
- 162. Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 2003; 10: 45–65. [DOI] [PubMed] [Google Scholar]
- 163. Zaremba J, Skrobanski P, Losy J. Tumour necrosis factor-alpha is increased in the cerebrospinal fluid and serum of ischaemic stroke patients and correlates with the volume of evolving brain infarct. Biomed Pharmacother 2001; 55: 258–263. [DOI] [PubMed] [Google Scholar]
- 164. Wang X, Yue TL, Barone FC, et al. Concomitant cortical expression of TNF-alpha and IL-1 beta mRNAs follows early response gene expression in transient focal ischemia. Mol Chem Neuropathol 1994; 23: 103–14. [DOI] [PubMed] [Google Scholar]
- 165. Gadani SP, Cronk JC, Norris GT, et al. Interleukin-4: a cytokine to remember. J Immunol 2013; 189: 4213–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Lively S, Hutchings S, Schlichter LC. Molecular and cellular responses to interleukin-4 treatment in a rat model of transient ischemia. J Neuropathol Exp Neurol 2016; 75: 1058–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Strle K, Zhou J-H, Shen W-H, et al. lnterleukin-10 in the brain. Crit Rev Immunol 2001; 21: 23. [PubMed] [Google Scholar]
- 168. Flanders KC, Ren RF, Lippa CF. Transforming growth factor-betas in neurodegenerative disease. Prog Neurobiol 1998; 54: 71–85. [DOI] [PubMed] [Google Scholar]
- 169. Furuya K, Takeda H, Azhar S, et al. Examination of several potential mechanisms for the negative outcome in a clinical stroke trial of enlimomab, a murine anti-human intercellular adhesion molecule-1 antibody: a bedside-to-bench study. Stroke 2001; 32: 2665–2674. [DOI] [PubMed] [Google Scholar]
- 170. Zhang J, Takahashi HK, Liu K, et al. Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke 2011; 42: 1420–1428. [DOI] [PubMed] [Google Scholar]
- 171. Michaluk P, Kaczmarek L. Matrix metalloproteinase-9 in glutamate-dependent adult brain function and dysfunction. Cell Death Differ 2007; 14: 1255–1258. [DOI] [PubMed] [Google Scholar]
- 172. Ramos-Fernandez M, Bellolio MF, Stead LG. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis 2011; 20: 47–54. [DOI] [PubMed] [Google Scholar]
- 173. Solito E, McArthur S, Christian H, et al. Annexin A1 in the brain - undiscovered roles? Trends Pharmacol Sci 2008; 29: 135–142. [DOI] [PubMed] [Google Scholar]
- 174. Albers GW, Goldstein LB, Hess DC, et al. Stroke treatment academic industry roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke 2011; 42: 2645–2650. [DOI] [PubMed] [Google Scholar]
- 175. Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR Guidelines: escalating STAIR and STEPS for Effective Translational Research. Transl Stroke Res 2013; 4: 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]