Abstract
Human mesenchymal stem cell (hMSC)-based chondrogenesis is a key process used to develop tissue engineered cartilage constructs from stem cells, but the resulting constructs have inferior biochemical and biomechanical properties compared to native articular cartilage. Transforming growth factor β containing medium is commonly applied to cell layers of hMSCs, which aggregate upon centrifugation to form 3-D constructs. The aggregation process leads to a high cell density condition, which can cause nutrient limitations during long-term culture and, subsequently, inferior quality of tissue engineered constructs. Our objective is to modulate the aggregation process by targeting RhoA/ROCK signaling pathway, the chief modulator of actomyosin contractility, to enhance the end quality of the engineered constructs. Through ROCK inhibition, repression of cytoskeletal tension in chondrogenic hMSCs was achieved along with less dense aggregates with enhanced transport properties. ROCK inhibition also led to significantly increased cartilaginous extracellular matrix accumulation. These findings can be used to create an improved microenvironment for hMSC-derived tissue engineered cartilage culture. We expect that these findings will ultimately lead to improved cartilaginous tissue development from hMSCs.
Keywords: : tissue engineering, chondrogenesis, mass transport, human mesenchymal stem cells, signaling
Introduction
Human mesenchymal stem cells (hMSCs) can be differentiated into a chondrogenic lineage and thus form an important cell source for cartilage tissue engineering.1 Chondrogenesis in hMSCs can be induced by transforming growth factor β (TGFβ) in aggregate culture, which leads to high cell density for cell–cell interactions, analogous to precartilage condensation during embryonic development.2–4 However in all cases, the biochemical properties of the resulting cartilaginous constructs are inferior compared to native tissue.5 Hence, our goal is to identify and manipulate cell-signaling pathways for optimizing hMSC-based cartilaginous tissue culture. The regulation of chondrogenesis in hMSCs is complex and poorly understood. Regulation of cytoskeleton tension is crucial for tissue development, and RhoA/ROCK signaling has been viewed as the critical regulators of this process particularly in hMSC commitment toward adipocyte or osteoblast fate.6 However, to our knowledge, the role of RhoA/ROCK signaling in hMSC chondrogenesis has not been investigated.7,8
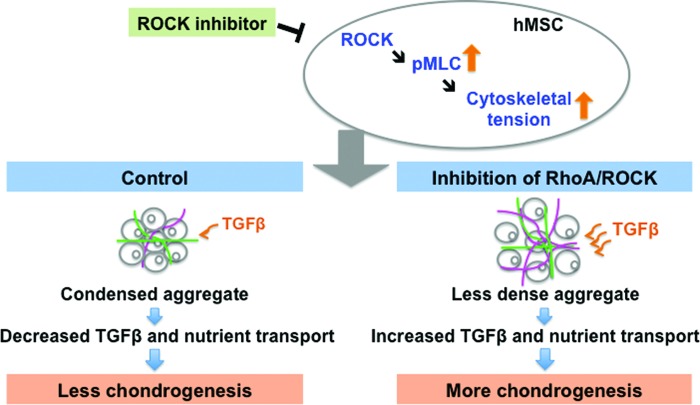
During chondrogenesis, hMSCs become rounded and have reduced stress fiber and focal adhesion formations,9 and these changes in cellular structure could be regulated by inhibition of RhoA/ROCK signaling as in adipogenic hMSCs.6 Treatment with the required chondrogenic inducer, TGFβ1, in human mesenchymal progenitor cell aggregates was found to activate the mitogen-activated protein kinases, which then downregulate the level of N-cadherin and β-catenin after peaking at the initial condensation stage on day 1.10 The subsequent decrease in intercellular adhesion after the aggregation stage is crucial for the progression of chondrogenic development.11,12 These findings imply that regulating intercellular space (interstitial space) within aggregates is necessary during chondrogenesis. It has been shown that ROCK inhibition in hMSC osteogenic aggregates can successfully decrease compaction by downregulating intracellular tension that could further lower the surface tension of cell aggregates.13,14 Therefore, we hypothesize that inhibition of RhoA/ROCK signaling will lead to reduced cytoskeletal tension in chondrogenic hMSCs and to enhanced intercellular space in aggregates. We further hypothesize that this will subsequently lead to enhanced chondrogenesis by improved transport properties within the less compact structure (Fig. 1).
FIG. 1.
Hypothesized effect of RhoA/ROCK signaling inhibition on the development of hMSC-based chondrogenic tissue. The chondrogenic aggregate under RhoA/ROCK signaling inhibition is hypothesized to be loosely formed through downregulation of cytoskeletal tension. The transport property within the tissue is therefore enhanced and that results in improvement in cartilaginous tissue development. (Lines in gray: hMSCs, in pink: glycosaminoglycan [GAG], in green: collagen.) hMSCs, human mesenchymal stem cells; TGFβ, transforming growth factor β. Color images available online at www.liebertpub.com/tea
In this study, we investigated the effect of ROCK inhibition on hMSC contraction and aggregation during chondrogenic induction. Furthermore, the biochemical properties of the resulting tissue were investigated. Optimal exposure regime of ROCK inhibition for chondrogenic enhancement in hMSC aggregates was determined. Improvement in mass transport was demonstrated upon ROCK inhibition and can explain observed enhancement in cartilaginous extracellular matrix (ECM) synthesis during chondrogenesis.
Materials and Methods
Materials
Fibroblast growth factor-2 (FGF-2) and TGFβ1 were obtained from PeproTech (Rocky Hill, NJ). l-Ascorbic acid phosphate was acquired from Wako Chemicals (Richmond, VA) and ITS+ premix culture supplement from Corning (Oneonta, NY). Dexamethasone, Triton X-100, DAPI (4′,6-Diamidino-2-phenylindole dihydrochloride), papain, calf thymus DNA, Hoechst 33258, chondroitin sulfate sodium salt, safranin-O, cetylpyridinium chloride, trans-4-hydroxy-l-proline (HYP), and protease from Streptomyces griseus were bought from Sigma-Aldrich (St. Louis, MO). All other components of culture medium, 0.2 μm carboxylate-modified fluorescent microspheres, human plasma fibronectin, collagen I antibody, and Texas Red-conjugated, lysine fixable dextran of 3000, 10,000, and 70,000 MW were obtained from Thermo Fisher Scientific (Grand Island, NY). Untreated 96-well conical V-bottomed polypropylene plates were sourced from Evergreen Scientific (Caplugs, CA). Y27632 dihydrochloride (Y27632) and (±)-blebbistatin (blebbistatin) were obtained from Tocris Bioscience (Avonmouth, Bristol, United Kingdom). Forty percent acrylamide solution and 2% bis-acrylamide solution were purchased from Bio-Rad (Hercules, CA). Paraformaldehyde was bought from Ted Pella (Redding, CA). Phalloidin was purchased from Cytoskeleton (Denver, CO). Collagen II antibody was purchased from Developmental Studies Hybridoma Bank (Iowa City, Iowa) and collagen X antibody from Abcam (Cambridge, MA). FITC (fluorescein isothiocyanate)-conjugated secondary antibody was from MP Biomedicals (Solon, OH).
Cell culture
hMSCs were isolated from bone marrow of four healthy donors through the Hematopoietic Biorepository & Cellular Therapy Core Facility of the Case Comprehensive Cancer Center. Informed consent under terms of an Institutional Review Board approved protocol was obtained from the donors before the harvesting of the aspirates. The hMSCs were selected based on the ability to adhere to cell culture plastic and proliferate to form colonies.15 hMSCs were then subcultured with growth medium consisting of Dulbecco's modified Eagle's medium, low glucose (1.5 g/L) supplemented with 10% lot-selected fetal bovine serum,15 and 10 ng/mL FGF-2.16,17 Passage 1 cells were used for chondrogenic tissue culture.
Chondrogenic tissue culture
Once 80–90% cell confluence was reached, hMSCs were trypsinized and resuspended at a density of 1.25 × 106 cells/mL in chondrogenic differentiation medium. (Dulbecco's modified Eagle's medium, high glucose [4.5 g/L] supplemented with 1% ITS+ premix culture supplement, 10−7 M dexamethasone, 50 μM ascorbate-2-phosphate, 1 mM sodium pyruvate, 2 mM l-glutamine, antibiotic-antimycotic and MEM nonessential amino acids at 1%, and 10 ng/mL TGFβ1).3,4 Of the cell suspension (250,000 cells), 0.2 mL aliquots were seeded into polypropylene 96-well, conical-bottom plates and centrifuged at 500 × g for 5 min to encourage cell condensation.18 To investigate the effect of ROCK inhibition on hMSC chondrogenesis, a ROCK inhibitor, Y27632, was added at a final concentration of 0 (control), 1, 5, 10, and 50 μM. The medium and ROCK inhibitor were replaced every other day for up to a maximum of 21 days until harvest.
Traction force microscopy
The contraction forces produced by hMSCs under the treatment of growth medium (control), chondrogenic differentiation medium, and RhoA/ROCK signaling inhibition by 10 μM Y27632 or a myosin II inhibitor and 10 μM blebbistatin in chondrogenic differentiation medium were measured by traction force microscopy (TFM), a technique for calculating traction stress generated by adherent cells on the underneath substrate.19,20 In brief, hMSCs were plated with growth medium onto a 5%/0.1% acrylamide/bis-acrylamide gel embedded with 0.5% 0.2 μm fluorescent microspheres and coated with 30 × 30 μm patterned fibronectin. After overnight cell attachment, growth medium was replaced with the chondrogenic differentiation medium. For analysis of cell traction stress on the acrylamide gel with an estimated Young's modulus of 5.8 kPa,21 the pattern-confined adherent hMSCs and fluorescent microbeads underneath the ventral surface of the hMSCs were imaged by an inverted microscopy (40 × /0.60 RC3; Olympus IX71, Center Valley, PA). hMSCs were then detached by trypsinization, and the relaxed positions of fluorescent microbeads were imaged. These images were computed using DIM and LIBTRC 2.4 software19 for microbead displacement fields and the corresponding traction stress maps, respectively, (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea).
Stress fiber imaging
The chondrogenic aggregates were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C overnight on day 7 of aggregate culture. After permeation in 0.5% Triton X-100 in PBS for 1 h at room temperature, the aggregates were incubated in staining reagent containing 0.1 μM phalloidin and DAPI (1:10,000) in PBS at 4°C overnight on a shaker in the dark. The stained aggregates were rinsed thrice in PBS over 3 h and then imaged every 2 μm from the tissue surface by Leica SP5 confocal microscope equipped with a 20 × 1 N.A. water immersion objective, a four channel NDD detector, and a tunable Ti/Sapphire I.R. laser (Coherent, Santa Clara, CA).
Aggregate size measurement
The aggregates at harvest were briefly rinsed with PBS. They were examined under a stereo microscope (Leica Microsystems GmbH, Buffalo Grove, IL) and then photographed by a camera equipped with macro lens (Nikon Nikkor 105 mm f/2.8G). The areas of aggregates were measured from the photographs using Image-Pro Plus software.
Glycosaminoglycan, hydroxyproline, and DNA assay
The quantitative assessment for glycosaminoglycan (GAG), hydroxyproline (HYP), and DNA in a single chondrogenic aggregate was performed as per published methods.18,22 In brief, each aggregate was digested in 200 μL papain solution (25 μg/mL), and the papain-digested extract was separated for the three assays. For GAG analysis, the extract was blended with Safranin-O reagent in a dot-blot apparatus (Bio-Rad), and the resulting dot was eluted in cetylpyridinium chloride. The absorbance of the eluate was read at 536 nm and compared to that of chondroitin sulfate standards. For HYP quantification, the hydrolyzed extract and HYP standards were incubated with oxidizing reagents and then Ehrlich's reagent. The absorbance of the products was measured at 505 nm for comparison. For DNA measurement, the extract was mixed with Hoechst 33258 dye to allow fluorescence quantification at an excitation wavelength of 340 nm and an emission wavelength of 465 nm and compared to the standards made with calf thymus DNA. The contents of GAG and HYP were normalized to DNA amounts for comparison between aggregates.
Mass transport analysis
To monitor the transport of glucose during chondrogenic aggregate culture, media glucose levels were measured by sampling medium daily and using a Contour Next USB Blood Glucose Monitoring System (Bayer AG, Leverkusen, Germany). A glucose standard curve generated from chondrogenic differentiation medium was used to calibrate the results. To demonstrate the diffusional transport properties over 21-day culture, fluorescent-labeled dextran of 3, 10, and 70 kDa was incubated with chondrogenic aggregates harvested at days 3, 7, or 21 for 1, 3, or 24 h and then fixed with 4% paraformaldehyde for histology. The images of two histological sections per pellet were analyzed using ImageJ software to obtain the fluorescence intensity profiles of dextran tracers within the aggregates. To minimize section size-dependent artifacts, the intensity was normalized by the section area and weighted by the projected aggregate area (Fig. 2C).
FIG. 2.
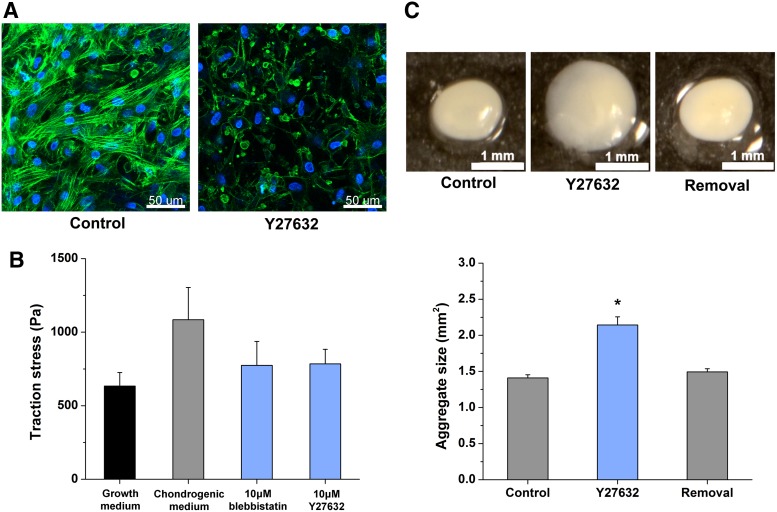
Inhibition of RhoA/ROCK signaling attenuates hMSC cytoskeleton tension and compactness of chondrogenic aggregation. (A) Evaluation of hMSC stress fiber formation under control and 10 μM Y27632 treatment during chondrogenic induction. Phalloidin (green) and DAPI (blue) were used for stress fiber and nuclear staining, respectively, and imaged at 50 μm depth from the surface of control and 10 μM Y27632 treated intact aggregates at Day 7. Scale bar 50 μm. (n = 8) (B) The change of contraction force generated by hMSCs in growth medium (control), chondrogenic differentiation medium, and chondrogenic medium with RhoA/ROCK signaling inhibitors, 10 μM blebbistatin or Y27632. (n ≥ 37) Student's t-test results for comparison to growth maintenance (control): chondrogenic differentiation medium p = 0.06; 10 μM blebbistatin p = 0.26; 10 μM Y27632 p = 0.22. (C) Assessment of the effect of 10 μM Y27632 treatment on hMSC chondrogenic aggregation. Images of aggregates at 48 h (top panel): untreated (left, control) and 10 μM Y27632 treated (middle, Y27632) aggregates. The aggregate on the right (removal) was exposed to 10 μM Y27632 treatment for the first 24 h only. Scale bar 1 mm. Bottom graph shows image analysis data. (n = 10) Data represent the mean ± SEM. ANOVA F-test; *p < 0.05. See also Supplementary Figure S1. ANOVA, analysis of variance; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride. Color images available online at www.liebertpub.com/tea
Histology and immunohistochemistry
To reveal GAG distribution, aggregates were fixed in 4% paraformaldehyde, paraffin-embedded, sectioned, and then stained with Safranin-O. For immunohistochemical evaluation, aggregates were embedded in optimal cutting temperature compound at −80°C and processed for cryosectioning. The sections were fixed in cold (4°C) acetone for 10 min and then blocked with 10% normal goat serum in PBS for 30 min. After the primary antibody against collagen type I (1:200), II (1:50), or X (1:100) diluted in 1% goat serum in PBS was incubated with the sections for 1 h, FITC-conjugated goat anti-mouse IgG, IgA, and IgM were applied at 1:400 in 1% normal goat serum in PBS for 45 min. For type X collagen staining, sections were treated with 1 mg/mL protease in PBS before blocking to expose antigens. The sections with no primary antibody treatment in parallel were used as negative controls. The stained sections were imaged under a 10 × objective using a SPOT RT digital camera on a Leica fluorescence microscope (Leica Microsystems).
Statistical analysis
Data are represented as mean ± SEM. To determine significance, Student's t-test was used for pair-wise comparisons, and analysis of variance with Fisher's test (Minitab®; Minitab, Inc., State College, PA) was used for multiple comparisons. Differences were considered significant when p-value <0.05.
Results
The effect of RhoA/ROCK signaling inhibition on hMSC aggregation
To investigate the effect of RhoA/ROCK signaling inhibition on cytoskeletal tension in hMSC chondrogenesis, chondrogenic aggregates were treated with a ROCK inhibitor, Y27632,23,24 at 10 μM. The diminished formation of stress fibers under ROCK inhibition was evident in intact day 7 aggregates (Fig. 2A).
hMSC aggregates form within 1 day under the conditions of high cell density and chondrogenic induction. Actin-myosin mediated contractile force is assumed to be enhanced upon chondrogenic induction, and inhibition of RhoA/ROCK signaling, as a critical modulator of actin-myosin mediated contraction,6 is inferred to downregulate the contractile force of hMSCs undergoing chondrogenesis. To quantify contractile forces generated by hMSCs under the growth maintenance condition (control), chondrogenic induction, and RhoA/ROCK signaling inhibition, we carried out TFM. The results of TFM experiments using single cells support our hypothesis and indicate that the utilization of Y27632 and blebbistatin25–28 for ROCK and myosin II inhibition, respectively, can effectively relieve the contraction stress to the level between stem cell status and chondrogenic induction (28% reduced) (Fig. 2B).
Since overnight treatment with 10 μM Y27632 downregulates cell contraction, we further examined its effect on cell condensation. The aggregates cultured under ROCK inhibition over 2 days were significantly enlarged. When ROCK inhibition was removed after 24 h, the aggregate size recovered to a level similar to control (Fig. 2C). This reversible effect on aggregate compactness at the initial stages of chondrogenesis further supports our assumption that the reduced contractile forces in hMSCs under ROCK inhibition lead to looser aggregation and, therefore, larger aggregates.
Assessment of hMSC-based chondrogenic differentiation under ROCK inhibition
The key to optimizing the cartilaginous tissue culture from hMSCs is to identify and supply the appropriate biochemical. We cultured hMSC aggregates over a period of 21 days with Y27632 at different levels and over different exposure times to evaluate the dose-dependency effect of RhoA/ROCK inhibition and then measured the size and biochemical properties of resulting aggregates to determine the best exposure regime.
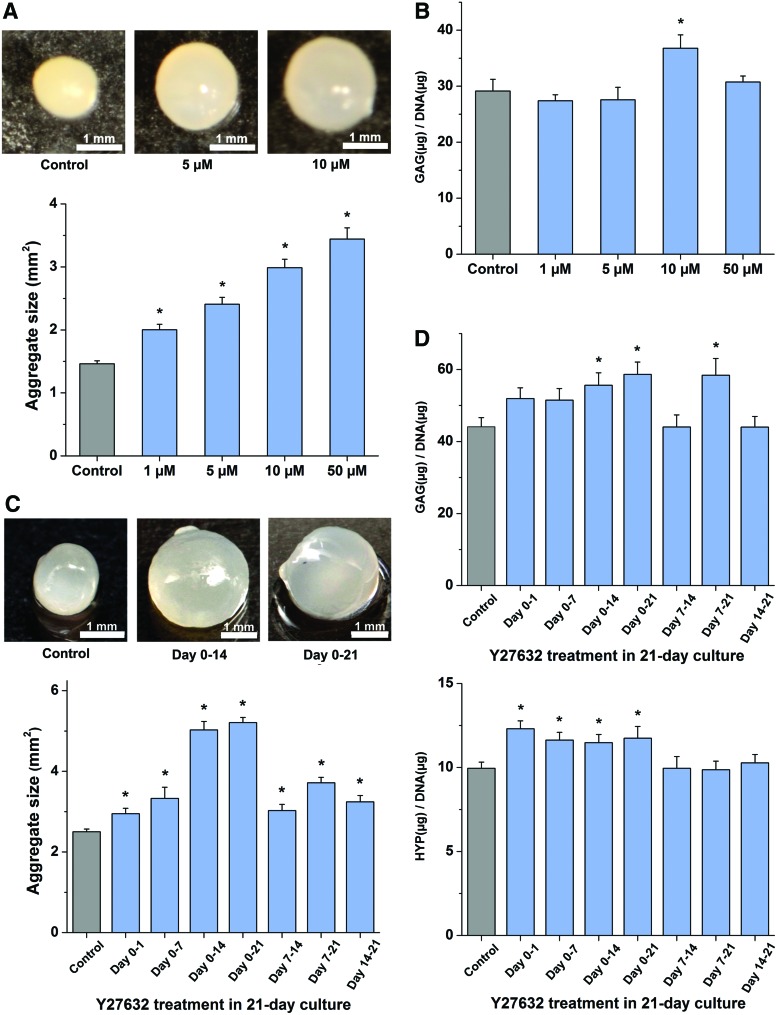
There has been no systematic study of Y27632 levels and exposure times on hMSC, although 10 μM was used in previous research.6,23,24 We tested concentrations ranging from 0 to 50 μM and different exposure times. All concentrations of Y27632 led to hMSC aggregation, but 50 μM led to a flat disc-like shape on day 2. This was verified under a stereo microscope. With maturation, the aggregates all became spherical by day 21. Histological results of all sections showed circular cross-sections suggesting that aggregates are spherical in nature (Supplementary Fig. S3). We show that there was a significant enlargement of aggregates in a dosage-dependent manner of Y27632 treatment starting at day 2 (Fig. 3A and Supplementary Fig. S2). Furthermore, the amount of glycosaminoglycans (GAG/DNA) in the resulting aggregates was significantly increased at 10 μM treatment after 21 days in culture (Fig. 3B, Supplementary Figs. S3 and S4). Hence, 10 μM Y27632 treatment was chosen for the subsequent exposure-time experiments.
FIG. 3.
Effect of dosage and exposure regime of Y27632 treatment on hMSC chondrogenic differentiation. (A) Morphological properties of hMSC aggregates after Y27632 treatment. Aggregates of control and Y27632 treatment at 1, 5, 10, or 50 μM were harvested after 21-day culture. Estimated size of aggregates was obtained from the projected areas of the images. Scale bar 1 mm. (n = 8) (B) Evaluation of Y27632 dosage effect on GAG accumulation after 21-day culture. The amount of GAG in digested aggregates was measured and normalized to DNA content for comparison. (n ≥ 8) (C) Morphological properties of hMSC aggregates after 10 μM Y27632 treatment in different periods of 21-day culture. The aggregates were imaged after harvest for size measurements. Scale bar 1 mm. (n ≥ 11) (D) The effect of different exposure regimes of 10 μM Y27632 on GAG and HYP depositions. The quantities of GAG and HYP in digested hMSC aggregates were normalized to DNA content to yield the result of GAG per DNA and HYP per DNA for comparison. (n ≥ 11) Data represent the mean ± SEM. ANOVA F-test; *p < 0.05. See also Supplementary Figures S2–S5. Color images available online at www.liebertpub.com/tea
All exposure regimes of 10 μM Y27632 treatment led to significant increase in size. The most significant effect was attained when aggregates were treated from day 0 to day 14 and from day 0 to day 21. These aggregates were about twofold larger than control aggregates after 21-day culture (Fig. 3C). GAG accumulation (GAG/DNA) upon ROCK inhibition (exposure regimes: day 0–14, 7–21, and 0–21) was significantly increased compared to control (up to 33% increase) (Fig. 3D). The amount of collagen in hMSC aggregates was determined by quantification of hydroxyproline (HYP), an amino acid exclusively present in collagen and elastin.29 The results show that ROCK inhibition from the onset of aggregate culture can significantly augment the deposition of collagen (up to 24% increase), and prolonged inhibition maintained the increase (Fig. 3D). Therefore, 10 μM Y27632 treatment of aggregates over the entire culture period of 21 days was chosen as the optimal regime. The corresponding DNA data are shown in Supplementary Figure S4. They show a slight but inconsistent change (8% increase to 12% decrease) in DNA under 10 μM ROCK inhibition.
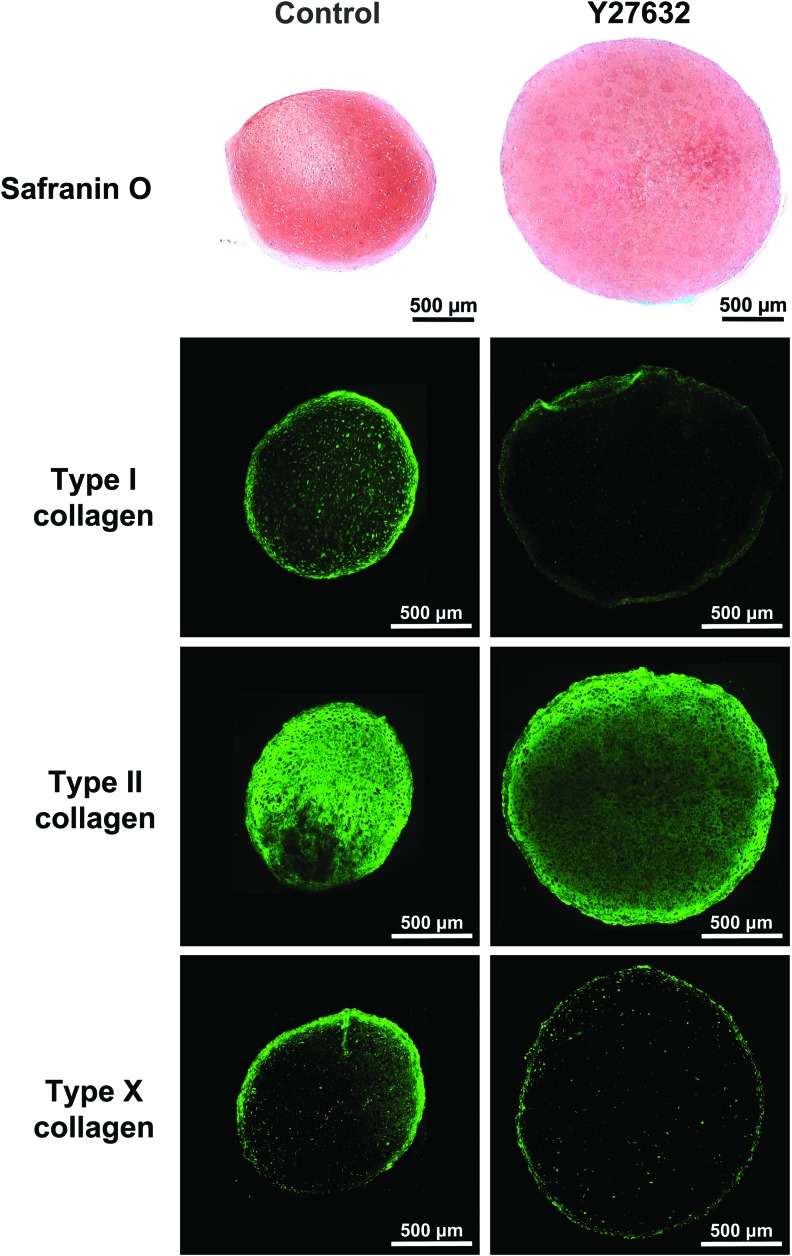
To further examine the chondrogenic response of hMSC aggregates under control and 10 μM Y27632 treatment, histology and immunohistochemistry were performed at day 21. Safranin-O staining demonstrated the presence of well-distributed GAG in both control and Y27362-treated aggregates. The progression of chondrogenesis can be determined by the deposition of different types of collagen. Type I, II, and X collagen are the indicators of initial, mature, and terminal stages of MSC chondrogenesis, respectively. In both control and Y27632 aggregates, type II collagen was present everywhere, and type I collagen was barely detectable at the periphery. Type X was weakly detected in control, but barely detectable in Y27632 treatment (Fig. 4).
FIG. 4.
Histology and immunohistochemistry results. Safranin-O and type I, II, and X collagen stained hMSC aggregates cultured with 10 μM Y27632 for 21 days were compared with control. Scale bar 500 μm. (n ≥ 4) Color images available online at www.liebertpub.com/tea
The susceptibility to Y27632 treatment in hMSC aggregates derived from three different donors was further evaluated by studying GAG and collagen accumulations as chondrogenic indicators. No negative effects were detected among three donors, but the increase in cartilaginous ECM varied. The 10 μM Y27632 treatment for 21-day culture significantly increased GAG synthesis in two of the three donors and promoted collagen deposition significantly only in one of the three donors (Supplementary Fig. S5). These results suggest that the Y27632 treatment for hMSCs from different donors needs to be optimized.
The enhanced cartilaginous ECM synthesis relative to improvement in transport properties
In light of the enlarged aggregates with increased deposition of chondrogenic markers, collagen, and GAG, we hypothesize that ROCK inhibition promotes the transport of chondrogenic factors and nutrients within the aggregates as a result of less compact aggregation. To test the hypothesis, the efficiency of molecular transport into hMSC aggregates was assessed by monitoring glucose consumption and detecting fluorescent-labeled dextran diffusion.
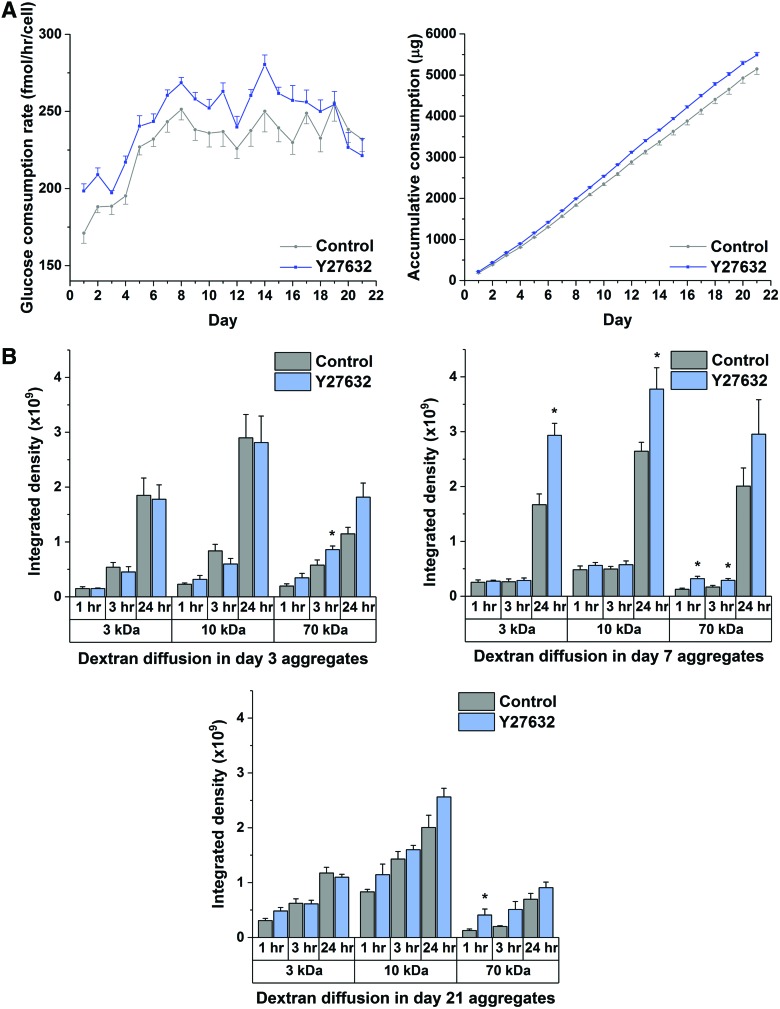
Glucose is an important metabolite and a structural precursor for chondrogenic differentiation. In particular, glucose is the primary substrate for GAG synthesis. The results show that the rate of glucose consumption under Y27632 treatment increased significantly from the onset of aggregate culture until day 19, which spanned the critical phases of chondrogenesis and ECM synthesis before the hypertrophic stage. Furthermore, the cumulative consumption under ROCK inhibition increased modestly (7%) compared to control during 21-day culture (Fig. 5A). The finding corresponds with the enhancement in matrix production and indicates the improved transport of small molecules like glucose (180 Da) upon ROCK inhibition.
FIG. 5.
Assessment of transport properties in hMSC chondrogenic aggregates under Y27632 treatment during 21-day culture. (A) Glucose consumption in hMSC aggregates after 10 μM Y27632 treatment. Daily glucose consumption rate and accumulative glucose consumption in 21-day culture were analyzed. (n = 6) (B) Diffusional status of hMSC aggregates under ROCK inhibition at different chondrogenic stages. The intensities of tissue sections in control and 10 μM Y27632 treated aggregates exposed to 3, 10, and 70 kDa fluorescent dextran tracers for 1, 3, and 24 h at day 3, 7, and 21 culture times were analyzed. (n ≥ 4) Data represent the mean ± SEM. ANOVA F-test; *p < 0.05. See also Supplementary Table S1. Color images available online at www.liebertpub.com/tea
To investigate diffusivity, we further studied the uptake of tracer molecules: 3, 10, and 70 kDa fluorescent-labeled neutral dextran—the molecular weights covering the range of large molecules in the chondrogenic differentiation medium (insulin, TGFβ, and BMPs).30 At day 3, the diffusion of 70 kDa dextran for 3 h in Y27632-treated aggregates was significantly increased. At day 7, the diffusion of not only 70 kDa dextran for 1 and 3 h but also smaller solutes of 3 and 10 kDa for 24 h were significantly improved under Y27632 treatment. At day 21, the diffusion of 70 kDa dextran for 1 h was significantly enhanced. Among all the chondrogenic stages, the diffusion of 70 kDa dextran under ROCK inhibition was steadily promoted supporting our hypothesis (Fig. 5B).
The diffusivity characteristics of the aggregates can be quantified by estimating diffusivity of the tracer in the aggregates. We used a mathematical model of unsteady diffusion of the tracer in a sphere and fitted the diffusivity to the time-dependent experimental data: normalized mean intensity and radius of the tissue section and aggregate radius evaluated through image analysis. The modeling allowed us to use the model to estimate diffusivity based on tissue section specific information (Supplementary Data). The results (Supplementary Table S1) showed enhanced diffusivity for 70 kDa dextran for days 3 and 21. For day 7, the diffusivities of 70 kDa dextran in inhibitor-treated and control aggregates were similar.
Discussion
For the first time, ROCK inhibition has been shown to lower cytoskeletal tension to a level that did not reverse the chondrogenic fate of hMSC aggregates. The downregulationof cell contraction upon ROCK inhibition in individual hMSCs can lead to a net reduction in aggregation force and, subsequently, less compacted aggregates. The relatively rapid reversal reinstatement of the normal aggregation process upon removal of ROCK inhibition at early chondrogenesis confirms this. In addition, our results suggest that ROCK inhibition affects cytoskeletal tension but not cell–cell adhesion, as lack of the latter leads to no aggregation.10 Long-term ROCK inhibition in aggregate culture was found to be beneficial for hMSC chondrogenesis. RhoA/ROCK signaling is also involved in the mediation of focal adhesion between cells and ECM proteins, and reduced focal adhesion is favored during chondrogenesis.31
Previous studies regarding RhoA/ROCK signaling on chondrogenic regulation suggested a context-dependent effect; its effect on different cell sources and culture environments was inconsistent.8 While Y27632 treatment in micromass culture of mouse chondrogenic cell line ATDC5 enhanced Sox9 transcript level and GAG synthesis,32 it decreased the transcript levels of collagen type II and aggrecan in micromass culture of primary mouse mesenchymal limb bud cells.8 Furthermore, Y27632 treatment in monolayer culture of human articular chondrocytes caused upregulation of Sox9 expression,33,34 but in rat synovium-derived MSCs led to downregulation of Sox9, type II collagen, and aggrecan expression.35 Due to this issue of cellular context dependence, there is a need to investigate the effect of RhoA/ROCK signaling on chondrogenesis in human MSC aggregates to manipulate this pathway for chondrogenic enhancement in cartilaginous tissue culture. In this study, we have shown that ROCK inhibition of chondrogenic differentiation in hMSC-based aggregates leads to enhanced chondrogenesis.
Relative to native adult articular cartilage, hMSC-based cartilaginous aggregates were reported to contain only 3% collagen type II and 40% GAG of native tissue.5 Results from this study showed that collagen and GAG deposition upon ROCK inhibition in hMSC aggregates undergoing chondrogenesis increased significantly compared to control aggregates. While this narrowed the gap between native tissue and aggregate significantly with respect to GAG deposition, collagen content was still significantly lower. ROCK inhibition increased the aggregate size and ECM (GAG ± collagen) but disproportionately; compared to control aggregates, the increase in ECM is not proportional to the increase in size although the starting cell number and the end DNA content were similar under both conditions. For 50 μM treatment, this effect was worse. Enhanced GAG along with reduced cytoskeletal tension can lead to increased water content in the aggregates,36 and the disproportionate GAG to water ratio at equilibrium can explain the corresponding disproportionate increases in GAG content and size of the aggregates upon ROCK inhibition.
Our results also suggest that ROCK inhibition can lead to enhanced mass transport due to the formation of less dense aggregates. Mass transport is important in nutrient delivery and signaling in the tissues. The efficiency of nutrient mass transfer in tissue-engineered cartilage can affect the ultimate size and ECM constitution. Throughout chondrogenesis in hMSC aggregates, various sizes and charges of molecules transport from the medium through the ECM into cells to maintain metabolism. These range from relatively low-molecular weight solutes such as glucose and larger solutes such as TGFβ1 (25 kDa).37 Glucose is a key metabolite during chondrogenesis due to the polysaccharides that form the basis of GAG. A moderate but consistent enhancement of glucose uptake upon ROCK inhibition is likely due to either the larger aggregate size and/or enhanced diffusivity of glucose within the aggregate; with the same number of cells, the uptake rate is proportional to the diameter of the aggregate.38 Enhanced diffusivity can occur if the ECM density is reduced. Indeed, the enhanced ECM synthesis in aggregates upon ROCK inhibition did not scale with enhanced size suggesting reduced ECM density in ROCK inhibited aggregates.
The significant differences noted in tracer experimental results between control and Y27632-treated aggregates were primarily for the higher molecular weight tracer, 70 kDa dextran. So, larger molecules such as biglycan (76 kDa), single type II procollagen chain (285 kDa), and proteoglycan (2500 kDa) were more likely to be obstructed in control than in Y27632-treated aggregates.30 In addition, dextran is a linear polymer of glucose, which may not well represent globular proteins. The higher flexibility in linear structure could render easier penetration into tissue than more rigid globular proteins of similar molecular weight.39 The tracer results also showed that control aggregates have improved transport at early culture times for smaller tracers: 3 and 10 kDa. The tissue components are not evenly distributed during early aggregation stage, which could lead to the discrepant results in smaller tracers at early time points.
The significant increase in aggregate size with the same number of hMSCs and its improved biochemical properties under ROCK inhibition could offer a strategy to prepare better chondrogenic aggregates for fusion into centimeter-sized constructs.40 We expect that these findings can provide the fundamental insight into the function of ROCK inhibition in hMSC chondrogenesis and will ultimately be applied to engineer cartilaginous tissue from hMSCs.
Supplementary Material
Acknowledgments
This research was supported, in part, by grants from the National Institutes of Health (EB021911 and GM050009). The authors thank Dr. Micah Dembo for generously sharing the LIBTRC 2.4 image analysis software and Dr. Dustin Thomas for help with TFM. The authors also thank Amad Awadallah at the Skeletal Research Center and Jim Berilla at the Department of Civil Engineering, Case Western Reserve University, Cleveland, OH for help with histology and machining for TFM, respectively. Finally, the authors thank Larissa Rizzi de Freitas, Emily Kwan, and Katerina Aris for their help with imaging and mass transport analysis.
Disclosure Statement
No competing financial interests exist.
References
- 1.Pittenger M.F., Mackay A.M., Beck S.C., et al. . Multilineage potential of adult human mesenchymal stem cells. Science 284, 143, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Fell H.B. The histogenesis of cartilage and bone in the long bones of the embryonic fowl. J Morphol 40, 417, 1925 [Google Scholar]
- 3.Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., and Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238, 265, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Yoo J.U., Barthel T.S., Nishimura K., et al. . The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am 80, 1745, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Hillel A.T., Taube J.M., Cornish T.C., et al. . Characterization of human mesenchymal stem cell-engineered cartilage: analysis of its ultrastructure, cell density and chondrocyte phenotype compared to native adult and fetal cartilage. Cells Tissues Organs 191, 12, 2010 [DOI] [PubMed] [Google Scholar]
- 6.McBeath R., Pirone D.M., Nelson C.M., Bhadriraju K., and Chen C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6, 483, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Kim M.J., Kim S., Kim Y., Jin E.J., and Sonn J.K. Inhibition of RhoA but not ROCK induces chondrogenesis of chick limb mesenchymal cells. Biochem Biophys Res Commun 418, 500, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Woods A., and Beier F. RhoA/ROCK signaling regulates chondrogenesis in a context-dependent manner. J Biol Chem 281, 13134, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Ichinose S., Tagami M., Muneta T., and Sekiya I. Morphological examination during in vitro cartilage formation by human mesenchymal stem cells. Cell Tissue Res 322, 217, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Tuli R., Tuli S., Nandi S., et al. . Transforming growth factor-β-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem 278, 41227, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Tufan A.C., Daumer K.M., DeLise A.M., and Tuan R.S. AP-1 Transcription factor complex is a target of signals from both WNT-7a and N-cadherin-dependent cell–cell adhesion complex during the regulation of limb mesenchymal chondrogenesis. Exp Cell Res 273, 197, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Tufan A.C., and Tuan R.S. Wnt regulation of limb mesenchymal chondrogenesis is accompanied by altered N-cadherin-related functions. FASEB J 15, 1436, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Tsai A.C., Liu Y., Yuan X., and Ma T. Compaction, fusion, and functional activation of three-dimensional human mesenchymal stem cell aggregate. Tissue Eng Part A 21, 1705, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning M.L., Foty R.A., Steinberg M.S., and Schoetz E.-M. Coaction of intercellular adhesion and cortical tension specifies tissue surface tension. Proc Natl Acad Sci U S A 107, 12517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lennon D.P., Haynesworth S.E., Bruder S.P., Jaiswal N., and Caplan A.I. Human and animal mesenchymal progenitor cells from bone marrow: identification of serum for optimal selection and proliferation. In Vitro Cell Dev Biol Anim 32, 602, 1996 [Google Scholar]
- 16.Solchaga L.A., Penick K., Porter J.D., Goldberg V.M., Caplan A.I., and Welter J.F. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol 203, 398, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi S., Shimazu A., Miyazaki K., et al. . Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun 288, 413, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Solchaga L., Penick K., and Welter J. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. In: Vemuri M., Chase L.G., Rao M.S., eds. Mesenchymal Stem Cell Assays and Applications. New York, NY: Humana Press, 2011, pp. 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dembo M., and Wang Y.-L. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J 76, 2307, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rape A.D., Guo W.-H., and Wang Y.-L. The regulation of traction force in relation to cell shape and focal adhesions. Biomaterials 32, 2043, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tse J.R., and Engler A.J. Preparation of hydrogel substrates with tunable mechanical properties. In: Current Protocols in Cell Biology. 47, 10.16.1, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Ham K.D., Oegema T.R., Loeser R.F., and Carlson C.S. Effects of long-term estrogen replacement therapy on articular cartilage IGFBP-2, IGFBP-3, collagen and proteoglycan levels in ovariectomized cynomolgus monkeys. Osteoarthritis Cartilage 12, 160, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Darenfed H., Dayanandan B., Zhang T., Hsieh S.H.K., Fournier A.E., and Mandato C.A. Molecular characterization of the effects of Y-27632. Cell Motil Cytoskeleton 64, 97, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Davies S.P., Reddy H., Caivano M., and Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351, 95, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allingham J.S., Smith R., and Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol 12, 378, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Kovacs M., Toth J., Hetenyi C., Malnasi-Csizmadia A., and Sellers J.R. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem 279, 35557, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Limouze J., Straight A., Mitchison T., and Sellers J. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil 25, 337, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Takács B., Billington N., Gyimesi M., et al. . Myosin complexed with ADP and blebbistatin reversibly adopts a conformation resembling the start point of the working stroke. Proc Natl Acad Sci U S A 107, 6799, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ignat'eva N.Y., Danilov N., Averkiev S., Obrezkova M., and Lunin V. Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J Anal Chem 62, 51, 2007 [Google Scholar]
- 30.Leddy H.A., and Guilak F. Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Ann Biomed Eng 31, 753, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Engler A.J., Sen S., Sweeney H.L., and Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Woods A., Wang G., and Beier F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem 280, 11626, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto E., Furumatsu T., Kanazawa T., Tamura M., and Ozaki T. ROCK inhibitor prevents the dedifferentiation of human articular chondrocytes. Biochem Biophys Res Commun 420, 124, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Tew S.R., and Hardingham T.E. Regulation of SOX9 mRNA in human articular chondrocytes involving p38 MAPK activation and mRNA stabilization. J Biol Chem 281, 39471, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Xu T., Wu M., Feng J., Lin X., and Gu Z. RhoA/Rho kinase signaling regulates transforming growth factor-beta1-induced chondrogenesis and actin organization of synovium-derived mesenchymal stem cells through interaction with the Smad pathway. Int J Mol Med 30, 1119, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Maroudas A., and Bannon C. Measurement of swelling pressure in cartilage and comparison with the osmotic pressure of constituent proteoglycans. Biorheology 18, 619, 1981 [DOI] [PubMed] [Google Scholar]
- 37.Bursac P.M., Freed L.E., Biron R.J., and Vunjak-Novakovic G. Mass transfer studies of tissue engineered cartilage. Tissue Eng 2, 141, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Crank J. The Mathematics of Diffusion, 2nd ed. Oxford, England: Clarendon Press, 1979 [Google Scholar]
- 39.Pluen A., Netti P.A., Jain R.K., and Berk D.A. Diffusion of macromolecules in agarose gels: comparison of linear and globular configurations. Biophys J 77, 542, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhumiratana S., Eton R.E., Oungoulian S.R., Wan L.Q., Ateshian G.A., and Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci U S A 111, 6940, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.