Abstract
Background:
Reviewing the neurosurgical literature demonstrated that spinal neurosurgeons rarely (0.78%) diagnose chiari-1 malformation (CM-1) in adults on magnetic resonance (MR) studies defined by tonsillar descent >5 mm below the foramen magnum (FM). Children, averaging 10 years of age, exhibit CM-1 in 96/100,000 cases. According to the literature, fewer spinal neurosurgeons additionally recognize and treat the low lying cerebellar tonsil (LLCT) syndrome.
Methods:
The normal location of the cerebellar tonsils on cranial/cervical MR averages 2.9 mm ± 3.4 mm above or up to 3 mm below the FM. The neurosurgical literature revealed that most neurosurgeons diagnose and treat CM-1 where the tonsils are >5 mm to an average of 12 mm below the FM. Fewer spinal neurosurgeons additionally diagnose and treat the LLCT syndrome defined by <5 mm of tonsillar descent below the FM.
Results:
According to the neurosurgical literature, many neurosurgeons perform cranial/spinal decompression with/without fusion and/or duraplasty for CM-1. Fewer neurosurgeons perform these procedures for CM-1 and the LLCT syndrome, for which they additionally perform preoperative cervical traction under anesthesia, and the postoperative placement of occipital neurostimulators (ONS) for intractable headaches following chiari-1/LLCT surgery.
Conclusion:
Reviewing the literature revealed that spinal neurosurgeons rarely diagnose CM-1, and treat them with decompressions with/without fusions and/or duraplasty. Fewer spinal neurosurgeons diagnose/treat both the CM-1 and LLCT syndromes, perform preoperative traction under anesthesia, and place ONS for persistent headaches following CM-1 surgery.
Keywords: Chiari-1 malformations (CM-1): low lying cerebellar tonsil syndrome, chiari-1 surgery, occipital neurostimulators, surgical indications, traction under anesthesia
INTRODUCTION
A review of the neurosurgical literature revealed that spinal neurosurgeons rarely (0.78%) diagnose chiari-1 malformations (CM-1) on magnetic resonance (MR) studies [Tables 1 and 2]. The diagnosis is based on the MR documentation of the cerebellar tonsils located >5 mm to an average of 12 mm below the foramen magnum (FM). Surgical intervention may include cranial/cervical decompression, with/without fusion, and/or duraplasty.
Table 1.
A review of the neurosurgical literature documented that spinal neurosurgeons typically define CM-1 malformations by tonsillar descent > 5 mm below the foramen magnum, while fewer define the low lying cerebellar tonsil syndrome
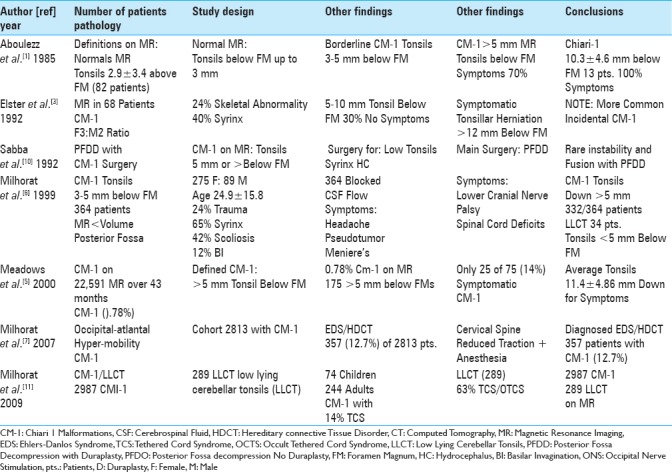
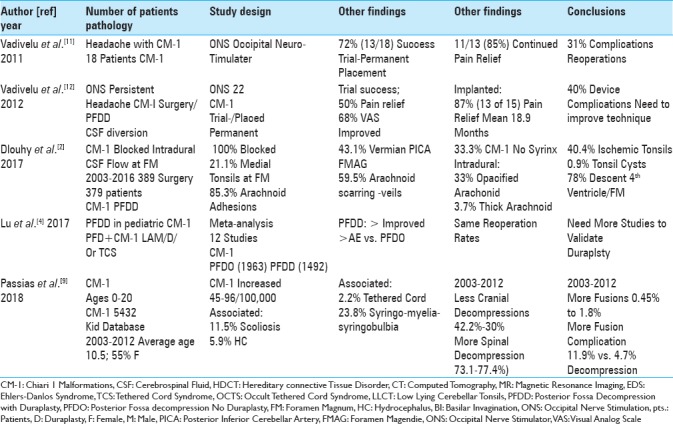
Table 2.
According to a review of the neurosurgical literature, spinal neurosurgeons typically defined chiari-1 malformations by tonsillar descent >5 mm below the foramen magnum vs. while fewer spinal neurosurgeons additionally defned the low lying cerebellar tonsil syndrome (LLCT:< 5 mm tonsillar descent) 2011-2018
The literature also showed that fewer spinal neurosurgeons additionally diagnose and treat the low lying cerebellar tonsil syndrome (LLCT) defined on MR by tonsils herniated <5 mm below the foramen magnum [Tables 1 and 2]. Besides performing cranial/cervical decompression, with/without fusion and/or duraplasty, they additionally perform preoperative traction under anesthesia, and may also postoperatively place occipital neurostimulators (ONS) for persistent headaches.
Here, we reviewed the diagnosis of the CM-1 and LLCT sydromes, and highlighted the different therapeutic options offered by spinal neurosurgeons based on a review of the neurosurgical literature.
Frequency of chiari-1 malformations (CM-1) diagnosed in the adult and pediatric populations
The frequency of CM-1 malformations is low in both the adult and pediatric age groups. In 2000, when Meadows et al. reviewed 22,591 MR studies performed over a 43-month period, they found only 0.78% (175 patients) of patients exhibited CM-1 malformations (e.g. defined by tonsils > 5 mm below the foramen magnum) [Table 1].[5] Of these 175 patients, only 25 (14%) were symptomatic with tonsils averaging 11.4 mm ± 4.86 mm below the FM. In children, Passias et al. (2018) diagnosed CM-1 in up to 96 per 100,000 children [e.g. 5432 Kid Database (2003–2012)]; here, patients averaged 10.5 years of age (range, 0–20), and 55% were females [Table 2].[9]
Classical definition of chiari-1 malformations (CM-1)
According to a review of the neurosurgical literature, spinal neurosurgeons typically define CM-1 utilizing MR scans that show >5 mm of tonsillar descent [Tables 1 and 2].[1,3,5,6,9] In 1985, Aboulezz et al. defined the normal location of cerebellar tonsils as 2.9 mm ± 3.4 mm above or up to 3 mm below the foramen magnum; borderline CM-1 tonsils were between 3 and 5 mm below the FM [Table 1].[1] In 1999, Milhorat et al. diagnosed CM-1 in 332/364 patients with tonsillar descent of >5 mm [Table 1].[6]
Extent of tonsillar descent correlates with symptomatic chiari-1 malformations (CM-1)
Different studies correlated the onset of chiari-1 symptoms with the extent of tonsillar descent below the FM [Table 1].[1,3] Aboulezz et al. (1985) found that 13 patients with CM-1 tonsillar herniation below an average of 10.3 ± 4.5 mm were symptomatic.[1] When Elster et al. (1992) evaluated 68 patients with CM-1, 70% with tonsils 5–10 mm, but 100% with tonsils >12 mm below the FM were symptomatic [Table 1].[3]
Fewer spinal neurosurgeons define low lying cerebellar tonsil syndrome (LLCT)
A review of the literature revealed that fewer spinal neurosurgeons additionally diagnosed the LLCT on MR [Table 1].[6,8] Milhorat et al. in 1999 found that 34 patients with MR-documented tonsils <5 mm below the FM had “chiari-1 like” clinical syndromes; for these patients, they newly defined the LLCT [Table 1].[6] In 2009, Milhorat et al. further described 289 patients with the LLCT.[8]
Associated abnormalities with adult and pediatric chiari-1 malformation (CM-1)
Multiple additional pathologies accompany CM-1 malformations [Tables 1 and 2].[3,6,7,9,10] These include the following: 24% skeletal abnormalities, 40%–65% syrinx formation, hydrocephalus, 42% scoliosis, 12% basilar invagination, and 12.7% occipital-atlantal hypermobility (e.g., associated with Ehlers–Danlos syndrome). In Passias et al. series involving 5432 CM-1 pediatric patients (Kid Database 2003–2012; ages 0–20), concurrent diagnoses included; 23.8% syringomyelia, 11.5% syringobulbia, 5.9% hydrocephalus, and 2.2% tethered cord syndromes [Table 2].[9]
Increased incidence of spinal decompression and fusion rates for chiari-1 malformation (CM-1)
Recently, higher spinal decompression and fusion rates have characterized CM-1 surgery in the pediatric population [Table 2].[9] For 5432 children with CM-1 malformations evaluated by Passias et al. from 2003–2012 (Kid Database), the incidence of cranial decompression decreased from 42.2% to 30%, whereas spinal decompressions increased from 73.1% to 77.4% [Table 2].[9] Fusion rates also increased over the same period from 0.45% to 1.8%, but were correlated with higher complication rates (11.9% for fusion vs. 4.7% for decompression alone).
Pros and cons for posterior fossa decompression only or with duraplasty for chiari-1 malformation
There are various pros and cons for performing posterior fossa decompression only (PFDO) vs. posterior fossa decompression with duraplasty (PFDD) for patients with CM-1 [Tables 1 and 2].[2,4,10] In 1992, Sabba et al. performed PFDD for CM-1 where the tonsils were >5 mm below the FM; they determined that fusion was not indicated [Table 1].[10] In Dlouhy et al. (2017), 389 CM-1 patients (both pediatric and adult) had PFDD; the authors observed a 100% obstruction rate of CSF at the level of the tonsils, with an added 21.1% incidence of obstruction at the Foramen of Magendie [Table 2].[2] Obstruction was also attributed to; 85.3% arachnoid adhesions, 43.1% vermian posterior inferior cerebellar artery, 59.5% obstruction of the Foramen of Magendie (arachnoidal scarring), 33% opacfied arachnoid, 3.7% thickened arachnoid, 40.4% ischemic/gliotic tonsils, 0.9% tonsillar cyst, and 78% inferior descent of the fourth ventricle [Table 2]. Lu et al. (2017) later performed a meta-analysis involving 12 studies of CM-1 comparing outcomes and complications for PFDO (1963 patients) vs. PFDD (1492 patients) [Table 2].[4] Those undergoing PFDD (e.g. with duraplasty) exhibited greater neurological improvement, but with higher complication rates. Interestingly, reoperation rates were similar for both groups. The authors concluded that future studies were warranted to document the safety/efficacy of duraplasty for these procedures.
Literature demonstrates fewer spinal neurosurgeons diagnose Ehlers-Danlos syndrome treated with cervical traction under anesthesia
The literature showed that fewer spinal neurosurgeons performed traction under anesthesia for patients with hereditary connective tissue disorder (HCTD)/Ehlers–Danlos syndrome (EDS) [Table 1].[7] In 2007, Milhorat et al. diagnosed 357 (12.7%) of 2813 CM-1 patients with HCTD/EDS and accomopanying craniocervical cranio/cervical ligamentous laxity.[7] Patients underwent cranio-cervical traction under general anesthesia to reduce their instability. This maneuver enabled the spinal neurosurgeons to determine if occipital-cervical fusion was indicated along with the CM-1 posterior fossa decompression.
Literature shows fewer spinal neurosurgeons perform occipital neurostimulator trials/permanent implants for persistent headache following chiari-1 malformation surgery
A review of the literature showed that fewer spinal neurosurgeons additionally placed ONS for patients with intractable headaches following CM-1 surgery [Table 2].[11,12] Vadivelu et al. (2011) evaluated 18 patients with CM-1; 72% (13/18) underwent successful trial placement, and received permanent ONS devices [Table 2].[11] Eleven (e.g. 13 or 85%) exhibited pain relief, but 31% developed complications requiring additional surgery. In 2012, Vadivelu et al. further acknowledged placing 22 trial stimulators defining success as a 50% resolution in pain (VAS score: Visual Analog Scale) [Table 2].[12] Fifteen of 22 (68%) trials were successful, and 13 had permanent ONS implants; 87% exhibited continued pain relief over the next 18.9 postoperative months. However, 40% required additional surgery for complications, prompting the authors to conclude that the surgical technique needed improvement.
CONCLUSION
According to our review of the neurosurgical literature, spinal neurosurgeons typically perform decompressions with/without fusions and/or duraplasty for CM-1 malformations. The literature, however, demonstrated that fewer spinal neurosurgeons additionally diagnosed and treated the LLCT syndrome, for which they also performed postoperative traction under anesthesia, and placed postoperative ONS for persistent headaches following CM-1 surgery (posterio decompressions with/without duraplasty and/or fusions with a 40% complication rate). Future studies should reexamine how spinal neurosurgeons define and treat the LLCT syndrome, the indications for preoperative traction under anesthesia, and the safety/efficacy of ONS placement.
Footnotes
REFERENCES
- 1.Aboulezz AO, Sartor K, Geyer CA, Gado MH. Position of cerebellar tonsils in the normal population and in patients with Chiari malformation: A quantitative approach to MR imaging. J Comput Assist Tomogr. 1985;9:1033–6. doi: 10.1097/00004728-198511000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Dlouhy BJ, Dawson JD, Menezes AH. Intradural pathology and pathophysiology associated with Chiari I malformation in children and adults with and without syringomyelia. J Neurosurg Pediatr. 2017;20:526–41. doi: 10.3171/2017.7.PEDS17224. [DOI] [PubMed] [Google Scholar]
- 3.Elster Ad, Chen MYM. Chiari I malformations; clinical and radiologic reappraisal. Radiology. 1992;183:347–53. doi: 10.1148/radiology.183.2.1561334. [DOI] [PubMed] [Google Scholar]
- 4.Lu VM, Phan K, Crowley SP, Daniels DJ. The addition of duraplasty to posterior fossa decompression in the surgical treatment of pediatric Chiari malformation Type I: A systematic review and meta-analysis of surgical and performance outcomes. J Neurosurg Pediatr. 2017;20:439–49. doi: 10.3171/2017.6.PEDS16367. [DOI] [PubMed] [Google Scholar]
- 5.Meadows J, Kraut M, Guarnieri M, Haroun RI, Carson BS. Asymptomatic Chiari Type I malformations identified on magnetic resonance imaging. J Neurosurg. 2000;92:920–6. doi: 10.3171/jns.2000.92.6.0920. [DOI] [PubMed] [Google Scholar]
- 6.Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C, et al. Chiari I malformation redefined: Clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005–17. doi: 10.1097/00006123-199905000-00042. [DOI] [PubMed] [Google Scholar]
- 7.Milhorat TH, Bolognese PA, Nishikawa M, McDonnell NB, Francomano CA. Syndrome of occipitoatlantoaxial hypermobility, cranial settling, and chiari malformation type I in patients with hereditary disorders of connective tissue. J Neurosurg Spine. 2007;7:601–9. doi: 10.3171/SPI-07/12/601. [DOI] [PubMed] [Google Scholar]
- 8.Milhorat TH, Bolognese PA, Nishikawa M, Francomano CA, McDonnell NB, Roonprapunt C, et al. Association of Chiari malformation type I and tethered cord syndrome: Preliminary results of sectioning filum terminale. Surg Neurol. 2009;72:20–35. doi: 10.1016/j.surneu.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Passias PG, Pyne A, Horn SR, Poorman GW, Janjua MB, Vasquez-Montes D, et al. Developments in the treatment of Chiari type 1 malformations over the past decade. J Spine Surg. 2018;4:45–54. doi: 10.21037/jss.2018.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabba MF, Renor BS, Ghizoni E, Tedeschi H, Joaquim AF. Posterior fossa decompression with duraplasty in Chiari surgery: A technical note. Rev Assoc Med Bras (1992) 2017;63:946–9. doi: 10.1590/1806-9282.63.11.946. [DOI] [PubMed] [Google Scholar]
- 11.Vadivelu S, Bolognese P, Milhorat TH, Mogilner AY. Occipital neuromodulation for refractory headache in the Chiari malformation population. Prog Neurol Surg. 2011;24:118–25. doi: 10.1159/000323044. [DOI] [PubMed] [Google Scholar]
- 12.Vadivelu S, Bolognese P, Milhorat TH, Mogilner AY. Occipital nerve stimulation for refractory headache in the Chiari malformation population. Neurosurgery. 2012;70:1430–6. doi: 10.1227/NEU.0b013e3182545a1c. discussion 1436-7. [DOI] [PubMed] [Google Scholar]


