Abstract
Significance: The concepts of junk DNA and transcriptional noise are long gone as the existence of noncoding RNAs (ncRNAs) has been tested extensively in recent years. Given that the epigenetic status of cells affects many biological processes, how ncRNAs mechanistically contribute to these processes is of great interest.
Recent Advances: Recent studies show that various ncRNAs interact with epigenetic and/or transcription factors to modulate the epigenetic status of cells directly and/or indirectly. There exists growing interest in the field of cardiovascular research to understand the roles of ncRNAs. Due to the large number of ncRNAs in the mammalian genome, only a handful of ncRNAs have been functionally elucidated, which makes it difficult to understand how ncRNAs interact with protein-coding genes and their encoded proteins.
Critical Issues: Although the canonical function of microRNAs (miRNAs) to inhibit the translation of protein-coding genes is well established, the number of functionally annotated long noncoding RNAs (lncRNAs) is still small, which is especially true in the heart.
Future Directions: Future studies must connect the epigenetic controls of various cellular phenomena by incorporating both miRNAs and lncRNAs. Antioxid. Redox Signal. 29, 832–845.
Keywords: : epigenetics, long noncoding RNAs, microRNAs
Introduction
By definition, epigenetics is the study of chromosomal changes that are not due to a change in DNA sequence (126). Epigenetics can alter gene expressions and can be inherited to the progeny of cells or of organisms. As such, epigenetic changes are important, and various techniques have been used to uncover their presence and contributions to biological processes, including those in the heart. Although many epigenetic studies have uncovered the contribution of proteins to such processes, a growing number of studies report that molecules other than proteins are important as well. Increasing evidence suggests that most of the mammalian genomes are transcribed as RNAs; however, not all RNAs are translated into proteins. The current estimate is that most of the mammalian genomes become RNAs (64), although only a very small part corresponds to the exons of protein-coding genes. Until recently, many of the remaining RNAs were considered as functionless transcriptional noise and experimental errors; however, recent studies show that noncoding RNAs (ncRNAs) are functional, as in the case of microRNAs (miRNAs). Besides miRNAs, longer ncRNAs have been identified. If their lengths are longer than 200 nucleotides (nt), these ncRNAs are collectively called “long noncoding RNAs (lncRNAs).” Generally speaking, lncRNAs are expressed at low levels compared to protein-coding genes but are more cell- and/or tissue-specific (15). Although lncRNAs are of great interest in the research community, their functions are largely unknown. This is especially true in the cardiovascular field, which we recently reviewed (117). Since our review, more lncRNAs have been studied in the heart. As it is estimated that the number of lncRNAs surpasses that of protein-coding genes (81) and hundreds of lncRNAs have been detected in the heart (63, 91–93), the number of functionally studied lncRNAs is still too small at the moment.
In this review, we will examine the current status of miRNAs and lncRNAs in the heart. Based on our expertise in bioinformatics, current problems in the annotation of lncRNAs will be discussed.
miRNAs and Epigenetics
As more and more miRNAs are functionally studied in the heart, it is not hard to imagine that there are numerous miRNAs that target epigenetic and/or transcriptional factors to control transcription in general. From the standpoint of translational inhibition, it is not hard to connect such relationship in the larger context. In reality, one miRNA targets more than one mRNA. Bioinformatic predictions list hundreds, even thousands, of potential targets of one miRNA, which complicates the whole study and the understanding of the relationship between miRNAs and epigenetic control. Rather than listing all the known (i.e., experimentally validated) miRNAs and their targets in the heart, we will briefly introduce the relationship between miRNAs and epigenetic controls aside from canonical mechanism of miRNAs.
MiRNAs in the nucleus
Given that translation takes place in ribosomes, it was originally thought that miRNAs loaded onto the RNA-induced silencing complex (RISC) bind to their targets outside of the nucleus. However, increasing evidence indicates the presence of RISC (90) and mature miRNAs in the nucleus (47, 54, 58, 60, 68, 99, 101, 115), which challenges the canonical mechanism of miRNAs in general. For example, both pre- and mature miR-373 bind to the promoters of E-cadherin and CSDC2 to induce their gene expressions (99). Instead of gene activation, miR-320 directs the association of AGO1 and EZH2 with the promoter of the nearby protein-coding gene POLR3D by silencing its promoter (60). At the level of RNA, miR-709 directly binds to the pri-miR-15a/16-1 to prevent maturation, which results in the suppression of miR-15a/16-1 to target the antiapoptotic gene Bcl-2 (115). A more detailed mechanism was elucidated recently in zebrafish, in which miR-9 and AGO are transported to the nucleus of quiescent adult neural stem cells via the RISC protein TNRC6, activating the expression of her4 with Notch3 for quiescence of adult neural stem cells, although the exact mechanism (direct or indirect) of the activation of Notch signaling is currently unknown (58). These examples suggest more epigenetic controls of miRNAs inside of the nucleus (Fig. 1).
FIG. 1.
Nuclear miRNAs. (A) Both pre- and mature miR-373 bind to the promoters of E-cadherin and CSDC2 in a human prostate cancer cell line “PC-3.” (B) miR-320 binds to the promoter of POLR3D to silence its expression in a human kidney cell line “HEK-293.” (C) Mouse miR-709 directly binds to the pri-miR-15a/16-1 to prevent their maturations. (D) miR-9 controls the Notch signaling pathway in the zebrafish adult neural stem cells for their quiescence status. miRNA, microRNA
miRNAs and super-enhancers
In 2013, a new enhancer domain was reported, which is called “super-enhancers” (45, 138). By definition, a super-enhancer encompasses a cluster of enhancers and is bound by several transcriptional regulators, which control the cell-type-specific expressions of a number of genes (138). Recent studies show that super-enhancers are involved in the regulation of miRNAs at the transcriptomic level (31, 109). Furthermore, a member of the bromodomain and extraterminal domain family of epigenetic readers BRD4, which marks super-enhancers (29), is suppressed by miR-9 through its binding to the 3′-UTR of BRD4 gene in cardiomyocytes (107). These studies suggest regulation of epigenetic remodeling by miRNAs.
LncRNAs and Epigenetics
Definition, identification, and functional characterization of lncRNAs
As we recently surveyed (134), the number of protein-coding genes is steadily decreasing in the annotation provided by the Ensembl database, while the number of lncRNAs is increasing (Fig. 2). This is mostly due to the fact that previously thought protein-coding genes, which are categorized as such based on the computational predictions, are not anymore protein coding as more evidence (again based mostly on the computational predictions) suggests that they are noncoding. Thus, Ensembl frequently updates its annotations. This is problematic as RNA sequencing (RNA-seq) data analysis changes depending on annotations, such as the biotype and length of a particular transcript. Such changes cause problems in the downstream analysis, especially in the quantification of transcripts (e.g., FPKM values) (134).
FIG. 2.
Numbers of human protein-coding genes and ncRNAs. The statistics is based on the gene counts in the primary assembly provided by the Ensembl database (www.ensembl.org/Homo_sapiens/Info/Annotation). Of note, the category for other ncRNAs (“miscellaneous other ncRNAs”) became available from the GRCh38.p2 assembly. In GRCH37.p7 and NCBI 36, only “RNA genes” category exists for all kinds of ncRNAs. ncRNA, noncoding RNA.
Although the abovementioned problems of RNA-seq exist, there is no other technology currently available to perform unbiased, genome-wide screening of lncRNAs. As the cost of next-generation sequencing (NGS) has been decreasing, RNA-seq is increasingly being used to analyze transcriptomes of various conditions, including those in the heart. Currently, there are numerous RNA-seq data sets available from the sequence read archive, most of which are already published in the literature. However, only few data sets have been analyzed for lncRNAs (63, 91–93). More importantly, many data sets were analyzed with the older version of genome assembly (i.e., hg19) rather than the newest version (i.e., GRCh38/hg38). As we have recently shown (134), the mapping rate is better using GRCh38/hg38 than hg19, which means that more sequencing reads can be utilized for further analysis. In other words, secondary analysis of RNA-seq is necessary to maximize the power of RNA-seq to identify lncRNAs and quantify them using the latest genome assembly and annotation.
As the cost of RNA-seq decreases and the protocol to perform it is standardized, the screening of transcripts using RNA-seq is no longer a challenge for laboratories around the world. Thus, various transcripts, including lncRNAs, can be identified and associated with a particular pathophysiological condition by analyzing the data as such (mostly guilt-by-association). The challenge now is to functionally characterize the identified lncRNAs. Despite the fact that lncRNAs interact with DNA, RNA, and proteins, the current consensus in the field of lncRNAs is that lncRNAs carry out their functions largely via interaction with proteins (36). Thus, it is essential that binding proteins be identified using biochemical assays, such as RNA pull-down (104) or chromatin isolation by RNA purification (22). Once the binding proteins are identified, research continues to find out how the interaction with the target lncRNAs and their associated proteins is affected on loss of the target lncRNA, which can be achieved with siRNAs and/or LNA GapmeRs. LNA GapmeRs is an especially important technology as it can be used in vivo (82, 121), which opens avenues for clinical use. Given that many lncRNAs are cell-type specifically expressed, LNA-GapmeR-based knockdown of lncRNAs might avoid off-target effects in other cell types. Given the power and accessibility of the CRISPR/Cas9 system, it is becoming more important to completely ablate the target lncRNA instead of transiently silencing it. However, there exists a challenge in such genetic tools as the complete deletion of the target lncRNA is required as interference of translation is not possible for lncRNAs as in the case of protein-coding gene. Thus, in the case of the CRISPR/Cas9 system, two guide RNAs designed to flank the target lncRNA are required to achieve complete ablation. Alternatively, poly-A signal can be inserted to truncate an lncRNA as in the case of Lockd (94). As more experimental tools are available, more functional studies are necessary to uncover the functions of lncRNAs and their associated proteins, which are much more challenging tasks to be achieved than simply screening by RNA-seq experiments.
LncRNAs and genomic imprinting
Classically, lncRNAs are known for their activity of epigenetic regulation via their action as genomic imprinting, which is an epigenetic phenomenon through which genes are expressed in a parent-of-origin dependent manner (98). Such imprinting lncRNAs include Airn (65), H19 (6, 103), MEG3 (83), and MEG8 (19) (the complete list of imprinting genes can be found in the Geneimprint database [www.geneimprint.com]). Besides the classic imprinting lncRNAs, the lncRNA XIST is known to silence one X chromosome during the development of female mammals (11–14). Recent studies show that XIST interacts with several proteins [e.g., a member of the SWI/SNF family of chromatin remodeling proteins ATRX (106), a member of the SPEN family of transcriptional repressors SHARP (79)] to directly regulate the exclusion of RNA polymerase II on the X chromosome, thereby suppressing the X chromosome [reviewed extensively in Ref. (25)]. Furthermore, other lncRNAs [i.e., XACT (119), TSIX (66, 67)] are also involved in controlling X chromosome activity via interacting or competing with XIST. As is the case for imprinting lncRNAs and XIST, many imprinting lncRNAs interact with epigenetic factors and other proteins to control the epigenetic status of the loci that they target, highlighting the importance of lncRNAs in the context of epigenetic control of the development of an organism.
LncRNAs in the heart
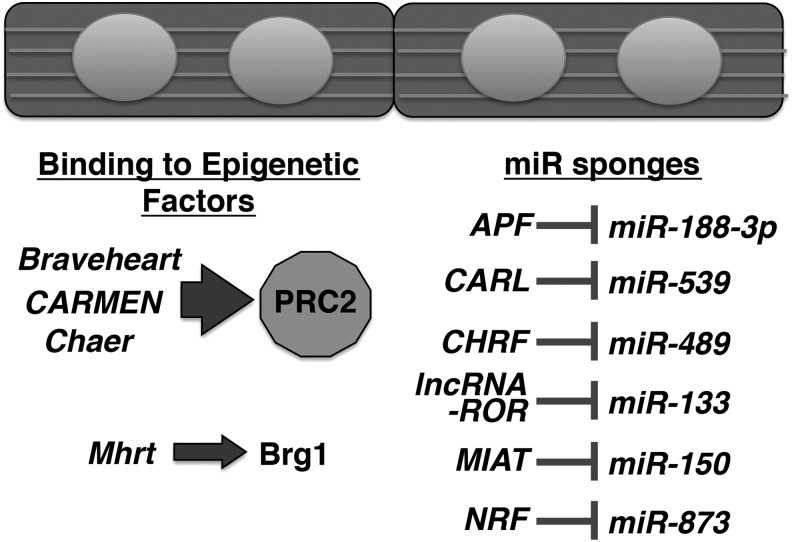
In the heart, only a handful lncRNAs have been elucidated with respect to their functions, which we have recently reviewed (117). Since our review, the functions of additional lncRNAs have been elucidated to a certain extent in the heart (Fig. 3 and Table 1). With regard to epigenetic regulation, some lncRNAs [e.g., Braveheart (61, 141), CARMEN (91), Chaer (132)] can directly bind to epigenetic factors (namely, PRC2) to exert gene regulations. However, caution should be used, as although PRC2 is known to specifically bind RNAs, the number of PRC2-bound RNAs is very high (23, 26, 27). Although such direct interaction of lncRNAs with epigenetic factors is easy to understand, some lncRNAs function as miRNA sponges to sequester otherwise available miRNAs (127, 129, 131, 149). For example, the lncRNA CARL binds miR-539, which targets one of the two subunits of prohibitin complex Phb2 for mitochondrial fission and apoptosis (131). Although this study investigated their effects in mitochondria, PHB2 is an estrogen receptor-selective coregulator and acts as a mediator of transcriptional repression (84), suggesting a potential mechanism in which lncRNA controls the epigenetic status via miRNA binding. More recently, a promoter-associated lncRNA called “Upperhand (Uph)” was reported to be important for its transcription (but not its RNA) in cis to promote cardiac transcription factor Hand2 expression via GATA4 binding and super-enhancer activity (4). In line with this study, recent bioinformatic analysis of long intergenic ncRNAs (lincRNAs) shows that many lincRNAs are associated with enhancers/promoters and control the expression of nearby protein-coding genes (especially, cis-regulation of disease-associated/suspected genes) (46, 113), suggesting that many lncRNAs may alter the epigenetic status of nearby protein-coding genes. In line with this, experimental evidence suggests that super-enhancers are also controlled by lncRNAs (97, 139) as in the case of the lncRNA CARMEN in the heart (91).
FIG. 3.
Functionally characterized lncRNAs in relation to interacting partners in the cardiomyocytes. APF, autophagy promoting factor; CARL, cardiac apoptosis-related lncRNA; CARMEN, CARdiac mesoderm enhancer-associated noncoding RNA; Chaer, cardiac hypertrophy-associated epigenetic regulator; CHRF, cardiac hypertrophy-related factor; Mhrt, myosin heavy chain-associated RNAtranscripts; PRC2, polycomb repressive complex 2.
Table 1.
List of Long Noncoding RNAs Known to be Functional in the Heart
| lncRNA | Organism(s) | Disease/condition | Functions in the heart | References |
|---|---|---|---|---|
| APF | Mouse | Myocardial infarction | Binds miR-188-3p, which targets Atg7 to control cardiac autophagy. | (127) |
| Braveheart | Mouse | ES-derived cardiomyocytes | Binds PRC2 and CNBP/ZNF9 to regulate cardiovascular lineage commitment. | (61, 141) |
| CARL | Mouse | Myocardial infarction | Binds miR-539, which targets Phb2 for mitochondrial fission and apoptosis. | (131) |
| CARMEN/MIR143HG | Human, mouse | Aortic stenosis, idiopathic dilated cardiomyopathy, myocardial infarction | Arises from the super-enhancer region and binds PRC2 to control the expression of cardiac genes; and controls MIR-143/145 expression and the smooth muscle cell fate in adult cardiac progenitor cells. | (91, 100) |
| Chaer | Human, Mouse | Hypertrophy | Binds PRC2 to inhibit H3K27 methylation at the promoter regions of genes involved in cardiac hypertrophy. | (132) |
| Chast | Human, mouse | Aortic stenosis, hypertrophy | Regulates neighboring Plekhm1 gene (cis-regulation), which is a regulator of autophagy. | (121) |
| CHRF | Human, mouse | Heart failure, hypertrophy | Binds miR-489, which targets prohypertrophic factor Myd88 | (129) |
| DWORF | Human, mouse | Ischemic heart failure | Encodes a 34aa peptide and enhances SERCA activity for myocyte contractility. | (86) |
| H19 | Human, rat, mouse | Calcific aortic valve disease, diabetic cardiomyopathy, hypertrophy, | Encodes miR-675, which targets CaMKIId for cardiac hypertrophy, VDAC1 for cardiomyocyte apoptosis, and UPSP10 in c-kit+ cardiac progenitor cells; prevents the recruitment of p53 to the promoter of NOTCH1. | (16, 39, 72, 74) |
| LINC00323/lnc-DSCAM-1 | Human | EHTs | Binds eIF4A3 to control the translation of GATA2 mRNA. | (33) |
| lncRNA-ROR | Mouse | Hypertrophy | Binds miR-133 to attenuate the effects of cardiac hypertrophy. | (55) |
| Mhrt | Human, mouse | Hypertrophy | Binds Brg1 to interfere its genomic DNA binding capability that causes aberrant gene expression and cardiac myopathy. | (40) |
| MIAT | Rat, mouse | Angiotensin II-induced H9c2 cells | Binds miR-150, which is possibly linked to the enhancement of cardiac hypertrophy. | (149) |
| MIR503HG/lnc-PLAC1-1 | Human | EHTs | Regulates neighboring miR-424 to control angiogenesis. | (33) |
| NRF | Mouse | Hydrogen peroxide-treated neonatal cardiomyocytes | Binds miR-873, which targets procardiomyocyte necrosis factors RIPK1 and RIPK3. | (128) |
| SMILR | Human | Aortic stenosis | Regulates neighboring HAS2 gene (cis-regulation). | (5) |
| Upperhand | Mouse | Knockout mice | Its transcription (but not its RNA) is required in cis to promote Hand2 expression via GATA4 binding and super-enhancer activity. | (4) |
“EHTs” stands for “human-induced pluripotent stem cell-derived engineered heart tissues.”
aa, amino acids; APF, autophagy promoting factor; CARL, cardiac apoptosis-related lncRNA; CARMEN, CARdiac mesoderm enhancer-associated noncoding RNA; Chaer, cardiac hypertrophy-associated epigenetic regulator; Chast, cardiac hypertrophy-associated transcript; CHRF, cardiac hypertrophy-related factor; DWORF, dwarf open reading frame; ES, embryonic stem cells; Mhrt, myosin heavy chain-associated RNAtranscripts; PRC2, polycomb repressive complex 2.
As it is estimated that the number of lncRNAs exceeds that of protein-coding genes (81) and hundreds of lncRNAs have been detected in the heart (63, 91–93), the number of functionally studied lncRNAs is too low at the moment. Although the usage of knockdown technologies (e.g., siRNAs, LNA GapmeRs) is convenient, all phenotypes must be confirmed genetically using knockout (KO) mice (7). In the cardiovascular field, only a handful of lncRNAs [e.g., Chaer (132), DWORF (86), and Uph (4)] have been validated via KO mice. Thus, extensive research is needed to firmly establish the importance of lncRNAs in cardiovascular biology; in particular, their potential connections to cardiovascular disease are still poorly understood (Figs. 4 and 5).
FIG. 4.
LncRNAs involved in myocardial infarction. (A) The lncRNA APF sequesters miR-188-3p to impede its binding to the target Atg7, which results in upregulation of Atg7 to control cardiac autophagy in cardiomyocytes. (B) CARL binds miR-539, which targets Phb2 for mitochondrial fission and apoptosis in cardiomyocytes. (C) CARMEN (also known as “MIR143HG”) is transcribed from the super-enhancer region and binds PRC2 to control expressions of cardiac genes in cardiomyocytes. Of note, although located on the same locus, CARMEN gives rise to its transcripts independently from the MIR-143/145 precursor. (D) DWORF encodes a 34aa peptide and enhances SERCA activity for myocyte contractility in cardiomyocytes. aa, amino acid; DWORF, dwarf open reading frame.
FIG. 5.
LncRNAs involved in cardiac hypertrophy. (A) Chaer binds PRC2 to inhibit H3K27 methylation at the promoter regions of hypertrophy-related genes in cardiomyocytes. (B) Chast regulates neighboring Plekhm1 gene (cis-regulation), which is a regulator of autophagy in cardiomyocytes. (C) CHRF binds miR-489, which targets prohypertrophic factor Myd88 in cardiomyocytes. (D) H19 harbors miR-675, which targets CaMKIId for cardiac hypertrophy and VDAC1 for cardiomyocyte apoptosis. (E) Mhrt binds Brg1 to interfere its genomic DNA binding capability that causes aberrant gene expression and cardiac myopathy in cardiomyocytes.
Circular RNAs
Aside from lncRNAs, an emerging field is that of circular RNAs (circRNAs), which are by-products of splicing events (more specifically, “backsplicing”) of mostly protein-coding genes (10, 52, 53). As early as the 1990s, it was known that circRNAs are stable and localized predominantly in the cytoplasm (24, 88). With the increased usage of NGS, bioinformatic tools have been built to detect circRNAs from RNA-seq data [reviewed recently in Refs. (42, 110)], including those in the heart (50, 59, 114, 130, 137). When some circRNAs are knocked down via siRNAs, it has been reported that there are phenotypes observed, which may not be observed when the parental transcripts of circRNAs (e.g., protein-coding genes whose exons [or introns] give rise to circRNAs) are knocked down (10, 35). Some studies suggest that circRNAs function as miRNA sponges (34, 41, 76, 80, 148). However, recent comprehensive bioinformatic analysis (38) and our biological validation experiments (10, 136) indicate that circRNAs acting as miRNA sponges are extremely rare. A recent study suggests yet another mechanism in which circRNAs might function; that is, they encode for proteins (143). By using bioinformatic techniques and validation by biological experiments, the authors demonstrated that 25 out of 7700 circRNAs detected in human fibroblasts possess coding potential, although their functions are currently unknown.
One interesting feature of circRNAs is that they are more stable than noncircular RNAs (e.g., lncRNAs) as RNases are not easily accessible due to absence of free ends of RNA. Because of their stability, they may exist in the blood; indeed, several studies reported the detection of circRNAs in the circulation, which points to possible use of circRNAs as biomarkers (62, 77). However, more functional studies are needed to uncover the potential functions of circRNAs and their possible relationship to cardiovascular disease.
LncRNAs and Bioinformatics
As already mentioned above, RNA-seq is the preferred technology to detect lncRNAs in various experimental contexts. Although there are several well-established bioinformatic tools available to analyze RNA-seq data to detect lncRNAs [reviewed extensively in Refs. (48, 120)], depending on the genome assembly/annotation and normalization method used, the results differ significantly in the identification of lncRNAs and their expression values (2, 71, 108). The problem arises from the reference genome to be used for mapping the sequencing reads and the annotation of transcripts/genes (i.e., gene transfer format [GTF] file) (134). Furthermore, transcripts that are not included in the annotation for assembling RNA-seq alignments also cause an additional problem. Our previous study (135) shows that 77,656 novel isoforms of annotated reference transcripts and 102,848 intergenic transcripts are identified, with 58,789 (75.70%) and 101,993 (99.17%) being predicted as noncoding, respectively, from 12 human tissues (87), while 181,434 annotated transcripts (87.13% out of 208,244 transcripts in Ensembl version 77) are expressed in at least 1 of 12 tissues analyzed. Although we could validate the presence of novel lncRNAs by reverse transcription polymerase chain reaction (RT-PCR) experiments, many novel lncRNAs contain repetitive elements, such as microsatellites (9) and short interspersed nuclear elements, including ALU elements (43), suggesting that novel lncRNAs detected from RNA-seq must be examined carefully before proceeding to biological experiments.
Since the presence of many annotations for a particular lncRNA constitutes a significant problem, we developed a bioinformatic tool called “UGAHash” web tool (134). UGAHash (“Universal Genomic Accession Hash”) is an algorithm for creating consistent nonconflicting accessions for genomic features asynchronously. In our UGAHash web tool, annotations from the Ensembl (versions 60–84), LNCipedia (versions 1.2, 2.1, and 3.1) (122, 123), NCBI (versions 103–107), NONCODE (versions 4 and 2016) (140, 144, 145), and lincRNA catalog (www.broadinstitute.org/genome_bio/human_lincrnas/?q=lincRNA_catalog) are included so that when a researcher identifies a novel transcript, it is possible to check its existence in major databases above.
Although the identification of lncRNAs is important, it is only the start of a project, especially for those lncRNAs that are identified in humans. The emergence of induced pluripotent stem cells (112) and the CRISPR/Cas9 system (44, 49, 51, 57) has led to advances in functional studies using human cells, but it is still not possible to understand the functions of lncRNAs in vivo without using model organisms. Given that the sequence conservation of lncRNAs from one species to another is lower than that of protein-coding genes (28), it is a challenge to identify a homolog of a human lncRNA. To this end, instead of searching for sequence conservation, genomic positional conservation has been used recently (118, 133), which is based on the presence of conserved protein pairs, defined as the set of all pairs of adjacent protein-coding genes within an organism's genome that are also adjacent when compared to orthologs of other species. Building on this conservation of lncRNAs, we introduced a series of knowledge databases to assist researchers interested in screening for lncRNAs in humans, mice, and zebrafish: “ANGIOGENES” (85) for expression profiles of transcripts in endothelial cells; “C-It-Loci” (133) for evolutionary-conserved, tissue-enriched, protein-coding genes and lncRNAs; and “RenalDB” (136) for enriched/specific transcripts with respect to nephrotic tissues and cells, developmental stages, and other metadata. Besides our knowledge databases, there are many excellent lncRNA databases currently available (Table 2). For example, besides the hallmark databases of lncRNAs NONCODE (145), LNCipedia (122), lncRNAdb (2a, 102), and NRED (30), ALDB (69) provides expression profiles of cow, pig, and chicken lncRNAs; LncRBase (17) for expression profiles of human and mouse lncRNAs from microarray data; and lncRNAtor (96) for flies, worms, and yeasts. In the case of disease-related lncRNAs, Catalogue of Cancer Genes/lncRNAs (CCG) (75) provides manually curated information for protein-coding genes and lncRNAs in various cancers; KTCNlncDB (111) for keratoconus (KTCN) and non-KTCN human corneas; Lnc2Cancer (89) for manually curated information regarding 666 human lncRNAs from 97 human cancers; LncRNADisease (20) for both manually curated and predicted relationship to various diseases; and TRANRIC (http://ibl.mdanderson.org/tanric/_design/basic/index.html) for expression profiles from over 8000 tumor samples of ∼20 The Cancer Genome Atlas cancer types. Although not necessary in the causal relationship to diseases in most cases, LncRNASNP (37) provides single-nucleotide polymorphisms in human/mouse lncRNAs; and LncVar (21) for lncRNA-associated genetic variations in six species (human, mouse, zebrafish, worm, fruit fly, and arabidopsis). Possibly related to functional characterization of lncRNAs, Co-LncRNA (147) provides Gene Ontology annotations and KEGG pathways inferred from the coexpressed protein-coding genes; DIANA-LncBase (95) for both experimentally validated and predicted binding between lncRNAs and miRNAs; LncATLAS (78) for subcellular localization information about lncRNAs; LongTarget for the predictions of DNA binding domains and their binding sites of lncRNAs (http://lncrna.smu.edu.cn); starBase (70) for binding sites of RNA-binding proteins and miRNAs; and TF2LncRNA for the possible relationship between transcription factors and lncRNAs (56). On top of these valuable tools, UCSC Genome Browser (116) provides almost one-stop-shop for much of the information mentioned above through a genome viewer, which users could explore for their favorite lncRNAs by entering the genomic coordinates. Readers are strongly encouraged to explore these resources when starting an lncRNA project. We anticipate that these bioinformatic tools will facilitate further research into lncRNAs as many lncRNAs are expressed in the heart, while others are specifically expressed in a certain cell type and/or disease condition. With the option to identify homologs in other species, it is possible to utilize model organisms to understand the functions of human lncRNAs, which are provided in our databases for mice and zebrafish.
Table 2.
List of Long Noncoding RNA Databases
Conclusion and Outlook
Given the potential (but yet to be proven) diverse functions of lncRNAs, the most optimistic speculation is that lncRNAs might be the missing link to understand the yet unknown pathogenesis of cardiovascular disease. However, since the number of lncRNAs with known functions is very limited, considerable additional work will be needed to fully understand the impact of lncRNAs on the pathophysiology of the heart. More importantly, a paradigm shift is necessary for lncRNA research because most of the published studies are focused on their binding protein partners or miRNAs, as some lncRNAs function as miRNA sponges, which target proteins. In other words, most of the published studies are in fact analyses of proteins and/or miRNAs that consider lncRNAs as scaffolds or components of protein-centered research, based on the concept that proteins are the ultimate products controlling the pathophysiology of our body. If this concept stands, then there is a concern that lncRNAs may not be as important as one wishes, which is still early to determine because this field is only emerging. This is a concern particularly because most lncRNAs are expressed at much lower levels than protein-coding genes and proteins. However, recent reports from Eric Olson's laboratory have demonstrated that some lncRNAs are not truly noncoding, since they may code for small peptides as in the case of DWORF (86) and myoregulin (3). Since it is very hard to detect peptides less than 38 amino acids by mass spectrometry (32), mass spectrometry-based screening may not reveal such small peptides encoded by lncRNAs. Further studies are needed to understand how such small peptides impact heart functions and physiology.
As research into lncRNAs intensifies, in near future, it will yield various aspects of lncRNAs by technologically advancing the field by introducing experimental techniques that better characterize lncRNAs. Furthermore, functions of lncRNAs other than molecular scaffolds will be elucidated, thereby enhancing our understanding of the pathophysiology of organs, including the heart. Further advances in NGS and bioinformatic tools will make it possible to identify links between additional lncRNAs, including circRNAs, and certain cardiovascular pathological conditions, which may lead to the use of lncRNAs as diagnostic biomarkers in clinical settings. Given that lncRNAs are generally more cell-type specific than mRNAs and miRNAs, by carefully selecting lncRNAs enriched in a certain cell type, it might be possible to identify a better therapeutic target for a disease as in the form of “RNA therapeutics.” Finally, building on the existing knowledge, systems biology will take center stage, enabling us to predict the cause and outcome of cardiovascular disease by incorporating genes, proteins, miRNAs, and lncRNAs into one large network.
Abbreviations Used
- aa
amino acid
- APF
autophagy promoting factor
- CARL
cardiac apoptosis-related lncRNA
- CARMEN
CARdiac mesoderm enhancer-associated noncoding RNA
- Chaer
cardiac hypertrophy-associated epigenetic regulator
- Chast
cardiac hypertrophy-associated transcript
- CHRF
cardiac hypertrophy-related factor
- circRNA
circular RNA
- DWORF
dwarf open reading frame
- KO
knockout
- KTCN
keratoconus
- lincRNA
long intergenic noncoding RNA
- lncRNA
long noncoding RNA
- Mhrt
myosin heavy chain-associated RNAtranscripts
- miRNA
microRNA
- ncRNA
noncoding RNA
- NGS
next-generation sequencing
- PRC2
polycomb repressive complex 2
- RISC
RNA-induced silencing complex
- RNA-seq
RNA sequencing
- Uph
upperhand
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (UC 67/2-1 to Dr. Uchida); the startup funding from the Mansbach Family, the Gheens Foundation, and other generous supporters at the University of Louisville (to Dr. Uchida); and the National Institutes of Health grants P01 HL078825 and UM1 HL113530 (to Dr. Bolli).
References
- 1.Database Resources of the National Center for Biotechnology Information
- 2.A comprehensive assessment of RNA-seq accuracy, reproducibility and information content by the Sequencing Quality Control Consortium. Nat Biotechnol 32: 903–914, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Amaral PP, Clark Mb, Gascoigne DK, Dinger ME, and Mattick JS. lncRNAdb: a reference database for long noncoding RNAs. Nucleic Acids Res 39: D146–D151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DM, Anderson KM, Chang CL, Makarewich CA, Nelson BR, McAnally JR, Kasaragod P, Shelton JM, Liou J, Bassel-Duby R, and Olson EN. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 160: 595–606, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KM, Anderson DM, McAnally JR, Shelton JM, Bassel-Duby R, and Olson EN. Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 539: 433–436, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballantyne MD, Pinel K, Dakin R, Vesey AT, Diver L, Mackenzie R, Garcia R, Welsh P, Sattar N, Hamilton G, Joshi N, Dweck MR, Miano JM, McBride MW, Newby DE, McDonald RA, and Baker AH. Smooth muscle enriched long noncoding RNA (SMILR) regulates cell proliferation. Circulation 133: 2050–2065, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartolomei MS, Zemel S, and Tilghman SM. Parental imprinting of the mouse H19 gene. Nature 351: 153–155, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Bassett AR, Akhtar A, Barlow DP, Bird AP, Brockdorff N, Duboule D, Ephrussi A, Ferguson-Smith AC, Gingeras TR, Haerty W, Higgs DR, Miska EA, and Ponting CP. Considerations when investigating lncRNA function in vivo. Elife 3: e03058, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhartiya D, Pal K, Ghosh S, Kapoor S, Jalali S, Panwar B, Jain S, Sati S, Sengupta S, Sachidanandan C, Raghava GPS, Sivasubbu S, Scaria V, and Scaria V. lncRNome: a comprehensive knowledgebase of human long noncoding RNAs. Database (Oxford) 2013: bat034, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bidichandani SI, Ashizawa T, and Patel PI. The GAA triplet-repeat expansion in Friedreich ataxia interferes with transcription and may be associated with an unusual DNA structure. Am J Hum Genet 62: 111–121, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boeckel JN, Jae N, Heumuller AW, Chen W, Boon RA, Stellos K, Zeiher AM, John D, Uchida S, and Dimmeler S. Identification and characterization of hypoxia-regulated endothelial circular RNA. Circ Res 117: 884–890, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Borensztein M, Syx L, Ancelin K, Diabangouaya P, Picard C, Liu T, Liang JB, Vassilev I, Galupa R, Servant N, Barillot E, Surani A, Chen CJ, and Heard E. Xist-dependent imprinted X inactivation and the early developmental consequences of its failure. Nat Struct Mol Biol 24: 226–233, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borsani G, Tonlorenzi R, Simmler MC, Dandolo L, Arnaud D, Capra V, Grompe M, Pizzuti A, Muzny D, Lawrence C, Willard HF, Avner P, and Ballabio A. Characterization of a murine gene expressed from the inactive X chromosome. Nature 351: 325–329, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, and Willard HF. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349: 38–44, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Brown CJ, Lafreniere RG, Powers VE, Sebastio G, Ballabio A, Pettigrew AL, Ledbetter DH, Levy E, Craig IW, and Willard HF. Localization of the X inactivation centre on the human X chromosome in Xq13. Nature 349: 82–84, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, and Rinn JL. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev 25: 1915–1927, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai B, Ma W, Bi C, Yang F, Zhang L, Han Z, Huang Q, Ding F, Li Y, Yan G, Pan Z, Yang B, and Lu Y. Long noncoding RNA H19 mediates melatonin inhibition of premature senescence of c-kit(+) cardiac progenitor cells by promoting miR-675. J Pineal Res 61: 82–95, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Chakraborty S, Deb A, Maji RK, Saha S, and Ghosh Z. LncRBase: an enriched resource for lncRNA information. PLoS One 18: 9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan WL, Huang HD, and Chang JG. lncRNAMap: a map of putative regulatory functions in the long non-coding transcriptome. Comput Biol Chem 50: 41–49, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Charlier C, Segers K, Karim L, Shay T, Gyapay G, Cockett N, and Georges M. The callipyge mutation enhances the expression of coregulated imprinted genes in cis without affecting their imprinting status. Nat Genet 27: 367–369, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Wang Z, Wang D, Qiu C, Liu M, Chen X, Zhang Q, Yan G, and Cui Q. LncRNADisease: a database for long-non-coding RNA-associated diseases. Nucleic Acids Res 41: D983–D986, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Hao Y, Cui Y, Fan Z, He S, Luo J, and Chen R. LncVar: a database of genetic variation associated with long non-coding genes. Bioinformatics 33: 112–118, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Chu C, Qu K, Zhong FL, Artandi SE, and Chang HY. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol Cell 44: 667–678, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cifuentes-Rojas C, Hernandez AJ, Sarma K, and Lee JT. Regulatory interactions between RNA and polycomb repressive complex 2. Mol Cell 55: 171–185, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cocquerelle C, Mascrez B, Hetuin D, and Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J 7: 155–160, 1993 [DOI] [PubMed] [Google Scholar]
- 25.da Rocha ST. and Heard E. Novel players in X inactivation: insights into Xist-mediated gene silencing and chromosome conformation. Nat Struct Mol Biol 24: 197–204, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Davidovich C, Wang X, Cifuentes-Rojas C, Goodrich KJ, Gooding AR, Lee JT, and Cech TR. Toward a consensus on the binding specificity and promiscuity of PRC2 for RNA. Mol Cell 57: 552–558, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidovich C, Zheng L, Goodrich KJ, and Cech TR. Promiscuous RNA binding by Polycomb repressive complex 2. Nat Struct Mol Biol 20: 1250–1257, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, and Guigo R. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 22: 1775–1789, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Micco R, Fontanals-Cirera B, Low V, Ntziachristos P, Yuen SK, Lovell CD, Dolgalev I, Yonekubo Y, Zhang G, Rusinova E, Gerona-Navarro G, Canamero M, Ohlmeyer M, Aifantis I, Zhou MM, Tsirigos A, and Hernando E. Control of embryonic stem cell identity by BRD4-dependent transcriptional elongation of super-enhancer-associated pluripotency genes. Cell Rep 9: 234–247, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinger ME, Pang Kc, Mercer TR, Crowe ML, Grimmond SM, and Mattick JS. NRED: a database of long noncoding RNA expression. Nucleic Acids Res 37: D122–D126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan Q, Mao X, Xiao Y, Liu Z, Wang Y, Zhou H, Zhou Z, Cai J, Xia K, Zhu Q, Qi J, Huang H, Plutzky J, and Yang T. Super enhancers at the miR-146a and miR-155 genes contribute to self-regulation of inflammation. Biochim Biophys Acta 1859: 564–571, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Ezkurdia I, Juan D, Rodriguez JM, Frankish A, Diekhans M, Harrow J, Vazquez J, Valencia A, and Tress ML. Multiple evidence strands suggest that there may be as few as 19,000 human protein-coding genes. Hum Mol Genet 23: 5866–5878, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiedler J, Breckwoldt K, Remmele CW, Hartmann D, Dittrich M, Pfanne A, Just A, Xiao K, Kunz M, Muller T, Hansen A, Geffers R, Dandekar T, Eschenhagen T, and Thum T. Development of long noncoding RNA-based strategies to modulate tissue vascularization. J Am Coll Cardiol 66: 2005–2015, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geng HH, Li R, Su YM, Xiao J, Pan M, Cai XX, and Ji XP. The circular RNA Cdr1as promotes myocardial infarction by mediating the regulation of miR-7a on its target genes expression. PLoS One 11: e0151753, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerstner S, Kohler W, Heidkamp G, Purbojo A, Uchida S, Ekici AB, Heger L, Luetke-Eversloh M, Schubert R, Bader P, Klingebiel T, Koehl U, Mackensen A, Romagnani C, Cesnjevar R, Dudziak D, and Ullrich E. Specific phenotype and function of CD56-expressing innate immune cell subsets in human thymus. J Leukoc Biol 100: 1297–1310, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Goff LA. and Rinn JL. Linking RNA biology to lncRNAs. Genome Res 25: 1456–1465, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong J, Liu W, Zhang J, Miao X, and Guo AY. lncRNASNP: a database of SNPs in lncRNAs and their potential functions in human and mouse. Nucleic Acids Res 43: D181–D186, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo JU, Agarwal V, Guo H, and Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol 15: 409, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadji F, Boulanger MC, Guay SP, Gaudreault N, Amellah S, Mkannez G, Bouchareb R, Tremblay-Marchand J, Nsaibia MJ, Guauque-Olarte S, Pibarot P, Bouchard L, Bosse Y, and Mathieu P. Altered DNA methylation of long non-coding RNA H19 in calcific aortic valve disease promotes mineralization by silencing NOTCH1. Circulation 134: 1848–1862, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Han P, Li W, Lin CH, Yang J, Shang C, Nurnberg ST, Jin KK, Xu W, Lin CY, Lin CJ, Xiong Y, Chien HC, Zhou B, Ashley E, Bernstein D, Chen PS, Chen HS, Quertermous T, and Chang CP. A long noncoding RNA protects the heart from pathological hypertrophy. Nature 514: 102–106, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, and Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature 495: 384–388, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Hansen TB, Veno MT, Damgaard CK, and Kjems J. Comparison of circular RNA prediction tools. Nucleic Acids Res 44: e58, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasler J. and Strub K. Alu elements as regulators of gene expression. Nucleic Acids Res 34: 5491–5497, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, and Doudna JA. Sequence- and structure-specific RNA processing by a CRISPR endonuclease. Science 329: 1355–1358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-Andre V, Sigova AA, Hoke HA, and Young RA. Super-enhancers in the control of cell identity and disease. Cell 155: 934–947, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hon CC, Ramilowski JA, Harshbarger J, Bertin N, Rackham OJ, Gough J, Denisenko E, Schmeier S, Poulsen TM, Severin J, Lizio M, Kawaji H, Kasukawa T, Itoh M, Burroughs AM, Noma S, Djebali S, Alam T, Medvedeva YA, Testa AC, Lipovich L, Yip CW, Abugessaisa I, Mendez M, Hasegawa A, Tang D, Lassmann T, Heutink P, Babina M, Wells CA, Kojima S, Nakamura Y, Suzuki H, Daub CO, de Hoon MJ, Arner E, Hayashizaki Y, Carninci P, and Forrest AR. An atlas of human long non-coding RNAs with accurate 5' ends. Nature 543: 199–204, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang HW, Wentzel EA, and Mendell JT. A hexanucleotide element directs microRNA nuclear import. Science 315: 97–100, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Ilott NE. and Ponting CP. Predicting long non-coding RNAs using RNA sequencing. Methods 63: 50–59, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Ishino Y, Shinagawa H, Makino K, Amemura M, and Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol 169: 5429–5433, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jakobi T, Czaja-Hasse LF, Reinhardt R, and Dieterich C. Profiling and validation of the circular RNA repertoire in adult murine hearts. Genomics Proteomics Bioinformatics 14: 216–223, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jansen R, Embden JD, Gaastra W, and Schouls LM. Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol 43: 1565–1575, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Jeck WR. and Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol 32: 453–461, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, and Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19: 141–157, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeffries CD, Fried HM, and Perkins DO. Nuclear and cytoplasmic localization of neural stem cell microRNAs. RNA 17: 675–686, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang F, Zhou X, and Huang J. Long non-coding RNA-ROR mediates the reprogramming in cardiac hypertrophy. PLoS One 11: e0152767, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang Q, Wang J, Wang Y, Ma R, Wu X, and Li Y. TF2LncRNA: identifying common transcription factors for a list of lncRNA genes from ChIP-Seq data. Biomed Res Int 2014: 317642, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, and Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katz S, Cussigh D, Urban N, Blomfield I, Guillemot F, Bally-Cuif L, and Coolen M. A Nuclear role for miR-9 and argonaute proteins in balancing quiescent and activated neural stem cell states. Cell Rep 17: 1383–1398, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan MA, Reckman YJ, Aufiero S, van den Hoogenhof MM, van der Made I, Beqqali A, Koolbergen DR, Rasmussen TB, van der Velden J, Creemers EE, and Pinto YM. RBM20 regulates circular RNA production from the titin gene. Circ Res 119: 996–1003, 2016 [DOI] [PubMed] [Google Scholar]
- 60.Kim DH, Saetrom P, Snove O, Jr., and Rossi JJ. MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc Natl Acad Sci U S A 105: 16230–16235, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, Abo R, Tabebordbar M, Lee RT, Burge CB, and Boyer LA. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 152: 570–583, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kulcheski FR, Christoff AP, and Margis R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J Biotechnol 238: 42–51, 2016 [DOI] [PubMed] [Google Scholar]
- 63.Kurian L, Aguirre A, Sancho-Martinez I, Benner C, Hishida T, Nguyen TB, Reddy P, Nivet E, Krause MN, Nelles DA, Rodriguez Esteban C, Campistol JM, Yeo GW, and Izpisua Belmonte JC. Identification of novel long noncoding RNAs underlying vertebrate cardiovascular development. Circulation 131: 1278–1290, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Raymond C, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowski J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, and Chen YJ. Initial sequencing and analysis of the human genome. Nature 409: 860–921, 2001 [DOI] [PubMed] [Google Scholar]
- 65.Latos PA, Pauler FM, Koerner MV, Senergin HB, Hudson QJ, Stocsits RR, Allhoff W, Stricker SH, Klement RM, Warczok KE, Aumayr K, Pasierbek P, and Barlow DP. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science 338: 1469–1472, 2012 [DOI] [PubMed] [Google Scholar]
- 66.Lee JT, Davidow LS, and Warshawsky D. Tsix, a gene antisense to Xist at the X-inactivation centre. Nat Genet 21: 400–404, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Lee JT. and Lu N. Targeted mutagenesis of Tsix leads to nonrandom X inactivation. Cell 99: 47–57, 1999 [DOI] [PubMed] [Google Scholar]
- 68.Leucci E, Patella F, Waage J, Holmstrom K, Lindow M, Porse B, Kauppinen S, and Lund AH. microRNA-9 targets the long non-coding RNA MALAT1 for degradation in the nucleus. Sci Rep 3: 2535, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li A, Zhang J, Zhou Z, Wang L, Liu Y, and Liu Y. ALDB: a domestic-animal long noncoding RNA database. PLoS One 10: e0124003, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li JH, Liu S, Zhou H, Qu L-H, and Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res 42: D92–D97, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li P, Piao Y, Shon HS, and Ryu KH. Comparing the normalization methods for the differential analysis of Illumina high-throughput RNA-Seq data. BMC Bioinformatics 16: 347, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li X, Wang H, Yao B, Xu W, Chen J, and Zhou X. lncRNA H19/miR-675 axis regulates cardiomyocyte apoptosis by targeting VDAC1 in diabetic cardiomyopathy. Sci Rep 6: 36340, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liao Q, Xiao H, Bu D, Xie C, Miao R, Luo H, Zhao G, Yu K, Zhao H, Skogerbo G, Chen R, Wu Z, Liu C, and Zhao Y. ncFANs: a web server for functional annotation of long non-coding RNAs. Nucleic Acids Res 39: W118–W124, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu L, An X, Li Z, Song Y, Li L, Zuo S, Liu N, Yang G, Wang H, Cheng X, Zhang Y, Yang X, and Wang J. The H19 long noncoding RNA is a novel negative regulator of cardiomyocyte hypertrophy. Cardiovasc Res 111: 56–65, 2016 [DOI] [PubMed] [Google Scholar]
- 75.Liu M, Yang YT, Xu G, Tan C, and Lu ZJ. CCG: an integrative resource of cancer protein-coding genes and long noncoding RNAs. Discov Med 22: 351–359, 2016 [PubMed] [Google Scholar]
- 76.Liu Q, Zhang X, Hu X, Dai L, Fu X, Zhang J, and Ao Y. Circular Rna related to the chondrocyte ECM regulates MMP13 expression by functioning as a MiR-136 ‘sponge’ in human cartilage degradation. Sci Rep 6: 22572, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lyu D. and Huang S. The emerging role and clinical implication of human exonic circular RNA. RNA Biol 14: 1000–1006, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mas-Ponte DA-OhooX, Carlevaro-Fita JA-Ohoo, Palumbo E, Hermoso Pulido TA-Ohoo, Guigo RA-Ohoo, and Johnson RA-Ohoo. LncATLAS database for subcellular localisation of long noncoding RNAs. RNA 23: 1080–1087, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McHugh CA, Chen CK, Chow A, Surka CF, Tran C, McDonel P, Pandya-Jones A, Blanco M, Burghard C, Moradian A, Sweredoski MJ, Shishkin AA, Su J, Lander ES, Hess S, Plath K, and Guttman M. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 521: 232–236, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, and Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495: 333–338, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Mercer TR, Gerhardt DJ, Dinger ME, Crawford J, Trapnell C, Jeddeloh JA, Mattick JS, and Rinn JL. Targeted RNA sequencing reveals the deep complexity of the human transcriptome. Nat Biotechnol 30: 99–104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Michalik KM, You X, Manavski Y, Doddaballapur A, Zornig M, Braun T, John D, Ponomareva Y, Chen W, Uchida S, Boon RA, and Dimmeler S. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res 114: 1389–1397, 2014 [DOI] [PubMed] [Google Scholar]
- 83.Miyoshi N, Wagatsuma H, Wakana S, Shiroishi T, Nomura M, Aisaka K, Kohda T, Surani MA, Kaneko-Ishino T, and Ishino F. Identification of an imprinted gene, Meg3/Gtl2 and its human homologue MEG3, first mapped on mouse distal chromosome 12 and human chromosome 14q. Genes Cells 5: 211–220, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Montano MM, Ekena K, Delage-Mourroux R, Chang W, Martini P, and Katzenellenbogen BS. An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc Natl Acad Sci U S A 96: 6947–6952, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muller R, Weirick T, John D, Militello G, Chen W, Dimmeler S, and Uchida S. ANGIOGENES: knowledge database for protein-coding and noncoding RNA genes in endothelial cells. Sci Rep 6: 32475, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nelson BR, Makarewich CA, Anderson DM, Winders BR, Troupes CD, Wu F, Reese AL, McAnally JR, Chen X, Kavalali ET, Cannon SC, Houser SR, Bassel-Duby R, and Olson EN. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 351: 271–275, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nielsen MM, Tehler D, Vang S, Sudzina F, Hedegaard J, Nordentoft I, Orntoft TF, Lund AH, and Pedersen JS. Identification of expressed and conserved human noncoding RNAs. RNA 20: 236–251, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, Kinzler KW, and Vogelstein B. Scrambled exons. Cell 64: 607–613, 1991 [DOI] [PubMed] [Google Scholar]
- 89.Ning S, Zhang J, Wang P, Zhi H, Wang J, Liu Y, Gao Y, Guo M, Yue M, Wang L, and Li X. Lnc2Cancer: a manually curated database of experimentally supported lncRNAs associated with various human cancers. Nucleic Acids Res 44: D980–D985, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohrt T, Mutze J, Staroske W, Weinmann L, Hock J, Crell K, Meister G, and Schwille P. Fluorescence correlation spectroscopy and fluorescence cross-correlation spectroscopy reveal the cytoplasmic origination of loaded nuclear RISC in vivo in human cells. Nucleic Acids Res 36: 6439–6449, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ounzain S, Micheletti R, Arnan C, Plaisance I, Cecchi D, Schroen B, Reverter F, Alexanian M, Gonzales C, Ng SY, Bussotti G, Pezzuto I, Notredame C, Heymans S, Guigo R, Johnson R, and Pedrazzini T. CARMEN, a human super enhancer-associated long noncoding RNA controlling cardiac specification, differentiation and homeostasis. J Mol Cell Cardiol 89: 98–112, 2015 [DOI] [PubMed] [Google Scholar]
- 92.Ounzain S, Micheletti R, Beckmann T, Schroen B, Alexanian M, Pezzuto I, Crippa S, Nemir M, Sarre A, Johnson R, Dauvillier J, Burdet F, Ibberson M, Guigo R, Xenarios I, Heymans S, and Pedrazzini T. Genome-wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart-specific long non-coding RNAs. Eur Heart J 36: 353–368a, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ounzain S, Pezzuto I, Micheletti R, Burdet F, Sheta R, Nemir M, Gonzales C, Sarre A, Alexanian M, Blow MJ, May D, Johnson R, Dauvillier J, Pennacchio LA, and Pedrazzini T. Functional importance of cardiac enhancer-associated noncoding RNAs in heart development and disease. J Mol Cell Cardiol 76: 55–70, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paralkar VR, Taborda CC, Huang P, Yao Y, Kossenkov AV, Prasad R, Luan J, Davies JO, Hughes JR, Hardison RC, Blobel GA, and Weiss MJ. Unlinking an lncRNA from its associated cis element. Mol Cell 62: 104–110, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paraskevopoulou MD, Vlachos IS, Karagkouni D, Georgakilas G, Kanellos I, Vergoulis T, Zagganas K, Tsanakas P, Floros E, Dalamagas T, and Hatzigeorgiou AG. DIANA-LncBase v2: indexing microRNA targets on non-coding transcripts. Nucleic Acids Res 44: D231–D238, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park C, Yu N, Choi I, Kim W, and Lee S. lncRNAtor: a comprehensive resource for functional investigation of long non-coding RNAs. Bioinformatics 30: 2480–2485, 2014 [DOI] [PubMed] [Google Scholar]
- 97.Pefanis E, Wang J, Rothschild G, Lim J, Kazadi D, Sun J, Federation A, Chao J, Elliott O, Liu ZP, Economides AN, Bradner JE, Rabadan R, and Basu U. RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell 161: 774–789, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peters J. The role of genomic imprinting in biology and disease: an expanding view. Nat Rev Genet 15: 517–530, 2014 [DOI] [PubMed] [Google Scholar]
- 99.Place RF, Li LC, Pookot D, Noonan EJ, and Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci U S A 105: 1608–1613, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Plaisance I, Perruchoud S, Fernandez-Tenorio M, Gonzales C, Ounzain S, Ruchat P, Nemir M, Niggli E, and Pedrazzini T. Cardiomyocyte lineage specification in adult human cardiac precursor cells via modulation of enhancer-associated long noncoding RNA expression. JACC 1: 472–493, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Politz JC, Hogan EM, and Pederson T. MicroRNAs with a nucleolar location. Rna 15: 1705–1715, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quek XC, Thomson DW, Maag JL, Bartonicek N, Signal B, Clark MB, Gloss BS, and Dinger ME. lncRNAdb v2.0: expanding the reference database for functional long noncoding RNAs. Nucleic Acids Res 43: D168–D173, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rachmilewitz J, Goshen R, Ariel I, Schneider T, de Groot N, and Hochberg A. Parental imprinting of the human H19 gene. FEBS Lett 309: 25–28, 1992 [DOI] [PubMed] [Google Scholar]
- 104.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, and Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129: 1311–1323, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ruffier M, Kahari A, Komorowska M, Keenan S, Laird M, Longden I, Proctor G, Searle S, Staines D, Taylor K, Vullo A, Yates A, Zerbino D, and Flicek P. Ensembl core software resources: storage and programmatic access for DNA sequence and genome annotation. Database (Oxford) 2017. DOI: 10.1093/database/bax020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sarma K, Cifuentes-Rojas C, Ergun A, Del Rosario A, Jeon Y, White F, Sadreyev R, and Lee JT. ATRX directs binding of PRC2 to Xist RNA and Polycomb targets. Cell 159: 869–883, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stratton MS, Lin CY, Anand P, Tatman PD, Ferguson BS, Wickers ST, Ambardekar AV, Sucharov CC, Bradner JE, Haldar SM, and McKinsey TA. Signal-dependent recruitment of BRD4 to cardiomyocyte super-enhancers is suppressed by a microRNA. Cell Rep 16: 1366–1378, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun Z. and Zhu Y. Systematic comparison of RNA-Seq normalization methods using measurement error models. Bioinformatics 28: 2584–2591, 2012 [DOI] [PubMed] [Google Scholar]
- 109.Suzuki HI, Young RA, and Sharp PA. Super-enhancer-mediated RNA Processing revealed by integrative microRNA network analysis. Cell 168: 1000–1014.e15, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Szabo L. and Salzman J. Detecting circular RNAs: bioinformatic and experimental challenges. Nat Rev Genet 17: 679–692, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Szczesniak MW, Kabza M, Karolak JA, Rydzanicz M, Nowak DM, Ginter-Matuszewska B, Polakowski P, Ploski R, Szaflik JP, and Gajecka M. KTCNlncDB-a first platform to investigate lncRNAs expressed in human keratoconus and non-keratoconus corneas. Database (Oxford) 2017: baw168, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, and Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872, 2007 [DOI] [PubMed] [Google Scholar]
- 113.Tan JY, Smith AA, Ferreira da Silva M, Matthey-Doret C, Rueedi R, Sonmez R, Ding D, Kutalik Z, Bergmann S, and Marques AC. cis-acting complex-trait-associated lincRNA expression correlates with modulation of chromosomal architecture. Cell Rep 18: 2280–2288, 2017 [DOI] [PubMed] [Google Scholar]
- 114.Tan WL, Lim BT, Anene-Nzelu CG, Ackers-Johnson M, Dashi A, See K, Tiang Z, Lee DP, Chua WW, Luu TD, Li PY, Richards AM, and Foo RS. A landscape of circular RNA expression in the human heart. Cardiovasc Res 113: 298–309, 2017 [DOI] [PubMed] [Google Scholar]
- 115.Tang R, Li L, Zhu D, Hou D, Cao T, Gu H, Zhang J, Chen J, Zhang CY, and Zen K. Mouse miRNA-709 directly regulates miRNA-15a/16-1 biogenesis at the posttranscriptional level in the nucleus: evidence for a microRNA hierarchy system. Cell Res 22: 504–515, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tyner C, Barber GP, Casper J, Clawson H, Diekhans M, Eisenhart C, Fischer CM, Gibson D, Gonzalez JN, Guruvadoo L, Haeussler M, Heitner S, Hinrichs AS, Karolchik D, Lee BTA-Ohoo, Lee CMA-Ohoo, Nejad P, Raney BJ, Rosenbloom KR, Speir ML, Villarreal C, Vivian J, Zweig AS, Haussler D, Kuhn RM, and Kent WJ. The UCSC Genome Browser database: 2017 update. Nucleic Acids Res 45: D626–D634, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Uchida S. and Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circ Res 116: 737–750, 2015 [DOI] [PubMed] [Google Scholar]
- 118.Ulitsky I, Shkumatava A, Jan CH, Sive H, and Bartel DP. Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell 147: 1537–1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vallot C, Patrat C, Collier AJ, Huret C, Casanova M, Liyakat Ali TM, Tosolini M, Frydman N, Heard E, Rugg-Gunn PJ, and Rougeulle C. XACT noncoding RNA competes with XIST in the control of X chromosome activity during human early development. Cell Stem Cell 20: 102–111, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Veneziano D, Nigita G, Ferro A, Ilott NE, and Ponting CP. Computational approaches for the analysis of ncRNA through deep sequencing techniques. Front Bioeng Biotechnol 3: 77, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Viereck J, Kumarswamy R, Foinquinos A, Xiao K, Avramopoulos P, Kunz M, Dittrich M, Maetzig T, Zimmer K, Remke J, Just A, Fendrich J, Scherf K, Bolesani E, Schambach A, Weidemann F, Zweigerdt R, de Windt LJ, Engelhardt S, Dandekar T, Batkai S, and Thum T. Long noncoding RNA Chast promotes cardiac remodeling. Sci Transl Med 8: 326ra22, 2016 [DOI] [PubMed] [Google Scholar]
- 122.Volders PJ, Verheggen K, Menschaert G, Vandepoele K, Martens L, Vandesompele J, and Mestdagh P. An update on LNCipedia: a database for annotated human lncRNA sequences. Nucleic Acids Res 43: D174–D180, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Volders PJ, Helsens K, Wang X, Menten B, Martens L, Gevaert K, Vandesompele J, and Mestdagh P. LNCipedia: a database for annotated human lncRNA transcript sequences and structures. Nucleic Acids Res 41: D246–D251, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. This reference has been deleted.
- 125. This reference has been deleted.
- 126.Waddington CH. Organisers and Genes. Cambridge, UK University Press, 1940. x + 160 p [Google Scholar]
- 127.Wang JX, Gao J, Ding SL, Wang K, Jiao JQ, Wang Y, Sun T, Zhou LY, Long B, Zhang XJ, Li Q, Liu JP, Feng C, Liu J, Gong Y, Zhou Z, and Li PF. Oxidative modification of miR-184 enables it to target Bcl-xL and Bcl-w. Mol Cell 59: 50–61, 2015 [DOI] [PubMed] [Google Scholar]
- 128.Wang K, Liu F, Liu CY, An T, Zhang J, Zhou LY, Wang M, Dong YH, Li N, Gao JN, Zhao YF, and Li PF. The long noncoding RNA NRF regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ 23: 1394–1405, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang K, Liu F, Zhou LY, Long B, Yuan SM, Wang Y, Liu CY, Sun T, Zhang XJ, and Li PF. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res 114: 1377–1388, 2014 [DOI] [PubMed] [Google Scholar]
- 130.Wang K, Long B, Liu F, Wang JX, Liu CY, Zhao B, Zhou LY, Sun T, Wang M, Yu T, Gong Y, Liu J, Dong YH, Li N, and Li PF. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J 37: 2602–2611, 2016 [DOI] [PubMed] [Google Scholar]
- 131.Wang K, Long B, Zhou LY, Liu F, Zhou QY, Liu CY, Fan YY, and Li PF. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun 5: 3596, 2014 [DOI] [PubMed] [Google Scholar]
- 132.Wang Z, Zhang XJ, Ji YX, Zhang P, Deng KQ, Gong J, Ren S, Wang X, Chen I, Wang H, Gao C, Yokota T, Ang YS, Li S, Cass A, Vondriska TM, Li G, Deb A, Srivastava D, Yang HT, Xiao X, Li H, and Wang Y. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat Med 22: 1131–1139, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weirick T, John D, Dimmeler S, and Uchida S. C-It-Loci: a knowledge database for tissue-enriched loci. Bioinformatics 31: 3537–3543, 2015 [DOI] [PubMed] [Google Scholar]
- 134.Weirick T, John D, and Uchida S. Resolving the problem of multiple accessions of the same transcript deposited across various public databases. Brief Bioinform 18: 226–235, 2016 [DOI] [PubMed] [Google Scholar]
- 135.Weirick T, Militello G, Muller R, John D, Dimmeler S, and Uchida S. The identification and characterization of novel transcripts from RNA-seq data. Brief Bioinform 17: 678–685, 2016 [DOI] [PubMed] [Google Scholar]
- 136.Weirick T, Militello G, Ponomareva Y, John D, Doring C, Dimmeler S, and Uchida S. Logic programming to infer complex RNA expression patterns from RNA-seq data. Brief Bioinform 2016. DOI: 10.1093/bib/bbw117 [DOI] [PubMed] [Google Scholar]
- 137.Werfel S, Nothjunge S, Schwarzmayr T, Strom TM, Meitinger T, and Engelhardt S. Characterization of circular RNAs in human, mouse and rat hearts. J Mol Cell Cardiol 98: 103–107, 2016 [DOI] [PubMed] [Google Scholar]
- 138.Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, and Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153: 307–319, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xiang JF, Yin QF, Chen T, Zhang Y, Zhang XO, Wu Z, Zhang S, Wang HB, Ge J, Lu X, Yang L, and Chen LL. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res 24: 513–531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xie C, Yuan J, Li H, Li M, Zhao G, Bu D, Zhu W, Wu W, Chen R, and Zhao Y. NONCODEv4: exploring the world of long non-coding RNA genes. Nucleic Acids Res 42: D98–D103, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xue Z, Hennelly S, Doyle B, Gulati AA, Novikova IV, Sanbonmatsu KY, and Boyer LA. A G-rich motif in the lncRNA braveheart interacts with a zinc-finger transcription factor to specify the cardiovascular lineage. Mol Cell 64: 37–50, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang JH, Li JH, Jiang S, Zhou H, and Qu LH. ChIPBase: a database for decoding the transcriptional regulation of long non-coding RNA and microRNA genes from ChIP-Seq data. Nucleic Acids Res 41: D177–D187, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen L, Wang Y, Wong CC, Xiao X, and Wang Z. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res 27: 626–641, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao Y, Li H, Fang S, Kang Y, Wu W, Hao Y, Li Z, Bu D, Sun N, Zhang MQ, and Chen R. NONCODE 2016: an informative and valuable data source of long non-coding RNAs. Nucleic Acids Res 44: D203–D208, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao Y, Yuan J, and Chen R. NONCODEv4: annotation of noncoding RNAs with emphasis on long noncoding RNAs. Methods Mol Biol 1402: 243–254, 2016 [DOI] [PubMed] [Google Scholar]
- 146. This reference has been deleted.
- 147.Zhao Z, Bai J, Wu A, Wang Y, Zhang J, Wang Z, Li Y, Xu J, and Li X. Co-LncRNA: investigating the lncRNA combinatorial effects in GO annotations and KEGG pathways based on human RNA-Seq data. Database (Oxford) 2015: pii:, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, Luo Y, Lyu D, Li Y, Shi G, Liang L, Gu J, He X, and Huang S. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun 7: 11215, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhu XH, Yuan YX, Rao SL, and Wang P. LncRNA MIAT enhances cardiac hypertrophy partly through sponging miR-150. Eur Rev Med Pharmacol Sci 20: 3653–3660, 2016 [PubMed] [Google Scholar]