Abstract
Background/Aim:
Bleeding risk among patients with acute or chronic liver disease after invasive procedures is a common concern in clinical practice. This retrospective study aimed to explore whether the presence of coagulopathy increased the risk of major bleeding after invasive procedures in cirrhosis.
Patients and Methods:
A total of 874 cirrhotic patients underwent invasive procedures. Coagulopathy was defined as international normalized ratio (INR) ≥1.5 and/or platelets (PLTs) ≤50 × 109/L. Severe thrombocytopenia was defined as PLTs ≤ 50 × 109/L. Invasive procedures, major bleeding after invasive procedures, and in-hospital deaths were recorded.
Results:
In all, 296 patients (33.9%) had coagulopathy. Major bleeding after invasive procedures occurred in 21 patients (2.4%). Major bleeding after invasive procedures was more frequent in patients with coagulopathy than those without coagulopathy (4.1% vs 1.6%, P = 0.023). Major bleeding after invasive procedures was more frequent in patients with severe thrombocytopenia than those without severe thrombocytopenia (4.9% vs 1.6%, P = 0.008). Incidence of major bleeding after invasive procedures was not significantly different between patients with INR ≥ 1.5 and INR < 1.5 (4.5% vs 2.0%, P = 0.065). Patients with INR ≥1.5 had a significantly higher in-hospital mortality than those with INR < 1.5 (6.4% vs 1.3%, P < 0.001).
Conclusion:
Severe thrombocytopenia significantly increased the risk of major bleeding after invasive procedures in cirrhosis. INR ≥ 1.5 significantly increased in-hospital mortality.
Keywords: In-hospital mortality, international normalized ratio, platelets, prothrombin time, thrombocytopenia
INTRODUCTION
Prothrombin time (PT), international normalized ratio (INR), and platelet (PLT) counts are conventional coagulation tests. Patients with cirrhosis have higher PT/INR and lower PLT. Thus, cirrhotic patients are traditionally at a high risk of bleeding. However, current evidence regarding the association between conventional coagulation tests and the risk of bleeding in cirrhotic patients remains controversial.[1,2,3,4,5,6,7,8,9]
In an Italian study, Napolitano et al. found that post-procedural bleeding was rare in cirrhotic patients with abnormal INR and/or low PLT who underwent invasive investigations and could not be predicted by abnormal INR or PLT.[10] In an Indian study, Shah et al. also found that abnormal conventional coagulation parameters did not predict clinically significant bleeding in cirrhosis.[11] Tripodi et al. indicated no causal relationship between coagulopathy associated with chronic liver diseases and bleeding[5] and further suggested that PT and INR could reflect the severity of liver dysfunction and predict the mortality of acute and chronic liver diseases, but not the risk of bleeding.[6]
In contrast, Cocero et al. found that patients with chronic liver disease who had a PLT of >40 × 103/μL and an INR of <2.5 had a relatively low risk of bleeding, but an INR of ≥2.5 and a PLT of ≤40 × 103/μL represented significant risk factors of bleeding after extractions.[12] Giannini et al. also found that bleeding risk after invasive procedures was associated with the degree of thrombocytopenia in patients with advanced liver diseases, but not PT/INR.[13]
Considering that conventional coagulation tests used to assess the risk of bleeding in cirrhosis has been largely challenged, we explored whether the presence of coagulopathy increased the risk of major bleeding after invasive procedures in liver cirrhosis.
PATIENTS AND METHODS
Patients
This study retrospectively screened all cirrhotic patients who were consecutively admitted to our hospital from 1st January 2011 to 30th June 2014. Patients were diagnosed with cirrhosis on the basis of clinical presentations (decompensated events), liver function tests [i.e., total bilirubin, albumin (ALB), PT, etc.], and abdominal ultrasound and computed tomography (CT) scans (liver contour, spleen size, portal vein diameter, and gastroesophageal varices),[14,15,16,17] or histological evidence of cirrhosis, if necessary. All patients with cirrhosis undergoing invasive procedures were included in the study. Exclusion criteria were as follows: (1) patients diagnosed with malignancy, especially hepatocellular carcinoma; (2) incomplete regular coagulation tests, such as PLT, PT, and INR; (3) incomplete medical records; (4) patients receiving anticoagulation and antiplatelet drugs during the past 7 days; (5) patients with a history of hematological diseases; and (6) patients who developed acute hemorrhage 5 days before invasive procedures. The study protocol was approved by the Medical Ethical Committee of our hospital [number k (2016) 39]. The patients’ informed consent was waived.
Data collection
The primary data items included sex, age, etiology of liver diseases, ascites, hepatic encephalopathy (HE), laboratory tests, Child–Pugh class/score,[18] and Model for End-Stage Liver Disease (MELD) score.[19] We recorded all invasive procedures carried out during hospitalizations, such as endoscopic band ligation, endoscopic glue injection, endoscopic sclerotherapy, abdominocentesis, pleurocentesis, endoscopic retrograde cholangiopancreatography (ERCP), cholecystectomy, splenectomy, stem-cell therapy, endoscopic polypectomy, central vein cannulation, bone marrow puncture, splenic arterial embolization, and percutaneous liver biopsy. We also recorded the PLTs or plasma transfusion before invasive procedures, the presence of major bleeding secondary to invasive procedures, and in-hospital deaths.
Definitions and classifications
Coagulopathy was defined as INR ≥1.5 and/or PLT ≤50 × 109/L.[11,13,20] Severe thrombocytopenia was defined as PLT ≤50 × 109/L.[11,13,20] Major bleeding after invasive procedures was defined as overt bleeding or decrease in hemoglobin to less than 80 g/L after invasive procedures.[21] They were divided into patients with and without coagulopathy, patients with and without severe thrombocytopenia, and patients with INR ≥1.5 and INR <1.5.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median (range) and were compared using independent sample t-test. Categorical variables were expressed as frequency (percentage), and were compared using Chi-squared test. A two-tailed P value of <0.05 was considered statistically significant. All statistical analyses were used using SPSS Statistics 17.0 (SPSS, Chicago IL).
RESULTS
A total of 874 patients with cirrhosis who underwent invasive procedures were included. The average age was 55.08 years; 65.9% of patients were male; 38.7%, 43.7%, and 14.6% of patients were Child–Pugh class A, B, and C, respectively; 33.9% of patients had coagulopathy; 23.3% of patients had severe thrombocytopenia; and 18.0% of patients had INR ≥1.5. The most common type of invasive procedures was endoscopic band ligation, followed by abdominocentesis.
In all, 21 (2.4%) patients developed major bleeding after endoscopic band ligation (n = 4), endoscopic sclerotherapy (n = 3), large volume abdominocentesis (n = 2), endoscopic sclerotherapy combined with glue injection (n = 2), splenectomy in combination with cholecystectomy (n = 2), endoscopic glue injection (n = 1), endoscopic band ligation combined with glue injection (n = 1), ERCP (n = 1), splenectomy (n = 1), splenectomy in combination with abdominocentesis (n = 1), endoscopic band ligation and sclerotherapy in combination with phlebotomy (n = 1), gastrointestinal polypectomy in combination with abdominocentesis (n = 1), and artificial hip replacement (n = 1). Among them, 16 patients received red blood cell (RBC) transfusions, and 2 patients received PLTs and plasma transfusion.
Nineteen patients died during hospitalization because of multiple organ failure (n = 7), liver failure (n = 5), gastrointestinal bleeding (n = 3), HE with spontaneous bacterial peritonitis (n = 1), HE with renal failure and metabolic acidosis (n = 1), uremia (n = 1), and fungal pneumonia with hyperkalemia (n = 1).
Major bleeding after invasive procedures
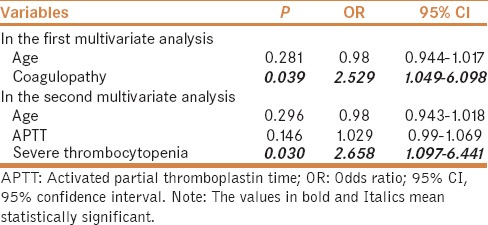
In univariate analysis, the factors significantly associated with major bleeding after invasive procedures were lower age, higher activated partial thromboplastin time (APTT), and larger proportions of coagulopathy and severe thrombocytopenia [Table 1]. The colinearity between APTT and coagulopathy and that between severe thrombocytopenia and coagulopathy should be acknowledged. We performed logistic multivariate analysis twice. In the first multivariate analysis including age and coagulopathy, we found that coagulopathy was the only independent predictor for major bleeding after invasive procedures [odds ratio (OR) = 2.529; 95% confidence interval (CI) = 1.049–6.098, P = 0.039). In the second multivariate analysis including age, APTT, and severe thrombocytopenia, we found that severe thrombocytopenia was the only independent predictor for major bleeding after invasive procedures (OR = 2.658; 95% CI = 1.097–6.441, P = 0.030) [Table 2].
Table 1.
Comparison between patients with and without bleeding after invasive procedures
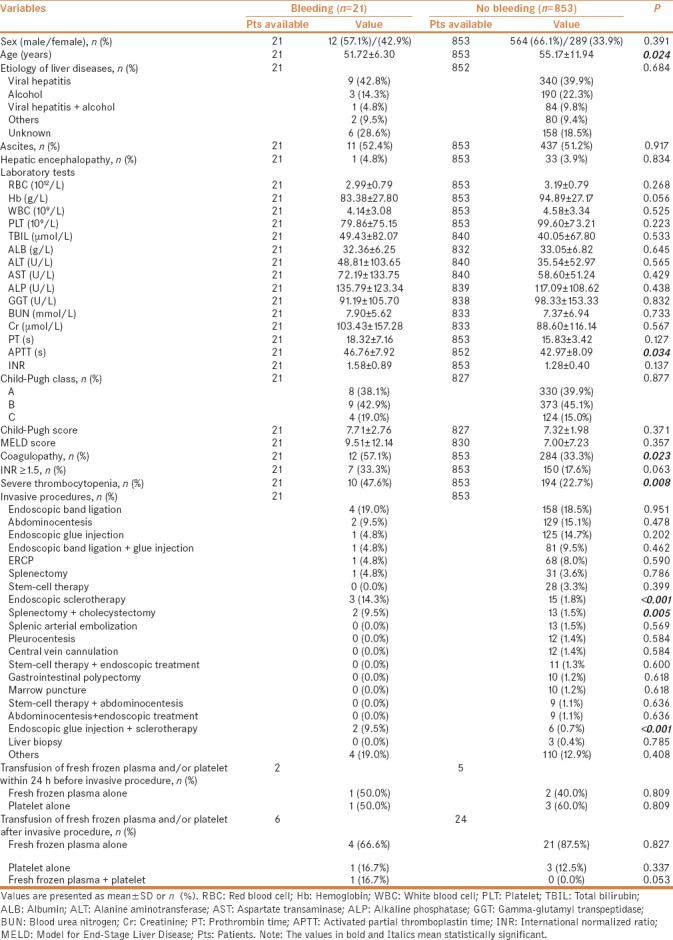
Table 2.
Multivariate analysis of predictors of major bleeding after invasive procedures
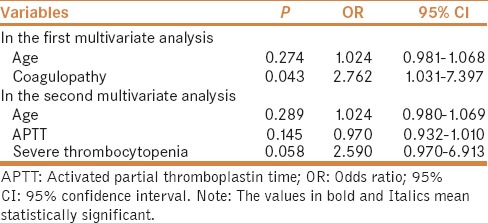
In univariate analysis after excluding patients who did not receive blood transfusion, the factors significantly associated with major bleeding after invasive procedures were lower age, higher APTT, and larger proportions of coagulopathy and severe thrombocytopenia [Table 3]. Similarly, considering the colinearity among variables, we performed logistic multivariate analysis twice. In the first multivariate analysis including age and coagulopathy, we found that coagulopathy was the only independent predictor for major bleeding after invasive procedures (OR = 2.762; 95% CI = 1.031–7.397, P = 0.043). In the second multivariate analysis including age, APTT, and severe thrombocytopenia, we did not find any independent predictors for major bleeding after invasive procedures [Table 4].
Table 3.
Subgroup analysis after excluding patients who received blood transfusion: comparison between patients with and without bleeding after invasive procedures
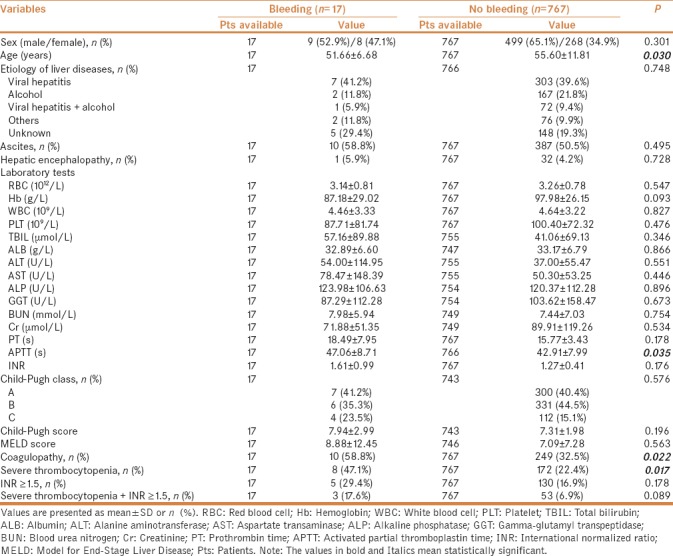
Table 4.
Subgroup analysis after excluding patients who received blood transfusion: multivariate analysis of predictors of major bleeding after invasive procedures
In-hospital death after invasive procedures
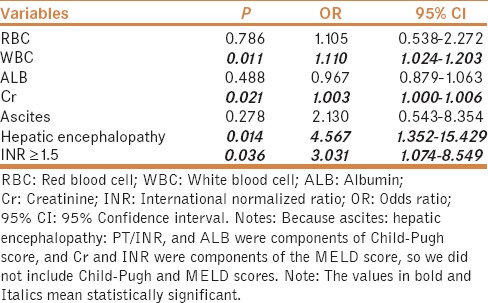
In univariate analysis, factors significantly associated with in-hospital mortality were larger proportions of ascites, HE, Child–Pugh class C, post-procedural bleeding, higher white blood cell (WBC), blood urea nitrogen (BUN), creatinine (Cr), PT, APTT, INR, Child–Pugh score, MELD score, lower RBC and ALB, larger proportions of coagulopathy, and INR ≥1.5 [Table 5]. Notably, ascites, HE, PT/INR, and ALB were components of Child–Pugh score. Cr and INR were components of MELD score. The colinearity between PT/INR and APTT and that between BUN and Cr should be acknowledged. We performed logistic multivariate analysis including ascites, HE, WBC, Cr, RBC, ALB, and INR ≥1.5, we found that WBC, Cr, HE, and INR ≥1.5 were the independent predictors for in-hospital mortality (OR = 1.110; 95% CI = 1.024–1.203, P = 0.011; OR = 1.003; 95% CI = 1.000–1.006, P = 0.021; OR = 4.567; 95% CI = 1.352–15.429, P = 0.014; OR = 3.031; 95% CI = 1.074–8.549, P = 0.036, respectively) [Table 6].
Table 5.
Comparison between patients with and without in-hospital death after invasive procedures
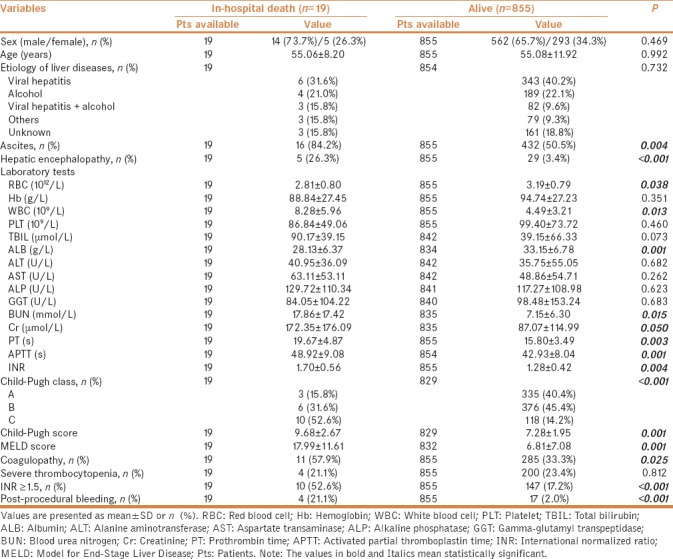
Table 6.
Multivariate analysis of predictors of in-hospital mortality
Impact of coagulopathy
Major bleeding after invasive procedures was more frequent in patients with coagulopathy than those without coagulopathy (12/296, 4.1% vs 9/578, 1.6%, P = 0.023). In-hospital mortality was significantly higher in patients with coagulopathy than those without coagulopathy (11/296, 3.7% vs 8/568, 1.4%, P = 0.025). In-hospital mortality was significantly higher in patients with coagulopathy who developed major bleeding after invasive procedures than those who did not develop major bleeding after invasive procedures (2/12, 16.7% vs 9/284, 3.2%, P = 0.015).
Impact of severe thrombocytopenia
Ten and 11 patients with and without severe thrombocytopenia developed major bleeding after invasive procedures, respectively. Major bleeding after invasive procedures was more frequent in patients with severe thrombocytopenia than those without severe thrombocytopenia (10/204, 4.9% vs 11/670, 1.6%, P = 0.008), but in-hospital mortality was not significantly different between them (4/204, 2.0% vs 15/670, 2.2%, P = 0.812).
INR ≥1.5 versus INR <1.5
Seven and 14 patients with INR ≥1.5 and INR <1.5 developed major bleeding after invasive procedures, respectively (7/157, 4.5% vs 14/717, 2.0%, P = 0.063). However, in-hospital mortality was significantly higher in patients with INR ≥1.5 than those with INR <1.5 (10/157, 6.4% vs 9/717, 1.3%, P < 0.001).
DISCUSSION
Cirrhotic patients with coagulopathy need to be carefully assessed for the risk of bleeding before invasive procedures. At present, in addition to PT/INR and PLT, no coagulation tests have been formally recommended for the assessment of coagulation status in clinical practice. However, the relationship between PT/INR and the risk of bleeding in cirrhosis has been frequently questioned. In this study, we collected data of patients with cirrhosis to further explore the value of PT/INR and PLT in predicting the risk of major bleeding after invasive procedures.
In the overall analysis, 2.4% of patients developed major bleeding after invasive procedures. Similarly, some prospective studies also showed that cirrhotic patients rarely developed major bleeding after invasive procedures and that the incidence of major bleeding after invasive procedures was 0%–2.3%.[10,11,22]
In addition, 4.1% of patients with coagulopathy developed major bleeding after invasive procedures. Notably, the most common type of invasive procedures in our patients was endoscopic treatment, which carries a relatively high risk of bleeding. In a randomized controlled trial by De Pietri et al.,[21] the incidence of bleeding after invasive procedures in 60 patients with severe coagulopathy appeared to be lower (1/60, 1.7%). By comparison, several features of the randomized controlled trial should be noted: (1) large volume paracentesis was the most common type of invasive procedure, which carried a relatively low risk of bleeding (19/60, 31.7%) and (2) 58% (35/60) of patients received fresh frozen plasma or PLT transfusion before invasive procedures.
In this study, 33.9% of cirrhotic patients who underwent invasive procedures had coagulopathy. The incidence of major bleeding after invasive procedures was more frequent in patients with coagulopathy than those without coagulopathy. In contrast, De Pietri et al. indicated that post-procedural bleeding risk is not related to coagulopathy itself, but the occurrence of local procedure-related complications.[21] However, the following issues should be noted: (1) the number of patients included in De Pietri's trial was relatively small (n = 60); (2) all included patients were diagnosed with coagulopathy, and no control group without coagulopathy was established; (3) only one patient developed major bleeding after an invasive procedure which carried a low risk of bleeding, and therefore, the statistical power of the study is questionable.
Our study demonstrated that the presence of INR ≥1.5 alone was not significantly associated with an increased risk of major bleeding, which might confirm Baveno VI consensus recommendations that PT/INR might not be a reliable indicator of assessing the risk of major bleeding after invasive procedures in patients with cirrhosis.[3,5,8,13]
Our study also found that patients with coagulopathy had a significantly higher in-hospital mortality than those without coagulopathy. In addition, if patients with coagulopathy developed major bleeding after invasive procedures, the in-hospital mortality would be higher. In particular, INR ≥1.5, but not severe thrombocytopenia, was significantly associated with an increased in-hospital mortality. Therefore, INR could have a closer relationship with mortality of cirrhotic patients who carried out invasive procedures.
Our study has some drawbacks. First, this was a retrospective study, the data were not available for some patients, and selection bias was inevitable. Second, the cutoff values to define coagulopathy and severe thrombocytopenia were derived from a previous study carried out in a similar setting. However, there is no consensus regarding this definition.[7,13] Third, we did not assess other coagulation and fibrinolytic parameters. Fourth, international guidelines did not recommend the correction of INR and PLT by blood product transfusion.[23] The data regarding patients with cirrhosis who received the transfusion of blood products before invasive procedures were heterogeneous and not collected in our study. Unlike De Pietri's trial, we did not explore the significance of blood product transfusion before invasive procedures.
CONCLUSION
Assessment of bleeding risk is one of the most important challenges in clinical management of patients with liver diseases. Severe thrombocytopenia significantly increased the risk of major bleeding after invasive procedures in cirrhosis. INR ≥1.5 significantly increased in-hospital mortality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Muciño-Bermejo J, Carrillo-Esper R, Uribe M, Méndez-Sánchez N. Coagulation abnormalities in the cirrhotic patient. Ann Hepatol. 2013;12:713–24. [PubMed] [Google Scholar]
- 2.Northup PG, Caldwell SH. Coagulation in liver disease: A guide for the clinician. Clin Gastroenterol Hepatol. 2013;11:1064–74. doi: 10.1016/j.cgh.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Tripodi A, Primignani M, Mannucci PM. Abnormalities of hemostasis and bleeding in chronic liver disease: The paradigm is challenged. Intern Emerg Med. 2010;5:7–12. doi: 10.1007/s11739-009-0302-z. [DOI] [PubMed] [Google Scholar]
- 4.De Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–52. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Tripodi A. The coagulopathy of chronic liver disease: Is there a causal relationship withbleeding? No. Eur J Intern Med. 2010;21:65–9. doi: 10.1016/j.ejim.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Tripodi A, Caldwell SH, Hoffman M, Trotter JF, Sanyal AJ. Review article: The prothrombintime test as a measure of bleeding risk and prognosis in liver disease. Aliment Pharmacol Ther. 2007;26:141–8. doi: 10.1111/j.1365-2036.2007.03369.x. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Redy KR, et al. Coagulation disorders and hemostasis in liver disease: Pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039–46. doi: 10.1002/hep.21303. [DOI] [PubMed] [Google Scholar]
- 8.Mannucci PM. Abnormal hemostasis tests and bleeding in chronic liver disease: Are they related? No. J Thromb Haemost. 2006;4:721–3. doi: 10.1111/j.1538-7836.2006.01886.x. [DOI] [PubMed] [Google Scholar]
- 9.Reverter JC. Abnormal hemostasis tests and bleeding in chronic liver disease: Are they related? Yes. J Thromb Haemost. 2006;4:717–20. doi: 10.1111/j.1538-7836.2006.01887.x. [DOI] [PubMed] [Google Scholar]
- 10.Napolitano G, Iacobellis A, Merla A, Niro G, Valvano MR, Terracciano F, et al. Bleeding after invasive procedures is rare and unpredicted by platelet counts in cirrhotic patients with thrombocytopenia. Eur J Intern Med. 2017;38:79–82. doi: 10.1016/j.ejim.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Shah A, Amarapurkar D, Dharod M, Chandnani M, Baijal R, Kumar P, et al. Coagulopathy in cirrhosis: A prospective study to correlate conventional tests of coagulation and bleeding following invasive procedures in cirrhotics. Indian J Gastroenterol. 2015;34:359–64. doi: 10.1007/s12664-015-0584-1. [DOI] [PubMed] [Google Scholar]
- 12.Cocero N, Bezzi M, Martini S, Carossa S. Oral surgical treatment of patients with chronic liver disease: Assessments of bleeding and its relationship with thrombocytopenia and blood coagulation parameters. J Oral Maxillofac Surg. 2017;75:28–34. doi: 10.1016/j.joms.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Giannini EG, Greco A, Marenco S, Andorno E, Valente U, Savarino V. Incidence of bleeding following invasive procedures in patients with thrombocytopenia and advanced liver disease. Clin Gastroenterol Hepatol. 2010;8:899–902. doi: 10.1016/j.cgh.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Deng H, Qi X, Zhang Y, Peng Y, Li J, Guo X. Diagnostic accuracy of contrast-enhanced computed tomography for esophageal varices in liver cirrhosis: A retrospective observational study. J Evid Based Med. 2017;10:46–52. doi: 10.1111/jebm.12226. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Qi X, Deng H, Peng Y, Shao L, Ma J, et al. Coagulation tests with the risk of acute upper gastrointestinal bleeding in liver cirrhosis: A retrospective study. Gastroenterol Rep (Oxf) 2016;4:315–9. doi: 10.1093/gastro/gov059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Qi X, Peng Y, Deng H, Li J, Ning Z, et al. Association of umbilical hernia with volume of ascites in liver cirrhosis: A retrospective observational study. J Evid Based Med. 2016;9:170–80. doi: 10.1111/jebm.12225. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Qi X, De Stefano V, Hou F, Ning Z, Zhao J, et al. Epidemiology, risk Factors, and in-hospital mortality of venous thromboembolism in liver cirrhosis: A single-center retrospective observational study. Med Sci Monit. 2016;22:969–76. doi: 10.12659/MSM.896153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 19.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 20.Malloy PC, Grassi CJ, Kundu S, Gervais DA, Miller DL, Osnis RB, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2009;20:240–9. doi: 10.1016/j.jvir.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 21.De Pietri L, Bianchini M, Montalti R, De Maria N, Di Maira T, Begliomini B, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: A randomized, controlled trial. Hepatology. 2016;63:566–73. doi: 10.1002/hep.28148. [DOI] [PubMed] [Google Scholar]
- 22.Townsend JC, Heard R, Powers ER, Reuben A. Usefulness of international normalized ratio to predict bleeding complications in patients with end-stage liver disease who undergo cardiac catheterization. Am J Cardiol. 2012;110:1062–5. doi: 10.1016/j.amjcard.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–38. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]


