Abstract
Background/Aim:
We studied the expression of interleukin-17 and interleukin-22 in the serum and the lower esophageal sphincter (LES) in healthy individuals and in patients diagnosed with achalasia (AC) to gain a better understanding of the etiopathogenesis of AC.
Patients and Methods:
Our study comprised 14 randomly selected patients with AC who underwent peroral endoscopic myotomy and 14 randomly selected healthy individuals who served as controls. Venous blood samples were evaluated in all study subjects to detect the expression of interleukin-17 and interleukin-22 in the serum using an enzyme-linked immunosorbent assay. Immunohistochemistry studies were performed to evaluate LES myofilaments obtained from both groups, as well as from 12 patients diagnosed with a subendothelial non-invasive tumor and who had undergone submucosal tunneling endoscopic resection, to assess the expression of interleukin-17 and interleukin-22 in LES myofilaments.
Results:
Compared with that in the control group, the expression of interleukin-17 and interleukin-22 in the serum and LES, in patients with AC, was significantly increased and was positively correlated.
Conclusion:
Interleukin-17 and interleukin-22 are upregulated in the serum and LES in patients with AC, suggesting that both interleukin-17 and interleukin-22 are involved in the pathogenesis of AC, and that AC may be an immune-mediated inflammatory disease.
Keywords: Achalasia, autoimmune, interleukin-17, interleukin-22
INTRODUCTION
Achalasia (AC) is an esophageal motility disorder caused by a defective mechanism of lower esophageal sphincter (LES) relaxation secondary to weakening/disappearance of the esophageal peristaltic waves. Clinical manifestations of AC include dysphagia, vomiting, chest pain, heartburn, and weight loss, among others. This disease entity is primarily caused by a disorder of LES relaxation because of a reduction or complete absence of inhibitory neurons of the myenteric nerve plexus of LES.[1,2] Although this disease entity has been described and named as early as 300 years ago, the cause of inhibitory neuron impairment in the myenteric nerve plexus of LES remains undetermined. Infectious, autoimmune, and genetic factors may be closely related to the pathogenesis of AC. Presently, AC is understood to be a disease associated with a multifactorial etiology in which the infection causes inflammation of the myenteric nerve plexus, followed by activation of an inflammation-induced autoimmune response, particularly noted in individuals with genetic susceptibility. This sequence of events destroys the inhibitory neuron and causes AC.[3] Therefore, AC may be viewed as an immune-mediated inflammatory disease (IMID).
Interleukin-17 (IL-17) is considered a key mediator in the pathomechanisms involved with IMIDs. It promotes neutrophil aggregation and the activation of innate immune cells, enhances the function of B cells, and induces the release of inflammatory factors. Interleukin22 (IL-22) belongs to the Interleukin-10(IL-10) family, which participates in initiating the innate immune response to pathogens and regulates the production of antibodies and is closely related to the development of autoimmune diseases. Several Chinese and global studies have demonstrated that IL-17 and IL-22 play a key role in the pathogenesis of a large number of autoimmune diseases.[4,5,6,7,8]
As the two key mediators associated with autoimmune inflammatory diseases, the expression of IL-17 and IL-22 in patients with AC may play an important role in understanding the pathogenesis of AC. Few studies have described the expression and significance of IL-17 and IL-22 in patients with AC in China and globally. This study investigated the expression of IL-17 and IL-22 in the serum and LES in patients with AC and healthy individuals (controls), aiming to explore the significance of IL-17 and IL-22 in the pathogenesis of AC to gain a better understanding of the etiopathogenesis of AC.
PATIENTS AND METHODS
Participants
The experimental group (EXP) comprised 14 patients with AC who were randomly selected and underwent a peroral endoscopic myotomy (POEM) procedure at the Second Hospital of Hebei Medical University between May 2015 and December 2015 and included eight men and six women between 28 and 56 years of age (median age 41.5 years). The diagnosis of AC had been confirmed in all patients following assessment of preoperative clinical symptoms, upper gastrointestinal imaging, high-resolution esophageal manometry and endoscopy. We confirmed that AC did not exist concomitantly with other systemic diseases in any patients. No immunosuppressant had been used in any patients within a month before sample collection, and no symptoms of infection were observed in any patients. Peripheral blood samples were obtained from each patient before and during the POEM. In addition, intraoperatively, LES was exposed by establishing an esophageal submucosal tunnel, and samples of LES myofilaments were obtained using a biopsy forceps. The control group (CON) comprised 14 healthy individuals including eight men and six women between 28 and 58 years of age (median age 43.5 years) from whom peripheral blood samples were obtained. LES tissue samples were obtained from 12 patients who underwent gastric cardia submucosal tunneling endoscopic resection (STER) for a subendothelial non-invasive tumor at the Second Hospital of Hebei Medical University, between May 2015 and December 2015 and included 7 men and 5 women between 32 and 64 years of age (median age 50.5 years). We confirmed that all 12 patients had not been diagnosed with any concomitant systemic diseases, had not been administrated immunosuppressants or other drugs, and had not reported the occurrence of any infectious disease within a month before enrollment in our study. During STER, the tumor masses were removed through the established tunnel, and LES myofilaments were exposed and samples were obtained using a biopsy forceps. There was no significant difference between groups EXP and CON with respect to gender and age (P < 0.05).
Reagents, kits, and antibodies
IL-17 Human ELISA Kit and IL-22 Human ELISA Kit were purchased from Abcam (Cambridge, UK). IL-17 polyclonal antibody and IL-22 polyclonal antibody were purchased from Bioworld Technology (Minnesota, CA, USA). Reagents were all purchased from Hebei Bio-High Technology Company (Hebei, China).
Determination of serum concentrations of IL-17 and IL-22
We obtained a fasting sample of 4 mL of peripheral venous blood from each patient and control and placed it into a pyrogen-free coagulation tube. The tube was allowed to stand for approximately 30 min to allow the sample to coagulate, followed by centrifugation at 3000 rpm. Serum was removed from the clot as soon as possible after clotting and separation. Aliquots of serum samples were stored at −20°C and thawed several times, and the human IL-17 and IL-22 levels were determined. All reagents, working standards, and serum samples were prepared. The number of microplate strips required to test the desired number of serum samples plus appropriate number of wells needed for running blanks and standards were determined. The microplate was washed twice with approximately 400 μL1× Wash Buffer per well with thorough aspiration of microplate contents between washes. After the final wash step, wells were emptied and microplate was tapped on absorbent pad or paper towel to remove excess 1× Wash Buffer. The microplate strips were used immediately after washing. About 100 μL of each standard dilution was pipetted into appropriate wells, 50 μL of sample diluent was pipetted to sample wells, 50 μL of each sample was pipetted to appropriate wells, and 50 μL of biotin conjugate was pipetted to all wells. They were covered with adhesive film and incubated at room temperature (18°C to 25°C) for 2 h (microplate can be incubated on a shaker set at 400 rpm). Adhesive film was removed and wells were emptied. Microplate strips were washed four times. About 100 μL of streptavidin–horseradish peroxidase (HRP) was added to all wells, including the blank wells. They were covered with adhesive film and incubated at room temperature (18° C to 25° C) for 1 h (microplate can be incubated on a shaker set at 400 rpm). Adhesive film was removed and wells were emptied. Microplate strips were washed four times. About 100 μL of TMB substrate solution was added to all wells. The microplate strips were incubated at room temperature (18°C to 25°C) for 10 min. Direct exposure to intense light was avoided. The enzyme reaction was stopped by adding 100 μL of Stop solution into each well. Absorbance of each microplate was read on a spectrophotometer using 450 nm as the primary wave length.
Immunohistochemistry
Each LES myofilament sample was fixed in 10% formalin immediately after it was obtained, followed by dehydration, waxing, and embedding. The wax block was sliced in a 4mm continuous slice. It was incubated with blocking buffer (normal goat serum at room temperature for 20 min). The goat serum was discarded, and IL-17 and IL-22 polyclonal antibodies were dropped with dilution in phosphate-buffered saline (PBS; 0.01M PBS, pH 7.4). The sections were incubated for 2 h at 37°C. These were rinsed in PBS-T (3 × 5 min). The goat anti-mouse IgG was dropped, and the sections were incubated for 30 min at 37°C. They were rinsed in PBS-T (3 × 5 min) and incubated with S-A/HRP at 37°C for 30 min, followed by rinsing (3 × 5 min) in PBS-T. Coloration was done with 3,3-diaminobenzidin (DAB) and kept at room temperature without light for 10 min. Coloration was finished with distilled water. It was stained with hematoxylin. It was then dehydrated, cleared and mounted with neutral gums. The negative control group was carried out with the same steps as described above, but IL-17 and IL-22 polyclonal antibodies were replaced by PBS. Immunohistochemistry evaluation was performed to detect the expression of IL-17 and IL-22 in LES. A multi-function true color cell image analysis system (Image-Pro) was used to analyze the positive expression of IL-17 and IL-22, and their intensities were calculated and expressed as the integrated optical density.
Statistical analysis
SPSS19.0 software IBM (New York, USA) was used for all statistical analysis and data processing. The data were expressed as means ± standard deviations (± s). The expression of IL-17 and IL-22 in the peripheral blood of the studied groups was compared using t-test of independent sample, with P < 0.05 considered statistically significant. The expression of IL-17 and IL-22 in LES between the groups was compared using t-test of independent sample, with P < 0.05 considered statistically significant. Pearson's test was used to detect the possible correlation between the expression of IL-17 and IL-22 in the serum and that in LES, and the correlation coefficient was expressed as r.
RESULTS
Expression of IL-17 and IL-22 in serum
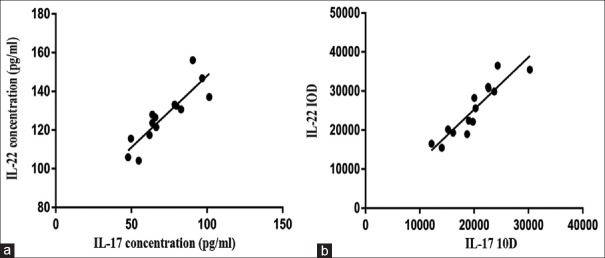
The expression of IL-17 in the serum of EXP is 71.69 ± 16.94 pg/mL and it was 46.90 ± 13.86 pg/mL in CON. Compared with that in CON, the expression of IL-17 in the serum of EXP was significantly increased the two groups of comparison have a significant difference (P < 0.0001). The expression of IL-22 in the serum of EXP is 127.11 ± 14.27 pg/mL and it was 49.88 ± 13.37 pg/mL in CON. Compared with that in CON, the expression of IL-22 in the serum of EXP was significantly increased the two groups of comparison have a significant difference (P < 0.0001) [Table 1]. The expression of IL-17 and IL-22 in serum of AC was positively correlated (r = 0.876, P < 0.0001) [Figure 1a].
Table 1.
Expressions of IL-17 and IL-22 in serum (pg/mL)

Figure 1.
(a) The correlation between IL-17 and IL-22 in serum of AC. (b) The correlation between IL-17 and IL-22 in LES of AC
Expression of IL-17 and IL-22 in LES
The expression of IL-17 is mainly in the cytoplasm and nucleus [Figure 2b]. The IOD value of IL-17 in AC group was 19913.27 ± 4718.99, and it was 5111.80 ± 1223.38 in CON (The integral optical density value is the sum of the optical density of each pixel within the measured scope). The expression of IL-17 in LES myofilaments of EXP was higher [Table 2] than that observed in CON the two groups of comparison have a significant difference (P < 0.0001). The expression of IL-22 is mainly in the cytoplasm and nucleus [Figure 2d]. The IOD value of IL-22 in AC group was 25193.05 ± 6879.05, and it was 6563.54 ± 2127.91 in CON. The expression of IL-22 in LES myofilaments of EXP was significantly higher than that observed in CON [Table 2] the two groups of comparison have a significant difference (P < 0.0001). The expression of IL-17 and IL-22 in LES of AC was positively correlated (r = 0.916, P < 0.0001) [Figure 1b].
Figure 2.
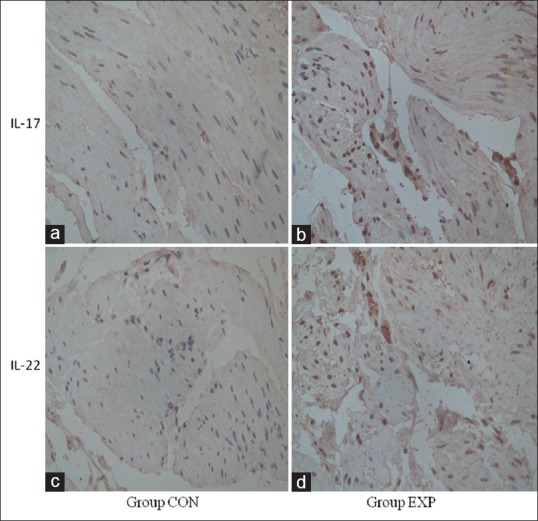
(a) Immunohistochemistry of IL-17 in LES of control group (×400). (b) Immunohistochemistry of IL-17 in LES of AC (×400). (c) Immunohistochemistry of IL-22 in LES of control group (×400). (d) Immunohistochemistry of IL-22 in LES of AC (×400)
Table 2.
Expressions of IL-17 and IL-22 in LES (IOD)

DISCUSSION
Although the incidence of AC is relatively low as an esophageal motility disorder,[9,10] it produces a long-term impact on a patient's quality of life. To date, there is no complete effective radical treatment available; thus, in recent times, this disease entity has been gaining attention from researchers in China, and globally.
Presently, infection, autoimmune, and genetic factors have been considered among the etiological agents involved in the incidence and progress of AC. However, over the past few decades, a large body of research in China and even globally has demonstrated inconsistent results regarding the relationship between AC and infection.[11,12,13] In fact, a few studies have not detected any relationship between infection and AC.[14] To date, there is inadequate evidence available to prove that AC is associated with present or previous infection. Therefore, it could be reasoned that infection may function as a disease initiator in the pathogenesis of AC, and the immune-mediated inflammatory response might be the definitive causative factor leading to impairment of inhibitory neurons. Therefore, AC is likely to be an IMID. A number of reports from China and abroad have described an association between AC and a variety of IMIDs.[15,16,17] As discussed earlier, AC has been observed to be closely related to other IMIDs, and a large number of immune complexes have been identified in patients with AC. Storch et al.[18] reported in a study comprising eight patients with AC and six controls, that terminal complement complex C5b-9 was detected in LES myofilaments in all patients with AC. Moreover, two patients among these eight also showed the presence of immunoglobulin M, whereas only one control showed the presence of C5b-9. In addition, five patients with AC demonstrated positive results for the presence of complement C9 in their LES myofilament samples, whereas all members of the control group demonstrated negative results, suggesting that activation of the complement system may be related to the autoimmune component of AC. In addition, Storch et al. reported the presence of antibodies against the myenteric plexus in the serum of four patients with AC among the eight studied, whereas the control group did not show these antibodies. Several other studies have also reported the presence of anti-neuronal antibodies.[16,19] In a recent study describing 14 patients with AC, Furuzawa-Carballeda et al.[13] reported that all patients with AC demonstrated positive myenteric plexus antibodies, whereas members of the control group demonstrated negative results. Furthermore, a large number of immune cells and inflammatory factors were also identified in the serum and LES of patients with AC. Similarly, several studies performed in China and globally have reported the local infiltration of a large number of eosinophils, lymphocytes, inflammatory cells, and inflammatory factors.[20,21] The above-mentioned studies support the hypothesis that AC may be an IMID.
IL-17, a key mediator in IMIDs, promotes neutrophil aggregation and activation of innate immune cells, enhances the function of B cells, and induces the release of inflammatory factors. IL-22, as a member of the IL-10 family, initiates the innate immune response to pathogens in vivo, regulates the production of antibodies, and is also closely related to IMIDs. IL-17 and IL-22 have been identified by many Chinese and global studies as being involved in the pathogenesis of several IMIDs and are known to play an important role in various autoimmune diseases. Therefore, this study examined the significance of the expression of IL-17 and IL-22 in the serum and LES of patients with AC and healthy controls and discussed the relationship between the expression of these two inflammatory biomarkers and the pathogenesis of AC, aiming to explore the possible etiopathogenesis of AC.
Relationship between AC and IL-17
As an important mediator of the inflammatory response, IL-17 plays a key role in the defense mechanism of the body against infection and is also closely related to chronic inflammatory injury and autoimmune regulation. IL-17 can promote the release of inflammatory factors from monocytes and also promote neutrophil aggregation and activation of various immune cells. Therefore, overexpression of IL-17 is known to cause chronic inflammatory injury in vivo.
Increased serum levels of IL-17 are reflected in a variety of autoimmune diseases. For example, Menon et al.[22] have demonstrated significantly increased levels of T-helper 17 (Th17) and IL-17 + T cells in the peripheral blood and synovial fluid of patients diagnosed with rheumatoid arthritis (RA), and these were observed to be associated with disease activity. In studies performed on patients diagnosed with multiple sclerosis, the expression of IL-17 was observed to be significantly increased in the brain tissue of patients.[23,24] It has also been shown that the expression of inflammatory mediators such as Th17 and IL-17 is significantly increased in the histopathological tissue sections obtained from patients with Crohn's disease and that these inflammatory biomarkers are associated with the activity of Crohn's disease.[25,26,27] Similarly, Kristensen et al.[7] observed an increased expression of IL-17 in patients with autoimmune thyroid disease. Aldahlawi et al.[28] reported that serum levels of IL-17 were significantly higher in 44 patients diagnosed with systemic lupus erythematosus than those observed in 20 healthy controls, and that these serum levels were associated with the activity of this disease. These and other related studies demonstrate that IL-17 is closely related to a variety of IMIDs.
In this study, we found that IL-17 was upregulated in the serum and LES of EXP compared with CON, suggesting that IL-17 may be associated with the etiopathogenesis of AC. On the basis of previous studies performed, we identified the pro-inflammatory effect of IL-17 and its close relationship with a variety of IMIDs; thus, the results of this study suggested that AC may be an autoimmune-mediated neuronal inflammatory injury, which is in agreement with the results of previous studies related to AC.
Relationship between AC and IL-22
IL-22 is a member of the IL-10 family and is primarily produced by/derived from Th17, although other cells such as Th22 or natural killer cells can also express IL-22. As a cytokine, IL-22 is closely related to inflammatory and autoimmune diseases. In this study, the expression of IL-22 in the serum and LES myofilaments of EXP was observed to be significantly increased compared with that in CON, indicating that IL-22 is closely related to the incidence of AC, which is in agreement with the results demonstrated by previous studies.
Presently, there is limited research available regarding the relationship between IL-22 and the etiopathogenesis of AC, and the exact role of IL-22 in the causation and development of AC remains unclear. However, previous studies relating to IL-22 and its role in other autoimmune and inflammatory diseases can provide a better understanding of its association with AC. The etiology of Guillain-Barre syndrome (GBS) is now attributed to viral infection-induced activation of immune cells, which leads to an immune-mediated inflammatory injury of peripheral nerves, similar to the mechanism that is currently presumed to be the etiology of AC. Li et al.[29] examined the levels of IL-17 and IL-22 in the cerebrospinal fluid and plasma of 22 patients with GBS and 18 healthy controls and observed that IL-17 and IL-22 are significantly upregulated in the cerebrospinal fluid and plasma of patients with GBS, indicating that these two cytokines are closely linked with the etiology of GBS. Human autoimmune myasthenia gravis (MG) is an antibody-mediated, T-cell-dependent, organ-specific autoimmune disease characterized by neuromuscular junction damage as its primary pathological feature. A large number of studies have shown a close association between IL-22 and MG.[30,31] Studies have shown that IL-22 is a key cytokine associated with the pathogenesis of RA, a well-known chronic autoimmune disease. For example, Kim et al.[32] reported in a study that the concentration of IL-22 was higher in the serum and articular effusion in patients with RA than that observed in the control group. These studies suggest that IL-22 is closely related to autoimmune inflammatory diseases, which also provides a new concept in this study to discuss and determine the relationship between IL-22 and AC.
Correlation between IL-17 and IL-22 in AC
The results of this study showed that both IL-17 and IL-22 are positively correlated in the serum and LES myofilaments of patients with AC and that IL-17 and IL-22 are concomitantly involved in the pathogenesis of AC, which is supported by the results obtained from previous studies. Previous studies have shown, both at the protein and the messenger RNA levels, that helper T cells that can express IL-17 can also produce IL-22, and that both these inflammatory cytokines (IL-17 and IL-22) are regulated by transforming growth factor β and IL-6. This increases the possibility of a synergistic effect of IL-17 and IL-22.[33] Moreover, IL-17 and IL-22 can simultaneously induce a tissue inflammatory response, and several autoimmune diseases are known to show an increased expression of both these inflammatory biomarkers. For example, the expression of IL-17 and IL-22 is significantly increased in the peripheral blood of patients diagnosed with acute generalized rash-type impetigo, and both these can induce production of IL-8, thereby promoting neutrophil aggregation in the epidermis of such patients.[34] A few studies have shown that recombinant human IL-17A can also regulate the pro-inflammatory and tissue protective effects of IL-22, as well as the expression of IL-22. An inflammatory model and an airway injury model have demonstrated that IL-17A can regulate the tissue-protective or the pro-inflammatory role of IL-22.[35] These studies provide a basis for the reasoning that IL-17 and IL-22 may play a synergistic role in the pathogenesis of AC.
In this study, we determined that IL-17 and IL-22 are closely related to AC. These two inflammatory biomarkers play important roles in the pathogenesis of AC, suggesting that AC could be an auto IMID with IL-17/IL-22 being actively involved in its etiopathogenesis. This study expands the idea of AC research, contributing to research regarding the etiology of AC and provides new perspectives that would aid in the clinical diagnosis and treatment of AC. However, the pathomechanism that could explain the synergistic effect of IL-17 and IL-22 in the pathogenesis of AC remains unknown, and this subject requires further studies and discussion.
CONCLUSION
Interleukin-17 and interleukin-22 are upregulated in the serum and LES in patients with AC, suggesting that both interleukin-17 and interleukin-22 are involved in the pathogenesis of AC, and that AC may be an immune-mediated inflammatory disease (IMID).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This study was conducted in accordance with the Declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Hebei Medical University. Written informed consent was obtained from all participants.
REFERENCES
- 1.Gockel I, Bohl JR, Junginger T. Achalasia: New insights in pathogenesis. Am J Gastroenterol. 2006;101:202–3. doi: 10.1111/j.1572-0241.2006.00393_4.x. [DOI] [PubMed] [Google Scholar]
- 2.Boeckxstaens GE. Achalasia. Best Pract Res Clin Gastroenterol. 2007;21:595–608. doi: 10.1016/j.bpg.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Gockel HR, Schumacher J, Gockel I, Lang H, Haaf T, Nöthen MM. Achalasia: Will genetic studies provide insights? Hum Genet. 2010;128:353–64. doi: 10.1007/s00439-010-0874-8. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Jin T, Zhang HL, Yu H, Meng F, Concha Quezada H, et al. Circulating Th17, Th22, and Th1 cells are elevated in the Guillain-Barre syndrome and downregulated by IVIg treatments. Mediators Inflamm. 2014;2014:740947. doi: 10.1155/2014/740947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang ZY, Zhang Z, Fauser U, Schluesener HJ. Improved outcome of EAN, an animal model of GBS, through amelioration of peripheral and central inflammation by minocycline. J Cell Mol Med. 2009;13:341–51. doi: 10.1111/j.1582-4934.2008.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciccia F, Guggino G, Rizzo A, Ferrante A, Raimondo S, Giardina A, et al. Potential involvement of IL-22 and IL-22-producing cells in the inflamed salivary glands of patients with Sjogren's syndrome. Ann Rheum Dis. 2012;71:295–301. doi: 10.1136/ard.2011.154013. [DOI] [PubMed] [Google Scholar]
- 7.Kristensen B. Regulatory B and T cell responses in patients with autoimmune thyroid disease and healthy controls. Dan Med J. 2016;63:pii: B5177. [PubMed] [Google Scholar]
- 8.Stanisavljević S, Lukić J, Momčilović M, Miljković M, Jevtić B, Kojić M, et al. Gut-associated lymphoid tissue, gut microbes and susceptibility to experimental autoimmune encephalomyelitis. Benef Microbes. 2016;7:363–73. doi: 10.3920/BM2015.0159. [DOI] [PubMed] [Google Scholar]
- 9.Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet. 2014;383:83–93. doi: 10.1016/S0140-6736(13)60651-0. [DOI] [PubMed] [Google Scholar]
- 10.Marlais M, Fishman JR, Fell JM, Haddad MJ, Rawat DJ. UK incidence of achalasia: An 11-year national epidemiological study. Arch Dis Child. 2011;96:192–4. doi: 10.1136/adc.2009.171975. [DOI] [PubMed] [Google Scholar]
- 11.Facco M, Brun P, Baesso I, Costantini M, Rizzetto C, Berto A, et al. T cells in the myenteric plexus of achalasia patients show a skewed TCR repertoire and react to HSV-1 antigens. Am J Gastroenterol. 2008;103:1598–609. doi: 10.1111/j.1572-0241.2008.01956.x. [DOI] [PubMed] [Google Scholar]
- 12.Lau KW, McCaughey C, Coyle PV, Murray LJ, Johnston BT. Enhanced reactivity of peripheral blood immune cells to HSV-1 in primary achalasia. Scand J Gastroenterol. 2010;45:806–13. doi: 10.3109/00365521003587804. [DOI] [PubMed] [Google Scholar]
- 13.Furuzawa-Carballeda J, Aguilar-León D, Gamboa-Dominguez A, Valdovinos MA, Nuñez-Álvarez C, Martín-del-Campo LA, et al. Achalasia—An autoimmune inflammatory disease: A cross sectional study. J Immunol Res. 2015;2015:729217. doi: 10.1155/2015/729217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birgisson S, Galinski MS, Goldblum JR, Rice TW, Richter JE. Achalasia is not associated with measles or known herpes and human papilloma viruses. Dig Dis Sci. 1997;42:300–6. doi: 10.1023/a:1018805600276. [DOI] [PubMed] [Google Scholar]
- 15.Quidute AR, Freitas EV, Lima TG, Feitosa AM, Santos JP, Correia JW. Achalasia and thyroid disease: Possible autoimmune connection? Arq Bras Endocrinol Metabol. 2012;56:677–82. doi: 10.1590/s0004-27302012000900013. [DOI] [PubMed] [Google Scholar]
- 16.Kraichely RE, Farrugia G, Pittock SJ, Castell DO, Lennon VA. Neural autoantibody profile of primary achalasia. Dig Dis Sci. 2010;55:307–11. doi: 10.1007/s10620-009-0838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Booy JD, Takata J, Tomlinson G, Urbach DR. The prevalence of autoimmune disease in patients with esophageal achalasia. Dis Esophagus. 2012;25:209–13. doi: 10.1111/j.1442-2050.2011.01249.x. [DOI] [PubMed] [Google Scholar]
- 18.Storch WB, Eckardt VF, Junginger T. Complement components and terminal complement complex in oesophageal smooth muscle of patients with achalasia. Cell Mol Biol (Noisy-le-grand) 2002;48:247–52. [PubMed] [Google Scholar]
- 19.Moses PL, Ellis LM, Anees MR, Ho W, Rothstein RI, Meddings JB, et al. Antineuronal antibodies in idiopathic achalasia and gastro-oesophageal reflux disease. Gut. 2003;52:629–36. doi: 10.1136/gut.52.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sodikoff JB, Lo AA, Shetuni BB, Kahrilas PJ, Yang GY, Pandolfino JE. Histopathologic patterns among achalasia subtypes. Neurogastroenterol Motil. 2016;28:139–45. doi: 10.1111/nmo.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cools-Lartigue J, Chang SY, McKendy K, Mayrand S, Marcus V, Fried GM, et al. Pattern of esophageal eosinophilic infiltration in patients with achalasia and response to Heller myotomy and Dor fundoplication. Dis Esophagus. 2013;26:766–75. doi: 10.1111/j.1442-2050.2012.01385.x. [DOI] [PubMed] [Google Scholar]
- 22.Menon B, Gullick NJ, Walter GJ, Rajasekhar M, Garrood T, Evans HG, et al. Interleukin-17+CD8+T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol. 2014;66:1272–81. doi: 10.1002/art.38376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durelli L, Conti L, Clerico M, Boselli D, Contessa G, Ripellino P, et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann Neurol. 2009;65:499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- 24.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–55. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang W, Su J, Zhang X, Cheng X, Zhou J, Shi R, et al. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflamm Res. 2014;63:943–50. doi: 10.1007/s00011-014-0768-7. [DOI] [PubMed] [Google Scholar]
- 26.Sahin A, Calhan T, Cengiz M, Kahraman R, Aydin K, Ozdil K, et al. Serum interleukin 17 levels in patients with Crohn's disease: Real life data. Dis Markers. 2014;2014:690853. doi: 10.1155/2014/690853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueno A, Jijon H, Chan R, Ford K, Hirota C, Kaplan GG, et al. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+T cells and defective suppressive function of circulating Foxp3+regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:2522–34. doi: 10.1097/MIB.0b013e3182a85709. [DOI] [PubMed] [Google Scholar]
- 28.Aldahlawi AM, Elshal MF, Damiaiti LA, Damanhori LH, Bahlas SM. Analysis of CD95 and CCR7 expression on circulating CD4(+) lymphocytes revealed disparate immunoregulatory potentials in systemic lupus erythematosus. Saudi J Biol Sci. 2016;23:101–7. doi: 10.1016/j.sjbs.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S, Yu M, Li H, Zhang H, Jiang Y. IL-17 and IL-22 in cerebrospinal fluid and plasma are elevated in Guillain-Barre syndrome. Mediators Inflamm. 2012;2012:260473. doi: 10.1155/2012/260473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche JC, Capablo JL, Larrad L, Gervas-Arruga J, Ara JR, Sánchez A, et al. Increased serum interleukin-17 levels in patients with myasthenia gravis. Muscle Nerve. 2011;44:278–80. doi: 10.1002/mus.22070. [DOI] [PubMed] [Google Scholar]
- 31.Dalakas MC. The role of high-dose immune globulin intravenous in the treatment of dermatomyositis. Int Immunopharmacol. 2006;6:550–6. doi: 10.1016/j.intimp.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Kim KW, Kim HR, Park JY, Park JS, Oh HJ, Woo YJ, et al. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum. 2012;64:1015–23. doi: 10.1002/art.33446. [DOI] [PubMed] [Google Scholar]
- 33.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabashima R, Sugita K, Sawada Y, Hino R, Nakamura M, Tokura Y. Increased circulating Th17 frequencies and serum IL-22 levels in patients with acute generalized exanthematous pustulosis. J Eur Acad Dermatol Venereol. 2011;25:485–8. doi: 10.1111/j.1468-3083.2010.03771.x. [DOI] [PubMed] [Google Scholar]
- 35.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]