Abstract
Significance: Mesenchymal stem cells (MSCs), adult stem cells with the potential of differentiation into mesodermal lineages, play an important role in tissue homeostasis and regeneration. In different organs, a subpopulation of MSCs is located near the vasculature and possibly represents the original source of lineage-committed mesenchymal progenitors.
Recent Advances: The plasticity and immune characteristics of MSCs render them a preferential tool for regenerative cell therapy.
Critical Issues: The culture expansion needed before MSC transplantation is associated with cellular senescence. Moreover, accelerated senescence of the total and perivascular MSC pool has been observed in humans and mouse models of premature aging disorders. MSC dysfunction is acknowledged as a culprit for the aging-associated degeneration of mesodermal tissues, but the underlying epigenetic pathways remain elusive. This article reviews current understanding of mechanisms impinging on MSC health, including oxidative stress, Nrf2-antioxidant responsive element activity, sirtuins, noncoding RNAs, and PKCs.
Future Directions: We provide evidence that epigenetic profiling of MSCs is utilitarian to the prediction of therapeutic outcomes. In addition, strategies that target oxidative stress-associated mechanisms represent promising approaches to counteract the detrimental effect of age and senescence in MSCs.—Antioxid. Redox Signal. 29, 864–879.
Keywords: : cell therapy, mesenchymal stem cells, pericytes, reactive oxygen species, senescence
Introduction
Understanding the aging process and the mechanisms underpinning the development of aging-associated diseases represents one of the most important endeavors of modern medical research. The death rate at all ages has been dramatically reduced in recent decades, resulting in a remarkable increase in life expectancy. However, increased lifespan also correlates with an increasingly old population and an increased number of individuals with chronic pathologies often requiring hospitalization.
Accumulating evidence suggests that stem cells play a crucial role in controlling physiological homeostasis, and rate of aging and stem cell exhaustion is considered one of the hallmarks of aging (67, 69). Hence, maintenance of stem cell health may translate into postponing aging-related diseases. The loss of stem cell activity and acquisition of a senescent phenotype is due to both intrinsic factors, such as DNA damage, telomeres shortening, and chromatin modifications [as reviewed in Ref. (9)], and external factors involving the stem cell microenvironment [cytokine stimulation (74)] and widespread damage in tissues. Senescence has a dualistic function: from one side, it protects cells from damage and oncogene activation but from the other, it limits the possibility for tissue regeneration triggering aging-related deterioration. In the past decades, mesenchymal stem cells (MSCs) have been investigated as therapeutic tools for regenerative medicine, especially for treating chronic diseases. However, the possible impact of pathologic conditions on MSC viability and function has to be taken into account. In addition, MSCs' numerical reduction or dysfunction can per se cause pathologies (101). For instance, accelerated attrition of the MSCs pool has been observed in premature aging disorders, including Werner syndrome and Hutchinson-Gilford progeria syndrome (66, 124). It is, thus, clear that these cells, although harboring multilineage regeneration potential, may manifest intrinsic defects compromising their use in regenerative medicine. Conversely, transplantation of young MSCs increases the lifespan and fitness of progeroid phenotype as observed in mice (62). However, the genetic and epigenetic mechanisms underpinning MSC-mediated senescence have not been fully elucidated.
This article reviews current understanding of the contribution of oxidative stress as a trigger of modifications at DNA and RNA leading to MSC aging and senescence (Fig. 1). We also discuss how a quality and quantity assessment of these aging-related modifications can help upgrade current regenerative medicine approaches.
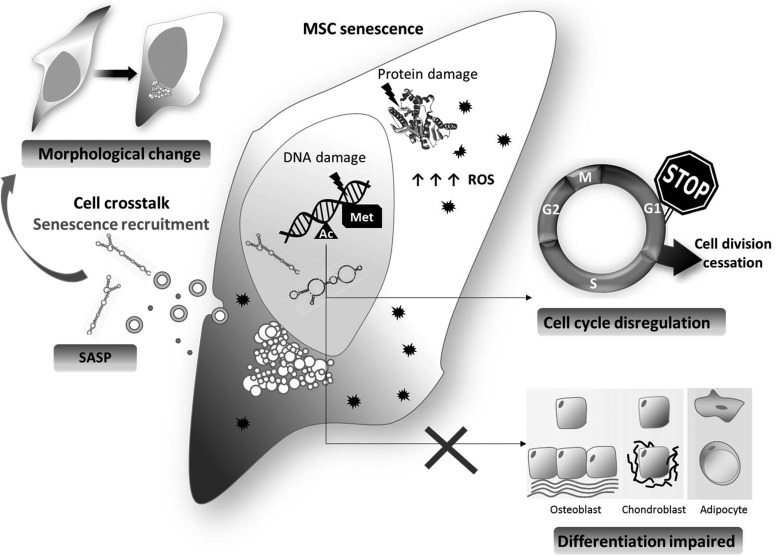
FIG. 1.
Overview of senescence in MSCs. MSC senescence is driven by diverse events, which occur as the cells, proliferates, such as epigenetic modifications, DNA damage, and ROS accumulation. Those events cause an irreversible cell cycle arrest, a change in morphology (spread and enlarged), and an impairment in differentiation ability. In addition, senescent cells produce and secrete a series of SASP paracrinally mediating senescence of close MSCs. MSC, mesenchymal stem cell; ROS, reactive oxygen species; SASP, senescence-associated secretory phenotype.
The Nature of MSCs and Their Potential for Therapy
In humans, MSCs can be isolated from different adult tissues, including bone marrow where they were first discovered in 1970, skeletal muscle, adipose tissue, umbilical cord, synovium, the circulatory system, dental pulp, amniotic fluid, fetal blood, liver, and lung [as reviewed in Ref. (85)]. Minimal characterization criteria helped to standardize MSC isolation and allowed the definition of MSCs as plastic adherent lineage-negative cells, expressing CD105, CD73, CD90 and potentially able to differentiate at least to osteocytes, chondrocytes, and adipocytes (42). Recently, new criteria, including markers of potency, have been recommended (33). Despite their low abundance [in the bone marrow, their yield spans between 0.01% and 0.001% of nucleated cells (86)], MSCs are believed to be one of the most useful cell sources for clinical application in tissue regeneration. Indeed, compared with embryonic stem cells, MSCs are safe and non-immunogenic.
Although normally in a quiescent state, MSCs can re-enter the cell cycle and differentiate following specific stimuli such as tissue injury. Thus, MSCs are important in guiding the processes of healing and tissue regeneration (83). In addition, MSCs are not only able to give rise to the cell types found in the tissue they were isolated from, but they can also differentiate into a variety of mesodermal cell types and into cell types of other germinal layers through a process known as transdifferentiation (85).
Great relevance has been attributed to perivascular mesenchymal cells, which possibly represents the original source of lineage-committed mesenchymal progenitors. These cells are better known as pericytes. In vitro, pericytes show typical mesenchymal properties with the capacity to attach to tissue culture plastic, expand for multiple passages, and differentiate into osteogenic, chondrogenic, or adipogenic lineages (23). In vivo lineage tracing studies have reported pericytes as progenitors of white adipocytes (102), follicular dendritic cells (59), and skeletal muscle (25, 26). Pericytes have also been proposed to give rise to neurons, astrocytes, and oligodendrocytes (27) and to play a major role as fibroblast progenitors in fibrotic responses (34, 38). However, in a very recent paper from Guimaraes-Camboa, the mesenchymal origin and properties of pericytes were called into question while using fate mapping experiments in murine models. Using the transcription factor Tbx18 as an embryonic pericyte marker, Guimaraes-Camboa et al. demonstrated that Tbx18-positive cells also co-express pericyte markers CD146 and neural/glial antigen 2, but they do not differentiate during the development in any mesenchymal lineage (40). These results challenge the current view of endogenous pericytes as multipotent tissue-resident progenitors and suggest that the plasticity observed in vitro or after transplantation in vivo arises from artificial cell manipulations ex vivo.
MSCs in Regenerative Medicine: State-of-the-Art
MSCs represent a great promise for regenerative medicine. They can help in the repair of injured organs and their functional recovery by migrating and homing at the injury site (71, 109). This can be achieved by both transplanting purified MSCs from a donor (especially in a syngeneic fashion) and stimulating their activation in vivo from the reservoir located in situ. Moreover, MSCs can repair injured tissues by directly differentiating to functional cells of the tissue, favoring angiogenesis or paracrinally, stimulating resident progenitor activation (90). Paracrine factors released from transplanted or resident MSCs can also contribute to immunosuppression of the host to avoid an immune response. This effect seems to be exerted by both paracrine suppression of lymphocyte T and favoring the transition of macrophages from subtype M1 (pro-inflammatory) to M2 (anti-inflammatory). Moreover, MSCs secretome is also involved in paracrine protection against apoptosis and oxidative stress [reviewed in Ref. (64)]. In recent years, the role of microparticles as a component of MSCs secretome has been envisioned as a new tool in regenerative medicine. Those particles promote the horizontal transfer of mRNAs, microRNAs (miRs), and proteins modulating the activity of target cells (84). For instance, studies defined the important role of exosomes in mediating paracrine information transfer to healing myocardial ischemia/reperfusion injury in mice (61) and pigs (105). Despite the bulk of information available on the feasibility and therapeutic outcomes, MSC-based cell therapy did not obtain the expected success in clinical practice. This is due to several factors such as documented recovery of immunogenicity associated with MSC differentiation causing late rejection (45), the poor engraftment (58), the short-term therapeutic benefits (95), and the acquisition of a senescent phenotype after several cycles of expansion in vitro. Senescence, in particular, is due to the interplay between stochastic and programmed events (41). MSC senescence is triggered by a plethora of events that are mostly regulated by epigenetic phenomena, which will be herein discussed.
MSCs are not Exempted by Experiencing Aging and Senescence: Regulating Mechanisms
Cell vs. organism senescence
Despite their self-renewal ability, MSC expansion is restricted by the Hayflick limit, that is, the number of times a cell can divide until senescence occurs. At this point, cell cycle irreversibly stops even though MSCs are still metabolically active. Morphologically, senescent MSCs lose their typical spindle-like shape and become flat and enlarged with the formation of senescence-associated heterochromatic foci linked to the repression of proliferative genes and, hence, DNA synthesis. The mentioned morphological changes reflect a functional impairment since cells lose their differentiation potential, thus limiting their therapeutic capacities (114). In a sense, senescence is the recapitulation, in the cell, of aging in individuals. However, controversy still exists regarding the direct contribution of senescent MSCs to organism aging. In any case, senescence is a physiologic event and it is the expression of the lack of perfection in biologic systems. Indeed, every time a cell divides DNA damage accumulates at specific chromosomal regions known as telomeres. Telomeres are complexes made of proteins and single-stranded nucleotides located at the end of every chromosome and are more sensitive to replication damage stress than other chromosomal regions (96, 99). Their instability is due to the inability of DNA-polymerase to work on single-stranded sequences. The consequence is a shortening of telomeres at every cell division till reaching a critical threshold that triggers senescence. Telomeres attrition is generally counteracted by telomerase reverse transcriptase (TERT), an enzyme that is able to add DNA sequences at telomeric ends of chromosomes by using an RNA template. However, telomerase is often poorly expressed in human MSCs and in any case, it cannot counteract other causes of senescence (9) herein described.
Epigenetic regulation
Changes in the epigenetic regulation of gene expression have been recently related to the senescent phenotype. Koch et al. found that DNA methylation varied by 40% between early and late passages, with a general tendency to hypomethylation but, in some cases, also hypermethylation was detected. The same group proposed an Epigenetic-Senescence-Signature (ESS) based on the methylated state of specific CpG islets. In particular, they found that two CpG were hypermethylated whereas four were hypomethylated. This ESS significantly correlated with cell passage and was found with the same characteristics in MSCs from different origins (58). The ESS could, thus, be a helpful tool to evaluate when MSCs go toward senescence and standardize the good manufacturing practices (GMP) procedures of in vitro expansion before cell therapy as further discussed later in this article.
Senescence associated secretory phenotype
Other causes of MSC senescence are sustained cytokine stimulation and secreted autocrine or paracrine factors, including cytokines, growth factors, proteases, and soluble receptors called senescence-associated secretory phenotype (SASP). It has been demonstrated that chronic stimulation with antiproliferative cytokines such as interferon-β and transforming growth factor-β in MSCs induces ROS-mediated p53 or p16ink4a-dependent senescence (74, 118). More recently, Jin et al. showed that senescent human umbilical cord blood-derived MSCs secrete SASP molecules, among which the most dominant was monocyte chemoattractant potein-1 (MCP-1/CCL2) that was able to induce senescence through binding to its cognate receptor CCR2. In this case, the MCP-1 release is epigenetically regulated by a decreased level of BMI1, a member of the polycomb repressor complex-1 (49). Hence, MCP-1 could be exploited as a marker of cell senescence both in vitro and in vivo. Since MCP-1 is secreted also by other mural cells such as vascular smooth muscle cells (VSMCs), an MSC-specific secretome signature needs yet to be identified. Of note, we reported an increase of MCP-1 expression in aortic VSMCs from old rats and humans, pointing at a central role of this chemokine in age- and senescence-associated cellular dysfunctions (100). Lastly, SASP likely contributes to the correlation between senescent cell accumulation and disease onset (125).
Other mechanisms
Whatever the trigger is, the effectors of MSCs senescence are molecules governing cell cycle progressions such as p53 and p16ink4a-pRb. The two pathways act in both a concerted way and independently and different are the stimuli-activating one pathway, the other, or both (9, 65) (Fig. 2). In vitro, senescence is accelerated by the oxygen tension used for culturing conditions: MSCs are generally cultured at 21% O2, a percentage that is 4 to 10 times higher than the tension they experience inside the tissue of origin. The excess of oxygen increases the production of reactive oxygen species (ROS), which, in turn, activate senescence-associated pathways (30).
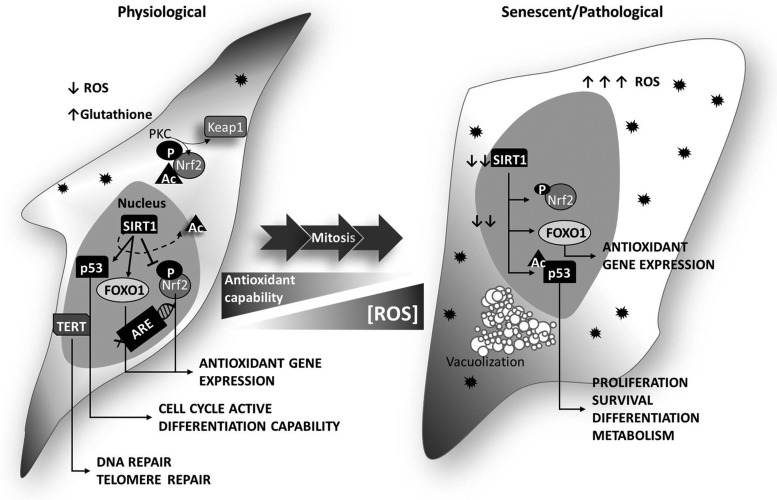
FIG. 2.
MSC senescence: correlation between ROS and senescence regulators. MSCs (left) show a physiologically low ROS content (black spiky dots in the figure), which is important to regulate cell proliferation and differentiation. After a series of cell divisions, ROS increase, due to an impairment of the radical defense system, and accumulate into the cell (right). ROS increase induces the overexpression of p53, which, once in the nucleus, is easily acetylated (Acp53). Although in functional MSCs nuclear p53 can be deacetylated by SIRT1, in senescent cells SIRT1 is downregulated, thus maintaining p53 acetylated and inactive. ARE, antioxidant responsive elements; SIRT1, sirtuin 1; TERT, telomerase reverse transcriptase.
The Oxidative Stress Theory
ROS are physiological byproducts of the oxidative metabolism produced during the passage of the reduction equivalents through the mitochondrial electron transport chain. Low physiological ROS concentration is beneficial to promote proliferation, DNA stability, and cell survival. This is particularly true for MSCs: at a steady state, MSCs rely on glycolysis and have a low content of ROS as reviewed in Ref. (92). In addition, they also have a high content of glutathione, and an active antioxidant machinery (111). However, as they replicate, intracellular and extracellular senescence-triggering stimuli induce a marked production of ROS. High ROS levels damage proteins and DNA, especially at a telomeric level inducing the so-called replicative senescence (48) that are otherwise known as stress-induced premature senescence (108). In this ROS-centric vision of MSC senescence reported as oxidative stress theory, ROS act as a molecular “grenade” affecting all cellular macromolecules at the multi-compartmental level, triggering senescence-associated pathways (15).
ROS-associated pathways
The increase in ROS content also correlates with a decrease in cell differentiation except for adipogenic differentiation that results in an increase. In addition, under high ROS content, the depotentiation of scavenger systems was observed. This phenomenon is due to a decline in the activity of the transcription factor Nrf2, which regulates ROS scavenger expression (70). Under physiological conditions, Nrf2 is sequestered in the cytoplasm bound to Keap1 protein. Increased ROS concentration induces Keap1 detachment and Nrf2 phosphorylation. Huang et al. demonstrated that protein kinase C (PKC) directly leads to Nrf2 activation by phosphorylation (44). Phosphorylated Nrf2 translocates to the nucleus where it promotes transcriptional activation of antioxidants scavenging enzymes (heme oxygenase-1 [HO-1], NAD(P)H:quinoneoxidoreductase 1 [NQO1], catalase, and superoxide dismutase [SOD]) binding to the antioxidant responsive elements (ARE) in their promoter regions (19). However, the Nrf2 expression is drastically reduced in senescent cells and aging in general (5), thus contributing to increased redox imbalance. Recently, our group found that a specific PKC isoform, PKCβII, plays a central role in diabetic complications. PKC is one of the members of a wide family of serine/threonine kinases. After activation by growth factors and ROS, PKC becomes active, thus regulating diverse pathways through a phosphorylation cascade. In our work, PKCβII amplifies oxidative stress in muscular pericytes (MPs) that are isolated from diabetic complicated skeletal muscle. Our results suggest that diabetic MPs share common traits with senescent cells since they showed: (1) a slowed replicative capacity, (2) a reduced myogenic differentiation potential counteracted by an increased propensity to adipogenesis, (3) a repression of antioxidant systems, and (4) increased ROS burden. Such a functional impairment is attributable to ROS-guided activation of the PKCβII-p66Shc signaling pathway (113). In this sense, diabetes not only recapitulates but also exacerbates cell senescence.
New Players in the Control of MSC Healthy Status: Noncoding RNAs
New post-transcriptional regulators associated with senescence include RNA binding proteins (RBPs) and noncoding RNAs of both miR and long noncoding RNA (lncRNA) classes. Indeed, it is becoming increasingly clear that in addition to coding genes, also noncoding RNAs regulate gene expression. MiRs are small, about 20 nucleotides in length, double-strand RNA found intracellularly and extracellularly, which interfere with RNA translation via different mechanisms, with the better known implying the binding to the 3’UTR sequence of the target gene RNA and the inhibition of translation via destabilization/degradation of the transcript. In the nucleus miR precursors, pri-miRs are digested by polymerase II and III to give rise to pre-miRs that are sequentially cut by the enzyme Drosha and transported to the cytoplasm where mature miRs are generated by the action of Dicer and enter the machinery of mRNA control. Aging and oxidative stress have been associated with dysregulation of the fine-tuned miR biogenesis process. Dicer, for example, is reportedly downregulated in old rat endothelial cells (ECs) compared with young donor cells (110). The consequent decrease in miRs content impacts cell capabilities to proliferating, adhering, migrating, and networking as shown in an experiment in which Dicer was reciprocally inhibited or overexpressed in young and old ECs, respectively. In mice, aging affects the composition of miRs in the adipose tissue, a central player in the control of lifespan and diseases. Of the total detected miRs by Mori et al., 51% decreased and only 10% of miRs increased when comparing 3-, 6-, and 24-month-old animals. The authors indicate a global decline of the miR-generating machinery, including Exportin-5 (the enzyme that transports pri-miRs from the nucleus to the cytoplasm) and Argonaute-2 (a miR-binding protein), but the most affected is Dicer. The same authors evidenced that Dicer Knockout cells show signs of senescence such as β-galactosidase positivity and upregulation of genes controlling mitochondrial stress pathways (75). Dicer down-modulation was confirmed in preadipocytes from old human donors and in the old nematodes model, pointing at an evolutionarily conserved mechanism. Dicer impairment was efficiently reverted by calorie restriction, a strategy that proved beneficial in extending lifespan cross-species (31). Of note, in isolated preadipocytes, oxidative stress induced by H2O2 treatment resulted in Dicer decrease, an effect prevented by insulin treatment.
Also, Exportin-5 and Drosha can be influenced by oxidative stress, although the associated mechanisms are still not completely clear (22, 103).
On the other hand, lncRNAs are RNAs of >200 nucleotides in length that do not code for proteins (72) comprehending antisense, intronic, and intergenic transcripts but also pseudogenes and retrotransposons. The question as to whether lncRNAs are just byproducts or are actively generated to control cell biology is still open. Growing evidence shows that they can influence gene expression in multiple complex manners, interacting with DNA, RNA, or RBPs. LncRNA effect is at the transcriptional and post-transcriptional level, and they can influence translation while acting as “sponges” for miRs and proteins (13, 63).
miRNAs: cellular effects
The role of noncoding RNAs in cellular senescence has been elegantly reviewed recently by Abdelmohsen and Gorospe (1); therefore, we will focus here only on some MSC-specific aspects (Fig. 3). Overall, miRs are known to control senescence-associated pathways such as the p16/Rb, the p53/p21, and secretory moieties related to the aging phenotype. A specific pattern of differentially expressed miRs has been associated with senescence in cells, including MSCs (35, 60). As mentioned earlier, one of the issues about noncoding RNA is still related to dissecting whether changes in their profile play an active role in the onset of pathologies or they just mirror the senescence of cell and tissue becoming dysfunctional. Some miRs have been validated for their crucial function in MSCs. miR-195 is one of them. It has been found to be elevated in association with MSCs aging and senescence together with miR-140, miR-146a/b, but miR-195 functional role analysis highlighted a direct control in telomere length as further discussed in this article (78). A functional role of the miR-141-3p increase in senescent MSCs was also demonstrated by linking it to prelamininA accumulation via miR-141-3p target ZMPSTE24 (122).
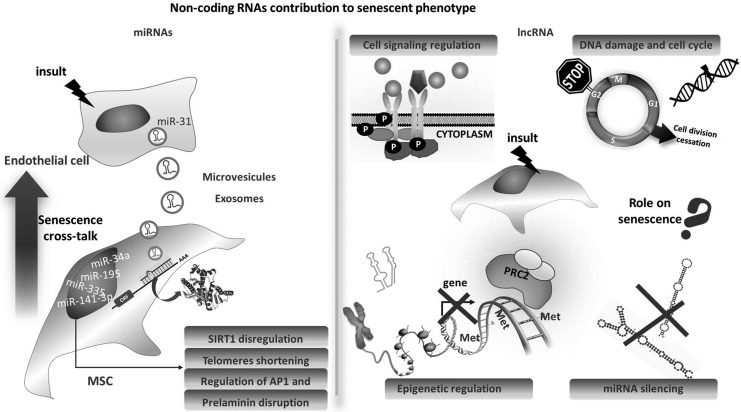
FIG. 3.
Noncoding RNAs' contribution to senescent phenotype. Several micro RNAs have been demonstrated to regulate senescence-related mechanisms (left). One of them, miR-31 has been reported to be shed from senescent endothelial cells as microparticle cargo. Once miR-31 is taken up by MSCs, it is sufficient to induce cellular senescence. Less clear is the contribution of lncRNAs to senescence for which many mechanisms of action are possible, as depicted here (right). lncRNA, long noncoding RNA; miR, microRNA.
miR-335 and miR-452 are two miRs that are upregulated in old MSCs from adipose tissue (81). MiR-335 has a central role in controlling MSCs proliferation and migration as further evidenced by the group of Tome et al. (106, 107). The authors link the cellular effect of miR-335 to AP-1 activity and to the regulation of members of the Fos family. Interestingly, the secretome of miR-335-MSCs was globally augmented, pointing at a transition toward the classical SASP condition.
The forced expression of miR-335 in MSCs in vitro recapitulates several aspects of senescence, including the impairment in the redox control system, such as the reduction in expression of SOD2 and an increase in superoxide generation. In this respect, it is crucial to note that miRs can themselves control the generation of ROS by regulating the Nrf2 pathway as reviewed in Ref. (21). On the other hand, in response to oxidative stress, a specific pattern of miRs is generated, with the prototype being miR-210 (14, 36). After an increase in the level of hypoxia-inducible factor-1 protein and of miR-210, the two factors regulate each other in a feedback loop. This virtuous cycle is needed for MSC survival in high ROS culture conditions (17, 54).
In a recent article, miR-210 modulation was linked to the beneficial effect in protecting MSCs from apoptosis of a redox controlling small molecule called zeaxanthin dipalmitate (68).
MiR-34a has been largely associated with aging and cardiovascular diseases in particularly affecting the function of cardiovascular cells (7). Confirmation that an miR-34a-dependent modulation of the SIRT-1/FOXO3a pathway is controlling MSCs vitality similarly to other cell types, including ECs, has been recently demonstrated (46, 119, 123).
miRNAs: paracrine functions
MiRs exert their action in the cell of origin but also in a paracrine fashion after secretion and transfer to a target cells/tissue. This concept has proved true also in the context of senescence where secreted miRs can vehicle their action from ECs to MSCs/pericytes via microparticle shuttling (117). The two cell types are in thigh connection and are reciprocally influenced as we also recently reviewed. ECs are strongly affected by age and senescence, and an SASP condition could apply to ECs too (11, 28) (Fig. 4). One such example of extracellular shuttled miR is miR-31, a senescence-increased miR known to regulate osteogenic differentiation (4) that has been shown to be taken up by MSCs after being released in EC-derived microparticles. The inhibition of miR-31 target gene Frizzled-3 is associated with osteogenic impairment. This effect was paired with an age-associated increase in circulating miR-31 in humans. A similar effect was described for miR-503, a miR that we found to be dysregulated in diabetes, a pathological condition associated with aging and senescence of ECs, which is transferred from ECs to pericytes inducing dysfunction (10, 12).
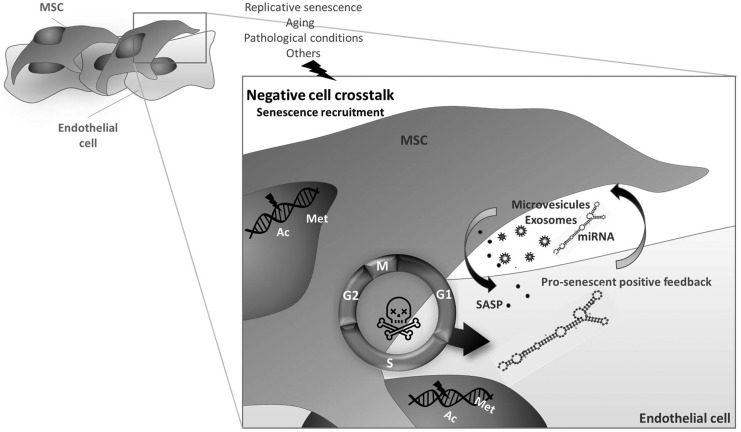
FIG. 4.
Negative influences. Endothelial cells are very sensitive to senescence and secrete molecules and microparticles that are paracrine hallmarks of senescence. Those include SASP, miRs, exosomes, and microvescicles that induce senescence of MSCs. miRs are also shed as microparticle cargo. Senescent MSCs, in turn, influence endothelial cells with similar mechanisms; however, the involvement of miRNA is not that clear.
LncRNAs
Several hundred lncRNAs are currently known, but their function is still not completely understood. A screening in fibroblast demonstrated that the short form of lncRNAs is upregulated in senescent cells compared with the long-sized ones. This result reinforces the hypothesis that mRNA stability is altered with aging (2, 29). Among the emerged lncRNAs associated with senescence, one that has been investigated in MSCs with functional consequences is HOTAIR, an lncRNA associated with a chromatin locus that is important for epigenetic changes and that is altered in cardiac disease of aging (37, 89). In the article by Kalwa et al., the expression level of HOTAIR is not changed with in vitro senescence, but its modulation alters the adipogenic differentiation ability of MSCs. The observed impact on the DNA methylation profile of HOTAIR was accompanied by triple helix formation (RNA-DNA-DNA) in the downregulated genes after HOTAIR overexpression (51).
We expect that several known and new lncRNAs will come into the list of senescence controlling factors in the next future since this class of molecules is the object of intense research.
Epigenetic Screening Integration with Clinical Data Has a Potential Predictive Value for Identification of Responders to Cell Therapy
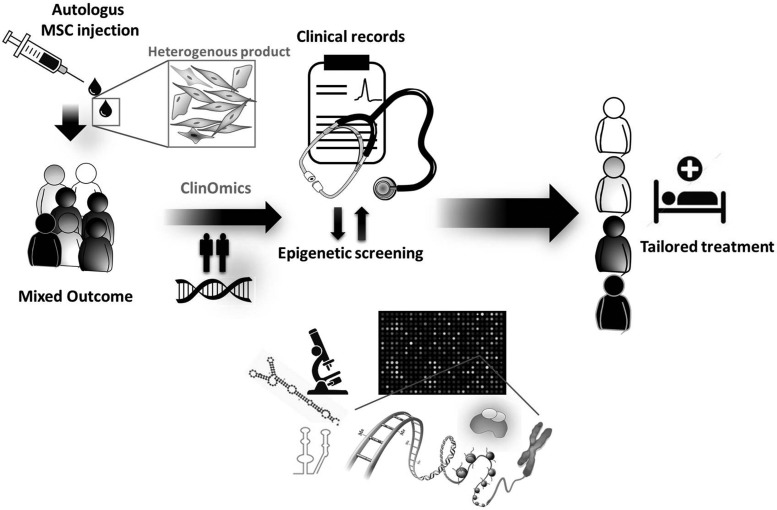
Since MSCs are poorly represented in human tissues, culture expansion is always necessary before the use of MSCs for cell therapy. This raises the question as to whether the occurrence of senescence can be monitored to avoid administration of senescent, non-mitotic cells, which would drive cell therapy failure. Patient-derived MSCs display heterogeneous reparative capacity, leading to unpredictable therapeutic outcomes and reducing a rigorous clinical application in the whole patient population. As mentioned earlier, attention is now focused on establishing quality control assessment at different levels of the standard operating procedure for harvesting, isolation, and expansion of cell populations, and on identifying predictors that are able to distinguish responders versus non-responders, thereby informing personalized therapies (Fig. 5). Hemodynamic predictors failed to pass the initial validation stage. For instance, the importance of basal contractility indexes such as left ventricular ejection fraction (LVEF) in influencing the outcome of cell therapy remains controversial. Two recent meta-analyses of bone marrow cell therapy trials in patients with acute MI indicate that patients experienced similar improvement in LVEF regardless of the baseline LVEF. However, improvements in LVESV were more pronounced in patients with lower baseline LVEF (3, 8). In contrast, in trials of chronic myocardial ischemia, the increase in LVEF elicited by cell therapy was significant only in the group with lower LVEF at baseline (8). Remarkable advancement in omics technology integrated by the interface with clinical data, for example, the clinomics, promises to bridge basic biological data and benefits on human health. Clinomics-based interrogation of stem cell heterogeneity may help deconvolute the heterogeneity of reparative performance, thus informing the development of new high-fidelity protocols (104).
FIG. 5.
Deconvolution of patient heterogeneity. Individual variability in drug efficacy and safety is a major challenge in current clinical practice. In the case of autologous cell therapy, the medicinal product is not homogeneous, and its therapeutic activity may vary among cell lines. Integration of clinical data with epigenetic screening can help allocate patients to the right treatment.
Biological markers of MSCs have been already validated as predictors of response to autologous stem cell transplantation in patients with neuronal degenerative diseases (55). There is still a paucity of data regarding the use of epigenetic screening to assess treatment response of cardiovascular MSC therapy. We found that a patient's age and smoking habit are negatively associated with therapeutic outcomes of pericyte cell therapy in a mouse model of limb ischemia, suggesting that intrinsic and environmental determinants of senescence could impact cell therapy performance (39).
Epigenetic screening
To identify new epigenetic predictors of therapeutic activity, we performed a whole genome DNA methylation array of the human pericyte populations (39). Methylation regulates gene expression by different mechanisms, acting at the promoter region and gene bodies. We identified 936 unique genes (106 of these involving promoter regions), whose methylation status is correlated with the blood flow recovery from ischemia. In addition, 5461 genes (930 in the promoter region) had a methylation status that correlates with capillary density in the ischemic muscle, and 784 unique genes (of these 89 in the promoter region) were associated with arteriole density. Integration of all the differentially methylated genes associated with the three outcomes identified 304 genes, of which 158 (52%) bear KROX-/EGR1-binding sites. The transcription factor KROX/EGR1 couples short-term changes in the extracellular environment to long-term changes in gene expression. It is induced by different growth factors and chemokines, including VEGF and stromal cell-derived factor (SDF-1), and stimulates microvascular neovascularization through FGF-mediated mechanisms. Moreover, the Arf-EGR-C/EBPβ axis is an important determinant of cellular responses (senescence or transformation) to oncogenic Ras signaling (91). An analysis of the genomic locations of the 304 genes shared by the three therapeutic outcomes identified a significant enrichment of the 6p21 loci for a gene network centered on CREB-binding protein. Such a nuclear protein binds to CREB, which restricts cellular senescence and apoptosis (94) and comprises Runt-related transcription factor 1, which belongs to a gene family that is implicated in stem cell plasticity (115).
The mechanisms that concentrate methylation to specific sequences and loci in the genome are unknown, although an interaction between DNA methyltransferases and other epigenetic factors has been proposed (87). Interestingly, a similar clustering has been observed in studies of gene polymorphisms. More than 90% of the genome lacks any disease-associate loci according to a meta-analysis of Genome-Wide Association studies of age-associated diseases (47). Surprisingly, a large spectrum of diseases maps to two specific loci 6p21 and INK4/ARF tumor suppressor locus. The former is where the major histocompatibility (MHC) locus resides. Genes at this locus determine a high susceptibility to a variety of auto-immune diseases and diabetes (47). It is not known whether mutations at the MHC locus can alter the immune privileged profile of MSCs, which usually express low levels of MHC class I (MHCI). In addition, 6p21 emerged as a new locus associated with coronary artery disease (CAD) at a genome-wide significance from a comprehensive analysis of the extent of pleiotropy of all CAD loci (116). Altogether, these reports indicate that polymorphic variants and epigenetic modifications at 6p21 loci may have a strong impact on age-associated diseases and regenerative processes.
In our study on human pericytes, the majority of differentially methylated CpG sites were associated with a known transcript. Hence, we next investigated the expressional profile by using gene arrays (GEO accession number: GSE57964) and reverse transcriptase-polymerase chain reaction (RT-PCR) analyses. This was followed by a gene set enrichment analysis to identify transcription factors whose targets are significantly enriched among genes correlating with cell therapy outcomes in the limb ischemia model (39). MAZ, a transcription factor that emerged from the DNA methylation analysis described earlier, was associated with a high number (139) of differentially expressed genes. MAZ is a zinc finger transcription factor that binds to GpC-rich cis-elements in the promoter regions of numerous mammalian genes and is also able to act as a recruiting scaffold for different proteins, such as methylases and acetylases, to the transcriptional complex, thereby acting as an initiator or terminator of transcription (98). The transcription factor plays a role in VEGF-induced angiogenesis under the control of microRNA-125b, of which MAZ is an inhibitory target (88, 97). Intriguingly, the expression of microRNA-125b in pericytes is inversely correlated with their ability to induce reparative vascularization in the mouse limb ischemia model. The consensus sequence of MAZ-binding sites is very similar to that of Sp1-binding sites. In fact, MAZ and Sp1, an anti-senescence transcription factor, bind to the same cis-elements in the promoters of the genes for endothelial nitric-oxide synthase (eNOS), and the receptor for parathyroid hormone. However, MAZ acts as a repressor and Sp1 as an enhancer of eNOS levels, suggesting that they have dual functions in the regulation of gene expression. This contrasting action of different zinc-finger proteins binding to the same cis-elements is attributable to their capacity to recruit different proteins, such as methylases and acetylases, to the transcriptional complex (98). However, screening MSCs' epigenetic modification alone could be not sufficient to yield a homogeneous population. Attention should be also devoted to the microenvironment in which MSCs are grown. ROS load is strictly related to the composition of the culture medium and ROS amount holds the balance of power between cell differentiation or stemness, proliferation, or cell cycle. As the main nutrients involved in ROS production are glucose and oxygen, their concentration and combination should be precisely defined and maintained constant, especially during GMP procedures (32, 56).
The Long Road from Trigger Signals to Senescence Effectors Passes Through the Control of ROS
In senescent MSCs, ROS increase is paralleled by a decrease of Sirtuin 1 (SIRT1) mediated by its post-transcriptional modulation (93). Studies reveal that sirtuins (SIRT1-7), a family of histone deacetylase, act as life-span-regulating proteins and are master regulators of telomeres maintenance [SIRT1 (18) and SIRT6 (120)] and DNA repair after oxidative stress [SIRT6 (80)]. SIRT1, a nuclear isoform of SIRT proteins, modulates the activity of different proteins involved in the cell cycle. p53 is one of the main targets (121). In particular, SIRT1-dependent p53 deacetylation induces p53 inactivity and thus favors cell cycle progression (112). However, high ROS levels mediate, on the one hand, SIRT1 downregulation, and on the other hand, the upregulation of p53 protein. In this scenario, p53 is also kept in its acetylated and inactive state due to the lack of SIRT1. In such a condition, the cells experience the exit from the cell cycle and, hence, senescence (79).
Another target of SIRT1 activity is FOXO1, a transcription factor involved in the expression of antioxidants enzymes such as SODs and catalase. FOXO1 activity is regulated by the post-transcriptional mechanism such as acetylation. During senescence, the low SIRT1 levels impede FOXO1 deacetylation, which is necessary for its translocation to the nucleus. As a consequence, the antioxidant machinery under the senescence condition is repressed (57). Studies report a direct interaction between SIRT1 and Nrf2, the activity of which ends up with the upregulation of multiple antioxidant enzymes as previously reported. Analysis of the transcriptional complexes binding to ARE sequence detected histone acetyltransferase p300/CBP along with Nrf2 possibly mediating its acetylation. Acetylation of Nrf2 is important to enhance Nrf2 binding to ARE sequences. SIRT1, on the contrary, decreases acetylation of Nrf2 as well as Nrf2-dependent gene transcription (53) as a compensatory mechanism, even though conflicting results exist. Obviously, senescent mesenchymal cells lose such a regulatory mechanisms due to the reduced expression of both proteins. So different effectors, such as FOXO1 and Nrf2 contribute to the increase and maintenance of ROS during senescence, and this process is mediated by SIRT1. As discussed in the next paragraph, resveratrol has been proved to activate SIRT1 overexpression in senescent cells (50). It seems that acting at the SIRT1 level could be a strategy to potentiate SIRT1 and counteract senescence.
Strategies to Counteract Senescence and Improve MSC Health and Therapeutic Potentials
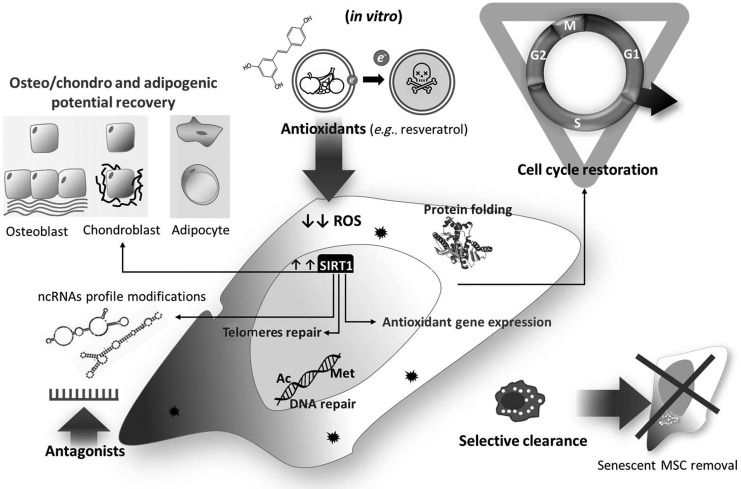
Taking into account all the mechanisms just mentioned, it appears evident that ROS are the main characters of a play (senescence) with a very intricate plot, regulated thanks to the interaction among sirtuins, p53, FOXO1, and Nrf2. Strategies to treat or reverse senescence of MSCs could help to counteract aging and cell senescence-associated diseases (Fig. 6). The efforts should be directed to both isolated cells for regenerative medicine and in vivo. A schematic summary of the mechanisms associated with senescence is presented in Table 1.
FIG. 6.
Strategies to reverse senescence. MSCs' senescence can be counteracted efficiently by using different approaches: potentiating antioxidant defense, inhibiting senescence-associated RNAs through specific antagonists, and increasing clearance of senescent cells by phagocytes. These intervention strategies induce the decrease of ROS and DNA damage, helping the cell to overcome the cell cycle blockade, improve MSC differentiation potential, and favor morphological recovery.
Table 1.
Documented Causes of Mesenchymal Stem Cell Senescence
| in vitro | Reference | in vivo | Reference |
|---|---|---|---|
| High O2 tension in culture | 29 | SASP | 87, 61 |
| DNA methylation variation | 55 | PROGERIA | 59, 63, 121 |
| Cytokines, growth factors | 71, 115 | Exosomes | 58, 102 |
| SASP | 47, 96–122 | ||
| Noncoding RNA | 20, 14–34 | ||
| Exosomes | 114 | ||
| ROS | 46, 105 |
ROS, reactive oxygen species; SASP, senescence-associated secretory phenotype.
Resveratrol
Recent studies indicate that resveratrol (3,5,4′-trihydroxy-trans-stilbene), a natural phenol produced by several plants in response to injury, does activate SIRT1 by allosteric interactions that increase SIRT1 affinity for both NAD+ and the acetylated substrate (43). In addition, resveratrol treatment improves the osteogenic and adipogenic differentiation potential of MSCs (24). In this sense, resveratrol mimics the effects of calorie restriction and, thus, could contribute to extending lifespan. Reversing SIRT1 activity could ameliorate different aspects linked to senescence, such as increased antioxidant enzyme expression through interaction with FOXO1 and reactivation of telomerase activity to counteracting telomeres attrition. Moreover, resveratrol treatment has been associated with changes in miRs' profile, supporting the central role of miRs tuning in controlling cellular fate (76, 77).
However, studies in animal models showed that resveratrol supplemented at a later life stage was ineffective (73). Moreover, long-term exposure to resveratrol increased MSCs' senescence. It is becoming clear that resveratrol effects can be beneficial or detrimental depending on the dosage and duration of the treatment. In addition, bioavailability after oral administration in mice and humans should be taken into account to evaluate the good or bad role of resveratrol treatments. Indeed, deeper analysis of blood resveratrol concentration, after oral administration both in mice and in humans, revealed that it is usually lower than the dosage proved to be effective in vitro, thus adding a bias in the interpretation of results (82).
Noncoding RNAs
Another approach to counteract senescent is modulation of miRs known to target molecules that directly or indirectly regulate senescence. Okada et al. recently identified in the bone marrow of old mice a population of mesenchymal cells with features of young mesenchymal cells (YMSCs). Microarray analysis identified a different pattern of miR in YMSCs with respect to their old counterpart (OMSCs). Relevant was the identification of miR-195 that was significantly overexpressed in OMSCs. Interestingly, a target gene of mir-195 is TERT. Thus, downregulating miR-195 rejuvenated OMSCs by counteracting telomeres attrition (78). MiR modulation can be employed to boost the therapeutic capabilities of MSCs as we showed studying human saphenous vein-isolated pericytes in a recent work. Transplantation of pericytes in mice infarcted heart resulted in improved cardiac function, blood flow recovery, and angiogenesis via paracrine signals activation dependent on miR-132 (52).
More controversial is the role of long noncoding RNA in mediating reversion of senescence. The lack of knowledge about their specific function and the multiple ways of interaction with nucleic acids and proteins render it difficult to target them.
Senescence clearance
Clearance of senescent stem cells (SCs) has been exploited in a progeroid mouse model using a transgenic approach, and it reportedly delays several age-associated disorders (6). Further, selective clearance of senescent SCs by a pharmacological agent (ABT263) resulted in the rejuvenation of the stem cell pool in bone marrow and skeletal muscles (16). In this context, new strategies to foster the clearance of damaged stem cells are emerging. Recently, Cheng et al. demonstrated that prolonged fasting followed by re-feeding exerts a pro-regenerative effect on bone marrow hematopoietic stem cells in mice and humans (20). Whether this strategy could be useful for mesenchymal stem cells remains unexplored.
Conclusions
The response of cells to time (organism age or passages in culture) by slowing proliferation/differentiation and acquiring a secretory phenotype represents the effort to counteract accumulating changes and damages. Senescence may, therefore, begin for protective reasons, but over time, it limits tissue and cells' regenerative capabilities. Since MSCs are increasingly recognized as the “guardians” of tissue homeostasis for their plastic response to injury, it is of great value to dissect the mechanisms that govern MSCs health. Moreover, MSCs can be exploited in regenerative medicine approaches after culture expansion. Reviewing the hallmarks of MSC changes associated with senescence highlights a central role of the redox control. The latter is associated with epigenetic changes that can provide a useful tool for selecting regenerative cells.
Abbreviations Used
- ARE
antioxidant responsive elements
- CAD
coronary artery disease
- EC
endothelial cell
- eNOS
endothelial nitric oxide synthase
- ESS
Epigenetic-Senescence-Signature
- GMP
good manufacturing practices
- lncRNA
long noncoding RNA
- LVEF
left ventricular ejection fraction
- MHC
major histocompatibility class
- MCP-1
monocyte chemoattractant potein-1
- miR
microRNA
- MPs
muscular pericytes
- PKC
protein kinase C
- RBP
RNA-binding protein
- ROS
reactive oxygen species
- SASP
senescence-associated secretory phenotype
- SC
stem cell
- SIRT1
sirtuin 1
- SOD
superoxide dismutase
- TERT
telomerase reverse transcriptase
- VSMCs
vascular smooth muscle cells
- YMSC
young mesenchymal cell
Acknowledgments
This work has been supported by the British Heart Foundation program grant (RG/13/17/30545) and the Italian Ministry of Health Ricerca Finalizzata (RF-2011-02346867).
References
- 1.Abdelmohsen K. and Gorospe M. Noncoding RNA control of cellular senescence. Wiley Interdiscip Rev RNA 6: 615–629, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelmohsen K, Panda A, Kang MJ, Xu J, Selimyan R, Yoon JH, Martindale JL, De S, Wood WH, 3rd, Becker KG, and Gorospe M. Senescence-associated lncRNAs: senescence-associated long noncoding RNAs. Aging Cell 12: 890–900, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afzal MR, Samanta A, Shah ZI, Jeevanantham V, Abdel-Latif A, Zuba-Surma EK, and Dawn B. Adult bone marrow cell therapy for ischemic heart disease: evidence and insights from randomized controlled trials. Circ Res 117: 558–575, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baglio SR, Devescovi V, Granchi D, and Baldini N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene 527: 321–331, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, and Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci 67: 313–329, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, and van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Treguer K, Carmona G, Bonauer A, Horrevoets AJ, Didier N, Girmatsion Z, Biliczki P, Ehrlich JR, Katus HA, Muller OJ, Potente M, Zeiher AM, Hermeking H, and Dimmeler S. MicroRNA-34a regulates cardiac ageing and function. Nature 495: 107–110, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Brunskill SJ, Hyde CJ, Doree CJ, Watt SM, and Martin-Rendon E. Route of delivery and baseline left ventricular ejection fraction, key factors of bone-marrow-derived cell therapy for ischaemic heart disease. Eur J Heart Fail 11: 887–896, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Campisi J. and d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8: 729–740, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Caporali A. and Emanueli C. MicroRNA-503 and the extended microRNA-16 family in angiogenesis. Trends Cardiovasc Med 21: 162–166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caporali A, Martello A, Miscianinov V, Maselli D, Vono R, and Spinetti G. Contribution of pericyte paracrine regulation of the endothelium to angiogenesis. Pharmacol Ther 171: 56–64, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Caporali A, Meloni M, Nailor A, Mitic T, Shantikumar S, Riu F, Sala-Newby GB, Rose L, Besnier M, Katare R, Voellenkle C, Verkade P, Martelli F, Madeddu P, and Emanueli C. p75(NTR)-dependent activation of NF-kappaB regulates microRNA-503 transcription and pericyte-endothelial crosstalk in diabetes after limb ischaemia. Nat Commun 6: 8024, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, and Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147: 358–369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan YC, Banerjee J, Choi SY, and Sen CK. miR-210: the master hypoxamir. Microcirculation 19: 215–223, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandrasekaran A, Idelchik MD, and Melendez JA. Redox control of senescence and age-related disease. Redox Biol 11: 91–102, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, and Zhou D. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22: 78–83, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang W, Lee CY, Park JH, Park MS, Maeng LS, Yoon CS, Lee MY, Hwang KC, and Chung YA. Survival of hypoxic human mesenchymal stem cells is enhanced by a positive feedback loop involving miR-210 and hypoxia-inducible factor 1. J Vet Sci 14: 69–76, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Liu X, Zhu W, Hu X, Jiang Z, Xu Y, Wang L, Zhou Y, Chen P, Zhang N, Hu D, Zhang L, Wang Y, Xu Q, Wu R, Yu H, and Wang J. SIRT1 ameliorates age-related senescence of mesenchymal stem cells via modulating telomere shelterin. Front Aging Neurosci 6: 103, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Zhang Z, and Cai L. Diabetic cardiomyopathy and its prevention by nrf2: current status. Diabetes Metab J 38: 337–345, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, Da Sacco S, Mirisola M, Quinn DI, Dorff TB, Kopchick JJ, and Longo VD. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 14: 810–823, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng X, Ku CH, and Siow RC. Regulation of the Nrf2 antioxidant pathway by microRNAs: new players in micromanaging redox homeostasis. Free Radic Biol Med 64: 4–11, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Crampton N, Kodiha M, Shrivastava S, Umar R, and Stochaj U. Oxidative stress inhibits nuclear protein export by multiple mechanisms that target FG nucleoporins and Crm1. Mol Biol Cell 20: 5106–5116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, and Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Dai Z, Li Y, Quarles LD, Song T, Pan W, Zhou H, and Xiao Z. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine 14: 806–814, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, and Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2: 499, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, and Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9: 255–267, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Dore-Duffy P, Katychev A, Wang X, and Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab 26: 613–624, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol (1985) 106: 326–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng L. and Niu DK. Relationship between mRNA stability and length: an old question with a new twist. Biochem Genet 45: 131–137, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Finkel T. and Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Fontana L, Partridge L, and Longo VD. Extending healthy life span—from yeast to humans. Science 328: 321–326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forristal CE, Christensen DR, Chinnery FE, Petruzzelli R, Parry KL, Sanchez-Elsner T, and Houghton FD. Environmental oxygen tension regulates the energy metabolism and self-renewal of human embryonic stem cells. PLoS One 8: e62507, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, DeBruijn J, Dominici M, Fibbe WE, Gee AP, Gimble JM, Hematti P, Koh MB, LeBlanc K, Martin I, McNiece IK, Mendicino M, Oh S, Ortiz L, Phinney DG, Planat V, Shi Y, Stroncek DF, Viswanathan S, Weiss DJ, and Sensebe L. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy 18: 151–159, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, and Frisen J. A pericyte origin of spinal cord scar tissue. Science 333: 238–242, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Gorospe M. and Abdelmohsen K. MicroRegulators come of age in senescence. Trends Genet 27: 233–241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greco S, Gaetano C, and Martelli F. HypoxamiR regulation and function in ischemic cardiovascular diseases. Antioxid Redox Signal 21: 1202–1219, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greco S, Zaccagnini G, Perfetti A, Fuschi P, Valaperta R, Voellenkle C, Castelvecchio S, Gaetano C, Finato N, Beltrami AP, Menicanti L, and Martelli F. Long noncoding RNA dysregulation in ischemic heart failure. J Transl Med 14: 183, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenhalgh SN, Iredale JP, and Henderson NC. Origins of fibrosis: pericytes take centre stage. F1000Prime Rep 5: 37, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gubernator M, Slater SC, Spencer HL, Spiteri I, Sottoriva A, Riu F, Rowlinson J, Avolio E, Katare R, Mangialardi G, Oikawa A, Reni C, Campagnolo P, Spinetti G, Touloumis A, Tavare S, Prandi F, Pesce M, Hofner M, Klemens V, Emanueli C, Angelini G, and Madeddu P. Epigenetic profile of human adventitial progenitor cells correlates with therapeutic outcomes in a mouse model of limb ischemia. Arterioscler Thromb Vasc Biol 35: 675–688, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, and Evans SM. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20: 345–359 e5, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayflick L. Biological aging is no longer an unsolved problem. Ann N Y Acad Sci 1100: 1–13, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Horwitz EM. MSC: a coming of age in regenerative medicine. Cytotherapy 8: 194–195, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, and Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Huang HC, Nguyen T, and Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc Natl Acad Sci U S A 97: 12475–12480, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang XP, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, and Li RK. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation 122: 2419–2429, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Ito T, Yagi S, and Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun 398: 735–740, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Jeck WR, Siebold AP, and Sharpless NE. Review: a meta-analysis of GWAS and age-associated diseases. Aging Cell 11: 727–731, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeong SG. and Cho GW. Endogenous ROS levels are increased in replicative senescence in human bone marrow mesenchymal stromal cells. Biochem Biophys Res Commun 460: 971–976, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Jin HJ, Lee HJ, Heo J, Lim J, Kim M, Kim MK, Nam HY, Hong GH, Cho YS, Choi SJ, Kim IG, Shin DM, and Kim SW. Senescence-associated MCP-1 secretion is dependent on a decline in BMI1 in human mesenchymal stromal cells. Antioxid Redox Signal 24: 471–485, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joe IS, Jeong SG, and Cho GW. Resveratrol-induced SIRT1 activation promotes neuronal differentiation of human bone marrow mesenchymal stem cells. Neurosci Lett 584: 97–102, 2015 [DOI] [PubMed] [Google Scholar]
- 51.Kalwa M, Hanzelmann S, Otto S, Kuo CC, Franzen J, Joussen S, Fernandez-Rebollo E, Rath B, Koch C, Hofmann A, Lee SH, Teschendorff AE, Denecke B, Lin Q, Widschwendter M, Weinhold E, Costa IG, and Wagner W. The lncRNA HOTAIR impacts on mesenchymal stem cells via triple helix formation. Nucleic Acids Res 44: 10631–10643, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami AP, Angelini G, Emanueli C, and Madeddu P. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res 109: 894–906, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawai Y, Garduno L, Theodore M, Yang J, and Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem 286: 7629–7640, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim HW, Haider HK, Jiang S, and Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem 284: 33161–33168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim HY, Kim H, Oh KW, Oh SI, Koh SH, Baik W, Noh MY, Kim KS, and Kim SH. Biological markers of mesenchymal stromal cells as predictors of response to autologous stem cell transplantation in patients with amyotrophic lateral sclerosis: an investigator-initiated trial and in vivo study. Stem Cells 32: 2724–2731, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Kim YH, Heo JS, and Han HJ. High glucose increase cell cycle regulatory proteins level of mouse embryonic stem cells via PI3-K/Akt and MAPKs signal pathways. J Cell Physiol 209: 94–102, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Klotz LO, Sanchez-Ramos C, Prieto-Arroyo I, Urbanek P, Steinbrenner H, and Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol 6: 51–72, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koch CM, Joussen S, Schellenberg A, Lin Q, Zenke M, and Wagner W. Monitoring of cellular senescence by DNA-methylation at specific CpG sites. Aging Cell 11: 366–369, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Krautler NJ, Kana V, Kranich J, Tian Y, Perera D, Lemm D, Schwarz P, Armulik A, Browning JL, Tallquist M, Buch T, Oliveira-Martins JB, Zhu C, Hermann M, Wagner U, Brink R, Heikenwalder M, and Aguzzi A. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell 150: 194–206, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kundrotas G, Gasperskaja E, Slapsyte G, Gudleviciene Z, Krasko J, Stumbryte A, and Liudkeviciene R. Identity, proliferation capacity, genomic stability and novel senescence markers of mesenchymal stem cells isolated from low volume of human bone marrow. Oncotarget 7: 10788–10802, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, and Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4: 214–222, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Lavasani M, Robinson AR, Lu A, Song M, Feduska JM, Ahani B, Tilstra JS, Feldman CH, Robbins PD, Niedernhofer LJ, and Huard J. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun 3: 608, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JT. Epigenetic regulation by long noncoding RNAs. Science 338: 1435–1439, 2015 [DOI] [PubMed] [Google Scholar]
- 64.Liang X, Ding Y, Zhang Y, Tse HF, and Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant 23: 1045–1059, 2014 [DOI] [PubMed] [Google Scholar]
- 65.Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, and Lowe SW. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev 12: 3008–3019, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu GH, Barkho BZ, Ruiz S, Diep D, Qu J, Yang SL, Panopoulos AD, Suzuki K, Kurian L, Walsh C, Thompson J, Boue S, Fung HL, Sancho-Martinez I, Zhang K, Yates J, 3rd, and Izpisua Belmonte JC. Recapitulation of premature ageing with iPSCs from Hutchinson-Gilford progeria syndrome. Nature 472: 221–225, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu L, Cheung TH, Charville GW, Hurgo BM, Leavitt T, Shih J, Brunet A, and Rando TA. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep 4: 189–204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Xiong Y, Xing F, Gao H, Wang X, He L, Ren C, Liu L, So KF, and Xiao J. Precise regulation of miR-210 is critical for the cellular homeostasis maintenance and transplantation efficacy enhancement of mesenchymal stem cell in acute liver failure therapy. Cell Transplant 26: 805–820, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, and Kroemer G. The hallmarks of aging. Cell 153: 1194–1217, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mann GE, Niehueser-Saran J, Watson A, Gao L, Ishii T, de Winter P, and Siow RC. Nrf2/ARE regulated antioxidant gene expression in endothelial and smooth muscle cells in oxidative stress: implications for atherosclerosis and preeclampsia. Sheng Li Xue Bao 59: 117–127, 2007 [PubMed] [Google Scholar]
- 71.Mansilla E, Marin GH, Drago H, Sturla F, Salas E, Gardiner C, Bossi S, Lamonega R, Guzman A, Nunez A, Gil MA, Piccinelli G, Ibar R, and Soratti C. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc 38: 967–969, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Mercer TR, Dinger ME, and Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet 10: 155–159, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, and Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 66: 191–201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moiseeva O, Mallette FA, Mukhopadhyay UK, Moores A, and Ferbeyre G. DNA damage signaling and p53-dependent senescence after prolonged beta-interferon stimulation. Mol Biol Cell 17: 1583–1592, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mori MA, Raghavan P, Thomou T, Boucher J, Robida-Stubbs S, Macotela Y, Russell SJ, Kirkland JL, Blackwell TK, and Kahn CR. Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab 16: 336–347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mukhopadhyay P, Das S, Ahsan MK, Otani H, and Das DK. Modulation of microRNA 20b with resveratrol and longevinex is linked with their potent anti-angiogenic action in the ischaemic myocardium and synergestic effects of resveratrol and gamma-tocotrienol. J Cell Mol Med 16: 2504–2517, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mukhopadhyay P, Mukherjee S, Ahsan K, Bagchi A, Pacher P, and Das DK. Restoration of altered microRNA expression in the ischemic heart with resveratrol. PLoS One 5: e15705, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okada M, Kim HW, Matsu-ura K, Wang YG, Xu M, and Ashraf M. Abrogation of age-induced microRNA-195 rejuvenates the senescent mesenchymal stem cells by reactivating telomerase. Stem Cells 34: 148–159, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ota H. The mechanism of vascular senescence regulated by longevity gene, Sirt1. Nihon Ronen Igakkai Zasshi 44: 194–197, 2007 [DOI] [PubMed] [Google Scholar]
- 80.Pan H, Guan D, Liu X, Li J, Wang L, Wu J, Zhou J, Zhang W, Ren R, Li Y, Yang J, Hao Y, Yuan T, Yuan G, Wang H, Ju Z, Mao Z, Qu J, Tang F, and Liu GH. SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res 26: 190–205, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pandey AC, Semon JA, Kaushal D, O'Sullivan RP, Glowacki J, Gimble JM, and Bunnell BA. MicroRNA profiling reveals age-dependent differential expression of nuclear factor kappaB and mitogen-activated protein kinase in adipose and bone marrow-derived human mesenchymal stem cells. Stem Cell Res Ther 2: 49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peltz L, Gomez J, Marquez M, Alencastro F, Atashpanjeh N, Quang T, Bach T, and Zhao Y. Resveratrol exerts dosage and duration dependent effect on human mesenchymal stem cell development. PLoS One 7: e37162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perez-Campo FM. and Riancho JA. Epigenetic mechanisms regulating mesenchymal stem cell differentiation. Curr Genomics 16: 368–383, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Phinney DG. and Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 35: 851–858, 2017 [DOI] [PubMed] [Google Scholar]
- 85.Phinney DG. and Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells 25: 2896–2902, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, and Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147, 1999 [DOI] [PubMed] [Google Scholar]
- 87.Portela A. and Esteller M. Epigenetic modifications and human disease. Nat Biotechnol 28: 1057–1068, 2010 [DOI] [PubMed] [Google Scholar]
- 88.Ray A, Dhar S, and Ray BK. Control of VEGF expression in triple-negative breast carcinoma cells by suppression of SAF-1 transcription factor activity. Mol Cancer Res 9: 1030–1041, 2011 [DOI] [PubMed] [Google Scholar]
- 89.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, and Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129: 1311–1323, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salem HK. and Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 28: 585–596, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salotti J, Sakchaisri K, Tourtellotte WG, and Johnson PF. An Arf-Egr-C/EBPbeta pathway linked to ras-induced senescence and cancer. Mol Cell Biol 35: 866–883, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sart S, Song L, and Li Y. Controlling redox status for stem cell survival, expansion, and differentiation. Oxid Med Cell Longev 2015: 105135, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sasaki T, Maier B, Bartke A, and Scrable H. Progressive loss of SIRT1 with cell cycle withdrawal. Aging Cell 5: 413–422, 2006 [DOI] [PubMed] [Google Scholar]
- 94.Schauer IE, Knaub LA, Lloyd M, Watson PA, Gliwa C, Lewis KE, Chait A, Klemm DJ, Gunter JM, Bouchard R, McDonald TO, O'Brien KD, and Reusch JE. CREB downregulation in vascular disease: a common response to cardiovascular risk. Arterioscler Thromb Vasc Biol 30: 733–741, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, and Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg 73: 1919–1925; discussion 1926, 2002 [DOI] [PubMed] [Google Scholar]
- 96.Shin DM, Kucia M, and Ratajczak MZ. Nuclear and chromatin reorganization during cell senescence and aging—a mini-review. Gerontology 57: 76–84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smits M, Wurdinger T, van het Hof B, Drexhage JA, Geerts D, Wesseling P, Noske DP, Vandertop WP, de Vries HE, and Reijerkerk A. Myc-associated zinc finger protein (MAZ) is regulated by miR-125b and mediates VEGF-induced angiogenesis in glioblastoma. FASEB J 26: 2639–2647, 2012 [DOI] [PubMed] [Google Scholar]
- 98.Song J, Ugai H, Nakata-Tsutsui H, Kishikawa S, Suzuki E, Murata T, and Yokoyama KK. Transcriptional regulation by zinc-finger proteins Sp1 and MAZ involves interactions with the same cis-elements. Int J Mol Med 11: 547–553, 2003 [PubMed] [Google Scholar]
- 99.Sperka T, Wang J, and Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol 13: 579–590, 2012 [DOI] [PubMed] [Google Scholar]
- 100.Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, and Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol 24: 1397–1402, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Stenderup K, Justesen J, Clausen C, and Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33: 919–926, 2003 [DOI] [PubMed] [Google Scholar]
- 102.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, and Graff JM. White fat progenitor cells reside in the adipose vasculature. Science 322: 583–586, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tang X, Li M, Tucker L, and Ramratnam B. Glycogen synthase kinase 3 beta (GSK3beta) phosphorylates the RNAase III enzyme Drosha at S300 and S302. PLoS One 6: e20391, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Terzic A. and Behfar A. Stem cell therapy for heart failure: ensuring regenerative proficiency. Trends Cardiovasc Med 26: 395–404, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, and de Kleijn DP. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 1: 129–137, 2007 [DOI] [PubMed] [Google Scholar]
- 106.Tome M, Lopez-Romero P, Albo C, Sepulveda JC, Fernandez-Gutierrez B, Dopazo A, Bernad A, and Gonzalez MA. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ 18: 985–995, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tome M, Sepulveda JC, Delgado M, Andrades JA, Campisi J, Gonzalez MA, and Bernad A. miR-335 correlates with senescence/aging in human mesenchymal stem cells and inhibits their therapeutic actions through inhibition of AP-1 activity. Stem Cells 32: 2229–2244, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Toussaint O, Royer V, Salmon M, and Remacle J. Stress-induced premature senescence and tissue ageing. Biochem Pharmacol 64: 1007–1009, 2002 [DOI] [PubMed] [Google Scholar]
- 109.Uccelli A, Moretta L, and Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 8: 726–736, 2008 [DOI] [PubMed] [Google Scholar]
- 110.Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, Csiszar A, Losonczy G, Valcarcel-Ares MN, and Sonntag WE. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci 68: 877–891, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Valle-Prieto A. and Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev 19: 1885–1893, 2010 [DOI] [PubMed] [Google Scholar]
- 112.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, and Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107: 149–159, 2001 [DOI] [PubMed] [Google Scholar]
- 113.Vono R, Fuoco C, Testa S, Pirro S, Maselli D, Ferland McCollough D, Sangalli E, Pintus G, Giordo R, Finzi G, Sessa F, Cardani R, Gotti A, Losa S, Cesareni G, Rizzi R, Bearzi C, Cannata S, Spinetti G, Gargioli C, and Madeddu P. Activation of the pro-Oxidant PKCbetaII-p66Shc signaling pathway contributes to pericyte dysfunction in skeletal muscles of patients with diabetes with critical limb ischemia. Diabetes 65: 3691–3704, 2016 [DOI] [PubMed] [Google Scholar]
- 114.Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, and Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One 3: e2213, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang CQ, Mok MM, Yokomizo T, Tergaonkar V, and Osato M. Runx family genes in tissue stem cell dynamics. Adv Exp Med Biol 962: 117–138, 2017 [DOI] [PubMed] [Google Scholar]
- 116.Webb TR, Erdmann J, Stirrups KE, Stitziel NO, Masca NG, Jansen H, Kanoni S, Nelson CP, Ferrario PG, Konig IR, Eicher JD, Johnson AD, Hamby SE, Betsholtz C, Ruusalepp A, Franzen O, Schadt EE, Bjorkegren JL, Weeke PE, Auer PL, Schick UM, Lu Y, Zhang H, Dube MP, Goel A, Farrall M, Peloso GM, Won HH, Do R, van Iperen E, Kruppa J, Mahajan A, Scott RA, Willenborg C, Braund PS, van Capelleveen JC, Doney AS, Donnelly LA, Asselta R, Merlini PA, Duga S, Marziliano N, Denny JC, Shaffer C, El-Mokhtari NE, Franke A, Heilmann S, Hengstenberg C, Hoffmann P, Holmen OL, Hveem K, Jansson JH, Jockel KH, Kessler T, Kriebel J, Laugwitz KL, Marouli E, Martinelli N, McCarthy MI, Van Zuydam NR, Meisinger C, Esko T, Mihailov E, Escher SA, Alver M, Moebus S, Morris AD, Virtamo J, Nikpay M, Olivieri O, Provost S, AlQarawi A, Robertson NR, Akinsansya KO, Reilly DF, Vogt TF, Yin W, Asselbergs FW, Kooperberg C, Jackson RD, Stahl E, Muller-Nurasyid M, Strauch K, Varga TV, Waldenberger M, Zeng L, Chowdhury R, Salomaa V, Ford I, Jukema JW, Amouyel P, Kontto J, Nordestgaard BG, Ferrieres J, Saleheen D, Sattar N, Surendran P, Wagner A, Young R, Howson JM, Butterworth AS, Danesh J, Ardissino D, Bottinger EP, Erbel R, Franks PW, Girelli D, Hall AS, Hovingh GK, Kastrati A, Lieb W, Meitinger T, Kraus WE, Shah SH, McPherson R, Orho-Melander M, Melander O, Metspalu A, Palmer CN, Peters A, Rader DJ, Reilly MP, Loos RJ, Reiner AP, Roden DM, Tardif JC, Thompson JR, Wareham NJ, Watkins H, Willer CJ, Samani NJ, Schunkert H, Deloukas P, and Kathiresan S. Systematic evaluation of pleiotropy identifies 6 further loci associated with coronary artery disease. J Am Coll Cardiol 69: 823–836, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weilner S, Schraml E, Wieser M, Messner P, Schneider K, Wassermann K, Micutkova L, Fortschegger K, Maier AB, Westendorp R, Resch H, Wolbank S, Redl H, Jansen-Durr P, Pietschmann P, Grillari-Voglauer R, and Grillari J. Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell 15: 744–754, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu J, Niu J, Li X, Wang X, Guo Z, and Zhang F. TGF-beta1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev Biol 14: 21, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamakuchi M, Ferlito M, and Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A 105: 13421–13426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang B, Zwaans BM, Eckersdorff M, and Lombard DB. The sirtuin SIRT6 deacetylates H3 K56Ac in vivo to promote genomic stability. Cell Cycle 8: 2662–2663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yi J. and Luo J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim Biophys Acta 1804: 1684–1689, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu KR, Lee S, Jung JW, Hong IS, Kim HS, Seo Y, Shin TH, and Kang KS. MicroRNA-141-3p plays a role in human mesenchymal stem cell aging by directly targeting ZMPSTE24. J Cell Sci 126: 5422–5431, 2013 [DOI] [PubMed] [Google Scholar]
- 123.Zhang F, Cui J, Liu X, Lv B, Xie Z, and Yu B. Roles of microRNA-34a targeting SIRT1 in mesenchymal stem cells. Stem Cell Res Ther 6: 195, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang W, Li J, Suzuki K, Qu J, Wang P, Zhou J, Liu X, Ren R, Xu X, Ocampo A, Yuan T, Yang J, Li Y, Shi L, Guan D, Pan H, Duan S, Ding Z, Li M, Yi F, Bai R, Wang Y, Chen C, Yang F, Li X, Wang Z, Aizawa E, Goebl A, Soligalla RD, Reddy P, Esteban CR, Tang F, Liu GH, and Belmonte JC. Aging stem cells. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 348: 1160–1163, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu Y, Armstrong JL, Tchkonia T, and Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care 17: 324–328, 2014 [DOI] [PubMed] [Google Scholar]