Abstract
Background/Aims:
Gastroesophageal reflux disease (GERD) is a common condition that can lead to significant morbidity. Laryngopharyngeal reflux (LPR) is a distinct clinical entity that can occur simultaneously with GERD, necessitating additional treatment measures. The degree of overlap and clinical predictors of LPR among patients with GERD remains unknown. We aim to measure the prevalence of LPR in patients with GERD and identify clinical predictors.
Patients and Methods:
We performed a cross-sectional study involving patients with confirmed GERD according to the GERD questionnaire (GerdQ) using the reflux symptom index (RSI). Data on demographics, comorbidities, past and current medications, and GERD-related lifestyle measures were documented. The prevalence of LPR was calculated. Linear and logistic regression analyses were conducted to correlate GerdQ and RSI, and to identify clinical predictors of LPR, respectively.
Results:
A total of 80 patients with confirmed GERD were consecutively recruited and surveyed. Mean age was 43 (±16) and 60% were females. The majority of patients were Saudis (51%) and only 24% were smokers. The mean duration of GERD was 7 (±4.4) years and the average body mass index (BMI) was 36 ± 22. Sixty-six percent of the patients consumed coffee on regular basis. On simple and multiple linear regression analyses, a strong, positive correlation was observed between the GerdQ and RSI scores (coefficient = 1.13, 95%CI = 0.39–1.86), and ipratropium bromide inhaler was positively associated with RSI scores (coefficient = 13.12, 95%CI = 0.16–26.09). LPR was identified in 57 patients (71%). On simple and multiple logistic regression analyses, GerdQ scores (OR = 1.78, 95%CI = 1.13–2.80), BMI (OR = 1.07, 95%CI = 1.01–1.14), duration of GERD in years (OR = 1.42, 95%CI = 1.04–1.93), and the type of gender (OR = 49.67, 95%CI = 1.32–1870) appeared to increase the risk of LPR, whereas coffee consumption (OR = 0.0005, 95%CI = 1.82e–06, 0.13) appeared to be negatively associated with LPR.
Conclusions:
Contradictory to what is frequently reported, LPR commonly occurs and positively correlates with GERD. Several modifiable clinical predictors of LPR might exist, which highlight the importance of performing a complete clinical assessment of the patients with reflux symptoms.
Keywords: Correlation, gastroesophageal reflux disease, laryngopharyngeal reflux, severity
INTRODUCTION
Laryngopharyngeal reflux (LPR), which is also known as “extra-esophageal” or “silent” reflux, is described as retrograde reflux of gastro-duodenal contents into the larynx and pharynx, leading to severe damage of the upper aerodigestive tract.[1] Although LPR and gastroesophageal reflux disease (GERD) are both caused by the reflux of gastric contents, the two conditions show differences in clinical presentation and treatment modalities. In a number of studies, the incidence of classic reflux symptoms (heartburn and regurgitation) in patients with LPR is estimated to be 40%, and the incidence of esophagitis is estimated to be approximately 25%, which indicate that the majority of patients with LPR do not have esophagitis.[2] Both GERD and LPR are diagnosed based on clinical[3] characteristics but scoring systems and laryngoscopic findings are often used to confirm diagnosis and determine severity.[4] For example, to confirm the diagnosis of GERD, the GERD questionnaire (GerdQ), a six-item, easy-to-use questionnaire that was developed primarily as a diagnostic tool for GERD in primary care patients, consulting for upper gastrointestinal (GI) complaints with a sensitivity of 66%, specificity of 64%, positive predictive value of 92%, and negative predictive value of 22%, is commonly utilized as a complimentary tool.[5] Similarly, Belafsky et al.[6] developed the reflux symptom index (RSI), a validated, self-administered, nine-item scoring system, designed to assess the symptoms related to LPR (hoarseness, throat clearing, excess throat mucus or postnasal drip, dysphagia, coughing after eating or lying down, breathing difficulties, troublesome or annoying cough, globus pharyngeus, and heartburn). An RSI is scored on a scale of 0–5 to quantify the severity of LPR symptoms, with a maximum total score of 45. An RSI ≥13 is considered abnormal and strongly suggestive of LPR, which is why it is a commonly utilized diagnostic cut-off point.[6] An Arabic version of the RSI has been previously developed and validated.[7]
It is important to note that although most patients with LPR do not have GERD, some patients do indeed have both LPR and GERD. The Montreal consensus group established the following associations: (1) there is an association between GERD and extra-esophageal reflux symptoms; (2) the manifestations of extra-esophageal reflux symptoms rarely occur in the absence of GERD symptoms; (3) extra-esophageal symptoms are usually multifactorial, with GERD as one of the several potential aggravating cofactors; and (4) the data substantiating a beneficial effect of reflux treatments are weak.[8] A study by Groome et al. examined a large number of patients with endoscopically proven GERD and has demonstrated a correlation between the severity of GERD and the prevalence of LPR.[9] In a prospective study, 28–58% of the patients with endoscopic evidence of GERD were reported to have at least one item of the Comprehensive Reflux Symptom Scale questionnaire, which supports the Montreal consensus on an LPR-GERD continuum.[10]
The aim of this study is to correlate between the RSI and GerdQ, and to identify the clinical predictors of LPR among patient known to have GERD.
PATIENTS AND METHODS
The study protocol was reviewed and accepted by the institutional research ethics board at King Abdulaziz University (KAU).
We consecutively performed a cross-sectional survey of all patients with GERD seen at the gastroenterology, and ear, nose, and throat clinics at KAU Hospital. Pregnant patients, those who refused to participate through written informed consent and those who underwent upper GI or neck surgeries, were excluded.
Outcomes
To confirm the diagnosis of GERD, we used a score >8 on the validated Arabic version of the GerdQ[9] for patients not on active acid suppression treatment. Patients with clinical description of GERD already on acid suppression therapy (proton pump inhibitors [PPI] or H2blockers) instituted by a physician were also included even if GerdQ score was less than 9. Once GERD was confirmed, all study participants were asked to respond to an Arabic version of the RSI questionnaire to diagnose LPR, which was defined as an RSI ≥13.[7] Patient demographics and clinical data at the time of diagnosis, including age, gender, duration of LPR and typical GERD symptoms prior to diagnosis, past and present medications, dietary restrictions, cigarette smoking, and clinical symptoms of LPR and typical GERD, were collected.
Statistical analysis
Descriptive statistics were calculated for baseline characteristics for both continuous and categorical variables in which means, standard deviations, and minimum and maximum values were used for the former and frequencies were used for the latter. Chi square test or Fisher's exact test, and Student's t-test or Mann–Whitney U-test were used to compare the frequencies and means for categorical and continuous variables, respectively. Prevalence of LPR was calculated using the standard prevalence formula. Simple and multiple linear regression analyses were conducted to correlate RSI and GerdQ, where appropriate. Univariable logistic regression was used to examine the association between independent variables and LPR. Odds ratios (OR) and 95% confidence intervals (CIs) were estimated. STATA 11.2 (StataCorp, Texas, USA) was used in our analysis with statistical significance threshold set at 0.05 and precision boundaries set at 95%.
Sample size calculation
For sample size calculation, we hypothesized that the prevalence of LPR among patients with GERD is 40%. Assuming a type-1 error of 0.05 and 80% power to detect LPR in patients with GERD, we estimated that 65 patients would be needed to detect at least 20 cases of LPR, and 84 patients would be needed to detect an OR of 2 for the predictors of LPR.
RESULTS
Baseline characteristics
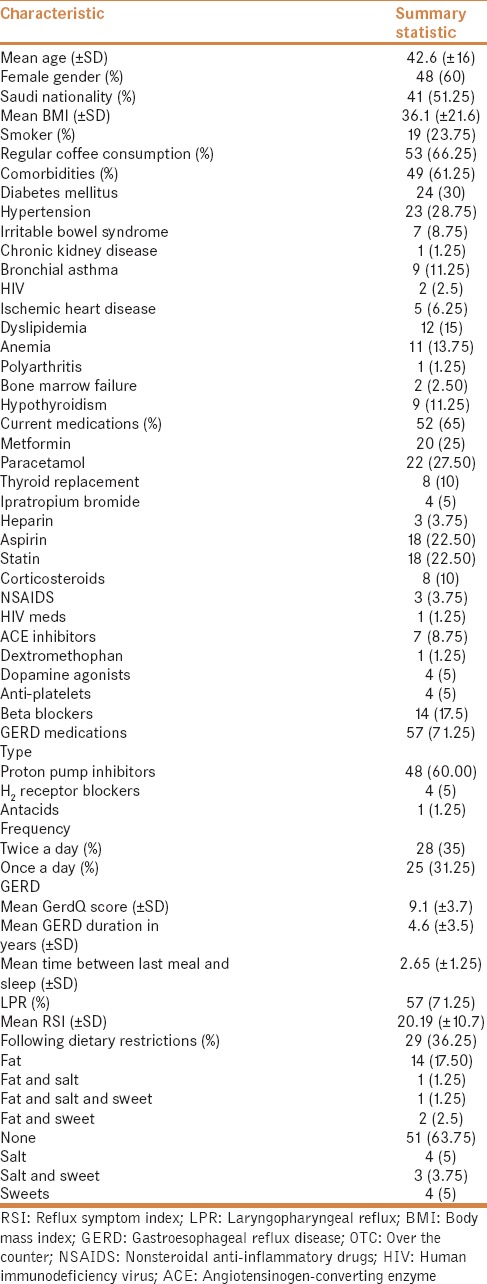
A total of 80 patients with confirmed GERD were recruited and surveyed. Mean age was 43 (±16) and 60% were females. Fifty-one percent of the patients were Saudis and only 24% were smokers. Mean duration of GERD was 7 (±4.4) years and the average body mass index (BMI) was 36 ± 22. Sixty-six percent of the patients consumed coffee on regular basis [Table 1].
Table 1.
Baseline characteristics of 80 patients with gastroesophageal reflux disease
Comorbidities
Sixty-one percent of the patients had chronic comorbid conditions. The most common comorbidity was diabetes mellitus (30%) followed by hypertension (29%). Sixty-five percent of the patients were on active medications [Table 1].
GERD treatments
The majority of patients (64%) did not follow any dietary restrictions as a treatment strategy for GERD. Medications to treat symptoms related to GERD were being utilized by 71% of patients [Table 1]. The most common type of reflux medication used was PPI (60%), which was mostly prescribed twice a day (54%) [Table 1].
GerdQ and RSI correlation
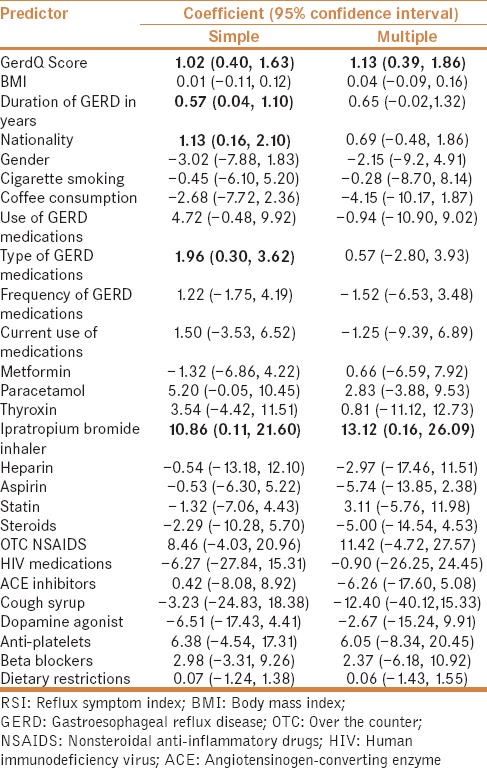
On simple and multiple linear regression analyses, a strong, positive correlation was observed between GerdQ and RSI scores (coefficient = 1.13, 95%CI = 0.39–1.86) [Figure 1]. Only ipratropium bromide inhalers appeared to be significantly and positively associated with RSI (coefficient = 13.12, 95%CI = 0.16–26.09) on multiple linear regression analysis [Table 2].
Figure 1.
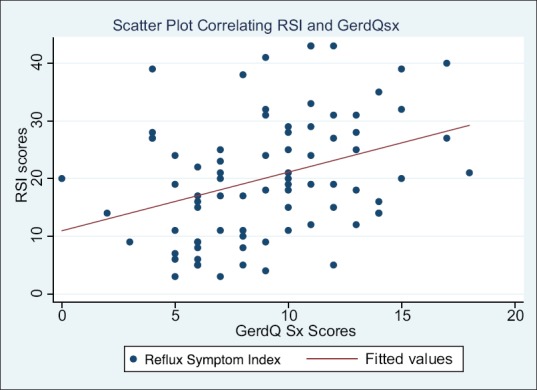
A scatter plot correlating reflux symptom index scores with GerdQ symptom scores
Table 2.
Simple and multiple linear regression analyses identifying predictors of RSI scores
LPR and predictors of LPR
On the basis of the preset definition, 57 patients (71%) were found to have LPR. Mean RSI was 20 ± 11. Mean GerdQ differed significantly between patients with and without LPR (10 ± 4 vs. 7 ± 2, P = 0.003).
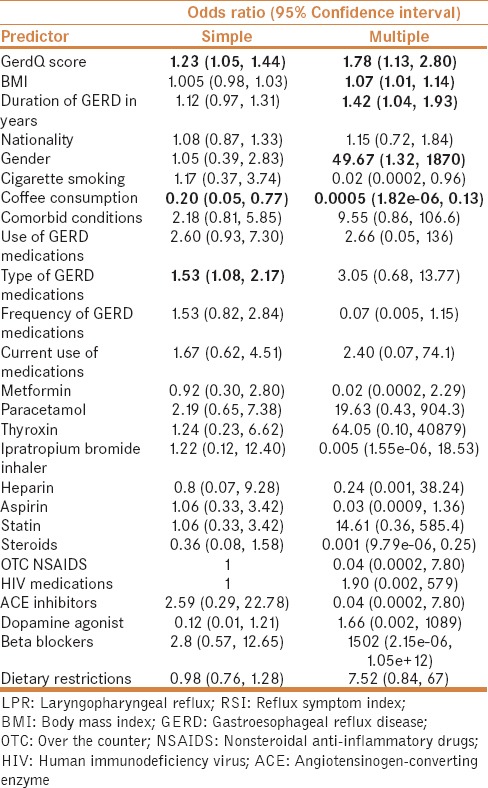
On simple and multiple logistic regression analyses, GerdQ score (OR = 1.78, 95%CI = 1.13–2.80), BMI (OR = 1.07, 95%CI = 1.01–1.14), duration of GERD in years (OR = 1.42, 95%CI = 1.04–1.93), and gender (OR = 49.67, 95%CI = 1.32–1870) appeared to increase the risk of LPR. Conversely, coffee consumption (OR = 0.0005, 95%CI = 1.82e–06, 0.13) appeared to be negatively associated with LPR [Table 3].
Table 3.
Simple and multiple logistic regression analyses identifying predictors of LPR according to RSI cut-off point of >12
DISCUSSION
LPR and GERD are the two conditions that differ in their clinical presentation, diagnosis, and treatment modalities; the most significant difference between LPR and GERD is that the majority of patients with LPR do not have esophagitis or its primary symptom, heartburn.[1] In recent studies, a stronger correlation was found between the two conditions. Tauber et al. reported that 69% of the GERD-positive patients had laryngitis based on inter-arytenoid erythema and edema seen during laryngoscopic examination.[11] A similar finding was demonstrated in a recent study by Vardar et al. in which LPR was reported in 70% of GERD patients.[12] Additionally, a meta-analysis by Joniau et al., which included 11 studies of 192 normal controls and 13 studies of 512 patients with reflux laryngitis, concluded that the prevalence of pharyngeal reflux events in patients with reflux laryngitis is only marginally higher than in normal controls (P = 0.079).[13] In this study, we examined a total of 80 patients with confirmed GERD and found that LPR was present in 57 patients (71%).
We utilized logistic regression analysis to identify the significant clinical predictors of LPR. GERDQ score, BMI, duration of GERD in years, and gender appeared to be positively associated with LPR occurrence. The positive association between LPR and BMI has been previously identified by Kamani et al. (P = 0.001).[14] Similarly, on the basis of our results, BMI is positively associated with LPR (OR = 1.07, 95%CI = 1.01–1.14). This is a clinically relevant observation given that BMI is a risk factor that can be modified mostly through diet and exercise, without the need for medical or surgical interventions.
Coffee, another modifiable risk factor, is highly consumed in many populations[15] and its effect on reflux remains controversial based on previous literature. Boekema et al. monitored esophageal pH for 24 h in two groups. The first group ingested water and the second group ingested coffee. GERD scores were higher in the group that consumed coffee but only during fasting time (median = 2.6, vs. 0; P = 0.028).[14] Additionally, no significant relationship was observed between coffee and reflux in a cross-sectional study of 5,451 coffee consumers reported by Shimamoto et al. (OR = 0.88, 95%CI = 0.74–1.04, P = 0.133).[16] Conversely, Wendl et al. reported that only caffeinated coffee significantly promoted GERD (P < 0.05), whereas caffeinated tea and decaffeinated coffee (P < 0.05) did not promote GERD symptoms.[17] Our results suggest that coffee consumption is negatively associated with the development of LPR (OR = 0.0005, 95%CI = 1.82e–06, 0.13); however, given the wide CI observed, we suggest interpreting these results with caution. Whether or not this can be translated into a clinical intervention for LPR remains unknown and merits further investigation.
We acknowledge that our study has many limitations including its cross-sectional design, the lack of direct laryngoscopic confirmation of LPR, relying on clinical rather than endoscopic criteria to diagnose GERD, and the lack of sufficient data regarding the amount and type of coffee consumed. Larger studies, preferably randomized controlled trials, are needed to further confirm our observations.
CONCLUSIONS
LPR is commonly encountered in patients with GERD. There is a positive correlation in severity between the two disorders. Many modifiable clinical predictors of LPR exist including BMI and coffee consumption.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): A clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101:1–78. doi: 10.1002/lary.1991.101.s53.1. [DOI] [PubMed] [Google Scholar]
- 2.Koufman JA, Aviv JE, Casiano RR, Shaw GY. Laryngopharyngeal reflux: Position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127:32–5. doi: 10.1067/mhn.2002.125760. [DOI] [PubMed] [Google Scholar]
- 3.Kamani T, Penney S, Mitra I, Pothula V. The prevalence of laryngopharyngeal reflux in the English population. Eur Arch Otorhinolaryngol. 2012;269:2219–25. doi: 10.1007/s00405-012-2028-1. [DOI] [PubMed] [Google Scholar]
- 4.Mahieu HF. Review article: The laryngological manifestations of reflux disease; why the scepticism? Aliment Pharmacol Ther. 2007;26(Suppl 2):17–24. doi: 10.1111/j.1365-2036.2007.03474.x. [DOI] [PubMed] [Google Scholar]
- 5.Jonasson C, Wernersson B, Hoff DA, Hatlebakk JG. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2013;37:564–72. doi: 10.1111/apt.12204. [DOI] [PubMed] [Google Scholar]
- 6.Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI) J Voice. 2002;16:274–7. doi: 10.1016/s0892-1997(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 7.Farahat M, Malki KH, Mesallam TA. Development of the Arabic version of Reflux Symptom Index. J Voice. 2012;26:814 e815–9. doi: 10.1016/j.jvoice.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. Globale Konsensusgruppe. [The Montreal definition and classification of gastroesophageal reflux disease: A global, evidence-based consensus paper] Z Gastroenterol. 2007;45:1125–40. doi: 10.1055/s-2007-963633. [DOI] [PubMed] [Google Scholar]
- 9.Groome M, Cotton JP, Borland M, McLeod S, Johnston DA, Dillon JF. Prevalence of laryngopharyngeal reflux in a population with gastroesophageal reflux. Laryngoscope. 2007;117:1424–8. doi: 10.1097/MLG.0b013e31806865cf. [DOI] [PubMed] [Google Scholar]
- 10.Drinnan M, Powell J, Nikkar-Esfahani A, Heading RC, Doyle J, Griffin SM, et al. Gastroesophageal and extraesophageal reflux symptoms: Similarities and differences. Laryngoscope. 2015;125:424–30. doi: 10.1002/lary.24950. [DOI] [PubMed] [Google Scholar]
- 11.Joniau S, Bradshaw A, Esterman A, Carney AS. Reflux and laryngitis: A systematic review. Otolaryngol Head Neck Surg. 2007;136:686–92. doi: 10.1016/j.otohns.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Vardar R, Varis A, Bayrakci B, Akyildiz S, Kirazli T, Bor S. Relationship between history, laryngoscopy and esophagogastroduodenoscopy for diagnosis of laryngopharyngeal reflux in patients with typical GERD. Eur Arch Otorhinolaryngol. 2012;269:187–91. doi: 10.1007/s00405-011-1748-y. [DOI] [PubMed] [Google Scholar]
- 13.Tauber S, Gross M, Issing WJ. Association of laryngopharyngeal symptoms with gastroesophageal reflux disease. Laryngoscope. 2002;112:879–86. doi: 10.1097/00005537-200205000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Boekema PJ, Samsom M, Smout AJ. Effect of coffee on gastro-oesophageal reflux in patients with reflux disease and healthy controls. Eur J Gastroenterol Hepatol. 1999;11:1271–6. doi: 10.1097/00042737-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Butt MS, Sultan MT. Coffee and its consumption: Benefits and risks. Crit Rev Food Sci Nutr. 2011;51:363–73. doi: 10.1080/10408390903586412. [DOI] [PubMed] [Google Scholar]
- 16.Noassociation of coffee consumption with gastric ulcer, duodenal ulcer, reflux esophagitis, and non-erosive reflux disease: A cross-sectional study of 8,013 healthy subjects in Japan. doi: 10.1371/journal.pone.0065996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pehl C, Pfeiffer A, Wendl B, Kaess H. Effect of decaffeination of coffee or tea on gastro-oesophageal reflux. Aliment Pharmacol Ther. 1997;11:483–6. doi: 10.1046/j.1365-2036.1997.00161.x. [DOI] [PubMed] [Google Scholar]


