Abstract
Background: The computational development of human monoamine oxidase (MAO) inhibitors led to advancement in drug design and the treatment of many neurodegenerative diseases and neuropsychiatric disorders. The computational development of human monoamine oxidase (MAO) inhibitors led to advancement in drug design and the treatment of many neurodegenerative diseases and neuropsychiatric disorders. Different natural heterocyclic structures are reported to display selective MAO inhibitory activity by preclinical and in-silico modeling.
Objective: Currently, the major interest is devoted to the study of natural based therapeutic agents from the different categories. Therefore, we presenting the review to critically discuss and outline the recent advances in our knowledge on the importance of natural and natural based ligand-MAO in-silico methods for novel MAO inhibitors.
Discussion: Several natural and related synthetic heterocyclic compounds such as coumarins, β-carboline, piperine, naphthoquinone, morpholine, caffeine, amphetamine moreover flavonoids, chalcones, xanthones, curcumin are discussed for their MAO inhibitory profile along with molecular docking and quantitative structure-activity relationship studies.
Conclusion: It is clear that, by this computational drug design approach, more particular, reversible and potent compounds can be proposed as MAO inhibitors by exact changes on the fundamental framework.
Keywords: 3D-QSAR, in-silico docking, monoamine oxidase, CoMFA, natural monoamine oxidase inhibitors
1. INTRODUCTION
The pharmaceutical ventures are confronting the enormous difficulties at each progression of the drug discovery and advancement. Computer technology-based drug development is essential factor for the R&D growth and productivity. Moreover, an extensive variety of molecular structure library from natural and synthetic origin accessible for in-silico design of novel drugs. Generally, lead molecules prepared form the herbal sources are more organically friendly due to their co-evolution along with protein target sites in natural systems [1-5].
Monoamine oxidases (MAOs; EC 1.4.3.4) are flavin-adenosine dinucleotide (FAD) containing mitochondrial membrane enzymes which oxidatively deaminate the xenobiotic and biogenic amines. The two isoforms of MAO are designated as MAO-A and MAO-B, which are recognized by their distinct substrate and inhibitor selectivity. MAO-B preferentially catalyzes the oxidation of benzylamine and phenylethylamine and is inhibited by selegiline, whereas MAO-A preferentially catalyzes the oxidation of serotonin and norepinephrine and is inhibited by clorgyline. Dopamine, tyramine and tryptamine are common substrates for both MAO isoforms [6-8]. Monoamine oxidases play a critical physiological role in the metabolism of neurotransmitters, selective and specific MAO inhibitors (MAOIs) were utilized for the treatment of depression and neurodegenerative syndromes, such as Alzheimer's disease and Parkinson's disease. The regulated degradation of these monoamines ensures the proper working of neurotransmission at synaptic level which is critical for the control of intellectual and other brain functions in the central nervous system (CNS). In the last years, many researches have been published for MAOIs by disclosure of the 3D-crystallographic structures of both MAO isoforms [9-11]. Human MAO inhibitors are currently used as anti-anxiety and antidepressants agents, whereas human MAO-B inhibitors are used alone or in combination with the therapy of Parkinson’s disease and Alzheimer’s disease [12-15]. Many natural and related synthetic derivatives like coumarins, β-carboline, piperine, naphthoquinone, morpholine, caffeine, amphetamine moreover flavonoids, chalcones, xanthones, curcumin showed appreciable activity towards the inhibition of MAO as well as neuroprotection [16-18]. Several natural MAO inhibitors along with targeted disorders that utilized molecular docking are listed below (Table 1).
Table 1.
Natural MAO inhibitors along with targeted disorders utilized molecular docking.
| Sr. No. | Enzyme | Inhibitors | Targeted Disease | Software Used | References |
|---|---|---|---|---|---|
| 1. | MAO-A | Eugenol and derivatives | Antidepressant | AutoDock 3.0 | Tao et al., 2005 [19] |
| 2. | MAO-B | 8-(3-Chlorostrylyl)caffeine | Neuroprotective | AutoDock 3.0.5 | Toprakci et al., 2005 [20] |
| 3. | MAO-A and MAO-B | Fucoxanthin | Parkinson’s disease | Autodock 4.0 | Jung et al., 2015 [21] |
| 4. | MAO-A and MAO-B | Eckol and dieckol | Parkinson’s disease | Autodock | Jung et al., 2017 [22] |
| 5. | MAO-A | Decursin Wogonin |
Neuroprotective | Autodock 4.2 | Lee et al., 2017 [23] |
| 6. | MAO-B | Homoisoflavonoid Mannich base derivatives | Alzheimer’s disease | Discovery Studio 2.5 | Li et al., 2017 [24] |
| 7. | MAO-A and MAO-B | Thioxanthone | Alzheimer’s disease | Discovery Studio 2.5 | Luo et al., 2017 [25] |
| 8. | MAO-A and MAO-B | Flavone Derivatives | Neurodegenerative disorders | Schrodinger | Jia et al., 2017 [26] |
2. Nataural products and related derivatives as MAO inhibitors
2.1. Chalcones
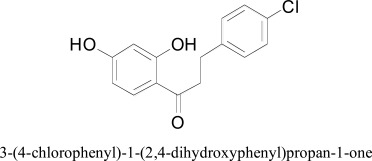
Chalcones (trans-1,3-diphenyl-2-propen-1-ones) are the biogenetic precursors of all known flavonoids and are abundant in edible plants. Chemically, they consist of open-chain flavonoids in which the two aromatic rings are joined by a three-carbon α,β-unsaturated carbonyl system [27, 28]. Natural two prenylated chalcones Xanthoangelol and 4-Hydroxyderricin from Angelica keiskei K were extracted by activity-guided fractionation and evaluated for their MAO inhibitory activity. Xanthoangelol is a nonselective MAO inhibitor which exhibited IC50 values as 43.4 μM, and 43.9 μM for MAO-A and MAO-B inhibition, respectively [29]. Other synthetic chalcone derivatives have been tested for human monoamine oxidases A and B (hMAO-A and hMAO-B) inhibition. Compounds consisting chlorine and hydroxyl or methoxyl substituents at the 4′-position of the chalcone moiety, showed hMAO-B selective activity in the micro- and nanomolar ranges. Molecular docking experiments were performed to visualize the enzyme-inhibitor interaction and to explain the selectivity of the potent compounds for hMAO-B. In all docking poses the hydroxy-phenyl ring was always situated near the flavin cofactor and hydrogen bonds stabilized the recognition of the ligands. The most active compound 3-(4-chlorophenyl)-1-(2,4-dihydroxyphenyl)-propan-1-one showed an H-π interaction with chlorophenyl ring and two hydrogen bonds with FAD and Tyr435, respectively [30].
3-(4-chlorophenyl)-1-(2,4-dihydroxyphenyl)propan-1-one
Carpéné et al., [31] reported another docking study of natural chalcone containing herbs for a comparison of different herbal components with the aid of molecular dynamics simulation. In docking methodology, resveratrol (cis, trans) was taken as a ligand and docking score was computed by Schrodinger’s software suite for both MAO isoforms. Most active resveratrol exhibited non-covalent docking interactions through XP and SP procedure. All the natural ligands interacted within substrate pocket near flavin moiety which was considered as dynamic site distant from MAO-A (about 4 Å) and MAO-B (about 5.5 Å), respectively. Important docking outcomes are represented in the following Table 2.
Table 2.
Molecular docking profile of natural ligands for MAO isoforms.
| Docking Specifications Evaluated | MAO-A | MAO-B |
|---|---|---|
| Docking Score (trough standard precision method | -8.400 and -5.726 | -9.411 to -6.739 |
| Docking Score (through standard precision method | -12.128 and -5.683 | -11.122 to -6.987. |
| π- π staking | Tyr444, Phe208, Tyr407, and Phe352 | Tyr398, Tyr435and Tyr326 |
| H-Bond | Asn181, Tyr197, and Tyr444 | Cys 172, Tyr188, and Tyr435 |
Some other important points observed from docking results are seen as follows:
i Resveratrol interacted noncovalently within the active site of MAO isoforms.
ii The Pro102 containing backbone oxygen atom appeared as H-bond acceptor for resveratrol.
iii The oxidation of tyramine was hindered by resveratrol that is an MAO-dependent oxidation.
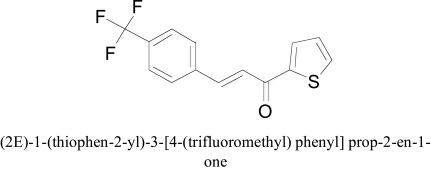
Design of the fluorinated thienylchalcones as potent MAO-B inhibitor was done by Mathew and co-workers. Among all the fluorinated thienylchalcones the, (2E)-1-(thiophen-2-yl)-3-[4-(trifluoromethyl) phenyl] prop-2-en-1-one was found as the most potent MAO-B inhibitor with Ki value for MAO-B of 0.90 ± 0.05 µM and a 5-fold selectivity towards MAO-B over the MAO-A isoform. The titled compounds showed reversible MAO-B inhibition; moreover, kinetic enzyme analysis determined the competitive mode of MAO-B binding. Molecular docking was used to validate the correct bounding and structure prediction. Autodock docking program was used for all docking calculations. Visual inspection of the docking pose of (2E)-1-(thiophen-2-yl)-3-[4-(trifluoromethyl) phenyl] prop-2-en-1-one the thiophene ring was found to be positioned between the phenolic side chains of Tyr435 and Tyr398 residues of MAO-B and it is anchoring from the surface of FAD. Moreover, the two π-π stacking interactions were also established with FAD and Tyr 435 [32].
(2E)-1-(thiophen-2-yl)-3-[4-(trifluoromethyl) phenyl] prop-2-en-1-one
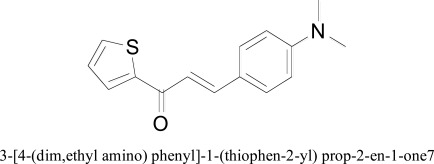
More recently, thiophene based chalcones were designed and evaluated for the MAO inhibition and ADMET studies were also carried out. The synthetic derivatives of chalcones were synthesized by the Claisen-Schmidt condensation reaction between 2-acetyl thiophene with differently substituted aromatic aldehydes. Initially, for docking studied the protein preparation was carried out using Protein Preparation Wizard of Maestro -8.4 (Schrodinger LLC) that assigned the protonation state and charges, finally, energy minimization was done through the OPLS2005 force field. The energy of synthetic chalcone based ligands was minimized through Molecular Orbital Package (MOPAC) suit until the RMS gradient attained a value smaller than 0.0001 kcal/mol Å. Docking calculations were performed by using AutoDock tools. From the docking studies, it was observed that thiophene chalcone derivatives posses comparatively more recognition potential towards MAO-B than MAO-A.
3-[4-(dim,ethyl amino) phenyl]-1-(thiophen-2-yl) prop-2-en-1-one7
Most active compound 3-[4-(dim,ethyl amino) phenyl]-1-(thiophen-2-yl) prop-2-en-1-one was found to be situated into the aromatic cage framed by Tyr 188, Tyr 326 Tyr398, Tyr435 and the FAD aromatic ring of MAO-B. Furthermore, binding interactions were stabilized through the π-π stacking between Tyr 188 residue and dimethyl amino substituted phenyl unit of thiophene chalcone. The synthetic scheme of substitution on thiophene based chalcones was designed to determine the impact of substituent to the phenyl system. Different electron donating groups such as dimethyl amino, methoxy, hydroxyl and electron withdrawing group such as chloro and nitro were selected as substituent on the phenyl system of thiophene based chalcones. It was observed that the substitution of phenyl ring at para position by electron releasing groups such as methoxyl, dimethyl amino and lipophilic halogen, contributed good binding interaction towards MAO-B [33].
2.2. Flavonoids
Epidemiological proof proposes that utilization of flavonoids (normally by means of foods grown from the ground) is related with a diminished danger of depression and mood alteration. The information gathered by latest surveys demonstrates that lifetime utilization of natural herbs (and subsequently higher flavonoid utilization) ensures a lower rate of depression in later life [34-37]. Comparative researches have been reported by different researchers to investigate the impacts of flavonoid-rich intercessions on mood [38-40].
To verify the effects of flavonoids on MAO enzyme inhibition a study was performed on powerful antioxidant known as quercetin. For the research work the active flavonoid quercetin isolated from Hypericum hircinum leaves was evaluated for MAO-A and B inhibitory activities by in vitro tests. A model of quercetin was designed by applying the graphical user interface by MacroModel (Maestro GUI), Schrodinger. The Monte Carlo reproduction through 1000 emphasis step was utilized for randomization of every single rotatable bond. Vitality minimization was accomplished for every confirmation by utilizing the forcefield AMBER joined particle and the GB/SA water verifiable model of salvation was actualized by a root-mean-square deviation in the nuclear directions. Calculation of the association energy of all substituents prior and then afterward full unwinding was computed by the MOLINE strategy. Molecular interactions indicated a great relationship with trial restraint information and affirmed the particular MAO-An acknowledgment in both configurational gatherings computed after MC docking experiments and full energy minimization. It was presumed that quercetin fits preferred in the hMAO-A over in the hMAO-B restricting pocket due to foundation of most extreme π-π connection and intermolecular hydrogen bonds. Chimenti et al. [41].
One comparative study to identify the effects of xanthones and quercetin on MAO A and MAO B by docking experiments has been performed by Zhang and co-workers. In earlier studies, we have found that quercetin (a flavonoid) was when simulated against hMAO B (with drug score of -61.5) was found to have maximum interaction with the targeted protein. The OH groups attached at 5th or 7th positions of quercetin were found to be involved in binding. The similar case was seen with the OH group of xanthones positioned at 1 and 3 in, along with the interaction of OHs at other positions. In case of quercetin, the ring B of catechol was found to be important in increasing enzyme inhibition and significantly contributes for the binding. The xanthones were found to be different in this case, which does not require catechol for the improved binding interaction with protein targets. Because of the ring B in flavonoids which is rotatable through C2-C10 bond, so the flavonoids alter their conformational changes to tie inside the dynamic locales of various target proteins, upheld by the superimposed quercetin compliances in the coupling site of MAO B [42].
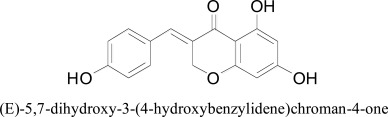
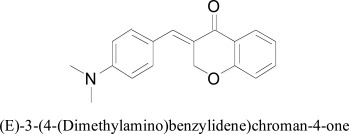
After studying the interaction pattern of flavonoids, a study was conducted on homoisoflavonoids and its derivatives. The synthesized compounds along with homoisoflavanoid were tested in vitro as inhibitors of human monoamine oxidase isoforms A and B. The docking experiments provided the insight into inhibitory action of homoisoflavonoids concerning both isoforms of human MAO. For this situation, the best postures of the (E)- 5,7-dihydroxy-3-(4-hydroxybenzylidene)chroman-4-one and (E)- 3-(4-(dimethylamino)benzylidene) chroman-4-one chromone rings were arranged close to the FAD in hMAO-A. The ligand-protein interactions were giving an impression of being indistinguishable in all derivatives. The distinction in hMAO-A coupling association was because of the nearness of a phenolic OH in the previous subordinate that framed a hydrogen bond with FAD N5 atom. Besides, (E)- 5,7-dihydroxy-3-(4-hydroxybenzylidene)chroman-4-one, close by the flavin cofactor, built up a selective bond with Tyr69. On the other hand the (E)- 3-(4-(dsimethylamino)ben-zylidene)chroman-4-one was observed to be included hydrophobic collaborations with Tyr444 and Asn181 [43].
(E)-5,7-dihydroxy-3-(4-hydroxybenzylidene)chroman-4-one
(E)-3-(4-(Dimethylamino)benzylidene)chroman-4-one
While determining the potential of flavonoids, a new class known as thioflavones was examined for its monoamine oxidase inhibitory potential by docking simulation. Docking studies were done by the Glide to perceive both (R)- 2j and (S)- 2j enantiomers of 2-(4-fluorophenyl)- 7-methyl-2,3 dihydrochromen-4-one and to assess the binding modes of flavonoid based structures within both isoforms of hMAO. The atomic displaying examination has indicated great connections to the exploratory information, demonstrated the conformational adaptability of both 2-(4-fluorophenyl)- 7-methyl-2,3 dihydrochromen-4-one enantiomers to fit inside both hMAO isomeric dynamic destinations with trademark liking. Most dynamic compound 2-(4-fluorophenyl)- 7-methyl-2,3-dihydrochromen-4-one that demonstrated the intense inhibitory action as racemate, was likewise the best inhibitor in the two enantiomeric frames [44].
Recently one researcher investigated hMAO inhibitory potencies of four Sideritis flavonoids, salvigenin, isoscutellarein 7-O-[6”'-O-acetyl-β-D-allopyranosyl-(1→2)]-6”-O acetyl-β-D-glucopyranoside, isoscutellarein 7-O-[6”'-O-acetyl-β-D-allopyranosyl-(1→2)]- β-D-glucopyranoside and xanthomicrol using recombinant hMAO isoenzymes. In the molecular docking tests, salvigenin was observed to be the most powerful hMAO-An inhibitor shaped different electrostatic and van der Waals connections with the coupling site of the hMAO-An and benzene ring of the coumarine moiety of the salvigenin formed two π-π collaborations with TYR444 and TYR407 deposits coating the depression. Besides, the xanthomicrol was observed to be the most particular inhibitor for hMAO-A shaped five hydrogen bonds with the side chains amino acids of the of the dynamic site hMAO-An isoform (between the -OH and GLY66, -OH and ASN181, -OH and LYS305 and -OCH3 and TYR444). The coumarine ring of xanthomicrol was discovered sandwiched between the TYR407and TYR444 deposits, which framed two π-π connections with TYR407 and constituted the hydrophobic pen of the dynamic site. Keeping in mind the end goal to break down more pertinent sub-atomic associations between the regular ligands and hMAO the stable edifices were subjected to 2-dimensional investigations [45].
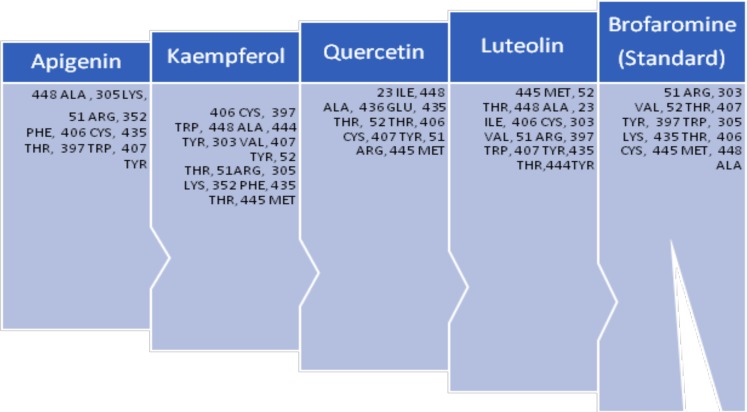
In a subsequent in silico docking study, inhibitory affinity of apigenin, kaempferol, quercetin and luteolin was analyzed with the aid of Auto Dock tools. The binding free energy and (ΔG) and inhibition constants (Ki) of the docked ligands were computed through the Lamarckian Genetic Algorithm (LGA) of AutoDock. Interaction with various residues is given in Fig. (1).
Fig. (1).
Various interacting residues of naturally occurring flavanoids.
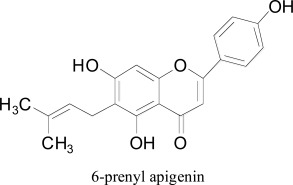
From Fig. (2) it was observed that luteolin formed docking interactions almost similar to that of brofaromine (standard) with amino acid residues 51 ARG, 303 VAL, 407 THR, 445 MET, 406 CYS, 448 ALA, 397 TRP and 435 THR. However, apigenin, kaempferol, quercetin and luteolin commonly showed binding interactions with 448 ALA, 406 CYS, 435 THR, 407 TYR amino acid residues. This indicated that the mode of interaction of four flavanoids was nearly similar, except some of the distinct bonds. In a later work, Beula et al. [46] isolated 6-prenyl apigenin from the seeds of Achyranthes aspera and performed molecular docking to have more insight into the binding modes of it towards monoamine oxidase-A enzyme. Docking experiments were performed using AutoDock, indicated the 6-prenyl apigenin as promising compound for MAO-A inhibition by having a docking score of -8.06 and calculated inhibition constant of about 1.23 µM. To better understand the structural role of the isolated flavonoid the structural was into three different fragments of the flavones skeleton, a distal side chain located at the 6th position and phenolic group at the second position of the nucleus. Interestingly, the π electrons of the phenolic group were sandwiched between phenolic side chains of TYR407 and TYR 444, constituted the ‘aromatic cage’ of the hydrophobic pocket of the enzyme. Moreover, one another π-π stacking interaction was appeared between flavone skeleton and TRP 441 residue in the lining of hMAO-A binding site.
New MAO-B inhibitors were identified from the ethanolic extract of Psoralea corylifolia seeds which is a medicinal plant well-known for its antiaging properties. Two one of a kind prenylflavanones, genistein (GST) and bavachinin (BNN) demonstrated the most astounding power and selectivity of MAO-B. Atomic docking assessed the coupling affinity for both flavonoids was performed. Zarmouh and associates additionally investigated flavanone bavachinin (BNN) and its simple bavachin (BVN) from the seeds of Psoralea corylifolia L. for their human MAO-A and MAO-B restraint in their earlier studies 2015 [47]. Sub-atomic docking was utilized to approve the right binding and mechanistic insight into docked parameters appeared in Table 3. The docking postures were approved through the bound ligands in the precious stone structures of human MAO-A-harmine mind boggling and human MAOB-2-(2-benzofuranyl)- 2-imidazoline complex [48].
Table 3.
MAO inhibition by natural components of psoralea corylifolia evaluated by docking.
| Sr. No. |
Natural
Compounds |
MAO-A active Site | MAO-B active Site | General Bonds |
Selectivity
Toward Single MAO Isoform |
|||
|---|---|---|---|---|---|---|---|---|
| Docking Score | H-bond Formed | Docking Score | H-bond Formed | H-bond Formed | Bonded Amino Acid Residues | |||
| 1. | Bavachin | -8.69 | H2O-726 | -3.95 | 0 | ... | ... | NA |
| 2. | Bavachinin | -1.54 | 0 | -6.82 | 2 | HO ... HN OH ... O |
THR: 201: A THR: 201: A |
B |
| 3. | Safinamide | -0.25 | 0 | -6.12 | 3 | NH ... O NH ... O NH ... O |
THR: 201: A GLU: 84: A PRO: 102: A |
B |
The same group further studied the isoflavone genistein (GST) and its analog daidzein (DZ) as potent MAO-A and MAO-B inhibitors. Molecular docking studies of GST and DZ were carried out within the active site cavity of MAO isoforms. Studies on MAO-A the chromone parts of two common ligands were situated within the minimal passage cavity close to the to the flavin cofactor (FAD), though their hydroxy-phenyl part was situated to the hydrophobic active site entrance surfaces. Both isoflavones showed indistinguishable and crossed orientation with the standard. In a best-coordinated pose to the standard was given by a slight draw of GST toward a hydrophilic zone at its C5-OH group. In case of hMAO-B, both analogs chromone moieties were situated altogether in the hydrophobic district of the dynamic site cavity (substrate-restricting area). Due to their hydroxyphenyl moiety near the passage depression, both analogs were found far from flavin cofactor or its encompassing tyrosine rsidues. Results inferred that GST C4'- OH group moiety set up more hydrogen bonds far from the hydrophobic sites than DZ. This system could build the greater reversibility in view of not influencing the flavin structure and having reversible hydrophobic and H-bond associations. The docking contemplated perceptions are given in Table 4 [49].
Table 4.
Molecular docking studies of DZ and GST within 3D crystallographic structure of monoamine oxidase-A and –B isoforms.
| Sr. No | Name of the Lead | MAO-A | MAO-B | Bond Length Distance Å | Amino Acid | ||
|---|---|---|---|---|---|---|---|
| Docking Score |
Predicted
H-bond |
Docking Score | Predicted H-bond | ||||
| 1. | Daidzein (DZ) | −6.8 | 0 | −12.8 | 1 (O … HN) | 2.32 | THR: 201: A |
| 2. | Genistein (GST) | −7.3 | 0 | −12.8 | 2 (OH … N) | 2.27 | THR: 201: A |
More recently, Margret and coworkers [50] investigated the MAO inhibitory potential of 26 isolated phytochemicals from the methanolic root extract of Clitoria ternatea. Computational docking was carried out on phyto components such as quercetin (quercetin 3-glucoside, quercetin 3-2 G-rhamnosyl rutinoside, Quercetin 3-rutinoside, quercetin 3-neohesperidoside), delphinidin 3,3,5-triglucoside, kaempferol (kaempferol 3-rutinoside, kaempferol 3-neohesperidoside, kaempferol 3-glucoside), Myricetin 3-glucoside, Myricetin 3-rutinoside and Myricetin 3-neohesperidoside was performed with the target proteins. The best connection between
2.3. Coumarins
Coumarins (cromen-2-ona) are a large family of compounds from both synthetic and natural origin and display numerous biological effects. Due to their structural variability and versatile synthetic methodologies, they occupy an important place in the realm of natural products and synthetic organic chemistry. Until now, numerous biological effects, especially in popular medicine, have been ascribed to this benzo-γ-pyrone nucleus such as anti-inflammatory, antitumor, and antimicrobial activities [51, 52].
Santana and coworkers used MARCH-INSIDE methodology to develop a QSAR for MAO inhibitory activity. Polarizability, refractivity, Molecular electron delocalization, and n-octanol/water partition coefficients were calculated by Markov model for a series of 1406 active/nonactive coumarin derivatives. Among all the evaluated compounds only 15 selected compounds were synthesized and evaluated for vitro MAO-A inhibition. The QSAR model showed 91.8% and 92.8% predictability and global accuracy in training and validation studies. Consequently, the theoretical predictions were compared with the experimental results where the in silico model correctly predicted 13 compounds [53].
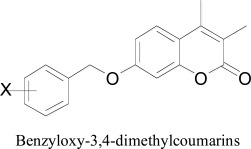
Quantitative Structure Activity Relationships (QSAR) has been developed to a large series of coumarin derivatives (71 compounds) for monoamine oxidase A and B (MAO-A and MAO-B) inhibitory activity. Good statistical results (q2 ˭ 0.72, r2 ˭ 0.86), were obtained by the QSAR study of substituted Benzyloxy-3,4-dimethylcoumarin derivatives acting on MAO-B, showed the significant lipophilic interactions to modulate the MAO inhibition while excluding dependence on any other electronic properties. CoMFA was carried out on two data sets of MAO-A and MAO-B inhibitors. Along with the GOLPE procedure, variable selection criteria, was applied to facilitate the graphical interpretation of results and to improve the predictions of the selected models [54].
Benzyloxy-3,4-dimethylcoumarins
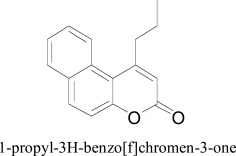
Moreover, a novel classifier by combining 0D, 1D and 2D molecular descriptors along with replacement method as well as linear discriminate analysis and the variable selection method were found to be useful tool for the prediction of hMAO-A and hMAO-B inhibitory potential and to inform about the selectivity toward hMAO-B of new coumarin derivatives. Some chemical features were inferred by interpretation of the models which can be considered important in the hMAO-B selectivity. For instance, the presence of five member rings in the chemical structure decreases the selectivity. The substitution with bulky groups at different positions of the 1-propyl-3H-benzo[f]chromen-3-one molecules helped to determine the steric requirements useful for MAO selectivity. Molecular interaction among Ile-199 in MAO-B and the corresponding Phe-208 in MAO-A with the 5 position of the coumarin ring was modeled through geometry optimization [55].
1-propyl-3H-benzo[f]chromen-3-one
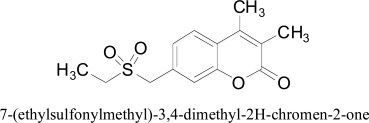
Veselovsky and coworkers molded substrate-binding region of monoamine oxidase A (MAO A) dynamic site by utilizing reversible competitive inhibitor interaction with three-dimensional protein structures. MAO A inhibitory activity was anticipated by using the method of spatial superposition of specific competitive reversible MAO inhibitors of the 3D-QSAR + CoMFA model. Compound 7-(ethyl-sulfonylmethyl)-3,4-dimethyl-2H-chromen-2-one shown an in-vitro IC50 estimation of 8.9 nM, while using 3D-QSAR + CoMFA models allowed prediction of favorable and unfavorable regions for substituents and to design the scheme for the active sites of MAO A and B [56].
7-(ethylsulfonylmethyl)-3,4-dimethyl-2H-chromen-2-one
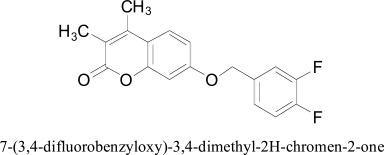
Another three-dimensional quantitative structure-activity relationship study using CoMFA and GOLPE procedures was carried out by Carrieri and coworkers. About 130 coumarin, tetrazole, oxadiazinone, and oxadiazolone derivatives were selected and studied. The lipophilic and steric fields, alone and in combination, given the valuable models with good predictions (q2 = 0.61). Flexible docking approach was used to validate the models provided by CoMFA against 3D crystallographic structure of MAO-B enzyme with the help of GRID and QXP computational programs. The docking experiments revealed that all derivatives interacted with MAO-B protein in an identical manner, irrespective of the docked heterocyclic structure [57].
7-(3,4-difluorobenzyloxy)-3,4-dimethyl-2H-chromen-2-one
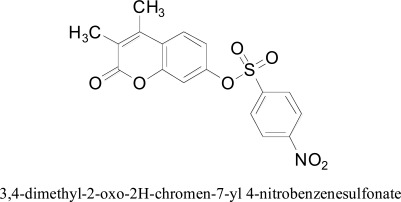
3,4-dimethyl-2-oxo-2H-chromen-7-yl 4-nitrobenzenesulfonate
One more QSAR study on simple coumarin analogues was carried out using MOE (version 2008.10) including instance walk and path counts, 2D-autocorrelations, connectivity indices, pure topological descriptors, and information indices. Linear Discriminant Analysis was applied to identify classification models while the robustness of a QSAR model was calculated through Y-randomization test. This study provided models to derivate different chemical characteristics which can be considered as crucial for the hMAO-B selectivity. It was observed that the presence of five member rings or the [CRX (coumarin carbon with double bonded oxygen) fragment (X: electronegative atom; and R: any functional group linked to carbon]: double bond) on the chemical structure decreases the selectivity [58].
Molecular docking studies on coumarin 3-Arylcoumarins derivatives were carried out by Matos and coworkers in 2011. A series of 6-substituted-3-arylcoumarin derivatives was synthesized and investigated for hMAO-A and h-MAO-B activity along with docking studies. Most of the studied compounds showed a high affinity and selectivity to the hMAO-B isoenzyme, with IC50 values in nanomolar and picomolar ranges. Ten of the 22 described compounds displayed higher MAO-B inhibitory activity and selectivity than selegiline. Coumarin 7 is the most active compound of this series, being 64 times more active than selegiline and also showing the highest hMAO-B specificity. In addition, docking experiments were carried out on hMAO-A and h-MAO-B structures [59].
2.4. Xanthones
Till date, only a few docking studies have been reported on the xanthones (9H-xanthen-9-ones) from the natural plants and synthetic origin although they are of biological and pharmacological interest because of their diverse pharmacological properties. Docking models were developed by Bevan and Harkcom in 2007 to evaluate MAO B inhibiting activity of a series of xanthone analogs with the aim of optimizing this functional group for better inhibition potential [60]. It was noticed that, among all the docked xanthone derivatives, there were no specific and consistent interactions among all of the analogs of the group. However, there were many hydrogen bonds appeared with amino acid residues Tyr 188, Tyr435, Tyr 326, Glu206, and Ile199 some of them also observed with residues Ile198, Cys172, and the flavin cofactor. Their findings revealed none of the specific docking interactions for the strong or weak MAO inhibitory activity. Moreover, some hydrophobic interactions were noticed, especially π-π stacking with Tyr435 and Tyr398 of the aromatic cage and with Tyr326 of MAO-A.
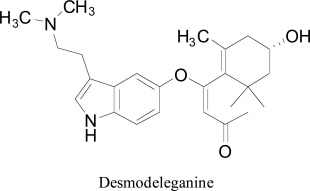
Zhi et al. [61] identified desmodeleganine from the leaves of Desmodiumelegans as promising monoamine oxidase inhibitor exhibited IC50 value of 13.92 ± 1.5μM, when the IC50 value of iproniazid as a standard was 6.5 ± 0.5μM. To gain further insight into the binding mode of desmodeleganine and bufotenin in active site of MAO isoforms, molecular docking calculations were performed using Schrödinger program. Both of titled compounds have shown hydrophobic interaction with the amino acid residues Ile207, Asn181, Phe352, Ile180, Gln215, and Tyr407 in MAO-A. The complexes seem to be stabilized by hydrophobic interactions of 3-hydroxy-β-ionone moiety of desmodeleganine with Ala111, Val210, Met324, Ile325, Phe209, Phe208, Ile335 and Leu337. Moreover, the hydrogen of hydroxyl on C-3′of desmodeleganine established hydrogen bonding with the carbonyl of Ala111. The mode of interaction between the ligands and the binding pocket of MAO-B was also found mainly hydrophobic. Both the titled compounds interacted with Cys172, Ile199, Leu171, Ile198, Phe168, Tyr326 and Gln206 residues of MAO The 3-hydroxy-β-ionone part of desmodeleganine showed hydrophobic interaction with Phe103, Ile316, Leu67, Pro102, Leu164 amino acid residues of MAO-B. Moreover, the hydrogen bonding of desmodeleganine with Pro102, Ile99 and Gln206 amino acid residues of MAO-B was also noticed. The docking analysis of both compound with MAO-A and MAO-B indicated that desmodeleganine with an extra 3-hydroxy-β-ionone group which increased the molecular surface area, volume and hydrophobicity led to the better affinity for both MAO-A and MAO-B than bufotenin.
Desmodeleganine
5. Alkaloids
Alkaloids possess a variety of biological activities, besides their effects on the central nervous system (CNS), as they are documented to interact strongly with receptors in the CNS. Apart from the synthetic analogs, isolated plant alkaloids also posse’s remarkable inhibition potential for MAO isoforms. Several subcategories of alkaloids have been found to inhibit both MAO-A and MAO-B which includes tetrahydroisoquinolines, naphthoquinones, tropanes, isoquinolines, tryptamines, piperidines, indoles, and quinazolines Table 2. Further derivatization of the natural structure of alkaloids reported to exhibiting interesting and effective CNS enzyme inhibitory activity which directly used as therapeutic in various neurological disorders implicated by overexpression of MAO [62, 63]. Various computational studies performed on alkaloids and their interaction with monoamine oxidase enzyme are illustrated below in Table 6.
Table 6.
Major binding interactions of Alkaloids resulted by docking studies.
| Sr. No | Alkaloid compounds | H-Bond interaction | π-π Stacking | Software Utilized | Refs. |
|---|---|---|---|---|---|
| 1. | Morpholine derivatives | Tyr326 | _ | Autodock 4.0 | Lühr et al., (2010) [64] |
| 2. | Piperine | Tyr 188, Gln 206 Cys 172, Tyr 326, Thr 201 and Ileu 199 | _ | Schrödinger | Mu et al., (2012) [73] |
| 3. | Menadione (vitamin K3) | Tyr398 and Tyr435 (for MAO-A) Tyr407 and Tyr444 (for MAO-B) |
_ | AutoDock 4.0 | Cerqueira et al., (2011) [65] |
| 4. | Amphetamine | Tyr197 | Tyr407 | AutoDockVina | Fresqui et al., (2013) [78] |
| 5. | Quinazoline | GLN 74, ILE 207, and TYR 444 SER 209 and GLU 216 | _ | MOE-Dock | Khattab et al., (2015) [81] |
| 6. | Nicotinamide | Tyr 444, Asn181 and Tyr 197 | Tyr407 | AUTODOCKTOOLS (ADT) | Shi et al., (2010) [73] |
| 7. | Caffeine | Tyr444, Tyr435 | _ | Autodock 4.0 | Petzer et al., (2016) [34] |
| 8. | Psychotria alkaloids | WAT-746 | Tyr-407 | GOLD | Son et al., (2013) [29] |
5.1. Morpholine Derivatives
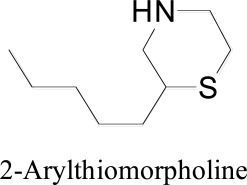
Morpholine and is derivatives were assessed through docking experiments for the monoamine oxidase inhibitory potential by Lühr et al. [64]. They outlined 2-Arylthiomorpholine and 2-arylthiomorpholin-5 one derivatives as non-basic and/or rigid phenylethylamine derivatives. Docking studies were done on both rodent and human MAO-B and MAO-A by utilizing Autodock 4.0 software. Docking revealed that the alkoxy bunches reach out into the passage depression of the catalyst and that the benzyloxy gather possesses a comparable position to the benzyloxy gathering of safinamide. The benzyloxy analogs were found as potent and selective for both rMAO-B and hMAO-B. The aromatic ring participated in a hydrogen boding with Tyr326. The sulfur atom in the heterocycle docked into the hMAO-B catalytic site interacts with Tyr398 and/or Tyr435. The benzyl substituent at the opposite end of the molecule interacted with the primarily aromatic residues of the entrance cavity. The rigid phenylethylamine scaffold was synthesized by attachment of the amine chain in an extended six-membered ring conformation increased MAO-B (but not MAO-A) inhibitory activity relative to the more flexible a methylated derivative.
2-Arylthiomorpholine
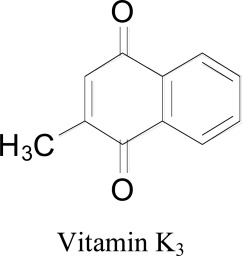
5.2. 1,4-naphthoquinones: vitamin K3 (Menadione)
A docking study on vitamin K3 for its MAO inhibitory activity was reported by Cerqueira and coworkers 2011 in which menadione showed a competitive and reversible inhibition of MAO. The molecular interactions of menadione were different within the both isoforms and specificity for MAO-B was found to be 60-fold higher Ki = 0.4µM than MAO-A Ki =26µM. Molecular docking by AutoDock 4.0, was used to validate the correct bounding and structure prediction [41]. Essential interposed amino acids were found as Tyr407 and Tyr444 (for MAO-A) or Tyr398 and Tyr435 (for MAO-B). Docking studies demonstrated that menadione connects with flavin moiety of the FAD through a hydrogen bond and shown close contacts with Gln206, Tyr326, Phe343, Tyr398, and Tyr435. In menadione-MAO-B docking, the calculated binding free energy was -5.50 kcal/mol. In docking with MAO-A, the free restricting energy was computed as - 5.40 kcal/mol and menadione ties to the flavin moiety through a system of hydrogen bonds with Tyr407, Gln215, Phe352, Tyr69, Leu337, and Tyr444 amino acid residues [65].
5.3. Isoquinoline
To attain a better understanding regarding the importance of natural isoquinoline alkaloid berberine, Hong-Fang Ji and Liang Shen isolated from the Chinese herb Rhizoma coptidis, and investigated for MAO inhibitory activity [66]. By using Surflex-Dock software the molecular docking studies were performed, when empirically derived scoring function was based on the binding affinities of protein and ligand complexes [67]. Hydrophobic interaction between the surface of berberine and neighboring hydrophobic residues is the principal force to establish the ligand-MAO interactions. Moreover, the cationic nature of berberine alkaloid was also found important for the electrostatic interaction with neighboring amino acid residues.
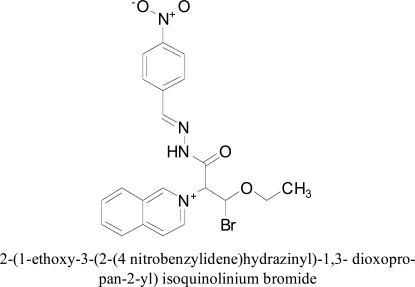
Hassan and coworkers developed N-malonyl-1,2-dihydroisoquinoline compounds as the CNS specific and shelf-stable inhibitors of hMAO using chemical delivery systems (DHIQCDSs). The reason for the shelf stability was due to dihydroisoquinoline consisting carbonyl group close to nitrogen. The in-vitro evaluation of MAO inhibition was competitive for MAO-A and MAO-B isoforms and specifically to hMAO-A. Molecular docking and simulations were performed with Molecular Operating Environment version 2013.08. Molecular docking simulation of compound 2-(1-ethoxy-3-(2-(4 nitrobenzylidene)hydrazinyl)-1,3- dioxopropan-2-yl) isoquinolinium bromide within MAO-A binding pocket formed three hydrogen bonds as with Gly-110 amino acid, carbonyl C=O ester of the compound and hydroxyl group and amide C=O of the side chain of Thr-211 and C=N and Val-93 backbone [68].
A 3-D QSAR study of several isoquinolines, N-methyl-1,2,3 and tetrahydroisoquinolines, N-methyl-1,2-dihydroiso-quinolines, 1,2,3,4-tetrahydroisoquinolines and N-methyliso-quinolinium ions was reported for monoamine oxidases A and B inhibitory potential by Thull and coworkers in 1995. Comparative molecular field analysis (CoMFA) was performed with the QSAR option of SYBYL software. By computing the lipophilic and steric fields of the isoquinolines, quantitative models having significant predictive ability showed point steric, whereas lipophilic field is essential for the polar interactions to modulate MAO-A inhibitory potential [69].
2-(1-ethoxy-3-(2-(4 nitrobenzylidene)hydrazinyl)-1,3- dioxopropan-2-yl) isoquinolinium bromide
5.4. Piperine
In the recent year’s many researchers investigated the biological properties of Piperine, an alkamide exists as an essential secondary metabolite in the fruits of long pepper (Piper longum Linn.), black pepper (Piper nigrum Linn.) and several other Piper species belonging to the plant family Piperaceae [70].
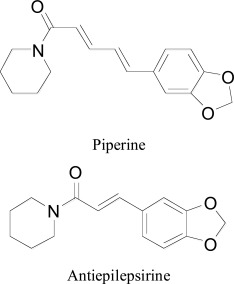
Al-Baghdadi et al. screened the piperine and antiepilepsirine compounds from the plant of both white and black pepper grains, Piper nigrum. The high-throughput screening (HTS) assay showed z-factor of piperine more than 0.8 for both isoforms. Docking studies were executed with MOE 2010 version to further explain the in-vitro results of the piperine and antiepilepsirine based compounds, and to establish the hypothetical binding poses for the compounds within the active site of MAO. Piperine docked mainly via methylenedioxyphenyl ring formed water-bridge with CYS172 and TYR188, with another hydrogen-bond-aromatic ring interaction was observed withTYR397 [71].
Rahman et al. researched and examined some piperine analogs for their capability to inhibit both MAO isoforms utilizing molecular docking through AutoDock programming [72]. From the experimental studies, they observed the estimation of inhibition constants (Ki) and the outcomes were observed to be in greatly related to the atomic docking estimations of Ki. The examination of binding pose analysis revealed the importance of three buried water molecules 726th, 746th and 805th in the active site of MAO-A. Besides, the carbonyl oxygen of the carbonyl amide established a similar interaction with the thiol of Cys 323. The visual inspection of docked piperine into the dynamic site of hMAO-B and extensive hydrophobic associations showed up at both passage and substrate-restricting pits. The principal amino acid residues required in the hydrophobic network were Tyr 435, Tyr 398, Cys172, Gln 206, Tyr 188, Gly 434 along with the aromatic ring of the FAD.
Another docking reported by Mu and coworkers, the binding affinity of the different piperine analogs through FlexX algorithm of SYBYL 7.2. Visual examination of docking pose of compounds within the MAO-B active site showed that the carbonyl oxygen of piperine derivatives established two hydrogen bonds with the amine group on the side chain of TYR60 and SER59. The two conjugated double bonds appeared as embedded in a large hydrophobic pocket formed by Asn181, Phe208, Ile180, Gln215, and Ile345. The results revealed that binding affinity mainly rendered by pyridine nuclei of piperine derivatives framed by Tyr407, Tyr197, Tyr444, and the aromatic ring helped to form π- π stacking interactions to Tyr407 of MAO-A [73].
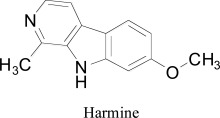
Conversely, in a later work, M. Thenmozhi et al. [74] extracted piperine from the fruit of Piper nigerum and evaluated for MAO-A inhibition. Molecular docking was computed by applying induced fit docking procedure of Schrödinger software [75]. The visual analysis of binding modes demonstrated the two hydrogen bond interactions with the ALA65, TYR68 residues. Another interesting binding mode of piperine was identical to MAO-A co-crystallized harmine as standard for docking studies, showed with TYR69. Moreover, many hydrophobic interactions were observed with amino acid residues as the top-ranked docking poses which included MET445, ILE23, GLY443, THR52, SER24, TYR444, PHE352, THR435, ARG51, LYS305, GLN215, TYR407, GLY67, and ALA 448. The calculated Glide score for the top ranked piperine analogues was observed as -11.5 kcal/mol.
5.5. Nicotinamides
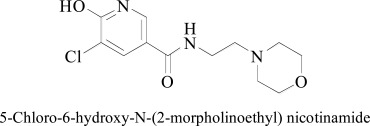
A single docking study was reported on nicotinamide by Shi et al., (2010), they synthesized N-(2-morpholinoethyl) nicotinamide and N-(3 morpholinopropyl) nicotinamide compounds as monoamine oxidase inhibitors [76]. Computational docking was carried out by Autodock tools selectively to most active derivative 5-Chloro-6-hydroxy-N-(2-morpholinoethyl) nicotinamide, exhibited significant MAO-A inhibitory potential (IC50(MAOA) = 0.045µM) and notable selectivity (IC50(MAO-B) = 26µM) [77]. The close inspection of the docking poses of MAO-A revealed that the pyridine ring is very important for binding as ‘aromatic cage’ enclosed by Tyr407, Tyr197, and Tyr444 amino acid residues. The Cl atom of ligand and the hydroxyl group of the side chain of Tyr444, Tyr197 and FAD established van der Waals interactions. Morpholine ring of the most active derivative was found sandwiched between hydrophobic pocket formed by Phe208, Gln215, Ile135, Asn181, and Ile180. The π-π stacking interactions of FAD aromatic ring with Tyr407 were observed in all docking experiments. Three hydrogen bonds were observed with Asn183, Tyr 447 and Tyr 198. In docking study of MAO-B binding poses showed that ‘aromatic cage’ was formulated by Tyr398, Tyr326, Tyr60, Tyr435 and the aromatic ring of the FAD. The stabilization of ligand was rendered by different hydrophobic interactions between the Gln206 and morpholine ring.
5-Chloro-6-hydroxy-N-(2-morpholinoethyl) nicotinamide
5.6. Amphetamines
Fresqui and collaborators assessed the impact of R and S configurations of a series of amphetamine derivatives on of monoamine oxidase A inhibitory movement through QSAR and molecular docking to indicate binding and molecular phenomena [78]. The set of 38 molecular descriptors, consisting steric, electronic, and hydrophobic factors, were computed. The selection of variable was carried out by the correlation coefficients along with ordered predictor selection (OPS) algorithm. Docking studies were performed for the slightest and most dynamic derivatives carried out utilizing AutoDock program. Docking experiments demonstrated the significance of π-π stacking formation with Tyr407 which is in charge of the response. Collaboration incorporates slanted vis-à-vis connection with Tyr444, while fragrant hydrogen-hydrogen communications with Tyr197 are ideal for R rather than S arrangements. Additionally highlighted by investigation of Fierro et al. [79] on amphetamine derivatives led four enantiomerically pure (S)-4-alkylthioamphetamine derivatives as monoamine oxidase (MAO) inhibitors. Molecular docking was performed utilizing Autodock against rMAO-A and hMAO-A in all experiments. On account of both hMAO-An and rMAO-A, enzymes demonstrated hydrogen restricting associations far from the flavin ring and near the carbonyl group of Phe208, The alkylthio bind was observed to be sandwiched amongst Tyr407 and Tyr444, an area that would good for the connections between very polarizable sulfur molecule and aromatic moieties along with hydroxyl groups of the tyrosines.
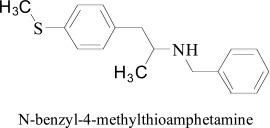
Finally, a series of N-benzyl-4-methylthioamphetamine and naphthylisopropylamine derivatives were tested for their MAO inhibition by Vilches-Herrera et al. [80]. In the docking profile of unsubstituted phenyl amphetamine the π- π interaction was appeared crucial for the MAO inhibition. The outcomes of their study revealed that none of N-benzyl-4-methylthioamphetamine analogue showed significant MAO inhibitory potential as compared with the standard.
N-benzyl-4-methylthioamphetamine
Moreover, it was also noticed that substitution by N-benzyl decreases the MAO inhibition of both naphthylisopropylamine and amphetamine derivatives. Though there are some studies clams that decrease of MAO-A inhibitory activity mainly rendered by small alkyl, alkoxy, methyl substituents to an amino group. Such property of reduction of inhibitory potential could be due to the hindrance of ligand in the path for the active site of the MAO and it is unable to form attractive interactions with important amino acid residues.
5.7. Quinazolines
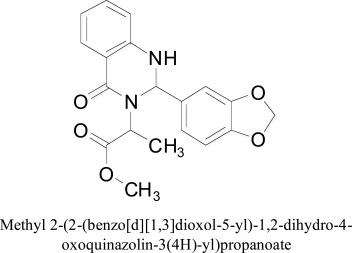
Quinazolines and its derivatives represent one of the important classes of alkaloids, which possess a wide range of CNS activities. To assess its MAO inhibitory effects Khattab and coworkers designed amino acid ester series of quinazoline by microwave irradiation [81]. The mechanism as well as the affinity for MAO was computed through molecular docking experiments utilizing MOE-Dock in the binding site of MAO-A [82]. The docking experiments of derivatives indicated hydrogen holding and hydrophobic connections through the MAO-A dynamic site and give a decent clarification to their intense inhibitory movement. Close examination of restricting stances better clarified the strong inhibitory movement of compounds through hydrogen bond interactions with the SER 209, GLN 74, ILE 207 and TYR 444 and GLU 216 residues of enzymes.
Methyl 2-(2-(benzo[d][1,3]dioxol-5-yl)-1,2-dihydro-4-oxoquinazolin-3(4H)-yl)propanoate
5.8. Caffeine
Caffeine is found in a large number of dietary sources consumed worldwide, i.e., candy bars, soft drinks, coffee, tea and cocoa beverages. There are many research studies published on caffeine scaffold for the MAO inhibitory activity. The literature supports the fact that structural modification by C8 position substitution of caffeine with a variety of moieties yields potential MAO inhibitors Petzer et al. [83]. In 2005 Toprakçí and coworkers reported the docking calculation on caffeine moiety by utilizing AutoDock software. The Lamarckian Genetic Algorithm (LGA) of AutoDockwas applied to compute the estimated free energy of ligand binding (ΔG binding, kcal/mol), the inhibition constant (Ki) for each ligand was calculated. A significant trend of correlation was found between estimated energies of the complexes and the experimental inhibition data [20].
On the basis of literature the (E)-8-styrylcaffeinyl analogs were designed as effective and reversible inhibitors of monoamine oxidase B by Strydom et al., [84]. Molecular docking was applied to validate the correct bounding mode and structure orientation prediction. The inhibitory action (IC50) of the docked compound against hMAO-A was discovered practically like hMAO-B, with estimations of 1.24 and 1.77 M, individually. The best restricting posture appeared by the derivatives with both MAO isoforms were comparative. The inclination of caffine was found toward the flavin ring in the substrate cavity, leaving the benzyloxy part into the passage cavity. Some stable van der Waals contacts also appeared with side chain hydrophobic amino acid at the passage cavity. An intriguing 180° revolution was seen in MAO-A in contrast with its introduction in MAO-B. This promotes both restricting modes which seemed balanced out by H-bonds between the carbonyl oxygen at C-6 of caffeinyl ring and phenolic hydrogen of Tyr435 in MAO-B, and between the carbonyl oxygen at C-2 of caffeinyl ring and phenolic hydrogen of Tyr444 in MAO-A. Their findings revealed that the ability of 8-benzyloxycaffeine analogs to interact with MAO-A binding site mainly depends on the degree of rotation freedom of the benzyloxy side chain at the C-O ether bond.
The same group further studied Strydom et al. [85] another series of 8-aryl and alkyloxy substituted caffeine compounds as more potent and specific MAO-B inhibitor more than earlier studied 8-benzyloxy derivatives. A similar mode of binding was observed in the docking poses in both MAO-A and MAO-B isoforms. Such orientation of caffeine ring was found crucial by the formation of three hydrogen bond in the active site water molecules and the phenolic hydrogen of Tyr-435, whereas a single hydrogen bond was established between the caffeine ring and MAO-A(with Tyr-407). The N1 methyl carbons of caffeine ring interacted nearer to the flavin ring and about parallel to the phenolic side chains of the Tyr-398 and Tyr-435 inside the substrate site of the MAO-B.
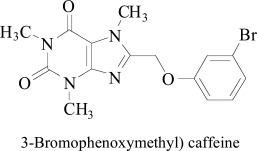
Finally, Okaecwe et al. [86] designed and studied a series of 8-benzyloxycaffeines derivatives as effective and reversible human monoamine oxidase (MAO) inhibitors. The most powerful inhibitor, 8-(3-bromophenoxymethyl) caffeine with IC50 estimated for MAO-A 34.0±31.5 and MAO-B 0.148 ± 0.002 reacted reversibly and intensely with MAO-B. The inhibitor indicated π-π and π-σ associations with Tyr-407 and Phe-208, separately. Hydrogen binding was observed to be shaped within dynamic site water particles. Strangely, bromophenoxymethyl-caffeine bond with the caffeine ring in the closeness of the FAD cofactor while the C8 phenoxymethyl side chain stretched out towards the passage of the MAO-A dynamic site. The most intriguing contrast between the coupling introductions of 8-(3-Bromophenoxy-methyl) caffeine in MAO-A and MAO-B was the collapsed adaptation found in MAO-A, while displaying an expanded compliance in the MAO-B dynamic site.
3-Bromophenoxymethyl) caffeine
Further, biological investigation of the caffeinyl center and substituents of the reference compound (E) 8-(3-chlorostyryl) caffeine prompted 9-deazaxanthines derivatives as MAO-B inhibitor was explored by Rivara et al. [87]. Computational docking experiments were carried out by Glide 5.7 program applying the SP scoring method. Results of the docking study demonstrated that the purine moiety fits amongst Tyr398 and Leu171 on one side and Tyr435 and Gln206 on the other one. This type of binding orientation seems identical to that reported for 8- benzyloxycaffeine analogs by Strydom and coworkers [84]. Derivatives substituted at meta position exhibited lipophilic substitution and had the most elevated MAO-B inhibitory potencies. These points support the effect of electron-withdrawing groups like m-chlorine and m-trifluoromethyl increased inhibitory potencies about 10-fold compared to the 4-fold observed for the 8-styryl-9- deazaxanthine analogs. The best outcomes were gotten for the methoxy and the trifluoromethyl the methoxy and the trifluoromethyl derivatives. Hydrophilic substituents were not found crucial for the interaction or restricting postures. Substitution at the para position with electron withdrawing groups, for example, fluorine delivered the best increment in MAO-B inhibitory intensity in nanomoles (IC50 = 200 nM).
The other report on 8-substituted caffeinyl analogs as MAO-A inhibitors was published by Azam et al. [88]. Molecular docking studies were executed to better explain the in-vitro results and mechanism of the inhibition of C8-substituted caffeinyl analogs to MAO-B. Molecular docking was performed on AutoDock program by appiying Lamarckian genetic algorithm. The most intrusting outcome of the study was that the polar compound caffeine was not accommodated within the active site of MAO-B. However, the C-8 substituted (E)-styryl and 4-phenylbutadien-1- yl derivatives shown significant MAO-B inhibitory activity because the decreases polarity of the compound which calculated through LogP. Visual inspection of the docking pose in MAO-B showed the Ile-199 as a “gate” between the entrance and substrate site. A similar orientation of hydrogen bonds was observed with Gln-206 as that of the safinamide which a native co-crystallized ligand. The results of docking experiments revealed that both cavities reacted for C-8 substituted (E)-styryl and 4-phenylbutadien-1-yl groups on caffeinyl ring that increased the MAO-B inhibitory activity. Moreover, Lipinski’s parameters were also computed that based on a set of parameters for molecular weight, lipophilicity and hydrophobicity, H bond donors and H bond acceptors [89].
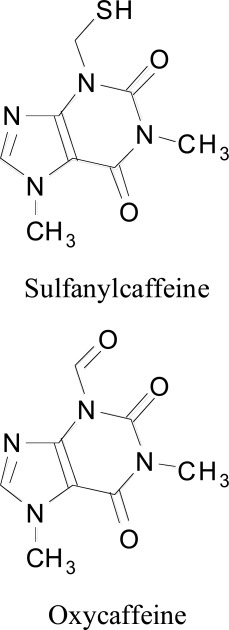
Conversely, another study was performed on a progression of 8-sulfanyl-and 8-aminocaffeine compounds as human monoamine oxidase inhibitors Booysen et al. [90]. The outcome of the study clarified the competitive and reversible inhibition of sulfanylcaffeine analogs for MAO-B hindrance with high action identical to those of the oxycaffeines. In the MAO-B dynamic site, the C8 side chains of the sulfanylcaffeine subordinates stretched out into the hydrophobic passage depression where they are settled by Van der Waals communications. Moreover, halogen substitution on the phenyl ring of the C8 side anchor prompts better Van der Waals and dipole collaborations between the MAO-B entrance depressions. A fascinating point about methoxy substitution on 8-(benzylsulfanyl) caffeine was its diminishing affinity towards both MAO-A and MAO-B inhibiton. These outcomes were found as per the discoveries of a past study, which demonstrated that MAO-B restraint potencies of a progression of benzyloxycaffeine analogs correspond with the electronegativity of substituents on the phenyl ring of the C8 side chain and that electron-pulling back gatherings improve MAO-B hindrance power Strydom et al. [84].
5.9. Indole Alkaloids
5.9.1. Psychotria Alkaloids
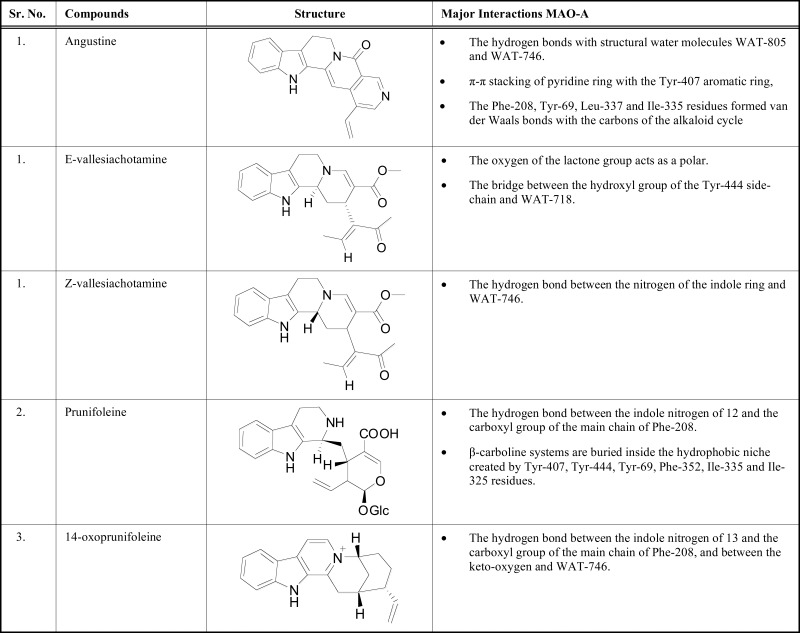
More recently, Psychotria alkaloids, principally monoterpene indoles, have been investigated for their inhibitory activity against central nervous system monoamine oxidase and cholinesterase enzymes. A study by Passos and collaborators tested thirteen Psychotria alkaloids angustine that is a vallesiachotamine lactone, E-vallesiachotamine, pauridianthoside, strictosidinic acid, vincosamide, lyaloside, strictosamide, psychollatine, brachycerine, prunifoleine, 14-oxoprune-foleine and Z-vallesiachotamine for their MAO-A inhibitory activity [91]. Docking simulations were performed on GOLD program CCDC, Cambridge, UK) version 5.0 with the MLP filter approach [92]. All the structural water molecules of MAO-A were taken into consideration because many important interactions were appeared with them (Table 7).
Table 7.
Docking profile of psychotria alkaloids and their major interactions.
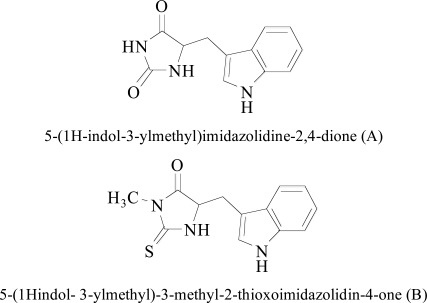
The docking study performed by Klein-Júnior et al. [93]. The three Psychotria indol alkaloids, namely bahienoside A, bufotenine, and desoxycordiofoline and two synthetic analogues 5-(1Hindol- 3-ylmethyl)-3-methyl-2-thioxoimida-zolidin-4-one and 5-(1H-indol-3-ylmethyl)imidazolidine-2,4-dione evaluated in-vitro regarding their interactions with different enzyme systems like AChE, BChE, MAO-A, and MAO-B. Docking experiments were carried out to insight the important interactions of indolyl-hydantoin and indolylmethyl-thiohydantoin compounds with the MAO-A. Interestingly, the indole moiety of both derivatives interacted with MAO-A binding cavity with an identical mode, forming van der Waals links with lipophilic amino acid residues. An important hydrogen bond appeared between methyl-thiohydantoin ring with the atom attached to the (N-5) of FAD cofactor. Moreover, the hydantoin scaffold of one indole compound bound by hydrogen bonds with two structural water molecules in the MAO-A binding pocket. The experimental IC50 value of two indole alkaloids were found as 8.23 μM and 0.07 μM for MAO-A.
5.9.2. β -Carboline Alkaloids
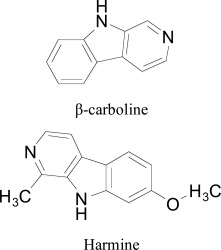
The β-carboline alkaloids are a large group of natural and synthetic indole alkaloids that possess a common tricyclic pyrido [3,4-b]indole ring structure. To evaluate β-carboline
alkaloids as MAO-A inhibitors Reniers et al. [94] used in silico molecular docking tool on a series of β -carboline analogues. The active residues of the cavity were observed as VAL210, PHE208, ASN181, TYR69, ILE335, GLN215, CYS323, LEU337, ILE325, PHE352, TYR444, TYR407, and FAD. Structural water molecules were found essential for binding. Furthermore, pyridine ring acted as a stabilizer to the aromatic cage (TYR407, TYR444, and FAD) and form π- π interaction. The amide group of the GLN215 side chain interacts tightly with harmine by a π- π interaction. Visual inspection of the binding modes showed that the lipophilic pocket formed by CYS323, LEU97, ILE325, VAL93, PHE208, ALA111, and PHE108 is surrounded by methoxy group of harmine which led the authors to introduce more bulky groups at the C7 position. One more finding of the research include O-alkylated compounds with lipophilic groups like cyclohexyl, phenyl and aliphatic chains increase the inhibition of MAO-A compared to harmine. Moreover, the flexible trifluorobutyl side chain showed productive interaction with lipophilic pocket residues, in accordance with an enhanced MAO-A inhibitor potency of substituted carboline compared to harmine. The authors also highlighted the role of Tyr326 in the MAO-B binding site. They partially attribute the low MAO-B binding affinity showed by this class of compounds to the pronounced restriction produced by Tyr326, position occupied by the less bulky Ile335 into MAO-A isoform.
β-carboline
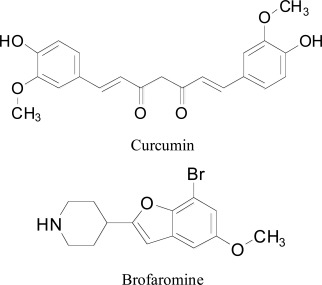
6. Curcumin
Curcumin (diferuloylmethane) has been used extensively studied for its CNS activities and MAO inhibition [95]. Docking profile of curcumin was reported by Sivaraman et al. [46] by using AutoDock software with aim to the further explain the in-vitro MAO inhibition results and to establish the hypothetical binding modes for the curcumin inside the active site of hMAO-A. Compound brofaromine is reported as an MAO-A reversible inhibitor and used in the treatment of depression and anxiety. Docking study showed the better accommodation of curcumin in the binding site of enzyme MAO-A shown in Table 8.
Table 8.
Docking studied of curcumin with MAO enzyme.
| S. No. | Compound | Free energy of Binding Kcal/mol | Inhibitory Constant Ki mM*/µM |
Hydrogen
Bond Formation |
Major Interactions |
|---|---|---|---|---|---|
| 1. | Curcumin | -3.20 | 12.98 | 12 | 274 PRO,51 ARG, 52THR, 43 GLU, 407 TYR, 23 ILE, 277 LEU, 45ARG, 402 TYR, 445 MET, 273 ILE, 448 ALA |
| 2. | Brofaromine (Standard) | -7.53 | 3.08 | 10 | 445 MET, 52 THR, 406 CYS,448 ALA, 303 VAL, 51 ARG,397, 305 LYS, TRP, 407 TYR, 435 THR |
While in another study by Singh et al., [95] different results were observed with the same software for binding energy and the interacting amino acid residues. Binding free energy along with predicted inhibitory constant and Ki were computed. The interaction pattern showed various modes with amino acid residues like hydrophobic, π-π interaction, cation-π, Ven der wall contacts and hydrogen bonding (Table 9).
Table 9.
Molecular docking profile of curcumin studied for MAO isoforms inhibition.
| S. No. | Compound | Free Energy of Binding Kcal/mol | Inhibitory Constant Ki µM | Hydrogen Bond Formation | Hydrophobic Interaction | π-π Stacking |
|---|---|---|---|---|---|---|
| 1. | Curcumin (MAO-B) | -7.80 | 1.89 µM | - | TYR398, MET436, TYR326, PHE343 | PHE343, TYR398 |
| 2. | Curcumin (MAO-A) | -7.96 | 1.56 µM | - | TYR69, ILE23, ILE180, ILE335, PHE352, TYR407 | TYR407, PHE352 |
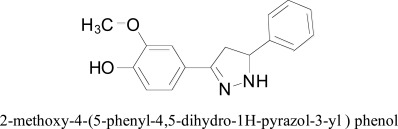
The enthusiasm for this curcuminoid was additionally sought after by Badavath's docking research group, they designed new 2-methoxy-4-(5-phenyl-4,5-dihydro-1H-pyrazol-3-yl) phenol analogs that almost similar to that of carbonyl site a curcumin (aryl-α,β-unsaturated) by conversion of the double bond position along with carbonyl group. The impact of chirality (C5 carbon of pyrazoline) and other structural components on the on binding affinities of the MAO was assessed through molecular docking methodology. They performed computational studies on the 3D crystal structure of MAO co-crystallized having native inhibitor structures, which led bad relationship among exploratory and computed values. Later on, other protocol was executed, as per computed binding energy and Ki value, the R-isomers were found to be 4-fold more active than S isomers for hMAO-A. For hMAO-B, both R-isomer and S-isomer estimated Ki values comparable to each other Badavath et al [96].
2-methoxy-4-(5-phenyl-4,5-dihydro-1H-pyrazol-3-yl) phenol
In a later paper, the same group contemplated synthetic curcumin-based ferulic acid amide derivatives, with a superior pharmacokinetic profile than natural curcumin, ferulic acid, and selegiline Badavath and co-workers. In hMAO-A, the amide group (tail) portion totally gets pulled in restricting pocket in binding pocket and the head portion obliged at the intersection of all the three pockets. However, in MAO-B the amide group was situated within the narrow cavity between the entrance and aromatic cage and 4-hydroxy-3-methoxy phenyl ring (head) was situated in the aromatic cage of hMAO-B. This experiment showed that binding energy of derivatives with hMAO-A lead to the steady increase in van der Waals interaction with an extension of carbon from C1-C4 in the side chain [97].
7. Other natural Compounds:
7.1. Eugenol
Eugenol (4-allyl-2-methoxyphenol) a natural phytoconstituent and essential component of many herbal plants exhibit various numerous neuropharmacological properties including MAO inhibition [98, 99].
Tao and et al, studied MAO inhibitory activity of and found this phytoconsituent as good antidepressant agent [100]. For the study eugenol was isolated from Rhizoma acori graminei, and its potential as monoamine oxidase inhibitor was assessed through in-vitro methods and further affirmation of mechanism was done by docking studies. For molecular docking study crystal structure of rodent MAO-A with (PDB 1O5W) was taken and AutoDock mole software was utilized to insight interaction pattern of eugenol and targeted enzyme. The dynamic buildups observed to be required in restricting cavity space of MAO-A was accounted for as Tyr69, Asn181, Tyr197, Tyr407, Gly443, Tyr444. The phenolic ring of eugenol was incorporated into the phenolic side chains of Tyr407 and Tyr444 and this geometric property of eugenol was observed to be in charge of p–p stacking connection. Be that as it may, in MAOB, phenolic ring of docked eugenol was stuffed by the side chains of Gln206 and Leu171. Findings of the study demonstrated that eugenol could hinder monoamine oxidase A, specifically with an (inhibition constant) Ki =26µM. MAO-B has also inhibited but at much higher concentrations (Ki = 211µM).
7.2. Sffron (Crocus sativus L.)
Saffron is the most valuable herb called as Kesar isolated as dried stigma from the flowers of Crocus sativus (Iridaceae) has been documented from ancient time for its pharmacological activities. Recently Monte and et al, evaluated the MAO inhibitory potential on a 3D-crystal structure of MAO by molecular docking studies of active principles of saffron (safranal and crocin) [101]. Molecular docking was carried on natural crocin, safranal and the semisynthetic derivatives using the Glide SP software. Among the all screened compounds thiosemicarbazone derivative was found to be more potent hMAO inhibitor by in-vitro and docking analysis. The derivatization of these active constituents was carried out and their structures were virtually docked for insight into inhibition mechanism of the hMAO (human MAO) isoforms. The most active hydrazothiazole based analog acted as a specific inhibitor of the hMAO-B enzyme. Chemical modification of crocin led to an increment of MAO inhibition due to the favorable orientation of them within the active site of the MAO. However, crocin established an interaction with the allosteric site of the protein surface. The docking profile was found to be correlated with the experimental inhibition data.
7.3. Trans,trans-Farnesol
Trans,trans-Farnesol (sesquiterpenoid alcohol) is extensively studied scaffold for its neurological properties and evidenced for MAO inhibition. To assess its MAO effects Karahan et al., examined its possible mechanism of action with aid of molecular docking using AutoDock 3.0.5 tools on a high-resolution crystal structure of monoamine oxidase-B (PDB entry code 1S3E, 1.6 Å resolution), with some critical adjustments done by GROMACS sub-atomic flow recreation program and the AutoDock ADDSOL utility program. Different properties like inhibition constants (Ki) and free energy of bindings (ΔGb) for the farnesol as MAO-B inhibitors. The isopropyl terminal of farnesol was appeared to be reached out toward the passageway depression framing contacts with the Ile199 side chain. Amino acid residues Tyr398 and Tyr435 appeared crucial for the inhibition mechanism [20].
CONCLUSION AND PERSPECTIVES
Presently monoamine oxidase (MAO) inhibitors are used for the treatment of various neurodegenerative disorders, including depression, Alzheimer’s disease and Parkinson’s disease. But these are associated with serious side effects due to lack of their efficacy and selectivity for a single MAO isoform. It is commonly accepted that the isolation of monoamine oxidase (MAO) inhibitors from herbal sources are the critical strategy for the drug design and development to cure various CNS diseases such as depression, anxiety, Parkinson’s disease and Alzheimer’s disease. Several medicinal researchers are actively working around there in this field of computational design of new potent and specific MAO inhibitors with better safety profile. The combination of the crystallographic structure of MAO, molecular docking, COMFA, lead hybridization and natural products provides the way for the discovery of new efficient and selective solutions for increasing neuronal health problems in developed nations. Moreover, this may provide an idea for the structure requirements and essential modifications on the basic moieties of different natural pounds for MAO inhibition. Based on these important reports, there are many ongoing research studies for the rational structure-based drug design of novel molecules. Among all the studied natural moieties the coumarins and alkaloids appeared as most promising natural targets for future chemical modification and for the pre-clinical and clinical studies.
Table 5.
Molecular docking of natural compounds isolated from Clitoria ternatea for MAO isoforms.
| S. No. | Compound | Binding Score Energy Value (Kcal/mol) | No. of Hydrogen Bonds | Interacting Amino Acid Residue |
|---|---|---|---|---|
| 1. | Kaempferol-monoglucoside | -14.9178 (MAO-A) -13.9653 (MAO-B) |
7(MAO-A) 3(MAO-B) |
ASN181, GLN443,GLN66,GLN443, MET445, TYR69, ALA 68 (MAO-A) LYS 296, TYR 60, GLY 434 (MAO-B) |
| 2. | Malvidin-3-0-glucoside | -7.86773 (MAO-A) - (MAO-B) |
3 (MAO-A) - (MAO-B) |
TYR 69, GLN215, ALA 68 (MAO-A) |
| 3. | n-Hexadecanoic acid | -5.4457 (MAO-A) -10.5192 (MAO-B) |
3 (MAO-A) 1 (MAO-B) |
ALA 68, MET 445, ALA 68 (MAO-A) TYR 60 (MAO-B) |
| 4. | Quercetin | -11.4556 (MAO-A) -10.9755(MAO-B) |
2 (MAO-A) 1 (MAO-B) |
ASN 181, PHE 208 (MAO-A) GLY 434 (MAO-B) |
(Z)- 9,17-octadecadienal and monoamine oxidase A contributed by the amino acid residues ALA68 and TYR69 though important collaboration between n-hexadecanoic acid and monoamine oxidase A were contributed by the amino acid residues MET 445 and ALA68. Moreover, docked kaempferol-3-monoglucoside showed a minimum score of - 13.90/ - 12.95 kcal/mol. The two compounds, (Z)-9,17-octadecadienal showed low restricting binding affinity energy estimation of -6.5/-7.71 kcal/mol against both the MAO isoforms whereas, n-hexadecanoic acid with a minimum docking score of - 10.5001 kcal/mol against MAO-B facilitated as potential lead molecules for further design of novel MAO inhibitors (Table 5).
ACKNOWLEDGEMENTS
Authors are thankful for the Library access provided by Maharshi Dayanand University, Rohtak, India.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Kapetanovic I.M. Computer-aided drug discovery and development (CADDD): in silico-chemico-biological approach. Chem. Biol. Interact. 2008;171(2):165–176. doi: 10.1016/j.cbi.2006.12.006. [http://dx.doi.org/10.1016/ j.cbi.2006.12.006]. [PMID: 17229415]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scotti T.M., Speck-Planche A., Fechine Tavares J., Sobral da Silva M., Cordeiro N.D., Scotti L. Virtual screening of alkaloids from apocynaceae with potential antitrypanosomal activity. Curr. Bioinform. 2015;10:509–519. [http://dx.doi.org/10.2174/ 1574893610666151008011042]. [Google Scholar]
- 3.Koutsoukas A., Simms B., Kirchmair J., Bond P.J., Whitmore A.V., Zimmer S., Young M.P., Jenkins J.L., Glick M., Glen R.C., Bender A. From in silico target prediction to multi-target drug design: current databases, methods and applications. J. Proteomics. 2011;74(12):2554–2574. doi: 10.1016/j.jprot.2011.05.011. [http://dx.doi.org/10.1016/ j.jprot.2011.05.011]. [PMID: 21621023]. [DOI] [PubMed] [Google Scholar]
- 4.Scotti L., Scotti M.T. Computer aided drug design studies in the discovery of secondary metabolites targeted against age-related neurodegenerative diseases. Curr. Top. Med. Chem. 2015;15(21):2239–2252. doi: 10.2174/1568026615666150610143510. [http://dx.doi.org/10.2174/1568026615666150610143510]. [PMID: 26059353]. [DOI] [PubMed] [Google Scholar]
- 5.Zoete V., Grosdidier A., Michielin O. Docking, virtual high throughput screening and in silico fragment-based drug design. J. Cell. Mol. Med. 2009;13(2):238–248. doi: 10.1111/j.1582-4934.2008.00665.x. [http://dx.doi.org/10.1111/ j.1582-4934.2008.00665.x]. [PMID: 19183238]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youdim M.B.H., Collins G.G.S., Sandler M., Bevan Jones A.B., Pare C.M.B., Nicholson W.J. Human brain monoamine oxidase: multiple forms and selective inhibitors. Nature. 1972;236(5344):225–228. doi: 10.1038/236225b0. [http://dx.doi.org/10.1038/236225b0]. [PMID: 4553640]. [DOI] [PubMed] [Google Scholar]
- 7.Shih J.C., Eiduson S. Monoamine oxidase (EC 1.4.3.4): isolation and characterization of multiple forms of the brain enzyme. J. Neurochem. 1973;21(1):41–49. doi: 10.1111/j.1471-4159.1973.tb04223.x. [http://dx.doi.org/10.1111/j.1471-4159.1973.tb04223.x]. [PMID: 4720902]. [DOI] [PubMed] [Google Scholar]
- 8.Tipton K.F., Boyce S., O’Sullivan J., Davey G.P., Healy J. Monoamine oxidases: certainties and uncertainties. Curr. Med. Chem. 2004;11(15):1965–1982. doi: 10.2174/0929867043364810. [http://dx.doi.org/10.2174/0929867043364810]. [PMID: 15279561]. [DOI] [PubMed] [Google Scholar]
- 9.Collins G.G.S., Sandler M. Human blood platelet monoamine oxidase. Biochem. Pharmacol. 1971;20(2):289–296. doi: 10.1016/0006-2952(71)90063-3. [http://dx. doi.org/10.1016/0006-2952(71)90063-3]. [PMID: 5150134]. [DOI] [PubMed] [Google Scholar]
- 10.Zhou C.X., Kong L.D., Ye W.C., Cheng C.H., Tan R.X. Inhibition of xanthine and monoamine oxidases by stilbenoids from Veratrum taliense. Planta Med. 2001;67(2):158–161. doi: 10.1055/s-2001-11500. [http://dx.doi.org/10. 1055/s-2001-11500]. [PMID: 11301865]. [DOI] [PubMed] [Google Scholar]
- 11.Youdim M.B., Edmondson D., Tipton K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006;7(4):295–309. doi: 10.1038/nrn1883. [http://dx.doi.org/10.1038/nrn1883]. [PMID: 16552415]. [DOI] [PubMed] [Google Scholar]
- 12.Fahn S., Cohen G. The oxidant stress hypothesis in Parkinson’s disease: evidence supporting it. Ann. Neurol. 1992;32(6):804–812. doi: 10.1002/ana.410320616. [http://dx.doi.org/10.1002/ana.410320616]. [PMID: 1471873]. [DOI] [PubMed] [Google Scholar]
- 13.Saura J., Luque J.M., Cesura A.M., Da Prada M., Chan-Palay V., Huber G., Löffler J., Richards J.G. Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience. 1994;62(1):15–30. doi: 10.1016/0306-4522(94)90311-5. [http://dx.doi.org/10.1016/0306-4522(94) 90311-5]. [PMID: 7816197]. [DOI] [PubMed] [Google Scholar]
- 14.Riederer P., Danielczyk W., Grünblatt E. Monoamine oxidase-B inhibition in Alzheimer’s disease. Neurotoxicology. 2004;25(1-2):271–277. doi: 10.1016/S0161-813X(03)00106-2. [http://dx.doi.org/10.1016/S0161-813X(03)00106-2]. [PMID: 14697902]. [DOI] [PubMed] [Google Scholar]
- 15.Thomas T. Monoamine oxidase-B inhibitors in the treatment of Alzheimer’s disease. Neurobiol. Aging. 2000;21(2):343–348. doi: 10.1016/s0197-4580(00)00100-7. [http:// dx.doi.org/10.1016/S0197-4580(00)00100-7]. [PMID: 10867219]. [DOI] [PubMed] [Google Scholar]
- 16.Ferino G., Vilar S., Matos M.J., Uriarte E., Cadoni E. Monoamine oxidase inhibitors: ten years of docking studies. Curr. Top. Med. Chem. 2012;12(20):2145–2162. doi: 10.2174/156802612805220048. [http://dx.doi.org/10.2174/ 156802612805220048]. [PMID: 23231393]. [DOI] [PubMed] [Google Scholar]
- 17.Viña D., Serra S., Lamela M., Delogu G. Herbal natural products as a source of monoamine oxidase inhibitors: a review. Curr. Top. Med. Chem. 2012;12(20):2131–2144. doi: 10.2174/156802612805219996. [http://dx.doi.org/10.2174/ 156802612805219996]. [PMID: 23231392]. [DOI] [PubMed] [Google Scholar]
- 18.Helguera A.M., Perez-Machado G., Cordeiro M.N., Borges F. Discovery of MAO-B inhibitors - present status and future directions part I: oxygen heterocycles and analogs. Mini Rev. Med. Chem. 2012;12(10):907–919. doi: 10.2174/138955712802762301. [http://dx.doi.org/10.2174/ 138955712802762301]. [PMID: 22420569]. [DOI] [PubMed] [Google Scholar]
- 19.Tao G., Irie Y., Li D.J., Keung W.M. Eugenol and its structural analogs inhibit monoamine oxidase A and exhibit antidepressant-like activity. Bioorg. Med. Chem. 2005;13(15):4777–4788. doi: 10.1016/j.bmc.2005.04.081. [http://dx.doi.org/10.1016/j.bmc.2005.04.081]. [PMID: 15936201]. [DOI] [PubMed] [Google Scholar]
- 20.Toprakçí M., Yelekçi K. Docking studies on monoamine oxidase-B inhibitors: estimation of inhibition constants (K(i)) of a series of experimentally tested compounds. Bioorg. Med. Chem. Lett. 2005;15(20):4438–4446. doi: 10.1016/j.bmcl.2005.07.043. [http://dx.doi.org/10.1016/j.bmcl.2005.07.043]. [PMID: 16137882]. [DOI] [PubMed] [Google Scholar]
- 21.Jung H.A., Roy A., Choi J.S. In vitro monoamine oxidase A and B inhibitory activity and molecular docking simulations of fucoxanthin. Fisheries. Sce. 2017;83:123–132. [Google Scholar]
- 22.Jung H.A., Roy A., Jung J.H., Choi J.S. Evaluation of the inhibitory effects of eckol and dieckol isolated from edible brown alga Eisenia bicyclis on human monoamine oxidases A and B. Arch. Pharm. Res. 2017;40(4):480–491. doi: 10.1007/s12272-017-0904-3. [http://dx.doi.org/10.1007/ s12272-017-0904-3]. [PMID: 28251489]. [DOI] [PubMed] [Google Scholar]
- 23.Lee H.W., Ryu H.W., Kang M.G., Park D., Lee H., Shin H.M., Oh S.R., Kim H. Potent inhibition of monoamine oxidase A by decursin from Angelica gigas Nakai and by wogonin from Scutellaria baicalensis Georgi. Int. J. Biol. Macromol. 2017;97:598–605. doi: 10.1016/j.ijbiomac.2017.01.080. [http://dx.doi.org/10.1016/j.ijbiomac.2017.01.080]. [PMID: 28109809]. [DOI] [PubMed] [Google Scholar]
- 24.Li Y., Qiang X., Luo L., Yang X., Xiao G., Zheng Y., Cao Z., Sang Z., Su F., Deng Y. Multitarget drug design strategy against Alzheimer’s disease: Homoisoflavonoid Mannich base derivatives serve as acetylcholinesterase and monoamine oxidase B dual inhibitors with multifunctional properties. Bioorg. Med. Chem. 2017;25(2):714–726. doi: 10.1016/j.bmc.2016.11.048. [http://dx.doi.org/10.1016/j.bmc.2016.11.048]. [PMID: 27923535]. [DOI] [PubMed] [Google Scholar]
- 25.Luo L., Li Y., Qiang X., Cao Z., Xu R., Yang X., Xiao G., Song Q., Tan Z., Deng Y. Multifunctional thioxanthone derivatives with acetylcholinesterase, monoamine oxidases and β-amyloid aggregation inhibitory activities as potential agents against Alzheimer’s disease. Bioorg. Med. Chem. 2017;25(6):1997–2009. doi: 10.1016/j.bmc.2017.02.027. [http://dx.doi.org/10.1016/j.bmc.2017.02.027]. [PMID: 28237559]. [DOI] [PubMed] [Google Scholar]
- 26.Luo L., Li Y., Qiang X., Cao Z., Xu R., Yang X., Xiao G., Song Q., Tan Z., Deng Y. Multifunctional thioxanthone derivatives with acetylcholinesterase, monoamine oxidases and β-amyloid aggregation inhibitory activities as potential agents against Alzheimer’s disease. Bioorg. Med. Chem. 2017;25(6):1997–2009. doi: 10.1016/j.bmc.2017.02.027. [http://dx.doi.org/10.1016/j.bmc.2017.02.027]. [PMID: 28237559]. [DOI] [PubMed] [Google Scholar]
- 27.Kumar D., Kumar M., Kumar A., Singh S.K. Chalcone and curcumin derivatives: a way ahead for malarial treatment. Mini Rev. Med. Chem. 2013;13(14):2116–2133. doi: 10.2174/13895575113136660101. [http://dx.doi.org/10. 2174/13895575113136660101]. [PMID: 24160709]. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt T.J., Khalid S.A., Romanha A.J., Alves T.M., Biavatti M.W., Brun R., Da Costa F.B., de Castro S.L., Ferreira V.F., de Lacerda M.V., Lago J.H., Leon L.L., Lopes N.P. das Neves Amorim, R.C.; Niehues, M.; Ogungbe, I.V.; Pohlit, A.M.; Scotti, M.T.; Setzer, W.N.; de N C Soeiro, M.; Steindel, M.; Tempone, A.G. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases - part II. Curr. Med. Chem. 2012;19(14):2176–2228. [http://dx.doi.org/10.2174/ 092986712800229087]. [PMID: 22414104]. [PubMed] [Google Scholar]
- 29.Kim J.H., Son Y.K., Kim G.H., Hwang K.H. Xanthoangelol and 4-hydroxyderricin are the major active principles of the inhibitory activities against monoamine oxidases on Angelica keiskei K. Biomol. Ther. (Seoul) 2013;21(3):234–240. doi: 10.4062/biomolther.2012.100. [http://dx.doi.org/10. 4062/biomolther.2012.100]. [PMID: 24265870]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chimenti F., Fioravanti R., Bolasco A., Chimenti P., Secci D., Rossi F., Yáñez M., Orallo F., Ortuso F., Alcaro S. Chalcones: a valid scaffold for monoamine oxidases inhibitors. J. Med. Chem. 2009;52(9):2818–2824. doi: 10.1021/jm801590u. [http://dx.doi.org/10.1021/jm801590u]. [PMID: 19378991]. [DOI] [PubMed] [Google Scholar]
- 31.Carpéné C., Hasnaoui M., Balogh B., Matyus P., Fernández-Quintela A., Rodríguez V., Mercader J., Portillo M.P. Dietary phenolic compounds interfere with the fate of hydrogen peroxide in human adipose tissue but do not directly inhibit primary amine oxidase activity. Oxid. Med. Cell. Longev. 2016;2016:2427618. doi: 10.1155/2016/2427618. [http://dx.doi.org/10.1155/2016/2427618]. [PMID: 26881018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathew B., Ucar G., Yabanogclu-Ciftci S., Baysal I., Suresh J., Elizabeth Mathew G., Kunjumon Vilapurathu J., Nadeena M.A., Nabeela P., Lakshmi V., Haridas A. Development of fluorinated thienylchalcones as monoamine oxidase-b inhibitors: Design, synthesis, biological evaluation and molecular docking studies. Lett. Org. Chem. 2015;12:605–613. [http://dx.doi.org/10.2174/ 1570178612666150903213416]. [Google Scholar]
- 33.Mathew B., Suresh J., Mathew G.E., Haridas A., Suresh G., Sabreena P. Synthesis, ADME studies, toxicity estimation, and exploration of molecular recognition of thiophene based chalcones towards monoamine oxidase-A and B. Beni-Seuf Univ. J. Appl. Sci. (Faisalabad) 2016;5:396–401. [Google Scholar]
- 34.Carradori S., Gidaro M.C., Petzer A., Costa G., Guglielmi P., Chimenti P., Alcaro S., Petzer J.P. Inhibition of human monoamine oxidase: Biological and molecular modeling studies on selected natural flavonoids. J. Agric. Food Chem. 2016;64(47):9004–9011. doi: 10.1021/acs.jafc.6b03529. [http://dx.doi.org/10.1021/acs.jafc.6b03529]. [PMID: 27933876]. [DOI] [PubMed] [Google Scholar]
- 35.Orhan I.E. Potential of natural products of herbal origin as monoamine oxidase inhibitors. Curr. Pharm. Des. 2016;22(3):268–276. doi: 10.2174/1381612822666151112150612. [http://dx.doi.org/10.2174/1381612822666151112150612]. [PMID: 26561069]. [DOI] [PubMed] [Google Scholar]
- 36.Chaurasiya N.D., Gogineni V., Elokely K.M., León F., Núñez M.J., Klein M.L., Walker L.A., Cutler S.J., Tekwani B.L. Isolation of Acacetin from Calea urticifolia with Inhibitory Properties against Human Monoamine Oxidase-A and -B. J. Nat. Prod. 2016;79(10):2538–2544. doi: 10.1021/acs.jnatprod.6b00440. [http://dx.doi.org/10.1021/acs.jnatprod.6b00440]. [PMID: 27754693]. [DOI] [PubMed] [Google Scholar]
- 37.Guo B., Zheng C., Cai W., Cheng J., Wang H., Li H., Sun Y., Cui W., Wang Y., Han Y., Lee S.M.Y., Zhang Z. Multifunction of chrysin in Parkinson’s model: anti-neuronal apoptosis, neuroprotection via activation of MEF2D, and inhibition of monoamine oxidase-B. J. Agric. Food Chem. 2016;64(26):5324–5333. doi: 10.1021/acs.jafc.6b01707. [http://dx. doi.org/10.1021/acs.jafc.6b01707]. [PMID: 27245668]. [DOI] [PubMed] [Google Scholar]
- 38.Mathew B., Suresh J., Mathew G.E., Parasuraman R., Abdulla N. Plant secondary metabolites- potent inhibitors of monoamine oxidase isoforms. Cent. Nerv. Syst. Agents Med. Chem. 2014;14(1):28–33. doi: 10.2174/1871524914666140826111930. [http://dx.doi.org/10.2174/1871524914666140826111930]. [PMID: 25142815]. [DOI] [PubMed] [Google Scholar]
- 39.Sloley B.D., Urichuk L.J., Morley P., Durkin J., Shan J.J., Pang P.K.T., Coutts R.T. Identification of kaempferol as a monoamine oxidase inhibitor and potential Neuroprotectant in extracts of Ginkgo biloba leaves. J. Pharm. Pharmacol. 2000;52(4):451–459. doi: 10.1211/0022357001774075. [http://dx.doi.org/10.1211/0022357001774075]. [PMID: 10813558]. [DOI] [PubMed] [Google Scholar]
- 40.van Diermen D., Marston A., Bravo J., Reist M., Carrupt P.A., Hostettmann K. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J. Ethnopharmacol. 2009;122(2):397–401. doi: 10.1016/j.jep.2009.01.007. [http://dx.doi. org/10.1016/j.jep.2009.01.007]. [PMID: 19168123]. [DOI] [PubMed] [Google Scholar]
- 41.Chimenti F., Cottiglia F., Bonsignore L., Casu L., Casu M., Floris C., Secci D., Bolasco A., Chimenti P., Granese A., Befani O., Turini P., Alcaro S., Ortuso F., Trombetta G., Loizzo A., Guarino I. Quercetin as the active principle of Hypericum hircinum exerts a selective inhibitory activity against MAO-A: extraction, biological analysis, and computational study. J. Nat. Prod. 2006;69(6):945–949. doi: 10.1021/np060015w. [http://dx.doi.org/10.1021/np060015w]. [PMID: 16792415]. [DOI] [PubMed] [Google Scholar]
- 42.Ji H.F., Zhang H.Y. Theoretical evaluation of flavonoids as multipotent agents to combat Alzheimer’s disease. J. Mol. Struct. THEOCHEM. 2006;767:3–9. [http://dx.doi.org/10.1016/j.theochem. 2006.04.041]. [Google Scholar]
- 43.Desideri N., Bolasco A., Fioravanti R., Monaco L.P., Orallo F., Yáñez M., Ortuso F., Alcaro S. Homoisoflavonoids: natural scaffolds with potent and selective monoamine oxidase-B inhibition properties. J. Med. Chem. 2011;54(7):2155–2164. doi: 10.1021/jm1013709. [http://dx.doi. org/10.1021/jm1013709]. [PMID: 21405131]. [DOI] [PubMed] [Google Scholar]
- 44.Chimenti F., Fioravanti R., Bolasco A., Chimenti P., Secci D., Rossi F., Yáñez M., Orallo F., Ortuso F., Alcaro S., Cirilli R., Ferretti R., Sanna M.L. A new series of flavones, thioflavones, and flavanones as selective monoamine oxidase-B inhibitors. Bioorg. Med. Chem. 2010;18(3):1273–1279. doi: 10.1016/j.bmc.2009.12.029. [http://dx.doi.org/ 10.1016/j.bmc.2009.12.029]. [PMID: 20045650]. [DOI] [PubMed] [Google Scholar]
- 45.Turkmenoglu F.P., Baysal İ., Ciftci-Yabanoglu S., Yelekci K., Temel H., Paşa S., Ezer N., Çalış İ., Ucar G. Flavonoids from Sideritis species: human monoamine oxidase (hMAO) inhibitory activities, molecular docking studies and crystal structure of xanthomicrol. Molecules. 2015;20(5):7454–7473. doi: 10.3390/molecules20057454. [http://dx.doi. org/10.3390/molecules20057454]. [PMID: 25915461]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sivaraman D., Vignesh G., Selvaraj R., Dare B.J. Identification of potential monoamine oxidase inhibitor from herbal source for the treatment of major depressive disorder: An in-silico screening approach. Pharma Chem. 2015;7:224–234. [Google Scholar]
- 47.Beula S.J., Raj V.B., Mathew B. Isolation and molecular recognization of 6-prenyl apigenin towards MAO-A as the active principle of seeds of Achyranthes aspera. Biomed. Prev. Nutr. 2014;4:379–382. [http://dx.doi.org/10.1016/j.bionut.2014.03.003]. [Google Scholar]
- 48.Zarmouh N., Eyunni S., Mazzio E., Messeha S., Elshami F., Soliman K. Bavachinin and genistein, two novel human monoamine Oxidase-B (MAO-B) inhibitors in the Psoralea Corylifolia seeds. FASEB J. 2015;29:771–772. [Google Scholar]
- 49.Zarmouh N.O., Messeha S.S., Elshami F.M., Soliman K.F. Evaluation of the Isoflavone Genistein as Reversible Human Monoamine Oxidase-A and -B Inhibitor. Evid. Based Complement. Alternat. Med. 2016;2016:1423052. doi: 10.1155/2016/1423052. [http://dx.doi.org/10.1155/ 2016/1423052]. [PMID: 27118978]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Margret A.A., Begum T.N., Parthasarathy S., Suvaithenamudhan S. A strategy to Employ Clitoria ternatea as a prospective brain drug confronting monoamine oxidase (MAO) against neurodegenerative diseases and depression. Nat. Prod. Bioprospect. 2015;5(6):293–306. doi: 10.1007/s13659-015-0079-x. [http://dx.doi.org/10.1007/s13659-015-0079-x]. [PMID: 26667936]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhiman P., Malik N., Khatkar A., Kulharia M. Antioxidant, xanthine oxidase and monoamine oxidase inhibitory potential of coumarins: A review. Curr. Org. Chem. 2017;21:294–304. [http://dx.doi.org/10.2174/1385272820666161021103547]. [Google Scholar]
- 52.Bubols G.B. Vianna, Dda.R.; Medina-Remon, A.; von Poser, G.; Lamuela-Raventos, R.M.; Eifler-Lima, V.L.; Garcia, S.C. The antioxidant activity of coumarins and flavonoids. Mini Rev. Med. Chem. 2013;13(3):318–334. doi: 10.2174/138955713804999775. [PMID: 22876957]. [DOI] [PubMed] [Google Scholar]
- 53.Santana L., Uriarte E., González-Díaz H., Zagotto G., Soto-Otero R., Méndez-Alvarez E., Carotti A., Testa B. A QSAR model for in silico screening of MAO-A inhibitors. Prediction, synthesis, and biological assay of novel coumarins. J. Med. Chem. 2006;49(3):1149–1156. doi: 10.1021/jm0509849. [http://dx.doi.org/10.1021/jm0509849]. [PMID: 16451079]. [DOI] [PubMed] [Google Scholar]
- 54.Gnerre C., Catto M., Leonetti F., Weber P., Carrupt P.A., Altomare C., Carotti A., Testa B. Inhibition of monoamine oxidases by functionalized coumarin derivatives: biological activities, QSARs, and 3D-QSARs. J. Med. Chem. 2000;43(25):4747–4758. doi: 10.1021/jm001028o. [http://dx.doi.org/10.1021/jm001028o]. [PMID: 11123983]. [DOI] [PubMed] [Google Scholar]
- 55.Morón J.A., Campillo M., Perez V., Unzeta M., Pardo L. Molecular determinants of MAO selectivity in a series of indolylmethylamine derivatives: biological activities, 3D-QSAR/CoMFA analysis, and computational simulation of ligand recognition. J. Med. Chem. 2000;43(9):1684–1691. doi: 10.1021/jm991164x. [http://dx.doi.org/10.1021/ jm991164x]. [PMID: 10794685]. [DOI] [PubMed] [Google Scholar]
- 56.Veselovsky A.V., Medvedev A.E., Tikhonova O.V., Skvortsov V.S., Ivanov A.S. Modeling of substrate-binding region of the active site of monoamine oxidase A. Biochemistry (Mosc.) 2000;65(8):910–916. [PMID: 11002183]. [PubMed] [Google Scholar]
- 57.Carrieri A., Carotti A., Barreca M.L., Altomare C. Binding models of reversible inhibitors to type-B monoamine oxidase. J. Comput. Aided Mol. Des. 2002;16(11):769–778. doi: 10.1023/a:1023815426730. [http://dx.doi.org/10. 1023/A:1023815426730]. [PMID: 12825788]. [DOI] [PubMed] [Google Scholar]
- 58.Helguera A.M., Pérez-Garrido A., Gaspar A., Reis J., Cagide F., Vina D., Cordeiro M.N.D., Borges F. Combining QSAR classification models for predictive modeling of human monoamine oxidase inhibitors. Eur. J. Med. Chem. 2013;59:75–90. doi: 10.1016/j.ejmech.2012.10.035. [http://dx.doi. org/10.1016/j.ejmech.2012.10.035]. [PMID: 23207409]. [DOI] [PubMed] [Google Scholar]
- 59.Matos M.J., Vilar S., García-Morales V., Tatonetti N.P., Uriarte E., Santana L., Viña D. Insight into the functional and structural properties of 3-arylcoumarin as an interesting scaffold in monoamine oxidase B inhibition. ChemMedChem. 2014;9(7):1488–1500. doi: 10.1002/cmdc.201300533. [http://dx.doi.org/10.1002/cmdc.201300533]. [PMID: 24715707]. [DOI] [PubMed] [Google Scholar]
- 60.Harkcom W.T., Bevan D.R. Molecular docking of inhibitors into monoamine oxidase B. Biochem. Biophys. Res. Commun. 2007;360(2):401–406. doi: 10.1016/j.bbrc.2007.06.055. [http://dx.doi.org/10.1016/j.bbrc.2007.06.055]. [PMID: 17597580]. [DOI] [PubMed] [Google Scholar]
- 61.Zhi K.K., Yang Z.D., Shi D.F., Yao X.J., Wang M.G. Desmodeleganine, a new alkaloid from the leaves of Desmodium elegans as a potential monoamine oxidase inhibitor. Fitoterapia. 2014;98:160–165. doi: 10.1016/j.fitote.2014.07.022. [http://dx.doi.org/10.1016/j.fitote.2014.07.022]. [PMID: 25102471]. [DOI] [PubMed] [Google Scholar]
- 62.Samoylenko V., Rahman M.M., Tekwani B.L., Tripathi L.M., Wang Y.H., Khan S.I., Khan I.A., Miller L.S., Joshi V.C., Muhammad I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J. Ethnopharmacol. 2010;127(2):357–367. doi: 10.1016/j.jep.2009.10.030. [http://dx.doi.org/10.1016/ j.jep.2009.10.030]. [PMID: 19879939]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao R., Peng W., Wang Z., Xu A. β-Carboline alkaloids: biochemical and pharmacological functions. Curr. Med. Chem. 2007;14(4):479–500. doi: 10.2174/092986707779940998. [http://dx.doi.org/10.2174/092986707779940998]. [PMID: 17305548]. [DOI] [PubMed] [Google Scholar]
- 64.Lühr S., Vilches-Herrera M., Fierro A., Ramsay R.R., Edmondson D.E., Reyes-Parada M., Cassels B.K., Iturriaga-Vásquez P. 2-Arylthiomorpholine derivatives as potent and selective monoamine oxidase B inhibitors. Bioorg. Med. Chem. 2010;18(4):1388–1395. doi: 10.1016/j.bmc.2010.01.029. [http://dx.doi.org/10.1016/j.bmc.2010.01.029]. [PMID: 20123154]. [DOI] [PubMed] [Google Scholar]
- 65.Coelho C.E., Netz P.A., Diniz C., Petry do Canto V., Follmer C. Molecular insights into human monoamine oxidase (MAO) inhibition by 1,4-naphthoquinone: evidences for menadione (vitamin K3) acting as a competitive and reversible inhibitor of MAO. Bioorg. Med. Chem. 2011;19(24):7416–7424. doi: 10.1016/j.bmc.2011.10.049. [http://dx.doi.org/10.1016/ j.bmc.2011.10.049]. [PMID: 22071524]. [DOI] [PubMed] [Google Scholar]
- 66.Ji H.F., Shen L. Molecular basis of inhibitory activities of berberine against pathogenic enzymes in Alzheimer’s disease. Sci. World J. 2012;2012:823201. doi: 10.1100/2012/823201. [http://dx.doi.org/10.1100/2012/823201]. [PMID: 22262957]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rarey M., Kramer B., Lengauer T., Klebe G. A fast flexible docking method using an incremental construction algorithm. J. Mol. Biol. 1996;261(3):470–489. doi: 10.1006/jmbi.1996.0477. [http://dx.doi.org/10.1006/jmbi. 1996.0477]. [PMID: 8780787]. [DOI] [PubMed] [Google Scholar]
- 68.Abd El-Gaber M.K., Hassan H.Y., Mahfouz N.M., Farag H.H., Bekhit A.A. Synthesis, biological investigation and molecular docking study of N-malonyl-1,2-dihydroisoquinoline derivatives as brain specific and shelf-stable MAO inhibitors. Eur. J. Med. Chem. 2015;93:481–491. doi: 10.1016/j.ejmech.2015.02.039. [http://dx.doi.org/10.1016/j.ejmech. 2015.02.039]. [PMID: 25732770]. [DOI] [PubMed] [Google Scholar]
- 69.Thull U., Kneubühler S., Gaillard P., Carrupt P.A., Testa B., Altomare C., Carotti A., Jenner P., McNaught K.S.P. Inhibition of monoamine oxidase by isoquinoline derivatives. Qualitative and 3D-quantitative structure-activity relationships. Biochem. Pharmacol. 1995;50(6):869–877. doi: 10.1016/0006-2952(95)00220-t. [http://dx.doi.org/10.1016/0006-2952 (95)00220-T]. [PMID: 7575650]. [DOI] [PubMed] [Google Scholar]
- 70.Lee S.A., Hong S.S., Han X.H., Hwang J.S., Oh G.J., Lee K.S., Lee M.K., Hwang B.Y., Ro J.S. Piperine from the fruits of Piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity. Chem. Pharm. Bull. (Tokyo) 2005;53(7):832–835. doi: 10.1248/cpb.53.832. [http://dx.doi.org/10.1248/cpb.53.832]. [PMID: 15997146]. [DOI] [PubMed] [Google Scholar]
- 71.Al-Baghdadi O.B., Prater N.I., Van der Schyf C.J., Geldenhuys W.J. Inhibition of monoamine oxidase by derivatives of piperine, an alkaloid from the pepper plant Piper nigrum, for possible use in Parkinson’s disease. Bioorg. Med. Chem. Lett. 2012;22(23):7183–7188. doi: 10.1016/j.bmcl.2012.09.056. [http://dx.doi.org/10.1016/j.bmcl.2012.09.056]. [PMID: 23102654]. [DOI] [PubMed] [Google Scholar]
- 72.Rahman T., Rahmatullah M. Proposed structural basis of interaction of piperine and related compounds with monoamine oxidases. Bioorg. Med. Chem. Lett. 2010;20(2):537–540. doi: 10.1016/j.bmcl.2009.11.106. [http://dx.doi.org/ 10.1016/j.bmcl.2009.11.106]. [PMID: 19969454]. [DOI] [PubMed] [Google Scholar]
- 73.Mu L.H., Wang B., Ren H.Y., Liu P., Guo D.H., Wang F.M., Bai L., Guo Y.S. Synthesis and inhibitory effect of piperine derivates on monoamine oxidase. Bioorg. Med. Chem. Lett. 2012;22(9):3343–3348. doi: 10.1016/j.bmcl.2012.02.090. [http://dx.doi.org/10.1016/j.bmcl.2012.02.090]. [PMID: 22475561]. [DOI] [PubMed] [Google Scholar]
- 74.Thenmozhi M. Sch. Acad. J. Biosci. 2014;2:119–123. [Google Scholar]
- 75.Friesner R.A., Banks J.L., Murphy R.B., Halgren T.A., Klicic J.J., Mainz D.T., Repasky M.P., Knoll E.H., Shelley M., Perry J.K., Shaw D.E., Francis P., Shenkin P.S. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004;47(7):1739–1749. doi: 10.1021/jm0306430. [http://dx.doi.org/10.1021/jm0306430]. [PMID: 15027865]. [DOI] [PubMed] [Google Scholar]
- 76.Shi L., Yang Y., Li Z.L., Zhu Z.W., Liu C.H., Zhu H.L. Design of novel nicotinamides as potent and selective monoamine oxidase a inhibitors. Bioorg. Med. Chem. 2010;18(4):1659–1664. doi: 10.1016/j.bmc.2009.12.065. [http://dx.doi.org/10.1016/j.bmc.2009.12.065]. [PMID: 20117937]. [DOI] [PubMed] [Google Scholar]
- 77.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [http://dx.doi.org/10. 1002/jcc.21256]. [PMID: 19399780]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fresqui M.A., Ferreira M.M., Trsic M. The influence of R and S configurations of a series of amphetamine derivatives on quantitative structure-activity relationship models. Anal. Chim. Acta. 2013;759:43–52. doi: 10.1016/j.aca.2012.11.004. [http://dx.doi.org/10.1016/j.aca.2012.11.004]. [PMID: 23260675]. [DOI] [PubMed] [Google Scholar]
- 79.Fierro A., Osorio-Olivares M., Cassels B.K., Edmondson D.E., Sepúlveda-Boza S., Reyes-Parada M. Human and rat monoamine oxidase-A are differentially inhibited by (S)-4-alkylthioamphetamine derivatives: insights from molecular modeling studies. Bioorg. Med. Chem. 2007;15(15):5198–5206. doi: 10.1016/j.bmc.2007.05.021. [http://dx.doi.org/10.1016/j.bmc.2007.05.021]. [PMID: 17521909]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vilches-Herrera M., Miranda-Sepúlveda J., Rebolledo-Fuentes M., Fierro A., Lühr S., Iturriaga-Vasquez P., Cassels B.K., Reyes-Parada M. Naphthylisopropylamine and N-benzylamphetamine derivatives as monoamine oxidase inhibitors. Bioorg. Med. Chem. 2009;17(6):2452–2460. doi: 10.1016/j.bmc.2009.01.074. [http://dx.doi.org/10.1016/j.bmc.2009.01. 074]. [PMID: 19243954]. [DOI] [PubMed] [Google Scholar]
- 81.Khattab S.N., Haiba N.S., Asal A.M., Bekhit A.A., Amer A., Abdel-Rahman H.M., El-Faham A. Synthesis and evaluation of quinazoline amino acid derivatives as mono amine oxidase (MAO) inhibitors. Bioorg. Med. Chem. 2015;23(13):3574–3585. doi: 10.1016/j.bmc.2015.04.021. [http://dx.doi.org/10.1016/j.bmc.2015.04.021]. [PMID: 25922182]. [DOI] [PubMed] [Google Scholar]
- 82.Molecular Operating Environment (MOE), 2011.10. Montreal, QC, Canada: Chemical Computing Group Inc.; 2011. [Google Scholar]
- 83.Petzer A., Pienaar A., Petzer J.P. The interactions of caffeine with monoamine oxidase. Life Sci. 2013;93(7):283–287. doi: 10.1016/j.lfs.2013.06.020. [http://dx.doi.org/10.1016/j.lfs.2013.06.020]. [PMID: 23850513]. [DOI] [PubMed] [Google Scholar]
- 84.Strydom B., Malan S.F., Castagnoli N., Jr, Bergh J.J., Petzer J.P. Inhibition of monoamine oxidase by 8-benzyloxycaffeine analogues. Bioorg. Med. Chem. 2010;18(3):1018–1028. doi: 10.1016/j.bmc.2009.12.064. [http://dx. doi.org/10.1016/j.bmc.2009.12.064]. [PMID: 20093036]. [DOI] [PubMed] [Google Scholar]
- 85.Strydom B., Bergh J.J., Petzer J.P. 8-Aryl- and alkyloxycaffeine analogues as inhibitors of monoamine oxidase. Eur. J. Med. Chem. 2011;46(8):3474–3485. doi: 10.1016/j.ejmech.2011.05.014. [http://dx.doi.org/10.1016/j.ejmech.2011. 05.014]. [PMID: 21621312]. [DOI] [PubMed] [Google Scholar]
- 86.Okaecwe T., Swanepoel A.J., Petzer A., Bergh J.J., Petzer J.P. Inhibition of monoamine oxidase by 8-phenoxymethylcaffeine derivatives. Bioorg. Med. Chem. 2012;20(14):4336–4347. doi: 10.1016/j.bmc.2012.05.048. [http://dx.doi.org/10.1016/j.bmc.2012.05.048]. [PMID: 22705191]. [DOI] [PubMed] [Google Scholar]
- 87.Rivara S., Piersanti G., Bartoccini F., Diamantini G., Pala D., Riccioni T., Stasi M.A., Cabri W., Borsini F., Mor M., Tarzia G., Minetti P. Synthesis of (E)-8-(3-chlorostyryl)caffeine analogues leading to 9-deazaxanthine derivatives as dual A(2A) antagonists/MAO-B inhibitors. J. Med. Chem. 2013;56(3):1247–1261. doi: 10.1021/jm301686s. [http://dx.doi.org/10.1021/jm301686s]. [PMID: 23281824]. [DOI] [PubMed] [Google Scholar]
- 88.Azam F., Madi A.M., Ali H.I. Molecular docking and prediction of pharmacokinetic properties of dual mechanism drugs that block MAO-B and adenosine A. J. Young Pharm. 2012;4:184–192. doi: 10.4103/0975-1483.100027. [http://dx.doi.org/10.4103/0975-1483.100027]. [PMID: 23112538]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012;64:4–17. doi: 10.1016/s0169-409x(00)00129-0. [http://dx.doi.org/10.1016/j. addr.2012.09.019]. [PMID: 11259830]. [DOI] [PubMed] [Google Scholar]
- 90.Booysen H.P., Moraal C. Terre’Blanche, G.; Petzer, A.; Bergh, J.J.; Petzer, J.P. Thio- and aminocaffeine analogues as inhibitors of human monoamine oxidase. Bioorg. Med. Chem. 2011;19(24):7507–7518. doi: 10.1016/j.bmc.2011.10.036. [http://dx.doi.org/10.1016/j.bmc.2011.10.036]. [PMID: 22055712]. [DOI] [PubMed] [Google Scholar]
- 91.Passos C.S., Simões-Pires C.A., Nurisso A., Soldi T.C., Kato L., de Oliveira C.M., de Faria E.O., Marcourt L., Gottfried C., Carrupt P.A., Henriques A.T. Indole alkaloids of Psychotria as multifunctional cholinesterases and monoamine oxidases inhibitors. Phytochemistry. 2013;86:8–20. doi: 10.1016/j.phytochem.2012.11.015. [http://dx.doi.org/10.1016/j. phytochem.2012.11.015]. [PMID: 23261030]. [DOI] [PubMed] [Google Scholar]
- 92.Jones G., Willett P., Glen R.C., Leach A.R., Taylor R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997;267(3):727–748. doi: 10.1006/jmbi.1996.0897. [http://dx.doi.org/10.1006/ jmbi.1996.0897]. [PMID: 9126849]. [DOI] [PubMed] [Google Scholar]
- 93.Klein L.C., Jr, Passos C.S., Moraes A.P., Wakui V.G., Konrath E.L., Nurisso A., Carrupt P.A., de Oliveira C.M., Kato L., Henriques A.T. Indole alkaloids and semisynthetic indole derivatives as multifunctional scaffolds aiming the inhibition of enzymes related to neurodegenerative diseases--a focus on Psychotria L. Genus. Curr. Top. Med. Chem. 2014;14(8):1056–1075. doi: 10.2174/1568026614666140324142409. [http://dx. doi.org/10.2174/1568026614666140324142409]. [PMID: 24660679]. [DOI] [PubMed] [Google Scholar]
- 94.Reniers J., Robert S., Frederick R., Masereel B., Vincent S., Wouters J. Synthesis and evaluation of β-carboline derivatives as potential monoamine oxidase inhibitors. Bioorg. Med. Chem. 2011;19(1):134–144. doi: 10.1016/j.bmc.2010.11.041. [http://dx.doi.org/10.1016/j.bmc.2010.11. 041]. [PMID: 21183355]. [DOI] [PubMed] [Google Scholar]
- 95.Singh R., Chaturvedi N., Singh V.K. In silico study of herbal compounds (baicalin, curcumin, dronabinol) as novel mao inhibitors for Parkinson’s disease treatment). Int, J. Life. Sci. Pharm. Res. 2012;2:81–98. [Google Scholar]
- 96.Badavath V.N., Baysal İ., Ucar G., Sinha B.N., Jayaprakash V. Monoamine oxidase inhibitory activity of novel Pyrazoline analogues: Curcumin based design and synthesis. ACS Med. Chem. Lett. 2015;7(1):56–61. doi: 10.1021/acsmedchemlett.5b00326. [http://dx.doi.org/10.1021/acsmedchemlett. 5b00326]. [PMID: 26819666]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Badavath V.N., Baysal İ., Uçar G., Mondal S.K., Sinha B.N., Jayaprakash V. Monoamine oxidase inhibitory activity of ferulic acid amides: curcumin-based design and synthesis. Arch. Pharm. (Weinheim) 2016;349(1):9–19. doi: 10.1002/ardp.201500317. [http://dx.doi.org/10.1002/ardp. 201500317]. [PMID: 26592858]. [DOI] [PubMed] [Google Scholar]
- 98.Kong L.D., Cheng C.H., Tan R.X. Inhibition of MAO A and B by some plant-derived alkaloids, phenols and anthraquinones. J. Ethnopharmacol. 2004;91(2-3):351–355. doi: 10.1016/j.jep.2004.01.013. [http://dx.doi.org/10. 1016/j.jep.2004.01.013]. [PMID: 15120460]. [DOI] [PubMed] [Google Scholar]
- 99.Irie Y. Effects of eugenol on the central nervous system: its possible application to treatment of alzheimer’s disease, depression, and parkinson’s disease. Curr. Bioact. Compd. 2006;2(1):57–66. [http://dx.doi.org/10.2174/1573407210602010057]. [Google Scholar]
- 100.Tao G., Irie Y., Li D.J., Keung W.M. Eugenol and its structural analogs inhibit monoamine oxidase A and exhibit antidepressant-like activity. Bioorg. Med. Chem. 2005;13(15):4777–4788. doi: 10.1016/j.bmc.2005.04.081. [http://dx.doi.org/10.1016/j.bmc.2005.04.081]. [PMID: 15936201]. [DOI] [PubMed] [Google Scholar]
- 101.De Monte C., Carradori S., Chimenti P., Secci D., Mannina L., Alcaro F., Petzer A., N’Da C.I., Gidaro M.C., Costa G., Alcaro S., Petzer J.P. New insights into the biological properties of Crocus sativus L.: chemical modifications, human monoamine oxidases inhibition and molecular modeling studies. Eur. J. Med. Chem. 2014;82:164–171. doi: 10.1016/j.ejmech.2014.05.048. [http://dx.doi.org/10.1016/j.ejmech. 2014.05.048]. [PMID: 24904963]. [DOI] [PubMed] [Google Scholar]