Abstract
A major challenge in tissue engineering is the generation of sufficient volumes of viable tissue for organ transplant. The development of a stable, mature vasculature is required to sustain the metabolic and functional activities of engineered tissues. Adipose stromal vascular fraction (SVF) cells are an easily accessible, heterogeneous cell system comprised of endothelial cells, macrophages, pericytes, and various stem cell populations. Collectively, SVF has been shown to spontaneously form vessel-like networks in vitro and robust, patent, and functional vasculatures in vivo. Capitalizing on this ability, we and others have demonstrated adipose SVF's utility in generating and augmenting engineered liver, cardiac, and vascular tissues, to name a few. This review highlights the scientific origins of SVF, the use of SVF as a clinically relevant vascular source, various SVF constituents and their roles, and practical considerations associated with isolating SVF for various tissue engineering applications.
Keywords: : stromal vascular fraction, vasculature, lipoaspirate, adipose tissue
Introduction
Efforts to engineer replacement tissues have yielded significant advances, from the production of simple conduits and reservoirs such as tracheas1 and urethras2 to the development of cardiac,3 kidney,4 and liver tissue mimics.5–7 However, because complex organ systems require an operative vasculature to meet their metabolic demands, a key challenge facing tissue-engineered structures is the development and presence of a stable, functional vascular supply.
As the largest endocrine organ in the body,8 adipose utilizes a complex vasculature to influence systemic processes, from insulin sensitivity to inflammation and immunological functions.9,10 Autologous adipose tissue has been used to fill soft tissue defects for decades11–14 and already has broad clinical relevance.15–17 It can also be processed to yield a distinct, heterogeneous cellular mixture devoid of adipocytes known as the stromal vascular fraction (SVF),18–21 which can self-assemble into complex vascular networks22 that are hierarchical, highly branched, and perfused6 (Fig. 1). This, in conjunction with the fact that SVF can be easily obtained from lipoaspirates at the point of care, makes it an attractive option for providing support to engineered tissues.23
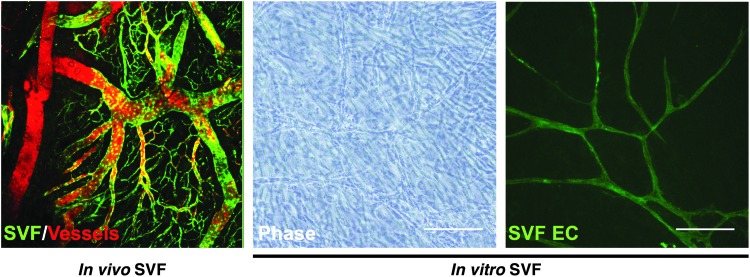
FIG. 1.
In vivo and In vitro SVF microvasculature. SVF cells from GFP+ rat were suspended in a three-dimensional collagen-I matrix and implanted subcutaneously for 4 weeks. SVF (green fluorescence) spontaneously self-assembled a hierarchical, mature, and patent vasculature. This vasculature anastomoses with the host circulation (GFP− Dextran-TRITC+) and demonstrates evidence of perfusion (GFP+ Dextran-TRITC+). The ability of the SVF cells to self-organize into a functional vasculature has therapeutic implications in a multitude of tissue engineering applications (Image credit: Dr. James Hoying). When SVF cells are plated in vitro, the SVF-derived endothelial cells (Ulex Europaeus Agglutinin-I-fluorescein+) spontaneously self-assemble an endothelial network within the overlying stromal cells. Scale bar = 10×, 200μm. SVF EC, stromal vascular fraction endothelial cells. Color images available online at www.liebertpub.com/teb
Although several efforts have used adipose-derived homogeneous cell populations for stromal and/or vascular support, it is important to recognize that SVF is a complex cellular system—and not simply a uniform cell type—that has clinically relevant, therapeutic potential. The purpose of this work is not only to highlight the utilities of the various cell types within SVF, but also to distinguish it from other similar cell types and sources. From this, we will also discuss future avenues to consider to further enhance SVF's relevance in research and clinical endeavors.
A Concise History of SVF
In the mid 1960s, nutrition and endocrinology chemist Rodbell performed the first experiments that isolated SVF from rats.18–21 Epididymal adipose tissue was exposed to collagenase before being centrifuged, resulting in three layers of cell populations with differing densities. The top layer was comprised of fat and buoyant adipocytes, whereas the middle layer contained tumescent fluid and excess collagenase solution. The cells at the very bottom—a pellet containing a mixture of cells (such as endothelial cells [EC], mast cells, stem cells, and macrophages)—formed the SVF.
While the subject of Rodbell's works during this time chiefly dealt with the metabolic properties of the adipocytes, his isolation of SVF led to subsequent pioneering efforts by Wagner et al. in the 1970s. In this setting, the adipose tissue was digested and centrifuged twice in an effort to isolate just the capillary EC, on which Wagner then performed studies to characterize the endothelial adenylate cyclase activity.24 Later that decade, in 1975, Wagner and Mathews further perfected their isolation of capillary endothelium from adipose SVF by adding thimerosal to further eliminate nonEC from the fraction.25 These polygonal cells formed a monolayer in two-dimensional culture characterized by vesicles and numerous intracellular junctions.
Although Rodbell, and Wagner et al. used adipose tissue or adipose SVF derivatives to study the biochemical processes of individual cell types, their methods facilitated efforts that utilized SVF in its entirety. For instance, in 1976, Van et al. characterized the doubling time of cultured SVF cells as between 40 and 60 h.26 In this setting, cultured SVF cells eventually formed a predominantly fibroblastic monolayer that also internalized lipids—a characteristic not observed with skin fibroblasts. Because these cells also synthesized and hydrolyzed triglycerides, a significant proportion of cultured SVF cells were thought to be adipocyte precursors.
In the late 1980s, Jarrell, Williams, and Rupnick, et al. isolated EC from human adipose SVF to endothelialize surgical vascular grafts.27,28 This research was later expanded to include point-of-care applications in which SVF could theoretically be isolated and delivered at the bedside perioperatively.29 Williams, Hoying, et al. also contributed to a greater understanding of the effects of enzymes on adipose digestion,30 differences in site-specific adipose isolation31 (also discussed in greater depth later-on in this review), and the formation of adipose-derived vascular networks in three-dimensional engineered structures32—all issues that critically affect the role and performance of SVF in tissue-engineered applications.
Thus, by the mid 1990s, SVF had been identified and several studies seeking to understand its capabilities and composition had already been conducted or were underway. The next section of this review will focus on several of the cell types present within SVF and their contributions to this dynamic mixture.
SVF Characterization and Isolation
SVF is a heterogeneous, versatile, and clinically relevant cell system. The interaction of these cell types contributes to SVF's overall therapeutic potential. Below, we discuss some of the cell types present as well as their discovered roles and mechanisms of action.
SVF composition
Today, SVF is known to have fibroblasts, mesenchymal stem cells (MSC), and EC, as well as smooth muscle cells, mural cells, macrophages, blood cells, and a whole cadre of other stem cell phenotypes (outlined in Table 1).6,16,22,33–38 While this mixed population more closely recapitulates the variety of cells seen in vivo, there is an overall lack of consensus regarding the specific proportions of these constituents to one another.39 Contributing to this is the fact that the SVF composition is dependent on a variety of factors, such as the adipose isolation site, processing methods, and the patient's own pathological status.40
Table 1.
Identified Multicellular Composition of Adipose Stromal Vascular Fraction
| Cell type | References in this review |
|---|---|
| Endothelial cells | 6,22,31,35,36,41,43,45,48 |
| Smooth muscle cells | 6,22,31 |
| Fibroblasts | 6,22,31 |
| Pericytes/perivascular cells/mural cells | 6,22,31,37 |
| Macrophages/monocytes/lymphocytes/immune cells | 6,22,31,35,41,75,76 |
| Adipose (-derived) stem cells/mesenchymal stem cells/other stem cell phenotypes | 6,31,33,35,36,49,50,67,68,69 |
In our initial analysis of the cell proportions within SVF, we processed rodent SVF cells through a fluorescence-activated cell sorting system to identify five populations: CD31+ EC, CD14+ monocytes and macrophages, CXCR4+/cMet+ multipotent cells, cKit+ progenitor cells, and PDGFR-B+ perivascular cells.6 We took this a step further by then examining differences in the proportions of these cells in fresh versus cultured SVF isolates. We observed that ∼33% of freshly isolated SVF cells were comprised of EC, and that culturing the SVF cells significantly reduced this number to ∼10% of the total population. Similar trends were observed with CD14+ monocytes/macrophages, which declined from ∼22% to ∼18%; with c-Kit+ progenitors, from ∼5% to ∼1%; with CXCR4+ multipotent cells, from ∼2.5% to ∼0%; and with PDGFR-B+ perivascular cells, from ∼20% to ∼18%—all due to culturing. From this preliminary study and in vivo experiments utilizing both SVF populations, it was abundantly clear that culturing the cells for as little as one passage, profoundly altered their cellular composition and resulting vascular phenotype.
Morris et al. examined the SVF stromal cells with greater specificity, focusing particularly on the proportions of CD11b+ innate immune cells, F4/80+ tissue macrophages, Gr-1+ myeloid cells, and CD2+ lymphocytes in relation to the amount of Tie2-GFP+ EC.41 Of these, ∼67% of the SVF cells were comprised of innate immune (∼20%), endothelial (∼25%), and myeloid cells (∼22%). To compare, Dong et al. reported that SVF is rich in blood-derived cells, adipose stromal cells (CD34+), and EC,35 whereas Klar et al. divided SVF into four populations—EC (CD31, CD34, and CD146), MSC (CD44, CD73, CD90, and CD105), stem cells (CD49f, CD117, CD133), and myeloid hematopoietic stem cells (CD14, CD15, CD45).36 To add to these descriptions, Silva et al. characterized the SVF adipose stromal/stem cell (ASC) population as being comprised of pericytes (CD45− CD146+ CD34−) and supra-adventitial preadipocyte-like cells (CD45− CD146− CD34+).37
This constellation of markers and designations makes for a somewhat confusing mosaic, highlighting the need for a standardized marker set by which different groups can compare the efficiencies and compositions of their SVF isolations. Part of this perplexity can be attributed to a lack of specific, unique markers for cells such as ASC39 or pericytes,42 for example. Additionally, the use of cultured cells over fresh isolates inherently selects for cells that adhere to tissue culture plastic within a defined timeframe, potentially skewing the distribution of the various constituents. Despite these challenges, what is clear is that the SVF is a dynamic population of cells with a potentially significant clinical utility.
Endothelial cells
One of the hallmark characteristics of adipose SVF cells is their ability to self-assemble into a hierarchical, branched, perfused vasculature in vivo6,22,43—characteristics that are associated with vascular maturity.44 While this capability requires a carefully orchestrated interplay between several cell types, the EC in SVF are critical to its formation of a functional vasculature.
In the early 1980s, Madri and Williams demonstrated that communications between the SVF EC and surrounding connective tissue dictated the cells' propensity to proliferate and form sheets, or to aggregate into tube-like structures.45 The introduction of interstitial collagen to SVF EC cultures precipitated monolayer formation and, with sufficient time, the formation of tube-like structures. Yet, the presence of basement membrane collagen promoted SVF EC tube-like formations over their proliferation into monolayers. These findings suggested that cultured SVF EC phenotypes are regulated by connective tissue—a point corroborated by Montesano et al., who used a clonal brain endothelial cell line instead of SVF EC.46 These findings also indicated that SVF EC can assemble into networks in vitro in the absence of other cell types, although these capillary-like networks carry no blood, and that blood flow and pressure are not required for basic SVF EC network assembly and architecture.6,47 Furthermore, with regard to in vivo assembly, Madri and Williams suggested that an angiogenic environment (such as a wound, for instance) may provide the variety of connective tissue profiles required to proliferate and assemble SVF EC into tubes.45
In addition to connective tissue influencing EC assembly, our own studies with human SVF cultures have shown that the endothelial subfraction can form robust networks in culture in the presence of other stromal cells, and that this process is also mediated by the Wnt signaling pathway, and particularly Wnt5a.48 While the introduction of a pan-Wnt inhibitor significantly decreased the density of SVF EC networks in vitro, we observed that the addition of recombinant Wnt5a could rescue SVF EC density, but not complexity, suggesting that other Wnt isoforms may work with Wnt5a to modulate other aspects of vascular assembly. Subsequent in vivo studies echoed our in vitro findings, further suggesting that Wnt5a mediates the in vivo vascular self-assembly characteristic of SVF. However, the source(s) of Wnt5a within SVF and the precise mechanistic axis by which it acts remain to be investigated.
Because SVF is a diverse population with various progenitor and stem cells, Koh et al. sought to determine if the EC in SVF were required to form vascular networks. After depleting EC from freshly isolated SVF, they demonstrated a complete abrogation of SVF vascular network formation in vivo.22 This suggests that the other cell types within SVF are, themselves, insufficient to recapitulate the vascular network formation seen with EC-enriched SVF cells in a defined time period.
SVF EC cells also play critical roles in promoting the survival and functionality of implanted parenchymal cells. We were the first to combine SVF with hepatocytes to form a liver tissue mimic.6 Using three-dimensional (3D) constructs comprised of SVF and HepG2 hepatocytes, we demonstrated that SVF EC self-assembled into highly intricate cages around HepG2 spheroids. Subsequent tests with a peripherally injected biomolecule (DiI-LDL) showed that the SVF EC vascular cages interacted with the HepG2 clusters, as DiI-LDL localized to these clusters over several hours; to the contrary, constructs lacking SVF were unable to localize DiI-LDL. Thus, SVF EC not only closely interacts and functionalizes parenchymal implants, but also interfaces with the host vasculature such that circulating biomolecules (e.g., DiI-LDL) can make their way toward implanted, metabolically active parenchyma. In a different application, we combined SVF with induced pluripotent stem cell-derived hepatocyte-like cells (iPSC-HLC) and demonstrated that SVF promoted the survival of iPSC-HLC compared with 3D implants devoid of SVF.7
In sum, EC are present in all vasculatures and their importance to SVF-derived vascular networks is equally as critical. Additionally, their ability to rapidly self-assemble into networks makes them attractive from a clinical standpoint, as they may offer therapeutic value in settings such as ischemia or with tissue-engineered implants requiring access to the circulation. Yet, it is also important to recognize that while SVF EC can self-assemble into tube-like structures in the absence of other SVF cells,45 it is likely the nonendothelial, stromal fraction of SVF confers signals that ultimately shape this network into a mature, functional vasculature. To that end, we present evidence supporting this idea in the following sections.
Adipose stem cells
Zuk et al. were the first to show that processed lipoaspirate (PLA) cells could differentiate into one of four lineages—adipogenic, osteogenic, chondrogenic, and myogenic—effectively demonstrating the multipotent potential of adipose tissue.49 A key parenchymal cell type within adipose tissue is the adipose-derived stromal cells (also known as adipose-derived or adipose stem cells; ASC), which are also considered to be a MSC subpopulation of SVF.50 In addition to the fact that ASC appear to be immune privileged, these cells have been shown to migrate to sites of injury and release paracrine secretions that reduce inflammation.51 This trait has been seen with both intraocular injections and tail vein injections, where the former stabilized the progression of streptozotocin-induced diabetic retinopathy51 and the latter mitigated systemic inflammation, improved glucose tolerance, preserved pancreatic β-cell mass, and increased β-cell proliferation in the face of streptozotocin treatment.52
ASC have also been shown to play significant roles with the vasculature. For instance, Cai et al. were among the first to demonstrate that ASC impact capillary density and perfusion, and that these actions are mediated by hepatocyte growth factor.53 When placed with human microvessel EC, ASC improved endothelial network assembly and stability.54 In trying to determine a mechanism for these outcomes, initial analyses revealed that these cells were positive for a full cadre of markers associated with stromal cells such as mesenchymal cells (CD10, CD13, CD90), pericytes (CD140a/b), and smooth muscle cells (alpha actin, calponin, and caldesmon). Immunohistochemical analysis of ASC-microvessel endothelial cell cocultures showed that after extensive culturing, the cells encased the vascular lumen in much the same way that pericytes did. Again, determining the density of ASC per SVF isolation remains challenging since specific markers have not been identified, although others such as PDGF-B and 3G533,38,54–56 and Pref157 have been implicated. Additionally, since ASC exhibit variable abundance—white adipose tissue (WAT) is enriched with ASC, for example50,58—the sourcing of SVF and its subpopulations needs to be taken into consideration.
To determine a mechanism of interaction between ASC and EC, Merfeld-Clauss et al. performed a series of coculture experiments, noting increases in overall extracellular matrix production, ASC expression of alpha-smooth muscle actin, and endothelial expression of CD31.59 Cocultures yielded the highest degree of endothelial network formation, followed by ASC-conditioned media with the next highest density and ASC fibroblast cocultures with the lowest density. Not only do these findings suggest that ASC endothelial cell interactions are contact dependent (a finding corroborated by Rohringer et al.60), but also that these interactions progress in both directions (as ASC increased alpha-smooth muscle actin expression as well). This is evidenced in later studies, implicating Activin A as an ASC endothelial cell contact-initiated means of (1) inducing ASC smooth muscle cell differentiation61 and (2) impairing vascular endothelial growth factor (VEGF) expression, reducing endothelial network formation.62 In a similar context, Freiman et al. cultured ASC progenitors—that is, MSC—with human adipose microvascular EC (HAMEC) in three-dimensional PLLA/PLGA scaffolds.63 A significantly complex, organized, and mature vasculature resulted after 2 weeks; such findings were supported by the increased expression of alpha-smooth muscle actin as well as increased tube alignment when compared with constructs containing MSC and dermal fibroblasts.
ASC also have the capacity to form vasculatures when interacting with other nonvascular EC types as well. In two separate studies, Strassburg et al. discussed the interface between ASC and endothelial cell progenitors (EPC)64 and lymphatic endothelium65 as well. The earlier of the two studies demonstrated that a direct interaction between ASC and EPC yielded capillary-like structures in a VEGF-dependent manner—notably, a finding not seen when ASC was cocultured with human EC. With respect to ASC influence on the lymphatic system, Strassburg et al. noted that both juxtacrine and paracrine interactions resulted in the increased expression of lymphoid markers and related lymph–vascular architecture. Holnthoner et al. described the interaction of ASC with EC isolated from peripheral blood (termed outgrowth endothelial cells) with a 3D fibrin environment, and noted the resulting vascular development and surrounding matrix degradation to be at least partly mediated by matrix metalloproteinase-14 (MMP-14).66 In totality, these findings further underscore the complex relationship and differences between ASC, progenitor cells, terminally differentiated cells (with particularly interesting findings concerning EPC vs. EC and ASC vs. MSC), and the extracellular environment (such as PLLA/PLGA, collagen, and fibrin scaffolding, for instance). Additionally, the precise mechanism by which the cell populations interact with one another is still a topic undergoing much development.
Even though several of the aforementioned studies assess the interactions between ASC and other ancillary cell types, a key point to emphasize is that ASC are a subpopulation within SVF,33,67–69 and that SVF not only includes ASC, but is also replete with EC, smooth muscle cells, macrophages, and fibroblasts, among others.6,22,31 The two populations, while related, are distinct and have unique characteristics; key differences between the ASC and SVF are attributable to their postisolation culture. While SVF is the heterogeneous cell pellet seen at the end of a centrifuged, postenzymatic adipose digestion, ASC are isolated by subjecting SVF to further mixing, lysing, washing, straining, and plating on tissue culture plastic; those cells that are adherent after 72 h in a basic media (such as Dulbecco's modified Eagle's medium [DMEM] with 10% fetal bovine serum [FBS], for instance) are then considered to be ASC.70 Plated, putative ASC are commonly subcultured to confluence and passaged several times to mitigate any influence by SVF EC and obtain a pure mesenchymal population.49,71
Furthermore, like SVF, ASC have demonstrated immense potential as a therapeutic cell source, demonstrating improved outcomes in lung injury,72 diabetic retinopathy,73 lymphedema,74 and vascularization.59 It should be recognized that the cellular heterogeneity of SVF is advantageous in that it contains the necessary cells to function as a regenerative therapeutic system. Many of these cell types interact with one another to produce complex vascular and tissue superstructures, and in many of the studies involving ASC, the ASC only yielded its effects when combined with other cell types such as vascular EC59,62 and lymphoid EC.65
Macrophages, monocytes, and immune cells
Adipose SVF contains a significant proportion of cells involved in immunoregulatory75 and vascular remodeling22 functions as well. Both functions play critical roles in SVF's ability to self-assemble into a mature vasculature. For example, efforts by Navarro et al. demonstrated that adipose monocytes contribute to angiogenesis and even differentiate into EC.76 These monocyte-derived EC then incorporated into the developing vascular network. Although they concluded that adipose monocytes may constitute a new angiogenic cell source, it is not clear if this monocyte phenotype is typically included in adipose SVF isolations, since others have demonstrated that EC-depleted adipose SVF cannot form vascular networks.22
Macrophages have been more extensively characterized in adipose SVF. Morris et al. show that CD11b+ macrophages constitute ∼20% of rodent adipose SVF isolations.41 Approximately 80% of these cells were also positive for F4/80, and ∼70% were positive for CD301 (a marker for M2 anti-inflammatory, proangiogenic macrophages75,77–79). The macrophages from SVF injected intravenously into system circulation were found in the adventitial layer of the saphenous artery, and controlled the artery's vascular tone in an H2O2-dependent manner. Thus, it is possible that these macrophages may control tonicity of SVF-derived vessels in a similar manner.
Koh et al. also described the role of SVF macrophages on vascular assembly,22 noting that macrophages were required for proper vascular structural organization. To support this, the depletion of CD11b+ and F4/80+ macrophages from SVF yielded vessels that were blunted and disconnected—findings supported by Fantin et al., who suggested that macrophages facilitate vessel anastomosis.80 Perhaps more interestingly, they also demonstrated the requirement for an interplay between SVF macrophages and host macrophages. In macrophage-depleted mice, SVF formed endothelial networks with higher vessel integrity at the core than at the periphery. In control mice, the integrity of SVF-derived vessels was uniform. Thus, while SVF can form networks in the absence of macrophages, the quality of these networks appears to critically depend on (1) their presence and (2) synergy between SVF- and host macrophage populations.
There are pathological and therapeutic scenarios that highlight the roles of SVF immune cells. For example, in fat-grafting procedures conducted by Dong et al., the inclusion of SVF in fat grafts lead to increases in CD206 expression (another M2 macrophage phenotype marker), and a downregulation of the proinflammatory agents, IL-1β and IL-6.35 In another example, rodents given high-fat diets had SVF profiles comprised of a higher proportion of macrophages and monocyte chemotactic protein-1 (MCP-1).81 In a disease such as obesity, portions of SVF taken from previously obese patients were found to have increased MCP-1 expression37; likewise, the hypoxic environment in fat seen with obesity has been shown to increase the amount of SVF-derived inflammatory cytokines that potentiate their effect through a P38-mediated mechanism.82 Thus, the environment in which SVF cells are situated, that is, in obesity or metabolic syndrome,83 can have significant effects on their cell proportions (and particularly macrophage subsets) and propensity to incite or propagate inflammation.77
SVF isolation and processing
Clinically, SVF can be isolated from discarded human lipoaspirate. Yet, issues to consider include differences in the sites of isolation as well as how the isolated adipose tissue is processed. In addition to regional anatomical differences in fat composition, for instance, the molecular nature of adipose tissue is different with abdominal, medial thigh, lateral breast isolations,84 and differences exist between brown adipose tissue (BAT) and WAT. Prunet-Marcassus et al. demonstrated that the SVF cells isolated from WAT possessed more hematopoietic cells, macrophages, hematopoietic progenitors, and immature cells that, together, contributed to a higher degree of plasticity than SVF cells isolated from BAT.58
WAT is not strictly confined to subcutaneous regions. WAT found around the viscera, termed visceral adipose tissue (VAT), are often found in excessive quantities during obesity and metabolic disease. Increases in VAT stores can also raise one's risk of developing cardiovascular disease,85,86 digestive disorders,87 and certain urological diseases,88 to name a few. Given that excessive VAT is detrimental, it is reasonable to ask whether SVF isolated from VAT, compared with SVF from subcutaneous adipose tissue (SAT), is equally as detrimental or dysfunctional. Studies by O'Rourke et al. and Benencia et al. suggest that VAT SVF promotes inflammation,82,89 potentially due to a higher proportion of macrophages, natural killer cells, and T cells82 compared with SVF from SAT. Cohen et al. confirmed these findings, showing that SVF isolated from omental WAT or even the serous fluid of the peritoneum were rich in T cells and CD45+ leukocytes, respectively.
Other site-specific variations exist as well. For example, human omental fat was described to be replete with mesothelial cells as opposed to EC,90 whereas Williams et al. describe a predominance of EC in subcutaneous human lipoaspirates.31 Similar differences can be seen in rodents, in which SVF isolated from inguinal fat pads possessed a higher composition of macrophages and monocytes than SVF derived from epididymal fat stores.91 Furthermore, inguinal fat is thought to be more plastic and is easier to access.58 Thus, as EC are the backbone of the developing vasculature, and monocytes and macrophages play significant roles in vascular remodeling during angiogenesis, site-specific differences in SVF composition and plasticity can have profound consequences on the characteristics of the resulting, self-assembled vasculature. These effects should be accounted for during isolation as well as in experimental and clinical applications.
In addition to the differences that site-specific variations can impart on SVF, other considerations have varying effects as well. For instance, the pathological subtype of the patient (e.g., obesity, smokers, underlying genetic abnormalities, immunocompromised, aging, etc.) can have potentially devastating consequences on native vascular and immunological function,92–94 let alone the vasculature derived from patient adipose SVF. To that end, there is a relative paucity of high-quality studies clearly defining the impact of pathological disease on the therapeutic potential of SVF, although recent efforts have assessed the role of obesity37 and aging95,96 on SVF yields and function.
The isolation modality is another important consideration. Figure 2 depicts a general schematic for how our laboratory manually processes SVF. We, like many others, utilize collagenase for the chemical digestion of lipoaspirate, a technique still considered the gold standard of SVF processing.97 Still, one potential source of yield or functional variation could lie with the collagenase type, quantity, and/or lot. Williams et al. extensively evaluated commercially available brands and lots of collagenase, noting a reduction in collagenase efficacy with purification by dialysis and an enhancement of purified collagenase efficacy by the addition of trypsin.30 Complementing this, a variety of efforts (also reviewed in Refs.98,99) are now focused on the automated or semiautomated isolation of SVF,97,100–103 many of which eschew chemical digestion and are in clinical trials (e.g., NCT02234778, NCT01601353, and NCT01305863).
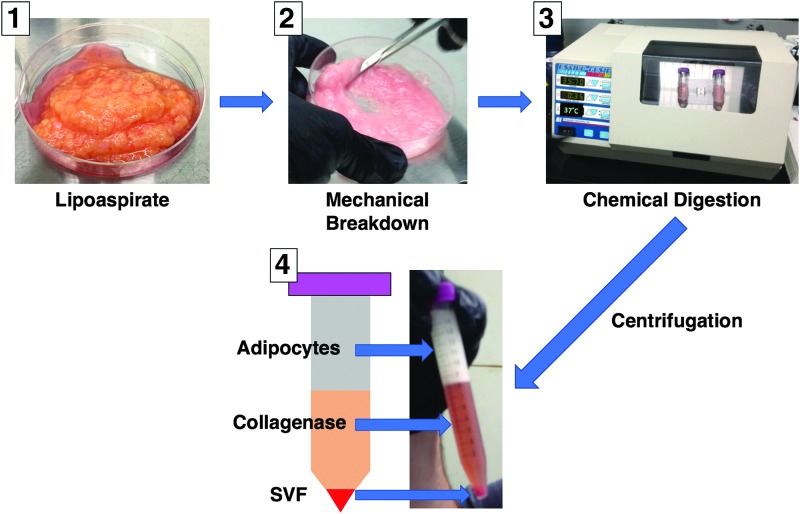
FIG. 2.
Manual Processing of lipoaspirate to yield SVF. In Photo 1, lipoaspirate is shown before mechanical breakdown using scissors (Photo 2). This breakdown provides a greater surface area on which the chemical digestion enzyme collagenase can act (Photo 3). After a defined time period of digestion, the resulting slurry is centrifuged, yielding a solution of three key parts as shown in Photo 4: a top layer of buoyant adipocytes, a middle layer of collagenase and remaining tumescent fluids, and a cellular pellet at the bottom containing SVF—a heterogeneous mix of various cell types. Color images available online at www.liebertpub.com/teb
Discussion and Concluding Remarks
The successful generation and survival of large volumes of engineered tissues are likely dependent on the presence of a stable and supportive vascular supply. Host tissues contain parenchyma that can access circulating proteins, hormones, factors, and products through a patent, well-distributed vascular supply. The absence of such a vasculature leads to cell death,104–108 as oxygen is only capable of diffusing over distances of less than a few hundred microns.109,110 Additionally, variations in the implant site can impair an engineered parenchyma's ability to interface with existing host vasculatures.111 An engineered vascular cell support system could serve as an ideal means of meeting the complex metabolic and functional requirements of various engineered parenchyma. One such system is the adipose-derived SVF, which is a heterogeneous mixture of EC, stem cells, macrophages, pericytes, and other vascular support and immunomodulatory cells. Collectively, these cells have functioned to spontaneously self-assemble into vessel-like networks in vitro48,61 and in vivo vasculatures that functionally anastomose with the host circulation.6,22 Such findings suggest that SVF could serve as a therapy for ischemic disease; to this end, preliminary studies have demonstrated that SVF promotes revascularization in peripheral ischemic disease22,112,113 and myocardial infarction.114,115
SVF contains the EC required for vascular network assembly as well as a full complement of support cells that likely play roles in stabilization and maturation.116–119 Furthermore, SVF may possess the ability to adapt to different clinical scenarios.120 It is thought to be more plastic than other vascular cell sources, although cell sourcing (e.g., from WAT vs. BAT) can yield site-specific differences.58 Additionally, SVF contains various populations of stem cells that may facilitate such plasticity and adaptation. In addition to forming a mature vascular network, SVF may also promote healing. Adipose stem cells (ASC) are a MSC subpopulation of SVF50 that appears to be immune privileged. These cells also migrate to sites of injury41 and release anti-inflammatory factors.51
A third consideration with SVF is that it can be isolated easily, bearing significant clinical and translational implications. Adipose tissue is routinely acquired in a minimally invasive fashion laparoscopically. The isolated adipose tissue is then subjected to enzymatic digestion and processing to yield the SVF. Although SVF isolation can be performed manually, there is a growing interest in automating this process102,103 for prompt application during an operation or at a patient's bedside.121 Such automation would enhance repeatability and reduce variability in clinical and experimental outcomes.
In sum, SVF contains a multitude of cell types that act to modulate the immune response, adapt to the host environment, and promote the formation of a robust, self-assembled, mature vasculature. Its relative ease of isolation makes SVF a clinically relevant, therapeutic cell source that is ideal for numerous applications in tissue engineering.
Acknowledgments
The authors sincerely thank Dr. James Hoying, colleagues in the University of Louisville Department of Physiology, the Department of Surgery, and elsewhere within the School of Medicine for their helpful discussions, administrative assistance, experimental provisions, and technical support. Funding for their work was provided by the AHA (11SDG7500025), NIH (1R21EB022185), and the Jewish Heritage Fund for Excellence. Additionally, they apologize to authors whose work they inadvertently excluded.
Disclosure Statement
No competing financial interests exist.
References
- 1.Macchiarini P., Jungebluth P., Go T., Asnaghi M.A., Rees L.E., Cogan T.A., Dodson A., Martorell J., Bellini S., Parnigotto P.P., Dickinson S.C., Hollander A.P., Mantero S., Conconi M.T., and Birchall M.A. Clinical transplantation of a tissue-engineered airway. Lancet 372, 2023, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Raya-Rivera A., Esquiliano D.R., Yoo J.J., Lopez-Bayghen E., Soker S., and Atala A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 377, 1175, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ott H.C., Matthiesen T.S., Goh S.K., Black L.D., Kren S.M., Netoff T.I., and Taylor D.A. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14, 213, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Song J.J., Guyette J.P., Gilpin S.E., Gonzalez G., Vacanti J.P., and Ott H.C. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 19, 646, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., Zhang R.R., Ueno Y., Zheng Y.W., Koike N., Aoyama S., Adachi Y., and Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Nunes S.S., Maijub J.G., Krishnan L., Ramakrishnan V.M., Clayton L.R., Williams S.K., Hoying J.B., and Boyd N.L. Generation of a functional liver tissue mimic using adipose stromal vascular fraction cell-derived vasculatures. Sci Rep 3, 2141, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramakrishnan V.M., Yang J.Y., Tien K.T., McKinley T.R., Bocard B.R., Maijub J.G., Burchell P.O., Williams S.K., Morris M.E., Hoying J.B., Wade-Martins R., West F.D., and Boyd N.L. Restoration of physiologically responsive low-density lipoprotein receptor-mediated endocytosis in genetically deficient induced pluripotent stem cells. Sci Rep 5, 13231, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen C.A., Shea A.A., Heffron C.L., Schmelz E.M., and Roberts P.C. Intra-abdominal fat depots represent distinct immunomodulatory microenvironments: a murine model. PLoS One 8, e66477, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant R.W., and Dixit V.D. Adipose tissue as an immunological organ. Obesity (Silver Spring) 23, 512, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X., and Godbey W.T. Viral vectors for gene delivery in tissue engineering. Adv Drug Deliv Rev 58, 515, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Ducic Y., Pontius A.T., and Smith J.E. Lipotransfer as an adjunct in head and neck reconstruction. Laryngoscope 113, 1600, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Fujino T., Tanino R., and Sugimoto C. Microvascular transfer of free deltopectoral dermal-fat flap. Plast Reconstr Surg 55, 428, 1975 [PubMed] [Google Scholar]
- 13.Rees T.D. The transfer of free composite grafts of skin and fat: a clinical study. Plast Reconstr Surg Transplant Bull 25, 556, 1960 [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura K., Sato K., Aoi N., Kurita M., Hirohi T., and Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg 32, 48, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lolli P., Malleo G., and Rigotta G. Treatment of chronic anal fissures and associated stenosis by autologous adipose tissue transplant: a pilot study. Dis Colon Rectum 53, 460, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Riordan N.H., Ichim T.E., Min W., Wang H., Solano F., Lara F., Alfaro M., Rodriguez J.P., Harman R.J., Patel A.N., Murphy M.P., Lee R.R., and Minev B. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med 7, 29, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hang-Fu L., Marmolya G., and Feigilin D.H. Liposuction fat-fillant implant for breast augmentation and reconstruction. Aesthetic Plast Surg 19, 427, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Rodbell M. Metabolism of isolated fat cells: I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 239, 375, 1964 [PubMed] [Google Scholar]
- 19.Rodbell M. Metabolism of isolated fat cells: II. The similar effects of phospholipase C (Clostridium perfringens Alpha Toxin) and of insulin on glucose and amino acid metabolism. J Biol Chem 241, 130, 1966 [PubMed] [Google Scholar]
- 20.Rodbell M. The metabolism of isolated fat cells: IV. Regulation of release of protein by lipolytic hormones and insulin. J Biol Chem 241, 3909, 1966 [PubMed] [Google Scholar]
- 21.Rodbell M., and Jones A.B. Metabolism of isolated fat cells: III. The similar inhibitory action of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. J Biol Chem 241, 140, 1966 [PubMed] [Google Scholar]
- 22.Koh Y.J., Koh B.I., Kim H., Joo H.J., Jin H.K., Jeon J., Choi C., Lee D.H., Chung J.H., Cho C.H., Park W.S., Ryu J.K., Suh J.K., and Koh G.Y. Stromal vascular fraction from adipose tissue forms profound vascular network through the dynamic reassembly of blood endothelial cells. Arterioscler Thromb Vasc Biol 31, 1141, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Tsuji W., Rubin J.P., and Marra K.G. Adipose-derived stem cells: implications in tissue regeneration. World J Stem Cells 6, 312, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner R.C., Kreiner P., Barrnett R.J., and Bitensky M.W. Biochemical characterization and cytochemical localization of a catecholamine-sensitive adenylate cyclase in isolated capillary endothelium. Proc Natl Acad Sci U S A 69, 3175, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner R.C., and Matthews M.A. The isolation and culture of capillary endothelium from epididymal fat. Microvasc Res 10, 286, 1975 [DOI] [PubMed] [Google Scholar]
- 26.Van R.L., Bayliss C.E., and Roncari D.A. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J Clin Invest 58, 699, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarrell B.E., Williams S.K., Stokes G., Hubbard F.A., Carabasi R.A., Koolpe E., Greener D., Pratt K., Moritz M.J., and Radomski J. Use of freshly isolated capillary endothelial cells for the immediate establishment of a monolayer on a vascular graft at surgery. Surgery 100, 392, 1986 [PubMed] [Google Scholar]
- 28.Rupnick M.A., Hubbard A., Pratt K., Jarrell B.E., and Williams S.K. Endothelialization of vascular prosthetic surfaces after seeding or sodding with human microvascular endothelial cells. J Vasc Surg 9, 788, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Williams S.K., Jarrell B.E., Rose D.G., Pontell J., Kapelan B.A., Park P.K., and Carter T.L. Human microvessel endothelial cell isolation and vascular graft sodding in the operating room. Ann Vasc Surg 3, 146, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Williams S.K., McKenney S., and Jarrell B.E. Collagenase lot selection and purification for adipose tissue digestion. Cell Transplant 4, 281, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Williams S.K., Wang T.F., Castrillo R., and Jarrell B.E. Liposuction-derived human fat used for vascular graft sodding contains endothelial cells and not mesothelial cells as the major cell type. J Vasc Surg 19, 916, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Hoying J.B., Boswell C.A., and Williams S.K. Angiogenic potential of microvessel fragments established in three-dimensional collagen gels. In Vitro Cell Dev Biol 32, 409, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Zimmerlin L., Donnenberg V.S., Pfeifer M.E., Meyer E.M., Peault B., Rubin J.P., and Donnenberg A.D. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 77, 22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong Z., Fu R., Liu L., and Lu F. Stromal vascular fraction (SVF) cells enhance long-term survival of autologous fat grafting through the facilitation of M2 macrophages. Cell Biol Int 37, 855, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Dong Z., Peng Z., Chang Q., and Lu F. The survival condition and immunoregulatory function of adipose stromal vascular fraction (SVF) in the early stage of nonvascularized adipose transplantation. PLoS One 8, e80364, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klar A.S., Guven S., Zimoch J., Zapiorkowska N.A., Biedermann T., Bottcher-Haberzeth S., Meuli-Simmen C., Martin I., Scherberich A., Reichmann E., and Meuli M. Characterization of vasculogenic potential of human adipose-derived endothelial cells in a three-dimensional vascularized skin substitute. Pediatr Surg Int 32, 17, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Silva K.R., Liechocki S., Carneiro J.R., Claudio-da-Silva C., Maya-Monteiro C.M., Borojevic R., and Baptista L.S. Stromal-vascular fraction content and adipose stem cell behavior are altered in morbid obese and post bariatric surgery ex-obese women. Stem Cell Res Ther 6, 72, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zannettino A.C., Paton S., Arthur A., Khor F., Itescu S., Gimble J.M., and Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol 214, 413, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Gimble J.M., Bunnell B.A., Chiu E.S., and Guilak F. Concise review: adipose-derived stromal vascular fraction cells and stem cells: let's not get lost in translation. Stem Cells 29, 749, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Cousin B., Caspar-Bauguil S., Planat-Bénard V., Laharrague P., Pénicaud L., and Casteilla L. Adipose tissue: a subtle and complex cell system. J Soc Biol 200, 51, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Morris M.E., Beare J.E., Reed R.M., Dale J.R., LeBlanc A.J., Kaufman C.L., Zheng H., Ng C.K., Williams S.K., and Hoying J.B. Systemically delivered adipose stromal vascular fraction cells disseminate to peripheral artery walls and reduce vasomotor tone through a CD11b+ cell-dependent mechanism. Stem Cells Transl Med 4, 369, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geevarghese A., and Herman I.M. Pericyte-endothelial crosstalk: implications and opportunities for advanced cellular therapies. Transl Res 163, 296, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maijub J.G., Boyd N.L., Dale J.R., Hoying J.B., Morris M.E., and Williams S.K. Concentration-dependent vascularization of adipose stromal vascular fraction cells. Cell Transplant 24, 2029, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Goodwin A.M., and D'Amore P.A. Vessel maturation and perivascular cells. In: Marmé D., Fusenig N., eds. Tumor Angiogenesis. Heidelberg, Germany: Springer-Verlag, 2008 [Google Scholar]
- 45.Madri J.A., and Williams S.K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol 97, 153, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montesano R., Orci L., and Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol 97, 1648, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., and Walter P. Molecular Biology of the Cell, 6th ed. New York: Garland Science, 2014. [Google Scholar]
- 48.Ramakrishnan V.M., Tien K.T., McKinley T.R., Bocard B.R., McCurry T.M., Williams S.K., Hoying J.B., and Boyd N.L. Wnt5a regulates the assembly of human adipose derived stromal vascular fraction-derived microvasculatures. PLoS One 11, e0151402, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell W., Katz A.J., Benhaim P., Lorenz H.P., and Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng Part A 7, 211, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Ong W.K., and Sugii S. Adipose-derived stem cells: fatty potentials for therapy. Int J Biochem Cell Biol 45, 1083, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Rajashekhar G., Ramadan A., Abburi C., Callaghan B., Traktuev D.O., Evans-Molina C., Maturi R., Harris A., Kern T.S., and March K.L. Regenerative therapeutic potential of adipose stromal cells in early stage diabetic retinopathy. PLoS One 9, e84671, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kono T.M., Sims E.K., Moss D.R., Yamamoto W., Ahn G., Diamond J., Tong X., Day K.H., Territo P.R., Hanenberg H., Traktuev D.O., March K.L., and Evans-Molina C. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: analysis of hASC-derived paracrine effectors. Stem Cells 32, 1831, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai L., Johnstone B.H., Cook T.G., Liang Z., Traktuev D., Cornetta K., Ingram D.A., Rosen E.D., and March K.L. Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells 25, 3234, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Traktuev D.O., Merfeld-Clauss S., Li J., Kolonin M., Arap W., Pasqualini R., Johnstone B.H., and March K.L. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res 102, 77, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P.N., Traas J., Schugar R., Deasy B.M., Badylak S., Buhring H.J., Giacobino J.P., Lazzari L., Huard J., and Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Amos P.J., Shang H., Bailey A.M., Taylor A., Katz A.J., and Peirce S.M. IFATS collection: the role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype. Stem Cells 26, 2682, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smas C.M., and Sul H.S. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell 73, 725, 1993 [DOI] [PubMed] [Google Scholar]
- 58.Prunet-Marcassus B., Cousin B., Caton D., Andre M., Penicaud L., and Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp Cell Res 312, 727, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Merfeld-Clauss S., Gollahalli N., March K.L., and Traktuev D.O. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng Part A 16, 2953, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rohringer S., Hofbauer P., Schneider K.H., Husa A.M., Feichtinger G., Peterbauer-Scherb A., Redl H., and Holnthoner W. Mechanisms of vasculogenesis in 3D fibrin matrices mediated by the interaction of adipose-derived stem cells and endothelial cells. Angiogenesis 17, 921, 2014 [DOI] [PubMed] [Google Scholar]
- 61.Merfeld-Clauss S., Lupov I.P., Lu H., Feng D., Compton-Craig P., March K.L., and Traktuev D.O. Adipose stromal cells differentiate along a smooth muscle lineage pathway upon endothelial cell contact via induction of activin A. Circ Res 115, 800, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Merfeld-Clauss S., Lupov I.P., Lu H., March K.L., and Traktuev D.O. Adipose stromal cell contact with endothelial cells results in loss of complementary vasculogenic activity mediated by induction of activin A. Stem Cells 33, 3039, 2015 [DOI] [PubMed] [Google Scholar]
- 63.Freiman A., Shandalov Y., Rozenfeld D., Shor E., Segal S., Ben-David D., Meretzki S., Egozi D., and Levenberg S. Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Res Ther 7, 5, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strassburg S., Nienhueser H., Stark G.B., Finkenzeller G., and Torio-Padron N. Human adipose-derived stem cells enhance the angiogenic potential of endothelial progenitor cells, but not of human umbilical vein endothelial cells. Tissue Eng Part A 19, 166, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Strassburg S., Torio-Padron N., Finkenzeller G., Frankenschmidt A., and Stark G.B. Adipose-derived stem cells support lymphangiogenic parameters in vitro. J Cell Biochem 117, 2620, 2016 [DOI] [PubMed] [Google Scholar]
- 66.Holnthoner W., Hohenegger K., Husa A.M., Muehleder S., Meinl A., Peterbauer-Scherb A., and Redl H. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. J Tissue Eng Regen Med 9, 127, 2015 [DOI] [PubMed] [Google Scholar]
- 67.Cawthorn W.P., Scheller E.L., and MacDougald O.A. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res 53, 227, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cousin B., Andre M., Arnaud E., Penicaud L., and Casteilla L. Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem Biophys Res Commun 301, 1016, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Han J., Koh Y.J., Moon H.R., Ryoo H.G., Cho C.H., Kim I., and Koh G.Y. Adipose tissue is an extramedullary reservoir for functional hematopoetic stem and progenitor cells. Blood 115, 957, 2010 [DOI] [PubMed] [Google Scholar]
- 70.Bunnell B.A., Flaat M., Gagliardi C., Patel B., and Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods 45, 115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin G., Garcia M., Ning H., Banie L., Guo Y.L., Lue T.F., and Lin C.S. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev 17, 1053, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schweitzer K.S., Johnstone B.H., Garrison J., Rush N.I., Cooper S., Traktuev D.O., Feng D., Adamkowicz J.J., Van Denmark M., Fisher A.J., Kamocki K., Brown M.B., Presson R.G.J., Broxmeyer H.E., March K.L., and Petrache I. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med 183, 215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rajashekhar G., Traktuev D.O., Roell W.C., Johnstone B.H., Merfeld-Clauss S., Van Natta B., Rosen E.D., March K.L., and Clauss M. IFATS collection: adipose stromal cell differentiation is reduced by endothelial cell contact and paracrine communication: role of canonical Wnt signaling. Stem Cells 26, 2674, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Hayashida K., Yoshida S., Yoshimoto H., Fujioka M., Saijo H., Migita K., Kumaya M., and Akita S. Adipose-derived stem cells and vascularized lymph node transfers successfully treat mouse hindlimb secondary lymphedema by early reconnection of the lymphatic system and lymphangiogenesis. Plast Reconstr Surg 139, 639, 2017 [DOI] [PubMed] [Google Scholar]
- 75.Morris D.L., Oatmen K.E., Wang T., DelProposto J.L., and Lumeng C.N. CX3CR1 deficiency does not influence trafficking of adipose tissue macrophages in mice with diet-induced obesity. Obesity (Silver Spring) 20, 1189, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Navarro A., Marin S., Riol N., Carbonell-Uberos F., and Miñana M.D. Human adipose tissue-resident monocytes exhibit an endothelial-like phenotype and display angiogenic properties. Stem Cell Res Ther 5, 50, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ploeger D.T., van Putten S.M., Koerts J.A., van Luyn M.J., and Harmsen M.C. Human macrophages primed with angiogenic factors show dynamic plasticity, irrespective of extracellular matrix components. Immunobiology 217, 299, 2012 [DOI] [PubMed] [Google Scholar]
- 78.Potente M., Gerhardt H., and Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873, 2011 [DOI] [PubMed] [Google Scholar]
- 79.Lumeng C.N., DelProposto J.L., Westcott D.J., and Saltiel A.R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57, 3239, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fantin A., Vieira J.M., Gestri G., Denti L., Schwarz Q., Prykhozhij S., Peri F., Wilson S.W., and Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116, 829, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hamaguchi K., Itabashi A., Kuroe Y., Nakano M., Fujimoto E., Kato T., Satoi K., Utsuyama M., and Sato K. Analysis of adipose tissues and stromal vascular cells in a murine arthritis model. Metabolism 61, 1687, 2012 [DOI] [PubMed] [Google Scholar]
- 82.O'Rourke R.W., White A.E., Metcalf M.D., Olivas A.S., Mitra P., Larison W.G., Cheang E.C., Varlamov O., Corless C.L., Roberts C.T., Jr., and Marks D.L. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia 54, 1480, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Monteiro R., and Azeveido I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm 2010, 1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Phinney S.D., Stern J.S., Burke K.E., Tang A.B., Miller G., and Holamn R.T. Human subcutaneous adipose tissue shows site-specific differences in fatty acid composition. Am J Clin Nutr 60, 725, 1994 [DOI] [PubMed] [Google Scholar]
- 85.Peiris A.N., Sothmann M.S., Hoffmann R.G., Hennes M.I., Wilson C.R., Gustafson A.B., and Kissebah A.H. Adiposity, fat distribution, and cardiovascular risk. Ann Intern Med 110, 867, 1989 [DOI] [PubMed] [Google Scholar]
- 86.Després J.P., Moorjani S., Lupien P.J., Tremblay A., Nadeau A., and Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 10, 497, 1990 [DOI] [PubMed] [Google Scholar]
- 87.Büning C., von Craft C., Hermsdorf M., Gentz E., Wirth E.K., Valentini L., and Haas V. Visceral adipose tissue in patients with Crohn's disease correlates with disease activity, inflammatory markers, and outcome. Inflamm Bowel Dis 21, 2590, 2015 [DOI] [PubMed] [Google Scholar]
- 88.Akarken I., Tarhan H., Ekin R.G., Çakmak Ö., Koç G., Ilbey Y.Ö., and Zorlu F. Visceral obesity: a new risk factor for stone disease. Can Urol Assoc J 9, E795, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benencia F., Harshman S., Duran-Ortiz S., Lubbers E.R., List E.O., Householder L., Al-Naeeli M., Liang X., Welch L., Kopchick J.J., and Berryman D.E. Male bovine GH transgenic mice have decreased adiposity with an adipose depot-specific increase in immune cell populations. Endocrinology 156, 1794, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takahashi K., Goto T., Mukai K., Sawasaki Y., and Hata J. Cobblestone monolayer cells from human omental adipose tissue are possibly mesothelial, not endothelial. In Vitro Cell Dev Biol 25, 109, 1989 [DOI] [PubMed] [Google Scholar]
- 91.Du Z.Y., Ma T., Lock E.J., Hao Q., Kristiansen K., Frøyland L., and Madsen L. Depot-dependent effects of adipose tissue explants on co-cultured hepatocytes. PLoS One 6, e20917, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.El Husseny M.W., Mamdouh M., Shaban S., Ibrahim Abushouk A., Zaki M.M., Ahmed O.M., and Abdel-Daim M.M. Adipokines: potential therapeutic targets for vascular dysfunction in type II diabetes mellitus and obesity. J Diabetes Res 2017, 8095926, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Al Rifai M., DeFillippis A.P., McEvoy J.W., Hall M.E., Acien A.N., Jones M.R., Keith R., Magid H.S., Rodriguez C.J., Barr G.R., Benjamin E.J., Robertson R.M., Bhatnagar A., and Blaha M.J. The relationship between smoking intensity and subclinical cardiovascular injury: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 258, 119, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boratyńska M., Obremska M., Małecki R., Gacka M., Magott M., Kamińska D., Banasik M., Kusztal M., Chełmoński A., Jablecki J., and Klinger M. Impact of immunosuppressive treatment on the cardiovascular system in patients after hand transplantation. Transplant Proc 46, 2890, 2014 [DOI] [PubMed] [Google Scholar]
- 95.Aird A.L., Nevitt C.D., Christian K., Williams S.K., Hoying J.B., and LeBlanc A.J. Adipose-derived stromal vascular fraction cells isolated from old animals exhibit reduced capacity to support the formation of microvascular networks. Exp Gerontol 63, 18, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quaade M.L., Jensen C.H., Andersen D.C., and Sheikh S.P. A 3-month age difference profoundly alters the primary rat stromal vascular fraction phenotype. Acta Histochem 118, 513, 2016 [DOI] [PubMed] [Google Scholar]
- 97.Chaput B., Bertheuil N., Escubes M., Grolleau J.L., Garrido I., Laloze J., Espagnolle N., Casteilla L., Sensebé L., and Varin A. Mechanically isolated stromal vascular fraction provides a valid and useful collagenase-free alternative technique: a comparative study. Plast Reconstr Surg 138, 807, 2016 [DOI] [PubMed] [Google Scholar]
- 98.Oberbauer E., Steffenhagen C., Wurzer C., Gabriel C., Redl H., and Wolbank S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. Cell Regen (Lond) 4, 7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Dongen J.A., Tuin A.J., Spiekman M., Jansma J., van der Lei B., and Harmsen M.C. Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: a systematic review. J Tissue Eng Regen Med 2017. [Epub ahead of print]; DOI: 10.1002/term.2407 [DOI] [PubMed] [Google Scholar]
- 100.Hanke A., Prantl L., Wenzel C., Nerlich M., Brockhoff G., Loibl M., and Gemhert S. Semi-automated extraction and characterization of Stromal Vascular Fraction using a new medical device. Clin Hemorheol Microcirc 64, 403, 2016 [DOI] [PubMed] [Google Scholar]
- 101.Brown J.C., Shang H., Li Y., Yang N., Patel N., and Katz A.J. Isolation of adipose-derived stromal vascular fraction cells using a novel point-of-care device: cell characterization and review of the literature. Tissue Eng Part C Methods 23, 125, 2017 [DOI] [PubMed] [Google Scholar]
- 102.Williams S.K., Kosnik P.E., Kleinert L.B., Vossman E.M., Lye K.D., and Shine M.H. Adipose stromal vascular fraction cells isolated using an automated point of care system improve the patency of expanded polytetrafluoroethylene vascular grafts. Tissue Eng Part A 19, 1295, 2013 [DOI] [PubMed] [Google Scholar]
- 103.Katz A.J., Hedrick M.H., Llull R., and Futrell J.W. A novel device for the simple and efficient refinement of liposuctioned tissue. Plast Reconstr Surg 107, 595, 2001 [DOI] [PubMed] [Google Scholar]
- 104.Vogt M.T., and Farber E. On the molecular pathology of ischemic renal cell death. Reversible and irreversible cellular and mitochondrial metabolic alterations. Am J Pathol 53, 1, 1968 [PMC free article] [PubMed] [Google Scholar]
- 105.Cammermeyer J. “Ischemic neuronal disease” of Spielmeyer. A reevaluation. Arch Neurol 29, 391, 1973 [DOI] [PubMed] [Google Scholar]
- 106.Jennings R.B., and Ganote C.E. Structural changes in myocardium during acute ischemia. Circ Res 35, 156, 1974 [PubMed] [Google Scholar]
- 107.Jones R.T., and Trump B.F. Cellular and subcellular effects of ischemia on the pancreatic acinar cell: in vitro studies of rat tissue. Virchow's Arch B Cell Pathol 19, 325, 1975 [DOI] [PubMed] [Google Scholar]
- 108.Delva E., Camus Y., Nordlinger B., Hannoun L., Parc R., Deriaz H., Lienhart A., and Huguet C. Vascular occlusions for liver resections. Operative management and tolerance to hepatic ischemia: 142 cases. Ann Surg 209, 211, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Folkman J., and Hochberg M. Self-regulation of growth in three dimensions. J Exp Med 138, 745, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thomlinson R.H., and Gray L.H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 9, 539, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Griffith L.G., and Naughton G. Tissue engineering—current challenges and expanding opportunities. Science 295, 1009, 2002 [DOI] [PubMed] [Google Scholar]
- 112.Rehman J., Traktuev D.O., Li J., Merfeld-Clauss S., Temm-Grove C.J., Bovenkerk J.E., Pell C.L., Johnstone B.H., Considine R.V., and March K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292, 2004 [DOI] [PubMed] [Google Scholar]
- 113.Cervelli V., Gentile P., De Angelis B., Calabrese C., Di Stefani A., Scioli M.G., Curcio B.C., Felici M., and Orlandi A. Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post-traumatic lower extremity ulcers. Stem Cell Res 6, 103, 2011 [DOI] [PubMed] [Google Scholar]
- 114.Leblanc A.J., Touroo J.S., Hoying J.B., and Williams S.K. Adipose stromal vascular fraction cell construct sustains coronary microvascular function after acute myocardial infarction. Am J Physiol Heart Circ Physiol 302, H973, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Premaratne G.U., Ma L.P., Fujita M., Lin X., Bollano E., and Fu M. Stromal vascular fraction transplantation as an alternative therapy for ischemic heart failure: anti-inflammatory role. J Cardiothorac Surg 6, 43, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boyd N.L., Nunes S.S., Krishnan L., Jokinen J.D., Ramakrishnan V.M., Bugg A.R., and Hoying J.B. Dissecting the role of human embryonic stem cell-derived mesenchymal cells in human umbilical vein endothelial cell network stabilization in three-dimensional environments. Tissue Eng Part A 19, 211, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stratman A.N., Malotte K.M., Mahan R.D., Davis M.J., and Davis G.E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114, 5091, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yoshimura K., Shigeura T., Matsumoto D., Sato T., Takaki Y., Aiba-Kojima E., Sato K., Inoue K., Nagase T., Koshima I., and Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 208, 64, 2006 [DOI] [PubMed] [Google Scholar]
- 119.Eto H., Ishimine H., Kinoshita K., Watanabe-Susaki K., Kato H., Doi K., Kuno S., Kurisaki A., and Yoshimura K. Characterization of human adipose tissue-resident hematopoietic cell populations reveals a novel macrophage subpopulation with CD34 expression and mesenchymal multipotency. Stem Cells Dev 22, 985, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rupnick M.A., Panigrahy D., Zhang C.Y., Dallabrida S.M., Lowell B.B., Langer R., and Folkman J. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A 99, 10730, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brey E.M. Vascularization: Regenerative Medicine and Tissue Engineering, 1st ed. Boca Raton, FL: CRC Press, 2014 [Google Scholar]