Abstract
Parkinson’s Disease (PD) is a long-term neurodegenerative brain disorder that mainly affects the motor system. The causes are still unknown, and even though currently there is no cure, several therapeutic options are available to manage its symptoms. The development of novel anti-parkinsonian agents and an understanding of their proper and optimal use are, indeed, highly demanding. For the last decades, L-3,4-DihydrOxyPhenylAlanine or levodopa (L-DOPA) has been the gold-standard therapy for the symptomatic treatment of motor dysfunctions associated to PD. However, the development of dyskinesias and motor fluctuations (wearing-off and on-off phenomena) associated with long-term L-DOPA replacement therapy have limited its antiparkinsonian efficacy. The investigation for non-dopaminergic therapies has been largely explored as an attempt to counteract the motor side effects associated with dopamine replacement therapy. Being one of the largest cell membrane protein families, G-Protein-Coupled Receptors (GPCRs) have become a relevant target for drug discovery focused on a wide range of therapeutic areas, including Central Nervous System (CNS) diseases. The modulation of specific GPCRs potentially implicated in PD, excluding dopamine receptors, may provide promising non-dopaminergic therapeutic alternatives for symptomatic treatment of PD. In this review, we focused on the impact of specific GPCR subclasses, including dopamine receptors, adenosine receptors, muscarinic acetylcholine receptors, metabotropic glutamate receptors, and 5-hydroxytryptamine receptors, on the pathophysiology of PD and the importance of structure- and ligand-based in silico approaches for the development of small molecules to target these receptors.
Keywords: Parkinson’s disease, G-protein-coupled receptors, drug design, ligand-docking, quantitative structure-activity relationships, pharmacophore
1. INTRODUCTION
Parkinson’s Disease (PD) was originally described by James Parkinson, in 1817, as a neurological disturbance consisting of resting tremor and a distinctive form of the progressive motor disorder, designated as shaking palsy or paralysis agitans in his monograph entitled An Essay On the Shaking Palsy [1]. Currently, it is considered the second most common neurodegenerative disorder after Alzheimer’s Disease (AD), affecting approximately 1% of the population worldwide over 55 years old. PD has been defined as a progressive, irreversible, and chronic neurological disorder characterized by increasingly disabling motor symptoms that are associated to impaired coordinated movements including bradykinesia (slowness of initiation of voluntary movements), resting tremor, cogwheel rigidity, postural instability, and gait disorders [2-4]. In addition, the majority of PD patients do not suffer from motor disabilities alone and numerous non-motor symptoms may lead to a decrease in the quality of life in patients: cognitive impairment, hallucinations, psychosis, anxiety, and depression [5, 6]. Another frequent anomalies related to autonomic (gastrointestinal and cardiovascular), sensory and Rapid Eye Movement (REM) and sleep behaviour dysfunctions are also clinically manifested in PD patients. Despite decades of comprehensive study and knowledge concerning the etiology and pathogenesis of PD, much has yet to be discovered in order to understand the pathophysiological mechanisms that contribute to the neuronal cell death (neurodegeneration) in PD. Although normal aging represents the most important risk factor, a combination of environmental (e.g. exposure to pesticides and herbicides, toxins, and organic solvents) and genetic factors may contribute to the onset of PD [7]. Two distinctive pathological manifestations have been associated to the clinical diagnosis of PD in post-mortem patients, including the selective and progressive degeneration of dopaminergic neuromelanin-containing neurons from the Substantia Nigra pars compacta (SNc) of the midbrain and striatum of the brain and the presence of Lewy bodies, intraneuronal inclusions of presynaptic protein α-synuclein in brain neurons [2-6].
The reduction of dopamine levels and the loss of SNc dopaminergic neurons have shown to influence directly the appearance of motor dysfunctions associated to PD in 6-HydroxyDopamine (6-OHDA)- and 1-Methyl-4-Phenyl-1,2,3,6-TetrahydroPyridine (MPTP)-treated animals. Since the degree of SNc dopaminergic neurodegeneration correlates positively with the severity of PD, dopamine replacement therapies have become the most effective therapeutic alternative to ameliorate daily function, quality of life, and survival in PD patients. However, the therapeutic strategy of direct administration of dopamine itself is not feasible, due to their inability to cross the Blood-Brain Barrier (BBB) [8]. An alternative BBB-permeable dopamine precursor, L-3,4-DihydrOxyPhenylAlanine or levodopa (L-DOPA), was conceived to effectively enhance dopamine concentration in the Central Nervous System (CNS). The antiparkinsonian effects of L-DOPA were first described by Carlsson and co-workers in reserpine-treated animals [9] and, later, in human PD patients after intravenous [10] and oral administration of low doses of L-DOPA [11]. Currently, L-DOPA is considered the most effective medication for correcting dopamine deficiency in PD, significantly attenuating the motor symptoms in human patients. Upon administration to systemic circulation, L-DOPA is converted into dopamine through a decarboxylation catalyzed by the naturally occurring enzyme Aromatic L-Amino acid DeCarboxylase (AADCs; EC 4.1.1.28) in both the CNS and Peripheral Nervous System (PNS). An excessive production of dopamine in the periphery can contribute to severe side effects that impair dopamine replacement therapy for PD patients. The administration of L-DOPA with AADC inhibitors as well as inhibitors of dopamine-metabolizing enzymes, including MonoAmine Oxidase-B (MAO-B; EC 1.4.3.4) and peripheral Catechol-O-MethylTransferase (COMT; EC 2.1.1.6) constitutes an alternative therapeutic approach to selectively increase dopamine levels in the CNS [12-14].
Despite the effectiveness of dopamine replacement therapies in symptomatic PD treatment, their clinical efficacy often decreases, particularly after chronic administration of L-DOPA, leading to the wearing-off [15, 16] and on-off phenomena [17, 18] due to oscillations of L-DOPA/drug levels, and to the development of long-term motor complications, such as the troublesome dyskinesias (involuntary muscle movements) [18, 19]. In addition, dopaminergic therapies focused on targeting dopamine receptors (DRs) with agonists have displayed favorable outcomes in early stages of PD, exhibiting antiparkinsonian effects with the lower risk of occurrence of problematic dyskinesias. DR agonists have also been used in combination with L-DOPA to delay the development of motor complications in late stages of the disease. Nevertheless, the use of DR agonists may result in non-motor complications (psychiatric disorders, nausea, vomiting, orthostatic hypotension, increased somnolence and sleep attacks, fatigue, and ankle edema) more severe than L-DOPA. Therefore, the occurrence of motor and non-motor complications associated to all types of dopamine replacement therapy suggested that the symptomatic treatment of PD focused on the re-establishment of dopaminergic neurotransmission may possess restricted therapeutic benefits for patients. Apart from dopaminergic therapies, the modulation of non-dopaminergic neurotransmission systems, including noradrenergic, cholinergic, adenosinergic, glutamatergic, and serotonergic, has been explored as alternative therapeutic approaches for symptomatic monotherapy and in combination with dopaminergic therapies. Interestingly, numerous studies have emphasized the relevance of pharmacological modulation of specific G-protein coupled receptors (GPCRs) for PD symptomatic therapy in preclinical PD animal models and clinical studies with PD patients. The present review highlights the impact of specific GPCR subclasses in the pathophysiology of PD, the structure-, and the ligand-based in silico approaches widely used in the identification of small-molecule modulators of these particular receptors.
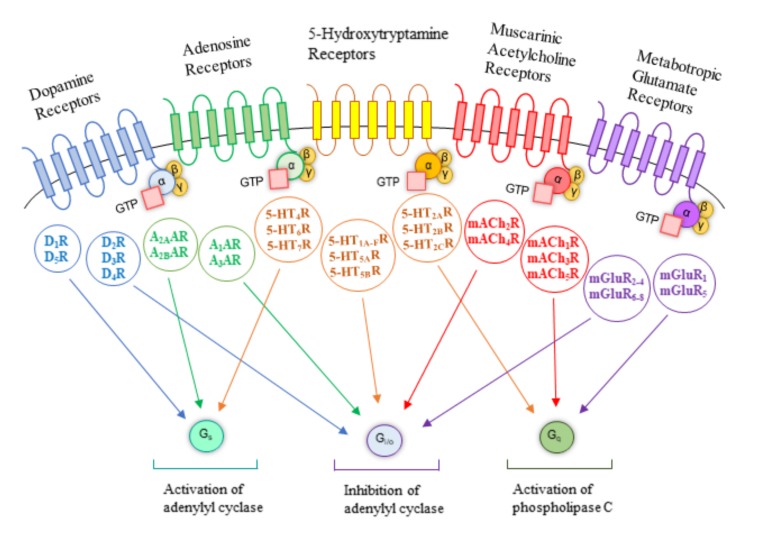
2. G-protein-coupled receptors as thera-peutic targets for Parkinson’s disease
With the increasing number of new cases per year of PD, there has been a considerable increase in the search for new therapeutic alternatives. While the research and development of promising drugs are demanding for all emerging therapeutic areas, the discovery of new therapeutic agents acting on PD and other CNS diseases has been particularly demanding and is associated to a very high attrition rate [20]. GPCRs-targeted agents represent approximately ~30-40% of currently marketed drugs for human therapeutics and these receptors have been subjected to a substantial number of computational studies [21] including as PD targets. GPCRs, also called seven TransMembrane (TM)-spanning receptors, represent the largest family of cell surface receptors of human genome and are characterized by a single polypeptide chain with a variable length that crosses the phospholipidic bilayer seven times adopting the typical structure of seven TM α-helices connected by three ExtraCellular (ECL) and three IntraCellular Loops (ICL) [22]. To date, over 800 human GPCRs have been identified and placed into five major families according to their amino acid sequence homology and phylogenetic analysis: glutamate (Class C, 22 members), rhodopsin (Class A, 672 members), adhesion (33 members), frizzled/taste2 (Class F, 36 members), and secretin (Class B, 15 members) [22]. Their members share >20% sequence identity in their TM domains and they mediate several downstream signaling pathways of physiological significance by responding to a plethora of structurally diverse ligands, particularly endogenous (biogenic amines, nucleotides, peptides, hormones, neurotransmitters, lipids, glycoproteins, and ions) and exogenous ligands (therapeutic agents, photons, tastants, and odorants) [23, 24]. The complexity of GPCR-mediated responses is determined by the association between the activated receptors and specific heterotrimeric Guanine nucleotide-binding-proteins (G-proteins). Heterotrimeric G-proteins are composed of two functional units, a Guanine-binding α-subunit (Gα) and a dimer consisting of the β- and γ- subunits (Gβγ). In the absence of a GPCR agonist, Gα is bound to Guanosine DiPhosphate (GDP) and associated with Gβγ. Agonist-mediated GPCR activation promotes conformational changes in GPCRs, contributing to the stabilization of active conformation of the receptor, and to the coupling and activation of heterotrimeric G-proteins. The coupling of GPCRs with G-proteins leads to GDP release and Guanosine TriPhosphate (GTP) binding to the Gα subunit. Subsequently, the GTP binding induces conformational alterations on Gα subunit, promoting the release of G-proteins from GPCR and the dissociation of heterotrimeric G-proteins into Gα and Gβγ subunits [25]. The Gα (Gαs, Gαi/o, Gαq, Gα12/13) and Gβγ subunits amplify and propagate their cell transduction signals through the modulation of various downstream effectors [26], including Adenylyl Cyclase (AC; EC 4.6.1.1) and PhosphoLipase C (PLC; EC 3.1.4.3), that, in turn, regulate the production of second messengers, such as Ca2+, cyclic Adenosine MonoPhosphate (cAMP), cyclic Guanosine MonoPhosphate (cGMP), DiAcylGlycerol (DAG), and Inositol-1,4,5-triPhosphate (IP3), the modulation of ion channels, and the activation of kinases cascades, triggering a wide array of cellular responses of physiological importance [27, 28] (Fig. 1). Nevertheless, not all GPCR signaling events are dependent on the activation of G-proteins. In fact, upon prolonged or repeated stimulation of GPCRs by agonists, a process of receptor desensitization induces a progressive attenuation of receptor responsiveness. Second-messenger-dependent protein kinases, Protein Kinase A (PKA; EC 2.7.11.11) and Protein Kinase C (PKC; EC 2.7.11.13), and G-protein coupled Receptor Kinases (GRKs; EC 2.7.11.14, EC 2.7.11.15, and EC 2.7.11.16) are the two families of regulatory proteins that participate in the receptor signaling desensitization, a mechanism independent of the activation of G-proteins. While second-messenger-dependent protein kinases promote phosphorylation of multiple GPCRs, suppressing agonist responsiveness to these receptors even in the absence of agonist occupation (heterologous desensitization), the recruitment of GRKs for receptor phosphorylation preferentially requires an agonist-bound conformation of GPCRs (homologous desensitization), leading to an attenuation of receptor signaling [29, 30]. GRK-dependent phosphorylation promotes GPCRs binding to a class of intracellular scaffolding proteins, β-arrestins, that sterically prevent further interactions between G-proteins and the activated receptors, causing desensitization [31] and ultimately internalization of GPCRs via clathrin-mediated endocytosis [32]. Additionally, β-arrestins are by themselves also able to stimulate different pathways, in particular, ligand-bias signaling [33]. Receptor proteolysis mediated by lysosomes [34] and GTP hydrolysis by Regulators of G-protein Signaling (RGS) proteins [35, 36] provide alternative mechanisms of GPCR downregulation. These regulatory mechanisms are critical not only for receptor desensitization but also for receptor resensitization for the next round of GPCR activation and signaling [37].
Fig. (1).
Signaling cascade of the distinct subtypes of GPCRs potentially involved in PD.
Drug discovery efforts targeting GPCRs have been made on the development of conventional agonists/antagonists that interact with the orthosteric binding site to modulate the activity of neurotransmitter receptors. However, the high conservation of orthosteric binding sites among subtypes of specific GPCR subfamilies has proven to be challenging for the design of therapeutic agents with high receptor subtype selectivity [38, 39]. Additionally, the ligands that interact with orthosteric sites for some GPCRs, in particular, peptide or protein receptors, possess physicochemical and pharmacokinetic properties that are unsuitable for drug discovery of small-molecule ligands [38, 39]. Recently, the identification of novel therapeutic agents acting as allosteric modulators of GPCRs has provided an alternative approach for the development of subtype-selective small molecules potentially useful for the treatment of CNS disorders, such as PD. Allosteric modulators interact to topographically distinct binding sites (allosteric sites) from the orthosteric sites of the endogenous ligands, to either increase (positive allosteric modulators, PAMs) or reduce (negative allosteric modulators, NAMs) receptor responsiveness to ligands. The presence of less highly conserved regions often present in allosteric sites of GPCRs enables the molecular optimization of modulators in order to achieve higher subtype selectivity [38, 39]. Overall, the exploration of allosteric sites of GPCRs for drug design is of utmost importance in medicinal chemistry, possessing several advantages including the possibility to target selective GPCR-signaling pathways without modulating others that may lead to adverse effects and to search for considerable diversity of chemical scaffolds to optimize the pharmacological profile of drug candidates (e.g. brain exposure) [38, 39].
Molecular Dynamics (MD) simulation studies have been widely employed as a useful complement to experimental methods in the determination of the atomic-level mechanisms for allostery, since these techniques can capture the motion of proteins in full atomic detail and predict the position of each atom in biomolecular systems as function of time using Newton’s second law [40]. The identification of atomic-level mechanisms for allostery is relevant not only for understanding protein function but also for the application of structure-based drug design approaches [41]. MD simulations were employed already in order to understand how allostery is responsible for modulation of the behavior of different receptors. Experimental studies comparing the action of both agonists and antagonists have shed light on conformational changes on β2AR upon binding to an irreversible agonist [42]. Another study used MD to compare the solved X-ray structure of an agonist-bound A2AAR [43] with the previously determined X-ray structure of an antagonist-bound structure of the same receptor [44]. By doing so, the researchers were able to determine how the structure was affected by both classes of orthosteric ligands and concluded that TM3, TM5, TM6, and TM7 were the most affected domains. ECL2 and ECL3 also showed considerable displacement, explained by their proximity to the binding site. Interestingly, ICL3, critical for several GPCR functions such as activation and recycling [45-47], was also shown to be considerably altered when comparing agonist- and antagonist-bound structures. This can highlight the ligand-bias mechanism and how it affects GPCR function. Only a few computational studies have addressed this problem. For example, a computational study by Dror et al. [48] using the M2 muscarinic receptor revealed that using cationic orthosteric ligands and cationic negative allosteric modulators hinders the binding of both, depending on the total charge and charge location of both ligand and modulator. This was found to be due to charge repulsion and, mostly, conformational rearrangements in orthosteric and allosteric binding sites upon allosteric modulator and orthosteric ligand binding, respectively. Additionally, the interaction between positive allosteric modulators and orthosteric ligands seem to increase binding affinity due to conformational changes as well, particularly binding site wider openings. Other computational studies addressed allostery only, such as the different conformational changes in 5-HT1A upon agonist, partial agonist and antagonist [49] – which pointed towards TM5 and TM6 as the most altered domains when comparing agonist- and antagonist-bound structures – or how buspirone, a 5-HT1A partial agonist, differently changes the 5-HT1A and 5-HT2A conformation upon binding [50]. To date, a substantial number of allosteric modulators of GPCRs have exhibited pharmacotherapeutic potential for the treatment of PD and other CNS diseases. Specifically, for PD, the development of allosteric modulators of muscarinic AcetylCholine Receptors (mAChRs) and metabotropic Glutamate Receptors (mGluRs) have demonstrated potential therapeutic applications in preclinical PD models.
2.1. Dopamine Receptors
Dopamine is an endogenous chemical belonging to catecholamine and phenethylamine families that functions as a catecolaminergic neurotransmitter and neurohormone, modulating various physiological actions on CNS such as voluntary movements, feeding, affect, reward, sleep, attention, working memory, and learning. In addition, hormonal regulation, cardiovascular functions, immune system, and renal functions are also influenced by dopamine [51, 52]. These physiological functions of dopamine are mainly mediated through activation of five subtypes of DRs, which are placed into two major classes, according to their ligand specificity, G-protein coupling, anatomical distribution, and physiological effects. The D1-like receptors, including D1R and D5R, promotes the activation of AC with concomitant stimulation of cAMP production via Gs/olf proteins, whereas the D2-like receptors, including D2R, D3R, and D4R, are preferentially coupled with Gi/o proteins to inhibit AC activity and, consequently, the production of cAMP [51, 52].
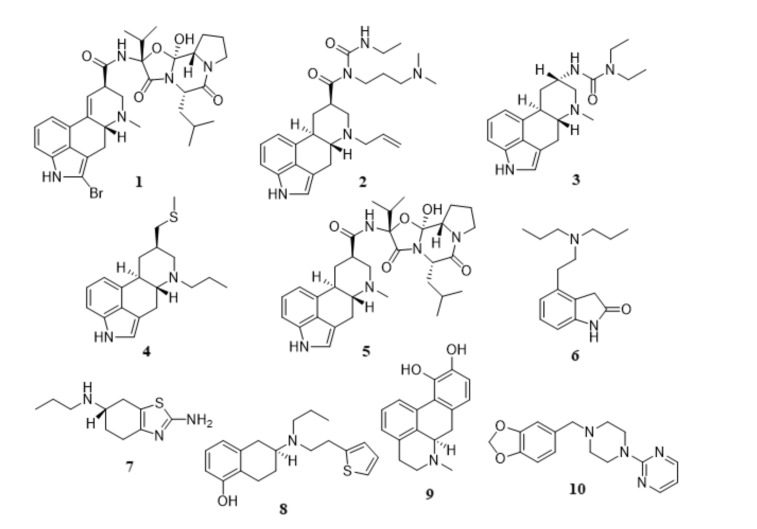
The evidence of selective loss of dopaminergic neurons in SNc as the most relevant pathological hallmark of PD has suggested that the use of dopamine replacement therapies as a therapeutic strategy to regulate dopamine levels in the brain may provide some symptomatic relief for PD patients. Despite the motor fluctuations and the occurrence of dyskinesias associated to a long-term administration of L-DOPA, alternative therapeutic opportunities have emerged as plausible approaches to counteract the prevalence of L-DOPA-induced motor complications. Apart from the combination of L-DOPA with AADC (EC 4.1.1.28), MAO-B (EC 1.4.3.4), and peripheral COMT (EC 2.1.1.6) inhibitors, the development of drug delivery systems and the use of long-acting drug candidates capable of stimulating presynaptic and postsynaptic DRs, such as the DR agonists may contribute to a more effective attenuation of motor fluctuations and reduction of induced dyskinesias. In MPTP-parkinsonian non-human primates, the administration of long-acting selective D2-like receptor agonists induced a significantly lower tendency to produce dyskinesia comparing to those treated with L-DOPA [53, 54]. In addition, DR agonists act directly on their receptors without the necessity of metabolic conversion. DR agonists may also provide a wider therapeutic window with reduced risk of dyskinesias, presumably due to their longer plasma half-lives and better pharmacokinetic profiles than L-DOPA, thereby producing more prolonged receptor stimulation. Moreover, DR agonists possess the ability to target certain receptor subtypes, resulting in more specific therapeutic effects and avoiding the occurrence of side effects derived from non-specific activation of receptors induced by L-DOPA. Unlike L-DOPA, their metabolism does not produce hazardous reactive oxygen species (ROS) on dopaminergic neurons. Several studies have suggested that DR agonists might present neuroprotective properties via direct scavenging of free radicals or enhancing the activity of enzymes that scavenge these radicals, increasing neurotrophic activity. Therefore, the involvement of DRs in the modulation of PD has led medicinal chemists to invest in the research of DR agonists with higher subtype selectivity. Until recently, several classes of small molecules targeting D2Rs and D3Rs have been discovered [55-67]. According to their chemical scaffolds, these DR agonists have been classified into two major classes (see Fig. 2): ergoline derivatives, including bromocriptine (1), cabergoline (2), lisuride (3), pergolide (4), and α-dihydroergocryptine (5), and non-ergoline derivatives, including ropinirole (6), pramipexole (7), rotigotine (8), apomorphine (9), and piribedil (10). Both classes exhibit comparable antiparkinsonian efficacy. However, ergoline-derived DR agonists have been associated with the occurrence of adverse effects such as cardiovascular, retroperitoneal, and pleuro-pulmonary fibrosis and, therefore, their use for clinical therapy has been significantly diminished. Alternatively, the development of non-ergoline derivatives may offer the same therapeutic benefits of ergoline derivatives without the mentioned side effects.
Fig. (2).
Chemical structures of ergoline (1-5) and non-ergoline derivatives (6-10): bromocriptine (1), cabergoline (2), lisuride (3), pergolide (4), α-dihydroergocryptine (5), ropinirole (6), pramipexole (7), rotigotine (8), apomorphine (9), and piribedil (10).
2.2. Adenosine Receptors
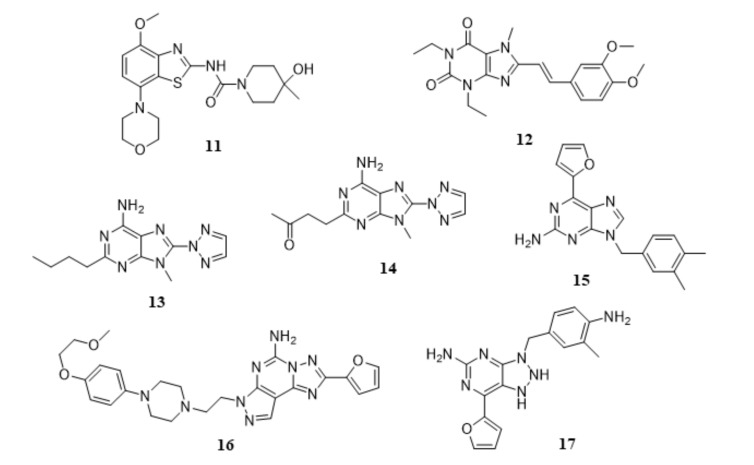
Adenosine is an endogenous neuromodulator involved in various pathophysiological functions through the interaction with four major subtypes of AR, A1 (A1AR), A2A (A2AAR), A2B (A2BAR), and A3 (A3AR) [68, 69]. While A1ARs and A3ARs are negatively coupled with AC via Gi/o proteins, exerting an inhibitory effect on the production of cAMP [69], the activation of A2AARs and A2BARs enhances AC activity via Gs proteins, causing an increase on cAMP levels [70]. In opposition to the ubiquitous distribution of A1ARs and A2BARs in the brain, A2AARs are densely expressed in restricted regions within the CNS and exist primarily in striatum, nucleus accumbens, and olfactory tubercles [71, 72], where they are coexpressed and physically interact with D2Rs, forming A2AAR-D2R heterodimers [73, 74]. A2AARs and D2Rs possess antagonistic effects on AC activity and experimental data have suggested the involvement of A2AARs-mediated adenosinergic neurotransmission on the negative regulation of D2Rs-dependent dopaminergic signaling [75, 76]. Preclinical studies have demonstrated that the pharmacological inhibition with selective A2AAR antagonists induce significant beneficial effects in animal models of PD, reversing catalepsy induced by haloperidol (D2R antagonist) in rodents [77] and cynomolgus monkeys [78], improving motor functions in 6-OHDA-lesioned rats [79], and attenuating PD-like lesions caused by administration of neurotoxin MPTP in mice [80] and cynomolgus monkeys [81]. Interestingly, coadministration of A2AAR antagonists with L-DOPA not only enhance the therapeutic effects of L-DOPA on the improvement of motor symptoms and on prevention of disease progression but also can minimize the incidence of L-DOPA-induced wearing-off and L-DOPA-related dyskinesias after long-term treatment [82]. Moreover, antiparkinsonian activity in preclinical animal models has been reported when A2AAR antagonists are administered in combination with selective D2R agonists. These evidences have led researchers to explore the inhibition of A2AAR with selective antagonists as potential enhancers of dopaminergic neurotransmission for symptomatic treatment of PD. Also, the selective and restricted localization of A2AAR in the basal ganglia provides a therapeutic opportunity for A2AAR antagonists to regulate motor functions without inducing non-specific effects in other brain regions. Therefore, A2AAR has been considered a promising therapeutic target for negative modulation by small-molecule drug candidates to be used either as monotherapy or in combination with dopaminergic drugs for PD therapeutics. A diverse plethora of chemical families of A2AAR antagonists have been identified [83-99] and among these small-molecule drug candidates, eight A2AAR antagonists have progressed to clinical studies (Fig. 3) to date by distinct pharmaceutical companies, including SYN-115 (11, Tozadenant) from Biotie Therapies and UCB Pharma S.A., KW-6002 (12, Istradefylline or NOURIAST®) from Kyowa Hakko Kirin Co. Ltd, ST1535 (13) and ST4206 (14) from Sigma-Tau, SCH-420814/MK-3814 (15, Preladenant) from Merck & Co. Inc., BIIB014/V2006 (16, Vipadenant) from Vernalis Plc-Biogen Idec Inc., V81444 (17) from Vernalis Plc, and PBF-509 (chemical structure not disclosed) from PaloBiofarma S.L., and [100]. Apart from their encouraging pharmacokinetic properties, safety and tolerability profile, the reported clinical studies have demonstrated that A2AAR antagonists significantly reduced L-DOPA-induced wearing-off effect, decreased the time spent by PD patients in a state of immobility (off-time) without impairing troublesome dyskinesia, and increased the time spent by PD patients in a state of mobility (on-time) with a moderate increase of non-troublesome dyskinesia, in L-DOPA-administered patients at an advanced stage of PD [100]. In 2013, KW6002 was approved for manufacturing and marketing in Japan as a novel therapeutic option for improvement of wearing-off effect in patients with PD when KW6002 is concomitantly administered with L-DOPA-containing products [101].
Fig. (3).
Chemical structures of A2AAR antagonists in clinical trails for PD treatment: SYN-115 or Tozadenant (11), KW-6002 or Istradefylline (12), ST1535 (13), ST4206 (14), BIIB014/V2006 (15), SCH-420814/MK-3814 or Preladenant (16), and V-81444 (17). The chemical structure of PBF-509 was not disclosed.
2.3. Muscarinic Receptors
Five distinct subtypes of muscarinic AcetylCholine Receptors (mAChR1-mAChR5 or M1-M5) [102] mediate the metabotropic actions of acetylcholine through regulation of distinct signaling pathways: mAChR1, mAChR3, and mAChR5 activate PLC via Gq proteins whereas mAChR2 and mAChR3 inhibit AC via Gi/o proteins [103]. The modulation of cholinergic system dependent on mAChRs plays a critical role in a wide plethora of central nervous system (CNS) functions, including the modulation of neuronal excitability and synaptic plasticity, sensorimotor gating, locomotor activity, memory and learning mechanisms, among other functions [104,105]. Due to their role in a number of CNS processes, mAChRs have been recognized as an attractive therapeutic target for discovery of drug candidates targeting CNS pathologies, namely PD, AD, Attention Deficit Hyperactivity Disorder (ADHD), and schizophrenia. Specifically, for PD, the characteristic dysfunction of dopaminergic neurotransmission in the stratium may contribute to a dysregulation of the dynamic equilibrum between cholinergic and dopaminergic systems. In fact, the loss of dopaminergic neurons triggers an excessive release of AcetylCholine (ACh) through activation of mACh autoreceptors and an overactivation of cholinergic system that results in the occurrence of serious motor and cognitive disturbances associated to PD [106]. Therefore, inhibition of mAChRs has been suggested as a promising strategy to overcome the increased cholinergic activity and to re-establish the cholinergic/dopaminergic balance. Among the five subtypes of mAChRs, mAChR1 and mAChR4 subtypes have been suggested to participate in modulation of PD pathophysiology. Pharmacological blockade of mAChR1 have shown therapeutic benefits in preclinical models of PD through blockade of carbachol-induced excitation of stratial medium spiny neurons, reversal of reserpine-induced akinesia and of haloperidol-induced catalepsy [107]. Additionally, selective mAChR1 and mAChR4 antagonists relieved unilateral 6-OHDA lesion-elicited motor deficits in mice [108] and mAChR4 antagonism suppressed pilocarpine- and pimozide-induced tremulous jaw movements [109,110]. The mAChR4 knockout enhanced dopaminergic neurotransmission [111], increased locomotor activity in D1R agonist-treated mice [112], and attenuated haloperidol- and risperidone-induced catalepsy in scopolamine-treated mice [113]. These results have suggested the inactivation of mAChR1 and mAChR4 with small-molecule antagonists as an encouraging approach for PD therapeutics.
The high sequence conservation within the orthosteric binding site of the five mAChRs has demonstrated to be unfavorable for the discovery and development of antagonists which specifically target the blockade of one mAChR subtype. A highly subtype-selective mAChR antagonist provides a more direct pharmacological effect, contributing to the elimination of potential side effects. Also, a mAChR antagonist could be used as a pharmacological tool and aid in the development of selective mAChR antagonists, which in turn, may be useful for treatment of PD. Numerous small-molecule antagonists acting on mAChR1 [114-126] and on mAChR4 [127-133] have been identified.
2.4. Metabotropic Glutamate Receptors
The amino acid glutamate is considered the major excitatory neurotransmitter in the brain. Glutamate elicits and modulates synaptic responses in CNS by activating two classes of glutamate receptors: ionotropic (iGluRs) and metabotropic glutamate receptors (mGluRs). IGluRs constitute a class of ligand-based ion channels subdivided into three families, including N-Methyl-D-Aspartate (NMDA), α-Amino-3-hydroxy-5-Methyl-4-isoxazole Propionic Acid (AMPA), and Kainate (KA) receptors. Belonging to glutamate-like or class C GPCRs, mGluRs are composed of eight subtypes which are classified into three groups according to the receptor structure, their pharmacological profile, and ligand binding specificity: Group I (mGluR1 and mGluR5), Group II (mGluR2 and mGluR3), and Group III (mGluR4, mGluR6, mGluR7, and mGluR8). The activation of group I mGluRs enhances the production of IP3 and DAG through stimulation of PLC via Gq proteins, whereas group II and group III mGluRs inhibit AC activity-dependent on Gi/Go proteins [134].
Several studies performed in preclinical animal PD models have suggested that the pharmacological blockade of group I mGluRs as well as the activation of group II and group III mGluRs may provide plausible therapeutic strategies for treatment of PD. Regarding the group I mGluRs, the administration of mGluR5 NAMs reversed parkinsonian symptoms, ranging from alleviation of akinesia in 6-OHDA-lesioned rats [135] to inhibition of muscle rigidity electromyographic activity, hypolocomotion, and catalepsy induced by haloperidol [136-138]. Interestingly, the coadministration of mGluR5 antagonists with A2AAR antagonists produces a synergystic effect on stimulation of locomotor activity in both untreated and reserpine-treated mice [139] and promoted a complete recovery of akinesia in 6-OHDA-lesioned rats in reaction time tasks [140]. Similarly, the combination of mGluR5 antagonists with NMDA receptor antagonists, at suboptimal doses, induced significant improvements on PD symptomatology, producing an anti-akinetic effect after 6-OHDA infusion in rats [141]. In rats with partial bilateral 6-OHDA lesions, the long-term administration of mGluR5 antagonists significantly reversed the overactive glutamatergic neurotransmission of striatum and subthalamic nucleus (STN) and SNr, thereby resulting in the alleviation of motor symptoms [135, 142, 143]. In addition, acute and chronic administration of mGluR5 NAMs significantly attenuate the development of L-DOPA induced dyskinesias in 6-OHDA-lesioned rats and in MPTP-treated monkeys chronically administered with L-DOPA [138, 144, 145]. In combination with L-DOPA, the mGluR5 NAMs induced antidyskinetic effects and prolonged the motor stimulant effects of L-DOPA in both rat and monkey PD models [145]. These evidences suggest that mGluR5 NAMs alone and/or coadministered with L-DOPA, A2AAR and NMDA receptor antagonists may provide a viable approach for symptomatic treatment of PD.
Interestingly, the mGluRs have shown to be implicated in processes of neurodegeneration/neuroprotection and to modulate excitatory synaptic neurotransmission, providing alternative therapeutic opportunities for neuroprotective drug candidates. More specifically, the mGluR5 knock-out in mice protected against MPTP-induced nigrostriatal damage, which suggested that blockade of mGluR5 may confer neuroprotective effects in animal models [146]. Additionally, in rodents, the administration of mGluR5 NAMs reduced the extent of nigrostriatal toxicity in rodents in response to MPTP [146, 147], 6-OHDA [148, 149], and methamphetamine [150], supporting the use of these drug candidates to exert neuroprotective activity and to slow the progression of neurodegeneration in PD. The relevance of pharmacological blockade of these receptors has inspired the researchers to discover antagonists and NAMs [151-164] targeting mGluR5.
The therapeutic benefits of activators of group II mGluRs have been demonstrated in several animal models. In fact, the intranigral or intracerebroventriular administration of mGluR2/mGluR3 agonists attenuated reserpine-induced akinesia and systemic administration of mGluR2/mGluR3 agonists impaired haloperidol-elicited catalepsy and muscle rigidity in rats [165, 166]. Interestingly, the activation of group II mGluRs may exert neuroprotective effects in rat
SNc neurons. Consistent with this hypothesis, administration of mGluR2/mGluR3 agonists decreased the extent of rat SNc neurodegeneration caused by 6-OHDA- [148] and MPTP-induced neurotoxicity [167, 168], underlying their role as disease-modifying agents in PD. Likewise, several evidences have suggested that the activation of group III mGluRs may alleviate PD symptoms by reducing glutamate and γ-aminobutyric acid (GABA) neurotransmission at both the stratiopallidal and STN-SNr synapses of the indirect pathway in the basal ganglia circuit [169-171]. Supporting this hypothesis, intracerebroventricular administration of group III mGluRs agonists has shown to reverse both acute (haloperidol-elicited catalepsy and reserpine-induced akinesia) and chronic (forelimb asymmetry caused by unilateral 6-OHDA-lesion) rat models of parkinsionism [170, 172]. Intrapallidal administration of group III mGluRs agonists alleviate both cataleptic and akinetic effects in rodents [172, 173], evidencing the importance of modulating synaptic neurotransmission as therapeutic strategy for symptomatic treatment of PD. In addition, the activation of group III mGluRs in the basal ganglia has displayed neuroprotective effects, reducing excitatory neurotransmission in the SNc by STN overactivity [174] and protecting against NMDA-elicited toxicity. The symptomatic and neuroprotective properties of group III mGluRs agonists are prevented in mGluR4 knockout mice, which suggest that the modulation of mGluR4 has the potential to exert antiparkinsonian activity in animal models. Overall, the pharmacological activation with mGluR2/mGluR3 [175-180] and mGluR4 [181-186] with agonists and PAMs may offer an alternative approach for PD therapeutics.
2.5. 5-Hydroxytryptamine Receptors
Being one of the most commonly studied neurotransmitters, 5-HydroxyTryptamine (5-HT) or serotonin regulates a wide array of physiological functions in the brain, particularly in emotion, modulation of sleep-wake cycles, cognition, memory, and motor behaviour. These functions are mediated through the interaction with 5-HydroxyTryptamine (5-HTRs) or serotonin Receptors, which are subdivided into thirteen subclasses: (i) 5-HT1AR, 5-HT1BR, 5-HT1DR, 5-HT1ER, 5-HT1FR, 5-HT5AR, and 5-HT5BR promote the downregulation of AC activity via Gi/Go proteins; (ii) 5-HT4R, 5-HT6R, and 5-HT7R stimulate the production of cAMP through activation of AC via Gs proteins; (iii) 5-HT2AR, 5-HT2BR, and 5-HT2CR induce the activation of PLC [187]. Several studies have suggested that the serotonergic activity is markedly impaired in PD. In fact, in PD patients, serotonin has shown to be reduced in the cortex, caudate nucleus, and hippocampus [188], the raphe nucleus and in the substance P-containing preganglionic neurons in the dorsal motor vagal nucleus [189], while serotonin markers are decreased in the caudate nucleus and putamen [190]. Interestingly, the pharmacological activation/blockade of specific 5-HTR subtypes with small molecules has displayed beneficial outcomes in preclinical PD models and clinical studies. Regarding the 5-HT1ARs, the administration of 5-HT1AR agonists has shown to attenuate/reverse 6-OHDA- [191] and haloperidol-induced catalepsy [192, 193], and to increase the motor activity in reserpine-administered rats [194]. In 6-OHDA-lesioned rats, 5-HT1AR agonists improve the performance in the forepaw adjusting steps test, an indicator of antiparkinsonian activity [195]. Pharmacological modulation of 5-HT1AR with agonists has shown to induce contraversive rotations in 6-OHDA-treated rats, to alleviate the parkinsonian symptoms in MPTP-lesioned common marmosets [196], and to attenuate L-DOPA induced motor complications [197-199]. Similarly to 5-HT1ARs, the activation of 5-HT1BRs mitigates the occurrence of dyskinesia and abnormal involuntary movements induced by L-DOPA [200-202]. Curiously, a synergystic effect on the decrease of abnormal involuntary movements was observed when 5-HT1BR agonists were co-administered with 5-HT1AR agonists [202]. Overall, targeting 5-HT1ARs [203-207] and 5-HT1BRs [208-211] with agonists may provide potential benefits on the reversal of PD symptoms.
On the other hand, the inactivation of 5-HT2AR and 5-HT2CR has demonstrated potential antiparkinsonian effects in human patients and animal models. In fact, trazodone, a dual 5-HT2AR/5-HT2CR antagonist, is effective on the treatment of PD-associated depression and on improvement of motor functions in human subjects [212]. Other dual 5-HT2AR/5-HT2CR antagonist, ritanserin, has shown to reduce bradykinesia and ameliorate gait disorder in human patients [213]. In MPTP-treated mice, peripheral administration of 5-HT2AR antagonists alone and in combination with dual 5-HT2AR/5-HT2CR antagonists enhanced motor performance on the beam walking apparatus [214, 215]. Regarding the 5-HT2CRs, the intracerebral administration of 5-HT2CR antagonists into the SNR elicited contraversive rotations to the injection side and enhanced antiparkinsonian action of D1R and D2R agonists in 6-OHDA-treated rats [216, 217]. To date, diverse chemical families of antagonists of 5-HT2AR [218-225] and 5-HT2CR [223-227] have been reported.
2.6. GPCR-based Drug Discovery for PD: An Overview of in silico Methodologies
Over the last years, the development of Computer-Assisted Drug Design (CADD) methodologies has been of extreme relevance for the identification of GPCR modulators targeting PD, contributing to increase the cost-efficiency and to speed up the drug discovery process. In silico drug design of GPCR modulators can be achieved by applying structure-based and ligand-based drug design methodologies.
2.6.1. Application of Structure-based Design Techniques for GPCR-based Drug Discovery
The field of structure-based drug design [228, 229] has been a rapidly growing area in which many advances in the drug discovery process have occurred in recent years. The explosive development of the structural biology focused on determination of high resolution three-dimensional (3D) structures of GPCRs has furnished a myriad of therapeutic opportunities for drug design of GPCR modulators inspired by structure-based drug design methodologies such as molecular docking simulations, structure-based, and fragment-based virtual screening techniques. Since GPCRs are membrane-bound protein receptors, experimental elucidation of their 3D structure, by X-ray crystallography and Nuclear
Magnetic Resonance (NMR) studies, has been a tremendously challenging task comparing to globular proteins due to problems associated to receptor purification, receptor instability, among others (reviewed in [230]). Until the elucidation of the X-ray diffraction structure at 2.8 Å resolution of bovine rhodopsin in 2000 (PDBid 1F88) [231], no X-ray structures of any GPCR were available. The 3D structural determination of bovine rhodopsin allowed a deeper understanding of GPCR functioning at the molecular level and, for many years, provided the only template for structure-based drug design approaches in homologous GPCRs for the study of drug-GPCR interactions. With the development of receptor crystallization strategies, a number of technical issues related to the low expression of GPCRs and their structural instability have been surmounted, thereby resulting in an accelerated increase of solved GPCR structures [230]. More than 200 3D structures of apo-GPCRs, protein-, natural ligand-, agonist-, and antagonist-bound GPCRs have been solved so far (Table 1), in which the rhodopsin-like or class A GPCRs have been the most reported class [42-44, 231-361]. Additionally, the 3D structures of four subfamilies of secretin-like or class B GPCRs (Corticotropin-Releasing Factor Receptors, CRFRs; Calcitonin Receptor-Like Receptors, CRLRs; Glucagon-Like Peptide Receptors, GLPRs; ParaThyroid Hormone-related peptide Receptors, PTHRs) [362-375], two subfamilies of glutamate-like or class C GPCRs (metabotropic Glutamate Receptors, mGluRs; γ-AminoButyric Acid Receptors, GABARs) [376-383], one subfamily of frizzled/taste2-like or class F GPCR (Smoothened receptors, Smo) [384-389], and two subfamilies of adhesion GPCRs (Adhesion G-protein coupled Receptor G1, ADGRG1; Adhesion G-protein coupled Receptor L3, ADGRL3) [390-392] have been disclosed on PDB (reported until February 2018).
Table 1.
Three-dimensional structures of GPCRs retrieved from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB) until February 2018.
| Rhodopsin-like or Class A GPCRs | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDBid | GPCR | Main Ligand(s) / Binding Partner(s) | Resolution / [Å] | Release Date | Refs. | ||||||||||||||||||
| 4IAQ | 5-HT1BR | Dihydroergotamine (agonist) | 2.80 | 2013 | [232] | ||||||||||||||||||
| 4IAR | 5-HT1BR | Ergotamine (agonist) | 2.70 | 2013 | [232] | ||||||||||||||||||
| 5V54 | 5-HT1BR | Methiothepin (inverse agonist) | 3.90 | 2018 | [233] | ||||||||||||||||||
| 4IB4 | 5-HT2BR | Ergotamine (agonist) | 2.70 | 2013 | [234] | ||||||||||||||||||
| 4NC3 | 5-HT2BR | Ergotamine (agonist) | 2.80 | 2013 | [235] | ||||||||||||||||||
| 5TUD | 5-HT2BR | Ergotamine (agonist) and anti-5-HT2B (antibody) | 3.00 | 2017 | [236] | ||||||||||||||||||
| 5TVN | 5-HT2BR | Lysergic acid diethylamide (agonist) | 2.90 | 2017 | [237] | ||||||||||||||||||
| 6BQG | 5-HT2CR | Ergotamine (agonist) | 3.00 | 2018 | [238] | ||||||||||||||||||
| 6BQH | 5-HT2CR | Ritanserin (inverse agonist) | 2.70 | 2018 | [238] | ||||||||||||||||||
| 5UEN | A1AR | DU172 (antagonist) | 3.20 | 2017 | [239] | ||||||||||||||||||
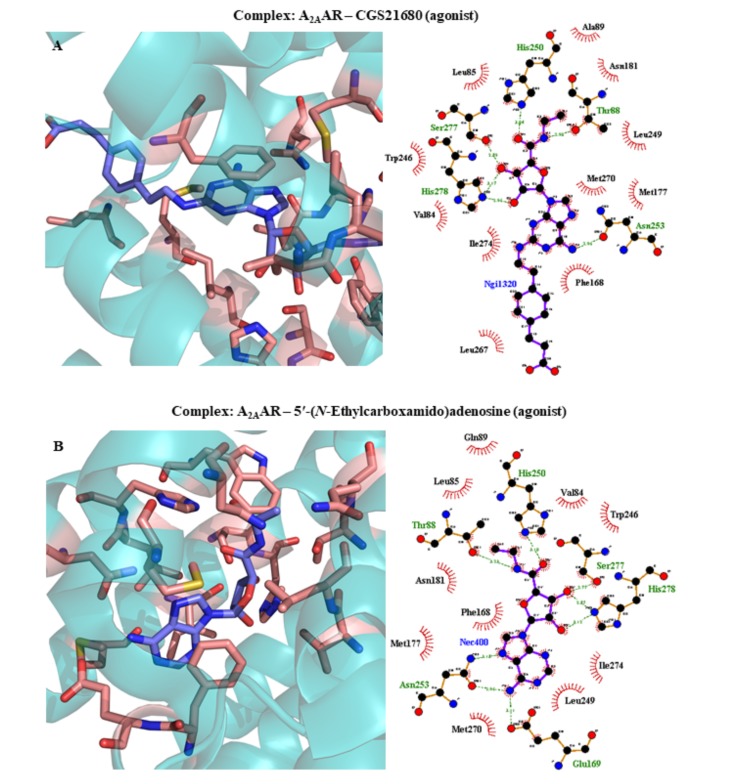
| 2YDO | A2AAR | Adenosine (agonist) | 3.00 | 2011 | [240] | ||||||||||||||||||
| 2YDV | A2AAR | 5′-(N-Ethylcarboxamido)adenosine (agonist) | 2.60 | 2011 | [240] | ||||||||||||||||||
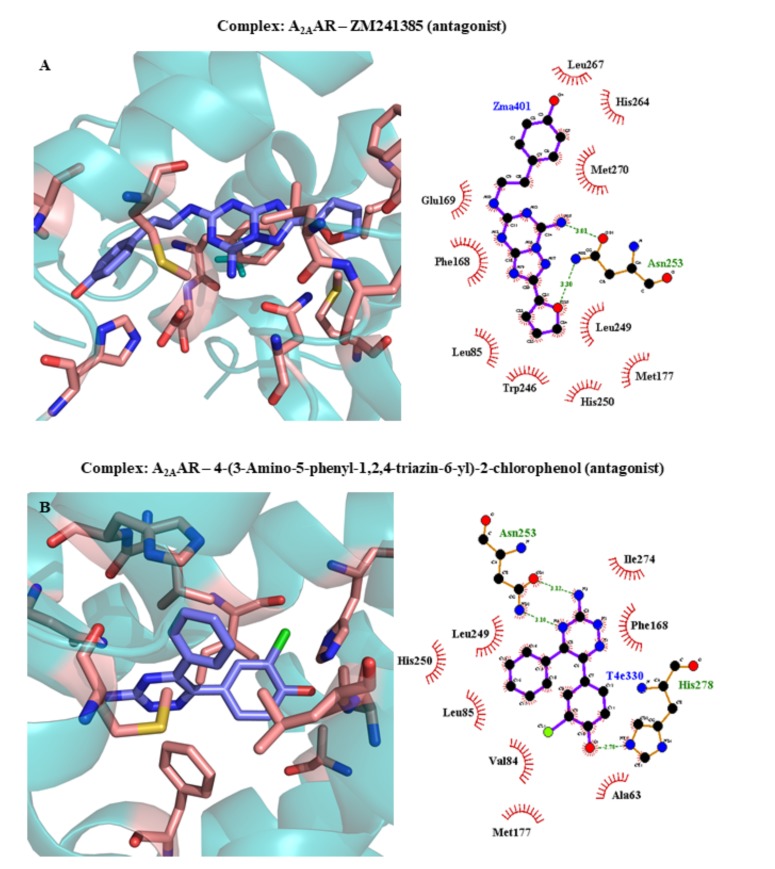
| 3EML | A2AAR | ZM241385 (antagonist) | 2.60 | 2008 | [44] | ||||||||||||||||||
| 3PWH | A2AAR | ZM241385 (antagonist) | 3.30 | 2011 | [241] | ||||||||||||||||||
| 3QAK | A2AAR | UK-432097 (agonist) | 2.71 | 2011 | [43] | ||||||||||||||||||
| 3REY | A2AAR | Xanthine amine congener (antagonist) | 3.31 | 2011 | [241] | ||||||||||||||||||
| 3RFM | A2AAR | Caffeine (antagonist) | 3.60 | 2011 | [241] | ||||||||||||||||||
| 3VG9 | A2AAR | ZM241385 (antagonist) and Fab2838 (inverse agonist) | 2.70 | 2012 | [242] | ||||||||||||||||||
| 3VGA | A2AAR | ZM241385 (antagonist) and Fab2838 (inverse agonist) | 3.10 | 2012 | [242] | ||||||||||||||||||
| 3UZA | A2AAR | 6-(2,6-Dimethylpyridin-4-yl)-5-phenyl-1,2,4-triazin-3-amine (antagonist) | 3.27 | 2012 | [243] | ||||||||||||||||||
| 3UZC | A2AAR | 4-(3-Amino-5-phenyl-1,2,4-triazin-6-yl)-2-chlorophenol (antagonist) | 3.34 | 2012 | [243] | ||||||||||||||||||
| 4EIY | A2AAR | ZM241385 (antagonist) | 1.80 | 2012 | [244] | ||||||||||||||||||
| 4UG2 | A2AAR | CGS21680 (agonist) | 2.60 | 2015 | [245] | ||||||||||||||||||
| 4UHR | A2AAR | CGS21680 (agonist) | 2.60 | 2015 | [245] | ||||||||||||||||||
| 5G53 | A2AAR | 5′-(N-Ethylcarboxamido)adenosine (agonist) and Gs proteins | 3.40 | 2016 | [246] | ||||||||||||||||||
| 5IU4 | A2AAR | ZM241385 (antagonist) | 1.72 | 2016 | [247] | ||||||||||||||||||
| 5IU7 | A2AAR | 2-(Furan-2-yl)-N5-(2-(4-phenylpiperidin-1-yl)ethyl)-1,2-dihydro-[1,2,4]triazolo[1,5-a][1,3,5]triazine-5,7-diamine (antagonist) | 1.90 | 2016 | [247] | ||||||||||||||||||
| 5IU8 | A2AAR | 2-(Furan-2-yl)-N5-(2-(4-methylpiperazin-1-yl)ethyl)-1,2-dihydro-[1,2,4]triazolo[1,5-a][1,3,5]triazine-5,7-diamine (antagonist) | 2.00 | 2016 | [247] | ||||||||||||||||||
| 5IUA | A2AAR | 2-(Furan-2-yl)-N5-(3-(4-phenylpiperazin-1-yl)propyl)-1,2-dihydro-[1,2,4]triazolo[1,5-a][1,3,5]triazine-5,7-diamine (antagonist) | 2.20 | 2016 | [247] | ||||||||||||||||||
| 5IUB | A2AAR | N5-(2-(4-(2,4-Difluorophenyl)piperazin-1-yl)ethyl)-2-(furan-2-yl)-1,2-dihydro-[1,2,4]triazolo[1,5-a][1,3,5]triazine-5,7-diamine (antagonist) | 2.10 | 2016 | [247] | ||||||||||||||||||
| 5JTB | A2AAR | ZM241385 (antagonist) | 2.80 | 2017 | [248] | ||||||||||||||||||
| 5K2A | A2AAR | ZM241385 (antagonist) | 2.50 | 2016 | [249] | ||||||||||||||||||
| Rhodopsin-like or Class A GPCRs | |||||||||||||||||||||||
| PDBid | GPCR | Main Ligand(s) / Binding Partner(s) | Resolution / [Å] | Release Date | Refs. | ||||||||||||||||||
| 5K2B | A2AAR | ZM241385 (antagonist) | 2.50 | 2016 | [249] | ||||||||||||||||||
| 5K2C | A2AAR | ZM241385 (antagonist) | 1.90 | 2016 | [249] | ||||||||||||||||||
| 5K2D | A2AAR | ZM241385 (antagonist) | 1.90 | 2016 | [249] | ||||||||||||||||||
| 5NLX | A2AAR | ZM241385 (antagonist) | 2.14 | 2017 | [250] | ||||||||||||||||||
| 5NM2 | A2AAR | ZM241385 (antagonist) | 1.95 | 2017 | [250] | ||||||||||||||||||
| 5OLG | A2AAR | ZM241385 (antagonist) | 1.87 | 2018 | [251] | ||||||||||||||||||
| 5OLH | A2AAR | Vipadenant (antagonist) | 2.60 | 2018 | [251] | ||||||||||||||||||
| 5OLO | A2AAR | Tozadenant (antagonist) | 3.10 | 2018 | [251] | ||||||||||||||||||
| 5OLV | A2AAR | LUAA47070 (antagonist) | 2.00 | 2018 | [251] | ||||||||||||||||||
| 5OLZ | A2AAR | 4-(3-Amino-5-phenyl-1,2,4-triazin-6-yl)-2-chlorophenol (antagonist) | 1.90 | 2018 | [251] | ||||||||||||||||||
| 5OM1 | A2AAR | 4-(3-Amino-5-phenyl-1,2,4-triazin-6-yl)-2-chlorophenol (antagonist) | 2.10 | 2018 | [251] | ||||||||||||||||||
| 5OM4 | A2AAR | 4-(3-Amino-5-phenyl-1,2,4-triazin-6-yl)-2-chlorophenol (antagonist) | 2.00 | 2018 | [251] | ||||||||||||||||||
| 5UIG | A2AAR | 5-Amino-N-[(2-methoxyphenyl)methyl]-2-(3-methylphenyl)-2H-1,2,3-triazole-4-carboximidamide (antagonist) | 3.50 | 2017 | [252] | ||||||||||||||||||
| 5UVI | A2AAR | ZM241385 (antagonist) | 3.20 | 2017 | [253] | ||||||||||||||||||
| 5VRA | A2AAR | ZM241385 (antagonist) | 2.35 | 2017 | [254] | ||||||||||||||||||
| 5WF5 | A2AAR | UK432097 (antagonist) | 2.60 | 2018 | [255] | ||||||||||||||||||
| 5WF6 | A2AAR | UK432097 (antagonist) | 2.90 | 2018 | [255] | ||||||||||||||||||
| 6AQF | A2AAR | ZM241385 (antagonist) | 2.51 | 2018 | [256] | ||||||||||||||||||
| 5VBL | APJR | AMG3054 (agonist) | 2.60 | 2017 | [257] | ||||||||||||||||||
| 4YAY | AT1R | ZD7155 (antagonist) | 2.90 | 2015 | [258] | ||||||||||||||||||
| 4ZUD | AT2R | Olmesartan (inverse agonist) | 2.80 | 2015 | [259] | ||||||||||||||||||
| 5UNF | AT2R | L-161,638 (antagonist) | 2.80 | 2017 | [260] | ||||||||||||||||||
| 5UNG | AT2R | L-161,638 (antagonist) | 2.80 | 2017 | [260] | ||||||||||||||||||
| 5UNH | AT2R | (N-[(Furan-2-yl)methyl]-N-(4-oxo-2-propyl-3-{[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl}-3,4-dihydroquinazolin-6-yl)benzamide) (antagonist) | 2.90 | 2017 | [260] | ||||||||||||||||||
| 6F3Y | B1R | Des-Arg10-kallidin (agonist) | NMR | 2018 | [261] | ||||||||||||||||||
| 6F3V | B2R | Bradykinin (agonist) | NMR | 2018 | [261] | ||||||||||||||||||
| 2VT4 | β1AR | Cyanopindolol (antagonist) | 2.70 | 2008 | [262] | ||||||||||||||||||
| 2Y00 | β1AR | Dobutamine (partial agonist) | 2.50 | 2011 | [263] | ||||||||||||||||||
| 2Y01 | β1AR | Dobutamine (partial agonist) | 2.60 | 2011 | [263] | ||||||||||||||||||
| 2Y02 | β1AR | Carmoterol (agonist) | 2.60 | 2011 | [263] | ||||||||||||||||||
| 2Y03 | β1AR | Isoprenaline (agonist) | 2.85 | 2011 | [263] | ||||||||||||||||||
| 2Y04 | β1AR | Salbutamol (partial agonist) | 3.05 | 2011 | [263] | ||||||||||||||||||
| 2YCY | β1AR | Cyanopindolol (antagonist) | 3.15 | 2011 | [264] | ||||||||||||||||||
| 2YCW | β1AR | Carazolol (antagonist) | 3.10 | 2011 | [264] | ||||||||||||||||||
| 2YCX | β1AR | Cyanopindolol (antagonist) | 3.25 | 2011 | [264] | ||||||||||||||||||
| 2YCZ | β1AR | Iodocyanopindolol (antagonist) | 3.65 | 2011 | [264] | ||||||||||||||||||
| 3ZPQ | β1AR | 4-(Piperazin-1-yl)-1H-indole (antagonist) | 2.80 | 2013 | [265] | ||||||||||||||||||
| Rhodopsin-like or Class A GPCRs | |||||||||||||||||||||||
| PDBid | GPCR | Main Ligand(s) / Binding Partner(s) | Resolution / [Å] | Release Date | Refs. | ||||||||||||||||||
| 3ZPR | β1AR | 4-Methyl-2-(piperazin-1-yl)quinoline (antagonist) | 2.70 | 2013 | [265] | ||||||||||||||||||
| 4AMI | β1AR | Bucindolol (agonist) | 3.20 | 2012 | [266] | ||||||||||||||||||
| 4AMJ | β1AR | Carvedilol (agonist) | 2.30 | 2012 | [266] | ||||||||||||||||||
| 4BVN | β1AR | Cyanopindolol (antagonist) | 2.10 | 2014 | [267] | ||||||||||||||||||
| 4GPO | β1AR | - | 3.50 | 2013 | [268] | ||||||||||||||||||
| 5A8E | β1AR | 7-Methylcyanopindolol (inverse agonist) | 2.40 | 2015 | [269] | ||||||||||||||||||
| 5F8U | β1AR | Cyanopindolol (antagonist) | 3.35 | 2015 | [270] | ||||||||||||||||||
| 2R4R | β2AR | Fab5 (antibody) and carazolol (inverse agonist) | 3.40 | 2007 | [271] | ||||||||||||||||||
| 2R4S | β2AR | Fab5 (antibody) and carazolol (inverse agonist) | 3.40 | 2007 | [271] | ||||||||||||||||||
| 2RH1 | β2AR | Carazolol (inverse agonist) | 2.40 | 2007 | [272] | ||||||||||||||||||
| 3D4S | β2AR | Timolol (partial inverse agonist) | 2.80 | 2008 | [273] | ||||||||||||||||||
| 3KJ6 | β2AR | Fab5 (antibody) | 3.40 | 2010 | [274] | ||||||||||||||||||
| 3NY8 | β2AR | ICI-118,551 (inverse agonist) | 2.84 | 2010 | [275] | ||||||||||||||||||
| 3NY9 | β2AR | Ethyl 4-({(2S)-2-hydroxy-3-[(1-methylethyl)amino]propyl}oxy)-3-methyl-1-benzofuran-2-carboxylate (inverse agonist) | 2.84 | 2010 | [275] | ||||||||||||||||||
| 3NYA | β2AR | Alprenolol (antagonist) | 3.16 | 2010 | [275] | ||||||||||||||||||
| 3P0G | β2AR | Nb80 (nanobody) and BI-167107 (agonist) | 3.50 | 2011 | [276] | ||||||||||||||||||
| 3PDS | β2AR | FAUC50 (agonist) | 3.50 | 2011 | [42] | ||||||||||||||||||
| 3SN6 | β2AR | Gs proteins | 3.20 | 2011 | [277] | ||||||||||||||||||
| 4GBR | β2AR | Carazolol (inverse agonist) | 3.99 | 2012 | [278] | ||||||||||||||||||
| 5D5A | β2AR | Carazolol (inverse agonist) | 2.48 | 2016 | [279] | ||||||||||||||||||
| 5D5B | β2AR | Carazolol (inverse agonist) | 3.80 | 2016 | [279] | ||||||||||||||||||
| 5JQH | β2AR | Nb60 (nanobody) and carazolol (inverse agonist) | 3.20 | 2016 | [280] | ||||||||||||||||||
| 5X7D | β2AR | Carazolol (inverse agonist) and 4-carbamoyl-N-[(2R)-2-cyclohexyl-2-phenylacetyl)]-L-phenylalanyl-3-bromo-N-methyl-L-phenylalaninamide (NAM) | 2.70 | 2017 | [281] | ||||||||||||||||||
| 5O9H | C5aR1 | NDT9513727 (inverse agonist) | 2.70 | 2018 | [282] | ||||||||||||||||||
| 5U09 | CB1R | Taranabant (inverse agonist) | 2.60 | 2016 | [283] | ||||||||||||||||||
| 5TGZ | CB1R | AM6538 (antagonist) | 2.80 | 2016 | [284] | ||||||||||||||||||
| 5T1A | CCR2 | BMS-681 (orthosteric antagonist) and CCR-RA-[R] (NAM) | 2.81 | 2016 | [285] | ||||||||||||||||||
| 2RRS | CCR5 | - | NMR | 2012 | [286] | ||||||||||||||||||
| 4MBS | CCR5 | Maraviroc (antagonist) | 2.71 | 2013 | [287] | ||||||||||||||||||
| 5LWE | CCR9 | Vercirnon (NAM) | 2.60 | 2016 | [288] | ||||||||||||||||||
| 2LNL | CXCR1 | - | NMR | 2012 | [289] | ||||||||||||||||||
| 3ODU | CXCR4 | IT1t (antagonist) | 2.50 | 2010 | [290] | ||||||||||||||||||
| 3OE0 | CXCR4 | CVX15 (antagonist) | 2.90 | 2010 | [290] | ||||||||||||||||||
| 3OE6 | CXCR4 | IT1t (antagonist) | 3.20 | 2010 | [290] | ||||||||||||||||||
| 3OE8 | CXCR4 | IT1t (antagonist) | 3.10 | 2010 | [290] | ||||||||||||||||||
| 3OE9 | CXCR4 | IT1t (antagonist) | 3.10 | 2010 | [290] | ||||||||||||||||||
| 4RWS | CXCR4 | vMIP-II complex (antagonist) | 3.10 | 2015 | [291] | ||||||||||||||||||
| 2N55 | CXCR5 | CXCL12 (agonist) | NMR | 2016 | [292] | ||||||||||||||||||
| Rhodopsin-like or Class A GPCRs | |||||||||||||||||||||||
| PDBid | GPCR | Main Ligand(s) / Binding Partner(s) | Resolution / [Å] | Release Date | Refs. | ||||||||||||||||||
| 6CM4 | D2R | Risperidone (inverse agonist) | 2.87 | 2018 | [293] | ||||||||||||||||||
| 3PBL | D3R | Eticlopride (antagonist) | 2.89 | 2010 | [294] | ||||||||||||||||||
| 5WIU | D4R | Nemonapride (antagonist) | 1.96 | 2017 | [295] | ||||||||||||||||||
| 5WIV | D4R | Nemonapride (antagonist) | 2.14 | 2017 | [295] | ||||||||||||||||||
| 4N6H | DOR | Naltrindole (antagonist) | 1.80 | 2013 | [296] | ||||||||||||||||||
| 4RWA | DOR | DIPP-NH2 (antagonist) | 3.28 | 2015 | [297] | ||||||||||||||||||
| 4RWD | DOR | DIPP-NH2 (antagonist) | 2.70 | 2015 | [297] | ||||||||||||||||||
| 5GLH | ETBR | Endothelin-1 (agonist) | 2.80 | 2016 | [298] | ||||||||||||||||||
| 5GLI | ETBR | - | 2.50 | 2016 | [298] | ||||||||||||||||||
| 4PHU | FFAR1 | TAK-875 (PAM) | 2.33 | 2014 | [299] | ||||||||||||||||||
| 5TZR | FFAR1 | MK-8666 (partial agonist) | 2.20 | 2017 | [300] | ||||||||||||||||||
| 5TZY | FFAR1 | AP8 (ago-PAM) and MK-8666 (partial agonist) | 3.22 | 2017 | [300] | ||||||||||||||||||
| 4AY9 | FSHR | FSH (agonist) | 2.50 | 2012 | [301] | ||||||||||||||||||
| 4MQW | FSHR | FSH (agonist) | 2.90 | 2014 | [302] | ||||||||||||||||||
| 3RZE | H1R | Doxepin (antagonist) | 3.10 | 2011 | [303] | ||||||||||||||||||
| 4DJH | KOR | JDTic (antagonist) | 2.90 | 2012 | [304] | ||||||||||||||||||
| 6B73 | KOR | Nb39 (nanobody) and MP1104 (agonist) | 3.10 | 2018 | [305] | ||||||||||||||||||
| 4Z34 | LPAR1 | ONO9780307 (antagonist) | 3.00 | 2015 | [306] | ||||||||||||||||||
| 4Z35 | LPAR1 | ONO9910539 (antagonist) | 2.90 | 2015 | [306] | ||||||||||||||||||
| 4Z36 | LPAR1 | ONO3080573 (antagonist) | 2.90 | 2015 | [306] | ||||||||||||||||||
| 5CXV | mAChR1 | Tiotropium (inverse agonist) | 2.70 | 2016 | [307] | ||||||||||||||||||
| 3UON | mAChR2 | R-(-)-Quinuclidinyl benzilate (inverse agonist) | 3.00 | 2012 | [308] | ||||||||||||||||||
| 4MQS | mAChR2 | Iperoxo (agonist) | 3.50 | 2013 | [309] | ||||||||||||||||||
| 4MQT | mAChR2 | Iperoxo (agonist) and LY2119620 (PAM) | 3.70 | 2013 | [309] | ||||||||||||||||||
| 4DAJ | mAChR3 | Tiotropium (inverse agonist) | 3.40 | 2012 | [310] | ||||||||||||||||||
| 4U14 | mAChR3 | Tiotropium (inverse agonist) | 3.57 | 2014 | [311] | ||||||||||||||||||
| 4U15 | mAChR3 | Tiotropium (inverse agonist) | 2.80 | 2014 | [311] | ||||||||||||||||||
| 4U16 | mAChR3 | N-Methylscopolamine (antagonist) | 3.70 | 2014 | [311] | ||||||||||||||||||
| 5DSG | mAChR3 | Tiotropium (inverse agonist) | 2.60 | 2016 | [307] | ||||||||||||||||||
| 4DKL | MOR | β-Funaltrexamine (antagonist) | 2.80 | 2012 | [312] | ||||||||||||||||||
| 5C1M | MOR | BU72 (agonist) | 2.10 | 2015 | [313] | ||||||||||||||||||
| 4EJ4 | N/OFQR | Naltrindole (antagonist) | 3.40 | 2012 | [314] | ||||||||||||||||||
| 4GRV | N/OFQR | Neurotensin 8-13 (agonist) | 2.80 | 2012 | [315] | ||||||||||||||||||
| 5DHG | N/OFQR | C35 (antagonist) | 3.00 | 2015 | [316] | ||||||||||||||||||
| 5DHH | N/OFQR | SB612111 (antagonist) | 3.00 | 2015 | [316] | ||||||||||||||||||
| 3ZEV | NTSR1 | TM86V-∆IC3A | 3.00 | 2014 | [317] | ||||||||||||||||||
| 4BUO | NTSR1 | TM86V-∆IC3B | 2.75 | 2014 | [317] | ||||||||||||||||||
| 4BV0 | NTSR1 | OGG7V-∆IC3A | 3.10 | 2014 | [317] | ||||||||||||||||||
| 4BWB | NTSR1 | HTGH4-∆IC3 | 3.57 | 2014 | [317] | ||||||||||||||||||
| 4XEE | NTSR1 | Neurotensin/Neuromedin N (agonist) | 2.90 | 2015 | [318] | ||||||||||||||||||
| 4XES | NTSR1 | Neurotensin/Neuromedin N (agonist) | 2.60 | 2015 | [318] | ||||||||||||||||||
| Rhodopsin-like or Class A GPCRs | |||||||||||||||||||||||
| PDBid | GPCR | Main Ligand(s) / Binding Partner(s) | Resolution / [Å] | Release Date | Refs. | ||||||||||||||||||
| 5T04 | NTSR1 | Neurotensin 8-13 (Arg-Arg-Pro-Tyr-Ile-Leu; agonist) | 3.30 | 2016 | [319] | ||||||||||||||||||
| 4ZJ8 | OX1R | Suvorexant (antagonist) | 2.75 | 2016 | [320] | ||||||||||||||||||
| 4ZJC | OX1R | SB-674042 (antagonist) | 2.83 | 2016 | [320] | ||||||||||||||||||
| 4S0V | OX2R | Suvorexant (antagonist) | 2.50 | 2015 | [321] | ||||||||||||||||||
| 4XNV | P2Y1R | BPTU (antagonist) | 2.20 | 2015 | [322] | ||||||||||||||||||
| 4XNW | P2Y1R | MRS2500 (antagonist) | 2.70 | 2015 | [322] | ||||||||||||||||||
| 4NTJ | P2Y12R | AZD1283 (agonist) | 2.62 | 2014 | [323] | ||||||||||||||||||
| 4PXZ | P2Y12R | 2MeSADP (agonist) | 2.50 | 2014 | [324] | ||||||||||||||||||
| 4PY0 | P2Y12R | 2MeSATP (partial agonist) | 3.10 | 2014 | [324] | ||||||||||||||||||
| 3VW7 | PAR1 | Vorapaxar (antagonist) | 2.20 | 2012 | [325] | ||||||||||||||||||
| 5NDD | PAR2 | AZ8838 (antagonist) | 2.80 | 2017 | [326] | ||||||||||||||||||
| 5NDZ | PAR2 | AZ3451 (antagonist) | 3.60 | 2017 | [326] | ||||||||||||||||||
| 5NJ6 | PAR2 | Fab3949 (antibody) and AZ7188 (antagonist) | 4.00 | 2017 | [326] | ||||||||||||||||||
| 1F88 | RHO | 11-cis-Retinal (agonist) | 2.80 | 2000 | [231] | ||||||||||||||||||
| 1GZM | RHO | 11-cis-Retinal (agonist) | 2.65 | 2003 | [327] | ||||||||||||||||||
| 1HZX | RHO | 11-cis-Retinal (agonist) | 2.80 | 2001 | [328] | ||||||||||||||||||
| 1JFP | RHO | 11-cis-Retinal (agonist) | NMR | 2001 | [329] | ||||||||||||||||||
| 1L9H | RHO | 11-cis-Retinal (agonist) | 2.60 | 2002 | [330] | ||||||||||||||||||
| 1LN6 | RHO | 11-cis-Retinal (agonist) | NMR | 2002 | [331] | ||||||||||||||||||
| 1U19 | RHO | 11-cis-Retinal (agonist) | 2.20 | 2004 | [332] | ||||||||||||||||||
| 2G87 | RHO | 11-cis-Retinal (agonist) | 2.60 | 2006 | [333] | ||||||||||||||||||
| 2HPY | RHO | 11-cis-Retinal (agonist) | 2.80 | 2006 | [334] | ||||||||||||||||||
| 2I35 | RHO | 11-cis-Retinal (agonist) | 3.80 | 2006 | [335] | ||||||||||||||||||
| 2I36 | RHO | - | 4.10 | 2006 | [335] | ||||||||||||||||||
| 2I37 | RHO | - | 4.15 | 2006 | [335] | ||||||||||||||||||
| 2J4Y | RHO | 11-cis-Retinal (agonist) | 3.40 | 2007 | [336] | ||||||||||||||||||
| 2PED | RHO | 11-cis-Retinal (agonist) | 2.95 | 2007 | [337] | ||||||||||||||||||
| 2X72 | RHO | Gαt1 and 11-cis-retinal (agonist) | 3.00 | 2011 | [338] | ||||||||||||||||||
| 2Z73 | RHO | 11-cis-Retinal (agonist) | 2.50 | 2008 | [339] | ||||||||||||||||||
| 2ZIY | RHO | 11-cis-Retinal (agonist) | 3.70 | 2008 | [340] | ||||||||||||||||||
| 3AYM | RHO | 11-cis-Retinal (agonist) | 2.80 | 2011 | [341] | ||||||||||||||||||
| 3AYN | RHO | 11-cis-Retinal (agonist) | 2.70 | 2011 | [341] | ||||||||||||||||||
| 3C9L | RHO | 11-cis-Retinal (agonist) | 2.65 | 2008 | [342] | ||||||||||||||||||
| 3C9M | RHO | 11-cis-Retinal (agonist) | 3.40 | 2008 | [342] | ||||||||||||||||||
| 3CAP | RHO | - | 2.90 | 2008 | [343] | ||||||||||||||||||
| 3DQB | RHO | Gαt1 | 3.20 | 2008 | [344] | ||||||||||||||||||
| 3PQR | RHO | Gαt1 and 11-cis-retinal (agonist) | 2.85 | 2011 | [345] | ||||||||||||||||||
| 3PXO | RHO | 11-cis-Retinal (agonist) | 3.00 | 2011 | [345] | ||||||||||||||||||
| 3OAX | RHO | β-Ionone and 11-cis-retinal (agonist) | 2.60 | 2011 | [346] | ||||||||||||||||||
| 4A4M | RHO | Gαt3 and 11-cis-retinal (agonist) | 3.30 | 2012 | [347] | ||||||||||||||||||
| 4BEY | RHO | Gαt2 | 2.90 | 2013 | [348] | ||||||||||||||||||
| Rhodopsin-like or Class B GPCRs | |||||||||||||||||||||||
| PDBid | GPCR | Ligand(s) / Binding Partner(s) | Resolution / [Å] | Release Date | Refs. | ||||||||||||||||||
| 4BEZ | RHO | - | 3.30 | 2013 | [348] | ||||||||||||||||||
| 4J4Q | RHO | Gαt1 | 2.65 | 2013 | [349] | ||||||||||||||||||
| 4PXF | RHO | Arrestin-1 | 2.75 | 2014 | [350] | ||||||||||||||||||
| 4X1H | RHO | Gαt1 | 2.29 | 2015 | [351] | ||||||||||||||||||
| 4ZWJ | RHO | - | 3.30 | 2015 | [352] | ||||||||||||||||||
| 5DGY | RHO | Visual arrestin-1 | 7.70 | 2016 | [353] | ||||||||||||||||||
| 5DYS | RHO | 11-cis-Retinal (agonist) | 2.30 | 2016 | [354] | ||||||||||||||||||
| 5EN0 | RHO | Gαt3 and 11-cis-retinal (agonist) | 2.81 | 2016 | [354] | ||||||||||||||||||
| 5TE3 | RHO | - | 2.70 | 2017 | [355] | ||||||||||||||||||
| 5TE5 | RHO | (2E)-{(4E)-4-[(3E)-4-(2,6,6-Trimethylcyclohex-1-en-1-yl)but-3-en-2-ylidene]cyclohex-2-en-1-ylidene}acetaldehyde | 4.01 | 2017 | [355] | ||||||||||||||||||
| 5W0P | RHO | Visual arrestin-1 | 3.01 | 2017 | [356] | ||||||||||||||||||
| 5WKT | RHO | Transducin Gα peptide (GαCT2) | 3.20 | 2017 | [254] | ||||||||||||||||||
| 3V2W | S1PR1 | ML056 (antagonist) | 3.35 | 2012 | [357] | ||||||||||||||||||
| 3V2Y | S1PR1 | CYM-5442 (agonist) | 2.80 | 2012 | [357] | ||||||||||||||||||
| 2XWT | TSHR | K1-70 (antagonist) | 1.90 | 2011 | [358] | ||||||||||||||||||
| 3G04 | TSHR | Thyroid-stimulating human monoclonal autoantibody (M22) | 2.55 | 2009 | [359] | ||||||||||||||||||
| 4XT1 | US28 | CX3CL1 (agonist) | 2.89 | 2015 | [360] | ||||||||||||||||||
| 4XT3 | US28 | CX3CL1 (agonist) | 3.80 | 2015 | [360] | ||||||||||||||||||
| 4JQI | V2R | Arrestin-2 | 2.60 | 2013 | [361] | ||||||||||||||||||
| Secretin-like or Class B GPCRs | |||||||||||||||||||||||
| PDBid | GPCR | Ligand(s) / Binding Partner(s) | Resolution / [Å] | Release Date | Refs. | ||||||||||||||||||
| 3EHS | CRFR1 | - | 2.76 | 2008 | [362] | ||||||||||||||||||
| 3EHT | CRFR1 | CRF (agonist) | 3.40 | 2008 | [362] | ||||||||||||||||||
| 3EHU | CRFR1 | CRF (agonist) | 1.96 | 2008 | [362] | ||||||||||||||||||
| 4K5Y | CRFR1 | CP-376395 (antagonist) | 2.98 | 2013 | [363] | ||||||||||||||||||
| 4Z9G | CRFR1 | CP-376395 (antagonist) | 3.18 | 2016 | [364] | ||||||||||||||||||
| 3AQF | CRLR | RAMP2 | 2.60 | 2011 | [365] | ||||||||||||||||||
| 3N7P | CRLR | RAMP1 | 2.80 | 2010 | [366] | ||||||||||||||||||
| 3N7R | CRLR | RAMP1 and telcagepant (antagonist) | 2.90 | 2010 | [366] | ||||||||||||||||||
| 3N7S | CRLR | RAMP1 and olcegepant (antagonist) | 2.10 | 2010 | [366] | ||||||||||||||||||
| 5UZ7 | CRLR | Gs proteins | 4.10 | 2017 | [367] | ||||||||||||||||||
| 5V6Y | CRLR | RAMP1 and adrenomedullin variant (antagonist) | 2.80 | 2018 | [368] | ||||||||||||||||||
| 4ERS | GLP1R | mAb1 (antagonist) | 2.64 | 2012 | [369] | ||||||||||||||||||
| 4L6R | GLP1R | Glucagon (agonist) | 3.30 | 2013 | [370] | ||||||||||||||||||
| 5EE7 | GLP1R | MK-0893 (antagonist) | 2.50 | 2016 | [371] | ||||||||||||||||||
| 5NX2 | GLP1R | Peptide 5 (agonist) | 3.70 | 2017 | [372] | ||||||||||||||||||
| 5VAI | GLP1R | Gs proteins | 4.10 | 2017 | [373] | ||||||||||||||||||
| 5VEW | GLP1R | PF-06372222 (NAM) | 2.70 | 2017 | [374] | ||||||||||||||||||
| 5VEX | GLP1R | NNC0640 (NAM) | 3.00 | 2017 | [374] | ||||||||||||||||||
| 3H3G | PTH1R | PTHrP | 1.94 | 2009 | [375] | ||||||||||||||||||
| Glutamate-like or Class C GPCRs | |||||||||||||||||||||||
| PDBid | GPCR | Ligand(s) / Binding Partner(s) | Resolution / [Å] | Release Date | Refs. | ||||||||||||||||||
| 4MQE | GABABR | - | 2.35 | 2013 | [376] | ||||||||||||||||||
| 4MQF | GABABR | 2-Hydroxysaclofen (antagonist) | 2.22 | 2013 | [376] | ||||||||||||||||||
| 4MR7 | GABABR | CGP54626 (antagonist) | 2.15 | 2013 | [376] | ||||||||||||||||||
| 4MR8 | GABABR | CGP35348 (antagonist) | 2.15 | 2013 | [376] | ||||||||||||||||||
| 4MR9 | GABABR | SCH50911 (antagonist) | 2.35 | 2013 | [376] | ||||||||||||||||||
| 4MRM | GABABR | Phaclofen (antagonist) | 2.86 | 2013 | [376] | ||||||||||||||||||
| 4MS1 | GABABR | CGP46381 (antagonist) | 2.25 | 2013 | [376] | ||||||||||||||||||
| 4MS3 | GABABR | γ-Aminobutyric acid (agonist) | 2.50 | 2013 | [376] | ||||||||||||||||||
| 4MS4 | GABABR | Baclofen (agonist) | 1.90 | 2013 | [376] | ||||||||||||||||||
| 4OR2 | mGluR1 | FITM (NAM) | 2.80 | 2014 | [377] | ||||||||||||||||||
| 1EWK | mGluR1 | Glutamate (agonist) | 2.20 | 2000 | [378] | ||||||||||||||||||
| 1EWT | mGluR1 | - | 3.70 | 2000 | [378] | ||||||||||||||||||
| 1EWV | mGluR1 | - | 4.00 | 2000 | [378] | ||||||||||||||||||
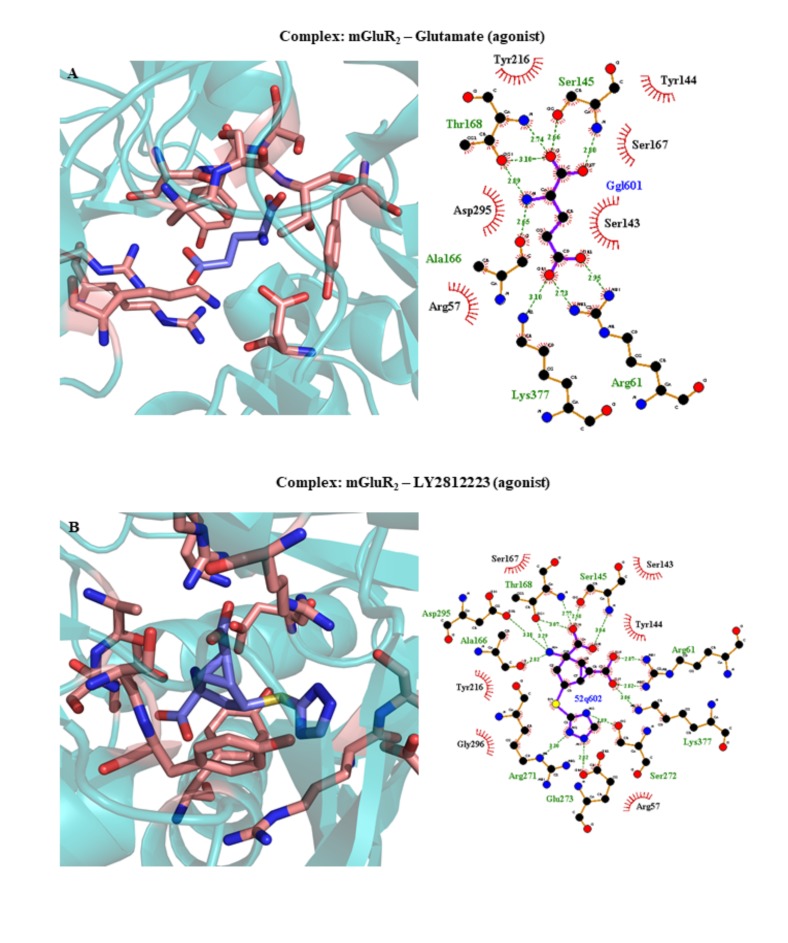
| 5CNI | mGluR2 | Glutamate (agonist) | 2.69 | 2015 | [379] | ||||||||||||||||||
| 5CNJ | mGluR2 | LY2812223 (agonist) | 2.65 | 2015 | [379] | ||||||||||||||||||
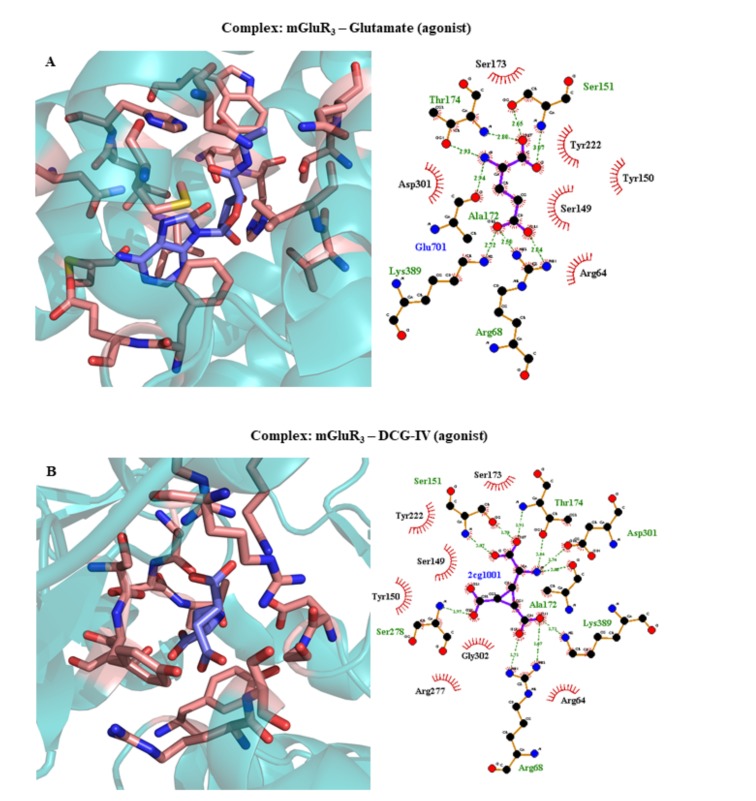
| 2E4U | mGluR3 | Glutamate (agonist) | 2.35 | 2007 | [380] | ||||||||||||||||||
| 2E4V | mGluR3 | DCG-IV (agonist) | 2.40 | 2007 | [380] | ||||||||||||||||||
| 2E4W | mGluR3 | 1S,3S-ACPD (agonist) | 2.40 | 2007 | [380] | ||||||||||||||||||
| 2E4X | mGluR3 | 1S,3R-ACPD (agonist) | 2.75 | 2007 | [380] | ||||||||||||||||||
| 2E4Y | mGluR3 | 2R,4R-APDC (agonist) | 3.40 | 2007 | [380] | ||||||||||||||||||
| 5CNK | mGluR3 | Glutamate (agonist) | 3.15 | 2015 | [379] | ||||||||||||||||||
| 5CNM | mGluR3 | LY2812223 (agonist) | 2.84 | 2015 | [379] | ||||||||||||||||||
| 4OO9 | mGluR5 | Mavoglurant (NAM) | 2.60 | 2014 | [381] | ||||||||||||||||||
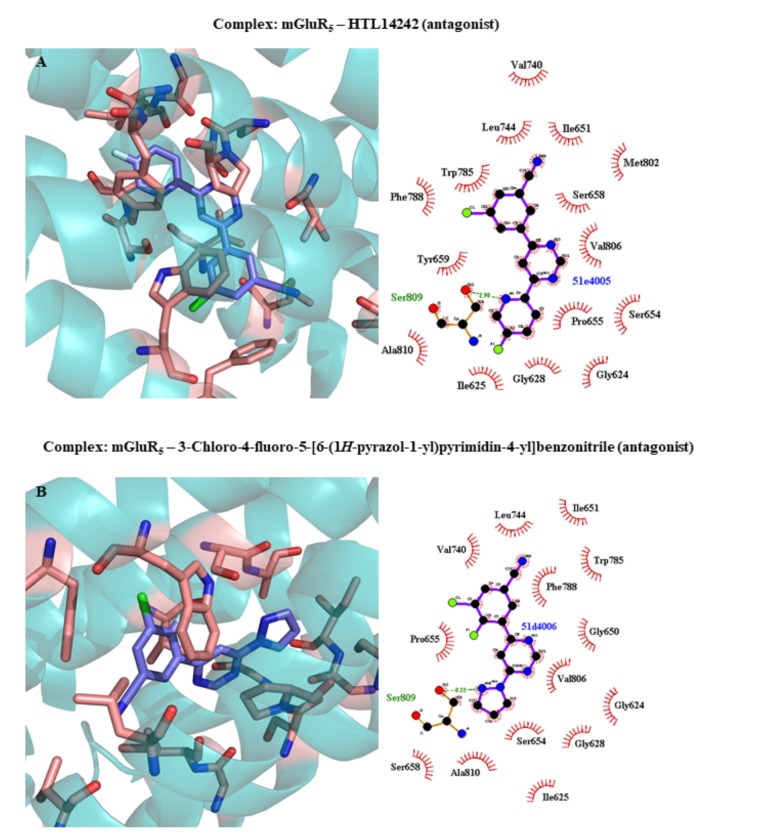
| 5CGC | mGluR5 | 3-Chloro-4-fluoro-5-[6-(1H-pyrazol-1-yl)pyrimidin-4-yl]benzonitrile (NAM) | 3.10 | 2015 | [382] | ||||||||||||||||||
| 5CGD | mGluR5 | HTL14242 (NAM) | 2.60 | 2015 | [382] | ||||||||||||||||||
| 6FFH | mGluR5 | Fenobam (NAM) | 2.65 | 2018 | [383] | ||||||||||||||||||
| 6FF1 | mGluR5 | MMPEP (NAM) | 2.20 | 2018 | [383] | ||||||||||||||||||
| 2E4Z | mGluR7 | - | 3.30 | 2007 | [380] | ||||||||||||||||||
| Frizzled/taste2-like or Class F GPCRs | |||||||||||||||||||||||
| PDBid | GPCR | Ligand(s) / Binding Partner(s) | Resolution / [Å] | Release Date | Reference | ||||||||||||||||||
| 4JKV | Smo | LY2940680 (antagonist) | 2.45 | 2013 | [384] | ||||||||||||||||||
| 4N4W | Smo | SANT-1 (antagonist) | 2.80 | 2014 | [385] | ||||||||||||||||||
| 4O9R | Smo | Cyclopamine (antagonist) | 3.20 | 2014 | [386] | ||||||||||||||||||
| 4QIM | Smo | Anta XV (antagonist) | 2.61 | 2014 | [385] | ||||||||||||||||||
| 4QIN | Smo | SAG1.5 (agonist) | 2.60 | 2014 | [385] | ||||||||||||||||||
| 5KZV | Smo | 20(S)-Hydroxycholesterol (agonist) | 1.62 | 2016 | [387] | ||||||||||||||||||
| 5KZY | Smo | Cyclopamine (antagonist) | 2.48 | 2016 | [387] | ||||||||||||||||||
| 5KZZ | Smo | - | 1.33 | 2016 | [387] | ||||||||||||||||||
| 5L7D | Smo | Cholesterol (agonist) | 3.20 | 2016 | [388] | ||||||||||||||||||
| 5L7I | Smo | Vismodegib (antagonist) | 3.30 | 2016 | [388] | ||||||||||||||||||
| Frizzled/taste2-like or Class F GPCRs | |||||||||||||||||||||||
| PDBid | GPCR | Ligand(s) / Binding Partner(s) | Resolution / [Å] | Release Date | Reference | ||||||||||||||||||
| 5V56 | Smo | TC114 (antagonist) | 2.90 | 2017 | [389] | ||||||||||||||||||
| 5V57 | Smo | TC114 (antagonist) | 3.00 | 2017 | [389] | ||||||||||||||||||
| Adhesion GPCRs | |||||||||||||||||||||||
| PDBid | GPCR | Ligand(s) / Binding Partner(s) | Resolution / [Å] | Release date | Reference | ||||||||||||||||||
| 5KVM | ADGRG1 | FN3 monobody | 2.45 | 2016 | [390] | ||||||||||||||||||
| 4RMK | ADGRL3 | - | 1.61 | 2015 | [391] | ||||||||||||||||||
| 4RML | ADGRL3 | - | 1.60 | 2015 | [391] | ||||||||||||||||||
| 5FTT | ADGRL3 | Unc5D and FLRT2 | 3.40 | 2016 | [392] | ||||||||||||||||||
| 5FTU | ADGRL3 | Unc5D and FLRT2 | 6.01 | 2016 | [392] | ||||||||||||||||||
Abbrevations: (5-HTR - 5-HydroxyTryptamine receptor; ADGRG1 - Adhesion G-protein coupled Receptor G1; ADGRL3 - Adhesion G-protein coupled Receptor L3; AR - Adenosine Receptor; APJR - Apelin Receptor; ATR - Angiotensin II Receptor; BR – Bradykinin Receptor; βAR: - β-Adrenergic Receptor; C5aR – C5a anaphylatoxin chemotactic Receptor; CBR - Cannabinoid Receptor; CCR - CC Chemokine Receptor; CRF - Corticotropin Releasing Factor; CRFR - Corticotropin Releasing Factor Receptor; CRLR - Calcitonin Receptor-Like Receptor; CXCR - CXC Chemokine Receptor; DOR - δ-Opioid Receptor; DR - Dopamine Receptor; ETR - Endothelin Receptor; FFAR - Free Fatty Acid Receptor; FLRT2 - Fibronectin Leucin-Rich Transmembrane protein 2; FN3 - FibroNectin type III domain; FSH - Follicle-Stimulating Hormone; FSHR - Follicle-Stimulating Hormone Receptor; GABAR - γ-AminoButyric Acid Receptor; GLPR - Glucagon-Like Peptide Receptor; HR - Histamine Receptor; KOR - κ-Opioid Receptor; LPAR - LysoPhosphatidic Acid Receptor; mAChR - muscarinic AcetylCholine Receptor; mGluR - metabotropic Glutamate Receptor; MOR - μ-Opioid Receptor; N/OFQR - Nociceptin/Orphanin FQ Receptor; NTSR - Neurotensin Receptor; OXR - Orexin Receptor; P2YR - Purinergic P2Y Receptor; PAR - Protease-Activated Receptor; PTHR - ParaThyroid Hormone-related peptide Receptor; PTHrP - ParaThyroid Hormone-related Peptide; RAMP - Receptor-Activity Modifying Protein; RHO - Rhodopsin; S1PR - Sphingosine-1-Phosphate Receptor; Smo - Smoothened Receptor; TSHR - Thyroid-Stimulating Hormone Receptor; Unc5D - Unc5D guidance receptor; US28 - Cytomegalovirus-encoded chemokine Receptor; VIPR - Vasoactive Intestinal Peptide Receptor; VR - Vasopressin Receptor).
Molecular docking is one of the most frequently used methods in structure-based drug design due to its ability to predict the conformation of ligands within an appropriate binding site with a considerable degree of accuracy [393-398]. Each ligand is docked onto the X-ray or NMR structure of the target protein or, if the 3D structure is not available, onto a model of the target (retrieved by homology modeling), applying molecular docking algorithms that explore the different binding poses of the ligands inside the binding site of the target. The identification of the most likely binding conformations involves the exploration of a large conformational space representing the various potential binding poses of the ligands and the prediction of the interaction energy associated to each of the predicted binding poses. Regarding the conformational search step, the structural parameters of ligands (translational, torsional, and rotational degrees of freedom) are increasingly modified, and several conformational search algorithms execute this stage by employing stochastic and systematic search methods [393-398]. Independently of the specificities of each search method, any conformational search algorithm should explore a broader range of energy landscape within an affordable computational time. Subsequently, the strength of the binding affinity of the predicted ligand-protein complexes is estimated by the use of scoring functions, which are given in most cases by the Gibbs free energy (ΔG) and the dissociation constant (Kd). Scoring functions are estimated mathematical functions that evaluate the most relevant physicochemical parameters involved in the ligand-protein interaction, in particular, the intermolecular interactions, desolvation, and entropic effects. The use of a high number of physicochemical parameters seems to increase the accuracy of the scoring function [393-398]. The molecular docking programs are executed through a cyclic and iterative process, in which the different ligand binding conformations are evaluated by the scoring functions until converging to a minimum energy conformation [393-398]. However, as the computational cost also increases proportionally with the number of included parameters, there should be a perfect combination between the accuracy and the speed of the calculation, which is crucial for databases containing a considerable number of chemical compounds. Nowadays, new scoring functions based on Machine-Learning (ML) algorithms are emerging [399, 400].
The determination of the scoring functions can be extremely useful in drug discovery for the virtual screening of commercially available compounds and in-house ligands that have been already synthesized and tested in vitro, or even pre-assembled databases of virtual drug candidates in order to identify the ligand structures that are most likely to interact to a protein target of interest, according to their docking scores. Apart from their applicability to virtual screening, the scoring functions can also be employed for de novo drug design of novel chemical structures targeting a specific protein and for hit-to-lead optimization of pharmacodynamic parameters of drug candidates [393-398]. Table 2 describes the most relevant docking studies performed for distinct chemical classes of modulators of GPCRs potentially involved in PD, using X-ray structures of GPCRs available on PDB and receptor models constructed from GPCR templates.
Table 2.
Structure-based drug design methodologies reported for ligands targeting GPCRs potentially involved in PD.
| Dopamine D2 Receptor (D2R) Agonists | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Method(s) | Ligand(s) | Relevant Ligand-receptor Interactions | ||||||||
| 1 | Docking onto D2R model using AutoDock 3.0.5 software package [401] (Template: X-ray structure of human β2AR; PDBid 2RH1 [272]) | Dopamine, 2-(aminomethyl)chromans | Dopamine: salt bridge interactions involving Asp114; hydrogen bond interactions with Ser193 and Ser197; π-π interactions involving Trp245, Phe248, and Phe249. 2-(Aminomethyl)chromans: salt bridge interactions involving Asp114; π-π interactions with Phe248; hydrogen bond interactions involving Ser193, Ser194, and/or Ser197 residues [402]. |
||||||||
| 2 | Docking onto D2R model using MOE software package [403] (Template: X-ray structure of human β2AR; PDBid 2RH1 [272]) | (R)-(-)-2-OH-NPA | R-(-)-2-OH-NPA: salt bridge interactions involving Asp114; hydrogen bond interactions with Asn186, Ser193, and Ser393; hydrophobic interactions involving Thr412 and π-π interactions with Phe390 [404]. | ||||||||
| 3 | Docking onto D2R model using GLIDE module from Schrödinger Suite [405-407] (Template: X-ray structure of D3R; PDBid 3PBL [294]) | Substituted piperidines, (2-methoxyphenyl) piperazines | Substituted piperidines: salt bridge interactions involving Asp114; π-π interactions with Phe393, His397, and the hydrophobic pocket composed by Phe386, Trp390, and Tyr420; hydrogen bond interactions with Ser193. (2-Methoxyphenyl) piperazines: salt bridge interactions involving Asp114; π-π interactions with Phe394 and the hydrophobic pocket composed by Phe386, Trp390, and Tyr420 [408]. |
||||||||
| 4 | Docking onto D2R model using GLIDE module of Schrödinger Suite [405-407] (Template: X-ray structure of D3R; PDBid 3PBL [294]) | 1-(2-Methoxyphenyl)-4-(1-phenethylpiperidin-4-yl)piperazines, 1-(2-methoxyphenyl)-4-[(1-phenethylpiperidin-4-yl)methyl]-piperazines | 1-(2-Methoxyphenyl)-4-(1-phenethylpiperidin-4-yl)piperazines: hydrophobic interactions with the hydrophobic pocket formed by Phe386, Trp390, and Tyr42 residues; salt bridge interactions involving Asp114; hydrogen bond interactions involving Asp114, Ser194, and Ser197 residues. Two possible binding conformations for 1-(2-Methoxyphenyl)-4-[(1-phenethylpiperidin-4-yl)methyl]-piperazines: (i) arylpiperazine moiety interacts with hydrophobic pocket of orthosteric binding site and the head part makes hydrogen bond interactions with Ser194 and Ser197; (ii) the head part interacts with hydrophobic pocket and arylpiperazine group makes hydrogen bond interactions with Ser194 and Ser197 [409]. |
||||||||
| 5 | Docking onto D2LR model using AutoDock Vina [410] and AutoDock 4.2 [411] softwares (Template: X-ray structure of D3R; PDBid 3PBL [294]) | (R)-7-OH-DPAT, (R)-7-OH-PIPAT, pramipexole, ropinirole, rotigotine, quinpirole, dopamine, PD128907 and cis-8-OH-PBZI | Hydrogen bond interactions involving Asp114, Val190, Ser193, and Ser194 residues; hydrophobic interactions involving Phe110, Val111, Val115, Ile184, Trp386, Phe389, Phe390, His393, Gly415, and Tyr416 residues [412]. | ||||||||
| 6 | Docking onto D2R model using AutoDock 3.0 software [401] (Template: X-ray structure of bovine RHO; PDBid 1F88 [231]) | Raclopride, L-741,626 | Raclopride: hydrogen bond interactions involving Asp114, Cys118, and Thr119; hydrophobic interactions with a hydrophobic region mainly composed by Tyr408 and Phe410 residues. L-741,626: Salt bridge interactions involving Asp114; hydrogen bond interactions with Ser221 and Thr412; π-π interactions involving Phe389 and Phe411 residues [413]. |
||||||||
| 7 | Docking onto D2R model using Docking module of INSIGHT II software (Accelerys Inc., Cambridge, UK) [414] (Template: X-ray structure of bovine RHO; PDBid 1F88 [231]) | 5-{2-[4-(2-Methoxyphenyl)piperazin-1-yl]ethoxy)-1,3-dihydro-2H-benzimidazole-2-thione | 5-{2-[4-(2-Methoxyphenyl)piperazin-1-yl]ethoxy)-1,3-dihydro-2H-benzimidazole-2-thione: salt bridge interactions involving Asp 86; hydrogen bond interactions with Trp115, Ser122, Ser141, Phe185, and His 189; π-π interactions involving Phe 178, Trp182, and Tyr216 residues [415]. | ||||||||
| 8 | Docking onto D2R using LIBDOCK module from Discovery Studio software [416] (Template: X-ray structure of bovine RHO; PDBid 1F88 [231]) | Arylpiperazines | Arylpiperazines: hydrophobic interactions involving Leu125, Leu126, Phe144, Val146, and Ile190 residues; hydrogen bond interactions involving Asn135 and Asn141; π-π interactions with Phe144 and His189 residues [417]. | ||||||||
| Dopamine D3 Receptor (D3R) Agonists | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 9 | Docking onto D3R model performed by CERIUS2 software (version 4.6) [418] (Template: X-ray structure of bovine RHO; PDBid 1F88 [231]) | (R)-(+)-7-OH-DPAT | R-(+)-7-OH-DPAT: salt bridge interactions involving Asp110; hydrogen bond interactions with Ser192; hydrophobic interactions with Phe106, Val107, Val111, Cys114, Phe345, Phe346, and Tyr373 residues [419]. | ||||||||
| Dopamine D3 Receptor (D3R) Agonists | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 10 | Docking onto X-ray structure of D3R (PDBid 3PBL [294]) using AutoDock Vina [410] and AutoDock 4.0 softwares [420, 421] | Glycyrrhetinic acid, E.resveratroloside, curcumin, hirsutanonol, glabridin, alloin, diacerein, bromocriptine, apomorphine, ropinirole | Hydrogen bond interactions with Asp110, Ser192, His349, Thr369, and Tyr373 residues; hydrophobic interactions involving the binding pocket Asp110, Ile183, Ser192, Phe346, His349, Thr369, and Tyr373 [422]. | ||||||||
| 11 | Docking onto X-ray structure of D3R (PDB id 3PBL [294]) using SurFlex Dock software method of SYBYL-X 1.3 package [423] | (E)-N-((1S,4r)-4-(2-(((S)-2-Amino-4,5,6,7-tetrahydro benzo[d]thiazol-6-yl)(propyl)amino)ethyl)cyclohexyl)-3-(4-chlorophenyl)acrylamide, N-((1S,4r)-4-(2-(((S)-2-amino-4,5,6,7-tetrahydrobenzo[d]thiazol-6-yl)(propyl) amino)ethyl)cyclohexyl)-3-(5-methyl-1,2,4-oxadiazol-3-yl)benzamide, N-((1S,4r)-4-(2-(((S)-2-amino-4,5,6,7-tetrahydrobenzo[d]thiazol-6-yl)(propyl)amino)ethyl) cyclohexyl)-2-chloropyridine-3-sulfonamide | (E)-N-((1S,4r)-4-(2-(((S)-2-Amino-4,5,6,7-tetrahydrobenzo[d]thiazol-6-yl)(propyl)amino)ethyl) cyclohexyl)-3-(4-chlorophenyl)acrylamide: hydrogen bond interactions with Asp110 and Ile183; van der Waals interactions with Phe106, Phe345, Tyr365, and Tyr373 residues; hydrophobic interactions involving Ser182, Phe345, and Ser366 residues. N-((1S,4r)-4-(2-(((S)-2-Amino-4,5,6,7-tetrahydrobenzo[d]thiazol-6-yl)(propyl)amino)ethyl) cyclohexyl)-3-(5-methyl-1,2,4-oxadiazol-3-yl)benzamide: hydrogen bond interactions with Asp110 and Ile183; hydrophobic interactions involving Val111, Ser182, Ile183, Val189, Ser192, Phe345, Phe346 residues. N-((1S,4r)-4-(2-(((S)-2-Amino-4,5,6,7-tetrahydrobenzo[d]thiazol-6-yl)(propyl)amino)ethyl) cyclohexyl)-2-chloropyridine-3-sulfonamide: hydrogen bond interactions with Glu90 and Ser192; hydrophobic interactions with Asp110, Ser182, Ile 183, Phe345, Phe346, and Tyr373 residues [424]. |
||||||||
| 12 | Docking onto X-ray structure of D3R (PDB id 3PBL [294]) using AutoDock Vina [410] and AutoDock 4.2 [411] softwares | R-7-OH-DPAT, R-7-OH-PIPAT, pramipexole, ropinirole, rotigotine, quinpirole, dopamine, PD128907 and cis-8-OH-PBZI | Hydrogen bond interactions involving Asp110, Val111, Thr115, Ser192, and Ser196 residues; hydrophobic interactions with Phe106, Val107, Val111, Ile183, Phe188, Trp342, Phe345, Phe346, His349, Thr369, and Tyr373 residues [412]. | ||||||||
| 13 | Docking onto X-ray structure of D3R (PDB id 3PBL [294]) using InducedFit docking software [425-427] | [4-(4-Carboxamidobutyl)]-1-arylpiperazines | N-(4-(4-(2-(tert-Butyl)-6-(trifluoromethyl)pyrimidin-4-yl)-piperazin-1-yl)butyl)imidazo[1,2-a]pyridine-2-carboxamide: hydrogen bond interactions involving Thr115 and Ser196; hydrophobic interactions with Ile183, Val189, and Val350 residues. The arylamide moiety of this ligand and other analogues may adopt three different conformations: (i) the arylamide group is docked onto Glu90; (ii) the arylamide moiety is placed in proximity to Val180 and Ser182 residues; (iii) the arylamide moiety is involved in π-π interactions with Tyr365 and in hydrogen bond interactions with Thr369 [66]. | ||||||||
| Adenosine A2A receptor (A2AAR) antagonists | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 14 | Docking onto A2AAR model using MOE software [428] (Template: X-ray structure of A2AAR; PDBid 3EML [44]) | 8-Substituted 9-ethyladenine derivatives | Two different ligand binding orientations towards A2AAR, interacting with key residues Phe163, Glu164, and Asn248 of A2AAR model (corresponding to Phe168, Glu169, and Asp253 of human A2AAR). Ligand binding orientation 1: π-π interactions involving Phe163; hydrogen bond interactions with Glu164 and Asn248. Ligand binding orientation 2: π-π interactions involving Phe163; hydrogen bond interactions with Asn248 [96]. | ||||||||
| 15 | Docking onto X-ray structure of A2AAR (PDBid 4EIY [244]) using AutoDock 4.2 software [411] | 3-[4-Amino-6-(2-chlorobenzyl)thieno[2,3-d] pyrimidin-2-yl]benzonitrile | 3-[4-Amino-6-(2-chlorobenzyl)thieno[2,3-d]pyrimidin-2-yl]benzonitrile: hydrophobic interactions involving Leu190, Leu194, Tyr197, Phe201, Ala236, Val239, and Ala243 residues [429]. | ||||||||
| 16 | Docking onto X-ray structure of A2AAR (PDBid 3EML [44]) using GLIDE module from Schrödinger suite [405-407] | 10 ligands with the best docking scores selected from a library of 46 A2AAR antagonists | Hydrogen bond interactions involving Asn253 for all ligands and Ile180, Ala81, and Tyr271 residues for some ligands; π-π interactions with Phe168 [430]. | ||||||||
| 17 | Docking onto X-ray structure of A2AAR (PDBid 3EML [44]) using DOCK 5.4 software [431] | 7-Substituted 5-amino-2-(2-furyl)pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines, piperazine derivatives of triazolotriazine and triazolopyrimidines | 7-Substituted 5-amino-2-(2-furyl)pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines: hydrogen bond interactions involving Thr88; π-π interactions involving Phe182 and Phe257 residues; hydrophobic interactions with Ile66, Ile92, Ile244, and Trp276 residues. Piperazine derivatives of triazolotriazine and triazolopyrimidines: hydrogen bond interactions involving Thr27; π-π stacking interactions with Phe182; hydrophobic interactions involving Ile92, Phe93, and Val186 residues [432]. |
||||||||
| Dopamine D3 Receptor (D3R) Agonists | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 18 | Docking onto X-ray structure of A2AAR (PDBid 4EIY [244]) using GLIDE module from Schrödinger suite [405-407] | 5’-Substituted amiloride derivatives | 5’-Substituted amiloride derivatives: hydrogen bond and salt bridge interactions with Asn52 and Thr88; π-π interactions with Trp246; hydrophobic interactions with Phe168, Met177, Leu249, and Ile274 residues [433]. | ||||||||
| 19 | Docking onto A2AAR model using GLIDE module from Schrödinger suite [405-407] (Template: X-ray structure of β1AR; PDBid 2VT4 [262]) | 4-(3-Aminoamino-5-phenyl-1,2,4-triazin-6-yl)-2-chlorophenol, 6-(2,6-dimethylpyridin-4-yl)-5-phenyl-1,2,4-triazin-3-amine | 4-(3-Amino-5-phenyl-1,2,4-triazin-6-yl)-2-chlorophenol: hydrogen bond interactions involving Asn253 and His278; π-π interactions with Phe168, and hydrophobic interactions involving Met270. 6-(2,6-Dimethylpyridin-4-yl)-5-phenyl-1,2,4-triazin-3-amine: hydrogen bond interactions involving Asn253; π-π interactions with Phe168; and hydrophobic interactions with Met270, with a pocket comprised by Leu84, Leu85, Met177, Asn181, Trp246, Leu249, and His250 and a pocket formed by Ala63, Ile66, Ser277, and His278 residues [243]. |
||||||||
| 20 | Docking onto A2AAR model using FlexiDock utility in the Biopolymer module of SYBYL 6.7.1 (Tripos, St Louis, MO) [434] (Template: X-ray structure of RHO; PDBid 1BRD [435]) | CGS15943 | CGS15943: hydrophobic interactions between quinazoline ring and Leu85, Ile135, Leu167, Phe168, Phe182, Val186, Trp246, and Leu249, and between furan ring and Ile80, Val84, and Ile274; hydrogen bond interactions involving Asn181 and Asn253 residues [436]. | ||||||||
| 21 | Docking onto X-ray structure of A2AAR (PDBid 3EML [44]) using GLIDE module from Schrödinger suite software [405-407], InducedFit docking software [425-427], and ICM molecular modeling software (Molsoft, LLC) [437] | ZM241385 | ZM241385: hydrogen bond interactions involving Glu169 and Asn253 residues; hydrophobic interactions with Phe168, His264, Ile267, Met270, and Ile274 residues [438-440]. | ||||||||
| 22 | Docking onto A2AAR model using GLIDE module from Schrödinger suite software [405-407] and InducedFit docking software [425-427] (Template: X-ray structure of β2AR; PDBid 2RH1 [272]) | ZM241385 | ZM241385: hydrogen bond interactions involving Asn253; hydrophobic interactions with Leu85, Thr88, Trp246, and Leu249 residues; π-π interactions with Phe168 [438]. | ||||||||
| 23 | Docking onto X-ray structure of A2AAR (PDBid 3EML [44]) using AutoDock Vina program [411]. | Caffeine | Five binding poses of caffeine to A2AAR were analysed and the most relevant residues involved in caffeine-A2AAR interaction are: Ala63, Val84, Leu85, Thr88, Phe168, Glu169, Met177, Trp246, Leu249, His250, Asn253, Ile274, and His278 [441]. | ||||||||
| 24 | Docking onto A2AAR model using CAChe 6.1 [442] and CAChe 7.5 softwares [442] (Template: X-ray structures of bovine RHO and β2AR; PDBid 1U19 [332] and 2RH1 [272]) | XAC, KW6002, ZM241385 | In bovine rhodopsin, the purine ring of XAC and the phenyl rings of KW6002 and ZM241385 are accommodated in a pocket formed by Leu85, Thr88, Gln89, Ile135, Leu167, Phe168, Val178, Asn181, and Phe182. The purine ring of KW6002 and ZM241385 and the aminoethylphenoxyacetamid group of XAC occupies a pocket comprised by Glu13, Val55, Ile60, Ala63, Phe80, Ile274, Ile275, and His278. The furan moiety of ZM-241385 interacts with a lipophilic pocket formed by Phe62, Ile66, and Val164. In β2AR, the purine ring of ligands is accommodated in a pocket formed by Leu48, Ala51, Asp52, Leu87, Thr88, Ser91, Leu95, Ile238, Phe242, Trp246, His250, Ser277, His278, Asn280, Ser281, and Asn285. The aromatic rings are placed in a pocket comprised by Val55, Ala59, Val60, Phe62, Ala63, Val84, Gln89, Glu169, Trp246, Ile274, and His278. XAC makes hydrogen bond interactions with Glu169, Ser277, His278, and Ser281 residues [443]. |
||||||||
| 25 | Docking onto a theoretical model of A2AAR (PDBid 1MMH) using DOCK 5.4 software [431] | Xanthine derivatives (KW6002, KF17837, BS-DMPX) Non-xanthine derivatives (7-substituted 5-amino-2-(2-furyl)pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines and piperazine derivatives of [1,2,4]triazolo[1,5-a]triazine) |
Xanthine derivatives: hydrogen bond interactions involving Ser277; π-π interactions involving Tyr179, His250, and Phe257 residues; hydrophobic interactions with hydrophobic domains located at the entrance and at the bottom of binding pocket formed by Ser91, Ile92, Trp246, Leu249, Ala273, and His278 residues. Non-xanthine derivatives: hydrogen bond interactions involving Thr88 and Thr271 residues; π-π interactions involving Phe82 and Phe257 residues. The furan ring accomodates in a hydrophobic pocket comprised by Ile66, Ile92, Ile244, and Trp276 in 7-substituted 5-amino-2-(2-furyl)pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines and in a hydrophobic pocket comprised by Ile92, Phe93, and Val186 in piperazine derivatives of [1,2,4]triazolo[1,5-a]triazine) [444]. |
||||||||
| Dopamine D3 Receptor (D3R) Agonists | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 26 | Docking onto TM2, TM3, TM5, TM6, and TM7 domains of A2AAR (PDBid 3PWH [241]) performed by Autodock using Lamarckian Genetic Algorithm [401] | Pyrimidine and triazine derivatives, pyrazolo[3,4-d]pyrimidines, pyrrolo[2,3-d]pyrimidines, triazolo[4,5-d]pyrimidines, 6-arylpurines, thieno[3,2-d]pyrimidines | Hydrogen bond interactions involving Glu169 and Asn253 residues; hydrophobic interactions with Val84, Leu249, Met270 and Ile274; π-π interactions with Phe168 of A2AAR [445]. | ||||||||
| 27 | Docking onto X-ray structure of A2AAR (PDBid 3PWH [241]) using GLIDE module from Schrödinger suite [405-407] | 2-(Furan-2-yl)-[1,2,4]triazolo[1,5-f]pyrimidin-5-amines, 2-(furan-2-yl)-[1,2,4]triazolo[1,5-a]pyrazin-8-amines, 2-(furan-2-yl)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-7-amines | Hydrogen bond interactions involving Glu169 and Asn253 residues; π-π interactions with Phe168 and His250. Weak interactions with Ser67 (hydrogen bond interactions) and Tyr271 (π-π interactions) for some derivatives [446]. | ||||||||
| M1 muscarinic acetylcholine receptor (mAChR1) antagonists / negative allosteric modulators (NAMs) | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 28 | Docking onto X-ray structure of mAChR1 (PDBid 5CXV [307]) using GLIDE module from Schrödinger suite [405-407] | Tiotropium | Tiotropium: hydrogen bond interactions involving Asp residue [307]. | ||||||||
| 29 | Docking onto mAChR1 model using DOCK 4.01 [447], FlexX 1.8 [448], and GOLD 1.1 softwares [449] (Template: X-ray structure of bovine RHO; PDBid 1F88 [231]) |
Pirenzepine | Pirenzepine: salt bridge interactions between the basic nitrogen atom and Asp residue at TM3, the lactam ring interacts with Asn residue at TM6 and its aromatic nitrogen atom with Thr residue at TM5 [450]. | ||||||||
| 30 | Docking onto mAChR1 model using GOLD software [449] (Template: X-ray structure of bovine RHO; PDBid 1F88 [231]) | N-Methyl-4-piperidyl benzylates | N-Methyl-4-piperidyl benzylates: salt bridge interactions involving Asp105; hydrophobic interactions involving Tyr404; hydrogen bond interactions with Tyr179 [116]. | ||||||||
| Metabotropic Glutamate Receptor 2 (MGluR2) agonists / positive allosteric modulators (PAMs) | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 31 | Docking onto mGluR2 model using GLIDE module from Schrödinger suite [405-407] (Template: X-ray structures of mGluR1, mGluR5, and β2AR (PDBid 4OR2 [377], 4OO9 [381], and 3SN6 [277]) | JNJ-42153605 | JNJ-42153605: hydrophobic interactions involving Leu639, Phe643, Leu732, Trp773, and Phe776 residues; hydrogen bond interactions involving Ser731 and Asn735; π-π interactions with His723 [451]. | ||||||||
| 32 | Docking onto X-ray structure of mGluR2 (PDBid 5CNJ [379]) using MOE v. 2013.08 [452] | LY2812223 | LY2812223: salt bridge interactions involving Arg61, Asp295, and Lys377 residues; hydrogen bond interaction involving Ser165, Ala 166, Thr168, and Glu273 residues; water-mediated hydrogen bond interactions with Asp301; π-H interactions with the Ser272 [379]. | ||||||||
| Metabotropic Glutamate Receptor 3 (MGluR3) agonists / positive allosteric modulators (PAMs) | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 33 | Docking onto X-ray structure of mGluR3 (PDBid 2E4U [380]) using Induced Fit algorithm [425-427] | Glutamate | Glutamate: hydrogen bond interactions with Ser151, Ala172, Ser173, and Thr174 residues; salt bridge interactions involving Arg64, Arg68, Asp301, and Lys389 residues [453]. | ||||||||
| 34 | Docking onto X-ray structures of mGluR3 (PDBid 2E4V [380], 2E4W [380], 2E4X [380], and 2E4Y [380]) | DCG-IV, 1S,3S-ACPD, 1S,3R-ACPD, 2R,4R-APDC | DCG-IV: hydrogen bond interactions involving Arg68, Ser151, Ala172, Thr174, Tyr222, Ser278, Asp301, and Lys389; water-mediated hydrogen bond interactions with Arg64; van der Walls interactions involving Tyr150. 1S,3S-ACPD: hydrogen bond interactions involving Arg68, Ser151, Ala172, Thr174, Asp301, and Lys389; water-mediated interactions with Arg64 and Ser278; van der Waals interactions involving Tyr222 and Gly302. 1S,3R-ACPD: hydrogen bond interactions involving Arg68, Ser151, Ala172, Thr174, Asp301, and Lys389; van der Waals interactions with Tyr222 and Gly302. 2R,4R-APDC: hydrogen bond interactions involving Arg68, Ser151, Ala172, Thr174, Asp301, and Lys389; van der Waals interactions with Tyr222 [380]. |
||||||||
| Metabotropic Glutamate Receptor 4 (MGluR4) agonists / positive allosteric modulators (PAMs) | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 35 | Docking onto mGluR4 model using AutoDock 3.0 software [401] (Template: X-ray structure of mGluR1; PDBid 1EWK [378]) | L-Glutamate, L-AP4, S-PPG | L-Glutamate, L-AP4, and S-PPG interact with the agonist binding site of mGluR4 composed by Arg78, Ser159, and Thr182 [454]. | ||||||||
| 36 | Docking onto VFT domain of mGluR4 using Discovery Studio 2.5.5 software (Accelerys Inc., Cambridge, UK) [455] | LSP4-2022 | The hydroxybenzilic moiety of LSP4-2022 interacts with Lys74 and Lys317 and the glutamate-like moiety interacts with Arg78, Ser159, Ala180, Thr182, Tyr230, Asp312, and Lys405. The phenoxyacetic acid group of LSP4-2022 is involved in hydrogen bond interactions with Thr108, Ser157, and Gly158 [456]. | ||||||||
| 37 | Docking onto mGluR4 model using Discover 3.0 and WHATIF softwares [413] (Template: X-ray structures of LIVBP, LBP, and AmiC; PDBid 2LIV [457], 2LBP [458], and 1PEA [459]) | ACTP-I | Docking to the open form of mGluR4 model, built from LIVBP and LBP templates, has shown that ACTP-I interacts with Lys74, Arg78, Ser159, and Thr182. Docking to the closed form of mGluR4 model, built from AmiC template, has shown that ACTP-1 interacts with Tyr230, Asp312, Ser313, and Lys317 [460]. | ||||||||
| Metabotropic Glutamate Receptor 5 (MGluR5) antagonists / negative allosteric modulators (NAMs) | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 38 | Docking onto X-ray structure of mGluR5 (PDBid 4OO9 [381]) using Maestro v.9.3 (Schrodinger) software [461] | Mavoglurant | Mavoglurant: hydrogen bond interactions involving Ser805, Ser809, and Asn747 residues; hydrophobic interactions involving two pockets (one hydrophobic pocket formed by Gly624, Ile625, Gly628, Ser654, Pro655, Ser658, Tyr659, Val806, Ser809, Ala810, and Ala813 residues, and other hydrophobic pocket consisted of Ile651, Val740, Pro743, and Leu744 residues) [381]. | ||||||||
| 39 | Docking onto X-ray structure of mGluR5 (PDBid 5CGC [382], 5CGD [382]) using PyMOL (Schrodinger) [462] | 3-Chloro-4-fluoro-5-[6-(1H-pyrazol-1-yl) pyrimidin-4-yl]benzonitrile, HTL14242 | 3-Chloro-4-fluoro-5-[6-(1H-pyrazol-1-yl) pyrimidin-4-yl]benzonitrile: hydrogen bond interactions involving Ser809; water-mediated hydrogen bond interaction with Val 740; π-π interactions with Trp785 and Phe788 residues. HTL14242: hydrogen bond interactions involving Ser809; water-mediated hydrogen bond interaction with Val 740; π-π interactions with Tyr659, Trp785, and Phe788 residues. The pyrazole and pyridyl rings of 3-chloro-4-fluoro-5-[6-(1H-pyrazol-1-yl) pyrimidin-4-yl]benzonitrile and HTL14242, respectively, are docked onto a pocket defined by Ile625, Gly628, Ser654, Pro655, Ser658, Tyr659 and Ser809. The pyrimidine linker of both ligands is located in a pocket formed between Pro655, Tyr659, Val806, and Ser809 residues [382]. |
||||||||
| 40 | Docking onto X-ray structure of mGluR5 (PDBid 4OO9 [381]) using CDocker [463] in Discovery Studio 3.1 (Accelrys Inc., Cambridge, UK) | 4-Bromo-N-(3-bromophenyl)thiazole-2-carboxamide, 4-bromo-N-(6-methylpyridin-2-yl)thiazole-2-carboxamide | 4-Bromo-N-(3-bromophenyl)thiazole-2-carboxamide: hydrogen bond interactions involving Tyr659; hydrophobic interactions involving two pockets (one hydrophobic pocket formed by Gly624, Ile625, Gly628, Ser654, Pro655, Ser658, Tyr659, Val806, Ser809, Ala810, and Ala813 residues, and other hydrophobic pocket defined by Pro655, Tyr659, Val806, and Ser809 residues. 4-Bromo-N-(6-methylpyridin-2-yl)thiazole-2-carboxamide: hydrogen bond interactions involving Tyr659; hydrophobic interactions with Ile625, Pro655, Ala810, and Ala813 residues [446] [464]. |
||||||||
| 41 | Docking onto X-ray structure of mGluR5 (PDBid 4OO9 [381]) using CDocker software [463] | Aglaiduline, 5-O-ethyl-hirsutanonol, yakuchinone A | Aglaiduline: hydrophobic interactions involving two pockets (one hydrophobic pocket formed by Arg648, Ile651, Val740, and Pro743 residues, and other hydrophobic pocket defined by Ser654, Pro655, and Ala810 residues; hydrogen bond interactions involving Asn747. Yakuchinone A: hydrophobic interactions involving two pockets (one hydrophobic pocket formed by Arg648, Ile651, Val740, and Pro743 residues, and other hydrophobic pocket defined by Ser654, Pro655, and Ala810); hydrogen bond interactions involving Ser805. 5-O-Ethyl-hirsutanonol: hydrogen bond interactions involving Ser805 and Ser809 [465]. |
||||||||
| 42 | Docking onto mGluR5 model using AutoDock 3.0.5 software [401] (Template: X-ray structures of mGluR1; PDBid 1EWK [378], 1EWV [378], 1EWT [378]) | S4-CPG, MCPG | Hydrogen bond interactions involving Tyr43, Ser152, and Thr154 residues [466]. | ||||||||
| 43 | Docking onto mGluR5 model using ROSETTA v3.4 software [467] (Template: X-ray structures of β2AR, S1PR1, and D3R; PDBid 2RH1 [272], 3V2Y [357], and 3PBL [294]) | CHPyEPC, 1,4-BisPEB, MPEP, 1,3-BisPEB | All NAM ligands make interactions with Leu630, Ile651, Gly652, Pro655, Trp785, Phe788, Tyr792, Val806, and Ser809 residues. CHPyEPC, 1,4-BisPEB, and 1,3-BisPEB interact with Val740, Pro743, Leu744, and Asn747 residues [468]. | ||||||||
| 5-Hydroxytryptamine Receptor 1A (5-HT1AR) Agonists | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 44 | Docking onto 5-HT1AR model using Affinity module from INSIGHT II software [414] (Template: X-ray structures of RHO; PDBid 1F88 [231], 1HZX [328], and 1L9H [330]) | N1-Substituted N-arylpiperazines | N1-Substituted N-arylpiperazines: salt bridge interactions involving Asp116; π-π interactions involving Phe361 and Tyr390 residues; hydrogen bond interactions with Thr188. Some ligands are involved in π-π interactions with hydrophobic pocket formed by Phe204, Leu 359, and Phe362 [469]. | ||||||||
| 45 | Docking onto 5-HT1AR model using Affinity module from INSIGHT II software [414] (Template: X-ray structures of RHO; PDBid 1F88 [231], 1HZX [328], and 1L9H [330]) | 1-Cinnamyl-4-(2-methoxyphenyl) piperazines | 1-Cinnamyl-4-(2-methoxyphenyl)piperazines: salt bridge interactions involving Asp116; π-π interactions involving Phe361 and Tyr390 residues; hydrogen bond interactions with Thr188 [470]. | ||||||||
| 46 | Docking onto 5-HT1AR model using Affinity from INSIGHT II software [414] (Template: X-ray structures of RHO; PDBid 1F88 [231], 1HZX [328], and 1L9H [330]) | 4-Halo-6-[2-(4-arylpiperazin-1-yl)ethyl]-1H-benzimidazoles | 4-Halo-6-[2-(4-arylpiperazin-1-yl)ethyl]-1H-benzimidazoles: salt bridge interactions involving Asp116; hydrogen bond interactions involving Ser199 and Trp358; π-π interactions involving Phe361 and Tyr390 residues [471]. | ||||||||
| 47 | Docking onto 5-HT1AR model using FlexX-Pharm from SYBYL 7.0 software [472] (Template: X-ray structure of bovine RHO; PDBid 1F88 [231]) | Arylpiperazines | Arylpiperazines: salt bridge interactions involving the Asp residue; π-π interactions with the Phe residue; hydrogen bond interactions with Asn, Cys, Ser, Thr, and Tyr residues [473]. | ||||||||
| 48 | Docking onto 5-HT1AR model using AutoDock 3.0.5 software [401] (Template: X-ray structure of RHO; PDBid 2R4R [271]) | 1-(Benzo[b]thiophen-2-yl)-3-(4-arylpiperazin-1-yl)-propan-1-one derivatives | 1-(Benzo[b]thiophen-2-yl)-3-(4-pyridin-2-yl)-piperazin-1-yl)-propan-1-one: salt bridge and hydrogen bond interactions involving Asp116; ion-dipole interactions with Asn385; π-π interactions involving Phe361 [474]. | ||||||||
| 49 | Docking onto 5-HT1AR model using GOLD 4.0 software [449] (Template: X-ray structure of β1AR; PDBid 2Y03 [263]) | Carboxamide and sulfonamide alkyl piperazine analogues | Carboxamide and sulfonamide alkyl piperazine analogues: salt bridge interactions involving Asp116; hydrogen bond interactions with Ser199 and Thr200; π-π interactions involving Tyr96, Phe361, Phe362, Trp387, and Tyr390 residues [475]. | ||||||||
| 50 | Docking onto 5-HT1AR model using AutoDock 4.0 software [411] (Template: X-ray structure of β2AR; PDBid 3P0G [276]) | 3-[3-(4-Aryl-1-piperazinyl)-propyl]-1H-indole derivatives | 3-[3-(4-Aryl-1-piperazinyl)-propyl]-1H-indole derivatives: salt bridge interactions involving Asp116; π-π interactions with Phe and Tyr residues; hydrogen bond interactions with the Ser residue [476]. | ||||||||
| 5-Hydroxytryptamine receptor 2A (5-HT2AR) antagonists | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 51 | Docking onto 5-HT2AR model using QXP software [477] (Template: X-ray structure of bovine RHO; PDBid 1F88 [231]) | (Aminoalkyl)benzo and heterocycloalkanones | (Aminoalkyl)benzo and heterocycloalkanones: hydrogen bond interactions involving Cys148, Asp155, and Ser159; hydrophobic interactions with residues located in TM2 and TM7 [478]. | ||||||||
| 52 | Docking onto X-ray structure of 5-HT2AR (PDBid 2VT4 [262]) using GLIDE module from Schrödinger suite [405-407] | 4-Aryl-2,7,7-trimethyl-5-oxo-N-phenyl- 1,4,5,6,7,8-hexahydroquinoline-3-carboxamides | 4-Aryl-2,7,7-trimethyl-5-oxo-N-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxamides: interaction with Cys199, Asn310, and Asn329 residues [479]. | ||||||||
| 53 | Docking onto 5-HT2AR model using GOLD 4.12 software [449] (Template: X-ray structure of β2AR; PDBid 2RH1 [272]) | Ketanserin, 05245768 | Ketanserin: salt bridge interactions involving the Asp residue; hydrogen bond interactions involving the Ser residue; hydrophobic interactions with Phe and Tyr residues. 05245768: salt bridge interactions involving the Asp residue; hydrogen bond interactions involving the Ser residue; hydrophobic interactions with Ile, Phe, and Tyr residues [480]. |
||||||||
| 54 | Docking onto 5-HT2AR model using FlexiDock from SYBYL-X 1.2 software (Tripos, St Louis, MO) [434] (Template: X-ray structure of β2AR; PDBid 2RH1 [272]) | (2R,4S)-PAT, (2S,4R)-PAT, (2R,4S)-4’-Cl-PAT, (2S,4R)-4’-Cl-PAT | (2S,4R)-PAT: salt bridge interactions involving the Asp residue; π-π interactions involving the Phe residue. (2R,4S)-PAT and (2S,4R)-4’-Cl-PAT: salt bridge interactions involving the Asp residue (2R,4S)-4’-Cl-PAT salt bridge interactions involving the Asp residue; hydrogen bond interactions with the Ser residue [481]. |
||||||||
| 5-Hydroxytryptamine Receptor 1A (5-HT1AR) Agonists | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 55 | Docking onto 5-HT2AR model using GLIDE module from Schrödinger suite [405-407] (Template: X-ray structure of β2AR; PDBid 2RH1 [272]) | Nefazodone, aripiprazole, haloperidol, cyproheptadine, trazodone, clozapine, ketanserin, spiperone, risperidone |
Interaction with a binding pocket comprised by Ile152, Asp155, Val156, Ser207, Met208, Pro211, Leu229, Asp231, Val235, Leu236, Ser239, Phe339, Asn343, and Asn363 residues [482]. | ||||||||
| 56 | Docking onto 5-HT2AR model using AutoDock Vina 1.1.1 [410] (Template: X-ray structure of β2AR; PDBid 3D4S [273]) | Arylpiperazines | Arylpiperazines: salt bridge interactions involving Asp155; hydrogen bond interactions with Asn343; hydrophobic interactions involving two hydrophobic pockets (one hydrophobic pocket comprised by Leu123, Ser159, Trp336, Phe339, Val366, and Tyr370 residues, and the other pocket formed by Trp151, Ile152, Leu229, Ala230, Phe234, and Val235 residues) [483]. | ||||||||
| 57 | Docking onto 5-HT2AR model using ICM Pro docking algorithm [437], GLIDE from Schrödinger suite [405-407], and GOLD programs [449] (Template: X-ray structures of bovine RHO and β2AR; PDBid 1U19 [332] and 2RH1 [272]) | Nantenine analogues | Nantenine analogues: salt bridge interactions involving Asp155; hydrogen bond interactions involving Asn343. The majority of nantenin analogus interacts with Asp155, Val156, Ser159, Ile210, Leu228, Phe234, Gly238, Ser242, and Ile341 residues. Other ligands interact with Ile152, Thr160, Ile163, Ile206, Ser239, Phe243, Ser244, Pro338, Phe339, Phe340, Thr342, Asn343, Met345, Val366, and Gly369 residues [484]. | ||||||||
| 58 | Docking onto 5-HT2AR model using GLIDE module from Schrödinger suite [405-407] (Template: X-ray structure of human A2AAR; PDBid 2YDV [240]) | Ketanserin, risperidone, ziprasidone | Ketanserin: salt bridge and hydrogen bond interactions involving Asp155; hydrogen bond interactions with Trp151; hydrophobic interactions with Phe125 and Val130; van der Waals interactions involving Leu126, Pro129, Leu154, Phe158, Val204, and Met208 residues. Risperidone: salt bridge and hydrogen bond interactions involving Asp155; hydrogen bond interactions with Trp151; van der Waals interactions involving Leu126, Val130, Ile152, Leu154, Phe158, and Met208 residues. Ziprasidone: hydrogen bond interactions involving Trp151; π-π interactions involving Phe158; hydrophobic interactions involving Leu122, Leu126, and Phe158 residues; van der Waals interactions involving Ile118, Phe193, Ile196, Ile197, and Trp200 residues [485]. |
||||||||
| 59 | Docking onto 5-HT2AR model using GLIDE module from Schrödinger suite [387-389] (Template: X-ray structure of human A2AAR; PDBid 2YDV [240]) | N-[3-(4-(1H-indol-2-yl)phenoxy)propyl]-4-chlorophenylamine | N-[3-(4-(1H-Indol-2-yl)phenoxy)propyl]-4-chlorophenylamine: hydrogen bond interactions involving Leu362 and Asn363 residues; π-π interactions involving Phe339; hydrophobic interactions with Thr342 and Asn343; van der Waals interactions with Leu136, Ala230, Phe339, Asn343, Ala346, Val347, Glu355 and Val366 residues [485]. | ||||||||
| 60 | Docking onto 5-HT2AR model using AutoDock 4.2 software [411] (Template: X-ray structure of β2AR; PDBid 2RH1 [272]) | (R)-Roemerine | (R)-Roemerine: salt bridge and hydrogen bond interactions involving Asp155; dipole-dipole interactions involving Ser159 and Tyr370, van der Waals interactions involving Ser239, Ala242, Trp336, Phe339, Phe340, Val366, and Tyr370 residues [486]. | ||||||||
| 5-Hydroxytryptamine Receptor 2C (5-HT2CR) Antagonists | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 61 | Docking onto 5-HT2CR model using QXP software [477] (Template: X-ray structure of bovine RHO; PDBid 1F88 [231]) | (Aminoalkyl)benzo and heterocycloalkanones | (Aminoalkyl)benzo and heterocycloalkanones: hydrogen bond interactions involving Cys148, Asp155, and Ser159; hydrophobic interactions with residues located in TM2 and TM7 [478]. | ||||||||
| 62 | Docking onto 5-HT2CR model using FlexX software [448] (Template: X-ray structure of β2AR; PDBid 2RH1 [272]) | N-(3-(4-Methylimidazolidin-1-yl)phenyl)-5,6-dihydrobenzo[h]quinazolin-4-amine, N-(4-methoxy-3-(4-methylpiperazin-1-yl)phenyl)-1,2-dihydro-3H-benzo[e] indole-3-carboxamide, 1-(3,5-difluoro phenyl)-3-(4-methoxy-3-(2-(piperidin-1-yl)ethoxy)phenyl) imidazolidin-2-one, N-(3-(2-((3-(piperazin-1-yl)pyrazin-2-yl) oxy)ethoxy) benzyl)propan-2-amine | Hydrophobic interactions involving Val106, Val135, Phe137, Phe214, Val215, Val221, Ala222, Phe223, Trp324, Phe327, Phe328, Leu350, and Leu354 residues. N-(3-(4-Methylimidazolidin-1-yl)phenyl)-5,6-dihydrobenzo[h]quinazolin-4-amine: hydrogen bond interactions with Asp134 and Arg195 residues. N-(4-Methoxy-3-(4-methylpiperazin-1-yl)phenyl)-1,2-dihydro-3H-benzo[e]indole-3-carboxamide: hydrogen bond interactions involving Asp134 and Tyr358 residues. 1-(3,5-Difluorophenyl)-3-(4-methoxy-3-(2-(piperidin-1-yl)ethoxy)phenyl) imidazolidin-2-one: hydrogen bond interactions involving Arg195 and Tyr358 residues. N-(3-(2-((3-(Piperazin-1-yl)pyrazin-2-yl)oxy)ethoxy)benzyl)propan-2-amine: hydrogen bond interactions involving Arg195, Val208, Asn351, and Tyr358 residues [487]. |
||||||||
| 5-Hydroxytryptamine Receptor 2C (5-HT2CR) Antagonists | |||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | ||||||||
| 63 | Docking onto 5-HT2CR model using FlexiDock from SYBYL-X 1.2 software (Tripos, St Louis, MO) [434] (Template: X-ray structure of β2AR; PDBid 2RH1 [272]) | (2R,4S)-PAT, (2S,4R)-PAT, (2R,4S)-4’-Cl-PAT, (2S,4R)-4’-Cl-PAT | (2S,4R)-PAT: salt bridge interactions involving the Asp residue; π-π interactions involving the Phe residue. (2R,4S)-PAT and (2S,4R)-4’-Cl-PAT: salt bridge interactions involving the Asp residue (2R,4S)-4’-Cl-PAT salt bridge interactions involving the Asp residue; hydrogen bond interactions with the Ser residue [481]. |
||||||||
| 64 | Docking onto 5-HT2CR model using GLIDE module from Schrödinger suite [387-389] [405-407] (Template: X-ray structure of β2AR; PDBid 2RH1 [272]) | (E)-3-(2-chlorophenyl)-1-(5-methoxy-6-(2-(piperidin-1-yl)ethoxy)indolin-1-yl)prop-2-en-1-one | (E)-3-(2-chlorophenyl)-1-(5-methoxy-6-(2-(piperidin-1-yl)ethoxy)indolin-1-yl)prop-2-en-1-one: salt bridge interactions involving Asn331, Ser334 and Val354 residues; hydrogen bond interactions involving Asn331; hydrophobic interactions involving Val135, Val208, Phe214, Ala222, Phe327, Phe328, and Val354 residues [488]. | ||||||||
2.6.2. Application of Ligand-based and Pharmacophore-based Design Techniques for GPCR-based Drug Discovery
The availability of structural data information for multiple GPCRs still remains scarce and, for that reason, computational drug design strategies have relied on theoretical models, in which the 3D model of a receptor structure is constructed by applying homology modeling techniques [489] and on ligand-based approaches [490]. The identification of an increasing number of small-molecule modulators with diverse chemical scaffolds together with experimental biological/pharmacological data (e.g. binding affinity values) has helped to the increased development of ligand-based drug design approaches. Among these ligand-based approaches, numerous CADD methodologies can be employed to large databases of compounds with drug-like properties ranging from pharmacophore modeling to quantitative structure-activity relationships (QSAR) and 3D-QSAR techniques such as Comparative Molecular Field Analysis (CoMFA) and Comparative Molecular Similarity Index Analysis (CoMSIA) [490].
The generation of pharmacophore models involves the identification of steric and electronic determining features that assure an optimal interaction towards a particular therapeutic target, triggering its pharmacological activity [491]. Thus, pharmacophore modeling is a computational tool that searches for common pharmacophoric patterns or chemical features (hydrogen bond acceptors and donors, hydrophobic groups, aromatic rings, etc.) shared by a set of active ligands with similar pharmacological activity and which may interact to the same binding sites. A pharmacophore model can be generated either in the absence of structural data information (ligand-based pharmacophore modeling) or based on the 3D structure of a therapeutic target (structure-based pharmacophore modeling) [491]. The development of structure-based pharmacophore models involves the examination of the pharmacophoric patterns in the binding site of the target and their spatial relationships. On the other hand, the ligand-based pharmacophore modeling implies the generation of a conformational space for each ligand of the training set to represent their conformational flexibility. A myriad of software tools and algorithms used for construction of a conformational space possess the ability to determine the different conformational geometries of the bioactive conformation and other similar geometries. An appropriate computational software for conformational search needs to generate all conformational geometries that the ligands can adopt when they are interacting with the therapeutic target. Subsequently, the multiple ligands of the training set are superimposed and the common 3D structural features crucial for biological/pharmacological activity are uncovered [491]. Once a pharmacophore model is constructed, it can be used for in silico screening of large databases of virtual and in-house drug candidates, in which a pharmacophore hypothesis is considered as a template for the discovery of hit ligands with similar pharmacophoric characteristics. In addition, the pharmacophore modeling can be applied to the generation of new chemical scaffolds and/or structures (de novo drug design), including the chemical features of a given pharmacophore hypothesis [492].
On the other hand, the investigation of QSAR has been a remarkably useful technique in early stages of the drug discovery process. The fundamental basis of this ligand-based methodology is that variations in the biological/pharmacological activity for congeneric and non-congeneric series of chemical compounds (training set) that target a common mechanism of action are correlated with variations in their physicochemical and structural properties [493-495]. Since structural features of chemical ligands can be efficiently uncovered by experimental or computational approaches, a statistically validated QSAR model is able to predict the biological/pharmacological activity of new chemical ligands, avoiding the time- and money-consuming chemical synthesis and biological evaluation of potentially uninteresting ligands. The generation of a QSAR model involves the collection of a statistical population of ligands and the determination of descriptor variables that can suitably explain the correlation of structural properties of ligands and their biological activity data (e.g. topological descriptors, electronic descriptors, geometrical descriptors, constitutional descriptors, etc.). Subsequently, various statistical methods within supervised ML techniques, including Partial Least Square (PLS) [496], Multiple (or multivariate) Linear Regression (MLR) [497], and Linear Discriminant Analysis (LDA) [498], and non-linear modeling, including Artificial Neural Networks (ANN) [499] and Support Vector Machines (SVM) [500] are applied to derive a robust mathematical correlation that explains the dependence of particular descriptor variables (independent variables) to the biological/pharmacological activity of a set of ligands (dependent variables). The resulting QSAR model is subjected to several validation tests to corroborate the reliability of the correlation models, in particular to internal validation or cross-validation and to external validation.
The internal validation or cross-validation consists in the elimination of one (Leave-One-Out cross-validation, LOO) or more (Leave Some-Out cross-validation, LSO; Leave-Many-Out cross-validation, LMO) ligands of the training set. The reconstruction of QSAR models is based on the remaining ligands of the training set using the combination of descriptor variables previously selected, and the pharmacological activity of the eliminated ligand(s) is predicted from the rebuilt QSAR model. Afterward, the same methodology is repeated until all or a definite portion of the ligands have been removed once. The external validation consists in the prediction of biological/pharmacological activity using a group of ligands that are not included in the training set (test set) and the same descriptor variables are employed in the generation of the QSAR model [501].
The 3D QSAR CoMFA and CoMSIA methodologies have emerged as fundamental tools for design and molecular optimization of drug candidates targeting GPCRs. The CoMFA methodology provides information of whether differences in steric (Lennard-Jones potential functions) and electrostatic components (Coulomb potential functions) for field calculation of a training set of molecules in a given alignment can be correlated with differences in biological/pharmacological activity [502-504]. A comparable 3D QSAR-based methodology, CoMSIA, was developed based on arbitrary descriptors named similarity indices. In opposition to CoMFA, CoMSIA applies a smoother potential based on Gaussian functions, allowing the calculation of various similarity indices, such as steric, electrostatic, hydrophobic, hydrogen bond acceptor, and hydrogen bond donor parameters, that covers more extensively than the steric and electrostatic fields calculated by CoMFA, the most significant contributing factors for the binding free energy of ligands towards a putative target [505]. In both methodologies, the 3D alignment of the chemical structures of ligands is required and should reveal the maximum superimposition of steric, electrostatic, hydrophobic, hydrogen bond acceptor, and hydrogen bond donor parameters that a database of ligands adopt when interacting with a specific therapeutic target. Each ligand on the training set is aligned to a template which exhibits a common molecular substructure and the aligned ligands are placed inside virtual 3D grid boxes. Subsequently, the interaction energies are calculated between the ligands and molecular fragments (molecular probes) at each grid point. Applying an appropriate statistical method for regression analysis, usually by PLS, the 3D-QSAR model is constructed to describe the variation of biological/pharmacological activity with the variation of CoMFA/CoMSIA interaction fields and the predictive ability of 3D-QSAR model is corroborated by cross-validation and prediction of activity of test set. The generated CoMFA/CoMSIA is typically represented as color-coded contoured 3D maps, which displays specific volumes of space where the magnitudes of steric, electrostatic, hydrophobic, hydrogen bond acceptor and hydrogen bond donor parameters are positively or negatively correlated with the pharmacological activity [502-505]. This type of graphical representation can be presumed as a model of the binding site in which a training set of ligands is supposed to interact. Table 3 emphasizes the distinct ligand- and pharmacophore-based design approaches applied for the aforementioned GPCR-derived therapeutic targets potentially involved in PD.
Table 3.
Ligand- and pharmacophore-based drug design methodologies reported for ligands targeting GPCRs potentially involved in PD.
| Dopamine D2 Receptor (D2R) Agonists | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Method(s) | Ligand(s) | Results/Conclusions | |||||||
| 1 | CoMFA using SYBYL 6.8 QSAR module [434] | Aminoindans, aminotetralins, dopamine analogs, ergolines, apomorphine, phenylpiperidines, benzo[f]quinolines | CoMFA maps showed that bulky substituents with low electronegativity on the fused piperidine ring nitrogen and small substituents with low electronegativity on the aromatic ring are favorable features for agonist activity [506]. | |||||||
| 2 | CoMFA and CoMSIA using SYBYL 8.0 [434] and MOE v. 2011.10 [507] programs | (S)-6-((2-(4-Phenylpiperazine-1-yl)-ethyl)(propyl)amino)-5,6,7,8-tetrahydronaphthalene-1-ol analogs | The key features for ligand activity are divided into two groups: near the aminotetralin head group and at/near the phenyl ring bound to piperazine moiety. Near the aminotetralin head group, the presence of bulky groups on 5-methoxy group of aminotetralin moiety is beneficial for activity; the introduction of bulky groups near the N-propyl group of aminotetralin moiety is expected to decrease the activity; the presence of electronegative substituents at 7-position of aminotetralin group and hydrophilic groups near the N-propyl group is predicted to enhance activity; a beneficial effect for activity is also expected upon the introduction of hydrogen bond donor groups near the 5- and 7-positions and near the N-propyl group of aminotetralin moiety. The presence of bulky substituents at 6-, 7-, and 8- positions of quinoline ring, electropositive groups on 3-position, and hydrophilic substituents on quinoline ring is favorable for activity. The introduction of hydrophilic substituents around the piperazine ring and hydrogen bond donor groups on the nitrogen atom of piperazine ring enhances the binding affinity [508]. | |||||||
| 3 | Pharmacophore modeling using Discovery Studio software [416] | Arylpiperazines | Pharmacophore model including key features for ligand activity: (i) salt bridge interactions between the basic nitrogen atom of piperazine ring and the receptor; (ii) one or more aromatic interactions involving arylpiperazine substructure; (iii) hydrogen bond interaction between the oxygen atom of methoxy group and the receptor; (iv) possibility of hydrogen bond interaction involving the linker part [417]. | |||||||
| 4 | Pharmacophore modeling using MOE v. 2005.06 [509] | Aminotetralins, apomorphine, quinolines | Pharmacophore model including key features for ligand activity: (i) one excluded volume covering the projected feature Asp; (ii) one positively charged nitrogen atom interacting with Ser; (iii) one hydrogen bond donor feature interacting with Asp; (iv) one aromatic ring feature [510]. | |||||||
| Dopamine D3 Receptor (D3R) Agonists | ||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | |||||||
| 5 | HQSAR and CoMFA using SYBYL 6.6 program [434] | Piperazinylalkylisoxazole analogues | The best HQSAR model was constructed using Atom, Bond, Connectivity, Donor and Acceptor (A/B/C/DA) as fragment type, 257 as hologram length, and 4–7 as fragment size. CoMFA models showed that the electrostatic parameters are the most contributing factor for D3R agonist affinity of these ligands [511]. |
|||||||
| 6 | CoMFA using SYBYL-X 1.3 program [434] | Library of 34 structurally diverse D3R agonists | Two representative molecules were used to analyze the key features for D3R binding affinity. N-((1S,4r)-4-(2-(((S)-2-Amino-4,5,6,7-tetrahydrobenzo[d]thiazol-6-yl)(propyl) amino)ethyl)cyclohexyl)-3-(5-methyl-1,2,4-oxadiazol-3-yl)benzamide: (i) the presence of bulky groups near the 2- and 3-position in pyridine ring enhances the binding affinity and near the 4-position of pyridine ring is expected to reduce the activity; (ii) the presence of electropositive groups near the 2-position and electronegative groups near the 4- and 5-position in pyridine ring may enhance activity. N-((1S,4r)-4-(2-(((S)-2-Amino-4,5,6,7-tetrahydrobenzo[d]thiazol-6-yl)(propyl) amino)ethyl)cyclohexyl)-2-chloropyridine-3-sulfonamide: (i) the introduction of bulky and electronegative substituents near the 5-position of the benzene ring is expected to increase agonist activity; (ii) the introduction of electropositive groups near methyl position of 1,2,4-oxadiazole ring is favorable for activity [424]. |
|||||||
| 7 | CoMFA and CoMSIA using SYBYL 7.2 program [434] | Library of 41 structurally diverse D3R agonists | The hydrophobic interactions are the most important contributing factor for D3R agonist activity. Hydrogen bonding also contributes largely to the binding affinity and may confer receptor subtype selectivity [512]. | |||||||
| Dopamine D3 Receptor (D3R) Agonists | ||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | |||||||
| 8 | CoMFA and CoMSIA using SYBYL 8.0 [434] and MOE v. 2011.10 [507] programs | (S)-6-((2-(4-Phenylpiperazine-1-yl)-ethyl)(propyl)amino)-5,6,7,8-tetrahydronaphthalene-1-of analogs | The key features for ligand activity are divided into two groups: near the aminotetralin head group and at/near the phenyl ring bound to piperazine moiety. Regarding the aminotetralin head group, the introduction of bulky substituents around 7- and 8-positions, hydrophobic and hydrophilic substituents on phenyl and cyclohexyl rings of aminotetralin moiety, respectively, is favorable for activity. The presence of bulky groups near the N-propyl group of aminotetralin moiety is expected to reduce the binding affinity to D3R. The introduction of both hydrogen bond donor and acceptor substituents near piperazine ring is predicted to be favorable for agonist activity [508]. | |||||||
| 9 | Pharmacophore modeling using Chem-X software | (R)-(+)-PD-128907, (R)-(+)-7-OH-DPAT, BP-897, (S)-(-)-3-PPP, pramipexole, (+)-UH-232, (S)-(-)-DS-121, quinelorane, (-)-quinpirole, ropinirole | Pharmacophore model including common key features for these ligands: (i) one aromatic ring and one sp3 nitrogen bound to a propyl group and to two additional sp3 carbons; (ii) the distance between the aromatic ring center and the basic sp3 nitrogen within these compounds was found to be on approximately 5.16 ± 0.16 Å [419]. | |||||||
| Adenosine A2A Receptor (A2AAR) Antagonists | ||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | |||||||
| 10 | HQSAR using SYBYL 7.2 program [434] | 2-(Furan-2-yl)-[1,2,4]triazolo[1,5-f]pyrimidin-5-amines, 2-(furan-2-yl)-[1,2,4]triazolo[1,5-a]pyrazin-8-amines, 2-(furan-2-yl)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-7-amines | The best HQSAR model includes the combination of fragment parameters A/B/C/DA, with a size fragment of 7-10 and a hologram length of 199. The structure features that contribute positively for the activity are: (i) for 2-(furan-2-yl)-[1,2,4]triazolo[1,5-f]pyrimidin-5-amines, the presence of a substituent at C3 of phenyl ring; (ii) for 2-(furan-2-yl)-[1,2,4]triazolo[1,5-a]pyrazin-8-amines, a short length of carbon chain between the piperazine moiety and the methylated nitrogen atom [446]. | |||||||
| 11 | CoMFA using SYBYL 6.3 program [434] | Flavonoids | The key features for high affinity to A2AAR are: (i) the presence of bulky substituents at C2, C7, and C8 of chromone ring; (ii) the absence of high electron density groups at the para position of phenyl ring [513]. | |||||||
| 12 | CoMFA using SYBYL-X 1.1.1 program [434] | Substituted thieno[2,3-d]pyrimidines | The key features for A2AAR antagonistic activity are: (i) the presence of bulky substituents in the thiophene ring and small substituents in the pyrimidine ring; (ii) electropositive substituents between positions 1 and 2 and at position 4 of benzene ring located at pyrimidine ring; (iii) electronegative substituents at position 2 of the benzene ring located at the pyrimidine ring [429]. | |||||||
| 13 | CoMFA and CoMSIA using DRAGON software [514] | Pyrimidine and triazine derivatives, pyrazolo[3,4-d]pyrimidines, pyrrolo[2,3-d]pyrimidines, triazolo[4,5-d]pyrimidines, 6-arylpurines, thieno[3,2-d]pyrimidines | For pyrimidine and triazine derivatives, the key features for A2AAR antagonistic activity are: (i) the presence of a limitedly bulky, electronegative, and hydrophobic group at C6; (ii) the presence of a small, electronegative, and hydrophilic group at C2; (iii) a limitedly bulky, electronegative, and hydrophilic group (non hydrogen bond donor group) at C4. For pyrazolo[3,4-d]pyrimidines, pyrrolo[2,3-d]pyrimidines, triazolo[4,5-d]pyrimidines, and 6-arylpurines, the key features for A2AAR antagonistic activity are: (i) the presence of a small, and electronegative group (hydrogen bond acceptor group) at C6; (ii) a hydrogen bond donor group at C2; (iii) a hydrogen bond acceptor group at N3. For thieno[3,2-d]pyrimidines, the key features for A2AAR antagonistic activity are: (i) the presence of a limitedly bulky, electronegative, and hydrophilic group (hydrogen bond donor group) at C6, a small and electronegative group (hydrogen bond donor group) [445]. | |||||||
| 14 | Pharmacophore modeling and GFA-based QSAR using Cerius2 [418] and LigandScout programs [515] | 4-Arylthieno[3,2-d]-pyrimidines | Molecular connectivity índex (SC-2), molecular surface area (AREA), graph-theoretical (WIENER), and molecular flexibility (PHI-MAG) descriptors influenced the activity of ligands against A2AAR. Pharmacophore model including key features for ligand activity: (i) the presence of one hydrogen bond donor feature (amino group interacts with Asn253), two hydrophobic features (one group interacts with Leu85, Phe168, Met177, Trp246, and Leu249 residues; other group interacts with Leu269 and Met270 residues) [98]. |
|||||||
| 15 | Pharmacophore modeling and QSAR using PHASE 3.2 module of Schrodinger suite [516] | Library of 46 A2AAR antagonists | Pharmacophore model including key features for ligand activity: (i) one hydrogen bond acceptor feature; (ii) one hydrogen bond donor feature; (iii) one hydrophobic feature; (iv) two aromatic ring features [430]. | |||||||
| Adenosine A2A Receptor (A2AAR) Antagonists | ||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | |||||||
| 16 | Pharmacophore modeling using CATALYST 4.11 software package [517] | Xanthine derivatives (KW6002, KF17837, BS-DMPX) Non-xanthine derivatives (7-substituted 5-amino-2-(2-furyl)pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines and piperazine derivatives of [1,2,4]triazolo[1,5-a]triazine) |
Pharmacophore models for xanthine and non-xanthine derivatives including key features for ligand activity: (i) two hydrophobic features; (ii) one hydrogen bond acceptor feature; (iii) one aromatic ring feature [445]. | |||||||
| 17 | Pharmacophore modeling using PHASE 2.0 module of Schrodinger suite [516] | Library of 68 A2AAR antagonists | Pharmacophore model 1 including key features for ligand activity: (i) two hydrogen bond acceptor features; (ii) two aromatic ring features; (iii) one hydrogen bond donor feature. Pharmacophore model 2 including key features for ligand activity: (i) two hydrogen bond acceptor features; (ii) one hydrogen bond donor feature; (iii) one aromatic ring; (iv) one hydrophobic feature [518]. | |||||||
| 18 | Pharmacophore modeling using CATALYST 4.10 software package [517] | 7-Substituted 5-amino-2-(2-furyl)pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines | Pharmacophore model including key features for ligand activity: (i) one ring aromatic feature; (ii) one positively ionizable feature; (iii) one hydrogen bond acceptor lipid feature; (iv) one hydrophobic feature [519]. | |||||||
| 19 | Pharmacophore modeling using CATALYST 4.11 program [517] | 1,2,4-Triazole derivatives, pyrazolotriazolopyrimidines, trifluoropyrimidines, 9-ethyladenine derivatives, thioacyhydrazones | Pharmacophore model including key features for ligand activity: (i) one hydrogen bond donor feature; (ii) three hydrophobic features; (iii) one aromatic ring feature [520]. | |||||||
| 20 | Pharmacophore modeling using PHASE module of Schrodinger suite [516] | Library of 751 A2AAR antagonists | Pharmacophore model including key features for ligand activity: (i) one hydrogen bond acceptor feature; (ii) one hydrogen bond donor feature; (iv) two aromatic ring features [521]. | |||||||
| 21 | Pharmacophore modeling using FLAPPharm program [522] | Istradefyline, MSX-2, SYN-115, BIIB014, SCH-442416, ZM-241385, ST-1535, preladenant | Pharmacophore model including key features for ligand activity: (i) three hydrogen bond acceptor features; (ii) one hydrogen bond donor feature; (iii) hydrophobic features. The proposed pharmacophore is predicted to interact Glu169 and Asn253 [523]. | |||||||
| 22 | Pharmacophore modeling using CATALYST 4.11 program [517] | 7-Substituted 5-amino-2-(2-furyl)pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines, piperazine derivatives of triazolotriazine and triazolopyrimidines | Pharmacophore model including key features for ligand activity: (i) two hydrophobic features, and one aromatic ring hydrophobic feature for 7-substituted 5-amino-2-(2-furyl)pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines; (ii) one hydrogen bond acceptor feature, two hydrophobic features, and one aromatic ring hydrophobic feature for piperazine derivatives of triazolotriazine and triazolopyrimidines [432]. | |||||||
| M1 muscarinic acetylcholine receptor (mAChR1) antagonists / negative allosteric modulators (NAMs) | ||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | |||||||
| 23 | Pharmacophore modeling and 3D-QSAR using CATALYST 4.10 program [517] | α-Substituted 2,2-diphenylpropionates | The stereoelectronic properties including total energies, bond distances, valence angles, torsion angles, HOMO–LUMO energies, reactivity indices, vibrational frequencies of ether and carbonyl moieties, and nitrogen atom proton influences the binding affinity of these ligands. Pharmacophore model including key features for ligand activity: (i) one aromatic ring feature; (ii) one hydrogen bond donor; (iii) basic nitrogen species at a distance of ~4Å from the hydrogen bond acceptor [524]. |
|||||||
| 24 | Pharmacophore modeling using PHASE module of Schrodinger suite [516] | Trihexylphenidyl, atropine, darifenacin, 4-DAMP, propantheline, pirenzepine | Pharmacophore model including the common molecular features: (i) molecular weight: 289.37–426.55; (ii) polar surface area: 23.47–68.78 Å2; (iii) hydrogen bond acceptors: 1–3; (iv) hydrogen bond donors: 0–1; (v) rotatable bonds: 2–7; (vi) AlogP: 1.68–4.53 [525]. | |||||||
| 25 | Pharmacophore modeling using CATALYST 4.10 program [517] | Caramiphen, indocaramiphen, nitrocaramiphen, atropine, dicyclomine, methoctramine, oxybutynin | Pharmacophore model including key features for ligand activity: (i) two hydrogen bond acceptor features; (ii) one aliphatic hydrophobic feature; (iii) one aromatic ring feature [526]. | |||||||
| Metabotropic Glutamate Receptor 4 (MGluR4) agonists / positive allosteric modulators (PAMs) | ||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | |||||||
| 26 | Pharmacophore modeling using APEX-3D software (MSI) [527] | (S)-Glu, (S)-Asp, (S)-AP4, (S)-SOP, (2S)-4CH2Glu, (S)-Gla, (2S)-CCG-I, (2SR,3RS)-CPrAP4, (2SR,3SR)-CPrAP4, (1S,3S)-ACPD, (2SR,4SR)-CpeAP4, (2SR,4RS)-CpeAP4, ACPT-I, (+)-ACPT-III | Pharmacophore model including key features for ligand activity: (i) the presence of two hydrogen bond donor groups; (ii) the presence of three hydrogen bond acceptor groups. Additional regions are predicted to interact with mGluR4, including the oxygen atom of phosphonic and phosphoric groups of (S)-Glu analogues, the carboxylic groups of (S)-Glu, ACPT-I, and (+)-ACPT-III, and the presence of cycloalkyl rings of CPrAP4, CpeAP4, ACPT-I, and (+)-ACPT-III [527]. | |||||||
| Metabotropic Glutamate Receptor 5 (MGluR5) Antagonists / Negative Allosteric Modulators (NAMs) | ||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | |||||||
| 27 | Pharmacophore modeling using Accelrys Discovery Studio 4.0 (DS) software [528] | Pyridyl and phenyl substituted oxadiazoles, 5-aryl-3-acylpyridinyl-pyrazoles, 1-aryl-4-acylpyridinyl-imidazoles, aryl azetidinyl oxadiazoles, N-aryl pyrrolidinonyl oxadiazoles | Pharmacophore model including common key features: (i) one hydrogen bond acceptor feature; (ii) one hydrophobic feature; (iii) two hydrophobic aromatic features; (iv) two excluded volumes [465]. | |||||||
| 5-Hydroxytryptamine Receptor 1A (5-HT1AR) Agonists | ||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | |||||||
| 28 | QSAR using 2D molecular descriptors by MOE v. 2004.0314 programs [529] | 2-[ω-(4-Arylpiperazin-1-yl)alkyl]perhydropyrrolo-[1,2-c]imidazoles, 2-[ω-(4-arylpiperazin-1-yl)alkyl]perhydroimidazo[1,5-a]pyridines | Steric parameters contribute most significantly to the 5-HT1AR agonist activity. The number of rotatable bonds, partial charge descriptors, subdivided surface area descriptors, and an indicator variable for carbonyl oxygens also influence the 5-HT1AR agonist activity of the ligands [530]. | |||||||
| 29 | CoMFA using SYBYL 5.5 program [434] | Arylpiperazines, (aryloxy)propanolamines, tetrahydropyridyl indoles | Arylpiperazines: the introduction of steric groups is favorable close to the aromatic ring and unfavorable near the basic nitrogen atom. The presence of electronegative substituents near the ortho position of the aromatic ring is beneficial for activity. (Aryloxy)propanolamines: the introduction of bulky and electronegative substituents close to the ortho and meta positions of the aromatic ring is beneficial for the activity. The presence of bulky and electropositive groups near the para position of the aromatic ring is favorable as well as the introduction of electropositive substituents near the basic nitrogen atom. Tetrahydropyridylindoles: the steric parameters contribute most significantly to the activity. The introduction of bulky and hydrogen bond donor groups at 5-position of índole ring is predicted to be beneficial for the activity [531]. |
|||||||
| 30 | CoMFA using SYBYL 5.5 program [434] | 3-(1,2,5,6-Tetrahydropyridin-4-yl)índole derivatives | The steric parameters contribute most significantly to the 5-HT1AR agonist activity. The presence of bulky substituents in the plane of índole 5-position is beneficial for 5-HT1AR activity, whereas bulky substituents located out of a plane is expected to decrease the activity. There is also a preference for coplanarity between indole and tetrahydropyridine rings. Using as a reference a 5-CONH2 analog, the presence of oxygen and hydrogen atoms of carboxamide moiety contributes positively to 5-HT1AR activity [532]. | |||||||
| 31 | CoMFA using SYBYL 6.0 program [434] | Bicyclohydantoin-phenylpiperazines | The electrostatic parameters contribute most significantly to the 5-HT1AR agonist activity. The key features for agonist activity are: (i) the introduction of bulky substituents at ortho and meta positions of the phenyl ring; (ii) the introduction of electron-withdrawing substituents at ortho and meta positions of the phenyl ring. The presence of bulky and electron-withdrawing substituents is expected to have a negative effect on activity [533]. | |||||||
| 32 | Pharmacophore modeling and CoMFA using DISCO [534] and SYBYL programs [434] | Pyridazinothiazepines, pyridazinooxazepines | Pharmacophore model including common key features for ligand activity: (i) two hydrophobic ring features (phenyl and 3(2H)-pyridazinone rings); (ii) four hydrogen bond donor features (N atom of protonated amine, N1 atom and the two lone pairs of O atom of carbonyl group of 3(2H)-pyridazinone ring); (iii) two hydrogen bond acceptor features (O atom of carbonyl group and N1 atom of 3(2H)-pyridazinone ring; (iv) O atom of carbonyl group interacting to the N atom of oxazepine or thiazepine rings), using as reference the ligand GYKI16527 [535]. | |||||||
| 33 | Pharmacophore modeling and CoMFA using GALAHAD program [536-538] | Arylpiperazines | Pharmacophore model including common key features for ligand activity: (i) one hydrogen bond acceptor feature; (ii) one positively charged group; (iii) one aromatic ring feature; (iv) one hydrophobic feature. The steric parameters contribute most significantly to the 5-HT1AR agonist activity. The introduction of bulky substituents, as benzothiophene, and electropositive substituents attached to benzothiophene ring may enhance activity. Moreover, the presence of electronegative substituents surrounding the dihydrobenzodioxepin ring may have a positive effect on activity [539]. |
|||||||
| 5-Hydroxytryptamine Receptor 1A (5-HT1AR) Agonists | ||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | |||||||
| 34 | Pharmacophore modeling using CATALYST 4.6 program [517] | (2-Alkoxy)phenylpiperazine derivatives of 1-(2-hydroxy-3-(4-arylpiperazin-1-yl)propyl)-5,5-diphenylimidazolidine-2,4-dione with alkyl or ester substituents at N3 of the hydantoin ring | Pharmacophore model including common key features for ligand activity: (i) one hydrogen bond acceptor; (ii) one positively charged group; (iii) one aromatic ring feature; (iv) one hydrophobic feature. The inter-feature distance range between hydrogen bond acceptor and hydrophobic features is [5.53-5.62 Å], between hydrogen bond acceptor and positively charged group is [5.13-6.00 Å], between hydrogen bond acceptor and aromatic ring is [10.08-11.68 Å], between hydrophobic feature and positively charged group is [7.60-8.08 Å], between hydrophobic and aromatic ring features is [12.24-13.91 Å], and between positively charged group and aromatic ring feature is [5.68-5.69 Å] [540, 541]. | |||||||
| 35 | Pharmacophore modeling using Accelrys Discovery Studio versions 3.5 (DS) software [542] | (R)-8-OH-DPAT, quetiapine, olanzapine, ziprasidone, 5-methyl urapidil, BMY14802, JB788, S14671, F15599, F13714, 1,3-dioxolane derivatives, pyridyl-fused 3-amino chroman derivatives, 1-(meta-trifluoromethylphenyl) piperazines | Pharmacophore modeling including common key features for ligand activity: (i) one positively ionizable group; (ii) one hydrogen bond acceptor feature; (iii) three hydrophobic features; (iv) seven excluded volumes [543]. | |||||||
| 36 | Pharmacophore modeling using GRID 22 program [544] | 8-OH-DPAT, buspirone, vilazodone, aripiprazole, F-13640 | Pharmacophore modeling including common key features for ligand activity: (i) two hydrophobic features surrounded by TM5/TM6 and by TM2/TM7/ECL2; (ii) one positive ionizable feature interacting with Asp; (iii) one hydrogen bond acceptor feature interacting with Tyr; (iv) three excluded volumes [162]. | |||||||
| 5-Hydroxytryptamine Receptor 2A (5-HT2AR) Antagonists | ||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | |||||||
| 37 | QSAR performed by GRID 22 [544] and GOLPE 4.6.0 programs [545] and using GRID/GOLPE descriptors | Butyrophenones | The interaction of hydrophobic groups in two areas, one at the bottom of the pocket near Leu163 and the other in the center near the side chains of Val and Phe residues, is expected to increase the binding affinity. The interaction of these ligands near the oxygen atom of Asp residue, the indolic nitrogen of Trp residue, at the bottom near the Ser residue, the side chain of Asn residue, and close to other Ser and Trp residues are favorable for antagonistic activity [546]. | |||||||
| 38 | QSAR performed by MDL QSAR software package, version 2.2 (Symyx) using electronic and molecular parameters [547] | 1-Benzhydryl-piperazines and 1-arylpiperazines with xanthine moiety at N4 | The binding affinity of these ligands to 5-HT2AR correlates positively with the lipophilicity of ligands, the largest negative charge associated to O6 atom of xanthine moiety, and with the partial atomic charge of N4 atom of piperazine moiety [547]. | |||||||
| 39 | QSAR by DRAGON software (version 1.11-2001) [514] and using DRAGON descriptors | 2-Alkyl-4-aryl-pyrimidine fused heterocycles | A lower number of rotatable bonds, a more hydrophobic nature of the ligands, and lower polar surface area are expected to be favorable for binding affinity [548]. | |||||||
| 40 | CoMFA using SYBYL 5.5 program [434] | 3-(1,2,5,6-Tetrahydropyridine-4-yl)índole derivatives | The introduction of bulky substituents around the 5-position of indole ring is unfavorable for activity, whereas the presence of bulky groups at the N1 position of pyridine ring and at the N1 position of the indole ring is expected to be beneficial for activity. The introduction of electronegative substituents around the 5-position of the indole ring and of electropositive substituents above 5- and 6-carbons of the indole ring may increase the activity [532]. | |||||||
| 41 | CoMFA using SYBYL 7.0 program [434] | Hexahydro- and octahydropyrido[1,2-c]pyrimidine derivatives | The introduction of bulky substituents in the proximity to the para position in the benzene ring attached to the piperazine ring and the absence of substituents at ortho position may enhance activity. The presence of bulky groups placed in the proximity of piperazine ring and at the C4 position of the imide moiety is also beneficial for activity. The introduction of electronegative substituents in imide and piperazine moieties may increase the affinity [549]. | |||||||
| 5-Hydroxytryptamine Receptor 2A (5-HT2AR) Antagonists | ||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | |||||||
| 42 | CoMSIA using SYBYL 7.2 program [434] | Tetrahydrofuran derivatives, benzamides, 3-aminoethyl-1-tetralones, piperazines, benzothiazepines, pyrrolobenzodiazepines, clozapine, flupentixol, haloperidol, loxapine, mesoridazine, olanzapine, quetiapine, risperidone, sertindole, thiothixene, thioridazine, compazine, ziprasidone | The electrostatic, hydrophobic, and hydrogen bond donor parameters contribute most significantly to the binding affinity towards 5-HT2A, comparing to steric and hydrogen bond acceptor parameters [550]. | |||||||
| 43 | Pharmacophore modeling using MOE v. 2007.09 program [551] | Ketanserin, risperidone, ritanserin, spiperone, clozapine, sertindole, setoperone, chlorpromazine, cyproheptadine, tefludazine, 5H-thiazolo[3,2-a]pyrimidine-5-one derivatives | Pharmacophore model including common key features for 5-HT2A antagonist activity: (i) two hydrogen bond acceptor features; (ii) one aromatic ring or hydrophobic feature with two parallel features for π orbital accommodation; (iii) one aromatic ring feature with one hydrogen bond donor group; (iv) one hydrophobic feature [552]. | |||||||
| 44 | Pharmacophore modeling using MOE v. 2008.10 program [553] | Thiophene derivatives | Pharmacophore model including the common key features for ligand activity: (i) one aromatic ring feature; (ii) one positively ionizable group containing a nitrogen atom; (iii) one hydrogen bond acceptor feature; (iv) one hydrophobic or aromatic feature attached to the positively ionizable group. The inter-feature distance range between the positively ionizable group and the hydrogen acceptor group is [6.6-10.3 Å], between aromatic ring feature and the hydrogen bond acceptor feature is [3.1-3.8 Å], between aromatic ring feature and the positively ionizable group is [7.4-8.8 Å], and the angle between the positively ionizable group and the plane of aromatic ring feature is [141.0-152.9 Å]o [554]. | |||||||
| 45 | Pharmacophore modeling using MOE v. 2008.10 programs [553] | 4-Nitroindole derivatives | Pharmacophore model including the common key features for ligand activity: (i) two aromatic ring features; (ii) one positively ionizable group; (iii) one hydrophobic feature [555]. | |||||||
| 46 | Pharmacophore modeling using GRID 22 software [544] | Ketanserin | Pharmacophore model including the key features for ligand activity: (i) one positively ionizable amino group; (ii) two hydrophobic features; (iii) one hydrophobic feature [480]. | |||||||
| 5-Hydroxytryptamine receptor 2C (5-HT2CR) antagonists | ||||||||||
| Entry | Method(s) | Ligand(s) | Results/Conclusions | |||||||
| 47 | QSAR by DRAGON software (v.1.11-2001) [514] and using Dragon descriptors | Isoindoline | The binding affinity of these ligands are correlated to several atomic descriptors, in particular, the eigenvalue n.2 and the eigenvalue n.5 of the Burden matrix, atomic van der Waals volume to path length 2 and 8 of the Moran autocorrelations and path length 2 of Geary autocorrelation, polarizability to the highest eigenvalue n.1 and n.6 of the Burden matrix, and Sanderson electronegativity to path length 4 of Geary autocorrelation descriptors [556]. | |||||||
| 48 | QSAR by D-CENT QSAR program [557, 558] using quantum chemical parameters | N-Benzylphenethylamines | The binding affinity was correlated negatively with total atomic electrophilic superdelocalizability of atom 3 and atom 13, the orbital electrophilic superdelocalizability of the highest occupied molecular orbital localized on atom 20, and the local atomic electronic chemical potential of atom 22. On the other hand, the binding affinity was correlated positively with Fukui index of the second highest occupied molecular orbital localized on atom 15 and Fukui index of the lowest vacant molecular orbital localized on atom 17 [559]. | |||||||
| 49 | CoMFA using SYBYL 7.0 program [434] | Library of 24 structurally diverse 5-HT2CR antagonists | The introduction of bulky substituents in the regions where methyl and propyl groups are located may enhance activity, whereas the presence of bulky groups in the proximity of índole group is expected to decrease activity. The presence of electronegative substituents near the amide moiety and positively charged groups is favorable for antagonistic activity [488]. | |||||||
| 50 | Pharmacophore modeling using Accelrys Discovery Studio version 2.1 (DS) software [560] | Library of 24 structurally diverse 5-HT2CR antagonists | Pharmacophore model including the common key features for ligand activity: (i) three hydrophobic features; (ii) one positively ionizable group; (iii) one hydrogen acceptor feature [488]. | |||||||
| 51 | Pharmacophore modeling using CATALYST 4.6 program [517] | Diaryl substituted pyrrolidinones and pyrrolones | Pharmacophore model including the common key features for ligand activity: (i) one positively ionizable group; (ii) one hydrogen bond acceptor feature; (iii) one aromatic ring feature; (iv) three hydrophobic features [561]. | |||||||
3. Relevant features in GPCR-ligand interactions
3.1. Impact of Structural Water Molecules in GPCR-mediated Interactions
Ubiquitous when considering biological interactions, water molecules display different and important roles in protein-protein interactions in general, and in GPCR associated interactions in particular. These molecules are not to be overlooked when considering drug design for either orthosteric or allosteric purpose. By forming a ‘water pocket’ network, they can play an important role on the transition between active and inactive states, by deforming TM7 and potentiating hydrogen bonds that stabilize the structure on the inactive state [22]. In the case of rhodopsin, water molecules tend to be clustered in the vicinity of the binding pockets, near highly conserved residues [330]. Furthermore, internal water networks connecting to the ligand binding site have found to be highly conserved, even for receptors from different subfamilies of GPCRs, in particular, A2AAR and DOR, with associated recurring motifs [562]. The direct involvement of the structural water molecules in the interaction of ligands with GPCRs can be a major bottleneck for an efficient design of drug candidates without the proper structural data information, since the replacement of water molecules in the ligand binding site may induce a dramatic modification of Structure-Activity Relationships (SAR) [562]. Interestingly, the inclusion of structural water molecules in crystallographic structures without the knowledge of structural water positions can lead to an improvement of virtual screening enrichment of active drug candidates over decoys [563]. Apart from experimental approaches, such as X-ray crystallography and NMR, MD simulations have been employed to predict the involvement of water molecules in GPCR-ligand interactions and to study the water networks within receptors in different activation states [564].
Homology modeling approaches, in particular, have found to significantly decrease in accuracy when the participation of structural water molecules is not considered. Hence, it is essential to always assess the number and the characteristics of the water-contacts of the GPCR in study [565]. In docking, the water molecules associated to the binding pockets can be determinant in the development of drug candidates that may establish distinct types of interactions with the protein target [44]. Several docking tools take into account the intervention of water molecules in protein-ligand interaction, such as Yet Another Docking Approach (YADA) [566], which considers the explicitly structural water molecules at the binding site and improves the prediction scores by up to 24%. Another docking software, Molegro Virtual Docker (MVD) [567], incorporates water molecules in protein-ligand complexes and considers that water molecules exhibit the same flexibility of the ligand. During the docking simulations, MVD solvates the ligands with the maximum number of water molecules, and these are then retained or excluded depending on energy contributions. Each water molecule is flexible on/off part of the ligand and is treated with the same flexibility as the ligand. To facilitate the removal of the water molecules, a constant (positive) entropy penalty value is introduced per included water. This approach has found to be successful in docking simulations when the water molecules are included in ligands containing hydrogen bonding groups [567]. Also, a more recent approach incorporated Quantum Mechanics/Molecular Mechanics (QM/MM) calculations with an implicit solvent for application to GPCR-targeted drug discovery, by considering the ligands and protein residues in the binding site as QM described regions [568].
In addition to the above-mentioned tools, alternative software packages are used to model water molecules, such as GRID [544], Hydrophobic INTeractions (HINT) [569], SuperStar [570], Just Add Water Molecules (JAWM) [571], WaterMap [572, 573], water Potential of Mean Forces (wPMF) [574], Water Fingerprints for Ligand And Proteins (WaterFLAP) [522], among others. Overall, water molecules should be taken into consideration, since they may influence the ligand binding affinity, the binding free energy, and the protein mobility.
3.2. Analysis of the Ligand-binding Site of Potential GPCR-derived Therapeutic Targets
Even though computational methods have proven to be effective to solve structural aspects of GPCR-ligand complexes, they rely on experimental work as both validations and as a starting point. Recently, the determination of 3D structures of GPCR-ligand complexes had substantially increased, shedding light on important patterns governing the interactions that lead to the formation and stabilization of these complexes. Among all the aforementioned GPCR-derived therapeutic targets of PD, the X-ray structure of five GPCRs (D3R, A2AAR, mGluR2, mGluR3, and mGluR5) has been determined in complex with small-molecule modulators. Table 4 represents an overview of all known 3D structures of ligands in complex with GPCR-derived therapeutic targets of PD available on PDB and the important interacting residues involved in the GPCR-ligand interaction. Relevant interactions were retrieved from the G-Protein-Coupled Receptor database (GPCRdb) [575] for D3R, A2AAR and mGluR5, and from the PDBeMOTIF for mGluR2 and mGluR3. The analysis of the ligand-binding site of the distinct GPCR-derived therapeutic targets of PD, reveals the presence of residue patterns underlying the GPCR-ligand interaction. The majority of A2AAR ligands makes hydrophobic interactions with Leu85 in TM3. Interestingly, the A2AAR agonists and the A2AAR antagonist 4-(3-amino-5-phenyl-1,2,4-triazin-6-yl)-2-chlorophenol interact with Val83 in TM3. In ECL2, the A2AAR modulators establish aromatic interactions with Phe168. More specifically, the A2AAR agonists make π-π interactions with the Phe168 and hydrogen bond interactions with Glu169. Considering the TM6 domain, the analysis of the Table 4 shows that the A2AAR ligands make hydrophobic interactions with Trp248 and Leu249, π-π interactions with His250, and hydrogen bond interactions with Asn253. Some A2AAR antagonists establish hydrophobic interactions with Met177 in TM5 and π-π interactions with His264 located on ECL2. Similarly, to TM6, a considerable number of residues located in the TM7 domain are involved in the A2AAR-ligand interaction. Interesting, the A2AAR agonists make hydrogen bond interactions with Ser277 and His278, while the A2AAR antagonists make hydrophobic interactions with Met270. In addition, some antagonists bind to Leu267, to Tyr271, and to Ile274. The structural details of agonists CGS21680 and 5′-(N-ethylcarboxamido)adenosine, and the antagonists ZM241385 and 4-(3-amino-5-phenyl-1,2,4-triazine-6-yl)-2-chlorophenol in complex with A2AAR are represented in Fig. (4A, 4B), Fig. (5A, and 5B), respectively. The mGluR2 orthosteric agonists mentioned on Table 4 make hydrogen bond interactions with Arg61, Ser145, Ala166, Tyr168, and Asp295 residues and van der Waals interactions with Arg57, Ser167, Tyr216, and Lys377 residues mainly located on the extracellular Amino-Terminal Domain (ATD). The structural details of the binding modes of mGluR2 orthosteric agonists are represented in Fig. (6A and 6B). Similarly, the mGluR3 orthosteric agonists interact with residues located on the extracellular ATD. The mGluR3 agonists make hydrogen bond and van der Waals interactions with Arg64, Arg68, Tyr150, Ser151, Ala172, Ser173, Thr174, and Lys389 residues. The structural details of the binding pose of glutamate (Fig. 7A) and DCG-IV (Fig. 7B) in complex with mGluR3 are represented. While mGluR2 and mGluR3 orthosteric agonists interact with their receptors on extracellular ATD, the mGluR5 NAM binds exclusively to the TM domains (TM2, TM3, TM4, and TM7 domains for mavoglurant; TM3, TM5, TM6, and TM7 domains for HTL14242 and 3-chloro-4-fluoro-5-[6-(1H-pyrazole-1-yl)pyrimidin-4-yl]benzonitrile). In TM3, the mGluR5 NAM makes hydrophobic interactions with Ile651 and Pro655.The structural details of the binding modes of mGluR2 orthosteric agonists are represented in Fig. (6A and 6B). Similarly, the mGluR3 orthosteric agonists interact with residues located on the extracellular ATD. The mGluR3 agonists make hydrogen bond and van der Waals interactions with Arg64, Arg68, Tyr150, Ser151, Ala172, Ser173, Thr174, and Lys389 residues. The structural details of the binding pose of glutamate (Fig. 7A) and DCG-IV (Fig. 7B) in complex with mGluR3 are represented. While mGluR2 and mGluR3 orthosteric agonists interact with their receptors on extracellular ATD, the mGluR5 NAM binds exclusively to the TM domains (TM2, TM3, TM4, and TM7 domains for mavoglurant; TM3, TM5, TM6, and TM7 domains for HTL14242 and 3-chloro-4-fluoro-5-[6-(1H-pyrazole-1-yl)pyrimidin-4-yl]benzonitrile). In TM3, the mGluR5 NAM makes hydrophobic interactions with Ile651 and Pro655. Additionally, in TM7 domains, the mGluR5 NAM makes hydrophobic interactions with Val806 and hydrogen bond interactions with Ser809. A representation of the binding conformation of HTL14242 and 3-chloro-4-fluoro-5-[6-(1H-pyrazol-1-yl)pyrimidin-4-yl]benzonitrile is shown in Fig. (8A and 8B), respectively.
Table 4.
Residues involved in the interaction of ligands with potential GPCR-derived therapeutic targets of PD for which structural data information is available on PDB.
| Receptor | Main Ligand(s)/ Binding Partners |
Localization of the Interacting Residues on GPCR Structure | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dopamine D3 Receptor (D3R) | ||||||||||||||||
| 3PBL [294] | Eticlopride (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Asp110 (HB and SB); Val111 (HP) | TM4: - | ECL2: Ile183 (HP) | TM5: - | TM6: Phe345 (AR); Phe346 (HP); His349 (HB) | ECL3: - | TM7: - | |||||||
| Adenosine A2A receptor (A2AAR) | ||||||||||||||||
| 2YDO [240] |
Adeonosine (agonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: - | TM6: Trp246 (HP); Leu249 (HP); Asn253 (HB) | ECL3: - | TM7: Ser277 (HB); H278 (2HB) | |||||||
| 2YDV [240] |
5′-(N-Ethylcarboxamido)adenosine (agonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Val84 (HP); Val85 (HP); Thr88 (HB); | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: - | TM6: Trp246 (HP); Leu249 (HP); His250 (HB); Asn253 (2HB) | ECL3: - | TM7: Ser277 (HB); His278 (HB) | |||||||
| 3EML [44] | ZM241385 (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Leu85 (AR) | TM4: - | ECL2: - | TM5: Met177 (HP) | TM6: Trp246 (HP); Leu249 (HP); His250 (AR); Asn253 (HB) | ECL3: His264 (AR) | TM7: Lys267 (HP); Met270 (HP) | |||||||
| 3PWH [241] | ZM241385 (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: Phe168 (AR) | TM5: Met177 (HP) | TM6: Leu249 (HP); His250 (AR); Asn253 (HB) | ECL3: - | TM7: Tyr271 (HP); Ile274 (HP) | |||||||
| 3QAK [43] | UK-432097 (agonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Val84 (HP); Leu85 (HP) | TM4: - | ECL2: Phe168 (AR); Glu169 (2HB) | TM5: - | TM6: Trp246 (HP); Leu249 (HP); His250 (HB); Asn253 (HB) | ECL3: - | TM7: Met270 (HP); Tyr271 (HB and AR); Ile274 (HP); Ser277 (HB); His278 (2HB) | |||||||
| 3REY [241] | Xanthine amine congener (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: Phe168 (HP) | TM5: Met177 (HP) | TM6: Asn253 (HB) | ECL3: - | TM7: Met270 (HP); Ile274 (HP) | |||||||
| 3RFM [241] | Caffeine (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: Phe168 (HP) | TM5: - | TM6: - | ECL3: - | TM7: Ile274 (HP) | |||||||
| Receptor | Main Ligand(s)/ Binding Partners |
Localization of the Interacting Residues on GPCR Structure | ||||||||||||||
| Adenosine A2A Receptor (A2AAR) | ||||||||||||||||
| 3UZA [243] | 6-(2,6-Dimethylpyridin-4-yl)-5-phenyl-1,2,4-triazin-3-amine (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: Phe168 (HP) | TM5: Met177 (HP) | TM6: Leu249 (HP); His250 (AR); Asn253 (2HB) | ECL3: - | TM7: Ile274 (HP) | |||||||
| 3UZC [243] |
4-(3-Amino-5-phenyl-1,2,4-triazin-6-yl)-2-chlorophenol (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Val84 (HP); Leu85 (HP) | TM4: - | ECL2: Phe168 (AR) | TM5: Met177 (HP) | TM6: Trp246 (HP); Leu249 (HP); His250 (AR); Asn253 (2HB) | ECL3: - | TM7: Ile274 (HP); His278 (HB) | |||||||
| 3VG9 [242] |
ZM241385 (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Leu85 (HP) | TM4: - | ECL2: Phe168 (HP) | TM5: - | TM6: Trp246 (HP); Leu249 (HP); His250 (AR); Asn253 (HB) | ECL3: - | TM7: Met270 (HP); Tyr271 (HP) | |||||||
| 3VGA [242] | ZM241385 (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Leu85 (HP) | TM4: - | ECL2: Phe168 (AR) | TM5: Met177 (HP) | TM6: Leu249 (HP); His250 (AR) | ECL3: - | TM7: Met270 (HP) | |||||||
| 4EIY [244] | ZM241385 (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1:- | TM3: - | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: Met177 (HP) | TM6: Trp246 (HP); Leu249 (HP); His250 (AR); Asn253 (3HB) | ECL3: His264 (AR) | TM7: Leu267 (HP); Met270 (HP) | |||||||
| 4UG2 [245] | CGS21680 (agonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: Ser67 (HB) | ECL1: - | TM3: Val84 (HP); Leu85 (HP); Thr88 (HB) | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: - | TM6: Trp246 (HP); Leu249 (HP); His250 (HB); Asn253 (2HB) | ECL3: - | TM7: Met270 (HP); Ile274 (HP); Ser277 (HB); His278 (2HB) | |||||||
| 4UHR [245] | CGS21680 (agonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Val84 (HP); Leu85 (HP); Thr88 (HB) | TM4: - | ECL2: Phe168 (AR) | TM5: - | TM6: Trp246 (HP); Leu249 (HP); His250 (HB); Asn253 (HB) | ECL3: - | TM7: Ile274 (HP); Ser277 (HB); His278 (2HB) | |||||||
| 5G53 [246] | 5′-(N-Ethylcarboxamido)adenosine (agonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Val84 (HP); Leu85 (HP); Thr88 (HB) | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: - | TM6: Trp246 (HP); Leu249 (HP); His250 (HB); Asn253 (2HB) | ECL3: - | TM7: Ser277 (HB); His278 (2HB) | |||||||
| 5IU4 [247] | ZM241385 (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Leu85 (HP) | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: Met177 (HP) | TM6: Trp246 (HP); Leu249 (HP); His250 (AR); Asn253 (3HB) | ECL3: His264 (AR) | TM7: Met270 (HP) | |||||||
| Receptor | Main Ligand(s)/ Binding Partners |
Localization of the Interacting Residues on GPCR Structure | ||||||||||||||
| Adenosine A2A Receptor (A2AAR) | ||||||||||||||||
| 5IU7 [247] | 2-(Furan-2-yl)-N5-(2-(4-phenylpiperidin-1-yl)ethyl)-1,2-dihydro-[1,2,4]triazolo[1,5-a][1,3,5]triazine-5,7-diamine (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Leu85 (HP) | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: Met177 (HP) | TM6: Trp246 (HP); Leu249 (HP); His250 (AR); Asn253 (3HB) | ECL3: His264 (AR) | TM7: Met270 (HP) | |||||||
| 5IU8 [247] | 2-(Furan-2-yl)-N5-(2-(4-phenylpiperidin-1-yl)ethyl)-1,2-dihydro-[1,2,4]triazolo[1,5-a][1,3,5]triazine-5,7-diamine (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Leu85 (HP) | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: Met177 (HP) | TM6: Trp246 (HP); Leu249 (HP); His250 (AR); Asn253 (2HB) | ECL3: - | TM7: Met270 (HP) | |||||||
| 5IUA [247] | 2-(Furan-2-yl)-N5-(3-(4-phenylpiperazin-1-yl)propyl)-1,2-dihydro-[1,2,4]triazolo[1,5-a][1,3,5]triazine-5,7-diamine (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Leu85 (HP) | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: Met177 (HP) | TM6: Trp246 (HP); Leu249 (HP); His250 (AR); Asn253 (HB) | ECL3: - | TM7: Leu267 (HP); Met270 (HP) | |||||||
| 5IUB [247] | N5-(2-(4-(2,4-Difluorophenyl)piperazin-1-yl)ethyl)-2-(furan-2-yl)-1,2-dihydro-[1,2,4]triazolo[1,5-a][1,3,5]triazine-5,7-diamine (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Leu85 (HP) | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: Met177 (HP) | TM6: Trp246 (HP); Leu249 (HP); His250 (AR); Asn253 (3HB) | ECL3: His264 (AR); Ala265 | TM7: Leu267 (HB); Met270 (HP); Ile274 (HP) | |||||||
| 5K2A [249] | ZM241385 (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: Met177 (HP) | TM6: Trp246 (HP); Leu249 (HP); His250 (AR); Asn253 (3HB) | ECL3: His264 (AR); Ala265 | TM7: Leu267 (HP); Met270 (HP) | |||||||
| 5K2B [249] | ZM241385 (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: - | TM6: Trp246 (HP); Leu249 (HP); His250 (AR); Asn253 (3HB) | ECL3: - | TM7: Leu267 (HP); Met270 (HP) | |||||||
| 5K2C [249] | ZM241385 (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: Met177 (HP) | TM6: Leu249 (HP); His250 (AR); Asn253 (3HB) | ECL3: His264 (AR) | TM7: Met270 (HP) | |||||||
| 5K2D [249] | ZM241385 (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: Phe168 (AR); Glu169 (HB) | TM5: Met177 (HP) | TM6: Leu249 (HP); His250 (AR); Asn253 (3HB) | ECL3: His264 (AR) | TM7: Met270 (HP) | |||||||
| 5UIG [252] | 5-Amino-N-[(2-methoxyphenyl)methyl]-2-(3-methylphenyl)-2H-1,2,3-triazole-4-carboximidamide (antagonist) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Leu85 (HP) | TM4: - | ECL2: Phe168 (AR) | TM5: Met177 (HP) | TM6: Trp246 (HP); Leu249 (HP); His250 (AR) | ECL3: - | TM7: Tyr271 (HP); Ile274 (HP) | |||||||
| Receptor | Main Ligand(s)/ Binding Partners |
Localization of the Interacting Residues on GPCR Structure | ||||||||||||||
| Metabotropic Glutamate Receptor 2 (MGluR2) | ||||||||||||||||
| 5CNI [379] |
Glutamate (agonist) | Extracellular ATD: Arg57 (VdW); Arg61 (HB); Tyr144 (VdW); Ser145 (HB); Ala166 (HB); Ser167 (VdW); Thr168 (HB); Tyr216 (VdW); Asp295 (HB); Lys377 (VdW) | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: - | TM5: - | TM6: - | ECL3: - | TM7: - | |||||||
| 5CNJ [379] | LY2812223 (agonist) | Extracellular ATD: Arg57 (VdW); Arg61 (HB); Ser143 (VdW); Tyr144 (SB); Ser145 (HB); Ala166 (HB); Ser167 (VdW); Thr168 (HB); Tyr216 (VdW); Arg271 (VdW); Ser272 (VdW); Glu273 (VdW); Asp295 (HB); Gly296 (VdW); Lys377 (VdW) – chain A Arg57 (VdW); Arg61 (HB); Ser143 (VdW); Tyr144 (VdW); Ser145 (HB); Ala166 (VdW); Ser167 (VdW); Thr168 (HB); Tyr216 (VdW); Arg271 (VdW); Ser272 (VdW); Glu273 (VdW); Asp295 (HB); Gly296 (VdW); Lys377 (VdW) – chain B |
||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: - | TM5: - | TM6: - | ECL3: - | TM7: - | |||||||
| Metabotropic Glutamate Receptor 3 (MGluR3) | ||||||||||||||||
| 2E4U [380] | Glutamate (agonist) | Extracellular ATD: Arg64 (VdW); Arg68 (HB); Ser149 (VdW); Tyr150 (VdW); Ser151 (HB); Ala172 (VdW); Ser173 (VdW); Thr174 (HB); Tyr222 (VdW); Asp301 (HB); Lys389 (VdW) | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: - | TM5: - | TM6: - | ECL3: - | TM7: - | |||||||
| 2E4V [380] | DCG-IV (agonist) | Extracellular ATD: Arg64 (VdW); Arg68 (HB); Ser149 (VdW); Tyr150 (VdW); Ser151 (HB); Ala172 (VdW); Ser173 (VdW); Thr174 (HB); Tyr222 (VdW); Arg277 (VdW); Ser278 (HB); Asp301 (HB); Gly302 (VdW); Lys389 (VdW) | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: - | TM5: - | TM6: - | ECL3: - | TM7: - | |||||||
| 2E4W [380] | 1S,3S-ACPD (agonist) | Extracellular ATD: Arg64 (VdW); Arg68 (HB); Ser149 (VdW); Tyr150 (VdW); Ser151 (HB); Ala172 (HB); Ser173 (VdW); Thr174 (HB); Tyr222 (VdW); Asp301 (HB); Gly302 (VdW); Lys389 (VdW) | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: - | TM5: - | TM6: - | ECL3: - | TM7: - | |||||||
| 2E4X [380] | 1S,3R-ACPD (agonist) | Extracellular ATD: Arg64 (VdW); Arg68 (HB); Ser149 (VdW); Tyr150 (VdW); Ser151 (HB); Ala172 (HB); Ser173 (VdW); Thr174 (HB); Tyr222 (VdW); Asp301 (HB); Gly302 (VdW); Lys389 (VdW) | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: - | TM5: - | TM6: - | ECL3: - | TM7: - | |||||||
| 2E4Y [380] | 2R,4R-APDC (agonist) | Extracellular ATD: Arg64 (SB); Arg68 (HB); Ser149 (HB); Tyr150 (VdW); Ser151 (HB); Ala172 (HB); Ser173 (VdW); Thr174 (VdW); Tyr222 (VdW); Asp301 (HB) – chain A Arg64 (HB); Arg68 (HB); Tyr150 (VdW); Ser151 (HB); Ala172 (VdW); Ser173 (VdW); Thr174 (HB); Tyr222 (SB); Asp301 (HB); Gly302 (VdW); Lys389 (VdW) – chain B |
||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: - | TM5: - | TM6: - | ECL3: - | TM7: - | |||||||
| 5CNK [379] | Glutamate (agonist) | Extracellular ATD: Arg64 (VdW); Arg68 (HB); Ser149 (VdW); Tyr150 (VdW); Ser151 (HB); Ala172 (HB); Thr174 (VdW); Tyr222 (VdW); Asp301 (HB); Lys389 (HB) – chain A Arg64 (VdW); Arg68 (HB); Ser149 (VdW); Tyr150 (VdW); Ser151 (HB); Ala172 (HB); Thr174 (VdW); Tyr222 (VdW); Asp301 (HB); Lys389 (HB) – chain B |
||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: - | TM5: - | TM6: - | ECL3: - | TM7: - | |||||||
| 5CNM [379] | LY2812223 (agonist) | Extracellular ATD: Arg64 (VdW); Arg68 (HB); Tyr150 (VdW); Ser151 (HB); Ala172 (HB); Ser173 (VdW); Thr174 (VdW); Lys389 (HB) | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: - | TM4: - | ECL2: - | TM5: - | TM6: - | ECL3: - | TM7: - | |||||||
| Metabotropic Glutamate Receptor 5 (MGluR5) | ||||||||||||||||
| 4OO9 [381] | Mavoglurant (NAM) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: Ile625 (HP) | ECL1: - | TM3: Ile651 (HP); Pro655 (HP); Tyr659 (HP) |
TM4: Leu744 (HP); Asn747 (HB) | ECL2: - | TM5: - | TM6: - | ECL3: - | TM7: Ser805 (2HB); Val806 (HP); Ser809 (HB); Ala810 (HP) | |||||||
| 5CGC [382] | 3-Chloro-4-fluoro-5-[6-(1H-pyrazol-1-yl)pyrimidin-4-yl]benzonitrile (NAM) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: - | ECL1: - | TM3: Ile651 (HP); Pro655 (HP) | TM4: - | ECL2: - | TM5: Leu744 (HP) | TM6: Trp785 (AR); Phe788 (HP) | ECL3: - | TM7: Val806 (HP); Ser809 (HB); Ala810 (HP) | |||||||
| Receptor | Main Ligand(s)/ Binding Partners |
Localization of the Interacting Residues on GPCR Structure | ||||||||||||||
| Metabotropic Glutamate Receptor 5 (MGluR5) | ||||||||||||||||
| 5CGD [382] | HTL14242 (NAM) | Extracellular ATD: - | ||||||||||||||
| TM1: - | TM2: Ile625 (HP) | ECL1: - | TM3: Ile651 (HP); Pro655 (HP) | TM4: - | ECL2: - | TM5: Leu744 (HP) | TM6: Trp785 (HP); Phe788 (AR) | ECL3: - | TM7: Val806 (HP); Ser809 (HB) | |||||||
Among all the aforementioned GPCR-derived therapeutic targets of PD, the X-ray structure of five GPCRs (D3R, A2AAR, mGluR2, mGluR3, and mGluR5) has been determined in complex with small-molecule modulators. Relevant interactions were retrieved from the GPCRdb [575] and are divided into Hydrogen Bonds (HB), Salt Bridges (SB), HydroPhobic (HP), and ARomatic interactions (AR). For mGluR2 and mGluR3, the information related to ligand interaction was gathered using PDBeMOTIF [576], an online tool designed to analyse protein-ligand interaction by detecting Hydrogen Bonds (HB) and Van der Waals (VdW) interactions.
Fig. (4).
Structural detail of the interaction between A2AAR and agonists. A) Complex between A2AAR and CGS21680 (PDBid 4UHR [245]); B) Complex between A2AAR and 5′-(N-ethylcarboxamido)adenosine (PDBid 5G53 [246]).
Fig. (5).
Structural detail of the interaction between A2AAR and antagonists. A) Complex between A2AAR and ZM241385 (PDBid 3EML [44]); B) Complex between A2AAR and 4-(3-amino-5-phenyl-1,2,4-triazin-6-yl)-2-chlorophenol (PDBid 3UZC [243]).
Fig. (6).
Structural detail of the interaction between mGluR2 and orthosteric agonists. A) Complex between mGluR2 and glutamate (PDBid 5CNI [379]); B) Complex between mGluR2 and LY2812223 (PDBid 5CNJ [379]).
Fig. (7).
Structural detail of the interaction between mGluR3 and orthosteric agonists. A) Complex between mGluR3 and glutamate (PDBid 2E4U [380]); B) Complex between mGluR3 and DCG-IV (PDBid 2E4V [380]).
Fig. (8).
Structural detail of the interaction between mGluR5 and antagonists/NAM. A) Complex between mGluR5 and HTL14242 (PDBid 5CGD [382]); B) Complex between mGluR2 and 3-chloro-4-fluoro-5-[6-(1H-pyrazol-1-yl)pyrimidin-4-yl]benzonitrile (PDBid 5CGC [382]).
CONCLUSION
GPCRs are practically ubiquitous proteins and drug targets, making them of high interest when dealing with a wide range of emerging diseases as PD. Drug discovery efforts targeting alternative therapeutic targets have been made to reduce the occurrence of these side effects. In fact, the pharmacological activation/inhibition of all the aforementioned GPCR subtypes with small-molecule drug candidates may reduce L-DOPA induced dyskinesias. This raises the need for novel and subtype-selective drug candidates useful for PD therapy.
The design of receptor subtype ligands that interact with the orthosteric binding site of GPCRs has proven to be ineffective, specifically for muscarinic acetylcholine receptors and metabotropic glutamate receptors, because of the high homology across binding sites of different GPCR subtypes, leading to a decreased subtype selectivity and specificity and unfavorable side effect profiles. Taking this into account, allosteric modulators are preferable to target subtype specific GPCRs by interacting with a protein region that is both larger and more diverse. Experimentally, these structure-based drug design methodologies have the advantage of understanding drug-GPCR interactions at a molecular level, which is vital for the development of new and reliable pharmacophore models. Nevertheless, the drug design of GPCR modulators based on orthosteric or allosteric binding site requires prior structural data information, which is scarce for the majority of GPCRs. In fact, future drugs acting on GPCRs are likely to rely on ligand-based computational methodologies to tackle missing structural data information. Overall, these in silico approaches have been extremely relevant in early stages of drug discovery, particularly in lead optimization of drug candidates, in order to determine the most favorable molecular modifications for the identification of more potent and subtype-selective GPCR modulators targeting PD. Another aspect of extreme importance in drug discovery process of GPCR modulators resides in their pharmacokinetic and toxicological profile since usually, drug candidates with a favorable pharmacodynamic profile fail to advance at late stages of drug discovery process due to their unfavorable pharmacokinetic properties and toxicity. A drug design strategy that perfectly combines favorable pharmacodynamic properties of small molecule GPCR modulators with encouraging pharmacokinetic properties (e.g. blood-brain barrier permeability, brain exposure, etc) is crucial for the development of promising antiparkinsonian agents with potential clinical efficacy.
Acknowledgements
This work had the financial support of Fundação para a Ciência e a Tecnologia (FCT/MEC) through national funds and co-financed by FEDER, under the Partnership Agreement PT2020 (projects UID/QUI/50006/2013 and POCI/01/0145/FEDER/007265). Irina S. Moreira acknowledges support by the FCT-Investigator programme - IF/00578/2014 (co-financed by European Social Fund and Programa Operacional Potencial Humano), a Marie Skłodowska-Curie Individual Fellowship MSCA-IF-2015 [MEMBRANEPROT 659826], the FEDER (Programa Operacional Factores de Competitividade - COMPETE 2020) and FCT-project: UID/NEU/04539/2013. Rita Melo acknowledges support from the FCT (SFRH/BPD/97650/2013 and UID/Multi/04349/2013 project). MNDSC further acknowledges FCT for the sabbatical grant SFRH/BSAB/127789/2016.
LIST OF ABBREVIATIONS
- 5-HTR
5-HydroxyTryptamine Receptor
- 6-OHDA
6-HydroxyDopamine
- AADC
Aromatic L-Amino acid DeCarboxylase
- AC
Adenylyl Cyclase
- AD
Alzheimer’s Disease
- ADGRG1
ADhesion G-protein coupled Receptor G1
- ADGRL3
ADhesion G-protein coupled Receptor L3
- ADHD
Attention Deficit Hyperactivity Disorder
- AMPA
α-Amino-3-hydroxy-5-Methyl-4-isoxazolePropionic Acid
- ANN
Artificial Neural Networks
- APEX-3D
Activity Prediction EXpert system-3D
- APJR
Apelin Receptor
- AR
Adenosine Receptor
- ATD
Amino-Terminal Domain
- ATR
AngioTensin II Receptor
- AutoDock
Automated Docking
- βAR
β-Adrenergic Receptor
- BBB
Blood-Brain Barrier
- CAChe
Computer-Aided Chemistry consulting
- cAMP
cyclic Adenosine MonoPhosphate
- CADD
Computer-Assisted Drug Design
- CBR
Cannabinoid Receptor
- CCR
CC Chemokine Receptor
- CDocker
CHARMm-based Docker
- cGMP
cyclic Guanosine MonoPhosphate
- CNS
Central Nervous System
- CoMFA
Comparative Molecular Field Analysis
- CoMSIA
Comparative Molecular Similarity Index Analysis
- COMT
Catechol-O-MethylTransferase
- CRF
Corticotropin Releasing Factor
- CRFR
Corticotropin Releasing Factor Receptor
- CRLR
Calcitonin Receptor-Like Receptor
- CXCR
CXC Chemokine Receptor
- CV
Cross-Validation
- DAG
DiAcylGlycerol
- DISCO
DIscrete Surface Charge Optimization
- DOR
δ-Opioid Receptor
- DR
Dopamine Receptor
- ECL
ExtraCellular Loop
- ETR
EndoThelin Receptor
- FFAR
Free Fatty Acid Receptor
- FLAP
Fingerprints for Ligands And Proteins
- FlexiDock
Flexible Docking
- FLRT2
Fibronectin Leucin-Rich Transmembrane protein 2
- FN3
FibroNectin type III domain
- FSH
Follicle-Stimulating Hormone
- FSHR
Follicle-Stimulating Hormone Receptor
- GABA
γ-AminoButyric Acid
- GABAR
γ-AminoButyric Acid Receptor
- GALAHAD
Genetic Algorithm with Linear Assignment of Hypermolecular Alignment of Database
- GDP
Guanosine DiPhosphate
- GFA
Genetic Function Approximation
- GLIDE
Grid-based LIgand-Docking with Energetics
- GLPR
Glucagon-Like Peptide Receptor
- GOLD
Genetic Optimization for Ligand Docking
- GOLPE
General Optimal Linear PLS Estimation
- GPCR
G-Protein Coupled Receptors
- GPCRdb
G-Protein Coupled Receptors database
- GRK
G-protein coupled Receptor Kinase
- GTP
Guanosine TriPhosphate
- HINT
Hydrophobic INTeractions
- HQSAR
Hologram Quantitative Structure-Activity Relationship
- HR
Histamine Receptor
- ICL
IntraCellular Loop
- ICM
Internal Coordinate Mechanics
- iGluR
Ionotropic Glutamate Receptor
- IP3
Inositol-1,4,5-triPhosphate
- JAWM
Just Add Water Molecules
- KA
Kainic Acid
- KOR
κ-Opioid Receptor
- LDA
Linear Discriminant Analysis
- LibDock
Library Docking
- LPAR
LysoPhosphatidic Acid Receptor
- L-DOPA
L-3,4-Dihydroxyphenylalanine or LevoDOPA
- mAChR
Muscarinic AcetylCholine Receptor
- MAO-B
MonoAmino Oxidase-B
- MD
Molecular Dynamics
- mGluR
Metabotropic Glutamate Receptor
- ML
Machine Learning
- MLR
Multiple Linear Regression
- MOE
Molecular Operating Environment
- MOR
μ-Opioid Receptor
- MPTP
1-Methyl-4-Phenyl-1,2,3,6-TetrahydroPyridine
- MVD
Molegro Virtual Docker
- N/OFQR
Nociceptin/Orphanin FQ Receptor
- NAM
Negative Allosteric Modulator
- NMDA
N-Methyl-D-Aspartate
- NMR
Nuclear Magnetic Resonance
- NTSR
Neurotensin Receptor
- OXR
OreXin Receptor
- P2YR
Purinergic P2Y Receptor
- PAM
Positive Allosteric Modulator
- PAR
Protease-Activated Receptor
- PD
Parkinson’s Disease
- PHASE
PHarmacophore Alignment and Scoring Engine
- PKA
Protein Kinase A
- PKC
Protein Kinase C
- PLC
PhosphoLipase C
- PLS
Partial Least Square
- PNS
Peripheral Nervous System
- PTHR
ParaThyroid Hormone-related peptide Receptor
- PTHrP
ParaThyroid Hormone-related Peptide
- QM/MM
Quantum Mechanics/Molecular Mechanics
- QSAR
Quantitative Structure-Activity Relationship
- RAMP
Receptor-Activity Modifying Protein
- REM
Rapid Eye Movement
- RGS
Regulators of G-protein Signaling
- RHO
Rhodopsin
- S1PR
Sphingosine-1-Phosphate Receptor
- SAR
Structure-Activity Relationships
- Smo
Smoothened Receptor
- SNc
Substantia Nigra pars compacta
- SNr
Substantia Nigra pars reticulata
- STN
SubThalamic Nucleus
- SVM
Support Vector Machines
- TM
TransMembrane
- TSHR
Thyroid-Stimulating Hormone Receptor
- Unc5D
Unc5D guidance receptor
- US28
Cytomegalovirus-encoded chemokine Receptor
- VFT
Venus FlyTrap
- VIPR
Vasoactive Intestinal Peptide Receptor
- VR
Vasopressin Receptor
- wPMF
water Potential of Mean Forces
- YADA
Yet Another Docking Approach
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Parkinson J. An Essay On The Shaking Palsy. London, UK: Whittingham and Rowland; 1817. [Google Scholar]
- 2.Dauer W., Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [http://dx.doi.org/10.1016/ S0896-6273(03)00568-3]. [PMID: 12971891]. [DOI] [PubMed] [Google Scholar]
- 3.Lew M. Overview of Parkinson's Disease. 2007. [DOI] [PubMed]
- 4.Schapira A.H. Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol. Sci. 2009;30(1):41–47. doi: 10.1016/j.tips.2008.10.005. [http://dx.doi.org/10. 1016/j.tips.2008.10.005]. [PMID: 19042040]. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri K.R., Healy D.G., Schapira A.H. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5(3):235–245. doi: 10.1016/S1474-4422(06)70373-8. [http://dx.doi.org/10.1016/S1474-4422(06)70373-8]. [PMID: 16488379]. [DOI] [PubMed] [Google Scholar]
- 6.Poewe W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008;15(S1) Suppl. 1:14–20. doi: 10.1111/j.1468-1331.2008.02056.x. [http://dx.doi.org/10.1111/ j.1468-1331.2008.02056.x]. [PMID: 18353132]. [DOI] [PubMed] [Google Scholar]
- 7.Warner T.T., Schapira A.H.V. Genetic and environmental factors in the cause of Parkinson’s disease. Ann. Neurol. 2003;53(S3) Suppl. 3:S16–S23. doi: 10.1002/ana.10487. [http://dx.doi.org/10.1002/ana.10487]. [PMID: 12666095]. [DOI] [PubMed] [Google Scholar]
- 8.Smith Y., Wichmann T., Factor S.A., DeLong M.R. Parkinson’s disease therapeutics: new developments and challenges since the introduction of levodopa. Neuropsychopharmacology. 2012;37(1):213–246. doi: 10.1038/npp.2011.212. [http://dx.doi.org/10.1038/npp.2011.212]. [PMID: 21956442]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsson A., Lindqvist M., Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180(4596):1200–1200. doi: 10.1038/1801200a0. [http://dx.doi.org/ 10.1038/1801200a0]. [PMID: 13483658]. [DOI] [PubMed] [Google Scholar]
- 10.Birkmayer W., Hornykiewicz O. Wien. Klin. Wochenschr. 1961;73:787–788. [The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia]. [PMID: 13869404]. [PubMed] [Google Scholar]
- 11.Barbeau A., Sourkes T.L., Murphy C.F. Les catecholamines de la maladie de parkinsons. In: de Ajuriaguerra J., editor. Monoa-mines et Systeme Nervoux Central. Geneve: Goerg & Cie SA; 1962. pp. 247–262. [Google Scholar]
- 12.Baas H., Beiske A.G., Ghika J., Jackson M., Oertel W.H., Poewe W., Ransmayr G. 1998.
- 13.Youdim M.B., Edmondson D., Tipton K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006;7(4):295–309. doi: 10.1038/nrn1883. [http://dx.doi.org/10.1038/nrn1883]. [PMID: 16552415]. [DOI] [PubMed] [Google Scholar]
- 14.Porras G., De Deurwaerdere P., Li Q., Marti M., Morgenstern R., Sohr R., Bezard E., Morari M., Meissner W.G. L-dopa-induced dyskinesia: beyond an excessive dopamine tone in the striatum. Sci. Rep. 2014;4:3730. doi: 10.1038/srep03730. [http://dx.doi.org/10.1038/ srep03730]. [PMID: 24429495]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pahwa R., Lyons K.E. Levodopa-related wearing-off in Parkinson’s disease: identification and management. Curr. Med. Res. Opin. 2009;25(4):841–849. doi: 10.1185/03007990902779319. [http://dx.doi.org/10.1185/ 03007990902779319]. [PMID: 19228103]. [DOI] [PubMed] [Google Scholar]
- 16.Jenner P. Wearing off, dyskinesia, and the use of continuous drug delivery in Parkinson’s disease. Neurol. Clin. 2013;31(3) Suppl.:S17–S35. doi: 10.1016/j.ncl.2013.04.010. [http://dx.doi.org/10.1016/j.ncl.2013.04.010]. [PMID: 23931952]. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson T., Magnusson T., Carlsson A., Linde A., Granérus A.K. “On-off” phenomenon in Parkinson’s disease: correlation to the concentration of dopa in plasma. J. Neural Transm. (Vienna) 1984;59(3):229–240. doi: 10.1007/BF01250010. [http://dx.doi.org/10.1007/BF01250010]. [PMID: 6429277]. [DOI] [PubMed] [Google Scholar]
- 18.Schrag A., Quinn N. Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain. 2000;123(Pt 11):2297–2305. doi: 10.1093/brain/123.11.2297. [http://dx.doi.org/10.1093/brain/123.11.2297]. [PMID: 11050029]. [DOI] [PubMed] [Google Scholar]
- 19.Thanvi B., Lo N., Robinson T. Levodopa-induced dyskinesia in Parkinson’s disease: clinical features, pathogenesis, prevention and treatment. Postgrad. Med. J. 2007;83(980):384–388. doi: 10.1136/pgmj.2006.054759. [http://dx. doi.org/10.1136/pgmj.2006.054759]. [PMID: 17551069]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pangalos M.N., Schechter L.E., Hurko O. Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat. Rev. Drug Discov. 2007;6(7):521–532. doi: 10.1038/nrd2094. [http://dx.doi.org/ 10.1038/nrd2094]. [PMID: 17599084]. [DOI] [PubMed] [Google Scholar]
- 21.Moreira I.S. Structural features of the G-protein/GPCR interactions. Biochim. Biophys. Acta. 2014;1840(1):16–33. doi: 10.1016/j.bbagen.2013.08.027. [http://dx.doi. org/10.1016/j.bbagen.2013.08.027]. [PMID: 24016604]. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum D.M., Rasmussen S.G., Kobilka B.K. The structure and function of G-protein-coupled receptors. Nature. 2009;459(7245):356–363. doi: 10.1038/nature08144. [http://dx.doi.org/10.1038/nature08144]. [PMID: 19458711]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiöth H.B., Fredriksson R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen. Comp. Endocrinol. 2005;142(1-2):94–101. doi: 10.1016/j.ygcen.2004.12.018. [http://dx.doi.org/10. 1016/j.ygcen.2004.12.018]. [PMID: 15862553]. [DOI] [PubMed] [Google Scholar]
- 24.Marinissen M.J., Gutkind J.S. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol. Sci. 2001;22(7):368–376. doi: 10.1016/s0165-6147(00)01678-3. [http://dx.doi.org/10.1016/S0165-6147(00) 01678-3]. [PMID: 11431032]. [DOI] [PubMed] [Google Scholar]
- 25.Lang M., Beck-Sickinger A.G. Structure-activity relationship studies: methods and ligand design for G-protein coupled peptide receptors. Curr. Protein Pept. Sci. 2006;7(4):335–353. doi: 10.2174/138920306778017981. [http://dx. doi.org/10.2174/138920306778017981]. [PMID: 16918448]. [DOI] [PubMed] [Google Scholar]
- 26.Gilman A.G. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [http://dx.doi.org/ 10.1146/annurev.bi.56.070187.003151]. [PMID: 3113327]. [DOI] [PubMed] [Google Scholar]
- 27.Birnbaumer L. The discovery of signal transduction by G proteins: a personal account and an overview of the initial findings and contributions that led to our present understanding. Biochim. Biophys. Acta. 2007;1768(4):756–771. doi: 10.1016/j.bbamem.2006.09.027. [http://dx.doi.org/10.1016/j.bbamem. 2006.09.027]. [PMID: 17141178]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kontoyianni M., Liu Z. Structure-based design in the GPCR target space. Curr. Med. Chem. 2012;19(4):544–556. doi: 10.2174/092986712798918824. [http://dx. doi.org/10.2174/092986712798918824]. [PMID: 22204332]. [DOI] [PubMed] [Google Scholar]
- 29.Ghadessy R.S., Kelly E. Second messenger-dependent protein kinases and protein synthesis regulate endogenous secretin receptor responsiveness. Br. J. Pharmacol. 2002;135(8):2020–2028. doi: 10.1038/sj.bjp.0704655. [http://dx.doi.org/10.1038/sj.bjp.0704655]. [PMID: 11959806]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shukla A.K., Xiao K., Lefkowitz R.J. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem. Sci. 2011;36(9):457–469. doi: 10.1016/j.tibs.2011.06.003. [http://dx.doi.org/10.1016/ j.tibs.2011.06.003]. [PMID: 21764321]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lefkowitz R.J. G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J. Biol. Chem. 1998;273(30):18677–18680. doi: 10.1074/jbc.273.30.18677. [http://dx.doi. org/10.1074/jbc.273.30.18677]. [PMID: 9668034]. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe B.L., Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8(5):462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [http://dx.doi.org/10.1111/j.1600-0854.2007.00551.x]. [PMID: 17376169]. [DOI] [PubMed] [Google Scholar]
- 33.Reiter E., Ahn S., Shukla A.K., Lefkowitz R.J. Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu. Rev. Pharmacol. Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [http://dx.doi.org/10.1146/annurev.pharmtox.010909.105800]. [PMID: 21942629]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsao P., von Zastrow M. Downregulation of G protein-coupled receptors. Curr. Opin. Neurobiol. 2000;10(3):365–369. doi: 10.1016/s0959-4388(00)00096-9. [http://dx. doi.org/10.1016/S0959-4388(00)00096-9]. [PMID: 10851176]. [DOI] [PubMed] [Google Scholar]
- 35.De Vries L., Zheng B., Fischer T., Elenko E., Farquhar M.G. The regulator of G protein signaling family. Annu. Rev. Pharmacol. Toxicol. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. [http://dx.doi.org/10.1146/annurev. pharmtox.40.1.235]. [PMID: 10836135]. [DOI] [PubMed] [Google Scholar]
- 36.Ross E.M., Wilkie T.M. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [http://dx.doi.org/10.1146/annurev.biochem.69.1.795]. [PMID: 10966476]. [DOI] [PubMed] [Google Scholar]
- 37.Mohan M.L., Vasudevan N.T., Gupta M.K., Martelli E.E., Naga Prasad S.V. G-protein coupled receptor resensitization-appreciating the balancing act of receptor function. 2012 https://www.ncbi. nlm.nih.gov/ pmc/articles/PMC4607669/ [PMC free article] [PubMed]
- 38.Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat. Rev. Drug Discov. 2002;1(3):198–210. doi: 10.1038/nrd746. [http://dx.doi.org/10.1038/nrd746]. [PMID: 12120504]. [DOI] [PubMed] [Google Scholar]
- 39.Conn P.J., Christopoulos A., Lindsley C.W. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009;8(1):41–54. doi: 10.1038/nrd2760. [http://dx.doi.org/ 10.1038/nrd2760]. [PMID: 19116626]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karplus M., Kuriyan J. Molecular dynamics and protein function. Proc. Natl. Acad. Sci. USA. 2005;102(19):6679–6685. doi: 10.1073/pnas.0408930102. [http://dx. doi.org/10.1073/pnas.0408930102]. [PMID: 15870208]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nussinov R., Tsai C-J. Allostery in disease and in drug discovery. Cell. 2013;153(2):293–305. doi: 10.1016/j.cell.2013.03.034. [http://dx.doi.org/10.1016/j.cell. 2013.03.034]. [PMID: 23582321]. [DOI] [PubMed] [Google Scholar]
- 42.Rosenbaum D.M., Zhang C., Lyons J.A., Holl R., Aragao D., Arlow D.H., Rasmussen S.G.F., Choi H.J., Devree B.T., Sunahara R.K., Chae P.S., Gellman S.H., Dror R.O., Shaw D.E., Weis W.I., Caffrey M., Gmeiner P., Kobilka B.K. Structure and function of an irreversible agonist-β(2) adrenoceptor complex. Nature. 2011;469(7329):236–240. doi: 10.1038/nature09665. [http://dx.doi.org/10.1038/ nature09665]. [PMID: 21228876]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu F., Wu H., Katritch V., Han G.W., Jacobson K.A., Gao Z.G., Cherezov V., Stevens R.C. Structure of an agonist-bound human A2A adenosine receptor. Science. 2011;332(6027):322–327. doi: 10.1126/science.1202793. [http://dx.doi.org/10.1126/science.1202793]. [PMID: 21393508]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaakola V.P., Griffith M.T., Hanson M.A., Cherezov V., Chien E.Y., Lane J.R., Ijzerman A.P., Stevens R.C. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322(5905):1211–1217. doi: 10.1126/science.1164772. [http://dx.doi. org/10.1126/science.1164772]. [PMID: 18832607]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozcan O., Uyar A., Doruker P., Akten E.D. Effect of intracellular loop 3 on intrinsic dynamics of human β2-adrenergic receptor. BMC Struct. Biol. 2013;13:29. doi: 10.1186/1472-6807-13-29. https://bmcstructbiol. biomedcentral. com/articles/10.1186/1472-6807-13-29 [http://dx.doi.org/10.1186/ 1472-6807-13-29]. [PMID: 24206668]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pydi S.P., Singh N., Upadhyaya J., Bhullar R.P., Chelikani P. The third intracellular loop plays a critical role in bitter taste receptor activation. Biochim. Biophys. Acta. 2014;1838(1 Pt B):231–236. doi: 10.1016/j.bbamem.2013.08.009. [http://dx.doi.org/10.1016/j.bbamem.2013.08.009]. [PMID: 23994601]. [DOI] [PubMed] [Google Scholar]
- 47.Gómez-Moutón C., Fischer T., Peregil R.M., Jiménez-Baranda S., Stossel T.P., Nakamura F., Mañes S. Filamin A interaction with the CXCR4 third intracellular loop regulates endocytosis and signaling of WT and WHIM-like receptors. Blood. 2015;125(7):1116–1125. doi: 10.1182/blood-2014-09-601807. [http://dx.doi.org/10.1182/blood-2014-09-601807]. [PMID: 25355818]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dror R.O., Green H.F., Valant C., Borhani D.W., Valcourt J.R., Pan A.C., Arlow D.H., Canals M., Lane J.R., Rahmani R., Baell J.B., Sexton P.M., Christopoulos A., Shaw D.E. Structural basis for modulation of a G-protein-coupled receptor by allosteric drugs. Nature. 2013;503(7475):295–299. doi: 10.1038/nature12595. [http://dx.doi.org/10. 1038/nature12595]. [PMID: 24121438]. [DOI] [PubMed] [Google Scholar]
- 49.Sylte I., Bronowska A., Dahl S.G. Ligand induced conformational states of the 5-HT(1A) receptor. Eur. J. Pharmacol. 2001;416(1-2):33–41. doi: 10.1016/s0014-2999(01)00860-3. [http://dx.doi.org/10.1016/S0014-2999(01)00860-3]. [PMID: 11282110]. [DOI] [PubMed] [Google Scholar]
- 50.Bronowska A., Leś A., Chilmonczyk Z., Filipek S., Edvardsen O., Ostensen R., Sylte I. Molecular dynamics of buspirone analogues interacting with the 5-HT1A and 5-HT2A serotonin receptors. Bioorg. Med. Chem. 2001;9(4):881–895. doi: 10.1016/s0968-0896(00)00307-2. [http://dx.doi.org/10. 1016/S0968-0896(00)00307-2]. [PMID: 11354671]. [DOI] [PubMed] [Google Scholar]
- 51.Beaulieu J-M., Gainetdinov R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [http://dx.doi.org/10.1124/pr.110.002642]. [PMID: 21303898]. [DOI] [PubMed] [Google Scholar]
- 52.Beaulieu J.M., Espinoza S., Gainetdinov R.R. Dopamine receptors - IUPHAR Review 13. Br. J. Pharmacol. 2015;172(1):1–23. doi: 10.1111/bph.12906. [http://dx.doi.org/10.1111/bph.12906]. [PMID: 25671228]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bédard P.J., Di Paolo T., Falardeau P., Boucher R. Chronic treatment with L-DOPA, but not bromocriptine induces dyskinesia in MPTP-parkinsonian monkeys. Correlation with [3H]spiperone binding. Brain Res. 1986;379(2):294–299. doi: 10.1016/0006-8993(86)90783-3. [http://dx.doi.org/ 10.1016/0006-8993(86)90783-3]. [PMID: 3488796]. [DOI] [PubMed] [Google Scholar]
- 54.Pearce R.K.B., Banerji T., Jenner P., Marsden C.D. De novo administration of ropinirole and bromocriptine induces less dyskinesia than L-dopa in the MPTP-treated marmoset. Mov. Disord. 1998;13(2):234–241. doi: 10.1002/mds.870130207. [http://dx.doi.org/10.1002/mds.870130207]. [PMID: 9539335]. [DOI] [PubMed] [Google Scholar]
- 55.Chen X., McCorvy J.D., Fischer M.G., Butler K.V., Shen Y., Roth B.L., Jin J. Discovery of G Protein-Biased D2 Dopamine Receptor Partial Agonists. J. Med. Chem. 2016;59(23):10601–10618. doi: 10.1021/acs.jmedchem.6b01208. [http://dx.doi.org/10.1021/acs.jmedchem.6b01208]. [PMID: 27805392]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hiller C., Kling R.C., Heinemann F.W., Meyer K., Hübner H., Gmeiner P. Functionally selective dopamine D2/D3 receptor agonists comprising an enyne moiety. J. Med. Chem. 2013;56(12):5130–5141. doi: 10.1021/jm400520c. [http://dx.doi.org/10.1021/jm400520c]. [PMID: 23730937]. [DOI] [PubMed] [Google Scholar]
- 57.Kühhorn J., Hübner H., Gmeiner P. Bivalent dopamine D2 receptor ligands: synthesis and binding properties. J. Med. Chem. 2011;54(13):4896–4903. doi: 10.1021/jm2004859. [http://dx.doi.org/10.1021/jm2004859]. [PMID: 21599022]. [DOI] [PubMed] [Google Scholar]
- 58.Weichert D., Banerjee A., Hiller C., Kling R.C., Hübner H., Gmeiner P. Molecular determinants of biased agonism at the dopamine D2 receptor. J. Med. Chem. 2015;58(6):2703–2717. doi: 10.1021/jm501889t. [http://dx.doi.org/10.1021/jm501889t]. [PMID: 25734236]. [DOI] [PubMed] [Google Scholar]
- 59.Mewshaw R.E., Kavanagh J., Stack G., Marquis K.L., Shi X., Kagan M.Z., Webb M.B., Katz A.H., Park A., Kang Y.H., Abou-Gharbia M., Scerni R., Wasik T., Cortes-Burgos L., Spangler T., Brennan J.A., Piesla M., Mazandarani H., Cockett M.I., Ochalski R., Coupet J., Andree T.H. New generation dopaminergic agents. 1. Discovery of a novel scaffold which embraces the D2 agonist pharmacophore. Structure-activity relationships of a series of 2-(aminomethyl)chromans. J. Med. Chem. 1997;40(26):4235–4256. doi: 10.1021/jm9703653. [http://dx.doi.org/10.1021/jm9703653]. [PMID: 9435894]. [DOI] [PubMed] [Google Scholar]
- 60.Boeckler F., Ohnmacht U., Lehmann T., Utz W., Hübner H., Gmeiner P. CoMFA and CoMSIA investigations revealing novel insights into the binding modes of dopamine D3 receptor agonists. J. Med. Chem. 2005;48(7):2493–2508. doi: 10.1021/jm049269+. [http://dx.doi.org/10.1021/ jm049269+]. [PMID: 15801839]. [DOI] [PubMed] [Google Scholar]
- 61.Hübner H., Haubmann C., Utz W., Gmeiner P. Conjugated enynes as nonaromatic catechol bioisosteres: synthesis, binding experiments, and computational studies of novel dopamine receptor agonists recognizing preferentially the D(3) subtype. J. Med. Chem. 2000;43(4):756–762. doi: 10.1021/jm991098z. [http://dx.doi.org/10.1021/jm991098z]. [PMID: 10691700]. [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Ladona F.J., Cox B.F. BP 897, a selective dopamine D3 receptor ligand with therapeutic potential for the treatment of cocaine-addiction. CNS Drug Rev. 2003;9(2):141–158. doi: 10.1111/j.1527-3458.2003.tb00246.x. [http://dx. doi.org/10.1111/j.1527-3458.2003.tb00246.x]. [PMID: 12847556]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tschammer N., Elsner J., Goetz A., Ehrlich K., Schuster S., Ruberg M., Kühhorn J., Thompson D., Whistler J., Hübner H., Gmeiner P. Highly potent 5-aminotetrahydropyrazolopyridines: enantioselective dopamine D3 receptor binding, functional selectivity, and analysis of receptor-ligand interactions. J. Med. Chem. 2011;54(7):2477–2491. doi: 10.1021/jm101639t. [http://dx.doi.org/10.1021/jm101639t]. [PMID: 21388142]. [DOI] [PubMed] [Google Scholar]
- 64.Hackling A., Ghosh R., Perachon S., Mann A., Höltje H.D., Wermuth C.G., Schwartz J.C., Sippl W., Sokoloff P., Stark H. N-(ω-(4-(2-methoxyphenyl)piperazin-1-yl)alkyl)carboxamides as dopamine D2 and D3 receptor ligands. J. Med. Chem. 2003;46(18):3883–3899. doi: 10.1021/jm030836n. [http://dx.doi.org/10.1021/jm030836n]. [PMID: 12930150]. [DOI] [PubMed] [Google Scholar]
- 65.Chen J., Collins G.T., Zhang J., Yang C.Y., Levant B., Woods J., Wang S. Design, synthesis, and evaluation of potent and selective ligands for the dopamine 3 (D3) receptor with a novel in vivo behavioral profile. J. Med. Chem. 2008;51(19):5905–5908. doi: 10.1021/jm800471h. [http://dx.doi.org/10.1021/jm800471h]. [PMID: 18785726]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ananthan S., Saini S.K., Zhou G., Hobrath J.V., Padmalayam I., Zhai L., Bostwick J.R., Antonio T., Reith M.E., McDowell S., Cho E., McAleer L., Taylor M., Luedtke R.R. Design, synthesis, and structure-activity relationship studies of a series of [4-(4-carboxamidobutyl)]-1-arylpiperazines: insights into structural features contributing to dopamine D3 versus D2 receptor subtype selectivity. J. Med. Chem. 2014;57(16):7042–7060. doi: 10.1021/jm500801r. [http://dx.doi.org/ 10.1021/jm500801r]. [PMID: 25126833]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leopoldo M., Berardi F., Colabufo N.A., De Giorgio P., Lacivita E., Perrone R., Tortorella V. Structure-affinity relationship study on N-[4-(4-arylpiperazin-1-yl)butyl]arylcarboxamides as potent and selective dopamine D(3) receptor ligands. J. Med. Chem. 2002;45(26):5727–5735. doi: 10.1021/jm020952a. [http://dx.doi.org/10.1021/ jm020952a]. [PMID: 12477356]. [DOI] [PubMed] [Google Scholar]
- 68.Fredholm B.B. Adenosine receptors as drug targets. Exp. Cell Res. 2010;316(8):1284–1288. doi: 10.1016/j.yexcr.2010.02.004. [http://dx.doi.org/10.1016/j.yexcr.2010. 02.004]. [PMID: 20153317]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fredholm B.B., IJzerman A.P., Jacobson K.A., Linden J., Müller C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol. Rev. 2011;63(1):1–34. doi: 10.1124/pr.110.003285. [http://dx.doi. org/10.1124/pr.110.003285]. [PMID: 21303899]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen J.F., Eltzschig H.K., Fredholm B.B. Adenosine receptors as drug targets--what are the challenges? Nat. Rev. Drug Discov. 2013;12(4):265–286. doi: 10.1038/nrd3955. [http://dx.doi.org/10.1038/nrd3955]. [PMID: 23535933]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moreau J-L., Huber G. Central adenosine A(2A) receptors: an overview. Brain Res. Brain Res. Rev. 1999;31(1):65–82. doi: 10.1016/s0165-0173(99)00059-4. [http:// dx.doi.org/10.1016/S0165-0173(99)00059-4]. [PMID: 10611496]. [DOI] [PubMed] [Google Scholar]
- 72.Svenningsson P., Le Moine C., Fisone G., Fredholm B.B. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog. Neurobiol. 1999;59(4):355–396. doi: 10.1016/s0301-0082(99)00011-8. [http://dx.doi.org/10. 1016/S0301-0082(99)00011-8]. [PMID: 10501634]. [DOI] [PubMed] [Google Scholar]
- 73.Torvinen M., Kozell L.B., Neve K.A., Agnati L.F., Fuxe K. Biochemical identification of the dopamine D2 receptor domains interacting with the adenosine A2A receptor. J. Mol. Neurosci. 2004;24(2):173–180. doi: 10.1385/JMN:24:2:173. [http://dx.doi.org/10.1385/JMN:24:2:173]. [PMID: 15456930]. [DOI] [PubMed] [Google Scholar]
- 74.Canals M., Marcellino D., Fanelli F., Ciruela F., de Benedetti P., Goldberg S.R., Neve K., Fuxe K., Agnati L.F., Woods A.S., Ferré S., Lluis C., Bouvier M., Franco R. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Biol. Chem. 2003;278(47):46741–46749. doi: 10.1074/jbc.M306451200. [http://dx.doi.org/10.1074/jbc.M306451200]. [PMID: 12933819]. [DOI] [PubMed] [Google Scholar]
- 75.Ferré S., Fuxe K., von Euler G., Johansson B., Fredholm B.B. Adenosine-dopamine interactions in the brain. Neuroscience. 1992;51(3):501–512. doi: 10.1016/0306-4522(92)90291-9. [http://dx.doi.org/10.1016/0306-4522(92)90291-9]. [PMID: 1488111]. [DOI] [PubMed] [Google Scholar]
- 76.Ferré S., Fredholm B.B., Morelli M., Popoli P., Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20(10):482–487. doi: 10.1016/s0166-2236(97)01096-5. [http://dx.doi.org/10.1016/S0166-2236(97)01096-5]. [PMID: 9347617]. [DOI] [PubMed] [Google Scholar]
- 77.Trevitt J., Vallance C., Harris A., Goode T. Adenosine antagonists reverse the cataleptic effects of haloperidol: implications for the treatment of Parkinson’s disease. Pharmacol. Biochem. Behav. 2009;92(3):521–527. doi: 10.1016/j.pbb.2009.02.001. [http://dx.doi.org/10.1016/j.pbb.2009.02.001]. [PMID: 19463269]. [DOI] [PubMed] [Google Scholar]
- 78.Varty G.B., Hodgson R.A., Pond A.J., Grzelak M.E., Parker E.M., Hunter J.C. The effects of adenosine A2A receptor antagonists on haloperidol-induced movement disorders in primates. Psychopharmacology (Berl.) 2008;200(3):393–401. doi: 10.1007/s00213-008-1214-8. [http://dx.doi. org/10.1007/s00213-008-1214-8]. [PMID: 18594798]. [DOI] [PubMed] [Google Scholar]
- 79.Lundblad M., Vaudano E., Cenci M.A. Cellular and behavioural effects of the adenosine A2a receptor antagonist KW-6002 in a rat model of l-DOPA-induced dyskinesia. J. Neurochem. 2003;84(6):1398–1410. doi: 10.1046/j.1471-4159.2003.01632.x. [http://dx.doi.org/10.1046/j.1471-4159.2003.01632.x]. [PMID: 12614340]. [DOI] [PubMed] [Google Scholar]
- 80.Chen J.F., Xu K., Petzer J.P., Staal R., Xu Y.H., Beilstein M., Sonsalla P.K., Castagnoli K., Castagnoli N., Jr, Schwarzschild M.A. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease. J. Neurosci. 2001;21(10):RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [http://dx.doi.org/10.1523/JNEUROSCI.21-10-j0001.2001]. [PMID: 11319241]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grondin R., Bédard P.J., Hadj Tahar A., Grégoire L., Mori A., Kase H. Antiparkinsonian effect of a new selective adenosine A2A receptor antagonist in MPTP-treated monkeys. Neurology. 1999;52(8):1673–1677. doi: 10.1212/wnl.52.8.1673. [http://dx.doi.org/10.1212/WNL.52.8.1673]. [PMID: 10331698]. [DOI] [PubMed] [Google Scholar]
- 82.Kanda T., Jackson M.J., Smith L.A., Pearce R.K., Nakamura J., Kase H., Kuwana Y., Jenner P. Combined use of the adenosine A(2A) antagonist KW-6002 with L-DOPA or with selective D1 or D2 dopamine agonists increases antiparkinsonian activity but not dyskinesia in MPTP-treated monkeys. Exp. Neurol. 2000;162(2):321–327. doi: 10.1006/exnr.2000.7350. [http://dx.doi.org/10.1006/exnr.2000.7350]. [PMID: 10739638]. [DOI] [PubMed] [Google Scholar]
- 83.Baraldi P.G., Cacciari B., Spalluto G. Pineda de las Infantas y Villatoro, M.J.; Zocchi, C.; Dionisotti, S.; Ongini, E. Pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine derivatives: potent and selective A(2A) adenosine antagonists. J. Med. Chem. 1996;39(5):1164–1171. doi: 10.1021/jm950746l. [http://dx.doi.org/10.1021/jm950746l]. [PMID: 8676354]. [DOI] [PubMed] [Google Scholar]
- 84.Baraldi P.G., Cacciari B., Spalluto G., Bergonzoni M., Dionisotti S., Ongini E., Varani K., Borea P.A. Design, synthesis, and biological evaluation of a second generation of pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines as potent and selective A2A adenosine receptor antagonists. J. Med. Chem. 1998;41(12):2126–2133. doi: 10.1021/jm9708689. [http://dx.doi.org/10.1021/jm9708689]. [PMID: 9622554]. [DOI] [PubMed] [Google Scholar]
- 85.Neustadt B.R., Hao J., Lindo N., Greenlee W.J., Stamford A.W., Tulshian D., Ongini E., Hunter J., Monopoli A., Bertorelli R., Foster C., Arik L., Lachowicz J., Ng K., Feng K.I. Potent, selective, and orally active adenosine A2A receptor antagonists: arylpiperazine derivatives of pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines. Bioorg. Med. Chem. Lett. 2007;17(5):1376–1380. doi: 10.1016/j.bmcl.2006.11.083. [http://dx.doi.org/10.1016/j.bmcl.2006.11.083]. [PMID: 17236762]. [DOI] [PubMed] [Google Scholar]
- 86.Vu C.B., Peng B., Kumaravel G., Smits G., Jin X., Phadke D., Engber T., Huang C., Reilly J., Tam S., Grant D., Hetu G., Chen L., Zhang J., Petter R.C. Piperazine derivatives of [1,2,4]triazolo[1,5-a][1,3,5]triazine as potent and selective adenosine A2a receptor antagonists. J. Med. Chem. 2004;47(17):4291–4299. doi: 10.1021/jm0498405. [http://dx.doi.org/10.1021/jm0498405]. [PMID: 15294001]. [DOI] [PubMed] [Google Scholar]
- 87.Vu C.B., Pan D., Peng B., Kumaravel G., Smits G., Jin X., Phadke D., Engber T., Huang C., Reilly J., Tam S., Grant D., Hetu G., Petter R.C. Novel diamino derivatives of [1,2,4] triazolo[1,5-a][1,3,5]triazine as potent and selective adenosine A2a receptor antagonists. J. Med. Chem. 2005;48(6):2009–2018. doi: 10.1021/jm0498396. [http://dx.doi.org/10.1021/jm0498396]. [PMID: 15771443]. [DOI] [PubMed] [Google Scholar]
- 88.Gillespie R.J., Bamford S.J., Botting R., Comer M., Denny S., Gaur S., Griffin M., Jordan A.M., Knight A.R., Lerpiniere J., Leonardi S., Lightowler S., McAteer S., Merrett A., Misra A., Padfield A., Reece M., Saadi M., Selwood D.L., Stratton G.C., Surry D., Todd R., Tong X., Ruston V., Upton R., Weiss S.M. Antagonists of the human A(2A) adenosine receptor. 4. Design, synthesis, and preclinical evaluation of 7-aryltriazolo[4,5-d]pyrimidines. J. Med. Chem. 2009;52(1):33–47. doi: 10.1021/jm800961g. [http://dx.doi.org/10.1021/ jm800961g]. [PMID: 19072055]. [DOI] [PubMed] [Google Scholar]
- 89.Jacobson K.A., Gallo-Rodriguez C., Melman N., Fischer B., Maillard M., van Bergen A., van Galen P.J., Karton Y. Structure-activity relationships of 8-styrylxanthines as A2-selective adenosine antagonists. J. Med. Chem. 1993;36(10):1333–1342. doi: 10.1021/jm00062a005. [http://dx.doi.org/10.1021/jm00062a005]. [PMID: 8496902]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mantri M., de Graaf O., van Veldhoven J., Göblyös A., von Frijtag Drabbe Künzel J.K., Mulder-Krieger T., Link R., de Vries H., Beukers M.W., Brussee J., Ijzerman A.P. 2-Amino-6-furan-2-yl-4-substituted nicotinonitriles as A2A adenosine receptor antagonists. J. Med. Chem. 2008;51(15):4449–4455. doi: 10.1021/jm701594y. [http://dx. doi.org/10.1021/jm701594y]. [PMID: 18637670]. [DOI] [PubMed] [Google Scholar]
- 91.Müller C.E., Geis U., Hipp J., Schobert U., Frobenius W., Pawłowski M., Suzuki F., Sandoval-Ramírez J. Synthesis and structure-activity relationships of 3,7-dimethyl-1-propargylxanthine derivatives, A2A-selective adenosine receptor antagonists. J. Med. Chem. 1997;40(26):4396–4405. doi: 10.1021/jm970515+. [http://dx.doi.org/10.1021/ jm970515+]. [PMID: 9435909]. [DOI] [PubMed] [Google Scholar]
- 92.Shook B.C., Rassnick S., Osborne M.C., Davis S., Westover L., Boulet J., Hall D., Rupert K.C., Heintzelman G.R., Hansen K., Chakravarty D., Bullington J.L., Russell R., Branum S., Wells K.M., Damon S., Youells S., Li X., Beauchamp D.A., Palmer D., Reyes M., Demarest K., Tang Y., Rhodes K., Jackson P.F. In vivo characterization of a dual adenosine A2A/A1 receptor antagonist in animal models of Parkinson’s disease. J. Med. Chem. 2010;53(22):8104–8115. doi: 10.1021/jm100971t. [http://dx.doi.org/10.1021/jm100971t]. [PMID: 20973483]. [DOI] [PubMed] [Google Scholar]
- 93.Shook B.C., Rassnick S., Wallace N., Crooke J., Ault M., Chakravarty D., Barbay J.K., Wang A., Powell M.T., Leonard K., Alford V., Scannevin R.H., Carroll K., Lampron L., Westover L., Lim H.K., Russell R., Branum S., Wells K.M., Damon S., Youells S., Li X., Beauchamp D.A., Rhodes K., Jackson P.F. Design and characterization of optimized adenosine A2A/A1 receptor antagonists for the treatment of Parkinson’s disease. J. Med. Chem. 2012;55(3):1402–1417. doi: 10.1021/jm201640m. [http://dx.doi.org/10.1021/ jm201640m]. [PMID: 22239465]. [DOI] [PubMed] [Google Scholar]
- 94.Slee D.H., Zhang X., Moorjani M., Lin E., Lanier M.C., Chen Y., Rueter J.K., Lechner S.M., Markison S., Malany S., Joswig T., Santos M., Gross R.S., Williams J.P., Castro-Palomino J.C., Crespo M.I., Prat M., Gual S., Díaz J.L., Wen J., O’Brien Z., Saunders J. Identification of novel, water-soluble, 2-amino-N-pyrimidin-4-yl acetamides as A2A receptor antagonists with in vivo efficacy. J. Med. Chem. 2008;51(3):400–406. doi: 10.1021/jm070623o. [http://dx.doi.org/ 10.1021/jm070623o]. [PMID: 18189346]. [DOI] [PubMed] [Google Scholar]
- 95.Zhang X., Tellew J.E., Luo Z., Moorjani M., Lin E., Lanier M.C., Chen Y., Williams J.P., Saunders J., Lechner S.M., Markison S., Joswig T., Petroski R., Piercey J., Kargo W., Malany S., Santos M., Gross R.S., Wen J., Jalali K., O’Brien Z., Stotz C.E., Crespo M.I., Díaz J.L., Slee D.H. Lead optimization of 4-acetylamino-2-(3,5-dimethylpyrazol-1-yl)-6-pyridylpyrimidines as A2A adenosine receptor antagonists for the treatment of Parkinson’s disease. J. Med. Chem. 2008;51(22):7099–7110. doi: 10.1021/jm800851u. [http://dx.doi.org/10.1021/jm800851u]. [PMID: 18947224]. [DOI] [PubMed] [Google Scholar]
- 96.Volpini R., Dal Ben D., Lambertucci C., Marucci G., Mishra R.C., Ramadori A.T., Klotz K.N., Trincavelli M.L., Martini C., Cristalli G. Adenosine A2A receptor antagonists: new 8-substituted 9-ethyladenines as tools for in vivo rat models of Parkinson’s disease. ChemMedChem. 2009;4(6):1010–1019. doi: 10.1002/cmdc.200800434. [http://dx.doi.org/ 10.1002/cmdc.200800434]. [PMID: 19343763]. [DOI] [PubMed] [Google Scholar]
- 97.Zhou G., Aslanian R., Gallo G., Khan T., Kuang R., Purakkattle B., De Ruiz M., Stamford A., Ting P., Wu H., Wang H., Xiao D., Yu T., Zhang Y., Mullins D., Hodgson R. Discovery of aminoquinazoline derivatives as human A(2A) adenosine receptor antagonists. Bioorg. Med. Chem. Lett. 2016;26(4):1348–1354. doi: 10.1016/j.bmcl.2015.11.048. [http://dx.doi.org/10.1016/j.bmcl.2015.11.048]. [PMID: 26781932]. [DOI] [PubMed] [Google Scholar]
- 98.Ahmed S.S.S.J., Ahameethunisa A., Santos W. QSAR and pharmacophore modeling of 4-arylthieno [3, 2-d] pyrimidine derivatives against adenosine receptor of Parkinson’s Disease. J. Theor. Comput. Chem. 2010;9(6):975–991. [http://dx.doi.org/10.1142/ S0219633610006146]. [Google Scholar]
- 99.Ghatol S.P., Verma S., Agarwal K., Sharon A. 2010.
- 100.Preti D., Baraldi P.G., Moorman A.R., Borea P.A., Varani K. History and perspectives of A2A adenosine receptor antagonists as potential therapeutic agents. Med. Res. Rev. 2015;35(4):790–848. doi: 10.1002/med.21344. [http://dx.doi.org/10.1002/med.21344]. [PMID: 25821194]. [DOI] [PubMed] [Google Scholar]
- 101.Dungo R., Deeks E.D. Istradefylline: first global approval. Drugs. 2013;73(8):875–882. doi: 10.1007/s40265-013-0066-7. [http://dx.doi.org/10.1007/s40265-013-0066-7]. [PMID: 23700273]. [DOI] [PubMed] [Google Scholar]
- 102.Caulfield M.P., Birdsall N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998;50(2):279–290. [PMID: 9647869]. [PubMed] [Google Scholar]
- 103.Langmead C.J., Watson J., Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 2008;117(2):232–243. doi: 10.1016/j.pharmthera.2007.09.009. [http://dx.doi.org/10.1016/j.pharmthera.2007.09.009]. [PMID: 18082893]. [DOI] [PubMed] [Google Scholar]
- 104.Hasselmo M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006;16(6):710–715. doi: 10.1016/j.conb.2006.09.002. [http://dx.doi.org/ 10.1016/j.conb.2006.09.002]. [PMID: 17011181]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maehara S., Hikichi H., Ohta H. Behavioral effects of N-desmethylclozapine on locomotor activity and sensorimotor gating function in mice-Possible involvement of muscarinic receptors. Brain Res. 2011;1418:111–119. doi: 10.1016/j.brainres.2011.08.056. [http://dx.doi.org/10.1016/j. brainres.2011.08.056]. [PMID: 21917240]. [DOI] [PubMed] [Google Scholar]
- 106.Lehmann J., Langer S.Z. The striatal cholinergic interneuron: synaptic target of dopaminergic terminals? Neuroscience. 1983;10(4):1105–1120. doi: 10.1016/0306-4522(83)90102-1. [http://dx.doi.org/10.1016/0306-4522(83)90102-1]. [PMID: 6320043]. [DOI] [PubMed] [Google Scholar]
- 107.Xiang Z., Thompson A.D., Jones C.K., Lindsley C.W., Conn P.J. Roles of the M1 muscarinic acetylcholine receptor subtype in the regulation of basal ganglia function and implications for the treatment of Parkinson’s disease. J. Pharmacol. Exp. Ther. 2012;340(3):595–603. doi: 10.1124/jpet.111.187856. [http://dx.doi.org/10.1124/jpet.111.187856]. [PMID: 22135383]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ztaou S., Maurice N., Camon J., Guiraudie-Capraz G., Kerkerian-Le Goff L., Beurrier C., Liberge M., Amalric M. Involvement of Striatal Cholinergic Interneurons and M1 and M4 Muscarinic Receptors in Motor Symptoms of Parkinson’s Disease. J. Neurosci. 2016;36(35):9161–9172. doi: 10.1523/JNEUROSCI.0873-16.2016. [http://dx.doi.org/10.1523/ JNEUROSCI.0873-16.2016]. [PMID: 27581457]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mayorga A.J., Cousins M.S., Trevitt J.T., Conlan A., Gianutsos G., Salamone J.D. Characterization of the muscarinic receptor subtype mediating pilocarpine-induced tremulous jaw movements in rats. Eur. J. Pharmacol. 1999;364(1):7–11. doi: 10.1016/s0014-2999(98)00811-5. [http://dx.doi.org/10. 1016/S0014-2999(98)00811-5]. [PMID: 9920179]. [DOI] [PubMed] [Google Scholar]
- 110.Betz A.J., McLaughlin P.J., Burgos M., Weber S.M., Salamone J.D. The muscarinic receptor antagonist tropicamide suppresses tremulous jaw movements in a rodent model of parkinsonian tremor: possible role of M4 receptors. Psychopharmacology (Berl.) 2007;194(3):347–359. doi: 10.1007/s00213-007-0844-6. [http://dx.doi.org/10.1007/s00213-007-0844-6]. [PMID: 17594079]. [DOI] [PubMed] [Google Scholar]
- 111.Tzavara E.T., Bymaster F.P., Davis R.J., Wade M.R., Perry K.W., Wess J., McKinzie D.L., Felder C., Nomikos G.G.M. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18(12):1410–1412. doi: 10.1096/fj.04-1575fje. [http://dx.doi.org/10.1096/fj.04-1575fje]. [PMID: 15231726]. [DOI] [PubMed] [Google Scholar]
- 112.Gomeza J., Zhang L., Kostenis E., Felder C.C., Bymaster F.P., Brodkin J., Shannon H., Xia B., Duttaroy A., Deng C.X., Wess J. Generation and pharmacological analysis of M2 and M4 muscarinic receptor knockout mice. Life Sci. 2001;68(22-23):2457–2466. doi: 10.1016/s0024-3205(01)01039-6. [http://dx.doi.org/10.1016/S0024-3205(01)01039-6]. [PMID: 11392613]. [DOI] [PubMed] [Google Scholar]
- 113.Fink-Jensen A., Schmidt L.S., Dencker D., Schülein C., Wess J., Wörtwein G., Woldbye D.P.D. Antipsychotic-induced catalepsy is attenuated in mice lacking the M4 muscarinic acetylcholine receptor. Eur. J. Pharmacol. 2011;656(1-3):39–44. doi: 10.1016/j.ejphar.2011.01.018. [http://dx. doi.org/10.1016/j.ejphar.2011.01.018]. [PMID: 21269601]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Augelli-Szafran C.E., Blankley C.J., Jaen J.C., Moreland D.W., Nelson C.B., Penvose-Yi J.R., Schwarz R.D., Thomas A.J. Identification and characterization of m1 selective muscarinic receptor antagonists1. J. Med. Chem. 1999;42(3):356–363. doi: 10.1021/jm980067l. [http://dx.doi. org/10.1021/jm980067l]. [PMID: 9986705]. [DOI] [PubMed] [Google Scholar]
- 115.Eberlein W.G., Engel W.W., Trummlitz G., Schmidt G., Hammer R. Tricyclic compounds as selective antimuscarinics. 2. Structure-activity relationships of M1-selective antimuscarinics related to pirenzepine. J. Med. Chem. 1988;31(6):1169–1174. doi: 10.1021/jm00401a016. [http://dx.doi. org/10.1021/jm00401a016]. [PMID: 3373484]. [DOI] [PubMed] [Google Scholar]
- 116.Selent J., Brandt W., Pamperin D., Göber B. Enantiomeric N-methyl-4-piperidyl benzilates as muscarinic receptor ligands: Radioligand binding studies and docking studies to models of the three muscarinic receptors M1, M2 and M3. Bioorg. Med. Chem. 2006;14(6):1729–1736. doi: 10.1016/j.bmc.2005.10.030. [http://dx.doi.org/10.1016/j.bmc.2005.10.030]. [PMID: 16290166]. [DOI] [PubMed] [Google Scholar]
- 117.Miller N.R., Daniels R.N., Lee D., Conn P.J., Lindsley C.W. Synthesis and SAR of N-(4-(4-alklylpiperazin-1-yl)phenyl) benzamides as muscarinic acetylcholine receptor subtype 1 (M1) anatgonists. Bioorg. Med. Chem. Lett. 2010;20(7):2174–2177. doi: 10.1016/j.bmcl.2010.02.041. [http://dx.doi.org/10.1016/j.bmcl.2010.02.041]. [PMID: 20202841]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weaver C.D., Sheffler D.J., Lewis L.M., Bridges T.M., Williams R., Nalywajko N.T., Kennedy J.P., Mulder M.M., Jadhav S., Aldrich L.A., Jones C.K., Marlo J.E., Niswender C.M., Mock M.M., Zheng F., Conn P.J., Lindsley C.W. Discovery and development of a potent and highly selective small molecule muscarinic acetylcholine receptor subtype I (mAChR 1 or M1) antagonist in vitro and in vivo probe. Curr. Top. Med. Chem. 2009;9(13):1217–1226. doi: 10.2174/156802609789753635. [http://dx.doi.org/10.2174/156802609789753635]. [PMID: 19807667]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Melancon B.J., Lamers A.P., Bridges T.M., Sulikowski G.A., Utley T.J., Sheffler D.J., Noetzel M.J., Morrison R.D., Daniels J.S., Niswender C.M., Jones C.K., Conn P.J., Lindsley C.W., Wood M.R. Development of a more highly selective M(1) antagonist from the continued optimization of the MLPCN Probe ML012. Bioorg. Med. Chem. Lett. 2012;22(2):1044–1048. doi: 10.1016/j.bmcl.2011.11.110. [http://dx.doi. org/10.1016/j.bmcl.2011.11.110]. [PMID: 22197142]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Melancon B.J., Utley T.J., Sevel C., Mattmann M.E., Cheung Y-Y., Bridges T.M., Morrison R.D., Sheffler D.J., Niswender C.M., Daniels J.S., Conn P.J., Lindsley C.W., Wood M.R. Development of novel M1 antagonist scaffolds through the continued optimization of the MLPCN probe ML012. Bioorg. Med. Chem. Lett. 2012;22(15):5035–5040. doi: 10.1016/j.bmcl.2012.06.018. [http://dx.doi.org/10.1016/j.bmcl. 2012.06.018]. [PMID: 22749871]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Poslusney M.S., Sevel C., Utley T.J., Bridges T.M., Morrison R.D., Kett N.R., Sheffler D.J., Niswender C.M., Daniels J.S., Conn P.J., Lindsley C.W., Wood M.R. Synthesis and biological characterization of a series of novel diaryl amide M1 antagonists. Bioorg. Med. Chem. Lett. 2012;22(22):6923–6928. doi: 10.1016/j.bmcl.2012.09.011. [http://dx.doi. org/10.1016/j.bmcl.2012.09.011]. [PMID: 23062550]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ramos A.C., Andersen M.L., Oliveira M.G., Soeiro A.C., Galduróz J.C. Biperiden (M1 antagonist) impairs the expression of cocaine conditioned place preference but potentiates the expression of cocaine-induced behavioral sensitization. Behav. Brain Res. 2012;231(1):213–216. doi: 10.1016/j.bbr.2012.03.030. [http://dx.doi.org/10.1016/j.bbr.2012.03.030]. [PMID: 22469627]. [DOI] [PubMed] [Google Scholar]
- 123.Giachetti A., Giraldo E., Ladinsky H., Montagna E. Binding and functional profiles of the selective M1 muscarinic receptor antagonists trihexyphenidyl and dicyclomine. Br. J. Pharmacol. 1986;89(1):83–90. doi: 10.1111/j.1476-5381.1986.tb11123.x. [http://dx.doi.org/10.1111/j.1476-5381.1986.tb11123.x]. [PMID: 2432979]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thomas D.R., Dada A., Jones G.A., Deisz R.A., Gigout S., Langmead C.J., Werry T.D., Hendry N., Hagan J.J., Davies C.H., Watson J.M. N-desmethylclozapine (NDMC) is an antagonist at the human native muscarinic M(1) receptor. Neuropharmacology. 2010;58(8):1206–1214. doi: 10.1016/j.neuropharm.2010.02.017. [http://dx.doi.org/10.1016/j. neuropharm.2010.02.017]. [PMID: 20206188]. [DOI] [PubMed] [Google Scholar]
- 125.Cazzola M., Matera M.G., Liccardi G., Sacerdoti G., D’Amato G., Rossi F. Effect of telenzepine, an M1-selective muscarinic receptor antagonist, in patients with nocturnal asthma. Pulm. Pharmacol. 1994;7(2):91–97. doi: 10.1006/pulp.1994.1010. [http://dx.doi.org/10.1006/pulp.1994.1010]. [PMID: 8081076]. [DOI] [PubMed] [Google Scholar]
- 126.Hammer R., Berrie C.P., Birdsall N.J.M., Burgen A.S.V., Hulme E.C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980;283(5742):90–92. doi: 10.1038/283090a0. [http:// dx.doi.org/10.1038/283090a0]. [PMID: 7350532]. [DOI] [PubMed] [Google Scholar]
- 127.Augelli-Szafran C.E., Jaen J.C., Moreland D.W., Nelson C.B., Penvose-Yi J.R., Schwarz R.D. Identification and characterization of m4 selective muscarinic antagonists. Bioorg. Med. Chem. Lett. 1998;8(15):1991–1996. doi: 10.1016/s0960-894x(98)00351-5. [http://dx.doi.org/10.1016/S0960-894X (98)00351-5]. [PMID: 9873472]. [DOI] [PubMed] [Google Scholar]
- 128.Varoli L., Andreani A., Burnelli S., Granaiola M., Leoni A., Locatelli A., Morigi R., Rambaldi M., Bedini A., Fazio N., Spampinato S. Diphenidol-related diamines as novel muscarinic M4 receptor antagonists. Bioorg. Med. Chem. Lett. 2008;18(9):2972–2976. doi: 10.1016/j.bmcl.2008.03.061. [http://dx.doi.org/10.1016/j.bmcl.2008.03.061]. [PMID: 18395442]. [DOI] [PubMed] [Google Scholar]
- 129.Varoli L., Angeli P., Buccioni M., Burnelli S., Fazio N., Marucci G., Recanatini M., Spampinato S. Synthesis and pharmacological profile of a series of 1-substituted-2-carbonyl derivatives of Diphenidol: novel M4 muscarinic receptor antagonists. Med. Chem. 2008;4(2):121–128. doi: 10.2174/157340608783789211. [http://dx.doi.org/10.2174/157340608783789211]. [PMID: 18336331]. [DOI] [PubMed] [Google Scholar]
- 130.Lewis L.M., Sheffler D., Williams R., Bridges T.M., Kennedy J.P., Brogan J.T., Mulder M.J., Williams L., Nalywajko N.T., Niswender C.M., Weaver C.D., Conn P.J., Lindsley C.W. Synthesis and SAR of selective muscarinic acetylcholine receptor subtype 1 (M1 mAChR) antagonists. Bioorg. Med. Chem. Lett. 2008;18(3):885–890. doi: 10.1016/j.bmcl.2007.12.051. [http://dx.doi.org/10.1016/j.bmcl.2007.12.051]. [PMID: 18178088]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Böhme T.M., Augelli-Szafran C.E., Hallak H., Pugsley T., Serpa K., Schwarz R.D. Synthesis and pharmacology of benzoxazines as highly selective antagonists at M(4) muscarinic receptors. J. Med. Chem. 2002;45(14):3094–3102. doi: 10.1021/jm011116o. [http://dx.doi.org/10. 1021/jm011116o]. [PMID: 12086495]. [DOI] [PubMed] [Google Scholar]
- 132.Croy C.H., Chan W.Y., Castetter A.M., Watt M.L., Quets A.T., Felder C.C. Characterization of PCS1055, a novel muscarinic M4 receptor antagonist. Eur. J. Pharmacol. 2016;782:70–76. doi: 10.1016/j.ejphar.2016.04.022. [http:// dx.doi.org/10.1016/j.ejphar.2016.04.022]. [PMID: 27085897]. [DOI] [PubMed] [Google Scholar]
- 133.Veeraragavan S., Bui N., Perkins J.R., Yuva-Paylor L.A., Paylor R. The modulation of fragile X behaviors by the muscarinic M4 antagonist, tropicamide. Behav. Neurosci. 2011;125(5):783–790. doi: 10.1037/a0025202. [http://dx.doi.org/10.1037/a0025202]. [PMID: 21942438]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hovelsø N., Sotty F., Montezinho L.P., Pinheiro P.S., Herrik K.F., Mørk A. Therapeutic potential of metabotropic glutamate receptor modulators. Curr. Neuropharmacol. 2012;10(1):12–48. doi: 10.2174/157015912799362805. [http://dx.doi.org/10.2174/157015912799362805]. [PMID: 22942876]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Breysse N., Amalric M., Salin P. Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basal-ganglia structures in parkinsonian rats. J. Neurosci. 2003;23(23):8302–8309. doi: 10.1523/JNEUROSCI.23-23-08302.2003. [http://dx.doi.org/10.1523/JNEUROSCI. 23-23-08302.2003]. [PMID: 12967992]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ossowska K., Konieczny J., Wolfarth S., Pilc A. MTEP, a new selective antagonist of the metabotropic glutamate receptor subtype 5 (mGluR5), produces antiparkinsonian-like effects in rats. Neuropharmacology. 2005;49(4):447–455. doi: 10.1016/j.neuropharm.2005.04.002. [http://dx.doi.org/10.1016/ j.neuropharm.2005.04.002]. [PMID: 15919101]. [DOI] [PubMed] [Google Scholar]
- 137.Ossowska K., Konieczny J., Wolfarth S., Wierońska J., Pilc A. Blockade of the metabotropic glutamate receptor subtype 5 (mGluR5) produces antiparkinsonian-like effects in rats. Neuropharmacology. 2001;41(4):413–420. doi: 10.1016/s0028-3908(01)00083-1. [http://dx.doi.org/10.1016/ S0028-3908(01)00083-1]. [PMID: 11543761]. [DOI] [PubMed] [Google Scholar]
- 138.Dekundy A., Pietraszek M., Schaefer D., Cenci M.A., Danysz W. Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson’s disease. Brain Res. Bull. 2006;69(3):318–326. doi: 10.1016/j.brainresbull.2005.12.009. [http://dx.doi.org/10.1016/j.brainresbull.2005. 12.009]. [PMID: 16564428]. [DOI] [PubMed] [Google Scholar]
- 139.Kachroo A., Orlando L.R., Grandy D.K., Chen J.F., Young A.B., Schwarzschild M.A. Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J. Neurosci. 2005;25(45):10414–10419. doi: 10.1523/JNEUROSCI.3660-05.2005. [http://dx.doi.org/ 10.1523/JNEUROSCI.3660-05.2005]. [PMID: 16280580]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Coccurello R., Breysse N., Amalric M. Simultaneous blockade of adenosine A2A and metabotropic glutamate mGlu5 receptors increase their efficacy in reversing Parkinsonian deficits in rats. Neuropsychopharmacology. 2004;29(8):1451–1461. doi: 10.1038/sj.npp.1300444. [http://dx.doi. org/10.1038/sj.npp.1300444]. [PMID: 15039773]. [DOI] [PubMed] [Google Scholar]
- 141.Turle-Lorenzo N., Breysse N., Baunez C., Amalric M. Functional interaction between mGlu 5 and NMDA receptors in a rat model of Parkinson’s disease. Psychopharmacology (Berl.) 2005;179(1):117–127. doi: 10.1007/s00213-005-2202-x. [http://dx.doi.org/10.1007/s00213-005-2202-x]. [PMID: 15726332]. [DOI] [PubMed] [Google Scholar]
- 142.Armentero M.T., Fancellu R., Nappi G., Bramanti P., Blandini F. Prolonged blockade of NMDA or mGluR5 glutamate receptors reduces nigrostriatal degeneration while inducing selective metabolic changes in the basal ganglia circuitry in a rodent model of Parkinson’s disease. Neurobiol. Dis. 2006;22(1):1–9. doi: 10.1016/j.nbd.2005.09.010. [http://dx. doi.org/10.1016/j.nbd.2005.09.010]. [PMID: 16289868]. [DOI] [PubMed] [Google Scholar]
- 143.Oueslati A., Breysse N., Amalric M., Kerkerian-Le Goff L., Salin P. Dysfunction of the cortico-basal ganglia-cortical loop in a rat model of early parkinsonism is reversed by metabotropic glutamate receptor 5 antagonism. Eur. J. Neurosci. 2005;22(11):2765–2774. doi: 10.1111/j.1460-9568.2005.04498.x. [http://dx.doi.org/10.1111/j.1460-9568.2005.04498.x]. [PMID: 16324110]. [DOI] [PubMed] [Google Scholar]
- 144.Levandis G., Bazzini E., Armentero M.T., Nappi G., Blandini F. Systemic administration of an mGluR5 antagonist, but not unilateral subthalamic lesion, counteracts l-DOPA-induced dyskinesias in a rodent model of Parkinson’s disease. Neurobiol. Dis. 2008;29(1):161–168. doi: 10.1016/j.nbd.2007.08.011. [http://dx.doi.org/10.1016/j.nbd.2007.08.011]. [PMID: 17933546]. [DOI] [PubMed] [Google Scholar]
- 145.Rylander D., Iderberg H., Li Q., Dekundy A., Zhang J., Li H., Baishen R., Danysz W., Bezard E., Cenci M.A. A mGluR5 antagonist under clinical development improves L-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol. Dis. 2010;39(3):352–361. doi: 10.1016/j.nbd.2010.05.001. [http://dx.doi.org/10.1016/j.nbd.2010.05. 001]. [PMID: 20452425]. [DOI] [PubMed] [Google Scholar]
- 146.Battaglia G., Busceti C.L., Molinaro G., Biagioni F., Storto M., Fornai F., Nicoletti F., Bruno V. Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigro-striatal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J. Neurosci. 2004;24(4):828–835. doi: 10.1523/JNEUROSCI.3831-03.2004. [http://dx.doi.org/10.1523/JNEUROSCI.3831-03.2004]. [PMID: 14749427]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aguirre J.A., Kehr J., Yoshitake T., Liu F.L., Rivera A., Fernandez-Espinola S., Andbjer B., Leo G., Medhurst A.D., Agnati L.F., Fuxe K. Protection but maintained dysfunction of nigral dopaminergic nerve cell bodies and striatal dopaminergic terminals in MPTP-lesioned mice after acute treatment with the mGluR5 antagonist MPEP. Brain Res. 2005;1033(2):216–220. doi: 10.1016/j.brainres.2004.11.040. [http://dx. doi.org/10.1016/j.brainres.2004.11.040]. [PMID: 15694927]. [DOI] [PubMed] [Google Scholar]
- 148.Vernon A.C., Palmer S., Datla K.P., Zbarsky V., Croucher M.J., Dexter D.T. Neuroprotective effects of metabotropic glutamate receptor ligands in a 6-hydroxydopamine rodent model of Parkinson’s disease. Eur. J. Neurosci. 2005;22(7):1799–1806. doi: 10.1111/j.1460-9568.2005.04362.x. [http://dx. doi.org/10.1111/j.1460-9568.2005.04362.x]. [PMID: 16197521]. [DOI] [PubMed] [Google Scholar]
- 149.Vernon A.C., Zbarsky V., Datla K.P., Croucher M.J., Dexter D.T. Subtype selective antagonism of substantia nigra pars compacta Group I metabotropic glutamate receptors protects the nigrostriatal system against 6-hydroxydopamine toxicity in vivo. J. Neurochem. 2007;103(3):1075–1091. doi: 10.1111/j.1471-4159.2007.04860.x. [http://dx.doi.org/10.1111/j. 1471-4159.2007.04860.x]. [PMID: 17714448]. [DOI] [PubMed] [Google Scholar]
- 150.Battaglia G., Fornai F., Busceti C.L., Aloisi G., Cerrito F. ; De Blasi A., Melchiorri D., Nicoletti F. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity. J. Neurosci. 2002;22(6):2135–2141. doi: 10.1523/JNEUROSCI.22-06-02135.2002. [http://dx.doi.org/10.1523/JNEUROSCI.22-06-02135.2002]. [PMID: 11896153]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang L., Balan G., Barreiro G., Boscoe B.P., Chenard L.K., Cianfrogna J., Claffey M.M., Chen L., Coffman K.J., Drozda S.E., Dunetz J.R., Fonseca K.R., Galatsis P., Grimwood S., Lazzaro J.T., Mancuso J.Y., Miller E.L., Reese M.R., Rogers B.N., Sakurada I., Skaddan M., Smith D.L., Stepan A.F., Trapa P., Tuttle J.B., Verhoest P.R., Walker D.P., Wright A.S., Zaleska M.M., Zasadny K., Shaffer C.L. Discovery and preclinical characterization of 1-methyl-3-(4-methylpyridin-3-yl)-6-(pyridin-2-ylmethoxy)-1H-pyrazolo-[3,4-b]pyrazine (PF470): a highly potent, selective, and efficacious metabotropic glutamate receptor 5 (mGluR5) negative allosteric modulator. J. Med. Chem. 2014;57(3):861–877. doi: 10.1021/jm401622k. [http://dx.doi.org/10.1021/jm401622k]. [PMID: 24392688]. [DOI] [PubMed] [Google Scholar]
- 152.Burdi D.F., Hunt R., Fan L., Hu T., Wang J., Guo Z., Huang Z., Wu C., Hardy L., Detheux M., Orsini M.A., Quinton M.S., Lew R., Spear K. Design, synthesis, and structure-activity relationships of novel bicyclic azole-amines as negative allosteric modulators of metabotropic glutamate receptor 5. J. Med. Chem. 2010;53(19):7107–7118. doi: 10.1021/jm100736h. [http://dx.doi.org/10.1021/jm100736h]. [PMID: 20809633]. [DOI] [PubMed] [Google Scholar]
- 153.Keck T.M., Zou M-F., Zhang P., Rutledge R.P., Newman A.H. Metabotropic glutamate receptor 5 negative allosteric modulators as novel tools for in vivo investigation. ACS Med. Chem. Lett. 2012;3(7):544–549. doi: 10.1021/ml3000726. [http://dx.doi.org/10.1021/ml3000726]. [PMID: 22924094]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cosford N.D., Tehrani L., Roppe J., Schweiger E., Smith N.D., Anderson J., Bristow L., Brodkin J., Jiang X., McDonald I., Rao S., Washburn M., Varney M.A. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J. Med. Chem. 2003;46(2):204–206. doi: 10.1021/jm025570j. [http://dx.doi.org/10.1021/ jm025570j]. [PMID: 12519057]. [DOI] [PubMed] [Google Scholar]
- 155.Sharma S., Kedrowski J., Rook J.M., Smith R.L., Jones C.K., Rodriguez A.L., Conn P.J., Lindsley C.W. Discovery of molecular switches that modulate modes of metabotropic glutamate receptor subtype 5 (mGlu5) pharmacology in vitro and in vivo within a series of functionalized, regioisomeric 2- and 5-(phenylethynyl) pyrimidines. J. Med. Chem. 2009;52(14):4103–4106. doi: 10.1021/jm900654c. [http://dx. doi.org/10.1021/jm900654c]. [PMID: 19537763]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hirose W., Kato Y., Yamamoto T., Kassai M., Takata M., Hayashi S., Arai Y., Imai S., Yoshida K. Synthesis, structure-activity relationships and biological evaluation of 4,5,6,7-tetrahydropyrazolopyrazines as metabotropic glutamate receptor 5 negative allosteric modulators. Bioorg. Med. Chem. Lett. 2016;26(16):3866–3869. doi: 10.1016/j.bmcl.2016.07.019. [http://dx.doi.org/10.1016/j.bmcl.2016.07.019]. [PMID: 27432763]. [DOI] [PubMed] [Google Scholar]
- 157.Yoshikawa K., Ohyama T., Takahashi E., Numajiri Y., Konno M., Moriyama M., Takemi N., Kunita K., Nishimura K., Hayashi R. Identification of α-substituted acylamines as novel, potent, and orally active mGluR5 negative allosteric modulators. Bioorg. Med. Chem. Lett. 2015;25(16):3135–3141. doi: 10.1016/j.bmcl.2015.06.008. [http://dx.doi.org/ 10.1016/j.bmcl.2015.06.008]. [PMID: 26112438]. [DOI] [PubMed] [Google Scholar]
- 158.Nógrádi K., Wágner G., Domány G., Bobok A., Magdó I., Kiss B., Kolok S., Fónagy K., Gyertyán I., Háda V., Kóti J., Gál K., Farkas S., Keserű G.M., Greiner I., Szombathelyi Z. Thieno[2,3-b]pyridines as negative allosteric modulators of metabotropic GluR5 receptors: hit-to-lead optimization. Bioorg. Med. Chem. Lett. 2014;24(16):3845–3849. doi: 10.1016/j.bmcl.2014.06.057. [http://dx.doi.org/10.1016/j.bmcl. 2014.06.057]. [PMID: 25017030]. [DOI] [PubMed] [Google Scholar]
- 159.Kubas H., Meyer U., Hechenberger M., Klein K.U., Plitt P., Zemribo R., Spexgoor H.W., van Assema S.G.A., Abel U. Scaffold hopping approach towards various AFQ-056 analogs as potent metabotropic glutamate receptor 5 negative allosteric modulators. Bioorg. Med. Chem. Lett. 2013;23(23):6370–6376. doi: 10.1016/j.bmcl.2013.09.059. [http://dx.doi. org/10.1016/j.bmcl.2013.09.059]. [PMID: 24125886]. [DOI] [PubMed] [Google Scholar]
- 160.Kubas H., Meyer U., Krueger B., Hechenberger M., Vanejevs M., Zemribo R., Kauss V., Ambartsumova R., Pyatkin I., Polosukhin A.I., Abel U. Discovery, synthesis, and structure-activity relationships of 2-aminoquinazoline derivatives as a novel class of metabotropic glutamate receptor 5 negative allosteric modulators. Bioorg. Med. Chem. Lett. 2013;23(16):4493–4500. doi: 10.1016/j.bmcl.2013.06.049. [http://dx.doi. org/10.1016/j.bmcl.2013.06.049]. [PMID: 23856046]. [DOI] [PubMed] [Google Scholar]
- 161.Chae E., Shin Y.J., Ryu E.J., Ji M.K., Ryune Cho N., Lee K.H., Jeong H.J., Kim S.J., Choi Y., Seok Oh K., Park C.E., Soo Yoon Y. Discovery of biological evaluation of pyrazole/imidazole amides as mGlu5 receptor negative allosteric modulators. Bioorg. Med. Chem. Lett. 2013;23(7):2134–2139. doi: 10.1016/j.bmcl.2013.01.116. [http://dx.doi.org/10. 1016/j.bmcl.2013.01.116]. [PMID: 23434029]. [DOI] [PubMed] [Google Scholar]
- 162.Xu L., Zhou S., Yu K., Gao B., Jiang H., Zhen X., Fu W. Molecular modeling of the 3D structure of 5-HT(1A)R: discovery of novel 5-HT(1A)R agonists via dynamic pharmacophore-based virtual screening. J. Chem. Inf. Model. 2013;53(12):3202–3211. doi: 10.1021/ci400481p. [http://dx.doi.org/10.1021/ci400481p]. [PMID: 24245825]. [DOI] [PubMed] [Google Scholar]
- 163.Koller M., Carcache D.A., Orain D., Ertl P., Behnke D., Desrayaud S., Laue G., Vranesic I. Discovery of 1H-pyrrolo[2,3-c]pyridine-7-carboxamides as novel, allosteric mGluR5 antagonists. Bioorg. Med. Chem. Lett. 2012;22(20):6454–6459. doi: 10.1016/j.bmcl.2012.08.053. [http://dx.doi. org/10.1016/j.bmcl.2012.08.053]. [PMID: 22963764]. [DOI] [PubMed] [Google Scholar]
- 164.Packiarajan M., Ferreira C.G.M., Hong S.P., White A.D., Chandrasena G., Pu X., Brodbeck R.M., Robichaud A.J. Azetidinyl oxadiazoles as potent mGluR5 positive allosteric modulators. Bioorg. Med. Chem. Lett. 2012;22(20):6469–6474. doi: 10.1016/j.bmcl.2012.08.044. [http://dx.doi. org/10.1016/j.bmcl.2012.08.044]. [PMID: 22975301]. [DOI] [PubMed] [Google Scholar]
- 165.Konieczny J., Ossowska K., Wolfarth S., Pilc A. LY354740, a group II metabotropic glutamate receptor agonist with potential antiparkinsonian properties in rats. Naunyn Schmiedebergs Arch. Pharmacol. 1998;358(4):500–502. doi: 10.1007/pl00005284. [http://dx.doi.org/10.1007/ PL00005284]. [PMID: 9826074]. [DOI] [PubMed] [Google Scholar]
- 166.Bradley S.R., Marino M.J., Wittmann M., Rouse S.T., Awad H., Levey A.I., Conn P.J. Activation of group II metabotropic glutamate receptors inhibits synaptic excitation of the substantia Nigra pars reticulata. J. Neurosci. 2000;20(9):3085–3094. doi: 10.1523/JNEUROSCI.20-09-03085.2000. [http://dx. doi.org/10.1523/JNEUROSCI.20-09-03085.2000]. [PMID: 10777772]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Matarredona E.R., Santiago M., Venero J.L., Cano J., Machado A. Group II metabotropic glutamate receptor activation protects striatal dopaminergic nerve terminals against MPP+-induced neurotoxicity along with brain-derived neurotrophic factor induction. J. Neurochem. 2001;76(2):351–360. doi: 10.1046/j.1471-4159.2001.00056.x. [http://dx.doi.org/10.1046/ j.1471-4159.2001.00056.x]. [PMID: 11208898]. [DOI] [PubMed] [Google Scholar]
- 168.Battaglia G., Busceti C.L., Pontarelli F., Biagioni F., Fornai F., Paparelli A., Bruno V., Ruggieri S., Nicoletti F. Protective role of group-II metabotropic glutamate receptors against nigro-striatal degeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Neuropharmacology. 2003;45(2):155–166. doi: 10.1016/s0028-3908(03)00146-1. [http://dx.doi.org/10.1016/S0028-3908(03)00146-1]. [PMID: 12842121]. [DOI] [PubMed] [Google Scholar]
- 169.Matsui T., Kita H. Activation of group III metabotropic glutamate receptors presynaptically reduces both GABAergic and glutamatergic transmission in the rat globus pallidus. Neuroscience. 2003;122(3):727–737. doi: 10.1016/j.neuroscience.2003.08.032. [http://dx.doi.org/10.1016/j.neuroscience.2003.08. 032]. [PMID: 14622916]. [DOI] [PubMed] [Google Scholar]
- 170.Valenti O., Marino M.J., Wittmann M., Lis E., DiLella A.G., Kinney G.G., Conn P.J. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J. Neurosci. 2003;23(18):7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [http://dx.doi.org/10.1523/JNEUROSCI. 23-18-07218.2003]. [PMID: 12904482]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wittmann M., Marino M.J., Bradley S.R., Conn P.J. Activation of group III mGluRs inhibits GABAergic and glutamatergic transmission in the substantia nigra pars reticulata. J. Neurophysiol. 2001;85(5):1960–1968. doi: 10.1152/jn.2001.85.5.1960. [http://dx.doi.org/10.1152/jn.2001.85.5. 1960]. [PMID: 11353013]. [DOI] [PubMed] [Google Scholar]
- 172.MacInnes N., Messenger M.J., Duty S. Activation of group III metabotropic glutamate receptors in selected regions of the basal ganglia alleviates akinesia in the reserpine-treated rat. Br. J. Pharmacol. 2004;141(1):15–22. doi: 10.1038/sj.bjp.0705566. [http://dx.doi.org/10.1038/sj.bjp. 0705566]. [PMID: 14597605]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lopez S., Turle-Lorenzo N., Acher F., De Leonibus E., Mele A., Amalric M. Targeting group III metabotropic glutamate receptors produces complex behavioral effects in rodent models of Parkinson’s disease. J. Neurosci. 2007;27(25):6701–6711. doi: 10.1523/JNEUROSCI.0299-07.2007. [http://dx. doi.org/10.1523/JNEUROSCI.0299-07.2007]. [PMID: 17581957]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Valenti O., Mannaioni G., Seabrook G.R., Conn P.J., Marino M.J. Group III metabotropic glutamate-receptor-mediated modulation of excitatory transmission in rodent substantia nigra pars compacta dopamine neurons. J. Pharmacol. Exp. Ther. 2005;313(3):1296–1304. doi: 10.1124/jpet.104.080481. [http://dx.doi.org/10.1124/jpet.104.080481]. [PMID: 15761115]. [DOI] [PubMed] [Google Scholar]
- 175.Zhang L., Brodney M.A., Candler J., Doran A.C., Duplantier A.J., Efremov I.V., Evrard E., Kraus K., Ganong A.H., Haas J.A., Hanks A.N., Jenza K., Lazzaro J.T., Maklad N., McCarthy S.A., Qian W., Rogers B.N., Rottas M.D., Schmidt C.J., Siuciak J.A., Tingley F.D., III, Zhang A.Q. 1-[(1-methyl-1H-imidazol-2-yl)methyl]-4-phenylpiperidines as mGluR2 positive allosteric modulators for the treatment of psychosis. J. Med. Chem. 2011;54(6):1724–1739. doi: 10.1021/jm101414h. [http://dx.doi.org/10.1021/jm101414h]. [PMID: 21366332]. [DOI] [PubMed] [Google Scholar]
- 176.Andrés J.I., Alcázar J., Cid J.M., De Angelis M., Iturrino L., Langlois X., Lavreysen H., Trabanco A.A., Celen S., Bormans G. Synthesis, evaluation, and radiolabeling of new potent positive allosteric modulators of the metabotropic glutamate receptor 2 as potential tracers for positron emission tomography imaging. J. Med. Chem. 2012;55(20):8685–8699. doi: 10.1021/jm300912k. [http://dx.doi.org/10.1021/ jm300912k]. [PMID: 22992024]. [DOI] [PubMed] [Google Scholar]
- 177.Trabanco A.A., Duvey G., Cid J.M., Macdonald G.J., Cluzeau P., Nhem V., Furnari R., Behaj N., Poulain G., Finn T., Lavreysen H., Poli S., Raux A., Thollon Y., Poirier N., D’Addona D., Andrés J.I., Lutjens R., Le Poul E., Imogai H., Rocher J.P. New positive allosteric modulators of the metabotropic glutamate receptor 2 (mGluR2): identification and synthesis of N-propyl-8-chloro-6-substituted isoquinolones. Bioorg. Med. Chem. Lett. 2011;21(3):971–976. doi: 10.1016/j.bmcl.2010.12.048. [http://dx.doi.org/10.1016/j.bmcl.2010.12.048]. [PMID: 21232953]. [DOI] [PubMed] [Google Scholar]
- 178.Cid J.M., Duvey G., Cluzeau P., Nhem V., Macary K., Raux A., Poirier N., Muller J., Boléa C., Finn T., Poli S., Epping-Jordan M., Chamelot E., Derouet F., Girard F., Macdonald G.J., Vega J.A., de Lucas A.I., Matesanz E., Lavreysen H., Linares M.L., Oehlrich D., Oyarzábal J., Tresadern G., Trabanco A.A., Andrés J.I., Le Poul E., Imogai H., Lutjens R., Rocher J.P. Discovery of 1,5-disubstituted pyridones: a new class of positive allosteric modulators of the metabotropic glutamate 2 receptor. ACS Chem. Neurosci. 2010;1(12):788–795. doi: 10.1021/cn1000638. [http://dx.doi.org/10.1021/ cn1000638]. [PMID: 22778815]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Garbaccio R.M., Brnardic E.J., Fraley M.E., Hartman G.D., Hutson P.H., O’Brien J.A., Magliaro B.C., Uslaner J.M., Huszar S.L., Fillgrove K.L., Small J.H., Tang C., Kuo Y., Jacobson M.A. Discovery of oxazolobenzimidazoles as positive allosteric modulators for the mGluR2 receptor. ACS Med. Chem. Lett. 2010;1(8):406–410. doi: 10.1021/ml100115a. [http://dx.doi.org/10.1021/ml100115a]. [PMID: 24900224]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brnardic E.J., Fraley M.E., Garbaccio R.M., Layton M.E., Sanders J.M., Culberson C., Jacobson M.A., Magliaro B.C., Hutson P.H., O’Brien J.A., Huszar S.L., Uslaner J.M., Fillgrove K.L., Tang C., Kuo Y., Sur S.M., Hartman G.D. 3-Aryl-5-phenoxymethyl-1,3-oxazolidin-2-ones as positive allosteric modulators of mGluR2 for the treatment of schizophrenia: Hit-to-lead efforts. Bioorg. Med. Chem. Lett. 2010;20(10):3129–3133. doi: 10.1016/j.bmcl.2010.03.089. [http://dx.doi.org/10.1016/j.bmcl.2010.03.089]. [PMID: 20409708]. [DOI] [PubMed] [Google Scholar]
- 181.Tresadern G., Cid J.M., Macdonald G.J., Vega J.A., de Lucas A.I., García A., Matesanz E., Linares M.L., Oehlrich D., Lavreysen H., Biesmans I., Trabanco A.A. Scaffold hopping from pyridones to imidazo[1,2-a]pyridines. New positive allosteric modulators of metabotropic glutamate 2 receptor. Bioorg. Med. Chem. Lett. 2010;20(1):175–179. doi: 10.1016/j.bmcl.2009.11.008. [http://dx.doi.org/10.1016/ j.bmcl.2009.11.008]. [PMID: 19932615]. [DOI] [PubMed] [Google Scholar]
- 182.Huynh T.H.V., Erichsen M.N., Tora A.S., Goudet C., Sagot E., Assaf Z., Thomsen C., Brodbeck R., Stensbøl T.B., Bjørn-Yoshimoto W.E., Nielsen B., Pin J.P., Gefflaut T., Bunch L. New 4-functionalized glutamate analogues are selective agonists at metabotropic glutamate receptor subtype 2 or selective agonists at metabotropic glutamate receptor group III. J. Med. Chem. 2016;59(3):914–924. doi: 10.1021/acs.jmedchem.5b01333. [http://dx.doi.org/10.1021/acs.jmedchem.5b01333]. [PMID: 26814576]. [DOI] [PubMed] [Google Scholar]
- 183.Engers D.W., Niswender C.M., Weaver C.D., Jadhav S., Menon U.N., Zamorano R., Conn P.J., Lindsley C.W., Hopkins C.R. Synthesis and evaluation of a series of heterobiarylamides that are centrally penetrant metabotropic glutamate receptor 4 (mGluR4) positive allosteric modulators (PAMs). J. Med. Chem. 2009;52(14):4115–4118. doi: 10.1021/jm9005065. [http://dx.doi.org/10.1021/jm9005065]. [PMID: 19469556]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jones C.K., Engers D.W., Thompson A.D., Field J.R., Blobaum A.L., Lindsley S.R., Zhou Y., Gogliotti R.D., Jadhav S., Zamorano R., Bogenpohl J., Smith Y., Morrison R., Daniels J.S., Weaver C.D., Conn P.J., Lindsley C.W., Niswender C.M., Hopkins C.R. Discovery, synthesis, and structure-activity relationship development of a series of N-4-(2,5-dioxopyrrolidin-1-yl)phenylpicolinamides (VU0400195, ML182): characterization of a novel positive allosteric modulator of the metabotropic glutamate receptor 4 (mGlu(4)) with oral efficacy in an antiparkinsonian animal model. J. Med. Chem. 2011;54(21):7639–7647. doi: 10.1021/jm200956q. [http://dx. doi.org/10.1021/jm200956q]. [PMID: 21966889]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Niswender C.M., Johnson K.A., Weaver C.D., Jones C.K., Xiang Z., Luo Q., Rodriguez A.L., Marlo J.E., de Paulis T., Thompson A.D., Days E.L., Nalywajko T., Austin C.A., Williams M.B., Ayala J.E., Williams R., Lindsley C.W., Conn P.J. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol. Pharmacol. 2008;74(5):1345–1358. doi: 10.1124/mol.108.049551. [http://dx.doi.org/ 10.1124/mol.108.049551]. [PMID: 18664603]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Niswender C.M., Lebois E.P., Luo Q., Kim K., Muchalski H., Yin H., Conn P.J., Lindsley C.W. Positive allosteric modulators of the metabotropic glutamate receptor subtype 4 (mGluR4): Part I. Discovery of pyrazolo[3,4-d]pyrimidines as novel mGluR4 positive allosteric modulators. Bioorg. Med. Chem. Lett. 2008;18(20):5626–5630. doi: 10.1016/j.bmcl.2008.08.087. [http://dx.doi.org/10.1016/j.bmcl.2008.08.087]. [PMID: 18793851]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pithadia A.B., Jain S.M. 5-Hydroxytryptamine receptor subtypes and their modulators with therapeutic potentials. J. Clin. Med. Res. 2009;1(2):72–80. doi: 10.4021/jocmr2009.05.1237. [PMID: 22505971]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Scatton B., Javoy-Agid F., Rouquier L., Dubois B., Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275(2):321–328. doi: 10.1016/0006-8993(83)90993-9. [http://dx.doi.org/10.1016/0006-8993(83)90993-9]. [PMID: 6626985]. [DOI] [PubMed] [Google Scholar]
- 189.Halliday G.M., Blumbergs P.C., Cotton R.G., Blessing W.W., Geffen L.B. Loss of brainstem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Res. 1990;510(1):104–107. doi: 10.1016/0006-8993(90)90733-r. [http://dx.doi.org/10.1016/0006-8993(90)90733-R]. [PMID: 1691042]. [DOI] [PubMed] [Google Scholar]
- 190.Kish S.J., Tong J., Hornykiewicz O., Rajput A., Chang L.J., Guttman M., Furukawa Y. Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain. 2008;131(Pt 1):120–131. doi: 10.1093/brain/awm239. [PMID: 17956909]. [DOI] [PubMed] [Google Scholar]
- 191.Nayebi A.M., Rad S.R., Saberian M., Azimzadeh S., Samini M. Buspirone improves 6-hydroxydopamine-induced catalepsy through stimulation of nigral 5-HT(1A) receptors in rats. Pharmacol. Rep. 2010;62(2):258–264. doi: 10.1016/s1734-1140(10)70264-4. [http://dx.doi.org/10.1016/S1734-1140(10) 70264-4]. [PMID: 20508280]. [DOI] [PubMed] [Google Scholar]
- 192.Kleven M.S., Barret-Grévoz C., Bruins Slot L., Newman-Tancredi A. Novel antipsychotic agents with 5-HT(1A) agonist properties: role of 5-HT(1A) receptor activation in attenuation of catalepsy induction in rats. Neuropharmacology. 2005;49(2):135–143. doi: 10.1016/j.neuropharm.2005.02.005. [http://dx.doi.org/10.1016/j.neuropharm.2005.02.005]. [PMID: 15996562]. [DOI] [PubMed] [Google Scholar]
- 193.Ohno Y., Shimizu S., Imaki J. Effects of tandospirone, a 5-HT1A agonistic anxiolytic agent, on haloperidol-induced catalepsy and forebrain Fos expression in mice. J. Pharmacol. Sci. 2009;109(4):593–599. doi: 10.1254/jphs.08313fp. [http://dx.doi.org/10.1254/jphs.08313FP]. [PMID: 19352073]. [DOI] [PubMed] [Google Scholar]
- 194.Mignon L., Wolf W.A. Postsynaptic 5-HT(1A) receptors mediate an increase in locomotor activity in the monoamine-depleted rat. Psychopharmacology (Berl.) 2002;163(1):85–94. doi: 10.1007/s00213-002-1121-3. [http://dx.doi. org/10.1007/s00213-002-1121-3]. [PMID: 12185404]. [DOI] [PubMed] [Google Scholar]
- 195.Dupre K.B., Eskow K.L., Barnum C.J., Bishop C. Striatal 5-HT1A receptor stimulation reduces D1 receptor-induced dyskinesia and improves movement in the hemiparkinsonian rat. Neuropharmacology. 2008;55(8):1321–1328. doi: 10.1016/j.neuropharm.2008.08.031. [http://dx.doi.org/10.1016/ j.neuropharm.2008.08.031]. [PMID: 18824001]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jones C.A., Johnston L.C., Jackson M.J., Smith L.A., van Scharrenburg G., Rose S., Jenner P.G., McCreary A.C. An in vivo pharmacological evaluation of pardoprunox (SLV308)--a novel combined dopamine D(2)/D(3) receptor partial agonist and 5-HT(1A) receptor agonist with efficacy in experimental models of Parkinson’s disease. Eur. Neuropsychopharmacol. 2010;20(8):582–593. doi: 10.1016/j.euroneuro.2010.03.001. [http://dx.doi.org/10.1016/j.euroneuro.2010.03.001]. [PMID: 20434890]. [DOI] [PubMed] [Google Scholar]
- 197.Carta M., Carlsson T., Kirik D., Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130(Pt 7):1819–1833. doi: 10.1093/brain/awm082. [http://dx.doi.org/10.1093/brain/awm082]. [PMID: 17452372]. [DOI] [PubMed] [Google Scholar]
- 198.Lindgren H.S., Andersson D.R., Lagerkvist S., Nissbrandt H., Cenci M.A. L-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson’s disease: temporal and quantitative relationship to the expression of dyskinesia. J. Neurochem. 2010;112(6):1465–1476. doi: 10.1111/j.1471-4159.2009.06556.x. [http://dx.doi.org/10.1111/ j.1471-4159.2009.06556.x]. [PMID: 20050978]. [DOI] [PubMed] [Google Scholar]
- 199.Tayarani-Binazir K., Jackson M.J., Rose S., McCreary A.C., Jenner P. The partial dopamine agonist pardoprunox (SLV308) administered in combination with l-dopa improves efficacy and decreases dyskinesia in MPTP treated common marmosets. Exp. Neurol. 2010;226(2):320–327. doi: 10.1016/j.expneurol.2010.09.007. [http://dx.doi.org/10.1016/j.expneurol. 2010.09.007]. [PMID: 20843474]. [DOI] [PubMed] [Google Scholar]
- 200.Zhang X., Andren P.E., Greengard P., Svenningsson P. Evidence for a role of the 5-HT1B receptor and its adaptor protein, p11, in L-DOPA treatment of an animal model of Parkinsonism. Proc. Natl. Acad. Sci. USA. 2008;105(6):2163–2168. doi: 10.1073/pnas.0711839105. [http://dx.doi.org/10. 1073/pnas.0711839105]. [PMID: 18256188]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jackson M.J., Al-Barghouthy G., Pearce R.K., Smith L., Hagan J.J., Jenner P. Effect of 5-HT1B/D receptor agonist and antagonist administration on motor function in haloperidol and MPTP-treated common marmosets. Pharmacol. Biochem. Behav. 2004;79(3):391–400. doi: 10.1016/j.pbb.2004.07.015. [http://dx.doi.org/10.1016/j.pbb.2004.07.015]. [PMID: 15582011]. [DOI] [PubMed] [Google Scholar]
- 202.Muñoz A., Carlsson T., Tronci E., Kirik D., Björklund A., Carta M. Serotonin neuron-dependent and -independent reduction of dyskinesia by 5-HT1A and 5-HT1B receptor agonists in the rat Parkinson model. Exp. Neurol. 2009;219(1):298–307. doi: 10.1016/j.expneurol.2009.05.033. [http://dx. doi.org/10.1016/j.expneurol.2009.05.033]. [PMID: 19500572]. [DOI] [PubMed] [Google Scholar]
- 203.Dhanawat M., Das N., Shrivastava S.K. Design, synthesis, anticonvulsant screening and 5HT<sub>1A/2A</sub> receptor affinity of N(3)-substituted 2,4-imidazolidinediones and oxazolidinediones. Drug Discov. Ther. 2011;5(5):227–237. doi: 10.5582/ddt.2011.v5.5.227. [http://dx.doi.org/10. 5582/ddt.2011.v5.5.227]. [PMID: 22466369]. [DOI] [PubMed] [Google Scholar]
- 204.Pytka K., Walczak M., Kij A., Rapacz A., Siwek A., Kazek G., Olczyk A., Gałuszka A., Waszkielewicz A., Marona H., Sapa J., Filipek B. The antidepressant-like activity of 6-methoxy-2-[4-(2-methoxyphenyl)piperazin-1-yl]-9H-xanthen-9-one involves serotonergic 5-HT(1A) and 5-HT(2A/C) receptors activation. Eur. J. Pharmacol. 2015;764:537–546. doi: 10.1016/j.ejphar.2015.07.041. [http://dx.doi.org/10.1016/j. ejphar.2015.07.041]. [PMID: 26210317]. [DOI] [PubMed] [Google Scholar]
- 205.Partyka A., Chłoń-Rzepa G., Wasik A., Jastrzębska-Więsek M., Bucki A., Kołaczkowski M., Satała G., Bojarski A.J., Wesołowska A. Antidepressant- and anxiolytic-like activity of 7-phenylpiperazinylalkyl-1,3-dimethyl-purine-2,6-dione derivatives with diversified 5-HT1A receptor functional profile. Bioorg. Med. Chem. 2015;23(1):212–221. doi: 10.1016/j.bmc.2014.11.008. [http://dx.doi.org/10.1016/j.bmc. 2014.11.008]. [PMID: 25435254]. [DOI] [PubMed] [Google Scholar]
- 206.Bollinger S., Hübner H., Heinemann F.W., Meyer K., Gmeiner P. Novel pyridylmethylamines as highly selective 5-HT(1A) superagonists. J. Med. Chem. 2010;53(19):7167–7179. doi: 10.1021/jm100835q. [http://dx.doi. org/10.1021/jm100835q]. [PMID: 20860381]. [DOI] [PubMed] [Google Scholar]
- 207.Franchini S., Prandi A., Baraldi A., Sorbi C., Tait A., Buccioni M., Marucci G., Cilia A., Pirona L., Fossa P., Cichero E., Brasili L. 1,3-Dioxolane-based ligands incorporating a lactam or imide moiety: structure-affinity/activity relationship at α1-adrenoceptor subtypes and at 5-HT1A receptors. Eur. J. Med. Chem. 2010;45(9):3740–3751. doi: 10.1016/j.ejmech.2010.05.023. [http://dx.doi.org/10.1016/j.ejmech.2010.05.023]. [PMID: 20605276]. [DOI] [PubMed] [Google Scholar]
- 208.Carr M.A., Creviston P.E., Hutchison D.R., Kennedy J.H., Khau V.V., Kress T.J., Leanna M.R., Marshall J.D., Martinelli M.J., Peterson B.C., Varie D.L., Wepsiec J.P. Synthetic Studies toward the Partial Ergot Alkaloid LY228729, a Potent 5HT1A Receptor Agonist. J. Org. Chem. 1997;62(25):8640–8653. [http:// dx.doi.org/10.1021/jo971256z]. [Google Scholar]
- 209.Neale R.F., Fallon S.L., Boyar W.C., Wasley J.W.F., Martin L.L., Stone G.A., Glaeser B.S., Sinton C.M., Williams M. Biochemical and pharmacological characterization of CGS 12066B, a selective serotonin-1B agonist. Eur. J. Pharmacol. 1987;136(1):1–9. doi: 10.1016/0014-2999(87)90772-2. [http://dx.doi.org/10.1016/0014-2999(87)90772-2]. [PMID: 3496228]. [DOI] [PubMed] [Google Scholar]
- 210.Edmeads J.G., Millson D.S. 1997.
- 211.Jandu K.S., Barrett V., Brockwell M., Cambridge D., Farrant D.R., Foster C., Giles H., Glen R.C., Hill A.P., Hobbs H., Honey A., Martin G.R., Salmon J., Smith D., Woollard P., Selwood D.L. Discovery of 4-[3-(trans-3-dimethylaminocyclobutyl)-1H-indol-5-ylmethyl]-(4S)-oxazolidin-2-one (4991W93), a 5HT(1B/1D) receptor partial agonist and a potent inhibitor of electrically induced plasma extravasation. J. Med. Chem. 2001;44(5):681–693. doi: 10.1021/jm000956k. [http://dx.doi.org/10.1021/jm000956k]. [PMID: 11262079]. [DOI] [PubMed] [Google Scholar]
- 212.Werneck A.L., Rosso A.L., Vincent M.B. The use of an antagonist 5-HT2a/c for depression and motor function in Parkinson’ disease. Arq. Neuropsiquiatr. 2009;67(2B):407–412. doi: 10.1590/s0004-282x2009000300007. [http://dx.doi. org/10.1590/S0004-282X2009000300007]. [PMID: 19623435]. [DOI] [PubMed] [Google Scholar]
- 213.Henderson J., Yiannikas C., Graham J.S. Effect of ritanserin, a highly selective 5-HT2 receptor antagonist, on Parkinson’s disease. Clin. Exp. Neurol. 1992;29:277–282. [PMID: 1343870]. [PubMed] [Google Scholar]
- 214.Ferguson M.C., Nayyar T., Deutch A.Y., Ansah T.A. 5-HT2A receptor antagonists improve motor impairments in the MPTP mouse model of Parkinson’s disease. Neuropharmacology. 2010;59(1-2):31–36. doi: 10.1016/j.neuropharm.2010.03.013. [http://dx.doi.org/10.1016/j.neuropharm.2010.03.013]. [PMID: 20361986]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ansah T.A., Ferguson M.C., Nayyar T. The 5-HT(2A) Receptor Antagonist M100907 Produces Antiparkinsonian Effects and Decreases Striatal Glutamate. Front. Syst. Neurosci. 2011;5(48):48. doi: 10.3389/fnsys.2011.00048. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3117200/ [PMID: 21716656]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fox S.H., Moser B., Brotchie J.M. Behavioral effects of 5-HT2C receptor antagonism in the substantia nigra zona reticulata of the 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Exp. Neurol. 1998;151(1):35–49. doi: 10.1006/exnr.1998.6792. [http://dx.doi.org/10.1006/exnr.1998. 6792]. [PMID: 9582253]. [DOI] [PubMed] [Google Scholar]
- 217.Fox S.H., Brotchie J.M. 5-HT(2C) receptor antagonists enhance the behavioural response to dopamine D(1) receptor agonists in the 6-hydroxydopamine-lesioned rat. Eur. J. Pharmacol. 2000;398(1):59–64. doi: 10.1016/s0014-2999(00)00238-7. [http://dx.doi.org/10.1016/S0014-2999(00)00238-7]. [PMID: 10856448]. [DOI] [PubMed] [Google Scholar]
- 218.Watt A.P., Hitzel L., Mortishire-Smith R.J. Enantiomeric separation of substituted 2-arylindoles on derivatised polysaccharide chiral stationary phases. J. Biochem. Biophys. Methods. 2002;54(1-3):275–286. doi: 10.1016/s0165-022x(02)00121-5. [http://dx.doi.org/10.1016/S0165-022X(02)00121-5]. [PMID: 12543504]. [DOI] [PubMed] [Google Scholar]
- 219.Campiani G., Butini S., Fattorusso C., Catalanotti B., Gemma S., Nacci V., Morelli E., Cagnotto A., Mereghetti I., Mennini T., Carli M., Minetti P., Di Cesare M.A., Mastroianni D., Scafetta N., Galletti B., Stasi M.A., Castorina M., Pacifici L., Vertechy M., Di Serio S., Ghirardi O., Tinti O., Carminati P. Pyrrolo[1,3]benzothiazepine-based serotonin and dopamine receptor antagonists. Molecular modeling, further structure-activity relationship studies, and identification of novel atypical antipsychotic agents. J. Med. Chem. 2004;47(1):143–157. doi: 10.1021/jm0309811. [http://dx.doi.org/10. 1021/jm0309811]. [PMID: 14695828]. [DOI] [PubMed] [Google Scholar]
- 220.Johnsen M., Rehse K., Pertz H., Stasch J.P., Bischoff E. New antithrombotic 1-phthalazinamines with serotonin antagonistic properties. Arch. Pharm. (Weinheim) 2003;336(12):591–597. doi: 10.1002/ardp.200300775. [http://dx.doi.org/10.1002/ardp.200300775]. [PMID: 14677153]. [DOI] [PubMed] [Google Scholar]
- 221.González-Gómez J.C., Santana L., Uriarte E., Brea J., Villazón M., Loza M.I., De Luca M., Rivas M.E., Montenegro G.Y., Fontenla J.A. New arylpiperazine derivatives with high affinity for α1A, D2 and 5-HT2A receptors. Bioorg. Med. Chem. Lett. 2003;13(2):175–178. doi: 10.1016/s0960-894x(02)00933-2. [http://dx.doi.org/10.1016/S0960-894X(02)00933-2]. [PMID: 12482418]. [DOI] [PubMed] [Google Scholar]
- 222.Swain C.J., Teran A., Maroto M., Cabello A. Identification and optimisation of 5-amino-7-aryldihydro-1,4-diazepines as 5-HT2A ligands. Bioorg. Med. Chem. Lett. 2006;16(23):6058–6062. doi: 10.1016/j.bmcl.2006.08.108. [http://dx.doi.org/10.1016/j.bmcl.2006.08.108]. [PMID: 16971119]. [DOI] [PubMed] [Google Scholar]
- 223.Awouters F., Niemegeers C.J.E., Megens A.A.H.P., Meert T.F., Janssen P.A.J. Pharmacological profile of ritanserin: A very specific central serotonin S2-antagonist. Drug Dev. Res. 1988;15(1):61–73. [http://dx.doi.org/10.1002/ddr.430150107]. [Google Scholar]
- 224.Akhondzadeh S., Malek-Hosseini M., Ghoreishi A., Raznahan M., Rezazadeh S.A. Effect of ritanserin, a 5HT2A/2C antagonist, on negative symptoms of schizophrenia: a double-blind randomized placebo-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(8):1879–1883. doi: 10.1016/j.pnpbp.2008.08.020. [http://dx.doi.org/10.1016/j. pnpbp.2008.08.020]. [PMID: 18801405]. [DOI] [PubMed] [Google Scholar]
- 225.Carro L., Torrado M., Raviña E., Masaguer C.F., Lage S., Brea J., Loza M.I. Synthesis and biological evaluation of a series of aminoalkyl-tetralones and tetralols as dual dopamine/serotonin ligands. Eur. J. Med. Chem. 2014;71:237–249. doi: 10.1016/j.ejmech.2013.10.066. [http://dx.doi.org/ 10.1016/j.ejmech.2013.10.066]. [PMID: 24316025]. [DOI] [PubMed] [Google Scholar]
- 226.Tosh D.K., Ciancetta A., Warnick E., Crane S., Gao Z.G., Jacobson K.A. Structure-based scaffold repurposing for G protein coupled receptors: Transformation of adenosine derivatives into 5HT2B/5HT2C serotonin receptor antagonists. J. Med. Chem. 2016;59(24):11006–11026. doi: 10.1021/acs.jmedchem.6b01183. [http://dx.doi.org/10.1021/acs.jmedchem. 6b01183]. [PMID: 27933810]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Di Matteo V., Di Giovanni G., Esposito E. SB 242084: A selective 5-HT2C receptor antagonist. CNS Drug Rev. 2000;6(3):195–205. [http://dx.doi.org/10.1111/j.1527-3458.2000.tb00147.x]. [Google Scholar]
- 228.de Azevedo W.F., Jr, Canduri F., Basso L.A., Palma M.S., Santos D.S. Determining the structural basis for specificity of ligands using crystallographic screening. Cell Biochem. Biophys. 2006;44(3):405–411. doi: 10.1385/CBB:44:3:405. [http://dx.doi.org/10.1385/CBB:44:3:405]. [PMID: 16679527]. [DOI] [PubMed] [Google Scholar]
- 229.Levin N.M.B., Pintro V.O., de Avila M.B., de Mattos B.B., De Azevedo W.F. Jr Understanding the Structural Basis for Inhibition of Cyclin-Dependent Kinases. New Pieces in the Molecular Puzzle. Curr. Drug Targets. 2017;18(9):1104–1111. doi: 10.2174/1389450118666161116130155. [http://dx.doi.org/ 10.2174/1389450118666161116130155]. [PMID: 27848884]. [DOI] [PubMed] [Google Scholar]
- 230.Ghosh E., Kumari P., Jaiman D., Shukla A.K. Methodological advances: the unsung heroes of the GPCR structural revolution. Nat. Rev. Mol. Cell Biol. 2015;16(2):69–81. doi: 10.1038/nrm3933. [http://dx.doi.org/10. 1038/nrm3933]. [PMID: 25589408]. [DOI] [PubMed] [Google Scholar]
- 231.Palczewski K., Kumasaka T., Hori T., Behnke C.A., Motoshima H., Fox B.A., Le Trong I., Teller D.C., Okada T., Stenkamp R.E., Yamamoto M., Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289(5480):739–745. doi: 10.1126/science.289.5480.739. [http://dx.doi.org/10.1126/science.289.5480.739]. [PMID: 10926528]. [DOI] [PubMed] [Google Scholar]
- 232.Wang C., Jiang Y., Ma J., Wu H., Wacker D., Katritch V., Han G.W., Liu W., Huang X.P., Vardy E., McCorvy J.D., Gao X., Zhou X.E., Melcher K., Zhang C., Bai F., Yang H., Yang L., Jiang H., Roth B.L., Cherezov V., Stevens R.C., Xu H.E. Structural basis for molecular recognition at serotonin receptors. Science. 2013;340(6132):610–614. doi: 10.1126/science.1232807. [http://dx.doi.org/10.1126/ science.1232807]. [PMID: 23519210]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yin W., Zhou X.E., Yang D., de Waal P.W., Wang M., Dai A., Cai X., Huang C.Y., Liu P., Wang X., Yin Y., Liu B., Zhou Y., Wang J., Liu H., Caffrey M., Melcher K., Xu Y., Wang M.W., Xu H.E., Jiang Y. Crystal structure of the human 5-HT1B serotonin receptor bound to an inverse agonist. Cell Discov. 2018;4:12. doi: 10.1038/s41421-018-0009-2. [http://dx.doi.org/10.1038/s41421-018-0009-2]. [PMID: 29560272]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wacker D., Wang C., Katritch V., Han G.W., Huang X.P., Vardy E., McCorvy J.D., Jiang Y., Chu M., Siu F.Y., Liu W., Xu H.E., Cherezov V., Roth B.L., Stevens R.C. Structural features for functional selectivity at serotonin receptors. Science. 2013;340(6132):615–619. doi: 10.1126/science.1232808. [http://dx.doi.org/10.1126/science. 1232808]. [PMID: 23519215]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Liu W., Wacker D., Gati C., Han G.W., James D., Wang D., Nelson G., Weierstall U., Katritch V., Barty A., Zatsepin N.A., Li D., Messerschmidt M., Boutet S., Williams G.J., Koglin J.E., Seibert M.M., Wang C., Shah S.T., Basu S., Fromme R., Kupitz C., Rendek K.N., Grotjohann I., Fromme P., Kirian R.A., Beyerlein K.R., White T.A., Chapman H.N., Caffrey M., Spence J.C., Stevens R.C., Cherezov V. Serial femtosecond crystallography of G protein-coupled receptors. Science. 2013;342(6165):1521–1524. doi: 10.1126/science.1244142. [http://dx.doi.org/10.1126/science.1244142]. [PMID: 24357322]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ishchenko A., Wacker D., Kapoor M., Zhang A., Han G.W., Basu S., Patel N., Messerschmidt M., Weierstall U., Liu W., Katritch V., Roth B.L., Stevens R.C., Cherezov V. Structural insights into the extracellular recognition of the human serotonin 2B receptor by an antibody. Proc. Natl. Acad. Sci. USA. 2017;114(31):8223–8228. doi: 10.1073/pnas.1700891114. [http://dx.doi.org/10.1073/pnas.1700891114]. [PMID: 28716900]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wacker D., Wang S., McCorvy J.D., Betz R.M., Venkatakrishnan A.J., Levit A., Lansu K., Schools Z.L., Che T., Nichols D.E., Shoichet B.K., Dror R.O., Roth B.L. Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell. 2017;168(3):377–389.e12. doi: 10.1016/j.cell.2016.12.033. [http://dx.doi.org/10.1016/j.cell.2016.12.033]. [PMID: 28129538]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Peng Y., McCorvy J.D., Harpsøe K., Lansu K., Yuan S., Popov P., Qu L., Pu M., Che T., Nikolajsen L.F., Huang X.P., Wu Y., Shen L., Bjørn-Yoshimoto W.E., Ding K., Wacker D., Han G.W., Cheng J., Katritch V., Jensen A.A., Hanson M.A., Zhao S., Gloriam D.E., Roth B.L., Stevens R.C., Liu Z.J. 5-HT2C Receptor Structures Reveal the Structural Basis of GPCR Polypharmacology. Cell. 2018;172(4):719–730.e14. doi: 10.1016/j.cell.2018.01.001. [http://dx.doi.org/10. 1016/j.cell.2018.01.001]. [PMID: 29398112]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Glukhova A., Thal D.M., Nguyen A.T., Vecchio E.A., Jörg M., Scammells P.J., May L.T., Sexton P.M., Christopoulos A. Structure of the Adenosine A1 Receptor Reveals the Basis for Subtype Selectivity. Cell. 2017;168(5):867–877.e13. doi: 10.1016/j.cell.2017.01.042. [http://dx.doi.org/ 10.1016/j.cell.2017.01.042]. [PMID: 28235198]. [DOI] [PubMed] [Google Scholar]
- 240.Lebon G., Warne T., Edwards P.C., Bennett K., Langmead C.J., Leslie A.G.W., Tate C.G. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature. 2011;474(7352):521–525. doi: 10.1038/nature10136. [http://dx.doi.org/10.1038/nature10136]. [PMID: 21593763]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Doré A.S., Robertson N., Errey J.C., Ng I., Hollenstein K., Tehan B., Hurrell E., Bennett K., Congreve M., Magnani F., Tate C.G., Weir M., Marshall F.H. Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure. 2011;19(9):1283–1293. doi: 10.1016/j.str.2011.06.014. [http://dx. doi.org/10.1016/j.str.2011.06.014]. [PMID: 21885291]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hino T., Arakawa T., Iwanari H., Yurugi-Kobayashi T., Ikeda-Suno C., Nakada-Nakura Y., Kusano-Arai O., Weyand S., Shimamura T., Nomura N., Cameron A.D., Kobayashi T., Hamakubo T., Iwata S., Murata T. G-protein-coupled receptor inactivation by an allosteric inverse-agonist antibody. Nature. 2012;482(7384):237–240. doi: 10.1038/nature10750. [http://dx.doi.org/10.1038/nature10750]. [PMID: 22286059]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Congreve M., Andrews S.P., Doré A.S., Hollenstein K., Hurrell E., Langmead C.J., Mason J.S., Ng I.W., Tehan B., Zhukov A., Weir M., Marshall F.H. Discovery of 1,2,4-triazine derivatives as adenosine A(2A) antagonists using structure based drug design. J. Med. Chem. 2012;55(5):1898–1903. doi: 10.1021/jm201376w. [http://dx.doi.org/10.1021/ jm201376w]. [PMID: 22220592]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Liu W., Chun E., Thompson A.A., Chubukov P., Xu F., Katritch V., Han G.W., Roth C.B., Heitman L.H., IJzerman A.P., Cherezov V., Stevens R.C. Structural basis for allosteric regulation of GPCRs by sodium ions. Science. 2012;337(6091):232–236. doi: 10.1126/science.1219218. [http://dx.doi.org/10.1126/science.1219218]. [PMID: 22798613]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lebon G., Edwards P.C., Leslie A.G.W., Tate C.G. Molecular Determinants of CGS21680 Binding to the Human Adenosine A2A Receptor. Mol. Pharmacol. 2015;87(6):907–915. doi: 10.1124/mol.114.097360. [http://dx.doi. org/10.1124/mol.114.097360]. [PMID: 25762024]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Carpenter B., Nehmé R., Warne T., Leslie A.G.W., Tate C.G. Structure of the adenosine A(2A) receptor bound to an engineered G protein. Nature. 2016;536(7614):104–107. doi: 10.1038/nature18966. [http://dx.doi.org/10. 1038/nature18966]. [PMID: 27462812]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Segala E., Guo D., Cheng R.K., Bortolato A., Deflorian F., Doré A.S., Errey J.C., Heitman L.H., IJzerman A.P., Marshall F.H., Cooke R.M. Controlling the Dissociation of Ligands from the Adenosine A2A Receptor through Modulation of Salt Bridge Strength. J. Med. Chem. 2016;59(13):6470–6479. doi: 10.1021/acs.jmedchem.6b00653. [http://dx.doi. org/10.1021/acs.jmedchem.6b00653]. [PMID: 27312113]. [DOI] [PubMed] [Google Scholar]
- 248.Melnikov I., Polovinkin V., Kovalev K., Gushchin I., Shevtsov M., Shevchenko V., Mishin A., Alekseev A., Rodriguez-Valera F., Borshchevskiy V., Cherezov V., Leonard G.A., Gordeliy V., Popov A. Fast iodide-SAD phasing for high-throughput membrane protein structure determination. Sci. Adv. 2017;3(5):e1602952. doi: 10.1126/sciadv.1602952. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5429034/ [http://dx.doi.org/10.1126/sciadv.1602952]. [PMID: 28508075]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Batyuk A., Galli L., Ishchenko A., Han G.W., Gati C., Popov P.A., Lee M.Y., Stauch B., White T.A., Barty A., Aquila A., Hunter M.S., Liang M., Boutet S., Pu M., Liu Z.J., Nelson G., James D., Li C., Zhao Y., Spence J.C., Liu W., Fromme P., Katritch V., Weierstall U., Stevens R.C., Cherezov V. Native phasing of x-ray free-electron laser data for a G protein-coupled receptor. Sci. Adv. 2016;2(9):e1600292. doi: 10.1126/sciadv.1600292. [http://dx.doi.org/10.1126/ sciadv.1600292]. [PMID: 27679816]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Weinert T., Olieric N., Cheng R., Brünle S., James D., Ozerov D., Gashi D., Vera L., Marsh M., Jaeger K., Dworkowski F., Panepucci E., Basu S., Skopintsev P., Doré A.S., Geng T., Cooke R.M., Liang M., Prota A.E., Panneels V., Nogly P., Ermler U., Schertler G., Hennig M., Steinmetz M.O., Wang M., Standfuss J. Serial millisecond crystallography for routine room-temperature structure determination at synchrotrons. Nat. Commun. 2017;8(1):542. doi: 10.1038/s41467-017-00630-4. [http://dx.doi.org/10.1038/s41467-017-00630-4]. [PMID: 28912485]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rucktooa P., Cheng R.K.Y., Segala E., Geng T., Errey J.C., Brown G.A., Cooke R.M., Marshall F.H., Doré A.S. Towards high throughput GPCR crystallography: In Meso soaking of Adenosine A2A Receptor crystals. Sci. Rep. 2018;8(1):41. doi: 10.1038/s41598-017-18570-w. [http:// dx.doi.org/10.1038/s41598-017-18570-w]. [PMID: 29311713]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sun B., Bachhawat P., Chu M.L., Wood M., Ceska T., Sands Z.A., Mercier J., Lebon F., Kobilka T.S., Kobilka B.K. Crystal structure of the adenosine A2A receptor bound to an antagonist reveals a potential allosteric pocket. Proc. Natl. Acad. Sci. USA. 2017;114(8):2066–2071. doi: 10.1073/pnas.1621423114. [http://dx.doi.org/10.1073/pnas. 1621423114]. [PMID: 28167788]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Martin-Garcia J.M., Conrad C.E., Nelson G., Stander N., Zatsepin N.A., Zook J., Zhu L., Geiger J., Chun E., Kissick D., Hilgart M.C., Ogata C., Ishchenko A., Nagaratnam N., Roy-Chowdhury S., Coe J., Subramanian G., Schaffer A., James D., Ketawala G., Venugopalan N., Xu S., Corcoran S., Ferguson D., Weierstall U., Spence J.C.H., Cherezov V., Fromme P., Fischetti R.F., Liu W. Serial Millisecond Crystallography of Membrane and Soluble Protein Micro-crystals using Synchrotron Radiation. 2017 doi: 10.1107/S205225251700570X. https://doi.org/10 [DOI] [PMC free article] [PubMed]
- 254.Broecker J., Morizumi T., Ou W.L., Klingel V., Kuo A., Kissick D.J., Ishchenko A., Lee M.Y., Xu S., Makarov O., Cherezov V., Ogata C.M., Ernst O.P. High-throughput in situ X-ray screening of and data collection from protein crystals at room temperature and under cryogenic conditions. Nat. Protoc. 2018;13(2):260–292. doi: 10.1038/nprot.2017.135. [http://dx.doi.org/10.1038/nprot.2017.135]. [PMID: 29300389]. [DOI] [PubMed] [Google Scholar]
- 255.White K.L., Eddy M.T., Gao Z.G., Han G.W., Lian T., Deary A., Patel N., Jacobson K.A., Katritch V., Stevens R.C. Structural Connection between Activation Microswitch and Allosteric Sodium Site in GPCR Signaling. Structure. 2018;26(2):259–269.e5. doi: 10.1016/j.str.2017.12.013. [http://dx.doi.org/10.1016/j.str.2017.12.013]. [PMID: 29395784]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Eddy M.T., Lee M.Y., Gao Z.G., White K.L., Didenko T., Horst R., Audet M., Stanczak P., McClary K.M., Han G.W., Jacobson K.A., Stevens R.C., Wüthrich K. Allosteric Coupling of Drug Binding and Intracellular Signaling in the A2A Adenosine Receptor. Cell. 2018;172(1-2):68–80.e12. doi: 10.1016/j.cell.2017.12.004. [http://dx.doi.org/10.1016/ j.cell.2017.12.004]. [PMID: 29290469]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ma Y., Yue Y., Ma Y., Zhang Q., Zhou Q., Song Y., Shen Y., Li X., Ma X., Li C., Hanson M.A., Han G.W., Sickmier E.A., Swaminath G., Zhao S., Stevens R.C., Hu L.A., Zhong W., Zhang M., Xu F. Structural Basis for Apelin Control of the Human Apelin Receptor. Structure. 2017;25(6):858–866.e4. doi: 10.1016/j.str.2017.04.008. [http:// dx.doi.org/10.1016/j.str.2017.04.008]. [PMID: 28528775]. [DOI] [PubMed] [Google Scholar]
- 258.Zhang H., Unal H., Gati C., Han G.W., Liu W., Zatsepin N.A., James D., Wang D., Nelson G., Weierstall U., Sawaya M.R., Xu Q., Messerschmidt M., Williams G.J., Boutet S., Yefanov O.M., White T.A., Wang C., Ishchenko A., Tirupula K.C., Desnoyer R., Coe J., Conrad C.E., Fromme P., Stevens R.C., Katritch V., Karnik S.S., Cherezov V. Structure of the Angiotensin receptor revealed by serial femtosecond crystallography. Cell. 2015;161(4):833–844. doi: 10.1016/j.cell.2015.04.011. [http://dx.doi.org/10.1016/j.cell.2015.04. 011]. [PMID: 25913193]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhang H., Unal H., Desnoyer R., Han G.W., Patel N., Katritch V., Karnik S.S., Cherezov V., Stevens R.C. Structural Basis for Ligand Recognition and Functional Selectivity at Angiotensin Receptor. J. Biol. Chem. 2015;290(49):29127–29139. doi: 10.1074/jbc.M115.689000. [http://dx.doi. org/10.1074/jbc.M115.689000]. [PMID: 26420482]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhang H., Han G.W., Batyuk A., Ishchenko A., White K.L., Patel N., Sadybekov A., Zamlynny B., Rudd M.T., Hollenstein K., Tolstikova A., White T.A., Hunter M.S., Weierstall U., Liu W., Babaoglu K., Moore E.L., Katz R.D., Shipman J.M., Garcia-Calvo M., Sharma S., Sheth P., Soisson S.M., Stevens R.C., Katritch V., Cherezov V. Structural basis for selectivity and diversity in angiotensin II receptors. Nature. 2017;544(7650):327–332. doi: 10.1038/nature22035. [http://dx.doi.org/10.1038/nature22035]. [PMID: 28379944]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Joedicke L., Mao J., Kuenze G., Reinhart C., Kalavacherla T., Jonker H.R.A., Richter C., Schwalbe H., Meiler J., Preu J., Michel H., Glaubitz C. The molecular basis of subtype selectivity of human kinin G-protein-coupled receptors. Nat. Chem. Biol. 2018;14(3):284–290. doi: 10.1038/nchembio.2551. [http://dx.doi.org/10.1038/nchembio.2551]. [PMID: 29334381]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Warne T., Serrano-Vega M.J., Baker J.G., Moukhametzianov R., Edwards P.C., Henderson R., Leslie A.G., Tate C.G., Schertler G.F. Structure of a β1-adrenergic G-protein-coupled receptor. Nature. 2008;454(7203):486–491. doi: 10.1038/nature07101. [http://dx.doi.org/10.1038/nature 07101]. [PMID: 18594507]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Warne T., Moukhametzianov R., Baker J.G., Nehmé R., Edwards P.C., Leslie A.G.W., Schertler G.F.X., Tate C.G. The structural basis for agonist and partial agonist action on a β(1)-adrenergic receptor. Nature. 2011;469(7329):241–244. doi: 10.1038/nature09746. [http://dx. doi.org/10.1038/nature09746]. [PMID: 21228877]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Moukhametzianov R., Warne T., Edwards P.C., Serrano-Vega M.J., Leslie A.G.W., Tate C.G., Schertler G.F.X. Two distinct conformations of helix 6 observed in antagonist-bound structures of a β1-adrenergic receptor. Proc. Natl. Acad. Sci. USA. 2011;108(20):8228–8232. doi: 10.1073/pnas.1100185108. [http://dx.doi.org/10.1073/pnas.1100185108]. [PMID: 21540331]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Christopher J.A., Brown J., Doré A.S., Errey J.C., Koglin M., Marshall F.H., Myszka D.G., Rich R.L., Tate C.G., Tehan B., Warne T., Congreve M. Biophysical fragment screening of the β1-adrenergic receptor: identification of high affinity arylpiperazine leads using structure-based drug design. J. Med. Chem. 2013;56(9):3446–3455. doi: 10.1021/jm400140q. [http://dx.doi.org/10.1021/jm400140q]. [PMID: 23517028]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Warne T., Edwards P.C., Leslie A.G., Tate C.G. Crystal structures of a stabilized β1-adrenoceptor bound to the biased agonists bucindolol and carvedilol. Structure. 2012;20(5):841–849. doi: 10.1016/j.str.2012.03.014. [http://dx.doi.org/10.1016/j.str.2012.03.014]. [PMID: 22579251]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Miller-Gallacher J.L., Nehmé R., Warne T., Edwards P.C., Schertler G.F.X., Leslie A.G.W., Tate C.G. The 2.1 Å resolution structure of cyanopindolol-bound β1-adrenoceptor identifies an intramembrane Na+ ion that stabilises the ligand-free receptor. PLoS One. 2014;9(3):e92727. doi: 10.1371/journal.pone.0092727. [http://dx.doi.org/10.1371/journal.pone. 0092727]. [PMID: 24663151]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Huang J., Chen S., Zhang J.J., Huang X.Y. Crystal structure of oligomeric β1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat. Struct. Mol. Biol. 2013;20(4):419–425. doi: 10.1038/nsmb.2504. [http://dx. doi.org/10.1038/nsmb.2504]. [PMID: 23435379]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sato T., Baker J., Warne T., Brown G.A., Leslie A.G. ; Congreve M., Tate C.G. Pharmacological Analysis and Structure Determination of 7-Methylcyanopindolol-Bound β1-Adrenergic Receptor. Mol. Pharmacol. 2015;88(6):1024–1034. doi: 10.1124/mol.115.101030. [http://dx.doi. org/10.1124/mol.115.101030]. [PMID: 26385885]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Leslie A.G.W., Warne T., Tate C.G. Ligand occupancy in crystal structure of β1-adrenergic G protein-coupled receptor. Nat. Struct. Mol. Biol. 2015;22(12):941–942. doi: 10.1038/nsmb.3130. [http://dx.doi.org/10.1038/nsmb. 3130]. [PMID: 26643842]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rasmussen S.G.F., Choi H.J., Rosenbaum D.M., Kobilka T.S., Thian F.S., Edwards P.C., Burghammer M., Ratnala V.R., Sanishvili R., Fischetti R.F., Schertler G.F., Weis W.I., Kobilka B.K. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature. 2007;450(7168):383–387. doi: 10.1038/nature06325. [http://dx.doi. org/10.1038/nature06325]. [PMID: 17952055]. [DOI] [PubMed] [Google Scholar]
- 272.Cherezov V., Rosenbaum D.M., Hanson M.A., Rasmussen S.G., Thian F.S., Kobilka T.S., Choi H.J., Kuhn P., Weis W.I. ; Kobilka B.K., Stevens R.C. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science. 2007;318(5854):1258–1265. doi: 10.1126/science.1150577. [http://dx.doi.org/10.1126/ science.1150577]. [PMID: 17962520]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hanson M.A., Cherezov V., Griffith M.T., Roth C.B., Jaakola V.P., Chien E.Y., Velasquez J., Kuhn P., Stevens R.C. A specific cholesterol binding site is established by the 2.8 A structure of the human β2-adrenergic receptor. Structure. 2008;16(6):897–905. doi: 10.1016/j.str.2008.05.001. [http://dx.doi.org/10.1016/j.str.2008.05.001]. [PMID: 18547522]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bokoch M.P., Zou Y., Rasmussen S.G., Liu C.W., Nygaard R., Rosenbaum D.M., Fung J.J., Choi H.J., Thian F.S., Kobilka T.S., Puglisi J.D., Weis W.I., Pardo L., Prosser R.S., Mueller L., Kobilka B.K. Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature. 2010;463(7277):108–112. doi: 10.1038/nature08650. [http://dx.doi.org/10.1038/nature08650]. [PMID: 20054398]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wacker D., Fenalti G., Brown M.A., Katritch V., Abagyan R., Cherezov V., Stevens R.C. Conserved binding mode of human β2 adrenergic receptor inverse agonists and antagonist revealed by X-ray crystallography. J. Am. Chem. Soc. 2010;132(33):11443–11445. doi: 10.1021/ja105108q. [http://dx.doi.org/10.1021/ja105108q]. [PMID: 20669948]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rasmussen S.G., Choi H.J., Fung J.J., Pardon E., Casarosa P., Chae P.S., Devree B.T., Rosenbaum D.M., Thian F.S., Kobilka T.S., Schnapp A., Konetzki I., Sunahara R.K., Gellman S.H., Pautsch A., Steyaert J., Weis W.I., Kobilka B.K. Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature. 2011;469(7329):175–180. doi: 10.1038/nature09648. [http://dx.doi.org/10.1038/nature09648]. [PMID: 21228869]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Rasmussen S.G., DeVree B.T., Zou Y., Kruse A.C., Chung K.Y., Kobilka T.S., Thian F.S., Chae P.S., Pardon E., Calinski D., Mathiesen J.M., Shah S.T., Lyons J.A., Caffrey M., Gellman S.H., Steyaert J., Skiniotis G., Weis W.I., Sunahara R.K., Kobilka B.K. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477(7366):549–555. doi: 10.1038/nature10361. [http://dx.doi. org/10.1038/nature10361]. [PMID: 21772288]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zou Y., Weis W.I., Kobilka B.K. N-terminal T4 lysozyme fusion facilitates crystallization of a G protein coupled receptor. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0046039. https://doi.org/10 [http://dx.doi.org/10.1371/journal. pone.0046039]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Huang C.Y., Olieric V., Ma P., Howe N., Vogeley L., Liu X., Warshamanage R., Weinert T., Panepucci E., Kobilka B., Diederichs K., Wang M., Caffrey M. In meso in situ serial X-ray crystallography of soluble and membrane proteins at cryogenic temperatures. Acta Crystallogr. D Struct. Biol. 2016;72(Pt 1):93–112. doi: 10.1107/S2059798315021683. [http://dx.doi.org/10.1107/S2059798315021683]. [PMID: 26894538]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Staus D.P., Strachan R.T., Manglik A., Pani B., Kahsai A.W., Kim T.H., Wingler L.M., Ahn S., Chatterjee A., Masoudi A., Kruse A.C., Pardon E., Steyaert J., Weis W.I., Prosser R.S., Kobilka B.K., Costa T., Lefkowitz R.J. Allosteric nanobodies reveal the dynamic range and diverse mechanisms of G-protein-coupled receptor activation. Nature. 2016;535(7612):448–452. doi: 10.1038/nature18636. [http://dx.doi.org/10.1038/nature18636]. [PMID: 27409812]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Liu X., Ahn S., Kahsai A.W., Meng K.C., Latorraca N.R., Pani B., Venkatakrishnan A.J., Masoudi A., Weis W.I., Dror R.O., Chen X., Lefkowitz R.J., Kobilka B.K. Mechanism of intracellular allosteric β2AR antagonist revealed by X-ray crystal structure. Nature. 2017;548(7668):480–484. doi: 10.1038/nature23652. [http://dx.doi.org/10.1038/ nature23652]. [PMID: 28813418]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Robertson N., Rappas M., Doré A.S., Brown J., Bottegoni G., Koglin M., Cansfield J., Jazayeri A., Cooke R.M., Marshall F.H. Structure of the complement C5a receptor bound to the extra-helical antagonist NDT9513727. Nature. 2018;553(7686):111–114. doi: 10.1038/nature25025. [http://dx.doi.org/10.1038/nature25025]. [PMID: 29300009]. [DOI] [PubMed] [Google Scholar]
- 283.Shao Z., Yin J., Chapman K., Grzemska M., Clark L., Wang J., Rosenbaum D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature. 2016;540(7634):602–606. doi: 10.1038/nature20613. [http://dx.doi.org/10.1038/nature20613]. [PMID: 27851727]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Hua T., Vemuri K., Pu M., Qu L., Han G.W., Wu Y., Zhao S., Shui W., Li S., Korde A., Laprairie R.B., Stahl E.L., Ho J.H., Zvonok N., Zhou H., Kufareva I., Wu B., Zhao Q., Hanson M.A., Bohn L.M., Makriyannis A., Stevens R.C., Liu Z.J. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell. 2016;167(3):750–762.e14. doi: 10.1016/j.cell.2016.10.004. [http://dx.doi.org/10.1016/j.cell.2016.10.004]. [PMID: 27768894]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zheng Y., Qin L., Zacarías N.V., de Vries H., Han G.W., Gustavsson M., Dabros M., Zhao C., Cherney R.J., Carter P., Stamos D., Abagyan R., Cherezov V., Stevens R.C., IJzerman A.P., Heitman L.H., Tebben A., Kufareva I., Handel T.M. Structure of CC chemokine receptor 2 with orthosteric and allosteric antagonists. Nature. 2016;540(7633):458–461. doi: 10.1038/nature20605. [http://dx.doi.org/10. 1038/nature20605]. [PMID: 27926736]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Miyamoto K., Togiya K. Solution structure of LC4 transmembrane segment of CCR5. PLoS One. 2011;6(5):e20452. doi: 10.1371/journal.pone.0020452. [http:// dx.doi.org/10.1371/journal.pone.0020452]. [PMID: 21647380]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Tan Q., Zhu Y., Li J., Chen Z., Han G.W., Kufareva I., Li T., Ma L., Fenalti G., Li J., Zhang W., Xie X., Yang H., Jiang H., Cherezov V., Liu H., Stevens R.C., Zhao Q., Wu B. Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science. 2013;341(6152):1387–1390. doi: 10.1126/science.1241475. [http://dx.doi.org/10. 1126/science.1241475]. [PMID: 24030490]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Oswald C., Rappas M., Kean J., Doré A.S., Errey J.C., Bennett K., Deflorian F., Christopher J.A., Jazayeri A., Mason J.S., Congreve M., Cooke R.M., Marshall F.H. Intracellular allosteric antagonism of the CCR9 receptor. Nature. 2016;540(7633):462–465. doi: 10.1038/nature20606. [http://dx.doi.org/10.1038/nature20606]. [PMID: 27926729]. [DOI] [PubMed] [Google Scholar]
- 289.Park S.H., Das B.B., Casagrande F., Tian Y., Nothnagel H.J., Chu M., Kiefer H., Maier K., De Angelis A.A., Marassi F.M., Opella S.J. Structure of the chemokine receptor CXCR1 in phospholipid bilayers. Nature. 2012;491(7426):779–783. doi: 10.1038/nature11580. [PMID: 23086146]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wu B., Chien E.Y., Mol C.D., Fenalti G., Liu W., Katritch V., Abagyan R., Brooun A., Wells P., Bi F.C., Hamel D.J., Kuhn P., Handel T.M., Cherezov V., Stevens R.C. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330(6007):1066–1071. doi: 10.1126/science.1194396. [http://dx.doi. org/10.1126/science.1194396]. [PMID: 20929726]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Qin L., Kufareva I., Holden L.G., Wang C., Zheng Y., Zhao C., Fenalti G., Wu H., Han G.W., Cherezov V., Abagyan R., Stevens R.C., Handel T.M. Structural biology. Crystal structure of the chemokine receptor CXCR4 in complex with a viral chemokine. Science. 2015;347(6226):1117–1122. doi: 10.1126/science.1261064. [http://dx.doi. org/10.1126/science.1261064]. [PMID: 25612609]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ziarek J.J., Kleist A.B., London N., Raveh B., Montpas N., Bonneterre J., St-Onge G., DiCosmo-Ponticello C.J., Koplinski C.A., Roy I., Stephens B., Thelen S., Veldkamp C.T., Coffman F.D., Cohen M.C., Dwinell M.B., Thelen M., Peterson F.C., Heveker N., Volkman B.F. Structural basis for chemokine recognition by a G protein-coupled receptor and implications for receptor activa-tion. Sci. Signal. 2017;10(471) doi: 10.1126/scisignal.aah5756. http://stke.sciencemag.org/content/ 10/471/eaah5756.long [http://dx.doi.org/10.1126/scisignal.aah5756]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wang S., Che T., Levit A., Shoichet B.K., Wacker D., Roth B.L. Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature. 2018;555(7695):269–273. doi: 10.1038/nature25758. [http://dx.doi.org/10.1038/nature25758]. [PMID: 29466326]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chien E.Y., Liu W., Zhao Q., Katritch V., Han G.W., Hanson M.A., Shi L., Newman A.H., Javitch J.A., Cherezov V., Stevens R.C. Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science. 2010;330(6007):1091–1095. doi: 10.1126/science.1197410. [http://dx.doi.org/10.1126/science.1197410]. [PMID: 21097933]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wang S., Wacker D., Levit A., Che T., Betz R.M., McCorvy J.D., Venkatakrishnan A.J., Huang X.P., Dror R.O., Shoichet B.K., Roth B.L.D.D. 4 dopamine receptor high-resolution structures enable the discovery of selective agonists. Science. 2017;358(6361):381–386. doi: 10.1126/science.aan5468. [http://dx.doi.org/10.1126/science.aan5468]. [PMID: 29051383]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Fenalti G., Giguere P.M., Katritch V., Huang X.P., Thompson A.A., Cherezov V., Roth B.L., Stevens R.C. Molecular control of δ-opioid receptor signalling. Nature. 2014;506(7487):191–196. doi: 10.1038/nature12944. [http://dx.doi.org/10.1038/nature12944]. [PMID: 24413399]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Fenalti G., Zatsepin N.A., Betti C., Giguere P., Han G.W., Ishchenko A., Liu W., Guillemyn K., Zhang H., James D., Wang D., Weierstall U., Spence J.C., Boutet S., Messerschmidt M., Williams G.J., Gati C., Yefanov O.M., White T.A., Oberthuer D., Metz M., Yoon C.H., Barty A., Chapman H.N., Basu S., Coe J., Conrad C.E., Fromme R., Fromme P., Tourwé D., Schiller P.W., Roth B.L., Ballet S., Katritch V., Stevens R.C., Cherezov V. Structural basis for bifunctional peptide recognition at human δ-opioid receptor. Nat. Struct. Mol. Biol. 2015;22(3):265–268. doi: 10.1038/nsmb.2965. [http://dx.doi.org/10.1038/nsmb.2965]. [PMID: 25686086]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Shihoya W., Nishizawa T., Okuta A., Tani K., Dohmae N., Fujiyoshi Y., Nureki O., Doi T. Activation mechanism of endothelin ETB receptor by endothelin-1. Nature. 2016;537(7620):363–368. doi: 10.1038/nature19319. [http://dx.doi.org/10.1038/nature19319]. [PMID: 27595334]. [DOI] [PubMed] [Google Scholar]
- 299.Srivastava A., Yano J., Hirozane Y., Kefala G., Gruswitz F., Snell G., Lane W., Ivetac A., Aertgeerts K., Nguyen J., Jennings A., Okada K. High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875. Nature. 2014;513(7516):124–127. doi: 10.1038/nature13494. [http://dx.doi.org/10.1038/nature13494]. [PMID: 25043059]. [DOI] [PubMed] [Google Scholar]
- 300.Lu J., Byrne N., Wang J., Bricogne G., Brown F.K., Chobanian H.R., Colletti S.L., Di Salvo J., Thomas-Fowlkes B., Guo Y., Hall D.L., Hadix J., Hastings N.B., Hermes J.D., Ho T., Howard A.D., Josien H., Kornienko M., Lumb K.J., Miller M.W., Patel S.B., Pio B., Plummer C.W., Sherborne B.S., Sheth P., Souza S., Tummala S., Vonrhein C., Webb M., Allen S.J., Johnston J.M., Weinglass A.B., Sharma S., Soisson S.M. Structural basis for the cooperative allosteric activation of the free fatty acid receptor GPR40. Nat. Struct. Mol. Biol. 2017;24(7):570–577. doi: 10.1038/nsmb.3417. [http://dx.doi.org/10.1038/nsmb.3417]. [PMID: 28581512]. [DOI] [PubMed] [Google Scholar]
- 301.Jiang X., Liu H., Chen X., Chen P.H., Fischer D., Sriraman V., Yu H.N., Arkinstall S., He X. Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor. Proc. Natl. Acad. Sci. USA. 2012;109(31):12491–12496. doi: 10.1073/pnas.1206643109. [http:// dx.doi.org/10.1073/pnas.1206643109]. [PMID: 22802634]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Jiang X., Fischer D., Chen X., McKenna S.D., Liu H., Sriraman V., Yu H.N., Goutopoulos A., Arkinstall S., He X. Evidence for Follicle-stimulating Hormone Receptor as a Functional Trimer. J. Biol. Chem. 2014;289(20):14273–14282. doi: 10.1074/jbc.M114.549592. [http://dx.doi. org/10.1074/jbc.M114.549592]. [PMID: 24692546]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shimamura T., Shiroishi M., Weyand S., Tsujimoto H., Winter G., Katritch V., Abagyan R., Cherezov V., Liu W., Han G.W., Kobayashi T., Stevens R.C., Iwata S. Structure of the human histamine H1 receptor complex with doxepin. Nature. 2011;475(7354):65–70. doi: 10.1038/nature10236. [http://dx.doi.org/10.1038/nature10236]. [PMID: 21697825]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wu H., Wacker D., Mileni M., Katritch V., Han G.W., Vardy E., Liu W., Thompson A.A., Huang X.P., Carroll F.I., Mascarella S.W., Westkaemper R.B., Mosier P.D., Roth B.L., Cherezov V., Stevens R.C. Structure of the human κ-opioid receptor in complex with JDTic. Nature. 2012;485(7398):327–332. doi: 10.1038/nature10939. [http://dx.doi.org/10.1038/nature10939]. [PMID: 22437504]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Che T., Majumdar S., Zaidi S.A., Ondachi P., McCorvy J.D., Wang S., Mosier P.D., Uprety R., Vardy E., Krumm B.E., Han G.W., Lee M.Y., Pardon E., Steyaert J., Huang X.P., Strachan R.T., Tribo A.R., Pasternak G.W., Carroll F.I., Stevens R.C., Cherezov V., Katritch V., Wacker D., Roth B.L. Structure of the Nanobody-Stabilized Active State of the Kappa Opioid Receptor. Cell. 2018;172(1-2):55–67.e15. doi: 10.1016/j.cell.2017.12.011. [http://dx.doi.org/10.1016/j.cell. 2017.12.011]. [PMID: 29307491]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Chrencik J.E., Roth C.B., Terakado M., Kurata H., Omi R., Kihara Y., Warshaviak D., Nakade S., Asmar-Rovira G., Mileni M., Mizuno H., Griffith M.T., Rodgers C., Han G.W., Velasquez J., Chun J., Stevens R.C., Hanson M.A. Crystal Structure of Antagonist Bound Human Lysophosphatidic Acid Receptor 1. Cell. 2015;161(7):1633–1643. doi: 10.1016/j.cell.2015.06.002. [http://dx.doi.org/10.1016/j.cell. 2015.06.002]. [PMID: 26091040]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Thal D.M., Sun B., Feng D., Nawaratne V., Leach K., Felder C.C., Bures M.G., Evans D.A., Weis W.I., Bachhawat P., Kobilka T.S., Sexton P.M., Kobilka B.K., Christopoulos A. Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature. 2016;531(7594):335–340. doi: 10.1038/nature17188. [http://dx.doi.org/10.1038/nature 17188]. [PMID: 26958838]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Haga K., Kruse A.C., Asada H., Yurugi-Kobayashi T., Shiroishi M., Zhang C., Weis W.I., Okada T., Kobilka B.K., Haga T., Kobayashi T. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature. 2012;482(7386):547–551. doi: 10.1038/nature10753. [http://dx.doi.org/10.1038/nature10753]. [PMID: 22278061]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kruse A.C., Ring A.M., Manglik A., Hu J., Hu K., Eitel K., Hübner H., Pardon E., Valant C., Sexton P.M., Christopoulos A., Felder C.C., Gmeiner P., Steyaert J., Weis W.I., Garcia K.C., Wess J., Kobilka B.K. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504(7478):101–106. doi: 10.1038/nature12735. [http://dx.doi.org/10.1038/nature12735]. [PMID: 24256733]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Kruse A.C., Hu J., Pan A.C., Arlow D.H., Rosenbaum D.M., Rosemond E., Green H.F., Liu T., Chae P.S., Dror R.O., Shaw D.E., Weis W.I., Wess J., Kobilka B.K. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature. 2012;482(7386):552–556. doi: 10.1038/nature10867. [http://dx.doi.org/10.1038/nature10867]. [PMID: 22358844]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Thorsen T.S., Matt R., Weis W.I., Kobilka B.K. Modified T4 Lysozyme Fusion Proteins Facilitate G Protein-Coupled Receptor Crystallogenesis. Structure. 2014;22(11):1657–1664. doi: 10.1016/j.str.2014.08.022. [http://dx. doi.org/10.1016/j.str.2014.08.022]. [PMID: 25450769]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Manglik A., Kruse A.C., Kobilka T.S., Thian F.S., Mathiesen J.M., Sunahara R.K., Pardo L., Weis W.I., Kobilka B.K., Granier S. Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature. 2012;485(7398):321–326. doi: 10.1038/nature10954. [http://dx. doi.org/10.1038/nature10954]. [PMID: 22437502]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Huang W., Manglik A., Venkatakrishnan A.J., Laeremans T., Feinberg E.N., Sanborn A.L., Kato H.E., Livingston K.E., Thorsen T.S., Kling R.C., Granier S., Gmeiner P., Husbands S.M., Traynor J.R., Weis W.I., Steyaert J., Dror R.O., Kobilka B.K. Crystal structure of the μ-opioid receptor bound to a morphinan antagonist. Nature. 2015;524(7565):315–321. doi: 10.1038/nature14886. [http://dx.doi. org/10.1038/nature14886]. [PMID: 26245379]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Granier S., Manglik A., Kruse A.C., Kobilka T.S., Thian F.S., Weis W.I., Kobilka B.K. Structure of the δ-opioid receptor bound to naltrindole. Nature. 2012;485(7398):400–404. doi: 10.1038/nature11111. [http://dx.doi. org/10.1038/nature11111]. [PMID: 22596164]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.White J.F., Noinaj N., Shibata Y., Love J., Kloss B., Xu F., Gvozdenovic-Jeremic J., Shah P., Shiloach J., Tate C.G., Grisshammer R. Structure of the agonist-bound neurotensin receptor. Nature. 2012;490(7421):508–513. doi: 10.1038/nature11558. [http://dx.doi.org/10.1038/ nature11558]. [PMID: 23051748]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Miller R.L., Thompson A.A., Trapella C., Guerrini R., Malfacini D., Patel N., Han G.W., Cherezov V., Caló G., Katritch V., Stevens R.C. The Importance of Ligand-Receptor Conformational Pairs in Stabilization: Spotlight on the N/OFQ G Protein-Coupled Receptor. Structure. 2015;23(12):2291–2299. doi: 10.1016/j.str.2015.07.024. [http://dx.doi.org/ 10.1016/j.str.2015.07.024]. [PMID: 26526853]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Egloff P., Hillenbrand M., Klenk C., Batyuk A., Heine P. ; Balada S., Schlinkmann K.M., Scott D.J., Schütz M., Plückthun A. Structure of signaling-competent neurotensin receptor 1 obtained by directed evolution in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2014;111(6):E655–E662. doi: 10.1073/pnas.1317903111. [http://dx.doi.org/10.1073/pnas. 1317903111]. [PMID: 24453215]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Krumm B.E., White J.F., Shah P., Grisshammer R. Structural prerequisites for G-protein activation by the neurotensin receptor. Nat. Commun. 2015;6:7895. doi: 10.1038/ncomms8895. [http://dx.doi.org/10.1038/ncomms 8895]. [PMID: 26205105]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Krumm B.E., Lee S., Bhattacharya S., Botos I., White C.F., Du H., Vaidehi N., Grisshammer R. Structure and dynamics of a constitutively active neurotensin receptor. Sci. Rep. 2016;6:38564. doi: 10.1038/srep38564. [http://dx.doi.org/10.1038/srep38564]. [PMID: 27924846]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Yin J., Babaoglu K., Brautigam C.A., Clark L., Shao Z., Scheuermann T.H., Harrell C.M., Gotter A.L., Roecker A.J., Winrow C.J., Renger J.J., Coleman P.J., Rosenbaum D.M. Structure and ligand-binding mechanism of the human OX1 and OX2 orexin receptors. Nat. Struct. Mol. Biol. 2016;23(4):293–299. doi: 10.1038/nsmb.3183. [http://dx.doi.org/10.1038/nsmb.3183]. [PMID: 26950369]. [DOI] [PubMed] [Google Scholar]
- 321.Yin J., Mobarec J.C., Kolb P., Rosenbaum D.M. Crystal structure of the human OX2 orexin receptor bound to the insomnia drug suvorexant. Nature. 2015;519(7542):247–250. doi: 10.1038/nature14035. [http://dx.doi.org/ 10.1038/nature14035]. [PMID: 25533960]. [DOI] [PubMed] [Google Scholar]
- 322.Zhang D., Gao Z.G., Zhang K., Kiselev E., Crane S., Wang J., Paoletta S., Yi C., Ma L., Zhang W., Han G.W., Liu H., Cherezov V., Katritch V., Jiang H., Stevens R.C., Jacobson K.A., Zhao Q., Wu B. Two disparate ligand-binding sites in the human P2Y1 receptor. Nature. 2015;520(7547):317–321. doi: 10.1038/nature14287. [http://dx.doi.org/ 10.1038/nature14287]. [PMID: 25822790]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Zhang K., Zhang J., Gao Z-G., Zhang D., Zhu L., Han G.W., Moss S.M., Paoletta S., Kiselev E., Lu W., Fenalti G., Zhang W., Müller C.E., Yang H., Jiang H., Cherezov V., Katritch V., Jacobson K.A., Stevens R.C., Wu B., Zhao Q. Structure of the human P2Y12 receptor in complex with an antithrombotic drug. Nature. 2014;509(7498):115–118. doi: 10.1038/nature13083. [http://dx.doi.org/10.1038/nature 13083]. [PMID: 24670650]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zhang J., Zhang K., Gao Z.G., Paoletta S., Zhang D., Han G.W., Li T., Ma L., Zhang W., Müller C.E., Yang H., Jiang H., Cherezov V., Katritch V., Jacobson K.A., Stevens R.C., Wu B., Zhao Q. Agonist-bound structure of the human P2Y12 receptor. Nature. 2014;509(7498):119–122. doi: 10.1038/nature13288. [http://dx.doi.org/10.1038/ nature13288]. [PMID: 24784220]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhang C., Srinivasan Y., Arlow D.H., Fung J.J., Palmer D., Zheng Y., Green H.F., Pandey A., Dror R.O., Shaw D.E., Weis W.I., Coughlin S.R., Kobilka B.K. High-resolution crystal structure of human protease-activated receptor 1. Nature. 2012;492(7429):387–392. doi: 10.1038/nature11701. [http://dx.doi.org/10.1038/nature11701]. [PMID: 23222541]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Cheng R.K.Y., Fiez-Vandal C., Schlenker O., Edman K., Aggeler B., Brown D.G., Brown G.A., Cooke R.M., Dumelin C.E., Doré A.S., Geschwindner S., Grebner C., Hermansson N.O., Jazayeri A., Johansson P., Leong L., Prihandoko R., Rappas M., Soutter H., Snijder A., Sundström L., Tehan B., Thornton P., Troast D., Wiggin G., Zhukov A., Marshall F.H., Dekker N. Structural insight into allosteric modulation of protease-activated receptor 2. Nature. 2017;545(7652):112–115. doi: 10.1038/nature22309. [http://dx.doi.org/10. 1038/nature22309]. [PMID: 28445455]. [DOI] [PubMed] [Google Scholar]
- 327.Li J., Edwards P.C., Burghammer M., Villa C., Schertler G.F.X. Structure of bovine rhodopsin in a trigonal crystal form. J. Mol. Biol. 2004;343(5):1409–1438. doi: 10.1016/j.jmb.2004.08.090. [http://dx.doi.org/10.1016/j.jmb. 2004.08.090]. [PMID: 15491621]. [DOI] [PubMed] [Google Scholar]
- 328.Teller D.C., Okada T., Behnke C.A., Palczewski K., Stenkamp R.E. Advances in determination of a high-resolution three-dimensional structure of rhodopsin, a model of G-protein-coupled receptors (GPCRs). Biochemistry. 2001;40(26):7761–7772. doi: 10.1021/bi0155091. [http://dx.doi.org/10.1021/bi0155091]. [PMID: 11425302]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Yeagle P.L., Choi G., Albert A.D. Studies on the structure of the G-protein-coupled receptor rhodopsin including the putative G-protein binding site in unactivated and activated forms. Biochemistry. 2001;40(39):11932–11937. doi: 10.1021/bi015543f. [http://dx.doi.org/10.1021/bi015543f]. [PMID: 11570894]. [DOI] [PubMed] [Google Scholar]
- 330.Okada T., Fujiyoshi Y., Silow M., Navarro J., Landau E.M., Shichida Y. Functional role of internal water molecules in rhodopsin revealed by X-ray crystallography. Proc. Natl. Acad. Sci. USA. 2002;99(9):5982–5987. doi: 10.1073/pnas.082666399. [http://dx.doi.org/10.1073/pnas.082666399]. [PMID: 11972040]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Choi G., Landin J., Galan J.F., Birge R.R., Albert A.D., Yeagle P.L. Structural studies of metarhodopsin II, the activated form of the G-protein coupled receptor, rhodopsin. Biochemistry. 2002;41(23):7318–7324. doi: 10.1021/bi025507w. [http://dx.doi.org/10.1021/bi025507w]. [PMID: 12044163]. [DOI] [PubMed] [Google Scholar]
- 332.Okada T., Sugihara M., Bondar A.N., Elstner M., Entel P., Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J. Mol. Biol. 2004;342(2):571–583. doi: 10.1016/j.jmb.2004.07.044. [http://dx.doi.org/10.1016/j.jmb.2004.07.044]. [PMID: 15327956]. [DOI] [PubMed] [Google Scholar]
- 333.Nakamichi H., Okada T. Crystallographic analysis of primary visual photochemistry. Angew. Chem. Int. Ed. Engl. 2006;45(26):4270–4273. doi: 10.1002/anie.200600595. [http://dx.doi.org/10.1002/anie.200600595]. [PMID: 16586416]. [DOI] [PubMed] [Google Scholar]
- 334.Nakamichi H., Okada T. Local peptide movement in the photoreaction intermediate of rhodopsin. Proc. Natl. Acad. Sci. USA. 2006;103(34):12729–12734. doi: 10.1073/pnas.0601765103. [http://dx.doi.org/10.1073/pnas.0601765103]. [PMID: 16908857]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Salom D., Lodowski D.T., Stenkamp R.E., Le Trong I., Golczak M., Jastrzebska B., Harris T., Ballesteros J.A., Palczewski K. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc. Natl. Acad. Sci. USA. 2006;103(44):16123–16128. doi: 10.1073/pnas.0608022103. [http://dx.doi.org/10.1073/pnas.0608022103]. [PMID: 17060607]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Standfuss J., Xie G., Edwards P.C., Burghammer M., Oprian D.D., Schertler G.F.X. Crystal structure of a thermally stable rhodopsin mutant. J. Mol. Biol. 2007;372(5):1179–1188. doi: 10.1016/j.jmb.2007.03.007. [http:// dx.doi.org/10.1016/j.jmb.2007.03.007]. [PMID: 17825322]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Nakamichi H., Buss V., Okada T. Photoisomerization mechanism of rhodopsin and 9-cis-rhodopsin revealed by x-ray crystallography. Biophys. J. 2007;92(12):L106–L108. doi: 10.1529/biophysj.107.108225. [http://dx.doi.org/10. 1529/biophysj.107.108225]. [PMID: 17449675]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Standfuss J., Edwards P.C., D’Antona A., Fransen M., Xie G., Oprian D.D., Schertler G.F.X. The structural basis of agonist-induced activation in constitutively active rhodopsin. Nature. 2011;471(7340):656–660. doi: 10.1038/nature09795. [http://dx.doi.org/10.1038/nature09795]. [PMID: 21389983]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Murakami M., Kouyama T. Crystal structure of squid rhodopsin. Nature. 2008;453(7193):363–367. doi: 10.1038/nature06925. [http://dx.doi.org/10.1038/ nature06925]. [PMID: 18480818]. [DOI] [PubMed] [Google Scholar]
- 340.Shimamura T., Hiraki K., Takahashi N., Hori T., Ago H., Masuda K., Takio K., Ishiguro M., Miyano M. Crystal structure of squid rhodopsin with intracellularly extended cytoplasmic region. J. Biol. Chem. 2008;283(26):17753–17756. doi: 10.1074/jbc.C800040200. [http://dx.doi.org/10. 1074/jbc.C800040200]. [PMID: 18463093]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Murakami M., Kouyama T. Crystallographic analysis of the primary photochemical reaction of squid rhodopsin. J. Mol. Biol. 2011;413(3):615–627. doi: 10.1016/j.jmb.2011.08.044. [http://dx.doi.org/10.1016/j.jmb.2011.08. 044]. [PMID: 21906602]. [DOI] [PubMed] [Google Scholar]
- 342.Stenkamp R.E. Alternative models for two crystal structures of bovine rhodopsin. Acta Crystallogr. D Biol. Crystallogr. 2008;D64(Pt 8):902–904. doi: 10.1107/S0907444908017162. [http://dx.doi.org/10.1107/S0907444908017162]. [PMID: 18645239]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Park J.H., Scheerer P., Hofmann K.P., Choe H-W., Ernst O.P. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454(7201):183–187. doi: 10.1038/nature07063. [http://dx.doi.org/10.1038/ nature07063]. [PMID: 18563085]. [DOI] [PubMed] [Google Scholar]
- 344.Scheerer P., Park J.H., Hildebrand P.W., Kim Y.J., Krauss N., Choe H-W., Hofmann K.P., Ernst O.P. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455(7212):497–502. doi: 10.1038/nature07330. [http://dx.doi.org/10.1038/nature07330]. [PMID: 18818650]. [DOI] [PubMed] [Google Scholar]
- 345.Choe H.W., Kim Y.J., Park J.H., Morizumi T., Pai E.F., Krauss N., Hofmann K.P., Scheerer P., Ernst O.P. Crystal structure of metarhodopsin II. Nature. 2011;471(7340):651–655. doi: 10.1038/nature09789. [http://dx. doi.org/10.1038/nature09789]. [PMID: 21389988]. [DOI] [PubMed] [Google Scholar]
- 346.Makino C.L., Riley C.K., Looney J., Crouch R.K., Okada T. Binding of more than one retinoid to visual opsins. Biophys. J. 2010;99(7):2366–2373. doi: 10.1016/j.bpj.2010.08.003. [http://dx.doi.org/10.1016/j.bpj.2010.08.003]. [PMID: 20923672]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Deupi X., Edwards P., Singhal A., Nickle B., Oprian D., Schertler G., Standfuss J., Stabilized G. Stabilized G protein binding site in the structure of constitutively active metarhodopsin-II. Proc. Natl. Acad. Sci. USA. 2012;109(1):119–124. doi: 10.1073/pnas.1114089108. [http://dx. doi.org/10.1073/pnas.1114089108]. [PMID: 22198838]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Singhal A., Ostermaier M.K., Vishnivetskiy S.A., Panneels V., Homan K.T., Tesmer J.J., Veprintsev D., Deupi X., Gurevich V.V., Schertler G.F., Standfuss J. Insights into congenital stationary night blindness based on the structure of G90D rhodopsin. EMBO Rep. 2013;14(6):520–526. doi: 10.1038/embor.2013.44. [http://dx.doi.org/10.1038/ embor.2013.44]. [PMID: 23579341]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Park J.H., Morizumi T., Li Y., Hong J.E., Pai E.F., Hofmann K.P., Choe H.W., Ernst O.P. Opsin, a structural model for olfactory receptors? Angew. Chem. Int. Ed. Engl. 2013;52(42):11021–11024. doi: 10.1002/anie.201302374. [http://dx.doi.org/10.1002/anie.201302374]. [PMID: 24038729]. [DOI] [PubMed] [Google Scholar]
- 350.Szczepek M., Beyrière F., Hofmann K.P., Elgeti M., Kazmin R., Rose A., Bartl F.J., von Stetten D., Heck M., Sommer M.E., Hildebrand P.W., Scheerer P. Crystal structure of a common GPCR-binding interface for G protein and arrestin. Nat. Commun. 2014;5:4801–4801. doi: 10.1038/ncomms5801. [http://dx.doi.org/10.1038/ncomms5801]. [PMID: 25205354]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Blankenship E., Vahedi-Faridi A., Lodowski D.T. The High-Resolution Structure of Activated Opsin Reveals a Conserved Solvent Network in the Transmembrane Region Essential for Activation. Structure. 2015;23(12):2358–2364. doi: 10.1016/j.str.2015.09.015. [http://dx.doi.org/10. 1016/j.str.2015.09.015]. [PMID: 26526852]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Kang Y., Zhou X.E., Gao X., He Y., Liu W., Ishchenko A., Barty A., White T.A., Yefanov O., Han G.W., Xu Q., de Waal P.W., Ke J., Tan M.H., Zhang C., Moeller A., West G.M., Pascal B.D., Van Eps N., Caro L.N., Vishnivetskiy S.A., Lee R.J., Suino-Powell K.M., Gu X., Pal K., Ma J., Zhi X., Boutet S., Williams G.J., Messerschmidt M., Gati C., Zatsepin N.A., Wang D., James D., Basu S., Roy-Chowdhury S., Conrad C.E., Coe J., Liu H., Lisova S., Kupitz C., Grotjohann I., Fromme R., Jiang Y., Tan M., Yang H., Li J., Wang M., Zheng Z., Li D., Howe N., Zhao Y., Standfuss J., Diederichs K., Dong Y., Potter C.S., Carragher B., Caffrey M., Jiang H., Chapman H.N., Spence J.C., Fromme P., Weierstall U., Ernst O.P., Katritch V., Gurevich V.V., Griffin P.R., Hubbell W.L., Stevens R.C., Cherezov V., Melcher K., Xu H.E. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature. 2015;523(7562):561–567. doi: 10.1038/nature14656. [http://dx.doi.org/10.1038/nature14656]. [PMID: 26200343]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Zhou X.E., Gao X., Barty A., Kang Y., He Y., Liu W., Ishchenko A., White T.A., Yefanov O., Han G.W., Xu Q., de Waal P.W., Suino-Powell K.M., Boutet S., Williams G.J., Wang M., Li D., Caffrey M., Chapman H.N., Spence J.C., Fromme P., Weierstall U., Stevens R.C., Cherezov V., Melcher K., Xu H.E. X-ray laser diffraction for structure determination of the rhodopsin-arrestin complex. Sci. Data. 2016;3:160021–160021. doi: 10.1038/sdata.2016.21. [http://dx. doi.org/10.1038/sdata.2016.21]. [PMID: 27070998]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Singhal A., Guo Y., Matkovic M., Schertler G., Deupi X., Yan E.C., Standfuss J. Structural role of the T94I rhodopsin mutation in congenital stationary night blindness. EMBO Rep. 2016;17(10):1431–1440. doi: 10.15252/embr.201642671. [http://dx.doi.org/10.15252/embr.201642671]. [PMID: 27458239]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Gulati S., Jastrzebska B., Banerjee S., Placeres A.L., Miszta P., Gao S., Gunderson K., Tochtrop G.P., Filipek S., Katayama K., Kiser P.D., Mogi M., Stewart P.L., Palczewski K. Photocyclic behavior of rhodopsin induced by an atypical isomerization mechanism. Proc. Natl. Acad. Sci. USA. 2017;114(13):E2608–E2615. doi: 10.1073/pnas.1617446114. [http://dx.doi.org/10.1073/pnas.1617446114]. [PMID: 28289214]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Zhou X.E., He Y., de Waal P.W., Gao X., Kang Y., Van Eps N., Yin Y., Pal K., Goswami D., White T.A., Barty A., Latorraca N.R., Chapman H.N., Hubbell W.L., Dror R.O., Stevens R.C., Cherezov V., Gurevich V.V., Griffin P.R., Ernst O.P., Melcher K., Xu H.E. Identification of Phosphorylation Codes for Arrestin Recruitment by G Protein-Coupled Receptors. Cell. 2017;170(3):457–469.e13. doi: 10.1016/j.cell.2017.07.002. [http://dx.doi.org/10.1016/j.cell.2017.07.002]. [PMID: 28753425]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Hanson M.A., Roth C.B., Jo E., Griffith M.T., Scott F.L., Reinhart G., Desale H., Clemons B., Cahalan S.M., Schuerer S.C., Sanna M.G., Han G.W., Kuhn P., Rosen H., Stevens R.C. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335(6070):851–855. doi: 10.1126/science.1215904. [http://dx.doi.org/10.1126/science.1215904]. [PMID: 22344443]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Sanders P., Young S., Sanders J., Kabelis K., Baker S., Sullivan A., Evans M., Clark J., Wilmot J., Hu X., Roberts E., Powell M., Núñez Miguel R., Furmaniak J., Rees Smith B. Crystal structure of the TSH receptor (TSHR) bound to a blocking-type TSHR autoantibody. J. Mol. Endocrinol. 2011;46(2):81–99. doi: 10.1530/JME-10-0127. [PMID: 21247981]. [DOI] [PubMed] [Google Scholar]
- 359.Sanders J., Chirgadze D.Y., Sanders P., Baker S., Sullivan A., Bhardwaja A., Bolton J., Reeve M., Nakatake N., Evans M., Richards T., Powell M., Miguel R.N., Blundell T.L., Furmaniak J., Smith B.R. Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid. 2007;17(5):395–410. doi: 10.1089/thy.2007.0034. [http://dx.doi.org/10.1089/thy.2007.0034]. [PMID: 17542669]. [DOI] [PubMed] [Google Scholar]
- 360.Burg J.S., Ingram J.R., Venkatakrishnan A.J., Jude K.M., Dukkipati A., Feinberg E.N., Angelini A., Waghray D., Dror R.O., Ploegh H.L., Garcia K.C. Structural biology. Structural basis for chemokine recognition and activation of a viral G protein-coupled receptor. Science. 2015;347(6226):1113–1117. doi: 10.1126/science.aaa5026. [http://dx.doi. org/10.1126/science.aaa5026]. [PMID: 25745166]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Shukla A.K., Manglik A., Kruse A.C., Xiao K., Reis R.I., Tseng W.C., Staus D.P., Hilger D., Uysal S., Huang L.Y., Paduch M., Tripathi-Shukla P., Koide A., Koide S., Weis W.I., Kossiakoff A.A., Kobilka B.K., Lefkowitz R.J. Structure of active β-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497(7447):137–141. doi: 10.1038/nature12120. [http://dx.doi.org/ 10.1038/nature12120]. [PMID: 23604254]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Pioszak A.A., Parker N.R., Suino-Powell K., Xu H.E. Molecular recognition of corticotropin-releasing factor by its G-protein-coupled receptor CRFR1. J. Biol. Chem. 2008;283(47):32900–32912. doi: 10.1074/jbc.M805749200. [http://dx.doi.org/10.1074/jbc.M805749200]. [PMID: 18801728]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Hollenstein K., Kean J., Bortolato A., Cheng R.K., Doré A.S., Jazayeri A., Cooke R.M., Weir M., Marshall F.H. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature. 2013;499(7459):438–443. doi: 10.1038/nature12357. [http://dx.doi.org/10.1038/nature12357]. [PMID: 23863939]. [DOI] [PubMed] [Google Scholar]
- 364.Dore A.S., Bortolato A., Hollenstein K., Cheng R.K.Y., Read R.J., Marshall F.H. Decoding Corticotropin-Releasing Factor Receptor Type 1 Crystal Structures. Curr. Mol. Pharmacol. 2017;10(4):334–344. doi: 10.2174/1874467210666170110114727. [PMID: 28183242]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Kusano S., Kukimoto-Niino M., Hino N., Ohsawa N., Okuda K., Sakamoto K., Shirouzu M., Shindo T., Yokoyama S. Structural basis for extracellular interactions between calcitonin receptor-like receptor and receptor activity-modifying protein 2 for adrenomedullin-specific binding. Protein Sci. 2012;21(2):199–210. doi: 10.1002/pro.2003. [http://dx.doi.org/10.1002/pro.2003]. [PMID: 22102369]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.ter Haar E., Koth C.M., Abdul-Manan N., Swenson L., Coll J.T., Lippke J.A., Lepre C.A., Garcia-Guzman M., Moore J.M. Crystal structure of the ectodomain complex of the CGRP receptor, a class-B GPCR, reveals the site of drug antagonism. Structure. 2010;18(9):1083–1093. doi: 10.1016/j.str.2010.05.014. [http://dx.doi.org/10.1016/j.str.2010.05. 014]. [PMID: 20826335]. [DOI] [PubMed] [Google Scholar]
- 367.Liang Y.L., Khoshouei M., Radjainia M., Zhang Y., Glukhova A., Tarrasch J., Thal D.M., Furness S.G.B., Christopoulos G., Coudrat T., Danev R., Baumeister W., Miller L.J., Christopoulos A., Kobilka B.K., Wootten D., Skiniotis G., Sexton P.M. Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature. 2017;546(7656):118–123. doi: 10.1038/nature22327. [http://dx.doi.org/10.1038/ nature22327]. [PMID: 28437792]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Booe J.M., Warner M.L., Roehrkasse A.M., Hay D.L., Pioszak A.A. Probing the Mechanism of Receptor Activity-Modifying Protein Modulation of GPCR Ligand Selectivity through Rational Design of Potent Adrenomedullin and Calcitonin Gene-Related Peptide Antagonists. Mol. Pharmacol. 2018;93(4):355–367. doi: 10.1124/mol.117.110916. [http:// dx.doi.org/10.1124/mol.117.110916]. [PMID: 29363552]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Koth C.M., Murray J.M., Mukund S., Madjidi A., Minn A., Clarke H.J., Wong T., Chiang V., Luis E., Estevez A., Rondon J., Zhang Y., Hötzel I., Allan B.B. Molecular basis for negative regulation of the glucagon receptor. Proc. Natl. Acad. Sci. USA. 2012;109(36):14393–14398. doi: 10.1073/pnas.1206734109. [http://dx.doi.org/10.1073/pnas. 1206734109]. [PMID: 22908259]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Siu F.Y., He M., de Graaf C., Han G.W., Yang D., Zhang Z., Zhou C., Xu Q., Wacker D., Joseph J.S., Liu W., Lau J., Cherezov V., Katritch V., Wang M.W., Stevens R.C. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013;499(7459):444–449. doi: 10.1038/nature12393. [http://dx.doi.org/10.1038/nature12393]. [PMID: 23863937]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Jazayeri A., Doré A.S., Lamb D., Krishnamurthy H., Southall S.M., Baig A.H., Bortolato A., Koglin M., Robertson N.J., Errey J.C., Andrews S.P., Teobald I., Brown A.J., Cooke R.M., Weir M., Marshall F.H. Extra-helical binding site of a glucagon receptor antagonist. Nature. 2016;533(7602):274–277. doi: 10.1038/nature17414. [http://dx. doi.org/10.1038/nature17414]. [PMID: 27111510]. [DOI] [PubMed] [Google Scholar]
- 372.Jazayeri A., Rappas M., Brown A.J.H., Kean J., Errey J.C., Robertson N.J., Fiez-Vandal C., Andrews S.P., Congreve M., Bortolato A., Mason J.S., Baig A.H., Teobald I., Doré A.S., Weir M., Cooke R.M., Marshall F.H. Crystal structure of the GLP-1 receptor bound to a peptide agonist. Nature. 2017;546(7657):254–258. doi: 10.1038/nature22800. [http://dx.doi.org/10.1038/nature22800]. [PMID: 28562585]. [DOI] [PubMed] [Google Scholar]
- 373.Zhang Y., Sun B., Feng D., Hu H., Chu M., Qu Q., Tarrasch J.T., Li S., Sun Kobilka T., Kobilka B.K., Skiniotis G. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature. 2017;546(7657):248–253. doi: 10.1038/nature22394. [http://dx.doi.org/10.1038/ nature22394]. [PMID: 28538729]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Song G., Yang D., Wang Y., de Graaf C., Zhou Q., Jiang S., Liu K., Cai X., Dai A., Lin G., Liu D., Wu F., Wu Y., Zhao S., Ye L., Han G.W., Lau J., Wu B., Hanson M.A., Liu Z.J., Wang M.W., Stevens R.C. Human GLP-1 receptor transmembrane domain structure in complex with allosteric modulators. Nature. 2017;546(7657):312–315. doi: 10.1038/nature22378. [http://dx.doi.org/10.1038/nature 22378]. [PMID: 28514449]. [DOI] [PubMed] [Google Scholar]
- 375.Pioszak A.A., Parker N.R., Gardella T.J., Xu H.E. Structural basis for parathyroid hormone-related protein binding to the parathyroid hormone receptor and design of conformation-selective peptides. J. Biol. Chem. 2009;284(41):28382–28391. doi: 10.1074/jbc.M109.022905. [http://dx. doi.org/10.1074/jbc.M109.022905]. [PMID: 19674967]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Geng Y., Bush M., Mosyak L., Wang F., Fan Q.R. Structural mechanism of ligand activation in human GABA(B) receptor. Nature. 2013;504(7479):254–259. doi: 10.1038/nature12725. [http://dx.doi.org/10.1038/nature 12725]. [PMID: 24305054]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Wu H., Wang C., Gregory K.J., Han G.W., Cho H.P., Xia Y., Niswender C.M., Katritch V., Meiler J., Cherezov V., Conn P.J., Stevens R.C. Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science. 2014;344(6179):58–64. doi: 10.1126/science.1249489. [http://dx.doi.org/10.1126/science.1249489]. [PMID: 24603153]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Kunishima N., Shimada Y., Tsuji Y., Sato T., Yamamoto M., Kumasaka T., Nakanishi S., Jingami H., Morikawa K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407(6807):971–977. doi: 10.1038/35039564. [http://dx.doi.org/10. 1038/35039564]. [PMID: 11069170]. [DOI] [PubMed] [Google Scholar]
- 379.Monn J.A., Prieto L., Taboada L., Hao J., Reinhard M.R., Henry S.S., Beadle C.D., Walton L., Man T., Rudyk H., Clark B., Tupper D., Baker S.R., Lamas C., Montero C., Marcos A., Blanco J., Bures M., Clawson D.K., Atwell S., Lu F., Wang J., Russell M., Heinz B.A., Wang X., Carter J.H., Getman B.G., Catlow J.T., Swanson S., Johnson B.G., Shaw D.B., McKinzie D.L. Synthesis and Pharmacological Characterization of C4-(Thiotriazolyl)-substituted-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylates. Identification of (1R,2S,4R,5R,6R)-2-Amino-4-(1H-1,2,4-triazol-3-ylsulfanyl)bicyclo[3.1.0]hexane-2,6-dicarboxylic Acid (LY2812223), a highly potent, functionally selective mGlu2 receptor agonist. J. Med. Chem. 2015;58(18):7526–7548. doi: 10.1021/acs.jmedchem.5b01124. [http:// dx.doi.org/10.1021/acs.jmedchem.5b01124]. [PMID: 26313429]. [DOI] [PubMed] [Google Scholar]
- 380.Muto T., Tsuchiya D., Morikawa K., Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA. 2007;104(10):3759–3764. doi: 10.1073/pnas.0611577104. [http://dx.doi.org/10.1073/pnas.0611577104]. [PMID: 17360426]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Doré A.S., Okrasa K., Patel J.C., Serrano-Vega M., Bennett K., Cooke R.M., Errey J.C., Jazayeri A., Khan S., Tehan B., Weir M., Wiggin G.R., Marshall F.H. Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature. 2014;511(7511):557–562. doi: 10.1038/nature13396. [http://dx.doi.org/10.1038/nature13396]. [PMID: 25042998]. [DOI] [PubMed] [Google Scholar]
- 382.Christopher J.A., Aves S.J., Bennett K.A., Doré A.S., Errey J.C., Jazayeri A., Marshall F.H., Okrasa K., Serrano-Vega M.J., Tehan B.G., Wiggin G.R., Congreve M. Fragment and structure-based drug discovery for a class C GPCR: Discovery of the mGlu5 negative allosteric modulator HTL14242 (3-chloro-5-[6-(5-fluoropyridin-2-yl)pyrimidin-4-yl]benzonitrile). J. Med. Chem. 2015;58(16):6653–6664. doi: 10.1021/acs.jmedchem.5b00892. [http://dx.doi.org/10.1021/acs.jmedchem. 5b00892]. [PMID: 26225459]. [DOI] [PubMed] [Google Scholar]
- 383.Christopher J.A., Orgován Z., Congreve M., Doré A.S., Errey J.C., Marshall F.H., Mason J.S., Okrasa K., Rucktooa P., Serrano-Vega M.J., Ferenczy G.G., Keserű G.M. Structure-based optimization strategies for G protein-coupled receptor (GPCR) allosteric modulators: A case study from analyses of new metabotropic glutamate receptor 5 (mGlu5) X-ray structures. J. Med. Chem. 2018 doi: 10.1021/acs.jmedchem.7b01722. [http://dx.doi.org/10.1021/acs.jmedchem.7b01722]. [PMID: 29455526]. [DOI] [PubMed] [Google Scholar]
- 384.Wang C., Wu H., Katritch V., Han G.W., Huang X.P., Liu W., Siu F.Y., Roth B.L., Cherezov V., Stevens R.C. Structure of the human smoothened receptor bound to an antitumour agent. Nature. 2013;497(7449):338–343. doi: 10.1038/nature12167. [http://dx.doi.org/10.1038/nature12167]. [PMID: 23636324]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Wang C., Wu H., Evron T., Vardy E., Han G.W., Huang X.P., Hufeisen S.J., Mangano T.J., Urban D.J., Katritch V., Cherezov V., Caron M.G., Roth B.L., Stevens R.C. Structural basis for Smoothened receptor modulation and chemoresistance to anticancer drugs. Nat. Commun. 2014;5:4355. doi: 10.1038/ncomms5355. [PMID: 25008467]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Weierstall U., James D., Wang C., White T.A., Wang D., Liu W., Spence J.C., Bruce Doak R., Nelson G., Fromme P., Fromme R., Grotjohann I., Kupitz C., Zatsepin N.A., Liu H., Basu S., Wacker D., Han G.W., Katritch V., Boutet S., Messerschmidt M., Williams G.J., Koglin J.E., Marvin Seibert M., Klinker M., Gati C., Shoeman R.L., Barty A., Chapman H.N., Kirian R.A., Beyerlein K.R., Stevens R.C., Li D., Shah S.T., Howe N., Caffrey M., Cherezov V. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography. Nat. Commun. 2014;5:3309. doi: 10.1038/ncomms4309. [http://dx.doi.org/10.1038/ncomms 4309]. [PMID: 24525480]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Huang P., Nedelcu D., Watanabe M., Jao C., Kim Y., Liu J., Salic A. Cellular cholesterol directly activates smoothened in hedgehog signaling. Cell. 2016;166(5):1176–1187.e14. doi: 10.1016/j.cell.2016.08.003. [http:// dx.doi.org/10.1016/j.cell.2016.08.003]. [PMID: 27545348]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Byrne E.F.X., Sircar R., Miller P.S., Hedger G., Luchetti G., Nachtergaele S., Tully M.D., Mydock-McGrane L., Covey D.F., Rambo R.P., Sansom M.S.P., Newstead S., Rohatgi R., Siebold C. Structural basis of Smoothened regulation by its extracellular domains. Nature. 2016;535(7613):517–522. doi: 10.1038/nature18934. [http://dx.doi.org/10. 1038/nature18934]. [PMID: 27437577]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Zhang X., Zhao F., Wu Y., Yang J., Han G.W., Zhao S., Ishchenko A., Ye L., Lin X., Ding K., Dharmarajan V., Griffin P.R., Gati C., Nelson G., Hunter M.S., Hanson M.A., Cherezov V., Stevens R.C., Tan W., Tao H., Xu F. Crystal structure of a multi-domain human smoothened receptor in complex with a super stabilizing ligand. Nat. Commun. 2017;8:15383. doi: 10.1038/ncomms15383. [http://dx.doi. org/10.1038/ncomms15383]. [PMID: 28513578]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Salzman G.S., Ackerman S.D., Ding C., Koide A., Leon K., Luo R., Stoveken H.M., Fernandez C.G., Tall G.G., Piao X., Monk K.R., Koide S., Araç D. Structural basis for regulation of GPR56/ADGRG1 by its alternatively spliced extracellular domains. Neuron. 2016;91(6):1292–1304. doi: 10.1016/j.neuron.2016.08.022. [http://dx.doi.org/10.1016/j. neuron.2016.08.022]. [PMID: 27657451]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Ranaivoson F.M., Liu Q., Martini F., Bergami F., von Daake S., Li S., Lee D., Demeler B., Hendrickson W.A., Comoletti D. Structural and mechanistic insights into the latrophilin3-FLRT3 complex that mediates glutamatergic synapse development. Structure. 2015;23(9):1665–1677. doi: 10.1016/j.str.2015.06.022. [http://dx.doi.org/10.1016/j.str. 2015.06.022]. [PMID: 26235031]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Lima C.D., Klein M.G., Hendrickson W.A. Super-Complexes of Adhesion GPCRs and Neural Guidance Receptors. Nat. Commun. 2016;7:•••. doi: 10.1038/ncomms11184. https://www.nature.com/articles/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Kitchen D.B., Decornez H., Furr J.R., Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat. Rev. Drug Discov. 2004;3(11):935–949. doi: 10.1038/nrd1549. [http://dx. doi.org/10.1038/nrd1549]. [PMID: 15520816]. [DOI] [PubMed] [Google Scholar]
- 394.Lemos A., Leão M., Soares J., Palmeira A., Pinto M., Saraiva L., Sousa M.E. Medicinal chemistry strategies to disrupt the p53-MDM2/MDMX interaction. Med. Res. Rev. 2016;36(5):789–844. doi: 10.1002/med.21393. [http://dx.doi.org/10.1002/med.21393]. [PMID: 27302609]. [DOI] [PubMed] [Google Scholar]
- 395.Lemos A., Melo R., Moreira I.S., Cordeiro M.N.D.S. Computational approaches to target key therapeutical targets in Alzheimer disease. In: Roy K., editor. Neuromethods. Springer; 2018. pp. 61–106. [Google Scholar]
- 396.Azevedo L.S., Moraes F.P., Xavier M.M., Pantoja E.O., Villavicencio B., Finck J.A., Proença A.M., Rocha K.B., De Azevedo W.F., Jr Recent progress of molecular docking simulations applied to development of drugs. Curr. Bioinform. 2012;7(4):352–365. [http://dx.doi.org/10.2174/157489312803901063]. [Google Scholar]
- 397.Xavier M.M., Heck G.S., Avila M.B., Levin N.M.B., Pintro V.O., Carvalho N.L., Azevedo W.F. SAnDReS a computational tool for statistical analysis of docking results and development of scoring functions. Comb. Chem. High Throughput Screen. 2016;19(10):801–812. doi: 10.2174/1386207319666160927111347. [http://dx.doi.org/10.2174/1386207319666160927111347]. [PMID: 27686428]. [DOI] [PubMed] [Google Scholar]
- 398.Ferreira L.G., Dos Santos R.N., Oliva G., Andricopulo A.D. Molecular docking and structure-based drug design strategies. Molecules. 2015;20(7):13384–13421. doi: 10.3390/molecules200713384. [http://dx.doi.org/10.3390/ molecules200713384]. [PMID: 26205061]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Ericksen S.S., Wu H., Zhang H., Michael L.A., Newton M.A., Hoffmann F.M., Wildman S.A. Machine Learning Consensus Scoring Improves Performance Across Targets in Structure-Based Virtual Screening. 2017 doi: 10.1021/acs.jcim.7b00153. http://pubs.acs.org/doi/abs/10.1021/acs [DOI] [PMC free article] [PubMed]
- 400.Wójcikowski M., Ballester P.J., Siedlecki P. Performance of machine-learning scoring functions in structure-based virtual screening. Sci. Rep. 2017;7:46710. doi: 10.1038/srep46710. https://www.nature.com/ articles/srep46710 [http://dx.doi.org/10.1038/srep46710]. [PMID: 28440302]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Morris G.M., Goodsell D.S., Halliday R.S., Huey R., Hart W.E., Belew R.K., Olson A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998;19(14):1639–1662. [http://dx. doi.org/10.1002/(SICI)1096-987X(19981115)19:14<1639:AID-JCC10>3.0.CO;2-B]. [Google Scholar]
- 402.Ostopovici-Halip L., Rad-Curpan R. Modeling of ligand binding to dopamine D2 receptor. J. Serb. Chem. Soc. 2014;79(2):175–183. [http://dx.doi.org/10.2298/JSC130208046O]. [Google Scholar]
- 403.Molecular Operating Environment (MOE) 2009. [Google Scholar]
- 404.Malo M., Brive L., Luthman K., Svensson P. 2012.
- 405.Friesner R.A., Banks J.L., Murphy R.B., Halgren T.A., Klicic J.J., Mainz D.T., Repasky M.P., Knoll E.H., Shelley M., Perry J.K., Shaw D.E., Francis P., Shenkin P.S. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004;47(7):1739–1749. doi: 10.1021/jm0306430. [http://dx.doi.org/10.1021/jm0306430]. [PMID: 15027865]. [DOI] [PubMed] [Google Scholar]
- 406.Friesner R.A., Murphy R.B., Repasky M.P., Frye L.L., Greenwood J.R., Halgren T.A., Sanschagrin P.C., Mainz D.T. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006;49(21):6177–6196. doi: 10.1021/jm051256o. [http://dx.doi.org/10.1021/jm 051256o]. [PMID: 17034125]. [DOI] [PubMed] [Google Scholar]
- 407.Halgren T.A., Murphy R.B., Friesner R.A., Beard H.S., Frye L.L., Pollard W.T., Banks J.L. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004;47(7):1750–1759. doi: 10.1021/jm030644s. [http://dx.doi. org/10.1021/jm030644s]. [PMID: 15027866]. [DOI] [PubMed] [Google Scholar]
- 408.Penjisevic J.Z., Sukalovic V.V., Andric D.B., Roglic G.M., Novakovic I., Soskic V., Kostic-Rajacic S.V. Synthesis, biological evaluation and docking analysis of substituted piperidines and (2-methoxyphenyl) piperazines. J. Serb. Chem. Soc. 2016;81(4):347–356. [http://dx.doi.org/10.2298/JSC151021097P]. [Google Scholar]
- 409.Penjišević J.Z., Šukalović V.V., Andrić D.B., Roglić G.M., Šoškić V., Kostić-Rajačić S.V. Synthesis, Biological, and Computational Evaluation of Substituted 1-(2-Methoxyphenyl)-4-(1-phenethylpiperidin-4-yl)piperazines and 1-(2-Methoxyphenyl)-4-[(1-phenethylpiperidin-4-yl)methyl]piperazines as Dopaminergic Ligands. Arch. Pharm. (Weinheim) 2016;349(8):614–626. doi: 10.1002/ardp.201600081. [http:// dx.doi.org/10.1002/ardp.201600081]. [PMID: 27335270]. [DOI] [PubMed] [Google Scholar]
- 410.Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [PMID: 19499576]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [http://dx.doi.org/10. 1002/jcc.21256]. [PMID: 19399780]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Platania C.B.M., Salomone S., Leggio G.M., Drago F., Bucolo C. Homology modeling of dopamine D2 and D3 receptors: molecular dynamics refinement and docking evaluation. PLoS One. 2012;7(9):e44316. doi: 10.1371/journal.pone.0044316. [http://dx.doi.org/10.1371/journal.pone.0044316]. [PMID: 22970199]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Ortore G., Tuccinardi T., Bertini S., Martinelli A. A theoretical study to investigate D2DAR/D4DAR selectivity: receptor modeling and molecular docking of dopaminergic ligands. J. Med. Chem. 2006;49(4):1397–1407. doi: 10.1021/jm051046b. [http://dx.doi.org/10.1021/jm051046b]. [PMID: 16480275]. [DOI] [PubMed] [Google Scholar]
- 414.2000.
- 415.Sukalović V., Andrić D., Roglić G., Kostić-Rajacić S., Schrattenholz A., Soskić V. Synthesis, dopamine D2 receptor binding studies and docking analysis of 5-[3-(4-arylpiperazin-1-yl)propyl]-1H-benzimidazole, 5-[2-(4-arylpiperazin-1-yl)ethoxy]-1H-benzimidazole and their analogs. Eur. J. Med. Chem. 2005;40(5):481–493. doi: 10.1016/j.ejmech.2004.10.006. [http://dx.doi.org/10.1016/j.ejmech.2004.10.006]. [PMID: 15893022]. [DOI] [PubMed] [Google Scholar]
- 416.Accelrys Software Inc . 2009. [Google Scholar]
- 417.Sukalovic V., Soskic V., Andric D., Roglic G., Kostic-Rajacic S. Modeling key interactions between dopamine D2 receptor second extracellular loop and arylpiperazine ligands. J. Serb. Chem. Soc. 2012;77(3):259–277. [http://dx.doi.org/10.2298/JSC111028212S]. [Google Scholar]
- 418.2005.
- 419.Varady J., Wu X., Fang X., Min J., Hu Z., Levant B., Wang S. Molecular modeling of the three-dimensional structure of dopamine 3 (D3) subtype receptor: discovery of novel and potent D3 ligands through a hybrid pharmacophore- and structure-based database searching approach. J. Med. Chem. 2003;46(21):4377–4392. doi: 10.1021/jm030085p. [http://dx.doi.org/10.1021/jm030085p]. [PMID: 14521403]. [DOI] [PubMed] [Google Scholar]
- 420.Huey R., Goodsell D.S., Morris G.M., Olson A.J. Grid-Based Hydrogen Bond Potentials with Improved Directionality. Lett. Drug Des. Discov. 2004;1(2):178–183. [http://dx.doi.org/10. 2174/1570180043485581]. [Google Scholar]
- 421.Huey R., Morris G.M., Olson A.J., Goodsell D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007;28(6):1145–1152. doi: 10.1002/jcc.20634. [http://dx.doi.org/10.1002/ jcc.20634]. [PMID: 17274016]. [DOI] [PubMed] [Google Scholar]
- 422.Mirza M.U., Mirza A.H., Ghori N-U-H., Ferdous S. Glycyrrhetinic acid and E.resveratroloside act as potential plant derived compounds against dopamine receptor D3 for Parkinson’s disease: a pharmacoinformatics study. Drug Des. Devel. Ther. 2014;9:187–198. doi: 10.2147/DDDT.S72794. [http://dx.doi.org/10.2147/DDDT.S72794]. [PMID: 25565772]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Jain A.N. Surflex-Dock 2.1: robust performance from ligand energetic modeling, ring flexibility, and knowledge-based search. J. Comput. Aided Mol. Des. 2007;21(5):281–306. doi: 10.1007/s10822-007-9114-2. [http://dx.doi. org/10.1007/s10822-007-9114-2]. [PMID: 17387436]. [DOI] [PubMed] [Google Scholar]
- 424.Duan X., Zhang X., Xu B., Wang F., Lei M. Computational study and modified design of selective dopamine D3 receptor agonists. Chem. Biol. Drug Des. 2016;88(1):142–154. doi: 10.1111/cbdd.12743. [http://dx. doi.org/10.1111/cbdd.12743]. [PMID: 26851125]. [DOI] [PubMed] [Google Scholar]
- 425.Farid R., Day T., Friesner R.A., Pearlstein R.A. New insights about HERG blockade obtained from protein modeling, potential energy mapping, and docking studies. Bioorg. Med. Chem. 2006;14(9):3160–3173. doi: 10.1016/j.bmc.2005.12.032. [http://dx.doi.org/10.1016/j.bmc.2005.12.032]. [PMID: 16413785]. [DOI] [PubMed] [Google Scholar]
- 426.Sherman W., Beard H.S., Farid R. Use of an induced fit receptor structure in virtual screening. Chem. Biol. Drug Des. 2006;67(1):83–84. doi: 10.1111/j.1747-0285.2005.00327.x. [http://dx.doi.org/10.1111/j.1747-0285.2005.00327.x]. [PMID: 16492153]. [DOI] [PubMed] [Google Scholar]
- 427.Sherman W., Day T., Jacobson M.P., Friesner R.A., Farid R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006;49(2):534–553. doi: 10.1021/jm050540c. [http://dx.doi.org/10.1021/jm 050540c]. [PMID: 16420040]. [DOI] [PubMed] [Google Scholar]
- 428.Molecular Operating Environment (MOE), Chemical Compu-ting Group Inc . 2005. [Google Scholar]
- 429.Pourbasheer E., Shokouhi Tabar S., Masand V.H., Aalizadeh R., Ganjali M.R. 3D-QSAR and docking studies on adenosine A2A receptor antagonists by the CoMFA method. SAR QSAR Environ. Res. 2015;26(6):461–477. doi: 10.1080/1062936X.2015.1049666. [http://dx.doi.org/10.1080/1062936X. 2015.1049666]. [PMID: 26055215]. [DOI] [PubMed] [Google Scholar]
- 430.Mustyala K.K., Chitturi A.R., Naikal James P.S., Vuruputuri U. Pharmacophore mapping and in silico screening to identify new potent leads for A(2A) adenosine receptor as antagonists. J. Recept. Signal Transduct. Res. 2012;32(2):102–113. doi: 10.3109/10799893.2012.660532. [http://dx.doi. org/10.3109/10799893.2012.660532]. [PMID: 22384789]. [DOI] [PubMed] [Google Scholar]
- 431.Moustakas D.T., Lang P.T., Pegg S., Pettersen E., Kuntz I.D., Brooijmans N., Rizzo R.C. Development and validation of a modular, extensible docking program: DOCK 5. J. Comput. Aided Mol. Des. 2006;20(10-11):601–619. doi: 10.1007/s10822-006-9060-4. [http://dx.doi.org/10.1007/ s10822-006-9060-4]. [PMID: 17149653]. [DOI] [PubMed] [Google Scholar]
- 432.Wei J., Qu W., Ye Y., Gao Q. 3D pharmacophore based virtual screening of A 2A adenosine receptor antagonists. Protein Pept. Lett. 2010;17(3):332–339. doi: 10.2174/092986610790780260. [http://dx.doi.org/10.2174/0929866 10790780260]. [PMID: 20236086]. [DOI] [PubMed] [Google Scholar]
- 433.Massink A., Louvel J., Adlere I., van Veen C., Huisman B.J.H., Dijksteel G.S., Guo D., Lenselink E.B., Buckley B.J., Matthews H., Ranson M., Kelso M., IJzerman A.P. 5′-Substituted Amiloride Derivatives as Allosteric Modulators Binding in the Sodium Ion Pocket of the Adenosine A2A Receptor. J. Med. Chem. 2016;59(10):4769–4777. doi: 10.1021/acs.jmedchem.6b00142. [http://dx.doi.org/10.1021/acs.jmedchem. 6b00142]. [PMID: 27124340]. [DOI] [PubMed] [Google Scholar]
- 434.Vanopdenbosch N., Cramer R., Giarrusso F.F. SYBYL, the integrated molecular modeling system. J. Mol. Graph. 1985;3:110–111. [Google Scholar]
- 435.Henderson R., Baldwin J.M., Ceska T.A., Zemlin F., Beckmann E., Downing K.H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J. Mol. Biol. 1990;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [http://dx.doi.org/10.1016/S0022-2836(05) 80271-2]. [PMID: 2359127]. [DOI] [PubMed] [Google Scholar]
- 436.Kim S-K., Gao Z-G., Van Rompaey P., Gross A.S., Chen A., Van Calenbergh S., Jacobson K.A. Modeling the adenosine receptors: comparison of the binding domains of A2A agonists and antagonists. J. Med. Chem. 2003;46(23):4847–4859. doi: 10.1021/jm0300431. [http://dx.doi. org/10.1021/jm0300431]. [PMID: 14584936]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Abagyan R.A., Orry A., Raush E., Budagyan L., Totrov M. ICM Manual, 3.0. La Jolla, CA: MolSoft LLC; 2009. [Google Scholar]
- 438.Ivanov A.A., Barak D., Jacobson K.A. Evaluation of homology modeling of G-protein-coupled receptors in light of the A(2A) adenosine receptor crystallographic structure. J. Med. Chem. 2009;52(10):3284–3292. doi: 10.1021/jm801533x. [http://dx.doi.org/10.1021/jm801533x]. [PMID: 19402631]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Katritch V., Jaakola V-P., Lane J.R., Lin J., Ijzerman A.P., Yeager M., Kufareva I., Stevens R.C., Abagyan R. Structure-based discovery of novel chemotypes for adenosine A(2A) receptor antagonists. J. Med. Chem. 2010;53(4):1799–1809. doi: 10.1021/jm901647p. [http://dx. doi.org/10.1021/jm901647p]. [PMID: 20095623]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Jaakola V-P., Ijzerman A.P. The crystallographic structure of the human adenosine A2A receptor in a high-affinity antagonist-bound state: implications for GPCR drug screening and design. Curr. Opin. Struct. Biol. 2010;20(4):401–414. doi: 10.1016/j.sbi.2010.05.002. [http://dx.doi.org/ 10.1016/j.sbi.2010.05.002]. [PMID: 20538452]. [DOI] [PubMed] [Google Scholar]
- 441.Liu Y., Burger S.K., Ayers P.W., Vöhringer-Martinez E. Computational study of the binding modes of caffeine to the adenosine A2A receptor. J. Phys. Chem. B. 2011;115(47):13880–13890. doi: 10.1021/jp2022049. [http://dx.doi.org/10.1021/jp2022049]. [PMID: 21970461]. [DOI] [PubMed] [Google Scholar]
- 442.Crouch R.D., Holden M.S., Samet C. CAChe molecular modeling: A visualization tool early in the undergraduate chemistry curriculum. J. Chem. Educ. 1996;73(10):916. [http://dx.doi.org/ 10.1021/ed073p916]. [Google Scholar]
- 443.Yuzlenko O., Kieć-Kononowicz K. Molecular modeling of A1 and A2A adenosine receptors: comparison of rhodopsin- and β2-adrenergic-based homology models through the docking studies. J. Comput. Chem. 2009;30(1):14–32. doi: 10.1002/jcc.21001. [http://dx.doi.org/10.1002/ jcc.21001]. [PMID: 18496794]. [DOI] [PubMed] [Google Scholar]
- 444.Ye Y., Wei J., Dai X., Gao Q. Computational studies of the binding modes of A 2A adenosine receptor antagonists. Amino Acids. 2008;35(2):389–396. doi: 10.1007/s00726-007-0604-2. [http://dx.doi.org/10.1007/s00726-007-0604-2]. [PMID: 17978889]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Zhang L., Liu T., Wang X., Wang J., Li G., Li Y., Yang L., Wang Y. Insight into the binding mode and the structural features of the pyrimidine derivatives as human A2A adenosine receptor antagonists. Biosystems. 2014;115:13–22. doi: 10.1016/j.biosystems.2013.04.003. [http://dx.doi.org/10. 1016/j.biosystems.2013.04.003]. [PMID: 23665268]. [DOI] [PubMed] [Google Scholar]
- 446.Muñoz-Gutiérrez C., Caballero J., Morales-Bayuelo A. HQSAR and molecular docking studies of furanyl derivatives as adenosine A2A receptor antagonists. Med. Chem. Res. 2016;25(7):1316–1328. [http://dx.doi.org/10.1007/s00044-016-1575-1]. [Google Scholar]
- 447.Ewing T.J.A., Kuntz I.D. Critical evaluation of search algorithms for automated molecular docking and database screening. J. Comput. Chem. 1997;18(9):1175–1189. [http://dx.doi.org/10.1002 /(SICI)1096-987X(19970715)18:9<1175:AID-JCC6>3.0.CO;2-O]. [Google Scholar]
- 448.Rarey M., Kramer B., Lengauer T., Klebe G. A fast flexible docking method using an incremental construction algorithm. J. Mol. Biol. 1996;261(3):470–489. doi: 10.1006/jmbi.1996.0477. [http://dx.doi.org/10.1006/jmbi. 1996.0477]. [PMID: 8780787]. [DOI] [PubMed] [Google Scholar]
- 449.Jones G., Willett P., Glen R.C., Leach A.R., Taylor R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997;267(3):727–748. doi: 10.1006/jmbi.1996.0897. [http://dx.doi.org/10. 1006/jmbi.1996.0897]. [PMID: 9126849]. [DOI] [PubMed] [Google Scholar]
- 450.Bissantz C., Bernard P., Hibert M., Rognan D. Protein-based virtual screening of chemical databases. II. Are homology models of G-Protein Coupled Receptors suitable targets? Proteins. 2003;50(1):5–25. doi: 10.1002/prot.10237. [http://dx.doi.org/10.1002/prot.10237]. [PMID: 12471595]. [DOI] [PubMed] [Google Scholar]
- 451.Ahnaou A., Lavreysen H., Tresadern G., Cid J.M., Drinkenburg W.H. mGlu2 Receptor agonism, but not positive allosteric modulation, elicits rapid tolerance towards their primary efficacy on sleep measures in rats. PLoS One. 2015;10(12):e0144017. doi: 10.1371/journal.pone.0144017. [http://dx. doi.org/10.1371/journal.pone.0144017]. [PMID: 26658273]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Molecular Operating Environment (MOE) 2013. [Google Scholar]
- 453.Huynh T.H., Erichsen M.N., Tora A.S., Goudet C., Sagot E., Assaf Z., Thomsen C., Brodbeck R., Stensbøl T.B., Bjørn-Yoshimoto W.E., Nielsen B., Pin J.P., Gefflaut T., Bunch L. New 4-functionalized glutamate analogues are selective agonists at metabotropic glutamate receptor subtype 2 or selective agonists at metabotropic glutamate receptor group III. J. Med. Chem. 2016;59(3):914–924. doi: 10.1021/acs.jmedchem.5b01333. [http://dx.doi.org/10.1021/acs.jmedchem.5b01333]. [PMID: 26814576]. [DOI] [PubMed] [Google Scholar]
- 454.Macchiarulo A., Costantino G., Sbaglia R., Aiello S., Meniconi M., Pellicciari R. The role of electrostatic interaction in the molecular recognition of selective agonists to metabotropic glutamate receptors. Proteins. 2003;50(4):609–619. doi: 10.1002/prot.10301. [http://dx.doi.org/10. 1002/prot.10301]. [PMID: 12577267]. [DOI] [PubMed] [Google Scholar]
- 455.Accelrys Software Inc . 2010. [Google Scholar]
- 456.Goudet C., Vilar B., Courtiol T., Deltheil T., Bessiron T., Brabet I., Oueslati N., Rigault D., Bertrand H.O., McLean H., Daniel H., Amalric M., Acher F., Pin J.P. A novel selective metabotropic glutamate receptor 4 agonist reveals new possibilities for developing subtype selective ligands with therapeutic potential. FASEB J. 2012;26(4):1682–1693. doi: 10.1096/fj.11-195941. [http://dx.doi.org/10.1096/fj. 11-195941]. [PMID: 22223752]. [DOI] [PubMed] [Google Scholar]
- 457.Sack J.S., Saper M.A., Quiocho F.A. Periplasmic binding protein structure and function. Refined X-ray structures of the leucine/ isoleucine/valine-binding protein and its complex with leucine. J. Mol. Biol. 1989;206(1):171–191. doi: 10.1016/0022-2836(89)90531-7. [http://dx.doi.org/10.1016/0022-2836(89)90531-7]. [PMID: 2649682]. [DOI] [PubMed] [Google Scholar]
- 458.Sack J.S., Trakhanov S.D., Tsigannik I.H., Quiocho F.A. Structure of the L-leucine-binding protein refined at 2.4 A resolution and comparison with the Leu/Ile/Val-binding protein structure. J. Mol. Biol. 1989;206(1):193–207. doi: 10.1016/0022-2836(89)90532-9. [http://dx.doi.org/10.1016/0022-2836(89)90532-9]. [PMID: 2649683]. [DOI] [PubMed] [Google Scholar]
- 459.Pearl L., O’Hara B., Drew R., Wilson S. Crystal structure of AmiC: the controller of transcription antitermination in the amidase operon of Pseudomonas aeruginosa. EMBO J. 1994;13(24):5810–5817. doi: 10.1002/j.1460-2075.1994.tb06924.x. [PMID: 7813419]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Bessis A.S., Bertrand H.O., Galvez T., De Colle C., Pin J.P., Acher F. Three-dimensional model of the extracellular domain of the type 4a metabotropic glutamate receptor: new insights into the activation process. Protein Sci. 2000;9(11):2200–2209. doi: 10.1110/ps.9.11.2200. [http://dx.doi.org/10.1110/ps.9.11.2200]. [PMID: 11152130]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Release S. 2013-2: Maestro. New York, NY: Schrödinger, LLC; 2013. [Google Scholar]
- 462.The PyMOL Molecular Graphics System, version 1.7.4; Schrodinger, LLC. [Google Scholar]
- 463.Wu G., Robertson D.H., Brooks C.L., III, Vieth M. Detailed analysis of grid-based molecular docking: A case study of CDOCKER-A CHARMm-based MD docking algorithm. J. Comput. Chem. 2003;24(13):1549–1562. doi: 10.1002/jcc.10306. [http://dx.doi.org/10.1002/ jcc.10306]. [PMID: 12925999]. [DOI] [PubMed] [Google Scholar]
- 464.Vu H.N., Kim J.Y., Hassan A.H.E., Choi K., Park J-H., Park K.D., Lee J.K., Pae A.N., Choo H., Min S-J., Cho Y.S. Synthesis and biological evaluation of picolinamides and thiazole-2-carboxamides as mGluR5 (metabotropic glutamate receptor 5) antagonists. Bioorg. Med. Chem. Lett. 2016;26(1):140–144. doi: 10.1016/j.bmcl.2015.11.012. [http:// dx.doi.org/10.1016/j.bmcl.2015.11.012]. [PMID: 26598462]. [DOI] [PubMed] [Google Scholar]
- 465.Jiang L., Li Y., Qiao L., Chen X., He Y., Zhang Y., Li G. Discovery of potential negative allosteric modulators of mGluR5 from natural products using pharmacophore modeling, molecular docking, and molecular dynamics simulation studies. Can. J. Chem. 2015;93(11):1199–1206. [http://dx.doi.org/10.1139/cjc-2015-0197]. [Google Scholar]
- 466.Casoni A., Clerici F., Contini A. Molecular dynamic simulation of mGluR5 amino terminal domain: essential dynamics analysis captures the agonist or antagonist behaviour of ligands. J. Mol. Graph. Model. 2013;41:72–78. doi: 10.1016/j.jmgm.2013.02.002. [http://dx.doi.org/10.1016/j. jmgm.2013.02.002]. [PMID: 23500630]. [DOI] [PubMed] [Google Scholar]
- 467.Misura K.M., Chivian D., Rohl C.A., Kim D.E., Baker D. Physically realistic homology models built with ROSETTA can be more accurate than their templates. Proc. Natl. Acad. Sci. USA. 2006;103(14):5361–5366. doi: 10.1073/pnas.0509355103. [http://dx.doi.org/10.1073/pnas. 0509355103]. [PMID: 16567638]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Dalton J.A., Gómez-Santacana X., Llebaria A., Giraldo J. Computational analysis of negative and positive allosteric modulator binding and function in metabotropic glutamate receptor 5 (in)activation. J. Chem. Inf. Model. 2014;54(5):1476–1487. doi: 10.1021/ci500127c. [http://dx.doi.org/10.1021/ci500127c]. [PMID: 24793143]. [DOI] [PubMed] [Google Scholar]
- 469.Zlatovic M.V., Sukalovic V., Kostic-Rajacic S.V., Andric D.B., Roglic G.M. Influence of N-1 substituent properties on binding affinities of arylpiperazines to the binding site of 5-HT1A receptor. J. Serb. Chem. Soc. 2006;71(11):1125–1135. [http://dx.doi.org/10. 2298/JSC0611125Z]. [Google Scholar]
- 470.Penjisević J., Sukalović V., Andrić D., Kostić-Rajacić S., Soskić V., Roglić G. 1-cinnamyl-4-(2-methoxyphenyl)piperazines: synthesis, binding properties, and docking to dopamine (D(2)) and serotonin (5-HT(1A)) receptors. Arch. Pharm. (Weinheim) 2007;340(9):456–465. doi: 10.1002/ardp.200700062. [http://dx.doi.org/10.1002/ardp.200700062]. [PMID: 17763374]. [DOI] [PubMed] [Google Scholar]
- 471.Andrić D., Roglić G., Sukalović V., Soskić V., Kostić-Rajacić S. Synthesis, binding properties and receptor docking of 4-halo-6-[2-(4-arylpiperazin-1-yl)ethyl]-1H-benzimidazoles, mixed ligands of D2 and 5-HT1A receptors. Eur. J. Med. Chem. 2008;43(8):1696–1705. doi: 10.1016/j.ejmech.2007.09.027. [http://dx.doi.org/10.1016/j.ejmech.2007.09.027]. [PMID: 18006194]. [DOI] [PubMed] [Google Scholar]
- 472.Hindle S.A., Rarey M., Buning C., Lengaue T. Flexible docking under pharmacophore type constraints. J. Comput. Aided Mol. Des. 2002;16(2):129–149. doi: 10.1023/a:1016399411208. [http://dx.doi.org/10.1023/A:1016399411208]. [PMID: 12188022]. [DOI] [PubMed] [Google Scholar]
- 473.Nowak M., Kołaczkowski M., Pawłowski M., Bojarski A.J. Homology modeling of the serotonin 5-HT1A receptor using automated docking of bioactive compounds with defined geometry. J. Med. Chem. 2006;49(1):205–214. doi: 10.1021/jm050826h. [http://dx.doi.org/10.1021/ jm050826h]. [PMID: 16392805]. [DOI] [PubMed] [Google Scholar]
- 474.Pessoa-Mahana H., Recabarren-Gajardo G., Temer J.F., Zapata-Torres G., Pessoa-Mahana C.D., Barría C.S., Araya-Maturana R. Synthesis, docking studies and biological evaluation of benzo[b] thiophen-2-yl-3-(4-arylpiperazin-1-yl)-propan-1-one derivatives on 5-HT1A serotonin receptors. Molecules. 2012;17(2):1388–1407. doi: 10.3390/molecules17021388. [http://dx.doi.org/10.3390/molecules17021388]. [PMID: 22306829]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Dilly S., Scuvée-Moreau J., Wouters J., Liégeois J-F. The 5-HT(1A) agonism potential of substituted piperazine-ethyl-amide derivatives is conserved in the hexyl homologues: molecular modeling and pharmacological evaluation. J. Chem. Inf. Model. 2011;51(11):2961–2966. doi: 10.1021/ci200313r. [http://dx.doi.org/10.1021/ci200313r]. [PMID: 21957888]. [DOI] [PubMed] [Google Scholar]
- 476.Pessoa-Mahana H., Núñez C.U., Araya-Maturana R., Barría C.S., Zapata-Torres G., Pessoa-Mahana C.D., Iturriaga-Vasquez P., Mella-Raipán J., Reyes-Parada M., Celis-Barros C. Synthesis, 5-hydroxytryptamine1A receptor affinity and docking studies of 3-[3-(4-aryl-1-piperazinyl)-propyl]-1H-indole derivatives. Chem. Pharm. Bull. (Tokyo) 2012;60(5):632–638. doi: 10.1248/cpb.60.632. [http://dx.doi.org/10. 1248/cpb.60.632]. [PMID: 22689401]. [DOI] [PubMed] [Google Scholar]
- 477.McMartin C., Bohacek R.S. QXP: powerful, rapid computer algorithms for structure-based drug design. J. Comput. Aided Mol. Des. 1997;11(4):333–344. doi: 10.1023/a:1007907728892. [http://dx.doi.org/10.1023/A:1007907728892]. [PMID: 9334900]. [DOI] [PubMed] [Google Scholar]
- 478.Brea J., Rodrigo J., Carrieri A., Sanz F., Cadavid M.I., Enguix M.J., Villazón M., Mengod G., Caro Y., Masaguer C.F., Raviña E., Centeno N.B., Carotti A., Loza M.I. New serotonin 5-HT(2A), 5-HT(2B), and 5-HT(2C) receptor antagonists: synthesis, pharmacology, 3D-QSAR, and molecular modeling of (aminoalkyl)benzo and heterocycloalkanones. J. Med. Chem. 2002;45(1):54–71. doi: 10.1021/jm011014y. [http:// dx.doi.org/10.1021/jm011014y]. [PMID: 11754579]. [DOI] [PubMed] [Google Scholar]
- 479.Ahmed K., Dubey B., Shrivastava B., Sharma P., Nadeem S. Anxiolytic effects of newly synthesized derivatives in mice and molecular docking studies as serotonin 5HT2A receptor inhibitor. Pharm. Lett. 2015;7(6):93–101. [Google Scholar]
- 480.Du Xiong Z-J. P.; Li, B.; Zhen, X.-C.; Fu, U. Discovery of a Novel 5-HT2A Inhibitor by Pharmacophore-based Virtual Screening. Chem. Res. Chin. Univ. 2011;27:655–660. [Google Scholar]
- 481.Córdova-Sintjago T., Sakhuja R., Kondabolu K., Canal C.E., Booth R.G. Molecular determinants for ligand binding at serotonin 5-HT2A and 5-HT2C GPCRs: Experimental affinity results analyzed by molecular modeling and ligand docking studies. Int. J. Quantum Chem. 2012;112(24):3807–3814. doi: 10.1002/qua.24237. [http://dx.doi.org/10.1002/qua. 24237]. [PMID: 23913978]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Gandhimathi A., Sowdhamini R. Molecular modelling of human 5-hydroxytryptamine receptor (5-HT2A) and virtual screening studies towards the identification of agonist and antagonist molecules. J. Biomol. Struct. Dyn. 2016;34(5):952–970. doi: 10.1080/07391102.2015.1062802. [http://dx.doi. org/10.1080/07391102.2015.1062802]. [PMID: 26327576]. [DOI] [PubMed] [Google Scholar]
- 483.Sencanski M., Sukalovic V., Shakib K., Soskic V., Dosen-Micovic L., Kostic-Rajacic S. Molecular modeling of 5HT2A receptor - arylpiperazine ligands interactions. Chem. Biol. Drug Des. 2014;83(4):462–471. doi: 10.1111/cbdd.12261. [http://dx.doi.org/10.1111/cbdd.12261]. [PMID: 24772489]. [DOI] [PubMed] [Google Scholar]
- 484.Pecic S., Makkar P., Chaudhary S., Reddy B.V., Navarro H.A., Harding W.W. Affinity of aporphines for the human 5-HT2A receptor: insights from homology modeling and molecular docking studies. Bioorg. Med. Chem. 2010;18(15):5562–5575. doi: 10.1016/j.bmc.2010.06.043. [http://dx.doi. org/10.1016/j.bmc.2010.06.043]. [PMID: 20621490]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Bali A., Sen U., Peshin T. Synthesis, docking and pharmacological evaluation of novel indole based potential atypical antipsychotics. Eur. J. Med. Chem. 2014;74:477–490. doi: 10.1016/j.ejmech.2013.09.020. [http://dx.doi.org/10. 1016/j.ejmech.2013.09.020]. [PMID: 24495776]. [DOI] [PubMed] [Google Scholar]
- 486.Munusamy V., Yap B.K., Buckle M.J., Doughty S.W., Chung L.Y. Structure-based identification of aporphines with selective 5-HT(2A) receptor-binding activity. Chem. Biol. Drug Des. 2013;81(2):250–256. doi: 10.1111/cbdd.12069. [http://dx.doi.org/10.1111/cbdd.12069]. [PMID: 23039820]. [DOI] [PubMed] [Google Scholar]
- 487.Ahmad A., Nagarajan S., Doddareddy M.R., Cho Y-S., Pae A-N. Binding mode prediction of 5-hydroxytryptamine 2C receptor ligands by homology modeling and molecular docking analysis. Bull. Korean Chem. Soc. 2011;32(6):2008–2014. [http://dx.doi. org/10.5012/bkcs.2011.32.6.2008]. [Google Scholar]
- 488.Lu C., Jin F., Li C., Li W., Liu G., Tang Y. Insights into binding modes of 5-HT2c receptor antagonists with ligand-based and receptor-based methods. J. Mol. Model. 2011;17(10):2513–2523. doi: 10.1007/s00894-010-0936-9. [http://dx.doi.org/10.1007/s00894-010-0936-9]. [PMID: 21203788]. [DOI] [PubMed] [Google Scholar]
- 489.Cavasotto C.N., Phatak S.S. Homology modeling in drug discovery: current trends and applications. Drug Discov. Today. 2009;14(13-14):676–683. doi: 10.1016/j.drudis.2009.04.006. [http://dx.doi.org/10.1016/j.drudis.2009.04. 006]. [PMID: 19422931]. [DOI] [PubMed] [Google Scholar]
- 490.Bacilieri M., Moro S. Ligand-based drug design methodologies in drug discovery process: an overview. Curr. Drug Discov. Technol. 2006;3(3):155–165. doi: 10.2174/157016306780136781. [http://dx.doi.org/10.2174/157016306780136781]. [PMID: 17311561]. [DOI] [PubMed] [Google Scholar]
- 491.Yang S.Y. Pharmacophore modeling and applications in drug discovery: challenges and recent advances. Drug Discov. Today. 2010;15(11-12):444–450. doi: 10.1016/j.drudis.2010.03.013. [http://dx.doi.org/10.1016/j.drudis.2010. 03.013]. [PMID: 20362693]. [DOI] [PubMed] [Google Scholar]
- 492.Dror O., Shulman-Peleg A., Nussinov R., Wolfson H.J. Predicting molecular interactions in silico: I. A guide to pharmacophore identification and its applications to drug design. Curr. Med. Chem. 2004;11(1):71–90. doi: 10.2174/0929867043456287. [http://dx.doi.org/10.2174/0929867043456287]. [PMID: 14754427]. [DOI] [PubMed] [Google Scholar]
- 493.Winkler D.A. The role of quantitative structure--activity relationships (QSAR) in biomolecular discovery. Brief. Bioinform. 2002;3(1):73–86. doi: 10.1093/bib/3.1.73. [http://dx.doi.org/10.1093/bib/3.1.73]. [PMID: 12002226]. [DOI] [PubMed] [Google Scholar]
- 494.Perkins R., Fang H., Tong W., Welsh W.J. Quantitative structure-activity relationship methods: perspectives on drug discovery and toxicology. Environ. Toxicol. Chem. 2003;22(8):1666–1679. doi: 10.1897/01-171. [http://dx.doi.org/10.1897/01-171]. [PMID: 12924569]. [DOI] [PubMed] [Google Scholar]
- 495.Dudek A.Z., Arodz T., Gálvez J. Computational methods in developing quantitative structure-activity relationships (QSAR): a review. Comb. Chem. High Throughput Screen. 2006;9(3):213–228. doi: 10.2174/138620706776055539. [http://dx.doi.org/10.2174/138620706776055539]. [PMID: 16533155]. [DOI] [PubMed] [Google Scholar]
- 496.Boulesteix A.L., Strimmer K. Partial least squares: a versatile tool for the analysis of high-dimensional genomic data. Brief. Bioinform. 2007;8(1):32–44. doi: 10.1093/bib/bbl016. [http://dx.doi.org/10.1093/bib/bbl016]. [PMID: 16772269]. [DOI] [PubMed] [Google Scholar]
- 497.Alexopoulos E.C. Introduction to multivariate regression analysis. Hippokratia. 2010;14(Suppl. 1):23–28. [PMID: 21487487]. [PMC free article] [PubMed] [Google Scholar]
- 498.Speck-Planche A., Kleandrova V.V., Luan F., Cordeiro M.N.D.S. Multi-target inhibitors for proteins associated with Alzheimer: in silico discovery using fragment-based descriptors. Curr. Alzheimer Res. 2013;10(2):117–124. doi: 10.2174/1567205011310020001. [http://dx.doi.org/10.2174/ 1567205011310020001]. [PMID: 22515494]. [DOI] [PubMed] [Google Scholar]
- 499.Speck-Planche A., Kleandrova V.V. QSAR and molecular docking techniques for the discovery of potent monoamine oxidase B inhibitors: computer-aided generation of new rasagiline bioisosteres. Curr. Top. Med. Chem. 2012;12(16):1734–1747. [http://dx. doi.org/10.2174/1568026611209061734]. [PMID: 23030609]. [PubMed] [Google Scholar]
- 500.Chapelle O., Vapnik V., Bousquet O., Mukherjee S. Choosing Multiple Parameters for Support Vector Machines. Mach. Learn. 2002;46(1):131–159. [http://dx.doi.org/10.1023/A: 1012450327387]. [Google Scholar]
- 501.Roy K., Kar S., Das R.N. Statistical Methods in QSAR/QSPR. A Primer on QSAR/QSPR Modeling: Fundamental Concepts. Cham: Springer International Publishing; 2015. pp. 37–59. [Google Scholar]
- 502.Cramer R.D., Patterson D.E., Bunce J.D. Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J. Am. Chem. Soc. 1988;110(18):5959–5967. doi: 10.1021/ja00226a005. [http://dx.doi.org/10.1021/ja00226a005]. [PMID: 22148765]. [DOI] [PubMed] [Google Scholar]
- 503.Kubinyi H. 2008. [Google Scholar]
- 504.Ghemtio L., Zhang Y., Xhaard H. CoMFA/CoMSIA and pharmacophore modelling as powerful tools for efficient virtual screening: Application to anti-leishmanial betulin derivatives. Virtual Screen; 2012. pp. 55–82. [Google Scholar]
- 505.Klebe G., Abraham U., Mietzner T. Molecular similarity indices in a comparative analysis (CoMSIA) of drug molecules to correlate and predict their biological activity. J. Med. Chem. 1994;37(24):4130–4146. doi: 10.1021/jm00050a010. [http://dx.doi.org/10.1021/jm00050a010]. [PMID: 7990113]. [DOI] [PubMed] [Google Scholar]
- 506.Zhu N., Liang L., Stevens C.L.K. A CoMFA Study of Dopamine D2 Receptor Agonists and X-Ray Crystal Structure of Quinelorane Dihydrochloride Dihydrate, R(−)-Apomorphine Hydrochloride and R(−)-N-n-Propylnorapomorphine Hydrochloride. Struct. Chem. 2004;15(6):553–565. [http://dx.doi.org/10.1007/s11224-004-0730-3]. [Google Scholar]
- 507.Molecular Operating Environment (MOE) 2011. [Google Scholar]
- 508.Modi G., Sharma H., Kharkar P.S., Dutta A.K. Understanding the Structural Requirements of Hybrid (S)-6-((2-(4-Phenylpiperazin-1-yl)ethyl)(propyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol and its Analogs as D2/D3 Receptor Ligands: A Three-Dimensional Quantitative Structure-Activity Relationship (3D QSAR) Investigation. MedChemComm. 2014;5(9):1384–1399. doi: 10.1039/C4MD00159A. [http://dx.doi.org/10.1039/C4MD00159A]. [PMID: 25221669]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Molecular Operating Environment (MOE) 2005. [Google Scholar]
- 510.Malo M., Brive L., Luthman K., Svensson P. Selective pharmacophore models of dopamine D(1) and D(2) full agonists based on extended pharmacophore features. ChemMedChem. 2010;5(2):232–246. doi: 10.1002/cmdc.200900398. [http://dx.doi.org/10.1002/cmdc.200900398]. [PMID: 20077461]. [DOI] [PubMed] [Google Scholar]
- 511.Cha M.Y., Lee I.Y., Cha J.H., Choi K.I., Cho Y.S., Koh H.Y., Pae A.N. QSAR studies on piperazinylalkylisoxazole analogues selectively acting on dopamine D3 receptor by HQSAR and CoMFA. Bioorg. Med. Chem. 2003;11(7):1293–1298. doi: 10.1016/s0968-0896(02)00617-x. [http://dx.doi.org/10. 1016/S0968-0896(02)00617-X]. [PMID: 12628656]. [DOI] [PubMed] [Google Scholar]
- 512.Wang Q., Mach R.H., Luedtke R.R., Reichert D.E. Subtype selectivity of dopamine receptor ligands: insights from structure and ligand-based methods. J. Chem. Inf. Model. 2010;50(11):1970–1985. doi: 10.1021/ci1002747. [http://dx.doi.org/10.1021/ci1002747]. [PMID: 20936866]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Moro S., van Rhee A.M., Sanders L.H., Jacobson K.A. Flavonoid derivatives as adenosine receptor antagonists: a comparison of the hypothetical receptor binding site based on a comparative molecular field analysis model. J. Med. Chem. 1998;41(1):46–52. doi: 10.1021/jm970446z. [http://dx.doi.org/10.1021/jm970446z]. [PMID: 9438021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Mauri A., Consonni V., Pavan M., Todeschini R. DRAGON software: An easy approach to molecular descriptor calculations. MATCH Commun. Math. Comput. Chem. 2006;56:237–248. [Google Scholar]
- 515.Wolber G., Langer T. LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J. Chem. Inf. Model. 2005;45(1):160–169. doi: 10.1021/ci049885e. [http://dx. doi.org/10.1021/ci049885e]. [PMID: 15667141]. [DOI] [PubMed] [Google Scholar]
- 516.Dixon S.L., Smondyrev A.M., Rao S.N. PHASE: a novel approach to pharmacophore modeling and 3D database searching. Chem. Biol. Drug Des. 2006;67(5):370–372. doi: 10.1111/j.1747-0285.2006.00384.x. [http://dx.doi.org/ 10.1111/j.1747-0285.2006.00384.x]. [PMID: 16784462]. [DOI] [PubMed] [Google Scholar]
- 517.Sprague P.W., Hoffmann R. 1997. [Google Scholar]
- 518.Parenti M.D., Fioravanzo E., Mabilia M., Gallo G., Ciacci A. Induced fit and pharmacophore generation approach applied to A2A adenosine receptor antagonists. ARKIVOC. 2006;8:74–82. [Google Scholar]
- 519.Wei J., Wang S., Gao S., Dai X., Gao Q. 3D-pharmacophore models for selective A2A and A2B adenosine receptor antagonists. J. Chem. Inf. Model. 2007;47(2):613–625. doi: 10.1021/ci600410m. [http://dx.doi.org/10. 1021/ci600410m]. [PMID: 17330954]. [DOI] [PubMed] [Google Scholar]
- 520.Xu Z., Cheng F., Da C., Liu G., Tang Y. Pharmacophore modeling of human adenosine receptor A(2A) antagonists. J. Mol. Model. 2010;16(12):1867–1876. doi: 10.1007/s00894-010-0690-z. [http://dx.doi.org/10.1007/ s00894-010-0690-z]. [PMID: 20224910]. [DOI] [PubMed] [Google Scholar]
- 521.Bacilieri M., Ciancetta A., Paoletta S., Federico S., Cosconati S., Cacciari B., Taliani S., Da Settimo F., Novellino E., Klotz K.N., Spalluto G., Moro S. Revisiting a receptor-based pharmacophore hypothesis for human A(2A) adenosine receptor antagonists. J. Chem. Inf. Model. 2013;53(7):1620–1637. doi: 10.1021/ci300615u. [http://dx.doi. org/10.1021/ci300615u]. [PMID: 23705857]. [DOI] [PubMed] [Google Scholar]
- 522.Baroni M., Cruciani G., Sciabola S., Perruccio F., Mason J.S. A common reference framework for analyzing/comparing proteins and ligands. Fingerprints for Ligands and Proteins (FLAP): theory and application. J. Chem. Inf. Model. 2007;47(2):279–294. doi: 10.1021/ci600253e. [http://dx.doi.org/10.1021/ci600253e]. [PMID: 17381166]. [DOI] [PubMed] [Google Scholar]
- 523.Sirci F., Goracci L., Rodríguez D., van Muijlwijk-Koezen J., Gutiérrez-de-Terán H., Mannhold R. Ligand-, structure- and pharmacophore-based molecular fingerprints: a case study on adenosine A(1), A (2A), A (2B), and A (3) receptor antagonists. J. Comput. Aided Mol. Des. 2012;26(11):1247–1266. doi: 10.1007/s10822-012-9612-8. [http://dx. doi.org/10.1007/s10822-012-9612-8]. [PMID: 23065321]. [DOI] [PubMed] [Google Scholar]
- 524.Bhattacharjee A.K., Gordon J.A., Marek E., Campbell A., Gordon R.K. 3D-QSAR studies of 2,2-diphenylpropionates to aid discovery of novel potent muscarinic antagonists. Bioorg. Med. Chem. 2009;17(11):3999–4012. doi: 10.1016/j.bmc.2009.04.001. [http://dx.doi.org/10.1016/j.bmc. 2009.04.001]. [PMID: 19409797]. [DOI] [PubMed] [Google Scholar]
- 525.Bhandare R.R., Gao R., Canney D.J., Kharkar P.S. Novel γ-butyrolactone derivatives as muscarinic receptor antagonists: Pharmacophore elucidation and docking anal-yses. In: Ramasami P., Gupta B.M., Jhaumeer L.S., Li Kam Wah H., editors. Crystallizing Ideas – The Role of Chemistry. Cham: Springer International Publishing; 2016. pp. 155–179. [http://dx.doi.org/10.1007/978-3-319-31759-5_11] [Google Scholar]
- 526.Bhattacharjee A.K., Pomponio J.W., Evans S.A., Pervitsky D., Gordon R.K. Discovery of subtype selective muscarinic receptor antagonists as alternatives to atropine using in silico pharmacophore modeling and virtual screening methods. Bioorg. Med. Chem. 2013;21(9):2651–2662. doi: 10.1016/j.bmc.2013.01.072. [http://dx.doi.org/10.1016/j.bmc. 2013.01.072]. [PMID: 23523385]. [DOI] [PubMed] [Google Scholar]
- 527.Bessis A.S., Jullian N., Coudert E., Pin J.P., Acher F. Extended glutamate activates metabotropic receptor types 1, 2 and 4: selective features at mGluR4 binding site. Neuropharmacology. 1999;38(10):1543–1551. doi: 10.1016/s0028-3908(99)00096-9. [http://dx.doi.org/10.1016/S0028-3908(99)00096-9]. [PMID: 10530816]. [DOI] [PubMed] [Google Scholar]
- 528.Accelrys Software Inc . 2013. [Google Scholar]
- 529.Molecular Operating Environment (MOE) 2004. [Google Scholar]
- 530.Joshi U.J., Tikhele S.H., Shah F. 2D QSAR of arylpiperazines as 5-HT1A receptor agonists. Indian J. Pharm. Sci. 2007;69(6):800–804. [http://dx.doi.org/10.4103/0250-474X.39437]. [Google Scholar]
- 531.Gaillard P., Carrupt P-A., Testa B., Schambel P. Binding of arylpiperazines, (aryloxy)propanolamines, and tetrahydropyridylindoles to the 5-HT1A receptor: contribution of the molecular lipophilicity potential to three-dimensional quantitative structure-affinity relationship models. J. Med. Chem. 1996;39(1):126–134. doi: 10.1021/jm950410b. [http://dx.doi.org/10.1021/jm950410b]. [PMID: 8568799]. [DOI] [PubMed] [Google Scholar]
- 532.Agarwal A., Pearson P.P., Taylor E.W., Li H.B., Dahlgren T., Herslöf M., Yang Y., Lambert G., Nelson D.L., Regan J.W. Three-dimensional quantitative structure-activity relationships of 5-HT receptor binding data for tetrahydropyridinylindole derivatives: a comparison of the Hansch and CoMFA methods. J. Med. Chem. 1993;36(25):4006–4014. doi: 10.1021/jm00077a003. [http://dx.doi.org/10.1021/jm00077a003]. [PMID: 8258822]. [DOI] [PubMed] [Google Scholar]
- 533.López-Rodríguez M.L., Rosado M.L., Benhamú B., Morcillo M.J., Fernández E., Schaper K-J. Synthesis and structure-activity relationships of a new model of arylpiperazines. 2. Three-dimensional quantitative structure-activity relationships of hydantoin-phenylpiperazine derivatives with affinity for 5-HT1A and α 1 receptors. A comparison of CoMFA models. J. Med. Chem. 1997;40(11):1648–1656. doi: 10.1021/jm960744g. [http://dx.doi.org/10.1021/jm960744g]. [PMID: 9171874]. [DOI] [PubMed] [Google Scholar]
- 534.Patel Y., Gillet V.J., Bravi G., Leach A.R. A comparison of the pharmacophore identification programs: Catalyst, DISCO and GASP. J. Comput. Aided Mol. Des. 2002;16(8-9):653–681. doi: 10.1023/a:1021954728347. [http://dx.doi.org/10.1023/A:1021954728347]. [PMID: 12602956]. [DOI] [PubMed] [Google Scholar]
- 535.Borosy A., Morvay M., Mátyus P. 3D QSAR analysis of novel 5-HT1A receptor ligands. Chemom. Intell. Lab. Syst. 1999;47(2):239–252. [http://dx.doi.org/10.1016/S0169-7439(98)00213-5]. [Google Scholar]
- 536.Richmond N.J., Abrams C.A., Wolohan P.R., Abrahamian E., Willett P., Clark R.D. GALAHAD: 1. pharmacophore identification by hypermolecular alignment of ligands in 3D. J. Comput. Aided Mol. Des. 2006;20(9):567–587. doi: 10.1007/s10822-006-9082-y. [http://dx.doi.org/10.1007/ s10822-006-9082-y]. [PMID: 17051338]. [DOI] [PubMed] [Google Scholar]
- 537.Shepphird J.K., Clark R.D. A marriage made in torsional space: using GALAHAD models to drive pharmacophore multiplet searches. J. Comput. Aided Mol. Des. 2006;20(12):763–771. doi: 10.1007/s10822-006-9070-2. [http://dx.doi.org/10.1007/s10822-006-9070-2]. [PMID: 17016746]. [DOI] [PubMed] [Google Scholar]
- 538.Andrade C.H., Salum L.B., Pasqualoto K.F.M., Ferreira E.I., Andricopulo A.D. Three-dimensional quantitative structure-activity relationships for a large series of potent antitubercular agents. Lett. Drug Des. Discov. 2008;5(6):377–387. [http://dx.doi. org/10.2174/157018008785777289]. [Google Scholar]
- 539.Weber K.C., Salum L.B., Honório K.M., Andricopulo A.D., da Silva A.B. Pharmacophore-based 3D QSAR studies on a series of high affinity 5-HT1A receptor ligands. Eur. J. Med. Chem. 2010;45(4):1508–1514. doi: 10.1016/j.ejmech.2009.12.059. [http://dx.doi.org/10.1016/j.ejmech.2009.12.059]. [PMID: 20133028]. [DOI] [PubMed] [Google Scholar]
- 540.Lepailleur A., Bureau R., Paillet-Loilier M., Fabis F., Saettel N., Lemaître S., Dauphin F., Lesnard A., Lancelot J-Ch., Rault S. Molecular modeling studies focused on 5-HT7 versus 5-HT1A selectivity. Discovery of novel phenylpyrrole derivatives with high affinity for 5-HT7 receptors. J. Chem. Inf. Model. 2005;45(4):1075–1081. doi: 10.1021/ci050045p. [http://dx.doi.org/10.1021/ci050045p]. [PMID: 16045303]. [DOI] [PubMed] [Google Scholar]
- 541.Handzlik J., Szymańska E., Nędza K., Kubacka M., Siwek A., Mogilski S., Handzlik J., Filipek B., Kieć-Kononowicz K. Pharmacophore models based studies on the affinity and selectivity toward 5-HT1A with reference to α1-adrenergic receptors among arylpiperazine derivatives of phenytoin. Bioorg. Med. Chem. 2011;19(3):1349–1360. doi: 10.1016/j.bmc.2010.11.051. [http://dx.doi.org/10.1016/j.bmc.2010.11.051]. [PMID: 21232965]. [DOI] [PubMed] [Google Scholar]
- 542.Accelrys Software Inc . 2013. [Google Scholar]
- 543.Ngo T., Nicholas T.J., Chen J., Finch A.M., Griffith R. 5-HT1A receptor pharmacophores to screen for off-target activity of α1-adrenoceptor antagonists. J. Comput. Aided Mol. Des. 2013;27(4):305–319. doi: 10.1007/s10822-013-9647-5. [http://dx.doi.org/10.1007/s10822-013-9647-5]. [PMID: 23625023]. [DOI] [PubMed] [Google Scholar]
- 544.Goodford P.J. A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J. Med. Chem. 1985;28(7):849–857. doi: 10.1021/jm00145a002. [http://dx.doi.org/ 10.1021/jm00145a002]. [PMID: 3892003]. [DOI] [PubMed] [Google Scholar]
- 545.Accelrys Software Inc . 2013. [Google Scholar]
- 546.Dezi C., Brea J., Alvarado M., Raviña E., Masaguer C.F., Loza M.I., Sanz F., Pastor M. Multistructure 3D-QSAR studies on a series of conformationally constrained butyrophenones docked into a new homology model of the 5-HT2A receptor. J. Med. Chem. 2007;50(14):3242–3255. doi: 10.1021/jm070277a. [http://dx.doi.org/10.1021/jm070277a]. [PMID: 17579386]. [DOI] [PubMed] [Google Scholar]
- 547.Valkova I., Zlatkov A., Nedza K., Doytchinova I. Synthesis, 5-HT1A and 5-HT2A receptor affinity and QSAR study of 1-benzhydryl-piperazine derivatives with xanthine moiety at N4. Med. Chem. Res. 2012;21(4):477–486. [http://dx.doi.org/10.1007/ s00044-011-9555-y]. [Google Scholar]
- 548.Choudhary M., Pilania P., Sharma B.K. QSAR rationales for the 5-HT2A receptor antagonistic activity of 2-alkyl-4-aryl-pyrimidine fused heterocycles. Res. J. Pharm. Biol. Chem. Sci. 2015;6(2):326–338. [Google Scholar]
- 549.Maciejewska D., Zołek T., Herold F. CoMFA methodology in structure-activity analysis of hexahydro- and octahydropyrido[1,2-c]pyrimidine derivatives based on affinity towards 5-HT1A, 5-HT2A and α1-adrenergic receptors. J. Mol. Graph. Model. 2006;25(3):353–362. doi: 10.1016/j.jmgm.2006.02.002. [http://dx.doi.org/10.1016/j.jmgm.2006.02.002]. [PMID: 16542863]. [DOI] [PubMed] [Google Scholar]
- 550.Avram S., Duda-Seiman D., Borcan F., Wolschann P. QSAR-CoMSIA applied to antipsychotic drugs with their dopamine D2 and serotonine 5HT2A membrane receptors. J. Serb. Chem. Soc. 2011;76(2):263–281. [http://dx.doi.org/10.2298/JSC100806022A]. [Google Scholar]
- 551.Molecular Operating Environment (MOE) 2007. [Google Scholar]
- 552.Awadallah F.M. Synthesis, Pharmacophore modeling, and biological evaluation of novel 5H-thiazolo [3,2-a] pyrimidin-5-one derivatives as 5-HT2A receptor antagonists. Sci. Pharm. 2008;76:415–438. [http://dx.doi.org/10.3797/scipharm.0804-20]. [Google Scholar]
- 553.Molecular Operating Environment (MOE) 2008. [Google Scholar]
- 554.El-Kerdawy M.M., El-Bendary E.R., Abdel-Aziz A.A., El-wasseef D.R., El-Aziz N.I. Synthesis and pharmacological evaluation of novel fused thiophene derivatives as 5-HT2A receptor antagonists: molecular modeling study. Eur. J. Med. Chem. 2010;45(5):1805–1820. doi: 10.1016/j.ejmech.2010.01.013. [http://dx.doi.org/10.1016/j.ejmech.2010.01.013]. [PMID: 20149493]. [DOI] [PubMed] [Google Scholar]
- 555.Hayat F., Viswanath A.N., Pae A.N., Rhim H., Park W.K., Choo H.Y. Synthesis and biological evaluation of 4-nitroindole derivatives as 5-HT2A receptor antagonists. Bioorg. Med. Chem. 2015;23(6):1313–1320. doi: 10.1016/j.bmc.2015.01.032. [http://dx.doi.org/10.1016/j.bmc.2015.01. 032]. [PMID: 25684421]. [DOI] [PubMed] [Google Scholar]
- 556.Choudhary M., Sharma B.K. QSAR rationales for the isoindolone derivatives as 5-HT2C receptor antagonists. Res. J. Pharm. Biol. Chem. Sci. 2015;6(3):1725–1736. [Google Scholar]
- 557.Gómez-Jeria J.S. 2014.
- 558.Gómez-Jeria J.S. An empirical way to correct some drawbacks of mulliken population analysis. J. Chil. Chem. Soc. 2009;54:482–485. [http://dx.doi.org/10.4067/S0717-97072009000400036]. [Google Scholar]
- 559.Gómez-Jeria J.S., Robles-Navarro A. A quantum chemical study of the relationships between electronic structure and cloned rat 5-HT2C receptor binding affinity in N-benzylphenethylamines. Res. J. Pharm. Biol. Chem. Sci. 2015;6(3):1358–1373. [Google Scholar]
- 560.Accelrys Software Inc . 2004. [Google Scholar]
- 561.Micheli F., Pasquarello A., Tedesco G., Hamprecht D., Bonanomi G., Checchia A., Jaxa-Chamiec A., Damiani F., Davalli S., Donati D., Gallotti C., Petrone M., Rinaldi M., Riley G., Terreni S., Wood M. Diaryl substituted pyrrolidinones and pyrrolones as 5-HT2C inhibitors: synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2006;16(15):3906–3912. doi: 10.1016/j.bmcl.2006.05.034. [http://dx.doi. org/10.1016/j.bmcl.2006.05.034]. [PMID: 16730983]. [DOI] [PubMed] [Google Scholar]
- 562.Tautermann C.S. GPCR structures in drug design, emerging opportunities with new structures. Bioorg. Med. Chem. Lett. 2014;24(17):4073–4079. doi: 10.1016/j.bmcl.2014.07.009. [http://dx.doi.org/10.1016/j.bmcl.2014.07.009]. [PMID: 25086683]. [DOI] [PubMed] [Google Scholar]
- 563.Lenselink E.B., Beuming T., Sherman W., van Vlijmen H.W., IJzerman A.P. Selecting an optimal number of binding site waters to improve virtual screening enrichments against the adenosine A2A receptor. J. Chem. Inf. Model. 2014;54(6):1737–1746. doi: 10.1021/ci5000455. [http://dx. doi.org/10.1021/ci5000455]. [PMID: 24835542]. [DOI] [PubMed] [Google Scholar]
- 564.Tautermann C.S., Seeliger D., Kriegl J.M. What can we learn from molecular dynamics simulations for GPCR drug design? Comput. Struct. Biotechnol. J. 2014;13:111–121. doi: 10.1016/j.csbj.2014.12.002. [http://dx.doi. org/10.1016/j.csbj.2014.12.002]. [PMID: 25709761]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Michino M., Abola E., Brooks C.L., III, Dixon J.S., Moult J., Stevens R.C. Community-wide assessment of GPCR structure modelling and ligand docking: GPCR Dock 2008. Nat. Rev. Drug Discov. 2009;8(6):455–463. doi: 10.1038/nrd2877. [http://dx.doi.org/10.1038/nrd2877]. [PMID: 19461661]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Piotto S., Di Biasi L., Fino R., Parisi R., Sessa L., Concilio S. Yada: a novel tool for molecular docking calculations. J. Comput. Aided Mol. Des. 2016;30(9):753–759. doi: 10.1007/s10822-016-9953-9. [http://dx.doi.org/10.1007/ s10822-016-9953-9]. [PMID: 27565794]. [DOI] [PubMed] [Google Scholar]
- 567.Lie M.A., Thomsen R., Pedersen C.N., Schiøtt B., Christensen M.H. Molecular docking with ligand attached water molecules. J. Chem. Inf. Model. 2011;51(4):909–917. doi: 10.1021/ci100510m. [http://dx.doi.org/10. 1021/ci100510m]. [PMID: 21452852]. [DOI] [PubMed] [Google Scholar]
- 568.Kim M., Cho A.E. Incorporating QM and solvation into docking for applications to GPCR targets. Phys. Chem. Chem. Phys. 2016;18(40):28281–28289. doi: 10.1039/c6cp04742d. [http://dx.doi.org/10.1039/C6CP04742D]. [PMID: 27711562]. [DOI] [PubMed] [Google Scholar]
- 569.Amadasi A., Spyrakis F., Cozzini P., Abraham D.J., Kellogg G.E., Mozzarelli A. Mapping the energetics of water-protein and water-ligand interactions with the “natural” HINT forcefield: predictive tools for characterizing the roles of water in biomolecules. J. Mol. Biol. 2006;358(1):289–309. doi: 10.1016/j.jmb.2006.01.053. [http://dx.doi.org/10.1016/ j.jmb.2006.01.053]. [PMID: 16497327]. [DOI] [PubMed] [Google Scholar]
- 570.Verdonk M.L., Cole J.C., Taylor R. SuperStar: a knowledge-based approach for identifying interaction sites in proteins. J. Mol. Biol. 1999;289(4):1093–1108. doi: 10.1006/jmbi.1999.2809. [http://dx.doi.org/10.1006/jmbi. 1999.2809]. [PMID: 10369784]. [DOI] [PubMed] [Google Scholar]
- 571.Michel J., Tirado-Rives J., Jorgensen W.L. Prediction of the water content in protein binding sites. J. Phys. Chem. B. 2009;113(40):13337–13346. doi: 10.1021/jp9047456. [http://dx.doi.org/10.1021/jp9047456]. [PMID: 19754086]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Abel R., Young T., Farid R., Berne B.J., Friesner R.A. Role of the active-site solvent in the thermodynamics of factor Xa ligand binding. J. Am. Chem. Soc. 2008;130(9):2817–2831. doi: 10.1021/ja0771033. [http://dx. doi.org/10.1021/ja0771033]. [PMID: 18266362]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Young T., Abel R., Kim B., Berne B.J., Friesner R.A. Motifs for molecular recognition exploiting hydrophobic enclosure in protein-ligand binding. Proc. Natl. Acad. Sci. USA. 2007;104(3):808–813. doi: 10.1073/pnas.0610202104. [http://dx.doi.org/10.1073/pnas.0610202104]. [PMID: 17204562]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Zheng M., Li Y., Xiong B., Jiang H., Shen J. Water PMF for predicting the properties of water molecules in protein binding site. J. Comput. Chem. 2013;34(7):583–592. doi: 10.1002/jcc.23170. [http://dx.doi.org/10. 1002/jcc.23170]. [PMID: 23114863]. [DOI] [PubMed] [Google Scholar]
- 575.Isberg V., Mordalski S., Munk C., Rataj K., Harpsøe K., Hauser A.S., Vroling B., Bojarski A.J., Vriend G., Gloriam D.E. GPCRdb: an information system for G protein-coupled receptors. Nucleic Acids Res. 2016;44(D1):D356–D364. doi: 10.1093/nar/gkv1178. [http://dx.doi. org/10.1093/nar/gkv1178]. [PMID: 26582914]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Golovin A., Henrick K. MSDmotif: exploring protein sites and motifs. BMC Bioinformatics. 2008;9:312. doi: 10.1186/1471-2105-9-312. https://bmcbioinformatics. biomedcentral.com/articles/10.1186/1471-2105-9-312 [http://dx. doi.org/10.1186/1471-2105-9-312]. [PMID: 18637174]. [DOI] [PMC free article] [PubMed] [Google Scholar]