Abstract
Background:
Few studies have investigated the influence of patient-specific variables or procedure-specific factors on the overall cost of anterior cruciate ligament reconstruction (ACLR) in an ambulatory surgery setting.
Purpose:
To determine patient- and procedure-specific factors influencing the overall direct cost of outpatient arthroscopic ACLR utilizing a unique value-driven outcomes (VDO) tool.
Study Design:
Cohort study (economic and decision analysis); Level of evidence, 3.
Methods:
All ACLRs performed by 4 surgeons over 2 years were retrospectively reviewed. Cost data were derived from the VDO tool. Patient-specific variables included age, body mass index, comorbidities, American Society of Anesthesiologists (ASA) classification, smoking status, preoperative Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function Computerized Adaptive Testing (PF-CAT) score, and preoperative Single Assessment Numeric Evaluation (SANE) score. Procedure-specific variables included graft type, revision status, associated injuries and procedures, time from injury to ACLR, surgeon, and operating room (OR) time. Multivariate analysis determined patient- and procedure-related predictors of total direct costs.
Results:
There were 293 autograft reconstructions, 110 allograft reconstructions, and 31 hybrid reconstructions analyzed. Patient-specific factors did not significantly influence the ACLR cost. The mean OR time was shorter for allograft reconstruction (P < .001). Predictors of an increased direct cost included the use of an allograft or hybrid graft (44.5% and 33.1% increase, respectively; P < .001), increased OR time (0.3% increase per minute; P < .001), surgeon 3 or 4 (9.1% or 5.9% increase, respectively; P < .001 or P = .001, respectively), and concomitant meniscus repair (24.4% increase; P < .001). Within the meniscus repair cohort, all-inside, root, and combined repairs correlated with a 15.5%, 31.4%, and 53.2% increased mean direct cost, respectively, compared with inside-out repairs (P < .001).
Conclusion:
This study failed to identify modifiable patient-specific factors influencing direct costs of ACLR. Allografts and hybrid grafts were associated with an increased total direct cost. Meniscus repair independently predicted an increased direct cost, with all-inside, root, and combined repairs being costlier than inside-out repairs. The time-saving potential of all-inside meniscus repair was not realized in this study, making implant use a significant factor in the overall cost of ACLR with meniscus repair.
Keywords: value-driven outcomes, value-based care, meniscus repair, allograft, hybrid graft
Anterior cruciate ligament reconstruction (ACLR) is one of the most frequent arthroscopic procedures performed in the United States, with more than 130,000 cases each year.24 Additionally, the incidence of ACLRs increased from 40.9 cases per 10,000 patients in 2004 to 47.8 in 2009 and is likely to continue to increase.20 As a high-volume procedure, ACLR has previously been shown to be cost-effective.13,22,26 Mather et al27 estimated the lifetime cost to society of a typical ACLR procedure to be just over US$50,000 less costly and more effective based on quality-adjusted life years than rehabilitation alone. In an attempt to decrease costs, many health care systems are currently shifting services to value-based care models. As a consequence, the value of surgical procedures, or the outcome per dollars spent, becomes an important metric to consider in high-volume procedures such as ACLR. A number of studies have identified factors related to the cost of ACLR, including graft type, surgical timing, and nonoperative versus surgical management.2,12,15,19,26,27,31 A recent systematic review of ACLR economic studies concluded that early single-bundle, single-incision, outpatient ACLR using either a bone–patellar tendon–bone or hamstring autograft provides the most value to the health care system based on the current literature.31
As the cost of health care in the United States has continued to rise,30 the Centers for Medicare & Medicaid Services (CMS) has made efforts to decrease costs to the health care system and incentivize quality of care. A bundled care system has been estimated to have the potential to save the overall health care system as much as 5%.16 The orthopaedic community has recently become one of the first surgical specialties to test a bundled payment system for total joint replacement, certain spine procedures, and hip fracture surgery under the CMS Bundled Payments for Care Improvement (BPCI) initiative.5,6,30 The CMS has expressed interest in expanding the initiative to the outpatient setting as well.
Moving to a bundled care system means health care entities must take an objective look at factors that add to the direct cost of procedures and find ways to decrease costs and optimize outcomes throughout the episode of care. Recently, a tool called value-driven outcomes (VDO) has been developed at the University of Utah; it uses customizable cost methods to obtain accurate direct costs of a patient care episode. The VDO tool works by applying these methods to general ledgers of a health care system to identify costs related to direct patient care for an individualized patient encounter.18 The data created by this tool can be combined with clinical data from the electronic medical record to accurately investigate factors influencing the overall direct cost of a specified surgical procedure. The implementation of the VDO tool has been shown to reduce costs and improve quality within a large health care system.21
Considerable variance in the cost of ACLR exists throughout the country. For example, 1 study showed a nearly 12-fold difference in costs among surgeons in a single practice setting.1 Facility fees may also vary depending on the location of the performed ACLR procedure; ambulatory centers, for example, have been shown to decrease ACLR costs by as much as $1371 to $7390.11 While some degree of cost variance is to be expected based on specific patient and surgeon factors, it is a worthwhile exercise to analyze the overall cost of procedures within a health care system and to work toward identifying factors that may contribute to cost outliers. The major factors found to influence the cost of ACLR are thought to be operating room (OR) time and use of an allograft.2,3,9,15,28 Concomitant procedures such as meniscectomy or meniscus repair could theoretically increase costs; however, little has been written on the economics of additional procedures. Interestingly, Bonsell3 found no significant increase in cost or OR time with concomitant meniscectomy or meniscus repair.
Patient-specific factors can also influence the overall cost of care in the delivery of certain outpatient procedures. Tashjian et al32 showed that a high body mass index (BMI) and pre-existing comorbidities led to a higher cost in outpatient rotator cuff repair. To our knowledge, the role of patient-specific factors in ACLR costs has not been previously studied. It is possible that factors such as increased medical comorbidities or BMI, as was seen with rotator cuff repair, may complicate anesthesia or technical aspects of the procedure, leading to an increased OR time and increased cost of the procedure. If identified, efforts could be directed to modify the predictors of increased costs preoperatively. The purpose of this study was therefore to use the VDO tool to identify significant patient- and procedure-specific factors influencing the overall direct cost for a large cohort of outpatient ACLRs in a single orthopaedic-specific ambulatory surgery center. We hypothesized that patient- and procedure-specific factors would significantly influence the cost of ACLR.
Methods
Institutional review board approval was granted for this retrospective chart review at the University of Utah (#00071733). A search of all patients who underwent ACLR by 4 sports fellowship–trained orthopaedic surgeons (R.T.B., P.E.G., T.G.M., S.K.A.) at a single university hospital–based outpatient orthopaedic center was conducted between May 2014 and May 2016. The facility performs mainly outpatient surgery but is licensed as a hospital as opposed to an ambulatory surgery center. Current Procedural Terminology (CPT) code 29888 was used to query the VDO database to identify the patient cohort.
The University of Utah developed this unique VDO tool based on over 1 million unique data points derived from the electronic medical record.18 The VDO tool allocates general ledger expenditures to individual encounters within the health care system and reports total direct costs as a sum of the following categories: facility utilization cost, imaging, implant, laboratory, other services, pharmacy, and supply direct costs. Its use and validation have been previously reported and take into account both direct measures of items utilized during a patient encounter and a time-based assessment of labor for each cost center.21 This tool reports actual dollar amounts for pharmaceuticals and medical devices, but they cannot be published as they are the result of internal contracts with vendors. Therefore, in this study, we report cost data as a percentage relative to a defined reference value.
After the patient cohort was identified, a retrospective chart review was conducted. We examined patient-specific variables that could potentially influence costs, including age, BMI, medical comorbidities (cardiovascular, pulmonary, diabetic, alcohol or other substance abuse, anxiety, depression or other psychological disorder, or other metabolic disorder), American Society of Anesthesiologists (ASA) classification, smoking status, preoperative Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function Computerized Adaptive Testing (PF-CAT) score, and preoperative Single Assessment Numeric Evaluation (SANE) score. We also examined procedure-specific variables that could also potentially influence costs, including graft type, revision status, associated injuries, associated procedures, time from injury to ACLR, surgeon, and OR time.
Study inclusion criteria included the primary CPT code 29888 and both primary and revision single-bundle ACLRs. Associated procedures of chondroplasty, microfracture, osteochondral autograft transfer, meniscectomy, meniscus repair, removal of hardware, and bone grafting were included in this cohort, as their impact on the overall cost was of interest in this study. Exclusion criteria included multiligamentous knee injuries requiring either repair or reconstruction of intra- or extra-articular ligaments of the knee beyond ACLR. Utilized allografts included the gracilis, semitendinosus, tibialis anterior, tibialis posterior, and Achilles tendons from a single vendor and were all fresh, nonirradiated grafts. Utilized autografts included the hamstring and bone–patellar tendon–bone. In cases in which the treating surgeon determined the harvested autograft to be of insufficient diameter, the graft was augmented with a single hamstring allograft. These cases were separately categorized as a hybrid graft type.
The data from the VDO tool were from the episode of care that began and concluded on the day of surgery. There were no hospital admissions in this cohort of patients after surgery. Subsequent visits, rehabilitation, and complications requiring further treatment were not included in this cost analysis. Variables examined from the VDO database included the total direct cost of the case and are reported in US dollars as direct costs, defined as the costs of supplies and staff involved in the entire episode of care. The patient- and procedure-specific variables were then compared with the overall direct cost of the case to identify significant cost drivers within this cohort of outpatient ACLRs.
Statistical Analysis
Descriptive statistics including counts and percentages were utilized to summarize the patient demographic and clinical characteristics. Multivariate analyses were used to identify the predictors of costs for ACLR. A stepwise generalized linear model with a logarithmic link function was applied to account for the skewness of the cost distribution. The variance function was specified as gamma or Gaussian based on the modified Park test. Independent variables included age, BMI, medical comorbidities, ASA classification, smoking status, revision status, preoperative PROMIS PF-CAT score, preoperative SANE score, time from injury to ACLR, graft type, OR time (in minutes), surgeon, and associated injuries and associated procedures. Statistical significance of the multivariate analysis was set at P ≤ .05. A multivariate analysis of the overall cohort was utilized to identify predictors of the total direct cost, and a separate multivariate analysis of a cohort of ACLRs with a meniscal tear (167 isolated ACLRs, 97 ACLRs with meniscus repair) was utilized to examine the relationship between meniscus repair technique and total direct cost.
Results
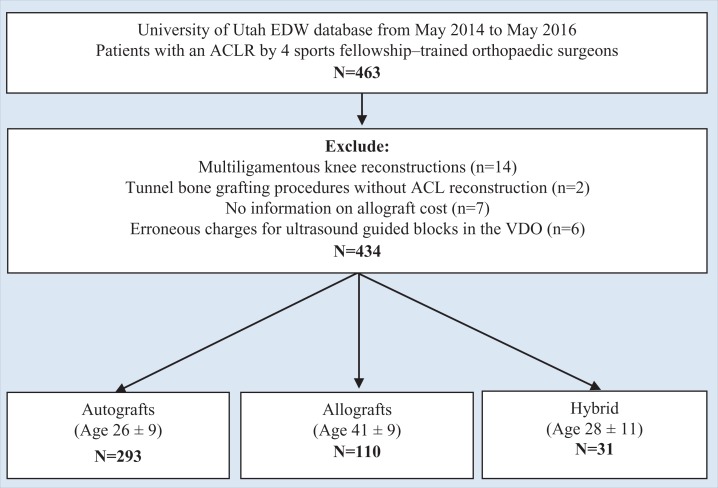
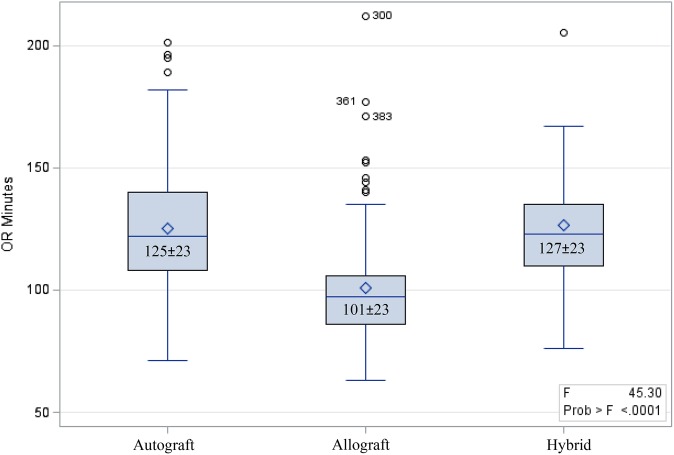
Figure 1 illustrates the patient selection in the electronic data warehouse. A total of 463 ACLRs were performed in our facility during the study period. Twenty-nine were excluded from the analysis; 14 were multiligamentous knee reconstructions, 2 were bone grafting procedures without ACLR that were incorrectly categorized in the facility’s coding database, 7 had absent allograft costs, and 6 had erroneous charges for ultrasound-guided blocks in the VDO tool that could not be adjudicated to verify correct charges. This left 394 primary and 40 revision ACLRs, for a total of 434 cases performed by 4 sports fellowship–trained orthopaedic surgeons. There were 293 autograft reconstructions with a mean patient age of 26 ± 9 years, 110 allograft reconstructions with a mean patient age of 41 ± 9 years, and 31 hybrid graft reconstructions with a mean patient age of 28 ± 11 years. The mean total OR time was significantly shorter for allografts (101 minutes) versus autografts (125 minutes) and hybrid grafts (127 minutes) (P < .001, 1-way analysis of variance) (Figure 2). Table 1 summarizes patient demographic and procedure characteristics. ACLRs were performed on normal weight (43.3%), nonsmoking patients (92.4%) who were under 30 years old (53.5%) with an ASA classification of 1 (71.4%) and no medical comorbidities (88.7%).
Figure 1.
Flowchart of patient selection in the electronic data warehouse. ACL, anterior cruciate ligament; ACLR, anterior cruciate ligament reconstruction; EDW, electronic data warehouse; VDO, value-driven outcomes.
Figure 2.
Mean operating room (OR) time (in minutes) by graft type.
TABLE 1.
Descriptive Statistics on Patient and Procedure Characteristics (N = 434)a
| n (%) | |
|---|---|
| Age | |
| <30 y | 232 (53.5) |
| ≥30 y | 202 (46.5) |
| Body mass index | |
| Underweight | 8 (1.8) |
| Normal weight | 188 (43.3) |
| Overweight | 148 (34.1) |
| Obese | 90 (20.7) |
| Medical comorbidities | 50 (11.3) |
| Smoking status | |
| Nonsmoker | 401 (92.4) |
| Smoker | 33 (7.6) |
| ASA classification | |
| 1 | 310 (71.4) |
| 2 | 122 (28.1) |
| 3 | 2 (0.5) |
| Time to surgery | |
| <10 wk | 245 (56.5) |
| ≥10 wk | 189 (43.6) |
| Surgeon | |
| 1 | 137 (31.6) |
| 2 | 32 (7.4) |
| 3 | 119 (27.4) |
| 4 | 146 (33.6) |
| Graft type | |
| Autograft | 293 (67.5) |
| Allograft | 110 (25.4) |
| Hybrid | 31 (7.1) |
| Associated injuries | |
| Lateral collateral ligament injury | 7 (1.6) |
| Medial collateral ligament injury | 53 (12.2) |
| Posterior cruciate ligament injury | 1 (0.2) |
| Meniscal injury | 267 (61.5) |
| Chondral injury | 1 (0.2) |
| Associated procedures | |
| Meniscus repair | 97 (22.4) |
| Microfracture | 7 (1.6) |
| Chondroplasty | 3 (0.7) |
| Osteochondral autograft transfer | 3 (0.7) |
| Meniscectomy | 143 (33.0) |
| Bone grafting | 1 (0.2) |
| Removal of hardware | 10 (2.3) |
| Meniscus repair technique (n = 97) | |
| Inside out | 20 (4.6) |
| All inside | 58 (13.4) |
| Root | 8 (1.8) |
| Combined | 11 (2.5) |
aASA, American Society of Anesthesiologists.
Table 2 presents the results from the multivariate analysis of the overall cohort on the total direct cost. None of the patient-specific variables were a predictor of direct costs. Procedure-specific predictors of an increased direct cost included the use of an allograft (44.5% increase; P < .001) or hybrid graft (33.1% increase; P < .001) compared with an autograft, as well as increased OR time (0.3% increase per minute; P < .001). There was a significant variation across surgeons with regard to the total cost. ACLR performed by surgeon 3 was associated with a 9.1% increase in costs (P < .001), and ACLR performed by surgeon 4 was associated with a 5.9% increase in costs (P = .001) compared with surgeon 1. Concomitant meniscus repair was associated with a 24.4% increase in costs (P < .001).
TABLE 2.
Results From Multivariate Analysis of Total Cohort on Total Direct Cost (N = 434)a
| Coefficient | % Change | Standard Error | P Value | |
|---|---|---|---|---|
| Age | ||||
| <30 y (reference) | ||||
| ≥30 y | 0.03 | 2.9 | 56 | .076 |
| Surgeon | ||||
| 1 (reference) | ||||
| 2 | 0.00 | 0.1 | 91 | .956 |
| 3 | 0.09 | 9.1 | 59 | <.001 |
| 4 | 0.06 | 5.9 | 60 | .001 |
| Graft type | ||||
| Autograft (reference) | ||||
| Allograft | 0.37 | 44.5 | 68 | <.001 |
| Hybrid | 0.29 | 33.1 | 92 | <.001 |
| Associated procedure | ||||
| Meniscus repair | 0.22 | 24.4 | 63 | <.001 |
| Microfracture | –0.17 | –15.5 | 177 | .001 |
| Removal of hardware | –0.08 | –7.6 | 152 | .068 |
| Other (chondroplasty, osteochondral autograft transfer, or bone grafting) | 0.15 | 15.9 | 178 | .003 |
| Operating room time | 0.00 | 0.3 | 1 | .000 |
aBody mass index, smoking status, revision status, American Society of Anesthesiologists classification, side of surgery, time from injury to surgery, medical comorbidities, associated injuries, associated procedure of meniscectomy, preoperative Patient-Reported Outcomes Measurement Information System Physical Function Computerized Adaptive Testing score, and preoperative Single Assessment Numeric Evaluation score were dropped from the stepwise analysis.
The results from the multivariate analysis of the meniscal tear cohort found that isolated ACLRs with a meniscal tear without repair (treated by meniscectomy, trephination, or no treatment) were associated with a 22.3% decrease in costs (P < .001) compared with the inside-out repair group. All-inside, root, and combined repairs correlated with a 15.5%, 31.4%, and 53.2% increased mean direct cost, respectively, compared with inside-out repairs (P < .001) (Table 3). In addition, when all-inside repairs were grouped based on the number of implants inserted, each repair implant added significant additional costs (P < .001). Other concomitant procedures (chondroplasty, osteochondral autograft transfer, bone grafting of tunnels with concomitant ACLR, microfracture) all occurred in such a low frequency that reporting an analysis of these was not felt to be meaningful.
TABLE 3.
Results From Multivariate Analysis of Patients With Meniscal Tears on Total Direct Cost (n = 264)
| Coefficient | % Change | Standard Error | P Value | |
|---|---|---|---|---|
| Meniscus repair technique | ||||
| Inside out (reference) | ||||
| All inside | 0.14 | 15.5 | 141 | <.001 |
| Root | 0.27 | 31.4 | 214 | <.001 |
| Combined | 0.43 | 53.2 | 254 | <.001 |
| No meniscus repair | –0.25 | –22.3 | 201 | <.001 |
| Surgeon | ||||
| 1 (reference) | ||||
| 2 | –0.02 | –1.6 | 123 | .640 |
| 3 | 0.11 | 12.0 | 84 | <.001 |
| 4 | 0.09 | 9.7 | 85 | <.001 |
| Graft type | ||||
| Autograft (reference) | ||||
| Allograft | 0.35 | 42.0 | 83 | <.001 |
| Hybrid | 0.25 | 29.0 | 127 | <.001 |
| Associated procedure | ||||
| Meniscus repair | –0.11 | –10.0 | 168 | .023 |
| Operating room time | 0.00 | 0.1 | 1 | .033 |
Discussion
A new finding of this investigation is the significant influence of concomitant procedures, particularly meniscus repair, on the direct cost of ACLR. Approximately 25% of meniscal tears are reported to be repaired at the time of ACLR.29 This study’s figure was slightly lower, with a 22.4% concomitant meniscus repair rate. Because of high repair rates, identifying meniscus repair techniques as independent predictors of increased costs becomes an important part of the equation in evaluating the cost of ACLR. In contrast to Bonsell,3 who found no significant difference in time (+4.5 vs +7 minutes, respectively) or cost with concomitant meniscectomy versus repair, we found that meniscus repair did add costs to ACLR. Inside-out repair added the least additional cost, while all-inside meniscus repair increased the cost nearly 16% compared with the traditional inside-out technique without a significant time-saving advantage.
In addition to costs, one must consider the effect that repair techniques have on healing rates and patient recovery. The literature would suggest no clear difference in healing rates of inside-out versus all-inside techniques.14 Therefore, from a cost and healing perspective, one would advocate for the inside-out technique. However, there is arguably some increased local morbidity associated with traditional inside-out meniscus repair and the incisions associated with it. Without clear differences in healing rates, the decreased morbidity and ease of use of an all-inside device may be justified despite the increase in costs. Additionally, as each implant used with the all-inside technique added additional significant costs, one could consider a selective approach to its use, in which tear size might dictate the use of only 1 or 2 implants.
This study did not characterize specific tear patterns, tear size, or number of sutures required to complete the repair, and there could be situations in which a smaller tear could be cost-effectively treated with the all-inside technique, which is deserving of further study. Furthermore, root repair was found to correlate with a 31.4% increase in costs compared with inside-out repair, which is a not insignificant amount. Although not specifically studied, the increase in costs is most likely related to the use of a proprietary disposable retrocutter drill to create a bony socket to allow for meniscal root healing, as healing to articular cartilage cannot be expected. The potential for alternative techniques to eliminate this added cost is deserving of attention.
A second important finding in this study was the failure to identify modifiable patient-specific variables contributing to increased ACLR costs in the ambulatory setting. To date, many studies have investigated surgeon or institutional factors influencing the cost of ACLR, but no study has looked at potential patient-specific variables influencing the cost of ACLR. Recently, Tashjian et al32 identified factors driving the direct cost of rotator cuff surgery. While the driving variable of costs appeared to be implant related, the authors found that a higher BMI and systemic illness were associated with increases in the facility cost and that systemic illness was associated with an increased pharmacy cost.32
Conceptually, sicker patients may influence cost increases through more intensive management by the anesthesia team, possibly leading to increased pharmacy requirements, increased time for induction or wake-up, or increased postanesthesia care unit time. While the majority of the patients in this study were of a low ASA classification (71.4% ASA 1) and without medical comorbidities (88.7%), we failed to identify any association of health markers influencing the cost of ACLR. Given the age and activity of patients requiring or desiring ACLR, this perhaps is not surprising. The cohort was largely healthy, and patients with a severe systemic risk, which would likely lead to increased costs, were not offered ACLR, chose not to undergo ACLR, or had a lower incidence of ACL tears.
Our results are similar to those found in previous investigations regarding the use of allografts in ACLR. We found, on average, a 44.5% increase in the total direct cost in allograft cases and a 33.1% increase in hybrid graft cases versus autografts. This was despite a significant time-saving reduction of a mean 24 minutes in the allograft cases. Multiple studies have identified that the time saved with allograft use is not offset by the cost of allograft tissue.2,9,12,15,28 However, Greis et al15 demonstrated an overall higher reimbursement for allograft cases, which effectively offset the cost of allograft tissue. This study did not investigate third-party reimbursement and cannot determine if current reimbursement schedules remain cost-effective with the use of allografts. Cole et al7 did report significantly lower costs in allograft cases; however, a high proportion of the autografts in that series had an inpatient stay, which can account for the increased costs not observed in previous investigations. In our study, all patients were discharged the day of surgery, and allografts demonstrated a clear association with increased direct costs.
The addition of an allograft to augment a hamstring autograft was also associated with increased costs. The use of a single hamstring tendon allograft to augment an autograft of insufficient size has been the practice for some surgeons at our center after reports emerged of increased failure of small-diameter hamstring autografts (<8 mm).8,23,25 In this series, a 33% increase in direct costs was found when a single hamstring allograft was used to augment small-diameter allografts. A recent study by Jacobs et al17 suggested that augmenting small-diameter hamstring autografts can decrease the higher failure rates of small-diameter grafts and have an incremental cost savings of $2765 compared with the hamstring autograft when considering the cost of revision. While our study identified hybrid grafts as a predictor of increased initial costs, we did not investigate revision rates or the cost of revision and cannot determine if the additional cost of the allograft was cost-effective in reducing the overall rate of revisions of small-diameter grafts. Others have found failure rates to be increased by the addition of allografts for small-diameter autografts.4,10 Future cost-effectiveness studies will be needed to determine if these findings of Jacobs et al17 remain true.
In this study, no increase in costs was seen in revision ACLR cases. This is somewhat surprising, as one would expect revision cases to be, on average, more complicated than primary cases and at the very least increase OR time, a primary factor in the cost of ACLR. However, if the revisions were in large part uncomplicated, the need for additional time or hardware would be limited and would not result in significant increases in the cost. This finding may also be partly due to our not counting staged procedures in which initial bone grafting had been performed at an earlier date. A more detailed analysis would be needed to directly compare primary versus revision cases, looking specifically at staged procedures to see if this remains true when including prior costs of bone grafting.
The cost differences that were seen for various surgeons were likely multifactorial. The multivariate analysis showed that the operating surgeon was an independent factor in costs, even after controlling for other variables. As each surgeon was able to choose implants, had his or her own pace of surgery, made decisions about spending time teaching, and made graft selection choices, this combination led to greater or fewer expenses, on average. As the primary goal during the time period in which this cohort of patients was operated on was not to be as inexpensive as possible, and because long-term outcomes were not examined in this cohort, no value judgment can be made other than physician choices, and technique can be a driver of costs.
The strengths of this study include a large cohort of patients among a 4-surgeon group and the implementation of an accurate and validated measure of direct costs from a hospital system perspective.18,21 Multiple variables related to both the patient and the surgeon were analyzed to determine the factors related to the direct cost of ACLR that had not been previously reported.
Several limitations do exist. First, because the focus of our study was on the cost of surgery and not surgical outcomes, we cannot comment on the cost-effectiveness of different ACLR procedures or the role that potential complications or revisions might play in the overall cost. The fact that no long-term outcome data were evaluated means that the overall effectiveness of treatment cannot be commented on. However, as ACLR has previously been determined to be a highly cost-effective procedure,13,22,26 the primary goal of this investigation was to isolate potentially modifiable variables affecting costs. In the longer term, partnering this VDO tool with patient-reported outcome data will hopefully enable us to provide a true sense of value of a surgical procedure. Second, we report only on the experience within a narrow 2-year time frame among a 4-surgeon single-institution group with a focus on cost containment. Variance in techniques, vendor contracts, or patient characteristics among different institutions may influence the relationships found in this study. Third, this was a rather homogenous, healthy population with minimal systemic illness. While no association of patient health and cost was found, patient cohorts with increasing medical complexity may identify associations not found in our investigation.
Conclusion
This study failed to identify modifiable patient-specific factors influencing the direct cost of ACLR. Allografts and hybrid grafts were associated with an increased total direct cost. Meniscus repair independently predicted increased direct costs, with all-inside, root, and combined repairs being costlier than inside-out repairs. The time-saving potential of all-inside meniscus repair was not realized in this study, making implant use a significant factor in the overall cost of ACLR with meniscus repair.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: T.G.M. has received educational support from Aesculap and Arthrex. S.K.A. is a consultant for Pivot Medical and Stryker and has received educational support from Aesculap. R.T.B. receives royalties from Arthrex and is a consultant for DePuy.
Ethical approval for this study was obtained from the University of Utah Institutional Review Board.
References
- 1. Archibald-Seiffer N, Jacobs JC, Jr, Saad C, Jevsevar DS, Shea KG. Review of anterior cruciate ligament reconstruction cost variance within a regional health care system. Am J Sports Med. 2015;43(6):1408–1412. [DOI] [PubMed] [Google Scholar]
- 2. Barrera Oro F, Sikka RS, Wolters B, et al. Autograft versus allograft: an economic cost comparison of anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(9):1219–1225. [DOI] [PubMed] [Google Scholar]
- 3. Bonsell S. Financial analysis of anterior cruciate ligament reconstruction at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2000;13(4):327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burrus MT, Werner BC, Crow AJ, et al. Increased failure rates after anterior cruciate ligament reconstruction with soft-tissue autograft-allograft hybrid grafts. Arthroscopy. 2015;31(12):2342–2351. [DOI] [PubMed] [Google Scholar]
- 5. Bushnell BD. Bundled payments in orthopedic surgery. Orthopedics. 2015;38(2):128–135. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Medicare & Medicaid Services. Bundled Payments for Care Improvement (BPCI) initiative. Available at: https://innovation.cms.gov/initiatives/bundled-payments. Accessed June 12, 2017.
- 7. Cole DW, Ginn TA, Chen GJ, et al. Cost comparison of anterior cruciate ligament reconstruction: autograft versus allograft. Arthroscopy. 2005;21(7):786–790. [DOI] [PubMed] [Google Scholar]
- 8. Conte EJ, Hyatt AE, Gatt CJ, Jr, Dhawan A. Hamstring autograft size can be predicted and is a potential risk factor for anterior cruciate ligament reconstruction failure. Arthroscopy. 2014;30(7):882–890. [DOI] [PubMed] [Google Scholar]
- 9. Cooper MT, Kaeding C. Comparison of the hospital cost of autograft versus allograft soft-tissue anterior cruciate ligament reconstructions. Arthroscopy. 2010;26(11):1478–1482. [DOI] [PubMed] [Google Scholar]
- 10. Darnley JE, Leger-St-Jean B, Pedroza AD, Flanigan DC, Kaeding CC, Magnussen RA. Anterior cruciate ligament reconstruction using a combination of autograft and allograft tendon: a MOON cohort study. Orthop J Sports Med. 2016;4(7):2325967116662249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrari D, Lopes TJ, Franca PF, Azevedo FM, Pappas E. Outpatient versus inpatient anterior cruciate ligament reconstruction: a systematic review with meta-analysis. Knee. 2017;24(2):197–206. [DOI] [PubMed] [Google Scholar]
- 12. Genuario JW, Faucett SC, Boublik M, Schlegel TF. A cost-effectiveness analysis comparing 3 anterior cruciate ligament graft types: bone-patellar tendon-bone autograft, hamstring autograft, and allograft. Am J Sports Med. 2012;40(2):307–314. [DOI] [PubMed] [Google Scholar]
- 13. Gottlob CA, Baker CL, Jr, Pellissier JM, Colvin L. Cost effectiveness of anterior cruciate ligament reconstruction in young adults. Clin Orthop Relat Res. 1999;(367):272–282. [PubMed] [Google Scholar]
- 14. Grant JA, Wilde J, Miller BS, Bedi A. Comparison of inside-out and all-inside techniques for the repair of isolated meniscal tears: a systematic review. Am J Sports Med. 2012;40(2):459–468. [DOI] [PubMed] [Google Scholar]
- 15. Greis PE, Koch BS, Adams B. Tibialis anterior or posterior allograft anterior cruciate ligament reconstruction versus hamstring autograft reconstruction: an economic analysis in a hospital-based outpatient setting. Arthroscopy. 2012;28(11):1695–1701. [DOI] [PubMed] [Google Scholar]
- 16. Hussey PS, Eibner C, Ridgely MS, McGlynn EA. Controlling U.S. health care spending: separating promising from unpromising approaches. N Engl J Med. 2009;361(22):2109–2111. [DOI] [PubMed] [Google Scholar]
- 17. Jacobs CA, Burnham JM, Makhni E, Malempati CS, Swart E, Johnson DL. Allograft augmentation of hamstring autograft for younger patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(4):892–899. [DOI] [PubMed] [Google Scholar]
- 18. Kawamoto K, Martin CJ, Williams K, et al. Value driven outcomes (VDO): a pragmatic, modular, and extensible software framework for understanding and improving health care costs and outcomes. J Am Med Inform Assoc. 2015;22(1):223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kiadaliri AA, Englund M, Lohmander LS, Carlsson KS, Frobell RB. No economic benefit of early knee reconstruction over optional delayed reconstruction for ACL tears: registry enriched randomised controlled trial data. Br J Sports Med. 2016;50(9):558–563. [DOI] [PubMed] [Google Scholar]
- 20. Leathers MP, Merz A, Wong J, Scott T, Wang JC, Hame SL. Trends and demographics in anterior cruciate ligament reconstruction in the United States. J Knee Surg. 2015;28(5):390–394. [DOI] [PubMed] [Google Scholar]
- 21. Lee VS, Kawamoto K, Hess R, et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316(10):1061–1072. [DOI] [PubMed] [Google Scholar]
- 22. Lubowitz JH, Appleby D. Cost-effectiveness analysis of the most common orthopaedic surgery procedures: knee arthroscopy and knee anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(10):1317–1322. [DOI] [PubMed] [Google Scholar]
- 23. Magnussen RA, Lawrence JT, West RL, Toth AP, Taylor DC, Garrett WE. Graft size and patient age are predictors of early revision after anterior cruciate ligament reconstruction with hamstring autograft. Arthroscopy. 2012;28(4):526–531. [DOI] [PubMed] [Google Scholar]
- 24. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363–2370. [DOI] [PubMed] [Google Scholar]
- 25. Mariscalco MW, Flanigan DC, Mitchell J, et al. The influence of hamstring autograft size on patient-reported outcomes and risk of revision after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) cohort study. Arthroscopy. 2013;29(12):1948–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mather RC, 3rd, Hettrich CM, Dunn WR, et al. Cost-effectiveness analysis of early reconstruction versus rehabilitation and delayed reconstruction for anterior cruciate ligament tears. Am J Sports Med. 2014;42(7):1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mather RC, 3rd, Koenig L, Kocher MS, et al. Societal and economic impact of anterior cruciate ligament tears. J Bone Joint Surg Am. 2013;95(19):1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagda SH, Altobelli GG, Bowdry KA, Brewster CE, Lombardo SJ. Cost analysis of outpatient anterior cruciate ligament reconstruction: autograft versus allograft. Clin Orthop Relat Res. 2010;468(5):1418–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noyes FR, Barber-Westin SD. Treatment of meniscus tears during anterior cruciate ligament reconstruction. Arthroscopy. 2012;28(1):123–130. [DOI] [PubMed] [Google Scholar]
- 30. Rana AJ, Bozic KJ. Bundled payments in orthopaedics. Clin Orthop Relat Res. 2015;473(2):422–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saltzman BM, Cvetanovich GL, Nwachukwu BU, Mall NA, Bush-Joseph CA, Bach BR., Jr Economic analyses in anterior cruciate ligament reconstruction: a qualitative and systematic review. Am J Sports Med. 2016;44(5):1329–1335. [DOI] [PubMed] [Google Scholar]
- 32. Tashjian RZ, Belisle J, Baran S, et al. Factors influencing direct clinical costs of outpatient arthroscopic rotator cuff repair surgery. J Shoulder Elbow Surg. 2018;27(2):237–241. [DOI] [PubMed] [Google Scholar]