Main Text
Safety notwithstanding, the immune system, as a sworn enemy of all things viral, has been seen as the enemy of oncolytic viruses (OVs). However, in this issue of Molecular Therapy, Ricca et al.1 challenge this doctrine. Not only do they show that oncolytic virotherapy with Newcastle Disease Virus (NDV) requires the immune system for efficacy, but they also show that actively ramping up anti-viral immunity before treatment enhances therapy.
The earliest gospels of oncolytic virotherapy envisioned an immaculately created, perfectly tumor-adapted virus that was engineered to replicate freely through the transformed cell population, which falls on its own sword and dies out rapidly in surrounding normal cells.2, 3, 4 When a virus infects a group of cells (tumor or not), the immune system swings into action to shut down virus replication and spread. From an oncolytic-centric viewpoint, these responses—when directed at an OV in a tumor—represent “the dark side,” reeking retribution upon creation.
However, seditious reports emerged from testing OVs in fully immune-competent animal models. In some cases, anti-tumor therapy depended upon (rather than was destroyed by) host immune effector cells.5, 6, 7, 8 More subversive still, anti-tumor therapy did not always necessarily correlate with levels of viral replication.5, 9, 10 These findings opened a schism between the oncolytic-centric “replication and lysis is everything” church and a new, breakaway immune-centric sect.11, 12 Immune-centrics preached that OVs may work more as indirect facilitators of immune-based killing (immunological adjuvants) than as direct killers (oncolysis). This was blasphemy. In its most extreme form, it suggested that OV therapy might be no different from injecting an immune-provoking, Toll-like receptor agonist, such as lipopolysaccharide (LPS) or CpG, to trigger an innate and adaptive immune response.13 Was oncolytic virotherapy simply a re-incarnation of Coley’s toxin? Such a creed was highly unpalatable because it argued against the need for “creationism”—generation of beautifully sculpted viruses engineered for tumor-specific infection, replication, and killing.
The schism between the oncolytic-centrics and immune-centrics has been healing. The more viral replication there is in a tumor, the greater the levels of immune stimulation to light the immunotherapeutic fire.12 Similarly, OVs represent a “living therapy”—just a few viral particles should amplify the therapeutic signal through replication. A virus-hostile immune system threatens this therapeutic amplification. However, a self-amplifying, anti-tumor innate, adaptive, and memory immune response, triggered by an OV, is also a living therapy. This keeps both sides happy and opens the way for the rational combination of OV with immune checkpoint inhibitors, which are all the rage at the moment.5, 14
OVs will be superior to small molecules and drugs like Coley’s toxin because they can, in principle, circulate and target and infect metastatic disease and then amplify.2, 3 However, pre-existing anti-viral immunity (B/T cell) remains the “evil guardian” that has to be defeated to reach this Holy Grail.15, 16 Thus, pre-existing immunity to the OV raises neutralizing antibody (NAb), which renders systemically delivered virus dead on arrival and prevents spread through the tumor. It also generates anti-viral memory T cells, which rapidly re-activate upon virus injection to quell viral infection. Doctrinally, no good could possibly come from pre-existing anti-viral immunity. Or could it?
In this issue, Ricca et al.1 do their level best to reopen the schism between the oncolytic- and immune-centrics. In the first instance, they re-capitulate their previous findings that intra-tumoral injection of Newcastle Disease Virus (NDV) leads to effective anti-tumor treatment, which is both dependent upon host immune effectors and occurs despite low levels of virus replication in tumors, showing their immune-centric colors that oncolytic virotherapy with NDV is actually oncolytic immunovirotherapy.5 They go on to show that, in mice with pre-existing anti-NDV immunity, viral replication in tumors is further reduced compared to levels in mice that are NDV-naive. This is as expected—NAbs, and anti-viral T cells, are, after all, virus killers. However, the real shocker is that mice pre-vaccinated with NDV have significantly better anti-tumor therapy following intra-tumoral injection than mice with no pre-existing anti-viral immunity. This is not just extreme immune-centrism; this is outright heresy.
Using immune depletions, they show that several immune subsets combine to lead a concerted attack against the virus in injected tumors. However, the presence of pre-primed, rapid anti-viral responders (principally CD8+ T cells, with help from natural killer [NK] cells) actually enhanced NDV oncolytic immunovirotherapy.
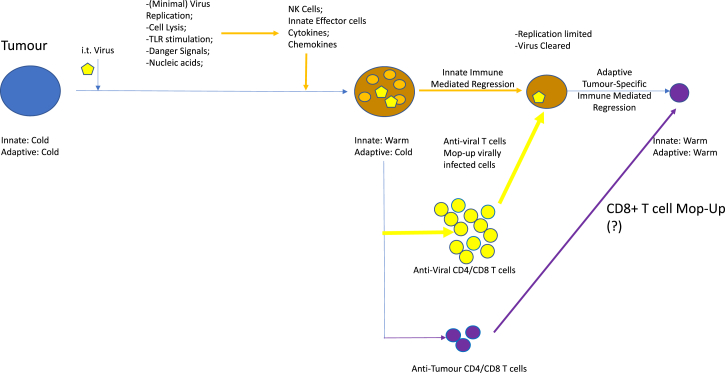
So how could this be working? An attractive working model is that, in virus-naive mice, therapy of injected tumors is mediated through innate anti-viral immune effectors (cytotoxic cells, cytokines) activated by virus in the tumors (Figure 1). Furthermore, tumor lysis primes anti-NDV CD4 and CD8 T cells, which track back to the site of viral protein expression (the tumor) to kill residual virus-expressing cells (Figure 1). A second T cell response involves the breaking of tolerance to self-tumor-associated antigens (TAAs) or the generation of de novo anti-tumor T cells targeted against neo-epitopes, against which the mice would not be tolerized (Figure 1). Both processes are facilitated by highly inflammatory signals provided by the virus acting as a potent immune adjuvant at the tumor and in the lymph nodes, with the anti-viral T cell response greatly outpacing the anti-tumor T cell response qualitatively and quantitatively (Figure 1).
Figure 1.
NDV Oncolytic Viroimmunotherapy in a Virus-Naive Host
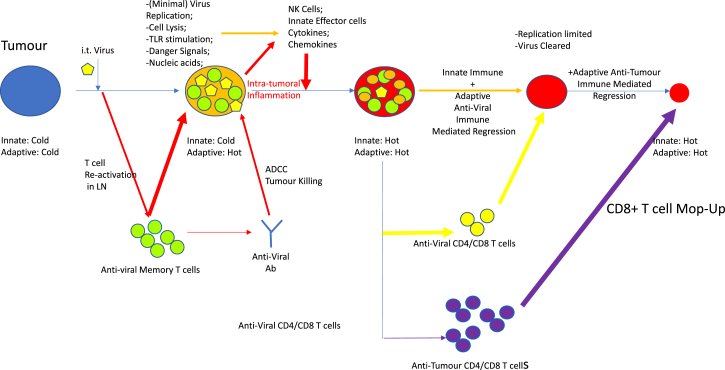
In the case of the pre-immunized mice, these anti-tumor immunotherapies are significantly enhanced because these same mechanisms would be augmented by a rapid influx of pre-primed anti-viral CD8, and maybe CD4, cells. These effectors recognize viral immunogens presented by tumor and stromal cells, adding to the general mayhem of intra-tumoral inflammation, resulting in better local therapy (Figure 2). In this respect, the authors point out clinical administration of the US Food and Drug Administration (FDA)-approved oncolytic T-Vec virus proceeded via initial virus priming for safety to prevent virus spread in virus-naive patients.17 Perhaps, given the new heresy, this regimen may have contributed to the therapy. Thus, once the virus was administered intra-tumorally it would self-boost the pre-existing anti-viral T cell response induced by vaccination and focus that T cell response upon the tumor as a site of virus infection.
Figure 2.
NDV Oncolytic Viroimmunotherapy Enhanced in a Virus Pre-immune Host
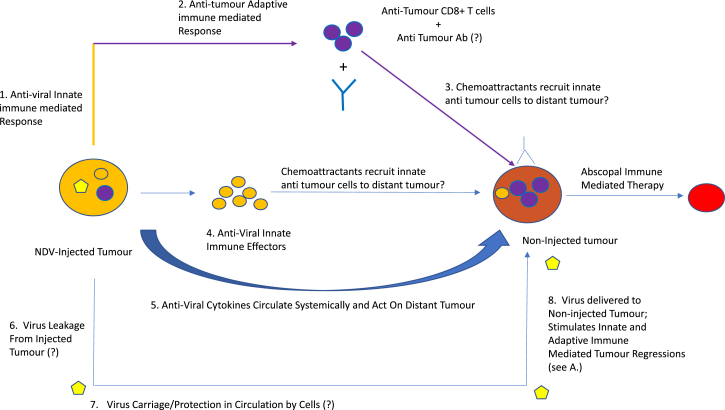
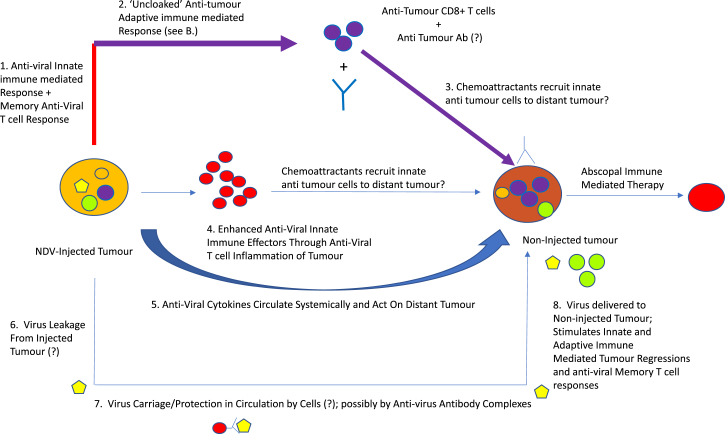
The authors addressed the relative roles of the anti-viral and anti-tumor T cell responses using mice bearing bilateral tumors. Intra-tumoral injection of one tumor led to regressions of both (Figure 3). Significantly, abscopal therapy was also improved in pre-immunized mice over virus-naive mice, dependent upon CD8+ effectors (Figure 4). If clearance of the un-injected tumors depended upon anti-viral responses (cells and NAbs), virus injected into one tumor must have trafficked via the circulation to the distant un-injected tumor to attract anti-viral effectors, leading to abscopal regressions (Figure 3). However, the investigators were unable to detect viral leakage between tumors, suggesting priming of anti-tumor T cell responses (Figure 3). Indeed, it may be that tolerance against self-TAA can be more readily broken in mice in which immuno-dominant anti-viral T cell responses have already been primed, thereby reducing competition for the de novo priming of immune-subdominant anti-TAA T cells (Figure 2).18 Consistent with such a model, the authors showed that virus-immune mice contained more tumor-reactive T cells than their non-immune counterparts.
Figure 3.
Local Intra-tumoral NDV Oncolytic Viroimmunotherapy in a Virus-Naive Host Is Effective against Distant Tumors
Figure 4.
Local Intra-tumoral NDV Oncolytic Viroimmunotherapy in a Virus Immunized Host Is Enhanced against Distant Tumors
These results should be interpreted cautiously. It is attractive for the immune-centrics that oncolysis and immune-mediated tumor killing primes T cell responses against TAA. However, the immune system is complex. For example, concomitant tumor immunity and resistance describes how seeding one tumor can lead to immune inflammation and perturbations, which make rejection of a second tumor more probable, without invoking breaking of tolerance to TAA.19 In addition, intense inflammation at one tumor (by intratumoral [ i.t.] virus) can activate innate immune cells and cytokine storms, which can circulate and attack a second tumor without involvement of a TAA-specific T cell response (Figures 3 and 4). Showing that and how oncolysis raises effective, adaptive TAA-specific T cell responses over and above concomitant anti-viral responses will be important to understand how OVs can be best combined with immune stimulators, such as immune checkpoint blockade.5, 14
Enhancement of therapy in pre-immunized mice may also derive from anti-viral antibody dependent cellular cytotoxicity (ADCC) through antibody binding to virus antigens expressed in the injected tumors (Figure 2). However, the lack of detectable virus in the distant tumors argues that the abscopal effects are not ADCC-mediated in the same way. In this respect, we have shown that antibody-(reo)virus complexes can hitchhike onto immune cells, which, when activated by granulocyte-macrophage colony-stimulating factor (GM-CSF), hand the virus off into tumors to trigger therapy in pre-immune mice.20, 21 As heresy goes, this is right up there, suggesting that not all anti-viral antibodies are necessarily catastrophic for OVs. Similarly, there are other reports in the scriptures in which pre-existing viral immunity has enhanced virus delivery and infection, supporting apparently heretical roles for the immune system in potentiating oncolytic viroimmunotherapy.22, 23, 24, 25, 26, 27
How one OV fights, or exploits, the immune system may be different from another. Nonetheless, the original gospel of oncolytic virotherapy has evolved to embrace, rather than villify, the immune system.28 The oncolytic- and immune-centrics mutually acknowledge that robust, tumor-targeted viral replication serves as an excellent immune adjuvant to prime innate, and adaptive, tumor killing. The new, improved doctrine of viroimmunotherapy sits well with most of us, whether we secretly remain closet oncolytic-centrics or immune-centrics. The results of Ricca et al.,1 and others, show that there are still mysteries to be uncovered in the field of viroimmunotherapy and that today’s heresy can sometimes be tomorrow’s new gospel.
Acknowledgments
The author thanks Toni L. Woltman for expert secretarial assistance.
References
- 1.Ricca J.M., Oseledchyk A., Walther T., Liu C., Mangarin L., Merghoub T., Wolchok J.D., Zamarin D. Pre-existing immnunity to oncolytic virus potentiates its immunotherapeutic efficacy. Mol. Ther. 2018;26:1008–1019. doi: 10.1016/j.ymthe.2018.01.019. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell S.J. Replicating vectors for cancer therapy: a question of strategy. Semin. Cancer Biol. 1994;5:437–443. [PubMed] [Google Scholar]
- 3.Kirn D., Martuza R.L., Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat. Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- 4.Cattaneo R., Miest T., Shashkova E.V., Barry M.A. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat. Rev. Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamarin D., Holmgaard R.B., Subudhi S.K., Park J.S., Mansour M., Palese P., Merghoub T., Wolchok J.D., Allison J.P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014;6:226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wongthida P., Diaz R.M., Galivo F., Kottke T., Thompson J., Pulido J., Pavelko K., Pease L., Melcher A., Vile R. Type III IFN interleukin-28 mediates the antitumor efficacy of oncolytic virus VSV in immune-competent mouse models of cancer. Cancer Res. 2010;70:4539–4549. doi: 10.1158/0008-5472.CAN-09-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wongthida P., Diaz R.M., Galivo F., Kottke T., Thompson J., Melcher A., Vile R. VSV oncolytic virotherapy in the B16 model depends upon intact MyD88 signaling. Mol. Ther. 2011;19:150–158. doi: 10.1038/mt.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz R.M., Galivo F., Kottke T., Wongthida P., Qiao J., Thompson J., Valdes M., Barber G., Vile R.G. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 9.Prestwich R.J., Ilett E.J., Errington F., Diaz R.M., Steele L.P., Kottke T., Thompson J., Galivo F., Harrington K.J., Pandha H.S. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin. Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galivo F., Diaz R.M., Wongthida P., Thompson J., Kottke T., Barber G., Melcher A., Vile R. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2010;17:158–170. doi: 10.1038/gt.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prestwich R.J., Errington F., Diaz R.M., Pandha H.S., Harrington K.J., Melcher A.A., Vile R.G. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum. Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melcher A., Parato K., Rooney C.M., Bell J.C. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol. Ther. 2011;19:1008–1016. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rommelfanger D.M., Compte M., Diaz R.M., Ilett E., Alvarez-Vallina L., Thompson J.M., Kottke T.J., Melcher A., Vile R.G. The efficacy versus toxicity profile of combination virotherapy and TLR immunotherapy highlights the danger of administering TLR agonists to oncolytic virus-treated mice. Mol Ther. 2013;21:348–357. doi: 10.1038/mt.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O., Olszanski A.J., Malvehy J., Cebon J., Fernandez E. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 2017;170:1109–1119 e1110. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson M.S., Lemoine N.R., Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv. Virol. 2012;2012:805629. doi: 10.1155/2012/805629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andtbacka R.H., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 18.Bridle B.W., Boudreau J.E., Lichty B.D., Brunellière J., Stephenson K., Koshy S., Bramson J.L., Wan Y. Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus. Mol. Ther. 2009;17:1814–1821. doi: 10.1038/mt.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prehn R.T. Two competing influences that may explain concomitant tumor resistance. Cancer Res. 1993;53:3266–3269. [PubMed] [Google Scholar]
- 20.Ilett E., Kottke T., Donnelly O., Thompson J., Willmon C., Diaz R., Zaidi S., Coffey M., Selby P., Harrington K. Cytokine conditioning enhances systemic delivery and therapy of an oncolytic virus. Mol. Ther. 2014;22:1851–1863. doi: 10.1038/mt.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adair R.A., Roulstone V., Scott K.J., Morgan R., Nuovo G.J., Fuller M., Beirne D., West E.J., Jennings V.A., Rose A. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci. Transl. Med. 2012;4:138ra77. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shashkova E.V., Doronin K., Senac J.S., Barry M.A. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 2008;68:5896–5904. doi: 10.1158/0008-5472.CAN-08-0488. [DOI] [PubMed] [Google Scholar]
- 23.Rodenhuis-Zybert I.A., van der Schaar H.M., da Silva Voorham J.M., van der Ende-Metselaar H., Lei H.Y., Wilschut J., Smit J.M. Immature dengue virus: a veiled pathogen? PLoS Pathog. 2010;6:e1000718. doi: 10.1371/journal.ppat.1000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leopold P.L., Wendland R.L., Vincent T., Crystal R.G. Neutralized adenovirus-immune complexes can mediate effective gene transfer via an Fc receptor-dependent infection pathway. J. Virol. 2006;80:10237–10247. doi: 10.1128/JVI.00512-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iankov I.D., Penheiter A.R., Griesmann G.E., Carlson S.K., Federspiel M.J., Galanis E. Neutralization capacity of measles virus H protein specific IgG determines the balance between antibody-enhanced infectivity and protection in microglial cells. Virus Res. 2013;172:15–23. doi: 10.1016/j.virusres.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geijtenbeek T.B., Kwon D.S., Torensma R., van Vliet S.J., van Duijnhoven G.C., Middel J., Cornelissen I.L., Nottet H.S., KewalRamani V.N., Littman D.R. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 27.Balathasan L., Tang V.A., Yadollahi B., Brun J., Labelle M., Lefebvre C., Swift S.L., Stojdl D.F. Activating Peripheral Innate Immunity Enables Safe and Effective Oncolytic Virotherapy in the Brain. Mol Ther Oncolytics. 2017;7:45–56. doi: 10.1016/j.omto.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichty B.D., Breitbach C.J., Stojdl D.F., Bell J.C. Going viral with cancer immunotherapy. Nat. Rev. Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]